Abstract
When electrical stimulation is applied over human muscle, the evoked force is generally considered to be of peripheral origin. However, in relaxed humans, stimulation (1 msec pulses, 100 Hz) over the muscles that plantarflex the ankle produced more than five times more force than could be accounted for by peripheral properties. This additional force was superimposed on the direct response to motor axon stimulation, produced up to 40% of the force generated during a maximal voluntary contraction, and was abolished during anesthesia of the tibial nerve proximal to the stimulation site. It therefore must have resulted from the activation of motoneurons within the spinal cord. The additional force could be initiated by stimulation of low-threshold afferents, distorted the classical relationship between force and stimulus frequency, and often outlasted the stimulation. The mean firing rate of 27 soleus motor units recorded during the sustained involuntary activity after the stimulation was 5.8 ± 0.2 Hz. The additional force increments were not attributable to voluntary intervention because they were present in three sleeping subjects and in two subjects with lesions of the thoracic spinal cord. The phenomenon is consistent with activation of plateau potentials within motoneurons and, if so, the present findings imply that plateau potentials can make a large contribution to forces produced by the human nervous system.
Keywords: human, motoneuron, muscle force, spinal cord, reflex, muscle contraction, plateau potential
The discharge of motoneurons is essential for all purposive movements. Traditionally, motoneurons are believed to summate linearly the various descending and reflex inputs that they receive (Eccles, 1957; Granit et al., 1966). However, some behavior of motoneurons does not easily fit within this view. First, there are situations in which motoneuronal discharge is excessively prolonged or inappropriately timed for the stimulus that initiated it. For example, soleus motoneurons in the cat may show prolonged self-sustained firing after activation of muscle spindle endings (Granit et al., 1957; Hultborn et al., 1975; Wada et al., 1989). In human subjects, motoneuron discharge may not be temporally locked to a large reflex input from muscle spindle afferents, despite the ability of monosynaptic pathways to phase-lock the discharge of motoneurons under other circumstances (Lang and Vallbo, 1967; Burke and Schiller, 1976). Second, animal experiments have demonstrated that motoneurons can develop plateau potentials that distort the relationship between input current and firing rate and can also produce self-sustained firing (Schwindt and Crill, 1980; Hounsgaard et al., 1988; Hounsgaard and Kiehn, 1989; Bennett et al., 1998a; Carlin et al., 2000) (for review, see Kiehn and Eken 1998; Hultborn, 1999; Hornby et al., 2000). There is indirect evidence that these mechanisms may influence motoneuron activity in conscious human subjects (Kiehn and Eken, 1997;Gorassini et al., 1998, 2000) and unrestrained animals (Eken and Kiehn, 1989; Gorassini et al., 1999). However, the potential “power” of any such assistance to the forces produced by human muscle has been difficult to gauge, partly because it is not possible to record intracellularly from human motoneurons in vivo and possibly because the conditions under which plateau potentials develop in experimental animals are dependent on the type and “state” of the experimental preparation (Wada et al., 1989). In humans, possible plateau activation must be inferred from more indirect measures such as single motor unit recordings (Kiehn and Eken, 1997; Gorassini et al., 1998, 2000).
Percutaneous electrical stimulation of human muscle is used extensively in both clinical and experimental settings. Such stimulation activates the muscle by directly stimulating the terminal branches of the motoneurons, and the resulting forces are thought to depend on the peripheral properties of the muscle and nerves under the stimulating electrodes. However, the stimulation will also activate sensory axons that can produce a “reflex” response in motoneurons with similarities to the tonic vibration reflex (De Gail et al., 1966; Lang and Vallbo, 1967). We have found that similar stimulation can produce surprisingly large forces and substantial distortions in the “normal” relation between stimulus frequency and the evoked force. This behavior cannot be explained by either peripheral properties of nerve and muscle or volitional drive to the motoneurons, and we propose the involvement of plateau-like properties of human motoneurons.
Some of these data have been presented in abstract form (Collins and Gandevia, 2000; Gandevia et al., 2001).
MATERIALS AND METHODS
Studies were performed on 15 able-bodied subjects (nine males, six females; age range, 24–56 years) and two paraplegic subjects with traumatic spinal cord lesions at the thoracic level (ages 39 and 43 years) studied 3 and 6 months after their accident. The procedures were approved by the local human research ethics committee. Subjects sat with the hips and knees comfortably flexed and the right foot strapped to a myograph to record torque about the ankle joint (hip, knee, and ankle 90–110°). Throughout the study subjects were reminded to relax, disregard the stimulation, and were encouraged to read. Sometimes the subjects were asked to confirm that they were relaxed, and they were often asked to “relax completely”, although they received no feedback about their performance during the experiment. Three subjects slept during parts of the experiment. Before the study, the maximal voluntary force (MVC) of ankle plantarflexion was measured in several attempts lasting 2–4 sec.
Electrical stimulation was applied over the calf muscles (triceps surae) via two flexible strip electrodes (10- to 18-cm-long × 3.5-cm-wide) positioned ∼10 and 20 cm distal to the popliteal fossa. Pulses of 1 msec duration were delivered from a stimulator that could be driven by a computer. The electrodes were positioned (with the cathode proximal) such that the stimuli produced minimal local discomfort. Stimulation intensity was adjusted based on the response to “test” trains (5 pulses; 1 msec duration; 100 Hz) producing 2–7% MVC. Several protocols were used in which trains of stimuli were delivered at constant intensity. (1) Trains at constant frequency: stimuli at 100 Hz for ≥7 sec with the trains separated by >1 min with the 5-pulse test trains before and after the long train. (2) Trains at low frequency with one or more abrupt intermittent periods of high-frequency stimulation (e.g., 2 sec at 25 Hz, then 100 Hz for 2 sec and finally 25 Hz for 3 sec). Control trains were delivered at 25 Hz only. (3) Trains with linearly changing frequencies (rising from ∼10–100 Hz over 10 sec and declining over 10 sec).
In many studies surface electromyographic (EMG) activity was recorded from medial gastrocnemius (MG) and lateral gastrocnemius (LG), soleus (Sol), and tibialis anterior (TA) muscle (sampling rate 1 kHz; bandpass 16 Hz-1 kHz). Torque, EMG, and stimulus parameters were recorded to disc. In seven subjects the activity of single motor units in soleus was recorded during self-sustained plantarflexion contractions via the surface EMG or a monopolar needle electrode (sampling rate 10 kHz; bandpass 16 Hz-3 kHz).
In two subjects the tibial nerve was blocked in the popliteal fossa (with 12–15 ml of 2% lidocaine with adrenaline). The nerve was initially localized with stimulation through a monopolar electrode, and the block was monitored clinically and by electrical stimulation. In one subject the block was complete, and in the other it reduced the force of maximal voluntary plantar flexion to <10% of control and abolished the Sol H reflex.
RESULTS
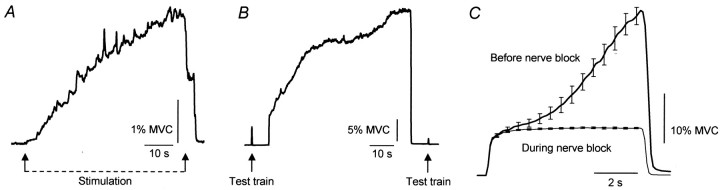
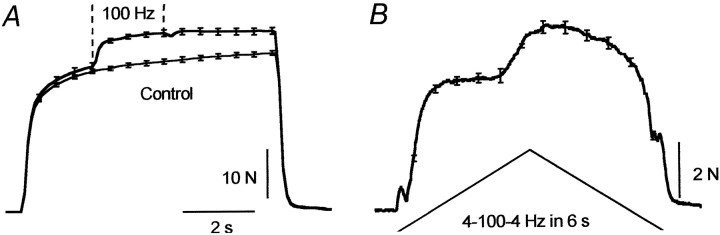
In all subjects electrical stimulation over the calf muscles produced an additional reflex-like plantarflexion force superimposed on the force arising from stimulation of the motor axons beneath the stimulating electrodes. This “additional” force could be evoked with a range of stimulus intensities, even below motor threshold. An example of the force that developed during a long stimulus train (100 Hz) delivered at motor threshold is shown for one subject in Figure 1A. When the stimulation was applied above motor threshold, the extra force that arose in addition to the direct response to motor axon stimulation could reach >40% MVC. Examples are shown in Figure 1, Band C, for trains of stimuli lasting 60 and 7 sec, respectively. The additional force arose at a variable latency after the onset of the trains and could be evoked using a variety of stimulation protocols. Across eight subjects the maximal force in a prolonged train lasting >10 sec (as in Fig. 1B) increased 1.6–5.7 fold over the force 0.5 sec into the train. This represented a mean increase of 21 ± 6% MVC (mean ± SEM; range, 7–43% MVC). Peak force occurred 44 ± 11 sec after stimulus onset (range, 8–106 sec). This additional force occurred even though the force due to the direct stimulation of motor axons declined. This is shown in Figure 1B by the reduction in the amplitude of the response to the brief test train (five stimuli at 100 Hz; mean reduction across the eight subjects, 56%). This decrease presumably reflects a reduced number of stimulated motor axons (Bergmans, 1970; Vagg et al., 1998) and peripheral fatigue in the muscle fibers activated by the stimulated motor axons. Sometimes the stimulation induced a cramp in the calf muscles that could be avoided by moving the stimulating electrodes or reducing the stimulus intensity.
Fig. 1.
Forces during 100 Hz stimuli over the calf muscles in relaxed subjects. A, Force during a stimulation for 55 sec delivered at the threshold for motor axon stimulation for one subject. B, Force during a train lasting 60 sec delivered above motor threshold for one subject. Note the gradual increase in force to ∼30% MVC and the reduction in response to the test train after the prolonged stimulation. C, Mean responses (± SEM) to five stimulus trains of 7 sec duration in one subject before and during a complete anesthetic block of the tibial nerve. Error bars (shown at 0.5 sec intervals throughout the stimulation) are very small on the “nerve block” trace.
To exclude the possibility that these unexpected forces reflected an odd peripheral property of the stimulated motor axons and the innervated muscle fibers, we assessed responses during block of the tibial nerve in two subjects. This prevented activation of motoneurons via reflex or antidromic paths and the resulting forces could only arise from peripheral properties of the motor axons and muscle fibers beneath the stimulating electrodes. When muscles below the knee were paralyzed and “disconnected” from the CNS in this way, the additional forces associated with sustained high-frequency stimulation were absent (Fig. 1C), and the evoked forces were much more consistent and predictable.
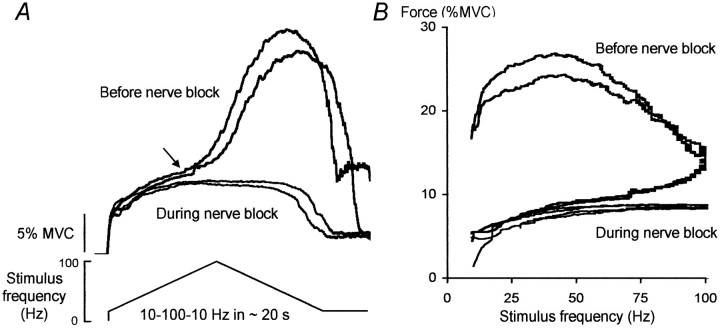
The relationship between stimulus frequency and the evoked force was investigated with a triangular pattern of stimulation with increasing then decreasing stimulus frequencies (Fig.2). The evoked force remained abnormally high or even increased as the stimulus frequency declined. In some instances a prominent “take off” for the extra force was identified (Fig. 2, arrow), although the stimulus frequency when this occurred was variable across subjects (between ∼30 and 100 Hz). This resulted in a marked distortion of the relationship between stimulus frequency and evoked force clearly evident by the hysteresis in the force-frequency plot in Figure 2B. This distortion was abolished during the tibial nerve block, thus confirming that the additional force arose from within the CNS.
Fig. 2.
Responses to “triangular-shaped” changes in stimulus frequency for one subject before and during complete tibial nerve block. A, Responses to trains of increasing then decreasing frequency (between 10 and 100 Hz) over ∼20 sec. Thearrow marks the onset of the “extra” force.B, Corresponding force–frequency relationship.
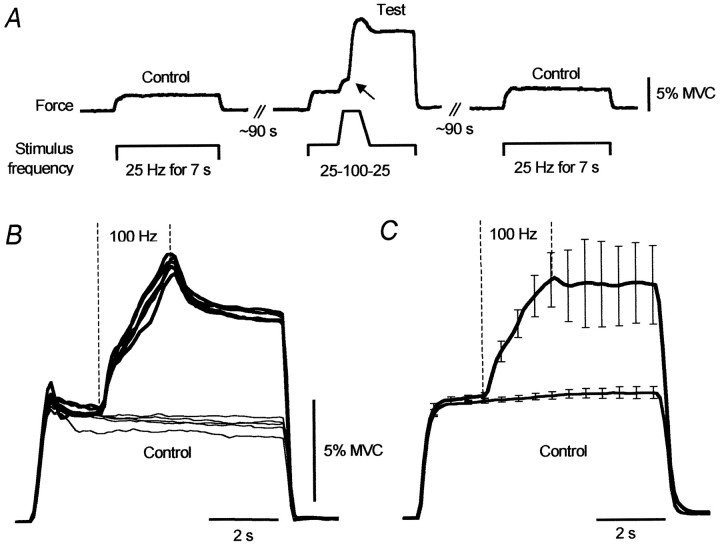
To assess the force increments that arose during the high-frequency (100 Hz) stimulation, we delivered brief periods (∼2 sec) of such stimulation during longer trains of stimuli at 25 Hz. These 100 Hz “bursts” not only increased the force much more than expected from the direct stimulation of motor axons, but when the frequency of stimulation returned to 25 Hz, the force remained inappropriately high. This is shown for one subject in Figure3A in which there was a clear take off in the force (arrow) during the 100 Hz burst, after which the force remained elevated compared to the same point during the two control stimulus trains. Figure 3B shows 10 superimposed responses (five control, five test) from a different subject, and the mean across the group of seven subjects is shown in Figure3C. For the group, the average force 3 sec after the end of the 100 Hz train was 188 ± 31% of that at the same time in the control train.
Fig. 3.
Forces during control trains (25 Hz) and during trains with additional 100 Hz stimulation. A, Force responses from one subject to three successive stimulus trains.B, Superimposed responses (n = 10) to control (thin lines) and test trains (thick lines) for one subject. C, Average responses for seven subjects (mean ± SEM). Data expressed relative to the force 0.5 sec after stimulus onset in control trains.
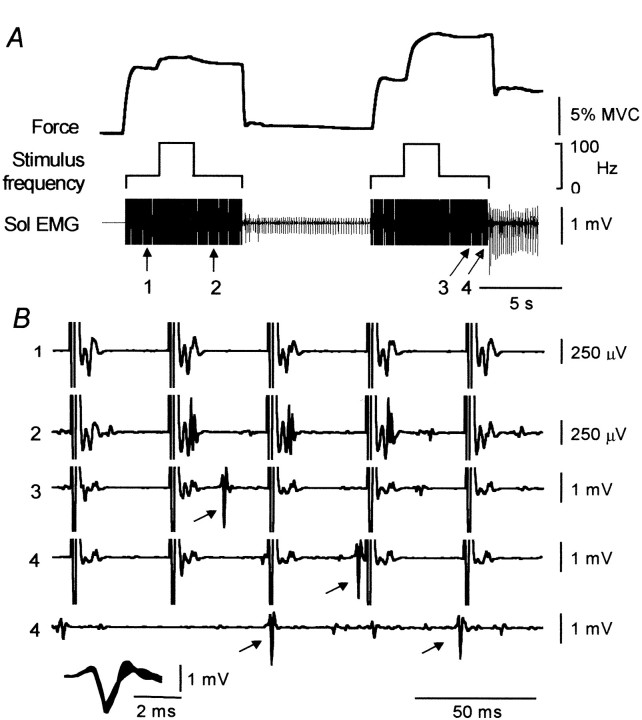
Figure 4A shows force and the activity of a single motor unit in Sol evoked by two successive 25 Hz stimulus trains with bursts at 100 Hz (as in Fig. 3). The initial 100 Hz burst (during the first stimulus train) resulted in only a small increase in force. In contrast, much more force was evoked during and after the 100 Hz burst of the second stimulation. Similarly, the sustained force and EMG that remained after the stimulation was turned off was much larger after the second stimulus train than the first. Figure 4B shows the EMG data on an expanded time scale (200 msec sections) from the four points indicated by thearrows in A. The large vertical lines in the first four traces show the truncated stimulus artifacts from the stimulation at 25 Hz. The first trace shows the EMG activity before the first 100 Hz burst (Fig. 4A, point 1). The only EMG activity present was time-locked to each stimulus pulse and reflected muscle activation caused by the direct stimulation of the motor axons. Part of this direct response was truncated with the stimulus artifact. The second trace is taken after the first 100 Hz burst (point 2) and shows the direct EMG response to the stimulation as well as the recruitment of other small motor units whose discharge was not time-locked to the stimulation. The data from the period after the second 100 Hz burst (Fig. 4A, point 3) show that a motor unit was recruited (at the arrow; note the reduction in gain). Trace 4 comprises the 400 msec period (in two parts) beginning with the last five pulses of the second stimulus train and shows that the large unit recruited at point 3 remained active throughout the remainder of the stimulus train. This unit also fired after the stimulus was turned off, initially at ∼10–12 Hz, and then the frequency declined to ∼6–8 Hz. The insert at the bottom of the Figure shows six superimposed spikes from the unit highlighted by thearrows in B (three recorded during the stimulation and three after).
Fig. 4.
Force and EMG activity during two successive stimulus trains of 25 Hz stimulus with a 100 Hz burst. Ashows the force and EMG activity (recorded with a monopolar recording electrode) before, during, and after the stimuli. Bshows the unitary activity at the four points (1–4) during the stimulation shown by the arrows in A. Note the change in gain. The data from point 4 encompass the last five stimulus pulses and the period immediately after that. Theinset at the bottom of Bshows the morphology of the spike highlighted by thearrows in traces 3 and 4(6 superimposed spikes, 3 during the stimulation and 3 after).
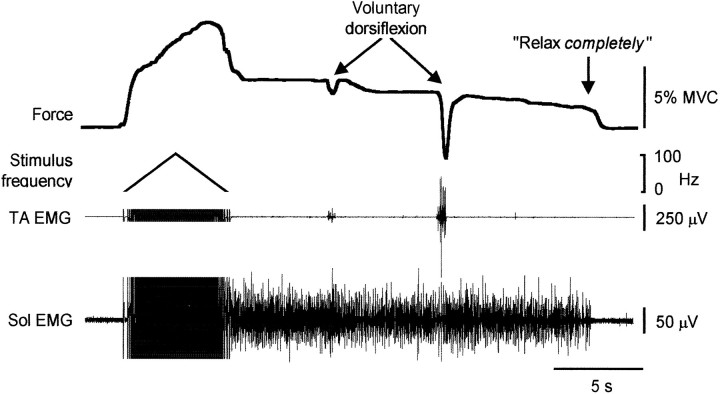
In all subjects there were instances when force was maintained after the stimulation was turned off. This sustained force could last for minutes and occurred although, when questioned, the subjects confirmed that they were “relaxed.” This occurred commonly when one or more 1 sec bursts of 100 Hz stimulation was delivered during 25 Hz trains and reflected the development of continuous EMG activity in the plantarflexor muscles, activity that had not been present before the stimulus train that evoked it (Figs. 4-6). The mean firing rate of 27 single motor units in Sol during such sustained involuntary activity was 5.8 ± 0.2 Hz (coefficient of variation; 12.9 ± 1.1%). An example of the force and EMG activity that remained after a triangular pattern of stimulation is shown in Figure5. In this example, brief voluntary dorsiflexion efforts failed to eliminate the sustained contraction of the plantarflexor muscles. Similar contractions (up to 10% maximum) also did not eliminate this activity in three of three subjects tested. Interestingly, if the sustained muscle contraction did not end spontaneously, a request to concentrate and relax completely usually terminated it, although when first asked subjects would typically state that they were relaxed. Figure 5 shows that the command to relax completely was not associated with activation of the antagonist muscles. No examples of sustained discharges after the stimulation was turned off were observed during the nerve blocks.
Fig. 5.
Recording from a subject during and after a train of increasing then decreasing frequency (between ∼4 and 100 Hz in 6 sec). Force continued to increase as the stimulus frequency declined, and sustained plantarflexion force and Sol EMG remained after stimulation. This persisted despite two brief efforts to dorsiflex the ankle but disappeared when the subject was asked to relax completely. EMG occurred in TA during the voluntary dorsiflexions but not when asked to relax completely. Stimulus artifacts have been truncated.
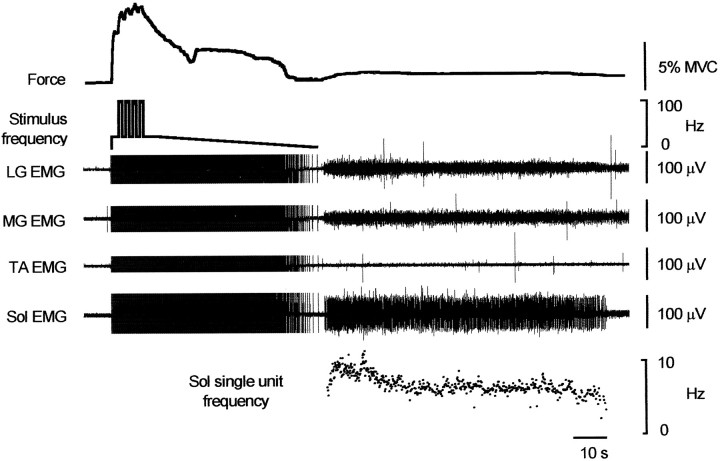
The additional forces were not attributable to inadvertent volitional descending drives to the motoneurons. They were recorded from subjects who were relaxed and often distracted by reading. They were also recorded in three subjects who went to sleep during the study (Fig. 6). Here force and EMG continued after a complex stimulus train in which the stimulus frequency declined slowly from 25 Hz after four 1 sec bursts at 100 Hz. In two paraplegic subjects with thoracic spinal cord lesions, the additional force responses were evident with the various protocols used to evoke them (Fig. 7), although these forces seemed smaller than in intact subjects.
Fig. 6.
Recordings from a sleeping subject to a complex stimulus train that included repeated bursts of 100 Hz stimulation followed by a decline in frequency. Force did not decline smoothly during the slow reduction in frequency and residual force developed after the train (accompanied by EMG in Sol and MG and LG, but not TA). Stimulus artifacts have been truncated. Instantaneous discharge rate of a single motor unit in soleus is shown.
Fig. 7.
Recordings from two patients with spinal cord injury (mean ± SEM, n = 5). A,Data from one subject with a complete spinal cord lesion at T12. Forces during trains of stimuli with 2 sec at 25 Hz, 2 sec at 100 Hz, and then 3 sec at 25 Hz. For the control sequence stimulus rate was constant at 25 Hz. B, Data from a subject with an incomplete lesion at T8. Force response to the triangular pattern of stimulation. Maximal voluntary plantarflexion force was <10% predicted for healthy subjects.
DISCUSSION
Electrical stimulation over the calf muscles at innocuous intensities can evoke substantial plantarflexion force, much more than attributable to direct stimulation of the motor axons. When this developed the force could increase more than fivefold. Some of this additional force often remained, despite lowering the stimulus frequency or even cessation of the stimulation. This force occurred despite activity-dependent hypoexcitability in the stimulated axons (Bergmans, 1970; Vagg et al., 1998), and the peripheral fatigue expected in the motor units with the largest (and most easily excited) motor axons (Burke, 1981) as seen in the responses to test stimuli before and after prolonged trains (Fig. 1B).
This behavior was of central origin because it was absent when the nerve was blocked proximal to the stimulation site. During the nerve block the evoked forces could only arise from direct activation of the motor axons, and the resulting contraction forces were consistent and predictable. The presence of the unexpected force increments during stimulation at intensities below the threshold for motor axons (see also De Gail et al., 1966; Lang and Vallbo, 1967) suggest that they probably arose from stimulation of large-diameter afferents and that the antidromic activation of motoneurons was not required. Muscle rather than cutaneous afferents are likely to be important because the behavior has been observed for muscles other than the calf such as tibialis anterior (Collins and Gandevia, 2000; Gandevia et al., 2001) and biceps brachii (D. F. Collins, and S. C. Gandevia, unpublished observations) where stimulation evoked little or no cutaneous sensation. When the stimulation was applied above motor threshold, motoneurons with large-diameter axons would be preferentially activated, and antidromic volleys traveling along those axons would abolish any orthodromic action potentials arising within the spinal cord. Thus, the additional force superimposed on the direct motor response must arise from the recruitment of the smaller, more fatigue-resistant, motoneurons.
The additional forces do not reflect voluntary drive to the motoneurons because they were present in sleeping subjects and in patients with spinal cord transection. We did not document sleep stage but expect that the subjects were in non-REM sleep, a state in which voluntary intent could not have interfered. The patient data also indicate that supraspinal projections are not required, a finding consistent with recent motor unit recordings from spinal cord-injured humans (Gorassini et al., 2000) and rats (Bennett et al., 2000). However, in our experiments these additional forces could be modified by descending commands because the instruction to relax completely could abolish the sustained involuntary activity that often remained after the stimulation was turned off. Surprisingly, these self-sustained contractions of the calf muscles were not abolished by brief isometric voluntary contractions of the antagonistic muscles, demonstrating a lack of responsiveness to a particular voluntary drive that is often reciprocally organized.
The presence of these additional forces dramatically shifts the force-frequency relation of the stimulated muscle and introduces nonlinearities far greater than known for “isolated” muscle (Partridge, 1966; Binder-Macleod and Clamann, 1989). Furthermore, it makes the responses to muscle stimulation more unpredictable because the size of the extra forces varies and the onset can be triggered at different points in a stimulus train. This contrasts with the stability of the responses to the different stimulus trains during the proximal nerve block.
Given the widespread use of electrical stimulation of human muscle, it is unclear why the potency of the phenomenon reported here has not been reported previously. Our use of large surface electrodes over the muscles, long-duration pulses that favor activation of afferent axons (Veale et al., 1973; Mogyoros et al., 1996), high stimulation frequencies, and the patterns of stimulation may account for this. However, inspection of published records suggests that the additional forces to electrical stimulation are present in some recordings (Bigland-Ritchie, 1981; Rafolt et al., 1999).
Previous studies with electrical stimulation have revealed that the discharge of human Sol motoneurons may be locked to the reflex stimulus at very low frequencies in the presence or absence of a weak voluntary contraction (as in the Hoffman or H reflex; Ashby and Zilm, 1982; Burke et al., 1984). However, in relaxed subjects the H reflex declines with increasing rates of stimulation (Burke and Schiller, 1976; Burke et al., 1989; Crone and Nielsen, 1989; Hultborn et al., 1996). It is often overlooked that at high stimulus frequencies this locking is absent and that the discharge of recruited motoneurons is not locked to the driving stimulus, as shown in Figure 4 (De Gail et al., 1966; Lang and Vallbo, 1967; Burke and Schiller, 1976). One explanation for the present results is that motoneurons are recruited by the high-frequency afferent bombardment and their discharge is then sustained via activation of plateau potentials within them. The motoneurons then become temporally uncoupled from the reflex inputs which, under other conditions, can drive them through largely monosynaptic reflex paths (Burke et al., 1984).
Plateau potentials leading to bistable states are generated in many central neurons (Fraser and MacVicar, 1996; Morisset and Nagy, 1999) and have been demonstrated in mammalian motoneurons (Schwindt and Crill, 1980; Hounsgaard et al., 1988; Hounsgaard and Kiehn, 1989;Bennett et al., 1998a; Lee and Heckman, 1998; Carlin et al., 2000) (for review, see Hornby et al., 2000). Several features of the present results are consistent with the involvement of plateau potentials in motoneurons. First, the additional force depended on the activation of the smaller, more fatigue-resistant motoneurons (Lee and Heckman, 1998,2000) by high-frequency synaptic input from large-diameter afferents (Hultborn et al., 1975; Bennett et al., 1998a; Lee and Heckman, 1998); second, it often became more prominent with repeated bursts of stimulation analogous to the “wind up” phenomenon (Bennett et al., 1998b); third, it could generate self-sustained firing (Hounsgaard et al., 1988; Wada et al., 1989; Kiehn and Eken, 1997; Gorassini et al., 1998); fourth, it could often be terminated by particular “inputs” to the motoneuron pool (Hultborn et al., 1975; Lee and Heckman, 1998), and finally, motor unit discharge frequencies were similar to those in a previous human study that probably involved plateau potentials (Kiehn and Eken, 1997). Alternatively, the abnormal force increments may derive from plateau-like activity in spinal interneurons, or a “reverberating” spinal circuit (Hultborn et al., 1975), although there is currently little evidence for such a circuit.
Regardless of the underlying mechanism, stimulation applied over human muscles can recruit motoneurons synaptically, presumably according to their natural recruitment order (small to large; Henneman, 1957;Henneman et al., 1965). This may have applications for functional electrical stimulation in which muscle fatigue is often a problem caused by the preferential activation of motoneurons with large-diameter axons. The additional recruitment of the smaller motoneurons that innervate more fatigue-resistant muscle fibers may be particularly useful for generating sustained contractions required for tasks such as standing. Similarly, because the stimulation activates a portion of the muscle not normally activated by direct motor axon stimulation, it may have applications in reducing the muscle atrophy resulting from disuse associated with acute or chronic injury conditions.
Plateau potentials have been invoked in other studies on human subjects to explain cramp-like behavior (Baldissera et al., 1991, 1994), self-sustained discharges (Kiehn and Eken, 1997; Gorassini et al., 1998), and the potential discrepancy between the forces at recruitment and derecruitment of motor units (Heckman and Lee, 1999). Whereas these studies focused on EMG recordings and the properties of single motor units, our study focused on the resulting forces and suggests that these intrinsic mechanisms can generate large forces and thereby make a substantial contribution to the control of voluntary movement. Thus, present understanding of the in vivo operation of human motoneuron pools may need revision.
Footnotes
This work was funded by the National Health and Medical Research Council of Australia (Grant number 3206). D.F.C. was supported by the National Sciences and Engineering Research Council of Canada and the Alberta Heritage Foundation for Medical Research. We are grateful to Drs. Elspeth McLachlan and James Brock for comments on this manuscript.
Correspondence should be addressed to S. C. Gandevia, Prince of Wales Medical Research Institute, Barker Street, Randwick, New South Wales, Australia 2031. E-mail: S.Gandevia@unsw.edu.au.
REFERENCES
- 1.Ashby P, Zilm D. Characteristics of postsynaptic potentials produced in single human motoneurons by homonymous group 1 volleys. Exp Brain Res. 1982;47:41–48. doi: 10.1007/BF00235884. [DOI] [PubMed] [Google Scholar]
- 2.Baldissera F, Cavallari P, Dworzak F. Cramps: a sign of motoneurone “bistability” in a human patient. Neurosci Lett. 1991;133:303–306. doi: 10.1016/0304-3940(91)90594-j. [DOI] [PubMed] [Google Scholar]
- 3.Baldissera F, Cavallari P, Dworzak F. Motor neuron “bistability”. A pathogenetic mechanism for cramps and myokymia. Brain. 1994;117:929–939. doi: 10.1093/brain/117.5.929. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998a;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998b;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DJ, Gorassini M, Yunru L, Sanelli L (2000) Plateaus and spasticity in chronic sacral spinal rats. Plateau potentials, and rhythmic firing in motoneurons, an international conference organized by R. M. Enoka and C. J. Heckman, Boulder, CO, June. (http://www.colorado.edu/kines/BoulderMeeting.html).
- 7.Bergmans J. The physiology of single human nerve fibres. University of Louvain; Vander: 1970. [Google Scholar]
- 8.Bigland-Ritchie B. EMG and fatigue of human voluntary and stimulated contractions. Ciba Found Symp. 1981;82:130–156. doi: 10.1002/9780470715420.ch9. [DOI] [PubMed] [Google Scholar]
- 9.Binder-Macleod SA, Clamann HP. Force output of cat motor units stimulated with trains of linearly varying frequency. J Neurophysiol. 1989;61:208–217. doi: 10.1152/jn.1989.61.1.208. [DOI] [PubMed] [Google Scholar]
- 10.Burke D, Schiller HH. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psychiatry. 1976;39:729–741. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to the human ankle jerk and H-reflex. J Neurophysiol. 1984;52:435–447. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- 12.Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. 1989;112:417–433. doi: 10.1093/brain/112.2.417. [DOI] [PubMed] [Google Scholar]
- 13.Burke RE. Motor units: anatomy, physiology and functional organisation. In: Brooks VB, editor. Handbook of Physiology. American Physiological Society; Bethesda, MD: 1981. pp. 345–421. [Google Scholar]
- 14.Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 15.Collins DF, Gandevia SC. Afferent activation contributes to force production during electrical stimulation of human muscle. Soc Neurosci Abstr. 2000;26:2215. [Google Scholar]
- 16.Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- 17.De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966;29:1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eccles JC. The physiology of nerve cells. Johns Hopkins UP; Baltimore: 1957. [Google Scholar]
- 19.Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol Scand. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- 20.Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandevia SC, Burke D, Collins DF. Possible role of plateau potentials in force increments produced by electrical stimulation over human muscles. Proc Austr Neurosci Soc. 2001;12:228. [Google Scholar]
- 22.Gorassini M, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–16. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- 23.Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- 24.Gorassini M, Bennett DJ, Yang JF, Harvey P (2000) Evidence for activation of IPICS from firing behaviour of motor unit pairs in uninjured and spinal cord injured human subjects. Plateau potentials and rhythmic firing in motoneurons, an international conference organized by R. M. Enoka and C. J. Heckman, Boulder, CO, June. (http://www.colorado.edu/kines/BoulderMeeting.html).
- 25.Granit R, Phillips CG, Skoglund S, Steg G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957;20:470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- 26.Granit R, Kernell D, Lamarre Y. Algebraical summation in synaptic activation of motoneurones firing within the “primary range” to injected currents. J Physiol (Lond) 1966;187:379–399. doi: 10.1113/jphysiol.1966.sp008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heckman CJ, Lee RH. Synaptic integration in bistable motoneurons. In: Binder MD, editor. Progress in brain research. Elsevier Science; Amsterdam: 1999. pp. 49–56. [DOI] [PubMed] [Google Scholar]
- 28.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 29.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 30.Hornby TG, Stauffer EK, Stuart DG. Open issues on the functional role of the plateau potential in the repetitive discharge of motoneurons in experimental animals and humans. In: Dengler R, Kossev AR, editors. Sensorimotor control. IOS; Amsterdam: 2000. [Google Scholar]
- 31.Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol (Lond) 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol (Lond) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- 34.Hultborn H, Wigstrom H, Wangberg B. Prolonged activation of soleus motoneurones following a conditioning train in soleus Ia afferents—a case for a reverberating loop? Neurosci Lett. 1975;1:147–152. doi: 10.1016/0304-3940(75)90030-0. [DOI] [PubMed] [Google Scholar]
- 35.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- 36.Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- 37.Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- 38.Lang AH, Vallbo ÅB. Motoneuron activation by low intensity tetanic stimulation of muscle afferents in man. Exp Neurol. 1967;18:383–391. doi: 10.1016/0014-4886(67)90056-8. [DOI] [PubMed] [Google Scholar]
- 39.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- 42.Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Partridge DL. Signal-handling characteristics of load-moving skeletal muscle. Am J Physiol. 1966;210:1178–1191. doi: 10.1152/ajplegacy.1966.210.5.1178. [DOI] [PubMed] [Google Scholar]
- 44.Rafolt D, Gallasch E, Mayr W, Lanmuller H. Dynamic force responses in electrically stimulated triceps surae muscles: effects of fatigue and temperature. Artif Organs. 1999;23:436–439. doi: 10.1046/j.1525-1594.1999.06373.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwindt P, Crill W. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- 46.Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol (Lond) 1998;507:919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veale JL, Mark RF, Rees S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry. 1973;36:75–86. doi: 10.1136/jnnp.36.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada N, Nakajima Y, Homma S. Long-lasting afterdischarge of alpha-motoneurons after muscle vibration or electrical stimulation of Group I afferent fibers in the anemically decerebrated cat. Neurosci Res. 1989;6:234–247. doi: 10.1016/0168-0102(89)90062-x. [DOI] [PubMed] [Google Scholar]