Abstract
Serotonin [5-hydroxytryptamine (5-HT)] 5-HT2A and 5-HT2C receptors (5-HT2ARs and 5-HT2CRs), which innervate the dopamine mesoaccumbens pathway, may play an important role in the behavioral effects of cocaine. To test this hypothesis, the present study measured cocaine-evoked locomotor activity after bilateral microinjection of selective 5-HT2AR and 5-HT2CR antagonists into the ventral tegmental area (VTA) or the nucleus accumbens (NAc) shell. Locomotor activity was measured after intracranial microinjection of saline (0.2 μl/side), the selective 5-HT2AR antagonistR-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol (M100907) (0.1 or 0.3 μg · 0.2 μl−1 · side−1), or the selective 5-HT2CR antagonist 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfon-amido)phenyl-5-oxopentyl)]-1,3,8-triazaspiro[4.5]decane-2,4-dione hydrochloride (RS 102221) (0.05–0.5 μg · 0.2 μl−1 · side−1) followed by an injection of saline (1 ml/kg, i.p.) or cocaine (10 mg/kg, i.p.). Microinjection of M100907 (0.1–0.3 μg/side) into the VTA or RS 102221 (0.15–0.5 μg/side) into the NAc shell attenuated cocaine-induced hyperactivity in a dose-related manner. However, hyperactivity evoked by cocaine was not altered by microinjection of RS 102221 into the VTA or M100907 into the NAc shell. No changes in basal activity were observed after microinjection of M100907 or RS 102221 into either brain region. These findings are the first to demonstrate that the behavioral effects of cocaine are generated in part by activation of 5-HT2ARs in the VTA and by activation of 5-HT2CRs in the NAc shell. The selective regulation of the mesoaccumbens circuit by 5-HT2ARs and 5-HT2CRs implicates these 5-HT receptors as important in the behavioral outcomes of systemic cocaine administration.
Keywords: behavior, cocaine, nucleus accumbens, serotonin, 5-HT2A receptor, 5-HT2C receptor, ventral tegmental area
An effective pharmacotherapy for cocaine dependence remains elusive (Klein, 1998) despite significant advances in unraveling the neurobiological mechanisms of cocaine. Cocaine inhibits dopamine (DA), serotonin [5-hydroxytryptamine (5-HT)], and norepinephrine reuptake into presynaptic nerve terminals (Koe, 1976), and thus indirectly stimulates multiple monoamine receptors. It has been established that elevated DA neurotransmission and indirect activation of DA D1- and D2-like receptors play a central role in the in vivo effects of cocaine (White and Kalivas, 1998). The importance of the DA mesoaccumbens pathway, which is composed of DA neurons in the ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc), has been underscored by observations that intra-NAc cocaine infusion mimics the reinforcing (McKinzie et al., 1999), discriminative stimulus (Callahan et al., 1994) and hypermotive properties (Delfs et al., 1990) of systemic cocaine.
Despite our understanding of the role of DA in the behavioral effects of cocaine, the utility of DA ligands as effective pharmacotherapeutic medications has been limited (Klein, 1998). Serotonin (5-HT) may represent another viable target in the search for such treatment medications. Cocaine inhibits 5-HT reuptake (Koe, 1976) and 5-HT manipulations have been shown to modulate the behavioral effects of cocaine (Walsh and Cunningham, 1997). Acutely abstinent cocaine abusers exhibit higher levels of midbrain 5-HT transporters relative to controls (Jacobsen et al., 2000), a finding which mirrors the increased density of 5-HT transporters seen in midbrains of rats treated chronically with cocaine (Cunningham et al., 1992). These observations establish a link between cocaine exposure and perturbations in 5-HT function.
Of the 14 characterized 5-HT receptors (Barnes and Sharpe, 1999), 5-HT2A and 5-HT2C receptors (5-HT2ARs and 5-HT2CRs) are localized to the mesoaccumbens DA pathway. In the VTA, a subpopulation of neurons expresses 5-HT2AR protein colocalized with tyrosine hydroxylase, the rate-limiting enzyme for DA (Doherty and Pickel, 2000). In contrast, 5-HT2CR mRNA is coexpressed in GABA neurons of the ventral mesencephalon, suggesting an indirect influence of this receptor on DA function (Eberle-Wang et al., 1997). Moderate levels of both 5-HT2ARs (Cornea-Hebert et al., 1999) and 5-HT2CRs (Clemett et al., 2000) are also found in neurons of the NAc. A sizeable amount of literature has accrued to support a functional role for 5-HT2ARs and 5-HT2CRs in the control of brain DA neurotransmission (see Discussion) (Di Matteo et al., 2001). Of particular note to the expression of cocaine-induced hyperactivity, the selective 5-HT2AR antagonist M100907 attenuated cocaine-induced hypermotility (O'Neill et al., 1999; McMahon and Cunningham, 2001), whereas the 5-HT2B/2CR antagonist SB 206553 dose-dependently inhibited or enhanced this hypermotility (McCreary and Cunningham, 1999). However, inconclusive studies with nonselective 5-HT2R antagonists (Meert and Janssen, 1992; Callahan and Cunningham, 1995) underscore the complexity of action for 5-HT receptors in the control of psychostimulant behaviors.
The present study used intracranial microinjection techniques and selective antagonists to examine the locus of action for 5-HT2ARs and 5-HT2CRs in regulating the mesoaccumbens pathway. Spontaneous and cocaine-stimulated activity were measured after microinjection of the selective 5-HT2AR antagonist M100907 or the selective 5-HT2CR antagonist RS 102221 into the VTA or NAc shell. M100907 was chosen for these studies because of its ∼100-fold greater affinity for 5-HT2ARs versus 5-HT2CRs (Kehne et al., 1996) and its efficacy in reversing cocaine-induced hyperactivity (O'Neill et al., 1999; McMahon and Cunningham, 2001). RS 102221 was chosen for its ∼30-fold greater affinity for 5-HT2CRs versus 5-HT2ARs (Bonhaus et al., 1997). The NAc shell was selected because a greater degree of 5-HT innervation (Brown and Molliver, 2000), and higher levels of 5-HT2ARs (Compan et al., 1998) and 5-HT2CRs (Clemett et al., 2000) are observed in the shell versus the core; the NAc shell has also been shown to be more sensitive to cocaine compared with the core (Pontieri et al., 1995).
MATERIALS AND METHODS
Animals. Male Sprague Dawley rats (n= 60; Harlan, Houston, TX) weighing 250–300 gm at the beginning of the experiment were used. The rats were housed three per cage in standard plastic rodent cages in a colony room maintained at 21 ± 2°C and at 40–50% humidity under a 12 hr light/dark cycle (lights on at 7:00 A.M.). Rats surgically fitted with indwelling bilateral guide cannulas were housed individually. Each rat was provided with tap water and rodent chow ad libitum except during experimental sessions. All experiments were conducted during the light phase of the light/dark cycle (between 9:00 A.M. and 2:00 P.M.). All experiments were performed in accordance with the National Institutes of HealthGuide for the Care and Use of Laboratory Animals.
Guide cannula surgery. Rats underwent surgical implantation of 22 gauge stainless steel bilateral guide cannulas (Small Parts Inc., Miami Lakes, FL) aimed 2 mm above the VTA (n = 32) or the NAc shell (n = 28). Each rat was anesthetized using an intramuscular injection of 43 mg/kg ketamine, 8.6 mg/kg xylazine, and 1.5 mg/kg acepromazine in physiological saline (0.9% NaCl). With the upper incisor bar of a Kopf stereotaxic instrument (David Kopf Instruments, Tujunga, CA) positioned at −3.8 mm below the interaural line and using the intersection of the bregma and the longitudinal sutures as the origin, the ventral surfaces of the bilateral guide cannulas were positioned 2 mm above the VTA [at a ±6° angle from the midsagittal plane; anteroposterior (AP), −6.0 mm; mediolateral (ML), ±1.3 mm; and dorsoventral (DV), −7 mm below the surface of the skull] or NAc shell (AP, +1.6 mm; ML, ±0.5 mm; and DV, −6 mm) (Paxinos and Watson, 1998). The guide cannulas were fastened to the skull with stainless steel screws (Small Parts) and cranioplastic cement (Plastics One Inc., Roanoke, VA) and were fitted with 28 gauge stainless steel bilateral obturators (Small Parts). Rats received a single injection of 300,000 U of sodium ampicillin intramuscularly after surgery and were allowed a 1 week recovery period during which they were handled and weighed daily.
Apparatus. Locomotor activity was monitored and quantified using an open-field activity system (San Diego Instruments, San Diego, CA). Each clear Plexiglas chamber (40 × 40 × 40 cm) was housed within a sound-attenuating enclosure and was surrounded with a 4 × 4 photobeam matrix located 4 cm from the floor surface. Interruptions of the photobeams resulted in counts of activity in the peripheral and central fields of the chamber. Activity recorded in the inner 16 × 16 cm of the open field was counted as central activity, whereas the field bounded by the outer 16 cm band registered peripheral activity. Separate counts of peripheral and central activity were made by the control software (Photobeam Activity Software; San Diego Instruments) and stored for subsequent statistical evaluation. Video cameras positioned above the chambers permitted continuous observation of behavior without disruption.
Intracranial microinjections. Surgically implanted rats were maintained in the colony room for a minimum of 1 week before behavioral testing for acclimation to daily handling procedures. In addition, all rats were habituated to the brief confinement associated with the intracranial microinjection technique by removing the 28 gauge internal obturators, gently restraining the rat for ∼3 min, and replacing the obturators. All rats were habituated to the testing environment for the 2 d (2 hr/daily) immediately preceding the start of the experiment. The animals were assigned to one of four groups according to antagonist treatment (M100907 or RS 102221) and brain locus (NAc shell or VTA). The groups received saline (0.2 μl/side) or M100907 (0.1 or 0.3 μg/side) into the NAc shell (n = 16) or VTA (n = 16) and saline (0.2 μl/side) or RS 102221 (0.05, 0.15, or 0.5 μg/side) into the NAc shell (n = 12) or VTA (n = 16). For each microinjection, the 28 gauge bilateral obturators were removed and two 33 gauge stainless steel bilateral internal cannulas (Small Parts) were positioned so as to extend 2 mm ventral to the bilateral guide cannula tips. The bilateral internal cannulas were attached to two 5 μl syringes (Hamilton Co., Reno, NV) via PE-50 tubing (Clay Adams, Parsippany, NJ). A microsyringe drive (Baby Bee; Bioanalytical Systems, Inc., West Lafayette, IN) driven by a programmable controller (Bee Hive Controller; Bioanalytical Systems) delivered a volume of 0.2 μl/side at a rate of 0.1 μl/min. After each microinjection, the bilateral internal cannulas were left in place for 1 min and the obturators were then replaced. Each microinjection was followed immediately by an injection of either saline (1 ml/kg, i.p.) or cocaine (10 mg/kg, i.p.). Horizontal locomotor activity counts were recorded and divided into 5 min bins for a total of 1 hr. Test sessions were conducted every 3 d and the order of microinjections for saline or the highest dose of antagonist was based on a Latin square design. Subsequent injections of antagonists were administered in descending order for a total of six (M100907) or eight (RS 102221) tests. Systemic cocaine injections were given every other test and only once per week.
Histology. At the end of the experiment, rats were overdosed with chloral hydrate (800 mg/kg, i.p.); brains were removed and stored in a 20% sucrose/10% formalin solution for at least 3 d before sectioning. Brain sections (50 μm) were mounted onto gelatin-coated glass slides. The brain sections were defatted, stained with cresyl violet, cleared with xylene, and coverslipped. The cannula placements were verified according to the atlas plates of Paxinos and Watson (1998) using a light microscope.
Data analysis. Peripheral and central activity counts were combined into a single measure of horizontal activity counts and were totaled across the 60 min session for each animal. Analyses were conducted with a two-way ANOVA for repeated measures for the factors of pretreatment (0, 0.1, or 0.3 μg/side M100907 or 0, 0.05, 0.15, or 0.5 μg/side RS 102221), treatment (0 or 10 mg/kg cocaine, i.p.), and pretreatment × treatment interaction (SAS for Windows, version 6.12; SAS Institute, Cary, NC). The Student–Newman–Keuls procedure was used to analyze preplanned, pairwise comparisons; all comparisons were conducted with an experimentwise α equal to 0.05. To assess whether the dose–effect nature of observed effects was dependent on the order of test in this repeated-measures design, a one-way ANOVA was conducted for the factor of order of test.
Drugs. Drugs were dissolved in sterile 0.9% NaCl. Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was injected intraperitoneally in a volume of 1 ml/kg.R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol (M100907) (Hoechst Marion Roussel, Cincinnati, OH) or 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl)]-1,3,8-triazaspiro[4.5]decane-2,4-dione hydrochloride (RS 102221) (Tocris, Ballwin, MO) were injected locally in a volume of 0.2 μl/side. The solutions of M100907 and RS 102221 were adjusted to a pH of 6–7. The control solutions adjusted to a pH of 6–7 did not affect basal locomotor activity during microinjection (data not shown).
RESULTS
Histology
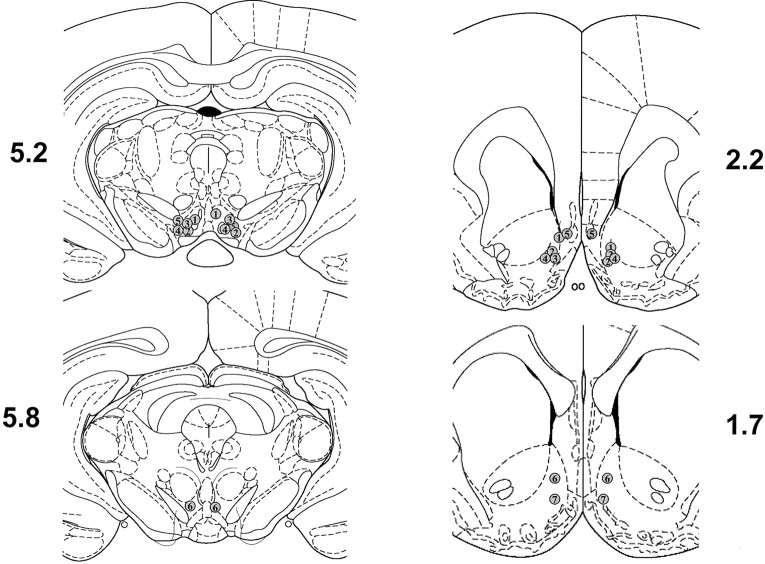
For each animal included in the analyses below, the injection cannulas projected bilaterally past the outer guide cannulas into the VTA or NAc. Figure 1 illustrates the cannula tip locations for the experiments in which M100907 was infused into the VTA (Fig. 1, left panels) and RS 102221 was infused into the NAc (Fig. 1, right panels). Inspection of brain tissue revealed slight evidence of gliosis at the site of injection, although surrounding tissue was generally intact.
Fig. 1.
Histological verification of infusion sites. The location of infusions is shown for all rats that received intra-VTA infusions of M100907 (left panels) and intra-NAc infusions of RS 102221 (right panels). Plates are taken fromPaxinos and Watson (1997), and the numbers beside each plate correspond to millimeters from bregma.
Intra-VTA or NAc shell microinjection of saline
Microinjection of saline into the VTA or NAc shell followed by systemic injection of saline resulted in levels of activity (∼500 counts/60 min) (Figs. 2-5) similar to those reported after systemic saline injection tested alone under identical conditions (McCreary and Cunningham, 1999; McMahon and Cunningham, 1999, 2001). Microinjection of saline into the VTA or NAc shell followed by systemic injection of cocaine (10 mg/kg, i.p.) resulted in levels of hyperactivity (∼2000 counts/60 min) that were significantly higher than saline–saline control values (p < 0.05) (Figs. 2-5) and similar to those reported after systemic cocaine injection in rats not implanted with guide cannulas tested under identical conditions (McCreary and Cunningham, 1999; McMahon and Cunningham, 1999, 2001).
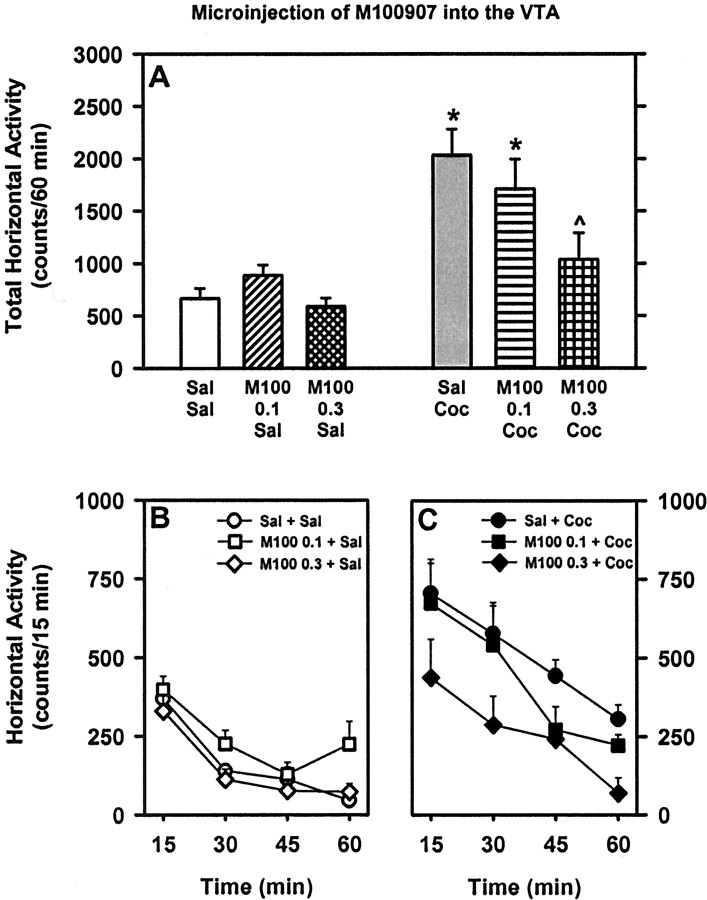
Fig. 2.
Basal and cocaine-stimulated locomotor activity after microinjection of the 5-HT2AR antagonist M100907 into the VTA. A, Mean (±SEM) total horizontal activity (counts per 60 min) after intra-VTA microinjection of saline (Sal) or M100907 (M100) (0.1 or 0.3 μg/side) followed by intraperitoneal injection of saline or cocaine (Coc) (10 mg/kg). *p < 0.05 versus Sal + Sal; ∧p < 0.05 versus Sal + Coc (Student–Newman–Keuls test). The time course of horizontal activity plotted in 15 min bins across the 60 min test session is depicted for basal (B) and Coc-stimulated (C) activity.
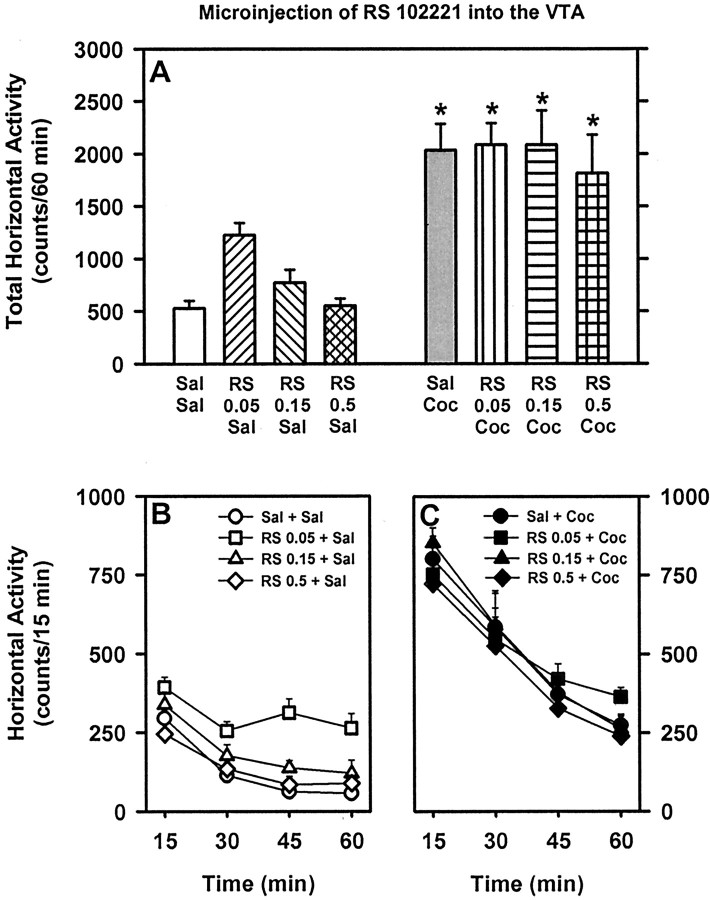
Fig. 3.
Basal and cocaine-stimulated locomotor activity after microinjection of the 5-HT2CR antagonist RS 102221 into the VTA. See legend to Figure 2 for explanation of this figure.
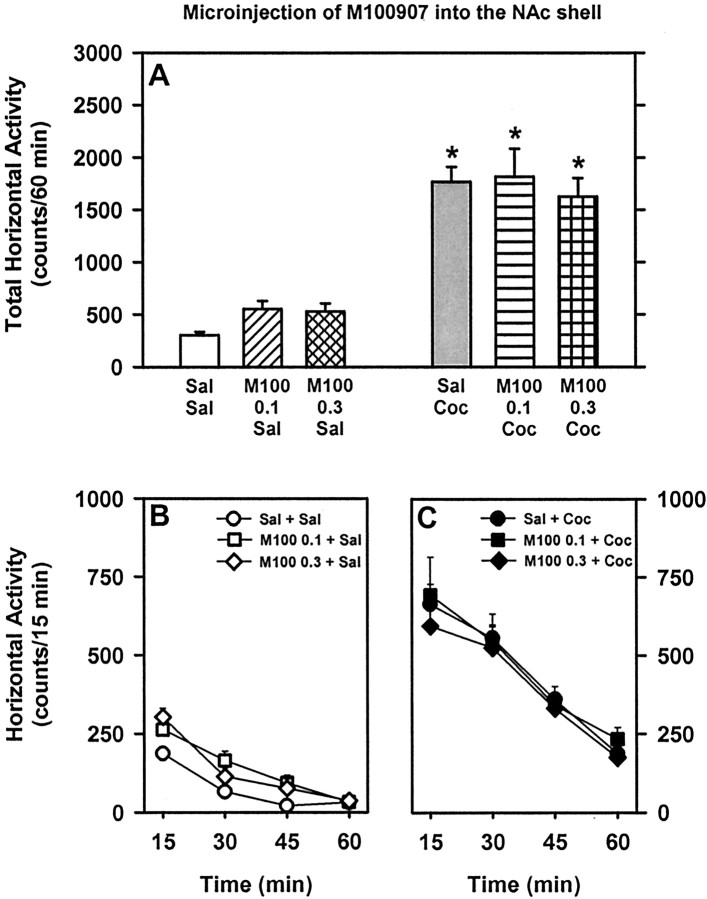
Fig. 4.
Basal and cocaine-stimulated locomotor activity after microinjection of the 5-HT2AR antagonist M100907 into the NAc shell. See legend to Figure 2 for explanation of this figure.
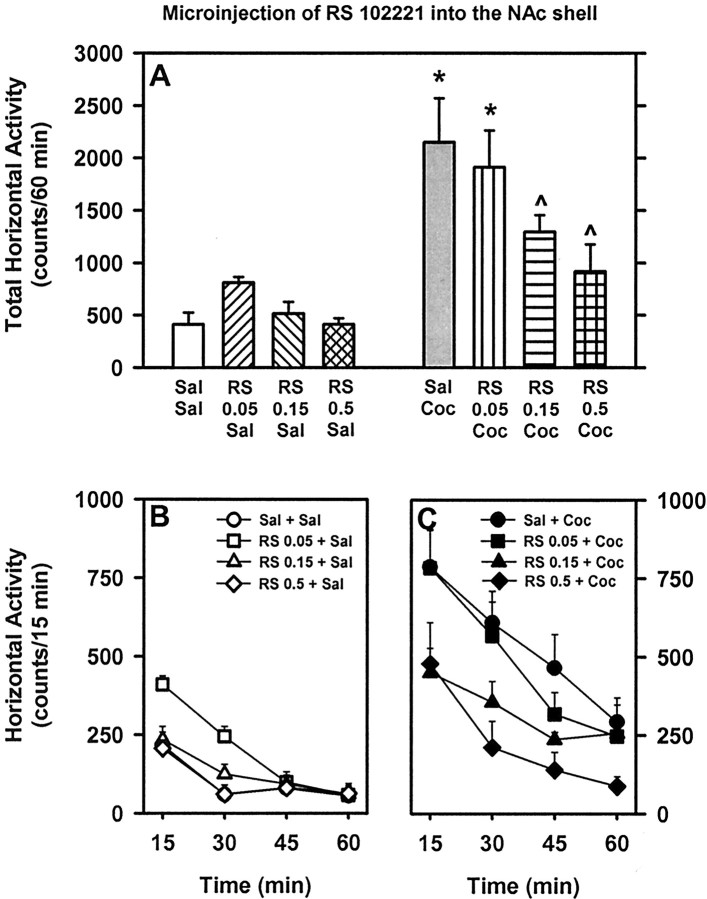
Fig. 5.
Basal and cocaine-stimulated locomotor activity after microinjection of the 5-HT2CR antagonist RS 102221 into the NAc shell. See legend to Figure 2 for explanation of this figure.
Intra-VTA microinjection of the 5-HT2AR antagonist M100907
To test the hypothesis that antagonism of 5-HT2ARs in the VTA would alter spontaneous or cocaine-stimulated horizontal activity, 16 rats were implanted with bilateral guide cannulas and received a microinjection of saline or M100907 (0.1 or 0.3 μg/side) followed by systemic injection of saline or cocaine (10 mg/kg). Of these, six rats exhibited cannula placements bilaterally positioned in the VTA at the level of the paranigral–parabrachial nuclei (Fig. 1, left panels). For these rats, a main effect of pretreatment (F(2,10) = 9.70; p < 0.01), treatment (F(1,5) = 18.39;p < 0.01), and a pretreatment × treatment interaction (F(2,10) = 7.55;p < 0.01) was observed for total horizontal activity summed across the 60 min session. Intra-VTA pretreatment with M100907 at a dose of 0.3 μg/side significantly attenuated horizontal activity stimulated by cocaine (p < 0.05) (Fig. 2) to levels that were not significantly different from saline–saline control levels (p > 0.05). In contrast, intra-VTA pretreatment with M100907 (0.1 or 0.3 μg/side) did not alter spontaneous horizontal activity (p > 0.05) (Fig. 2). The dose–effect nature of observed attenuation of cocaine-induced hyperactivity by intra-VTA M100907 was not dependent on the order of test as indicated by the one-way ANOVA conducted for the factor of order of test (F(5,30) = 1.35; p > 0.05).
In this experiment, five rats exhibited cannula placements at levels dorsal and dorsolateral to the VTA in the prerubral field and medial lemniscus, respectively, and these rats were assigned to an anatomical control group. For these rats, a significant main effect of treatment (F(1,4) = 20.48;p < 0.05) was observed for total horizontal activity summed across the 60 min session (data not shown). There was no main effect of pretreatment (F(2,8) = 3.19;p > 0.05) or a pretreatment × treatment interaction (F(2,8) = 0.57;p > 0.05).
Intra-VTA microinjection of the 5-HT2CR antagonist RS 102221
Sixteen rats received a microinjection of saline or the 5-HT2CR antagonist RS 102221 (0.05, 0.15, or 0.5 μg/side) followed by systemic injection of saline or cocaine (10 mg/kg). Of these, nine rats exhibited cannula placements bilaterally positioned in the VTA at the level of the paranigral–parabrachial nuclei. For these rats, a main effect of treatment (F(1,8) = 54.57; p < 0.001) was observed for total horizontal activity summed across the 60 min session (Fig. 3). There was no significant effect of pretreatment (F(3,24) = 2.27; p > 0.05) or a pretreatment × treatment interaction (F(3,24) = 1.05; p > 0.05).
In this experiment, five rats exhibited cannula placements at levels dorsal and dorsolateral to the VTA in the prerubral field and medial lemniscus, respectively, and these rats were assigned to an anatomical control group. For these rats, a main effect of treatment (F(1,3) = 15.89; p < 0.05) was observed for total horizontal activity summed across the 60 min session (data not shown). There was no main effect of pretreatment (F(3,9) = 1.98; p > 0.05) or a pretreatment × treatment interaction (F(3,9) = 0.81; p > 0.05).
Intra-NAc shell microinjection of the 5-HT2AR antagonist M100907
Sixteen rats received a microinjection of saline or the 5-HT2AR antagonist M100907 (0.1 or 0.3 μg/side) followed by systemic injection of saline or cocaine (10 mg/kg). Of these, nine rats exhibited cannula placements bilaterally positioned in the ventromedial portion of the NAc shell at +1.7 to +2.2 mm posterior to bregma. For these rats, a main effect of treatment (F(1,8) = 76.71; p < 0.001) was observed for total horizontal activity summed across the 60 min session (Fig. 4). There was no main effect of pretreatment (F(2,16) = 0.59; p > 0.05) or a pretreatment × treatment interaction (F(2,16) = 0.86;p > 0.05). Similarly, microinfusion of M100907 (0.1 or 0.3 μg/side) ventromedial to the NAc shell (n = 4) or into the NAc core (n = 1) did not alter basal or cocaine-stimulated activity levels (data not shown)
Intra-NAc shell microinjection of the 5-HT2CR antagonist RS 102221
To test the hypothesis that antagonism of 5-HT2CRs in the NAc shell would alter basal or cocaine-stimulated horizontal activity, 12 rats were implanted with bilateral guide cannulas and received a microinjection of saline or RS 102221 (0.05, 0.15, or 0.5 μg/side) followed by systemic injection of saline or cocaine (10 mg/kg). Of these, seven rats exhibited cannula placements bilaterally positioned in the ventromedial portion of the NAc shell at +1.7 to +2.2 mm posterior to bregma (Fig.1B). For these rats, a main effect of pretreatment (F(3,18) = 7.02; p < 0.01), treatment (F(1,6) = 62.44;p < 0.001), and a pretreatment × treatment interaction (F(3,18) = 3.55;p < 0.05) were observed for total horizontal activity summed across the 60 min session. Intra-NAc shell pretreatment with RS 102221 at doses of 0.15 or 0.5 μg/side significantly attenuated horizontal activity stimulated by cocaine (p < 0.05) (Fig. 5) to levels that were not significantly different from saline–saline control levels (p > 0.05). In contrast, intra-NAc shell pretreatment with RS 102221 (0.05, 0.15, or 0.5 μg/side) did not alter spontaneous horizontal activity (p > 0.05) (Fig. 5). In this experiment, two rats exhibited cannula placements in the core subregion, and there was no effect of microinjection of RS 102221 (0.05, 0.15, or 0.5 μg/side) on basal or cocaine-stimulated activity in these animals (data not shown). The dose–effect nature of observed attenuation of cocaine-induced hyperactivity by intra-NAc RS 102221 was not dependent on the order of test as indicated by the one-way ANOVA conducted for the factor of order of test (F(7,48) = 1.24;p > 0.05).
DISCUSSION
An effective antagonism of cocaine-induced hyperactivity was observed after microinjection of the selective 5-HT2AR antagonist M100907 (0.3 μg/side) into the VTA or the selective 5-HT2CR antagonist RS 102221 (0.15 or 0.5 μg/side) into the NAc shell. In contrast, cocaine-evoked hypermotility was unchanged after microinjection of RS 102221 into the VTA or M100907 into the NAc shell, and spontaneous locomotor activity was not altered by microinjection of M100907 or RS 102221 into either region. Based on previous findings that systemic administration of 5-HT2AR and 5-HT2B/2CR antagonists can influence some of the behavioral effects of cocaine (see the introductory remarks), the present study is the first to identify brain nuclei in which 5-HT2R subtypes function to control behavior evoked by cocaine.
The dose-related reduction of cocaine-stimulated activity but not spontaneous activity that was observed after microinjection of M100907 into the VTA, but not the NAc shell, suggests that 5-HT2ARs may exert control over the behavioral effects of cocaine through direct or indirect actions on DA cell bodies which serve as the origin of the mesoaccumbens pathway. Systemic administration of cocaine has been reported to increase 5-HT and DA efflux in the VTA in vivo (Chen and Reith, 1994), and 5-HT has been shown to depolarize VTA DA neuronsin vitro (Pessia et al., 1994). Therefore, cocaine may act through indirect stimulation of 5-HT2ARs on DA somata (Doherty and Pickel, 2000) to facilitate DA outflow from the VTA. Depolarization of VTA DA neurons in vitro has been observed after application of the nonselective 5-HT2R agonist 1-(2,5-dimethoxy-4-iodo)-2-aminopropane (DOI), and this effect is antagonized by the 5-HT2R antagonist ketanserin (Pessia et al., 1994). Because 5-HT2CR stimulation is thought to result in a depression of VTA cell firing (Prisco et al., 1994; Di Matteo et al., 2000), 5-HT2AR-dependent depolarization of DA somata may facilitate behavior elicited by stimulation of the mesoaccumbens DA pathway. Alternatively, 5-HT2ARs have been suggested to regulate the stimulated DA neurotransmission associated with increased DA synthesis, such as that induced by reverse transport of DA (e.g., amphetamine) (Schmidt et al., 1992) or antagonism of D2-like synthesis-modulating autoreceptors (Lucas and Spampinato, 2000). However, cocaine inhibits DA reuptake (Koe, 1976) and decreases DA synthesis, presumably via indirect stimulation of D2-like autoreceptors (Galloway, 1990), and it seems unlikely that blockade of a facilitatory role of 5-HT2ARs on DA synthesis accounts for the suppressive effect of M100907 on cocaine-induced behavior. Thus, we propose that mechanisms in addition to control over DA synthesis ascribed to 5-HT2ARs, such as direct excitation of DA somata, contribute to 5-HT2AR-mediated control of DA neurotransmission.
Microinjection of RS 102221 into the NAc shell, but not the VTA, antagonized the hypermotility evoked by cocaine in a dose-related manner, suggesting that functional 5-HT2CR control of mesoaccumbens pathways is distinctly regional. Systemic administration of cocaine has been shown to increase 5-HT and DA efflux in the NAc in vivo (Reith et al., 1997), presumably through reuptake inhibition via blockade of 5-HT and DA transporters, respectively. In addition to DA reuptake inhibition, the present study indicates that cocaine may also enhance DA efflux in the NAc shell indirectly through stimulation of 5-HT2CRs. Such a contention has received tentative support from neurochemical studies of DA efflux. For example, perfusion of DOI through a microdialysis probe increased DA efflux in the NAc, an effect that was reversed by co-perfusion with a nonselective 5-HT2R antagonist (Yan, 2000) or the selective 5-HT2B/2CR antagonist SB 206553 (Lucas and Spampinato, 2000). The mechanism whereby 5-HT2CRs in the NAc shell putatively regulate DA efflux likely involves a GABA–DA interface based on the distribution, localization, and morphology of neurons in the NAc containing 5-HT2cR mRNA (Eberle-Wang et al., 1997). However, the precise nature of this mechanism will require careful analyses of the synaptic connections in the NAc shell formed by these 5-HT2CR mRNA-positive neurons and DA and/or GABA nerve terminals (Eberle-Wang et al., 1997).
Tonic stimulation of 5-HT2CRs in the NAc shell does not appear to regulate basal locomotor activity and underlying DA function, because spontaneous activity was unaffected after intra-NAc shell microinjection of RS 102221. These observations are in contrast to other studies that suggest 5-HT2CRs inhibit basal DA neurotransmission (De Deurwaerdere and Spampinato, 1999; Di Matteo et al., 2001) and basal locomotor activity (Curzon and Kennett, 1990). Because most of these previous studies have used systemic administration of 5-HT2CR ligands, the neural locus of inhibitory 5-HT2CR actions is not determinable. The VTA may be a tentative site, because focal application of marginally selective 5-HT2CR agonists has been shown to decrease DA cell firing in this region (Prisco et al., 1994). Accordingly, antagonism of 5-HT2CRs in the VTA might be expected to increase basal locomotor activity. Despite a tendency for intra-VTA RS 102221 to increase spontaneous activity in the present study (Fig. 5), this marginal increase did not achieve statistical significance. However, antagonism of 5-HT2CRs in the VTA or other loci may not figure heavily into the regulation of basal locomotor activity, because systemic administration of the 5-HT2B/2CR antagonist SB 206553 did not alter basal activity (McCreary and Cunningham, 1999). Clarification of whether 5-HT2CR in the VTA inhibits DA neurotransmission and, as a consequence, normally suppresses spontaneous locomotor activity might be provided by future studies that measure locomotor activity after microinjection of 5-HT2CR agonists into the VTA and other sites.
The effective antagonism of cocaine-induced hyperactivity by intra-VTA M100907 occurred in the absence of changes in basal activity, a finding that has been consistently noted with systemic administration of M100907 (Kehne et al., 1996). This may reflect the relative absence of a tonic regulatory influence of 5-HT2ARs on DA function, a hypothesis supported by other neuropharmacological investigations. For instance, systemic administration of 5-HT2AR antagonists did not alter basal DA cell firing (Sorensen et al., 1993) or striatal (Schmidt et al., 1992) and accumbal (De Deurwaerdere and Spampinato, 1999) DA efflux. Similarly, striatal DA efflux was not evoked by co-perfusion of the 5-HT2A/2B/2CR agonist DOI and the 5-HT2B/2CR antagonist SB 206553, supporting the hypothesis that stimulation of 5-HT2ARs alone is not sufficient to increase DA efflux (Lucas and Spampinato, 2000).
Both M100907 and RS102221 are among the most selective antagonists at the 5-HT2AR and 5-HT2CR, respectively. However, both drugs do exhibit affinity for other receptors. For instance, M100907 possesses a moderate affinity for 5-HT2C, α1-adrenergic, and ς receptors (Ki = 88, 128, and 87 nm, respectively) (Kehne et al., 1996). However, M100907 did not share with the 5-HT2CR antagonist RS 102221 the ability to antagonize cocaine-stimulated activity after intra-NAc shell microinjection, suggesting that blockade of 5-HT2CRs does not mediate the observed effects of M100907.
Previous studies have shown that stimulation of α1-adrenergic receptors in the VTA can modulate DA cell firing in vitro (Grenhoff et al., 1995) and in vivo (Shi et al., 2000). Because the affinity of M100907 for α1-adrenergic receptors is even lower than that for 5-HT2CRs, antagonism of α1-adrenergic receptors in the VTA by M100907 is not likely to account for the reduction of cocaine-induced hyperactivity observed here. However, the possible role for α1-adrenergic receptors in cocaine-evoked alterations in DA neuronal function is worthy of further consideration given that recent findings suggest that α1-adrenergic receptors may provide stimulatory influences on DA neurons (Shi et al., 2000). The possibility that intra-VTA M100907 attenuated cocaine-induced hyperactivity via antagonism of ς receptors should also be entertained given that ς receptor activation in the VTA slightly increased DA cell firingin vivo (Gronier and Debonnel, 1999). However, unlike similar intra-NAc microinfusions of M100907 (present results), intra-NAc microinjection of a ς receptor ligand decreased spontaneous and apomorphine-evoked locomotor activity in the rat (Skuza et al., 1998). RS 102221 possesses a moderate affinity for 5-HT2ARs (Ki = 141 nm) (Bonhaus et al., 1997), with much less affinity for most other receptor subtypes, but because intra-VTA RS 102221, unlike M100907, did not attenuate cocaine-stimulated activity, prominent 5-HT2AR-blocking actions of RS 102221 may be ruled out. Therefore, it appears that in the present study M100907 and RS 102221 antagonized 5-HT2ARs and 5-HT2CRs, respectively, to affect changes in the behavioral effects of cocaine.
In summary, hyperactivity evoked by systemic administration of cocaine was blocked by antagonism of 5-HT2ARs in the VTA or 5-HT2CRs in the NAc shell. These results indicate that the behavioral profile for cocaine is regulated by 5-HT2R subtypes in separate regions of the mesoaccumbens DA pathway. To the extent that specific behaviors induced by cocaine are differentially mediated by 5-HT2R subtypes located in the origin or terminal field of the mesoaccumbens pathway, the consequences of giving a nonselective 5-HT2R antagonist systemically will depend on differential control of these regions by a specific 5-HT2R subtype. Finally, determination of the potential utility of selective manipulations of 5-HT2ARs and 5-HT2CRs in the treatment of cocaine dependence will require careful analysis of whether 5-HT2ARs in the VTA and 5-HT2CRs in the NAc shell modulate other behavioral effects of cocaine in the distinctly regional manner reported here.
Footnotes
This research was supported by the National Institute on Drug Abuse Grants DA05708 and DA06511 to K.A.C. and DA05879 to L.R.M., by a National Science Foundation–North Atlantic Treaty Organization Visiting Scientist Fellowship to M.F., and by the United States–Poland Joint Commission Maria Sklodowska-Curie Fund (M.F., K.A.C.).
L.R.M. and M.F. contributed equally to the research presented in this manuscript.
Correspondence should be addressed to Dr. Kathryn A. Cunningham, Department of Pharmacology and Toxicology, The University of Texas Medical Branch, Galveston, TX 77555-1031. E-mail: cunningham@utmb.edu.
REFERENCES
- 1.Barnes NM, Sharpe T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 3.Brown P, Molliver ME. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan PM, Cunningham KA. Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther. 1995;274:1414–1424. [PubMed] [Google Scholar]
- 5.Callahan PM, De La Garza R, Cunningham KA. Discriminative stimulus properties of cocaine: modification by dopamine D1 receptors in nucleus accumbens. Psychopharmacology. 1994;115:110–114. doi: 10.1007/BF02244759. [DOI] [PubMed] [Google Scholar]
- 6.Chen N-H, Reith MEA. Autoregulation and monoamine interactions in the ventral tegmental area in the absence and presence of cocaine: a microdialysis study in freely moving rats. J Pharmacol Exp Ther. 1994;271:1597–1610. [PubMed] [Google Scholar]
- 7.Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KCF. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 8.Compan V, Segu L, Buhot MC, Daszuta A. Selective increases in serotonin 5-HT1B/1D and 5-HT2A/2C binding sites in adult rat basal ganglia following lesions of serotonergic neurons. Brain Res. 1998;793:103–111. doi: 10.1016/s0006-8993(98)00168-1. [DOI] [PubMed] [Google Scholar]
- 9.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992;11:112–123. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- 11.Curzon G, Kennett GA : m-CPP: a tool for studying behavioural responses associated with 5-HT(1C) receptors. Trends Pharmacol Sci. 1990;11:181–182. doi: 10.1016/0165-6147(90)90109-l. [DOI] [PubMed] [Google Scholar]
- 12.De Deurwaerdere P, Spampinato U. Role of serotonin2A and serotonin2C receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- 13.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2c) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- 15.Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT2C receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- 16.Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- 17.Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet M-F. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384:233–247. [PubMed] [Google Scholar]
- 18.Galloway MP. Regulation of dopamine and serotonin synthesis by acute administration of cocaine. Synapse. 1990;6:63–72. doi: 10.1002/syn.890060108. [DOI] [PubMed] [Google Scholar]
- 19.Grenhoff J, North RA, Johnson SW. α1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 20.Gronier B, Debonnel G. Involvement of ς receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. Eur J Pharmacol. 1999;368:183–196. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB. Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry. 2000;157:1134–1140. doi: 10.1176/appi.ajp.157.7.1134. [DOI] [PubMed] [Google Scholar]
- 22.Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PLM, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- 23.Klein M. Research issues related to development of medications for treatment of cocaine addiction. Ann NY Acad Sci. 1998;844:75–91. [PubMed] [Google Scholar]
- 24.Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- 25.Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- 26.McCreary AC, Cunningham KA. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology. 1999;20:556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 27.McKinzie DL, Rodd ZA, Dagon CT, Murphy JM, McBride WJ. Cocaine is self-administered into the shell region of the nucleus accumbens in Wistar rats. In: McGinty JF, editor. Advancing from the ventral striatum to the extended amygdala: implications for neuropsychiatry and drug abuse. New York Academy of Sciences; New York: 1999. pp. 788–791. [DOI] [PubMed] [Google Scholar]
- 28.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine4 receptors attenuates hyperactivity induced by cocaine: putative role for 5-hydroxytryptamine4 receptors in the nucleus accumbens shell. J Pharmacol Exp Ther. 1999;291:300–307. [PubMed] [Google Scholar]
- 29.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine2A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- 30.Meert TF, Janssen PAJ. Ritanserin, a new therapeutic approach for drug abuse. Part 2: effects on cocaine. Drug Dev Res. 1992;25:39–53. [Google Scholar]
- 31.O'Neill MF, Heron-Maxwell CL, Shaw G. 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol Biochem Behav. 1999;63:237–243. doi: 10.1016/s0091-3057(98)00240-8. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- 33.Pessia M, Zhi-Gen J, North RA, Johnson SW. Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res. 1994;654:324–330. doi: 10.1016/0006-8993(94)90495-2. [DOI] [PubMed] [Google Scholar]
- 34.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prisco S, Pagannone S, Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J Pharmacol Exp Ther. 1994;271:83–90. [PubMed] [Google Scholar]
- 36.Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL. 5-HT2 receptors exert a state-dependent regulation of dopaminergic function: studies with MDL 100907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur J Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- 38.Shi W-X, Pun C-L, Zhang X-X, Jones MD, Bunney BS. Dual effects of d-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skuza G, Kolasiewicz W, Dziedzicka-Wasylewska M, Margas W. Effect of local intracerebral administration of EMD 57445, a selective ς receptor ligand, on the locomotor activity of the rat. Pol J Pharmacol. 1998;50:399–406. [PubMed] [Google Scholar]
- 40.Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological, and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- 41.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing, and subjective effects of cocaine. Psychopharmacology. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 42.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 43.Yan Q-S. Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain Res Bull. 2000;51:75–81. doi: 10.1016/s0361-9230(99)00208-7. [DOI] [PubMed] [Google Scholar]