Abstract
Selectivity to visual stimulus orientation is a basic cortical functional property believed to be crucial for normal vision. Maturation of this neuronal property requires neural activity. Still, it is unclear what might be the molecular basis for such activity-dependent processes and whether activity has an instructive or permissive role in development of orientation selectivity. There is strong evidence that the NMDA subtype of the glutamate receptor regulates activity-dependent mechanisms of ocular dominance plasticity during cortical development. For this reason, we have hypothesized that the NMDA receptor participates in activity-dependent mechanisms that sculpt orientation selectivity of cortical neurons. We used chronicin vivo infusion of antisense oligodeoxynucleotides (ODNs) to suppress NMDA receptor function in primary visual cortex during the period when orientation selectivity develops in ferrets. Chronic suppression of NMDA receptor function prevented the development of orientation and stimulus size selectivity in most cortical cells tested. In contrast, treatment with control sense or missense ODNs did not affect development of orientation selectivity, indicating specificity of effects. Importantly, antisense ODN treatment did not impair visually driven activity, which is required for development to occur. Moreover, orientation selectivity of cortical cells was not disrupted by antisense ODN treatment in mature animals, indicating developmental relevance of the effects. In conclusion, our findings document for the first time that cortical NMDA receptors are essential for the maturation of orientation selectivity. This result supports the notion that activity has an instructive role in sculpting the connections that underlie orientation selectivity in visual cortex.
Keywords: NMDA receptor, orientation selectivity, visual cortex, ferret, development, receptive field properties, antisense oligodeoxynucleotide
Selectivity to visual stimulus orientation is a functional property of cortical neurons believed to be crucial for normal vision. In ferrets and cats most cortical cells in primary visual cortex are orientation-selective (Hubel and Wiesel, 1962; Bishop et al., 1973; Chapman and Stryker, 1993), raising the issue of how this response property develops. Although vision is not necessary for the initial establishment of orientation selectivity (Wiesel and Hubel, 1974; Fregnac, 1979; Fregnac and Imbert, 1984), further maturation of this property is markedly affected by sensory stimulation (Blakemore and Van Sluyters, 1975; Buisseret and Imbert, 1976; Crair et al., 1998), and prevented by silencing cortical activity with tetrodotoxin (Chapman and Stryker, 1993). These findings indicate that the development of orientation selectivity requires neural activity. This activity dependence has led to the proposal of a correlation-based model according to which the development of cortical orientation tuning is instructed by activity (Miller, 1994; Miller et al., 1999), although a simple permissive role for activity cannot be excluded at present.
What might be the molecular basis for these activity-dependent processes during development of orientation selectivity? The prevailing theory used to explain activity-dependent neural plasticity suggests that mechanisms exist to strengthen synapses whose activity coincides with target depolarization beyond some threshold level (Hebb, 1949) and to eliminate synapses whose activity is not correlated with postsynaptic activation (Stent, 1973). This model requires a correlation detector that would signal synchronous presynaptic and postsynaptic depolarization. The biophysical properties of the NMDA subtype of the glutamate receptor (Mayer et al., 1984; Nowak et al., 1984; MacDermott et al., 1986) have led to the proposal that it functions as a correlation detector (Bear et al., 1987; Bourne and Nicoll, 1993; Fox and Daw, 1993), playing a critical role in activity-dependent increases in synaptic strength and in synapse stabilization. There is already strong evidence that the NMDA receptor is involved in ocular dominance plasticity in the developing visual cortex (Bear et al., 1990; Rauschecker et al., 1990; Roberts et al., 1998; Daw et al., 1999). Therefore, we have examined the attractive possibility that the NMDA receptor also participates in activity-dependent mechanisms that sculpt orientation selectivity and other functional properties of cortical neurons.
We have used chronic in vivo infusion of antisense oligodeoxynucleotides (ODNs) to suppress cortical NMDA receptor function from postnatal day 21 (P21) to P49, when orientation selectivity is known to develop in ferrets (Chapman and Stryker, 1993). Antisense ODN treatment reduced but did not eliminate NMDA receptor function in the visual cortex (Roberts et al., 1998). Additionally, treatment selectively reduced ocular dominance plasticity while preserving visual responsiveness and stimulus selectivity of cortical cells. Therefore, antisense techniques can be used to accomplish more selective manipulations of cortical function than is possible using traditional pharmacological agents, which are known to depress sensory cortical responses (Miller et al., 1989; Rauschecker et al., 1990; Daw, 1994; Kasamatsu et al., 1998). Antisense ODN treatment prevented the development of cortical cell orientation selectivity, indicating that the NMDA receptor is essential for the development of orientation-selective receptive fields.
MATERIALS AND METHODS
This study is based on a total of 416 cells that were examined by extracellular recordings conducted in vivo in ferrets. Ferrets were used for this study because they are born developmentally younger than cats and primates (Linden et al., 1981) and can, therefore, be used to study development of orientation selectivity before eye opening. Table 1 shows that a total of 21 ferrets were used in the visual physiology experiments: eight untreated animals, nine treated with antisense ODN, two treated with sense ODN, and two treated with missense ODN. Treatment with antisense ODN started at P21–P22 (n = 5 animals), P36 (n = 2), or P63 (n = 2), and treatment with control sense (n = 2) or missense (n = 2) ODN always started at P21–P22. Additionally, immunocytochemistry was conducted on antisense ODN and control ODN-treated animals to confirm the previous report (Roberts et al., 1998) that antisense ODN treatment reduces NMDAR1 subunit protein expression. The Institutional Animal Care and Use Committee at Virginia Commonwealth University approved all procedures described in this paper.
Table 1.
Groups of animals that were used in this study
| Age of injection | Age of study | Number of animals | #cells | |
|---|---|---|---|---|
| Untreated kit | P34–P38 | 3 | 40 | |
| Untreated adult | P50 | 5 | 133 | |
| AS-ODN | P21–P22 | P49–P50 | 5 | 86 |
| AS-ODN | P36 | P63 | 2 | 32 |
| AS-ODN | P63 | P90 | 2 | 31 |
| Sense-ODN | P21–22 | P49–P50 | 2 | 45 |
| Missense-ODN | P21–P22 | P49–P50 | 2 | 49 |
Three groups of animals were injected with antisense ODN (AS-ODN) at different ages. Control animals were injected with sense ODN or missense ODN at P21–P22. Two groups of untreated animals were studied around the time of eye opening (untreated kit) or around P50, when maturity of orientation selectivity is reached in the normal ferret.
Antisense ODN application. The ODN sequences used here were 5′ CAGCAGGTGCATGGTGCT (antisense), 5′ AGCACCATGCACCTGCTG (sense), and GATGCGTGACGATGCTCG (missense) (Oligos, Etc., Wilsonville, OR). The missense oligo is identical to the antisense oligo except for sequence rearrangements that maintain the original bases and GC ratio. The sequence of the antisense oligo was chosen to target the 5′ coding region of the NMDAR1 subunit mRNA, which is highly conserved in mammals (>99%). This sequence has been used successfully in previous studies (Wahlestedt et al., 1993; Roberts et al., 1998). In every case, we searched the available databases to assure that other genes do not share homologous regions. To increase stability, phosphorothioate bonds were incorporated at terminal nucleotides at the 5′ and 3′ ends. In the case shown in Figure 1, the antisense ODN was in addition labeled with fluorescein (Oligos, Etc; Whitesell et al., 1993). The ODNs were dissolved in PBS (0.9% NaCl in 0.1m phosphate buffer) to a concentration of 7 μg/μl. Fluorescent latex microspheres (Lumafluor, Naples, FL; 1 μl) were added to the solution for subsequent identification of the injection site. Infusion of ODNs (0.5 μl/hr in every case) was accomplished using osmotic minipumps (Alza 2002 or 2004; Alza, Palo Alto, CA) fitted with a catheter and cannula (28 gauge, beveled, stainless steel).
Fig. 1.
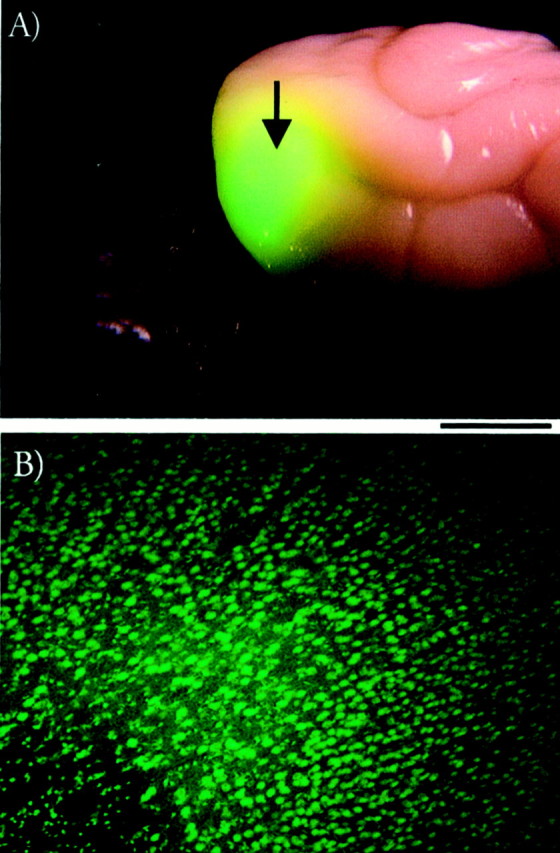
Distribution of antisense ODN labeled with fluorescein. A, Antisense ODN spreads from the injection site (indicated by the arrow) in prestriate cortex at least 5 mm to include a large portion of the binocular region of the primary visual cortex in the occipital pole. B, A very large number of cell bodies located in visual cortex were labeled with the ODN/fluorescein. Scale bar: 5 mm (A) and 250 μm (B).
Minipump implantations for in vivo recordings were performed in ferrets (P21–P63) weighing 200–500 gm. Animals were premedicated by subcutaneous injections of a tranquilizer (acepromazine, 1 mg/kg) and a muscarinic antagonist to reduce secretion (methyl atropine bromide, 0.2 mg/kg), anesthetized with intraperitoneal pentobarbital sodium (35 mg/kg), and placed in a stereotaxic frame. No procedures were started until animals were sufficiently anesthetized, as ascertained by the loss of withdrawal and cornea-blink reflexes. Body temperature, respiratory rate, and anesthesia level were monitored continuously during surgery. Additional doses of pentobarbital were given as needed. The cannula was positioned stereotaxically above the brain in the region that corresponds to the prestriate cortex, and a small craniotomy (∼1 mm diameter) was performed. The tip of the cannula was lowered stereotaxically into the cortex to a depth of ∼2.0 mm. The guide catheter and cannula were secured to the skull by using cyanoacrylate glue (Super Glue), and the minipump was placed under the skin of the neck. Extracellular in vivo recordings were conducted near the end of the 4 week infusion period.
Immunocytochemistry. Ferrets were deeply anesthetized with pentobarbital (120 mg/kg) and perfused transcardially with cold 0.9% saline, pH 7.2, followed by cold 4% paraformadehyde in 0.1m PBS, pH7.2. The brains were removed and post-fixed in 4% paraformaldehyde, 0.1 m PBS for 4 hr at 4°C. The caudal portion of the brain containing the primary visual cortex was vibratome sectioned (thickness, 30 μm) in the coronal plane. Free-floating tissue sections were incubated in 3% hydrogen peroxide for 20 min then washed with 0.01m PBS. (The remainder of the reaction was performed with 0.01 m PBS.) Sections were incubated in 10% normal horse serum, 2% bovine serum albumin (BSA), and PBS for 1 hr at room temperature as a blocking step. This was followed by incubation in a solution of primary antibody diluted (1:500) in 2% BSA–PBS for 48–72 hr at 4°C on a rotary shaker. The primary antibody was an anti-NMDAR1 monoclonal mouse IgG (clone 54.1; PharMingen, San Diego, CA). A washing step of four washes for 10 min each was performed with 2% BSA–PBS. Sections were incubated with a horse anti-mouse biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) diluted (1:500) in 2% BSA–PBS for 1 hr at room temperature. Washes were performed as above. Sections were incubated for 1 hr in the Vectastain Elite ABC complex (Vector Laboratories). Washes were performed again as above. Antibody staining was detected by incubating the sections in a solution of 0.06% cobalt-enhanced 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) in PBS for 1–3 min. Another set of washes was performed as above. Sections were mounted on chrome alum-subbed slides, dehydrated through graded alcohols (50, 70, 95, and 100% ethanol) and clearing agent, then mounted in Permount. Sections of treated and untreated tissue from the same animals always were processed and analyzed together.
Extracellular recordings in vivo. Animals were premedicated by subcutaneous injections of a tranquilizer (acepromazine, 1 mg/kg) and a muscarinic antagonist to reduce secretion (methyl atropine bromide, 0.2 mg/kg), anesthetized with intraperitoneal pentobarbital sodium (35 mg/kg), and placed in a stereotaxic frame. No procedures started until the animal was sufficiently anesthetized, as ascertained by the loss of withdrawal and cornea-blink reflexes. Body temperature, respiratory rate, and anesthesia level were monitored continuously during surgery. Additional doses of pentobarbital were given as needed. Surgery consisted of a craniotomy (∼3 mm diameter) over the region in primary visual cortex where recordings were conducted. After tracheal cannulation, anesthesia was maintained at surgical levels by using additional sodium thiopental (10–30 mg/kg, i.p.) and acepromazine (0.05 mg/kg, s.c.), and the animal was paralyzed with pancuronium bromide (0.4 mg/kg). Supplemental doses of pentobarbital, acepromazine, and pancuronium bromide were given along with subcutaneous injections of 10% dextrose and 0.9% saline every hour throughout the experiment or when heart rate or expired CO2 increased. Body temperature and expired CO2 were monitored continuously. The eyelids were opened, nictitating membranes were retracted with pseudoephedrine, the pupils were dilated with 1% atropine sulfate, and contact lenses were placed on the cornea.
A tungsten-in-glass microelectrode (Levick, 1972) was lowered into the primary visual cortex 2–4 mm away from the minipump using a microdrive device. After the isolation of a single unit, its receptive field, ocular dominance group, and preferred orientation, direction, and velocity were determined qualitatively with a moving bar of light. All data were collected from cells in the binocular region of the visual field. Ocular dominance, orientation, and direction selectivity were then quantitatively determined for each cell. Under computer control, a moving bar of light (0.5° wide and 20° long) was presented to each eye individually at 5–10 orientations centered around the optimal. One stimulus presentation consisted of the bar of light moving across the receptive field in one direction and back across in the opposite direction. Spikes were collected by the computer during the 10 stimulus presentations at each orientation, and peristimulus histograms were generated. Spontaneous activity was determined by recording activity in the absence of stimulation. To provide a quantitative estimate of response properties, a binocularity index, orientation selectivity index, and direction selectivity index were obtained for each cell as described in Results. Both the orientation and direction selectivity indices were determined using responses evoked by stimulus presentation to the dominant eye. The results were analyzed statistically by obtaining the median of the distribution for each animal, then using a Wilcoxon Mann–Whitney rank sum test.
At the conclusion of each experiment, the animal was given an overdose of pentobarbital (120 mg/kg). When CO2 began to fall, the animal was perfused transcardially with 0.9% saline followed by 10% formalin (or 4% paraformaldehyde for immunocytochemistry). The brain was then post-fixed in formalin for ∼12 hr (or in paraformaldehyde for 4 hr) at 4°C. The primary visual cortex was vibratome-sectioned (50 μm) in the coronal plane. Every other section was stained with cresyl violet and used to reconstruct electrode recording tracts and determine the effects of antisense ODN injection on cortical histology. The other sections were used for immunocytochemistry. Because of the placement of the cannula outside the primary visual cortex, mechanical damage near the recording site was avoided, and the area remained histologically indistinguishable from untreated cortex.
RESULTS
Ferrets received continuous antisense ODN treatment targeting the NMDAR1 subunit of the NMDA receptor or control sense–missense ODN. The injection cannula was located in prestriate cortex to avoid mechanical damage in striate cortex. The decision to implant the cannula in prestriate cortex was based on findings concerning the cortical distribution of the antisense ODN labeled with fluorescein. This is a standard procedure used to study distribution of antisense ODN within the CNS (Whitesell et al., 1993; Grzanna et al., 1998). Figure 1 shows that the labeled antisense ODN spreads from the injection site in prestriate cortex (Fig. 1A, arrow) to include a large portion of the primary visual cortex, which is located at the caudal pole of each hemisphere in ferrets. A very large number of striate cortical neurons take up the labeled antisense ODN, as shown in Figure1B. These findings are consistent with previous results showing that chronic infusion of antisense ODN affects protein expression, synaptic transmission, and visual plasticity over a large area of cortex ∼5 mm in diameter (Roberts et al., 1998).
Ferrets were injected with antisense, sense, or missense ODN starting on P21 and studied at P49–P50, when cortical orientation selectivity is adult-like (Chapman and Stryker, 1993). Additional animals received treatment starting around the time of eye opening or during maturity, and were studied 28 d later, at the end of treatment (Table 1). Extracellular recordings were conducted from 416 cortical cells with receptive fields located in the binocular region of the visual field representation in striate cortex. In every case, the microelectrode was located 2–4 mm from the injection cannula.
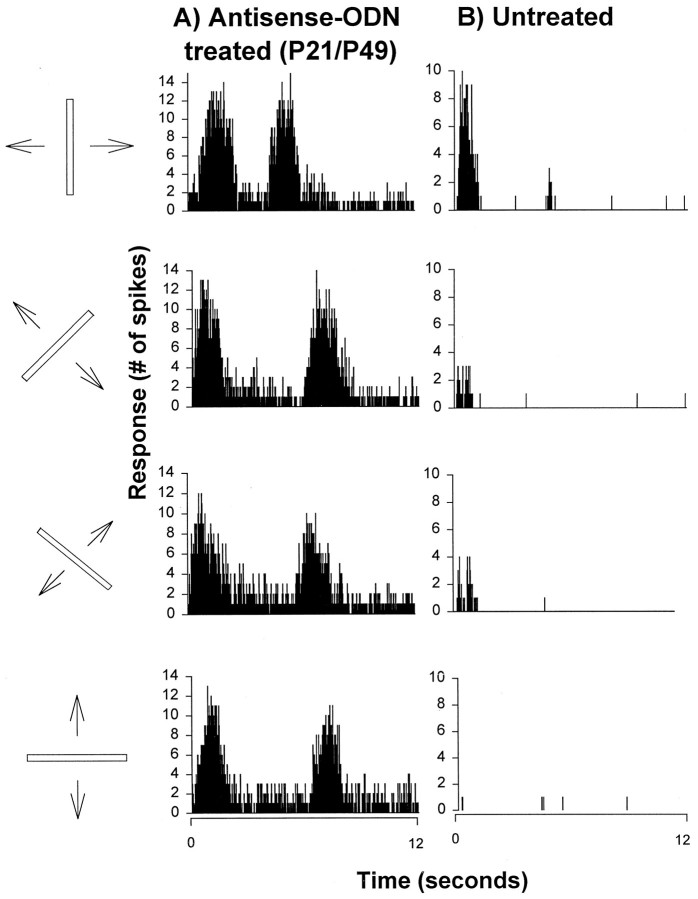
Microelectrode recordings in the striate cortex of ferrets treated with antisense ODN starting at P21 revealed that cortical cells were not selective to stimulus orientation. Examples of visual responses (e.g., peristimulus histograms) from a cortical cell located in a treated hemisphere are shown in Figure2A. They were obtained from a highly responsive cell that was not selective to the orientation of a moving bar of light. For comparison, the visual responses of a cortical cell from an untreated hemisphere studied at a similar age are shown in Figure 2B. This highly responsive cell was clearly orientation-selective, as most cortical neurons in the visual cortex of the ferret. These results illustrate the finding that antisense ODN treatment prevented the development of orientation selectivity in visual cortical neurons.
Fig. 2.
Antisense ODN treatment prevents development of orientation selectivity of individual cortical cells. A, A cortical neuron from an antisense ODN-treated ferret displayed robust response to every orientation of a moving bar of light tested.B, Normal orientation selectivity present in a cortical cell from an untreated animal.
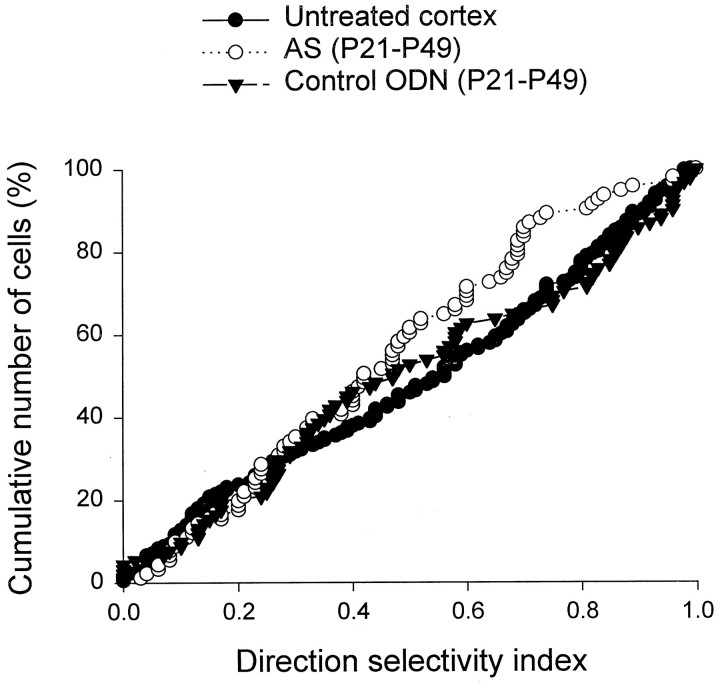
Orientation tuning histograms were compiled for a total of 354 cortical cells. To quantify the results, an orientation selectivity index (OSI) was obtained for each cell by dividing the response at 45° from the optimal, or the response obtained 90° from the optimal, by the response at the optimal orientation then subtracting the results from one. Figure 3 shows the cumulative percentage of cells plotted as a function of the orientation selectivity index for three different groups of animals: (1) antisense–ODN-treated animals studied at ∼P49 (filled circles), (2) untreated animals studied at approximately the same age (filled triangles), and (3) untreated ferrets studied at approximately the time of eye opening (open symbols). Indices of 1.0 indicate a high degree of selectivity, and indices of zero indicate lack of selectivity. Therefore, cumulative curves that are shifted to the left reflect a decreased neuronal orientation selectivity. The orientation selectivity indices at 90° as well as 45° were markedly reduced in the antisense ODN-treated cortex (n = 74 cells) relative to mature, untreated cortex (n = 83; p < 0.01; Wilcoxon-Mann–Whitney U test), reflecting a lowered orientation selectivity. Interestingly, stimulus specificity of treated cortical neurons at P49 resembled that present in kits around the time of eye opening (n = 40 cells), when orientation selectivity is quite immature. These results show that antisense ODN treatment targeting the NMDAR1 subunit of the NMDA receptor prevented the maturation of orientation selectivity in primary visual cortex, freezing the cortex in an immature state.
Fig. 3.
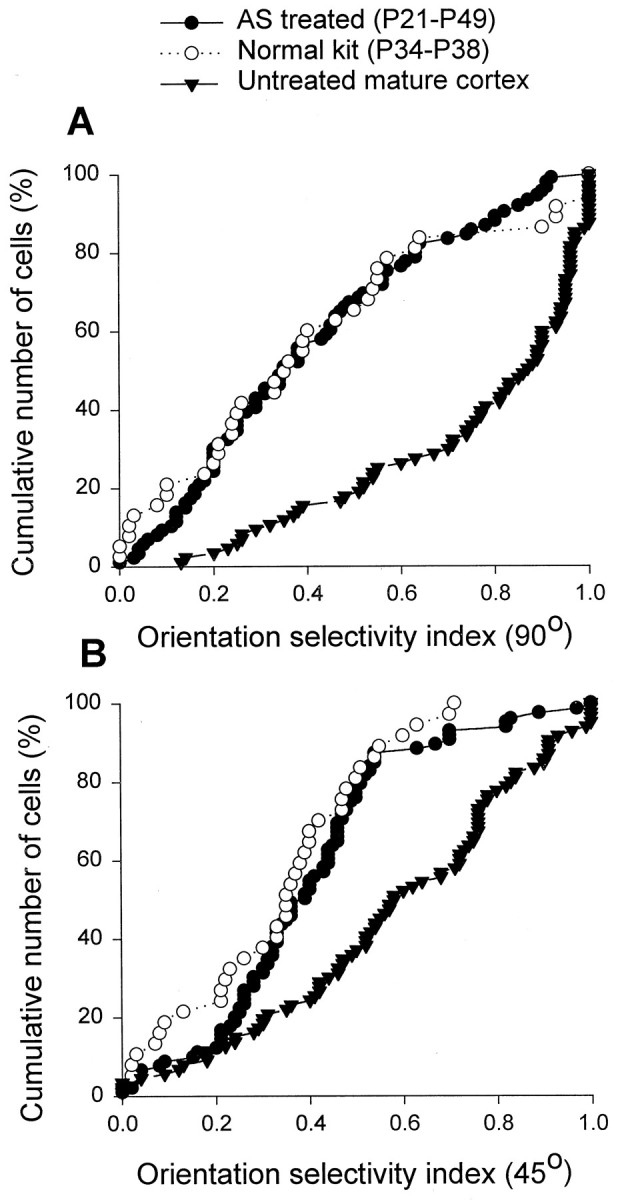
Antisense ODN treatment prevented the developmental changes in the orientation selectivity indices of cortical cells. The cumulative percentage of cells was plotted as a function of the orientation selectivity index at 90° (A) and 45° (B) for three groups of animals: (1) antisense ODN-treated and studied at ∼P49, (2) untreated kit studied around the time of eye opening, and (3) untreated mature animal studied around the same age as the antisense ODN-treated animals. The orientation selectivity indices for the cells studied in the untreated animals increased markedly from the time of eye opening until maturity at P49 (compare the open symbols andfilled triangles). In contrast, the antisense ODN-treated animals studied at P49 had orientation selectivity indices that were similar to those found in kits but markedly lower than those found in mature untreated ferrets. The distributions for antisense ODN-treated animals and untreated animals are different statistically (Wilcoxon–Mann–Whitney U test; p< 0.01). In contrast, the distributions for antisense ODN-treated animals and the untreated kittens are indistinguishable (p > 0.05).
To examine whether effects of antisense ODN treatment vary according to layer, cells were recorded at least 100 μm apart in each electrode tract. An example of the results (depth and OSI) observed along the recording tract in a treated animal is the following: cell 1 (150 μm, 0.11), cell 2 (250, 0.47), cell 3 (350, 0.43), cell 4(460, 0.56), cell 5 (560, 0.63), cell 6 (660, 0.63), cell 7 (750, 0.35), cell 8 (920, 0.46), cell 9 (1150, 0.27), cell 10 (1250, 0.91), and cell 11 (1450, 0.74). By comparison, higher OSI indices were observed along a recording tract in an untreated animal: cell 1(25 μm, 0.95), cell 2 (150, 0.74), cell 3 (250, 0.93), cell 4 (350, 0.94), cell 5 (550, 0.90), cell 6 (650, 0.52), cell 7 (900, 0.77), cell 8 (1180, 0.89), cell 9 (1280, 0.95), and cell 10 (1380, 0.87). We have assigned the cells along all recording tracts into two groups according to location: layers II-III and layers V-VI. This classification is based on the findings that the function of NMDA receptors in visual cortex varies according to layer (Fox et al., 1989). Orientation selectivity was not differentially affected in these two groups of cells (Wilcoxon rank sum test; p > 0.05).
To examine the specificity of the effects reported here, we studied orientation selectivity in animals treated with sense or missense ODNs from P21 to P49. Figure 4 compares the cumulative plots observed in these animals to those obtained in the antisense ODN-treated and untreated cortex. Microelectrode recordings in the striate cortex of these control animals at P49–P50 revealed that cortical cells were highly selective to stimulus orientation. Pooled together, the results observed in the control sense and missense ODN-treated animals were not significantly different from normal (p > 0.05). These results indicate specificity of effects obtained with the antisense techniques used here.
Fig. 4.
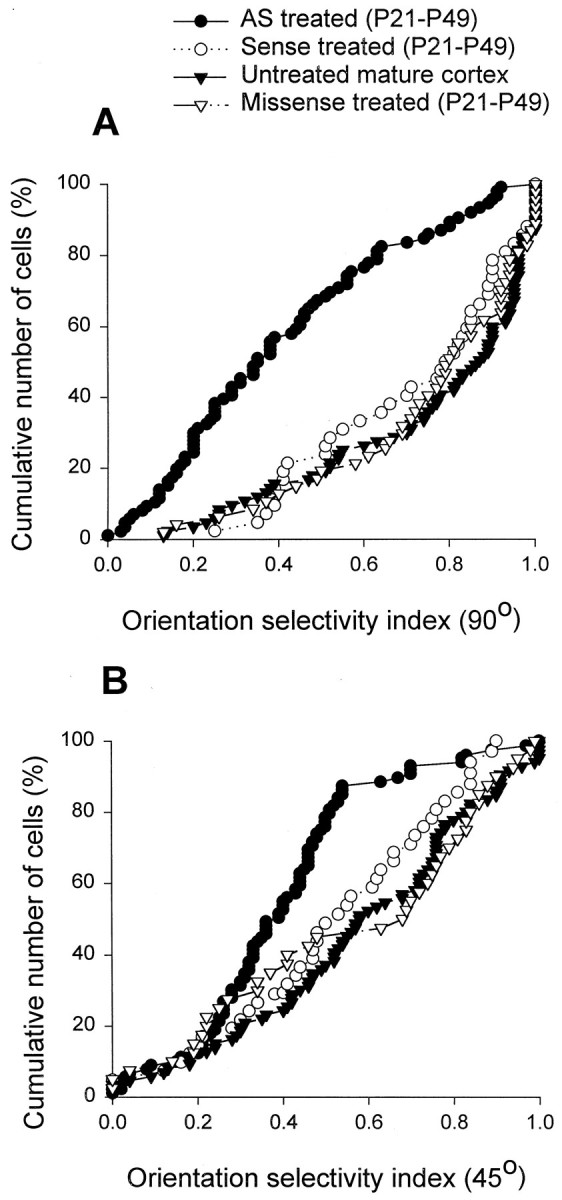
Intracortical infusion of control sense and missense ODN did not prevent development of orientation selectivity. The cumulative plots of orientation selectivity indices in the sense and missense ODN-treated animals were indistinguishable from normal (p > 0.05).
We have examined whether the remarkable effects of antisense ODN treatment on orientation selectivity are specific to an early developmental stage. Effects of antisense ODN treatment were compared in three groups of animals treated for 28 d starting at different ages. The cumulative plots in Figure 5show the results for ferrets treated starting before eye opening (P21,filled circles), a few days after eye opening (around P36,open circles), or at a time when orientation selectivity in the primary visual cortex is mature (P63, filled triangles). The cumulative plots indicate that antisense ODN treatment starting at maturity did not decrease orientation selectivity relative to normal (compare to plot for normal animals in Fig. 4). The Figure also shows that treatment starting at P21 induced a much greater disruption of orientation selectivity than treatment starting around P36. Together, these findings indicate that the observed effects of antisense ODN treatment only occur during the period when orientation selectivity is developing. Moreover, they suggest that cortical NMDA receptor function contributes to development of orientation selectivity before eye opening.
Fig. 5.
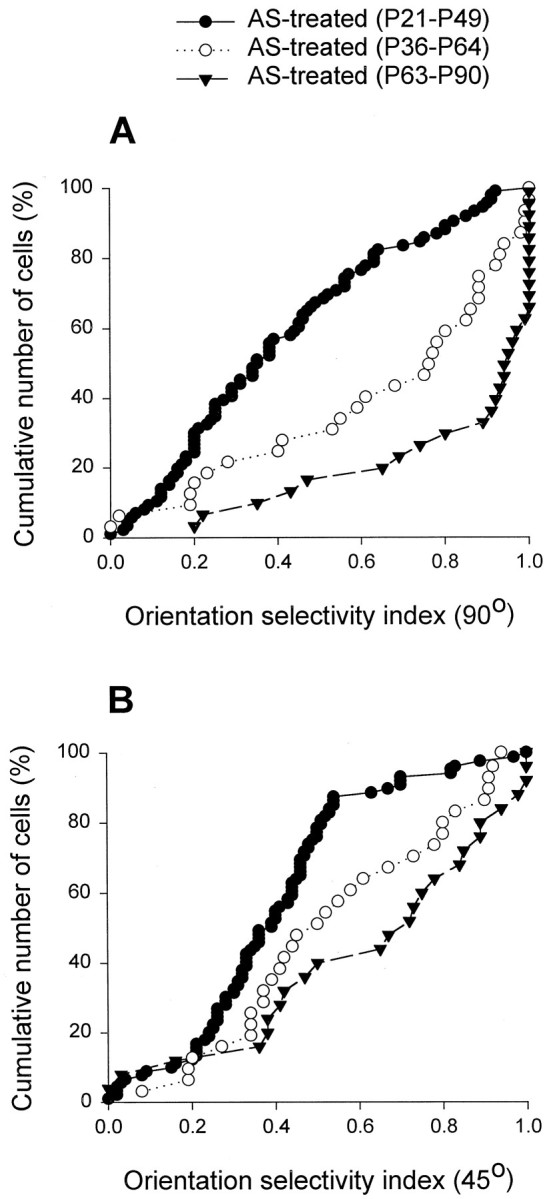
Age-dependent effects of antisense ODN treatment on cortical orientation selectivity. Strikingly different cumulative plots are shown of the orientation selectivity indices in animals treated with antisense ODN early in life, when orientation selectivity normally develops, and during maturity. Treatment at an intermediate age starting around P36 had less pronounced effects on the orientation selectivity indices than seen when treatment started earlier, at ∼P21.
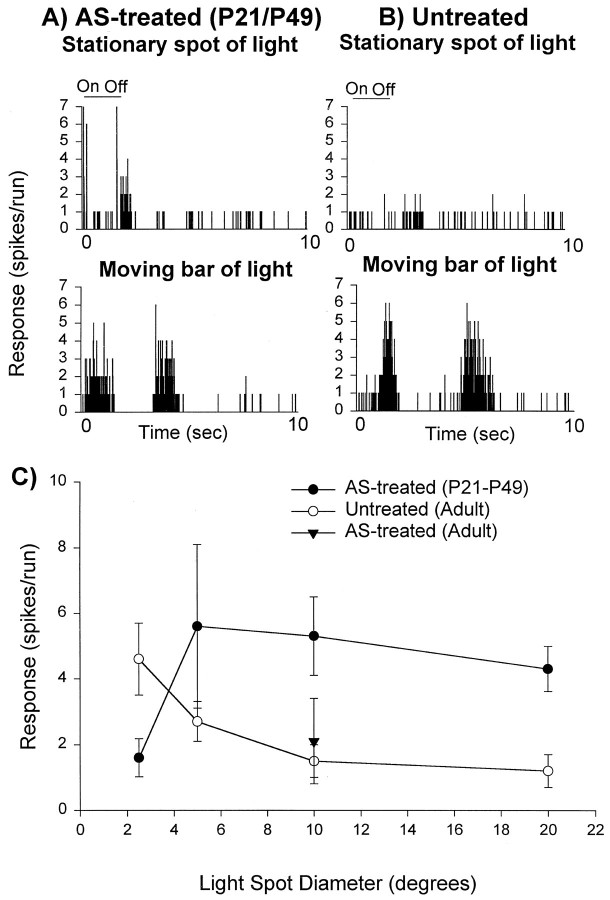
Orientation selectivity is thought to be intimately related to the receptive field structure of a cortical neuron and particularly the organization of its ON and OFF subregions (Hubel and Wiesel, 1962). Changes in receptive field structure may, therefore, provide the basis for the observed lack of orientation specificity in animals treated with antisense ODN. To provide a preliminary estimate of the spatial organization of excitatory and inhibitory influences to cortical cells, we used a simple test. It is well known that neurons in the primary visual cortex of cats do not respond to large spots of light flashing on their receptive fields (Hubel and Wiesel, 1962). To examine whether this also occurs in normal ferret visual cortex, we flashed spots of light of different dimensions centered on the receptive fields of cortical neurons. The peristimulus histogram in Figure6B illustrates the finding of a lack of response to large stimuli (10° diameter) presented on the cortical receptive fields of normal ferrets. In contrast, the peristimulus histogram in Figure 6Aillustrates the finding that cortical neurons in antisense ODN-treated cortex displayed a strong transient response followed by a sustained response to large stationary stimuli. The mean responses to stimuli of different sizes, varying from 2.5 to 20° diameter, observed in the population of untreated cortical cells is plotted in Figure6C (open circles). Note that normal cortical cells show very little response to a stimulus 10° in diameter. The same is true for the antisense ODN-treated mature cortex (filled triangle). In contrast, cells in ferrets treated from P21 (Fig. 6C, filled symbols) displayed robust responses to a large spot of light (10° diameter). The average cortical cell response to a spot of light 10° in diameter was substantially stronger in the animals treated with antisense ODN from P21 (n = 11 cells; open symbols) than in untreated animals (n = 13 cells; p < 0.05).
Fig. 6.
Suppression of cortical NMDA receptor function from P21 to P49 prevented maturation of stimulus size selectivity in cortical neurons. A, Example of a peristimulus time histogram from an antisense-ODN treated cortical neuron shows responses to a large stationary stimulus. B, Example from an untreated neuron illustrates lack of responses to large stimuli in most cells from normal animals. C, Pooled results (mean and SE).
In view of the profound effects of antisense ODN treatment on development of orientation selectivity, we have asked whether direction selectivity may have also been affected. A direction selectivity index was obtained for each cell by dividing the response elicited 180° away from the optimal direction by the response at the optimal direction and subtracting the result from 1. Indices of 1.0 indicate a high degree of selectivity, and indices of zero indicate lack of selectivity. As shown in Figure 7, direction selectivity remained relatively unaltered in antisense ODN-treated and control (sense and missense) ODN-treated cortex relative to normal, even after 28 d of treatment (p > 0.05). This finding raises the possibility that direction selectivity measured in cortical neurons may not be of cortical origin.
Fig. 7.
Effects of antisense ODN treatment on development of cortical direction selectivity. The cumulative plot of direction selectivity indices for the antisense ODN-treated animals was similar to the plots obtained from the control sense–missense ODN-treated and untreated animals. The small difference was not statistically significant (p > 0.05).
In view of the finding that ocular dominance bands in the primary visual cortex of the ferret develop during the time from P37 to P63 (Crair et al., 1998), we have also examined whether development of binocular responses in these animals may have been disrupted by the antisense ODN treatment. To quantify ocular dominance of cortical neurons, we calculated a binocularity index using the following equation: LE/(LE + RE), where LE stands for response to stimulation of the left eye and RE for right eye. A binocularity index of 1.0 indicates that a cell is responsive only to the left eye, whereas a binocularity index of 0.0 indicates that a cell is responsive only to the deprived eye. The histograms showing the distribution of cells into five binocularity ranges (Fig. 8) were compiled from: 67 cells from four untreated ferrets (A) and 49 cells from four antisense ODN-treated ferrets (B). All cells included in this study were located in the left hemisphere. Normal ferrets, and those treated with antisense ODN, had similar ocular dominance histograms and a large proportion of neurons that were binocularly driven. Although binocularity appears to be slightly enhanced in the antisense ODN animals, the differences are not significant. In conclusion, quantification of responses for each cortical cell indicated that suppression of cortical NMDA receptor function markedly affected orientation and size specificity while preserving direction selectivity and preserving or enhancing binocularity of normal cortical cells.
Fig. 8.
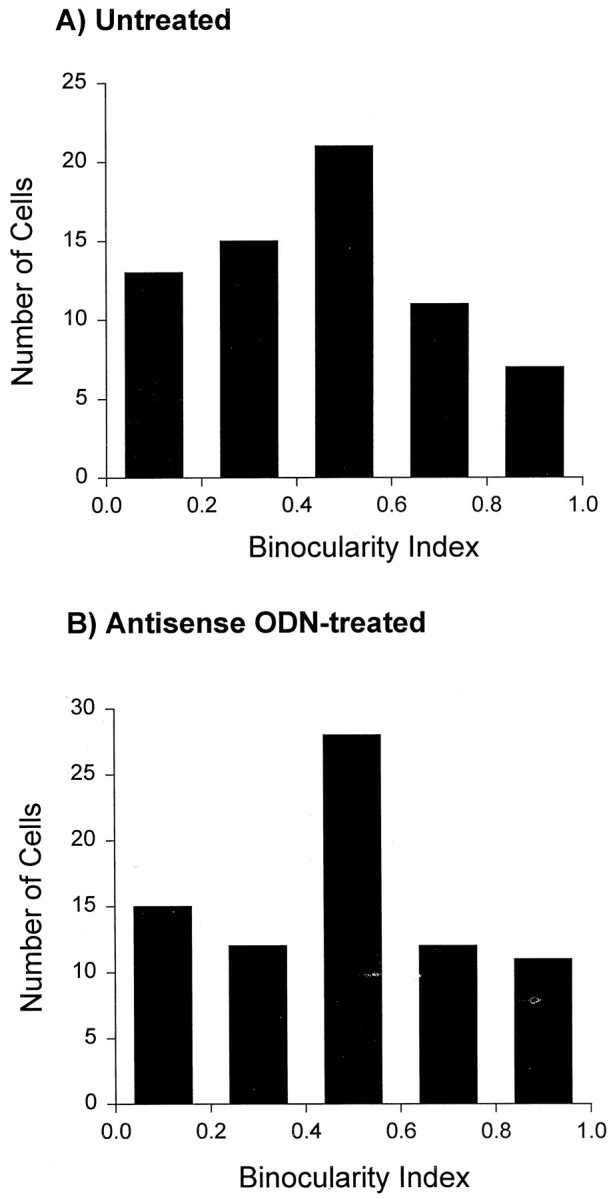
Knock down of NMDAR1 subunits during development did not affect the ocular dominance profile. The ocular dominance profiles in untreated (A) and antisense ODN-treated (B) ferrets were similar, as characterized by a large proportion of binocularly driven cells.
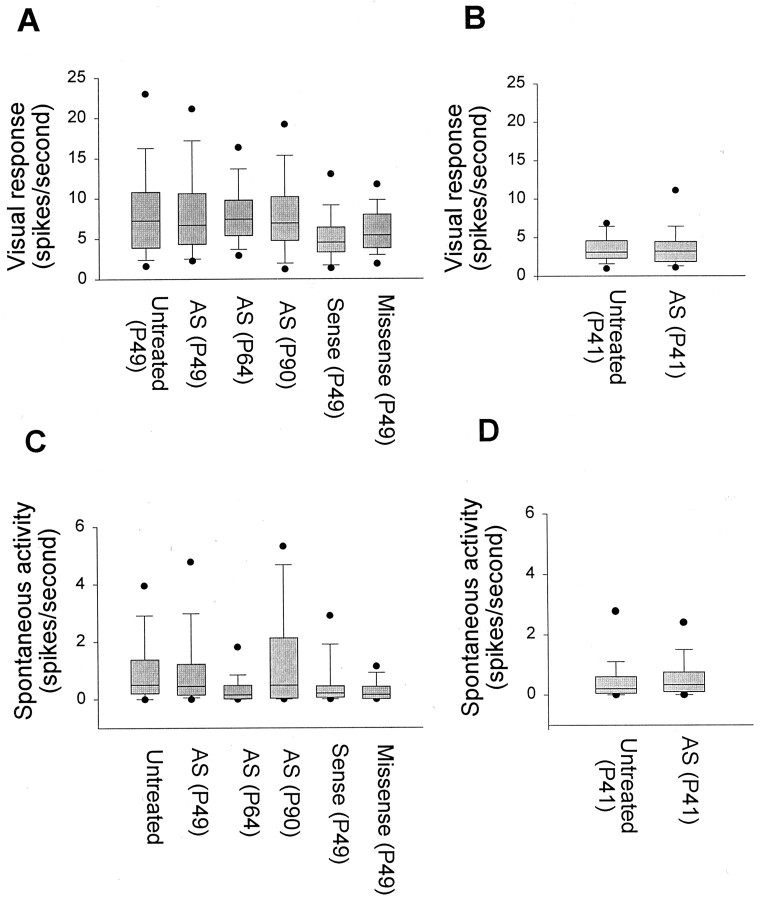
Finding that antisense ODN treatment blocks development of cortical functional properties raises the question of whether the effects result from a disruption of visual responses. Examples of peristimulus histograms shown in Figure 2 illustrate the finding that treatment did not reduce visual responses of cortical neurons. Population analysis also indicated that prolonged antisense ODN treatment did not affect visual responsiveness of the same cortical neurons that were examined in the above studies. Maximum response (in spikes/sec) to stimulation at the optimal orientation (Fig.9A) was not significantly affected by antisense ODN treatment compared with untreated cortex (p > 0.05). A small reduction in maximum response was observed in the sense ODN-treated cortex relative to the untreated cortex. However, most of the cells studied in the sense ODN-treated cortex were selective to stimulus orientation, indicating that a small reduction in responsiveness is not sufficient to disrupt development of cortical functional properties. Additional antisense-treated animals were studied at an earlier age, when orientation selectivity is developing. The findings, shown in Figure9B, support our conclusion that the antisense–ODN infusion does not affect visual responsiveness. Prolonged ODN treatment was found not to increase spontaneous activity (Fig. 9A), an effect that could conceivably also disrupt activity-dependent mechanisms of cortical maturation by decreasing signal-to-noise ratio. In conclusion, the effects of antisense ODN treatment on development of cortical receptive field properties did not result from disruption of sensory responses or spontaneous activity.
Fig. 9.
Antisense ODN treatment preserves maximum visual responses of striate cortical cells to a moving bar of light. Responses recorded at the optimal orientation (A, B) as well as the spontaneous activity (C, D) of cortical cells were relatively unaffected by antisense ODN treatment at different ages. There was no significant difference between the antisense ODN treatment group and untreated animals (p > 0.05). The box plots show the median, 10th, 25th, 75th, and 90th percentiles as vertical boxes witherror bars. The 5th and 95th percentiles are shown as dots.
To confirm our previous finding that antisense ODN treatment induced a reduction in the NMDAR1 subunit protein (Roberts et al., 1998), we conducted immunocytochemistry studies using a monoclonal antibody to this subunit protein. To reduce the possibility of artifacts from inadvertent differences in processing, sections of treated visual cortex always were processed together with sections obtained from the contralateral, untreated hemisphere of the same animals. The photomicrographs in Figure 10illustrate the finding that antisense ODN treatment (Fig.10B,D) induced a reduction in NMDAR1 subunit expression when compared with normal (Fig. 10A,C) ferret visual cortex. Additionally, the immunocytochemical micrographs, as well as adjacent Nissl-stained sections (data not shown), indicate the normal histological structure that was observed in ODN-treated cortex.
Fig. 10.
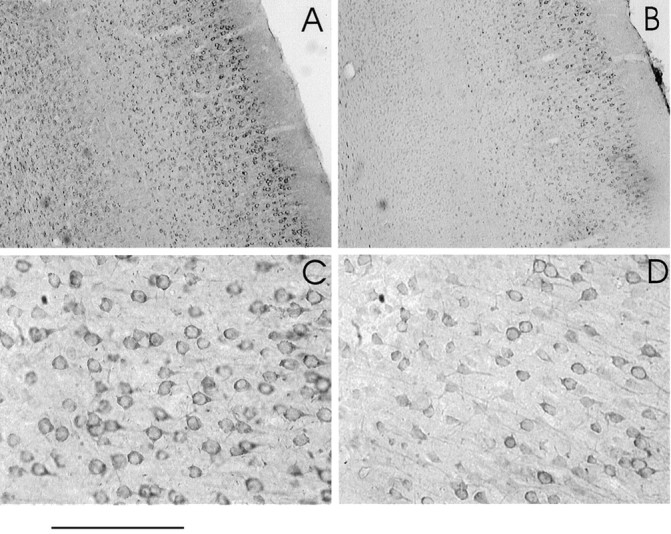
Effects of antisense ODN treatment on expression of the NMDAR1 subunit of the NMDA receptor. Immunocytochemistry of untreated (A,C) and antisense ODN-treated (B, D) ferret cortex using an anti-NMDAR1 antibody. Low-power (A, B) and high-power (C, D) photomicrographs show that the effects are widespread and affect a large number of cells. Normal and antisense ODN-treated tissue were processed together. Sections are from opposite hemispheres of the same animal. Scale bar: 0.5 mm (A, B) and 250 μm (C,D).
DISCUSSION
The development of cortical orientation selectivity is known to require neural activity (Chapman and Stryker, 1993). It has remained unclear, however, how activity contributes to the development of cortical orientation selectivity or what might be the molecular basis for such activity-dependent processes. We report two major findings concerning these mechanisms. First, we show for the first time that development of orientation selectivity is prevented when cortical NMDA receptor function is suppressed. This result cannot be explained by a nonspecific effect of the antisense ODN treatment because control sense and missense ODN infusion did not affect development of orientation selectivity. Moreover, response properties of cortical cells were not disrupted by antisense ODN treatment in mature animals, indicating that there is a specific period of development when suppressing NMDA receptor function affects orientation selectivity. Together, these findings indicate that NMDA receptors are essential for the development of orientation selectivity.
Our second major finding is that suppression of cortical NMDA receptor function starting before eye opening had a more disruptive effect on the development of orientation selectivity than suppression of NMDA receptors starting a few days after eye opening. These results suggest that NMDA receptor-mediated activity before eye opening contributes to the maturation of orientation selectivity.
How would activity before eye opening contribute to development of orientation selectivity in ferret primary visual cortex? Full maturation of orientation selectivity and the corresponding columnar system is markedly affected by sensory experience (Pettigrew, 1974;Thompson et al., 1983; Crair et al., 1998). However, the finding that some cells in visually naive animals show strong orientation preference (Wiesel and Hubel, 1974; Chapman and Stryker, 1993; Chapman et al., 1996) indicates that patterned visual experience is not required for the initial development of orientation selectivity. Rather, maturation of cortical selectivity before eye opening may rely on intrinsic neuronal activity (Katz and Shatz, 1996). Waves of retinal activity occur too early to explain development of orientation selectivity before eye opening (Maffei and Galli-Resta, 1990; Meister et al., 1991). However, neurons in the lateral geniculate nucleus display mature electrophysiological and synaptic properties and express an immature form of intrinsic oscillatory behavior (Ramoa and McCormick, 1994a,b; McCormick et al., 1995; Weliky and Katz, 1999) when orientation selectivity is developing. Thalamic patterns of intrinsic activity could, therefore, contribute to development of thalamocortical and intracortical connections. Another possibility is that retinal spontaneous activity can guide development of orientation selectivity even after waves of activity are no longer present. Consistent with this possibility, neighboring retinal ganglion cells are known to display correlated activity in mature animals (Mastronarde, 1983). Future studies will be required to elucidate the source of intrinsic neural activity that guides development of orientation selectivity before eye opening.
Notwithstanding the remarkable effects of antisense ODN treatment on development of orientation selectivity, this receptive field property was not completely eliminated; ferrets that received this treatment had orientation specificity similar to that present at the time of eye opening in normal animals. Interestingly, this finding is similar to results observed with intracortical infusion of tetrodotoxin to block cortical activity (Chapman and Stryker, 1993), and intraocular injections of d,l-2-amino-4-phosphonobutyric acid to block ON-center retinal ganglion cells activity (Chapman and Godecke, 2000). In both cases, development of orientation selectivity was markedly affected but not completely prevented. One possible explanation for this finding is that activity blockade in these studies was started well after the first axons from the lateral geniculate nucleus first invade cortex, which occurs as early as the second postnatal week in the ferret (Allendoerfer and Shatz, 1994). An alternative possibility is that the relevant activity is located in the geniculocortical circuit, or even cortex alone, before eye opening (Weliky and Katz, 1999). Therefore, the presence of some orientation selectivity in these neurons could reflect the contribution of spontaneous activity during earlier development. These alternative possibilities will have to be explored in future studies.
One major unresolved issue concerning activity dependence is whether activity guides development of cortical orientation selectivity or, alternatively, whether activity has a simple permissive role (Miller et al., 1999). Two recent studies have addressed this important issue. In the first study, altering patterns of neural activity using chronic electrical stimulation of the optic nerve during development resulted in disruption of orientation tuning in cortex (Weliky and Katz, 1997). In this study, electrical stimulation was only given ∼10% of the time. Surprisingly, normal input activity that was present the remaining 90% of the time was not sufficient to drive maturation of orientation selectivity. In another study, ferrets raised with the ON-center pathway silenced pharmacologically had development of cortical orientation selectivity frozen in an immature state, a finding that suggests that the balance of ON-and OFF-center activity is crucial for the development of orientation-selective receptive fields in cortical cells (Chapman and Godecke, 2000). These studies support an instructive role of activity during development of orientation selectivity. However, one major difficulty with attempts to distinguish between an instructive and permissive role of activity in most pharmacological studies of visual development and plasticity has been that overall activity is depressed by the treatment (Miller et al., 1989). Similarly, one potential problem with the Chapman and Godecke (2000) study is that by blocking ON-center activity, the investigators have presumably halved the overall levels of input activity to visual cortex. Under these conditions, orientation selectivity may fail to develop as a result of reduced input activity, which would support a permissive role of activity.
We have avoided this problem in our study by using a molecular approach that suppresses cortical NMDA receptor function. Antisense techniques were used to reduce expression of the NMDAR1 subunit, which is required in the functional assembly of the NMDA receptor (Kutsuwada et al., 1992; Meguro et al., 1992; Nakanishi et al., 1992; Ishii et al., 1993;Laurie and Seeburg, 1994). Our previous studies have shown that the selective reduction of NMDA receptor function using antisense techniques preserves normal cortical visual responsiveness and stimulus selectivity of cortical cells when treatment is conducted at a time when orientation selectivity is already mature (Roberts and Ramoa, 1998; also see present results). In contrast, pharmacological blockade of NMDA receptor function not only decreases sensory responses (Miller et al., 1989) but also leads to acute loss of stimulus specificities, including orientation selectivity (Bear et al., 1990; Rauschecker et al., 1990; Daw 1994). Moreover, a recent study has shown that pharmacological blockade of NMDA receptor function disrupts cortical binocularity, even in otherwise normal adult animals (Kasamatsu et al., 1998). For these reasons, use of antisense techniques provides a rigorous test for a specific role of NMDA receptors in development of cortical orientation selectivity. Therefore, our findings that animals treated under these conditions had their cortical orientation selectivity frozen at an immature state provide unambiguous evidence that NMDA receptors are essential for patterning connections that underlie orientation selectivity in primary visual cortex.
How would NMDA receptors contribute to the development of orientation selectivity? The model proposed to explain development of orientation selectivity is correlation-based, requiring that one set of inputs show greater correlation than another set; selection of the “most-correlated” set of inputs by a correlation detector would lead to the establishment of the neural circuitry underlying orientation selectivity (Miller et al., 1999). The NMDA receptor is thought to act as a correlation detector for presynaptic and postsynaptic activity (Bourne and Nicoll, 1993), because of its voltage-dependent blockade mediated by Mg2+. This blockade is relieved when synchronous firing of a large number of presynaptic fibers leads to sufficient depolarization of the postsynaptic membrane. The NMDA receptor could, therefore, be the correlation detector required in correlation-based development of orientation selectivity. This mechanism could be responsible for sculpting neural connections that underlie orientation selectivity based on patterns of afferent input. For instance, NMDA receptors could be responsible for sculpting the receptive fields of cortical cells based on the balance of ON- and OFF-center activity, thereby originating orientation tuning. In view of the postulated role of NMDA receptors as correlation detectors, our findings support the hypothesis that neural activity has an instructive role in the development of orientation selectivity.
Footnotes
This work was supported by the National Eye Institute Grant EY-11508 to A.S.R.
Correspondence should be addressed to Ary S. Ramoa, Department of Anatomy, Box 0709, Virginia Commonwealth University, 1101 East Marshall Street, Sanger Hall, Room 12-042, Richmond, VA 23298-0709. E-mail:aramoa@hsc.vcu.edu.
REFERENCES
- 1.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 2.Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- 3.Bear MF, Kleinschmidt A, Gu Q, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop PO, Coombs CS, Henry GH. Receptive fields of simple cells in the cat striate cortex. J Physiol (Lond) 1973;231:31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol (Lond) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72, Neuron 70[Suppl]:65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- 7.Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol (Lond) 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman B, Godecke I. Cortical cell orientation selectivity fails to develop in the absence of ON-center retinal ganglion cell activity. J Neurosci. 2000;20:1922–1930. doi: 10.1523/JNEUROSCI.20-05-01922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5261. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daw NW. Mechanisms of plasticity in the visual cortex. Invest Ophthalmol Vis Sci. 1994;35:4168–4179. [PubMed] [Google Scholar]
- 13.Daw NW, Gordon B, Fox KD, Flavin HJ, Kirsch JD, Beaver CJ, Ji KH, Reid SNM, Czepita D. Injection of MK-801 affects ocular dominance shifts more than visual activity. J Neurophysiol. 1999;81:204–215. doi: 10.1152/jn.1999.81.1.204. [DOI] [PubMed] [Google Scholar]
- 14.Fox K, Daw NW. Do NMDA receptors have a critical function in visual cortical plasticity? Trends Neurosci. 1993;16:116–122. doi: 10.1016/0166-2236(93)90136-a. [DOI] [PubMed] [Google Scholar]
- 15.Fox K, Sato H, Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989;9:2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fregnac Y. Development of orientation selectivity in the primary visual cortex of normal and dark reared kittens: I. Kinetics. Biol Cybern. 1979;34:187–193. doi: 10.1007/BF00337425. [DOI] [PubMed] [Google Scholar]
- 17.Fregnac Y, Imbert M. Development of neuronal selectivity in the primary visual cortex of the cat. Physiol Rev. 1984;64:325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- 18.Grzanna R, Dubin JR, Dent GW, Ji Z, Zhang W, Ho SP, Hartig PR. Intrastriatal and intraventricular injections of oligodeoxynucleotides in the rat brain: tissue penetration, intracellular distribution and c-fos antisense effects. Mol Brain Res. 1998;63:35–62. doi: 10.1016/s0169-328x(98)00238-1. [DOI] [PubMed] [Google Scholar]
- 19.Hebb DO. The organization of behavior. Wiley; New York: 1949. [Google Scholar]
- 20.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 22.Kasamatsu T, Iwama K, Mataga N, Hatveit E, Heggelund U, Heggelund P. Roles of N-methyl-d-aspartate receptors in ocular dominance plasticity in developing visual cortex: re-evaluation. Neuroscience. 1998;82:687–700. doi: 10.1016/s0306-4522(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 23.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;15:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 24.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 25.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levick WR. Another tungsten microelectrode. Med Electron Biol Eng. 1972;10:510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- 27.Linden DC, Guillery RW, Cucciaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- 28.MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 29.Maffei L, Galli-Resta Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc Natl Acad Sci USA. 1990;87:2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983;49:303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- 31.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg++ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 32.McCormick DA, Trent F, Ramoa AS. Postnatal development of synchronized network oscillations in the ferret dorsal lateral geniculate and perigeniculate nuclei. J Neurosci. 1995;15:5739–5752. doi: 10.1523/JNEUROSCI.15-08-05739.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 34.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 35.Miller KD. A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between ON- and OFF-Center inputs. J Neurosci. 1994;14:409–441. doi: 10.1523/JNEUROSCI.14-01-00409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller KD, Chapman B, Stryker MP. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1989;86:5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller KD, Erwin E, Kayser A. Is the development of orientation selectivity instructed by activity? J Neurobiol. 1999;41:44–57. doi: 10.1002/(sici)1097-4695(199910)41:1<44::aid-neu7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi N, Axel A, Schneider NA. Alternative splicing generates functionally dsitinct N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowak R, Bregestovski P, Ascher P, Herbert A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 40.Pettigrew JD. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol (Lond) 1974;237:49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramoa AS, McCormick D. Developmental changes in electrophysiological properties of LGN neurons during reorganization of retinogeniculate connections. J Neurosci. 1994a;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramoa AS, McCormick D. Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. J Neurosci. 1994b;14:2098–2105. doi: 10.1523/JNEUROSCI.14-04-02098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauschecker JP, Egert U, Kossel A. Effects of NMDA antagonists on developmental plasticity in kitten visual cortex. Int J Dev Neurosci. 1990;8:425–435. doi: 10.1016/0736-5748(90)90075-d. [DOI] [PubMed] [Google Scholar]
- 44.Roberts E, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA blocks ocular dominance plasticity while preserving visual responses. J Neurophysiol. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 45.Stent GS. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson ID, Kossut M, Blakemore C. Development of orientation columns in cat striate cortex revealed by 2-deoxyglucose autoradiography. Nature. 1983;24:712–715. doi: 10.1038/301712a0. [DOI] [PubMed] [Google Scholar]
- 47.Wahlestedt C, Golanov E, Yamamoto S, Yee F, Ericson H, Yoo H, Inturrisi CE, Reis DJ. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature. 1993;363:260–263. doi: 10.1038/363260a0. [DOI] [PubMed] [Google Scholar]
- 48.Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- 49.Weliky M, Katz LC. Correlational structure of spontaneous activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- 50.Whitesell L, Geselowitz D, Chavany C, Fahmy B, Walbridge S, Alger JR, Neckers LM. Stability, clearance, and disposition of intraventricularly administered oligodeoxynucleotides: implications for therapeutic application within the central nervous system. Proc Natl Acad Sci USA. 1993;90:4665–4669. doi: 10.1073/pnas.90.10.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]