Abstract
Mechanoelectrical transduction channels of hair cells allow for the entry of appreciable amounts of Ca2+, which regulates adaptation and triggers the mechanical activity of hair bundles. Most Ca2+ that enters transduction channels is extruded by the plasma membrane Ca2+-ATPase (PMCA), a Ca2+ pump that is highly concentrated in hair bundles and may be essential for normal hair cell function. Because PMCA isozymes and splice forms are regulated differentially and have distinct biochemical properties, we determined the identity of hair bundle PMCA in frog and rat hair cells. By screening a bullfrog saccular cDNA library, we identified abundant PMCA1b and PMCA2a clones as well as rare PMCA2b and PMCA2c clones. Using immunocytochemistry and immunoprecipitation experiments, we showed in bullfrog sacculus that PMCA1b is the major isozyme of hair cell and supporting cell basolateral membranes and that PMCA2a is the only PMCA present in hair bundles. This complete segregation of PMCA1 and PMCA2 isozymes holds for rat auditory and vestibular hair cells; PMCA2a is the only PMCA isoform in hair bundles of outer hair cells and vestibular hair cells and is the predominant PMCA of hair bundles of inner hair cells. Our data suggest that hair cells control plasma membrane Ca2+-pumping activity by targeting specific PMCA isozymes to distinct subcellular locations. Because PMCA2a is the only Ca2+ pump present at appreciable levels in hair bundles, the biochemical properties of this pump must account fully for the physiological features of transmembrane Ca2+pumping in bundles.
Keywords: hair cells, stereocilia, cochlea, calcium, calcium pump, isozymes
Ca2+ is a key modulator of mechanoelectrical transduction by hair cells, the sensory cells of the inner ear. Transduction is elicited by deflection of the hair bundle, the sensory organelle of the hair cell (for review, see Hudspeth et al., 2000). Bundle deflection leads directly to the opening of cation-selective transduction channels, which permit significant entry of Ca2+ (Lumpkin et al., 1997; Ricci and Fettiplace, 1998). Entering Ca2+ regulates both fast (Crawford et al., 1991) and slow (Eatock et al., 1987) adaptation in hair cells and also triggers bundle movements opposing the stimulus (Benser et al., 1996;Ricci et al., 2000). In addition, Ca2+ may regulate the formation of the transduction apparatus (Zhao et al., 1996).
Each hair bundle is composed of dozens to hundreds of actin-filled stereocilia and, with the exception of the mammalian cochlea, a single axonemal cilium called the kinocilium. Because the bundle is exposed to an unusual extracellular fluid, endolymph, which is high in K+ and low in Na+, Ca2+cannot be removed from bundles with Na+/Ca2+exchangers. Furthermore, stereocilia lack intracellular stores to sequester Ca2+. Consequently, hair bundles rely on mobile Ca2+ buffers (Ricci et al., 1998) and the plasma membrane Ca2+-ATPase (PMCA) to regulate Ca2+ levels in stereocilia (Lumpkin and Hudspeth, 1998; Ricci et al., 1998; Yamoah et al., 1998); indeed, the majority of the Ca2+ that enters through transduction channels is removed by PMCA (Lumpkin and Hudspeth, 1998).
Four PMCA genes (PMCA1–4) have been identified; further isoform diversity is generated by alternative splicing in two regions, A and C (Keeton et al., 1993). Although the functional consequences of alternative splicing within region A are poorly understood (Hilfiker et al., 1994), splicing in region C alters the affinity of PMCA for calmodulin (Enyedi et al., 1994) and removes or adds phosphorylation sites (Enyedi et al., 1997).
All PMCA isoforms and many of their splice variants are expressed in the mammalian cochlea (Crouch and Schulte, 1996; Furuta et al., 1998). Furthermore, hair cells contain high levels of PMCA (Crouch and Schulte, 1995; Apicella et al., 1997; Street et al., 1998; Yamoah et al., 1998); levels are particularly high in the hair bundle (Crouch and Schulte, 1995; Yamoah et al., 1998). Although the subcellular distribution of each isozyme and functional significance for expression of multiple PMCA genes remain unknown, at least one of these isozymes, PMCA2, is required for auditory and vestibular function (Kozel et al., 1998; Street et al., 1998; Takahashi and Kitamura, 1999). This requirement for PMCA2 in the inner ear, coupled with the high concentration of PMCA in bundles and essential functions of Ca2+ in transduction, led us to surmise that Ca2+ regulation in hair bundles was essential for auditory and vestibular function. To better understand the bundle Ca2+ regulation and the consequences of PMCA activity in stereocilia, we identified PMCA isoforms and splice variants in auditory and vestibular epithelia and determined that the hair bundle isoform was PMCA2a.
MATERIALS AND METHODS
Isolation of bullfrog PMCA clones. PMCA1-specific and PMCA2-specific DNA fragments were synthesized from bullfrog liver and lung RNA by RT-PCR. Total RNA was subjected to reverse transcription (Superscript II RNase H-reverse transcriptase, Life Technologies, Grand Island, NY) and was followed by PCR, using degenerate primers (5′ to 3′: Df1, GAYGCNTGYGARACNATG; Dr1, GGCGCAAGCTTRTCRTANACRTTNCKNCCCCACAT; Dr2, RAANGTRTANACYTTRTA). The amplification protocol comprised 5 min at 94°C, 30 cycles of 20 sec at 94°C, 2 min at 50°C (−0.5°C for each consecutive cycle), 2 min at 72°C, followed by 14 cycles of 20 sec at 94°C, 2 min at 50°C, 2 min at 72°C, and a final 10 min at 72°C. PCR products were cloned (TA Cloning Kit, Invitrogen, Carlsbad, CA) and sequenced. To generate probes for library screening, we amplified PMCA1 or PMCA2 fragments by PCR and labeled them with [32P]dCTP, using random hexamers (Amersham Pharmacia Biotech, Piscataway, NJ). The labeled PCR products were used to screen a bullfrog (Rana catesbeiana) sacculus cDNA library in Lambda ZAP Express (Stratagene, La Jolla, CA) constructed by Stefan Heller (Rockefeller University, New York, NY); ∼600,000 plaques were screened with each probe. To obtain insert-containing pBK-CMV phagemids, we performed in vivoexcisions on isolated clones by following the manufacturer's protocol (Stratagene).
Analysis of PMCA1 and PMCA2 splicing. Purified total RNA (RNeasy Kit, Qiagen, Valencia, CA) was primed with random hexamers and was reverse transcribed (ThermoScript RT-PCR System, Life Technologies). Nested primers that flank splice regions A and C in bullfrog PMCA1 (F1Cf1, GCAGAGAGGGAGTTACGCCG; F1Cf2, GGTCAGATCTTATGGTTTAG; F1Cr1, CATTTGAAGTCCAAGGAGC; F1Cr2, GCACTCTTCTGCCTGCTGC) and PMCA2 (F2Af1, CTCATGTGATGGAAGGCTC; F2Af2, GAAGAATGCTTGTAACAGC; F2Ar1, TGCCTA TCTGCACTGCCAG; F2Ar2, GGTCAGCTTGCCCTGTAGG; F2Cf1, CGCCTGACACAGAAGGAGG; F2Cf2, GGGAGTTAAGAAGAGGGC; F2Cr1, CTTCCTCTGCCGGGCATCC; F2Cr2, GCAGTGCCATCTCCAGC) or rat PMCA2 region A (R2A+1, GGACGGATGGTGGTGACTG; R2A+2, GCTGTGGGTGTCAACTCTC; R2A−1, AGATGGCTGTGGCGTTACC; R2A−2, ACCACCAGCACCGTCACAC) were used in PCR analysis for variability within these regions. The amplification protocol comprised 4 min at 94°C, 30 cycles of 30 sec at 94°C, 30 sec at 50–55°C, 45 sec at 72°C, and a final 10 min at 72°C.
Isozyme-selective antibodies. Antibody names, specificities, and antigens used for their generation are listed in Table1. Synthetic PMCA peptides, designed with an added N- or C-terminal cysteine residue, were used for the production of antisera (GeneMed Synthesis, South San Francisco, CA). To purify antipeptide antibodies, we coupled peptides to SulfoLink resin (Pierce, Rockville, IL) via their terminal cysteine residues at a density of 1 mg of peptide/ml of resin. Antipeptide antisera, diluted with 10 vol of 25 mm Tris, pH 8, were passed over a 0.5–1 ml peptide column three to four times. After washing the column with 20 vol of 25 mm Tris, pH 8, and 10 vol of 500 mmNaCl/25 mm Tris, pH 8, we eluted the antibodies with 100 mm glycine, pH 2.5, followed by 100 mm CAPS (3-(cyclohexylamino)-1-propane sulfonic acid), pH 11. Antibodies eluted with acid or base were neutralized, pooled, and dialyzed against PBS containing 0.02% sodium azide. The production of polyclonal antibodies specific for rat PMCA isoforms was described previously (Filoteo et al., 1997); they were subjected to affinity purification with methods similar to those described above. The monoclonal antibody NR4 (JA9) was raised against human erythrocyte PMCA and was directed toward an epitope very near the N terminus of isoform 4 (Adamo et al., 1992; Caride et al., 1996).
Table 1.
Isozyme-selective antibodies
| Name | Specificity | Antigen |
|---|---|---|
| F1N | Bullfrog PMCA1 | PMCA1 amino acids 1–96 |
| F2N | Bullfrog PMCA2 | PMCA2 amino acids 1–99 |
| F2a | Bullfrog PMCA2a, rat PMCA2a | CSSPTSASAAAAGQG (C terminus) |
| Fb | Bullfrog PMCA1b/2b, rat PMCA1b/2b/3b | CSSSPGSPIHSLETSL (C terminus) |
| F2v | Bullfrog PMCA2v | CSLPVGSTHPPSHPPAATDG (v variant) |
| NR1 | Rat PMCA1 | ANNSVAYSGVKNSIKEANC (N terminus) |
| NR2 | Rat PMCA2 | TNSDFYSKNQRNESSC (N terminus) |
| NR3 | Rat PMCA3 | ANSSIEFHPKPQQQREVPC (N terminus) |
| NR4 (JA9) | Rat PMCA4 | PMCA4 (mouse monoclonal) |
Glutathione S-transferase (GST) or Escherichia coli maltose-binding protein (MBP) was fused to N-terminal amino acids of PMCA1 (amino acids 1–96) and PMCA2 (amino acids 1–99) in the vectors pGEX-6P-1 (Amersham Pharmacia Biotech) and pMAL-p2 (New England Biolabs, Beverly, MA), respectively. Fusion proteins were expressed inE. coli and purified by glutathione or amylose affinity chromatography. Purified GST fusion proteins were injected into rabbits for the production of isoform-selective antibodies (Covance Research Products, Denver, PA). Antibodies were affinity purified on MBP-PMCA or GST-PMCA fusion protein columns, which were constructed by coupling fusion proteins to CNBr-Sepharose (Amersham Pharmacia Biotech) at a density of 1–5 mg of protein/ml of agarose. Antibodies against the N terminus of PMCA1 and PMCA2 were purified as described for antipeptide antibodies, with an additional negative selection step. To remove antibodies that recognize either PMCA1 and GST, first we passed GST-PMCA2 antiserum over a GST-PMCA1-Sepharose column. The flow through from this step subsequently was passed over an MBP-PMCA2-Sepharose column; bound antibodies were eluted as described above. Similarly, GST-PMCA1 antiserum first was passed over a GST-PMCA2-Sepharose precolumn and then purified on an MBP-PMCA1-Sepharose column.
Immunocytochemistry. Auditory and vestibular organs from postnatal day 21 (P21) or older rats were dissected in MEM (Life Technologies) with an added 25 mm HEPES, pH 7.5. Bullfrog sacculi were dissected in bullfrog saline solution [(in mm) 110 NaCl, 2 KCl, 2 MgCl2, 3d-glucose, 10 HEPES, pH 7.25] containing 4 mmCaCl2. Hair cells were isolated as described previously (Yamoah et al., 1998). Tissues or dissociated hair cells were fixed in 3% formaldehyde in saline for 30 min, washed in PBS, and then permeabilized and blocked for 1 hr in blocking solution (PBS, 0.2% saponin, 3% donkey serum, and 1% bovine serum albumin). Formaldehyde was obtained as a 16% solution in sealed ampoules from Electron Microscopy Sciences (Fort Washington, PA). Tissues were incubated overnight at room temperature with 2.5 μg/ml primary antibody in the blocking solution, washed in PBS, and then incubated with a secondary antibody (5 μg/ml Cy5-conjugated donkey anti-rabbit IgG or donkey anti-mouse IgG; Jackson ImmunoResearch Laboratories, West Grove, PA) and 0.25 μm FITC-phalloidin (Sigma, St. Louis, MO) in the blocking solution for 1–3 hr. In some experiments utriculi were colabeled with an antibody against the calcium-binding protein calretinin (1:200; Chemicon, Temecula, CA). Cells and tissues were washed in PBS, mounted with Vectashield (Vector, Burlingame, CA), and viewed with Plan Apochromat 40′ (numerical aperture, 1.00) and 60′ (numerical aperture, 1.40) oil lenses on a Nikon TE 300 inverted microscope with a Bio-Rad MRC 1024 confocal imaging system (Hercules, CA). Acquired images were processed with Scion Image (version 1.62a) and Photoshop (version 5.03; Adobe Systems, San Jose, CA).
SDS-PAGE and immunoblotting. Proteins were separated by SDS-PAGE that used 10% acrylamide gels with a 150:1 acrylamide-to-bisacrylamide ratio and were transferred to polyvinylidene fluoride blotting membranes in 7.5% methanol and 7.5 mm CAPS, pH 11, at 100 V for 2 hr with cooling. To enhance protein mobilization from the acrylamide gel, we added hemoglobin to the samples and to the pretransfer equilibration solution, as described previously (Gillespie and Gillespie, 1997). PMCA was detected with the 5F10 antibody (Affinity BioReagents, Golden, CO) as described previously (Yamoah et al., 1998), with a 5F10 dilution of 1:5000, alkaline phosphatase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) at 1:20,000, 0.1 mm of the chemiluminescence reagent CSPD [disodium 2-chloro-5-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)-tricyclo[3.3.1.13,7] decan}-4-yl)-1-phenyl phosphate; Tropix, Bedford, MA], and 10% Sapphire (Tropix), an enhancing reagent.
Immunoprecipitation. Hair bundles were purified from bullfrog sacculi by using the twist-off technique (Gillespie and Hudspeth, 1991). For each experiment we estimated the number of saccular equivalents of bundles that had been isolated so that one saccular equivalent is 100% recovery. Detergent-soluble proteins were extracted (Yamoah et al., 1998) from bullfrog residual maculae or purified hair bundles with 250 μl of immunoprecipitation solution [1% Triton X-100 and (in mm) 1 DTT, 150 NaCl, 25 HEPES, pH 7.5, plus 10 μm leupeptin, 10 μmpepstatin, 200 μm PMSF, and 0.1 mg/ml hemoglobin]. Extracts were incubated on ice for 4 hr with 6 μg of affinity-purified anti-PMCA antibody. Immune complexes were precipitated by adding 15 μl of donkey anti-rabbit IgG conjugated to Sepharose, incubating for 2 hr at 4°C with gentle agitation, and centrifuging to sediment the antibody–Sepharose complex. Donkey anti-rabbit IgG-Sepharose was prepared by coupling 2 mg of purified donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) to 1 ml of CNBr-Sepharose. After three 250 μl washes of immunoprecipitation solution, the immune complexes were recovered by incubating at 65°C for 20 min in an SDS-PAGE sample buffer that included 2% SDS and 100 mm DTT. For sequential immunoprecipitations additional anti-PMCA antibodies (6 μg) were added to the supernatant from the first immunoprecipitation, and the immune complexes were isolated as described above. In some cases a third immunoprecipitation was performed. Proteins from the final supernatant were precipitated with 6 vol of acetone at −20°C and then solubilized in SDS-PAGE sample buffer. Proteins were electrophoresed and transferred to blotting membranes; PMCA was detected with 5F10 as described above.
Immunoelectron microscopy. Postembedding immunoelectron microscopy was performed as described previously (Petralia et al., 1997). Rat cochleae were positioned gently onto a 400-mm-thick slice of Bacto-agar (Difco Laboratories, Detroit, MI) and mounted on filter paper glued to an aluminum specimen holder. Then the specimens were quick frozen by contact with a liquid nitrogen-cooled sapphire block of a Life Cell CF0100 quick-freezing machine (Research and Manufacturing, Tucson, AR) and promptly transferred to liquid nitrogen. Frog sacculi were fixed in 4% paraformaldehyde plus 0.5% glutaraldehyde in 0.1m phosphate buffer, cryoprotected in 30% glycerol, and frozen in liquid propane. All frozen samples were freeze substituted in 1.5% uranyl acetate in methanol at −90°C, infiltrated with Lowicryl HM-20 resin at −45°C, and polymerized with ultraviolet light. Ultrathin sections were obtained on a Ultracut microtome, collected in nickel grids, and used in immunocytochemistry. Sections were picked up on grids and were incubated in 0.1% sodium borohydride and 50 mm glycine in 5 mm Tris and 0.1% Triton X-100, pH 7.4 (TBST) for 10 min, blocked with 10% normal goat serum in TBST for 10 min, and then incubated for 2 hr in primary antibody in 1% normal goat serum/TBST. Grids were rinsed three times in TBST, blocked with 1% goat serum/TBST for 10 min, and then were incubated in gold-labeled secondary antibodies (10 nm gold particles conjugated to goat anti-rabbit IgG, diluted 1:20; Sigma) in 1% serum/0.5% polyethylene glycol (Mr = 20,000) in TBST for 1 hr. Grids were washed in TBST and water, stained with uranyl acetate, and observed in JEOL 1010 or Zeiss EM902 transmission electron microscopes.
Other methods. Amino acid alignments were created with the MegAlign software (DNAstar, Madison, WI). By convention (Carafoli and Stauffer, 1993; Strehler and Zacharias, 2001), PMCA isozymes are referred by their isoform numeral (1–4), splicing region C variant by small case letters (a–f), and splicing region A variants by small case letters (v–z) (e.g., PMCA2av). In some cases we dropped one or both of the splicing region letters (e.g., PMCA2 with the F2N antibody, which recognized all PMCA2 forms, or PMCA2a with the F2a antibody, which recognized the splicing region C form but did not distinguish splicing region A variants).
RESULTS
Isolation of bullfrog sacculus PMCA cDNA clones
To identify PMCA isoforms of bullfrog saccular hair cells, we first cloned several PMCA cDNAs from a saccular library. A vestibular organ ∼1 mm in diameter, the sacculus contains ∼2500 hair cells and twice as many supporting cells surrounded by a simple epithelium consisting of nonsensory cells (Jacobs and Hudspeth, 1990). Because no bullfrog PMCA sequences were available, we first aligned mammalian PMCA sequences and identified several regions of complete amino acid identity in all isozymes from all available species. Using degenerate oligonucleotides corresponding to these regions, we amplified PMCA fragments by using RT-PCR with RNA isolated from bullfrog tissues. Using Df1 and Dr1 primers, we amplified a 1.2 kb PCR product from bullfrog liver; all clones isolated from this PCR reaction were shown by sequencing to be closely related to mammalian PMCA1 (data not shown). Df1 and Dr2 primers produced a single 0.35 kb bullfrog lung PCR product, shown by cloning and sequencing to be PMCA2 (data not shown). Although not exhaustive, this PCR screen produced useful clones for the identification of bullfrog PMCA1 and PMCA2.
We used the cloned PCR products to screen a bullfrog sacculus cDNA library. Using the PMCA1 probe, we identified ∼150 positive clones (of 600,000 screened), one of which appeared to be full length (Figs.1, 2). At sequencing, this clone included a 1.3 kb unspliced intron with 39 stop codons, shared approximately equally by all three frames. To confirm that this insert was not present in the mature mRNA, we sequenced three other library clones, none of which had the insert. The corrected sequence of 4575 bases, tentatively identified as PMCA1, encoded a protein of 1214 amino acids (134,560 Da). Using the PMCA2 probe, we isolated ∼120 clones (of 700,000 screened), one of which appeared to be full length (Figs. 1,2). This clone also was spliced incompletely; to determine the correct sequence, we sequenced two other library clones and five PCR products that were amplified from bullfrog sacculus cDNA. This large clone also had a two nucleotide deletion in the coding sequence; we sequenced two additional library clones to correct the sequence. Because few cDNAs have been cloned from R. catesbeiana libraries, we are uncertain whether the high frequency of untranslatable clones reflects a species phenomenon or simply poor luck. The corrected sequence of 4416 bases, tentatively identified as PMCA2, encoded a protein of 1213 amino acids (132,801 Da).
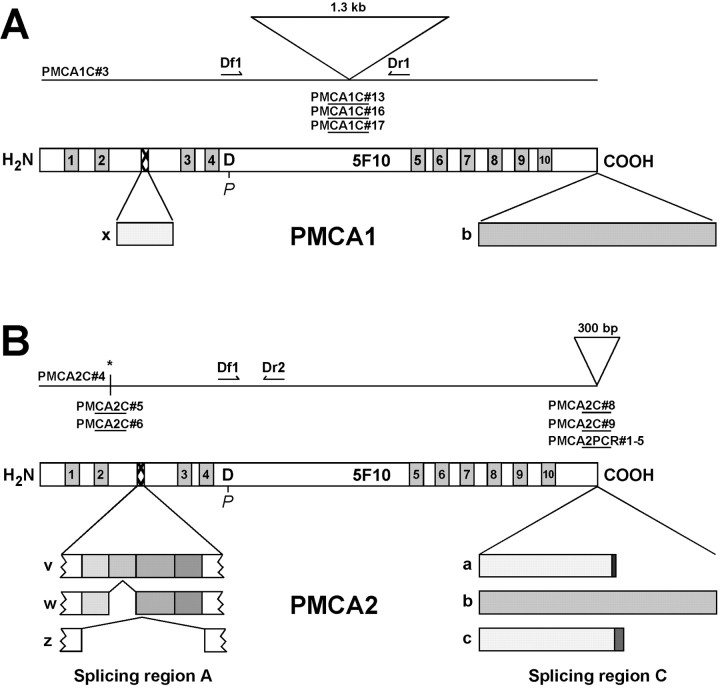
Fig. 1.
Cloning, structure, and alternative splicing of bullfrog PMCA isozymes. Horizontal lines indicate the extent of each cDNA that is sequenced, and the lines are aligned with a diagram of PMCA. Ten putative transmembrane domains (shaded and numbered), the phosphointermediate aspartate residue, and the 5F10 antibody binding site are indicated. A, Bullfrog sacculus PMCA1.PMCA1C#3 indicates the clone that was used to derived most of the sequence for PMCA1; at the indicated 1.3 kb insert three other clones were sequenced too. Df1 andDr1 indicate the positions of primers used in bullfrog liver RT-PCR to generate a PMCA1-specific probe. Splicing region A and C variants are indicated; we detected only x andb. B, Bullfrog sacculus PMCA2.PMCA2C#4 indicates the clone that was used to derived most of the sequence for PMCA2. At the position indicated with anasterisk, the site of a 2 bp deletion, we sequenced two additional clones to confirm the correct sequence. In addition, we sequenced at least two library clones and five PCR products at splicing region C; only PMA2C#4 included a 300 bp unspliced insert. Variants v, w, andz were detected at splicing region A; variantsa, b, and c were detected at splicing region C. Df1 and Dr2indicate positions of primers used in bullfrog lung RT-PCR to generate a PMCA2-specific probe.
Fig. 2.
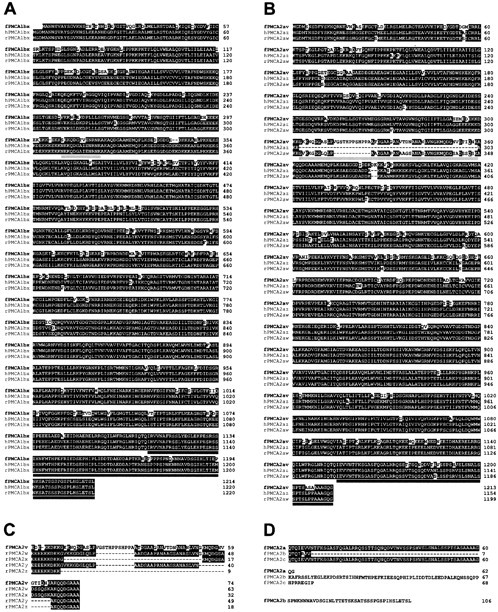
Sequence analysis of bullfrog sacculus PMCA1bx and PMCA1av isozymes. Highlighted residues are those with identity to the aligned residues in at least one other PMCA. Splicing region A is underlined in light gray; splicing region C is underlined indark gray. A, Bullfrog sacculus PMCA1bx (fPMCA1bx) aligned with human PMCA1bx (hPMCA1bx) and rat PMCA1bx (rPMCA1bx). Protein translation likely initiates at nucleotides 340–342; this is the first AUG codon of a long open reading frame and matches the second AUG of the mammalian PMCA1 sequence. B, Bullfrog sacculus PMCA2av (fPMCA2av) aligned with human PMCA2az (hPMCA2az) and rat PMCA2aw (rPMCA2aw). Nucleotides 328–330 correspond to the first AUG codon of a long open reading frame and match the known translation start site of mammalian PMCA2. C, Bullfrog sacculus splicing region A variant PMCA2av (fPMCA2av) aligned with rat PMCA2 splice region A variants w,x, y, and z(rPMCA2w, rPMCA2x,rPMCA2y, rPMCA2z, respectively).D, Bullfrog sacculus PMCA2 splice region C variants identified in the sacculus cDNA library.
We analyzed predicted amino acid sequences of bullfrog PMCA clones to determine their relationship to known PMCA isozymes. For each bullfrog sequence, high amino acid identity with other PMCAs extends throughout all cytoplasmic loops and the 10 putative transmembrane domains. The phosphoenzyme intermediate aspartate residue present in all P-type ATPases (Carafoli and Stauffer, 1993) is conserved in bullfrog PMCA1 (residue 469) and PMCA2 (residue 513). In addition, key residues in transmembrane domains that are critical for Ca2+ pumping in PMCA [P422, E423, P426, A854, N879, M882, D883, Q971, and E975 in human PMCA4 (Guerini et al., 1996, 2000)] were present in bullfrog PMCA1 (residues 426, 427, 430, 860, 885, 888, 889, 975, and 981) and PMCA2 (residues 470, 471, 474, 903, 928, 931, 932, 1020, and 1024). By examining the N-terminal ∼15 amino acids, which vary widely between different isozymes, we determined that our sequences were related most closely to PMCA1 and PMCA2 (Fig. 2A,B). At the amino acid level, bullfrog PMCA1 was 88.7% identical to rat PMCA1, and bullfrog PMCA2 was 90.1% identical to rat PMCA2 (excepting alternative splicing regions A and C). These high sequence similarities establish that these isozymes are the bullfrog orthologs of PMCA1 and PMCA2.
Because of the importance of alternative splicing for PMCA regulation, we examined bullfrog sacculus PMCA1 and PMCA2 library clones and RT-PCR products from sacculus RNA for alternative splicing in regions A and C. For PMCA1 we detected only x and b variants at sites A and C (Figs. 1, 2, Table 2).
Table 2.
PMCA splice variants in bullfrog sacculus
| Isozyme | Primers | Product size (bp) | Sacculus library | Sacculus cDNA |
|---|---|---|---|---|
| PMCA1x | F1A+2/F1A−2 | 326 | ++++ | ++++ |
| PMCA1b | F1C+2/F1C−2 | 439 | ++++ | ++++ |
| PMCA2v | F2A+2/F2A−2 | 410 | ++++ | ++++ |
| PMCA2w | F2A+2/F2A−2 | 374 | − | + |
| PMCA2z | F2A+2/F2A−2 | 239 | − | + |
| PMCA2a | F2C+2/F2C−2 | 613 | ++++ | ++++ |
| PMCA2b | F2C+2/F2C−2 | 386 | + | − |
| PMCA2c | F2C+2/F2C−2 | 559 | + | − |
Isozymes: PMCA isozymes cloned from the sacculus cDNA library or amplified by RT-PCR from bullfrog saccular RNA. Primers: The second pair of primers from a set of nested primers used for PCR. Product size: Expected PCR product sizes, given in base pairs, for each primer pair. Symbols signify abundant (++++; >80%), rare (+; <10%), and undetected (−) splice variants identified among saccular library clones or PCR products from saccular cDNA.
The slicing of PMCA2 was more complex. The full-length PMCA2 clone used a novel splicing region A variant, which we named v (Fig.2C). Interestingly, the cDNA sequence of this variant begins with GT, the consensus splice donor dinucleotide, and ends with AG, the consensus splice acceptor dinucleotide (Green, 1991), suggesting that this sequence can be removed by alternative splicing. PCR analysis of >50 library clones indicated that all were of splice formv; in addition, RT-PCR analysis on bullfrog sacculus RNA showed that, in addition to the abundant v, PMCA2 splicing region A variants w and z were also present (Table 2). The v splice form was not specific to the inner ear, however, but also was found in bullfrog PMCA2 in brain, kidney, lung, and retina (data not shown). The full-length PMCA2 clone incorporated the a splice variant in splicing region C (Fig.2D); in other library clones a was the predominant form (98 of 103 clones), although b (4 of 103) and c (1 of 103) also were detected (Table 2). Thea variant was the only splicing region C sequence identified by RT-PCR from saccular RNA (Table 2). The two major PMCA transcripts in bullfrog sacculus hence encoded PMCA1bx and PMCA2av.
Localization of PMCA isoforms in bullfrog sacculus
To localize saccular PMCA isoforms, we generated polyclonal antibodies selective for specific PMCA isozymes and splice variants (see Table 1) and used them for immunocytochemistry. Because fusion proteins used for generation of the F1N and F2N antibodies contained substantial sequence identity within the PMCA coding regions, we generated selective antibodies by performing sequential negative and positive affinity selection. Affinity-purified F1N recognized PMCA1, but not PMCA2, fusion proteins on immunoblots, whereas F2N recognized PMCA2, but not PMCA1, fusion proteins (data not shown). All antibodies also exhibited minimal cross-reactivity with other bullfrog saccular proteins on blots (see Fig. 5A) and therefore were useful tools for localizing PMCA isozymes in bullfrog saccular tissue.
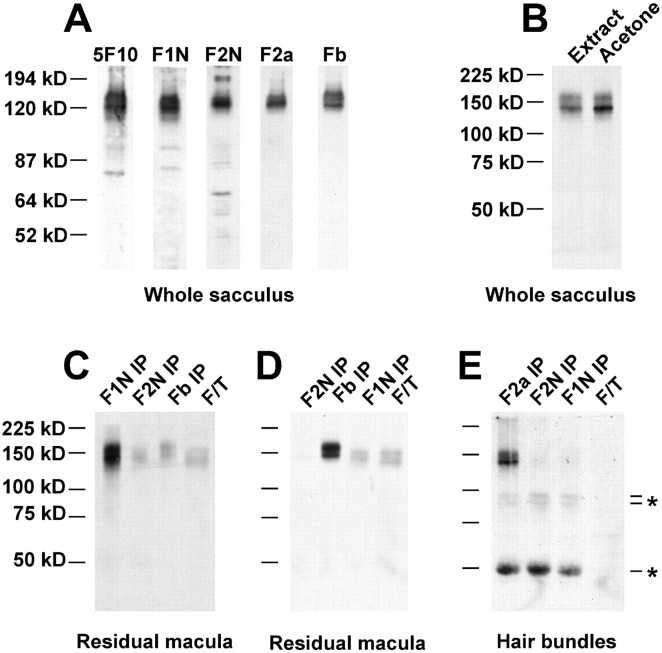
Fig. 5.
Protein immunoblot and immunoprecipitation analysis of bullfrog sacculus PMCA isozymes. A, Immunoblotting of whole bullfrog sacculus with 5F10 and isozyme-selective antibodies. One saccular equivalent was used for each lane. B, 5F10 immunoblot indicating efficiency of acetone precipitation. Extracts from bullfrog sacculi either were added directly to sample buffer (Extract) or were precipitated with acetone (Acetone). Band intensities were similar, indicating that acetone precipitation was efficient. C, 5F10 detection of PMCAs sequentially immunoprecipitated from a residual macula extract (five saccular equivalents) with PMCA-selective antibodies. F/T, Flow through, those proteins that did not bind to any of the precipitating antibodies. D, Same immunoprecipitation and detection conditions as in C, except that the antibody order is changed. E, 5F10 detection of PMCAs sequentially immunoprecipitated from a hair bundle extract (47 saccular equivalents) with PMCA-selective antibodies. Indicated bands (*) were derived from the precipitating antibodies (data not shown).
Antibodies listed in Table 2 were used to detect PMCA isozymes within the bullfrog sacculus (Fig. 3). The PMCA1 N terminus antibody (F1N) strongly labeled basolateral membranes of hair cells (Fig. 3A); although PMCA1 usually was excluded completely from apical membranes, in rare instances we observed faint hair bundle labeling. Plasma membranes of supporting cells also were labeled with PMCA1-selective antibodies. An identical labeling pattern was observed with the use of an antibody (Fb) that was raised against a peptide sequence within the b splice forms of bullfrog PMCA1 and PMCA2 (Fig. 3B,C); because rat PMCA3b contains an identical sequence, this antibody also may detect a putative bullfrog PMCA3b. Labeling with either antibody was usually more intense in hair cells with larger cell body diameters. No labeling was observed when an irrelevant affinity-purified antibody was used; furthermore, the peptide used to raise the Fb antiserum blocked labeling with Fb. Both observations indicate the specificity of the PMCA1 immunolabeling.
Fig. 3.
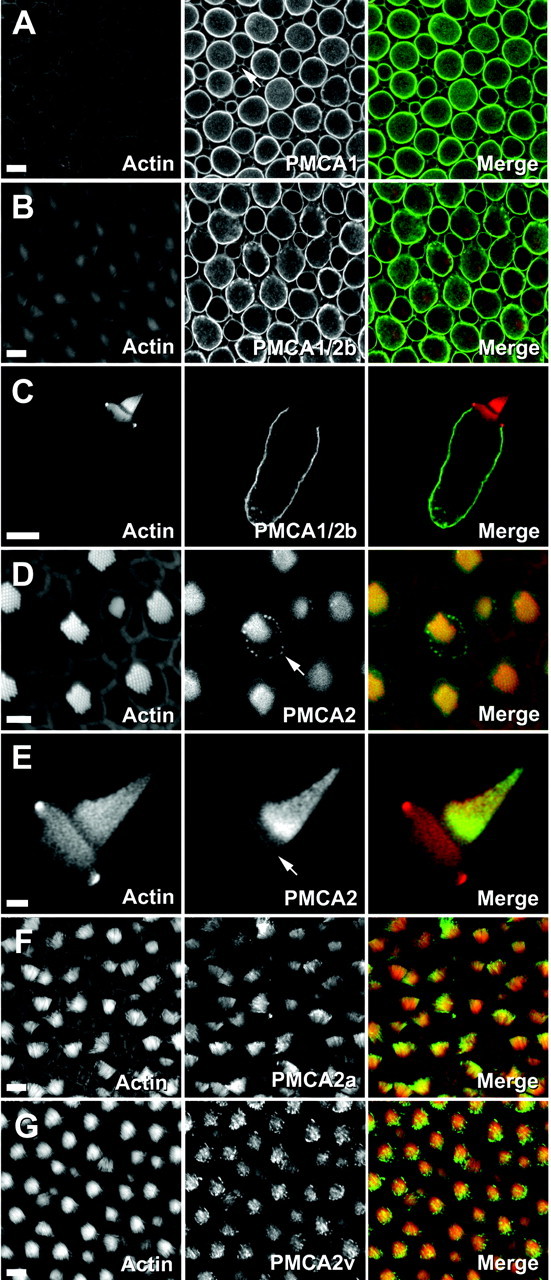
Localization of bullfrog sacculus PMCA isozymes by immunofluorescence. Left columns, Actin (FITC-phalloidin); middle columns, PMCA; right columns, combined actin (red) and PMCA (green). A, F1N labeling for PMCA1. Shown is a cross section through a bullfrog sacculus; plasma membranes of hair cells and supporting cells (arrow) are labeled. B, Fb labeling for PMCA1b and PMCA2b. Shown is a cross section through bullfrog sacculus hair cells and supporting cells. C, Fb labeling for PMCA1b and PMCA2b. The hair bundle is unlabeled. D, F2N labeling for PMCA2. Shown are apical surfaces of bullfrog sacculus hair cells and supporting cells; there is PMCA2 labeling in stereocilia and in the pericuticular necklace (arrow). E, F2N labeling for PMCA2. Shown is labeling in an isolated hair cell; there is strong labeling near the base of the tallest stereocilia and labeling along the apical surface (arrow). F, F2a labeling for PMCA2a. Shown is a whole-mount view of hair bundles. G, F2v for PMCA2v. Shown is a whole-mount view of hair bundles. Scale bars:A–C, F, G, 10 μm;D, 5 μm; E, 2 μm.
Hair bundles in bullfrog sacculi were labeled intensely by antibodies selective for the PMCA2 N terminus (F2N; Fig. 3D,E), for the PMCA2a splice form (F2a; Fig. 3F), or for the PMCA2v splice form (F2v; Fig. 3G). Labeling often appeared to be concentrated near stereociliary tips, although sacculus-to-sacculus variability was noted. In addition, PMCA2-selective antibodies labeled the apical surface of the hair cell and a punctate ring surrounding the cuticular plate (Fig. 3D). For each of these features the labeling was specific; no labeling was observed with an irrelevant primary antibody, and the peptide used to raise the F2v antibody blocked hair cell labeling with F2v. No PMCA2 labeling was detected in the basolateral membrane of hair cells, in supporting cells, or in extramacular nonsensory epithelial cells of the bullfrog sacculus.
We used immunoelectron microscopy to localize PMCA1 and PMCA2 at the ultrastructural level (Fig. 4). Using postembedding labeling on freeze-substituted saccular tissue, we found strong PMCA1 immunoreactivity along membranes of hair cells (Fig.4A) and supporting cells (data not shown). PMCA2 immunoreactivity was observed only along stereocilia membranes and at the apical surface of the hair cell (Fig. 4B–E). No PMCA2 labeling was seen along hair cell basolateral membranes or in supporting cells.
Fig. 4.
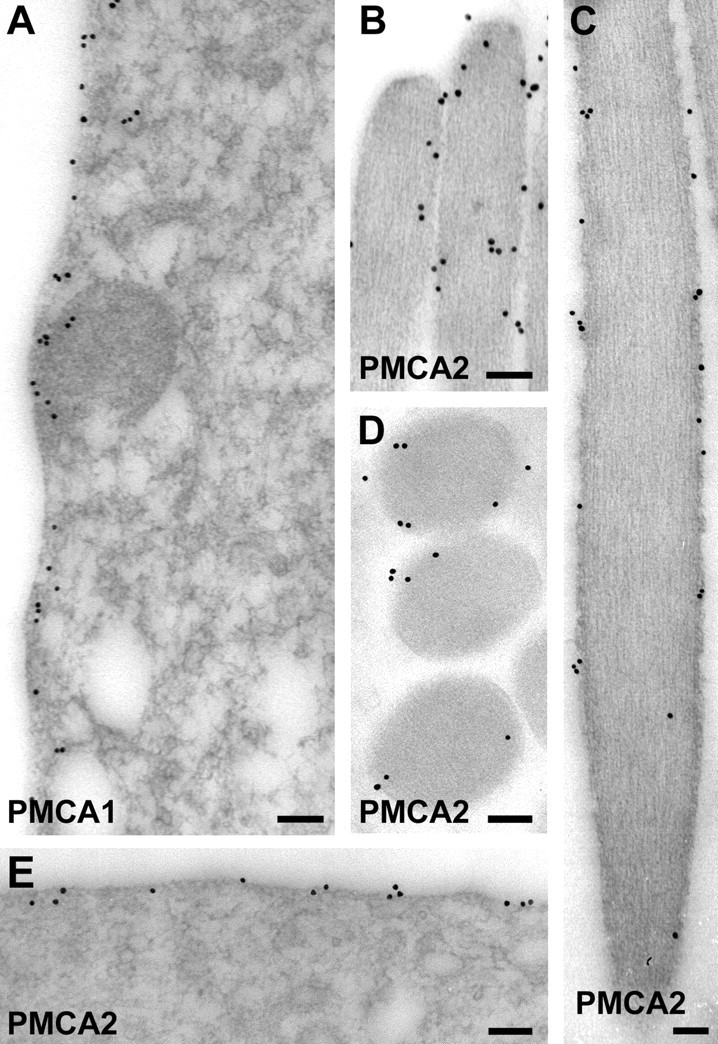
Localization of bullfrog sacculus PMCA isozymes by immunoelectron microscopy. A, F1N labeling for PMCA1. Shown is a hair cell basolateral membrane. B–E, F2N labeling for PMCA2. B, Stereociliary tips.C, Stereocilium shaft. D, Cross section through stereocilia. E, Hair cell apical surface. Scale bars, 100 nm.
These immunolabeling data indicate that PMCA1b is a major isozyme of hair cell basolateral membranes, and PMCA2a is a major isozyme of hair bundles. Nevertheless, other PMCA isozymes, not detected with our antibodies, also could be present in sacculi. Although the NR3 antibody, raised against rat PMCA3, did not label bullfrog sacculi (data not shown), its cross-reactivity with a putative bullfrog PMCA3 isozyme is unknown. Because immunolabeling cannot exclude the presence of PMCA3 or other isozymes, we turned to protein biochemistry to address the identity of hair bundle and somatic PMCA isozymes.
Protein immunoblot analysis of bullfrog sacculus PMCA isozymes
We analyzed PMCA1 and PMCA2 by protein immunoblotting, using each of the antibodies we previously had used for immunocytochemistry. With each antibody, as well as with the pan-PMCA antibody 5F10, we detected two to three bands of 130–170 kDa in whole bullfrog sacculus (Fig.5A). Only the antibody against the v form of PMCA2 (F2v) did not react efficiently with sacculus PMCAs on immunoblots. The higher molecular mass forms of PMCA were not unexpected; PMCA isozymes (particularly PMCA2) often migrate in SDS-PAGE with a second band ∼20 kDa larger than the expected protein product (Hilfiker et al., 1994).
Immunoprecipitation of bullfrog sacculus PMCA isozymes
We used an immunoprecipitation approach to confirm the identity of the principal PMCA isoforms of bullfrog hair cell somas and hair bundles. We precipitated PMCAs from saccular protein extracts with selective antibodies and then analyzed all precipitated and unprecipitated PMCAs by immunoblotting with the pan-PMCA antibody 5F10. Because 5F10 cross-reacts with all four mammalian PMCA isozymes (Caride et al., 1996) as well as with PMCAs in a wide range of species (Borke et al., 1989; de Talamoni et al., 1993; Benaim et al., 1995), we expected to analyze all saccular PMCA isozymes simultaneously in these experiments.
To perform these immunoprecipitation experiments, we solubilized PMCA from tissue extracts with Triton X-100 and then sequentially precipitated each extract with isoform-selective PMCA antibodies. Proteins remaining in the supernatant after immunoprecipitation were precipitated with acetone. For a comprehensive examination of PMCA isozymes, including those for which we had no specific antibody, we required complete acetone precipitation of remaining PMCAs; by examining total PMCA before and after acetone precipitation, we demonstrated that this precipitation was efficient (Fig.5B). PMCA was detected in immunoprecipitates and the final supernatant by SDS-PAGE and immunoblotting with 5F10.
We first determined which PMCA isozymes were present in bullfrog residual macula (Gillespie and Hudspeth, 1991), the saccular tissue remaining after hair bundle isolation (Fig. 5C,D). This tissue contains bundleless hair cells, supporting cells, and a few nonsensory extramacular cells. When F1N was used as the first precipitating antibody (Fig. 5C), most of the PMCA in the residual macula extract was precipitated. When Fb was used before F1N, Fb precipitated most PMCA (Fig. 5D). PMCA1 precipitated by F1N, but not Fb (Fig. 5D), may be another PMCA1 splicing region C variant, although our molecular cloning and RT-PCR experiments only detected the b form. Alternatively, proteolysis might have eliminated the Fb antibody epitope. Very little PMCA2 was present in the residual macula tissue (Fig. 5C,D). PMCA precipitated by Fb, but not F1N, in Figure 5C may be PMCA3b; in addition, there was a small amount of PMCA that was not precipitated by any of our antibodies, which may have been other PMCA3 splice variants or PMCA4. The results from these immunoprecipitation experiments thus demonstrate that the principal residual macula isozyme is PMCA1b, consistent with immunocytochemical results (see Fig. 3).
We next examined the identity of PMCA isozymes in purified hair bundles (Gillespie and Hudspeth, 1991). F2a immunoprecipitated all PMCA from a hair bundle protein extract; neither F2N nor F1N precipitated any additional PMCA, and no PMCA remained in the final supernatant (Fig.5E). Besides showing that the only bundle PMCA is PMCA2a, the data also demonstrated the effectiveness of the hair bundle purification. Although only a few saccular equivalents of PMCA1 give a strong 5F10 signal (e.g., Fig. 5C,D), we saw no significant 5F10 reactivity in 47 saccular equivalents of hair bundles when PMCA1 antibodies were used for immunoprecipitation (Fig. 5E).
Our experimental conditions insured that all 5F10-reactive PMCA isoforms from the sacculus, presumably all saccular PMCA molecules, could be analyzed in a single experiment. The data therefore established conclusively that PMCA2a is the hair bundle PMCA isozyme in the bullfrog sacculus and that PMCA1b accounts for a majority of the PMCA in the rest of the sacculus.
PMCA isoforms in rat organ of Corti and utriculus
To determine the generality of the bullfrog sacculus localization results, we examined PMCA isozyme distribution in auditory and vestibular organs of rat, where we could use antibodies selective for each of the four known PMCA isozymes (see Table 1; Filoteo et al., 1997). To confirm that PMCAs 1–4 are the only PMCAs present in vertebrates, we used the BLAST search algorithm (Altschul et al., 1990) to search for genes related to PMCA within the human genome (http://www.ncbi.nlm.nih.gov/genome/seq/HsBlast.html). Each human PMCA cDNA identified only its own gene and the other three PMCA genes and, in one case, a sarcoplasmic–endoplasmic reticulum Ca2+-ATPase (data not shown). These data suggest that, if any other PMCAs are present in the genome, they are related much more distantly to PMCAs 1–4 than each is to the others. By performing immunocytochemistry in rat tissues, we therefore were able to use antibodies against all PMCA gene products.
We first examined the organ of Corti, the sensory epithelium of the mammalian auditory system. In this tissue PMCA2 immunoreactivity was restricted in hair cells to bundles and apical surfaces (Fig.6B,F). Labeling was intense in bundles of outer hair cells; bundles of inner hair cells had far less immunoreactivity, which occasionally appeared to be concentrated at stereociliary tips. An identical labeling pattern was observed with F2a (Fig. 6C), indicating that PMCA2a is present in hair bundles.
Fig. 6.
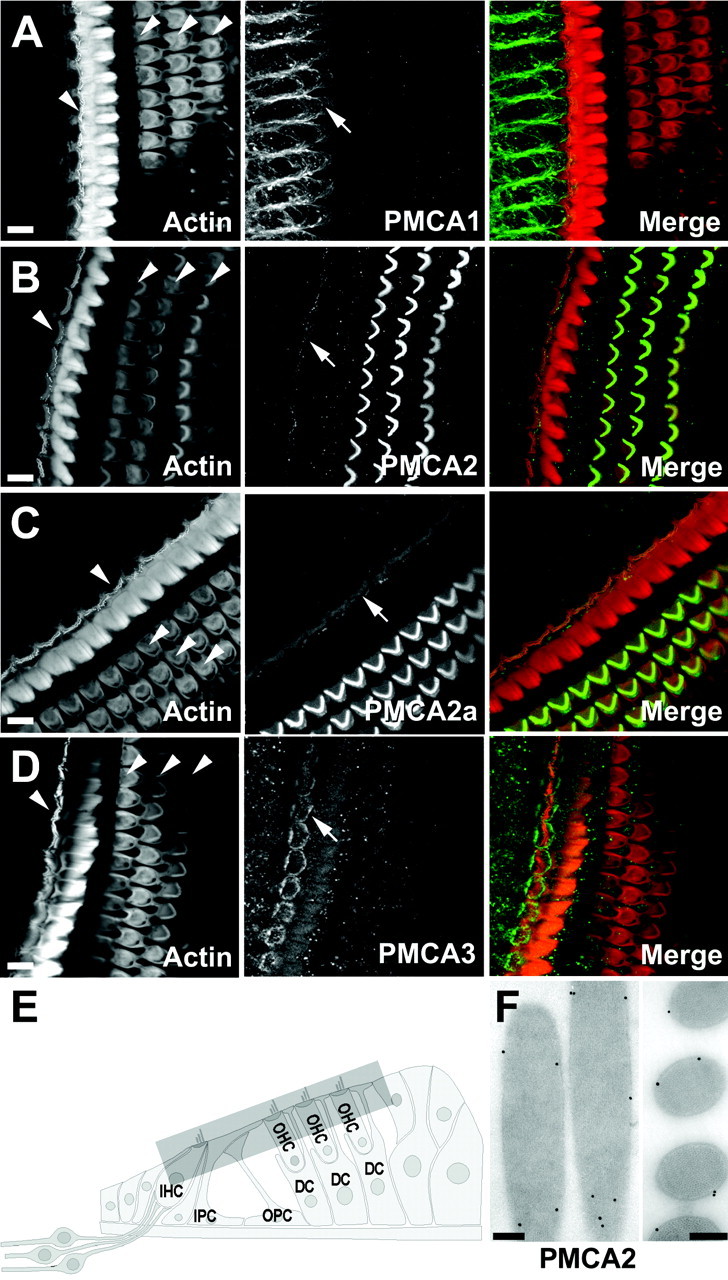
Localization of rat organ of Corti PMCA isozymes by immunofluorescence and immunoelectron microscopy. Shown are cross sections through the rat organ of Corti. Left columns, Actin (FITC-phalloidin); middlecolumns, PMCA immunolabeling; right columns, combined actin (red) and PMCA (green). In theleft columns the arrowheads point to the single row of inner hair cells and three rows of outer hair cells.A, NR1 labeling for PMCA1. Basolateral membranes of inner hair cells were labeled (arrow). B, NR2 labeling for PMCA2. Hair bundles of outer hair cells were labeled intensely; modest immunoreactivity was present on bundles of inner hair cells (arrow). C, F2a labeling for PMCA2a. Stereocilia of inner (arrow) and outer hair cells were labeled. D, NR3 labeling for PMCA3. Immunoreactivity was restricted to the pericuticular necklace (arrow) and bundles of inner hair cells. Bundle labeling was very low, when detectable. E, Diagram of the mammalian organ of Corti indicating the approximate region of projected confocal images. IHC, Inner hair cell;OHC, outer hair cell; IPC, inner pillar cell; OPC, outer pillar cell; DC, Deiter's cell. F, NR2 labeling for PMCA2; shown is postembedding immunoelectron microscopy on freeze-substituted rat cochlear tissue. Left, Stereociliary shafts and tip.Right, Cross section. PMCA2 is associated with plasma membranes. Scale bars: A–D, 10 μm; F, 200 nm.
PMCA1 was located on basolateral membranes of inner hair cells (Fig.6A) but was absent from their hair bundles. PMCA1 was never observed in basolateral membranes of outer hair cells, although on occasion we observed very low PMCA1 labeling on apical surfaces (data not shown). PMCA1 also was detected in the Deiter's cell cups, where these cells cradle the base of outer hair cells. PMCA3 was observed only in inner hair cells; this isozyme was located prominently in a ring near the inner hair cell cuticular plate and at lower levels on the apical surface of inner hair cells (Fig. 6D). PMCA3 labeling was detected occasionally in the basolateral plasma membrane of inner hair cells and, at very low levels, in bundles of inner hair cells. Fb, which recognizes PMCAs 1b, 2b, and 3b, labeled the plasma membrane of inner hair cell somas and Deiter's cell cups in a similar pattern to that obtained with NR1 (data not shown). Surprisingly, Fb also labeled plasma membranes of inner and outer pillar cells as well as phalanges of Dieter's cells. None of the other antibodies against rat PMCA, which recognize all known isozymes, labeled these structures. PMCA4 was not detected in the organ of Corti.
We also examined PMCAs in the rat utriculus, a vestibular organ. PMCA2 immunoreactivity was intense in hair bundles and was absent from the rest of the tissue (Fig.7B,C); furthermore, the vestibular bundle isoform was PMCA2a (Fig. 7D). F2N also strongly labeled hair bundles of the semicircular canal (Fig.7E). Although many vestibular hair bundles were labeled intensely with antibodies against PMCA2, a subset of bundles had lower levels of this isozyme. These bundles usually belonged to hair cells that were immunoreactive for the calcium-binding protein calretinin; consistent with their generally smaller bundles, these cells may be immature type II hair cells (Dechesne et al., 1991, 1994). Basolateral membranes of utricular hair cells were labeled with antibodies against PMCA1 and PMCA3. PMCA1 labeling was observed at substantial levels only in a fraction of hair cells (Fig. 7A). By contrast, PMCA3 labeling was apparent in nearly all hair cells, although it was usually considerably lower in calretinin-positive cells (Fig. 7D). Like the supporting cells of the bullfrog sacculus, supporting cells of the rat utriculus target PMCA1 to their plasma membranes, albeit at a significantly lower level than in hair cells. We did not observe PMCA4 expression in rat vestibular organs.
Fig. 7.
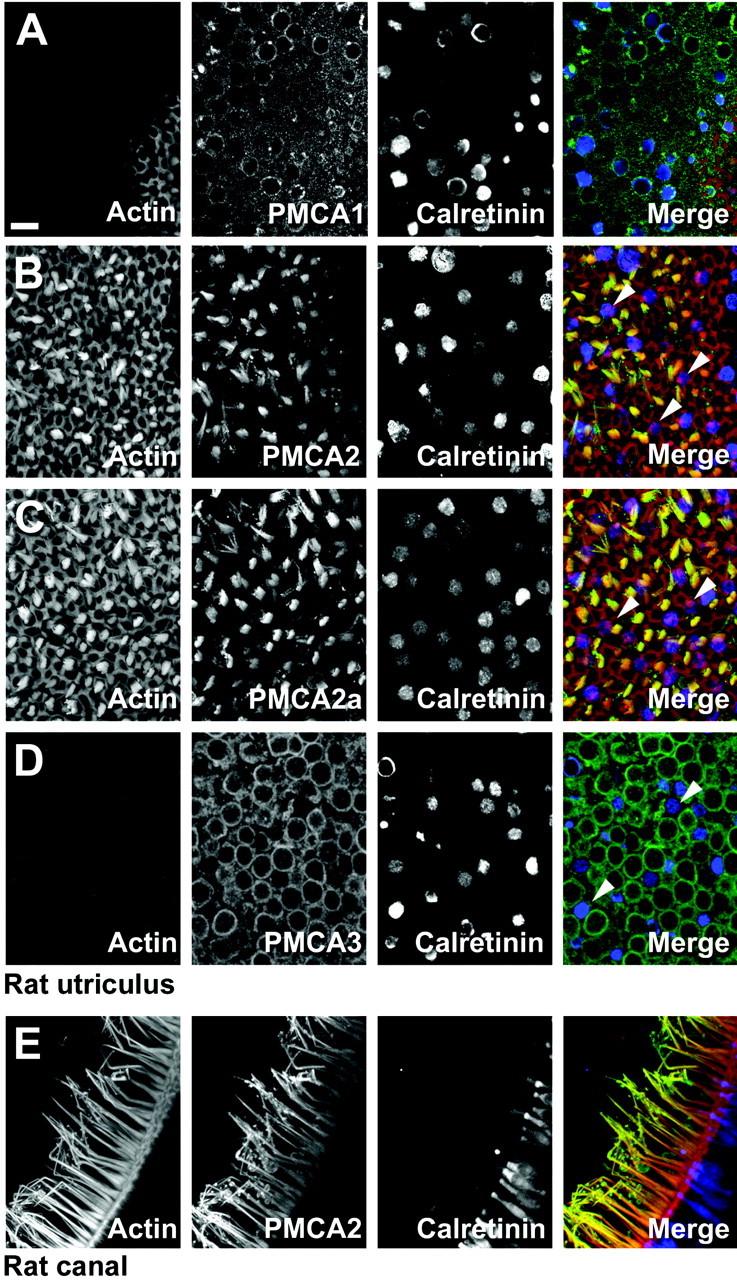
Localization of rat vestibular PMCA isozymes by immunofluorescence. Cross sections through the rat utriculus (A–D) and ampulla (E).Left columns, Actin (FITC-phalloidin); middle left columns, PMCA immunolabeling; middle right columns, calretinin immunolabeling; right columns, combined actin (red), PMCA (green), and calretinin (blue).A, NR1 labeling for PMCA1. Shown is a single confocal section. B, NR2 labeling for PMCA2. Shown is a projection of confocal sections covering ∼10 μm depth. Some hair bundles with low PMCA2 labeling belong to hair cells with high levels of calretinin (arrowheads). C, F2a labeling for PMCA2a. Shown is a projection of confocal sections covering ∼10 μm depth. Hair bundles with low PMCA2a and high calretinin labeling are indicated by arrowheads.D, NR3 labeling for PMCA3. Shown is a single confocal section. Hair cells with low PMCA3 expression have high calretinin levels (arrowheads). E, NR2 labeling for PMCA2. Scale bar, 10 μm (applies to all panels).
We observed no immunolabeling of rat vestibular organs or organ of Corti by using an antibody against the v splice variant of PMCA2 (data not shown). To confirm the absence of this splice form in rat hair cells, we performed RT-PCR on cochlea and utriculus RNA to examine the splicing in region A. Using primers that flank splicing region A, we found no evidence for the expression of a rat vform, although we did amplify, clone, and sequence w,x, and z forms (see Table 2).
Because we used antibodies against all four known PMCA isozymes (see Table 1), these data demonstrated that PMCA2a is the major bundle isoform in rat hair cells, as it is in bullfrog hair cells. By contrast, hair cells and supporting cells of the rat cochlea and vestibular system use a variety of PMCA isozymes in their basolateral membranes.
DISCUSSION
Hair cells target PMCA isozymes to discrete subcellular locations. In auditory and vestibular epithelia PMCA2a was the predominant calcium pump of hair bundles; by contrast, PMCA1b was observed only on plasma membranes of hair cell and supporting cell somas. Although we did not detect PMCA4 in auditory or vestibular epithelia, we did observe PMCA3 in mammalian hair cells. This differential localization of PMCA isoforms presumably reflects the need for independent Ca2+ regulation in different cells and within distinct subcellular compartments, such as the hair bundle (Fig.8).
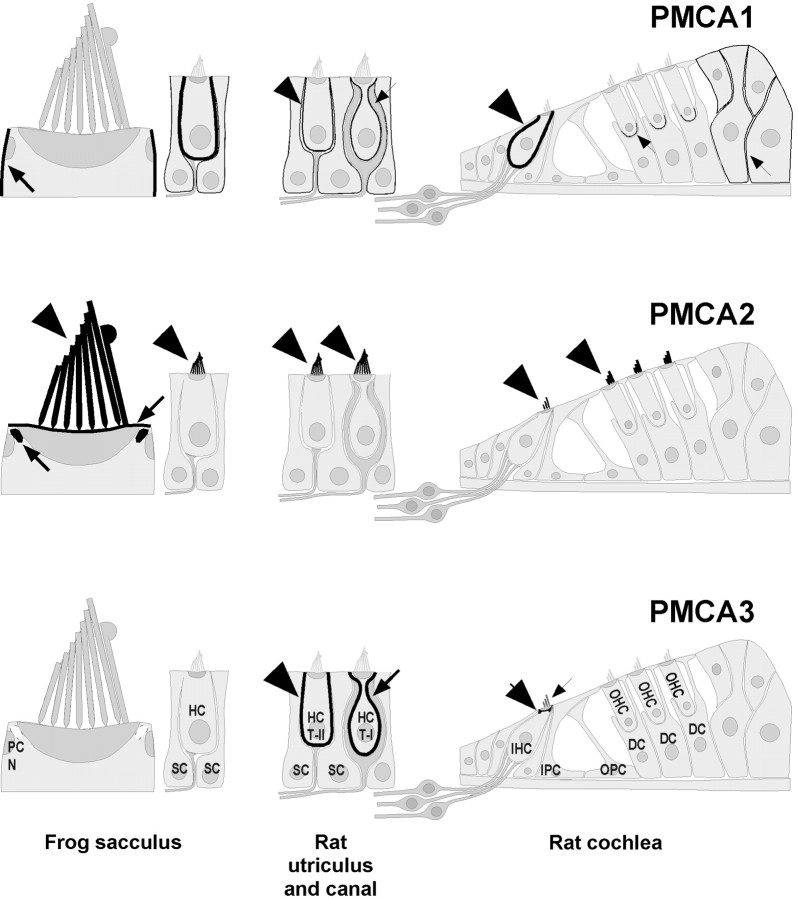
Fig. 8.
Schematic localization of PMCA isozymes in auditory and vestibular epithelia. PMCA isozyme location is indicated by black shading (intensity indicates relative amount) and by arrows or arrowheads.PMCA1 was located on basolateral membranes of bullfrog and rat vestibular hair cells as well as inner hair cells. Occasional labeling was seen on outer hair cell apical surfaces.PMCA2 was present at high levels on bundles and apical surfaces of vestibular hair cells and outer hair cells; levels were lower on bundles of inner hair cells. In bullfrog sacculus the labeling was also present in the pericuticular necklace. PMCA3labeling was strong in the basolateral membranes of rat utriculus hair cells; this isozyme was also present near the apical surface of inner hair cells and, in some instances, in their bundles. HC, Hair cell; PC N, pericuticular necklace;SC, supporting cell; HC T-I, type I vestibular hair cell; HC T-II, type II vestibular hair cell.
PMCA isoforms in the bullfrog sacculus
In bullfrog sacculus we found prominent expression of PMCA1bx and PMCA2av. Furthermore, sequential immunoprecipitation experiments suggested that PMCA2a is the only plasma membrane calcium pump in bullfrog hair bundles, and PMCA1b is the principal one of the basolateral membrane. This conclusion rests on the assumption that the monoclonal antibody 5F10 recognizes and binds all PMCA isoforms on protein immunoblots. Although true for all four mammalian PMCA isoforms (Caride et al., 1996), only when 5F10 can be tested against all bullfrog PMCAs can we determine conclusively that no other PMCA isoforms are located in hair bundles or hair cell plasma membranes. Nevertheless, the conclusion that PMCA2a is the bundle isoform was supported strongly by localization in the rat auditory and vestibular systems; with the use of antibodies against all four PMCA isozymes, PMCA2a was the only prominent hair bundle PMCA isozyme.
PMCA isoforms in the mammalian vestibular and auditory systems
In the organ of Corti the pan-PMCA monoclonal antibody 5F10 was used to localize PMCA to the surface of stereocilia of both inner and outer hair cells as well as along the basolateral membrane of inner hair cells (Crouch and Schulte, 1995; Apicella et al., 1997;Street et al., 1998). 5F10 immunoreactivity was absent from hair bundles of mutant mice that lacked PMCA2 expression, suggesting that this isozyme was prominent there (Street et al., 1998; Takahashi and Kitamura, 1999). RT-PCR experiments identified PMCA1–4 gene products, as well as many of their splice variants, in cochlea (Crouch and Schulte, 1996; Furuta et al., 1998); furthermore, in situhybridization experiments localized PMCA2 transcripts to outer hair cells and PMCA1 to inner hair cells (Furuta et al., 1998). Because PMCA labeling was prominent in outer hair cells and PMCA2 transcripts were localized there and because PMCA2b was the only PMCA2 splice variant identified in cochlea by RT-PCR, PMCA2b was hypothesized to be the stereocilia isoform (Crouch and Schulte, 1995; Furuta et al., 1998).
We show here, however, that PMCA2a is the major hair bundle PMCA not only in bullfrog sacculus but also in bundles of rat organ of Corti, utriculus, and semicircular canal. Furthermore, we did not observe hair bundle labeling using an antibody that recognizes the bsequence shared by PMCA1b, PMCA2b, and PMCA3b. Because PMCA2 protein levels in inner hair cells are very low, it is not surprising thatin situ hybridization experiments did not detect PMCA2 transcripts in inner hair cells (Furuta et al., 1998). Although we occasionally detected low levels of PMCA1 in bundles of outer hair cells and PMCA3 in bundles of inner hair cells, PMCA2a was detected consistently at moderate (inner hair cells) to very high (outer hair cells) levels, suggesting that this isozyme is the principal Ca2+ pump of cochlear hair bundles.
The presence of PMCA1b in the basolateral membrane of inner hair cells resembled its expression in the bullfrog and rat vestibular system. By contrast, no substantial amounts of PMCA were identified in basolateral membranes of outer hair cells (see Fig. 6A; Crouch and Schulte, 1995). This latter observation perhaps is not surprising, given the near-crystalline packing of outer hair cell intramembrane particles that are thought to correspond to the motor responsible for electromotility (Forge, 1991). PMCA3 was also absent from outer hair cells, although it was present in inner hair cells. We consistently observed PMCA3 in a ring below the apical surface that resembled the pericuticular necklace (Hasson et al., 1997), a region that is richly endowed with intracellular vesicles; in addition, PMCA3 was observed occasionally in bundles of inner hair cells. A possible interpretation of this localization is that PMCA3 is stored in the necklace and is mobilized into hair bundles of inner hair cells under appropriate conditions. Finally, we found no evidence for PMCA4 in the organ of Corti. Previous in situ hybridization experiments suggested that PMCA4 is expressed in the cochlea at very low levels, with the exception of a short period of expression in inner hair cells that peaked at P12 (Furuta et al., 1998). Because our observations were confined to older rats, our inability to detect this isozyme was unsurprising.
Localization and targeting of PMCA isozymes
Within hair cells and supporting cells PMCA isozymes are targeted to the apical domain exclusively or to the basolateral domain exclusively. Although splice variants may allow localization within a domain, these sequences may not be those that dictate basolateral or apical targeting. Conclusive determination of targeting mechanisms for individual PMCA isozymes must await closer experimental examination, for example by the expression of PMCA1bx–PMCA2av chimeras in hair cells.
Within a domain PMCA isozymes did not always appear to be distributed uniformly within the membrane. Localization may be specified by splicing region C alternative exons. Human PMCA2b and PMCA4b bind via their C termini to several membrane-associated scaffolding proteins that contain PDZ domains (Kim et al., 1998). Bullfrog PMCA1b contains the same C-terminal sequence (-ETSL) as human PMCA2b, suggesting that it too could bind PDZ proteins, which could localize this isozyme within the hair cell.
PMCA2 sometimes appeared to be concentrated near stereocilia tips (see, for example, Fig. 3G) and in a broad band near the base of the hair bundle (see, for example, Fig. 3E; Yamoah et al., 1998). Bundle PMCA uses the a splicing variant, however, which apparently does not bind any PDZ-containing proteins (Kim et al., 1998). Localization of PMCA2av within stereocilia might rely instead on the novel v variant within splicing region A. The 12 extra amino acids encoded by v resemble sequences that bind to src homology 3 (SH3) domains; although matching the minimal sequence, PxxP, required for SH3 binding, v does not match the more stringent general consensus ψPxψP, where ψ is a hydrophobic amino acid (Sudol, 1998). Although this putative SH3-binding domain conceivably could target PMCA2av to or localize it in bullfrog sacculus hair bundles, its absence from rat vestibular and auditory PMCA2 suggests that other localization mechanisms may be used in rat and perhaps in bullfrog hair cells.
PMCA2a as the stereocilia calcium pump
We have shown previously that PMCA is present in bullfrog hair bundles at the very high membrane density of ∼2000/μm2 (Yamoah et al., 1998). Furthermore, this PMCA can be sufficiently active during transduction to generate a measurable outward current (Yamoah et al., 1998) and extrude most of the Ca2+ that enters a stereocilium during transduction (Lumpkin and Hudspeth, 1998). The data presented here show that the calcium pump responsible for this activity is PMCA2a.
Why is PMCA2a restricted to the hair bundle? The hair cell may target PMCA2a to the hair bundle simply so that a high calcium pump concentration can be established there. If all PMCA2a travels to the hair bundle, the hair cell can regulate the membrane density of this protein in the stereocilia by controlling PMCA2a transcription and translation.
In addition, PMCA2 may have catalytic or regulatory properties that may be relevant for hair bundle physiology. First, PMCA2 isoforms have significantly lower values of Km for Ca2+ than do other isoforms, indicating that they can lower Ca2+ to substantially lower levels than can other isozymes (Elwess et al., 1997; Strehler and Zacharias, 2001). Resting hair bundle Ca2+is very low (Denk et al., 1995; Lumpkin and Hudspeth, 1998); bundles may use PMCA to lower Ca2+ to insulate them from Ca2+ fluxes in the soma (Lenzi and Roberts, 1994) or to permit specific bundle functions, such as tip link regeneration (Zhao et al., 1996). Second, calmodulin activates PMCA2 isozymes with a substantially greater affinity than with other PMCA isozymes; this affinity difference may be irrelevant, however, because of the high hair bundle calmodulin concentration (∼70 μm;Walker et al., 1993). Finally, the variant used at splicing region C may be relevant for hair bundle function. For example, protein kinase C phosphorylates the a form of PMCA2, preventing stimulation of the pump by calmodulin (Enyedi et al., 1997). Only when PMCA2a is expressed and purified can we determine all of the properties that are significant for hair bundle function, including the maximal pumping rate of the pump, H+ stoichiometry (Hao et al., 1994), and dependence on ATP, Ca2+, and calmodulin.
PMCA may have a broader role within hair bundles other than simply removing Ca2+ that enters during transduction or from the soma. Hair cells in lower vertebrates are capable of Ca2+-triggered bundle movements (Benser et al., 1996; Ricci et al., 2000), which may be required for sensitive detection of periodic stimuli (Hudspeth et al., 2000; Manley, 2000). Indeed, a PMCA2a-established spatiotemporal Ca2+ gradient at the Ca2+-sensitive site of the channel could provide the energy source for active bundle movements (Hudspeth, 1997). In the auditory system PMCA2a is present in an exceptionally high density in outer hair cells, which are responsible for local mechanical amplification (Dallos, 1992). Amplification, which is required for sensitive stimulus detection and sharp frequency discrimination, may be powered by somatic electromotility (Ashmore and Kolston, 1994) or hair bundle mechanical activity (Hudspeth et al., 2000). By establishing the necessary energy source, PMCA2a would play a central role in any bundle-based mechanism.
Footnotes
This work was supported by National Institutes of Health Grants DC00979, DC04571, and DC02368 (P.G.G.) and GM28835 and DC04200 (J.P.). We thank Stefan Heller for the bullfrog sacculus cDNA library and David Corey for the original image used for Figure 8.
Correspondence should be addressed to Dr. Peter G. Gillespie, Oregon Hearing Research Center and Vollum Institute, L335A/Oregon Health Sciences University, 3181 SW Sam Jackson Park Road, Portland OR 97201. E-mail: gillespp@ohsu.edu.
REFERENCES
- 1.Adamo HP, Caride AJ, Penniston JT. Use of expression mutants and monoclonal antibodies to map the erythrocyte Ca2+ pump. J Biol Chem. 1992;267:14244–14249. [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Apicella S, Chen S, Bing R, Penniston JT, Llinas R, Hillman DE. Plasmalemmal ATPase calcium pump localizes to inner and outer hair bundles. Neuroscience. 1997;79:1145–1151. doi: 10.1016/s0306-4522(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 4.Ashmore JF, Kolston PJ. Hair cell-based amplification in the cochlea. Curr Opin Neurobiol. 1994;4:503–508. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 5.Benaim G, Moreno SN, Hutchinson G, Cervino V, Hermoso T, Romero PJ, Ruiz F, de Souza W, Docampo R. Characterization of the plasma membrane calcium pump from Trypanosoma cruzi. Biochem J. 1995;306:299–303. doi: 10.1042/bj3060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benser ME, Marquis RE, Hudspeth AJ. Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus. J Neurosci. 1996;16:5629–5643. doi: 10.1523/JNEUROSCI.16-18-05629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borke JL, Epstein FH, Penniston JT, Kumar R. Localization of a plasma membrane Ca2+ pump in gill chloride cells and kidney distal tubule cells of the American eel, Anguilla rostrata. Bull Mt Desert Island Biol Lab. 1989;28:48–51. [Google Scholar]
- 8.Carafoli E, Stauffer T. The plasma membrane calcium pump: functional domains, regulation of activity, and tissue specificity of isoform expression. J Neurobiol. 1993;25:312–324. doi: 10.1002/neu.480250311. [DOI] [PubMed] [Google Scholar]
- 9.Caride AJ, Filoteo AG, Enyedi A, Verma AK, Penniston JT. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem J. 1996;316:353–359. doi: 10.1042/bj3160353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford AC, Evans MG, Fettiplace R. The actions of calcium on the mechanoelectrical transducer current of turtle hair cells. J Physiol (Lond) 1991;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crouch JJ, Schulte BA. Expression of plasma membrane Ca-ATPase in the adult and developing gerbil cochlea. Hear Res. 1995;92:112–119. doi: 10.1016/0378-5955(95)00201-4. [DOI] [PubMed] [Google Scholar]
- 12.Crouch JJ, Schulte BA. Identification and cloning of site C splice variants of plasma membrane Ca-ATPase in the gerbil cochlea. Hear Res. 1996;101:55–61. doi: 10.1016/s0378-5955(96)00132-3. [DOI] [PubMed] [Google Scholar]
- 13.Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechesne CJ, Winsky L, Kim HN, Goping G, Vu TD, Wenthold RJ, Jacobowitz DM. Identification and ultrastructural localization of a calretinin-like calcium-binding protein (protein 10) in the guinea pig and rat inner ear. Brain Res. 1991;560:139–148. doi: 10.1016/0006-8993(91)91224-o. [DOI] [PubMed] [Google Scholar]
- 15.Dechesne CJ, Rabejac D, Desmadryl G. Development of calretinin immunoreactivity in the mouse inner ear. J Comp Neurol. 1994;346:517–529. doi: 10.1002/cne.903460405. [DOI] [PubMed] [Google Scholar]
- 16.Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–1321. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 17.de Talamoni N, Smith CA, Wasserman RH, Beltramino C, Fullmer CS, Penniston JT. Immunocytochemical localization of the plasma membrane calcium pump, calbindin-D28k, and parvalbumin in Purkinje cells of avian and mammalian cerebellum. Proc Natl Acad Sci USA. 1993;90:11949–11953. doi: 10.1073/pnas.90.24.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987;7:2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elwess NL, Filoteo AG, Enyedi A, Penniston JT. Plasma membrane Ca2+ pump isoforms 2a and 2b are unusually responsive to calmodulin and Ca2+. J Biol Chem. 1997;272:17981–17986. doi: 10.1074/jbc.272.29.17981. [DOI] [PubMed] [Google Scholar]
- 20.Enyedi A, Verma AK, Heim R, Adamo HP, Filoteo AG, Strehler EE, Penniston JT. The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem. 1994;269:41–43. [PubMed] [Google Scholar]
- 21.Enyedi A, Elwess NL, Filoteo AG, Verma AK, Paszty K, Penniston JT. Protein kinase C phosphorylates the “a” forms of plasma membrane Ca2+ pump isoforms 2 and 3 and prevents binding of calmodulin. J Biol Chem. 1997;272:27525–27528. doi: 10.1074/jbc.272.44.27525. [DOI] [PubMed] [Google Scholar]
- 22.Filoteo AG, Elwess NL, Enyedi A, Caride A, Aung HH, Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem. 1997;272:23741–23747. doi: 10.1074/jbc.272.38.23741. [DOI] [PubMed] [Google Scholar]
- 23.Forge A. Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell Tissue Res. 1991;265:473–483. doi: 10.1007/BF00340870. [DOI] [PubMed] [Google Scholar]
- 24.Furuta H, Luo L, Hepler K, Ryan AF. Evidence for differential regulation of calcium by outer versus inner hair cells: plasma membrane Ca-ATPase gene expression. Hear Res. 1998;123:10–26. doi: 10.1016/s0378-5955(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie PG, Gillespie SK. Improved electrophoresis and transfer of picogram amounts of protein with hemoglobin. Anal Biochem. 1997;246:239–245. doi: 10.1006/abio.1997.2019. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie PG, Hudspeth AJ. High-purity isolation of bullfrog hair bundles and subcellular and topological localization of constituent proteins. J Cell Biol. 1991;112:625–640. doi: 10.1083/jcb.112.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green MR. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- 28.Guerini D, Foletti D, Vellani F, Carafoli E. Mutation of conserved residues in transmembrane domains 4, 6, and 8 causes loss of Ca2+ transport by the plasma membrane Ca2+ pump. Biochemistry. 1996;35:3290–3296. doi: 10.1021/bi952572f. [DOI] [PubMed] [Google Scholar]
- 29.Guerini D, Zecca-Mazza A, Carafoli E. Single amino acid mutations in transmembrane domain 5 confer to the plasma membrane Ca2+ pump properties typical of the Ca2+ pump of endo(sarco)plasmic reticulum. J Biol Chem. 2000;275:31361–31368. doi: 10.1074/jbc.M003474200. [DOI] [PubMed] [Google Scholar]
- 30.Hao L, Rigaud JL, Inesi G. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J Biol Chem. 1994;269:14268–14275. [PubMed] [Google Scholar]
- 31.Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Corey DP. Unconventional myosins in inner ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilfiker H, Guerini D, Carafoli E. Cloning and expression of isoform 2 of the human plasma membrane Ca2+-ATPase. J Biol Chem. 1994;269:26178–26183. [PubMed] [Google Scholar]
- 33.Hudspeth AJ. Mechanical amplification of stimuli by hair cells. Curr Opin Neurobiol. 1997;7:480–486. doi: 10.1016/s0959-4388(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 34.Hudspeth AJ, Choe Y, Mehta AD, Martin P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci USA. 2000;97:11765–11772. doi: 10.1073/pnas.97.22.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs RA, Hudspeth AJ. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb Symp Quant Biol. 1990;55:547–561. doi: 10.1101/sqb.1990.055.01.053. [DOI] [PubMed] [Google Scholar]
- 36.Keeton TP, Burk SE, Shull GE. Alternative splicing of exons encoding the calmodulin-binding domains and C termini of plasma membrane Ca2+-ATPase isoforms 1, 2, 3, and 4. J Biol Chem. 1993;268:2740–2748. [PubMed] [Google Scholar]
- 37.Kim E, DeMarco SJ, Marfatia SM, Chishti AH, Sheng M, Strehler EE. Plasma membrane Ca2+-ATPase isoform 4b binds to membrane-associated guanylate kinase (MAGUK) proteins via their PDZ (PSD-95/Dlg/ZO-1) domains. J Biol Chem. 1998;273:1591–1595. doi: 10.1074/jbc.273.3.1591. [DOI] [PubMed] [Google Scholar]
- 38.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 39.Lenzi D, Roberts WM. Calcium signaling in hair cells: multiple roles in a compact cell. Curr Opin Neurobiol. 1994;4:496–502. doi: 10.1016/0959-4388(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 40.Lumpkin EA, Hudspeth AJ. Regulation of free Ca2+ concentration in hair cell stereocilia. J Neurosci. 1998;18:6300–6318. doi: 10.1523/JNEUROSCI.18-16-06300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumpkin EA, Marquis RE, Hudspeth AJ. The selectivity of the hair cell's mechanoelectrical transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci USA. 1997;94:10997–11002. doi: 10.1073/pnas.94.20.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci USA. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petralia RS, Wang YX, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol (Lond) 1998;506:159–173. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci. 1998;18:8261–8277. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci. 2000;20:7131–7142. doi: 10.1523/JNEUROSCI.20-19-07131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 48.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 49.Sudol M. From src homology domains to other signaling modules: proposal of the “protein recognition code.”. Oncogene. 1998;17:1469–1474. doi: 10.1038/sj.onc.1202182. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Kitamura K. A point mutation in a plasma membrane Ca2+-ATPase gene causes deafness in Wriggle Mouse Sagami. Biochem Biophys Res Commun. 1999;261:773–778. doi: 10.1006/bbrc.1999.1102. [DOI] [PubMed] [Google Scholar]
- 51.Walker RG, Hudspeth AJ, Gillespie PG. Calmodulin and calmodulin-binding proteins in hair bundles. Proc Natl Acad Sci USA. 1993;90:2807–2811. doi: 10.1073/pnas.90.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci. 1998;18:610–624. doi: 10.1523/JNEUROSCI.18-02-00610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA. 1996;93:15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]