Abstract
The isomorphic representation of the contralateral whisker pad in the rodent cerebral cortex has served as a canonical example in primary somatosensory areas that the contralateral body surface is spatially represented as a topographic map. By characterizing responses evoked by multiwhisker stimuli, we provide direct evidence that the whisker region of the rat primary somatosensory cortex (SI) integrates information from both contralateral and ipsilateral whisker pads. The proportions of SI neurons responsive to ipsilateral whisker stimuli, as well as their response probabilities, increased with the number of ipsilateral whiskers stimulated. Under bilateral whisker stimulation, the responses of 95% of neurons recorded were affected by stimulation of ipsilateral whiskers. Contralateral tactile responses of SI neurons were profoundly influenced by preceding ipsilateral stimuli and vice versa. This effect depended on both the spatial location and the relative timing of bilateral whisker stimuli, leading to both spatial and temporal asymmetries of interaction. Bilateral whisker stimulation resulted in only modest changes in evoked response latency. Previous ipsilateral stimulation was also shown to affect tactile responses evoked by later ipsilateral stimuli. Inactivation of the opposite SI abolished ipsilaterally evoked responses as well as their influence on subsequently evoked contralateral responses in the intact SI. Based on these results, we conclude that the rat SI integrates information from both whisker pads and propose that such interactions may underlie the ability of rats to discriminate bilateral tactile stimuli.
Keywords: barrel cortex, bilateral, ipsilateral, integration, interhemispheric transfer, inactivation, topography
The role of the somatosensory cortex (SI) in integrating separate sources of tactile input has been investigated primarily by inferring from extracellular recordings the spatiotemporal transformations performed on convergent subcortical inputs. The whisker region of the SI in rodents is an ideal model for investigating the issue of cortical integration because of its modular topography, which purportedly reflects the arrangement of contralateral whiskers at the periphery (Woolsey and Van der Loos, 1970; Killackey, 1973). Recordings from SI neurons in temporal interaction studies have provided a basic description of the temporal and spatial attributes of cortical integration elicited by paired contralateral whisker stimuli (Simons, 1985; Simons and Carvell, 1989; Brumberg et al., 1996;Fanselow and Nicolelis, 1999). Such studies suggest that the SI may integrate information across multiple contralateral whiskers to generate behaviorally relevant information regarding the animal's surrounding environment.
If the rat is to use tactile information from both sides of its face, left and right whisker information must also be integrated. Comparisons between these separate sources of tactile input would then allow the animal to successfully detect the width of an aperture, or the orientation of an obstacle. A possible anatomical substrate for the integration of bilateral whisker information within the SI is provided by the approximately homotopic interconnection of the whisker barrel fields via the corpus callosum (White and DeAmicis, 1977; Olavarria et al., 1984; Koralek et al., 1990; Cauller et al., 1998) (for nonhomotopic connections, see Welker et al., 1988). Furthermore, field potential recordings provided evidence, more than two decades ago, for the existence of callosally mediated ipsilateral whisker responses in the rat SI (Pidoux and Verley, 1979). These previous studies suggest the existence of a homotopy between contralateral and ipsilateral whisker stimulation.
Despite these reports, however, the potential role that ipsilaterally evoked activity in the SI plays in processing whisker information remains unexplored. Indeed, the response attributes of single neurons to ipsilateral whisker stimuli have not been characterized; thus, it is not yet known whether individual SI neurons respond to both contralateral and ipsilateral whisker stimuli. Moreover, the spatial and temporal attributes of interaction between left- and right-side whisker stimuli in the SI, indicative of bilateral whisker integration, have not been characterized. Here, we address these issues and report evidence that neurons located in the SI integrate information from both the ipsilateral and contralateral whisker pads. Based on these findings, we propose that such interactions play a role in the processing of bilateral tactile information.
MATERIALS AND METHODS
Because the whisker-to-barrel pathway is completely crossed subcortically (Waite, 1969; Smith, 1973; Erzurumlu and Killackey, 1980), bilateral whisker stimulation can be used to explore spatiotemporal aspects of interaction between these initially separate streams of input to the SI (Fig.1A). Our results were obtained by measuring responses of neurons in layer V of the SI to ipsilateral and bilateral multiwhisker stimuli during multi-electrode recordings in anesthetized animals. We further addressed interhemispheric interactions in bilateral integration by pharmacological inactivation of one barrel field.
Fig. 1.
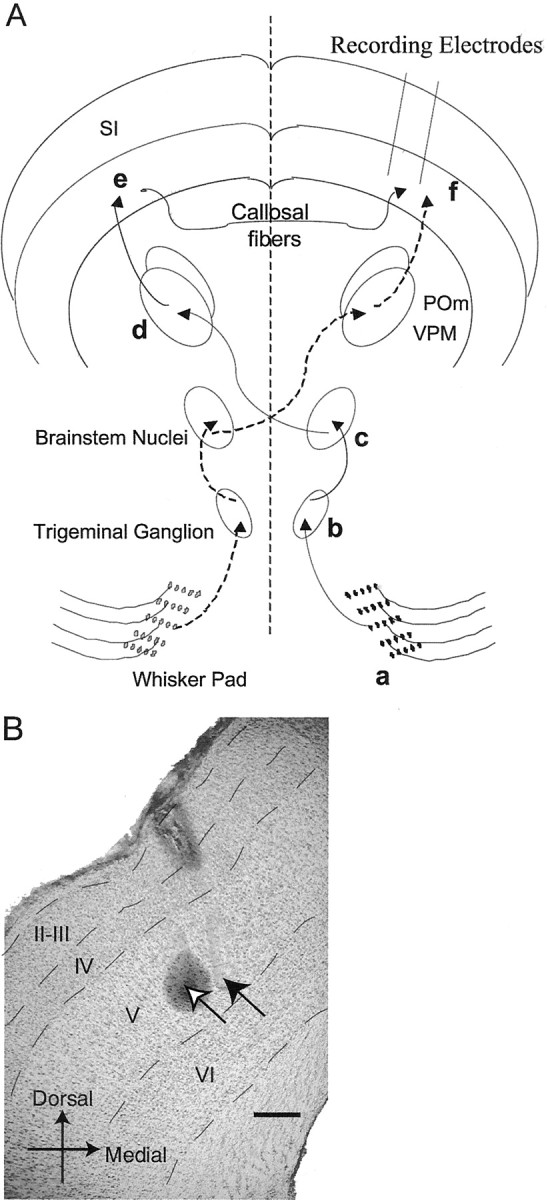
A, Ipsilateral and contralateral whisker-related pathways to the SI. Ipsilateral pathway is markeda–f (solid arrows); contralateral pathway is denoted by dashed arrows. Stimulation of the whisker pad (a) evokes activity in primary sensory neurons with cell bodies located in the trigeminal ganglion (b) that send projections to whisker-related, brainstem nuclei (c). Projections from these nuclei decussate, and, in turn, terminate in whisker-related thalamic nuclei (d). Thalamocortical projections to the SI (whisker barrel cortex) ramify throughout layers III–V, with dense terminations in layer IV forming what are known as “barrels” (e). Callosal connections arising throughout the SI (except layer IV barrels) sprout collaterals in layer V, as well as in layer I, of the contralateral SI (f). Using an array of electrodes, extracellular recordings are made within layer V, in which callosally mediated ipsilateral and thalamocortically ascending contralateral pathways converge. B, Photomicrograph of an 80 μm coronal section stained with cresyl violet and Prussian blue. The location of the tip of the electrode used to make a marking lesion is labeled with an open arrow and is in layer V. The “halo” surrounding the tip is the reaction product with Prussian blue. A filled arrow marks the location of a neighboring electrode tip (also in layer V) that can be detected by the presence of glial scarring. Dashed linesdemarcate the boundaries between layers. Scale bar, 200 μm.POm, Posterior medial nucleus; VPM, ventroposterior medial nucleus.
Experimental protocols. The subjects used in these experiments were 11 female Long–Evans rats (240–300 gm at surgery). Specifics of surgical and recording procedures can be found elsewhere (Nicolelis and Chapin, 1994; Nicolelis et al., 1997; Krupa et al., 1999). All methods were approved by the Duke University Animal Use Committee. Briefly, rats received chronic implants of 16 microwire arrays (NB Labs, Dennison, TX) located in layer V of both SIs (−3.0 mm caudal from bregma, 5.5 mm mediolateral, and ∼1300 μm depth from skull surface). During surgery, microwire arrays were slowly lowered (∼100 μm/min) into layer V of the SI slightly off perpendicular to the surface of the brain. Placement of arrays was guided by stereotaxic measurements and by continuous monitoring of electrophysiological signals evoked by whisker stimulation via audio monitor and oscilloscope. Arrays were arranged in two rows (spaced 0.5 mm apart) of eight microwires with an inter-microwire distance of 200 μm. All animals were also outfitted with a head bolt at the time of surgery. Postsurgical analgesia was provided by buprenorphine (0.1–0.5 mg/kg, s.c.), and a topical antibiotic was applied.
Five animals were implanted with a fine caliber infusion guide cannula attached to one of the microwire arrays to allow muscimol (500 ng in 500 μl of saline) or saline to be injected into the barrel cortex. Full recovery of cortical responses after muscimol injection has been demonstrated previously using this method, requiring >8 hr (Krupa et al., 1999). Muscimol or saline was slowly pressure injected using a microperfusion pump (Orien Research Inc., Beverly, MA). Inactivation was confirmed by assessing the spreading quiescence of single-unit and multiunit activity along electrodes of the injected SI. Across the course of the experiment, muscimol inactivation of one SI did not significantly affect the spontaneous activity level of the intact SI when compared with saline-injected controls.
Rats were allowed 2 weeks to recover from this surgical procedure. Before the start of recording sessions, rats were anesthetized with a single dose of pentobarbital (50 mg/kg, i.p.). The position of the head was affixed off the ground via the head bolt so that the whiskers were free of obstruction. Use of a multiwhisker deflector allowed rats to be left primarily unperturbed throughout the duration of the experiment, which lasted between 2.5 and 4 hr depending on the stimulus protocol used. Anesthetic state was assessed by a number of factors; rats did not exhibit a blink reflex or make any nonbreathing-related movement, including whisker movements, nor did we observe a substantive increase in background activity associated with awake states. After recording sessions, animals required several more hours to recover fully from anesthesia once placed into their home cage. At no time was there any indication that the animals were in any discomfort. In cases when animals were used in multiple stimulus protocols, several days were allowed to elapse between sessions.
A Many Neuron Acquisition Processor (MNAP; Plexon Inc., Dallas, TX) was used to simultaneously record neural activity from multiple microwires, digitizing waveforms at 40 kHz. Single-unit activity with a minimum 3:1 signal-to-noise ratio was discriminated using an amplitude threshold in combination with two time–voltage windows (Nicolelis et al., 1997). Off-line analysis of waveforms and interspike interval statistics were used to confirm on-line discriminations. All whisker stimuli were presented using a computer-controlled, multiwhisker deflector consisting of 16 independently drivable and positionable stimulators that deflected whiskers in the caudal to rostral direction (Krupa et al., 2001). This multiwhisker deflector was controlled by the program “Tempo” (Reflective Computing, St. Louis, MO), which sent time-stamped stimulus-related events to the MNAP.
Vibrissal whiskers (arranged in a matrix: rows, a to e dorsoventrally; and columns, 0–5 caudorostrally) were stimulated using three separate protocols. The first protocol involved stimulating all possible combinations of 1, 2, 3, and 4 whiskers within each of four ipsilateral whisker columns tested (0, 1, 2, and 3 columns tested on the b, c, d, and e whiskers). This protocol was used to investigate cortical activity that might arise from interactions between ipsilateral whiskers within a given whisker column. The second protocol tested whether interactions occurred between any two ipsilateral whisker columns (e.g., b3–e3 and b2–e2) by testing all paired combinations of the same four ipsilateral whisker columns in the first protocol. Paired combinations of ipsilateral whisker column stimuli were delivered either simultaneously or with a 60 msec interstimulus interval (ISI) between the first and the second whisker column stimuli.
To investigate the nature of bilateral interactions within the barrel cortex, we used a third stimulus protocol, which delivered a whisker column stimulus to one side of the face (the condition stimulus), followed by a whisker column stimulus to the other side of the face (the test stimulus). Condition and test stimuli were delivered to rostral (b3–e3) and caudal (b0–e0) whisker columns on both sides of the face, using ISIs that ranged from 0 to 210 msec. Three parameters of bilateral whisker stimuli were varied. The first parameter varied was the hemispheric sequence of stimulation (with respect to the recording site) of condition and test stimuli: either ipsilateral stimulation followed by contralateral stimulation or contralateral stimulation followed by ipsilateral stimulation. The second parameter varied was the ISI between condition and test stimuli. The final parameter of bilateral stimulation varied was the spatial location on the whisker pad, either rostral or caudal, of both the condition and test stimuli. Varying the spatial location of whisker stimuli resulted in four possible condition–test pairings; rostral–rostral (R–R), rostral–caudal (R–C), caudal–rostral (C–R), and finally, caudal–caudal (C–C) condition and test locations, respectively.
Stimulus configurations within a given protocol were delivered in a randomized order at an intertrial interval of 1 Hz until 200 (for ipsilateral only and inactivation protocols) or 300 (bilateral protocol) trials of each stimulus configuration were delivered. All whisker deflections were 5 msec in duration. Significant stimulus-induced responses in excess of a 95% confidence interval were identified by a semi-automated MATLAB (MathWorks Inc., Natick, MA) routine that allowed for the onset and offset of neuronal responses to be set by the user. The confidence interval was calculated using a bootstrap algorithm that used 10,000 replicates of the data, which included 100 msec of prestimulus and poststimulus time (Efron and Tibshirani, 1994). The interval between the onset and the offset of response, measured across all trials, was defined as the response window. The response probability of a neuron was defined as the number of trials that elicited a spike(s) in the response window divided by the total number of trials, multiplied by 100. Across trials that elicited at least one spike in the response window, the average number of spikes fired per trial was also calculated. Minimal latency was defined as the onset of the response window. All post hoctests were Fisher's PLSD unless otherwise specified. Animals were later killed and perfused, and electrolytic lesions were made on certain electrodes to allow placement of microwires to be confirmed histologically (Fig. 1B). Brains were sectioned coronally and then stained with cresyl violet and Prussian blue. All electrodes from which recordings were reported were localized to layer V.
Null hypotheses tested. Under bilateral stimulus conditions, two null hypotheses regarding the effect of condition stimuli on latter test-evoked responses were investigated. The first null hypothesis supposed that ipsilaterally and contralaterally evoked responses were non-interacting. Therefore, under bilateral stimulation, the expected response probability to the test stimulus after a condition stimulus was taken to be the response probability elicited by the test stimulus when given alone. The second null hypothesis supposed that neurons simply could not respond to the test stimulus when having already responded on the same trial to the condition stimulus. The expected probability of responding under this hypothesis was calculated by the following formula: t′ − (t′c), wheret′ is the probability of response to the test stimulus when given alone, and c is the observed probability of response to the condition stimulus during bilateral stimulation.
For simultaneous stimulation of pairs of ipsilateral whisker columns, the null hypothesis regarded the expected response probability attributable to pairing as the geometric sum (because probabilities do not add arithmetically) of the response probabilities elicited by either whisker column when given alone. The expected response probability in this condition was calculated by subtracting the product of the response probabilities elicited by the constituent whisker columns from the sum of their response probabilities.
RESULTS
A comparison of ipsilateral versus contralateral response characteristics
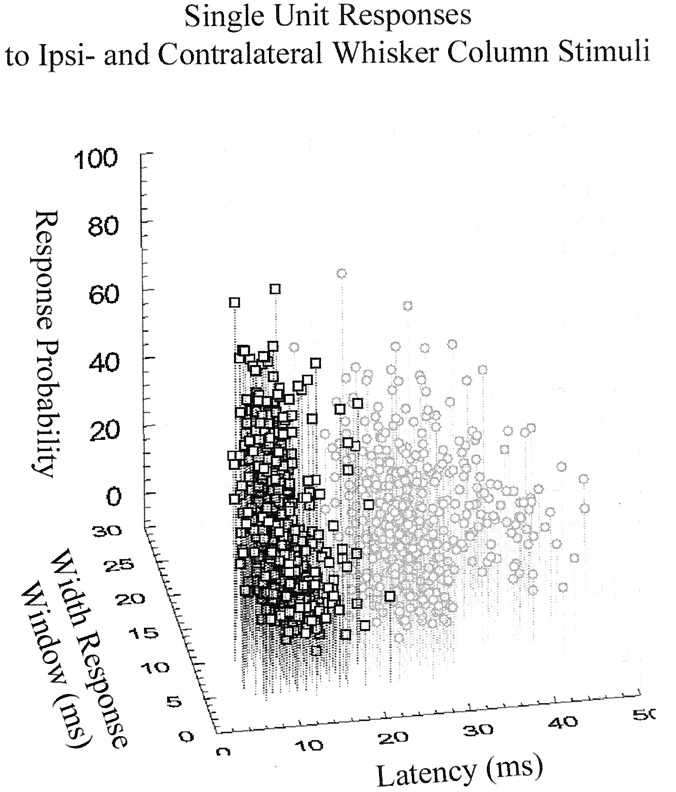
Overall, the responses to bilateral stimuli of 214 neurons (n = 11 rats, recorded from 174 electrodes) located in layer V of the SI were examined. One hundred fifty-four (72%) of these neurons responded to ipsilateral whisker column stimulation. Of these, the average evoked response probability across all ipsilateral whisker column stimuli given was 21.8 ± 13.0% (mean ± SD). In contrast, 210 neurons (98%) were responsive to contralateral whisker column stimulation, with an average evoked response probability of 30.2 ± 19.0%. On average, the minimal latency for a response elicited by an ipsilateral whisker column stimulus was 23 ± 4.7 msec compared with 11 ± 3.4 msec for responses elicited by contralateral whisker column stimuli. In trials with ipsilaterally evoked activity, SI neurons fired 1.1 ± 0.1 spikes in an average response window of 11 ± 4 msec, whereas in trials with contralaterally evoked activity, SI neurons fired 1.2 ± 0.2 spikes in a response window of 9 ± 3 msec. All neurons with identifiable ipsilateral responses were also responsive to contralateral whisker stimulation. For comparison, Figure2 plots single-unit responses to all ipsilateral and all contralateral whisker column stimuli by response probability, width of response window, and latency of response.
Fig. 2.
Single-unit responses in layer V of the rat SI to contralateral (squares) and ipsilateral (circles) whisker column stimuli. Responses evoked by contralateral and ipsilateral whisker column stimuli, taken from all animals and all neurons responding, are plotted by response probability, width of response window, and latency of response. Latency of response clearly demarcates contralaterally, from ipsilaterally evoked responses.
Response characteristics under parametrically varied ipsilateral whisker stimuli
To address what constitutes an effective suprathreshold ipsilateral whisker stimulus, we parametrically varied the number and location of simultaneously deflected whiskers ipsilateral to cortically implanted microwire arrays. The responsiveness of 40 single SI neurons (from three animals) was quantified while deflecting one, two, three, or four ipsilateral whiskers within a given whisker column. Two related, yet separate, issues were addressed: (1) whether the proportion of ipsilaterally responding neurons increased with the number of ipsilateral whiskers simultaneously stimulated, and (2) whether evoked response probabilities increased in ipsilaterally responsive neurons as additional whiskers were simultaneously deflected. Finally, the effect of simultaneously deflecting additional whiskers on response latency was also quantified.
With regard to the first issue, 35% of these SI neurons exhibited significant responses during stimulation of at least one of 16 individual ipsilateral whiskers. Stimulation of pairs of whiskers within an ipsilateral whisker column increased the percentage of neurons responding to 58%; stimulus combinations of three ipsilateral whiskers resulted in 65% responsive neurons, whereas stimulation of four whiskers within a column culminated in 75% of neurons displaying ipsilateral responses. Thus, a major effect of stimulating multiple whiskers within an ipsilateral whisker column was in the recruitment of an ever-larger proportion of responsive neurons. We also examined whether neurons were responsive to stimulation of multiple, ipsilateral whisker columns. On average, layer V SI neurons responded to stimulation of 2.4 ± 1.0 ipsilateral whisker columns.
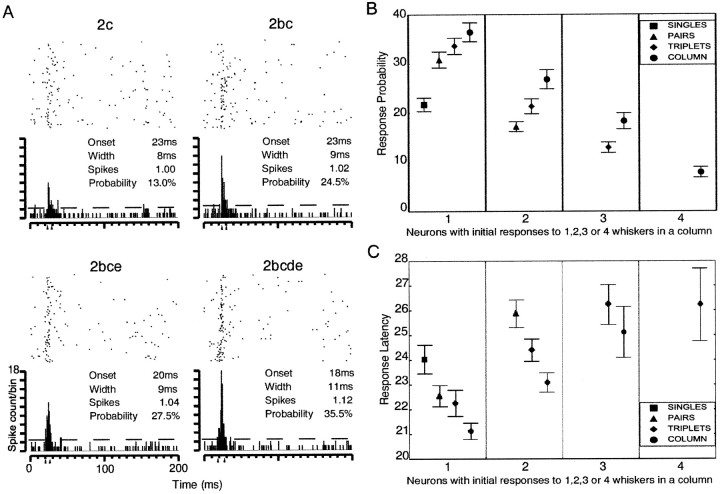
Because the proportion of responding neurons changed with the number of whiskers stimulated, to address the second issue, we first categorized neurons into those initially responsive to single, pairs, triplets, or all whiskers within a whisker column. Neurons that were initially responsive to single ipsilateral whisker stimuli tended to increase their firing probability as whiskers were added to the whisker column (Fig.3A,B). This observation also held for those neurons initially responsive to stimulation of pairs of whiskers (Fig. 3B). Furthermore, neurons responsive initially to only combinations of three whiskers within a column also increased their firing probabilities as the fourth whisker was added to the whisker column stimulus (Fig. 3B). Overall, whiskers stimulated as a column resulted in response probabilities that were greater than the strongest responding constituent whisker when stimulated alone (means ± SEM; column, 24.3 ± 1%; constituent, 15.7 ± 0.8%; paired ttest; t(179) = 13.476;p < 0.001).
Fig. 3.
Single-unit, layer V SI neuron responses to ipsilateral whisker stimuli. A, The response of a cortical neuron to ipsilateral stimuli of 1, 2, 3, and 4 whiskers within an ipsilateral whisker column shown as raster plots with accompanying peristimulus time histograms (PSTHs). Dashed line in PSTHs indicates 95% confidence interval;arrowheads indicate the onset of the response window and the offset of the response window. The onset and width of the response window, the average number of spikes fired across trials with spikes in the response window, and the probability of response are given.B, Adding whiskers to an ipsilateral column stimulus increases response probabilities of neurons. The mean response probability is shown on the y-axis. Neural records of ipsilateral responses are divided into four categories depending on whether the neuron first responded to 1, 2, 3, or 4 whiskers within a given whisker column. Where appropriate, the mean response ± SEM probability evoked by 1 (squares), 2 (triangles), 3 (diamonds), and 4 whiskers (circles) are plotted. C, The latency of response decreases as whiskers are added to a whisker column stimulus. Latency is shown on the y-axis (in milliseconds). Latencies are divided into four categories as in B. Mean ± SEM response latency evoked by 1 (squares), 2 (triangles), 3 (diamonds), and 4 whiskers (circles) are plotted.
Whereas response probabilities increased as more whiskers were stimulated within a whisker column, response latencies (Fig.3C) decreased as whiskers were added. Collectively, the response to whiskers stimulated as a column was significantly earlier than the earliest latency evoked by a constituent whisker (means ± SEM; column, 22.5 ± 0.2 msec; constituent, 24.2 ± 0.335 msec; paired t test; t(151)= −8.855; p < 0.001). Figure 3C also depicts how the average latency to a given number of whiskers being stimulated increases across neurons initially responsive to single, pairs, triplets, or all whiskers within a whisker column.
Bilateral interactions
The relationship of spatial and temporal attributes of bilateral stimuli to evoked cortical activity
Having identified ipsilaterally evoked whisker responses in SI neurons, we next addressed how such responses impinge on subsequent contralaterally evoked activity and vice versa. The major effect that previous stimulation had on responses evoked by subsequent stimulation was that of attenuating response probability (Fig.4). Figure 4 illustrates the condition–test stimulus paradigm used and provides the response profiles of two neurons, simultaneously recorded in either hemisphere during bilateral and (for comparison) unilateral stimulus conditions. Three parameters of bilateral whisker stimuli were varied: the hemispheric sequence, the ISI, and the spatial location of whisker stimuli. Parametric variation of these factors allowed us to test the null hypotheses that the hemispheric, temporal, and spatial relationships between bilateral whisker stimuli do not affect the firing probabilities of SI neurons to the test stimulus. The impact these parameters of bilateral stimulation exert on the responses probabilities of SI neurons to test stimuli are summarized statistically as a multifactor ANOVA in Table1 and are depicted graphically in Figure5A–D (ANOVA and figures use all 144 neurons recorded under these conditions, taken from seven animals). The percentage change from expected response probability is plotted separately in Figure 5 for responses evoked by contralateral test stimuli after ipsilateral condition stimuli (CIC responses) and, conversely, for responses evoked by ipsilateral test stimuli after contralateral condition stimuli (ICI responses).
Fig. 4.
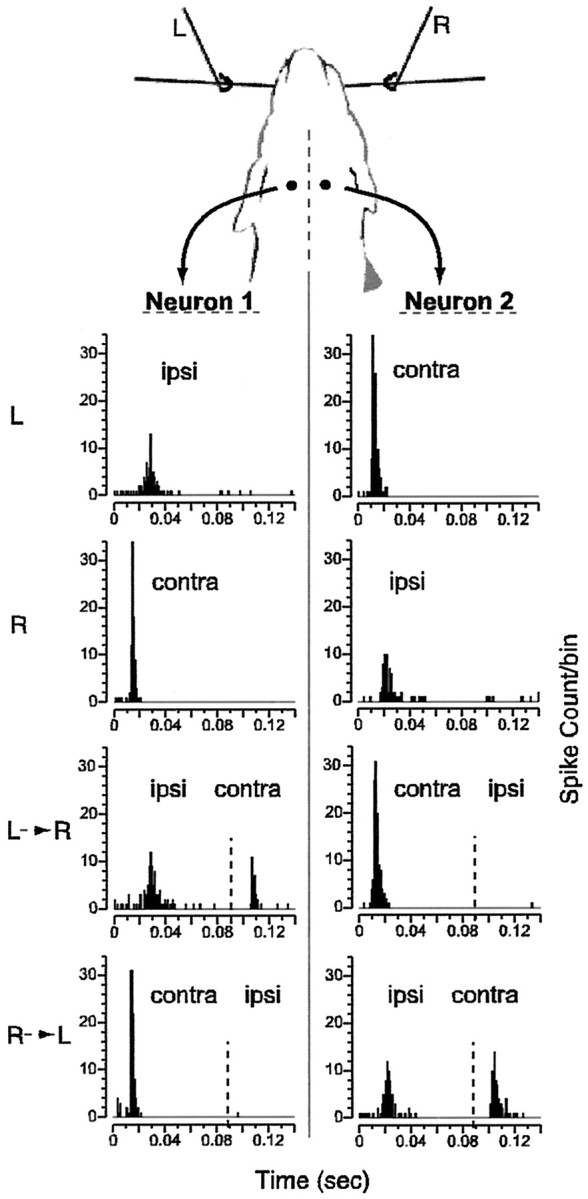
Schematic of bilateral whisker stimulation and neural responses. Left (L) and right (R) whisker columns (whiskers b3, c3, d3, and e3) are stimulated while simultaneously recording from left (Neuron 1) and right (Neuron 2) layer V, SI neurons. Evoked responses in neuron 1 and 2 are shown for four stimulus conditions: left whisker column alone (L), right whisker column alone (R), left then right whisker columns (L→R), and finally, right then left whisker columns (R→L) (dashed vertical line indicates time of second stimulus). Peaks in PSTHs labeled with ipsi andcontra where appropriate. Spike count per 1 msec bin of time is given along the y-axis (300 presentations of each stimulus configuration were given). Previous ipsilateral stimulation greatly reduced contralateral responses, whereas previous contralateral stimulation completely eliminated ipsilateral responses at this ISI (90 msec).
Table 1.
ANOVA for percentage chance from expected response probability
| DF | F value | p value | |
|---|---|---|---|
| Hemispheric sequence | 1 | 1635.850 | <0.0001 |
| ISI | 6 | 89.855 | <0.0001 |
| Spatial location | 3 | 25.351 | <0.0001 |
| Hemispheric sequence * ISI | 6 | 7.479 | <0.0001 |
| Hemispheric sequence * spatial location | 3 | 8.711 | <0.0001 |
| ISI * spatial location | 18 | 2.997 | <0.0001 |
| Hemispheric sequence * ISI * spatial | 18 | 4.022 | <0.0001 |
| Residual | 2116 |
Analysis of a three-way ANOVA of the data depicted graphically in Figure 5A–D demonstrates significant effects (p < 0.0001) of the three main factors (hemispheric sequence, ISI, and spatial location), as well as for their interaction terms (p < 0.0001).
Fig. 5.
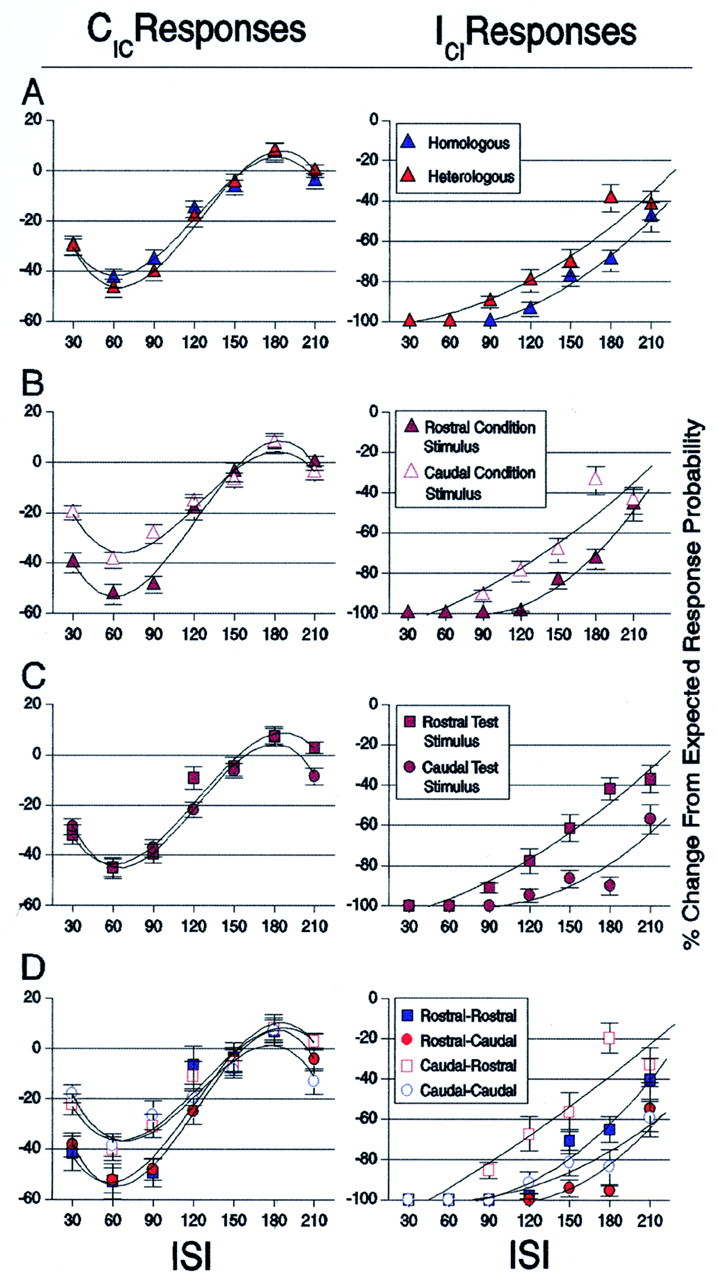
Bilateral interaction functions relating the influence that the spatial location of condition and test stimuli had on test-evoked responses. x-Axis, Interstimulus interval between condition and test stimuli. y-Axis, % Change From Expected Response Probability is defined as the percentage difference between the response evoked to the test stimulus when given alone by the response evoked to the test stimulus in the condition–test paradigm. Had the parameters of bilateral stimulation examined not affected response probabilities evoked by test stimuli, all observations would have been observed to fall at zero along theabscissa. CIC and ICI data (mean ± SEM) are fit with third- and second-order polynomials, respectively, for illustrative purposes only. A, The effect of homologous (R–R and C–C) versus heterologous (R–C and C–R) condition–test stimuli (blue vs red symbols) on CIC and ICI responses.B, The effect of rostral versus caudal condition stimuli (filled vs open symbols) on CIC and ICI responses. Test-evoked responses are plotted regardless of the position of test stimuli.C, The effect of rostral versus caudal test-evoked responses (squares vs circles) after condition stimulation. Rostral and caudal test-evoked responses are plotted regardless of the position of the condition stimulus.D, Bilateral interaction functions of test-evoked responses for all possible spatial configurations of condition–test stimuli (symbols follow the scheme used inA–C). Here, the spatial location of condition, as well as test stimuli, is taken into account when plotting response probability.
As shown in Table 1, all three parameters of bilateral stimulation significantly influenced test-evoked responses when compared with expected values. The first main factor tested, the hemispheric sequence of bilateral stimulation, indicated a significant difference between the amount of attenuation of CIC and ICI response probabilities. ICI responses exhibited a significantly greater attenuation (−82.0 ± 1.2%) than CICresponses (−20.2 ± 1.1%), with a mean difference of 61.8% (p < 0.0001). Both CICand ICI responses were significantly different from expected values under the first null hypothesis tested: that previous condition stimulation would have no effect on test-evoked responses (p < 0.0001). The second main factor tested was the effect of ISI on response probability. Response probability was greatly attenuated at short ISIs and showed a trend to recovery at longer ISIs (p < 0.0001). Finally, not all spatial configurations affected response probability equivalently, as indicated by the significant difference observed for the third main factor, that is, the spatial location of condition and test stimuli (p < 0.0001).
The three, two-way interaction terms of the ANOVA also yielded significant differences. The first two-way interaction term, “hemispheric sequence * ISI,” indicated that the functions of recovery are differently shaped for CIC and ICI responses. The second interaction term, “hemispheric sequence * spatial location,” indicated that the order and/or magnitude of attenuation observed with regard to the spatial location of condition and test stimuli was not the same for CIC and ICI responses. These interaction terms together indicate that the differences between CIC and ICI functions differ in the overall shape and not simply as a shift along thex-axis that might be expected because of the response latency differences alone. The third interaction term, “ISI * spatial location,” indicated that the shape of recovery functions was significantly different with respect to the spatial location of condition and test stimuli. Finally, the three-way interaction of the main factors tested indicated a significant difference in the shape of CIC and ICI functions of response recovery with respect to the spatial locations of condition and test stimuli.
In an effort to isolate the influences imposed on test-evoked responses by the various spatial attributes of bilateral stimuli, we plotted the same data analyzed in Table 1 under four different spatial groupings of condition and test stimuli (Fig. 5A–D). The first grouping, shown in Figure 5A, categorizes observations as arising from spatially homologous (R–R and C–C) or heterologous (R–C and C–R) condition–test pairings of whisker stimuli. This categorization allowed us to test the possibility that homologous bilateral stimuli would result in stronger attenuation of test-evoked response probability than attenuation resulting from heterologous bilateral stimuli. This hypothesis was based on previous experiments that demonstrated an anatomical and functional homotopy between the whisker barrel cortices (White and DeAmicis, 1977; Pidoux and Verley, 1979;Olavarria et al., 1984; Welker et al., 1988; Koralek et al., 1990; Cauller et al., 1998). To the contrary, no significant differences were observed between homologous and heterologous bilateral stimuli for CIC responses throughout the time course of recovery. However, ICI responses were more greatly attenuated under homologous, than heterologous stimulation, with a mean difference of 8.46% (F(1,847) = 19.424; p< 0.0001).
The second group (Fig. 5B) categorizes test-evoked responses after condition stimulation of rostral versus caudal whiskers, regardless of the location of the test stimuli. Division of the data in this manner was made to determine whether asymmetries exist regarding the magnitude of influence exerted by rostral versus caudal condition stimuli. Under such a categorization, the functions of recovery of CIC responses after rostral and caudal ipsilateral stimulation were significantly different (F(6,1297) = 4.287; p< 0.0003). CIC responses after rostral ipsilateral stimulation were more strongly attenuated (by a mean difference of 7.96%) than CIC responses after caudal ipsilateral stimulation (F(1,1297) = 12.184; p< 0.0005). The same result held for ICIresponses, because they too were more strongly attenuated by previous rostral, than by previous caudal, contralateral stimulation, with a mean difference of 8.36% (F(6,847) = 29.875; p < 0.0001).
The third group categorized observations that arose from rostral or caudal test stimuli, regardless of the spatial location of condition stimuli (Fig. 5C). Categorizing the data in this manner tested whether differences exist between the resilience of rostral and caudal test-evoked responses after condition stimulation. No significant differences were found for CICresponses evoked by rostral or caudal contralateral stimulation. However, rostrally evoked ICI responses appeared to be more resilient to previous contralateral stimulation than caudally evoked ICI responses, with a mean difference of 16.7% (F(1,847) = 60.340; p < 0.0001).
The fourth group categorized observations by all four possible spatial pairings of condition and test stimuli (Fig. 5D). Whereas the effect location of whisker stimuli exerted on test-evoked responses was identified separately for condition and for test stimuli in Figure5, B and C, Figure 5D illustrates the interaction between the various locations of condition and test stimuli in determining test-evoked responses. For CIC responses, both R–R and R–C pairings were significantly different from both C–R and C–C pairings, although they were not significantly different from each other (pvalues ≤ 0.0128 for above comparisons). Because no differences attributable to location of test stimuli were observed for CIC responses in Figure 5C, perhaps this result reflects only the difference of the placement of ipsilateral condition stimuli on CIC responses. For ICI responses, however, rostral whisker locations induced greater attenuation as condition stimuli and were more resilient as test stimuli than were caudal whisker locations. The interaction between the placement of condition and test stimuli for ICI responses, therefore, resulted in R–C and C–C condition–test pairs showing the greatest attenuation of response probability, followed by observations under R–R stimuli, and finally observations under C–R stimulation (p values ≤ 0.0026).
The impact of stimulus-induced inhibition in the SI
Given attenuation of test-evoked response probabilities after condition stimuli, we next postulated that such reductions could be explained by assuming that neurons, having fired previously to the condition stimulus, simply could not fire to the test stimulus within the same trial. By adjusting expected response probabilities to reflect this hypothesis (see Materials and Methods, second null hypothesis), CIC and ICI responses remained significantly different from expected values (F(6,2159) = 4.693; p≤ 0.0001). Therefore, even on those trials in which neurons did not fire to the condition stimulus, the probability of firing to the test stimulus was still diminished.
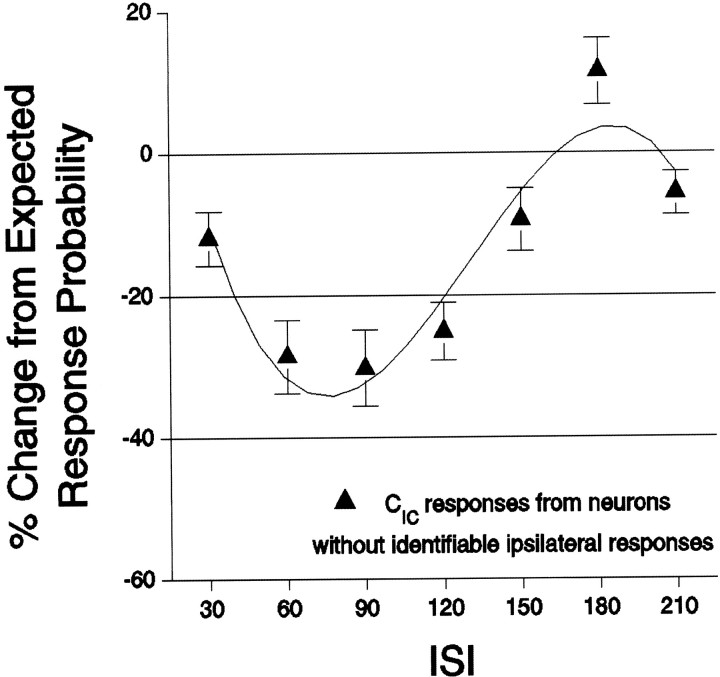
Even for neurons without any identifiable ipsilateral response, previous ipsilateral stimulation significantly reduced contralaterally evoked response probabilities, with a similar time course as that shown for CIC responses in Figure 5B(F(6,459) = 9.7; p ≤ 0.0001) (Fig. 6). These findings indicate that a far greater portion of SI neurons was affected by ipsilateral stimuli than would have been determined by measuring only suprathreshold ipsilateral responses. In fact, of 144 neurons recorded under bilateral whisker stimulation, 95% exhibited contralateral response probabilities after ipsilateral stimuli that were less than response probabilities evoked by the same contralateral stimulus before ipsilateral stimulation (one-sample sign test; p < 0.0001).
Fig. 6.
CIC responses are affected even in neurons without identifiable ipsilaterally evoked responses. Labeling conventions are the same as in Figure 5.
Impact on test-evoked response latencies
In addition to quantifying the impact of bilateral whisker stimulation on test-evoked response probability, the impact on the latency of response to test stimuli was also examined. The presence of a previous stimulus was observed to slow the response to a subsequent stimulus. Modest increases in response latency were observed for CIC and ICI test-evoked responses (p values < 0.001), and these increases were significantly different from each other (unpairedt test; unequal variance;t(210) = 8.15; p < 0.001). The mean increase in response latency for CIC responses was 0.98 ± 0.05 msec, whereas the mean increase in response latency for ICIresponses was 3.24 ± 0.27 msec across ISIs.
Response to bilaterally synchronous whisker stimulation
Given the strong attenuation of ICI so identified, perhaps it can be deduced from the difference in latencies of ipsilaterally and contralaterally evoked activity that bilaterally synchronous whisker stimulation would result in only contralateral responses in both SIs. Indeed, the probability and latency of response to a bilaterally synchronous whisker stimulus was similar to that evoked by the contralateral stimulus when given alone. The responses probability of bilaterally synchronous and contralateral only stimuli were 29.1 ± 0.7 versus 28.3 ± 0.7%, respectively, a mere 0.8% difference. Latencies were likewise 10.6 ± 0.007 versus 10.9 ± 0.007 msec for bilaterally synchronous and contralateral only stimuli, differing by 0.3 msec. However, although the effect size was very small in both instances, comparisons of both probability and latency were significant (paired t tests; means,t(501) = 3.208, p < 0.001; latencies, t(501) = −4.951,p < 0.001).
Ipsilateral–ipsilateral interactions
Considering the influence that ipsilateral stimulation exerts on contralaterally evoked responses, the possibility of interaction between dual, ipsilateral whisker column stimulation was investigated within the barrel cortex. We recorded the responses of 63 single units (from five animals, using 44 electrodes) to ipsilateral whisker column stimuli. Four ipsilateral whisker columns were stimulated individually and in all possible paired combinations using ISIs of 0 and 60 msec. The proportion of these neurons responding to at least one ipsilateral whisker column was 68%; this increased to 81% under synchronous stimulation of paired whisker columns.
Next, we addressed whether observed response probability evoked by synchronously paired whisker column stimuli differed from the geometric sum of response probabilities evoked by the constituent whisker columns when stimulated alone. Deviations from the geometric sum would indicate dependence between ipsilateral column stimuli, either facilitating or suppressing the response probabilities of the neurons. This comparison was performed for recordings from SI neurons that responded to both the constituent and paired whisker column stimuli. The average response probability evoked by constituent whisker columns was 20.5 ± 1.1% and significantly increased to 28.45 ± 1.5% with pairing (unpaired t test; t(139) = −3.938; p < 0.0001). However, this increase in evoked response probability was significantly less than the 36.4 ± 1.5% expected under the null hypothesis, which assumed constituent whisker columns to be independent of one another (paired t test;t(46) = −6.649; p < 0.0001).
In addition, previous ipsilateral whisker column stimulation strongly inhibited cortical responses to any subsequent ipsilateral whisker column stimulus, regardless of spatial location. This effect was so dominant that stimulus-evoked response to the second of the two ipsilateral whisker columns was rarely measurable; hence, no additional analyses were performed on these data. However, as similarly indicated for bilateral stimuli, even in cases in which ipsilateral stimulation was not observed to produce a suprathreshold response, responses to subsequent ipsilateral stimuli were nonetheless inhibited.
Effect of unilateral inactivation on intact SI responses to bilateral whisker stimulation
Because the two whisker barrel fields are connected by the corpus callosum, we tested the hypothesis that the opposite SI was the source of ipsilateral input observed in this study. According to this hypothesis, inactivation should not only eliminate ipsilaterally evoked activity, but in so doing, should negate the influences such activity would subsequently exert on the intact SI (Fig.7). Infusion of muscimol into the SI completely abolished all single-unit, and nearly abolished all multiunit, responses to ipsilateral stimuli in the intact hemisphere of five animals. Of 53 observations of ipsilaterally evoked neural responses (recorded from five animals, 43 electrodes), only two multiunit responses showed signs, although greatly reduced, of ipsilaterally evoked activity after infusion of muscimol into the opposite barrel cortex. In control experiments, infusion of saline in the SI resulted in no loss of ipsilaterally evoked responses in single-unit and multiunit recordings in the opposite hemisphere (17 single units, eight multiunits, two animals). In addition to eliminating ipsilateral responses, muscimol inactivation negated the influence wrought by previous ipsilateral stimulation on contralaterally evoked activity. Whereas contralateral response probabilities after ipsilateral stimulation were diminished by an average of 32.5 ± 3.2% (±SEM) before muscimol inactivation (135 observations, 17 neurons, two animals), responses from the same single units after muscimol inactivation resulted in a mere 2.6 ± 1.8% (±SEM) diminution across ISIs tested (30 and 120 msec) (pairedt test; t(135) = −9.693;p < 0.0001).
Fig. 7.
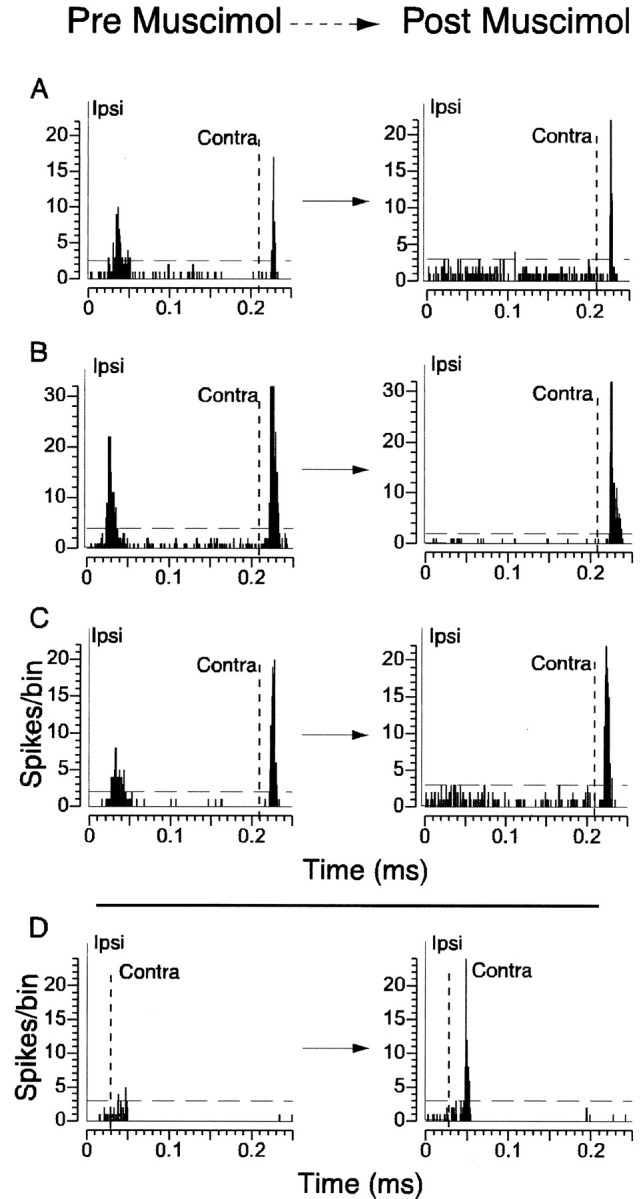
Infusion of muscimol into the barrel cortex abolishes ipsilaterally evoked responses in the intact barrel cortex and negates the influence ipsilaterally induced activity exerts on subsequent contralaterally evoked responses. PSTHs are shown for four neurons, recorded simultaneously from one animal, before and after muscimol injection into the opposite barrel cortex. Solid vertical line at 0 msec indicates time of the ipsilateral stimulus (Ipsi), and the dashed vertical line indicates time of the contralateral stimulus (Contra). A–C, After muscimol infusion, ipsilaterally evoked responses are completely abolished, whereas contralaterally evoked responses remain intact. D, Before muscimol, this neuron exhibited no response when ipsilateral preceded contralateral stimulation by 30 msec. After muscimol, the inhibition that had once been induced by ipsilateral stimulation is negated, resulting in the unmasking of the response of the neuron to the contralateral stimulus.
DISCUSSION
This study provides the first report characterizing the response probability, latency, spikes fired in response, and precision of firing of layer V SI neurons to ipsilateral and bilateral multiwhisker stimuli. The proportion of layer V neurons that responded to ipsilateral input, as well as their associated response probability, was found to increase as a function of the number of whiskers stimulated. These increases in response probability were accompanied by modest decreases in response latency. Furthermore, SI neurons were shown to integrate bilateral tactile information according to hemispheric sequence, spatial location, and relative timing of bilateral stimuli. Interactions occurred not only between bilateral stimuli but also between combinations of ipsilateral stimuli. Our results also indicated that the source of ipsilateral input is the opposite barrel cortex by demonstrating an almost complete abolishment of ipsilaterally evoked responses and their effects on contralaterally evoked activity after inactivation of the opposite barrel cortex.
Identifying ipsilaterally evoked activity and its nonlinear response properties
Since 1979, Pidoux and Verley's qualitative finding that the SI is responsive to ipsilateral whisker stimulation has garnered little consideration in models of barrel cortical function, presumably attributable to the lack of replication in single-unit recordings by others. Here, we provide strong confirmation and further expand on this earlier work. We attribute our ability to identify ipsilateral single-unit responses to a number of factors. First, the use of a computer-controlled stimulating device minimized perturbations to the animal, permitting recordings to be made during anesthesia. Ipsilaterally evoked responses were not observed if the animal was deeply anesthetized, as was the case during surgery or during preparation for recording sessions immediately after injection of anesthesia. Second, the use of multi-electrode recordings and the multiwhisker stimulator allowed greater numbers of neurons to be recorded under spatiotemporally varying stimuli drawn from a large portion of the whisker pad. By automating whisker stimulation, many more repetitions of nominally the same stimulus could be performed, increasing our statistical power. Furthermore, by randomly interleaving stimuli, the effect of any potential nonstationarities in anesthesia level was controlled.
Perhaps the absence of studies regarding ipsilaterally evoked activity in processing whisker information is also attributable to the pervading view that each SI represents only the contralateral whisker pad. Nonetheless, by characterizing the response properties of layer V SI neurons to numerous combinations of parametrically varied ipsilateral whisker stimuli, we provide evidence that the rat SI is involved in the integration of ipsilateral and bilateral whisker stimuli. Increasing the numbers of ipsilateral whiskers simultaneously stimulated resulted in an increase in the proportion of neurons that exhibited ipsilaterally evoked responses. Additionally, the simultaneous deflection of combinations of ipsilateral whisker columns increased response probability and decreased response latency when compared with that elicited by stimulating constituent whiskers separately. However, such increases in evoked response probability were decidedly sublinear to expected values, had the components of the whisker stimulus been independent of one another. Indeed, separating ipsilateral whisker column stimuli in time further identified a temporal dependency between ipsilateral stimuli. Whether these observed interactions are attributable to the cortex or to any combination of structures along the neuroaxis was, however, indeterminable. Nonetheless, the number and temporal order of stimulated ipsilateral whiskers nonlinearly affected the proportion of ipsilaterally responsive neurons, as well as their evoked-response probabilities.
Identifying the source of ipsilaterally evoked activity
Although the whisker-to-barrel pathway is generally believed to be lateralized subcortically, the presence of yet unidentified whisker-related ipsilateral pathways could confound the interpretation of the present results. For instance, the identification and impact of ipsilaterally evoked activity in the hindpaw region of the rat barrel cortex has been demonstrated by Armstrong-James and George (1988), yet these authors concluded that the pathways from the ipsilateral and contralateral paws converge subcortically. Their conclusion was based on the observation that inactivation of the homologous site in the opposite hemisphere failed to eliminate such responses. Additionally, retrograde axonal tracing failed to identify callosal connections between these hemispherically homologous cortical regions. However, subcortical, whisker-related ipsilateral pathways to the SI have not been found by either anatomical or electrophysiological methods. Thus, whereas the existence of callosal connections between the whisker barrel fields is well established, no subcortical ipsilateral pathways are known to exist. Also, there are no reports of either contralaterally evoked whisker responses in trigeminal whisker-related nuclei or ipsilaterally evoked responses in whisker-related thalamic nuclei. In other systems and species, ipsilaterally evoked activity in primary sensory cortices has been widely accounted for by transcallosal pathways (Berlucchi et al., 1967; Swadlow, 1974; Manzoni et al., 1989;Iwamura et al., 1994; Schnitzler et al., 1995; Clarey et al., 1996;Swadlow and Hicks, 1997).
In the present study, we demonstrated by pharmacological inactivation of one SI the elimination of ipsilaterally evoked whisker responses in the intact SI, supporting the proposition that the SIs provide one another with ipsilateral whisker information via callosal connections. We further provide evidence that unilateral inactivation of one SI removes, in the intact SI, the suppressive influence previous ipsilateral stimulation exerts on contralaterally evoked activity. Because callosal connections are thought to be excitatory in the rat cortex (Cipolloni and Peters, 1983; Giuffrida and Rustioni, 1989), it is not likely that callosally transmitted ipsilateral input directly contributes to the subsequent inhibition in the opposite hemisphere. Rather, we propose that such inhibitory influence arises locally as a consequence of callosal input, although other sources of inhibition dependent on callosal activity cannot be ruled out.
Bilateral interactions in SI
Although previous studies have provided important insights into the temporal and spatial dynamics of cortical interactions (Simons, 1985; Armstrong-James and George, 1988; Simons and Carvell, 1989;Brumberg et al., 1996; Ghazanfar and Nicolelis, 1997; Jancke et al., 1999), interpretations of these findings are limited because integration ascribed to the cortex may in part be attributable to subcortical processes. Whatever the nature of subcortical processes on ascending whisker information, cortical interactions may be addressed by exploiting the role the corpus callosum plays in integrating sensory information that is lateralized subcortically. Although important insights into the properties of callosal inputs have been made (Swadlow, 1974, 1977, 1990), the function of transcallosal activity remains primarily unknown. Experiments using gross manipulations, such as dennervation or reversible inactivation, have generally attributed such activity to a modulatory role (Clarey et al., 1996; Shin et al., 1997). We investigated directly the spatial and temporal characteristics of cortical interaction between thalamic and callosal inputs by parametrically varying the attributes of bilateral whisker stimuli. The identification of ipsilaterally and contralaterally evoked interactions, being shown to be dependent on the spatial and temporal inter-relationship of left- and right-side stimuli, strongly supports our notion that the barrel cortices integrate whisker stimuli bilaterally. Furthermore, the demonstration of differences in cortical activity in response to bilaterally heterologous whisker stimuli is a substantial departure from previous works that demonstrate bilateral influence on responses restricted to homotopic receptive fields (Pidoux, Verley, 1979; Armstrong-James, George, 1988; Calford and Tweedale, 1988; Iwamura et al., 1994; Clarey et al., 1996; Shin et al., 1997).
These findings present a challenge to the notion that the barrel cortices simply process contralateral aspects of whisker stimuli. Cortical responses evoked by multiwhisker stimulation differ substantially from those assumed previously to arise strictly as a result of the topographic superposition of individually evoked contralateral responses. We interpret such deviations from expected values as evidence of cortical integration of ipsilateral and bilateral whisker stimuli. This finding provides fertile ground for additional needed research regarding the contribution of intrinsic properties of neuronal excitability after subthreshold or suprathreshold depolarization under bilateral stimulation, such that has been determined for contralateral stimuli by Zhu and Connors (1999). We demonstrate that stimulating whiskers on one side of the face evokes contralateral activity that is followed by an ipsilateral “echo.” Contralateral and ipsilateral responses induce attenuation of subsequent responses as a function of timing, as well as spatial location of whisker stimuli. Hence, bilaterally asynchronous stimulation can result in a contralateral response in one hemisphere and an ipsilateral, then diminished, contralateral response in the other hemisphere. It is important to further note that the differences between the functions of response recovery cannot be accounted for by differences in response latency. Because, on average, ipsilaterally evoked responses were 12 msec later than contralaterally evoked responses, bilaterally synchronous stimulation resulted in contralateral responses in both hemispheres that occlude the ipsilateral echoes.
The myriad of interactions characterized can primarily be accounted for by proposing a small set of constraints supported by this and other works: (1) input to the SI is excitatory and homotopic for both contralateral and ipsilateral input, (2) contralateral input induces subsequent inhibition that has a spatial gradient, lessening in magnitude with distance from its center, (3) ipsilateral input evokes subsequent inhibition that is global with little or no spatial gradient, (4) ipsilaterally induced inhibition is of a greater magnitude if induced by rostral, rather than caudal, whisker stimuli, and similarly, (5) contralaterally induced inhibition is of a greater magnitude if induced by rostral, rather than caudal, whisker stimuli, (6) the strengths of rostral and caudal contralateral inputs are equivalent, in contrast to the final constraint, (7) the strength of rostral ipsilateral input is greater than the strength of caudal ipsilateral input.
ICI responses recorded under homologous–heterologous bilateral stimulation demonstrated the existence of a functional homotopy between contralateral and ipsilateral input to the barrel cortex (Fig. 5A), a finding in keeping with the notion that the SI sends approximately homotopic projections to the opposite SI. Suprathreshold responses evoked by callosal input conveying ipsilateral information are more strongly attenuated under bilaterally homologous whisker stimulation than under bilaterally heterologous whisker stimulation. This finding unifiesPidoux and Verley's (1979) conclusion that a homotopy exists between ipsilateral and contralateral whisker input (constraint 1) with that of Simons and Carvell's (1989) assertion that contralateral whisker stimulation induces a spatially diminishing gradient of inhibition (constraint 2).
In contrast to contralaterally induced inhibition, we determined that ipsilateral input induces attenuation of CICresponses globally across the SI, with little or no spatial gradient (constraint 3) (Fig. 5A). Because ipsilaterally induced attenuation seemingly lacks a spatial gradient, another area under the influence of callosal inputs from SI, such as SII, may also be involved in the spread of attenuation throughout the barrel cortex. Although ipsilaterally induced inhibition is global, rostral ipsilateral stimuli induced stronger inhibition of CIC responses than caudal ipsilateral stimuli (constraint 4) and exhibited a different time course of action (Fig.5B). This functional asymmetry in inhibition also holds for contralateral condition stimuli, with rostral condition stimuli inhibiting ICI responses more greatly than caudal condition stimuli (constraint 5) (Fig. 5B). An additional asymmetry regarding spatial location of test stimuli was determined to exist between CIC and ICIresponses. Whereas caudal and rostral test-evoked CIC responses were equivalently affected by previous ipsilateral stimulation, caudally evoked ICI responses were more greatly attenuated by condition stimuli than rostrally evoked ICIresponses (constraints 6 and 7) (Fig. 5C). These asymmetries were unexpected, and to our knowledge are not accounted for by any known anatomical asymmetries.
Changes in response probability caused by bilateral interactions could not be explained under the second null hypothesis tested, which postulates that neurons simply could not respond to the test stimulus on trials having responded previously to the condition stimulus. Furthermore, CIC responses were still attenuated even in neurons that did not have identifiable ipsilaterally evoked responses. By taking into account ipsilaterally induced suppression, 95% of neurons recorded were significantly affected by ipsilateral stimulation. These results indicate that the spatial extent of ipsilaterally induced attenuation is much greater than the spatial extent of ipsilaterally evoked suprathreshold activity.
We propose that interactions between thalamocortically ascending contralateral input and callosally converging ipsilateral input result in spatial and temporal asymmetries in activating excitatory and inhibitory elements within the SIs. Such processes may impart to the animal an ability to determine bilateral attributes of a stimulus, such as the orientation of an obstacle or diameter of an aperture. Indeed, preliminary behavioral evidence collected in our laboratory indicates that rats can, in fact, integrate bilateral whisker information to perform such tactile discrimination tasks and that such integration involves both SI cortices (Shuler et al., 2000).
Footnotes
This research was supported by National Institute of Dental Research Grant DE-11121-01 to M.A.L.N. and National Research Service Award Grant MH12570-01A1 to M.G.S. We thank Don Katz, Ben Rubin, Mark Laubach, Chris Stambaugh, Bill Hall, and David Fitzpatrick for their helpful insights and criticisms.
Correspondence should be addressed to Marshall G. Shuler, Department of Neurobiology, Box 3209, Duke University Medical Center, Durham, NC 27710. E-mail: mshuler@neuro.duke.edu.
REFERENCES
- 1.Armstrong-James M, George MJ. Bilateral receptive fields of cells in rat Sm1 cortex. Exp Brain Res. 1988;70:155–165. doi: 10.1007/BF00271857. [DOI] [PubMed] [Google Scholar]
- 2.Berlucchi G, Gazzaniga MS, Rizzolatti G. Microelectrode analysis of transfer of visual information by the corpus callosum. Arch Ital Biol. 1967;105:583–596. [PubMed] [Google Scholar]
- 3.Brumberg JC, Pinto DJ, Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. J Neurophysiol. 1996;76:130–140. doi: 10.1152/jn.1996.76.1.130. [DOI] [PubMed] [Google Scholar]
- 4.Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- 5.Cauller L, Clancy B, Connors B. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer 1. J Comp Neurol. 1998;390:297–310. [PubMed] [Google Scholar]
- 6.Cipolloni PB, Peters A. The termination of callosal fibres in the auditory cortex of the rat. A combined Golgi-electron microscope and degeneration study. J Neurocytol. 1983;12:713–726. doi: 10.1007/BF01258146. [DOI] [PubMed] [Google Scholar]
- 7.Clarey J, Tweedale R, Calford M. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- 8.Efron B, Tibshirani R. An introduction to the bootstrap. Chapman and Hall; New York: 1994. [Google Scholar]
- 9.Erzurumlu R, Killackey H. Diencephalic projections of the brainstem trigeminal complex in the rat. Neuroscience. 1980;5:1891–1901. doi: 10.1016/0306-4522(80)90037-8. [DOI] [PubMed] [Google Scholar]
- 10.Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghazanfar AA, Nicolelis MAL. Nonlinear processing of tactile information in the thalamocortical loop. J Neurophysiol. 1997;78:506–510. doi: 10.1152/jn.1997.78.1.506. [DOI] [PubMed] [Google Scholar]
- 12.Giuffrida R, Rustioni A. Glutamate and aspartate immunoreactivity in corticocortical neurons of the sensorimotor cortex of rats. Exp Brain Res. 1989;74:41–46. doi: 10.1007/BF00248278. [DOI] [PubMed] [Google Scholar]
- 13.Iwamura Y, Irki A, Tanaka M. Bilateral hand representation in the postcentral somatosensory cortex. Nature. 1994;369:554–556. doi: 10.1038/369554a0. [DOI] [PubMed] [Google Scholar]
- 14.Jancke D, Wolfram E, Dinse H, Akhaven A, Giese M, Steinhage A, Schoner G. Parametric population representation of retinal location: neuronal interaction dynamics in cat visual cortex. J Neurosci. 1999;19:9016–9028. doi: 10.1523/JNEUROSCI.19-20-09016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- 16.Koralek K, Olvarria J, Killackey H. Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol. 1990;299:133–150. doi: 10.1002/cne.902990202. [DOI] [PubMed] [Google Scholar]
- 17.Krupa D, Ghazanfar A, Nicolelis M. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupa DJ, Brisben AJ, Nicolelis MAL. A multi-channel whisker stimulator for producing spatiotemporally complex tactile stimuli. J Neurosci Methods. 2001;201:199–208. doi: 10.1016/s0165-0270(00)00345-9. [DOI] [PubMed] [Google Scholar]
- 19.Manzoni T, Barbaresi P, Conti F, Fabri M. The callosal connections of the primary somatosensory cortex and the neural bases of midline fusion. Exp Brain Res. 1989;76:251–266. doi: 10.1007/BF00247886. [DOI] [PubMed] [Google Scholar]
- 20.Nicolelis MA, Chapin JK. Spatiotemporal structure of somatosensory responses of many-neuron ensembles in the rat ventral posterior medial nucleus of the thalamus. J Neurosci. 1994;14:3511–3532. doi: 10.1523/JNEUROSCI.14-06-03511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 22.Olavarria J, van Sluyters R, Killackey H. Evidence for the complementary organization of callosal and thalamic connections within rat somatosensory cortex. Brain Res. 1984;291:364–368. doi: 10.1016/0006-8993(84)91270-8. [DOI] [PubMed] [Google Scholar]
- 23.Pidoux B, Verley R. Projections on the cortical somatic I barrel subfield from ipsilateral vibrissae in adult rodents. Electroencephalogr Clin Neurophysiol. 1979;46:715–726. doi: 10.1016/0013-4694(79)90111-1. [DOI] [PubMed] [Google Scholar]
- 24.Schnitzler A, Salmelin R, Salenius S, Jousmaki V, Hari R. Tactile information from the human hand reaches the ipsilateral primary somatosensory cortex. Neurosci Lett. 1995;200:25–28. doi: 10.1016/0304-3940(95)12065-c. [DOI] [PubMed] [Google Scholar]
- 25.Shin HC, Won CK, Jung SC, Oh S, Park S, Sohn JH. Interhemispheric modulation of sensory transmission in the primary somatosensory cortex of rats. Neurosci Lett. 1997;230:137–139. doi: 10.1016/s0304-3940(97)00486-2. [DOI] [PubMed] [Google Scholar]
- 26.Shuler MG, Krupa DJ, Nicolelis MAL. Discrimination of bilateral whisker stimuli in the freely behaving rat. Soc Neurosci Abstr. 2000;20:336.18. [Google Scholar]
- 27.Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- 28.Simons DJ, Carvell GE. Thalamocortical response transformations in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- 29.Smith R. The ascending fiber projections from the principle sensory trigeminal nucleus in the rat. J Physiol (Lond) 1973;148:423–446. doi: 10.1002/cne.901480403. [DOI] [PubMed] [Google Scholar]
- 30.Swadlow HA. Systematic variations in the conduction velocity of slowly conducting axons in the rabbit corpus callosum. Exp Neurol. 1974;43:445–451. doi: 10.1016/0014-4886(74)90183-6. [DOI] [PubMed] [Google Scholar]
- 31.Swadlow HA. Relationship of the corpus callosum to visual areas I and II of the awake rabbit. Exp Neurol. 1977;57:516–531. doi: 10.1016/0014-4886(77)90086-3. [DOI] [PubMed] [Google Scholar]
- 32.Swadlow HA. Efferent neurons and suspected interneurons in S-1 forelimb representation of the awake rabbit: receptive fields and axonal properties. J Neurophysiol. 1990;63:1477–1498. doi: 10.1152/jn.1990.63.6.1477. [DOI] [PubMed] [Google Scholar]
- 33.Swadlow HA, Hicks TP. Subthreshold receptive fields and baseline excitability of silent S1 callosal neurons in awake rabbits: contributions of AMPA/kainate and NMDA receptors. Exp Brain Res. 1997;115:403–409. doi: 10.1007/pl00005710. [DOI] [PubMed] [Google Scholar]
- 34.Waite PME. Organization of whisker responses in the rat thalamus. J Physiol (Lond) 1969;202:51–53. [PubMed] [Google Scholar]
- 35.Welker E, Hoogland P, Van der Loos H. Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res. 1988;73:411–435. doi: 10.1007/BF00248234. [DOI] [PubMed] [Google Scholar]
- 36.White E, DeAmicis R. Afferent and efferent projections of the region in mouse SmI cortex which contains the posteromedial barrel subfield. J Comp Neurol. 1977;175:455–482. doi: 10.1002/cne.901750405. [DOI] [PubMed] [Google Scholar]
- 37.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex: the description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol. 1999;81:1171–1183. doi: 10.1152/jn.1999.81.3.1171. [DOI] [PubMed] [Google Scholar]