Abstract
The phase of the mammalian circadian pacemaker, located in the suprachiasmatic nucleus (SCN), is modulated by a variety of stimuli, most notably the environmental light cycle. Light information is perceived by the circadian pacemaker through glutamate that is released from retinal ganglion cell terminals in the SCN. Other prominent modulatory inputs to the SCN include a serotonergic projection from the raphe nuclei and a neuropeptide Y (NPY) input from the intergeniculate leaflet. Light and glutamate phase-shift the SCN pacemaker at night, whereas serotonin (5-HT) and NPY primarily phase-shift the pacemaker during the day. In addition to directly phase-shifting the circadian pacemaker, SCN inputs have been shown to modulate the actions of one another. For example, 5-HT can inhibit the phase-shifting effects of light or glutamate applied to the SCN at night, and NPY and glutamate inhibit phase shifts of one another. In this study, we explored the possibility that glutamate can modulate serotonergic phase shifts during the day. For these experiments, we applied various combinations of 5-HT agonists, glutamate agonists, and electrical stimulation of the optic chiasm to SCN brain slices to determine the effect of these treatments on the rhythm of spontaneous neuronal activity generated by the SCN circadian pacemaker. We found that glutamate agonists and optic chiasm stimulation inhibit serotonergic phase advances and that this inhibition involves both AMPA and NMDA receptors. This inhibition by glutamate may be indirect, because it is blocked by both tetrodotoxin and the GABAA antagonist, bicuculline.
Keywords: suprachiasmatic, circadian, serotonin, glutamate, NMDA, AMPA, tetrodotoxin, DPAT, bicuculline, brain slice, rat
The suprachiasmatic nucleus (SCN) contains the primary circadian clock in mammals (Moore, 1995). The SCN pacemaker generates sustained near-24 hr oscillationsin vitro when maintained in culture in the absence of synchronizing stimuli (Shinohara et al., 1995;Yamazaki et al., 2000). Under normal conditions, however, SCN pacemaker phase is modulated by a variety of signals. These signals are generally divided into two categories: photic signals that modulate circadian phase when presented during the subjective night and nonphotic signals that modulate circadian phase when presented during the subjective day. The best characterized photic signal is light itself, which induces phase delays during the early night and phase advances during the late night (Takahashi and Zatz, 1982). These effects are mimicked by injecting glutamate or its agonists into the SCN (Mintz et al., 1999) or appliedin vitro (Ding et al., 1994, 1997). Light and glutamate are thought to modulate the clock through activating NMDA and non-NMDA glutamate receptors, increasing intracellular Ca2+, and activating nitric oxide synthase (Ding et al., 1994, 1997, 1998; Hamada et al., 1999; Mintz et al., 1999). Glutamate and light also activate cAMP response element-binding protein, increase c-fos levels, and increase levels of the circadian clock-associated gene products mPER1 and mPER2, any or all of which may be critical for photic phase shifts (Ding et al., 1997;Akiyama et al., 1999; Francois-Bellan et al., 1999; Obrietan et al., 1999; Field et al., 2000).
Nonphotic stimuli, conversely, phase-advance the SCN pacemaker when applied during the subjective day and generally have smaller effects at night. These stimuli include behavioral activity (Mrosovsky, 1995), sleep deprivation (Antle and Mistlberger, 2000; Grossman et al., 2000), and application of neuropeptide Y (NPY) (Biello et al., 1994;Golombek et al., 1996), melatonin (Cassone et al., 1985; Gillette and McArthur, 1996), GABA (Smith et al., 1989; Biggs and Prosser, 1998), or serotonin (5-HT) agonists (Medanic and Gillette, 1992;Shibata et al., 1992b; Tominaga et al., 1992; Edgar et al., 1993;Prosser et al., 1993) to the SCN.
Recent investigations have demonstrated interactions between phase-shifting stimuli. Most notably, several nonphotic stimuli (e.g., wheel-running behavior, 5-HT agonists, and NPY) have been shown to inhibit light- and/or glutamate-induced phase shifts (Ralph and Mrosovsky, 1992; Pickard et al., 1996; Biello et al., 1997; Pickard and Rea, 1997a; Mistlberger and Antle, 1998; Weber et al., 1998; Yannielli and Harrington, 2000). Glutamate and light, in turn, can block NPY-induced phase shifts (Biello et al., 1997), and light inhibits activity–arousal-induced phase shifts (Mrosovsky, 1991;Biello and Mrosovsky, 1995; Antle and Mistlberger, 2000; Grossman et al., 2000). To further explore photic–nonphotic interactions, the experiments presented here focus on whether glutamate can inhibit serotonergic phase shifts in vitro. The results indicate that glutamate inhibits serotonergic phase advances through stimulating both AMPA and NMDA receptors, and this effect is mimicked by electrical stimulation of the optic chiasm. In addition, the inhibition by glutamate appears to be indirect, possibly involving GABA interneurons.
MATERIALS AND METHODS
Brain slice preparation. Coronal brain slices (500 μm) containing the SCN were prepared during the daytime from adult, male Sprague Dawley rats housed in a 12 hr light/dark cycle as reported previously (Prosser and Gillette, 1989; Prosser et al., 1993;Prosser, 1998b). Slices were maintained at the interface of a Hatton-style brain slice chamber (Hatton et al., 1980) in which they were perfused continuously with warm (37°C), oxygenated (95% O2/5% CO2), glucose–bicarbonate-supplemented Earle's Balanced Salt Solution (EBSS; Sigma, St. Louis, MO), pH 7.4–7.5.
Single-unit recordings and data analysis. Single-unit recordings were obtained using methods described previously (Prosser et al., 1993; Prosser, 1998b). Briefly, the spontaneous activity of single SCN neurons was recorded using glass capillary microelectrodes filled with 3m NaCl. Each neuron was recorded for 5 min, and the data was stored for later determination of firing rate using a DataWave system (DataWave Technologies, Longmont, CO). In general, four to seven cells were recorded during each hour. These firing rates were used then to calculate 2 hr running averages, lagged by 1 hr, to obtain a measure of population neuronal activity. As in previous studies (Prosser et al., 1993; Prosser, 1998b), the time of peak neuronal activity was assessed visually by estimating, to the nearest quarter hour, the time of symmetrically highest activity.
Experimental treatments. All drugs used in phase-shifting experiments were bath-applied during the first day in vitroby stopping the perfusion and replacing the medium in the slice chamber with medium containing the test compound. At the end of the treatment period, the normal medium was reintroduced into the slice chamber, and perfusion was resumed. In most experiments, the treatment period lasted 1 hr, but in some experiments a 10 min application was used. In another group of experiments, the medium in the slice chamber was replaced three times with fresh medium containing the test compound(s) at 20 min intervals during the hour-long treatment. This method was initially used to test the blocking effects of glutamate after the 1 hr bath application was found ineffective (see Table 1). The reasoning behind this was that the glutamate might undergo rapid degradation and/or sequestration during the static bath conditions (Yudkoff et al., 1994;Hertz et al., 1999). This method of drug application produced positive results with glutamate, but not NMDA or kainate (see Table 1). For blocking experiments, the bathing medium was first replaced with medium containing the blocking compound. After 15 min (or after 5 min, if using the shorter, 10 min treatment paradigm), this solution was replaced with medium containing both compounds. This was followed by another 15 min (or 5 min) treatment with medium containing only the blocking agent, after which the normal medium was reintroduced to the slice chamber, and perfusion was resumed. These procedures have been shown not to induce phase shifts by themselves. Therefore, the times-of-peak for drug-treated slices were compared with the mean time-of-peak for untreated slices [zeitgeber time (ZT) 6.0 ± 0.3, n = 3, where ZT0 is the time of lights-on in the animal colony] to determine the amount of phase shift induced by the treatment. ANOVAs and Student's t tests were used, where appropriate, to test for significant differences between the means, with significance set at p < 0.05. Chemicals used in the study were (+)-8-hydroxy-dipropylaminotetralin HBr [(+)DPAT], tetrodotoxin (TTX), 5-hydroxytryptamine (5-HT), bicuculline methiodide,l-glutamate, NMDA, AMPA, and kainate (Research Biochemicals, Natick, MA; Sigma).
Table 1.
Summary of the phase-shifting effects of various compounds applied in vitro
| Treatment | Number | Length of treatment | Phase shift1-a |
|---|---|---|---|
| 5-HT (10 μm) | 3 | 1 hr | 4.3 ± 0.2* |
| (+)DPAT (10 μm)1-b | 3 | 1 hr | 3.7 ± 0.5* |
| (+)DPAT (10 μm) | 2 | 10 min | 4.0 ± 0.0* |
| Glutamate (10 mm) | 2 | 1 hr | 0.0 ± 0.0 |
| AMPA (10 μm) | 2 | 1 hr | 0.8 ± 0.4 |
| NMDA (100 μm) | 2 | 1 hr | 0.8 ± 0.4 |
| Kainate (100 μm) | 2 | 1 hr | 0.5 ± 0.0 |
| Optic chiasm stimulation | 3 | 15 min | −0.2 ± 0.2 |
| (+)DPAT (10 μm) + glutamate (10 μm–1 mm) | 2 | 1 hr | 2.8 ± 0.4* |
| (+)DPAT (10 μm) + glutamate (10 mm) | 3 | 1 hr | 2.2 ± 0.2* |
| [(+)DPAT (10 μm) + glutamate (10 mm)] ×31-c | 2 | 1 hr | 0.3 ± 0.4 |
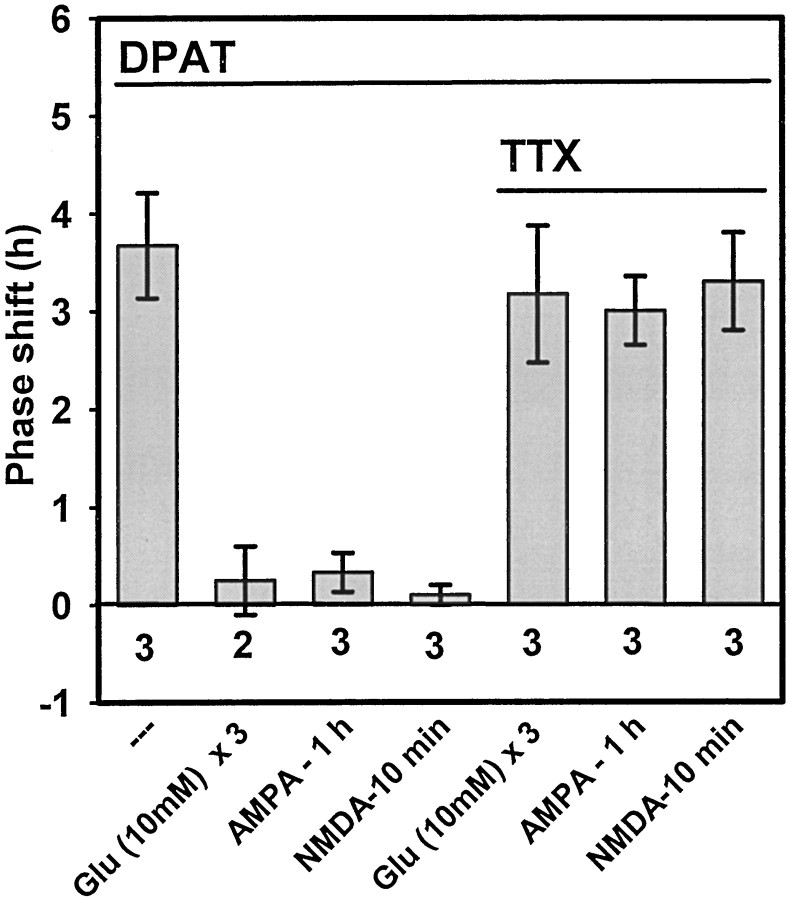
| [(+)DPAT (10 μm) + glutamate (10 mm) + TTX (1 μm)] ×31-c | 3 | 1 hr | 3.2 ± 0.1* |
| 5-HT (10 μm) + AMPA (10 μm) | 3 | 1 hr | 0.3 ± 0.2 |
| (+)DPAT (10 μm) + AMPA (10 μm) | 3 | 1 hr | 0.3 ± 0.2 |
| (+)DPAT (10 μm) + AMPA (10 μm) + TTX (1 μm) | 3 | 1 hr | 3.0 ± 0.4* |
| (+)DPAT (10 μm) + NMDA (10–100 mm) | 4 | 1 hr | 3.1 ± 0.3* |
| (+)DPAT (10 μm) + NMDA (100 μm) + AMPA (0.1 μm) | 2 | 1 hr | 3.1 ± 0.2* |
| [(+)DPAT (10 μm) + NMDA (10 μm)] ×31-c | 2 | 1 hr | 4.0 ± 0.7* |
| (+)DPAT (10 μm) + NMDA (100 μm) | 3 | 10 min | 0.1 ± 0.1 |
| (+)DPAT (10 μm) + NMDA (100 μm) + TTX (1 μm) | 3 | 10 min | 3.3 ± 0.5* |
| (+)DPAT (10 μm) + bicuculline (30 μm) | 3 | 10 min | 3.9 ± 0.1* |
| (+)DPAT (10 μm) + NMDA (100 μm) + bicuculline (30 μm) | 3 | 10 min | 3.8 ± 0.1* |
| (+)DPAT (10 μm) + kainate (10–100 μm) | 4 | 1 hr | 3.4 ± 0.3* |
| [(+)DPAT (10 μm) + kainate (100 μm)] ×31-c | 2 | 1 hr | 3.3 ± 0.4* |
| (+)DPAT (10 μm) + kainate (100 μm) | 2 | 10 min | 3.5 ± 0.0 |
| (+)DPAT (10 μm) + optic chiasm stimulation | 3 | 10 min | 0.8 ± 0.2 |
| (+)DPAT (10 μm) + optic chiasm stimulation + bicuculline | 3 | 10 min | 3.17 ± 0.2* |
Phase shifts calculated by comparing the times-of-peak in drug-treated versus control slices with the mean time-of-peak in control slices of ZT 6.0 ± 0.3 (n = 3).
Data from Prosser (1998b).
“×3” refers to three applications during the 1 hr treatment period. For details, see Materials and Methods.
p < 0.05 versus control.
Optic chiasm stimulation. Optic chiasm stimulation (OCS) was performed as described previously (Prosser, 1998a). Briefly, a bipolar, blunt-cut, insulated tungsten electrode was positioned in the optic chiasm ventrolateral to the SCN. Voltage (10 Hz, 10V, 3 msec duration) was applied for 15 min to determine the effect of stimulation alone. When combined with drug application, stimulation was applied first for 5 min, then in combination with the drug for 10 min, followed by a 5 min period of stimulation alone.
Multiunit activity recordings. Multiunit activity (MUA) recordings were used to determine the acute effects of experimental compounds on SCN neuronal activity. For these, a single 76 μm diameter blunt-cut, Teflon-coated metal electrode (90% platinum/10% iridium) was used as described previously (Prosser, 1998a). The electrode was first placed in the optic chiasm to determine the level of background electrical noise, and then it was moved to the SCN and lowered 50–100 μm into the slice. A threshold for counting electrical events (neuronal activity) was set to at least twice the level of background noise. Neuronal activity, expressed as the number of threshold crossings per second, was monitored continuously using a DataWave data collection and analysis system. After the MUA recording stabilized, drugs were rapidly perfused into the brain slice chamber (40 ml/hr; chamber volume, 3 ml) so that a complete exchange of the medium within the slice chamber occurred within 5 min. For each compound tested, the drug was applied for 15–30 min, followed by at least 30 min of perfusion with the normal medium. All drugs were applied during the first day in vitro, between ZT4 and ZT11. Although we have found MUA recordings provide a consistent and reliable record of acute changes in neuronal activity, we are unable to obtain reliable long-term (24–48 hr) recordings using MUA (Prosser, 1998a) and therefore do not use this technique for phase-shifting experiments.
RESULTS
5-HTergic phase advances at ZT6
Consistent with previous reports (Medanic and Gillette, 1992;Shibata et al., 1992b; Prosser et al., 1993), 5-HT (10 μm) and (+)DPAT (10 μm) induced robust phase advances when bath-applied to SCN slices for 1 hr at ZT6 (Fig.1). Similar phase advances were also seen after 10 min bath-applications of (+)DPAT (Table1). Conversely, neither glutamate nor any of its agonists (AMPA, NMDA, and kainate) altered the phase of the neuronal activity rhythm when applied at ZT6 (Table 1). All glutamatergic compounds were tested at concentrations that were either known to affect SCN activity during the day (Shibata et al., 1992a;Flett and Colwell, 1999) or shown previously to induce phase shifts during the night (Ding et al., 1994; Shibata et al., 1994;Forrest and Prosser, 2000) (our unpublished data). These data are summarized in Table 1.
Fig. 1.
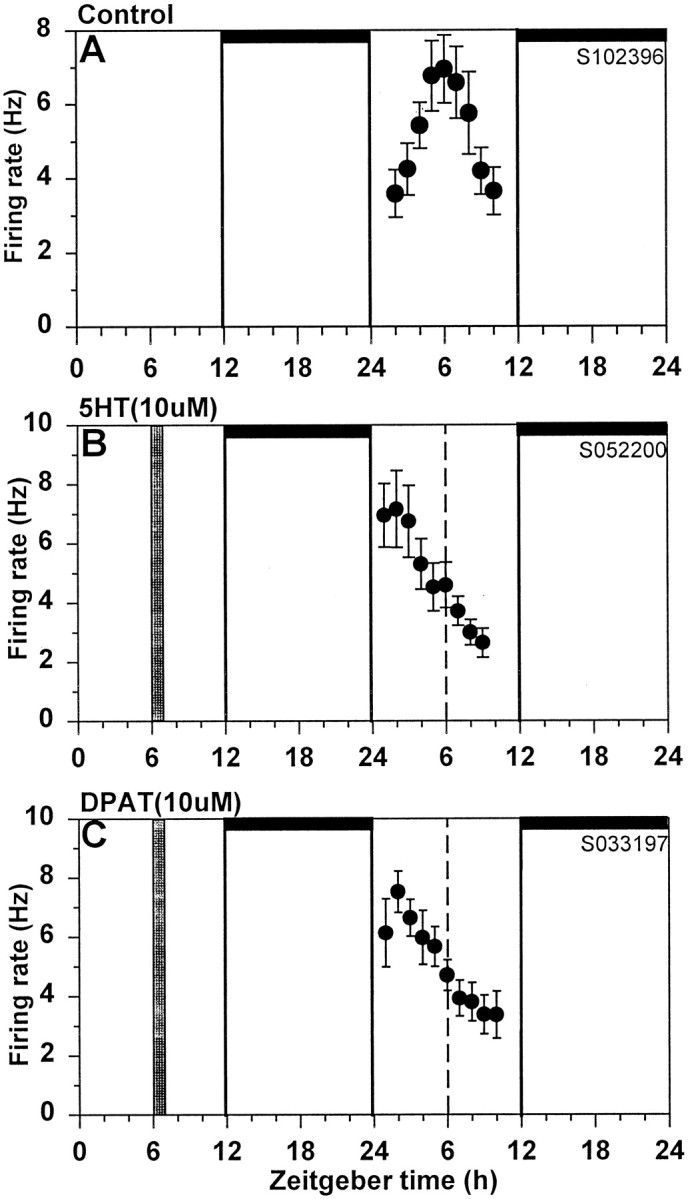
Serotonergic phase advances of the SCN neuronal activity rhythm. Shown are the 2 hr means ± SEM of SCN neuronal activity obtained in a control experiment (A), after treatment with 10 μm 5-HT (B), and after treatment with (+)DPAT (10 μm) (C). Neuronal activity peaked near ZT6 in the control experiment, whereas the peak in neuronal activity occurred at ZT2 after both the 5-HT and (+)DPAT treatment. Thus, both 5-HT and (+)DPAT phase-advanced the neuronal activity rhythm by 4 hr. Horizontal bars, Time of lights-off in the animal colony; vertical bar, time of drug treatment;dotted line, mean time-of-peak in control experiments.
Glutamatergic inhibition of 5-HTergic phase shifts
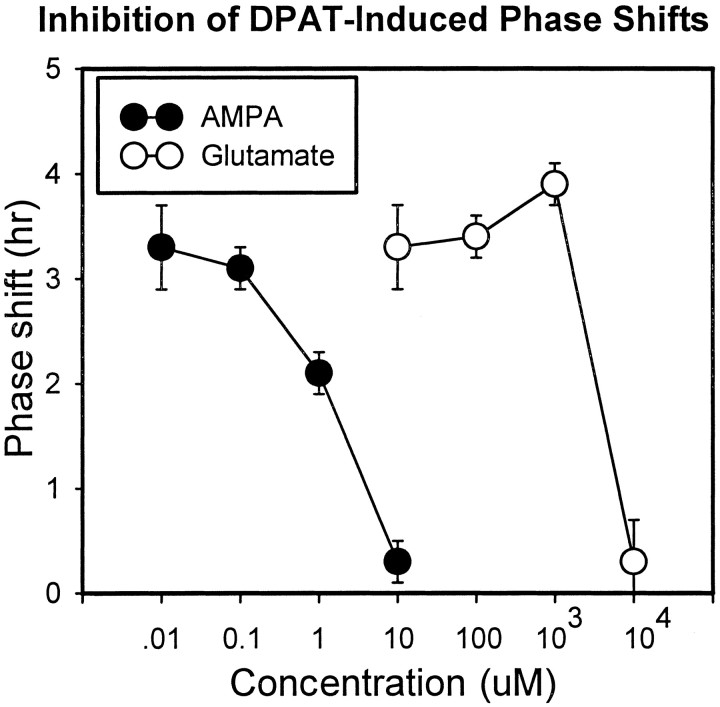
Although it did not induce phase shifts when applied alone at ZT6, AMPA (10 μm) completely abolished the phase advances induced by both 5-HT and (+)DPAT under 1 hr static bath conditions (Fig. 2). AMPA inhibition of (+)DPAT-induced phase shifts was dose-dependent, with an ED50 ∼1 μm (Fig.3). Glutamate, when reapplied three times during the course of the 1 hr treatment period (see Materials and Methods), also completely blocked (+)DPAT-induced phase advances. As with AMPA, this inhibition was dose-dependent, although much higher concentrations of glutamate were needed to block (+)DPAT-induced phase shifts (Fig. 3).
Fig. 2.
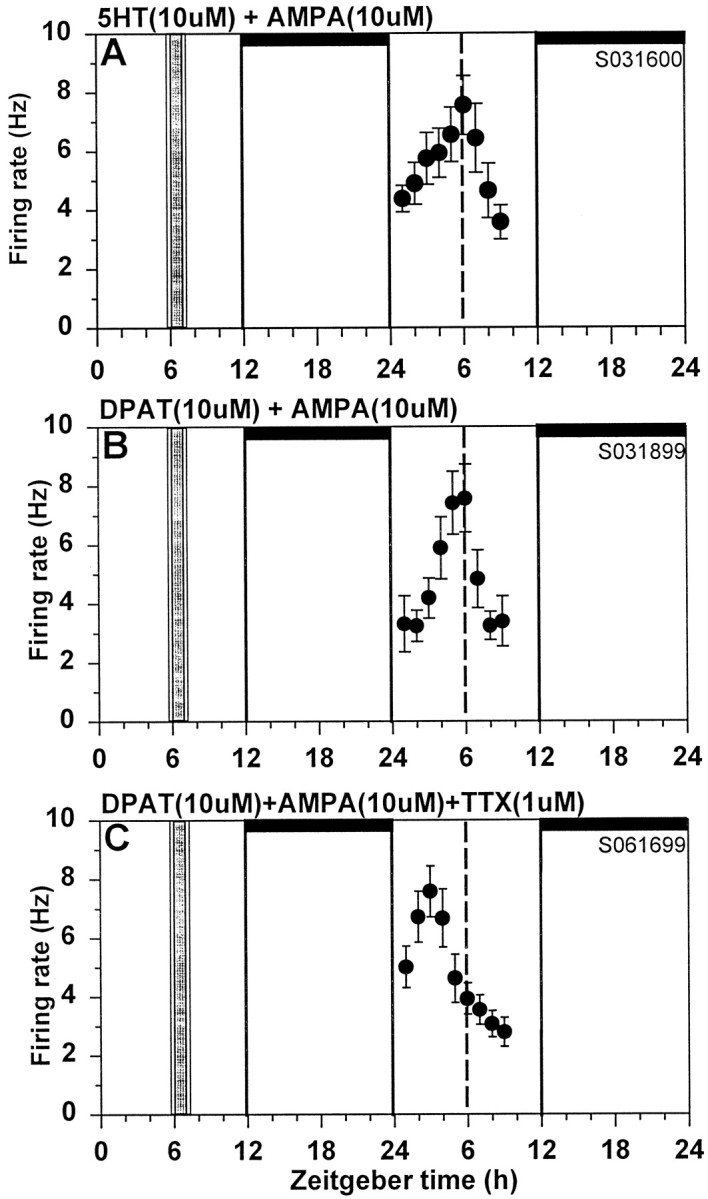
AMPA blocks serotonergic phase advances of the SCN neuronal activity rhythm. A, Coapplication of AMPA (10 μm) blocks the 5-HT-induced phase advance.B, Coapplication of AMPA (10 μm) completely abolished the (+)DPAT-induced phase advance.C, TTX (1 μm) prevents the inhibition by AMPA, thus restoring the (+)DPAT-induced phase advance. See Figure 1legend for details.
Fig. 3.
Dose dependence of glutamatergic inhibition. Shown are the mean phase advances (±SEM) induced by (+)DPAT application alone and in the presence of varying concentrations of AMPA (filled circles) and glutamate (open circles). Complete inhibition occurred with 10 μmAMPA and 10 mm glutamate (when glutamate is reapplied 3 times during the 1 hr treatment period). The ED50 for AMPA is near 1 μm and for glutamate is near 5 mm.
NMDA and kainate, at concentrations up to 100 μm, were completely ineffective at blocking phase advances induced by 1 hr (+)DPAT bath application at ZT6 (Table 1). These results were surprising because NMDA and kainate receptors are abundant in the SCN (van den Pol et al., 1994, 1996; Mikkelsen et al., 1993); NMDA and kainate increase SCN neuronal activity and intracellular Ca2+ levels (Shibata et al., 1992a; Dudek et al., 1993; Haak, 1999); and NMDA phase-shifts the SCN pacemaker when applied during the night (Ebling et al., 1991; Ding et al., 1994, 1997;Mintz and Albers, 1997; Mintz et al., 1999; Forrest and Prosser, 2000). However, in some systems, functional activation of NMDA receptors requires concurrent AMPA receptor-induced depolarization (van den Pol et al., 1996; Dingledine et al., 1999). Therefore, we tested the ability of a combined application of AMPA (0.1 μm) with 100 μm NMDA. This combination still did not block (+)DPAT-induced phase advances (Table 1).
Further investigation into the effects of AMPA and NMDA on SCN neuronal activity revealed that the excitation induced by NMDA dampened rapidly, so that activity often returned to near baseline levels within 15 min of its initial application. In contrast, the excitatory response to AMPA generally lasted much longer (Fig.4). Thus, we speculated that NMDA might be more effective when coapplied with (+)DPAT for a shorter length of time. In fact, NMDA completely blocked the phase advances induced by 10 min bath application of (+)DPAT (NMDA applied alone for 5 min before and after (+)DPAT–NMDA treatment). Similar inhibition still was not seen when kainate was coapplied with (+)DPAT for 10 min. (Table 1).
Fig. 4.
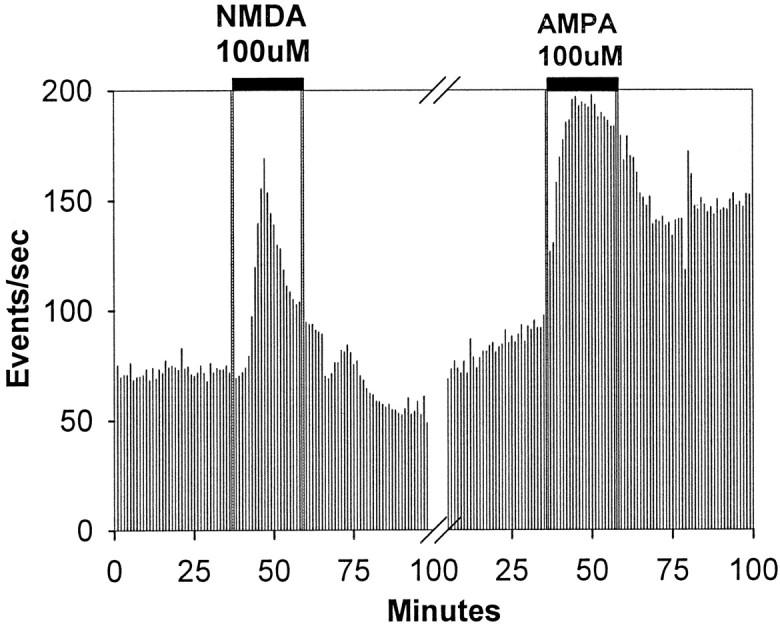
Multiunit activity recordings from the SCN showing acute effects of NMDA and AMPA on SCN neuronal activity. Perfusion application of NMDA to the SCN slice induced a large increase in activity that rapidly returned to near baseline levels. Conversely, neuronal activity remained high throughout the period of AMPA application.
Optic chiasm stimulation inhibits serotonergic phase shifts
Next, we investigated whether stimulation of endogenous glutamate release could block serotonergic phase advances. To test this, we applied electrical stimulation to the optic chiasm, which should stimulate release of glutamate from retinal terminals in the SCN (Liou et al., 1986). This treatment had no effect on the rhythm of SCN neuronal activity when applied alone at ZT6, but it inhibited phase advances induced by 10 min bath application of (+)DPAT (Fig.5, Table 1).
Fig. 5.
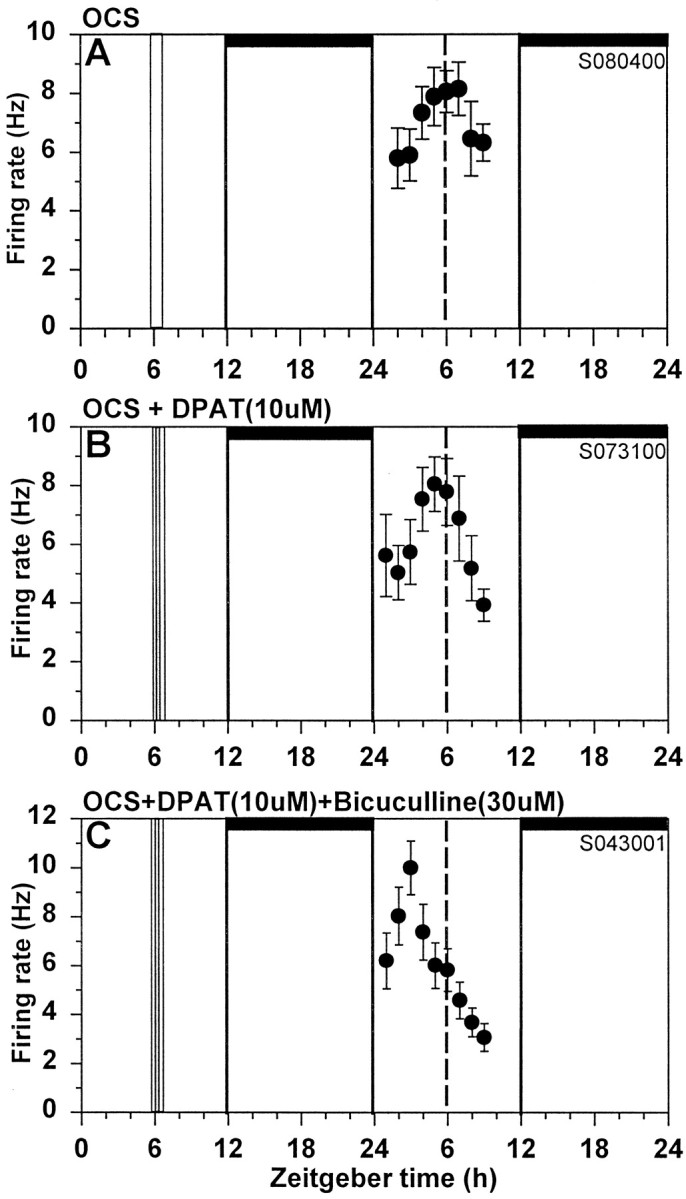
Optic chiasm stimulation inhibits serotonergic phase advances. A, Electrical stimulation of the optic chiasm at ZT6 for 15 min did not shift the rhythm of SCN neuronal activity. B, Optic chiasm stimulation inhibited the phase advance induced by 10 min application of (+)DPAT, so the peak in neuronal activity occurred near ZT6. C, Bicuculline coapplied with OCS reinstates the (+)DPAT-induced phase advance. See Figure 1 legend for details.
Glutamatergic inhibition is blocked by TTX and bicuculline
Finally, we investigated whether the glutamatergic inhibition involves direct or indirect interactions with 5-HT, that is, whether the 5-HT and glutamate agonists act on the same cells. To initially address this question, we applied TTX in conjunction with glutamate, AMPA, or NMDA under the experimental conditions in which each compound had been found to block (+)DPAT-induced phase advances. In all cases, TTX prevented the glutamatergic inhibition, so that the (+)DPAT-induced phase advance was reinstated (Figs. 2, 6; Table 1). Application of TTX alone at ZT6 does not induce phase shifts (Bergeron et al., 1999), and TTX does not block serotonergic phase shifts at ZT6 (Prosser et al., 1992).
Fig. 6.
Glutamate inhibition of (+)DPAT-induced phase advances is blocked by TTX. Shown are the mean phase advances (±SEM) induced by (+)DPAT alone or in the presence of glutamate agonists with and without TTX present. The inhibition by glutamate, AMPA, and NMDA are all prevented when TTX is coapplied. Numbers under the bars indicate the number of experiments.
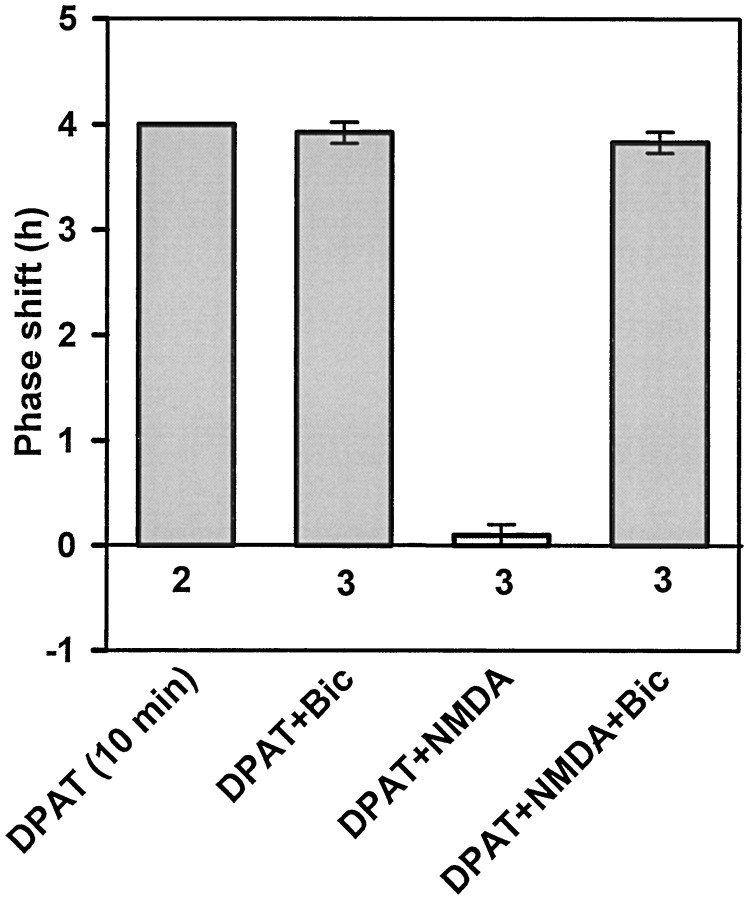
These results suggest that the inhibition by glutamate and its agonists requires Na+-dependent action potentials to be formed. If this is the case, then the inhibition by glutamate may involve SCN interneurons. To address this possibility, we tested whether the glutamatergic inhibition was sensitive to blockade of GABA receptors. We have previously shown that the selective GABAA antagonist, bicuculline, does not induce phase shifts when applied to the SCN in vitro at ZT6 (Bergeron et al., 1999). In these experiments we first tested whether bicuculline affects phase advances by (+)DPAT. As seen in Figure7, bicuculline (30 μm) did not block (+)DPAT-induced phase shifts, but it did block the inhibition by NMDA (Fig. 7). To further investigate this effect, we tested whether bicuculline also prevents OCS inhibition of (+)DPAT-induced phase shifts. As shown in Figure 5, coapplication of bicuculline with OCS and (+)DPAT reinstates the full 5-HTergic phase advance. These data are summarized in Table1.
Fig. 7.
Bicuculline (Bic) prevents glutamatergic inhibition of (+)DPAT-induced phase shifts. Shown are the mean phase advances (±SEM) induced by (+)DPAT in the presence of NMDA and/or bicuculline. Bicuculline did not affect the (+)DPAT-induced phase advance but did prevent the inhibition by NMDA. See Figure 6legend for details.
DISCUSSION
These data are the first to reveal glutamatergic inhibition of serotonergic phase shifts in vitro, demonstrating that this inhibition takes place in or near the SCN. As such, they extend the body of research showing a pattern of inhibitory interactions between photic and nonphotic stimuli. Furthermore, the results suggest that this inhibition may also occur in response to electrical stimulation of the optic chiasm.
In our experiments, we were able to inhibit the serotonergic phase shifts through stimulation of either NMDA or AMPA receptors, but not through stimulation of kainate receptors. This is similar to the results of studies investigating photic phase shifts, in which stimulation of both NMDA and AMPA receptors can mimic light-induced phase shifts. The ability of kainate to induce photic phase shifts has not, to our knowledge, been reported. Although we cannot completely rule out involvement of kainate receptors in the inhibitory actions seen here, none of the treatment regimens we tried were effective with kainate.
A large number of in vivo studies have shown that 5-HT can inhibit photic phase shifts. The evidence strongly supports a combination of presynaptic and postsynaptic sites of 5-HT action. Presynaptically, 5-HT stimulates 5-HT1B receptors that are located on retinal ganglion cell terminals in the SCN to inhibit light-induced glutamate release (Pickard and Rea, 1997b;Pickard et al., 1999). 5-HT also appears to act postsynaptically on either 5-HT1A or 5-HT7receptors to block the phase-shifting actions of glutamate (Rea et al., 1994, 1995; Moriya et al., 1996; Weber et al., 1998; Smith et al., 2001). The results of this study demonstrate the reverse situation, i.e., that glutamate can inhibit serotonergic phase shifts. These results are consistent with recent in vivo experiments showing that light can inhibit daytime phase advances induced by DPAT perfusion into the SCN (Ehlen et al., 2001). Interestingly, Challet et al. (1998) found that phase shifts induced by DPAT injection in the intergeniculate leaflet were blocked by light, whereas phase shifts induced by SCN injection of DPAT were not.
The mutual inhibition between light–glutamate and 5-HT is similar to that previously shown for light–glutamate and NPY (Biello et al., 1994, 1997; Yannielli and Harrington, 2000). Thus, it may be that in the SCN there is a general pattern of mutual inhibition between photic and nonphotic stimuli. The frequency with which interactions between phase-shifting stimuli have been observed raises interesting questions concerning how animals normally synchronize their daily rhythms to the environment. Results such as these lend support to the idea that synchronization involves a complex integration of multiple stimuli rather than an overriding reliance on a single environmental cue such as light. The interactions observed between phase-shifting stimuli acting within the SCN are also quite interesting because they now include two examples of a neurotransmitter (glutamate) modulating the actions of neuromodulators (5-HT and NPY). Results such as these raise the general issue of whether a clear functional distinction can be drawn between classical “neurotransmitters” and “neuromodulators”.
Previous studies have demonstrated that light can inhibit activity–arousal-induced phase shifts (Mrosovsky, 1991; Biello and Mrosovsky, 1995; Antle and Mistlberger, 2000; Grossman et al., 2000). It has yet to be resolved to what extent these phase shifts occur through 5-HT, NPY, or other neurotransmitters (Biello et al., 1994;Wickland and Turek, 1994; Biello, 1995; Bobrzynska et al., 1996; Antle et al., 1998; Mistlberger and Antle, 1998). It is likely that diverse neural substrates underlie these phase shifts and that their specific roles vary somewhat between different species and between the types of arousal being investigated. The data presented here show that photic inhibition of activity-induced phase shifts in rats could involve glutamate acting within the SCN to inhibit postsynaptic actions of 5-HT. Whether or not this occurs in vivo and whether this underlies some aspects of photic inhibition of activity-induced phase shifts remains to be determined.
The results presented here are especially interesting in light of a recent study showing that sleep deprivation, which induces robust daytime phase advances, increases 5-HT release in the SCN by 160%, and further, that light inhibits the sleep deprivation-induced phase shifts but not the release of 5-HT in the SCN (Grossman et al., 2000). Although the inhibition by light could involve neural substrates outside the SCN, our results indicate that the inability of light to suppress 5-HT release in the SCN does not exclude the SCN from being the site of light inhibition of the phase shifts. Light could be inhibiting the phase shifts by blocking postsynaptic actions of 5-HT in the SCN. This interpretation is also consistent with data showing that light inhibits DPAT-induced phase shifts in vivo(Ehlen et al., 2001).
Previous work has demonstrated that serotonergic phase shifts are not blocked by TTX (Prosser et al., 1992), suggesting that 5-HT may act directly on clock cells. Likewise, in vivo phase shifts in response to intra-SCN injections of glutamate are not blocked by TTX, indicating that glutamate may also act directly on clock cells to phase-shift the circadian pacemaker (Mintz et al., 1999). However, the results presented here show that the inhibition of serotonergic phase shifts by glutamate is blocked by TTX. This suggests that the inhibition by glutamate requires Na+-dependent action potentials and is indirect. This could mean that interneurons within the SCN are required to convey the glutamate signal to 5-HT-stimulated cells. If that is the case, then it is likely that the intervening neurons use GABA as their neurotransmitter, because of its ubiquitous presence in SCN cells (van den Pol et al., 1996). Our results showing that bicuculline prevents the glutamatergic inhibition are consistent with this hypothesis. To further test this hypothesis, one would like to determine whether GABA blocks 5-HT-induced phase shifts. This experiment will be difficult, however, because we have shown that activation of both GABAA and GABAB receptors in the SCN induces phase advances in the subjective day at the same circadian phases that 5-HT induces phase shifts (Biggs and Prosser, 1998; Bergeron et al., 1999).
Finally, our experiments show that electrical stimulation of the optic chiasm also blocks serotonergic phase shifts. One concern with this procedure is that the electrical stimulation may induce nonspecific release of neurotransmitters in the SCN slice. There are two reasons why we do not think that is occurring here. First, general depolarization would be expected to induce release of GABA, the most abundant neurotransmitter in the SCN (van den Pol and Dudek, 1993). However, GABA induces robust phase advances in the SCN at ZT6 (Biggs and Prosser, 1998; Bergeron et al., 1999), whereas optic chiasm stimulation does not. Second, we find that the electrical stimulation parameters used in these experiments induce phase shifts at night that mimic the phase-shifting effects of glutamate (T. Braden, V. McMillan, and R. A. Prosser, unpublished data). Together, we interpret these data as supporting the conclusion that optic chiasm stimulation blocks DPAT-induced phase shifts through inducing the release of endogenous glutamate.
In summary, this study presents evidence that glutamate, acting through both AMPA and NMDA receptors, can block the phase-modulating effects of serotonin in the SCN in vitro. This inhibition is prevented by coapplication of either TTX or the GABA antagonist, bicuculline, suggesting there is an indirect interaction between glutamate and serotonin with respect to phase-shifting the SCN pacemaker. These results are consistent with previous work showing mutually inhibitory interactions between photic and nonphotic stimuli in modulating the phase of the mammalian circadian pacemaker.
Footnotes
This research was supported by National Institutes of Health Grant MH53317.
Correspondence should be addressed to Dr. Rebecca A. Prosser, Department of Biochemistry and Cellular and Molecular Biology, M407 Walters Life Sciences Building, University of Tennessee, Knoxville, TN 37996. E-mail: rprosser@utk.edu.
REFERENCES
- 1.Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer2 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antle MC, Marchant EG, Niel L, Mistlberger RE. Serotonin antagonists do not attenuate activity-induced phase shifts of circadian rhythms in the Syrian hamster. Brain Res. 1998;813:139–149. doi: 10.1016/s0006-8993(98)01048-8. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron HE, Danielson B, Biggs KR, Prosser RA. TTX blocks baclofen-induced phase shifts of the mammalian circadian pacemaker in vitro. Brain Res. 1999;841:193–196. doi: 10.1016/s0006-8993(99)01791-6. [DOI] [PubMed] [Google Scholar]
- 5.Biello SM. Enhanced photic phase shifting after treatment with antiserum to neuropeptide Y. Brain Res. 1995;673:25–29. doi: 10.1016/0006-8993(94)01345-i. [DOI] [PubMed] [Google Scholar]
- 6.Biello SM, Mrosovsky N. Blocking the phase-shifting effect of neuropeptide Y with light. Proc R Soc Lond B Biol Sci. 1995;259:179–187. doi: 10.1098/rspb.1995.0026. [DOI] [PubMed] [Google Scholar]
- 7.Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 8.Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other's phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience. 1997;77:1049–1057. doi: 10.1016/s0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- 9.Biggs KR, Prosser RA. GABAB receptor stimulation phase-shifts the mammalian circadian clock in vitro. Brain Res. 1998;807:250–254. doi: 10.1016/s0006-8993(98)00820-8. [DOI] [PubMed] [Google Scholar]
- 10.Bobrzynska KJ, Vrang N, Mrosovsky N. Persistence of nonphotic phase shifts in hamsters after serotonin depletion in the suprachiasmatic nucleus. Brain Res. 1996;741:205–214. doi: 10.1016/s0006-8993(96)00913-4. [DOI] [PubMed] [Google Scholar]
- 11.Cassone VM, Chesworth M, Armstrong SM. Entrainment of rat circadian rhythms by daily injections of melatonin: synchronization in constant light and dependence upon the suprachiasmatic nuclei. Soc Neurosci Abstr. 1985;11:1140. [Google Scholar]
- 12.Challet E, Scarbrough K, Penev PD, Turek FW. Roles of suprachiasmatic nuclei and intergeniculate leaflets in mediating the phase-shifting effects of a serotonergic agonist and their photic modulation during subjective day. J Biol Rhythms. 1998;13:410–421. doi: 10.1177/074873098129000237. [DOI] [PubMed] [Google Scholar]
- 13.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 14.Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- 16.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 17.Dudek FE, Kim YI, Bouskila Y. Electrophysiology of the suprachiasmatic nucleus: synaptic transmission, membrane properties, and neuronal synchronization. J Biol Rhythms. 1993;8:S33–S37. [PubMed] [Google Scholar]
- 18.Ebling FJP, Maywood ES, Staley K, Humby T, Hancock DC, Waters CM, Evan GI, Hastings MH. The role of N-methyl-d-aspartate-type glutamatergic neurotransmission in the photic induction of immediate-early gene expression in the suprachiasmatic nuclei of the Syrian hamster. J Neuroendocrinol. 1991;3:641–652. doi: 10.1111/j.1365-2826.1991.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 19.Edgar DM, Miller JD, Prosser RA, Dean RR, Dement WC. Serotonin and the mammalian circadian system. II. Phase-shifting rat behavioral rhythms with serotonergic agonists. J Biol Rhythms. 1993;8:17–31. doi: 10.1177/074873049300800102. [DOI] [PubMed] [Google Scholar]
- 20.Ehlen JC, Grossman GH, Glass JD. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J Neurosci. 2001;21:5351–5357. doi: 10.1523/JNEUROSCI.21-14-05351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field MD, Maywood ES, O'Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- 22.Flett J, Colwell CS. Serotonin modulation of calcium transients in cells in the suprachiasmatic nucleus. J Biol Rhythms. 1999;14:354–363. doi: 10.1177/074873049901400502. [DOI] [PubMed] [Google Scholar]
- 23.Forrest JB, Prosser RA. Interactions between glutamate and serotonin agonists in phase-shifting the mammalian circadian clock in vitro at night. Soc Neurosci Abstr. 2000;26:189. [Google Scholar]
- 24.Francois-Bellan A-M, Deprez P, Becquet D. Light-induced variations in AP-1 binding activity and composition in the rat suprachiasmatic nucleus. J Neurochem. 1999;72:841–847. doi: 10.1046/j.1471-4159.1999.0720841.x. [DOI] [PubMed] [Google Scholar]
- 25.Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res. 1996;73:135–139. doi: 10.1016/0166-4328(96)00085-x. [DOI] [PubMed] [Google Scholar]
- 26.Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. NeuroReport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- 27.Grossman GH, Mistlberger RE, Antle MC, Ehlen JC, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport. 2000;11:1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- 28.Haak LL. Metabotropic glutamate receptor modulation of glutamate responses in the suprachiasmatic nucleus. J Neurophysiol. 1999;81:1308–1317. doi: 10.1152/jn.1999.81.3.1308. [DOI] [PubMed] [Google Scholar]
- 29.Hamada T, Liou SY, Fukushima T, Maruyama T, Watanabe S, Mikoshiba K, Ishida N. The role of inositol trisphosphate-induced Ca2+ release from IP3-receptor in the rat suprachiasmatic nucleus on circadian entrainment mechanism. Neurosci Lett. 1999;263:125–128. doi: 10.1016/s0304-3940(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 30.Hatton GI, Doran AD, Salm AK, Tweedle CD. Brain slice preparation: hypothalamus. Brain Res Bull. 1980;5:405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- 31.Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- 32.Liou SY, Shibata S, Iwasaki K, Ueki S. Optic nerve stimulation-induced increase of release of 3H-glutamate and 3H-aspartate but not 3H-GABA from the suprachiasmatic nucleus in slices of rat hypothalamus. Brain Res Bull. 1986;16:527–531. doi: 10.1016/0361-9230(86)90182-6. [DOI] [PubMed] [Google Scholar]
- 33.Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol (Lond) 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikkelsen JD, Larsen PJ, Ebling FJP. Distribution of N-methyl d-aspartate (NMDA) receptor mRNAs in the rat suprachiasmatic nucleus. Brain Res. 1993;632:329–333. doi: 10.1016/0006-8993(93)91171-n. [DOI] [PubMed] [Google Scholar]
- 35.Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res. 1997;758:245–249. doi: 10.1016/s0006-8993(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 36.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mistlberger RE, Antle MC. Behavioral inhibition of light-induced circadian phase resetting is phase and serotonin dependent. Brain Res. 1998;786:31–38. doi: 10.1016/s0006-8993(97)01269-9. [DOI] [PubMed] [Google Scholar]
- 38.Moore RY. Organization of the mammalian circadian system. Ciba Found Symp. 1995;183:88–106. [PubMed] [Google Scholar]
- 39.Moriya T, Yamanouchi S, Fukushima T, Shimazoe T, Shibata S, Watanabe S. Involvement of 5-HT1A receptor mechanisms in the inhibitory effects of methamphetamine on photic responses in the rodent suprachiasmatic nucleus. Brain Res. 1996;740:261–267. doi: 10.1016/s0006-8993(96)00860-8. [DOI] [PubMed] [Google Scholar]
- 40.Mrosovsky N. Double-pulse experiments with nonphotic and photic phase-shifting stimuli. J Biol Rhythms. 1991;6:167–179. doi: 10.1177/074873049100600207. [DOI] [PubMed] [Google Scholar]
- 41.Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Found Symp. 1995;183:154–174. doi: 10.1002/9780470514597.ch9. [DOI] [PubMed] [Google Scholar]
- 42.Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- 43.Pickard GE, Rea MA. Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms. Biol Cell. 1997a;89:513–523. doi: 10.1016/s0248-4900(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 44.Pickard GE, Rea MA. TFMPP, a 5HT1B receptor agonist, inhibits light-induced phase shifts of the circadian activity rhythm and c-Fos expression in the mouse suprachiasmatic nucleus. Neurosci Lett. 1997b;231:95–98. doi: 10.1016/s0304-3940(97)00534-x. [DOI] [PubMed] [Google Scholar]
- 45.Pickard GE, Weber ET, Scott PA, Riberdy AF, Rea MA. 5-HT1B receptor agonists inhibit light-induced phase shifts of behavioral circadian rhythms and expression of the immediate-early gene c-fos in the suprachiasmatic nucleus. J Neurosci. 1996;16:8208–8220. doi: 10.1523/JNEUROSCI.16-24-08208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci. 1999;19:4034–4045. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prosser RA. In vitro circadian rhythms of the mammalian suprachiasmatic nuclei: comparison of multi-unit and single-unit neuronal activity recordings. J Biol Rhythms. 1998a;13:30–38. doi: 10.1177/074873098128999899. [DOI] [PubMed] [Google Scholar]
- 48.Prosser RA. Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 1998b;808:31–41. doi: 10.1016/s0006-8993(98)00808-7. [DOI] [PubMed] [Google Scholar]
- 49.Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prosser RA, Heller HC, Miller JD. Serotonergic phase-shifts of the mammalian circadian clock: effects of tetrodotoxin and high Mg2+. Brain Res. 1992;573:336–340. doi: 10.1016/0006-8993(92)90784-7. [DOI] [PubMed] [Google Scholar]
- 51.Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system. I. In vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- 52.Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–359. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- 53.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rea MA, Barrera J, Glass JD, Gannon RL. Serotonergic potentiation of photic phase shifts of the circadian activity rhythm. NeuroReport. 1995;6:1289–1292. doi: 10.1097/00001756-199507100-00014. [DOI] [PubMed] [Google Scholar]
- 55.Shibata S, Tominaga K, Hamada T, Watanabe S. Excitatory effect of N-methyl-d-aspartate and kainate receptor on the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1992a;139:83–86. doi: 10.1016/0304-3940(92)90863-3. [DOI] [PubMed] [Google Scholar]
- 56.Shibata S, Tsuneyoshi A, Hamada T, Tominaga K, Watanabe S. Phase-resetting effect of 8-OH-DPAT, a serotonin1A receptor agonist, on the circadian rhythm of firing rate in the rat suprachiasmatic nuclei in vitro. Brain Res. 1992b;582:353–356. doi: 10.1016/0006-8993(92)90156-4. [DOI] [PubMed] [Google Scholar]
- 57.Shibata S, Watanabe A, Hamada T, Ono M, Watanabe S. N-methyl-d-aspartate induces phase shifts in circadian rhythm of neuronal activity of rat SCN in vitro. Am J Physiol. 1994;267:R360–R364. doi: 10.1152/ajpregu.1994.267.2.R360. [DOI] [PubMed] [Google Scholar]
- 58.Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith BN, Sollars PJ, Dudek FE, Pickard GE. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J Biol Rhythms. 2001;16:25–38. doi: 10.1177/074873040101600104. [DOI] [PubMed] [Google Scholar]
- 60.Smith RD, Inouye S-IT, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol. 1989;164:805–814. doi: 10.1007/BF00616752. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi JS, Zatz M. Regulation of circadian rhythmicity. Science. 1982;217:1104–1111. doi: 10.1126/science.6287576. [DOI] [PubMed] [Google Scholar]
- 62.Tominaga K, Shibata S, Ueki S, Watanabe S. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Eur J Pharmacol. 1992;214:79–84. doi: 10.1016/0014-2999(92)90099-p. [DOI] [PubMed] [Google Scholar]
- 63.van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- 64.van den Pol AN, Hermans-Borgmeyer I, Hofer M, Ghosh P, Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. J Comp Neurol. 1994;343:428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- 65.van den Pol AN, Strecker GJ, Dudek FE. Excitatory and inhibitory amino acids and synaptic transmission in the suprachiasmatic nucleus. In: Buijs RM, Kalsbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editors. Hypothalamic integration of circadian rhythms. Elsevier; Amsterdam: 1996. pp. 41–56. [DOI] [PubMed] [Google Scholar]
- 66.Weber ET, Gannon RL, Rea MA. Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J Biol Rhythms. 1998;13:209–218. doi: 10.1177/074873098129000057. [DOI] [PubMed] [Google Scholar]
- 67.Wickland C, Turek FW. Lesions of the thalamic intergeniculate leaflet block activity-induced phase shifts in the circadian activity rhythm of golden hamster. Brain Res. 1994;660:293–300. doi: 10.1016/0006-8993(94)91302-1. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R-I, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 69.Yannielli PC, Harrington ME. Neuropeptide Y applied in vitro can block the phase shifts induced by light in vivo. NeuroReport. 2000;11:1587–1591. [PubMed] [Google Scholar]
- 70.Yudkoff M, Nissim I, Daikhin Y, Lin Z-P, Nelson D, Pleasure D, Erecinska M. Brain glutamate metabolism: neuronal-astroglial relationships. Dev Neurosci. 1994;15:343–350. doi: 10.1159/000111354. [DOI] [PubMed] [Google Scholar]