Abstract
In adrenal chromaffin cells, a rise in cytosolic calcium concentration ([Ca2+]i) is a key event in the triggering of catecholamine exocytosis after splanchnic nerve activation. Action potential- or nicotine-induced [Ca2+]i transients are well described in individual chromaffin cells, but whether they remain spatially confined to the stimulated cell or propagate to adjacent cells is not yet known. To address this issue, the spatiotemporal organization of electrical and associated Ca2+ events between chromaffin cells was investigated using the patch-clamp technique and real-time confocal imaging in rat acute adrenal slices. Spontaneous or electrically evoked action potential-driven [Ca2+]i transients were simultaneously detected in neighboring cells. This was likely attributable to gap junction-mediated electrotonic communication, as shown by (1) the bidirectional reflection of voltage changes monitored between cell pairs, (2) Lucifer yellow (LY) diffusion between cells exhibiting spontaneous synchronized [Ca2+]i transients, and (3) the reduction of LY diffusion using the uncoupling agent carbenoxolone. Furthermore, transcripts encoding two connexins (Cx36 and Cx43) were found in single chromaffin cells. This gap junctional coupling was activated after a synaptic-like application of nicotine that mediated synchronous multicellular [Ca2+]i increases. In addition, nicotinic stimulation of a single cell triggered catecholamine release in coupled cells, as shown by amperometric detection of secretory events. Functional coupling between chromaffin cells in situ may represent an efficient complement to synaptic transmission to amplify catecholamine release after synaptic stimulation of a single excited chromaffin cell.
Keywords: electrical propagation, gap junction coupling, rat adrenal slices, chromaffin cells, real-time confocal microscopy, catecholamine release, nicotinic stimulation
In adrenal tissue, the physiological stimulus for catecholamine secretion from chromaffin cells is the synaptic release of acetylcholine, which increases membrane excitability leading to cytosolic Ca2+elevations (for review, see Douglas, 1968; Kidokoro and Ritchie, 1980;Wakade, 1981). To date, the relationships between electrical activity, [Ca2+]i increases, and exocytosis underlying stimulation-secretion coupling have been mainly studied at the single cell level, either in dissociated chromaffin cells in culture or more recently in chromaffin cells in situ using acute slice preparations (Voets et al., 1999; Albillos et al., 2000;Voets, 2000). Because of the anatomical organization of chromaffin cells into discrete cell complexes within the medulla (Hillarp, 1946), it is, however, of physiological interest to characterize stimulus-secretion coupling at the cell cluster level. It is currently proposed that each cluster functions as an independent unit to release catecholamines after activation of the splanchnic nerve (Iijima et al., 1992). But whether each chromaffin cell exocytoses in an autonomous manner after co-activation of its own synaptic inputs (Coupland, 1965) and/or whether chromaffin cells may also communicate between themselves to spatially and temporally harmonize their intracellular transduction signals remains an open question. As reported by Kajiwara et al. (1997), the spreading of electrical activity between chromaffin cells induced by transmural stimulation of the splanchnic nerve would be mostly dependent on nerve fiber activity rather than cell-to-cell coupling, leading to simultaneous excitation of all the chromaffin cells belonging to the same cluster. However, fast cell-to-cell communication mechanisms such as gap junction-mediated coupling may also be involved in the generation of simultaneous co-activation in adjoining chromaffin cells. Propagation of action potentials between chromaffin cells via electronic coupling was previously postulated to amplify the secretory signal to ensure massive release of catecholamines in the intact gland (Ceña et al., 1983). More recently, a junctional pathway coupling chromaffin cells in situ has been reported to potentially underlie the simultaneous firing of a cell cluster (Moser, 1998).
In the present study, we addressed the issue of the involvement of gap junctions between chromaffin cells and their role in catecholamine release. We therefore investigated the spatiotemporal organization of both electrical activity and ensuing [Ca2+]i increases between chromaffin cells in rat adrenal acute slices after single cell electrical or cholinergic stimulation. We showed that [Ca2+]i increases induced in response to spontaneous or evoked action potentials or to iontophoretic application of nicotine can be concomitantly observed in several chromaffin cells adjoining the stimulated one. This is likely to involve a gap junction-mediated pathway. Moreover, single cell stimulation led to spike-like secretory events resulting from catecholamine exocytosis in neighboring coupled cells. We propose here an additional mechanism mediating catecholamine secretion that might occur preferentially during episodes of low synaptic activity when only few chromaffin cells are synaptically stimulated.
MATERIALS AND METHODS
Adrenal slice preparation. Acute adrenal slices were prepared according to the technique previously described by Barbara and Takeda (1996). Briefly, the adrenal glands were removed from 12- to 16-week-old Wistar female rats that had been killed by decapitation after cervical dislocation. After keeping the glands in ice-cold saline for 2 min, a gland was glued onto an agarose cube and transferred to the stage of a vibratome (Microslicer, DTK-1000, D.S.K; Dosaka, Kyoto, Japan). Slices of 250 μm thickness were then cut with a razor blade and transferred to a storage chamber maintained at 32°C, containing Ringer's saline (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 12 glucose, buffered to pH 7.4. The saline was continuously bubbled with carbogen (95% O2 and 5% CO2). As reported for the anterior pituitary (Guérineau et al., 1998), acute adrenal slices were suitable for patch-clamp recording and Ca2+ signal imaging immediately after cutting. To obtain optical and/or electrophysiological recordings, slices were then transferred to a recording chamber attached to the stage of an upright microscope fitted with differential interference contrast optics (Axioskop FS; Zeiss, Le Pecq, France) and continuously superfused with Ringer's saline at 30°C.
Confocal imaging of cytosolic calcium. Ca2+ changes were routinely monitored with a real-time (30–480 frames/sec) confocal laser-scanning microscope equipped with an Ar–Kr laser (Odyssey XL with InterVision 1.5.1 software; Noran Instruments, Middleton, WI). Cells were viewed with a 63× 0.9 NA achroplan water-immersion objective (Zeiss). The largest detection slit (100 μm) of the confocal microscope was used for [Ca2+]i measurements, giving bright images with a 3.1 μm axial resolution. Slices were loaded with the Ca2+-sensitive fluorescent probe Oregon Green 488 BAPTA-1 by intermittent exposure to 15 μm Oregon Green 488 BAPTA-1 AM (Molecular Probes, Eugene, OR) for 20–30 min at 32°C, delivered onto a cell field with a blunt pipette (Bonnefont et al., 2000). Oregon Green 488 BAPTA-1 was excited through a 488 nm bandpass filter, and the emitted fluorescence was collected through a 515 nm barrier filter. To follow the time course of Oregon Green 488 BAPTA-1 emission changes, the bright overtime tool of the software package was applied to live images (120 images/sec, averaging four frames). Because Oregon Green 488 BAPTA-1 is a single-wavelength dye, its emission is a function of both intracellular Ca2+ and dye concentrations. [Ca2+]i changes were expressed as the F/Fminratio, where Fmin was the minimum fluorescence intensity measured during the recording (Mollard et al., 1995). No detectable difference was noted between slices used just after cutting or after several hours (up to 8 hr) in the storage chamber. Acquired data and images were then processed for analysis with IgorPro 3.16 (Wavemetrics, Lake Oswego, OR), NIH Image 1.6.0, and Adobe Photoshop 5.0.2 software.
Electrophysiology. All experiments were performed in the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were pulled to a resistance of 4–8 MΩ from borosilicate glass (1.5 mm outer diameter, 1.17 mm inner diameter) and filled with the following internal solution (in mm): 140 potassium-gluconate, 2 MgCl2, 1.1 EGTA, 5 HEPES, that was titrated to pH 7.2 with KOH. The membrane potential of a single chromaffin cell or cell pairs was recorded under current-clamp conditions using an EPC-9 dual patch-clamp amplifier (Heka Electronik, Lambrecht/Pfalz, Germany) and filtered at 3 kHz. Intercellular junctional currents were monitored under dual voltage-clamp conditions (Neyton and Trautmann, 1985) using a modified intrapipette solution (in mm: 140 Cs+-gluconate, 2 ATP, 2 MgCl2, 1.1 EGTA, 5 HEPES) and filtered off-line with a Kaiser low-pass filter (Igor Filter Design Laboratory software, version 3.1; Wavemetrics). For combined experiments in which membrane potential was simultaneously recorded with Ca2+ events, Oregon Green 488 BAPTA-1 in free acid form (10 μm) was directly added to the internal pipette solution. In some experiments, membrane potential was recorded in the perforated patch-clamp configuration using amphotericin B (0.5 mg/ml in the standard intrapipette solution), as described (Albillos et al., 2000). Cells with an access resistance >50 MΩ were discarded. Signals were acquired and analyzed using Pulse + PulseFit software (version 8.5; Heka Electronik) on a G4 Macintosh computer.
Dye transfer. The fluorescent dye Lucifer yellow (LY) (1 mm) was introduced into cells using sharp microelectrodes or via passive diffusion through the patch pipette. In this case, the cells were patched for few minutes before image acquisition. Dye transfer between co-active coupled cells was visualized with confocal microscopy using the 488 nm-centered wavelength of the laser beam.
Single cell RT-PCR. Single cell RT-PCR was performed as previously described (Lambolez et al., 1992). Patch pipettes were filled with 10 μl of autoclaved internal K+-gluconate solution. After breaking the patch, cell contents were collected by applying negative pressure to the patch pipette. The pipette's content was expelled into an Eppendorf microtube containing 20 U of ribonuclease (RNase) inhibitor (Roche, Meylan, France). The tube was then immediately frozen in liquid nitrogen and kept at −80°C until processed. The sample was denatured at 70°C for 5 min in the presence of 25 μmhexamers, and reverse transcription (RT) was performed in 20 μl of RT buffer containing: 200 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies, Cergy Pontoise, France), 20 U of RNase inhibitor, 10 mm dithiothreitol, and 0.6 mm of each dNTP (Roche), for 3 hr at 37°C. The reaction was stopped by heating at 95°C for 5 min. Because of the high amounts of dopamine β-hydroxylase (DβH) in chromaffin cells, a single PCR was sufficient to detect the messengers. However, the amplification of mRNA encoding connexins (Cxs) required a nested PCR. PCRs were performed using a T-gradient thermal cycler (Biometra, Göttingen, Germany). All PCR amplifications were achieved using 5–8 μl of RT product or first PCR product in 20 μl of PCR buffer containing 1.5 mm MgCl2, 0.2 mm dNTP, 1 μm of each primer, and 1U of DyNAzyme EXT polymerase (Finnzymes, Expoo, Finland). For the first PCR, the initial melt at 95°C (5 min) was followed by 35 cycles [92°C (1 min), 64°C (1 min), and 72°C (2 min)] and final extension at 72°C (7 min). For the unique DβH PCR and the nested Cx PCR, the protocol used was the following: after the initial melt at 95°C (2 min), 10 cycles were performed: 94°C (10 sec), 64°C for DβH or 57°C for Cxs (30 sec), 72°C (45 sec) followed by 40 identical cycles with 5 sec increments added to each cycle to an extension time (72°C), and a final extension at 72°C (7 min). For each experiment, a blank (without mRNA) and a positive control (total adrenal mRNA) were prepared. PCR products were submitted to electrophoresis on 2% agarose gel in Tris Borate EDTA buffer containing ethidium bromide for visualization. The primer sequences for specific amplifications of DβH (Melia et al., 1994), Cx36, and Cx43 (Oligo 4.0 software) were summarized in Table1. Pairs of primers contained an intronic sequence to discriminate cDNA amplification product versus genomic DNA amplification product. Note that for DβH and Cx36, the presence of an intronic sequence was inferred from the human and mouse sequences because rat cDNAs were not available.
Table 1.
Primer sequences used for the specific amplification of dopamine β-hydroxylase, connexin 43, and connexin 36 in single cells
| Primer sequence sense (5′–3′) | Primer sequence antisense (5′–3′) | Predicted product size (bp) | ||
|---|---|---|---|---|
| DβH | 1st PCR | AGTGCCGTGGATGATGGCTTCC | CAGCACTATTCTATGGAAGGGGGTC | 438 |
| Cx43 | 1st PCR | AACTTTGGCGGCGGCTTCAC | CAGGAAGGCCACCTCGAAGACAG | 560 |
| Nested PCR | TGGAGGGAAGGTGTGGCTG | TGAAGAGGATGCTGATGATGTAGG | 425 | |
| Cx36 | 1st PCR | ACAGCGATGGGGGAATGGAC | CACTTGGATGATGTAGAAGCGGG | 615 |
| Nested PCR | TCCTGTTGACTGTGGTGGTG | ACTTCTTATCTTCTCGCTTGCTC | 360 |
Real-time amperometric measurements of catecholamine release. Nicotine-driven catecholamine exocytosis from chromaffin cells was monitored by the electrochemical detection of secretory events (Chow et al., 1992). To perform single cell studies, carbon fibers with a 10 μm diameter tip and 50 μm length (WPI, Herts, UK) were chosen as previously reported (Barbara et al., 1998). The tip of the electrode was gently pressed against the cell surface. Amperometric currents were measured at a constant voltage of 800 mV with an EPC-9 patch-clamp amplifier and were analyzed and filtered using Igor Pro + Igor Filter Design Laboratory (Kaiser low-pass filter) software.
Test substances. To mimic a focal and brief stimulation, nicotine (nicotine chloride, 200 mm) was iontophoretically applied onto the cell of interest using a sharp microelectrode (100 MΩ if filled with acetate buffer), the tip of which was positioned near the cell. The concentration reported is that in the microelectrode. Nicotine was prepared from a stock solution in an acetate buffer (pH 4). At this pH value, nicotine was negatively charged and, consequently, could be delivered from the microelectrode after application of outward current pulses (50 nA intensity, 1 msec duration). Tetrodotoxin–cadmium-containing solution was pressure-ejected from an extracellular micropipette positioned in the vicinity of recorded cells. Nicotine, cadmium chloride, tetrodotoxin (TTX), Lucifer yellow (LY), and the gap junction blocker carbenoxolone were purchased from Sigma (St. Louis, MO).
Statistics. Numerical data are expressed as the mean ± SEM. Student's t test (paired or unpaired) was used to compare means. Percentages were compared using a contingency table and the χ2 test. Differences withp < 0.001 were considered significant.
RESULTS
Propagation of action potential-induced [Ca2+]i transients between neighboring chromaffin cells
Intercellular Ca2+ rises triggered by action potentials in a given chromaffin cell were imaged while simultaneously monitoring associated [Ca2+]i changes in neighboring cells. As illustrated in Figure1A, a 50 msec duration depolarizing current evoking a doublet of action potentials induced a simultaneous [Ca2+]i rise in the stimulated cell (cell 1) and in an adjacent cell (cell 2). The [Ca2+]i of other neighboring cells in the same optical plane remained at basal levels. The kinetics of both action potential-induced and propagated [Ca2+]i transients were not significantly different (time-to-peak: 160 ± 20 msec,n = 12 versus 150 ± 20 msec, n = 12, p > 0.001). However, the amplitude of the Ca2+ response in the unstepped cell was usually smaller than in the stimulated cell. The delay between the onset of both Ca2+ signals was very short (39.8 ± 9.4 msec; n = 12), leading to a speed of propagation of ∼500 μm/sec (based on a 20 μm distance between cell centers and the acquisition sample time). To determine whether the extent of propagation (one cell in the example shown in Fig. 1) depended on the amplitude of the [Ca2+]i rise in the stimulated cell, the depolarizing step duration was increased to 500 msec (Fig. 1B). A burst of 13 action potentials induced a larger [Ca2+]i increase but did not modify the number of responsive cells (n = 9 cell fields). Such a simultaneous [Ca2+]i increase between adjacent chromaffin cells (up to three cells in the same optical plane) on depolarization-evoked action potentials was observed in 39.5% of recorded cell fields (n = 15 of 38).
Fig. 1.
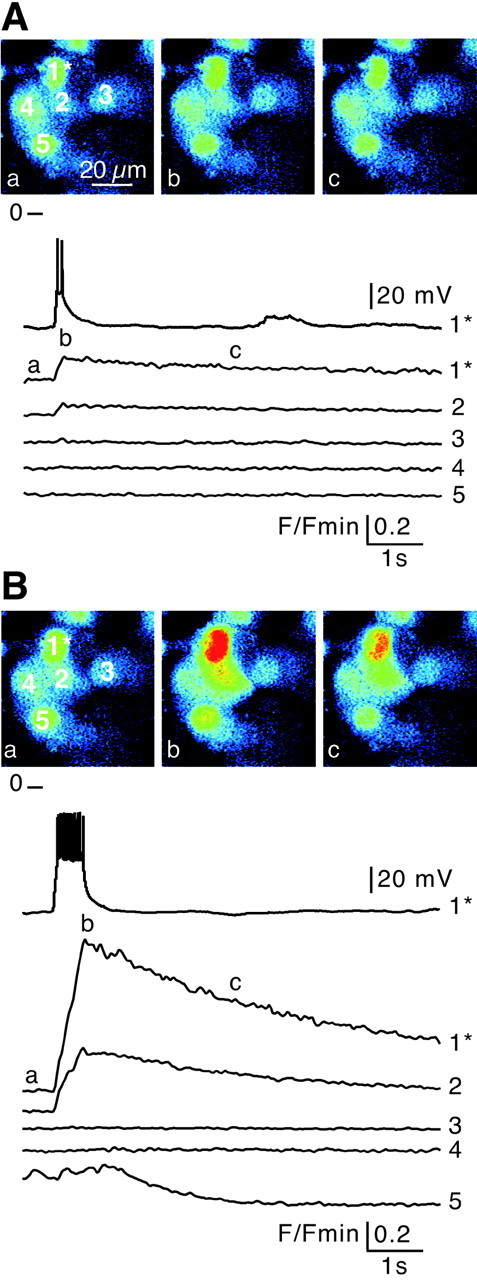
Propagation of action potential-induced [Ca2+]i transients between chromaffin cellsin situ. Electrical activity-driven multicellular [Ca2+]i increases were imaged by real-time scanning laser confocal imaging (120 images/sec with averaging 4 frames) in five chromaffin cells loaded with Oregon Green 488 BAPTA-1 as the Ca2+-sensitive fluorescent probe. Fluorescence emission changes were normalized to baseline fluorescenceF/Fmin. The stimulated cell is indicated by an asterisk. Each image corresponds to 10 averaged confocal images before (a), during (b), and after (c) depolarization. Action potentials were triggered by injecting depolarizing current into cell 1 (Vm = −75 mV). A brief depolarization (50 msec) inducing a doublet of action potentials (A) or a sustained depolarization (500 msec) evoking a burst of 13 action potentials (B) leads to a simultaneous transient [Ca2+]i increase in both the stimulated and a neighboring cell (cell 2). Note that cell 5 (B) spontaneously displayed a [Ca2+]i change during the recording that was not linked to the action potentials evoked in cell 1.
We then investigated whether simultaneous [Ca2+]i rises also occurred spontaneously between adjacent cells. As shown by time-lapse optical sequences of Oregon Green 488 BAPTA-1-loaded cells, ∼16% of chromaffin cells displaying spontaneous [Ca2+]i transients fired in synchrony with at least one neighboring cell (Fig.2A) (n= 195 cells from four different experiments). These spontaneously co-active chromaffin cells co-existed with asynchronous cells. Within a cluster, the synchronized cells were spatially confined (up to four cells in the same confocal plane). [Ca2+]i transients of co-active cells displayed similar kinetics (time-to-peak: 290 ± 65 msec vs 290 ± 61 msec for cell 1 and cell 2; n = 10 cell pairs; p > 0.001; paired t test). When [Ca2+]i transients were imaged for longer periods (>10 min), sequences of spontaneous synchronization–desynchronization were observed (data not shown).
Fig. 2.
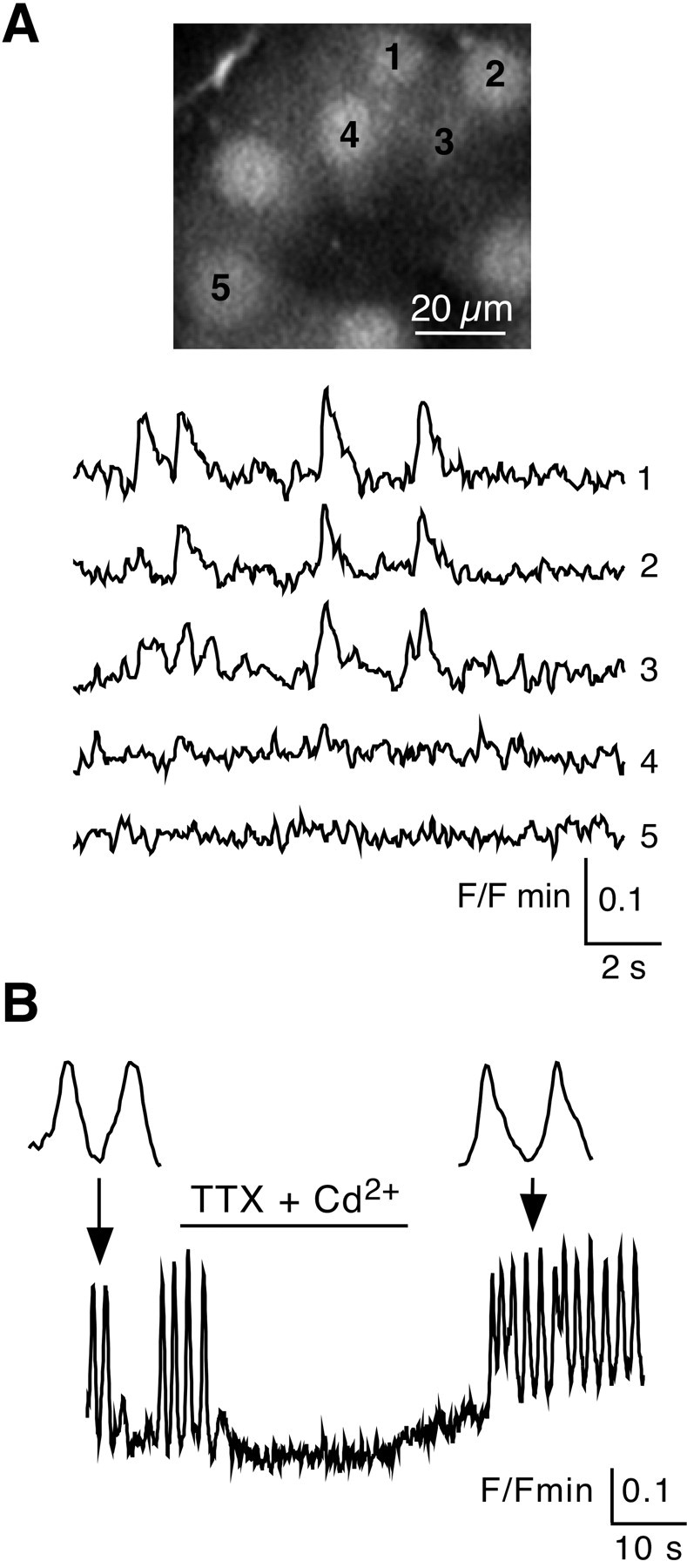
Synchronized spontaneous [Ca2+]i transients between chromaffin cells.A, Spontaneous [Ca2+]i changes were imaged in five chromaffin cells. Plots of relative Oregon Green 488 BAPTA-1 emission changes showing synchronized [Ca2+]i transients in three of five cells. Note that the two other cells remained silent. B, Reversible blocking effect of a 30 sec TTX (0.5 μm) + Cd2+ (0.5 mm) ejection on spontaneous [Ca2+]i transients. Insets,Detailed kinetics of two [Ca2+]i transients before and after application of blockers.
Synchronous and asynchronous [Ca2+]i transients probably resulted from spontaneous action potentials, because they were reversibly abolished in the presence of TTX (0.5 μm) + Cd2+ (0.5 mm), blockers of voltage-activated Na+ and Ca2+channels, respectively (Fig. 2B) (n = 22 of 29), TTX alone, or Cd2+ alone (n = 11 of 20 and 31 of 37, respectively, data not shown).
Taken together, these results indicate that in situ, either spontaneous or electrically evoked action potential-linked [Ca2+]i transients are not confined to the initially stimulated cells, but are transmitted to other chromaffin cells, indicating information transfer between adjoining cells.
Evidence for a gap junction-mediated electrical coupling
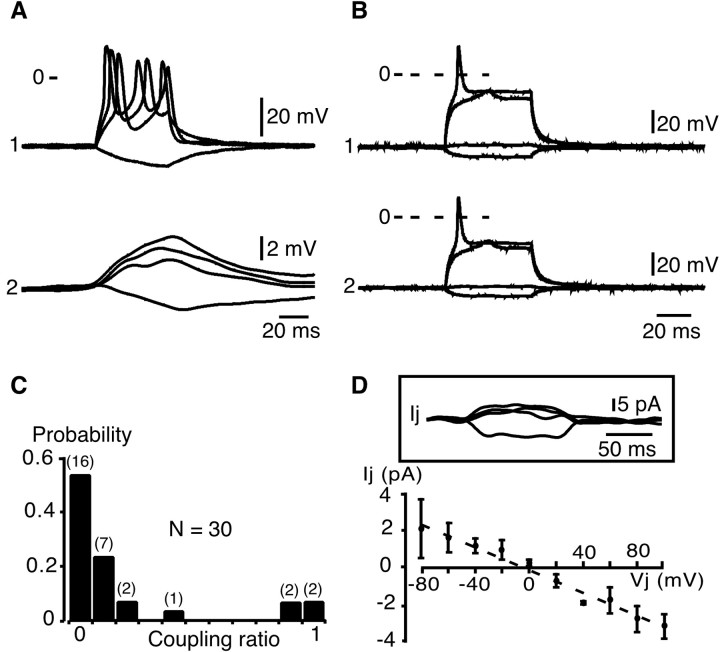
What are the mechanisms involved in the simultaneous [Ca2+]i rises occurring in neighboring chromaffin cells? Two cell-to-cell communication pathways (gap junctions or local release of compounds acting on neighboring cells) could explain this propagation. Two observations, (1) the rapid speed of propagation between two cells (500 μm/sec) and (2) the similar kinetics of both action potential-induced and propagated [Ca2+]i transients (Fig. 1), suggesting that action potentials are conducted to adjacent cells, support the hypothesis of junctional communication. To further confirm gap junctional coupling between chromaffin cells, the membrane potential of cell pairs was recorded in the whole-cell configuration with the dual patch-clamp technique (Fig. 3). The passive biophysical membrane properties of chromaffin cells in slices were: (1) resting membrane potential (−68 ± 1 mV;n = 172), input resistance (0.82 ± 0.04 GΩ; range, 0.13–3 GΩ; n = 172), and capacitance (11.64 ± 0.40 pF; n = 28). These results are in agreement with those reported by other groups (Barbara and Takeda, 1996; Kajiwara et al., 1997; Barbara et al., 1998). The fact that many chromaffin cells had a low membrane input resistance (≤500 MΩ in 32% of cells) was consistent with the idea that chromaffin cells could be coupled. In 40% of cell pairs (n = 68), the voltage changes in response to hyperpolarizing–depolarizing current injected in the stimulated cell were reflected in the unstepped cell (Fig.3A,B) and vice versa (data not shown). However, among the cell pairs, the response transmitted to the unstepped cell displayed variable amplitudes. In 75% of coupled pairs, the action potential was distorted, leading only to small depolarizations (Fig. 3A), whereas in the remaining pairs, the action potential was transmitted (Fig. 3B). Consequently, the coupling ratio exhibited a wide distribution range from 0.03 to 0.95 (mean 0.34 ± 0.10;n = 14 coupled pairs) (Fig. 3C). It is interesting to note that the coupling ratio value for a given cell pair remained constant independent of the amplitude or polarity of the current step. In accordance with gap junction-driven electrical coupling, junctional currents were recorded in 61% (8 of 13) of Cs+-loaded chromaffin cell pairs. Cell pairs were voltage clamped at −80 mV, and voltage steps were delivered from this potential with command pulses of both polarities to cell 1. The resulting junctional currents (Ij) recorded in the unstepped cell appeared to remain constant for the duration of the step (Fig. 3D, inset). The amplitude of Ij was then plotted as a function of the transjunctional potential (Fig.3D). The I–V curve displayed a linear relationship within the transjunctional membrane potential range of −80 to +100 mV. The curve used to fit the data were derived from a linear regression, given a macroscopic conductance of ∼3 nS.
Fig. 3.
Electrical coupling between chromaffin cell pairs. Membrane potential and macroscopic ionic currents were monitored in chromaffin cell pairs using the dual patch-clamp technique.A, Illustration of a cell pair in which the triggering of action potentials in the stimulated cell resulted in small membrane depolarizations in the unstepped cell. The two cells were current-clamped at −80 mV. Note that cell 2 was itself able to generate depolarization-evoked action potentials. B, Example of a cell pair in which action potentials were transmitted to the nonstimulated cell. The two cells were current-clamped at −80 mV.C, Histogram illustrating the wide distribution range of the coupling ratio calculated in 30 chromaffin cell pairs from current-clamp measurements of voltage amplitude (in response to a hyperpolarizing current injection) in both cell 1 (stepped cell) and cell 2 (target cell) (from 0 for noncoupled pairs to 1 for highly coupled pairs). The number of recorded cells is indicated inparentheses. D, Inset, Chart recordings of junctional currents (Ij) in a Cs+-loaded (140 mmCs+-gluconate) cell pair voltage clamped at −80 mV (voltage steps from −160 to +20 mV, 100 msec duration).Bottom, I–V relationship in which the junctional current amplitude (Ij) was plotted as a function of the transjunctional voltage (Vj) from −80 to +100 mV. The curves used to fit the data were derived from the linear regression y = 2.98× − 0.61 (dotted line). The correlation coefficientr2 was 0.98. Three to six cell pairs were used to determine the I–V curve at each transjunctional potential.
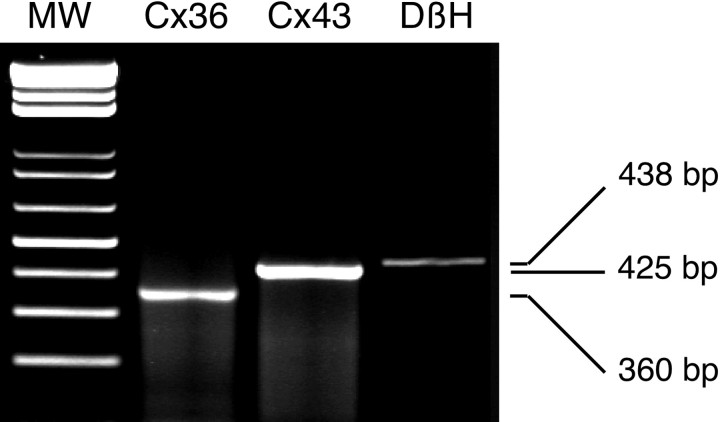
Further support for the presence of functional gap junctions in chromaffin cells was obtained in experiments to detect mRNA encoding connexins in randomly chosen cells. We focused on two connexins, Cx43 described in numerous endocrine–neuroendocrine tissues (Meda et al., 1993) and Cx36, a recently cloned connexin preferentially expressed in cell types of neural origin (Condorelli et al., 1998) such as chromaffin cells. All samples were first assessed for the expression of dopamine β-hydroxylase to confirm that harvested cells were indeed chromaffin cells. Because of the low expression level of mRNA encoding Cxs in single cells, the nested PCR procedure was required. The detection of Cx43 mRNA in 50% of harvested cortical cells was used as a control (n = 10), because rat cortical cells in the zona fasciculata and the zona reticularis are highly coupled by Cx43-containing gap junctions (Murray et al., 1995). Under the same experimental conditions, 20.6% and 25% of DβH-positive cells expressed at least Cx43 or Cx36 mRNA, respectively (n = 34 and 16). In some chromaffin cells (3 of 16), both Cx36 and Cx43 transcripts were found (Fig. 4). Although PCR experiments were done on different chromaffin cells than those from which we recorded, this result suggests that Cx36 and/or Cx43 may functionally and dynamically connect chromaffin cells in situ.
Fig. 4.
Expression of Cx36 and Cx43 mRNA in a single chromaffin cell. The harvested cell was identified as chromaffin cell by the presence of the DβH transcript (438 bp). Gel electrophoresis of RT-PCR products of the DβH-positive cell in which Cx36 mRNA (predicted size of 360 bp) and Cx43 mRNA (425 bp) were co-detected.MW, Size marker.
To further confirm the presence of functional gap junctions, chromaffin cells were impaled with a microelectrode filled with LY, a fluorescent tracer able to pass through gap junctions. LY diffusion was observed only between cells exhibiting synchronous [Ca2+]i transients (Fig.5A) (n = 4 cell fields). LY never diffused between nonsynchronized cells (n = 8 cell fields, data not shown). When LY was randomly injected into cells that were not Ca2+ imaged, the tracer diffused from the impaled cell to neighboring cells (up to 3) in 32.8% of the clusters impaled (n = 64). This percentage was not significantly different to that found for electrically coupled cells (40%, see Fig.3) and cells in which action potentials triggered simultaneous [Ca2+]i transients (39.5%) (Fig. 1) (p > 0.001). To assess the involvement of functional gap junctions in intercellular coupling, the fluorescent tracer was then injected in chromaffin cells chosen at random in the presence of bath-applied carbenoxolone (100 μm), a decoupling agent (Ishimatsu and Williams, 1996). Under these experimental conditions, the probability of observing an intercellular coupling (seen by LY diffusion) significantly decreased (0.06 versus 0.31 in control conditions;n = 46; p < 0.001) (Fig.5B).
Fig. 5.
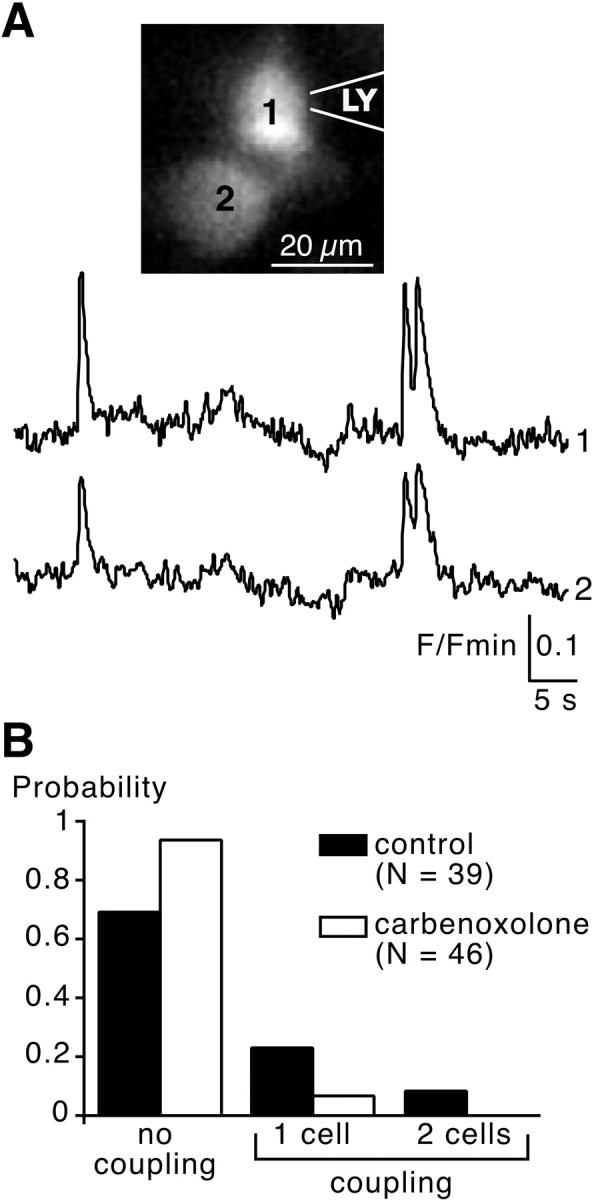
Lucifer yellow diffusion between spontaneously synchronized chromaffin cells and blockade by carbenoxolone.A, Optical measurements of two neighboring cells displaying synchronized spontaneous [Ca2+]i transients. After fluorimetric recording, cell 1 was patched with LY (1 mm in the intrapipette solution). A few seconds later, LY diffused into cell 2. B, Pooled data summarizing the probability of observing an intercellular coupling (seen by LY diffusion) between chromaffin cells injected at random in the presence or absence of the uncoupling agent carbenoxolone (100 μm, bath-application, 10–30 min). Control and treated slices came from the same adrenal glands and results are representative of three different experiments.
Involvement of gap junction-mediated coupling after nicotinic stimulation
Does the gap junction-driven response occur under physiological cholinergic stimulation? To mimic the in vivo release of transmitters into the synaptic cleft, the cholinergic secretagogue nicotine was iontophoretically applied onto a single chromaffin cell via a sharp microelectrode, leading to focal stimulation of a single cell. Subsequent multicellular [Ca2+]i changes were simultaneously imaged with real-time confocal microscopy. A 1 msec nicotinic application induced a transient [Ca2+]i rise in the stimulated cell as expected, but more interestingly, [Ca2+]i simultaneously increased in several adjacent cells (Fig.6A, left traces). Current polarity inversion (n = 10) or application of nicotine-free saline (n = 8) never induced [Ca2+]i changes in either stimulated or adjacent cells. Extracellular diffusion of nicotine could reasonably be ruled out because (1) a [Ca2+]i increase was not observed in all cells belonging to the same cluster, whereas all these cells were sensitive to pressure ejection of nicotine, (2) in some experiments, only the stimulated cell exhibited a [Ca2+]i rise, (3) the iontophoretic flux of nicotine was oriented in the opposite direction of the perfusion stream, and (4) saline was continuously ejected in the vicinity of the recorded cell field through a blunt macropipette, thus reinforcing the perfusion stream. The propagated signal between chromaffin cells reliably persisted after repetitive applications of nicotine, indicating that the intercellular mechanism underlying the propagation did not desensitize during the recording time (data not shown).
Fig. 6.
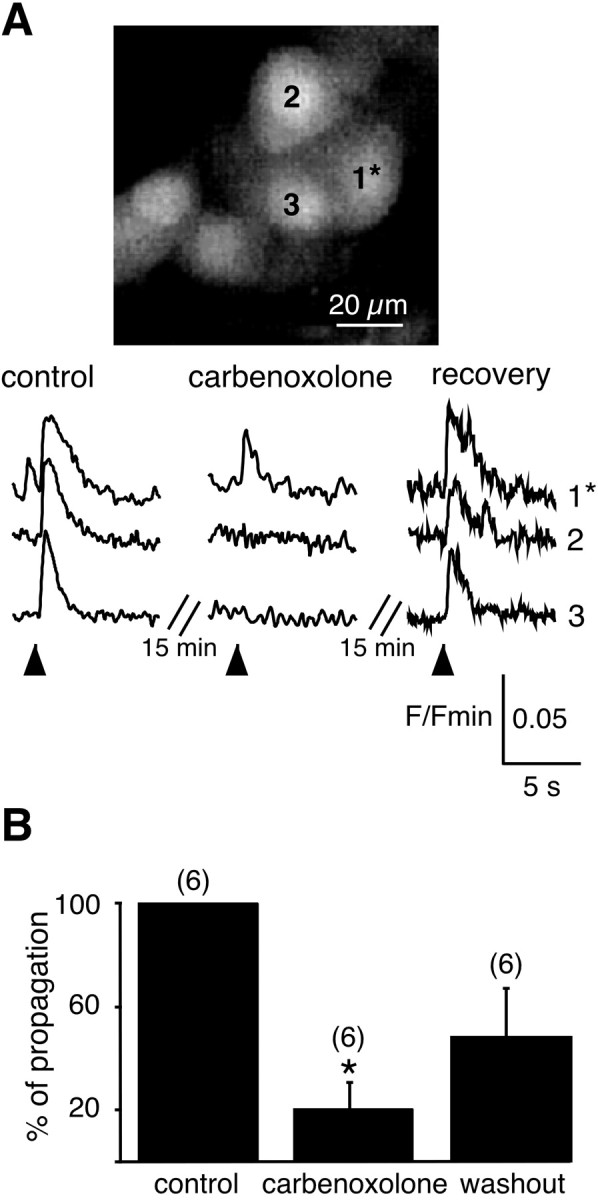
Blocking effect of carbenoxolone on nicotine-induced simultaneous [Ca2+]i increases within a chromaffin cell cluster. Nicotine (200 mm, 1 msec) was iontophoretically applied on cell 1 through a sharp microelectrode (the onset of the nicotinic stimulation is shown by anarrow). The [Ca2+]i increase originating in the stimulated cell was simultaneously detected in two adjacent cells. The [Ca2+]i rises in the nonstimulated cells (cell 2 and cell 3) were reversibly abolished in the presence of the gap junction blocker carbenoxolone (100 μm, 15 min bath application).B, Pooled data. The number of cell clusters recorded is indicated in parentheses. *p < 0.001, as compared with control values.
The functional implication of gap junctions in nicotine-induced signal propagation was further demonstrated by the blocking effect of carbenoxolone. Bath-application of carbenoxolone (100 μm, 15 min before nicotine iontophoresis) specifically suppressed the [Ca2+]i rises in coupled cells, without dramatically altering the Ca2+ response in the stimulated cell (Fig. 6A, middle and right traces). In separate experiments, we checked that carbenoxolone, per se, did not alter (1) the percentage of spontaneously active cells (44.3 ± 5.2% vs 44.6 ± 5.5% before and during carbenoxolone application, respectively; n = 17 cell clusters; p > 0.001; paired t test) and (2) the amplitude (0.49 ± 0.03 ΔF/Fmin vs 0.51 ± 0.03; p > 0.001; n = 41) and the duration (29.4 ± 1.4 sec vs 26.1 ± 1.3; p> 0.001; n = 41) of the nicotine-activated Ca2+ response. The effect of carbenoxolone was only partly (∼50% recovery) reversible after a 10–20 min washout (Fig. 6B).
Catecholamine secretion triggered after electrical and nicotinic stimulation in gap junction-coupled cells
Because an increase in [Ca2+]i is a prerequisite for exocytosis in chromaffin cells, we investigated the physiological relevance of action potential- and nicotine-triggered Ca2+ increases in coupled cells. To address this question, catecholamine exocytosis was monitored using the amperometric constant-voltage method (+800 mV) in cells neighboring the stimulated one. In 12.5% of cell clusters, a burst of action potentials induced catecholamine release from the stimulated cell as previously reported (Moser and Neher, 1997) and, more interestingly, also from nonstepped neighboring cells (perforated patch-clamp;n = 16) (Fig. 7). This percentage is similar to that found for highly coupled cell pairs (10%) (Fig. 3), in which unattenuated propagation of action potentials occurred.
Fig. 7.
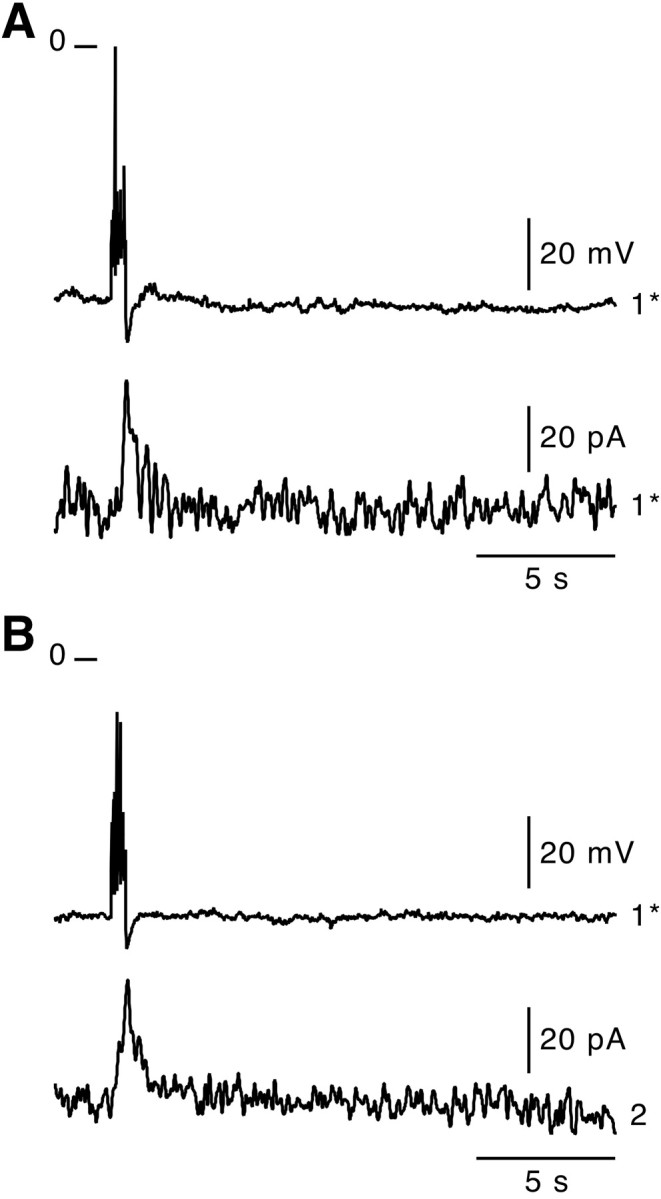
Action potential-induced catecholamine release in coupled chromaffin cells. A burst of action potentials (1 sec depolarization, perforated patch-clamp) was triggered in a chromaffin cell. Subsequent catecholamine exocytosis was simultaneously monitored by the amperometry technique at a constant voltage (+800 mV) in the stepped cell (A, cell 1*) and, during a second trial, in an adjacent cell (B, cell2). As shown by the outwardly directed current deflections, action potentials were effective in stimulating catecholamine release in both the stepped cell and the neighboring cell.
Similarly, an iontophoretic application of nicotine (1 msec) triggered spike-like secretory events in nonstimulated cells in which [Ca2+]i previously was shown to increase (Fig. 8, cell 2) (n = 8 of 11). The delay between nicotinic stimulation and the first secretory event was 1.04 ± 0.36 sec (n = 8; range, 0.02–3.1 sec). Catecholamine exocytosis was never detected in noncoupled cells (Fig. 8, cell 3). This result is in agreement with our data showing a that nicotine-driven Ca2+ signal was not observed in all the cells neighboring the stimulated cell (data not shown). Taken together, these results suggest that gap junction signaling activated by nicotine represents a functional mechanism leading to catecholamine secretion.
Fig. 8.
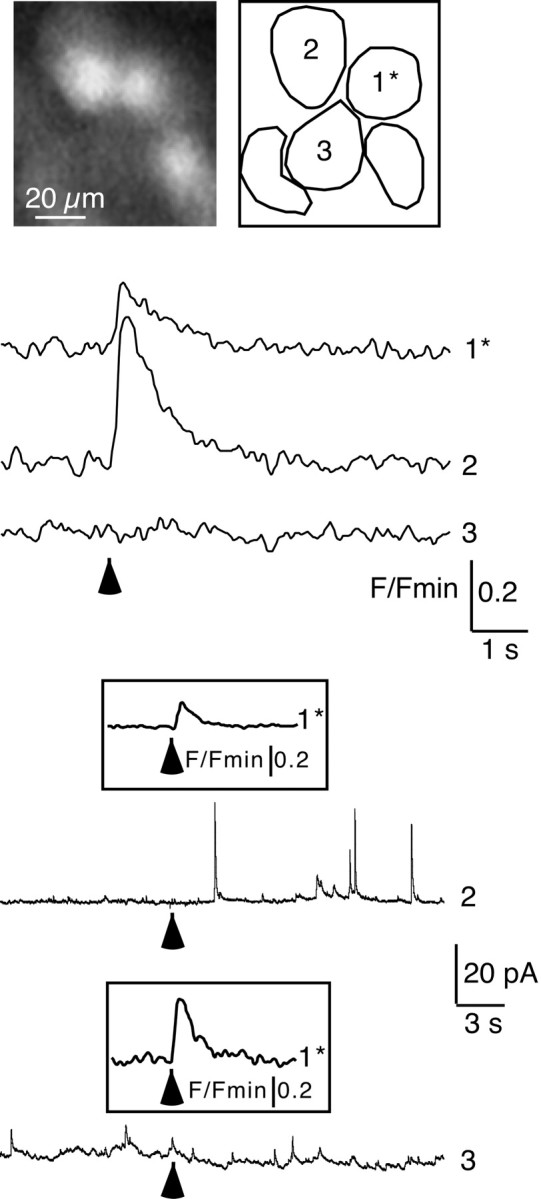
Nicotinic stimulation triggers catecholamine exocytosis in coupled chromaffin cells. The iontophoretic application of nicotine (1 msec; indicated by an arrow) on cell 1* evoked a simultaneous [Ca2+]i rise in cell 2 but was without effect on cell 3. Catecholamine exocytosis was sequentially followed by the amperometric detection of secretory events (constant voltage of +800 mV) in cell 2 and then cell 3, respectively. As shown by outward currents, nicotine triggered catecholamine release in cell 2 only. Insets, Nicotine-induced [Ca2+]i rises imaged in cell 1 during amperometric recordings of cells 2 and 3.
DISCUSSION
Based on anatomical data (Carmichael, 1986; Tomlinson and Coupland, 1990), the currently proposed concept for chromaffin cell secretion considers each cell as an independent functional unit releasing catecholamines after activation of its synaptic inputs (Iijima et al., 1992). Nevertheless, we describe here an additional mechanism involving gap junction-mediated coupling between chromaffin cells in rat adrenal slices. This cell-to-cell communication pathway underlies the propagation of electrical signal leading to simultaneous multicellular [Ca2+]i increases, thereby triggering catecholamine exocytosis.
Fast activation of simultaneous [Ca2+]i increases in adjoining chromaffin cells: the hallmark of electrotonic coupling via gap junctions
We show for the first time that the triggering of action potentials or the nicotinic stimulation of a single chromaffin cell results in simultaneous [Ca2+]i increases in adjoining cells caused by the propagation of a depolarizing voltage wave. As a general rule, excitable cells use distinct but not mutually exclusive pathways to communicate among themselves, i.e., synaptic transmission, gap junctions, and paracrine secretion. Based on our experimental results, gap junctions are likely to represent the anatomical correlate of the functional coupling observed here. At least four observations support this proposal: (1) bidirectional propagation of membrane depolarization between two cells, (2) junctional current recorded in cell pairs, (3) diffusion of LY between simultaneously active cells, and (4) reduction of signal propagation by the uncoupling agent carbenoxolone after nicotinic stimulation. Nevertheless, chromaffin cell granules are known to contain various compounds co-stored and co-released with catecholamines, such as ATP (Rojas et al., 1985), that could act locally on neighboring cells. Because of the slow velocity of ATP diffusion in the extracellular space (∼40 μm/sec; Guthrie et al., 1999), paracrine secretion of ATP is unlikely to be involved in the concomitant [Ca2+]i rises that we observed between chromaffin cells (speed of propagation of ∼500 μm/sec). A gap junction-mediated pathway usually ensures both electrotonic and metabolic communication between coupled cells, each of which is associated with distinct speeds of propagation. Although we cannot definitively rule out metabolic coupling (i.e., simple diffusion of second messengers), the speed of the propagation wave between coupled cells (∼500 μm/sec) strongly suggests the involvement of electrotonic coupling. In this way, the depolarization wave occurring either spontaneously or after stimulation rapidly propagates into the clusters of coupled chromaffin cells.
Functional electrical coupling mediated by gap junctions between rat chromaffin cells has not been demonstrated previously. Based on electrophysiological properties, the idea that chromaffin cells may be electrically coupled in situ has been hypothesized for various species, including rats (Ishikawa and Kanno, 1978), mouse (Nassar-Gentina et al., 1988; Moser, 1998) and guinea pigs (Holman et al., 1994). In addition, the presence of gap junctions has been reported in the rat adrenal medulla (Meda et al., 1993). Strengthening these findings, our dual patch-clamp recordings clearly show that gap junctions between chromaffin cells are functional and underlie the bidirectional transfer of voltage changes between coupled cells. In most cases, the amplitude of the voltage response induced in the unstepped cell was not sufficient to trigger action potentials, indicating weak coupling, as reported in mouse adrenal slices (Moser, 1998). In the remaining cell pairs, coupling strength was high enough to transmit action potentials. The distortion of the transmitted voltage signal compared with the original signal indicates significant low-pass filtering of the voltage response, as described in neurons (Venance et al., 2000). Several proposals can explain such filtering: (1) the presence of a few open gap junctions at the cell surface (Moser, 1998), (2) the expression of distinct connexin types with different unitary conductances, and (3) the expression of connexins with a low voltage sensitivity.
Our data on the connexins involved in the junctional communication between chromaffin cells are in agreement with previous reports showing that Cx43- or Cx36-containing gap junctions are expressed between neuroendocrine–endocrine cells (Meda et al., 1993; Munari-Silem and Rousset, 1996; Belluardo et al., 2000; Serre-Beinier et al., 2000). Two of our results indicate that Cx36 and Cx43 are present in chromaffin cells. First, the messengers encoding these two connexins were detected in single chromaffin cells. Second, experiments showing LY diffusion are in agreement with the possible involvement of Cx36 and/or Cx43, because both are dye-permeable (Veenstra, 1996; Srinivas et al., 1999). Assuming a unitary conductance of 61 and 10 pS for Cx43 and Cx36, respectively (Valiunas et al., 1997; Srinivas et al., 1999), the estimation of the junctional conductance in a rat chromaffin cell pair (3 nS maximum) would correspond to ∼50 and 300 open Cx43- and Cx36-built channels, adequate numbers to support significant voltage propagation when compared with only 7–10 open channels present between mouse chromaffin cells, leading to a junctional conductance that is too low to support spreading of electrical activity (Moser, 1998).
As shown by our results, ∼50% (depending on the technique used) of chromaffin cells in situ are functionally coupled via gap junctions to at least one apposing cell located in the same optical plane. To better assess the extent of coupling, a three-dimensional approach would be necessary. On the other hand, the dynamic aspects of coupling (occurrence of spontaneous coupling–decoupling) requiring long-lasting recordings merits further investigation. Nevertheless, in our view, intercellular signaling mediated through gap junctions most likely contributes to the mechanisms of communication within the adrenal medulla. The electrotonic coupling that we describe represents only one aspect of the role for gap junctions between chromaffin cells. As gap junctions are permeable to numerous low-molecular weight compounds, they are likely to subserve metabolic coupling, thereby propagating biological messages encoding different cell functions. With respect to the strength of the coupling, an unresolved issue raised by our findings is the functional consequences of strong and weak electrotonic coupling. Although [Ca2+]i changes were not simultaneously monitored during dual patch-clamp experiments, one could assume that strong and weak coupling are associated with the degree of the [Ca2+]i rise in the coupled cells. The propagation of action potentials triggers a Ca2+ signal that is sufficient to stimulate catecholamine exocytosis. In weakly coupled cell pairs, the depolarizations occurring near resting potential might also generate [Ca2+]i elevations, as reported for other endocrine cells (Mollard et al., 1994) and might control Ca2+-dependent cell functions other than exocytosis.
Physiological relevance of gap junctions in the adrenal medulla: complementing synaptic transmission to drive catecholamine exocytosis?
In endocrine glands in which secretagogues are delivered from the blood circulation, it is well known that gap junctions (mainly composed of Cx43) are required to ensure fast synchronized hormone release (Meda, 1996; Munari-Silem and Rousset, 1996). Although the synaptic boutons present on each chromaffin cell (Carmichael, 1986) themselves represent an efficient process to induce fast release of catecholamines, the gap junction-delineated route is also used for chromaffin cell exocytosis. The fact that simultaneous [Ca2+]i rises can occur spontaneously suggests that a spatiotemporal code between gap junction-coupled cells (Guérineau et al., 1998) coordinates basal exocytosis at the cell cluster level. During neurotransmission, the involvement of gap junctions in nicotine-induced multicellular [Ca2+]i rises and subsequent exocytosis suggests that in vivo direct chromaffin cell-to-cell interactions would be an important determinant in the regulation of secretory cell activity by acetylcholine. Although we show that gap junctions mainly ensure electrotonic coupling leading to exocytosis, we cannot rule out a role in other cell functions such as metabolic coupling, which would allow for the diffusion of chemical messengers (Ins(1,4,5)P3, ATP… ) between chromaffin cells.
Our study also raises the interesting issue that chromaffin cells are under the dual control of the splanchnic nerve (hitherto described as the major regulator of secretion) and of adjacent cells. We propose that within these anatomically well described cell complexes (Hillarp, 1946), both mechanisms might occur to activate catecholamine release depending on the catecholamine needs and/or the activation state of the synaptic pathway. Under basal conditions corresponding to low-frequency discharge of the splanchnic nerve, few synaptic boutons are stimulated and the propagation of the secretory events between gap junction-coupled cells may be sufficient to release catecholamines. In contrast, during stressful situations, the massive catecholamine secretion suddenly required might be mediated mostly by the synaptic pathway activated by sustained discharges of the splanchnic nerve, as reported earlier (Kajiwara et al., 1997). In particular physiological–pathological conditions, the functional state of synaptic transmission (presence of nonfunctional synapses as immature synapses, silent synapses… ) may also represent an intrinsic determinant requiring gap junctions, as an efficient aid to synapses leading chromaffin cells to exocytose catecholamines. In addition to this direct secretory role, the propagation of electrical activity and associated Ca2+ rises after activation of cholinergic nicotinic receptors might also stimulate other Ca2+-dependent cell activities (such as secretory vesicle trafficking, gene expression… ) to sensitize chromaffin cells to exocytosis after subsequent synaptic activation.
Footnotes
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Région Languedoc-Roussillon, Association pour la Recherche sur le Cancer, and Fondation pour la Recherche Médicale. We are indebted to Drs. O. Manzoni, P. Chavis, P. Mollard, and U. Gerber for critical reading and M. Passama and A. Carrette for their excellent technical assistance.
Correspondence should be addressed to Nathalie C. Guérineau, INSERM U469, CCIPE, 141 rue de la Cardonille, 34094 Montpellier CEDEX 5, France. E-mail: guerinea@u469.montp.inserm.fr.
REFERENCES
- 1.Albillos A, Neher E, Moser T. R-type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbara J-G, Takeda K. Quantal release at a neuronal nicotinic synapse from rat adrenal gland. Proc Natl Acad Sci USA. 1996;93:9905–9909. doi: 10.1073/pnas.93.18.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara J-G, Poncer J-C, McKinney RA, Takeda K. An adrenal slice preparation for the study of chromaffin cells and their cholinergic innervation. J Neurosci Methods. 1998;80:181–189. doi: 10.1016/s0165-0270(97)00200-8. [DOI] [PubMed] [Google Scholar]
- 4.Belluardo N, Mudò G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefont X, Fiekers J, Creff A, Mollard P. Rhythmic bursts of calcium transients in acute anterior pituitary slices. Endocrinology. 2000;141:868–875. doi: 10.1210/endo.141.3.7363. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael SW. Morphology and innervation of the adrenal medulla. In: Rosenheek K, Lelkes P, editors. Stimulus-secretion coupling. CRC; Boca Raton, FL: 1986. pp. 1–29. [Google Scholar]
- 7.Ceña V, Nicolas GP, Sanchez-Garcia P, Kirpekar SM, Garcia AG. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983;10:1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 8.Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 9.Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 10.Coupland RE. Electron microscopic observations on the structure of the rat adrenal medulla. I. The ultra-structure and organization of chromaffin cells in the normal adrenal medulla. J Anat. 1965;99:231–254. [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guérineau NC, Bonnefont X, Stoeckel L, Mollard P. Synchronized spontaneous Ca2+ transients in acute anterior pituitary slices. J Biol Chem. 1998;273:10389–10395. doi: 10.1074/jbc.273.17.10389. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Hillarp NA. Functional organization of the peripheral autonomic innervation. Acta Anat. 1946;4[Suppl]:1–153. doi: 10.1111/j.1748-1716.1949.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 16.Holman ME, Coleman HA, Tonta MA, Parkington HC. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. J Physiol (Lond) 1994;478:115–124. doi: 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iijima T, Matsumoto G, Kidokoro Y. Synaptic activation of rat adrenal medulla examined with a large photodiode array in combination with a voltage-sensitive dye. Neuroscience. 1992;51:211–219. doi: 10.1016/0306-4522(92)90486-l. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa K, Kanno T. Influences of extracellular calcium and potassium concentrations on adrenaline release and membrane potential in the perfused adrenal medulla of the rat. J Physiol (Lond) 1978;28:275–289. doi: 10.2170/jjphysiol.28.275. [DOI] [PubMed] [Google Scholar]
- 19.Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajiwara R, Sand O, Kidokoro Y, Barish ME, Iijima T. Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn J Physiol. 1997;47:449–464. doi: 10.2170/jjphysiol.47.449. [DOI] [PubMed] [Google Scholar]
- 21.Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol (Lond) 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambolez B, Audinat E, Bochet P, Crépel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 23.Meda P. The role of gap junction membrane channels in secretion and hormonal action. J Bioenerg Biomembr. 1996;28:369–377. doi: 10.1007/BF02110113. [DOI] [PubMed] [Google Scholar]
- 24.Meda P, Pepper MS, Traub O, Willecke K, Gros D, Beyer E, Nicholson B, Paul D, Orci L. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology. 1993;133:2371–2378. doi: 10.1210/endo.133.5.8404689. [DOI] [PubMed] [Google Scholar]
- 25.Melia KR, Trembleau A, Oddi R, Sanna PP, Bloom FE. Detection and regulation of tyrosine hydroxylase mRNA in catecholaminergic terminal fields: possible axonal compartmentalization. Exp Neurol. 1994;130:394–406. doi: 10.1006/exnr.1994.1219. [DOI] [PubMed] [Google Scholar]
- 26.Mollard P, Theler J-M, Guérineau N, Vacher P, Chiavaroli C, Schlegel W. Cytosolic Ca2+ of excitable pituitary cells at resting potentials is controlled by steady-state Ca2+ currents sensitive to dihydropyridines. J Biol Chem. 1994;269:25158–25164. [PubMed] [Google Scholar]
- 27.Mollard P, Seward EP, Nowycky MC. Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization. Proc Natl Acad Sci USA. 1995;92:3065–3069. doi: 10.1073/pnas.92.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser T. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol (Lond) 1998;506:195–205. doi: 10.1111/j.1469-7793.1998.195bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser T, Neher E. Rapid exocytosis in single chromaffin cells recorded from mouse adrenal slices. J Neurosci. 1997;17:2314–2323. doi: 10.1523/JNEUROSCI.17-07-02314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munari-Silem Y, Rousset B. Gap junction-mediated cell-to-cell communication in endocrine glands. Eur J Endocrinol. 1996;135:251–264. doi: 10.1530/eje.0.1350251. [DOI] [PubMed] [Google Scholar]
- 31.Murray SA, Oyoyo UA, Pharrams SY, Kumar NM, Gilula NB. Characterization of gap junction expression in the adrenal gland. Endocr Res. 1995;21:221–229. doi: 10.3109/07435809509030438. [DOI] [PubMed] [Google Scholar]
- 32.Nassar-Gentina V, Pollard HB, Rojas E. Electrical activity in chromaffin cells of intact mouse adrenal gland. Am J Physiol (Cell Physiol) 1988;254:C675–C683. doi: 10.1152/ajpcell.1988.254.5.C675. [DOI] [PubMed] [Google Scholar]
- 33.Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- 34.Rojas E, Pollard HB, Heldman E. Real-time measurements of acetylcholine-induced release of ATP from bovine medullary chromaffin cells. FEBS Lett. 1985;185:323–327. doi: 10.1016/0014-5793(85)80931-5. [DOI] [PubMed] [Google Scholar]
- 35.Serre-Beinier V, Le Gurun S, Belluardo N, Trovato-Salinaro A, Charollais A, Haefliger JA, Condorelli DF, Meda P. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49:727–734. doi: 10.2337/diabetes.49.5.727. [DOI] [PubMed] [Google Scholar]
- 36.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson A, Coupland RE. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age. A description and quantitative morphometric biochemical study of the innervation of the chromaffin cells and adrenal medullary neurons in Wistar rats. J Anat. 1990;169:209–236. [PMC free article] [PubMed] [Google Scholar]
- 38.Valiunas V, Bukauskas F, Weingart R. Conductances and selective permeability of connexin 43 gap junction channels examined in neonatal rat heart cells. Circ Res. 1997;80:708–719. doi: 10.1161/01.res.80.5.708. [DOI] [PubMed] [Google Scholar]
- 39.Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomembr. 1996;28:327–337. doi: 10.1007/BF02110109. [DOI] [PubMed] [Google Scholar]
- 40.Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci USA. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voets T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 42.Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- 43.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol (Lond) 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]