Abstract
Mesencephalic precursor cells may one day provide dopaminergic neurons for the treatment of Parkinson's disease. However, the generation of dopaminergic neurons from mesencephalic precursors has been difficult to follow, partly because an appropriate means for recognizing mesencephalic ventricular zone precursors has not been available. To visualize and isolate mesencephalic precursor cells from a mixed population, we used transgenic mice and rats carryinggreen fluorescent protein (GFP) cDNA under the control of the nestin enhancer.nestin-driven GFP was detected in the mesencephalic ventricular zone, and it colocalized with specific markers for neural precursor cells. In addition, data from flow-cytometry indicated that Prominin/CD133, a cell-surface marker for ventricular zone cells, was expressed specifically in these GFP-positive (GFP+) cells. After sorting by fluorescence-activated cell sorting, the GFP+ cells proliferated in vitro and expressed precursor cell markers but not neuronal markers. Using clonogenic sphere formation assays, we showed that this sorted population was enriched in multipotent precursor cells that could differentiate into both neurons and glia. Importantly, many neurons generated fromnestin-GFP-sorted mesencephalic precursors developed a dopaminergic phenotype in vitro. Finally,nestin-GFP+ cells were transplanted into the striatum of a rat model of Parkinson's disease. Bromodeoxyuridine–tyrosine hydroxylase double-labeling revealed that the transplanted cells generated new dopaminergic neurons within the host striatum. The implanted cells were able to restore dopaminergic function in the host striatum, as assessed by a behavioral measure: recovery from amphetamine-induced rotation. Together, these findings indicate that precursor cells harvested from the embryonic ventral mesencephalon can generate dopaminergic neurons able to restore function to the chemically denervated adult striatum.
Keywords: Parkinson's disease, green fluorescent protein (GFP), fluorescence-activated cell sorting (FACS), dopaminergic neuron, precursor cells, transplantation
Parkinson's disease is characterized by the selective degeneration of dopaminergic (DA) neurons in the substantia nigra of the midbrain (Nagatsu et al., 1984;Agid et al., 1987; Damier et al., 1999). Grafts of embryonic ventral mesencephalon (VM) can survive and reduce motor symptoms after transplantation into the brains of Parkinson's patients and animal models of Parkinson's disease (Brundin et al., 1988; Sauer and Brundin, 1991; Frodl et al., 1994; Lindvall, 1994; Nakao et al., 1994;Haque et al., 1997; Björklund and Lindvall, 2000). Although transplantation is a promising treatment for Parkinson's disease, its clinical application has been limited to a few cases, because it is very difficult to obtain large numbers of human embryonic mesencephalic tissues. To overcome this problem, various candidate cells have been investigated as possible donor cells for transplantation therapy for Parkinson's disease.
Mesencephalic precursor cells derived from the developing midbrain appear to be a good candidate cell source for dopaminergic precursors. Like ventricular zone (VZ) cells elsewhere throughout the neuraxis, mesencephalic precursor cells can expand, self-renew, and generate neuronal progeny. However, unlike neural progenitors derived from the telencephalic VZ, these cells have transcription factors characteristic of their midbrain origin (Smidt et al., 1997; Sakurada et al., 1999) and can respond to regional signals by rapidly differentiating as DA neurons (Ye et al., 1998). Because of these features, these cells should provide an especially appropriate cellular substrate for generating DA neurons in vitro (Ling et al., 1998; Studer et al., 1998; Daadi and Weiss, 1999; Potter et al., 1999). Nonetheless, in previous studies, mesencephalic precursor cells that were transplanted into the adult striatum differentiated into DA neurons to only a limited extent (Svendsen et al., 1996, 1997). Thus, it is unclear whether precursor cells taken from the ventral mesencephalic VZ have any particular bias or predisposition to differentiate into DA neurons. To better characterize the intrinsic biases and phenotypic potential of these cells, we needed to isolate a pure population of VZ precursor cells from fresh VM tissue.
As a first step toward isolating VZ precursors from the midbrain, we devised a method for labeling live mesencephalic precursor cells. Our plan was that, once labeled, the cells could be directly isolated and their differentiation could be followed over time, both in vitro and in vivo. For this purpose, we established transgenic lines of both mice and rats carrying green fluorescent protein (GFP) under the control of the neural-specific enhancer for thenestin gene (Zimmerman et al., 1994; Lothian and Lendahl, 1997), thereby tagging live neural precursor cells with a viable, real-time fluorescent reporter. We then used fluorescence-activated cell sorting (FACS) to identify and isolate these cells from the mesencephalic VZ. Finally, the differentiation of the cells was followed in vitro or the cells were directly implanted into chemically lesioned adult rat striatum in vivo.
We report here that these precursor cells retained the capacity to differentiate appropriately as DA neurons, to restore DA function to chemically deafferented striatum, and to reestablish dopamine-dependent motor behaviors.
MATERIALS AND METHODS
Transgenic mice and rats. To generate thenestin-GFP transgenic rats, fertilized eggs were microinjected with the previously described transgene (Roy et al., 2000a,b; Kawaguchi et al., 2001). The 2.7 kb SalI fragment of the E/nestin:EGFP plasmid was purified and then injected into the pronucleus of fertilized eggs. Sprague Dawley female rats were crossed to the nestin-GFP males to obtain transgenic rat embryos. The nestin-GFP transgenic mice used in this study were described previously (Yamaguchi et al., 2000). C57BL/6 female mice were crossed to the nestin-GFP male mice to obtain transgenic mouse embryos. Transgenic mice and rats were identified by Southern blot analysis or by PCR using tail DNA and primers specific for the GFP sequence.
Dissociated cell culture. Pregnancies were dated by inspection for the vaginal plug, and the day of the plug was defined as embryonic day 0 (E0). VMs were dissected from mouse (E11.5–E12.5) or rat (E13.5–E14.5) embryos in serum-free medium composed of a 1:1 mixture of DMEM and F-12 (Life Technologies, Gaithersburg, MD) and then digested in 0.25% trypsin (Life Technologies) at 37°C for 5 min. The cells were washed with fresh medium and then were triturated with a fire-polished Pasteur pipette. The cells were plated onto coverslips coated with polyethyleneimine (PEI) and cultured in DMEM–F-12 containing insulin (25 μg/ml), transferin (100 μg/ml), progesterone (20 nm), sodium selenate (30 ng), putrescine (60 μm), and 10% fetal bovine serum (FBS).
Sphere culture. Sorted cells were cultured as described previously (Reynolds and Weiss, 1992). In brief, the cells were washed with DMEM–F-12 and resuspended in DMEM–F-12 containing insulin (25 μg/ml), transferring (100 μg/ml), progesterone (20 nm), sodium selenate (30 ng), putrescine (60 μm), and basic FGF (bFGF) (20 ng/ml). Cells were plated at 5000 cells/200 μl per well in ultra-low attachment 96-well plates (Costar, Cambridge, MA). For the differentiation assay, spheres at 7–8 d in vitro were plated onto PEI-coated coverslips and cultured for another 7–10 d in DMEM–F-12 containing 10% FBS.
Immunofluorescence analyses for cryosections and cultured cells. Embryos were fixed overnight in 4% paraformaldehyde in PBS, pH 7.4, at 4°C, cryoprotected in 30% sucrose in PBS overnight, embedded in O.C.T. compound (Sakura Finetechinical Co. Ltd., Tokyo, Japan), and frozen in liquid nitrogen. Ten-micrometer-thick cryosections were cut and affixed to 3-aminopropyltriethoxysilane-coated glass slides (Matsunami Glass, Osaka, Japan). Cells attached to PEI-coated coverslips were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. Fixed cryosections and cultures were washed three times with PBS and permeabilized and blocked in PBS containing 0.3% Triton X-100 and 10% donkey serum for 30 min at room temperature, followed by an overnight incubation in the same buffer at 4°C with one or more of the following antibodies: mouse anti-tyrosine hydroxylase (TH) monoclonal antibody (obtained from Dr. Hiroshi Hatanaka, Osaka University, Osaka, Japan) (Hatanaka and Arimatsu, 1984) diluted 1:500; rabbit anti-TH antibody (obtained from Dr. Ikuko Nagatsu, Fujita Health University, Toyoake, Japan) (Nagatsu et al., 1989) diluted 1:10,000; mouse anti-βIII-tubulin monoclonal antibody (Sigma, St. Louis, MO) diluted 1:100; rat anti-Musashi1 monoclonal antibody (Kaneko et al., 2000) diluted 1:500; mouse anti-Ki67 monoclonal antibody (Novocastra Laboratories, Newcastle upon Tyne, UK) diluted 1:1000; mouse anti-Nestin monoclonal antibody rat 401 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) diluted 1:500; rabbit anti-dopamine transporter (DAT) polyclonal antibody (Chemicon, Temecula, CA) diluted at 1:1000; rabbit anti-GFAP polyclonal antibody (Dako, High Wycombe, UK) diluted 1:5; mouse anti-microtubule-associated protein 2 (MAP2) monoclonal antibody (Sigma) diluted at 1:100; and rat anti-Prominin monoclonal antibody 13A4 (obtained from Dr. Wieland B. Huttner, University of Heidelberg, Heidelberg, Germany) (Weigmann et al., 1997) diluted 1:300. After three washes with PBS, the sections or cells were incubated with secondary antibodies conjugated with FITC or Cy3 (Jackson ImmunoResearch, West Grove, PA) diluted 1:1000 in PBS, for 1 hr at room temperature. The cultured cells were also incubated briefly with 10 μm Hoechst 33342 (Sigma) to stain the nuclei. After three washes with PBS, the samples were mounted on slides and examined with a Zeiss (Oberkochen, Germany) LSM510 confocal imaging system or Zeiss Axioplan2.
Cell sorting. FACS sorting of GFP-positive (GFP+) cells was performed essentially as described previously (Wang et al., 1998; Roy et al., 2000a,b; Kawaguchi et al., 2001; Sawamoto et al., 2001a). VMs were dissected from embryos in cold DMEM–F-12 medium and digested in 0.25% trypsin (Life Technologies) at 37°C for 5 min. The cells were washed with fresh medium and then were triturated with a fire-polished Pasteur pipette. Dissociated cells were spun, resuspended in PBS containing 10 μg/ml propidium iodide (PI) and 0.05% sodium azide, and filtered through nylon mesh. Sorting and analyses of fluorescent cells were performed on an FACS Vantage flow cytometer/cell sorter (Becton Dickinson, Franklin Lakes, NJ). Data analyses were performed using CELLQuest software (Becton Dickinson). Cells (1–2 × 10−6/ml) were analyzed for forward scatter, side scatter, PI fluorescence, and GFP fluorescence with an argon laser (488 nm, 100 mW). Dead cells were excluded by gating on forward and side scatter and by eliminating PI-positive events. Cells harvested from wild-type mice were used to set the background fluorescence. Viable and fluorescent cells from the transgenic embryos were sorted into DMEM–F-12 medium at a speed of 3000 cells per second. For anti-Prominin staining, the VM cell suspension was incubated with the monoclonal antibody 13A4 for 30 min on ice. The cells were washed with PBS and then stained with phycoerythrin (PE)-conjugated anti-rat IgG, followed by washing. The stained cells were resuspended in PBS containing 10 μg/ml PI and 0.05% sodium azide and applied to the flow cytometer for analysis.
Unilateral 6-hydroxydopamine lesions and amphetamine-induced circling behavior. Young adult male Sprague Dawley rats (Charles River, Osaka, Japan), weighing 180–200 gm at the start of the experiment were used. The rats were maintained under a 12 hr dark/light cycle with ad libitum access to food and water. All surgical procedures were conducted under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). Unilateral lesions of the ascending mesostriatal DA pathway were made by intracerebral injections of 6-hydroxydopamine (6-OHDA) (3 mg/ml in 0.2 mg/ml ascorbate–saline; Sigma), as described in detail previously (Nakao et al., 1998). Two weeks after the lesion surgery, amphetamine (0.25 mg/kg, i.p.)-induced turning behavior was monitored over a period of 60 min. The rats that exhibited a net rotational asymmetry of at least six full turns per minute toward the lesioned side were selected for transplantation surgery, because this extent of amphetamine-induced rotational asymmetry corresponds, on average, to a 99% depletion of striatal DA neurons (Schmidt et al., 1983). The drug-induced rotational behavior was tested again 5 weeks after grafting to examine the functional effects of the implanted GFP+ cells.
Transplantation surgery. The sorted GFP+ cells were spun and resuspended in a final volume of 100–150 μl of HBSS. The cell viability, assessed with a trypan blue dye exclusion test, was over 95% just before grafting, and the cell concentration was 10,000–13,000 per microliter Lesioned rats were divided into two groups: lesion control (n = 4) and transplantation (n = 5). In the transplantation group, two stereotaxic deposits, of 2 μl of cell suspension each, were injected into the lesioned striatum as described previously (Nakao et al., 1994, 1998). All of the engrafted rats were given intraperitoneal injections of bromodeoxyuridine (BrdU) (50 mg/kg; Sigma) for 14 consecutive days, starting on the day of transplantation surgery.
Tissue processing. The animals were anesthetized with lethal doses of sodium pentobarbital and perfused transcardially with physiological saline, followed by 4% paraformaldehyde in 0.1m phosphate buffer, pH 7.4. After a 6 hr post-fixation incubation in the same fixative, the brains were immersed in 20% sucrose in 0.1 m phosphate buffer at 4°C until they sank. Sections were cut at 30 μm in a cryostat and collected in PBS.
For quantification of the number of grafted cells expressing the DA phenotype, free-floating sections were processed for TH immunohistochemistry as described previously (Nakao et al., 1994,1998). Briefly, the sections were preincubated in 10% blocking serum–0.2% Triton X-100–PBS for 1 hr at room temperature and then incubated overnight at room temperature with primary antibodies against tyrosine hydroxylase (1:500; Pel-Freeze). Subsequently, the tissue was exposed to biotinylated anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) for 1 hr at room temperature. The bound antibodies were visualized using an avidin–biotin–peroxidase complex system (Vectastain ABC Elite kit; Vector Laboratories), with 3,3-diaminobenzidine as the chromogen. The number of TH-immunoreactive neurons in each graft was counted under bright-field illumination on every third section. The raw counts of TH-immunoreactive cells were corrected according to the formula of Abercrombie (1946).
Some brain sections were also processed for immunofluorescence double-labeling to determine whether new DA neurons might be generated from grafted GFP+ cells by mitotic neurogenesis. Free-floating sections were pretreated in 50% formamide–2× SSC at 65°C for 2 hr, incubated in 2N HCl at 37°C for 30 min, and then rinsed for 10 min in 0.1 m boric acid, pH 8.5. Brain sections were incubated overnight with mouse monoclonal anti-BrdU (1:100; NeoMarkers, Fremont CA) and for 2 hr with biotinylated horse anti-mouse antibody (1: 200; Vector Laboratories). Sections were then incubated with ExtraAvidin-FITC conjugate (10 mg/ml; Sigma) for 2 hr. After three 5 min washes with PBS, the sections were incubated overnight with anti-TH antibody (1:500; Pel-Freeze), followed by a 2 hr incubation with Cy3-labeled sheep anti-rabbit IgG (1:50; Chemicon). Sections were thoroughly washed in PBS and then mounted on glass slides and covered with coverslips. Fluorescent signals were detected and processed using a confocal scanning laser microscope (Olympus Optical, Osaka, Japan).
Statistical analyses. All data were expressed as the mean ± SEM. A paired two-tailed Student's t test was used to compare amphetamine-induced rotation scores before and after grafting. p < 0.05 was considered significant.
RESULTS
Specific expression of the nestin-GFP transgene in the ventricular zone of the ventral mesencephalon
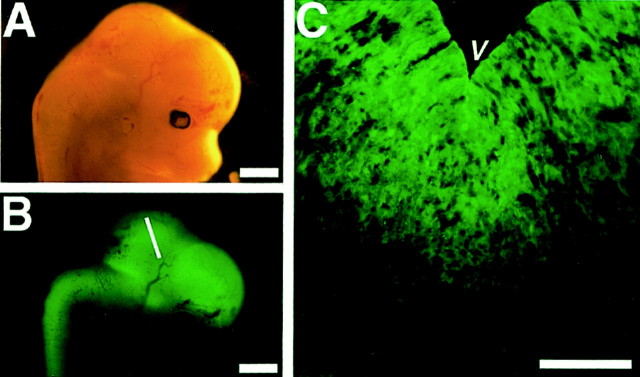
To label live precursor cells in the ventral mesencephalon, we used a GFP reporter gene placed under control of the regulatory elements of the nestin gene (Roy et al., 2000a,b; Yamaguchi et al., 2000; Kawaguchi et al., 2001; Sawamoto et al., 2001). We first examined the expression pattern of GFP in the VM of Nestin-GFP mouse embryos at E11.5. At this stage, DA neurons are generated in the ventricular zone of the VM by mitotic neurogenesis (Kawano et al., 1995). Under a fluorescence stereomicroscope, GFP fluorescence was detected throughout the developing CNS, including the mesencephalon (Fig.1A,B). To determine where GFP was expressed within the VM, coronal sections through the mesencephalon were examined. nestin-driven GFP fluorescence was brightest in ventricular zone cells in the VM and very weak outside of the VZ (Fig. 1C), which is an expression pattern similar to that of the Nestin protein and mRNA (Hockfield and McKay, 1985; Dahlstrand et al., 1995).
Fig. 1.
Mesencephalon of a nestin-GFPembryo. A, B, Head of an E12.5nestin-GFP embryo. A, Bright-field image.B, GFP fluorescence was detected throughout the CNS. C, A coronal section of the ventral mesencephalon (indicated by white line inB). GFP fluorescence was detected in the ventricular zone. Scale bars: A, B, 1 mm;C, 100 μm.
Sections of mesencephalon dissected from E11.5 nestin-GFPmouse embryos were then fixed and immunostained with antibodies against cell type-specific markers in combination with Cy3-conjugated secondary antibodies (Fig. 2). Figure2A–C shows a section stained with anti-TH antibody. In the VM, TH is expressed only in the DA neurons (Kalsbeek et al., 1992). The TH+ DA neurons were not located in the GFP+ ventricular zone. We next stained sections with an antibody against βIII-tubulin, a marker for postmitotic neurons (Fig. 2D–F). The pattern of GFP expression was essentially reciprocal to that of βIII-tubulin (Fig. 2D–F), suggesting that thenestin-GFP transgene was expressed by neural precursor cells in the VZ at a high level but was downregulated rapidly during neuronal differentiation. On the other hand, the expression pattern of GFP overlapped with the immunoreactivity of Musashi1 (Fig.2G–I), an RNA-binding protein that is highly enriched in CNS precursor cells (Sakakibara et al., 1996; Kaneko et al., 2000). We also examined whether GFP was expressed in mitotic cells. To label the nuclei of actively dividing cells in the VZ, we stained sections with an antibody against the Ki67 antigen, which is expressed specifically in proliferating cells (Gerdes et al., 1983). We found that a population of Ki67+ cells in the VZ expressed GFP (Fig. 2J–L). These results imply that the nestin-GFP is active in precursor cells in the VZ, including mitotic neural progenitor cells, but is downregulated during differentiation and departure from the VZ.
Fig. 2.
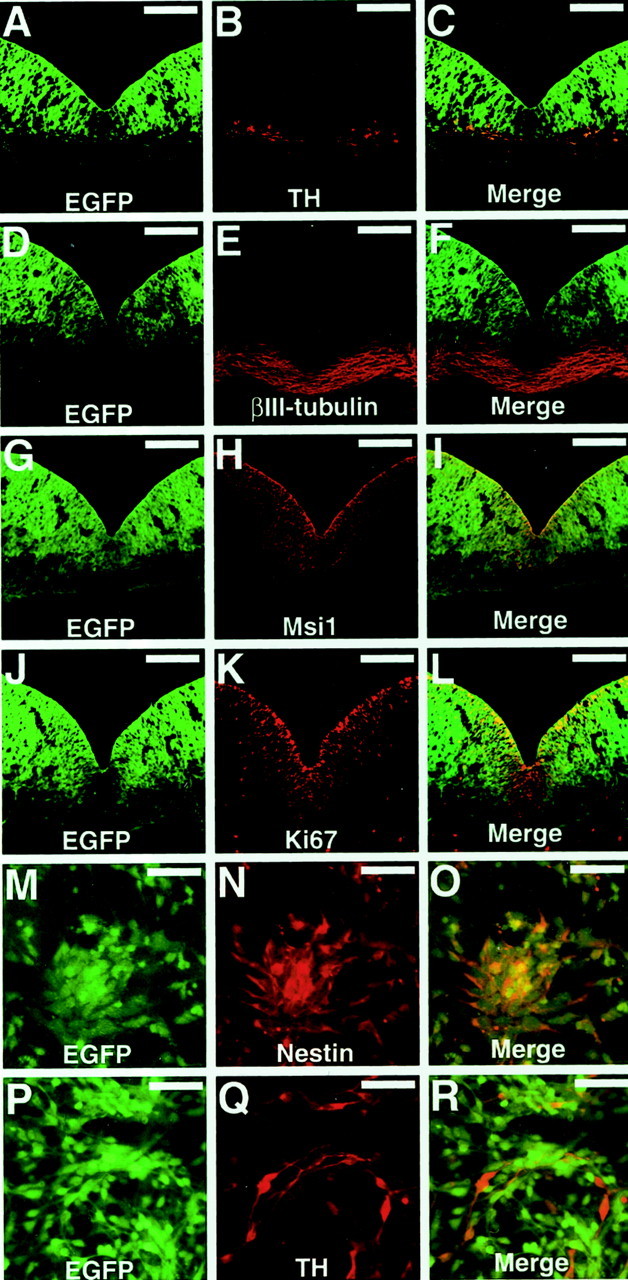
Expression pattern of thenestin-GFP transgene in the ventral mesencephalon.A–L, Coronal sections of the ventral mesencephalon fromnestin-GFP E11.5 embryos were stained with antibodies against tyrosine hydroxylase (A–C), βIII-tubulin (D–F), Musashi1 (G–I), or Ki67 (J–L).M–R, Dissociated cells from thenestin-GFP ventral mesencephalon were stained with antibodies against Nestin (M–O) or tyrosine hydroxylase (P–R). Each antibody was visualized with a Cy3-conjugated secondary antibody (red). Scale bars: A–L, 100 μm; M–R, 25 μm.
To more precisely define the antigenic phenotype of the GFP+ cells, dissociated VM cells derived from nestin-GFP embryos were cultured for 24 hr and then fixed and immunostained for cell type-specific markers. All GFP+ cells were immunoreactive to anti-Nestin (Fig. 2M–O) and to anti-Musashi1 (data not shown). In contrast, the GFP+ cells were negative for TH (Fig. 2P–R). The GFP+ cells were also negative for neuronal (βIII-tubulin and MAP2) and astrocytic (GFAP) markers (data not shown).
Together, these results suggest that, in the VM,nestin-driven GFP expression was more robust in undifferentiated progenitor cells than in their differentiated daughters, including TH+ DA neurons.
Direct isolation of VM precursor cells by FACS
The expression patterns of nestin-GFP in the embryo (Figs. 1, 2) raised the possibility that we could use flow cytometry to identify and isolate neural precursor cells in the VZ as a cell population expressing GFP. We first analyzed the GFP fluorescence intensity of dissociated VM cells from E11.5–E12.5 embryos. More than 80% of the total cells from nestin-GFP embryos showed a brighter green fluorescence than did any of the control cells, which were derived from the VMs of age- and strain-matched nontransgenic mice (Fig.3A,B). This was consistent with our observation that most of the cells outside of the VZ also had very weak but detectable green fluorescence (for example, see Fig. 1C).
Fig. 3.
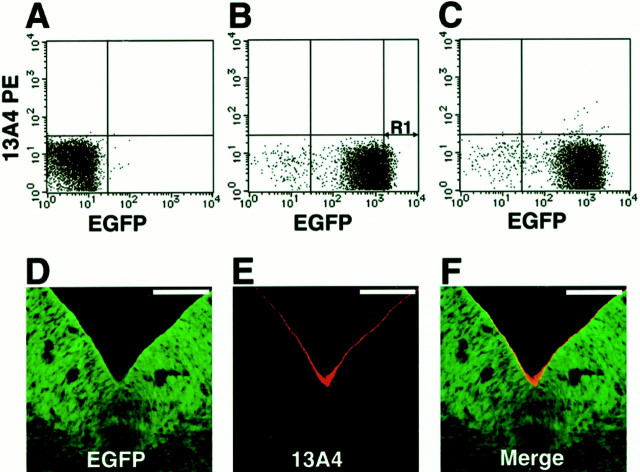
Isolation ofnestin-GFP+ cells from the embryonic VM. A–C, Flow-cytometric analyses of mesencephalic cells. A, VM cells from wild-type embryos were incubated with PE-conjugated anti-rat IgGs. B, VM cells fromnestin-GFP embryos were incubated with PE-conjugated anti-rat IgG. C, VM cells from nestin-GFPembryos were incubated with rat monoclonal antibody 13A4 (anti-Prominin) and PE-conjugated anti-rat IgG. D–F, A coronal section of E11.5 nestin-GFP ventral mesencephalon stained with anti-Prominin and a Cy3-conjugated secondary antibody (red). Scale bars, 100 μm.
To confirm that the GFP+ VM cell population isolated by flow cytometry contained cells from the ventricular zone, we labeled cells with the monoclonal antibody 13A4, which recognizes the extracellular portion of Prominin, a plasma membrane protein specifically expressed on the apical surface of epithelial cells (Weigmann et al., 1997). To examine the localization of Prominin in the VM, coronal sections of the mesencephalons fromnestin-GFP mice were stained with 13A4, followed by a Cy3-conjugated secondary antibody (Fig. 3D–F). Prominin expression was detected only on the apical surface of the ventricle, in which nestin-driven GFP was brightest.
We then stained a VM cell suspension from nestin-GFP VM tissue with 13A4 and a PE-conjugated secondary antibody and analyzed this binding simultaneously with GFP fluorescence (Fig. 3C). Almost all of the 13A4-positive cells showed bright GFP fluorescence, which was consistent with our observation that GFP fluorescence was brightest near the ventricular wall (Figs. 1C,2A–L), in which mitotic neurogenesis occurs. [We reported previously that the intensity of nestin-driven GFP expression in neural cells correlated with their mitotic and neurogenic potentials (Kawaguchi et al., 2001).]
Therefore, to obtain a highly purified population of precursor cells, the 10–15% of the total cell population (Fig. 3B,R1) that had the brightest relative fluorescence was sorted as GFP+ cells and used for the analyses described below. Reanalysis of the sorted GFP+ cell population by flow cytometry confirmed that all of the sorted cells expressed GFP (data not shown). The sorted GFP+ cells were allowed to attach to coverslips and stained with antibodies against Nestin, βIII-tubulin, and TH. As expected, virtually all of the GFP+ cells were positive for Nestin and negative for βIII-tubulin and TH (data not shown), suggesting that we had isolated a nearly pure population of precursor cells.
In vitro characterization of the sorted GFP+ cells
Because the anti-Ki67 staining of sections indicated that a subpopulation of the GFP+ cells was actively dividing in vivo (Fig. 2J–L), we next examined whether the isolated GFP+cells could also proliferate in vitro. Sorted cells were plated onto PEI-coated coverslips and cultured in DMEM–F-12-based serum-free media with epidermal growth factor (EGF) and bFGF (20 ng/ml each). Cells were treated for 1 hr with BrdU (10 μm) 1.5 hr after plating, fixed, and stained with anti-BrdU. Of the total cells counted (n = 2152), 7.8% (168 cells) had BrdU-labeled nuclei. In contrast, the sorted GFP-negative (GFP−) population incorporated BrdU into only 2% (27 cells) of the total cells (n = 1313). These results indicate that, under these culture conditions, fetal brain cells with bright GFP fluorescence were mitotic compared with those in which nestin transcription was no longer active.
We next examined whether the GFP+ VM cell population contained neural stem cells that were multipotent and had the capacity to self-renew. To evaluate these characteristics, we used a culture system in which neural stem cells selectively proliferate to form multicellular aggregates called neurospheres (Reynolds and Weiss, 1992). For this procedure, the sorted cells were counted and plated at a density of 5000 cells per well (Fig.4A). The cells were then cultured in serum-free medium supplemented with 20 ng/ml bFGF. We observed a number of small growing neurospheres that were composed of fluorescent cells as early as 2 d after plating (Fig.4B). Seven days after plating, we counted the number of fluorescent neurospheres whose diameter was >50 μm (Fig.4C). We found a striking difference between the potential of GFP+ cells and GFP− cells (cells without fluorescence or with a lower intensity of fluorescence than the GFP+ cells) to generate spheres. GFP+ cells generated 33 ± 8.6 spheres per well (n = 8), whereas GFP− cells generated only 2 ± 0.9 spheres per well (n = 8). In addition, the size of the spheres derived from the GFP+ cells was greater than that of the spheres from GFP− cells; GFP+ cells generated 9.2 ± 3.0 spheres with a diameter of >100 μm per well (n = 7), but no spheres of this size could be found in the GFP− cultures (n = 7).
Fig. 4.
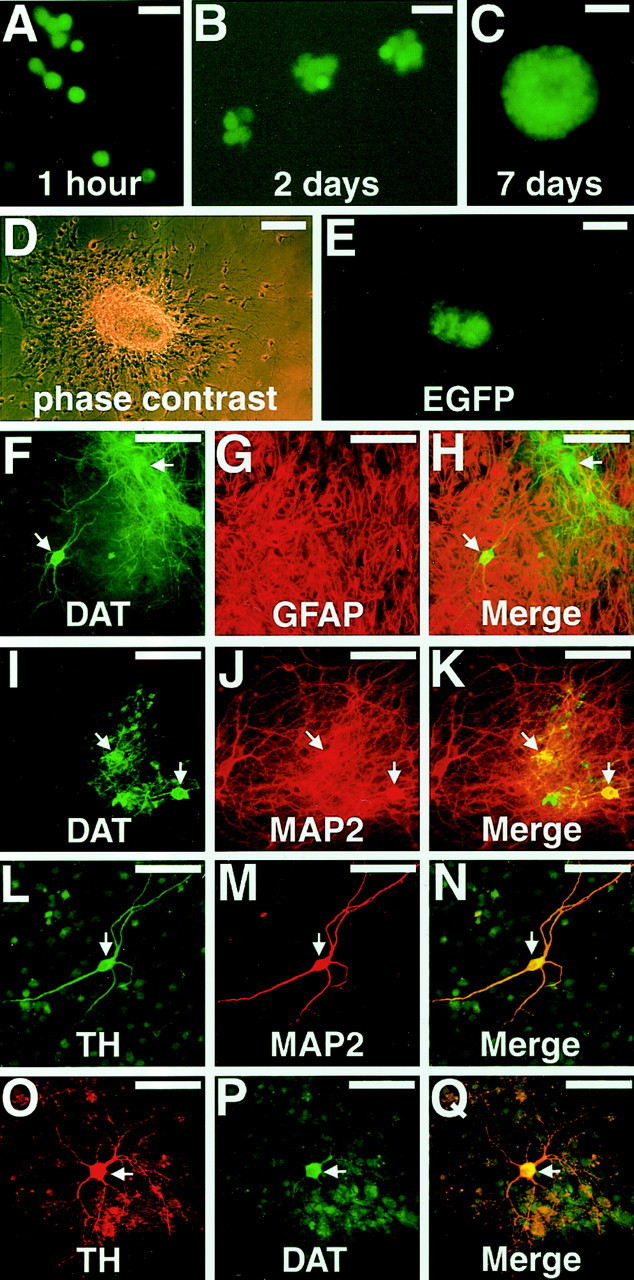
The sortednestin-GFP+ cells generated neurospheres. A–D, GFP fluorescent images of growing spheres at days 0 (A), 2 (B), and 7 (C).D–E, Cell sheet derived from a single neurosphere 16 hr after transferring it onto an adhesive coverslip. D, Phase contrast. E, GFP fluorescence was detectable only in the center of the colony in which cells still existed as multicellular aggregates. F–Q, Cell sheets after 10 d of differentiation stained with antibodies against DAT and GFAP (F–H), DAT and MAP2 (I–K), TH and MAP2 (L–N), or TH and DAT (O–Q). Putative DA neurons are indicated by arrows. Scale bars: A,B, 15 μm; C–E, 50 μm;F–Q, 25 μm.
We next examined the lineage potential of the sphere-initiating cells that were enriched in the GFP+ fraction. For this purpose, we transferred spheres formed in GFP+ cultures to PEI-coated coverslips and cultured them in medium containing FBS. After 16 hr, the spheres had flattened but incompletely; cells in the edge region of the colony spread well, but those in the center still formed multicellular aggregates (Fig. 4D). We then examined the GFP expression in the colonies using fluorescence microscopy. The cells in the aggregate at the center of the colony showed green fluorescence, but the spreading cells at the edge did not (Fig.4E). The colonies were then cultured as monolayers for 14 d, after which the cell sheets were stained with antibodies against neuronal (MAP2 and βIII-tubulin), astrocytic (GFAP), and oligodendrocytic (O4) markers. All of the colonies examined (n = 20) contained these three cell types (data not shown), indicating that the GFP+ cells that gave rise to the colonies were multipotent. Putative DA neurons that were positive for TH and/or DAT, as well as MAP2, were also observed in most of the colonies (Fig. 4F–Q). In parallel with these experiments, we also sorted GFP+ cells from the striatum. The striatum-derived GFP+ cells generated neurospheres as described previously (Kawaguchi et al., 2001); however, they never differentiated into neurons that were positive for TH or DAT (data not shown).
Finally, to examine the capacity of the sorted GFP+ cells for self-renewal, primary spheres were individually transferred to separate wells and then dissociated into single cells. These single sphere-derived cells were then cultured and assessed for secondary sphere formation. After 7 d, 50 ± 19 spheres per well (n = 7) showing GFP fluorescence similar to the primary spheres were generated. These results further indicated that the sphere-initiating cells sorted as GFP+ cells were both self-renewing and multipotent.
Transplantation of the sorted GFP+ cells into a rat model of Parkinson's disease
To study the function of the sorted GFP+ cells in vivo, we used a rat model of Parkinson's disease (for review, see Brundin et al., 1994). To obtain donor cells that could be transplanted into rats without immunosuppression, we generated transgenic rats carrying anestin-GFP transgene. The expression pattern of GFP in the ventricular zone and the nature of the sorted GFP+ cells in vitro were similar to those seen in mice. GFP expression in the ventricular zone was restricted to cells that were positive for Musashi1 and Nestin but negative for TH and βIII-tubulin (data not shown), and GFP+ cells sorted from the VM ofnestin-GFP rat embryos at E13.5–E14.5 (which corresponds to E11.5–E12.5 in mice) contained virtually no TH+ neurons. Furthermore, pilot transplantation experiments using mouse cells as donors gave data similar to those using rat cells (data not shown). Therefore, we show here only the results from experiments using the rat cells as donors.
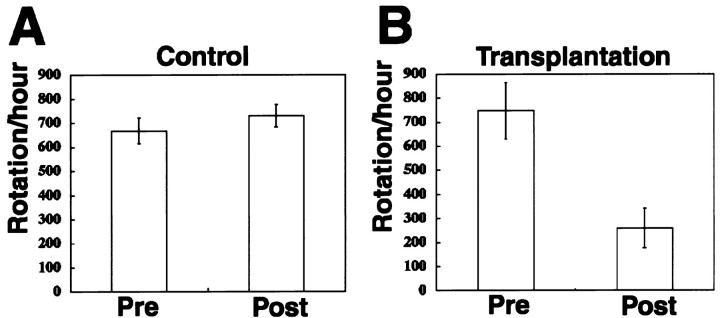
The chosen rat model permits a behavioral assessment (rotation response to amphetamine) of the extent to which the Parkinson's symptoms have been alleviated (Schmidt et al., 1983). In this experiment, all of the animals with a unilateral 6-OHDA lesion displayed a robust rotation response to amphetamine before transplantation, and there was no significant difference in rotation scores between the rats assigned to the lesion control and transplantation groups (p> 0.05; unpaired two-tailed Student t test). In the transplantation group, each rat received a total of ∼50,000 grafted cells in the lesioned striatum. Five weeks after grafting the cells, a significant reduction in rotational asymmetry was observed in the grafted rats (p < 0.01) (Fig.5B), whereas the lesion control animals did not show any improvement (p> 0.05) (Fig. 5A). The rotation scores of the grafted animals were reduced 65.2% on average compared with the pretransplantation scores.
Fig. 5.
Transplanted GFP+ cells caused functional recovery in a rat model of Parkinson's disease.A, B, Net ipsilateral amphetamine induced rotation asymmetry over the 60 min test session. A, Control animals (n = 4) showed no improvement.B, The animals with grafts (n = 5) showed steady improvement.
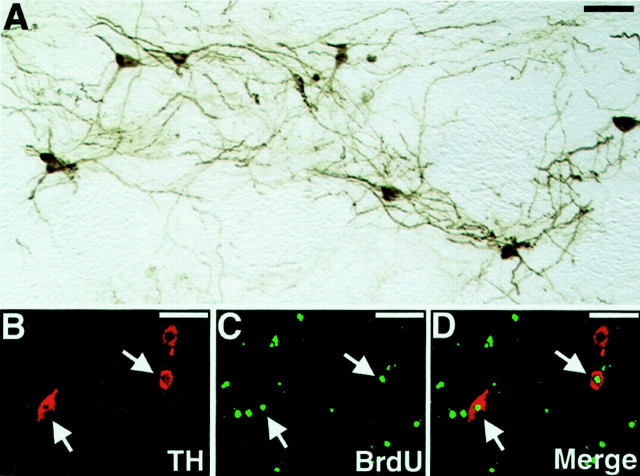
The 6-OHDA lesions of the mesostriatal bundle produced a virtually complete loss of TH+ DA neurons in the substantia nigra with a concomitant depletion of TH-immunoreactive fibers in the lesioned striatum. During examination of the brain tissues, each rat that had received GFP+cells was found to have two grafts containing TH+ cells in the rostral part of the host striatum. Individual TH+ cells within the grafts displayed morphological features suggestive of mature neurons and extended numerous TH+ fibers into the DA-depleted striatum (Fig.6A). Each rat had a mean number of 624 ± 123 TH+ cells in the two graft deposits. The percent reduction in net rotational asymmetry of individual animals was plotted against the number of surviving TH+ neurons, and linear regression analysis was performed. These two parameters were found to be significantly correlated (p < 0.005;r2 = 0.851).
Fig. 6.
Morphology of TH+ neurons in the graft. A, Brain sections of the 6-OHDA-lesioned animals that received sorted GFP+ cells were immunostained for TH. B–D, Evidence that transplanted GFP+ cells generated new TH+neurons. B, Anti-TH staining (red).C, Anti-BrdU staining (green).D, Merged image. Scale bars, 100 μm.
To label the new DA neurons generated from the donor cells by mitotic neurogenesis, the host animals were injected with BrdU daily for 2 weeks. The sections through the graft were stained with anti-BrdU and anti-TH antibodies (Fig. 6B–D). The rats contained an average of 47.2 ± 12.0 cells (n = 5) that were positive for both BrdU and TH in the striatum. Nuclei in 7.2 ± 0.8% of the TH+ cells (n= 5) were labeled with BrdU. Thus, enough transplanted precursor cells differentiated into functional DA neurons to improve the amphetamine-induced rotational asymmetry in this rat model of Parkinson's disease.
DISCUSSION
Midbrain DA neurons are generated from precursor cells in the ventricular zone of the developing VM. After mitosis in the ventricular zone, the precursor cells migrate radially, start to express specific markers including TH, and differentiate into mature DA neurons (Kawano et al., 1995). Because of the DA character of these cells, they are an interesting candidate source for donor cells that could be used in transplantation therapy for Parkinson's disease. Most studies of these precursor cells have been based on cells acquired by expanding dissociated cell cultures. However, after long-term culture under mitogenic stimulation, these expanded cell populations may become chromosomally abnormal and phenotypically unrepresentative of their founder populations. Therefore, large-scale expansion from a small number of progenitors may not be the best strategy for studying their native potential. The selection of mesencephalic precursor cells based on surface markers, a common and effective strategy in the hematopoetic system, is hampered in the neural system by the lack of surface antigens specific for the neural precursor phenotype. As an alternative strategy, we have developed a technique to visualize and isolate a specific cell type by labeling neural cells with GFP under the control of a specific promoter (for review, see Okano and Goldman, 2000). Using a nestin-GFP transgene, we demonstrated a strong correlation between GFP expression and the immature precursor cell phenotype in the forebrain (Kawaguchi et al., 2001). Here, we used thenestin-GFP transgene to visualize and identify mesencephalic precursor cells in the ventricular zone. As expected, GFP fluorescence was restricted to cells within the ventricular zone expressing Musashi1 and Nestin. Expression of GFP was near the background level in the postmitotic neurons that were positive for βIII-tubulin and/or TH. Almost all of the mitotic cells (Ki67+) expressed bright GFP fluorescence in the ventricular zone. Thus, we established that nestin-GFP was a useful and reliable marker for mesencephalic precursor cells.
To confirm that we could identify the precursor cells by flow cytometry as a population of cells with a bright GFP fluorescence, we used an antibody against Prominin. Prominin is a transmembrane protein that is expressed in the microvilli of the apical surface of epithelial cells, including those of the neuroepithelium (Weigmann et al., 1997). Recently, an antibody against the human homolog of Prominin, CD133, was used to enrich for human neural stem cells that initiate neurosphere generation (Uchida et al., 2000). Here, we found that Prominin was expressed on the ventricular surface in the developing mouse mesencephalon, indicating that Prominin could be used as a surface marker for neural precursor cells in mice. In flow cytometry, Prominin immunoreactivity was detected specifically on the cells that expressed the nestin-GFP transgene. The expression pattern ofnestin-GFP using frozen sections, cultured cells, and cell suspensions suggested that the sortednestin-GFP+ VM cells constituted a nearly pure population of precursor cells.
In vitro analysis indicated other characteristics that identified the sorted cell population as precursor cells. First, almost all of the sorted cells were positive for Nestin and Musashi1 but not for TH. Second, the sorting based on nestin-GFP fluorescence resulted in an enrichment of mitotically active cells in vitro. Third, this population included neural stem cells that could generate neurospheres. Finally, each sphere differentiated into multiple types of cells, including neurons that expressed DA markers such as TH and DAT (Lorang et al., 1994). It is known that virtually all spheres are clonally derived when cells are plated at a density <5 × 10−4 cells/ml (Hulpas et al., 1997). Therefore, these results imply that thenestin-GFP+ population contained multipotential precursor cells that could generate DA neurons. Thus, sorting nestin-GFP+ cells enabled us to obtain a nearly pure population of live VM precursor cells for the first time. The sorted cells should be a useful source of material for biological analyses of DA neurons and clinical studies for neurological disorders caused by defects in DA neurons.
Embryonic VM tissue used for transplantation contains a mixture of at least (1) differentiated neurons that have already undergone final mitosis, (2) immature neurons that have also undergone final mitosis but not started to express neuronal markers, and (3) neural progenitor cells that are still mitotic. However, it was unclear whether all of these cells have the potential to develop into functional DA neurons after transplantation. A recent study found that the great majority of TH+ neurons in nigral grafts were derived from postmitotic DA neurons that were already present in the VM tissue at the time of implantation (Sinclair et al., 1999). We also demonstrated that purified DA neurons could survive transplantation and improve Parkinson's disease (Sawamoto et al., 2001b). However, the potential of precursor cells to generate DA neurons in the adult brain was not studied sufficiently. Here, for the first time, we could evaluate the function of mesencephalic precursor cells isolated directly from the mixed population of cells in VM tissue. We transplanted ∼50,000 precursor cells in the striatum, and found that 1.25% of them (624 ± 123 cells) survived as TH+ neurons at the end of the experiments. Because the sorted GFP+ population did not contain any TH+ cells, we could ascertain that the TH+ cells detected in the grafts were generated after transplantation into the host brain.
The sorted cell population should include both mitotic and postmitotic precursors. Importantly, we obtained clear evidence that a subpopulation of the sorted precursor cells could generate new DA neurons by mitotic neurogenesis in the adult striatum, by BrdU labeling studies. Seven percent of the TH+ neurons in the grafts contained BrdU in their nuclei. Because the protocol for BrdU injection (once per day) used in this study probably did not label all of the DA neurons generated after transplantation, these data (7%) might underestimate the amount of mitosis that occurred in the host striatum. Thus, the present results indicate that primary precursor cells that have been isolated directly from fresh VM tissue possess a high potential to generate new DA neurons in the adult striatum. Furthermore, others have demonstrated recently that the adult striatum lesioned with 6-OHDA may provide a supportive environment for the differentiation of DA neurons (Zigova et al., 1998; Nishino et al., 2000).
The TH+BrdU−neurons in the grafts were likely to be implanted as immature postmitotic cells that began to express TH in the host striatum. There may be advantages to implanting postmitotic immature, rather than mature, DA neurons. For example, the dissociation of mature DA neurons in brain tissue is likely to cause serious damage to cells from the disruption of axons and dendrites. Because immature cells have a simple morphology, the dissociation process may not affect their viability and ability to innervate tissue after implantation. In addition, postmitotic cells should have already committed to a cell fate in the developing VM. Therefore, they ought to be able to develop into mature DA neurons even if they are transplanted into an ectopic environment. In fact, in this study, the TH+ neurons found in the grafts had long axons innervating the host tissue, a morphological feature they share with endogenous DA neurons.
The physiological function of the DA neurons generated from the sorted cell population was analyzed based on recovery from the amphetamine-induced rotation behavior in a rat model of Parkinson's disease. All of the animals that received the sorted precursors in their striatum showed significant recovery in the amphetamine-induced rotation behavior. We found a good correlation between the DA neuron cell number in the grafts and the degree of recovery, suggesting that dopamine secretion from the generated DA neurons was responsible for the improvement of the phenotype. Taking together the data on behavioral recovery and the successful transplantation of DA neurons, we conclude that the sorted precursors generated functional DA neuronsin vivo that could survive and innervate the adult striatum.
In contrast with our results, the transplantation of mesencephalic precursors that had been expanded in vitro as neurospheres did not result in sufficient differentiation into DA neurons to completely resolve the behavioral defects seen in the same rat model of Parkinson's disease (Svendsen et al., 1996, 1997). Although it is unknown why in vitro expansion of precursor cells might result in a loss of their potential to differentiate into DA neurons, it is possible that, under the conditions used for expansion, the cells were altered and lost their identity as DA precursors. Endogenous precursor cells have access to positional information that is important for adopting a specific fate. Long-term culture under stimulation with growth factors causes the loss of such positional information for unknown reasons (Santa-Olalla et al., 2000). For the application of precursor cells to transplantation therapy of Parkinson's disease, it is necessary to establish culture conditions that maintain their potential for DA differentiation, as well as their viability (Brundin and Björklund, 1998) after expansion in vitro. This work should stimulate future research aimed at the characterization of the VM precursor cells, as well as the molecular mechanisms by which precursor cells generate DA neurons.
Footnotes
This work was supported by grants-in-aid for scientific research and special grants for Strategic Promotion System for Brain Science from the Japanese Ministry of Education, Science, Sports, Culture, and Technology, Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation, and the Human Frontier Science Program. Work in the Goldman laboratory was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke and by grants from the National Multiple Sclerosis Society, the Mathers Charitable Foundation, and Project Amyotrophic Lateral Sclerosis. We thank Hiroshi Hatanaka and Ikuko Nagatsu for providing the anti-TH antibody, Wieland B. Huttner for the monoclonal antibody 13A4, and Abdellatif Benraiss, Ayano Kawaguchi, Yoshishiro Koyama, Takaki Miyata, Yohei Morita, Hiromitsu Nakauchi, Neeta Singh Roy, Shin-ichi Sakakibara, Kazuhiro Sakurada, and Takuya Shimazaki for their useful technical advice and discussions.
K.S. and N.N. contributed equally to this work.
Correspondence should be addressed to Dr. Hideyuki Okano, Department of Physiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku, Tokyo 160-8582, Japan. E-mail: hidokano@sc.itc.keio.ac.jp.
REFERENCES
- 1.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Agid Y, Javoy-Agid F, Ruberg M. Biochemistry of neurotransmitter in Parkinson's disease. In: Marsden CD, Fahn S, editors. Movement disorder, Vol 2. Butterworths; London: 1987. pp. 166–230. [Google Scholar]
- 3.Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 4.Brundin P, Björklund A. Survival of expanded dopaminergic precursors is critical for clinical trials. Nat Neurosci. 1998;1:537. doi: 10.1038/2773. [DOI] [PubMed] [Google Scholar]
- 5.Brundin P, Strecker RE, Widner H, Clarke DJ, Nilsson OG, Astedt B, Lindvall O, Björklund A. Human fetal dopamine neurons grafted in a rat model of Parkinson's disease: immunological aspects, spontaneous and drug-induced behavior, and dopamine release. Exp Brain Res. 1988;70:192–208. doi: 10.1007/BF00271860. [DOI] [PubMed] [Google Scholar]
- 6.Brundin P, Duan WM, Sauer H. Functional effects of mesencephalic dopamine neurons and adrenal chromaffin cells grafted to the rodent striatum. In: Dunnett S, Björklund A, editors. Functional neural transplantation. Raven; New York: 1994. pp. 157–195. [Google Scholar]
- 7.Daadi MM, Weiss S. Generation of tyrosine hydroxylase-producing neurons from precursors of the embryonic and adult forebrain. J Neurosci. 1999;19:4484–4497. doi: 10.1523/JNEUROSCI.19-11-04484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 9.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 10.Frodl EM, Duan WM, Sauer H, Kupsch A, Brundin P. Human embryonic dopamine neurons xenografted to the rat: effects of cyopreservation and varying regional source of donor cells on transplant survival, morphology and function. Brain Res. 1994;647:286–298. doi: 10.1016/0006-8993(94)91328-5. [DOI] [PubMed] [Google Scholar]
- 11.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 12.Haque NS, LeBlanc CJ, Isacson O. Differential dissection of the rat E16 ventral mesencephalon and survival and reinnervation of the 6-OHDA-lesioned striatum by a subset of aldehyde dehydrogenase-positive TH neurons. Cell Transplant. 1997;6:239–248. doi: 10.1177/096368979700600307. [DOI] [PubMed] [Google Scholar]
- 13.Hatanaka H, Arimatsu Y. Monoclonal antibodies to tyrosine hydroxylase from rat pheochromocytoma PC12h cells with special reference to nerve growth factor-mediated increase of the immunoprecipitable enzymes. Neurosci Res. 1984;1:253–263. doi: 10.1016/s0168-0102(84)80004-8. [DOI] [PubMed] [Google Scholar]
- 14.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulpas R, Tiarks C, Reilly J, Hsidh C-C, Recht L, Quesenberry PJ. In vitro cell-density dependent clonal growth of EGF-responsive murine neural progenitor cells under serum-free conditions. Exp Neurol. 1997;148:147–156. doi: 10.1006/exnr.1997.6672. [DOI] [PubMed] [Google Scholar]
- 16.Kalsbeek A, Voorn P, Buijs RM. Development of dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, Tohyama T, editors. Handbook of chemical neuroanatomy Vol 10. Elsevier; Amsterdam: 1992. pp. 63–112. [Google Scholar]
- 17.Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Musashi 1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:138–152. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman SA, Okano H. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Ohyama K, Kawamura K, Nagatsu I. Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res Dev Brain Res. 1995;86:101–113. doi: 10.1016/0165-3806(95)00018-9. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall O. Neural transplants in Parkinson's disease. In: Dunnett SB, Björklund A, editors. Functional neural transplantation. Raven; New York: 1994. pp. 103–137. [Google Scholar]
- 21.Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol. 1998;149:411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- 22.Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci. 1994;14:4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci. 1997;9:452–462. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 24.Nagatsu I, Komori K, Miura K, Sakai M, Karasawa N, Yamada K. Ontogeny of phenylethanolamine-N-methyltransferase and tyrosine hydroxylase-immunoreactive expression in the mouse anterior olfactory nucleus. Biomed Res. 1989;10:277–286. [Google Scholar]
- 25.Nagatsu T, Yamaguchi T, Rahman MK, Trocewicz J, Oka K, Hirata Y, Nagatsu I, Narabayashi H, Kondo T, Iizuka R. Catecholamine-related enzymes and the biopterin cofactor in Parkinson's disease and related extrapyramidal diseases. In: Hasseler RG, Christ J F, editors. Advances in neurology, Vol 40. Raven; New York: 1984. pp. 467–473. [PubMed] [Google Scholar]
- 26.Nakao N, Frodl EM, Duan WM, Widner H, Brundin P. Lazaroids improve the survival of grafted rat embryonic dopaminergic neurons. Proc Natl Acad Sci USA. 1994;91:12408–12412. doi: 10.1073/pnas.91.26.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakao N, Ogura M, Nakai K, Itakura T. Intrastriatal mesencephalic grafts affect neuronal activity in basal ganglia nuclei and their target structures in a rat model of Parkinson's disease. J Neurosci. 1998;18:1806–1817. doi: 10.1523/JNEUROSCI.18-05-01806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino H, Hida H, Takei N, Kumazaki M, Nakajima K, Baba H. Mesencephalic neural stem (progenitor) cells develop to dopaminergic neurons more strongly in dopamine-depleted striatum than in intact striatum. Exp Neurol. 2000;164:209–214. doi: 10.1006/exnr.2000.7426. [DOI] [PubMed] [Google Scholar]
- 29.Okano H, Goldman SA. Identification and selection of neural progenitor cells. NeuroScience News. 2000;3:27–31. [Google Scholar]
- 30.Potter ED, Ling ZD, Carvey PM. Cytokine-induced conversion of mesencephalic-derived progenitor cells into dopamine neurons. Cell Tissue Res. 1999;296:235–246. doi: 10.1007/s004410051285. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 32.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000a;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 33.Roy NS, Benraiss A, Wang S, Fraser RA, Goodman R, Couldwell WT, Nedergaard M, Kawaguchi A, Okano H, Goldman SA. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000b;59:321–331. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 35.Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126:4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 36.Santa-Olalla J, Cardenas-Aguayo MC, Fregoso-Lomas M, Covarrubias L. Positional identity is not preserved in neural precursor cells grown as neurospheres. Soc Neurosci Abstr. 2000;26:23.3. [Google Scholar]
- 37.Sauer H, Brundin P. Effects of cool storage on survival and function of intrastriatal ventral mesencephalic grafts. Restor Neurol Neurosci. 1991;2:123–135. doi: 10.3233/RNN-1991-2302. [DOI] [PubMed] [Google Scholar]
- 38.Sawamoto K, Yamamoto A, Kawaguchi A, Yamaguchi M, Mori K, Goldman SA, Okano H (2001a) Direct isolation of committed neuronal progenitor cells from transgenic mice co-expressing spectrally-distinct fluorescent proteins regulated by stage-specific neural promoters. J Neurosci Res, in press. [DOI] [PubMed]
- 39.Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H (2001b) Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- 40.Schmidt RH, Björklund A, Stenevi U, Dunnett SB, Gage FH. Intracerebral grafting of neuronal cell suspensions. III. Activity of intrastriatal nigral suspension implants as assessed by measurements of dopamine synthesis and metabolism. Acta Physiol Scand Suppl. 1983;522:19–28. [PubMed] [Google Scholar]
- 41.Sinclair SR, Fawcett JW, Dunnett SB. Dopamine cells in nigral grafts differentiate prior to implantation. Eur J Neurosci. 1999;11:4341–4348. doi: 10.1046/j.1460-9568.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- 42.Smidt MP, van Schaick HS, Lanctot C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci USA. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 44.Svendsen CN, Clarke DJ, Rosser AE, Dunnett SB. Survival and differentiation of rat and human epidermal growth factor-responsive precursor cells following grafting into the lesioned adult central nervous system. Exp Neurol. 1996;137:376–388. doi: 10.1006/exnr.1996.0039. [DOI] [PubMed] [Google Scholar]
- 45.Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 46.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang S, Wu H, Jiang J, Delohery TM, Isdell F, Goldman SA. Isolation of neuronal precursors by sorting embryonic forebrain transfected with GFP regulated by the T alpha 1 tubulin promoter. Nat Biotechnol 16 1998. 196 201[Erratum (1998) 16:478]. [DOI] [PubMed] [Google Scholar]
- 48.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 50.Ye W, Shimamura K, Rubenstein JL, Hunes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;29:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 51.Zigova T, Pencea V, Betarbet R, Wiegand SJ, Alexander C, Bakay RA, Luskin MB. Neuronal progenitor cells of the neonatal subventricular zone differentiate and disperse following transplantation into the adult rat striatum. Cell Transplant. 1998;7:137–156. doi: 10.1177/096368979800700209. [DOI] [PubMed] [Google Scholar]
- 52.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]