Abstract
During normal forward swimming, the swimmerets on neighboring segments of the crayfish abdomen make periodic power-stroke movements that have a characteristic intersegmental difference in phase. Three types of intersegmental interneurons that originate in each abdominal ganglion are necessary and sufficient to maintain this phase relationship. A cellular model of the intersegmental coordinating circuit that also produces the same intersegmental phase has been proposed. In this model, coordinating axons synapse with local interneurons in their target ganglion and form a concatenated circuit that links neighboring segmental ganglia. This model assumed that coordinating axons projected to their nearest-neighboring ganglion but not farther. We tested this assumption in two sets of experiments.
If the assumption is correct, then blocking synaptic transmission in an intermediate ganglion should uncouple swimmeret activity on opposite sides of the block. We bathed individual ganglia in a low Ca2+–high Mg2+ saline that effectively silenced both motor output from the ganglion and the coordinating interneurons that originated in it. With this block in place, other ganglia on opposite sides of the block could nonetheless maintain their normal phase difference. Simultaneous recordings of spikes in coordinating axons on opposite sides of the blocked ganglion showed that these axons projected beyond the neighboring ganglion. Selective bilateral ablation of the tracts in which these axons ran showed that they were necessary and usually sufficient to maintain coordination across a blocked ganglion.
We discuss revisions of the cellular model of the coordinating circuit that would incorporate these new results.
Keywords: coordination, crayfish, swimmeret, interneuron, pattern generation, locomotion
Effective coordination of limbs during locomotion requires that information about a limb be available to the circuits controlling other limbs and that this information be integrated with the ongoing activity of these other controllers. In segmented animals such as vertebrates and arthropods, the neural mechanisms that achieve this coordination during walking or swimming have not yet been described in any cellular detail (Bässler and Büschges, 1998; Skinner and Mulloney, 1998b). Coordination of swimmeret movements in crayfish is particularly promising in this regard because the motor program that drives swimmeret movements can reliably be elicited from isolated nervous systems (Ikeda and Wiersma, 1964; Mulloney, 1997). When a crayfish is swimming off the bottom, its four pairs of swimmerets make periodic power-stroke (PS) and return-stroke (RS) movements that thrust the animal forward. These movements are driven by separate pattern-generating modules (Murchison et al., 1993), one for each swimmeret, that produce bursts of impulses alternately in PS and RS motor neurons that drive each cycle of movement. These modules occur in pairs in each ganglion that innervates a pair of swimmerets. Modules in different segments are coordinated by a set of three identified interneurons that originate in each module. These neurons send their axons through the minuscule tract (MnT) and are referred to collectively as MnT coordinating interneurons. From each module, two MnT interneurons named ASCE and ASCL, send axons anteriorly to targets in the next anterior ganglion. A third MnT interneuron, named DSC, sends its axon posteriorly to targets in the next posterior ganglion (Namba and Mulloney, 1999). Each interneuron fires a burst of impulses at a characteristic phase in each cycle of activity in its home module. These impulses encode information about the phase and intensity of this activity and are the information that is necessary and sufficient to coordinate swimmerets on neighboring segments.
Previous experiments that asked how many ganglia were required to establish the normal intersegmental phase difference discovered that any two neighboring ganglia that innervated swimmerets could do so when their connections with the rest of the CNS were severed, and that longer-range connections were not required for normal coordination (Paul and Mulloney, 1986). This observation then led to the assumption that the intersegmental coordinating neurons formed a concatenated circuit in which these axons projected only to neighboring ganglia (Skinner and Mulloney, 1998a). In this paper, we tested this assumption in two sets of experiments.
We show that when the modules in a ganglion in the middle of the ventral nerve cord are silenced by blocking synaptic transmission, information sufficient to maintain intersegmental phase nonetheless does reach ganglia on opposite sides of the blocked ganglion. Moreover, the axon of each MnT coordinating interneuron projects farther than the nearest neighboring ganglion, and its impulses can be recorded in the interganglionic connectives more than two segments distant. Experiments in which these long axons were either selectively cut or selectively spared while one intermediate ganglion was blocked show that these axons also carry the information that accomplishes this longer-range coordination.
MATERIALS AND METHODS
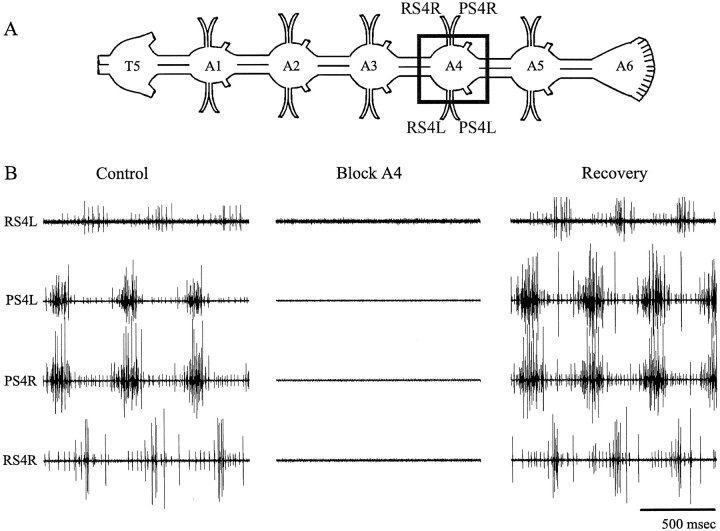
Crayfish (Pacifastacus leniusculus andProcambarus clarkii) were obtained from local suppliers and kept in freshwater aquaria at 15°C. Animals were anesthetized by chilling on ice and then exsanguinated by perfusing physiological saline into a wound created by removing one of the claws. The posterior part of the ventral nerve cord including the last thoracic ganglion (T5) and all six abdominal ganglia (A1–A6) was removed, pinned dorsal side up in a dish lined with Sylgard (Dow Corning, San Francisco, CA), and bathed in saline (Fig.1A).
Fig. 1.
Blocking chemical synaptic transmission also blocked expression of the normal swimmeret motor pattern.A, A diagram that illustrates the last thoracic ganglion (T5) and the six abdominal ganglia (A1–A6) of the isolated ventral nerve cord. Anterior is at the left. The squaresurrounding A4 shows the Vaseline well used to apply low Ca2+ saline just to that ganglion. Impulses in motor axons that projected to the pair of swimmerets innervated by A4 were recorded bilaterally with extracellular electrodes on the anterior (RS4L, RS4R) and posterior (PS4L, PS4R) branches of the swimmeret nerves. B, Three cycles of normal activity in A4 power-stroke (PS4L, PS4R) and return-stroke (RS4L, RS4R) motor axons recorded from branches of the left and right swimmeret nerves. Bathing the preparation in low Ca2+–high Mg2+ saline (Block A4) stopped all firing in these motor units. Restoring the normal extracellular concentrations of Ca2+ and Mg2+(Recovery) also restored coordinated firing of these units.
The normal saline solutions contained (in mm): 195 NaCl, 5.36 KCl, 2.6 MgCl2, 13.5 CaCl2, 10 TRIS base, and 4.7 maleic acid, at pH 7.4. The “low Ca2+–high Mg2+ ” saline had (in mm): 2.6 Ca2+, 52 Mg2+ (0.2 times normal Ca2+, 20 times normal Mg2+), and 117 NaCl (Sherff and Mulloney, 1996).
Electrophysiology. Each swimmeret is innervated by one nerve, N1, which splits into an anterior and posterior branch (Mulloney and Hall, 2000). The axons of RS motor neurons reach their target muscles through the anterior branch; axons of PS motor neurons reach their targets through the posterior branch (Mulloney and Hall, 2000). Action potentials of PS and RS motor neurons were recorded separately with extracellular pin electrodes on these two branches.
To record from axons in the MnT (Skinner, 1985), we removed the sheath from the dorsal side of the ganglion and placed conventional suction electrodes (inner diameter of 80–100 μm) on the dorsal surface of the lateral giant axon near the medial border of the lateral neuropil (LN) (Namba and Mulloney, 1999). To confirm that these MnT units were intersegmental interneurons, another suction electrode was placed on the medial surface of the desheathed hemiconnective at the boundary of Area 76 and Area 78 (Wiersma and Hughes, 1961). Each suction electrode could also be used to stimulate passing axons extracellularly. Electrophysiological recordings were collected on videocassette recording tape using a Neuro-Corder 886 (Neurodata Instruments, New York, NY). Recordings were transferred to a computer for analysis with pClamp (Axon Instruments, Foster City, CA).
Perfusion procedures. In most experiments used to observe MnT activity, preparations spontaneously expressed coordinated swimmeret motor patterns at the outset. To change the level of excitation in the swimmeret system (Mulloney, 1997), we superfused the isolated abdominal nerve cord with either 3 or 6 μm carbachol (Research Biochemicals, Natick, MA) in normal saline.
Description and analysis of recordings. To calculate parameters that describe the activity of the swimmeret system, we displayed activity recorded simultaneously by each electrode and measured the times at which each burst of impulses started or stopped, using a digitizing tablet. From these lists of times, we calculated the periods and durations of each burst and the phase at which each burst began relative to the motor output of the system (Mulloney and Hall, 1987). The period of each cycle of activity was the time from the start of one burst of impulses to the start of the next burst. The duration of each burst was the time from which it started to the time at which it ended. To compare directly the durations of bursts recorded under conditions in which the motor output had different periods, we calculated the relative durations of these bursts as duration/period (Skinner and Mulloney, 1998a).
Phase describes the point in a cycle of activity at which some event began. Normally, we used the start of each PS burst from the most posterior ganglion, A5, to define the start of each cycle (Mulloney, 1997). To measure the phase of an event, we measured its latency, the difference between the time at which it began and the time at which the preceding burst in PS5 began. We also measured the period of that cycle in the reference recording. The phase of theith burst was then calculated as latencyi/periodi. To compare the distributions of phases recorded under different conditions and different sample sizes, we plotted their relative cumulative frequencies. This procedure sorts the list of measured phases from smallest to largest and calculates for each phase the proportion of the sample smaller than or equal to it (Zar, 1996). To calculate probabilities that phases of bursts of impulses recorded in different ganglia occurred randomly relative to one another, we used the Kolmogorov–Smirnov test (Zar, 1996). To calculate descriptive statistics and ANOVA, we used SigmaStat (Jandel Scientific, Corte Madera, CA).
To measure the conduction velocities of individual MnT axons and the stability of these velocities, we used Clampex (Axon Instruments) to record a series of paired sweeps from the MnT electrode and an electrode on the connective. Each sweep was triggered either by a spontaneous impulse recorded by the MnT electrode (orthodromic) or by a stimulus pulse although the electrode on the connective (antidromic).
At the end of each experiment in which regions of the intersegmental connectives had been surgically interrupted, to see what parts of the connectives had been spared and what had been cut, the preparation was fixed in situ, stained with osmium ethylgallate (Leise and Mulloney, 1986), embedded, and sectioned using the protocol described by Namba and Mulloney (1999). Cross-sections of the connectives at the point at which cuts had been made and other sections from intact regions of the same connective were drawn using a camera lucida. Paired drawings were then overlaid to illustrate the extent of the ablation.
RESULTS
Low Ca2+–high Mg2+ blocked pattern generation in individual swimmeret modules
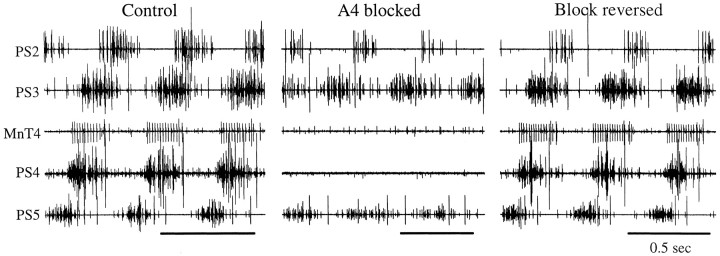
The bursts of impulses that occur in swimmeret motor neurons during expression of the swimmeret motor pattern are driven by synaptic currents from other neurons. When the control saline bathing an isolated nerve cord was replaced by a low Ca2+–high Mg2+ saline, all activity in both RS and PS branches of the nerves that innervate swimmerets disappeared (Sherff and Mulloney, 1996, 1997). If individual ganglia were selectively bathed in low Ca2+–high Mg2+ saline, the swimmeret motor neurons in that ganglion were silenced (Fig. 1), although the other ganglia continued to generate alternating bursts of impulses in their own PS and RS motor neurons. This effect was reversible (Fig. 1). From this evidence, we conclude that local superfusion with low Ca2+–high Mg2+ saline can inactivate the two swimmeret modules within a ganglion.
Modules on opposite sides of a blocked ganglion could maintain a stable intersegmental phase
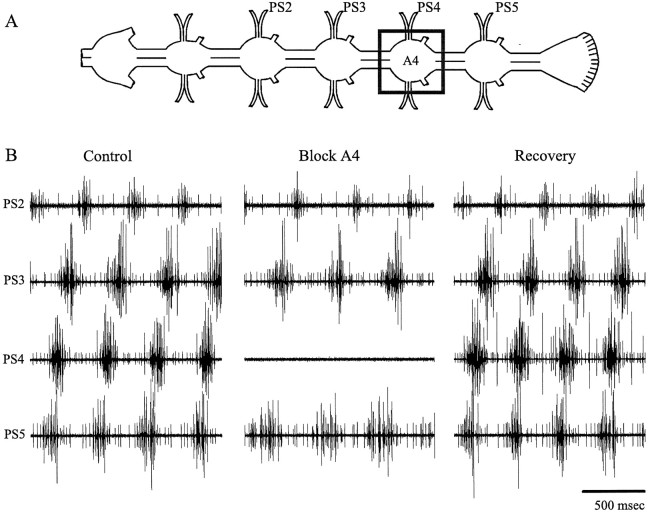
In both intact animals and isolated ventral nerve cords, the bursts of impulses in swimmeret motor neurons located in different ganglia have the same period. These bursts also have a posterior-to-anterior progression of intersegmental phase (Ikeda and Wiersma, 1964; Stein, 1971; Mulloney, 1997). When a ganglion in the middle of the chain of segmental ganglia was selectively blocked with low Ca2+–high Mg2+ saline, this coordination was sometimes maintained by anterior and posterior ganglia, on opposite sides of the block (Fig. 2) (Stein, 1971). Given our previous thinking (Paul and Mulloney, 1986; Skinner and Mulloney, 1998a), this observation was surprising, so we examined the performance of the swimmeret system when either A3 or A4 were selectively blocked.
Fig. 2.
Blocking chemical synaptic transmission in an intermediate ganglion in the ventral nerve cord did not necessarily disrupt coordination of the motor output from other ganglia on opposite sides of the blocked ganglion. A, A diagram that illustrates the positions of electrodes on branches of the four nerves that innervated swimmerets on the right side of the animal (PS2–PS5) and the position of the well that contained the low Ca2+–high Mg2+ saline bathing A4. Anterior is to the left. B, Recordings from the PS branches of ganglia A2 through A5 (PS2–PS5) under control conditions show the characteristic posterior-to-anterior progression of bursts of impulses in PS units in these different ganglia. When ganglion A4 was selectively bathed with low Ca2+–high Mg2+ saline, A4 was silenced but, nonetheless, bursts in PS5 and PS3 retained their normal phase relations (Block A4).
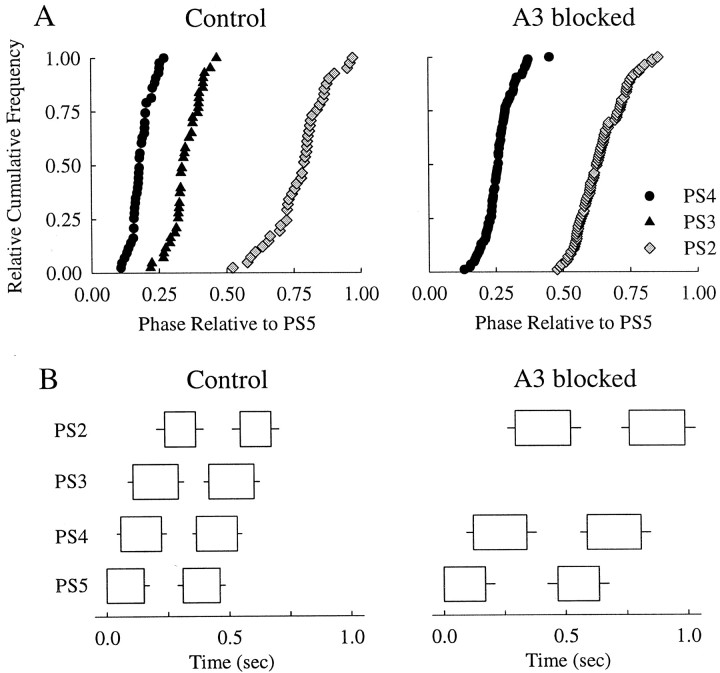
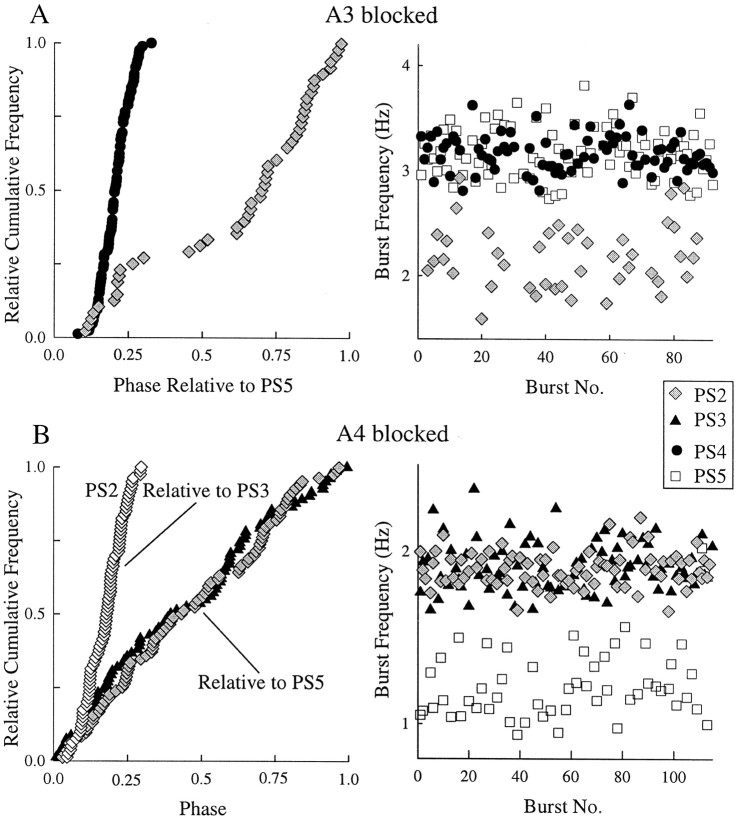
In four of eight experiments in which the modules in A3 were blocked with low Ca2+–high Mg2+ saline, bursts of impulses in PS axons (PS2) in A2 nonetheless maintained a stable phase relative to PS activity in A5; Figure 3 shows the results of one experiment. When A3 was blocked, the period of the output of the system did increase (Fig. 3B), but the relative durations of the PS bursts in the three ganglia that were not blocked did not change (Fig. 3B). Moreover, the distributions of PS2 and PS4 phases relative to PS5 did not change significantly (Fig. 3A). The other three experiments gave the same results.
Fig. 3.
A, Relative cumulative-frequency plots of intersegmental phase before and during a blockade of synaptic transmission in A3. B, Box plots that show the mean + SD durations of PS bursts in A2, A3, A4, and A5 under control conditions and after A3 had been blocked for 1 hr. These plots show the structure of two cycles of the motor pattern. Each box begins at the mean latency of the bursts it describes after the start of the PS5 burst; horizontal lines projecting to theleft show −SD of latency (PS2,PS3, PS4) or period (PS5). Horizontal lines projecting to theright show +SD of burst duration. With A3 blocked, there were no PS3 bursts.
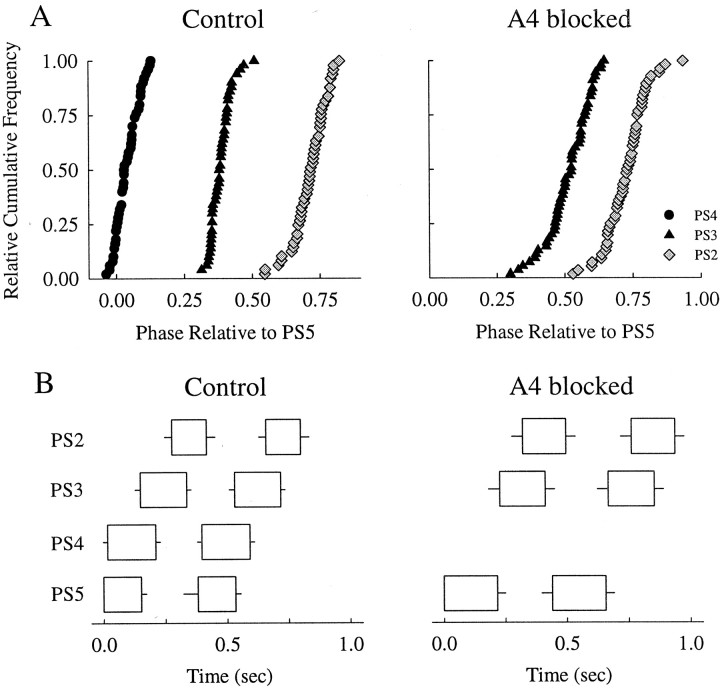
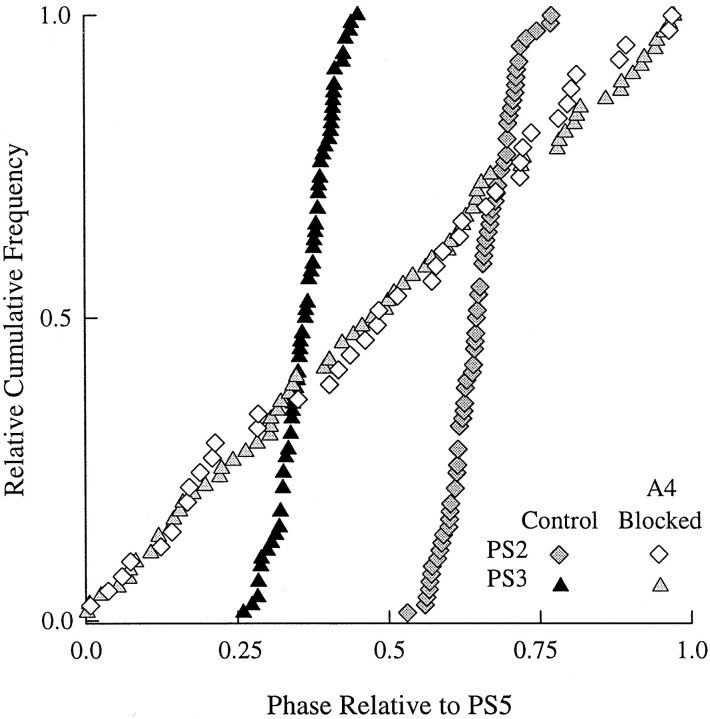
In 4 of 10 experiments in which A4 was blocked, bursts of impulses in PS2 and PS3 maintained the same phases relative to PS5 that they did under control conditions (Fig.4A). In the experiment illustrated in Figure 4, the period of the activity of the system increased slightly during the block, and the distributions of both PS2 and PS3 phases were somewhat broader than that in control, but the centers of the distributions did not shift, and the relative durations of the bursts did not change significantly. The other three experiments gave the same results. In the remaining six experiments, when the low Ca2+–high Mg2+ block was imposed, the preparations either stopped expressing the motor pattern altogether or stopped on one side of the block.
Fig. 4.
A, Relative cumulative-frequency plots of intersegmental phase before and during a blockade of synaptic transmission in A4. B, Box plots that show the mean + SD durations of PS bursts in A2, A3, A4, and A5 under control conditions and after A4 had been blocked for 1 hr. With A4 blocked, there were no PS4 bursts. The boxes have the same dimensions as those in Figure 3.
MnT coordinating interneurons sent axons beyond neighboring ganglia
Impulses in an MnT coordinating interneuron can be recorded from both the MnT in the home ganglion of the neuron and from the interganglionic connectives (Namba and Mulloney, 1999). When the system is actively expressing the swimmeret motor pattern, each MnT interneuron also fires bursts of impulses that occur at particular phases in each cycle of swimmeret activity in their home ganglion. In an active preparation, each type of MnT neuron could be identified physiologically from the structure and phase of its burst in the swimmeret cycle (Namba and Mulloney, 1999) and the conduction–velocity of its impulses. In most experiments, impulses in both ASCE and ASCL axons were recorded simultaneously by the same MnT electrode, and extracellular electrodes on the connectives usually recorded impulses in both units, so axons of both ascending units take approximately the same course.
We wondered whether these interneurons might be responsible for the persistent coordination of modules on opposite sides of a blocked ganglion. Stein (1969) had shown that “ascending coordinating fibers” extended at least two ganglia anterior to the ganglion in which their impulses originated. We began by looking for physiological evidence that axons of MnT interneurons extended beyond their nearest neighboring ganglion. Orthodromic impulses in axons of coordinating interneurons were recorded simultaneously from the MnT of a selected ganglion and from a selected interganglionic connective. Antidromic impulses were recorded by stimulating the axon through the electrode on the connective while recording with the MnT electrode. The results of these experiments for each type of coordinating interneuron are described next.
Ascending MnT interneuron ASCE
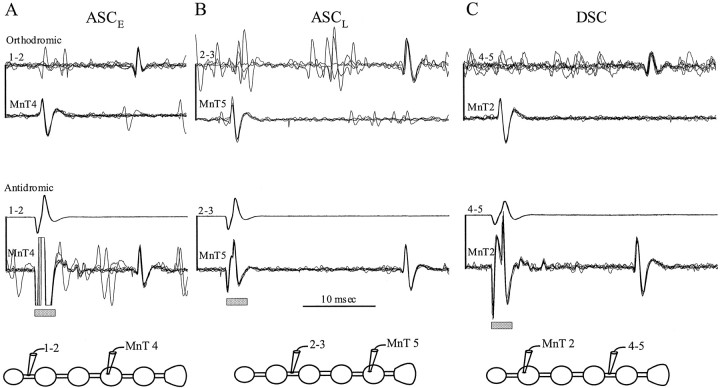
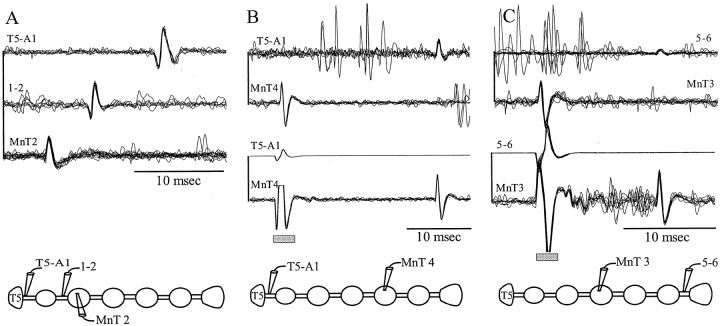
ASCE neurons fire bursts of impulses simultaneously with the PS bursts from their home ganglion. These neurons were active in every preparation that expressed the normal swimmeret motor pattern. Paired recordings from MnT and the connective beyond the nearest neighboring ganglion showed time-locked impulses propagating away from the home ganglion. Stimulation with the electrode on the connective elicited antidromic impulses that were recorded by the MnT electrode (Fig. 5A). The conduction velocities of orthodromic ASCEimpulses recorded from the connective, ∼0.6 m/sec, was the same as that of antidromic impulses that followed stimulation of the connective.
Fig. 5.
Axons of the MnT coordinating interneurons projected beyond their targets in adjacent ganglia. These paired recordings were made simultaneously in the two positions shown in the accompanying diagrams: from the MnT of the home ganglia of the neurons and from the ipsilateral connective between two ganglia.A, ASCE spikes recorded in MnT4 and in the connective between A1 and A2 (1–2). The top pair of traces are recordings of spontaneous orthodromic impulses; the bottom pair are antidromic impulses in response to stimulating the connective. Here and inB and C, the gray rectangle marks the stimulus artifacts. B, ASCL spikes recorded in MnT5 and in the connective between A2 and A3 (2–3). C, DSC spikes recorded in MnT2 and the connective between A4 and A5 (4–5). Time bar, 10 msec.
We were able to record from ASCE axons in intersegmental connectives two ganglia anterior (n = 6 preparations) and three ganglia anterior to the home ganglion of the neuron (n = 2 preparations). From these results, we conclude that axons of ASCE neurons can project anteriorly at least two ganglia beyond their nearest neighboring ganglion.
Ascending MnT interneuron ASCL
ASCL neurons fired bursts of impulses late in each PS burst in their home ganglion. When impulses in both ascending units were recorded simultaneously, ASCL impulses were normally larger than ASCE impulses. Their conduction velocity was ∼0.8 m/sec. ASCL was not active in every preparation, and its firing pattern was less regular than ASCE. Their axons also project beyond the nearest neighboring ganglion; we recorded ASCL axons in the connectives two ganglia anterior (n = 6 preparations) and three ganglia anterior (n = 3 preparations) to the home ganglion of the neuron (Fig.5B).
Descending MnT interneuron DSC
DSC interneurons fired bursts of impulses simultaneously with bursts in RS motor neurons in the home ganglion of each DSC. Their axons project from the LN into MnT and then posteriorly into the intersegmental connectives toward the posterior neighboring ganglion (Namba and Mulloney, 1999). The conduction velocity of DSC axons was ∼0.8 m/sec. In 10 preparations, we recorded impulses in DSC axons that originated in either A2, A3, or A4 at least one ganglion beyond the neighboring posterior ganglion. In one preparation, we were able to record from the axon of DSC from A2 in the connective between A4 and A5 (Fig. 5C), so this DSC sent its axon through the connective more than two ganglia away.
From this evidence, we conclude that each MnT coordinating interneuron sends its axon at least two ganglia beyond its nearest neighboring ganglion, farther than we had previously assumed (Skinner and Mulloney, 1998a). We then examined the possibility that these axons were responsible for the persistent coordination of PS bursts on opposite sides of a ganglion in which synaptic transmission was blocked.
Impulses in axons of MnT interneurons were not obstructed by a low Ca2+–high Mg2+ blockade of a ganglion through which they passed
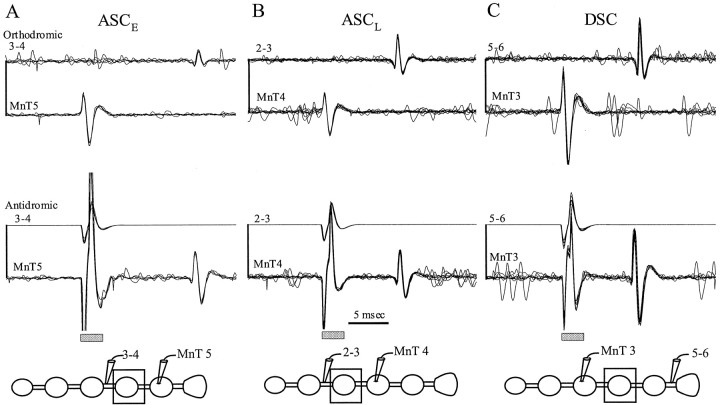
Impulses generated during active expression of the motor pattern could be recorded simultaneously on opposite sides of the blocked ganglion (Fig. 6). Stimulation through the electrode on the connective elicited antidromic impulses that arrived at the electrode on MnT after the same delay as the orthodromic impulses (Fig. 6). Therefore, information carried by MnT axons farther than neighboring ganglia was not affected by the low Ca2+–high Mg2+ block that revealed long-range coupling.
Fig. 6.
MnT impulses could traverse a blocked ganglion. Paired recordings of impulses in ASCE(A), ASCL (B), and DSC (C) axons made from the MnT in the home ganglion of the neuron and from the connective beyond a blocked ganglion (enclosed in box). Spontaneous impulses that occurred during expression of the swimmeret motor pattern were recorded first by the MnT electrode and later by the electrode on the connective (orthodromic). Antidromic impulses elicited by stimulation through the electrode on the connective were recorded by the MnT electrode, with the same conduction time as had the orthodromic impulses. Thegray rectangles beneath the antidromic recordings mark the stimulus artifacts.
Coordinating interneurons in a blocked ganglion fell silent during a low Ca2+--high Mg2+blockade
Although motor neurons were silenced by blocking synaptic transmission (Fig. 1), it was possible that MnT interneurons were not. In that case, persistent coordination might be accomplished by a circuit that required activity in the MnT interneurons of the blocked ganglion. To test for this possibility, we recorded impulses of coordinating interneurons from MnT in an intermediate ganglion before and during imposition of a block and after the block was washed out (Fig. 7). All three types of MnT interneurons were effectively silenced during the low Ca2+ block (DSC data not shown). These results mean that MnT activity in the blocked ganglion is not required to maintain coordination across a blocked ganglion. It also implies that the bursts of impulses in MnT interneurons are driven by synaptic currents from the pattern-generating kernel of the module in which they originate (Skinner and Mulloney, 1998a).
Fig. 7.
MnT axons that originate in a blocked ganglion were silenced when the block was imposed. These five simultaneous recordings show the coordinated PS output from ganglia A2, A3, A4, and A5 (PS2, PS3, PS4,PS5) and bursts of impulses in ASCE and ASCL axons recorded in MnT4. In the beginning (Control), the two MnT units fired impulses during each cycle of the motor output. Once the block of A4 was established, both of the MnT units originating in A4 and the PS motor neurons in A4 fell silent. These units resumed firing in phase with the rest of the system when A4 was again bathed in normal saline. Time bars, 0.5 sec.
MnT axons were necessary and sufficient to coordinate modules on opposite sides of a blocked ganglion
In preparations in which swimmeret modules in neighboring ganglia were functioning, the ascending and descending MnT axons that connected neighbors were necessary and sufficient to establish and maintain normal intersegmental coordination (Namba and Mulloney, 1999). However, they are not the only intersegmental axons that fire in phase with swimmeret activity (Wiersma and Hughes, 1961; Stein, 1971; Paul and Mulloney, 1986), and it seemed possible that long-distance coordination might be accomplished by information conducted by other interneurons. The axons of all three types of MnT coordinating interneurons lie close together in the interganglionic connectives, near the midline and just ventral to the median giant axon (Namba and Mulloney, 1999), but the axons of those other interneurons run far laterally in these connectives (Wiersma and Hughes, 1961; Stein, 1971). Therefore, we tested this possibility in two types of preparations: one in which the bundles of MnT axons in the connective were cut bilaterally but the rest of the connective was spared, and one in which all of each connective were cut bilaterally except the bundles that contained the MnT axons (cf. Namba and Mulloney, 1999). These preparations selectively eliminated either the information carried by the MnT axons or the information carried by other swimmeret interneurons. We then imposed a low Ca2+–high Mg2+ block on a ganglion next to the cuts and recorded the motor output from all of the ganglia. At the end of each experiment, the preparation was fixed and sectioned so that we could define the regions of each connective that remained intact.
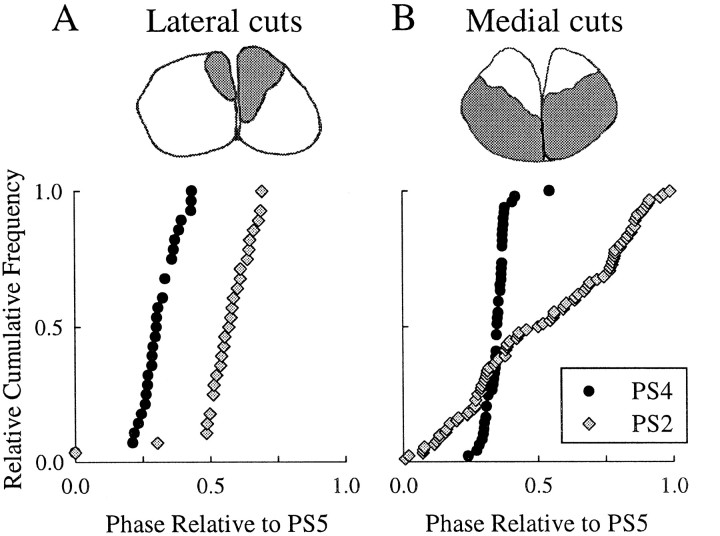
In every experiment in which the bundles of axons that contained MnT axons were severed bilaterally but the rest of the connectives were left intact (n = 5 preparations), coordination across a blocked ganglion collapsed (Fig.8B) (Stein, 1971). When all intersegmental axons projecting through a blocked ganglion were cut bilaterally except those in the immediate neighborhood of the MnT axons (Fig. 8A), PS bursts on opposite sides of the cut and the block retained the same phase differences they had when the system was intact (n = 4 preparations). In these experiments, once the connectives had been partially severed, we recorded MnT impulses from the remaining bundles of axons that continued to connect ganglia on opposite sides of the block (data not shown). Thus, the information carried in axons of MnT interneurons is necessary and sufficient to establish normal intersegmental phase differences between swimmeret motor output from ganglia separated by more than one segment.
Fig. 8.
Information carried by MnT axons that spanned a blocked ganglion was necessary and sufficient to coordinate swimmeret motor output on opposite sides of a blocked ganglion. These figures show the phases of PS2 bursts relative to PS5 recorded when A3 was blocked and either medial or lateral portions of the connectives between A2 and A3 had been cut bilaterally. A, When the dorsomedial regions that contained the axons of MnT interneurons were spared, normal coordination of PS2 bursts could still be achieved (n = 4 experiments). B, When the dorsomedial regions were selectively cut, sparing the rest of the connectives, stable phase relations of PS2 and PS5 bursts were not observed (n = 5 experiments). The drawings of cross-sections show in each case the regions of the connectives that remained intact (shaded) when the data plotted below were recorded. In each drawing, dorsal is to thetop.
Coordination across a blocked ganglion was not always stable
In approximately half of our preparations, the introduction of a selective blockade on one ganglion disrupted the system, and expression of the swimmeret motor pattern stopped in all ganglia. In several preparations, we observed a striking uncoupling of otherwise normal activity on opposite sides of the block (Fig.9). In some preparations, this uncoupling was transient and could be preceded and followed by sequences of normal coordination. In the experiment illustrated in Figure 9, PS2 and PS3 bursts were coordinated in a typical manner under control conditions. In a sequence of bursts recorded after the block of A4 had been in place for 63 min, PS2 and PS3 bursts were completely uncoordinated from PS5 bursts. The phases of both PS2 and PS3 bursts during this episode were random relative to PS5 activity, but PS2 was normal relative to PS3. However, during another sequence recorded 9 min later, PS2 and PS3 bursts were again normally coordinated with PS5 (data not shown). During this sequence of activity, the phases of PS bursts in these two ganglia were normally distributed relative to one another but randomly distributed relative to PS5, on the opposite side of the block. Ten minutes later, normal coupling had reappeared, although the block remained in place.
Fig. 9.
Transient uncoupling of activity anterior to and posterior to a blocked A4 ganglion. Relative cumulative-frequency plot of phases of PS2 and PS3 bursts recorded before A4 was blocked with low Ca2+ solution and 63 min after the block was first introduced.
In preparations in which intersegmental coordination failed during a selective blockade of one ganglion, the frequencies of PS bursts in ganglia on opposite sides of the block could differ, something that does not normally occur (Braun and Mulloney, 1995). This difference in period appeared both when A3 was blocked and when A4 was blocked (Fig.10). During the A3 block, PS2 bursts occurred approximately two-thirds as often as PS4 and PS5 bursts (Fig. 10A). During the A4 block, PS5 bursts occurred approximately one-half as often as PS2 and PS3 bursts (Fig.10B). These plots of instantaneous frequencies (the reciprocal of period) of individual PS bursts recorded simultaneously from three ganglia during blockade of A3 or A4 show that the pairs of ganglia that remained connected had similar frequencies, but the frequencies of bursts from the single ganglion on the opposite side of the block were lower and less stable. This difference could affect both PS2 bursts (A3 blocked) and PS5 bursts (A4 blocked).
Fig. 10.
The frequencies of PS bursts in ganglia on opposites sides of a blocked ganglion were sometimes different and, in these cases, intersegmental coordination across the block failed.A, When A3 was blocked, normal coordination of PS4 and PS5 continued, but PS2 bursts occurred less frequently, and the phases of these PS2 bursts relative to PS5 were unstable. B, When A4 was blocked, normal coordination of PS2 and PS3 continued, but PS5 bursts occurred less frequently, and phases of PS2 and PS3 relative to PS5 were random.
Projections of coordinating axons beyond the swimmeret system
In these species of crayfish, only four of six abdominal ganglia (A2–A5) innervate swimmerets used for forward swimming; females ofPacifastacus do not even have swimmerets on their first abdominal segment. Therefore, it was interesting to find axons of coordinating interneurons in the connectives anterior to and posterior to these four ganglia. In 11 preparations, we recorded from axons of ascending units in the connective between A1 and A2. Axons of ASCE from every abdominal ganglia A2–A5 occurred in the A1–A2 connective. Axons of ASCL neurons from every ganglion except A4 were also observed in the A1–A2 connective; it is likely that we missed the ASCLaxon from A4.
Axons of ASCE and ASCLinterneurons continued anterior toward the thorax (Fig.11A,B). In six preparations, we recorded from axons of the ascending interneurons in the connective between the thoracic ganglion T5 and A1. We were able to demonstrate ASCE axons from A2 and from A4 run in the T5–A1 connective.
Fig. 11.
A, Paired extracellular recordings of spontaneous orthodromic impulses in ASCE from MnT2, the connective between A1 and A2 (1–2), and the connective between thoracic ganglion T5 and A1 (T5-A1). The electrode on the connectives first recorded impulses in1–2 and then was moved to record T5-A1.B, Paired recordings of impulses in an ASCLaxon in MnT4 and in the connective between T5 and A1. The top pairs show spontaneous orthodromic impulses; the bottom pairs show antidromic impulses in response to stimulation of the T5–A1 connective. C, Axons of DSC interneurons projected posteriorly toward the terminal ganglion, A6. The top pairs of recordings show spontaneous orthodromic impulses from MnT4 and the connective between A5 and A6 (5–6); the bottom pairs show antidromic impulses in response to stimulation of the 5–6 connective. The gray rectangles beneath the antidromic recordings inB and C mark the stimulus artifacts.
Axons of DSC interneurons project posteriorly to the terminal abdominal ganglion, A6. In two preparations, the axons of a DSC originating in A4 were recorded in the connective between A5 and A6 (Fig.11C).
DISCUSSION
Effective coordination of movements of different limbs during locomotion is a fundamental criterion for effective behavior and a focal point for natural selection. Most modern research on intersegmental coordination of locomotion has focused on neural control of trunk muscles that produces undulatory swimming movements in lamprey, tadpoles, and leeches. The coordination of limbs on different segments is probably accomplished by neural circuits derived from those that control trunk musculature (Mulloney, 1993;Stein et al., 1995, 1998), but the phase relations of limb movements are usually more pronounced and more complex than those of trunk movements, even in the same animal. We do not have well proven explanations in cellular terms of how limbs in any animal are coordinated during locomotion (Skinner and Mulloney, 1998b).
To construct the current cellular model of the circuit that coordinates swimmeret modules in different segments (Skinner and Mulloney, 1998a), we drew on previous descriptions of local interneurons and intersegmental interneurons that were components of the swimmeret system. The dynamics of this model matched those of the swimmeret system itself in several important aspects, including stable intersegmental phase differences in the face of changes in period, so we are examining closely the assumptions and predictions of the model about the swimmeret system itself. The model assumed that axons of each coordinating interneuron project from their home module to targets in the nearest neighboring ganglia but not farther. This assumption was based on the experimental demonstration that any two neighboring abdominal ganglia that remained connected would maintain normal intersegmental phase difference, even when their connections to the rest of the nervous system were severed (Paul and Mulloney, 1986), so long-range projections were unnecessary for normal coordination. If this assumption were correct, then blocking synaptic transmission in one ganglion should uncouple swimmeret modules on opposite sides of the blocked ganglion. That is not what we observed (Figs. 2-4). When transmission in either A3 or A4 was selectively blocked, bursts of impulses in swimmeret motor neurons located in ganglia anterior to the blocked ganglion could nonetheless maintain a stable phase relative to PS bursts in A5.
One possibility that would save the original assumption would have been that MnT interneurons originating in the blocked ganglion continued to fire periodic bursts of impulses despite the silencing of all motor neurons. This did not happen (Fig. 7). By continuously recording from MnT axons in the blocked ganglion before and during the imposition of a block, we found that each MnT unit in the blocked ganglion was also silenced along with the motor neurons. This means that effective coordinating information can be conducted through a blocked ganglion to targets more remote than nearest neighbors.
Several results support that idea that this information is conducted to these more distant ganglia by the axons of these same MnT coordinating interneurons. Axons of each type of coordinating interneuron do project at least two ganglia beyond their nearest neighboring ganglion (Figs.5, 11). Conduction of impulses in these axons is unaffected by a local low Ca2+–high Mg2+ block (Fig. 6). Moreover, cutting bilaterally the regions of the intersegmental connective that contain these axons destroys coordination across a blocked ganglion (Fig. 8). Cutting all the rest of these connectives bilaterally except the regions in which these axons run spares coordination (Fig. 8). Stein (1969, 1971) had also observed that coordination failed when these median bundles were interrupted. These results mean that information conducted as bursts of impulses in axons of MnT coordinating interneurons is both necessary and sufficient to maintain long-range coordination.
The long-range connections are not, however, as effective at maintaining normal coordination as are nearest-neighbor connections. When an intermediate ganglion was blocked, we observed several episodes in which ganglia on opposite sides of the block expressed normal PS–RS alternation but at different frequencies and without a stable intersegmental phase (Figs. 9, 10). These episodes were followed by extended episodes in which coordination was again normal. In all previous experiments in which synaptic transmission was not blocked, including those in which excitation of the individual ganglia was not uniform (Braun and Mulloney, 1995), we have not observed this behavior. These episodes are not attributable to intrinsic differences in excitability of anterior and posterior ganglia (Mulloney, 1997). We observed this uncoupling in both experiments in which A3 was blocked and in which A4 was blocked (Fig. 10), so there was no evidence of any anterior versus posterior bias. One mechanism that might produce these differences would be that the patterns of synaptic connections made by each coordinating axon in each ganglion to which it projected was the same, but the strength of connections with targets in more distant ganglia was weaker than those in neighboring ganglia, a gradient of synaptic strength (Nakagawa and Mulloney, 2001). The episodic failures of coordination are also consistent with the assumption that intersegmental coupling between swimmeret modules is relatively weak (Skinner et al., 1997; Jones, 2001).
Another factor that might make the long-range connections less effective is that bursts of impulses from more distant ganglia arrive in each ganglion at a different phase in the cycle (earlier) than do those from neighboring ganglia. If the patterns and strengths of connections were identical, we would predict that this difference in timing should affect the phase whenever an intermediate ganglion was silenced. Working with a semi-intact preparation, Stein (1969) had observed that A5–A3 coordination did not change when he silenced A4 by manipulating sensory input to A4. We were nonetheless surprised not to see a systematic shift in intersegmental phases when one intermediate ganglion was blocked (Figs. 3, 4). These observations suggest that the connections of MnT axons with targets in more distant ganglia are not identical to their connections in neighboring ganglia.
Comparison of MnT interneurons with coordinating interneurons in other systems
The extent to which axons of coordinating interneurons project beyond neighboring segments has also vexed students of locomotion in other animals. The six types of intersegmental interneurons that coordinate contractions of the body wall during swimming in leeches originate in each segmental ganglion and project forward or backward through several neighboring ganglia, in which they synapse with the same targets in each ganglion (Friesen and Pearce, 1993). These interneurons are able to maintain intersegmental phase when as many as five intermediate ganglia are blocked.
At least two types of interneurons that originate in each spinal segment and project to neighboring segments are involved in coordination of movements in swimming lamprey (Buchanan et al., 1989;Ohta et al., 1991; Buchanan, 1999). The limits of their connections in segments anterior and posterior to their origin has been difficult to demonstrate in cellular terms, but systems-level experiments show that the ensemble of their activities can maintain coordination over tens of segments (Buchanan and Kasicki, 1999; McClellan and Hagevik, 1999;Miller and Sigvardt, 2000).
Walking in insects and crustaceans involves more pairs of limbs, three or four, but smaller numbers of neurons than it does in vertebrates. The details of limb movements during walking in various directions are well known at the behavioral level (Cruse, 1990;Müller and Cruse, 1991a,b; Domenici et al., 1999;Dickinson et al., 2000). Like the swimmerets, each walking leg has its own hemisegmental pattern-generating circuit (Sillar et al., 1987;Ronacher, 1989, 1991; Chrachri and Clarac, 1990), but coordination of neighboring legs is more variable in both intact animals and isolated preparations of thoracic ganglia than is coordination of swimmerets (Sillar et al., 1987; Ryckebusch and Laurent, 1994; Berkowitz and Laurent, 1996), and sensory input from each leg seems to play a major role in shaping each movement (Bässler and Büschges, 1998). Intersegmental interneurons that respond to proprioceptive input and that fire bursts of impulses during walking or grooming movements have been described in locust (Laurent, 1987; Ramirez and Pearson, 1988; Laurent and Burrows, 1989). These interneurons might coordinate legs during walking. Units in the connectives between thoracic ganglia of cockroaches fire in phase with leg elevation during walking (Pearson and Iles, 1973), and coordination of this levator activity in neighboring locust ganglia persists after proprioceptive feedback and descending input is eliminated (Berkowitz and Laurent, 1996), so it is likely that an intersegmental coordinating circuit establishes a framework within which proprioceptive information is interpreted. Our new results suggest that the neural circuit that coordinates swimmerets has many features in common with these other intersegmental coordinating circuits and might be evolutionarily homologous with them (Mulloney, 1993).
These results force us to rethink our cellular model of the swimmeret coordinating circuit. One of the simplest revisions of the model would assume that each coordinating axon makes the same pattern of synapses in each of its target modules but that these synapses are progressively weaker in each more distant module (cf. Williams, 1992). Additional progress will require physiological and anatomical identification of the neuronal targets of MnT interneurons and new computational analysis of this revised model.
Footnotes
This work was supported by National Science Foundation Grants IBN 95-14889 and IBN 97-28791 and by Human Frontier Science Program Grant RG 61/98 to B.M.
Correspondence should be addressed to B. Mulloney, Neurobiology, Physiology, and Behavior, University of California, Davis, One Shields Drive, Davis, CA 95616-8519. E-mail: bcmulloney@ucdavis.edu.
REFERENCES
- 1.Bässler U, Büschges A. Pattern generation for stick insect walking movements—multisensory control of a locomotor program. Brain Res Rev. 1998;27:65–88. doi: 10.1016/s0165-0173(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz A, Laurent G. Central generation of grooming motor patterns and interlimb coordination in locusts. J Neurosci. 1996;16:8079–8091. doi: 10.1523/JNEUROSCI.16-24-08079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun G, Mulloney B. Coordination in the crayfish swimmeret system: differential excitation causes changes in intersegmental phase. J Neurophysiol. 1995;73:880–885. doi: 10.1152/jn.1995.73.2.880. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan JT. Commissural interneurons in rhythm generation and intersegmental coupling in the lamprey spinal cord. J Neurophysiol. 1999;81:2037–2045. doi: 10.1152/jn.1999.81.5.2037. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan JT, Kasicki S. Segmental distribution of common synaptic inputs to spinal motoneurons during fictive swimming in the lamprey. J Neurophysiol. 1999;82:1156–1163. doi: 10.1152/jn.1999.82.3.1156. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan JT, Grillner S, Cullheim S, Risling M. Identification of excitatory interneurons contributing to generation of locomotion in lamprey: structure, pharmacology, and function. J Neurophysiol. 1989;62:59–69. doi: 10.1152/jn.1989.62.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Chrachri A, Clarac F. Fictive locomotion in the fourth thoracic ganglion of the crayfish. J Neurosci. 1990;10:707–719. doi: 10.1523/JNEUROSCI.10-03-00707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruse H. What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci. 1990;13:15–21. doi: 10.1016/0166-2236(90)90057-h. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. How animals move: an integrated view. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- 10.Domenici P, Schmitz J, Jamon M. The relationship between leg stepping pattern and yaw torque oscillations in curve walking of two crayfish species. J Exp Biol. 1999;202:3069–3080. doi: 10.1242/jeb.202.22.3069. [DOI] [PubMed] [Google Scholar]
- 11.Friesen WO, Pearce RA. Mechanisms of intersegmental coordination in leech locomotion. Semin Neurosci. 1993;5:41–47. [Google Scholar]
- 12.Ikeda K, Wiersma CAG. Autogenic rhythmicity in the abdominal ganglion of the crayfish: the control of swimmeret movements. Comp Biochem Physiol. 1964;12:107–115. doi: 10.1016/0010-406x(64)90053-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones SR. PhD thesis. Boston University; 2001. Rhythms in the neocortex and in CPG neurons: a dynamical systems analysis. [Google Scholar]
- 14.Laurent G. Parallel effects of joint receptors on motor neurones and intersegmental interneurones in the locust. J Comp Physiol [A] 1987;160:341–353. doi: 10.1007/BF00613023. [DOI] [PubMed] [Google Scholar]
- 15.Laurent G, Burrows M. Intersegmental interneurons can control the gain of reflexes in adjacent segments of the locust by their action on nonspiking local interneurons. J Neurosci. 1989;9:3030–3039. doi: 10.1523/JNEUROSCI.09-09-03030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leise EM, Mulloney B. The osmium-ethyl gallate procedure is superior to silver impregnations for mapping neuronal pathways. Brain Res. 1986;367:265–272. doi: 10.1016/0006-8993(86)91601-x. [DOI] [PubMed] [Google Scholar]
- 17.McClellan AD, Hagevik A. Coordination of spinal locomotor activity in the lamprey: long-distance coupling of spinal oscillators. Exp Brain Res. 1999;1:93–108. doi: 10.1007/s002210050719. [DOI] [PubMed] [Google Scholar]
- 18.Miller WL, Sigvardt KA. Extent and role of multisegmental coupling in the lamprey spinal locomotor pattern generator. J Neurophysiol. 2000;83:465–476. doi: 10.1152/jn.2000.83.1.465. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, Cruse H. The contralateral coordination of walking legs in the crayfish Astacus leptodactylus. I. Experimental results. Biol Cybern. 1991a;64:429–436. [Google Scholar]
- 20.Müller U, Cruse H. The contralateral coordination of walking legs in the crayfish Astacus leptodactylus. II. Model calculations. Biol Cybern. 1991b;64:437–446. [Google Scholar]
- 21.Mulloney B. Introduction: neural mechanisms of coordination during locomotion. Semin Neurosci. 1993;5:1–2. [Google Scholar]
- 22.Mulloney B. A test of the excitability-gradient hypothesis in the swimmeret system of crayfish. J Neurosci. 1997;17:1860–1868. doi: 10.1523/JNEUROSCI.17-05-01860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulloney B, Hall WM. The PD programs: a method for the quantitative description of motor patterns. J Neurosci Methods. 1987;19:47–59. doi: 10.1016/0165-0270(87)90020-3. [DOI] [PubMed] [Google Scholar]
- 24.Mulloney B, Hall WM. Functional organization of crayfish abdominal ganglia. III. Swimmeret motor neurons. J Comp Neurol. 2000;419:233–243. doi: 10.1002/(sici)1096-9861(20000403)419:2<233::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. J Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa H, Mulloney B. A presynaptic basis for gradients in strength of synapses between different abdominal stretch-receptor axons and their common target neurons. J Neurosci. 2001;21:1645–1655. doi: 10.1523/JNEUROSCI.21-05-01645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namba H, Mulloney B. Coordination of limb movements: three types of intersegmental interneurons in the swimmeret system, and their responses to changes in excitation. J Neurophysiol. 1999;81:2437–2450. doi: 10.1152/jn.1999.81.5.2437. [DOI] [PubMed] [Google Scholar]
- 28.Ohta Y, Dubuc R, Grillner S. A new population of neurons with crossed axons in the lamprey spinal cord. Brain Res. 1991;564:143–148. doi: 10.1016/0006-8993(91)91364-7. [DOI] [PubMed] [Google Scholar]
- 29.Paul DH, Mulloney B. Intersegmental coordination of swimmeret rhythms in isolated nerve cords of crayfish. J Comp Physiol [A] 1986;158:215–224. [Google Scholar]
- 30.Pearson KG, Iles JF. Nervous mechanisms underlying intersegmental coordination of leg movements during walking in the cockroach. J Exp Biol. 1973;58:725–744. [Google Scholar]
- 31.Ramirez J-M, Pearson KG. Generation of motor patterns for walking and flight in motoneurons supplying bifunctional muscles in the locust. J Neurobiol. 1988;18:257–282. doi: 10.1002/neu.480190307. [DOI] [PubMed] [Google Scholar]
- 32.Ronacher B. Stridulation of acridid grasshoppers after hemisection of thoracic ganglia: evidence for hemiganglionic oscillators. J Comp Physiol [A] 1989;164:723–736. [Google Scholar]
- 33.Ronacher B. Contribution of abdominal commissures in the bilateral coordination of the hindlegs during stridulation in the grasshopper Chorthippus dorsatus. J Comp Physiol [A] 1991;169:191–200. [Google Scholar]
- 34.Ryckebusch S, Laurent G. Interactions between segmental leg central pattern generators during fictive rhythms in the locust. J Neurophysiol. 1994;72:2771–2785. doi: 10.1152/jn.1994.72.6.2771. [DOI] [PubMed] [Google Scholar]
- 35.Sherff CM, Mulloney B. Tests of the motor neuron model of the local pattern-generating circuits in the swimmeret system. J Neurosci. 1996;16:2839–2859. doi: 10.1523/JNEUROSCI.16-08-02839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherff CM, Mulloney B. Passive properties of swimmeret motor neurons. J Neurophysiol. 1997;78:92–102. doi: 10.1152/jn.1997.78.1.92. [DOI] [PubMed] [Google Scholar]
- 37.Sillar KT, Clarac F, Bush BMH. Intersegmental coordination of central neural oscillators for rhythmic movements of the walking legs in crayfish, Pacifastacus leniusculus. J Exp Biol. 1987;131:245–264. [Google Scholar]
- 38.Skinner K. The structure of the fourth abdominal ganglion of the crayfish, Procambarus clarkii. II. Synaptic neuropils. J Comp Neurol. 1985;234:182–191. doi: 10.1002/cne.902340205. [DOI] [PubMed] [Google Scholar]
- 39.Skinner FK, Mulloney B. Intersegmental coordination of limb movements during locomotion: mathematical models predict circuits that drive swimmeret beating. J Neurosci. 1998a;18:3831–3842. doi: 10.1523/JNEUROSCI.18-10-03831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner FK, Mulloney B. Intersegmental coordination in invertebrates and vertebrates. Curr Opin Neurobiol. 1998b;8:725–732. doi: 10.1016/s0959-4388(98)80114-1. [DOI] [PubMed] [Google Scholar]
- 41.Skinner FK, Kopell N, Mulloney B. How does the crayfish swimmeret system work? Insights from nearest neighbor coupled oscillator models. J Comput Neurosci. 1997;4:151–160. doi: 10.1023/a:1008891328882. [DOI] [PubMed] [Google Scholar]
- 42.Stein PSG. PhD thesis. Stanford University; 1969. A neurophysiological study of two systems of coupled oscillators in crayfish. [Google Scholar]
- 43.Stein PSG. Intersegmental coordination of swimmeret motor neuron activity in crayfish. J Neurophysiol. 1971;34:310–318. doi: 10.1152/jn.1971.34.2.310. [DOI] [PubMed] [Google Scholar]
- 44.Stein PSG, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein PSG, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J Neurosci. 1998;18:467–479. doi: 10.1523/JNEUROSCI.18-01-00467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiersma CAG, Hughes GM. On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii. J Comp Neurol. 1961;116:209–228. doi: 10.1002/cne.901160209. [DOI] [PubMed] [Google Scholar]
- 47.Williams TL. Phase coupling in simulated chains of coupled oscillators representing the lamprey spinal cord. Neural Comput. 1992;4:546–558. [Google Scholar]
- 48.Zar JH. Biostatistical analysis, pp 471–479. Prentice-Hall; Englewood Cliffs, NJ: 1996. [Google Scholar]