Abstract
The cytokine interleukin-1 (IL-1) has been strongly implicated in the pathogenesis of ischemic brain damage. Evidence to date suggests that the major form of IL-1 contributing to ischemic injury is IL-1β rather than IL-1α, but this has not been tested directly.
The objective of the present study was to compare the effects of transient cerebral ischemia [30 min middle cerebral artery occlusion (MCAO)] on neuronal injury in wild-type (WT) mice and in IL-1α, IL-1β, or both IL-1α and IL-1β knock-out (KO) mice.
Mice lacking both forms of IL-1 exhibited dramatically reduced ischemic infarct volumes compared with wild type (total volume, 70%; cortex, 87% reduction). Ischemic damage compared with WT mice was not significantly altered in mice lacking either IL-1α or IL-1β alone. IL-1β mRNA, but not IL-1α or the IL-1 type 1 receptor, was strongly induced by MCAO in WT and IL-1α KO mice.
Administration (intracerebroventricularly) of recombinant IL-1 receptor antagonist significantly reduced infarct volume in WT (-32%) and IL-1α KO (-48%) mice, but had no effect on injury in IL-1β or IL-1α/β KO mice.
These data confirm that IL-1 plays a major role in ischemic brain injury. They also show that chronic deletion of IL-1α or IL-1β fails to influence brain damage, probably because of compensatory changes in the IL-1 system in IL-1α KO mice and changes in IL-1-independent mediators of neuronal death in IL-1β KO mice.
Keywords: interleukin-1, cytokines, stroke, brain, knock-out, mouse
Interleukin-1 (IL-1) is a proinflammatory cytokine that has been identified as an important mediator of neurodegeneration induced by experimental cerebral ischemia (stroke) or excitatory or traumatic brain injury in rodents (Touzani et al., 1999; Rothwell and Luheshi, 2000). Both IL-1 ligands (IL-1α and IL-1β) are produced rapidly in the brains of rodents exposed to cerebral ischemia (Wang et al., 1994; Hillhouse et al., 1998; Zhang et al., 1998), and recombinant IL-1β administered intracerebroventricularly, or directly into the brain, enhances ischemic and other forms of injury (Yamasaki et al., 1995; Loddick and Rothwell, 1996; Lawrence et al., 1998; Stroemer and Rothwell, 1998;Allan et al., 2000). Conversely, blocking IL-1 actions, by administration of the naturally occurring and selective IL-1 receptor antagonist (IL-1ra), markedly reduces neuronal loss and inflammation induced by a variety of experimental brain insults (Relton and Rothwell, 1992; Lin et al., 1995; Betz et al., 1995; Garcia et al., 1995; Yamasaki et al., 1995; Loddick and Rothwell, 1996; Stroemer and Rothwell, 1998; Yang et al., 1998).
Despite the extensive evidence implicating IL-1 in ischemic brain damage, little information is available on the relative contribution of IL-1α and IL-1β (Hill et al., 1999; Yu and Lau, 2000). Both cytokines are induced in response to cerebral ischemia (Touzani et al., 1999) or excitotoxicity, and central administration of IL-1β exacerbates damage (Allan et al., 1998, 2000; Lawrence et al., 1998).Yamasaki et al. (1995) reported that intracerebroventricular injection of neutralizing anti-IL-1β antibody to rats reduces ischemic brain damage, implicating IL-1β as the major mediator of injury. Comparatively, only little information is available in the literature concerning the role of IL-1α in ischemic brain injury (Hill et al., 1999; Touzani et al., 1999).
Furthermore, mice lacking the gene for caspase-1 or expressing a dominant negative form of caspase-1 (the enzyme that is required to cleave inactive pro-IL-1β to the mature active form), also exhibit reduced ischemic brain damage (Friedlander et al., 1997; Hara et al., 1997; Schielke et al., 1998; Liu et al., 1999). Again this suggests a primary role for IL-1β. However, caspase-1 also cleaves another cytokine pro IL-18 (Akita et al., 1997), and mice lacking the enzyme show reduced release of IL-1α as well as IL-1β (Li et al., 1997).
Horai et al. (1998) have reported that febrile responses to turpentine (which are modulated by IL-1 actions in the CNS) are diminished in mice lacking IL-1β, but not in those in which the IL-1α gene is deleted, and that IL-1α is markedly reduced in the brains of mice lacking IL-1β. This again suggests that IL-1β is the primary IL-1 ligand in the brain.
Thus, the objective of the present study was to identify the roles of IL-1α and IL-1β in ischemic brain damage by studying mice lacking either or both of these genes. A secondary aim was to determine whether deletion of one of these IL-1 ligands genes leads to a compensatory increase in the other and to test the effects of IL-1ra on ischemic brain damage in mice lacking IL-1α or β.
MATERIALS AND METHODS
Mice. All mice used were adult males. Mice, in which genes for IL-1α and/or IL-1β (IL-1α KO, IL-1β KO, and IL-1αβ KO) were deleted by homologous recombination [knock-out (KO) mice], as described previously (Horai et al., 1998) were raised on C57BL/6 background and bred in the local animal facilities. All KO mice were born healthy and no major differences in growth or weight were found (Horai et al., 1998 and present study). C57BL/6 wild-type (WT) mice were supplied by Charles River (Kent, UK). No overt differences were observed between IL-1 KO and WT mice. The animals were housed in a controlled environment with a 12 hr light/dark cycle (8:00 A.M./8:00 P.M.) at 22°C. Validation of IL-1 KO mice has been performed by RT-PCR (see Materials and Methods). All experiments were performed in accordance with United Kingdom legislation under the 1986 Animals (Scientific Procedures) Act.
Surgical procedures. The animals were studied at 3–6 months of age and weighed between 22 and 31 gm at the time of use. Anesthesia was induced by inhalation of 4% halothane in a NO2/O2 (70/30%) mixture and maintained by inhalation of 1.5∼2% halothane in a NO2/O2 (70/30%) mixture. Body temperature was monitored throughout surgery (via rectal probe), and animals were maintained normothermic (37.4 ± 0.4°C) by a heating blanket (Homeothermic Blanket Control Unit; Harvard Apparatus Limited).
For intracerebroventricular injection, guide cannulas were implanted stereotaxically (bregma −0.22 mm, lateral +1 mm, depth −2.5 mm) (Franklin and Paxinos, 1997) into the right lateral cerebral ventricle of the brain under halothane anesthesia (1.5% in NO2/O2, 70/30%), 5–7 d before middle cerebral artery occlusion (MCAO), to permit administration of substances intracerebroventricularly.
Temporary middle cerebral artery occlusion. Laser doppler flowmetry (Moor Instruments) was used to monitor cerebral blood flow (CBF) during the surgical procedure before and after MCAO for 7 min, and then the probe was disconnected to let the animals recover from the anesthesia. A small incision was made in the skin overlying the temporalis muscle, then a 0.7 mm, flexible, laser Doppler probe (model P10) was positioned on the superior portion of the temporal bone (6 mm lateral and 2 mm posterior from the bregma) and secured with glue. This position corresponded to the center of the ischemic territory.
Focal cerebral ischemia was induced by occlusion of the right middle cerebral artery (MCA) using the intraluminal filament technique (Clark et al., 1997) with the following modifications. After a midline, neck incision, the common carotid artery (CCA) and external carotid artery were isolated and ligated with 6.0 silk suture. A 6.0 silk suture was placed around the internal carotid artery to avoid bleeding through the arteriotomy when introducing the filament into the CCA. A nylon monofilament (Drennan; diameter, 83 μm) coated with “thermomelting” glue (1.5∼2 mm long, diameter 180 μm) was introduced through an incision in the CCA and advanced gently up to the origin of the MCA. Cerebral blood flow was monitored for the following 7 min. Thereafter, to limit the effect of the anesthesia on infarct size, animals were allowed to recover from anesthesia. Thirty minutes after induction of MCAO, mice were reanesthetized, and the occluding filament was withdrawn gently back into common carotid to allow reperfusion. In sham-operated mice, the same surgical procedure was performed, except that the filament was not advanced to occlude the MCA.
Intracardiac perfusion. Intracardiac perfusion was performed for cerebrovasculature assessment and PCR studies. Mice were anesthetized with pentobarbital (Pentobarbitone sodium; 250 mg/kg, i.p.). An incision was performed along the thorax to expose the heart, and the left cardiac ventricle was cannulated.
For cerebrovasculature assessment, animals were perfused with 4% PFA for 5 min (2 ml/min), followed by ink (Quink Parker) diluted in 4% PFA (1:5 v/v) (n = 8 per strain). Brains were removed carefully, left in 4% PFA overnight, and the Circle of Willis and major arteries were carefully examined under microscope (Meiji, Encinitas, CA and SV Micro digital camera, SoundVision).
For PCR studies, 24 hr after induction of MCAO, mice were perfused with a solution of diethylpyrocarbonate (DEPC 0.1%/NaCl 0.9%) for 5 min (2 ml/min) to limit RNA degradation in brain samples to be used for PCR measurement.
Administration of substances. Vehicle (NaCl 0.9%/BSA 0.1%) or IL-1ra (5 gm/l in NaCl 0.9%/BSA 0.1%) were administered randomly into the cerebral ventricles 30 min before occlusion and 10 min after reperfusion (i.e., 40 min after induction of MCAO). For each injection, a volume of 0.5 μl was infused over 5 min. Human recombinant IL-1ra was a generous gift from Dr. Steve Poole (National Institute for Biological Standards and Control, Herts, UK)
Measurement of infarct volume. Twenty-four hours after MCAO, mice were killed by anesthetic overdose with halothane and decapitated. Brains were removed and frozen in cooled (−40°C) isopentane. Coronal brain sections were cut serially (20 μm at 300 μm intervals) on frozen brains by cryostat and stained with cresyl fast violet to identify viable tissue. Infarcted areas were assessed blindly and delineated by the relative paleness of histological staining in the ischemic tissue. Infarct volumes were calculated by the integration of infarcted areas on each brain slice, as quantified with a computer-assisted image analyzer (SigmaScan 5.0; SPPS, Chicago, IL). To correct for the effect of edema, the total infarcted area was also determined indirectly by subtracting the area of the healthy tissue in the ipsilateral hemisphere from the area of the contralateral hemisphere on each section, edema was then estimated by the difference between volumes corrected and not corrected for edema (Osborne et al., 1987; Lin et al., 1993).
Semiquantitative PCR. After DEPC–saline perfusion (as described above), ipsilateral and contralateral cortices were dissected on ice under a surgical microscope (Stemi SV11; Leica-Zeiss), and frozen on dry ice. RNA Trizol (Life Technologies, Gaithersburg, MD) extraction and RT-PCR were then performed on the tissue samples. To quantify expression of IL-1α, IL-1β, and IL-1RI genes, their PCR products were compared with constitutively expressed genes: β-actin for IL-1α and IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for IL-1RI (sequences of the primers are described in Table 1).
Table 1.
Primer sequences used for PCR
| Gene | Primer | Sequence (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| β-Actin | Forward | CAGATCATGTTTGAGACCTTC | 493 |
| Reverse | ACTTCATGATGGAATTGAATG | ||
| GAPDH | Forward | TGAATGACATCAAGAAGGTGGTGGAG | 239 |
| Reverse | TCCTTGGAGGCCATGTAGGCCAT | ||
| IL-1α | Forward | CAAACTGATGAAGCTCGTCA | 225 |
| Reverse | TCTCCTTGAGCGCTCACGAA | ||
| IL-1β | Forward | CTGTGTCTTTCCCGTGGACC | 200 |
| Reverse | CAGCTCATATGGGTCCGACA | ||
| IL-1RI | Forward | ACCCCCATATCAGCGGACCG | 429 |
| Reverse | TTGCTTCCCCCGGAACGTAT |
Primers for IL-1α, IL-1β, and IL-1RI are from Gabellec et al (1997).
The PCR program was 5 min at 95°C, then cycles of denaturation at 95°C for 30 sec, annealing at 55°C (IL-1α, IL-1β, β-actin, and GAPDH) or 58°C (IL-1RI) for 45 sec, and extension at 72°C for 40 sec repeated for 35 cycles, with a 5 min final extension period at 72°C. Thereafter, PCR products were loaded on 2% agarose gel and quantified using computer-assisted image analyzer (SigmaScan 5.0; SPPS) and expressed as a ratio of the control gene. The absence of PCR products in control samples without reverse transcription of RNA and size of PCR products as described by Gabellec et al. (1997a) were confirmed (Table 1).
Statistical analyses. All data are presented as mean ± SEM. To determine differences between groups, a one-way ANOVA (Statview 5.0; SAS Institute, Cary, NC) was performed for infarct volumes and CBF data with one factor: strain (WT, IL-1α KO, IL-1β KO, or IL-1αβ KO), or a multiparametric ANOVA with two factors: strain and treatment (saline or IL-1ra), when treatment was applied. Because such analysis for infarct volume revealed a significant (p < 0.05) principal effect for the two factors studied and interactions between them, a subsequent one-way ANOVA was therefore performed, followed by Scheffé post hoc test when required. Incidence of subcortical damage was assessed through the use of χ2 test.
PCR results were analyzed by the mean of a Kruskal–Wallis nonparametric test followed, when significant differences between groups were highlighted, by a Mann–Whitney U test to determine differences between control versus MCAO and sham versus MCAO. For all statistical analyses, the significance level accepted wasp < 0.05.
RESULTS
Cerebral blood flow measurement and cerebrovascular anatomy
Examination of circle of Willis and gross cerebrovascular anatomy did not reveal any differences among any of the strains studied (data not shown).
Doppler monitoring showed that reductions in CBF caused by insertion of the thread were similar in all strains (percentage fall in CBF from preischemic value: WT, 79 ± 0.1; IL-1α KO, 76 ± 0.1; IL-1β KO, 77 ± 0.1; and IL-1αβ KO, 80 ± 0.1%). Similar results were observed in animals injected with saline or IL-1ra (decrease in CBF expressed as a percentage of preischemic value: WT/saline, 86 ± 0.1; WT/IL-1ra, 80 ± 0.1; IL-1α KO/saline, 82 ± 0.1; IL-1α KO/IL-1ra, 83 ± 0.1; IL-1β KO/saline, 85 ± 0.1;IL-1β/IL-1ra: 85 ± 0.1%).
Effect of IL-1α and/or IL-1β gene deletion on cerebral ischemia
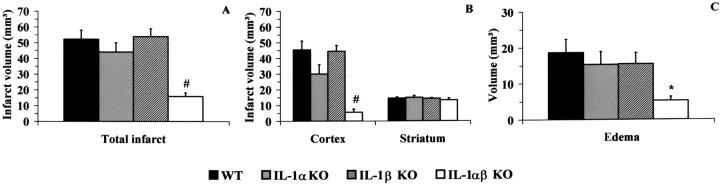
No significant differences in total infarct volumes and edema (Figs. 1, 2A,C) were found between WT mice and mice in which IL-1α or IL-1β genes had been deleted. Cortical infarct volumes were also similar in the WT, IL-1α KO, and IL-1β KO mice (Fig. 2B). Conversely, in mice lacking genes for both IL-1α and IL-1β (IL-1αβ KO), total infarct volume was significantly smaller than WT (−70%) or either IL-1α KO (−64%) or IL-1β KO mice (−71%; p < 0.01) (Figs. 1, 2A). Cortical infarct volumes were also markedly reduced in the IL-1αβ KO mice (−87% vs WT and IL-1β KO mice and −81% vs IL-1α KO mice) (Fig. 2B). Similarly, edema was significantly smaller in IL-1αβ KO mice compared with WT (−71%,p < 0.05), but the latter did not reach statistical significance when compared with IL-1α KO and IL-1β KO despite a similar reduction of edema (−66% IL-1αβ KO vs IL-1α KO and IL-1β KO). No significant change in striatal infarct volume was observed between any of the strains studied (Fig.2B). Similar results were observed in total and cortical infarct after correction for edema.
Fig. 1.
Representative coronal brain sections (20 μm) of WT, IL-1α KO, IL-1β KO, and IL-1αβ KO mice 24 hr after 30 min of middle cerebral artery occlusion.
Fig. 2.
Effect of 30 min middle cerebral artery occlusion on infarct volume (A, total; B, cortex and striatum) and edema (C) in WT, IL-1α KO, IL-1β KO, and IL-1αβ KO mice (n = 9 per group). Volumes are expressed in cubic millimeters (mean ± SEM). *Significantly different to WT, and #significantly different to WT, IL-1α KO, and IL-1β KO mice, respectively (p < 0.05, ANOVA followed by Scheffepost hoc test).
IL-1α and IL-1β mRNA expression in WT, IL-1α KO, and IL-1β KO mice
In cortices ipsilateral to MCAO, IL-1α mRNA was expressed constitutively in WT, but not in IL-1β KO mice (Fig.3A). Cerebral ischemia did not induce significant changes in IL-1α expression in WT animals. The level of IL-1α mRNA in IL-1β KO mice was increased in sham-operated groups and MCAO groups when compared with control group, but the overall changes were not statistically significant (p = 0.06; Kruskal–Wallis test), and no difference was observed between sham-operated and MCAO groups (Fig.3A).
Fig. 3.

Representative PCR gels, with corresponding image analysis quantification for IL-1α (A), IL-1β (B), and IL-1RI (C) in ipsilateral cortices in WT (control, n = 4; sham,n = 3; MCAO, n = 4), IL-1α KO (control, n = 3; sham, n = 3; MCAO, n = 4), and IL-1β KO mice (control,n = 3; sham, n = 4; MCAO,n = 5). Data are expressed as the ratio of the gene of interest to the relevant control gene (β-actin for IL-1α and IL-1β and GAPDH for IL-1RI; mean ± SEM). Significantly different from control (*) and sham-operated (#) groups (p < 0.05; Mann–WhitneyU test).
Conversely, RT-PCR for IL-1β mRNA showed that IL-1β was not expressed constitutively in WT or IL-1α KO mice, but was induced strongly by cerebral ischemia in both WT (+323% in MCAO group vs sham group) and IL-1α KO mice (Fig. 3B). IL-1RI mRNA expression was not altered by cerebral ischemia (Fig. 3C). Similar results were found in the striatum and other subcortical brain areas (data not shown).
Effect of intracerebroventricular injection of IL-1ra in WT, IL-1α KO, and IL-1β KO mice
To assess the influence of endogenous IL-1 on ischemic brain damage in IL-1α KO and IL-1β KO mice, the effect of injection of IL-1ra on brain damage was compared with vehicle treatment in separate groups of animals exposed to MCAO.
Only minor differences in infarct volume were observed between WT and IL-1α KO and IL-1β KO mice receiving saline. Cortical infarct size, but not total infarct volume, was slightly reduced in IL-1α KO (Fig.4B) when compared with WT. In IL-1β KO mice receiving saline, only cortical infarct volume not corrected for edema was significantly reduced (−27%; data not shown) compared with the WT. When treated with saline, edema was reduced in IL-1α and IL-1β KO mice compared with WT but reached statistical significance only in IL-1β KO mice (Fig. 4C).
Fig. 4.
Comparison of total (A) and cortical infarct (B) volumes, corrected for edema (expressed in cubic millimeters; mean ± SEM) and edema (C) between saline and IL-1ra treatments, in WT, IL-1α KO, and IL-1β KO mice (WT/saline, n = 13; WT/IL1-ra,n = 10; IL-1α KO/saline, n = 10; IL-1α KO/IL-1ra, n = 10; IL-1β KO/saline,n = 12; IL-1β KO/IL-1ra, n = 11). *Indicates significant difference from saline-treated group. #Indicates a significant difference from WT receiving identical treatment (p < 0.05, ANOVA followed by Scheffe post hoc test).
Intracerebroventricular injection of IL-1ra reduced significantly total and cortical infarct size in WT (−32 and −37%) and IL-1α KO (−48 and −56%), but not in IL-1β KO mice (Fig. 4). Similar results were observed for infarct volumes without correction for edema (data not shown). Moreover, cortical infarct volume was reduced significantly in IL-1α KO mice receiving IL-1ra when compared with IL-1β KO (Fig.4). IL-1ra did not significantly affect the size of the lesion in the striatum (data not shown). No differences were observed in subcortical infarct size between strains or treatment, although IL-1ra reduced the incidence of subcortical damage in WT mice (mice with subcortical damage/total number of mice: WT/IL-1ra, 4/10 vs WT/saline, 12/13;p < 0.01; χ2 test). IL-1ra had no effect in IL-1β KO (Fig. 4) or IL-1αβ KO mice [total infarct: IL-1αβ KO/saline, 12.1 ± 1.9 mm3 (n = 4) vs IL-1αβ KO/IL-1ra 22.4 ± 4.7 mm3(n = 5) mean ± SEM].
DISCUSSION
Occlusion of the MCAO induced reproducible brain damage in wild-type mice, resulting in neuronal loss in the cortex and striatum that was accompanied by edema (Figs. 1, 2). Subcortical (hippocampus and/or thalamus) brain areas were also infarcted in ∼50% of mice, but no statistical differences in subcortical infarct size were observed between strains.
In the present study, we observed no differences in the cerebrovascular architecture (defined from injection of carbon black) or the severity of the ischemic insult (assessed from cerebral blood flow) between IL-1 knock out (IL-1α, IL-1β, or IL-1αβ KO) and wild-type mice. Consequently, we demonstrated that simultaneous deletion of both IL-1α and IL-1β (IL-1αβ KO) genes caused a marked reduction (approximately −85% for cortical areas and approximately −70% for total infarct volume) in ischemic brain damage (Figs. 1, 2). Similarly, brain edema was significantly reduced in IL-1αβ KO mice (−70%) when compared with WT mice. These observations support a major role for endogenous IL-1 in ischemic brain damage. It seems unlikely that this dramatic effect might be related to changes in body temperature or CBF because such parameters were not significantly different between strains.
Relatively few publications have reported changes in expression of the IL-1 family, such as IL-1α and IL-1RI, after stroke (Hill et al., 1999; Yu and Lau, 2000), yet it has been assumed that IL-1β is the primary form of IL-1 involved in ischemic brain damage (Buttini et al., 1994; Davies et al., 1999; Touzani et al., 1999), and in other local and systemic responses to peripheral injury (Luheshi et al., 1997;Eriksson et al., 1999). Indeed Horai et al. (1998) studying the same mice as those used in the present study, report that IL-1β KO mice, but not those lacking IL-1α, exhibit reduced responses to the effects of systemic injection of turpentine on fever. Similarly, Kozak et al. (1995) have reported a reduced febrile response to lipopolysaccharide in IL-1β KO mice. Conversely Alheim et al. (1997) have shown a potential hyper-responsive fever in IL-1β-deficient mice, suggesting that in fever induction as well as in cerebral ischemia, IL-1β could have an important, but not obligatory role in the brain as a proinflammatory cytokine.
IL-1β appears to regulate IL-1α expression because the latter is reduced in mice lacking IL-1β or caspase-1 (Dinarello, 1997; Horai et al., 1998). This has been confirmed in the present study by RT-PCR in IL-1β KO mice. Similarly, we also observed markedly reduced expression of IL-1α in microglia from mice in which IL-1β gene is deleted (D. Brough, R. Le Feuvre, and N. Rothwell, unpublished data), which is consistent with reports that IL-1β regulates IL-1α expression. On the basis of these observations we predicted that deletion of IL-1β (but perhaps not IL-1α) would limit ischemic brain damage. However, we found in the present study that infarct volume and edema were almost identical in mice lacking IL-1α or IL-1β when compared with WT mice.
Thus, the effects of chronic inhibition of IL-1β through gene deletion appear to differ from acute inhibition of IL-1β by immunoneutralization (Yamasaki et al., 1995). It seems most likely that chronic deletion of IL-1α or IL-1β failed to influence brain damage because of a compensatory increase in the other form of IL-1. To test this hypothesis, we first studied the expression of IL-1α, IL-1β, and IL-1RI mRNA after MCAO by semiquantitative RT-PCR. As described previously (Touzani et al., 1999; Legos et al., 2000), IL-1β mRNA was strongly induced by cerebral ischemia in WT (fourfold increase; MCAO vs sham groups) and in IL-1α KO mice (Fig. 3B). IL-1α was induced in sham-operated and MCAO groups when compared with the control group, however because there was no difference between sham-operated and ischemic group, it seems that the induction of IL-1α observed here was more likely related to the surgery. Conversely, no significant changes in IL-1RI expression were observed in any strain studied. Previous studies have reported increased expression of IL-1α mRNA after focal ischemia in mice (Hill et al., 1999) and IL-1RI after global ischemia or focal ischemia in rats (Sairanen et al., 1997; Wang et al., 1997). The apparent discrepancy between the results we describe here and these reports could be attributable to differences between the models of cerebral ischemia (global vs focal), techniques used (in situ hybridization), and/or the time point investigated. However, the latter seems unlikely for IL-1RI because the previous reports have shown that the induction of IL-1RI was prolonged for up to 24 hr after ischemia (Sairanen et al., 1997).
It is possible that deletion of one form of IL-1 results in compensatory changes in some other aspects of the IL-1 system or in postreceptor signaling such that ischemic brain damage is not affected. We attempted to test this possibility by studying the effects of IL-1ra in mice lacking IL-1α, IL-1β, or both IL-1α and β. We predicted that if the IL-1 system was upregulated in IL-1α KO or IL-1β KO mice, IL-1ra treatment should reduce damage in these mice. As reported previously in slightly different models of cerebral ischemia in rats and mice (Garcia et al., 1995; Loddick and Rothwell, 1996; Stroemer and Rothwell, 1997; Touzani et al., 1999), injection of recombinant IL-1ra (intracerebroventricularly) in wild-type mice reduced ischemic infarct volume by ∼35% (Fig. 4). Reduction of infarct size by IL-1ra was not associated with changes in physiological parameters such as body temperature in the present or earlier studies (Betz et al., 1995;Loddick and Rothwell, 1996) or CBF (see Touzani et al., 1999). This finding supports the involvement of IL-1 in ischemic brain damage. The reduction in ischemic brain damage in these IL-1α/β KO mice (approximately −70 to −80%) was greater than the effect of IL-1ra reported in this and in earlier studies (approximately −30 to −60%; for review, see Touzani et al., 1999). Because we have used the optimal dose of IL-1ra (Stroemer and Rothwell, 1997; Touzani et al., 1999), the difference in ischemic damage between IL-1ra-treated animals and deletion of IL-1α and IL-1β may reflect poor brain penetration of IL-1ra or instability of the protein in the brain. However, it could also suggest that IL-1ra does not fully block effects of endogenous IL-1 on type I IL-1 receptor or another unidentified IL-1 signaling receptor or receptors. Intracerebroventricular injection of IL-1ra significantly reduced total (−48%) and cortical (−54%; IL-1ra vs saline) infarct sizes in IL-1α KO mice, but had no effect in IL-1β KO mice (Fig. 4). In contrast to untreated mice in which no difference between strains was observed, a slight decrease in cortical infarct size was observed in IL-1α and IL-1β KO mice receiving saline compared with WT saline-treated animals, probably because of reduction in edema, which can be modified by intracerebroventricular cannulation and/or injection, when compared with untreated mice. Total infarct volumes in mice receiving saline were not significantly different.
These data suggest that in IL-1α KO mice, there was some compensatory upregulation in IL-1 system, possibly at the level of translation, cleavage, or release of IL-1β, expression of IL-1ra, or downstream of IL-1R1, which was inhibited by IL-1ra treatment. In contrast, IL-1ra was ineffective in IL-1β KO mice, indicating that the IL-1 system might not contribute to the ischemic injury in these mice, but that there may have been compensatory changes in other mediators of injury, such as other cytokines, glutamate, or free radicals. This potential compensation for gene deletion is important in considering all data from knock-out animals. In parallel, the tendency of IL-1α KO mice to have a smaller infarct than the WT or IL-1β KO mice could suggest that any actions of endogenous IL-1α in the IL-1β KO mice were not fully antagonized by administration of IL-1ra. It could also suggest that IL-1α is acting on another subtype of IL-1 receptor than the type I. These data suggest that chronic deletion of IL-1β (and probably IL-1α) has different effects on ischemic brain damage to acute inhibition of IL-1 and that IL-1β KO mice differ from those lacking the caspase-1 gene.
Summary
Overall, the data suggest that IL-1 is a major contributor to ischemic brain damage because deletion of both IL-1α and IL-1β leads to a massive reduction in injury. Deletion of IL-1α apparently results in some compensatory changes in the IL-1 system such that overall damage is unaffected. Chronic deletion of IL-1β (unlike acute intervention) does not result in reduced ischemic brain damage presumably because of upregulation of other mediators of injury.
Footnotes
This work was supported by the Medical Research Council (United Kingdom). We thank Drs. Sarah Loddick and Stuart Allan for their expert comments, and Catherine Smith, Anthea Hughes, and Sally Shepperd for their contribution to this work.
Correspondence should be addressed to Prof. N. J. Rothwell, School of Biological Sciences, 1.124 Stopford Building, University of Manchester, Oxford Road, Manchester M13 9PT, UK. Email:Nancy.Rothwell@man.ac.uk
REFERENCES
- 1.Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MS, Fujii M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M, Kurimoto M. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;272:26595–26603. doi: 10.1074/jbc.272.42.26595. [DOI] [PubMed] [Google Scholar]
- 2.Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Malinowsky D, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T. Hyperresponsive febrile reactions to interleukin (IL)-1alpha and IL-1beta, and altered brain cytokine mRNA and serum cytokine levels, in IL-1beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:2681–2686. doi: 10.1073/pnas.94.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan SM, Lawrence CB, Grundy RP, Stroemer RP, Rothwell NJ. Sites and mechanisms of interleukin-1 action. In: Krieglstein J, Oberpichler-Schwenk H, editors. Pharmacology of cerebral ischemia. Medpharm Scientific; Stuttgart: 1998. pp. 395–399. [Google Scholar]
- 4.Allan SM, Parker LC, Collins B, Davies R, Luheshi GN, Rothwell NJ. Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat. Proc Natl Acad Sci USA. 2000;97:5580–5585. doi: 10.1073/pnas.090464197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15:547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- 6.Buttini M, Sauter A, Boddeke HW. Induction of interleukin-1 beta mRNA after focal cerebral ischaemia in the rat. Mol Brain Res. 1994;23:126–134. doi: 10.1016/0169-328x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 7.Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- 8.Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson C, van Dam AM, Lucassen PJ, Bol JG, Winblad B, Schultzberg M. Immunohistochemical localization of interleukin-1beta, interleukin-1 receptor antagonist and interleukin-1beta converting enzyme/caspase-1 in the rat brain after peripheral administration of kainic acid. Neuroscience. 1999;93:915–930. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 11.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Franklin; San Diego: 1997. [Google Scholar]
- 12.Friedlander RM, Gagliardini V, Hara H, Fink KB, Li W, MacDonald G, Fishman MC, Greenberg AH, Moskowitz MA, Yuan J. Expression of a dominant negative mutant of interleukin-1 beta converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J Exp Med. 1997;185:933–940. doi: 10.1084/jem.185.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabellec MM, Griffais R, Fillion G, Haour F. Analysis of brain mRNA by reverse transcription-polymerase chain reaction and hybridization with digoxigenin-labeled DNA probe. Brain Res Brain Res Protoc. 1997a;1:145–151. doi: 10.1016/s1385-299x(96)00023-2. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JH, Liu KF, Relton JK. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am J Pathol. 1995;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- 15.Hara H, Fink K, Endres M, Friedlander RM, Gagliardini V, Yuan J, Moskowitz MA. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hill JK, Gunion-Rinker L, Kulhanek D, Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP, Eckenstein FP. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res. 1999;820:45–54. doi: 10.1016/s0006-8993(98)01140-8. [DOI] [PubMed] [Google Scholar]
- 17.Hillhouse EW, Kida S, Iannotti F. Middle cerebral artery occlusion in the rat causes a biphasic production of immunoreactive interleukin-1beta in the cerebral cortex. Neurosci Lett. 1998;249:177–179. doi: 10.1016/s0304-3940(98)00392-9. [DOI] [PubMed] [Google Scholar]
- 18.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak W, Zheng H, Conn CA, Soszynski D, van der Ploeg LH, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am J Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1β and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 21.Legos JJ, Whitmore RG, Erhardt JA, Parsons AA, Tuma RF, Barone FC. Quantitative changes in interleukin proteins following focal stroke in the rat. Neurosci Lett. 2000;282:189–192. doi: 10.1016/s0304-3940(00)00907-1. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Allen H, Banerjee S, Seshadri T. Characterization of mice deficient in interleukin-1 beta converting enzyme. J Cell Biochem. 1997;64:27–32. doi: 10.1002/(sici)1097-4644(199701)64:1<27::aid-jcb5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Lin MT, Kao TY, Jin YT, Chen CF. Interleukin-1 receptor antagonist attenuates the heat stroke-induced neuronal damage by reducing the cerebral ischemia in rats. Brain Res Bull. 1995;37:595–598. doi: 10.1016/0361-9230(95)00046-h. [DOI] [PubMed] [Google Scholar]
- 24.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 25.Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JDE. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Luheshi GN, Stefferl A, Turnbull AV, Dascombe MJ, Brouwer S, Hopkins SJ, Rothwell NJ. Febrile response to tissue inflammation involves both peripheral and brain IL-1 and TNF-alpha in the rat. Am J Physiol. 1997;272:R862–R868. doi: 10.1152/ajpregu.1997.272.3.R862. [DOI] [PubMed] [Google Scholar]
- 28.Osborne KA, Shigeno T, Balarsky A-M, Ford I, McCulloch J, Teasdale GM, Graham DI. Quantitative assessment of early brain damage in a rat model of focal cerebral ischemia. J Neurol Neurosurg Psychiatry. 1987;50:402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 31.Sairanen TR, Lindsberg PJ, Brenner M, Siren AL. Global forebrain ischemia results in differential cellular expression of interleukin-1beta (IL-1beta) and its receptor at mRNA and protein level. J Cereb Blood Flow Metab. 1997;17:1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injection of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Stroemer RP, Rothwell NJ. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1 beta in the rat. J Cereb Blood Flow Metab. 1998;18:833–839. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Barone FC, Aiyar NV, Feuerstein GZ. Interleukin-1 receptor and receptor antagonist gene expression after focal stroke in rats. Stroke. 1997;28:155–161. doi: 10.1161/01.str.28.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 39.Yang GY, Liu XH, Kadoya C, Zhao YJ, Mao Y, Davidson BL, Betz AL. Attenuation of ischemic inflammatory response in mouse brain using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist. J Cereb Blood Flow Metab. 1998;18:840–847. doi: 10.1097/00004647-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Yu AC, Lau LT. Expression of interleukin-1 alpha, tumor necrosis factor alpha and interleukin-6 genes in astrocytes under ischemic injury. Neurochem Int. 2000;36:369–377. doi: 10.1016/s0197-0186(99)00145-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Chopp M, Goussev A, Powers C. Cerebral vessels express interleukin 1beta after focal cerebral ischemia. Brain Res. 1998;784:210–217. doi: 10.1016/s0006-8993(97)01317-6. [DOI] [PubMed] [Google Scholar]