Abstract
Using whole-cell patch-clamp recording and intracellular Ca2+ imaging of rat cultured DRG neurons, we studied the cross talk between GABAA and P2X receptors. A rapidly fading current was the main response to ATP, whereas GABA elicited slowly desensitizing inward currents. Coapplication of these agonists produced a total current much smaller than the linear summation of individual responses (68 ± 5% with 10 μm ATP plus 100 μm GABA). Occlusion was observed regardless of ATP response type. Neurons without functional P2X receptors manifested no effect of ATP on GABA currents (and vice versa). Occlusion was also absent in the presence of the P2X blocker trinitrophenyl-ATP (TNP-ATP) or of the GABA blocker picrotoxin, indicating a lack of involvement by metabotropic ATP or GABA receptors. Less occlusion was obtained when ATP was applied 2 sec after GABA than when GABA was applied after ATP. Changing the polarity of GABA currents by using intracellular SO42− instead of Cl− significantly reduced the occlusion of ATP currents by GABA, suggesting an important role for Cl− efflux in this phenomenon. Occlusion was enhanced whenever intracellular Ca2+([Ca2+]i) was not buffered, indicating the cross talk-facilitating role of this divalent cation. Ca2+ imaging showed that ATP (but not GABA) increased [Ca2+]i in voltage-clamped or intact neurons. Our data demonstrated a novel Cl− and Ca2+-dependent interaction between cationic P2X and anionic GABAAreceptors of DRG neurons. Such negative cross talk might represent a model for a new mechanism to inhibit afferent excitation to the spinal cord as GABA and ATP are coreleased within the dorsal horn.
Keywords: ATP, GABA, occlusion, calcium imaging, chloride channels, DRG
Although the activation of neurotransmitter receptors by their own transmitter has been thought to be a discrete, independent process, this phenomenon recently has been shown to be modulated by distinct receptors when they are activated simultaneously by their own transmitter. This mechanism, generally termed cross talk, provides a fast and efficient way to adapt transmitter signaling to changing functional needs. Cross talk between ATP P2X receptors and acetylcholine (ACh) nicotinic receptors is manifested as occlusion whereby the single-cell response to the combined application of the two agonists is much less than the sum of the two individual responses (Nakazawa, 1994; Barajas-Lopez et al., 1998; Searl et al., 1998; Zhou and Galligan, 1998; Khakh et al., 2000). ATP/ACh cross talk is interesting because ATP can be co-released with ACh from the same cholinergic terminals (Silinsky and Redman, 1996). The molecular mechanisms responsible for such interactions are unknown. More recent studies have demonstrated that receptor cross talk between dopamine and adenosine receptors (Gines et al., 2000) or GABAA and dopamine receptors (Liu et al., 2000) involves direct protein–protein intramembrane interaction.
In the spinal cord ATP is coreleased together with GABA (Jo and Schlichter, 1999; Hugel and Schlichter, 2000). The ionotropic receptors activated by ATP (P2X receptors) or by GABA (GABAA receptors) have distinct structures (Nicke et al., 1998; Torres et al., 1999) and gate either nonselective cationic channels permeable to Na+, K+, and Ca2+(P2X receptors) or anionic Cl−-permeable channels (GABAA receptors). Although GABA is believed to be the main transmitter for presynaptic inhibition in the spinal cord (Sivilotti and Nistri, 1991; Kaila, 1994), ATP induces glutamate release (Gu and MacDermott, 1997; Li et al., 1998) via the activation of ionotropic P2X receptors of spinal neurons (Bardoni et al., 1997; Jo and Schlichter, 1999) and thus would contrast the inhibitory action of GABA.
Several subtypes of ATP receptors have been found in sensory dorsal root ganglion (DRG) cells (Chen et al., 1995; Collo et al., 1996;Vulchanova et al., 1998), which express at least three subunits (P2X1, P2X2, P2X3) assembled into the corresponding homomeric receptors or in the heteromeric P2X2/3 receptors (Lewis et al., 1995; Burgard et al., 1999; Grubb and Evans, 1999). In DRG neurons of small and medium size ATP evokes kinetically distinct membrane currents depending on the activated receptor type (Burgard et al., 1999; Grubb and Evans, 1999; Labrakakis et al., 2000). GABA receptors found on DRG neurons mainly belong to the ionotropic GABAA and metabotropic GABAB subclasses (Sivilotti and Nistri, 1991).
Despite strong evidence suggesting a functional role for ATP and GABA in spinal neurotransmission, there is no demonstration that they can cross talk at membrane level. Because cultured DRG cells that project primary afferents to the spinal cord are useful models to study the neuronal action of ATP or GABA, the major aim of the present study was investigating any functional interaction between ATP-activated ionotropic P2X receptors and ionotropic GABAAreceptors on DRG neurons. We report for the first time negative cross talk between cationic (P2X) and anionic (GABAA) ionotropic receptors.
MATERIALS AND METHODS
Cell preparation. Rat DRG neurons were cultured according to the method of Hu and Li (1997) with modifications. Ether-anesthetized rats (2–3 weeks old) of both sexes were decapitated, a procedure (including animal handling and care) in accordance with the Animal Welfare Act and approved by the Local Authority Veterinary Service. The thoracic and lumbar ganglia were excised and placed into DMEM containing penicillin and streptomycin. After removing roots and surrounding connective tissue, we minced and incubated the DRGs with trypsin (0.5 mg/ml), collagenase (1.0 mg/ml), and DNase (0.1 mg/ml) in 5 ml of DMEM at 35°C in a shaking bath for 35–40 min. After enzymatic treatment the cells were drawn gently up and down a 2 ml plastic pipette. Enzymatic digestion was stopped by adding 10% fetal calf serum (FCS). Cells were centrifuged at 250–300 × g at room temperature for 5 min, and the supernatant was removed. Cells were resuspended with an adequate amount of DMEM (plus 10% FCS). Finally, DRG neurons were plated on poly-l-lysine-coated (5 mg/ml) Petri dishes and cultured for 1–2 d under an atmosphere containing 5% CO2. Cells were used within 2 d of plating when the neurons were devoid of neurite growth.
Patch-clamp recording. Cells were superfused continuously (3–5 ml/min) with physiological solution containing (in mm) 152 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH-adjusted to 7.4 with NaOH; osmolarity was adjusted to 300 mOsm with glucose. The Ca2+-free solution contained 2 mm Mg2+ instead of Ca2+ and 5 mm EGTA. Experiments in extracellular low Cl−solution were performed by equimolar replacement (50%) of NaCl with Na2SO4. To aid Na2SO4 solubility, we warmed up and continuously stirred the stock solution. The extracellular low Cl− solution was checked for osmolarity and gave a junction potential of ∼2 mV. Patch pipettes were pulled from thin glass capillary tubes and had resistances of ∼3–5 MΩ when filled (in mm) with 120 CsCl, 20 HEPES, 1 MgCl2, 3 Mg2ATP3, and 5 EGTA, pH-adjusted to 7.2 with CsOH. Osmolarity of the pipette solution was 270 mOsm. In some experiments BAPTA (10 mm) or fluo-4 (25 μm) was added instead of EGTA, or exogenous buffers were omitted altogether. For experiments to change the GABA reversal potential, pipette CsCl was substituted with 75 mmCs2SO4 (Hugel and Schlichter, 2000). Junction potential was −24.5 mV and corrected accordingly.
Whole-cell currents were recorded from cells of 20–35 μm in diameter, thus considered to be small and medium-sized neurons (Burgard et al., 1999; Grubb and Evans, 1999; Ueno et al., 1999). In most cells series resistance was compensated by 80%. Cells were voltage clamped at −70 mV (unless otherwise indicated). After whole-cell configuration was obtained, an equilibration period of 5 min was used for establishing full solution exchange between the patch pipette and the cell. Currents were filtered at 1 kHz and acquired on an IBM personal computer by means of pClamp 6.0 software (Axon Instruments, Foster City, CA).
Drug delivery. Agonists or antagonists of P2X and GABAA receptors were diluted with extracellular solution to final concentration and applied by a rapid superfusion system (Rapid Solution Changer RSC-200, BioLogic Science Instruments, Grenoble, France) placed 100–150 μm near the cell. Time for the solution exchange across the cell (estimated by the change in liquid junction potential) was 30 msec. Agonist applications were 2 sec in duration and typically were spaced every 5–6 min to avoid desensitization of the ATP receptors. Antagonists were applied via the bathing solution as well as via the agonist-superfusing flow line.
With the exception of trinitrophenyl-ATP (TNP-ATP, Molecular Probes, Eugene, OR), all drugs including enzymes for cell culture were obtained from Sigma (Milan, Italy).
Confocal microscopy imaging of [Ca2+]i. For confocal microscopy imaging in the visible light range we used two Ca2+-sensitive dyes. For voltage-clamp experiments when [Ca2+]i was measured together with membrane currents, we applied 25 μm fluo-4 (cell-impermeable form, pentapotassium salt; Molecular Probes) via the patch pipette. For experiments on intact cells, these were loaded with 5 μm fluo-3 (cell-permeable form, AM ester compound; Molecular Probes) by preincubation for 45 min (Khiroug et al., 1998). Fluo-4 is similar in structure and spectral properties to fluo-3, but it has a greater absorption near 488 nm, brighter fluorescence emission, and larger dynamic range for [Ca2+]i near the dye Kd for Ca2+ (345 nm; seeGee et al., 2000). In these experiments EGTA was omitted from the patch pipette. Fluorescence emission of Ca2+-sensitive dyes was excited by an argon–krypton laser (488 nm) and detected with the photomultiplier tube of a MultiProbe 2001 confocal-scanning microscope (Molecular Dynamics, Sunnyvale, CA) that used a combination of 510 nm high-pass and 530 nm bandpass filters. Fluorescence signals were digitized with the 16-line rapid scan mode (temporal resolution, 160 msec per scan; pixel size, 0.6 μm; confocal aperture, 200 μm). [Ca2+]i transients were analyzed in terms of fractional amplitude increase (ΔF/F0, whereF0 is the baseline fluorescence level and ΔF is the rise over baseline).
Data analysis. All data are presented as the mean ± SEM (n = number of cells), with statistical significance assessed by paired Student's t test (for parametric data) or the Mann–Whitney rank sum test (for nonparametric data). A p value of <0.05 was accepted as indicative of statistically significant difference. Concentration–response curves for ATP or GABA were fit with the logistic equation (Origin 6.0, Microcal, Northampton, MA).
RESULTS
Currents mediated by ATP or GABA
We first examined whether under our experimental conditions we could observe responses mediated by ATP or GABA in accordance with the pharmacological profiles of receptors described for these transmitters on DRG cells. On neurons held at −70 mV, ATP (0.1–100 μm range; applied for 2 sec via a fast perfusion system) elicited inward currents on 184 (90%) of 204 cells. The response pattern to ATP was heterogeneous because three types of response could be identified, as shown by the examples of cells (depicted in Fig.1Aa–Ac) exposed to paired pulses of 10 μm ATP (20 sec interval between applications).
Fig. 1.
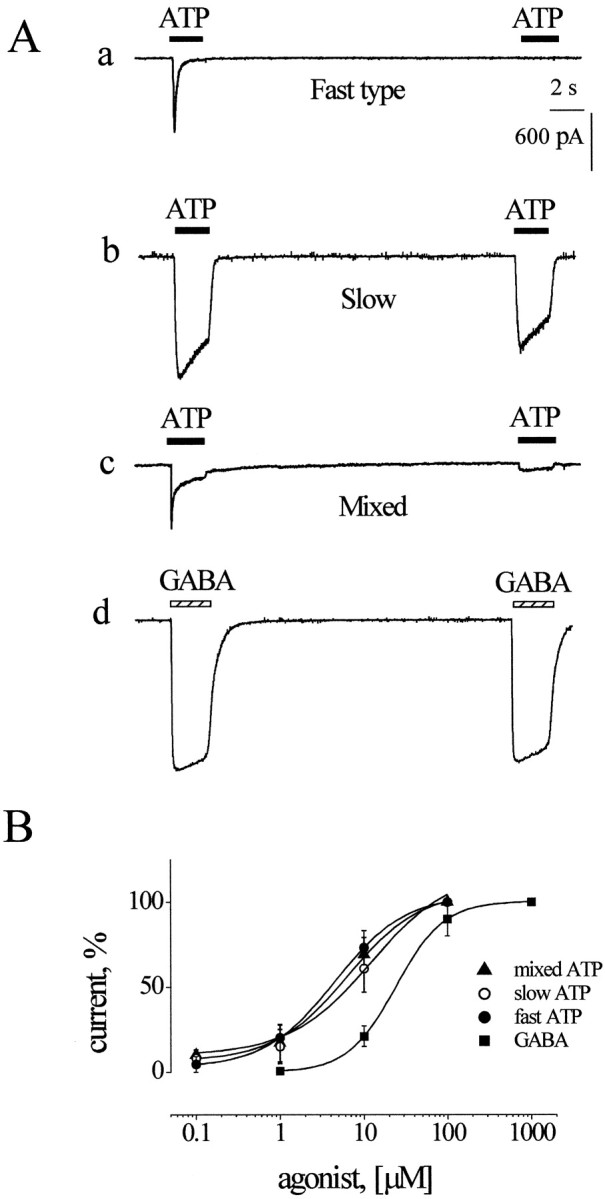
Characteristics of inward currents generated by ATP or GABA on DRG neurons. Aa, Example of fast-type ATP current with strong desensitization (note complete current fade during agonist application), which prevents current generation 20 sec later by the same application of ATP. Ab, Example of slow ATP currents with minimal desensitization. Ac, Example of mixed-type ATP response whereby current fades to plateau during agonist application; plateau response can be reproduced 20 sec later.Ad, Example of GABA-induced inward currents with small desensitization. B, Mean log concentration–response curves for ATP or GABA. Data are given as a percentage of the maximum response (ATP, n = 3–6 cells; GABA,n = 5 cells). Curves are fitted with the logistic equation.
The first type of current (exemplified in Fig. 1Aa), termed “fast-type” response in accordance with the classification by Burgard et al. (1999), peaked at −770 pA and, because of receptor desensitization, rapidly decayed back to baseline (residual current was 1% of the peak one) despite the continuous presence of ATP. Further application of the same pulse of ATP yielded virtually no response 20 sec later, indicating a very slow rate of recovery from the desensitized state. Full recovery was observed after at least a 4 min washout (data not shown). Thus in all subsequent experiments to avoid cumulative desensitization, we set the rate of ATP application for once every 5–6 min. In a random sample of 15 neurons displaying fast-type responses, the current activated by 10 μm ATP decayed with 62 ± 9 msec τfast and 622 ± 143 msec τslow. Because the ratio between the fast/slow current components was 5.4 ± 1.5, the slow component made a rather small contribution to the overall ATP current.
A different type of current evoked by the same concentration of ATP (similar to the “slow type” reported by Burgard et al., 1999) is exemplified in Figure 1Ab. The peak amplitude of the first ATP current (paired-pulse protocol) was −1478 pA, from which it faded quite slowly (τ = 3207 msec). The second pulse (applied 20 sec later) elicited an inward current only slightly smaller (−1121 pA) than the first one. In a sample of seven cells the average desensitization rate of the slow-type current was fitted by a single exponential (τ = 2756 ± 629 msec).
A third “mixed-type” current induced by 10 μm ATP is exemplified in Figure 1Ac, where it peaked at −602 pA and decayed biphasically (τfast = 41 and τslow = 500 msec) to a shallow plateau. In this case the relative amplitude of the plateau current was clearly larger than the one observed in Figure 1Aa, because the ratio between fast response peak and slow component amplitude was now 1.2. Note also that paired-pulse application revealed only the slow component 20 sec later (Fig. 1Ac). In five cells the values for τfast and τslow were 52 ± 12 and 500 ± 116 msec, respectively. The ratio of amplitudes of fast/slow components was 2.5 ± 0.2. Thus the principal difference between fast and mixed response types was not their time course but the amplitude of the residual, slow current at the end of agonist application (see alsoBurgard et al., 1999).
On average, 61% of the cells displayed fast currents, whereas the proportion of cells showing slow or mixed currents was 18 and 21%, respectively. Changing ATP concentrations applied to the same cell did not convert one response type into another one, indicating that the response pattern was cell-specific. Furthermore, fast and mixed responses usually were observed in cells with somatic diameters of 20–30 μm, whereas slow responses were typical of larger (30–35 μm) neurons.
In contrast to the heterogeneous ATP responses, the current responses elicited by GABA were stereotypic. Figure 1Adpresents an example of typical GABA currents (paired 2 sec pulses; 100 μm GABA). The first current peaked at −1455 pA, whereas the second one, generated 20 sec later, was depressed only slightly (Fig. 1Ad). Only 1% of DRG neurons in our study did not respond to GABA. Figure 1B shows the log dose–response curves for peak inward currents evoked by ATP (fast, slow, or mixed-type responses) or GABA. EC50values for ATP were 4.4 μm (fast response;n = 6 cells), 11.2 μm (slow response; n = 5 cells), or 6.1 μm (mixed response; n = 3 cells), whereas for GABA the EC50 value was 24 μm (n = 5 cells).
Thus the characteristics of ATP or GABA responses observed in the present study fully accord with those described in similar cells (Hu and Li, 1997; Burgard et al., 1999; Grubb and Evans, 1999; Piper and Docherty, 2000).
Occlusion of currents with the coapplication of ATP and GABA
Because ATP and GABA are thought to be coreleased (Jo and Schlichter, 1999; Hugel and Schlichter, 2000), we studied whether the simultaneous presence of these two transmitters at membrane level might induce cross talk between their different receptor systems. For this reason we studied responses of individual DRG neurons to a separate or combined application of ATP and GABA. Figure2A presents examples of membrane inward currents induced by 10 μm ATP or 100 μm GABA (left panel) or by the coapplication of these two agonists (via the same tube of fast perfusion system; right panel). To assess any interaction between the ATP and GABA receptor systems, we compared the total current predicted by a simple summation of the two individual currents with the experimentally observed current evoked by coapplied ATP and GABA. Figure 2A, right, shows that the observed current evoked by ATP plus GABA was smaller in peak amplitude than the predicted one, indicating current occlusion during the simultaneous activation of these two receptor systems. Thus the observed peak current was 64% of the predicted one for the fast-type ATP responses (Fig. 2Aa), 59% for the slow-type ATP responses (Fig. 2Ab), and 66% for the mixed-type ATP responses (Fig. 2Ac). It is thus clear that occlusion was present regardless of the ATP current type. Occlusion was not attributable to gradual current run-down because we used only reproducible ATP and GABA currents with full recovery after washout.
Fig. 2.
Cross talk between ATP and GABA responses.Aa, On a cell expressing fast-type ATP response and standard response to GABA, the coapplication of these agents generates a total current (right) much smaller than the one predicted by the linear summation of individual responses.Ab, Ac, Similar observations are obtained with cells expressing slow or mixed ATP responses. B, Histograms indicating occlusion (expressed as a percentage of the current predicted with the linear summation) of peak current amplitude (left) or charge integral (right). Bars with asterisks are significantly (p < 0.05) different from the predicted value (indicated by a horizontal line). Gand A denote GABA and ATP, respectively, with corresponding micromolar concentrations; n = 6–7 cells. Ca, GABA (100 μm) induces an inward current (left) in a cell insensitive to 10 μm ATP (middle); the coapplication of these agents produces a GABA response (right) similar to that of the control. Cb, ATP (10 μm) evokes a rapid inward current (left) in a cell insensitive to 100 μm GABA (middle); the coapplication of these agents elicits a response (right) similar to the control ATP one.
A quantitative analysis of the occlusion phenomenon and its dependence on agonist concentrations is presented in Figure 2B, in which data pertaining to all three ATP response types were pooled. Using low concentrations of GABA (10 μm) plus ATP (1 μm), which separately generated responses ∼20% of the maximum (see Fig. 1B), we found no significant occlusion of the peak response (89 ± 7% of predicted amplitude; n = 7; p > 0.05). Even when ATP was raised to 10 μm (which per se evoked responses 75% of the maximum; see Fig. 1B), no significant occlusion took place (89 ± 6%; n= 7; p > 0.05). However, when GABA was 100 μm (plus 10 μm ATP), significant occlusion developed (68 ± 5%; n = 6;p < 0.05), indicating that the concentration of GABA was apparently a critical element to observe this phenomenon. Further concentration increments (1 mm GABA plus 1 mm ATP) did not intensify occlusion significantly (63 ± 6%; n = 7; p < 0.05).
The distinct rise times of fast ATP and GABA currents may have led to an incorrect estimate of responses measured simply in terms of current peaks (Rogers et al., 1997). To circumvent this problem, we also measured the charge integrals, as shown in Figure 2B,right. Like peak amplitudes, the charge integral of the observed combined response to low agonist concentrations was not different from the predicted one (Fig. 2B). However, a combination of 100 μm GABA plus 10 μm ATP yielded a total charge equivalent to 81 ± 4% of the predicted value (n = 6;p < 0.05). For higher combined concentrations (1 mm GABA plus 1 mm ATP) the observed charge reduction was even stronger (57 ± 10%;n = 7; p < 0.05).
The main finding of these experiments was that the coapplication of ATP and GABA could produce nonadditive responses, probably because of the inhibition of one current by another. We next explored whether this was a receptor-mediated phenomenon and whether such receptors could be identified pharmacologically.
Occlusion was a receptor-mediated phenomenon
A restricted number of DRG cells appeared to lack responses to either ATP or GABA, thus providing a valuable tool to explore whether one agonist, unable to generate measurable responses per se, could cross-react with the receptors for the other agonist to influence cell sensitivity. Figure 2C shows examples of such extreme cases. When a cell responded to 100 μmGABA without any response to a separate application of 10 μm ATP, there was no change in the GABA response repeated in the presence of ATP (Fig. 2Ca). Similar data were obtained from five cells. Likewise, when a cell was insensitive to 100 μm GABA and responded to 10 μm ATP, the combined application of these substances yielded an inward current that did not differ from the control ATP-mediated one (Fig. 2Cb). Similar data were observed in two cells. These observations suggest that the occlusion phenomenon required the expression of functional receptors for GABA as well as for ATP, prompting further experiments to assess their pharmacological profile.
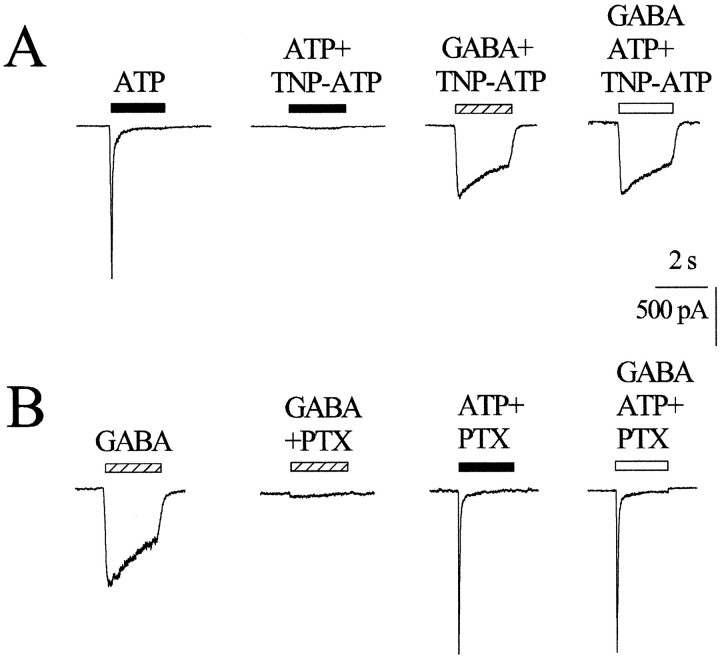
TNP-ATP (10 nm; 3 min application), a selective blocker of P2X1, P2X3, and P2X2/3 receptors (Virginio et al., 1998b; Burgard et al., 1999, 2000), strongly reduced the 10 μm ATP current amplitude to 3% of the control value (Fig.3A). Note that TNP-ATP selectively depressed the fast component of the ATP current. On average, the ATP fast currents in the presence of TNP-ATP were reduced significantly to 14% of the controls (p < 0.05;n = 5). The receptor selectivity of TNP-ATP was confirmed by the fact that it did not change responses that were mediated by GABA (−1019 ± 370 or −941 ± 207 pA in control or after 10 nm TNP-ATP; n = 7;p > 0.05). As shown in Figure 3A, the occlusion phenomenon was lost in the presence of TNP-ATP. In fact, the inward current (−728 pA) produced by the coapplication of 10 μm ATP plus 100 μm GABA in the presence of TNP-ATP was almost the same as the current (−759 pA) evoked by GABA in the presence of TNP-ATP. On average, the peak current during the application of the two agonists and TNP-ATP did not differ significantly from the one predicted for the linear summation of agonist currents (109 ± 11%; n = 5;p > 0.05), but it was significantly different from the values observed when TNP-ATP was omitted (68 ± 5%;n = 6; p < 0.05). Because the occlusion phenomenon was absent when most P2X receptors were blocked or when no ATP receptors were demonstrable, we reasoned that occlusion could not be attributable to a direct inhibitory action of ATP on GABA receptors.
Fig. 3.
Pharmacological blockers prevent occlusion.A, The rapid inward current evoked by 10 μm ATP is blocked strongly by 10 nm TNP-ATP, leaving only a shallow plateau. The current induced by 100 μm GABA in the presence of TNP-ATP does not differ from the control GABA current (data not shown) and is not occluded by coapplied ATP. B, Picrotoxin (PTX; 100 μm) strongly blocks the current evoked by 100 μm GABA. ATP (10 μm) is effective to induce an inward current in the presence of picrotoxin; however, when ATP is coapplied with GABA and picrotoxin, there is no occlusion of the total current.
Because in DRG cells bicuculline is a relatively weak blocker of GABAA receptors (Gallagher et al., 1978), we tested whether the noncompetitive GABA receptor blocker picrotoxin could interfere with cross talk. Figure 3B shows an example of the sensitivity of the occlusion phenomenon to GABA receptor antagonism. Picrotoxin (100 μm; 3 min application) reduced the amplitude of the control GABA response from −820 to −63 pA (Fig. 3B). The inward current produced by 10 μm ATP plus picrotoxin (−1350 pA) was similar to the one (−1341 pA) elicited by the coapplication of 100 μm GABA plus 10 μm ATP in the presence of picrotoxin (Fig. 3B, last two panels). On average, the current caused by the coapplication of 100 μm GABA and 10 μmATP in the presence of picrotoxin was 110 ± 4% of the predicted one (n = 8; p > 0.05) and was significantly (p < 0.05) different from the occluded current. These results indicate that the occlusion phenomenon was blocked fully by pharmacological antagonists of ionotropic receptors, thus making it unlikely that metabotropic GABA or ATP receptors were implicated in this phenomenon.
The sequence of agonist application determines the extent of occlusion
The experiments conducted so far relied on the coapplication of GABA and ATP and could not clarify issues such as, for instance, whether staggering the agonist application by changing the order of drug delivery could produce the same degree of occlusion. This approach also should help to understand the relative contribution by ATP and GABA to the occlusion phenomenon. Figure4Aa shows how ATP could influence subsequent inward currents because of GABA (or vice versa). The current induced by the first application of ATP (10 μm) peaked at −260 pA and almost completely faded (4.6% of peak) just before 100 μm GABA, which in turn evoked a −264 pA current. After wash, 100 μm GABA alone (Fig. 4Ab) induced a 13% larger response (compare GABA peak amplitudes in Fig.4Aa,Ab). However, the peak current (−114 pA) evoked by ATP (Fig. 4Bb), applied 2 sec after GABA, was only 44% of the previous ATP response (see Fig. 4Aa). Thus occlusion was not a bidirectional process, independent from agonist application timing; GABA occluded ATP responses more strongly than the other way around.
Fig. 4.

Phased application of GABA and ATP shows a different extent of occlusion. A, When 100 μm GABA is applied after 2 sec of 10 μm ATP application (Aa), the GABA peak current is 13% smaller than the value observed in Ab. Note that, when ATP is applied 2 sec after GABA, the peak of its fast current is only 44% of control (compare Ab with Aa).B, On a cell displaying a slow inward current to 10 μm ATP, 100 μm GABA applied 2 sec later induces a current (Ba) that differs from the control one (Bb) by only 10%. Conversely, the ATP current after 2 sec of GABA application is 60% of the control ATP current (compareBb with Ba). C, D, Histograms of occlusion data when GABA is applied 2 sec after ATP (C) or when ATP is applied 2 sec after GABA (D). Ordinates represent the percentage of peak current values generated by GABA (C) or ATP (D) in the continuous presence of the other agonist and calculated with respect to control. A andG denote ATP and GABA, respectively, with values expressed as micromolar concentrations. Note the much stronger reduction of ATP currents by GABA than vice versa. *p < 0.05; **p < 0.001;n = 6–11 cells.
This argument, however, did not take into account the fact that, because the ATP response had faded by the time of GABA application, most ATP receptors would have been in an inactive state, perhaps refractory to GABA receptor modulation. To explore this possibility, we studied slow ATP responses so that GABA could be applied when the ATP receptors were still operational. This approach is shown in Figure4Ba, in which the residual ATP current at 2 sec was still 37% of the peak one (Fig. 4Ba). The subsequent GABA current (Fig. 4Ba) was −370 pA, which grew by 10% when retested in the absence of ATP (Fig. 4Bb). The slow ATP current generated during the GABA current (Fig.4Bb) was 60% of the control ATP current (Fig.4Ba). It therefore appears that the earlier application of GABA was a more potent conditioning test to occlude the subsequent response to ATP than applying ATP first and testing GABA later.
Figure 4C summarizes averaged data for a wide range of agonist concentrations. No significant occlusion of GABA currents applied in the presence of ATP was observed with low concentrations of these agonists (10 μm GABA after 1 μm ATP or 10 μm GABA after 10 μm ATP). Thus simply increasing the ATP concentration while maintaining constant the subsequent GABA one did not produce occlusion. A slight, yet significant occlusion of GABA responses was observed when 100 μm GABA was applied 2 sec after the 10 μm ATP pulse (83 ± 6% of control; n = 10; p< 0.05). Occlusion was more pronounced with saturating concentrations of GABA (1 mm) after 1 mmATP because the depression of GABA current in this case was 59 ± 13% (n = 6; p < 0.05).
In contrast, a much stronger occlusion of ATP responses was obtained when ATP was applied in the presence of GABA (Fig.4D). Like in the case of agonist coapplication, low concentrations (such as 1 μm ATP after 10 μm GABA or 10 μm ATP after 10 μm GABA) were ineffective (n = 5, p > 0.05; n = 6, p > 0.05, respectively). However, a strong reduction of the current produced by 10 μm ATP after 100 μm GABA was observed. This ATP response was 57 ± 7% of control (n = 11). An even more dramatic occlusion of the ATP response was seen with 1 mm ATP applied after 1 mmGABA (28 ± 9%; n = 6; p < 0.001).
These results collectively indicate that GABA could decrease greatly the responses to ATP, whereas ATP was less efficient in depressing GABA currents. One possibility to account for this differential degree of occlusion would be that GABA generated a much larger increase in input conductance than did ATP. Accordingly, the application of ATP to a cell with greatly increased input conductance might yield strongly attenuated ATP responses. To examine this possibility, we measured the net input conductance values produced by 100 μm GABA or 10 μm ATP by calculating (after leak subtraction) the slope of the current–voltage (I–V) relations in the range between −70 and −50 mV; in the case of GABA the conductance value was 12.8 ± 3.5 nS for GABA and 17.6 ± 4.1 nS for ATP, indicating that these agents induced comparable increases in cell input conductance.
Direction of current flow determines occlusion
At −70 mV holding potential the ATP currents are attributable to the opening of nonselective cationic channels permeable to Na+, Ca2+, and K+ (Virginio et al., 1998a; Koshimizu et al., 2000), whereas GABA currents are produced by the efflux of Cl− (Sivilotti and Nistri, 1991; Kaila, 1994). Increasing the extracellular Cl−concentration is known to block ionotropic P2X receptors of NG 108-15 cells, probably by impairing agonist binding (Kaiho et al., 1997). We therefore investigated whether, on DRG neurons, Cl− efflux via open GABA channels might have been involved in the depression of ATP currents by GABA. For this purpose we substituted Cl− in the pipette solution with the relatively impermeant SO42− (Jo and Schlichter, 1999; Hugel and Schlichter, 2000) so that at negative potentials GABA elicited Cl− influx and thus outward currents. As shown in Figure5A, left, the substitution of Cl− with SO42− shifted theI–V relation for GABA currents upward in a parallel manner and moved the EGABA from 4.6 to −96 mV (n = 5). The ATP I–V relation was unaffected, however (Fig. 5A, right), and the ATP calculated reversal potential remained at 2 mV (n = 5; see also Jo and Schlichter, 1999).
Fig. 5.
Occlusion depends on the reversal potential of GABA. A, Average current–voltage plots of GABA (left) or ATP (right) responses obtained with either Cs2SO4 (filled circles) or CsCl (open circles) in the patch pipette. Calculated GABA reversal potentials are −96 and 4.6 mV, respectively, whereas values for ATP are 2 mV in either case.B, On a cell clamped at −50 mV (with a Cs2SO4-filled pipette), 100 μmGABA applied 2 sec after ATP (10 μm) induces an outward current (Ba) that is 96% of the control GABA current peak (Bb). Likewise, the ATP current 2 sec after GABA is 99% (Bb) of its control (Ba). Thus reversing GABA current polarity leads to the disappearance of occlusion.
On a cell clamped at −50 mV, the usual agonist concentrations (100 μm GABA; 10 μm ATP) induced ATP and GABA currents of different polarity (inward and outward, respectively; Fig.5B). Note that the GABA current was almost the same in the presence of ATP (Fig. 5Ba) as before applying ATP (Fig.5Bb; 190 and 198 pA, respectively). On average, the GABA current was (p < 0.05) depressed significantly by ATP to 85 ± 5% of control (n = 9). The ATP current amplitude was −307 pA when applied alone (Fig. 5Ba) and −304 pA 2 sec after the application of GABA (Fig. 5Bb). The average ATP current after the application GABA was 88 ± 4% (n = 9; p < 0.05) of control; that is, occlusion was much smaller than in the symmetrical Cl− solution (see Fig.4D). Thus it appears that reversing the direction of Cl− flux through GABA receptors greatly reduced the depression of ATP currents.
We also checked whether the presence of SO42− rather than the current polarity was responsible for attenuating the occlusion phenomenon. To this end we clamped cells at +30 mV when the pipette solution contained CsCl and made the direction of both ATP and GABA currents outward. When 100 μm GABA was applied 2 sec after 10 μm ATP, the resulting GABA current was 73 ± 11% of the GABA current before ATP (n = 6;p < 0.05). However, the depression of ATP by previous application of GABA was virtually absent (97 ± 13% of control;n = 6; compare it with 57% at −70 mV). Depression of ATP by GABA thus strongly depended on the direction of Cl− flow rather than on the intracellular anion species, suggesting that a rapid rise in extracellular Cl− might have been responsible for the observed phenomenon. To validate a role of external Cl− in modulating ATP responses of DRG cells, we performed experiments (n = 8 cells) in solutions in which 50% of the external NaCl was replaced by Na2SO4. In this case the GABA reversal potential shifted to ∼10 mV, which is close to the 8.5 mV value predicted by the Nernst equation, indicating that SO42− was relatively impermeant through Cl− channels. In low Cl− solution the peak inward current generated by 10 μm ATP was enhanced by 48 ± 13% (n = 8; p < 0.05). Thus these observations support the notion that external Cl− has a depressant action on ATP receptors (Kaiho et al., 1997). If this anion were allowed to build up transiently via the opening of GABA channels, it apparently could induce a temporary intensification of the ATP receptor depression.
Effect of Ca2+
Because cross talk between certain ionotropic receptors is known to depend on intracellular Ca2+-mediated mechanisms (Chen and Wong, 1995), we explored whether this condition applied also to the ATP/GABA interaction. Ca2+ is known to modulate the activity of GABAA (Inoue et al., 1986) and ATP (Khiroug et al., 1997b; Cook et al., 1998; Piper and Docherty, 2000) receptors.
To reduce the influx of Ca2+, we first used a Ca2+-free external solution. Omitting this divalent cation dramatically curtailed the interaction between the two receptor systems, as shown in Figure6A. In fact, the control 10 μm ATP current (−527 pA) was as large as the ATP current (−534 pA) observed 2 sec after 100 μm GABA. Likewise, the inward current (−599 pA) induced by GABA in the presence of 10 μmATP was indistinguishable from the subsequent control GABA current (−600 pA). These data suggested the importance of Ca2+ for the cross talk between ATP and GABA receptor systems and led us to testing this hypothesis further.
Fig. 6.
Role of Ca2+ in the occlusion phenomenon. A, In Ca2+-free extracellular solution (superfused for at least 20 min) the peak current induced by 100 μm GABA applied 2 sec after ATP (10 μm; left) is like the control GABA current (right). Similarly, the ATP current is the same before (left) or after the application of GABA (right). B, On a cell recorded without the addition of exogenous buffers to the pipette solution, the GABA current in the presence of ATP (left) is 74% of the one recorded without ATP (right). The ATP current in the presence of GABA (right) is only 29% of the value observed without GABA (left). These data indicate a strong potentiation of occlusion when intracellular Ca2+ is not buffered exogenously. C, D, Effect of various [Ca2+]ibuffering treatments on ATP (C) or GABA (D) current amplitude. Each agonist is applied 2 sec after the application of the other one is started. Data are expressed as a percentage of current produced by a single agonist under the same recording conditions. Note the incremental size of agonist currents as conditions range from no exogenous buffering to Ca2+-free medium. Asterisks indicatep < 0.05; n = 6–11 cells.E, F, Lack of significant change in ATP (E) or GABA (F) control current amplitude with various degrees of [Ca2+]i buffering, indicating that these conditions did not affect cell ability to respond to separately applied agonists; n = 6–11 cells.
Exposure of cells to Ca2+-free medium leads to a depletion of internal Ca2+stores (D'Andrea et al., 1993). To assess the role of intracellular Ca2+([Ca2+]i) in cross talk, we explored the effects of various degrees of [Ca2+]i buffering (Khiroug et al., 1997a,b) and compared the results against the standard condition (5 mm EGTA in the pipette solution). Omitting Ca2+ buffers from the pipette solution (as shown by the example of Fig. 6B) strongly enhanced the occlusion between GABA and ATP, especially when GABA was first applied. In fact, the 10 μm ATP current became very small in the presence of 100 μm GABA (29% of the control ATP response; Fig. 6B,right). The GABA current also was depressed in the presence of ATP because it became 74% of the control GABA current (Fig.6B, left).
Because EGTA is a relatively slow buffer, we also used a pipette solution containing the more efficient buffer BAPTA (10 mm). The histograms of Figure 6, C andD, summarize the data obtained with BAPTA as well as with other [Ca2+]ibuffering conditions. Depression of ATP currents after a 2 sec GABA application was 40 ± 10% (n = 6;p < 0.05) when [Ca2+]i was not buffered, 57 ± 7% (n = 11; p < 0.05) with 5 mm EGTA, and 73 ± 8% (n = 8; p < 0.05) with 10 mm BAPTA. In Ca2+-free medium the depression was insignificant (97 ± 11% of control; n = 8;p > 0.05). These data strongly suggest a role for [Ca2+]i in the development of the occlusion of ATP responses by GABA. Increased [Ca2+]i therefore might be a factor promoting the negative action of GABA on ATP currents.
The ATP-produced depression of subsequent GABA responses was, to some extent, dependent on [Ca2+]i (Fig.6D). No significant depression of GABA by the 2 sec ATP pulse was found in Ca2+-free conditions (94 ± 6% of control; n = 9;p > 0.05). Depression of GABA was 71 ± 7% (n = 6; p < 0.05) without the addition of internal Ca2+ buffers, 85 ± 6% (n = 11; p < 0.05) with 5 mm EGTA, and 86 ± 4% (n = 10; p < 0.05) with 10 mm BAPTA. Figure 6, E and F, also shows that changes in the intensity of the occlusion phenomenon with various Ca2+ buffering conditions were not a mere manifestation of altered receptor activity brought out by these experimental conditions. In fact, there was no significant change in the average amplitude of currents induced by ATP (Fig.6E) or GABA (Fig. 6F) with conditions ranging from no addition of intracellular buffer to Ca2+-free medium.
Calcium imaging
The Ca2+ dependence of the occluding action by GABA toward ATP responses raised the issue of whether GABA could have produced [Ca2+]i rises sufficiently large to affect the function of P2X receptors. In fact, GABA can increase [Ca2+]i in developing or adult rat neurons (Cherubini et al., 1991; Leinekugel et al., 1995; Frech et al., 1999).
Thus we used [Ca2+]i imaging of DRG cells loaded with a fluorescent dye and activated by GABA and/or ATP. First, we tested intact DRG neurons (without concomitant recording of the membrane currents) loaded with the membrane-permeable dye fluo-3 AM. A 2 sec application of 10 μm ATP produced Ca2+ signals in the majority of cells (23 of 30), as shown by the example of Figure7Aa. In this case ATP increased [Ca2+]iby a factor of 0.53 with respect to baseline. In a sample of 23 intact DRG neurons the fluorescence increase was 0.73 ± 0.20 (Fig.7Ca). Unlike ATP, 100 μm GABA usually did not evoke significant [Ca2+]i changes (see Fig. 7Ab for a typical case and 7Ca for pooled data). The GABAA receptor agonist isoguvacine (100 μm) was equally ineffective because it induced a 6% increase in basic fluorescence in only 1 of 10 cells.
Fig. 7.
[Ca2+]i changes after the application of ATP or GABA. A, Representative example of [Ca2+]i imaging from an intact cell exposed to 10 μm ATP (Aa) or 100 μm GABA (Ab). Note that ATP increases [Ca2+]i, whereas GABA does not.B, On a cell that was patch clamped at −70 mV, ATP (10 μm) evokes a fast inward current (Bc) associated with a small increase in [Ca2+]i (Ba). Conversely, GABA (100 μm) fails to raise [Ca2+]i (Bb) despite a large inward current (Bd). C, Summary of [Ca2+]i changes in the presence of ATP or GABA for intact (Ca) or voltage-clamped (Cb) cells. Note the stronger effect of ATP on [Ca2+]i in intact cells versus voltage-clamped cells. Asterisks indicatep < 0.05; n = 12–30.
Combining Ca2+ imaging with the simultaneous recording of membrane currents (via a pipette containing 25 μm fluo-4) allowed us to check whether any Ca2+ signals could be produced by GABA or ATP in conditions reproducing more closely our previous observations. In the presence of fluo-4 we confirmed current occlusion when 10 μm ATP was applied 2 sec after 100 μm GABA. Occluded ATP currents were thus 43 ± 9% of control (n = 5; p < 0.05), a value close to one found without the addition of internal Ca2+ buffers (see Fig. 6C). When GABA was tested 2 sec after ATP, the GABA current was depressed to 80 ± 8% of control (n = 5), a value again similar to the one found with no added buffers (see Fig.6D). Although ATP currents as large as −1338 pA (Fig. 7Bc) induced a slight increase in [Ca2+]i (by 0.16; Fig. 7Ba), even larger GABA currents (Fig. 7Bd; note also larger charge integral) produced no change in basal cell fluorescence (Fig. 7Bb). In an average of 12 voltage-clamped DRG cells, the generation of 10 μm ATP currents (−861 ± 170 pA) was accompanied by a concomitant significant increase in [Ca2+]i by a factor of 0.18 ± 0.06 (n = 12; p< 0.05; Fig. 7Cb). When we compare histograms in Figure 7,Ca and Cb, it appears that the ATP-evoked [Ca2+]i rise observed in intact cells is 4.3 times larger than the one detected in voltage-clamped neurons. This result indicates that the main source for [Ca2+]i rise is the activation of voltage-dependent Ca2+channels. Despite robust membrane currents (mean value −995 ± 288 pA) induced by 100 μm GABA, there was no accompanying change in [Ca2+]i (0.06 ± 0.01; n = 12; p > 0.05; Fig.7Cb). Thus these data did not reveal any [Ca2+]i transients produced by GABA that might be responsible for the depression of ATP signals by this agonist.
DISCUSSION
The main finding of the present study is the novel mechanism of negative interaction between native ionotropic receptors activated by ATP or GABA on DRG neurons. This finding is important not only because it demonstrates a new type of interaction between receptors with different structure and function but also because it raises the possibility of a similar cross talk by ATP and GABA coreleased on spinal cord neurons (Jo and Schlichter, 1999).
Occlusion was a receptor-mediated phenomenon
In small and medium-sized DRG neurons ATP generates membrane currents of distinct shape (Burgard et al., 1999; Grubb and Evans, 1999) apparently depending on the homo- or heteromeric combination of P2X2 and P2X3 subunits (Labrakakis et al., 2000; Liu et al., 2001). Although the role and relative preponderance of different receptor subtypes were not the focus of the present study, it became clear that fast, slow, and mixed responses to ATP all could undergo significant occlusion in conjunction with the action of GABA, which is mediated mainly by GABAA receptors (Kaila, 1994). Occlusion of ATP and GABA responses therefore appeared to be a general phenomenon, not linked to any particular ATP receptor group. In cells lacking ATP responses the action of GABA was not depressed by ATP; similarly, cells unresponsive to GABA did not show any GABA-mediated occlusion of ATP responses. Furthermore, during a pharmacological block of ATP receptors by TNP-ATP, ATP could not depress GABA currents. Likewise, no occlusion by GABA of ATP currents was observed during GABA receptor block by picrotoxin. These data indicate that occlusion is a receptor-based process and that there is no apparent direct action by ATP on GABA receptors (or vice versa).
Characteristics of occlusion
When GABA and ATP were coapplied, the observed membrane current was much smaller than the sum of the two individual ones. This is a novel phenomenon because it involves an interaction between cationic and anionic channels. Occlusion between cationic channels has been reported recently with ATP and acetylcholine (Nakazawa, 1994;Barajas-Lopez et al., 1998; Searl et al., 1998; Zhou and Galligan, 1998; Khakh et al., 2000). In the present study the ATP/GABA receptor occlusion was manifested with relatively high concentrations of these agonists, implying the need for activating a large fraction of membrane receptors to produce such an interaction, in analogy with some reports on ATP/acetylcholine interaction (Zhou and Galligan, 1998; Khakh et al., 2000). This phenomenon occurred rapidly in terms of either depressed peak amplitude or reduced charge integral.
The coapplication of agonists could not clarify whether each agent contributed equally to the occlusion process. This issue was examined by phasing the application of one drug after the start of the application of the other one. Thus preapplied GABA more strongly depressed ATP currents than preapplied ATP did to GABA currents. When the same concentrations of GABA and ATP were coapplied, the combined current depression was intermediate between the values obtained with phased applications. Dishomogeneity in occluding properties was not attributable to greatly different changes in cell input conductance induced by ATP or GABA.
Occlusion by phased application of agents might have been caused by cross-desensitization (Ariens and Simonis, 1964) between these two receptor systems. However, this possibility seems unlikely for at least two reasons: (1) although GABA was more effective to depress ATP than vice versa, desensitization of GABA receptors (as judged by current fade) was usually less intense than ATP receptor desensitization; (2) although various ATP receptors displayed differing degree of desensitization (observed as current fading), they all had a similar ability to depress subsequent GABA responses. Nakazawa (1994) also noted that negative cross talk between ATP and acetylcholine receptors was not attributable to desensitization.
Inhibition by GABA of ATP currents depended on ionic current polarity
Previous work on NG108-15 cells has indicated that extracellular Cl− may depress P2X receptor function, probably via the inhibition of agonist binding to the receptor site (Kaiho et al., 1997). The present experiments on DRG cells also indicated that decreasing external Cl−(replaced by SO42−) significantly enhanced ATP-mediated inward currents. We therefore considered the possibility that Cl−efflux via open GABA channels could rise transiently to occlude ATP responses. For this purpose, we used the relatively impermeant SO42− instead of Cl− (Jo and Schlichter, 1999) in the pipette solution. This approach changedEGABA without changingEATP (see also Jo and Schlichter, 1999) and made GABA-evoked currents outward at the negative holding potential, whereas ATP-mediated currents remained inwardly directed. Under these conditions ATP still could depress subsequent GABA currents, whereas GABA greatly lost its ability to occlude ATP responses. The loss of GABA-occluding properties was not attributable to SO42− itself, because similar results were obtained at +30 mV holding potential with standard CsCl in the patch pipette and outward GABA currents. Thus it appears that the GABA-induced occlusion of ATP currents chiefly (although not exclusively) required Cl− efflux through activated GABAA receptors. Nevertheless, because Cl− was present in high concentrations on both sides of the cell membrane, it would be necessary to envisage that ATP and GABA receptors were coupled very closely and were located in membrane areas of restricted access to the extracellular milieu to be sensitive to any extracellular Cl− rise by GABA channel activity. Within the framework of this hypothesis, it is possible to view Cl− as a functional coupling agent between two distinct ionotropic receptor types.
One alternative possibility is that the GABA/ATP receptor interaction was an example of protein–protein interaction like the case of GABAA and dopamine receptors (Liu et al., 2000) or ATP and nicotinic receptors (Khakh et al., 2000). In the present case any protein–protein interaction would be indirect because it requires Cl− as a coupling agent rather than relying on the physical intramembrane interaction between distinct receptors (Liu et al., 2000). Because the present study is just the first report of the ATP/GABA receptor interaction, further experiments clearly are needed to identify the mechanisms underlying this phenomenon.
How can Ca2+ modulate the inhibition of ATP receptors by GABA?
The strong Ca2+ dependence of the depressant GABA action on ATP currents was unexpected because GABAA receptors are not known to be permeable to Ca2+, and no [Ca2+]i increase induced by GABA could be observed in our experiments. The inhibitory action of GABA was magnified when [Ca2+]i was elevated by the lack of Ca2+ buffers in the pipette solution and was eliminated in Ca2+-free medium (with a consequent fall in [Ca2+]i;D'Andrea et al., 1993). The degree of occlusion by GABA also could be graded, depending on the buffering ability of EGTA or BAPTA (Khiroug et al., 1997a,b, 1998). These data suggest that steady-state [Ca2+]i (rather than transient rises in [Ca2+]i) is important to control the susceptibility of ATP receptors to GABA-mediated Cl−-dependent inhibition. Although [Ca2+]ican, for example, downregulate the activity of NMDA channels (Umemiya et al., 2001) or upregulate AMPA channels (Liu and Cull-Candy, 2000), in the present study [Ca2+]i appeared to modulate ATP receptors only when they were subjected to Cl−-dependent occlusion.
Ca2+-dependent inhibition by ATP of GABAA receptor function
Because increased [Ca2+]i depresses GABAA receptor function (Inoue et al., 1986), for instance as a consequence of NMDA receptor activation (Chen and Wong, 1995), a similar mechanism might be used for explaining the ATP-induced depression of subsequent GABA currents because activated P2X receptors are permeable to Ca2+ (Rogers et al., 1997). In our voltage-clamp experiments [Ca2+]i signals evoked by ATP were considerably smaller than those recorded from intact cells and could account for the comparatively modest occlusion by ATP of GABA currents. This observation accords with the report that Ca2+ influx through P2X3 receptors, the most abundant type in DRG neurons, is relatively limited (Virginio et al., 1998a). The present study could not quantify precisely the contribution by Ca2+ influx (via ATP channels) to the total ATP current because [Ca2+]i imaging was performed with a nonratiometric method. Nevertheless, in intact cells [Ca2+]itransients induced by ATP were larger because they presumably included influx via depolarization-activated Ca2+channels (see also Koshimizu et al., 2000). Should the cell membrane be allowed to depolarize, any occlusive effect by ATP on GABA currents might become stronger.
Physiological implications
Because in the spinal cord ATP is coreleased together with GABA (Jo and Schlichter, 1999), it is interesting to consider the possibility of ATP/GABA interaction also in this tissue. DRG afferent terminals are a physiologically important target for ATP and GABA (Sivilotti and Nistri, 1991; Gu and MacDermott, 1997). Although the action of ATP is to facilitate glutamate release (Bardoni et al., 1997;Gu and MacDermott, 1997; Li et al., 1998; Jo and Schlichter, 1999), GABA produces a presynaptic inhibition of afferent terminals (Sivilotti and Nistri, 1991) via multiple mechanisms, including presynaptic membrane shunting (Graham and Redman, 1994) and GABAB receptor-mediated depression of Ca2+ channels (Deuchars et al., 2000). Rapid reduction in the excitatory action of ATP by GABA might be an additional, new mechanism of sensory information processing. Interestingly, DRG neurons themselves can release ATP (Stevens and Fields, 2000), thus providing the physiological condition for GABA/ATP interplay.
Footnotes
This work was supported by grants from Ministero dell'Universita' e della Ricerca Scientifica e Tecnologica (cofin 2000), from Istituto Nazionale di Fisica della Materia (PRA CADY), and from International Association (INTAS). We thank Dr. Massimo Righi for his support with cell cultures.
Correspondence should be addressed to Andrea Nistri, International School for Advanced Studies (SISSA), Via Beirut 4, 34014 Trieste, Italy. E-mail: nistri@sissa.it.
REFERENCES
- 1.Ariens EJ, Simonis AM. A molecular basis for drug action. The interaction of one or more drugs with different receptors. J Pharm Pharmacol. 1964;16:289–312. doi: 10.1111/j.2042-7158.1964.tb07461.x. [DOI] [PubMed] [Google Scholar]
- 2.Barajas-Lopez C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J Physiol (Lond) 1998;513:671–683. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgard EC, Niforatos W, Van Beissen T, Lynch KJ, Touma E, Metzger RE, Kowaluk EA, Jarvis MF. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- 5.Burgard EC, Niforatos W, Van Beissen T, Lynch KJ, Kage KL, Touma E, Kowaluk EA, Jarvis MF. Competitive antagonism of recombinant P2X2/3 receptors by 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate (TNP-ATP). Mol Pharmacol. 2000;58:1502–1510. doi: 10.1124/mol.58.6.1502. [DOI] [PubMed] [Google Scholar]
- 6.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen QX, Wong RKS. Suppression of GABAA receptor responses by NMDA application in hippocampal neurons acutely isolated from the adult guinea-pig. J Physiol (Lond) 1995;482:353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 9.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook SP, Rodland KD, McCleskey EW. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci. 1998;18:9238–9244. doi: 10.1523/JNEUROSCI.18-22-09238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Andrea P, Zacchetti D, Meldolesi J, Grohovaz F. Mechanism of [Ca2+]i oscillations in rat chromaffin cells: the intracellular oscillator operates within a useful range of [Ca2+]i. J Biol Chem. 1993;268:15213–15220. [PubMed] [Google Scholar]
- 12.Deuchars SA, Trippenbach T, Spyer KM. Dorsal column nuclei neurons recorded in a brain stem–spinal cord preparation: characteristics and their responses to dorsal root stimulation. J Neurophysiol. 2000;84:1361–1368. doi: 10.1152/jn.2000.84.3.1361. [DOI] [PubMed] [Google Scholar]
- 13.Frech MJ, Deitmer JW, Backus KH. Intracellular chloride and calcium transients evoked by γ-aminobutyric acid and glycine in neurons of the rat inferior colliculus. J Neurobiol. 1999;40:386–396. doi: 10.1002/(sici)1097-4695(19990905)40:3<386::aid-neu10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurons. J Physiol (Lond) 1978;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- 16.Gines S, Hilion J, Torvinen M, Le Crom S, Casado V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Vernier F, Liuis P, Ferre S, Fuxe K, Franco R. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham B, Redman S. A simulation of action potentials in synaptic boutons during presynaptic inhibition. J Neurophysiol. 1994;71:538–549. doi: 10.1152/jn.1994.71.2.538. [DOI] [PubMed] [Google Scholar]
- 18.Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 19.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 20.Hu HZ, Li ZW. Modulation by adenosine of GABA-activated current in rat dorsal root ganglion neurons. J Physiol (Lond) 1997;501:67–75. doi: 10.1111/j.1469-7793.1997.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue M, Oomura Y, Yakushiji T, Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986;324:156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- 23.Jo YH, Schlichter R. Synaptic co-release of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–246. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- 24.Kaiho H, Kimura J, Matsuoka I, Nakahishi H. Effects of anions on ATP-activated nonselective cation current in NG108-15 cells. J Neurophysiol. 1997;77:2717–2722. doi: 10.1152/jn.1997.77.5.2717. [DOI] [PubMed] [Google Scholar]
- 25.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 26.Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–410. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- 27.Khiroug L, Giniatullin R, Sokolova E, Talantova M, Nistri A. Imaging of intracellular calcium during desensitization of nicotinic acetylcholine receptors of rat chromaffin cells. Br J Pharmacol. 1997a;122:1323–1332. doi: 10.1038/sj.bjp.0701518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khiroug L, Giniatullin R, Talantova M, Nistri A. Role of intracellular calcium in fast and slow desensitization of P2 receptors in PC12 cells. Br J Pharmacol. 1997b;120:1552–1560. doi: 10.1038/sj.bjp.0701060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khiroug L, Sokolova E, Giniatullin R, Afzalov R, Nistri A. Recovery from desensitization of neuronal nicotinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. J Neurosci. 1998;18:2458–2466. doi: 10.1523/JNEUROSCI.18-07-02458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koshimizu TA, Van Goor F, Tomic M, Wong OLA, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor channels expressed in excitable cells. Mol Pharmacol. 2000;58:936–945. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 31.Labrakakis C, Gerstner E, MacDermott AB. Adenosine triphosphate-evoked currents in cultured dorsal root ganglion neurons obtained from rat embryos: desensitization kinetics and modulation of glutamate release. Neuroscience. 2000;101:1117–1126. doi: 10.1016/s0306-4522(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 32.Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from neonatal hippocampal slices. J Physiol (Lond) 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Calejesan AA, Zhou M. ATP P2X receptors and sensory synaptic transmission between primary afferent fibres and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Wan QI, Pristupa ZB, Yu XM, Wang YT, Niznik HB. Direct protein–protein coupling enables cross talk between dopamine D5 and γ-aminobutyric acid A receptors. Nature. 2000;403:274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A, Burnstock G. Coexpression of P2X3 and P2X2 receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- 37.Liu SQL, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 38.Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piper AS, Docherty RJ. One-way cross-desensitization between P2X purinoceptors and vanilloid receptors in adult rat dorsal root ganglion neurons. J Physiol (Lond) 2000;523:685–696. doi: 10.1111/j.1469-7793.2000.t01-1-00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers M, Colquhoun LM, Patrick JW, Dani JA. Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. J Neurophysiol. 1997;77:1407–1417. doi: 10.1152/jn.1997.77.3.1407. [DOI] [PubMed] [Google Scholar]
- 42.Searl TJ, Redman RS, Silinsky EM. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. J Physiol (Lond) 1998;510:783–791. doi: 10.1111/j.1469-7793.1998.783bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J Physiol (Lond) 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- 45.Stevens B, Fields RD. Response of Schwann cells to action potential in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 46.Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 47.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umemiya M, Chen N, Raymond LA, Murphy TH. A calcium-dependent feedback mechanism participates in shaping single NMDA miniature EPSCs. J Neurosci. 2001;21:1–9. doi: 10.1523/JNEUROSCI.21-01-00001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virginio C, North RA, Surprenant A. Calcium permeability and block at heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurons. J Physiol (Lond) 1998a;510:27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998b;53:969–973. [PubMed] [Google Scholar]
- 51.Vulchanova L, Riedl MS, Shuster SJ, Stone KM, Hargreaves KM, Buell G, Surprenant A, North RA, Edle R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol (Lond) 1998;513:685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]