Abstract
In the striatum, dopamine D1 and adenosine A2A receptors stimulate the production of cAMP, which is involved in neuromodulation and long-lasting changes in gene expression and synaptic function. Positive coupling of receptors to adenylyl cyclase can be mediated through the ubiquitous GTP-binding protein GαS subunit or through the olfactory isoform, Gαolf, which predominates in the striatum. In this study, using double in situ hybridization, we show that virtually all striatal efferent neurons, identified by the expression of preproenkephalin A, substance P, or D1 receptor mRNA, contained high amounts of Gαolf mRNA and undetectable levels of Gαs mRNA. In contrast, the large cholinergic interneurons contained both Gαolf and Gαstranscripts. To assess the functional relationship between dopamine or adenosine receptors and G-proteins, we examined G-protein levels in the striatum of D1 and A2A receptor knock-out mice. A selective increase in Gαolf protein was observed in these animals, without change in mRNA levels. Conversely, Gαolf levels were decreased in animals lacking a functional dopamine transporter. These results indicate that Gαolf protein levels are regulated through D1and A2A receptor usage. To determine the functional consequences of changes in Gαolf levels, we used heterozygous Gαolf knock-out mice, which possess half of the normal Gαolf levels. In these animals, the locomotor effects of amphetamine and caffeine, two psychostimulant drugs that affect dopamine and adenosine signaling, respectively, were markedly reduced. Together, these results identify Gαolf as a critical and regulated component of both dopamine and adenosine signaling.
Keywords: Golf, Gs, G-protein, D1 receptor, A2A receptor, knock-out mice, striatum, dopamine, adenosine, dopamine transporter, homologous recombination
The nigrostriatal dopaminergic neurons are essential for proper motor function in both humans and rodents. In the dorsal striatum, dopaminergic influence is predominantly mediated through D1(D1R) and D2(D2R) dopamine receptors that stimulate and inhibit cAMP production, respectively (Sibley and Monsma, 1992; Jaber et al., 1996). cAMP signaling contributes largely to the acute effects of dopamine as well as to long-lasting changes in gene expression and synaptic plasticity (for review, see Greengard et al., 1999; Berke and Hyman, 2000). In the striatum, D1R and D2R are enriched in distinct populations of efferent GABAergic spiny neurons (Gerfen et al., 1990; Hersch et al., 1995; Le Moine and Bloch, 1995; Yung et al., 1996), although sensitive measurements reveal some degree of overlap in receptor distribution (Surmeier et al., 1996). The D1R-enriched population, referred to as striatonigral, projects to the substantia nigra pars reticulata and to the entopeduncular nucleus and contains substance P and dynorphin as cotransmitters, whereas the D2R-enriched population, referred to as striatopallidal, projects to the external globus pallidus and contains enkephalins (Beckstead and Cruz, 1986; Gerfen and Young, 1988; Gerfen et al., 1990; Le Moine et al., 1990, 1991; Le Moine and Bloch, 1995). In the striatopallidal neurons, which possess little or no D1R, adenosine A2Areceptors (A2ARs) are the main receptor type stimulating the cAMP production (Premont et al., 1977; Schiffmann et al., 1991; Svenningsson et al., 1997). Because of this “strategic” location, A2ARs are potential therapeutic targets for treating psychosis and Parkinson's disease (Ledent et al., 1997;Svenningsson et al., 1999).
In spite of the strong effects of D1R and A2AR on cAMP production, the striatum contains small amounts of Gαs, the G-protein subunit responsible for adenylyl cyclase stimulation in most cell types, but high concentrations of the olfactory isoform Gαolf (Drinnan et al., 1991; Herve et al., 1993). Gαolf shares 80% amino acid identity with Gαs and mediates olfactory receptor signaling in the olfactory epithelium (Jones and Reed, 1989; Belluscio et al., 1998). Cocaine responses are abolished in Gαolf knock-out mice, indicating that Gαolf may be necessary for dopamine action (Zhuang et al., 2000), and recent data provide strong evidence that Gαolf couples A2AR to adenylyl cyclase (Kull et al., 2000). Moreover, we have shown that D1R- and A2AR-stimulated cAMP production is blocked in the striatum of Gαolf knock-out mice (Corvol et al., 2001).
The first aim of the present study was to characterize in detail the cellular localization of Gαs and Gαolf in the striatum using in situhybridization. We then examined the functional relationship between striatal Gαolf levels and dopamine or adenosine transmission, using mutant mice in which the genes for D1R, A2AR, or dopamine transporter (DAT) are disrupted. Finally, we examined the functional consequences of decreasing Gαolf levels, by measuring the locomotor responses to amphetamine and caffeine in mice, which have reduced levels of Gαolf. Our results indicate thus that Gαolf is a crucial site for the regulation of D1R and A2AR efficacy in the striatum and a potential locus for dysfunction in dopamine and adenosine neurotransmission.
MATERIALS AND METHODS
Tissue preparation for in situhybridization. Brains from adult male Sprague Dawley rats (200–280 gm; Centre d'élevage Janvier, Le Genest. Saint-Isle, France) were dissected out, frozen in liquid nitrogen, and sectioned at the level of the striatum following the atlas of Swanson (1992) into 10-μm-thick sections that were stored at −80°C until use. All experiments were performed in accordance with the guidelines of the French Agriculture and Forestry Ministry for handling animals (decree 87849, license 01499) and with the Centre National de la Recherche Scientifique approval.
Probe synthesis. 35S-labeled or digoxigenin-labeled cRNA probes were prepared by in vitrotranscription from rat or mouse (Gαs) cDNA clones corresponding to fragments of Gαolf, Gαs, preproenkephalin A (Yoshikawa et al., 1984), substance P (Bonner et al., 1987), D1R (Monsma et al., 1990), and choline acetyltransferase (Ibanez et al., 1991) cDNAs. The Gαolf cDNA clone corresponded to the clone 8 described by Herve et al. (1995), and the Gαs cDNA clone corresponded to the most 3′ 550 bp fragment of the murine Gαs cDNA sequence (Sullivan et al., 1986). The sequence identity between Gαolf and Gαs probes was <30%. Transcription was performed from 50 ng of linearized plasmid using either 35S-UTP (>1000 Ci/mmol; NEN Life Science, Paris, France) or digoxigenin-11-UTP (Roche Diagnostic, Meylan, France) and SP6, T3, or T7 RNA polymerases as described (Le Moine and Bloch, 1995). After alkaline hydrolysis to obtain cRNA fragments of ∼250 bp, the35S-labeled probes were purified on Sephadex-G50. The 35S-labeled purified probes and the digoxigenin-labeled probes were precipitated in 3m sodium acetate, pH 5, and absolute ethanol (0.1/2.5 vol).
In situ hybridization: single detection of Gαolf and Gαs mRNAs on cryostat sections.Cryostat sections were post-fixed in 4% paraformaldehyde (PFA) for 5 min at room temperature, rinsed twice in 4× SSC, and placed into 0.25% acetic anhydride with 0.1 mtriethanolamine in 4× SSC, pH 8, for 10 min at room temperature. After dehydration, the sections were hybridized overnight at 55°C under coverslips with 106 cpm of35S-labeled Gαolfor Gαs cRNA probes in 50 μl of hybridization solution (20 mm Tris-HCl, 1 mm EDTA, 300 mm NaCl, 50% formamide, 10% dextran sulfate, 1× Denhardt's reagent, 250 μg/ml yeast tRNA, 100 μg/ml salmon sperm DNA, 100 mmDTT, 0.1% SDS, and 0.1% sodium thiosulfate). Coverslips were removed by rinsing in 4× SSC. After 20 min of RNase A treatment (20 μg/ml) at 37°C, the sections were washed sequentially in 2× SSC (5 min, twice), 1× SSC (5 min), 0.5× SSC (5 min) at room temperature, and rinsed in 0.1× SSC at 65°C (30 min, twice) before dehydration (the latter SSC washes contained 1 mm DTT). Sections were used to expose x-ray films (Kodak Biomax; Eastman Kodak, Rochester, NY) for 3–6 d, and then dipped into Ilford K5 emulsion (diluted 1:3 in 1× SSC), which was developed after an 8 week exposition and stained with toluidine blue.
In situ hybridization: simultaneous detection of two mRNAs on the same sections. Different combinations of cRNA probes were used for the simultaneous detection of two mRNAs on the same sections. Cryostat sections were post-fixed, acetylated, and dehydrated as described above. Combinations of 35S- and digoxigenin-labeled probes (106 cpm of35S-labeled probe plus 10–20 ng of digoxigenin-labeled probe) were hybridized in the same hybridization solution as described above. After elimination of coverslips, the slides were treated with RNase A and washed in decreasing concentrations of SSC as mentioned above, but without DTT. At the end of the washes, the slides were cooled in 0.1× SSC at room temperature and then processed directly for detection of the digoxigenin signal. The sections were rinsed twice for 5 min in buffer A (1m NaCl, 0.1 m Tris, and 2 mm MgCl2, pH 7.5), and then for 30 min in buffer A containing 3% normal goat serum and 0.3% Triton X-100. After a 5 hr incubation at room temperature with alkaline phosphatase-conjugated anti-digoxigenin antiserum (1:1000 in buffer A containing 3% normal goat serum and 0.3% Triton X-100; Roche Diagnostic), the sections were rinsed twice 5 min in buffer A, twice 10 min in 1 m STM buffer (1 mNaCl, 0.1 m Tris, and 5 mmMgCl2, pH 9.5), and twice for 10 min in 0.1m STM buffer (0.1 m NaCl, 0.1 m Tris, and 5 mmMgCl2, pH 9.5). The sections were then incubated overnight in the dark at room temperature in 0.1m STM buffer containing 0.34 mg/ml nitroblue tetrazolium and 0.18 mg/ml bromo-chloro-indolylphosphate. The sections were rinsed briefly in 0.1 m STM buffer and 2 hr in 1× SSC, dried, and dipped into Ilford K5 emulsion (diluted 1:3 in 1× SSC). After being exposed 10–14 weeks in the dark, the emulsions were developed, and the sections were mounted without counterstaining. Labeled neurons both from single-labeling and double-labeling experiments were counted on sections from three different animals as previously described on similar material (Le Moine and Bloch, 1995).
Mutant mice. Pairs of heterozygous mice with a disrupted gene of Gαolf (Belluscio et al., 1998) and a hybrid 129 and C57Bl/6 genetic background were crossed. The genomic DNA of the progeny was extracted from tail tissue, digested byHindIII, and hybridized with a Gαolfprobe corresponding 1.2 kb Pst/Kpn fragment of mouse Gαolf gene located 0.5 kb upstream to the ATG codon translational start site. The probe hybridizes with fragments of 2.8 kb when the gene is mutated or 15 kb fragments in wild-type (Belluscio et al., 1998). Pairs of heterozygous mice bearing a null mutation for D1R or DAT gene and having a hybrid 129 and C57BL/6 genetic background were mated to obtain wild-type and homozygous mutant mice and were typed by Southern blot, as described byDrago et al. (1994) or by Giros et al. (1996). Male wild-type controls and homozygous A2AR (Ledent et al., 1997) or CB1 cannabinoid receptor (Ledent et al., 1999) knock-out mice used for the experiments were cousins, and their common grandparents were heterozygous mutant mice backcrossed on CD1 background for 10 generations. Mice were kept in stable conditions of temperature (22°C) and humidity (60%) with a constant cycle of 12 hr light and dark and had ad libitum access to food and water.
Gα protein antibodies. Specific antibodies against Gαolf (SL48SP) were obtained by immunizing a rabbit against a recombinant Gαolfprotein and by purifying the obtained serum with Gαolf and Gαs columns, as described previously (Corvol et al., 2001). Specific antibodies against Gαs (SL22AP) were raised against a Gαs-selective peptide and affinity-purified on a peptide column (Penit-Soria et al., 1997). The other antibodies were mouse monoclonal antibodies from commercial sources against Gαo (clone L5.6; Neomarkers, Union City, CA), Gαi2 (clone 2A.3; Neomarkers), and Gβ (clone 3, Transduction Laboratories, Lexington, KY).
Immunoblot analysis. Wild-type and mutant mice (2–10 month old, age-matched) were killed by decapitation, and their brains were immediately dissected out from the skull and frozen on dry ice. Microdiscs of tissue were punched out from frozen slices (500-μm-thick) within the striatum using a stainless steel cylinder (1.4 mm diameter). Samples were homogenized in 1% SDS, equalized for their content in protein, and analyzed by Western blot as described previously (Herve et al., 1993). Antibody dilutions were 1:1000, 1:500, 1:300, 1:600, and 1:1000 for antibodies against Gαolf, Gαs, Gαi2, Gαo, and Gβ, respectively. Antibodies were revealed by the peroxidase–chemiluminescence method (ECL; Amersham, Orsay, France) and autoradiography. The antibodies were stripped one or two times to detect other antigens on the same membranes (Erickson et al., 1982;Herve et al., 1993). Quantification was performed by optical density measurement on autoradiographic films using a computer-assisted densitometer and the NIH Image software. In each membrane, samples from control and mutant mice were alternatively loaded, and the results were normalized as percentage of the mean of controls in each membrane.
cDNA probes and Northern analysis. The Gαolf cDNA probe corresponded to a PCR fragment containing the 1156 bp of the coding region of rat cDNA clone (Jones and Reed, 1989; Herve et al., 1995) and the Gαsprobe was the same as described above. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was a 1.3 kbp full length of rat GAPDH cDNA (Fort et al., 1985). Total RNA was extracted by the guanidinium thiocyanate–phenol-chloroform method (Chomczynski and Sacchi, 1987) from tissue microdiscs taken on frozen slices, and Northern blots were generated as previously described (Herve et al., 1993). The filters were hybridized successively with Gαolf, Gαs, and GAPDH probes, with a dehybridation step between two hybridations consisting in the incubation of membrane in boiling 0.5% SDS. Radioactivity of positive bands was measured using Instant Imager (Packard, Downers Groves, IL). Gαolf and Gαs mRNA concentrations were expressed as the counts per minute ratio of Gαolf/GAPDH and Gαs/GAPDH, respectively.
(3H)SCH23390, (125I)iodosulpride, (3H)CGS21680, and (3H)WIN35428 binding. Twenty micrometer coronal brain sections from three adult heterozygous Gαolf +/− mice and three wild-type littermates were thaw-mounted onto SuperFrost microscope slides. D1R, D2R, A2AR, and DATs were analyzed by incubating the tissue sections with 2.5 nm(3H)SCH23390 (91 Ci/mmol), 0.2 nm(125I)iodosulpride (2000 Ci/mmol), 5 nm (3H)CGS21680 (47 Ci/mmol,) and 4 nm(3H)WIN35428 (86 Ci/mmol), respectively, in conditions as previously described (Bouthenet et al., 1987; Savasta et al., 1988; Jarvis and Williams, 1989; Herbert et al., 1999). The radioligands were obtained from Amersham Pharmacia Biotech (Saclay, France) or from NEN (Boston, MA). Nonspecific binding was determined from adjacent brain sections in the presence of 1 μm SCH23390, 10 μmsulpiride, 10 μm CGS21680, and 30 μm benztropine for blocking D1R, D2R, A2AR, and DATs, respectively. After washing in ice-cold buffer, brain sections were rapidly dried and used to expose tritium-sensitive film (Hyperfilm 3H; Amersham). In autoradiographs, the binding in caudate putamen and nucleus accumbens was evaluated by measuring the optical density with a computer-assisted image analyzer and the NIH image software, and the data were expressed in femtomoles of bound ligand per milligram of tissue using standards (3H-microscales; Amersham Pharmacia Biotech).
Locomotor activity. Male heterozygous Gαolf +/− mice and wild-type littermates were introduced in a circular corridor (4.5 cm width, 17 cm external diameter) crossed by four infrared beams (1.5 cm above the base) placed at every 90° (Imetronic, Pessac, France). The locomotion was counted when the animals interrupted two successive beams and, thus, had traveled one-fourth of the circular corridor. In each session, the spontaneous activity was recorded for 50 min, before the animals were injected with saline or drugs, and their activity was recorded for an additional 60 or 120 min period. In the two first sessions, the animals received saline injections (5 ml/kg, i.p.), and in the third session, they received amphetamine (1, 2, or 3 mg/kg, i.p) or caffeine (25 mg/kg, i.p.) dissolved in saline. For each session an equal number of heterozygous and wild-type mice was studied, and for each drug treatment, groups of 7–14 animals were compared. The tests were performed between 12:00 and 6:00 P.M. in stable conditions of temperature and humidity.
RESULTS
Characterization of striatal neurons expressing Gαsand Gαolf mRNAs
As previously reported (Drinnan et al., 1991; Herve et al., 1993;Kull et al., 2000), the striatum was the brain region in which the contrast between the levels of Gαs and Gαolf mRNAs was the most dramatic (Fig.1). At low magnification, high levels of Gαolf mRNAs were observed in all the striatal areas, including caudate putamen (Cp), nucleus accumbens (NA), and olfactory tubercle (OT) (Fig. 1A,C,E,G), which appeared almost devoid of Gαs mRNAs (Fig.1D,F). A strong signal for both Gαolf and Gαs was detected in a few regions, including piriform cortex (Pir), islands of Calleja, medial habenula (Hb), and dentate gyrus (DG) (Fig.1A–H), whereas in most other brain areas an intense labeling for Gαs mRNAs was associated with low or no labeling for Gαolf mRNA (Fig.1A–H). The use of sense probes showed no labeling for either Gαolf (Fig. 1I) or Gαs (Fig. 1J) mRNAs.
Fig. 1.
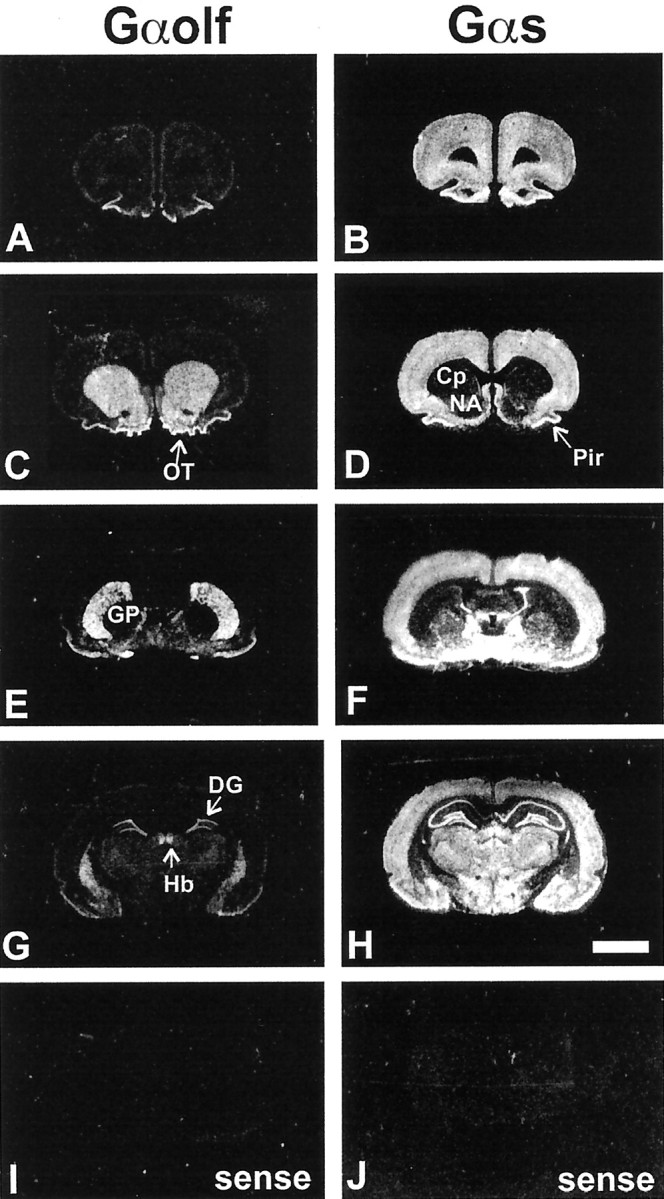
Distribution of Gαolf and Gαs mRNAs in forebrain. Negative of x-ray films exposed to rat brain adjacent sections hybridized with 35S-labeled probes for Gαolf and Gαs mRNAs.A, C, E, G, Gαolf mRNA was highly expressed in striatal areas, including caudate putamen, nucleus accumbens, and olfactory tubercle (C, E, G). A signal was also found in the piriform cortex, the islands of Calleja, the medial habenula, and the dentate gyrus (A, C,E, G). All the other areas showed very low or no labeling for Gαolf mRNA. B,D, F, H, On the contrary, Gαs mRNA was highly expressed in many brain areas including all cortical areas, septum, globus pallidus, most of the hypothalamic and thalamic nuclei, hippocampus, and amygdala (B, D, F,H). Caudate putamen, nucleus accumbens, and olfactory tubercles showed almost no labeling, except for some sparse neurons (D, F). A total absence of labeling was observed in control experiments using sense probes for Gαolf and Gαs mRNAs (I, J). Cp, Caudate putamen;NA, nucleus accumbens; OT, olfactory tubercle; GP, globus pallidus; Hb, medial habenula; DG, dentate gyrus; Pir,piriform cortex. Scale bar, 5 mm.
At the cellular level Gαolf mRNA was expressed in 96.5 ± 1.3% (n = 1250) of the striatal neurons, mostly medium-sized. Conversely, GαsmRNA-positive neurons were scattered all over the striatal areas, including 13.4 ± 1.2% (n = 1300) of the caudate putamen neurons, from which approximately one-third were large-sized. Double-labeling experiments were performed using various combinations of Gαolf and Gαs probes labeled with 35S or digoxigenin. Virtually all Gαs mRNA-positive neurons expressed Gαolf mRNA levels above background, whereas Gαolf mRNA was present in numerous neurons with no labeling for Gαs mRNA (Fig.2A,B). In particular, Gαolf mRNA was present in almost all striatal medium-sized neurons, very few of which also expressed high levels of Gαs mRNA (Fig. 2B, arrow). Simultaneous detection of Gαolf mRNA and D1R mRNA showed that virtually all cells expressing D1R mRNA were positive for Gαolf mRNA (Fig. 2C, arrows). However, approximately half of Gαolf-positive neurons did not contain D1R mRNA (Fig.2C, white arrowheads). Large-sized neurons were positive for both Gαolf and Gαs (Fig. 2B, arrowhead). These large neurons, which contained GαolfmRNA, as well as Gαs mRNA, were cholinergic interneurons, as demonstrated with a digoxigenin-labeled probe for choline acetyltransferase mRNA (Fig. 2D,E, arrows).
Fig. 2.
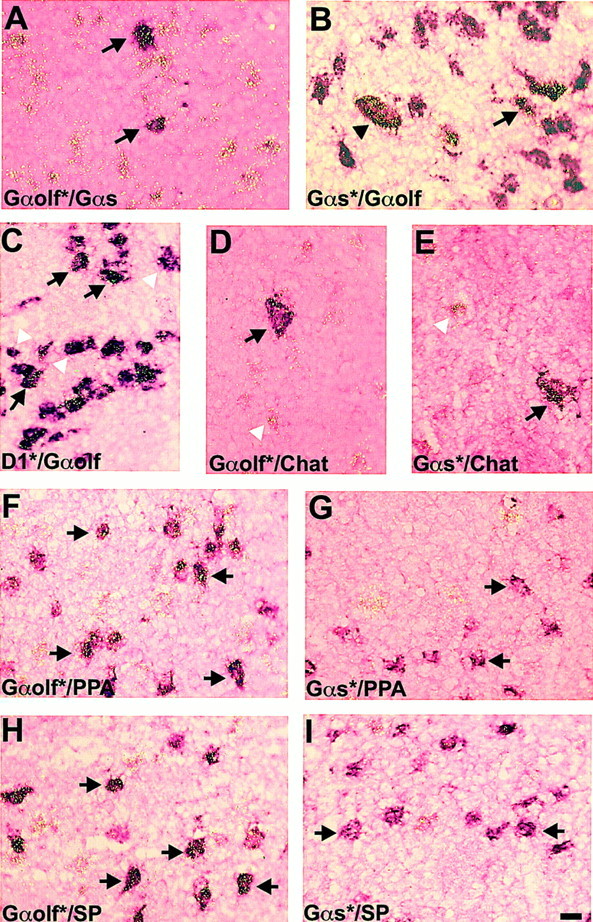
Phenotypical characterization of striatal neurons expressing Gαolf and Gαs mRNAs. Double-labeling in situ hybridization of rat brain sections was performed with various combinations of35S-labeled probes (indicated by asterisksin the figure) and digoxigenin-labeled probes. In all photomicrographs, silver grains for 35S-labeling are visualized using epi-illumination and appear as bright yellow grains, whereas digoxigenin labeling appears as purple staining.A, Most Gαolf-positive neurons (silver grains) did not contain Gαs mRNA (digoxigenin), which was present in only a few large- or medium-sized neurons (which also contained Gαolf mRNA;35S, arrows). B, The reverse combination of probe labeling showed that Gαolf mRNA (digoxigenin) was present in numerous medium-sized neurons, few of which also expressed significant levels of Gαs mRNA (35S, arrow). Most of the large-sized neurons contained both mRNAs (arrowhead).C, Simultaneous detection of Gαolf mRNA (digoxigenin) and D1R mRNA (silver grains) showed that Gαolf mRNA was colocalized with D1R mRNA (arrows), but approximately half of Gαolf mRNA-positive neurons did not contain D1R mRNA (white arrowheads).D, E, Double labeling for choline acetyltransferase mRNA (digoxigenin) and Gαolf mRNA (35S; D) or Gαs mRNA (35S; E). Both Gαolf and Gαs mRNAs were present in choline acetyltransferase-positive large neurons (arrows). Numerous medium-sized neurons expressed only Gαolf mRNA (D, white arrowhead), whereas very few expressed Gαs mRNA (E, white arrowhead).F, G, Double labeling for PPA (digoxigenin) and Gαolf mRNA (35S;F) or Gαs mRNA (35S;G). H, I, Double labeling for substance P mRNA (SP; digoxigenin) and Gαolf mRNA (35S; H) or Gαs mRNA (35S; I). Gαolf mRNA was present both in preproenkephalin A-positive (F, arrows) and substance P-positive (H, arrows) neurons, as well as in negative cells (F, H, silver grains). Gαs mRNA was mainly absent in these digoxigenin-labeled neurons (G, I, arrows). Scale bar, 12 μm.
In the dorsal striatum, the striatopallidal and striatonigral medium-sized spiny neurons were identified using digoxigenin-labeled probes for preproenkephalin A mRNA (PPA) (Fig. 2F,G) or for substance P mRNA (SP) (Fig. 2H,I), respectively. Gαolf mRNA (35S-labeled probe) was present in both preproenkephalin A- and substance P mRNA-containing neurons (Fig.2F,H, arrows), whereas the levels of silver grains for Gαs mRNA (35S-labeled probe) in these neurons were close to background (Fig. 2G,I, arrows).
Alterations of striatal G-protein levels in mice lacking D1 receptors
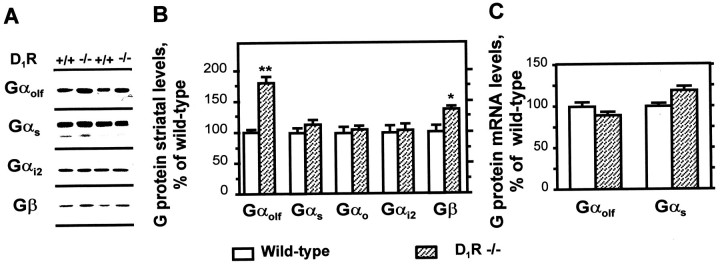
Previous studies had shown that destruction of dopamine neurons resulted in an increase in Gαolfprotein levels (Herve et al., 1993; Marcotte et al., 1994; Penit-Soria et al., 1997). The simplest explanation of these observations was that the increase in Gαolf was the consequence of the chronic absence of dopamine receptor stimulation. We therefore examined whether the complete absence of D1R was able to alter Gαolf, using mice in which the D1R gene had been disrupted (Drago et al., 1994). As shown in Figure 3, the striatal Gαolf concentrations were higher in mutant mice than in wild-type littermates, whereas the levels of other G-protein α subunits Gαs, Gαo, and Gαi2 were not altered (Fig.3A,B). The levels of Gβ subunit were also significantly increased in D1R knock-out animals (Fig.3A,B), probably reflecting the stoichiometric increase in Gβ subunits participating in heterotrimeric complexes with Gαolf. The lower increase in Gβ (+36 ± 5%) than in Gαolf (+80 ± 10%) levels can be accounted for by the association of Gβ subunits with other α subunits, which remained unaffected. Interestingly, a slight but significant increase in Gαolf protein was also detected in the striatum of heterozygous animals having only one null allele for the D1R gene (116 ± 5% of wild-type; n = 15; p < 0.05), whereas Gαs protein levels were unchanged in these animals (103 ± 5% of controls).
Fig. 3.
Levels of G-proteins in the striatum of D1 receptor knock-out mice. A, Western blot analysis of representative striatal homogenates from D1R knock-out (D1R −/−) mice or wild-type controls (D1R +/+), with antibodies that recognize specifically Gαolf, Gαs, Gαi2, and Gβ. B, The optical density of immunoreactive bands was quantified on films and normalized taking as 100% of the mean optical density of controls on each film (see Materials and Methods). Results correspond to the mean ± SEM of data obtained in 6–16 animals studied in three independent experiments. *p < 0.05 and **p< 0.01 by two-tailed Student's t test.C, Northern analysis of RNA isolated from the striata of D1R knock-out (D1R −/−) mice and wild-type control (D1R +/+) mice. In each lane, the amounts of32P-labeled Gαolf, Gαs, and GAPDH probes bound to the membranes were measured with an Instant Imager. The levels of Gαolf and Gαs mRNA were normalized to those of GAPDH mRNA and were expressed as a percentage of mean value measured in wild-type controls (n = 14–16).
We examined Gαolf mRNA levels by Northern blot to determine whether the increase in Gαolfprotein observed in the striatum of mutant mice resulted from an increase in the transcription of the Gαolf gene and/or a stabilization of its transcript (Fig. 3C). At least two species of Gαolf mRNA were detected in the striatum of wild-type and mutant mice resulting from variations in the length of 5′- and 3′-untranslated regions of Gαolf mRNAs, as described previously (Herve et al., 1995). When we measured the total amount of radioactive probe hybridized with all the Gαolf mRNA species in Northern blots, the concentration of Gαolftranscripts was not significantly different in the striatum of D1R −/− mutant mice or wild-type mice (Fig.3C). When radioactivity was measured independently for the two main bands, no significant variation was observed in the striatum of D1R-deficient mice, suggesting that the size pattern of Gαolf mRNA was unaffected in these mice (data not shown). Thus, the increase in Gαolf protein observed in the striatum of D1R −/− mutant mice was not the consequence of alterations in Gαolf mRNA levels. Gαs mRNA concentrations were also not significantly altered in the striatum of D1R knock-out mice (Fig. 3C).
Alterations of striatal Gαolf protein levels in mice lacking dopamine transporter
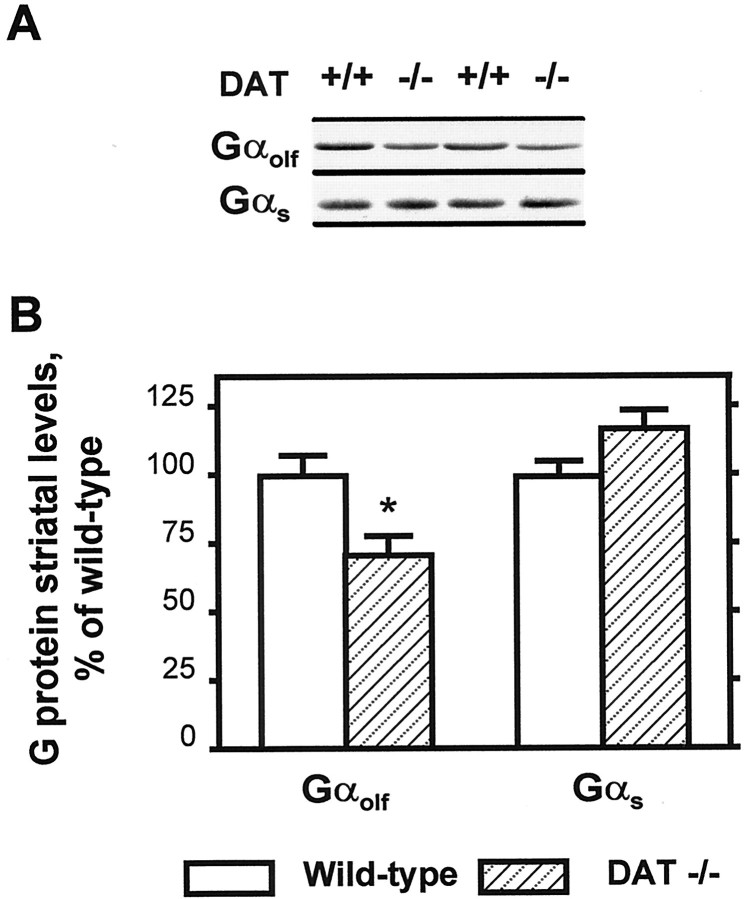
The results obtained in D1R-deficient mice, as well as previous experiments using dopamine-depleted animals, indicated that an impairment in dopamine transmission could increase the levels of Gαolf in the striatum. To test if constitutive upregulation of dopamine neurotransmission could affect Gαolf in the opposite direction, we examined the striatal concentrations of Gαolf in mutant mice lacking DAT, responsible for the largest part of dopamine clearance from the extracellular space (Giros et al., 1996). The levels of Gαolf, but not Gαs, were decreased in the striatum of these mice (Fig.4). Thus, the chronic increase in the stimulation of D1R on the one hand, and the absence of D1R or of dopamine stimulation on the other hand, altered Gαolf protein levels in opposite directions.
Fig. 4.
Levels of Gαolf and Gαs proteins in the striatum of dopamine transporter knock-out mice. A, Western blot analysis of representative striatal homogenates from DAT knock-out mice (DAT−/−) or wild-type littermates (DAT +/+). Blots were incubated with antibodies that recognize specifically Gαolf or Gαs. B, The optical density of immunoreactive bands was quantified on films as in Figure3B (n = 8; *p < 0.02; two-tailed Student's t test).
Alterations of striatal Gαolf protein levels in mice lacking A2A receptors
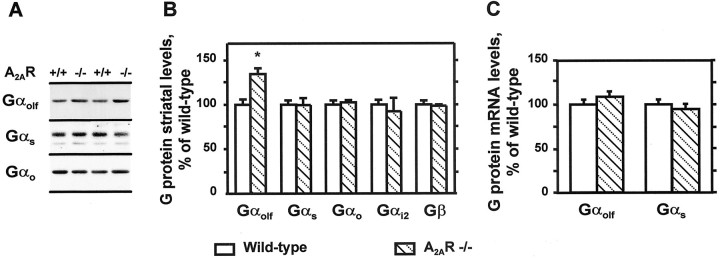
Because Gαolf was expressed in neurons containing A2AR and because the lack of Gαolf abolished the cAMP production induced by an A2 agonist (Corvol et al., 2001), we examined whether the absence of A2AR could alter Gαolflevels in a manner similar to D1R. To evaluate this possibility, we measured Gαolf protein and other G-protein subunits in the striatum of mutant mice lacking A2AR (Ledent et al., 1997). By comparison to wild-type controls, an increase in Gαolfprotein levels was observed in A2AR knock-out mice, whereas Gαs, Gαo, and Gαi2 subunits were not significantly altered in the same striatal samples (Fig.5A,B). In contrast to what was seen in D1R knock-out mice, no change in striatal concentrations of Gβ was detected in A2AR knock-out mice (Fig. 5B). This may be attributable to the fact that the increase in the concentration of Gαolf was less pronounced than in D1R −/− mice, and not sufficient to enhance significantly the total levels of Gβ. As in the case of D1R, the total amounts of Gαolf and Gαs mRNA (Fig. 5C) and the expression pattern of the various forms of Gαolf mRNA (data not shown) were not altered in A2AR knock-out mice.
Fig. 5.
Levels of G-proteins in striatum of A2A receptor knock-out mice. A, Western blot analysis of representative striatal homogenates from A2AR knock-out mice (A2AR −/−) or wild-type controls (A2AR +/+), with antibodies that recognize specifically Gαolf, Gαs, and Gαo. B, The optical density of immunoreactive bands was quantified on films as in Figure3B (n = 4–7; *p< 0.01; two-tailed Student's t test).C, Northern analysis of RNA isolated from the striata of A2AR knock-out (A2AR −/−) mice and wild-type control (A2AR +/+) mice. Results were obtained as described in the legend to C (n = 10–11).
The observed increases in Gαolf protein levels seen in D1R and A2AR knock-out mice could be an unspecific consequence of the absence of any G-protein-coupled receptor. To rule out this possibility, we measured the G-protein subunits levels in knock-out mice lacking CB1 cannabinoid receptors, which are expressed at very high levels in medium-sized spiny neurons, but are negatively coupled to adenylyl cyclase unlike the D1R and A2AR (Ledent et al., 1999). The lack of expression of CB1 receptors in the striatum induced no significant modification in the striatal concentrations of Gαolf (93 ± 5% of wild-type;n = 4). The striatal levels of Gαs, Gαo, Gαi1, Gαi2, and Gβ subunits were also unchanged in these mice (data not shown). It is noteworthy that the deficiency in functional D2R was reported to have no influence on Gαolf mRNA expression in the striatum (Zahniser et al., 2000).
Locomotor responses to amphetamine and caffeine in mice heterozygous for a null mutation of Gαolf
Because alterations of dopamine or adenosine transmission affected Gαolf levels, we investigated whether changes in G-protein levels could have functional consequences on behavioral responses mediated through D1R and A2AR. In the striatum of mice heterozygous for a null mutation of Gαolf gene (Gαolf +/−), the levels of Gαolf were approximately half of those in wild-type mice, and the D1R- and A2AR-stimulated adenylyl cyclase responses were significantly reduced (Corvol et al., 2001). Thus, these mice provided an excellent model to test the effects of reduced amounts of Gαolf, such as those observed in DAT-deficient mice, without the interference of other alterations of dopamine neurotransmission.
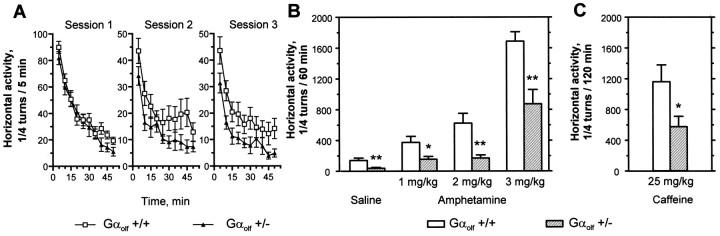
We examined the locomotor activity of Gαolf+/− mice and wild-type littermates in response tod-amphetamine and caffeine, two drugs whose actions depend on D1R and A2AR, respectively (Crawford et al., 1997; Ledent et al., 1997). The locomotor activity of mice was tested in three sessions, in which their basal activity was first monitored (Fig.6A). During the second and third sessions, Gαolf +/− mice had a slightly lower spontaneous activity than wild-type littermates (Fig.6A). The response to drugs was studied during the third session. When Gαolf +/− mice were challenged with 1, 2, or 3 mg/kg of d-amphetamine (Fig. 6B) or with 25 mg/kg of caffeine (Fig.6C), their locomotor activity was stimulated but remained significantly lower than that of wild-type mice receiving the same treatments (Fig. 6B,C). Interestingly, the slight increase in locomotor activity induced by saline injection was also decreased in Gαolf +/− mice (Fig.6B).
Fig. 6.
Effects of amphetamine and caffeine on the locomotor activity of Gαolf +/− mice. The horizontal activity of male heterozygous (Gαolf +/−) and wild-type (Gαolf +/+) mice was measured in three sessions. In each session, animals were allowed to habituate to the testing apparatus for 50 min and received saline injections in the two first sessions and amphetamine (1, 2, or 3 mg/kg) or caffeine (25 mg/kg) in the third session. A, Spontaneous activity of Gαolf+/− and Gαolf +/+ mice during the 50 min habituation period in the three sessions. Activities were significantly lower in mutant mice during sessions 2 and 3 (two-way ANOVA;F(1,260) = 22.3 andF(1,260) = 33.1, respectively;p < 0.001). B, Effects of amphetamine or saline injections on the locomotor activity of Gαolf +/− mice. Data for saline correspond to the results obtained in the second session. C, Effects of caffeine on the locomotor activity of Gαolf +/− mice. The results are the mean ± SEM of data obtained with 7–14 animals. *p < 0.05 and **p < 0.01, significantly different from wild-type controls with the same treatment.
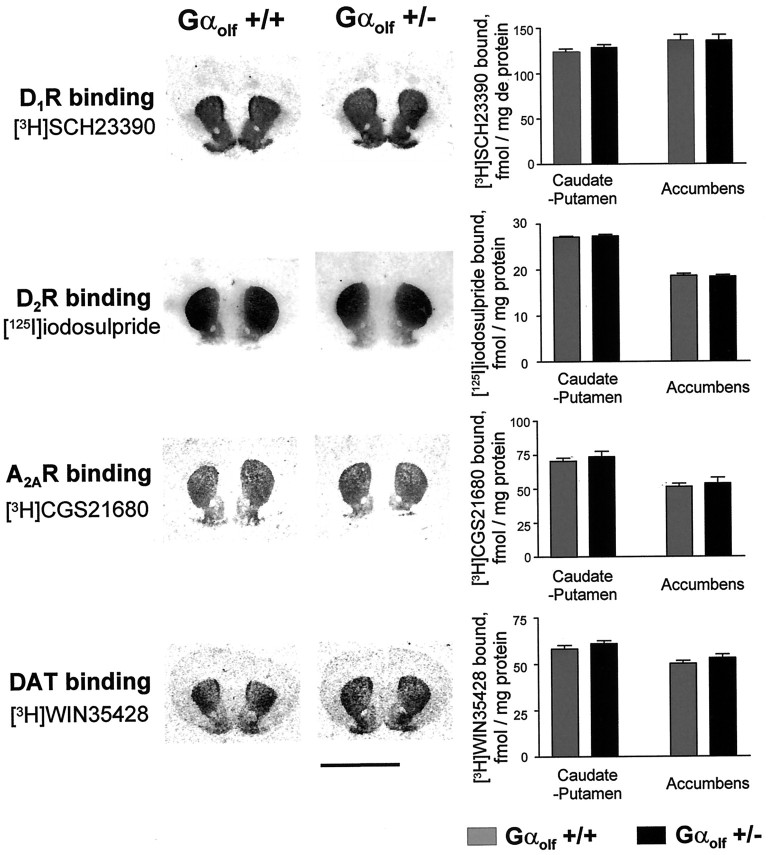
In Gαolf +/− mice, the alterations in responses to amphetamine and caffeine were not caused by changes in D1R, D2R, or A2AR levels in the caudate putamen or nucleus accumbens because the binding of (3H)SCH23390, (125I)iodosulpride, and (3H)CGS21680 was unchanged in these brain regions (Fig. 7). The measurements of the (3H)WIN35428 binding in the caudate putamen and nucleus accumbens of Gαolf +/− mice (Fig. 7) indicated also a normal density of DAT, which is known to be crucial for the psychostimulant effect of d-amphetamine in mouse (Giros et al., 1996).
Fig. 7.
Lack of change in D1,D2, and A2A receptors and in dopamine transporter in the caudate putamen and nucleus accumbens of heterozygous Gαolf knock-out mice. Coronal brain sections at the level of the striatum were incubated with (3H)SCH23390, (125I)iodosulpride, (3H)CGS21680, or (3H)WIN35428 to analyze the D1R, D2R, A2AR, and DAT, respectively. The optical densities in the caudate putamen and the nucleus accumbens were measured in at least six different sections in each mouse and compared with standards. The results correspond to the mean ± SEM of data obtained in three mutant mice and three wild-type littermates. Scale bar, 5 mm.
DISCUSSION
Neuronal expression of Gαolf and Gαs in the striatum
Receptor-regulated production of cAMP in the striatum plays a central role in the physiology of the basal ganglia as well as in the long-term modifications that take place after repeated administration of drugs of abuse (for review, see Greengard et al., 1999; Berke and Hyman, 2000). The striatum contains high levels of Gαolf and very low levels of Gαs (Drinnan et al., 1991; Herve et al., 1993). Recent studies have demonstrated the role of Gαolf in receptor-regulated cAMP production of the striatum and its functional consequences (Zhuang et al., 2000;Corvol et al., 2001). We confirm here that most medium-sized neurons, which correspond to GABAergic output spiny neurons and represent the vast majority of striatal neurons (Kemp and Powell, 1971), express Gαolf and very little or no Gαs transcripts (Kull et al., 2000). In the rat dorsal striatum these output neurons are divided in two populations identified on the basis of the neuropeptides they express (Gerfen and Young, 1988). We found that GαolfmRNA was expressed in both types of neurons, which did not contain significant amounts of Gαs mRNA. Moreover, D1R-positive neurons expressed Gαolf transcripts, but no detectable Gαs. The rare medium-sized neurons containing Gαs mRNA also expressed Gαolf in double-labeling experiments. Because substance P- or preproenkephalin A-positive neurons did not express Gαs, the medium-sized neurons containing both Gαs and Gαolf mRNA are likely to be aspiny interneurons (Kawaguchi et al., 1995). Interestingly, we show here that large-sized cholinergic interneurons, identified by the presence of choline acetyltransferase mRNAs, expressed both Gαolf and Gαs mRNAs. Striatal cholinergic neurons contain no A2AR (Svenningsson et al., 1997), but a few express low amounts of D1R (Le Moine et al., 1991; Surmeier et al., 1996). Recent experiments using in situ hybridization have also confirmed the presence of D5 receptor mRNA in these neurons (C. Le Moine, unpublished data), as previously indicated by single-cell RT-PCR experiments (Yan and Surmeier, 1997) and an immunohistochemical study in primate (Bergson et al., 1995). Our results indicate that both Gαolf and Gαs may couple D1R and D5 receptors to adenylyl cyclase in striatal cholinergic neurons. Thus, it appears that virtually all the striatal output neurons express only Gαolf, whereas interneurons express both Gαolf and Gαs. The presence of Gαolf transcripts, but not of Gαs, in D1R- and A2AR-rich output neurons, accounts for the fact that cAMP formation by stimulation of these receptors was virtually abolished in Gαolf mutant mice (Corvol et al., 2001).
Regulation of Gαolf levels in mice with genetically altered D1 or A2A signaling
In a previous study we have shown that the disappearance of striatal dopamine increased Gαolf levels by 40–50% (Herve et al., 1993; Penit-Soria et al., 1997). In mutant mice lacking either D1R or A2AR we found a selective increase in Gαolf protein. Together these observations reveal that a lack of stimulation of D1R, as well as the absence of either D1R or A2AR, result in an upregulation of Gαolf levels, suggesting that receptor usage may control Gαolf levels. This hypothesis is corroborated by the study of DAT −/− mice, which provide a model of chronic hyperstimulation of dopamine receptorsin vivo (Giros et al., 1996). In these mice the levels of Gαolf protein were decreased in the striatum. These variations are reminiscent, at the level of a G-protein, of the classical “denervation hypersensitivity” and “agonist-induced desensitization,” well characterized at the level of receptors (Freedman and Lefkowitz, 1996; Bloch et al., 1999).
Our results show that in A2AR and D1R mutant mice, the changes in Gαolf occur at a post-transcriptional level and do not result from a change in gene expression. Preliminary evidence suggests that regulation of Gαolf levels may also be independent of cAMP-regulated protein phosphorylation pathways, because these levels were unchanged in mice lacking functional DARPP-32 (D. Herve, A. Fienberg, and J. A. Girault, unpublished observations), a key mediator in striatal cAMP-dependent protein phosphorylation (Fienberg et al., 1998). A more attractive possibility is that alterations in Gαolf protein levels result directly from changes in its rate of activation. This hypothesis is supported by several cell culture studies on Gαs. In NG108–15 cells, stimulation of receptors activating adenylyl cyclase induced a downregulation of Gαs at a post-translational level, which was independent of cAMP production (McKenzie and Milligan, 1990; Adie and Milligan, 1994). Similarly, strong activation of Gαs by cholera toxin or by an activating mutation dramatically decreased its cellular concentration, possibly by an increased degradation (Levis and Bourne, 1992; Milligan, 1993). We suggest that analogous changes in Gαolfdegradation rate may account for the changes in its levels observed here in vivo. Normal stimulation of D1R and A2AR by endogenous dopamine and adenosine would result in a basal rate of Gαolf degradation, which is reduced in mice lacking dopamine or functional D1R or A2AR. Conversely, in mice lacking DAT, overstimulation of D1R would increase Gαolf rate of degradation. According to this hypothesis, the changes in Gαolf concentration are expected to occur only in the striatal cells affected by the mutation, i.e., striatonigral cells in mice lacking functional D1R or DAT or striatopallidal cells in mice lacking functional A2AR.
Role of Gαolf in locomotor activity
We have recently shown that reduced levels of Gαolf in heterozygous mutant mice (Gαolf +/−) resulted in a reduced stimulation of adenylyl cyclase by D1R and A2AR in the striatum (Corvol et al., 2001). The present study demonstrates that reduced availability of Gαolf in these mice has also important functional consequences on spontaneous and drug-stimulated locomotor activity, revealing a novel phenotype of haplo-insufficiency.
The ventral striatum, including the nucleus accumbens, is involved in spontaneous and drug-stimulated motor activity (Pennartz et al., 1994). A role for D1R in the control of locomotor activity is supported by several studies. Microinjection of D1R antagonist in the ventral striatum of rats reduces their locomotor activity, whereas D1R agonists have the opposite effect (Meyer, 1993). Accordingly, the decreased coupling of D1R to adenylyl cyclase in the ventral striatum of Gαolf +/− mice could account for their slightly lower locomotor activity. By contrast it should be noted that an increase in basal activity has been reported in Gαolf −/− mice (Belluscio et al., 1998;Zhuang et al., 2000) and D1R −/− mice (Xu et al., 1994). These surprising observations contrasted with the pharmacological evidence for a stimulatory role on motor activity of D1R in the nucleus accumbens (Meyer, 1993; Vezina et al., 1994). These paradoxical behavioral effects in Gαolf −/− and D1R −/− mice could be caused by differences in the methods used for measuring locomotor activity or alternatively may be the consequences of developmental changes and/or compensatory actions of other neuromodulatory systems such as serotoninergic or noradrenergic systems (Tassin et al., 1992; Trovero et al., 1992; Gainetdinov et al., 1999). Nevertheless, in both Gαolf−/− and D1R −/− mice, the locomotor responses to cocaine or amphetamine are lost, unambiguously showing that both Gαolf and D1R are necessary for the locomotor responses induced by these psychostimulants (Xu et al., 1994; Crawford et al., 1997; Zhuang et al., 2000). Interestingly, the acute effects of d-amphetamine on motor activity diminished in Gαolf +/− mice, further demonstrating that Gαolf levels determine the amplitude of D1R-mediated responses.
In Gαolf +/− mice, the effects of caffeine on motor activity were reduced, indicating that Gαolf levels are an important factor for caffeine-induced behavioral response. The psychostimulant effects of caffeine are mediated through A2AR and are absent in mutant mice lacking these receptors (El Yacoubi et al., 2000). Caffeine is a nonselective antagonist of A2AR, and caffeine could have a reduced effect in Gαolf +/− mice because the ongoing A2AR signaling, which is produced by endogenous adenosine, is blunted in these mice. This explanation is likely because endogenous levels of adenosine are sufficient to cause the activation of adenosine receptors in brain (for review, see Svenningsson et al., 1999). However, D1R activity is required for caffeine-induced motor effect (Garrett and Holtzman, 1994), and the lower D1R signaling in Gαolf +/− mice could also contribute to the attenuation of caffeine effects. Further experiments are needed to explore the respective contribution of these two mechanisms, both leading to a diminished effect of caffeine.
Functional implications
The present study demonstrates that experimental alterations in Gαolf concentrations affect two important types of neurotransmission in basal ganglia and provides strong evidence that Gαolf levels in striatal neurons are regulatedin vivo by its use. Whereas much effort has been devoted to understand desensitization and downregulation of G-protein-coupled receptors in vitro and in vivo (Freedman and Lefkowitz, 1996; Bloch et al., 1999), little is known about the regulations that take place at the level of G-proteins. Our previous results in 6-hydroxydopamine-lesioned rats, as well as the present results in mice with genetically altered neurotransmission, show that a loss of D1R activation or the absence of D1R or A2AR result in an upregulation of Gαolf. Thus, Gαolf constitutes an attractive locus for the regulation of D1R and A2AR signaling in the striatum, either by modulation of its levels, or, possibly, by functionally relevant post-translational modifications. Alterations in Gαolf levels and/or activity are parameters that will have to be carefully considered in the function of the basal ganglia in physiological and pathological conditions. In this respect, our results introduce Gαolfheterozygous mutant mice as a potential animal model for studying striatum-dependent behavioral dysfunctions.
Footnotes
This work has been supported by Institut National de la Santé et de la Recherche Médicale, by a grant from Mission Interministérielle de lutte contre la Drogue et la Toxicomanie addiction program, and by Grant 97H0003 from Ministère de Education Nationale de l'Enseignement Supérieur et de la Recherche. We thank F. Laujay for his help with in situ hybridization experiments, R. Axel, J. Drago, M. Parmentier, B. Giros, and M. Caron for kindly allowing the use of Gαolf, D1R, A2AR, and DAT mutant mice, respectively, F. Gonon and E. Borrelli for providing us with some mutant mice, P. Ingrassia, R. Urbe, and H. Ibrahim for their help with animal breeding, and C. Drouin and J. P. Tassin for their advice about the measure of locomotor activity. We thank J. Glowinski and his colleagues of INSERM U114 for their support.
Correspondence should be addressed to D. Hervé, INSERM U536, Collège de France, 11, place Marcelin Berthelot, 75231 Paris, France. E-mail: denis.herve@infobiogen.fr.
REFERENCES
- 1.Adie EJ, Milligan G. Agonist regulation of cellular Gs alpha-subunit levels in neuroblastoma x glioma hybrid NG108–15 cells transfected to express different levels of the human beta 2 adrenoceptor. Biochem J. 1994;300:709–715. doi: 10.1042/bj3000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckstead RM, Cruz CJ. Striatal axons to the globus pallidus, entopeduncular nucleus and substantia nigra come mainly from separate cell populations in cat. Neuroscience. 1986;19:147–158. doi: 10.1016/0306-4522(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 3.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 4.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 6.Bloch B, Dumartin B, Bernard V. In vivo regulation of intraneuronal trafficking of G-protein-coupled receptors for neurotransmitters. Trends Pharmacol Sci. 1999;20:315–319. doi: 10.1016/s0165-6147(99)01360-7. [DOI] [PubMed] [Google Scholar]
- 7.Bonner TI, Affolter HU, Young AC, Young WS. A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Brain Res. 1987;388:243–249. doi: 10.1016/0169-328x(87)90031-3. [DOI] [PubMed] [Google Scholar]
- 8.Bouthenet ML, Martres MP, Sales N, Schwartz JC. A detailed mapping of dopamine D2 receptors in rat central nervous system by autoradiography with (125I)iodosulpride. Neuroscience. 1987;20:117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Corvol J-C, Studler J-M, Schonn J-S, Girault J-A, Hervé D. Gαolf is necessary for coupling D1 and A2 receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 11.Crawford CA, Drago J, Watson JB, Levine MS. Effect of repeated amphetamine treatment on the locomotor activity of dopamine D1a-deficient mouse. NeuroReport. 1997;8:2523–2527. doi: 10.1097/00001756-199707280-00021. [DOI] [PubMed] [Google Scholar]
- 12.Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, SP H, Bartlett PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drinnan SL, Hope BP, Snutch TP, Vincent SR. Golf in the basal ganglia. Mol Cell Neurosci. 1991;2:66–70. doi: 10.1016/1044-7431(91)90040-u. [DOI] [PubMed] [Google Scholar]
- 14.El Yacoubi M, Ledent C, Ménard J-F, Parmentier M, Costentin J, Vaugeois J-M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson PF, Minier LN, Lasher RS. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamine gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982;51:241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- 16.Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 17.Fort P, Marty L, Piechaczyk M, El Sabrouty S, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman NJ, Lefkowitz RJ. Desensitization of G-protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. [PubMed] [Google Scholar]
- 19.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 20.Garrett BE, Holtzman SG. D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav. 1994;47:89–94. doi: 10.1016/0091-3057(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 21.Gerfen CR, Young WS. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- 22.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR., Jr D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 23.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 24.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 25.Herbert MA, Larson GA, Zahniser NR, Gerhardt GA. Age-related reductions in (3H) WIN35428 binding to the dopamine transporter in nigrostriatal and mesolimbic brain regions of the Fisher 344 rat. J Pharmacol Exp Ther. 1999;288:1334–1339. [PubMed] [Google Scholar]
- 26.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herve D, Rogard M, Levi-Strauss M. Molecular analysis of the multiple Golf alpha subunit mRNAs in the rat brain. Brain Res Mol Brain Res. 1995;32:125–134. doi: 10.1016/0169-328x(95)00070-9. [DOI] [PubMed] [Google Scholar]
- 29.Ibanez CF, Ernfors P, Persson H. Developmental and regional expression of choline acetyltransferase mRNA in rat central nervous system. J Neurosci Res. 1991;29:163–171. doi: 10.1002/jnr.490290205. [DOI] [PubMed] [Google Scholar]
- 30.Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- 32.Jones DT, Reed RR. Golf: an olfactory neuron specific-G-protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 34.Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- 35.Kull B, Svenningsson P, Fredholm BB. Adenosine A2A receptors are colocalized with and activate Golf in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 36.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzi T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Agressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 37.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knock-out mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 38.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 39.Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci USA. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levis MJ, Bourne HR. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcotte ER, Sullivan RM, Mishra RK. Striatal G-proteins: Effects of unilateral 6-hydroxydopamine lesions. Neurosci Lett. 1994;169:195–198. doi: 10.1016/0304-3940(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie FR, Milligan G. Prostaglandin E1-mediated, cyclic AMP-independent, down-regulation of Gs alpha in neuroblastoma x glioma hybrid cells. J Biol Chem. 1990;265:17084–17093. [PubMed] [Google Scholar]
- 44.Meyer ME. Effects of intraaccumbens dopamine agonist SK&F38393 and antagonist SCH23390 on locomotor activities in rats. Pharmacol Biochem Behav. 1993;45:843–847. doi: 10.1016/0091-3057(93)90130-l. [DOI] [PubMed] [Google Scholar]
- 45.Milligan G. Agonist regulation of cellular G-protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci. 1993;14:413–418. doi: 10.1016/0165-6147(93)90064-Q. [DOI] [PubMed] [Google Scholar]
- 46.Monsma FJ, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci USA. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penit-Soria J, Durand C, Besson MJ, Hervé D. Levels of stimulatory G-protein are increased in the rat striatum after neonatal lesion of dopamine neurons. NeuroReport. 1997;8:829–833. doi: 10.1097/00001756-199703030-00005. [DOI] [PubMed] [Google Scholar]
- 48.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 49.Premont J, Perez M, Bockaert J. Adenosine-sensitive adenylate cyclase in rat striatal homogenates and its relationship to dopamine- and Ca2+-sensitive adenylate cyclases. Mol Pharmacol. 1977;13:662–670. [PubMed] [Google Scholar]
- 50.Savasta M, Dubois A, Benavides J, Scatton B. Different plasticity changes in D1 and D2 receptors in rat striatal subregions following impairment of dopaminergic transmission. Neurosci Lett. 1988;85:119–124. doi: 10.1016/0304-3940(88)90440-5. [DOI] [PubMed] [Google Scholar]
- 51.Schiffmann SN, Jacobs O, Vanderhaeghen J-J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- 52.Sibley DR, Monsma FJ. Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan KA, Liao YC, Alborzi A, Beiderman B, Chang FH, Masters SB, Levinson AD, Bourne HR. Inhibitory and stimulatory G-protein of adenylate cyclase: cDNA and amino acid sequences of the α chain. Proc Natl Acad Sci USA. 1986;83:6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 56.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Biochemistry, distribution and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 57.Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- 58.Tassin JP, Trovero F, Herve D, Blanc G, Glowinski J. Biochemical and behavioural consequences of interactions between dopaminergic and noradrenergic systems in rat prefrontal cortex. Neurochem Int [Suppl] 1992;20:225S–230S. doi: 10.1016/0197-0186(92)90243-k. [DOI] [PubMed] [Google Scholar]
- 59.Trovero F, Blanc G, Herve D, Vezina P, Glowinski J, Tassin JP. Contribution of an alpha 1-adrenergic receptor subtype to the expression of the “ventral tegmental area syndrome.”. Neuroscience. 1992;47:69–76. doi: 10.1016/0306-4522(92)90121-h. [DOI] [PubMed] [Google Scholar]
- 60.Vezina P, Blanc G, Glowinski J, Tassin JP. Blockade of D-1 dopamine receptors in the medial prefrontal cortex produces delayed effects on pre- and postsynaptic indices of dopamine function in the nucleus accumbens. Synapse. 1994;16:104–112. doi: 10.1002/syn.890160204. [DOI] [PubMed] [Google Scholar]
- 61.Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 62.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 63.Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- 64.Yung KK, Smith AD, Levey AI, Bolam JP. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. Eur J Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 65.Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, Larson GA, Kelly MA, Grandy DK, Rubinstein M, Low MJ, Fredholm BB. Functional uncoupling of adenosine A2A receptors and reduced response to caffeine in mice lacking dopamine D2 receptors. J Neurosci. 2000;20:5949–5957. doi: 10.1523/JNEUROSCI.20-16-05949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhuang X, Belluscio L, Hen R. Golfα mediates dopamine D1 receptor signaling. J Neurosci 20 2000. RC91:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]