Abstract
Serotonin (5-HT) has been strongly implicated in the regulation of the mammalian circadian clock located in the suprachiasmatic nuclei (SCN); however, its role in behavioral (nonphotic) circadian phase resetting remains elusive. Central to this issue are divergent lines of evidence that the SCN may, or may not, be a target for the phase-resetting effects of 5-HT. We have addressed this question using a novel reverse-microdialysis approach for timed perfusions of serotonergic and other agents to the Syrian hamster SCN with durations equivalent to the increases in in vivo 5-HT release during phase-resetting behavioral manipulations. We found that 3 hr perfusions of the SCN with either 5-HT or the 5-HT1A,7receptor agonist 2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydro-naphthalene (8-OH-DPAT) at midday advanced the phase of the free-running circadian rhythm of wheel-running assessed using an Aschoff type II procedure. Phase shifts induced by 8-OH-DPAT were enhanced more than threefold by pretreatment with the 5-HT synthesis inhibitorpara-chlorophenylalanine. Phase advances induced by SCN 8-OH-DPAT perfusion were significantly inhibited by the 5-HT2,7 receptor antagonist ritanserin and by the more selective 5-HT7 receptor antagonist DR4004, implicating the 5-HT7 receptor in mediating this phase resetting. Concurrent exposure to light during the 8-OH-DPAT perfusion abolished the phase advances. Furthermore, coperfusion of the SCN with TTX, which blocked in vivo 5-HT release, did not suppress intra-SCN 8-OH-DPAT-induced phase advances. These results indicate that 5-HT7 receptor-mediated phase resetting in the SCN is markedly influenced by the degree of postsynaptic responsiveness to 5-HT and by photic stimulation. Finally, 5-HT may act directly on SCN clock cells to induce in vivo nonphotic phase resetting.
Keywords: suprachiasmatic nucleus, serotonin, 8-OH-DPAT, DR4004, ritanserin, circadian rhythm, hamster, in vivo brain microdialysis, phase-resetting, behavioral rhythm
The suprachiasmatic nuclei (SCN) are the primary center for the generation of circadian rhythms in mammals (Rusak and Zucker, 1979; Moore, 1983; Klein et al., 1991). The timing of the SCN clock is synchronized to the light/dark cycle by photic input supplied directly from the retina to the SCN via the retinohypothalamic tract (RHT) (Moore and Lenn, 1972; Pickard, 1982;Johnson et al., 1988) and indirectly via the projection from the intergeniculate leaflet (IGL), the geniculohypothalamic tract (Card and Moore, 1982; Johnson et al., 1989). The IGL also conveys nonphotic (behavioral) entraining input to the SCN (Rusak et al., 1989; Biello et al., 1994; Janik et al., 1995). A third input to the SCN is a dense serotonergic projection from the midbrain raphe (Moore et al., 1978;Meyer-Bernstein and Morin, 1996).
Although the SCN contain one of the highest concentrations of serotonin (5-HT) in the forebrain, a circadian clock-related function of comparative magnitude for this neurotransmitter has not been firmly established (Morin, 1999; Mistlberger et al., 2000). One proposed role of 5-HT is the modulation of RHT-mediated photic signaling in the SCN. Support for this comes from findings that 5-HT receptor agonists attenuate RHT-mediated responses in the SCN, including light-activated neuronal activity (Miller and Fuller, 1990; Ying and Rusak, 1994) and immediate-early gene activation (Selim et al., 1993) (for review, seeRea and Pickard, 2000).
There is also evidence that 5-HT may act in the SCN to regulate nonphotic circadian phase resetting. For example, 5-HT receptor agonists reset the SCN clock in vitro (Prosser et al., 1990;Medanic and Gillette, 1992) (for review, see Prosser, 2000) andin vivo (Tominaga et al., 1992; Edgar et al., 1993;Bobrzynska et al., 1996a; Penev et al., 1997; Challet et al., 1998;Horikawa et al., 2000). Correspondingly, depletion of central 5-HT inhibits the phase resetting evoked by locomotor activity–arousal (Sumova et al., 1996; Marchant et al., 1997) and prevents entrainment to scheduled activity regimens (Edgar et al., 1997; Marchant et al., 1997). Finally, nonphotic phase-resetting stimuli, including wheel running (Dudley et al., 1998) and sleep deprivation (Grossman et al., 2000), stimulate 5-HT release in the SCN.
Nevertheless, there is an impressive body of evidence arguing against an in vivo phase-resetting action of 5-HT in the SCN. This includes reports that unilateral SCN injection of 5-HT agonist lacks phase-shifting effect (Mintz et al., 1997), and lesions of SCN 5-HT innervation do not prevent nonphotic phase resetting (Bobrzynska et al., 1996b; Meyer-Bernstein and Morin, 1997). Also, enhanced 5-HT release in the SCN does not potentiate nonphotic phase advances (Antle et al., 2000), and SCN administration of 5-HT antagonists does not prevent behavioral phase resetting (Antle et al., 1998). In view of the mixed evidence for the SCN being a target for serotonergic phase resetting, the present study was undertaken to examine this important issue by exploiting a novel reverse-microdialysis approach for delivering timed perfusions of serotonergic agents to the SCN. This technique facilitates the delivery of agents for durations approximating the increases in 5-HT output during phase-resetting behavioral manipulations (Dudley et al., 1998; Grossman et al., 2000). Results from these experiments would provide fundamental information on the role of 5-HT in nonphotic entrainment and its actions in the SCN.
MATERIALS AND METHODS
Animals
Adult male Syrian hamsters (Mesocricetus auratus), raised from breeder pairs obtained from Harlan Sprague Dawley (Madison, IL), were used in these studies. The animals were housed at 22°C in a climate-controlled vivarium under a 14/10 hr light/dark cycle (LD) (250 lux illuminance). Before experimentation, animals were individually housed in a rectangular polycarbonate cage equipped with a 14-inch-diameter running wheel. Rodent chow (Prolab 3000; PMI Feeds, St. Louis, MO) and water were provided ad libitum.
Microdialysis
The microdialysis procedures developed in this laboratory for use in studying the SCN of Syrian hamsters have been described previously (Dudley et al., 1998). Under sodium pentobarbital anesthesia (50 mg/kg Nembutal), the animals received a microdialysis probe reentry guide cannula, with the distal end stereotaxically aimed such that, when inserted, the dialysis probe extended 3 mm beyond the guide cannula to the lateral margin of the SCN. The coordinates used were as follows: anteroposterior (AP), +0.4 mm from bregma; lateral, +0.3 mm from the midsagittal suture; and height, 5.0 mm from dura, with head level. One group of anatomical controls received an implant located 2 mm caudal to these coordinates (AP, −1.6 mm from bregma; the other coordinates were the same). The guide cannulas were anchored by three stainless steel screws in the skull and secured with dental acrylic. When not in use, a 26 gauge wire stilette (extending 1 mm from the cannula end) was used to seal the reentry cannula. The microdialysis probes were constructed from hemicellulose dialysis tubing with 12 kDa cutoff (230 μm outer diameter; Spectra-por; Fisher Scientific, Pittsburgh, PA) glued to a 26 gauge stainless steel outer cannula containing a fused silica inner cannula (Polymicro Technologies, Phoenix, AZ). The dialysis membrane tip length of the probes was 1.0 mm. During the microdialysis experiments, probes were perfused with artificial CSF (ACSF) (in mm: 147.2 NaCl, 4.0 KCl, and 1.8 CaCl2, pH 7.2) at a flow rate of 1.2 μl/min. Insertion of the microdialysis probes was undertaken 24 hr before the start of perfusion without anesthetizing the animal. Probe position was verified histologically from 20-μm-thick frozen sections stained with cresyl violet at the end of the experiment (described below).
Circadian activity measurements
Daily wheel-running activity was recorded by a magnetic switch attached to the running wheel assembly with its output interfaced with a computerized data acquisition system (Dataquest III; Mini-Mitter Co., Sunriver, OR). Circadian phase resetting was assessed using a modified Aschoff type II procedure (Aschoff, 1965), in which animals maintained under LD up to the time of experimentation were released into total darkness (DD) at the beginning of microdialysis perfusion zeitgeber time 6 (ZT 6)] or at the time of intraperitoneal drug administration. Phase shifts of the circadian wheel-running rhythm were calculated as the difference between the averaged activity onset for 5 d preceding drug treatment and onsets predicted by least-squares regression analysis of activity onsets from days 3–10 after treatment. Activity onset was defined as the first 10 min period in which the total number of wheel revolutions exceeded 50% of the maximum number of revolutions per 10 min measured that day and that was followed by at least 1 hr of sustained activity.
Experimental protocols
p-Chlorophenylalanine-induced inhibition of serotonergic activity. After reentry cannula implantation, wheel-running activity was monitored under LD for 5 d. The animals received two treatments with p-chlorophenylalanine (p-CPA) (each treatment, 150 mg/kg; Sigma, St. Louis, MO) administered by subcutaneous injection 4 d and then 1 d preceding the microdialysis experiments. Controls received similarly timed subcutaneous injections of vehicle. Animals were used either in the phase-resetting experiments described below or for determination ofp-CPA effects on SCN serotonergic activity. For such determination, animals were killed by Nembutal overdose the day after the last p-CPA or vehicle treatment at ZT 6 (ZT 12 was designated as the time of lights off), which corresponds to the time of the onset of microdialysis perfusion in the phase-resetting experiments. The brains were removed, and frozen 0.8-mm-thick coronal cryostat sections containing the SCN were prepared. The SCN tissue was obtained using a stainless steel punch, and the tissues were weighed and sonicated in 0.05 m perchlorate in distilled water (1 mg/30 μl). Extracts were centrifuged at 34,000 ×g at 4°C for 20 min, and the contents of 5-HT and its principal metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the supernatant were measured using the validated HPLC procedures described below.
Microdialysis perfusion of serotonergic agents. Starting at 1 d after the last p-CPA or vehicle injection, 3 hr SCN perfusions began at ZT 6 in animals receiving the 5-HT1A,7 receptor agonist 2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydro-naphthalene (8-OH-DPAT) (Sigma) or 5-HT creatinine sulfate (Sigma) in 94.5:5.5% ACSF/DMSO v/v [the perfusate free-base concentration of each agonist was 1.2 mm; with an ∼2.4% in vitro probe efficiency for indoleamines (Glass et al., 1992), the external concentrations of both agonists was estimated at ∼30 μm]. Control animals received ACSF/DMSO vehicle. Systemic (intraperitoneal) administration of 8-OH-DPAT was also undertaken in a separate group of animals. In the receptor antagonist trials, the selective 5-HT7 receptor antagonist DR4004 (Meiji Seika Kaisha Ltd., Yokohama, Japan) was dissolved directly in the ACSF at concentrations of 120 or 240 μm. The DR4004 was administered for 4 hr, beginning 30 min before 8-OH-DPAT perfusion and extending 30 min after the agonist perfusion. The effects of intraperitoneal administration of the 5-HT2,7 receptor antagonist ritanserin on intra-SCN 8-OH-DPAT phase resetting were also evaluated in a separate group of animals. For all microdialysis procedures, the animals were kept in their home cage, with the running wheel locked.
Microdialysis perfusion of tetrodotoxin. The effect of TTX on the phase-resetting effect of intra-SCN 8-OH-DPAT perfusion was assessed using a 1 hr microdialysis perfusion of the SCN region with TTX (0.5 μm perfusate concentration; Sigma) extending 20 min before, during, and 20 min after a 20 min perfusion with 8-OH-DPAT. Effectiveness of this dose of TTX in blocking sodium channel-dependent action potentials was determined by measuring the content of 5-HT in SCN microdialysate of animals perfused with the TTX. A 40 min baseline collection began at ZT 6, and TTX was subsequently delivered over a 1 hr period, after which time posttreatment collection was undertaken for 1.6 hr. The content of 5-HT in the SCN microdialysates was measured using the validated HPLC procedures described below.
HPLC. Microdialysates were analyzed for 5-HT using a high-performance liquid chromatograph with an amperometric radial flow electrochemical detector [Bioanalytical Systems (BAS), West Lafayette, IN] described by Dudley et al. (1998). The detector was set at a potential of 590 mV relative to a silver reference electrode. A 10 μl aliquot of microdialysate was injected directly onto a 1.0 × 100 mm reverse-phase 3 μm C-18 column (BAS). Mobile phase consisted of (in gm): 9.45 monochloroacetic acid, 3.6 NaOH, 0.25 Na2EDTA, and 0.2 octane sulfonic acid in 1.0 l of purified distilled water, pH 3.1. Tetrahydrofuran (6 ml) was added after filtration. Flow rate through the column was 90 μl/min, and sensitivity (the minimal amount of 5-HT producing a signal four times that of background) was ∼500 fg (average baseline content of 5-HT in a 20 μl sample of SCN microdialysate ranged between 5 and 10 pg). The 5-HT reuptake inhibitor citalopram (4.0 μm; Farmitalia, Milano, Italy) was added to the ACSF perfusate in the experiments. Authenticity of the 5-HT peak has been verified in previous studies by the following: (1) coelution with authentic standard; (2) increases after electrical stimulation of the median raphe or localized perfusion with ACSF containing citalopram or 100 mm KCl; and (3) decreases after localized perfusion with TTX or systemic treatment with 8-OH-DPAT or the 5-HT1A mixed autoreceptor agonist BMY 7378 (Sigma) (Dudley et al., 1998, 1999).
Histology. After experimentation, hamsters were anesthetized with Nembutal, and a dialysis probe was inserted into the reentry cannula. After 1 hr, the probe was filled with methylene blue solution for 20 min to help visualize probe tip location. The animal was then killed with an overdose of Nembutal, the brain was removed, and 20-μm-thick cryostat sections were prepared. The sections were mounted on glass slides and stained with cresyl violet. Apart from the anatomical control group, animals whose dialysis probe was >500 μm from the SCN were excluded from the data analysis. Locations of the implants included in the statistical analyses are represented diagrammatically in Figure 1.
Fig. 1.
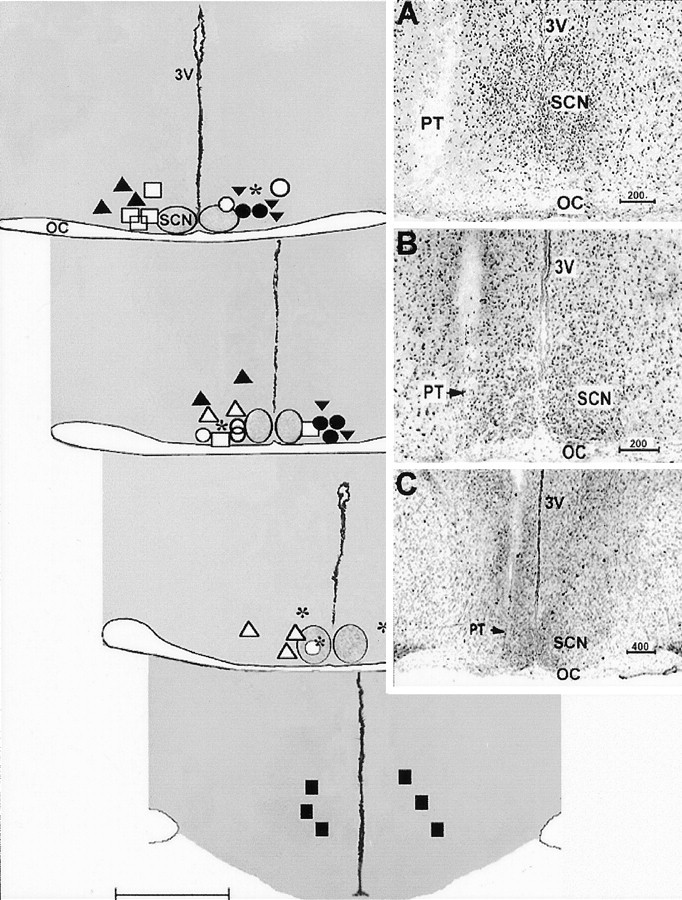
Diagrammatic coronal sections showing histologically verified locations of the microdialysis probes tips of different groups included in the study. Coronal planes extend from the anterior SCN (top left section) to the caudal hypothalamus (bottom right section).Symbols represent the ventral extent of the probe implants for the treatment groups as follows: *, p-CPA plus SCN 8-OH-DPAT perfusion; ▾, no p-CPA plus SCN 8-OH-DPAT perfusion; ○, p-CPA plus SCN ACSF vehicle perfusion; ●, p-CPA plus SCN 8-OH-DPAT perfusion in light; ▴, p-CPA plus SCN 5-HT perfusion; ▵, p-CPA plus SCN 8-OH-DPAT plus DR4004 perfusion; ■, p-CPA plus SCN 8OH-DPAT plus ritanserin perfusion; ▪, p-CPA plus 8-OH-DPAT perfusion 2 mm caudal to SCN.OC, Optic chiasma; 3V, third ventricle. Scale bar, 1.0 mm. Insets are photomicrographs of cresyl violet-stained coronal hypothalamic sections showing the location of the probe tip (PT) relative to the SCN in three animals under higher (A, B) and lower (C) magnification. Note the positioning of the microdialysis tip against the lateral margin of the SCN. Values shown above the scale bars are in micrometers.
Statistics
Drug treatment effects on circadian phase-resetting responses to the pharmacological treatments were analyzed using a repeated-measures ANOVA, followed by the Student–Newman–Keuls post hoc mean comparison test. The level set for statistical significance wasp < 0.05.
RESULTS
p-CPA enhances serotonergic phase resetting
The p-CPA treatment protocol reduced SCN tissue content of 5-HT estimated at the time of microdialysis perfusion by 87.5% (7.5 ± 1.6 ng per SCN for controls vs 0.9 ± 0.5 ng per SCN for p-CPA; p < 0.03;n = 4 per group). Serotonergic activity estimated by the 5-HIAA/5-HT ratio of 0.239 ± 0.030 for controls versus 0.048 ± 0.010 for p-CPA (p < 0.005) was 80%. The p-CPA treatment had little effect on the circadian wheel-running rhythm under initial LD conditions or on the phase or period of the free-running rhythm after release to DD. Treatment with p-CPA, however, markedly potentiated the phase-resetting effect of the 3 hr microdialysis perfusion of the SCN with 8-OH-DPAT. Animals pretreated with p-CPA exhibited 8-OH-DPAT-induced phase advances that were 325% larger than those of animals pretreated with oil vehicle alone (98.8 ± 22.0 vs 30.5 ± 9.9 min, respectively; p < 0.05) (Table1, Fig. 2). Pretreatment with p-CPA also caused a significant (220%) potentiation of the phase-resetting effect of systemically administered 8-OH-DPAT (71.4 ± 10.4 vs 32.4 ± 6.1 min for vehicle controls; p < 0.05). The phase-resetting effect of 8-OH-DPAT in p-CPA-pretreated control animals with perfusion sites 2 mm caudal to the SCN was significantly reduced compared with that in the SCN perfused animals (46.2 ± 7.8 min;p < 0.05 vs SCN perfusion) (Table 1). The phase-advancing effect of 3 hr SCN perfusion ofp-CPA-pretreated animals with 5-HT (98.6 ± 22.4 min) was equivalent to that caused by 8-OH-DPAT. The 3 hr perfusion with ACSF alone had a negligible effect on circadian phase (−4.9 ± 11.3 min) (Table 1). It must be noted that some animals of the control and 8-OH-DPAT groups exhibited transient arousal (moving about the cage) for short periods during the microdialysis perfusion. Because the arousal had little phase-resetting effect in the controls, it should have not have affected the 8-OH-DPAT-induced shifts.
Table 1.
Circadian phase-resetting effects of serotonin agonists administered at midday by microdialysis perfusion or intraperitoneal injection after pretreatment with or without p-CPA
| Treatment | p-CPA | Nop-CPA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 hr SCN DPAT (6) | caud. to SCN 3 hr DPAT (6) | 20 min SCN DPAT (6) | 3 hr SCN ACSF (6) | 3 hr SCN 5-HT (5) | Light+ 3 hr SCN DPAT (5) | TTX+ 20 min SCN DPAT (5) | Intraperitoneal DPAT (9) | 3 hr SCN DPAT (5) | Intraperitoneal DPAT (6) | |
| Phase shift (min) | 98.8 ± 22.0 | 46.2 ± 7.8 | 42.4 ± 11.2 | −4.9 ± 11.3 | 98.6 ± 22.4 | 14.6 ± 13.3 | 71.6 ± 21.0 | 71.4 ± 10.4 | 30.5 ± 9.9 | 32.4 ± 6.1 |
| a | b, c | b, c | d | a | b, d | a, b, c | a, c | b, e | b, e | |
Values are means ± SEM. Treatment groups with different letters are significantly different; p < 0.05. Values in parentheses are the number of animals per group. “3 hr SCN DPAT” denotes the group that received a 3 hr perfusion with 8-OH-DPAT in the SCN. “Caud. to SCN 3 hr DPAT” denotes the control group that received a 3 hr perfusion with 8-OH-DPAT 2 mm caudal to the SCN.
Fig. 2.

Double-plotted wheel-running activity records of two representative profiles from three treatment groups. Days are indicated vertically from top to bottom, and time is indicated horizontally. The shaded horizontal bars represent the 3 hr microdialysis perfusion from ZT 6 to ZT 9. A, B, SCN 8-OH-DPAT perfusion in darkness without p-CPA pretreatment; C,D, SCN 8-OH-DPAT perfusion in darkness withp-CPA pretreatment (2 additional profiles of this treatment group are shown in Fig.4A,B); E,F, SCN 8-OH-DPAT perfusion in the light withp-CPA pretreatment. See Materials and Methods for details of the various treatment protocols.
5-HT7 antagonists inhibit 8-OH-DPAT phase resetting in the SCN
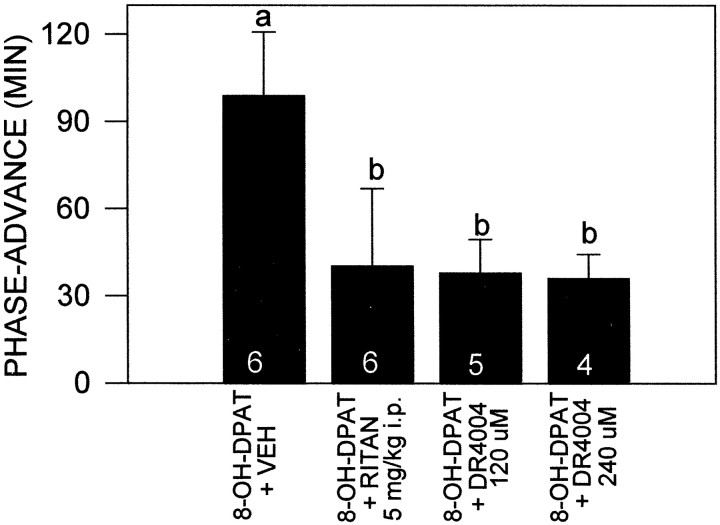
Effects of the 5-HT antagonists ritanserin and DR4004 on 8-OH-DPAT phase resetting were assessed in groups pretreated withp-CPA. Pretreatment with a single intraperitoneal injection of the 5-HT2,7 receptor antagonist ritanserin significantly inhibited the phase-advancing effect of 3 hr perfusion of the SCN with 8-OH-DPAT (n = 6; 40.3 ± 26.5 vs 98.8 ± 22.0 min; p < 0.05) (Figs.3, 4). The dosage of ritanserin used (5 mg/kg) has been shown to be more than sufficient to maximally occupy its receptors for extended periods (Sumova et al., 1996). Coperfusion of the SCN with two different concentrations of the highly selective 5-HT7 receptor antagonist DR4004 also significantly attenuated the phase-advancing effect of SCN 8-OH-DPAT perfusion [120 μm (n= 5), 37.8 ± 11.6 min; 240 μm(n = 4), 36.0 ± 8.3; p < 0.05 vs 8-OH-DPAT) (Fig. 4).
Fig. 3.
Double-plotted wheel-running activity records of two representative profiles from three 5-HT antagonist treatment groups. All were pretreated with p-CPA. Theshaded horizontal bars represent the 3 hr microdialysis perfusion from ZT 6 to ZT 9. A, B, SCN 8-OH-DPAT perfusion in darkness; C, D, SCN 8-OH-DPAT plus intraperitoneal ritanserin injection in darkness;E, F, SCN 8-OH-DPAT plus DR4004 perfusion in darkness.
Fig. 4.
The inhibitory effects of intraperitoneal administration of the 5-HT2,7 receptor antagonist ritanserin or coperfusion with two concentrations of the highly selective 5-HT7 receptor antagonist DR4004 on the phase-advancing effect of SCN perfusion with 8-OH-DPAT. These treatments significantly attenuated the phase-advancing response to the 8-OH-DPAT. Bars with different lettersare significantly different. Numbers in thebars are the number of animals per group.
Phase resetting is induced by short-duration 8-OH-DPAT SCN perfusion
The shorter 20 min perfusions of the SCN with 8-OH-DPAT inp-CPA-pretreated animals were also effective in inducing significant phase advances (42.4 ± 11.2 min; p < 0.05 vs vehicle perfusion) (Table 1). This effect, however, was significantly less than the 98.8 ± 22.0 min phase advances produced by the 3 hr SCN 8-OH-DPAT perfusions (p< 0.05).
Light exposure abolishes 8-OH-DPAT phase resetting in the SCN
Performing the 3 hr SCN 8-OH-DPAT perfusion in the presence of room light (250 lux) and then releasing the animals into DD at the end of perfusion abolished the phase-resetting response of the 8-OH-DPAT inp-CPA-pretreated animals (14.6 ± 13.3 vs 98.8 ± 22.0 min; p < 0.05) (Table 1, Fig. 2).
8-OH-DPAT-induced phase resetting in the SCN is TTX insensitive
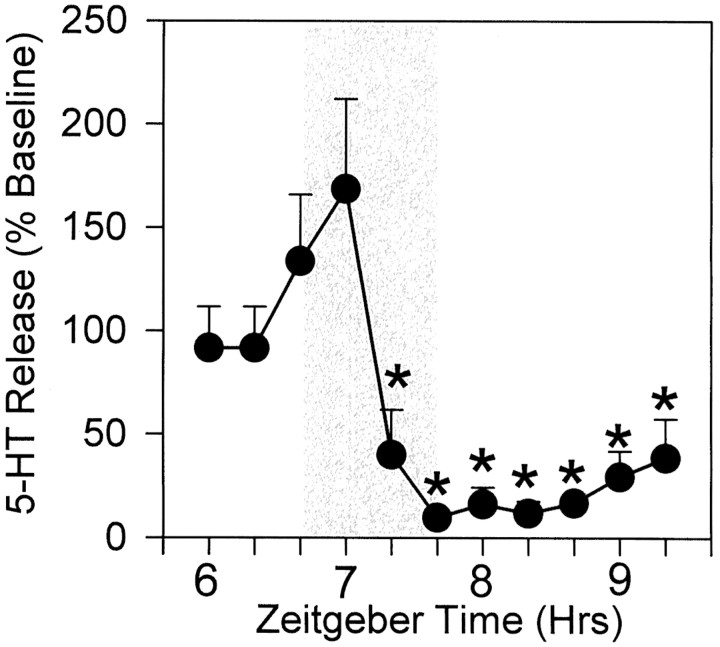
The present data reveal that TTX treatment (0.5 μmperfusate concentration) does not block phase resetting induced by 20 min perfusion of the SCN with 8-OH-DPAT in p-CPA-pretreated animals (Table 1). Perfusion of the SCN with this concentration of TTX for 1 hr, however, caused a maximal 93 ± 4% reduction in extracellular SCN 5-HT concentration (n = 4;p < 0.05 vs baseline) (Fig. 5) perfusion. From this data, it is inferred that this TTX treatment would have significantly blocked sodium channel-mediated action potential activity in the same region as the coperfused 8-OH-DPAT for a period exceeding the 20 min 8-OH-DPAT perfusion.
Fig. 5.
The inhibitory effect of 1 hr microdialysis perfusion with TTX (0.5 μm; shaded region) on in vivo 5-HT release in the SCN, measured from the microdialysis probe. A significant suppression of 5-HT release occurred within 40 min of the onset of TTX perfusion and persisted for at least 1.6 hr after cessation of perfusion. *p < 0.05 versus pretreatment baseline level (n = 4).
DISCUSSION
The present results confirm that neural elements in or near the SCN can respond directly to the phase-resetting action of 5-HT receptor agonists in vivo. Timed microdialysis perfusions of the SCN with the 5-HT1,7 receptor agonist 8-OH-DPAT or 5-HT itself at midday significantly advanced the phase of the Syrian hamster circadian clock. The suppression of 8-OH-DPAT-induced phase advances by the 5-HT2,7 antagonist ritanserin or by the highly selective 5-HT7 antagonist DR4004 (Kikuchi et al., 1999) confirms further that this clock-resetting action is mediated by 5-HT7 receptors. This supports results from the in vitro rat SCN slice preparation, which first implicated 5-HT7receptors in serotonergic circadian clock resetting (Lovenburg et al., 1993). The identities of the 5-HT7receptor-expressing elements involved in this phase resetting are unknown; however, immunocytochemical labeling studies have revealed a widespread distribution of 5-HT7 receptors in the mouse (Pickard and Belenky, 2000) and hamster (for review, seeMistlberger et al., 2000) SCN. These receptors exist on presynaptic and postsynaptic processes of SCN neurons and on astroglia, suggesting that the functions of this receptor in the mammalian clock are diverse. Its colocalization with vasopressin-, VIP-, and GABA-containing neurons (Pickard and Belenky, 2000) denotes multiple neuronal roles of the 5-HT7 receptor in SCN function.
The marked potentiation of 8-OH-DPAT-induced phase advances using the 5-HT synthesis inhibitor p-CPA is evidence that serotonergic phase shifting is enhanced by depletion-induced sensitization of SCN postsynaptic response to 5-HT. Depletion-induced 5-HT hypersensitization by other approaches, including 5,7-DHT lesioning of 5-HT neurons, also enhances hypothalamic neuroendocrine responses to 5-HT agonists (Van de Kar et al., 1998) and upregulates 5-HT receptor binding in the SCN (Manrique et al., 1993). From these findings, a mechanism is hypothesized in which fluctuations in SCN serotonergic activity [i.e., circadian rhythmicity of SCN 5-HT release and its behavioral induction (Dudley et al., 1998; Grossman et al., 2000)] could endogenously modulate postsynaptic sensitivity to 5-HT. Thus, the extent of phase-shifting response could be influenced by postsynaptic, as well as by presynaptic, 5-HT-related actions, with the extent of a shift being determined by the circadian period of delivery of the nonphotic stimulus, as well as by the pattern of 5-HT release preceding the stimulus. Although a relationship between physiological change in 5-HT release and postsynaptic responsiveness to 5-HT has received little study, it is suggested that circadian fluctuations in postsynaptic neurophysiological and behavioral responses to 8-OH-DPAT (Mason, 1986; Currie and Coscina, 1993; Lu and Nagayama, 1996) may be keyed to rhythmic daily changes in 5-HT release modulating daily changes in 5-HT1A receptor sensitivity.
The present results obtained with p-CPA also fit with the view that the robust phase advancing induced by 5-HT agonists in the SCN slice preparation (∼3 hr) is attributable to deafferentation-induced postsynaptic hypersensitivity. However, because the p-CPA-potentiated advances (∼1.5 hr) were only approximately half the size of those obtained in the SCN slice (Prosser, 2000), it may be that the p-CPA treatment did not fully sensitize the phase-resetting response. It is also possible that the greater sensitivity of the SCN slice preparation to 5-HT agonists could be attributable to the removal of other innervation that normally inhibits SCN responses to 5-HT [e.g., glutamate or NPY (Biello et al., 1997; Prosser, 1998)], which would further increase the response of the slice to depolarizing drug and/or neurotransmitter actions.
Nevertheless, it is evident that in vivo phase shifts induced by applications of 8-OH-DPAT to the SCN region, without sensitizing manipulations, are absent or relatively small (Mintz et al., 1997; Challet et al., 1998; present results). These findings alone would suggest that the SCN are not a major target for the phase-resetting action of 5-HT. However, it is reasonable that the modest shifting effects are a consequence of various methodological limitations that are not necessarily mutually exclusive. For example, the duration of exogenous serotonergic agonists delivered by microinjection may be insufficient. This is supported by our finding that the shorter 8-OH-DPAT perfusions caused smaller phase advances than did the longer perfusions [however, application of 8-OH-DPAT in the SCN slice for durations as short as 10 min is sufficient to induce large shifts of neuronal activity (R. Prosser, personal communication)]. It must be noted that the 3 hr perfusions of the SCN with 8-OH-DPAT produced only ∼30 min phase advances in non-p-CPA-treated animals, which suggests that receptor sensitivity could also be an important issue. Another consideration is that there may be limited in vivo accessibility of relevant 5-HT receptors to exogenous antagonists. The large serotonergic phase-shifting responses registered in the SCN slice could be attributable in part to increased receptor accessibility caused by degeneration of fibers of innervation or other neural elements. Along these lines, it is possible that astroglial processes closely associated with numerous SCN synaptic complexes (Shen et al., 1999) could block the accessibility of postsynaptic receptors to exogenous 5-HT agonists under in vivo conditions.
A notable aspect of photic and nonphotic clock resetting is that the former appears to suppress the latter. At the behavioral level, light exposure during an activity pulse blocks or attenuates the phase-shifting response to the activity pulse (Mrosovsky, 1991; Biello and Mrosovsky, 1995). Also, the phase-resetting effect of sleep deprivation is attenuated by concurrent light exposure (Antle and Mistlberger, 2000). At the neurochemical level, light inhibits phase advances induced by systemic (Penev et al., 1997) or intra-IGL (Challet et al., 1998) administration of 8-OH-DPAT and intra-SCN injection of NPY (Biello and Mrosovsky, 1995). Moreover, glutamate blocks NPY- and 5-HT-induced phase-advances in the SCN slice (Biello et al., 1997;Prosser, 1999). Our present data showing that concurrent light exposure blocks the phase-resetting action of SCN 8-OH-DPAT perfusions confirm and extend the above findings; they substantiate the impressive inhibitory effect of light on 8-OH-DPAT-induced phase resetting and corroborate recent experiments in the SCN slice preparation in which glutamate suppressed serotonergic phase-resetting in the SCN (Prosser, 1999). The present results also suggest that, as light inhibits the phase-resetting action of a 5-HT agonist, it likely blocks postsynaptic, or related downstream, actions of 5-HT in the SCN. A study using bilateral microinjections of 8-OH-DPAT has provided alternative evidence that the IGL, and not the SCN, is the primary locus for the photic inhibition of 8-OH-DPAT-induced phase resetting (Challet et al., 1998). Nevertheless, that demonstration of an ∼45% suppression of 8-OH-DPAT shifting in the SCN by light [34 min (no light) vs 19 min (light)], although not reaching statistical significance, leans toward an inhibitory action of light on serotonergic phase resetting in the SCN.
The present finding that 8-OH-DPAT phase-advancing effects are resistant to TTX parallels observations from the in vivo SCN slice preparation, in which coadministration of TTX with the nonselective 5-HT agonist quipazine did not block the phase shifts induced by this agonist (Prosser et al., 1992). In those experiments, as in the present study, the dosages of TTX administered were effective in eliminating action potentials and greatly reducing the synaptic release of serotonin, respectively, arguing that TTX significantly attenuated Na+ channel-dependent action potentials during the period of 5-HT agonists application. Therefore, the TTX-resistant phase-resetting effect of 5-HT agonists must either be exerted via direct action on the clock cells or by upstream stimulation of elements that influence clock cells by nonaction potential-dependent communication, such as ephaptic or gap junctional routes. In the latter regard, it is possible that such distal effects of serotonergic stimulation could likely be mediated via gap junctional communication among astroglia. This contention is supported by the findings that SCN astroglia express the 5-HT7receptor (Mistlberger et al., 2000) and that their activity is significantly affected by 8-OH-DPAT treatment (Glass and Chen, 1999).
Finally, it must be noted that the treatments with ritanserin and DR4004 did not fully block the phase-resetting effect of the 8-OH-DPAT perfusion. There are at least two possibilities for this: either the residual shifts were mediated by a 5-HT receptor other than the 5-HT7 subtype (which, by using the 5-HT1A,7 agonist 8-OH-DPAT, most likely would be 5-HT1A receptors) or the doses of the antagonists were insufficient to block the 8-OH-DPAT shifts. With respect to the former consideration, there is little mRNA for the 5-HT1A receptor in the SCN (Roca et al., 1993), and the pharmacological profile for phase shifting from work in the SCN slice strongly points to the 5-HT7 and not the 5-HT1A subtype. Considering the latter possibility, with respect to ritanserin, the systemic dosage used here (5 mg/kg) has been shown to be sufficient to maximally occupy its receptors for extended periods (Leysen et al., 1985; Sumova et al., 1996).
In conclusion, the present results confirm that elements in or near the SCN have the potential for responding directly to the phase-advancing action of serotonin in vivo. The extent of the phase-resetting response may be regulated by the degree of SCN postsynaptic sensitivity to 5-HT. Also, the phase-advancing action of 5-HT agonists at subjective midday is likely mediated by 5-HT7 receptors and in a TTX-insensitive manner. The phase-resetting action of 5-HT may thus be by a direct action on the SCN clock cells or on elements that communicate to the clock cells using gap junctional or other nonsynaptic transmission processes. It will be of interest to determine whether p-CPA treatment as used here will reveal other circadian phases for 8-OH-DPAT phase-resetting response.
Footnotes
This research was supported by National Institutes of Health Grant NS35229 to J.D.G. We are grateful to Meiji Seika Kaisha Ltd. (Yokohama, Japan) for supplying DR4004.
Correspondence should be addressed to J. David Glass, Department of Biological Sciences, Kent State University, Kent, OH 44242. E-mail:jglass@kent.edu.
REFERENCES
- 1.Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antle MC, Marchant EG, Niel L, Mistlberger RE. Serotonin antagonists do not attenuate activity-induced phase-shifts of circadian rhythms in the Syrian hamster. Brain Res. 1998;813:139–149. doi: 10.1016/s0006-8993(98)01048-8. [DOI] [PubMed] [Google Scholar]
- 3.Antle MC, Glass JD, Mistlberger RE. 5-HT1A antagonist-induced 5-HT release in the hamster suprachiasmatic nuclei: effects on circadian clock resetting. Neurosci Lett. 2000;282:97–100. doi: 10.1016/s0304-3940(00)00873-9. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian clocks. North-Holland; Amsterdam: 1965. pp. 95–111. [Google Scholar]
- 5.Biello SM, Mrosovsky N. Blocking the phase-shifting effect of neuropeptide Y with light. Proc R Soc Lond B Biol Sci. 1995;259:179–187. doi: 10.1098/rspb.1995.0026. [DOI] [PubMed] [Google Scholar]
- 6.Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviourally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 7.Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other's phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience. 1997;77:1049–1057. doi: 10.1016/s0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- 8.Bobrzynska KJ, Godfrey MH, Mrosovsky N. Serotonergic stimulation and nonphotic phase-shifting in hamsters. Physiol Behav. 1996a;59:221–230. doi: 10.1016/0031-9384(95)02130-2. [DOI] [PubMed] [Google Scholar]
- 9.Bobrzynska KJ, Vrang N, Mrosovsky N. Persistence of nonphotic phase shifts in hamsters after serotonin depletion in the suprachiasmatic nucleus. Brain Res. 1996b;741:205–214. doi: 10.1016/s0006-8993(96)00913-4. [DOI] [PubMed] [Google Scholar]
- 10.Card JP, Moore RY. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J Comp Physiol. 1982;206:390–396. doi: 10.1002/cne.902060407. [DOI] [PubMed] [Google Scholar]
- 11.Challet E, Scarbrough K, Penev PD, Turek FW. Roles of the suprachiasmatic nuclei and intergeniculate leaflets in mediating the phase-shifting effects of a serotonin agonist and their photic modulation during subjective day. J Biol Rhythms. 1998;13:410–421. doi: 10.1177/074873098129000237. [DOI] [PubMed] [Google Scholar]
- 12.Currie PJ, Coscina DV. Diurnal variations in the feeding response to 8-OH-DPAT injected into the dorsal or median raphe. NeuroReport. 1993;4:1105–1107. [PubMed] [Google Scholar]
- 13.Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18:5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley TE, DiNardo LA, Glass JD. In vivo assessment of the midbrain raphe nuclear regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurophysiol. 1999;81:1469–1477. doi: 10.1152/jn.1999.81.4.1469. [DOI] [PubMed] [Google Scholar]
- 15.Edgar DM, Miller JD, Prosser RA, Dean RR, Dement WC. Serotonin and the mammalian circadian system. II. Phase-shifting rat behavioral rhythms with serotonergic agonists. J Biol Rhythms. 1993;8:17–31. doi: 10.1177/074873049300800102. [DOI] [PubMed] [Google Scholar]
- 16.Edgar DM, Reid MS, Dement WC. Serotonergic afferents mediate activity-dependent entrainment of the mouse circadian clock. Am J Physiol. 1997;273:R265–R269. doi: 10.1152/ajpregu.1997.273.1.R265. [DOI] [PubMed] [Google Scholar]
- 17.Glass JD, Chen L. Serotonergic modulation of astrocytic activity in the hamster suprachiasmatic nucleus. Neuroscience. 1999;94:1253–1259. doi: 10.1016/s0306-4522(99)00369-3. [DOI] [PubMed] [Google Scholar]
- 18.Glass JD, Randolph WW, Ferreira SA, Rea MA, Hauser UE, Blank JL, De Vries MJ. Diurnal variation in 5-hydroxyindoleacetic acid output in the suprachiasmatic region of the Siberian hamster assessed by in vivo microdialysis: evidence for nocturnal activation of serotonin release. Neuroendocrinology. 1992;56:582–590. doi: 10.1159/000126277. [DOI] [PubMed] [Google Scholar]
- 19.Grossman GH, Mistlberger RE, Antle MC, Ehlen JC, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport. 2000;11:1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- 20.Horikawa K, Yokota S-I, Fuji K, Akiyama M, Moriya T, Okamura H, Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J Neurosci. 2000;20:5867–5873. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janik D, Mikkelsen JD, Mrosovsky N. Cellular colocalization of Fos and neuropeptide Y in the intergeniculate leaflet after nonphotic phase-shifting events. Brain Res. 1995;698:137–145. doi: 10.1016/0006-8993(95)00878-t. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RF, Moore RY, Morin LP. Lateral geniculate lesions alter circadian activity rhythms in the hamster. Brain Res Bull. 1989;22:411–422. doi: 10.1016/0361-9230(89)90068-3. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi C, Nagaso H, Hiranuma T, Koyama M. Tetrahydrobenzindoles: selective antagonists of the 5-HT7 receptor. J Med Chem. 1999;42:533–535. doi: 10.1021/jm980519u. [DOI] [PubMed] [Google Scholar]
- 25.Klein DC, Moore RM, Reppert SM. Suprachiasmatic nucleus: the minds clock. Oxford UP; New York: 1991. [Google Scholar]
- 26.Leysen JE, Gommeren W, van Gompel P, Wynants J, Janssen PFM, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin a very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- 27.Lovenburg TW, Baron BM, deLecea L, Miller JD, Prosser RA, Rea MA, Foy PE, Rake M, Stone AL, Siegel BM, Danielson PE, Sutcliie JG, Erlander MG. A novel adenylate cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 28.Lu J-Q, Nagayama H. Circadian rhythm in the response of central 5-HT1A receptors to 8-OH-DPAT in rats. Psychopharmacology. 1996;123:42–45. doi: 10.1007/BF02246279. [DOI] [PubMed] [Google Scholar]
- 29.Manrique C, Segu L, Hery F, Hery M, Faudon M, Francois-Bellan AM. Increase of central 5-HT1B binding sites following 5,7-dihydroxytryptamine axotomy in the adult rat. Brain Res. 1993;623:345–348. doi: 10.1016/0006-8993(93)91452-x. [DOI] [PubMed] [Google Scholar]
- 30.Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J Neurosci. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason R. Circadian variation is sensitivity of suprachiasmatic and lateral geniculate neurones to 5-hydroxytryptamine in the rat. J Physiol (Lond) 1986;377:1–13. doi: 10.1113/jphysiol.1986.sp016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol (Lond) 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-Bernstein EL, Morin LP. The serotonergic projection from the median raphe nucleus to the suprachiasmatic nucleus modulates activity onset, but not other circadian rhythm parameters. Brain Res. 1997;755:112–120. doi: 10.1016/s0006-8993(97)00111-x. [DOI] [PubMed] [Google Scholar]
- 35.Miller JD, Fuller CA. The response of suprachiasmatic neurons of the rat hypothalamus to photic and serotonergic stimulation. Brain Res. 1990;515:155–162. doi: 10.1016/0006-8993(90)90590-8. [DOI] [PubMed] [Google Scholar]
- 36.Mintz EM, Gillespie CF, Marvel CL, Huhman KL, Albers HE. Serotonergic regulation of circadian rhythms in Syrian hamsters. Neuroscience. 1997;79:563–569. doi: 10.1016/s0306-4522(96)00696-3. [DOI] [PubMed] [Google Scholar]
- 37.Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biol Rhythm Res. 2000;31:240–283. [Google Scholar]
- 38.Moore RY. Organization and function of a central nervous system oscillator: the suprachiasmatic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- 39.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 40.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 41.Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Ann Med. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- 42.Mrosovsky N. Double-pulse experiments with nonphotic and photic phase-shifting stimuli. J Biol Rhythms. 1991;6:167–179. doi: 10.1177/074873049100600207. [DOI] [PubMed] [Google Scholar]
- 43.Penev PD, Zee PC, Turek FW. Serotonin in the spotlight. Nature. 1997;385:123. doi: 10.1038/385123a0. [DOI] [PubMed] [Google Scholar]
- 44.Pickard GE. The afferent connection of the suprachiasmatic nucleus of the golden hamster with emphasis on retinohypothalamic projection. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- 45.Pickard GE, Belenky M. Subcellular distribution of 5-HT7 receptors in the suprachiasmatic nucleus. Soc Neurosci Abstr. 2000;20:76.39. [Google Scholar]
- 46.Prosser RA. Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 1998;808:31–41. doi: 10.1016/s0006-8993(98)00808-7. [DOI] [PubMed] [Google Scholar]
- 47.Prosser RA. Serotonergic phase-advances of the suprachiasmatic circadian pacemaker in vitro are blocked by glutamate agonists. Soc Neurosci Abstr. 1999;19:750.15. [Google Scholar]
- 48.Prosser RA. Serotonergic actions and interactions on the SCN circadian pacemaker: in vitro investigations. Biol Rhythms Res. 2000;31:315–339. [Google Scholar]
- 49.Prosser RA, Miller JD, Heller HC. A serotonin agonist phase-shifts the circadian clock in the suprachiasmatic nuclei in vitro. Brain Res. 1990;534:336–339. doi: 10.1016/0006-8993(90)90153-3. [DOI] [PubMed] [Google Scholar]
- 50.Prosser RA, Miller JD, Heller HC. Serotonergic phase shifts of the mammalian circadian clock: effects of tetrodotoxin and high Mg+2. Brain Res. 1992;573:336–340. doi: 10.1016/0006-8993(92)90784-7. [DOI] [PubMed] [Google Scholar]
- 51.Rea MA, Pickard GE. Serotonergic modulation of photic entrainment in the Syrian hamster. Biol Rhythm Res. 2000;31:284–314. [Google Scholar]
- 52.Roca AL, Weaver DR, Reppert SM. Serotonin receptor gene expression in the rat suprachiasmatic nuclei. Brain Res. 1993;608:159–165. doi: 10.1016/0006-8993(93)90789-p. [DOI] [PubMed] [Google Scholar]
- 53.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 54.Rusak B, Meijer JH, Harrington ME. Hamster circadian rhythms are phase-shifted by electrical stimulation of the geniculo-hypothalamic tract. Brain Res. 1989;493:283–291. doi: 10.1016/0006-8993(89)91163-3. [DOI] [PubMed] [Google Scholar]
- 55.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibition of light-induced Fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Res. 1993;621:181–188. doi: 10.1016/0006-8993(93)90105-v. [DOI] [PubMed] [Google Scholar]
- 56.Shen H, Glass JD, Seki T, Watanabe M. Ultrastructural analysis of polysialylated neural cell adhesion molecule in the suprachiasmatic nuclei of the adult mouse. Anat Rec. 1999;256:448–457. doi: 10.1002/(SICI)1097-0185(19991201)256:4<448::AID-AR11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Sumova A, Maywood ES, Selvage D, Ebling FJP, Hastings MH. Serotonergic antagonists impair arousal-induced phase shifts of the circadian system of the Syrian hamster. Brain Res. 1996;709:88–96. doi: 10.1016/0006-8993(95)01314-8. [DOI] [PubMed] [Google Scholar]
- 58.Tominaga K, Shibata S, Ueki S, Watanabe S. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Eur J Pharmacol. 1992;214:79–84. doi: 10.1016/0014-2999(92)90099-p. [DOI] [PubMed] [Google Scholar]
- 59.Van de Kar LD, Li Q, Cabrera TM, Brownfield MS, Battaglia G. Alterations in 8-hydroxy-2-(diproylamino)tetralin-induced neuroendocrine responses after 5,7-dihydroxytryptamine-induced denervation of serotonergic neurons. J Pharmacol Expt Ther. 1998;286:256–262. [PubMed] [Google Scholar]
- 60.Ying S-W, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]