Abstract
NMDA receptors within the amygdala play an important role in the acquisition and expression of conditioned fear. Because amygdaloid injections of NMDA receptor antagonists did not block the expression of every behavioral sign of fear, a discussion arose as to whether amygdaloid NMDA receptors play different roles in different kinds of fear-conditioning tasks. To clarify the exact role of amygdaloid NMDA receptors, the present study measured the effects of amygdaloid NMDA receptor blockade on the two major animal models of conditioned fear. An experimental design was used that allowed simultaneous measurement of fear-potentiated startle and freezing during the same test session after animals had undergone identical training procedures. The present study clearly demonstrates that injections of the NMDA receptor antagonist AP-5 into the lateral nucleus of the amygdala significantly attenuated both behavioral fear responses (i.e., the amygdaloid NMDA receptors are necessary for the expression of fear-potentiated startle and freezing). The present results together with others from the literature indicate that NMDA receptors within the lateral amygdala are critically involved in normal synaptic transmission. It appears then that NMDA receptor antagonists may block the acquisition of fear conditioning by directly interfering with normal synaptic transmissions in the amygdala. Possible reasons for some discrepant results in earlier studies are also discussed.
Keywords: amygdala, AP-5, conditioned fear, expression, freezing, glutamate, NMDA receptor, plasticity, startle
Fear conditioning is the learning of an association between an initially neutral stimulus and a potentially threatening stimulus [unconditioned stimulus (US)]. After a few pairings of these stimuli, the neutral stimulus becomes a conditioned stimulus (CS) capable of eliciting behavioral, autonomic, and endocrine fear responses that normally occur in threatening situations. (In rats, these responses include freezing, ultrasonic vocalization, potentiation of reflexes, defecation, etc.) Because fearful experiences are rapidly learned and long remembered, fear conditioning also has become an excellent model to investigate the processes and mechanisms underlying learning (Davis et al., 1993; Fendt and Fanselow, 1999; LeDoux, 2000).
A variety of studies have painted a relatively clear picture of the neuroanatomy and neurochemistry of conditioned fear. The amygdala is thought to play an essential role in the acquisition and expression of conditioned fear (Blanchard and Blanchard, 1972; Davis et al., 1993;Lavond et al., 1993; Fendt and Fanselow, 1999; LeDoux, 2000). Specifically, the amygdala has been hypothesized as the interface between the sensory systems carrying the information about the CS and the US and the different motor and autonomic pathways eliciting the conditioned fear responses. The sensory inputs to the amygdala mainly terminate in the lateral nucleus of the amygdala (LA) (Doron and LeDoux, 1999), and lesions of the LA blocked acquisition as well as the expression of conditioned fear (Blanchard and Blanchard, 1972;Hitchcock and Davis, 1986, 1987; Kim and Davis, 1993; Fox and Sorenson, 1994; Kim et al., 1994; Maren et al., 1996a; Killcross et al., 1997;Maren, 1999). The LA appears to be the site of plasticity in fear conditioning: extracellular recordings in awake, behaving animals have demonstrated that long-term potentiation (LTP) occurs in the LA during fear conditioning (Rogan and LeDoux, 1995; Rogan et al., 1997). This is also supported by various studies investigating LTP in the LA using intracellular recordings in in vitro brain slices (LeDoux, 2000).
Given the evidence implicating the LA as a critical site of synaptic plasticity in fear conditioning, the acquisition of conditioned fear should be blocked by intra-LA injections of drugs that block LTP. The competitive NMDA receptor antagonist (±)-2-amino-5-phosphonopentanoic acid (AP-5) is known to block LTP in the CA1 region of the hippocampus (Collingridge and Bliss, 1987), so injections of AP-5 into the LA should block the acquisition of conditioned fear. Several studies have demonstrated a very effective blockade of fear conditioning after AP-5 injections into the LA (Miserendino et al., 1990; Campeau et al., 1992;Maren et al., 1996b; Gewirtz and Davis, 1997; Lee and Kim, 1998). Although the results regarding acquisition of conditioned fear have been to a vast extent consistent, there has been a great controversy regarding the effects of NMDA receptor blockade on expression of conditioned fear. Studies using the two major animal models of fear revealed contradictory results: AP-5 injections into the LA did not affect expression of fear-potentiated startle (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997), whereas expression of freezing was blocked by AP-5 injections into the LA (Maren et al., 1996b; Lee and Kim, 1998). Lee and Kim (1998) suggested two possible reasons for this discrepancy: First, there may be different outputs within the amygdala mediating different aspects of fear. The output mediating conditioned freezing might be NMDA receptor dependent, whereas the output mediating fear-potentiated startle might be NMDA receptor independent. Second, these different fear measures also act in different time windows. Short fear responses such as the potentiation of the startle response might be NMDA receptor independent, whereas long-lasting fear responses such as freezing might be NMDA receptor dependent. Furthermore, they speculated about possible differences within the conditioning procedures.
The debate regarding whether NMDA receptors within the amygdala are essential for the expression of conditioned fear or not is not just a methodological discussion; it is of theoretical importance because conclusions about the neural mechanisms of acquisition and expression of conditioned fear hinge on the answer (cf. LeDoux, 2000). For example, do different labeled sensory-motor lines or a single output of the amygdala control different fear responses? Do amygdaloid NMDA receptors control different things in different kinds of fear-conditioning tasks?
The aim of the present study was to clarify the discrepancy between the different studies investigating the role of amygdaloid NMDA receptors in the expression of conditioned fear. Therefore, different doses of the NMDA receptor antagonist AP-5 were injected into the lateral nucleus of the amygdala, and the expression of fear-potentiated startle as well as conditioned freezing was tested. An experimental design was used in which both fear-potentiated startle and conditioned freezing can be simultaneously measured within the same experimental session so that potential differences in experimental condition can be excluded.
MATERIALS AND METHODS
Subjects. Twenty-eight male Sprague Dawley rats (240–280 gm at the beginning of the experiments) were anesthetized with ketamine–xylazine (9:1; 100 mg/kg, i.p.) and placed into a stereotaxic frame. Two stainless steel guide cannulas with a diameter of 0.7 mm were implanted bilaterally into the brain, aiming at the lateral nucleus of the amygdala [−2.8 mm caudal, ±5.0 mm lateral, −7.2 mm ventral from bregma, according to the coordinates of Paxinos and Watson (1997)], and fixed to the skull with dental cement and three anchoring screws. To maintain patency, stylets were inserted into the guide cannulas. The rats were housed in groups of four to six animals under a continuous light/dark cycle (lights on from 7:00 A.M. to 7:00 P.M.). Food and water were available ad libitum.
Conditioning procedure. One week after surgery, the rats were trained in one of two identical dark boxes (38 × 60 × 28 cm3), the floors of which were composed of steel bars spaced ∼15 mm apart. The CS was a white light, produced by a 15 W bulb located at the top of the box. The US was a 0.6 mA foot shock, produced by a shock generator (custom made at the University of Tübingen). The rats were placed into the training box; after an acclimatization time of 5 min, they received pairings of the light CS and foot shock US. The US was presented during the last 0.5 sec of the 3.7 sec light CS at an average intertrial interval of 3 min (range 2–4 min). After an initial training session on day 1 (10 pairings), the animals were tested daily (day 2–5) (see below for testing procedure) for the effects of different doses of AP-5 on fear-potentiated startle. To avoid extinction of fear conditioning during the test days, the animals were retrained once daily 4 hr before testing. During the retraining procedures, only five CS–US pairings were presented.
Testing procedure. To measure fear-potentiated startle, the animals were tested in two identical test chambers. The rats were placed in wire mesh cages (20 × 10 × 12 cm3) with a steel floor, which were put up on a piezoelectric accelerometer (custom made at the University of Tübingen). The accelerometer was located inside a sound-attenuated test chamber (100 × 80 × 60 cm3). Movements of the rats resulted in changes of the voltage output of the accelerometer. These signals were amplified, digitized, and fed into a computer for additional analysis. A loudspeaker, set up at a distance of 40 cm from the wire mesh cage, delivered the acoustic startle stimuli and a continuous white background noise [55 dB sound pressure level (SPL), root mean square (RMS)]. The presentation of the acoustic stimuli was controlled by a microcomputer and an appropriate interface (Hortmann universal function synthesizer; Hortmann, Neckartenzlingen, Germany). The whole-body startle amplitude was calculated from the difference between the peak-to-peak voltage output of the accelerometer within time windows of 80 msec after and 80 msec before the startle stimulus onset. The motor activity of the animals was calculated using the RMS value of the accelerometer output measured during the last second of the light CS (before the onset of the startle stimulus). The difference between this value and the baseline motor activity (measured during the habituation period, see above) was used as a measure of “freezing.” Preliminary studies in this laboratory as well as in other laboratories (Leaton and Borszcz, 1985; Gewirtz et al., 1997; McNish et al., 1997) showed that this kind of measure is highly correlated with freezing quantified by an observer.
Two injection cannulas with a diameter of 0.4 mm were inserted into the guide cannulas of the rats. The injection cannulas were connected to two 1 μl syringes (Scientific Glass Engineering, Weiterstadt, Germany). Injections of 0.5 μl of AP-5 solution were given at a rate of 0.3 μl/min, and the injection cannulas remained in the brain for 2 additional minutes. Immediately after AP-5 injections, the animals were placed into the test cage, and after an acclimatization time of 5 min, 10 initial startle stimuli (10 kHz, 20 msec duration including 0.4 msec rise and fall times, 100 dB SPL, 30 sec interstimulus interval) were presented to obtain a habituated startle amplitude. After the 10 initial startle stimuli, each animal received 10 additional startle stimuli, with one-half presented alone and one-half presented 3.2 sec after the onset of the light CS. All trial types were presented in a pseudorandom order (30 sec interstimulus interval).
Drug. Each rat received 0, 12.5, 25, and 50 nmol of AP-5 (Research Biochemicals, Natick, MA), dissolved in saline, pH 7.4, in a counterbalanced order across 4 subsequent days.
Histology. After completion of the tests, the animals were killed by an overdose of Nembutal. The animals were decapitated, and their brains were removed and immersion-fixed with 8% paraformaldehyde in PBS with 20% sucrose. Coronal sections of 60 μm were taken on a freezing microtome and stained with thionine. The injection sites were drawn onto plates taken from the atlas ofPaxinos and Watson (1997).
Statistical analysis. Statistical analysis of the data was accomplished by ANOVA using a repeated-measure design, followed bypost hoc Tukey's tests. For all statistical comparisons, ap value of <0.05 was taken as the criterion for statistical significance.
RESULTS
Histology
The locations of the injection cannulas in the LA are schematically shown in Figure 1. Eighteen animals had injection sites bilaterally located in the LA. Ten animals were excluded from additional analysis because of misplaced injections (caudate putamen, cortex; six animals) or because they had amygdaloid lesions that were probably mechanically caused by the injection cannulas (four animals).
Fig. 1.
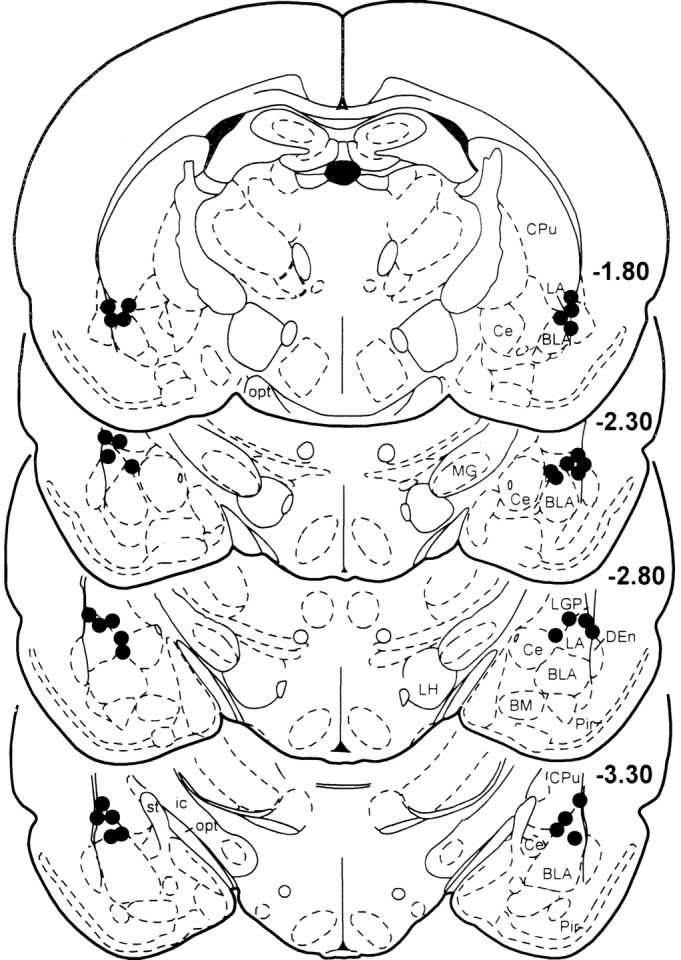
Serial drawing of coronal sections depicting the injection sites of AP-5 into the lateral nucleus of the amygdala.Numbers on the sections indicate the rostrocaudal level in millimeters relative to bregma, according to Paxinos and Watson (1997). BLA, Basolateral nucleus of the amygdala;Ce, central nucleus of the amygdala; CPu, caudate putamen; DEn, dorsal endopiriform nucleus;ic, internal capsule LH, lateral hypothalamus; MG, medial geniculate nucleus;opt, optical nerve; Pir, piriform cortex;st, stria terminalis.
General observation
The general health of the animals after the surgery was good. Earlier experiments in this laboratory showed that the LA is very vulnerable (i.e., injection cannulas located in the middle of the LA often cause lesions of the amygdala). For the present study, I therefore tried to hit the LA on its very dorsal or lateral region. Nevertheless, four animals showed lesions of the amygdala and were excluded from the analysis.
The integrity of the eardrums in general is an additional prerequisite for the analysis of the startle data. In the present study, two animals had damaged eardrums. Only their motor activity data but not their startle test data were used for analysis.
Fear-potentiated startle
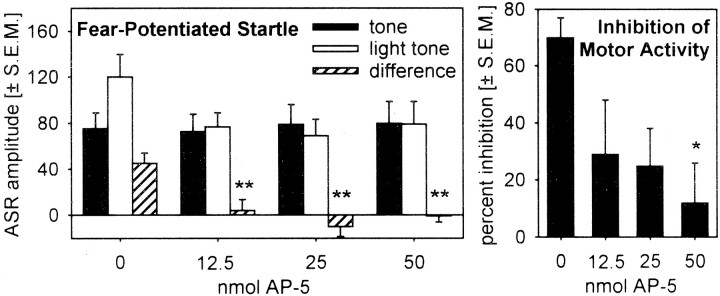
Figure 2, left, shows the mean startle amplitudes on the tone alone and light–noise trials as a function of drug treatment (saline or 12.5, 25, and 50 nmol of AP-5). The ANOVA found no effect of treatment on the baseline startle amplitude (tone alone trials), as indicated by a p value of 0.90 (F(3,42) < 1.0). However, AP-5 injections into the LA highly significantly block the expression of fear potentiation (ANOVA, F(3,52) = 7,26; p < 0.001). Post hoc comparisons showed significant differences between injections of saline and all concentrations of AP-5 (Tukey's tests, p values of <0.01). Furthermore, there are no effects of the factor test day on the effects of saline or AP-5 (all doses were pooled) on fear-potentiated startle (ANOVA, F(3,45) = 1.556;p = 0.231).
Fig. 2.
Bar graph depicting the mean acoustic startle response amplitudes (left) and the mean percentage of inhibition of motor activity (right) during the light CS in arbitrary accelerometer readings after injections of 0, 12.5, 25, and 50 nmol of AP-5 into the lateral nucleus of the amygdala. *p < 0.05, **p < 0.01 for the comparison between saline and AP-5 injections (post hoc Tukey's t test after a significant main effect of the ANOVA).
Inhibition of motor activity
Figure 2, right, depicts the mean inhibition of the motor activity during the last second of the light CS. AP-5 injections into the LA reduce this inhibition, as indicated by a significant ANOVA (F(3,51) = 3,433; p = 0.024). Post hoc comparisons of the different groups revealed a significant difference between saline and 50 nmol of AP-5 (p = 0.020). The general motor activity measured in the acclimatization period at the beginning of each test was not affected by AP-5 injections (ANOVA,F(3,51) = 0.648; p = 0.588).
DISCUSSION
The present study tested whether NMDA receptors within the LA are involved in the expression of fear-potentiated startle and conditioned freezing. Because in previous studies contradictory effects were measured with these two major animal models of fear, and the exact role of amygdaloid NMDA receptors was therefore unclear, the present study was necessary to understand the neural control of expression of conditioned fear. The present results clearly demonstrate that NMDA receptors within the LA are critically involved in the expression of both fear responses. Injections of AP-5, an NMDA receptor antagonist, significantly block the expression of fear-potentiated startle and attenuate the inhibition of motor activity in the presence of a fear CS. This is in contrast to previous studies (Miserendino et al., 1990;Campeau et al., 1992; Gewirtz and Davis, 1997) showing that the expression of fear-potentiated startle is not affected by amygdaloid injections of AP-5, but confirms other studies demonstrating an important role of amygdaloid NMDA receptors in the expression of conditioned freezing (Maren et al., 1996b; Lee and Kim, 1998).
It is well accepted that amygdaloid NMDA receptors play a crucial role in the acquisition of conditioned fear (Miserendino et al., 1990;Campeau et al., 1992; Maren et al., 1996b; Gewirtz and Davis, 1997; Lee and Kim, 1998). It is important for the understanding of the role of amygdaloid NMDA receptors in fear-related behaviors to know whether amygdaloid glutamate acts via NMDA receptors selectively during acquisition and/or expression of fear. Basically, there are two main possible roles of the NMDA receptors: first, if they are selectively involved only in the acquisition but not in the expression of conditioned fear, this indicates that NMDA receptors within the amygdala are involved in the plasticity of the amygdala. Second, if blockade of the NMDA receptors inhibits both acquisition and expression of conditioned fear, then they are likely to be involved in the glutamatergic transmission of visual or auditory signals through the amygdala (cf. LeDoux, 2000). This “transmission hypothesis” is supported by various electrophysiological studies showing that AP-5 affects the glutamatergic transmission within the amygdala (Li et al., 1995, 1996; Maren and Fanselow, 1995; Weisskopf et al., 1999). Additional studies have shown that those doses of AP-5 which inhibited LTP within the LA also blocked glutamatergic transmission; lower doses of AP-5 that did not affect glutamatergic transmission within the LA also did not affect LTP (Chapman and Bellavance, 1992). This is in contrast to the findings in the hippocampus, in which lower AP-5 concentrations that blocked LTP did not affect glutamatergic transmission (Collingridge and Bliss, 1987).
The present study demonstrated a clear blockade of expression of fear-potentiated startle and freezing after AP-5 injections into the LA. It should be noted that freezing was not directly observed, but attenuation of motor activity in the startle test chamber was measured, which is highly correlated with freezing (Leaton and Borszcz, 1985;Gewirtz et al., 1997; McNish et al., 1997). The question now is why the studies of Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997) revealed contradictory results. There are three main experimental differences in the studies that could account for these discrepancies:
(1) In contrast to the studies by Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997), a repeated-measure design was used in this study. For this design, every animal received all doses of the drug and served as its own control. The advantage of this procedural design is that one can examine whether each animal is able to express fear-potentiated startle after saline injections (i.e., verify that the function of the amygdala is not impaired by the implantation of the injection cannulas). I think that this is a very critical point of experiments using amygdaloid injection cannulas because I (and also other laboratories) have observed that the amygdala is very sensitive to mechanical and pharmacological manipulations (i.e., in some animals the cannulas itself or injections of saline prevent expression of fear) [see the very low level of fear-potentiation in Miserendino et al. (1990)]. All animals in the present study showed expression of fear-potentiated startle during control conditions (and no visible lesions in histological analysis); therefore, I can conclude that the injection sites used in this study did not disturb the regular amygdaloid functions under control conditions but were able to affect amygdaloid function by drug applications.
Because the control injections of the studies of Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997) showed a low level of fear-potentiation but did not prevent expression of fear-potentiated startle, this experimental difference obviously is not the main reason for the different results of the studies.
(2) In contrast to the studies by Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997), the fear-conditioning training in this study was made in a different context than the test session [i.e., only conditioning to a discrete cue (the light CS) was tested]. Because a fear-conditioned context enhances the baseline startle response (McNish et al., 1997; Frankland et al., 1998), the amount of fear potentiation by the discrete CS could be attenuated by a mixed conditioning procedure. This could be an important point because the injection sites of the studies by Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997) were located in the basolateral nucleus of the amygdala (also see below), and the basolateral nucleus is a critical nucleus for contextual fear conditioning (LeDoux, 2000). The AP-5 injections of the present study into the LA should only affect conditioning to a discrete cue, whereas AP-5 injections into the basolateral amygdala might also affect fear to the context and might lead to other results in a mixed conditioning (and test) design.
However, because there was weak fear potentiation by the discrete CS in the studies of Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997), this cannot be the main reason for the different results. Furthermore, Lee and Kim (1998) showed that both context and cue (tone and light) conditioning is affected by AP-5 injections into the amygdala.
(3) An important difference of the studies is the precise location of the injection sites. In most studies [except those by LeDoux and colleagues (Li et al., 1995, 1996; Quirk et al., 1995, 1997; Rogan et al., 1995, 1997; Weisskopf et al., 1999)] investigating the effects of drug injections into the amygdala, injections were made into the basolateral nucleus of the amygdala (Miserendino et al., 1990;Campeau et al., 1992; Gewirtz and Davis, 1997) (but see Lee and Kim, 1998; Lee et al., 2001). The injection sites of the present study were located in the lateral nucleus of the amygdala, ∼1 mm dorsal to the basolateral amygdala, exactly on the site for which electrophysiological studies showed physiological plasticity with the shortest latency (Quirk et al., 1995, 1997). Consistent with the electrophysiological data, Amorapanth et al. (2000) showed that lesions of the lateral but not of the basolateral nucleus of the amygdala block the acquisition of a fear CS. These data suggest that the projection from the LA to the central nucleus of the amygdala is sufficient to mediate fear conditioning and would explain why injections of AP-5 into the LA but not into the basolateral nucleus of the amygdala prevent expression of conditioned fear. It is possible that the NMDA receptors within both nuclei have different roles: for example, the NMDA receptors in the LA might play an important role in expression of conditioned fear (e.g., the present study), whereas the NMDA receptors within the basolateral nucleus of the amygdala are not necessary for mediating conditioned fear [e.g., studies by Davis and colleagues (see above)]. After strong fear conditioning (the present study used several retrainings), the projection from the LA to the central nucleus might be sufficient for expression (cf. Amorapanth et al., 2000), and AP-5 injections block expression. Although the basolateral nucleus is necessary for expression of conditioned fear after more weak conditioning [studies by Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997)], the NMDA receptors within the basolateral nucleus play no role in mediating conditioned fear, so AP-5 injections have no effect.
We think that this difference might be the main reason for the different results of this study and the studies of Davis and colleagues (Miserendino et al., 1990; Campeau et al., 1992; Gewirtz and Davis, 1997). However, in other studies (Lee and Kim, 1998) in which AP-5 was injected into the basolateral amygdala, effects on expression were observed that were similar to those seen in the present study.
Taken together, the present study shows that amygdaloid NMDA receptors critically contribute to the expression of conditioned fear as measured by fear-potentiated startle and freezing. This is supported by previous studies (Maren et al., 1996b; Lee and Kim, 1998) demonstrating similar effects. It is important to note that Lee et al. (2001) also show a blockade of the postshock freezing response, which is also believed to be a conditioned response (Fanselow, 1980), after NMDA receptor blockade within the amygdala. Possible reasons for different effects of amygdaloid AP-5 injections in different animal models (Lee and Kim, 1998) could be disproved by the present study, because the same drug effects were observed after identical manipulations in the two different animal models. In my opinion, the differences of the studies investigating the effects of NMDA receptor antagonists on expression of fear-potentiated startle must be explained by some idiosyncratic aspects of the procedures (mainly the injection sites) or some other experimental difference (see above).
The fact that NMDA receptors within the amygdala play a role in the expression of conditioned fear supports the transmission hypothesis ofLeDoux (2000), which states that amygdaloid NMDA receptors make an important contribution to synaptic transmission in the pathways that provide sensory input to the amygdala (compare also Discussion of Lee et al., 2001). If the amygdaloid plasticity underlying fear conditioning cannot explained by amygdaloid NMDA receptors, the question is whether there are possible additional sites of plasticity within the amygdala. Studies in this laboratory have shown that injections of the metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine into the lateral nucleus of the amygdala block the acquisition but not the expression of conditioned fear as measured by the fear-potentiated startle paradigm as well as freezing (Fendt et al., 2000). Therefore, I propose that the metabotropic glutamate receptor might be more important for amygdaloid plasticity than the NMDA receptor.
In conclusion, the present study shows that NMDA receptors within the LA are critical for the expression of conditioned fear in the paradigms of fear-potentiated startle and freezing. I therefore conclude that amygdaloid NMDA receptors are more important for the synaptic transmission that provides sensory inputs to the amygdala and less important for the encoding of conditioned fear.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 550/C8). I am grateful to T. H. Brown, M. Davis, M. S. Fanselow, J. J. Kim, M. Koch, J. Ostwald, C. F. Plappert, H.-U. Schnitzler, and the three unknown reviewers for helpful discussions and/or comments pertaining to the manuscript. A special thank you to Helga Zillus for excellent technical assistance.
Correspondence should be addressed to Markus Fendt, Tierphysiologie, Universität Tübingen, Auf der Morgenstelle 28, D-72076 Tübingen, Germany. E-mail: markus.fendt@uni-tuebingen.de.
REFERENCES
- 1.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 3.Campeau S, Miserendino MJD, Davis M. Intra-amygdaloid infusion of the N-methyl-d-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PF, Bellavance LL. Induction of long-term potentiation in the basolateral amygdala does not depend on NMDA receptor activation. Synapse. 1992;11:310–318. doi: 10.1002/syn.890110406. [DOI] [PubMed] [Google Scholar]
- 5.Collingridge GL, Bliss TVP. NMDA receptors: their role in long-term potentiation. Trends Neurosci. 1987;10:288–293. [Google Scholar]
- 6.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 7.Doron NN, LeDoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- 8.Fanselow MS. Conditioned and unconditioned components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 9.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 10.Fendt M, Schwienbacher I, Koch M. Injections of the metabotropic glutamate receptor antagonist MPEP into the lateral amygdala prevent acquisition of conditioned fear in rats. Soc Neurosci Abstr. 2000;26:75.3. [Google Scholar]
- 11.Fox RJ, Sorenson CA. Bilateral lesions of the amygdala attenuate analgesia induced by diverse environmental challenges. Brain Res. 1994;648:215–221. doi: 10.1016/0006-8993(94)91120-7. [DOI] [PubMed] [Google Scholar]
- 12.Frankland PW, Dockstader CL, McDonald RJ. Discriminative and nondiscriminative contextual fear conditioning potentiate the acoustic startle response. Psychobiology. 1998;26:267–274. [Google Scholar]
- 13.Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- 14.Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- 17.Killcross SS, Robbins TW, Everitt BJ. Different types of fear-conditioned behavior mediated by separate nuclei within the amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1994;107:1088–1092. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- 20.Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- 21.Leaton RN, Borszcz GS. Potentiated startle: its relation to freezing and shock intensity in rats. J Exp Psychol Anim Behav Process. 1985;11:421–428. [Google Scholar]
- 22.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Choi J-S, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XF, Phillips RG, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105:87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- 26.Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- 27.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditioned fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maren S, Aharanov G, Fanselow MS. Retrograde abolition of conditioned fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996a;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 30.Maren S, Aharanov G, Stote DL, Fanselow MS. N-methyl-d-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditioned fear in rats. Behav Neurosci. 1996b;110:1365–1375. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 31.McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: a disruption of freezing but not fear-potentiated startle. J Neurosci. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- 34.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 35.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 36.Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 37.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 38.Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]