Abstract
Many molecular accounts of long-term memory storage postulate that the synthesis of new proteins is necessary for long-term changes in neuronal function. These experiments generally have examined the learning that occurs as associations are acquired between neutral and biologically important stimuli. Little is known about the importance of protein synthesis in the establishment of memories for extinction, which occurs as the relations established during acquisition are severed. Extinction appears to be an active learning process that results in the formation of new memories rather than in the simple erasure or forgetting of memories from acquisition. Furthermore, under certain circumstances, extinction can result in long-term changes in behavior lasting for days to weeks. Here we show that although memories for the acquisition of spatial and contextual learning required protein synthesis, memories for extinction formed in the absence of protein synthesis. These results suggest that acquisition and extinction are mediated by distinct molecular mechanisms and that long-term memories can form in the absence of protein synthesis.
Keywords: memory, extinction, protein synthesis, spatial learning, fear conditioning, hippocampus
As memories are acquired and consolidated, a cascade of events involving a variety of intracellular signaling molecules occurs (see Abel and Lattal, 2001). Some of these transduction cascades ultimately result in gene induction and protein synthesis, which are thought to be necessary for long-term memory (Flood et al., 1973; Davis and Squire, 1984; Abel et al., 1997). The requirement for protein synthesis during acquisition has been demonstrated in many forms of learning, including spatial learning in the Morris water maze and Pavlovian associative learning in contextual fear conditioning (Abel et al., 1997; Bourtchouladze et al., 1998;Meiri and Rosenblum, 1998). In the water maze, animals must learn and remember the location of an escape platform hidden just beneath the surface of a pool of opaque water. In contextual fear conditioning, animals learn that a conditioning context signals the occurrence of a foot shock. Memories for spatial learning and contextual fear conditioning are revealed in behavior as a search preference for the platform location and as a context-evoked freezing response, respectively. The importance of protein synthesis in spatial learning and fear conditioning has been demonstrated during the learning that occurs as animals form memories of excitatory relations among environmental stimuli.
Little is known about the involvement of protein synthesis in extinction, which occurs when the relations among stimuli established during acquisition are severed. During extinction, previously established responses are suppressed, resulting in long-term changes in behavior. Preferences for a spatial location decrease in the water maze as the animal learns that the cues no longer predict the location of that platform, and fear evoked by a context decreases as animals learn that the context is no longer predictive of shock. Many experiments have shown that extinction is an active learning process that results in new memories rather than in the erasure or forgetting of memories established during acquisition (Pavlov, 1927; Bouton, 1993; Rescorla, 2001). Indeed, although the behavior in the presence of a previously conditioned stimulus is attenuated during extinction, the original association is surprisingly unaffected (for review, see Rescorla, 2001). This suggests that the processes that operate during extinction act to suppress rather than erase the original learning. Defining the nature of this depressive process has led to many important behavioral theories about the extinction process (e.g., Pavlov, 1927; Konorski, 1967; Rescorla and Wagner, 1972; Bouton, 1993; Rescorla, 1993).
Although much is now known about the behavioral properties of extinction, very little is known about the underlying molecular mechanisms and the extent to which they are similar to mechanisms of acquisition. Several experiments have shown that the NMDA type of glutamate receptor, which appears to be critical for certain types of associative learning, also may play a role in extinction (Falls et al., 1992; Baker and Azorlosa, 1996; Johnson et al., 2000). Two other processes, protein synthesis and gene transcription, appear to be critical for acquisition. Because extinction, like acquisition, results in long-term memories and requires NMDA receptor activation, one might expect that the requirement for protein synthesis would be similar in establishing memories for acquisition and extinction.
In the following experiments, we assessed the role of protein synthesis in acquisition and extinction of spatial learning and contextual fear conditioning in C57BL/6 mice. The protein synthesis inhibitor anisomycin was administered either during acquisition or during extinction to isolate the effects of protein synthesis inhibition on acquisition and extinction independently. Anisomycin blocked the initial acquisition of a spatial preference and also blocked the formation of a new preference trained during a reversal procedure; however, anisomycin had no effect on the extinction of a previously established preference. Similarly, although anisomycin blocked the acquisition of context-evoked fear, it had no effect on the extinction of previously established fear. These experiments show that acquisition and extinction of hippocampus-dependent spatial and contextual tasks may be mediated by fundamentally distinct molecular mechanisms, and they suggest that protein synthesis-independent mechanisms can mediate long-term changes in behavior.
MATERIALS AND METHODS
Subjects. Male and female C57BL/6 mice bred in our animal facility from mice originally obtained from The Jackson Laboratory (Bar Harbor, ME) were used in the experiments. They were 8–12 weeks old and had free access to food and water in their home cages. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Injections. Anisomycin (Sigma, St. Louis, MO) was diluted in saline and dissolved in 1N HCl. NaOH (1N) was added to the solution until the pH was ∼7. Mice received subcutaneous injections of 150 mg of anisomycin/kg of body weight or an equivalent volume of saline. This amount of anisomycin has been shown to yield >90% protein synthesis inhibition in the brain during the first 2 hr and >60% inhibition during the next 2 hr (Flood et al., 1973). All injections occurred 30 min before each session or immediately after each session, depending on the experiment.
Water maze acquisition. The water maze was a circular pool (120 cm in diameter). White tempera nontoxic paint was mixed with water to make the surface opaque. Hidden 0.5 cm beneath the surface of the water was a circular platform (11.2 cm in diameter), which was placed in a constant location throughout acquisition training. During acquisition and reversal training, mice received 4 trials/d, in which they searched for the hidden platform for a maximum of 60 sec. After finding the platform, mice remained there for 20 sec. These trials were separated by an intertrial interval (ITI) of 4–6 min (which was used in all subsequent water maze experiments). The path of the mouse was recorded using a video tracking system (HVS Image). In the acquisition experiment (Fig. 1), mice received injections of anisomycin (n = 8) or saline (n = 16) before each training session. On the day after the fifth acquisition session, all mice received injections of saline and then received a probe trial in which swimming paths in the absence of the platform were recorded for 60 sec. Preference for a target was assessed by analyzing time spent searching in the target quadrant compared with the other three quadrants. Preference for the target quadrant was also compared with time spent in the most-preferred nontarget location [Maximum Nontarget (MN)] calculated for each individual mouse. The MN measure also allows us to assess the searching abilities of mice that show no target preference. Mice that engage in a directed search should show a preference for one of the quadrants, but if they swim randomly, no preference should be evident in any quadrant (Riedel et al., 1999).
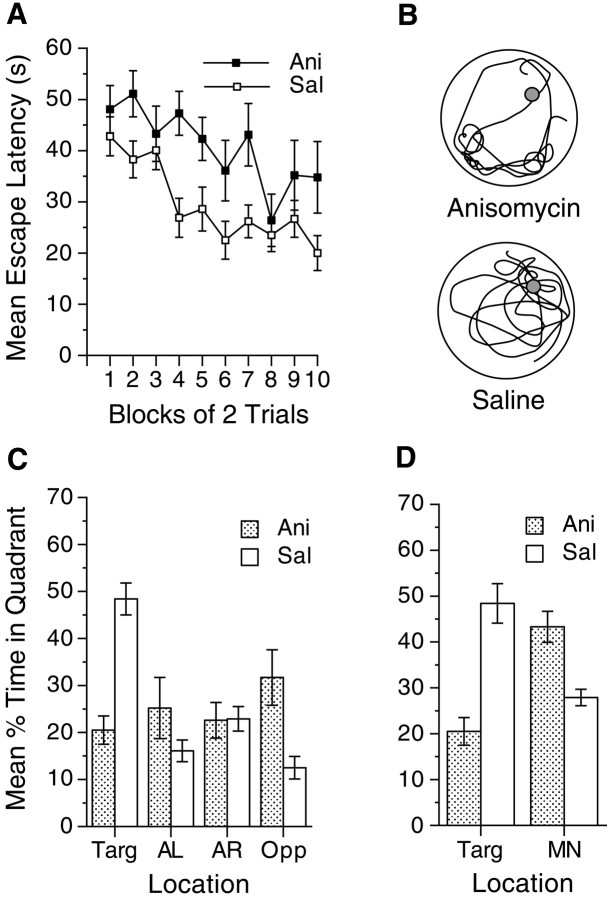
Fig. 1.
Protein synthesis is required for the acquisition of spatial preferences in the Morris water maze. A, Mean escape latencies during the 5 d of acquisition of spatial learning under anisomycin (filled squares) or saline (open squares). Data are presented in blocks of two trials. B, Representative paths during the probe trial shown for mice that received anisomycin or saline during reversal. The path is shown for the mouse from each group closest to the median of time spent searching in the target quadrant, the number of target platform crossings, total path length, percentage of time floating, and percentage of time thigmotactic. C, Preference during a 60 sec probe trial for the training quadrant location (Targ) for mice given anisomycin (shaded bars) or saline (open bars) during training. Time spent in the adjacent left (AL), adjacent right (AR), and opposite (Opp) quadrants also is shown. D, Preference during the probe for the training quadrant location (Targ) compared with the maximum nontarget (MN). The MN was calculated by determining each animal's most preferred nontarget quadrant. Error bars indicate SE.
The effects of anisomycin on general performance were assessed in a nonspatial version of the water maze, in which mice given anisomycin (n = 8) or saline (n = 8) searched for a platform that had a visible cue attached to it (Fig. 2). During visible platform training, the platform was placed in different locations on each of the 4 trials/d for 3 consecutive days.
Fig. 2.
Protein synthesis inhibition does not disrupt the acquisition of a nonspatial visible platform task. Mice given anisomycin (filled squares) or saline (open squares) acquired the task at the same rate. Data are presented in blocks of two trials over the 3 d of training. Error bars indicate SE.
Water maze reversal. In the reversal experiment (Fig. 3), mice were trained to find the platform in the original location for 7 d. No injections were administered during the initial training. Preference during a probe trial on the day after the end of acquisition (P1) was used to assign mice to groups that would receive anisomycin or saline during reversal. Any mouse that showed <30% preference for the target location during P1 was dropped from the experiment before reversal training (n = 3). Before each reversal session, mice received injections of anisomycin (n = 8) or saline (n = 11). During reversal, the platform was moved to the opposite side of the pool. Mice received 4 trials/d of reversal training for 8 d. Two probe trials assessed preferences during reversal training, one after the fourth session of reversal training (P2) and another after the eighth session of reversal training (P3). After reversal training, all mice were retrained to the original location for four sessions, during which they received no injections, followed by a fourth probe trial (P4). All mice received injections of saline 30 min before the probe trials, which occurred 24 hr after the most recent training session. The next training session began 24 hr after the probe trial.
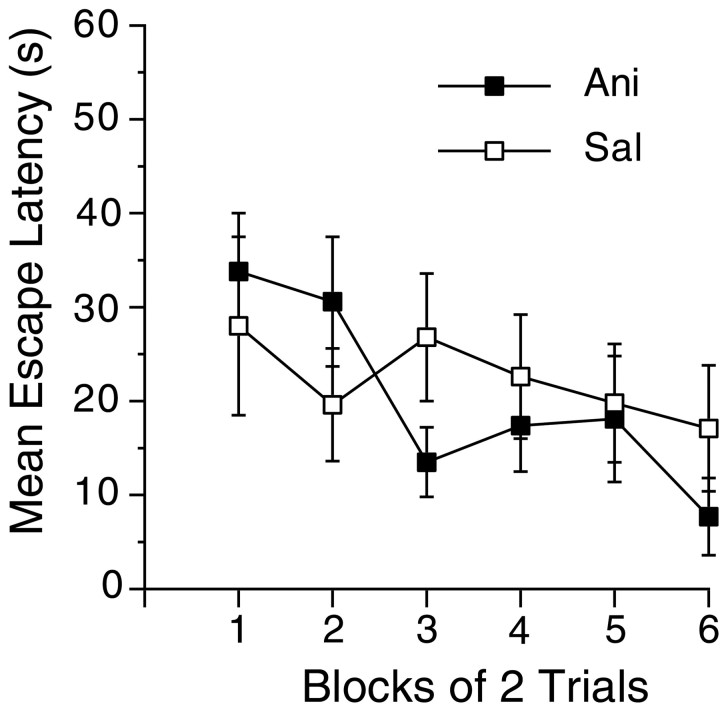
Fig. 3.
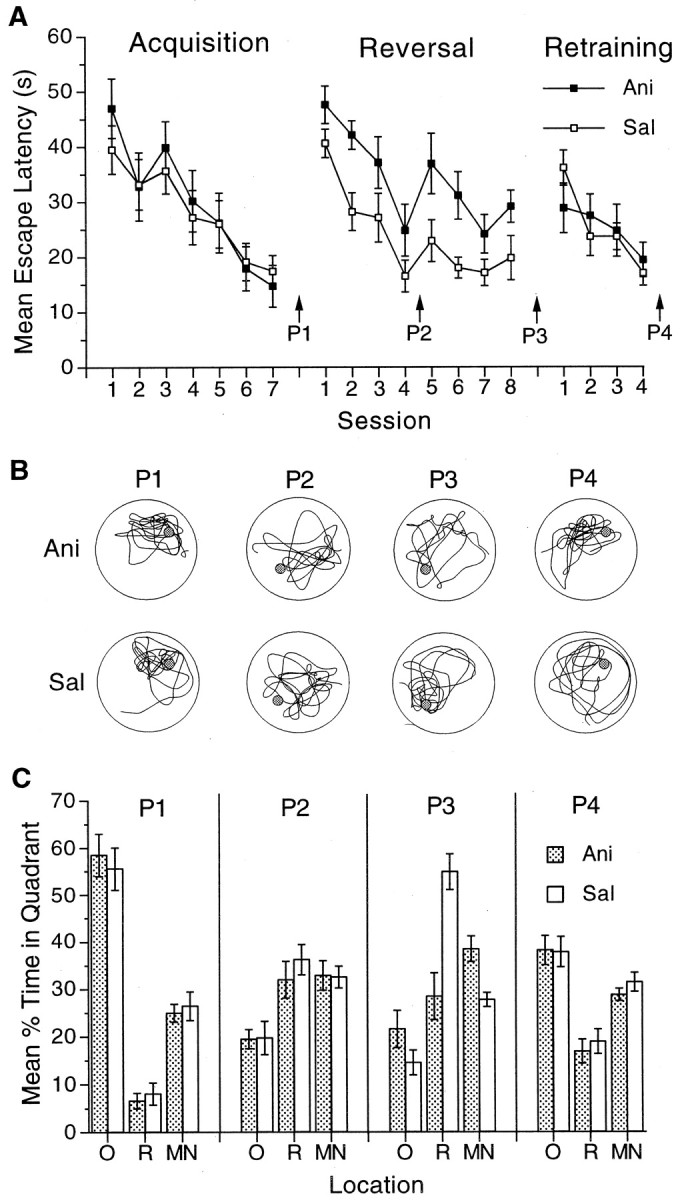
Protein synthesis is not required for extinction of a previously established preference during reversal training.A, Mean escape latencies during the seven sessions of acquisition training without injections, the eight sessions of reversal training under either anisomycin (filled squares) or saline (open squares), and the four sessions of retraining without injections. B, Representative paths during the probe trials for mice that received anisomycin or saline during reversal. The paths from each probe trial were chosen on the basis of the criteria described in the legend to Figure 1.C, Preference for the original (O), reversal (R), and maximum nontarget (MN) locations during the four probe trials (P1–P4) in groups that received anisomycin (shaded bars) or saline (open bars) during reversal. Error bars indicate SE.
Water maze extinction. In the simple extinction experiment (Fig. 4), mice were trained to find the platform in the original location for 10 d in the absence of anisomycin or saline injections. They were assigned to groups on the basis of their performance during the probe trial after acquisition, and mice that spent <30% of the probe trial in the target quadrant were dropped from the experiment before extinction (n = 3). During extinction training, mice received injections of anisomycin (n = 10) or saline (n = 8) before each session and received four 60 sec trials/d in which they swam in the pool in the absence of the platform. On the day after the fourth session of extinction, a probe trial was run in which all mice swam after saline injections.
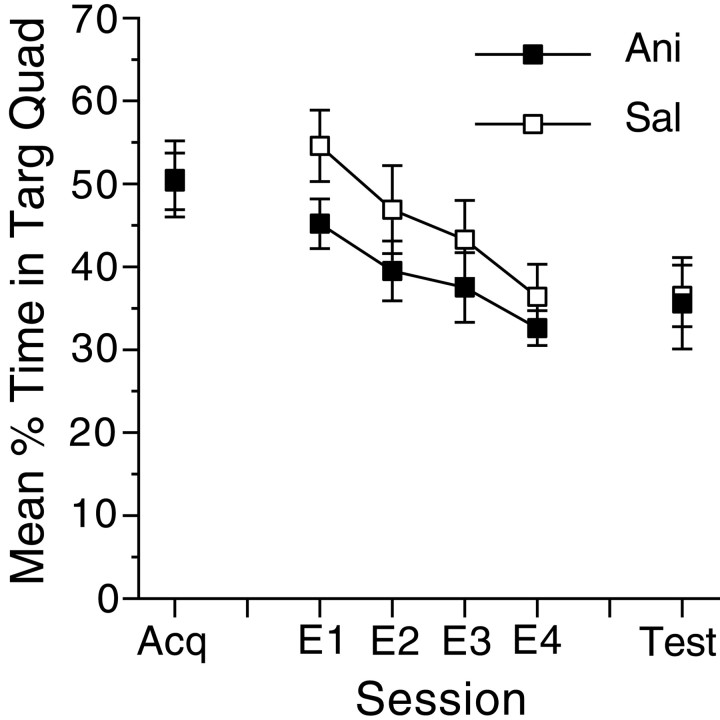
Fig. 4.
Extinction in the absence of the platform does not require protein synthesis. Preference for the original target location is shown at the end of acquisition, during extinction, and after extinction. No injections occurred during acquisition. Mice received injections of either anisomycin (filled squares) or saline (open squares) during extinction. Error bars indicate SE. The test occurred on the day after the final extinction session. E1–E4, Extinction sessions 1–4.
Contextual fear conditioning. Three contextual fear conditioning experiments used a total of 102 mice. In the first (Fig.5A), 30 min before fear conditioning, mice received injections of saline (n = 24) or anisomycin (n = 7). Mice were placed into a conditioning chamber made of Plexiglas (either a 23 × 23 × 23 cm cube or a 21.5-cm-diameter × 23-cm-high circular chamber) and received a 2 sec 1.5 mA scrambled foot shock from a grid floor (Med Associates, Inc.) 2 and 2.5 min after placement into the chamber. Mice were removed from the chamber after a total of 3 min. Extinction began the following day. All of the mice that received injections of anisomycin before conditioning received injections of saline before extinction (group Ani/Sal, n = 7); mice that received injections of saline before conditioning received injections of anisomycin (group Sal/Ani, n = 12) or saline (group Sal/Sal;n = 12) before extinction sessions. Extinction consisted of two 3 min exposures to the conditioning chamber in the absence of shock for 3 d. Each exposure was separated by a 15–20 min ITI. The fourth day was a test session, in which all mice received injections of saline and received two additional extinction trials. Mice received an identical test 10 d later. Conditioning was assessed by measuring freezing behavior (Fanselow, 1980). The behavior of each mouse was sampled at 5 sec intervals, and the percentage of those intervals in which the mouse froze was calculated.
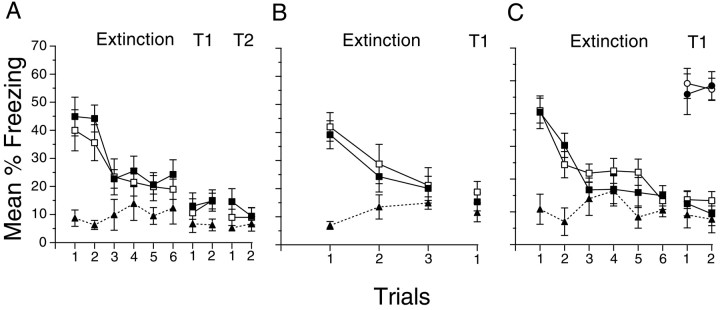
Fig. 5.
Protein synthesis is required for the acquisition but not the extinction of context-evoked fear. Mean percent freezing throughout the course of extinction after contextual fear conditioning is shown for groups that received anisomycin during acquisition and saline during extinction (Ani/Sal; filled triangles), saline during conditioning and anisomycin during extinction (Sal/Ani; filled squares), and saline during both conditioning and extinction (Sal/Sal; open squares). A, Two massed shocks during conditioning and two spaced context exposures per day during extinction. Injections occurred before conditioning and extinction. Test 1 (T1) occurred the day after the final extinction session. Test 2 (T2) occurred 10 d after extinction. B, Two massed shocks with postconditioning injections and one context exposure per day during extinction with post-trial injections. C, Two spaced shocks during conditioning with postconditioning injections and two spaced context exposures during extinction with post-trial injections. Groups Ani No Ext (filled circles) and Sal No Ext (open circles) were conditioned and received injections with the other groups but did not receive extinction. Error bars indicate SE.
The second contextual fear conditioning experiment (Fig. 5B) was identical to the first, except mice received injections of anisomycin (n = 8) or saline (n = 8) immediately after conditioning and after extinction and received only one extinction trial per day.
In the third contextual fear conditioning experiment (Fig.5C), mice received spaced conditioning, which consisted of two 3 min exposures to the context with a shock 2.5 min after placement into the chamber. Extinction was identical to conditioning in this experiment, except that no shock was presented. The ITI during conditioning and extinction was 15–20 min. As in the second fear conditioning experiment, injections occurred immediately after conditioning or extinction. To determine whether there were effects of anisomycin on the long-term consolidation of the memory for acquisition, additional groups of mice received injections but remained in their home cages during extinction [groups Ani No Ext (n = 8) and Sal No Ext (n = 8)]. They were tested for retention with the other groups.
Statistical analysis. ANOVAs were performed in all experiments. Simple planned comparisons were made using Student'st test.
RESULTS
Spatial learning: acquisition
The requirement for protein synthesis during the acquisition of spatial preferences is evident in Figure1. Injection of the protein synthesis inhibitor anisomycin before each training session in the hidden version of the Morris water maze caused higher latencies to find the platform (Fig. 1A) (F(1,22) = 20.3; p < 0.001) and resulted in no reliable improvement in latency to find the platform from the first to last block of trials. The probe trial, in which spatial preferences were recorded in the absence of the platform, revealed that anisomycin blocked the formation of a spatial preference for the target location (Fig. 1B–D). During this probe trial, mice that received injections of saline spent more time searching the target quadrant than did mice that received injections of anisomycin (F(1,32) = 9.4; p < 0.01), which showed no preference for the target quadrant. It is not clear from Figure 1C whether the anisomycin-treated mice swam randomly in the pool or engaged in a directed search in the wrong location. Calculating the most preferred nontarget quadrant (Fig.1D, MN) for each individual mouse revealed that anisomycin-treated mice spent time swimming preferentially in a particular quadrant (Fig. 1D), but they were no better than chance at searching in the correct quadrant (Fig. 1C). Saline-treated mice showed a preference for the target relative to the maximum nontarget quadrant (t(16) = 4.1; p < 0.001), indicating that they had acquired a spatial preference for the target location. Anisomycin had no effect on the acquisition of a nonspatial version of this task, suggesting that it is unlikely that the spatial learning deficit is caused by significant deficits in performance (Fig. 2). Both groups acquired the visual task, as evidenced by their decreased latencies (F(5,70) = 6.2; p < 0.001).
Spatial learning: reversal
Having established that anisomycin blocks the acquisition of spatial learning, we next examined whether protein synthesis inhibition during spatial extinction would impair memory for extinction. When the relation between a specific location and the distal cues established during acquisition is severed, mice given anisomycin should perseverate in their preference for the originally trained location if they remember nothing from the extinction experience. There are several ways to extinguish an established preference in the water maze (Lattal and Abel, 2000). We first used a reversal procedure in which mice initially were trained to form a spatial preference for one location with no saline or anisomycin injections and then were trained under anisomycin or saline to swim to the platform after it had been moved to the opposite side of the pool. This reversal procedure has the advantage of allowing a simultaneous comparison of the effects of anisomycin on the acquisition of a preference for the reversal location with its effects on the extinction of a previously established preference for the original location. For mice that receive injections of saline during reversal training, preference for the original location should extinguish as preference for the reversal location is acquired (Lattal and Abel, 2000). For mice that receive injections of anisomycin, the originally established preference might remain during reversal training, because anisomycin might be expected to block the formation of all memories of the reversal experience.
As can be seen in Figure 3, although protein synthesis inhibition blocked the acquisition of a preference for a reversal location, it had no effect on the extinction of a preference for an originally trained location. After seven sessions of acquisition in the absence of injections, mice showed a robust preference for the original location (Fig. 3C,P1, O). On the first reversal trial, the two groups did not differ in latency to reach the reversal target (Ani, 53.7 sec; Sal, 49.5 sec; p > 0.05) or in the time spent searching in the original target quadrant (Ani, 48.6%; Sal, 49.4%; p > 0.05), again suggesting that anisomycin did not adversely affect performance. Although there were no differences in latencies during acquisition under no drug (F(1,17) < 1.0), there were reliable differences during reversal (F(1,17)=15.9; p< 0.001) as well as reliable decreases across sessions (Fig.3A) (F(7,119) = 11.3,p < 0.001). A probe trial during reversal revealed that the latency decreases during the first four sessions of reversal did not reflect the development of preferences for the reversal quadrant (Fig. 3C, P2, R) but instead likely reflected a wider search strategy as the preference for the original location was extinguished. This probe trial also revealed that the preference for the original location had decreased by the same amount in both groups, which demonstrates that protein synthesis was not required for the formation of memories for the absence of the platform in its original location. After an additional four sessions of reversal training, mice that received injections of saline acquired a preference for the reversal location (Fig. 3C,P3, R) compared with the maximum nontarget location (t(10) = 3.6;p < 0.001) and differed reliably from anisomycin-treated mice in preference for the reversal location (t(17) = 2.5; p < 0.05). Mice that received injections of anisomycin failed to acquire a preference for the reversal location, although they continued to remember that the target was no longer located in the original position.
After reversal training, all mice were retrained to the original location with no injections. There was a large change in latencies from the last reversal session to the first retraining session in the saline group (t(8) = 2.6; p< 0.01) but not in the anisomycin group, although the difference in latencies during the first retraining session was not reliable (Fig.3A). Because the anisomycin group did not acquire a new preference during reversal, that group may have been in a better position to relearn the original location, but by the end of the fourth session of retraining, the two groups showed a similar preference for the original location (Fig. 3C, P4) (F(1,17) > 21.0; p < 0.001), suggesting that anisomycin injections did not cause long-term changes in learning ability (Fig. 3C, P4).
These findings suggest a dissociation between the requirement for protein synthesis during acquisition of new spatial preferences and extinction of old spatial preferences. A demonstration of this with the reversal procedure is particularly powerful, because this task results in two simultaneously trained long-term memories within each subject, one for the absence of the platform in the original location and another for the presence of the platform in the reversal location. During reversal, mice given anisomycin remembered that the platform was no longer placed in the original position (as demonstrated by their decreased preference for the original quadrant), but they could not learn the new location of the platform (as demonstrated by their failure to preferentially search in the reversal quadrant). Thus, although anisomycin blocked acquisition of a new preference, it had no effect on the extinction of a previously established preference, suggesting that acquisition and extinction are mediated by distinct molecular mechanisms.
Spatial learning: simple extinction
Although there are many advantages to the reversal procedure, one of the difficulties in assessing the contribution of protein synthesis to extinction in this procedure is that the rate of extinction of the original preference is confounded with the rate of acquisition of the new preference. To examine the effects of anisomycin on the course of extinction more directly, we extinguished preferences by repeatedly placing mice into the pool in the absence of the hidden platform, which had been placed in a constant location throughout acquisition training. Analyses of preferences for the training quadrant during extinction (Fig. 4) revealed only a reliable main effect of session (F(3,51) = 14.3;p < 0.001), suggesting that the groups did not differ during extinction. A comparison of preferences during the acquisition probe trial with preferences during the probe trial after extinction found that target quadrant preference in both groups decreased after extinction (F(1,17) = 11.3;p < 0.01) and that there were no group differences in target preference after extinction (F(1,17) < 1.0). This suggests that the extinction treatment resulted in a decreased preference for the target quadrant that was independent of protein synthesis. Taken together, the findings from the reversal and simple extinction experiments show that memories during extinction of spatial preferences can form in the absence of protein synthesis.
Contextual fear conditioning
The generality of the findings from the Morris water maze experiments was examined by assessing the effects of anisomycin on the acquisition and extinction of context-evoked fear. Although these two tasks have different requirements for performance, they share a common dependence on the hippocampus (e.g., Abel et al., 1997). As can be seen in Figure 5, three contextual fear conditioning experiments using different conditioning and extinction parameters revealed a similar pattern of results: anisomycin disrupted the establishment of memories for contextual fear but had no effect on memories for extinction when it was administered to mice that were conditioned in the presence of saline. During the first extinction trial, which served as a memory test for acquisition, mice that received injections of anisomycin before or immediately after fear conditioning (group Ani/Sal) froze less than did mice that received injections of saline (groups Sal/Ani and Sal/Sal; p < 0.001). This is consistent with previous findings that the memory for the acquisition of contextual fear conditioning is dependent on protein synthesis (Abel et al., 1997).
Fear decreased similarly during extinction in groups given saline or anisomycin during extinction in each experiment (p < 0.001), suggesting that anisomycin did not block memories for extinction. There also were no differences among the groups when tested under saline (Fig. 5, T1) (F < 1.0), which shows that the memory established during extinction was independent of protein synthesis. Levels of freezing were similar during a retention test 10 d after extinction, showing that the memories for extinction were retained for long periods (Fig. 5A, T2). Although conditioned responding often shows spontaneous recovery after long retention intervals after extinction (e.g., Rescorla, 1997), all groups that received extinction in our experiments continued to show low levels of freezing after the 10 d retention interval. The failure to observe spontaneous recovery is not necessarily surprising, because spontaneous recovery often is a transient phenomenon and may depend critically on the conditions for performance as well as the amount of extinction and the retention interval (e.g., Rosas and Bouton, 1996). The observation that context-evoked freezing continued to be low 10 d after extinction suggests that the changes in behavior that occurred during extinction were long-lasting and independent of protein synthesis.
The similar pattern of results found in these three fear conditioning experiments is important because they occurred with different training and injection protocols. The results shown in Figure 5A were obtained using a spaced extinction protocol with pretrial injections. Neither decreasing the number of extinction trials per day nor administering post-trial injections revealed an effect of anisomycin on the rate of extinction (Fig. 5B). Spaced extinction trials with post-trial injections also resulted in no differences between mice that received injections of anisomycin or saline (Fig. 5C). Thus, the same general pattern of results occurred under a variety of extinction protocols and with both pretrial and post-trial injections of anisomycin, which controls for potential effects of anisomycin on performance during extinction.
The fear conditioning results shown in Figure 5C also demonstrate that the decrement observed during extinction was attributable to a learning process and not to simple forgetting or erasure of the previously established memory. Mice that received injections but not extinction did not differ and showed higher levels of freezing than the groups that underwent extinction (Fig.5C) (p < 0.001), suggesting that anisomycin injections over 3 d did not disrupt long-term consolidation or retention of the memory for contextual fear established during acquisition. This demonstrates that the decrement during extinction evident in Figure 5 cannot be attributable to forgetting the original learning or to the interference of anisomycin with long-term consolidation of the original learning (Riedel et al., 1999) but instead is attributable to the long-term changes in behavior caused by extinction. Thus, as in spatial learning, there is a dissociation between the requirement for protein synthesis in the memories formed during acquisition and extinction of context-evoked fear.
DISCUSSION
The critical finding from these experiments is that long-term memories can form in the absence of protein synthesis. Although protein synthesis was required for the acquisition of spatial preferences and contextual fear conditioning, memories for extinction occurred in the absence of protein synthesis. Many experiments have shown that instead of erasing the original memory for acquisition, extinction results in the establishment of new memories that can be retrieved long after the original learning has occurred (Bouton, 1993). Our findings therefore suggest that these long-term changes in memory can occur through protein synthesis-independent mechanisms.
These experiments also demonstrate a strong requirement for protein synthesis during acquisition of spatial learning and context-evoked fear. This is consistent with many findings from experiments using a variety of subjects and preparations (e.g., Abel et al., 1997;Bourtchouladze et al., 1998; Meiri and Rosenblum, 1998). In a spatial learning experiment, Meiri and Rosenblum (1998) found that anisomycin administered during acquisition blocked decreases in latencies to find the hidden platform, but because there was not a probe trial in that study, it is difficult to know the degree to which spatial learning was affected. Our probe trials revealed profound deficits in search paths after acquisition had occurred in the presence of anisomycin, thus reinforcing the idea that protein synthesis is important for spatial learning.
The effect on initial acquisition is strengthened by the similar effect on the acquisition of a reversal preference in the water maze. The deficit in the reversal experiment is important because it shows that mice that have formed a spatial preference before anisomycin injections do not form a new preference when subsequently trained in the presence of anisomycin. Cain and colleagues (1998) have found that the role of certain neurotransmitter systems in spatial learning is dependent on whether the animals are familiar with the behavioral requirements of the task. A similar conclusion can be drawn from the work of Bannerman et al. (1995), who found that the formation of a spatial preference in one swimming pool protects animals against the deleterious effects of NMDA receptor blockade. In our reversal experiment, the initial training established the basic search behaviors necessary for forming a preference, which means that any effect during reversal cannot be caused by unfamiliarity with the task. Instead, the failure of mice to develop a reversal preference under anisomycin demonstrates a learning or memory deficit as opposed to a performance deficit.
The procedures used in our experiments allow us to make strong inferences about the role of protein synthesis particularly on the extinction process without being confounded by residual effects on acquisition. In many experiments that use the reversal technique, the manipulation of interest occurs before initial acquisition, meaning that groups that differ in reversal learning often differ in acquisition learning, which makes performance during reversal difficult to interpret. A similar problem has been faced in experiments examining the effects of a neurobiological manipulation on extinction of fear conditioning (see Falls and Davis, 1995). In our experiments, extinction was investigated in groups that had acquired the task under normal conditions. Thus, in each experiment, performance during extinction was not confounded by residual effects of different acquisition treatments. Similarly, because the duration of acquisition and extinction trials was identical, the effects of protein synthesis inhibition were not confounded with differential exposure to the stimuli during acquisition and extinction. We also used both pretrial and post-trial injections, which strengthens the idea that our results do not reflect differential effects on performance during acquisition and extinction. Thus, the striking difference between the effectiveness of anisomycin injections on acquisition and extinction appears to be attributable specifically to differential effects on acquisition and extinction processes.
In addition to effects on acquisition and extinction, these experiments also provide an opportunity to assess the necessity of protein synthesis for the retrieval of previously formed memories. In each of our experiments, mice that received acquisition with saline showed normal retrieval of acquisition learning during the first trial of extinction in the presence of anisomycin. On the first trial of reversal learning in the water maze, mice that received injections of anisomycin showed retrieval similar to that of mice that received injections of saline. Similarly, groups given anisomycin or saline did not differ in performance on the initial extinction trials or in overall rate of extinction, suggesting that the memories formed during acquisition and extinction could be retrieved independent of protein synthesis.
This finding of protein synthesis-independent retrieval is consistent with previous findings from spatial learning and contextual fear conditioning (Bourtchouladze et al., 1998; Meiri and Rosenblum, 1998). There are suggestions, however, that retrieval may induce a period of reconsolidation that depends on protein synthesis (Nader et al., 2000). Using a cued fear conditioning procedure, Nader et al. (2000) found that injections of anisomycin into the amygdala after a retrieval test decreased performance to the cue during a subsequent retrieval test. In our experiments, post-trial injections of anisomycin and saline resulted in similar decrements in performance, suggesting that the decremental process that occurred during extinction was independent of protein synthesis. It is important to note that our spatial-learning and contextual fear conditioning tasks are sensitive to hippocampal lesions, whereas the cued fear conditioning studied by Nader et al. (2000) is not. However, it also should be noted that both contextual and cued fear conditioning are sensitive to amygdala lesions, and our systemic injections would affect the amygdala as well as the hippocampus (e.g., Abel et al., 1997).
A recent paper by Berman and Dudai (2001) suggests that protein synthesis may be important for extinction of conditioned taste aversions. They found that anisomycin injected into the insular cortex blocked memories for extinction of taste aversion learning. One explanation for the different pattern of results found by our experiments and those by Berman and Dudai is that behavioral preparations that involve different brain structures might have unique requirements for protein synthesis during extinction. It also is possible that the requirement for protein synthesis depends on the nature of the task (also see Flood et al., 1977). Another difference is the amount of exposure to the stimulus that occurs during extinction. In conditioned taste aversion, the animal generally samples a much smaller amount of the flavor on the first extinction trial than it does during acquisition trials. It is therefore possible that brief exposures to stimuli as in the study by Berman and Dudai (2001) will result in a dependence on protein synthesis, but longer exposures may not. By holding exposure constant during acquisition and extinction, we can conclude that the protein synthesis requirements of acquisition and extinction differ in our tasks.
The major implication from these experiments is that the molecular processes that underlie long-term behavioral changes following acquisition and extinction may be quite different. Behavioral experiments have shown that the changes in behavior that occur during extinction do not reflect changes in the strength of the original memory but instead reflect the superimposition of a depressive process on that original association (for review, see Rescorla, 2001). Our experiments suggest that this depressive process may have different molecular properties from the process that underlies acquisition. Experiments at the systems level also have suggested differences in the neurobiology of acquisition and extinction. The ventromedial prefrontal cortex, which does not appear to play a critical role in acquisition, may be important for retaining memories of extinction after fear conditioning (Quirk et al., 2000), although other findings question the importance of this structure in extinction (Gewirtz et al., 1997).
On a molecular level, there is evidence that the NMDA receptor may be important for extinction, suggesting that calcium may be a key second messenger in this process (Falls et al., 1992; Baker and Azorlosa, 1996; Johnson et al., 2000). A molecular account of extinction therefore may need to incorporate mechanisms that are calcium-dependent but protein synthesis-independent. Candidate molecular processes that might mediate long-term changes in neural function independent of gene induction and new protein synthesis include alterations in the neuronal cytoskeleton (Kennedy, 1997; Craven and Bredt, 1998; van Rossum and Hanisch, 1999), autophosphorylation of protein kinases (Lisman, 1985), and proteolysis (Lynch and Baudry, 1984). Cytoskeletal changes could result in changes in the postsynaptic density, thereby altering the subcellular localization of NMDA and AMPA receptors and changing the morphology and efficiency of the synapse (Kennedy, 1997; Craven and Bredt, 1998; van Rossum and Hanisch, 1999). Whatever the mechanism, these experiments demonstrate that the requirement for protein synthesis in the acquisition and extinction of spatial preferences and context-evoked fear differs. Whereas memories for the acquisition of spatial locations and context–shock associations failed to form in the presence of anisomycin, memories for extinction formed readily and persisted across days. These findings suggest that the study of the neurobiological basis of extinction may reveal novel cellular regulatory mechanisms involved in mediating long-lasting changes in memory and behavior.
Footnotes
This research was supported by a National Research Service Award postdoctoral fellowship and a National Institutes of Health neuropsychopharmacology training grant to K.M.L. and by grants from the Merck Foundation, National Institutes of Health, University of Pennsylvania Research Foundation, and Whitehall Foundation to T.A. We thank Mike Mullen for assistance with data collection and Michael Nusbaum for comments on this manuscript.
Correspondence should be addressed to Ted Abel, Department of Biology, 3740 Hamilton Walk, University of Pennsylvania, Philadelphia, PA 19104. E-mail: abele@sas.upenn.edu.
REFERENCES
- 1.Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 2.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 3.Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 5.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 6.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- 7.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Cain DP. Testing the NMDA, long-term potentiation, and cholinergic hypotheses of spatial learning. Neurosci Biobehav Rev. 1998;22:181–193. doi: 10.1016/s0149-7634(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 9.Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 10.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 11.Falls WA, Davis M. Behavioral and physiological analysis of fear inhibition. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and clinical consequences of stress: from normal adaptation to PTSD. Lippincott-Raven; Philadelphia: 1995. pp. 177–202. [Google Scholar]
- 12.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 14.Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiol Behav. 1973;10:555–562. doi: 10.1016/0031-9384(73)90221-7. [DOI] [PubMed] [Google Scholar]
- 15.Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. Protein synthesis inhibition and memory for pole jump active avoidance and extinction. Pharmacol Biochem Behav. 1977;7:71–77. doi: 10.1016/0091-3057(77)90013-2. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DM, Baker JD, Azorlosa JL. Acquisition, extinction, and reinstatement of Pavlovian fear conditioning: the roles of the NMDA receptor and nitric oxide. Brain Res. 2000;857:66–70. doi: 10.1016/s0006-8993(99)02388-4. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 19.Konorski J. Integrative activity of the brain, an interdisciplinary approach. University of Chicago; Chicago: 1967. [Google Scholar]
- 20.Lattal KM, Abel T (2000) The role of protein synthesis in extinction of spatial preferences. Paper presented at the Annual Meeting of the Society for Neuroscience, New Orleans, LA, October.
- 21.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci USA. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- 23.Meiri N, Rosenblum K. Lateral ventricle injection of the protein synthesis inhibitor anisomycin impairs long-term memory in a spatial memory task. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 24.Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 25.Pavlov IP. Conditioned reflexes, an investigation of the physiological activity of the cerebral cortex. Oxford UP; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rescorla RA. Inhibitory associations between S and R in extinction. Anim Learn Behav. 1993;21:327–336. [Google Scholar]
- 28.Rescorla RA. Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Anim Learn Behav. 1997;25:99–107. [Google Scholar]
- 29.Rescorla RA. Experimental extinction. In: Mowrer RR, Klein S, editors. Handbook of contemporary learning theories. Erlbaum; Mahwah, NJ: 2001. pp. 119–154. [Google Scholar]
- 30.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning. II. Current research and theory. Appleton-Century-Croft; New York: 1972. pp. 64–99. [Google Scholar]
- 31.Riedel G, Micheau J, Lam AG, Roloff E, Martin SJ, Bridge H, Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 32.Rosas JM, Bouton ME. Spontaneous recovery after extinction of a conditioned taste aversion. Anim Learn Behav. 1996;24:341–348. [Google Scholar]
- 33.van Rossum D, Hanisch UK. Cytoskeletal dynamics in dendritic spines: direct modulation by glutamate receptors? Trends Neurosci. 1999;22:290–295. doi: 10.1016/s0166-2236(99)01404-6. [DOI] [PubMed] [Google Scholar]