Abstract
We examine the role of synaptic activity in the development of identified Drosophila embryonic motorneurons. Synaptic activity was blocked by both pan-neuronal expression of tetanus toxin light chain (TeTxLC) and by reduction of acetylcholine (ACh) using a temperature-sensitive allele of choline acetyltransferase (Chats2). In the absence of synaptic activity, aCC and RP2 motorneurons develop with an apparently normal morphology and retain their capacity to form synapses. However, blockade of synaptic transmission results in significant changes in the electrical phenotype of these neurons. Specifically, increases are seen in both voltage-gated inward Na+ and voltage-gated outward K+ currents. Voltage-gated Ca2+currents do not change. The changes in conductances appear to promote neuron excitability. In the absence of synaptic activity, the number of action potentials fired by a depolarizing ramp (−60 to +60 mV) is increased and, in addition, the amplitude of the initial action potential fired is also significantly larger. Silencing synaptic input to just aCC, without affecting inputs to other neurons, demonstrates that the capability to respond to changing levels of synaptic excitation is intrinsic to these neurons. The alteration to electrical properties are not permanent, being reversed by restoration of normal synaptic function. Whereas our data suggest that synaptic activity makes little or no contribution to the initial formation of embryonic neural circuits, the electrical development of neurons that constitute these circuits seems to depend on a process that requires synaptic activity.
Keywords: aCC, activity, connectivity, Drosophila, neurogenesis, synaptic activity, synaptogenesis
The extent to which the properties of neurons are regulated by neural activity and by synaptic communication with neighboring cells is a central issue for our understanding of the nervous system and the way it develops. Although the initial growth and guidance of axons are thought to be largely independent of activity, there is a great deal of evidence that the refinement and stabilization of connections are subject to activity-dependent control (Goodman and Shatz, 1993; Katz and Shatz, 1996; Tessier-Lavigne and Goodman, 1996). Mechanisms of this kind lead to a refinement of the retinotectal projection, allow for the proper alignment of central representations of different sensory modalities and, on the output side, underlie the competitive sorting and selective elimination of motor terminals on muscle fibers (Hall and Sanes, 1993;Goodman and Shatz, 1993; Katz and Shatz, 1996; Colman et al., 1997).
Much of the evidence for activity-based tuning of connectivity comes from sensory systems and leads to the general conclusion that, in the absence of activity, the developmental machinery generates a roughly appropriate pattern of connections, but that subsequent refinement and pruning of inappropriate connections fail to occur. By contrast there have been very few comparable experiments with developing motor circuitry, although it is clear that activity is pervasively present in maturing embryonic motor systems as it is in some developing sensory projections (Moody, 1998; Bate, 1999). Those experiments that have been done suggest that, in the absence of activity, fundamental motor circuitry underlying, for example, undulatory swimming in amphibians develops quite normally and that “many early motor patterns are hardwired into the developing anatomy” (Haverkamp, 1986; Haverkamp and Oppenheim, 1986, Sanes et al., 2000).
Whereas appropriate connectivity is essential to function, it is the electrical properties of nerve cells that dictate their signaling characteristics within the network. We know little about how these characteristics are determined, despite the fact that emerging function depends both on developing connections and on the progressive acquisition of electrical properties by immature nerve cells. In theDrosophila embryo we have shown that the electrical phenotype of central neurons develops as a result of the sequential appearance of ionic conductances during the later phases of embryogenesis when movement begins (Baines and Bate, 1998). InXenopus, well defined changes in motorneuron K+ conductances accompany the transition from an embryonic to a larval pattern of swimming (Sun and Dale, 1998). That the orderly acquisition of such conductances is a prerequisite for normal function is shown by the phenotype of mutant, touch-insensitive, zebra fish (Ribera and Nüsslein-Volhard, 1998). In such mutants Rohon Beard cells specifically fail to undergo a developmentally regulated increase in Na+ conductance that is essential for the normal maturation of the touch response. Is the acquisition of such electrical phenotypes an autonomous property of individual neurons or does it depend on communication with neighboring cells?
In this paper we address the question of whether communication between synaptic partners is essential to the structural and physiological differentiation of identified motorneurons in the embryonic CNS of Drosophila. We show that the morphology of these neurons and the synaptic connections that they receive are unaffected by the absence of synaptic transmission. The electrical properties of the same cells, however, are abnormal. Our results suggest that synaptic communication between neurons is an essential regulator of electrical phenotype and that synaptic activity is, therefore, required for the normal functional development of the network.
MATERIALS AND METHODS
Fly stocks
Flies were fed on apple juice agar supplemented with yeast. Wild type was Oregon-R. Scabrous GAL4 was used to express tetanus toxin light chain (TeTxLC) throughout the entire CNS (Mlodzik et al., 1990). RRC-GAL4 was used to selectively express UAS-driven transgenes in aCC (Fujioka et al., 1999). This construct drives GAL4 expression in only three neurons in the embryonic CNS; strongly in aCC, more variably in RP2 and very weakly in pCC (Baines et al., 1999). Flies carrying a ts allele of choline acetyltransferase(Chats2) were used to reduce ACh (Salvaterra and McCaman, 1985). These flies were maintained at 18°C. Expression of TeTxLC (TNT-G) was confirmed using antibodies (Sweeney et al., 1995). pUAS-EGFP-Kir2.1 was constructed from an Nhe/Hpal fragment containing EGFP-Kir2.1(Johns et al., 1999), which was subcloned into an EcoRV/Xbal site of pMartini (A gift from S. Findley. University of Washington) to generate pMEGFPKir2.1. A NotI fragment containing EGFPKir2.1 was then subcloned into theNotI site of pUAST (Brand and Perrimon, 1993) to generate pUAST-EGFPKir2.1. This construct was injected (Spradling, 1986) in conjunction with helper P element phs-Δ-2–3 (Misra and Rio, 1990) in to y w embryos. A number of independent transformants were generated one of which, a homozygous viable second chromosome insert (Kir1), was used in this study.
Embryo dissection
Eggs were dechorionated in commercial bleach, and embryos were removed from their vitelline membrane using a glass micropipette. Embryos were dissected, and central neurons were accessed as described in Baines and Bate (1998). The embryo was viewed using a 63× water immersion lens combined with Nomarski optics (Olympus BX50WI microscope).
DiI labeling and microscopy
Embryos were dissected, fixed in formaldehyde [8% in 75 mm phosphate buffer (PB), pH 7.2, 1 hr], washed in PB and aCC-labeled by applying a droplet of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate (DiI) (Molecular Probes, Eugene, OR) to its neuromuscular junction (NMJ) on muscle DA1. After overnight incubation at 4°C, embryos were examined with epifluorescence, and those preparations in which only aCC was labeled were either prepared for confocal microscopy or electron microscopy.
Confocal microscopy. Embryos were labeled using a monoclonal antibody (mAb) against Fasciclin II (FasII; mAb 1D4, 1:5) to delineate the axon scaffold. All reagents used were diluted in PB containing Tween 20 (0.1%). FasII staining was visualized using a CY-5-conjugated secondary antibody (1:100; Molecular Probes). Preparations were mounted in Vectashield (Vector Laboratories, Burlingame, CA), coverslipped, and viewed using a 60× oil-immersion lens on a Leica (Nussloch, Germany) TCS SP confocal microscope. Z-series were collected at 0.5–1 μm intervals and projected to a single plane for morphometric analysis. The area of arborization was determined using a grid-based system based on that described by Käethner and Stüermer (1992), but using a grid spacing of 0.39 μm2. Maximum spread of arborization was measured as the distance between the two most extreme dendrite tips across either the anteroposterior or mediolateral axes of the neuropil.
Electron microscopy. Embryos were fixed again in formaldehyde (8%, 8 min, 75 mm PB, pH 7.2), washed, and transferred into Tris buffer (0.1 m, pH. 7.5) before photoconversion using 3,3′-diaminobenzidine tetrachloride (Fisher Scientific; 3 mg/ml in Tris buffer). After washing in Tris buffer followed by H2O, embryos were post-fixed in osmium (1% in H2O, 1 hr), stained with aqueous uranyl acetate (2%, 30 min), dehydrated, and embedded in Araldite resin. Embryos were sectioned at 2 μm thickness until labeled profiles were encountered, at which point a series of ultrathin sections (30–50 nm, silver–gray) were taken. Labeled neurons were not serially sectioned in their entirety, but instead were sampled at 2 μm intervals with 20–30 consecutive ultrathin sections taken at each successive level. Sections were stained with lead citrate (5 min) and analyzed on a Philips EM 300.
Electrophysiology
The procedure for whole-cell recordings and composition of salines used are described in Baines and Bate (1998). The only modification was to include charybdotoxin (Tocris Cookson) in the K+ isolation saline to maximize the block to IK(Ca). Only cells with an input resistance >1 GΩ (average, 4.05 ± 0.46 GΩ; n= 100; mean ± SE) were accepted for analysis. Typical cell capacitance (determined by integration of the area under the capacitative transients for the average of 50 steps from −60 to −90 mV) were 2.6 ± 0.06 pF, (n = 50; mean ± SE) and were not compensated for on the patch amplifier. Current traces were sampled at 20 kHz and filtered at 2 kHz. All recordings were made at room temperature (22–24°C). Cells were unequivocally identified by labeling with carboxyfluoroscein (0.25%), which was included in the patch saline (Baines et al., 1999). Muscle recordings were performed as described in Broadie and Bate (1993).
Statistics
Data were compared using the nonparametric Mann–WhitneyU test. Results were deemed significant at p≤ 0.05. All values shown are mean ± SE.
RESULTS
aCC and RP2 receive identical synaptic input
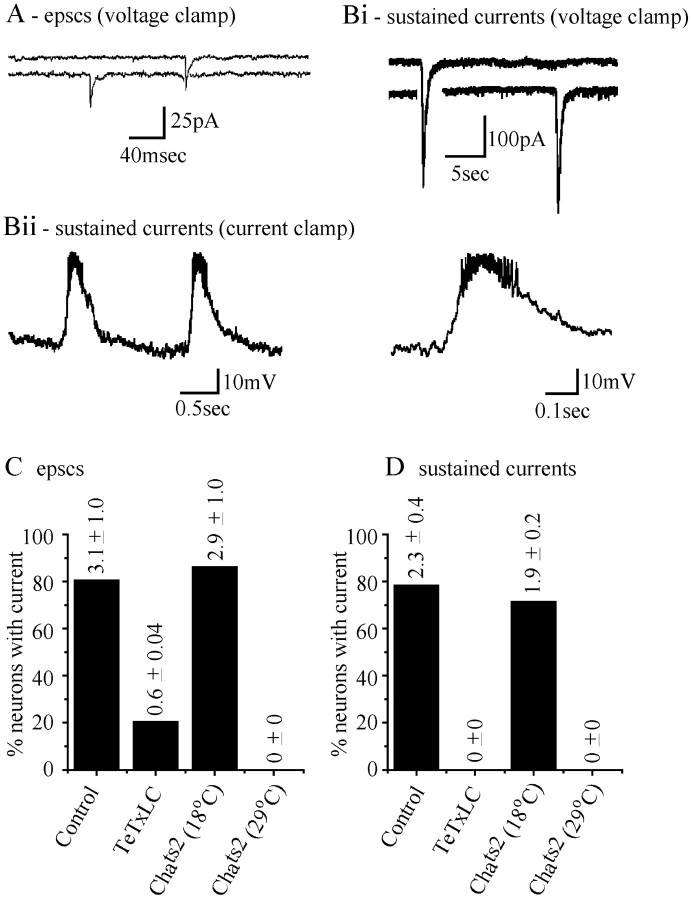
Two motorneurons, aCC and RP2, which innervate dorsal muscles DA1 and DA2, respectively, are situated dorsally in the CNS, where they are accessible to patch electrodes (Baines and Bate, 1998, Baines et al., 1999). Recordings from either aCC or RP2 (voltage-clamped at −60 mV), at 20–21 hr after egg laying (AEL) (hatching occurs at ∼21 hr), reveal two kinds of inward currents that are identical in both neurons. The first type consists of discrete currents lasting between 20 and 40 msec with an average amplitude of 18 ± 1 pA (n = 90; mean ± SE; Fig.1A). These currents, which were present in the majority of neurons recorded (22 of 27 neurons; Fig. 1C) have previously been shown to fulfill the criteria expected of EPSCs (Baines and Bate, 1998; Baines et al., 1999). In wild-type aCC/RP2, EPSCs occur at a frequency of ∼3.1 ± 1 per minute (no difference between aCC and RP2). The second type of synaptic input consists of longer duration (0.5–2 sec), larger amplitude (50–300 pA), excitatory currents, at a frequency of ∼2.3 ± 0.4 per minute (Fig. 1B,D). These events, which we call sustained currents, occurred in 21 of 27 neurons and often initiate the firing of action potentials by aCC/RP2 (Baines et al., 1999). This is more clearly seen when recording in current-clamp mode (Fig. 1Bii). The appearance of sustained currents in aCC/RP2 coincides with the onset of peristaltic motor activity in the embryo, and the development of this synaptic drive probably underlies the emergence of coordinated locomotion.
Fig. 1.
Synaptic input to aCC and RP2 is identical. Passive recordings from wild-type aCC and RP2 neurons voltage clamped at −60 mV show the presence of two types of synaptic input.A, Discrete inward currents of small amplitude (18 pA average) and short duration (20–40 msec) are visible in the majority of recordings. These events have previously been shown to fulfill the criteria expected of EPSCs (Baines and Bate, 1998). Bi, Recordings from aCC/RP2 also reveal the presence of relatively slow- and large-amplitude sustained inward currents. Bii, In current-clamp mode, sustained currents produce large depolarizations that trigger the firing of action potentials in aCC/RP2. Action potentials are clearly visible at a slower time base (right panel). C and D show the proportion of aCC/RP2 neurons that exhibit EPSCs (C) and sustained inward currents (D) in the various genetic backgrounds used in this study; control (including WT, scabrous GAL4, and UAS-TeTxLC), TeTxLC expression, and Chats2 at either 18°C (permissive temperature) or 29°C (restrictive temperature). Bars represent the percentage of neurons in which these respective currents were observed, whereas values given represent the average frequency (per minute) observed in each case (n = 27, 10, 7, and 7 neurons, respectively). Frequency of EPSCs and sustained currents in TeTxLC andChats2 (29°C) are significantly different from control and Chats2(18°C), respectively (p ≤ 0.01).
Suppression of synaptic activity
To assess the possible function of synaptic transmission in the development of embryonic neurons, we suppressed synaptic activity throughout the CNS and determined the consequences in aCC/RP2. We used two different methods. First, we used a pan-neuronal GAL4 driver,scabrous GAL4 (Mlodzik et al., 1990) to target expression of TeTxLC throughout the nervous system. TeTxLC, which enzymatically cleaves the synaptic vesicle-associated proteinn-Synaptobrevin, results in embryonic paralysis by blocking the evoked release of neurotransmitter and, in addition, reduces spontaneous release (i.e., minis) by 50–75% (Sweeney et al., 1995;Deitcher et al., 1998). Second, we usedChats2, a temperature-sensitive allele of the choline acetyltransferase gene, which encodes the synthetic enzyme for ACh (Greenspan, 1980). ACh, which is the predominant excitatory neurotransmitter in the insect CNS (Burrows, 1996), is significantly reduced inChats2 at temperatures >22°C, [<10% of wild type (WT)], and this results in embryonic paralysis (Greenspan, 1980; Salvaterra and McCaman, 1985). This phenotype is reversed if the embryos are shifted to temperatures <22°C. Blocking synaptic activity by expression of TeTxLC throughout the CNS or by raising Chats2 embryos at 29°C does not produce any obvious structural defects in the embryonic CNS (Chase and Kankel, 1988; Sweeney et al., 1995).
Expression of TeTxLC in all neurons significantly reduces the appearance and frequency of EPSCs and totally abolishes sustained currents in aCC/RP2. In the presence of TeTxLC, only 2 of 10 aCC/RP2 neurons showed EPSCs, and in these two neurons the frequency of EPSCs was significantly reduced (0.6 ± 0.04 per minute, these events may even be large-amplitude spontaneous minis). None of the neurons showed sustained currents (p ≤ 0.01; Fig.1C,D). Recordings from aCC/RP2 inChats2 embryos raised at 29°C (the maximum temperature tolerated by developing embryos in our experiments) show that this manipulation mimics the effect of TeTxLC. Under these conditions zero of seven neurons have EPSCs or sustained currents (p ≤ 0.01; Fig. 1C,D). InChats2 embryos maintained at the permissive temperature (18°C), both types of input are present in aCC/RP2 at levels not significantly different to WT: six of seven neurons showed EPSCs (2.9 ± 1.0 per min), and five of seven neurons showed sustained currents (1.9 ± 0.2 per minute; Fig.1C,D). We conclude that the use of either TeTxLC orChats2 is an effective method of removing synaptic excitatory input from aCC/RP2.
Neuronal morphology and synaptic inputs develop normally in the absence of synaptic transmission
To assess the effects of suppressing synaptic transmission on neuronal morphology, we used a retrograde filling technique to label individual aCC motorneurons. DiI was applied to the aCC/DA1 NMJ at the end of embryogenesis (20–21 hr AEL). Figure2A compares the morphology of aCC in a control CNS and in a CNS where synaptic transmission has been blocked by pan-neuronal TeTxLC expression. An analysis of the arborization of aCC, including total area, maximum spread, and position relative to the axon scaffold (delineated by antibodies to FasII), reveals no apparent differences in the presence or absence of synaptic transmission (TeTxLC orChats2 raised at 29°C; Table1).
Fig. 2.
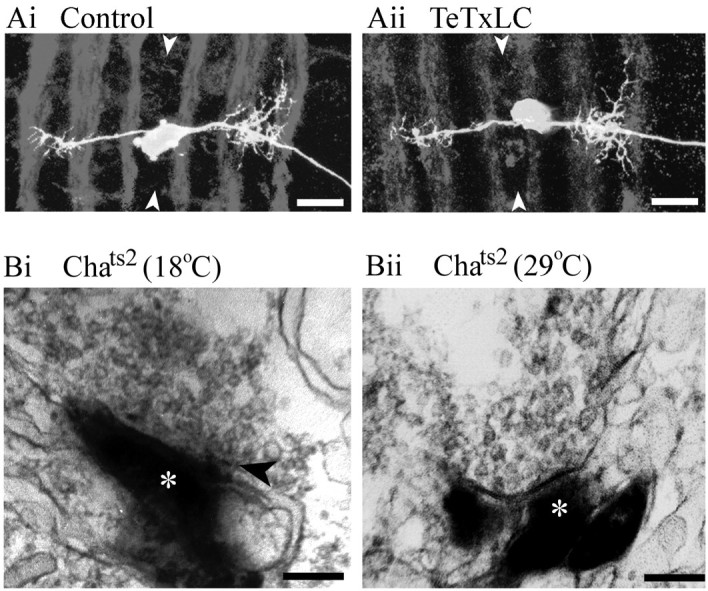
Morphology of aCC and presynaptic terminals develop independently of synaptic activity. A, Confocal projections of aCC neurons retrogradely labeled by DiI in both control (inactive TeTxLC; Ai) and when evoked synaptic activity is blocked (active TeTxLC; Aii). No differences in morphology are attributable to the absence of synaptic activity (Table1). Neurons were labeled at 20–21 hr AEL, and preparations have been counterstained with antibody to FasII to visualize the axon scaffold.Arrowheads indicate the position of the midline. Scale bar, 10 μm; anterior is topmost. B, Electron micrographs of photoconverted DiI-labeled aCC neurons show labeled profiles (asterisks) in control (Chats2 at 18°C; Bi) and in CNS in which evoked synaptic activity is absent (Chats2 at 29°C;Bii). Sites of synaptic input to aCC were identified by an accumulation of clear synaptic vesicles, some of which are docked to the presynaptic membrane, immediately adjacent to such labeled profiles. At this stage of development, synaptic elements such as T-bars are rare (Bi, arrow), although an increased electron density of the presynaptic membrane is often visible (Bii). Scale bar, 200 nm.
Table 1.
Morphological measurements of the arborization of aCC in control CNS (Chats2 at 18°C and expression of inactive TeTxLC) and when evoked synaptic activity is absent (Chats2 at 29°C and active TeTxLC)
| A-P axis (μm) | M-L axis (μm) | Area (μm2) | ||||
|---|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| TeTxLC (inactive) | 19.2 ± 1.2 | 8.4 ± 0.7 | 11.8 ± 0.8 | 12.8 ± 1.15 | 76.7 ± 7.5 | 31.0 ± 2.0 |
| TeTxLC (active) | 18.3 ± 1.2 | 9.0 ± 0.8 | 11.5 ± 0.7 | 11.1 ± 0.7 | 68.4 ± 4.8 | 32.5 ± 2.75 |
| Chats2 (18°C) | 16.1 ± 1.25 | 10.0 ± 0.5 | 10.8 ± 0.7 | 12.1 ± 0.5 | 69.3 ± 2.7 | 40.9 ± 1.9 |
| Chats2(29°C) | 16.2 ± 0.7 | 9.95 ± 0.7 | 12.0 ± 0.4 | 12.6 ± 0.55 | 75.8 ± 2.4 | 38.8 ± 2.8 |
A-P, Anterioposterior axis; M-L, mediolateral axis. All values are mean ± SE for n ≥ 8. No data pairs show significant differences.
Although these results suggest that embryonic neurons develop a normal pattern of dendritic branching and arborization without synaptic transmission, they do not reveal whether normal synaptic inputs can develop under these conditions. To address this question we photoconverted the DiI label in aCC to produce an electron-dense product visible in the electron microscope. This technique allows the unequivocal identification of labeled profiles as belonging to the arborization of aCC (Fig. 2B). Sites of presynaptic input to aCC were identified by the presence of a cluster of clear synaptic vesicles, with a requirement that some vesicles be docked to the presynaptic membrane immediately adjacent to the labeled profile (Baines et al., 1999). In control neurons (Chats2 at 18°C), such synaptic vesicle accumulations were seen immediately adjacent to labeled profiles in 15% of profiles examined (37 of 252 profiles; seven cells sectioned from four embryos; Fig. 2B). In a background where synaptic activity was absent (Chats2 at 29°C) the frequency of presynaptic endings adjacent to aCC remained unchanged (37 of 250 profiles; eight cells sectioned from four embryos; Fig.2B). Thus, the presence of synaptic activity does not seem to be a requirement either for the initial growth or for a normal frequency of presynaptic terminals on embryonic neurons.
Suppression of synaptic activity alters the electrical properties of aCC/RP2
Because aCC and RP2 are accessible to patch electrodes, we could extend our analysis to ask whether these cells can develop a normal electrical phenotype in the absence of synaptic transmission. Using whole-cell voltage clamp we compared the electrical properties of aCC and RP2 in embryos where synaptic activity was absent with controls in which synaptic function was normal.
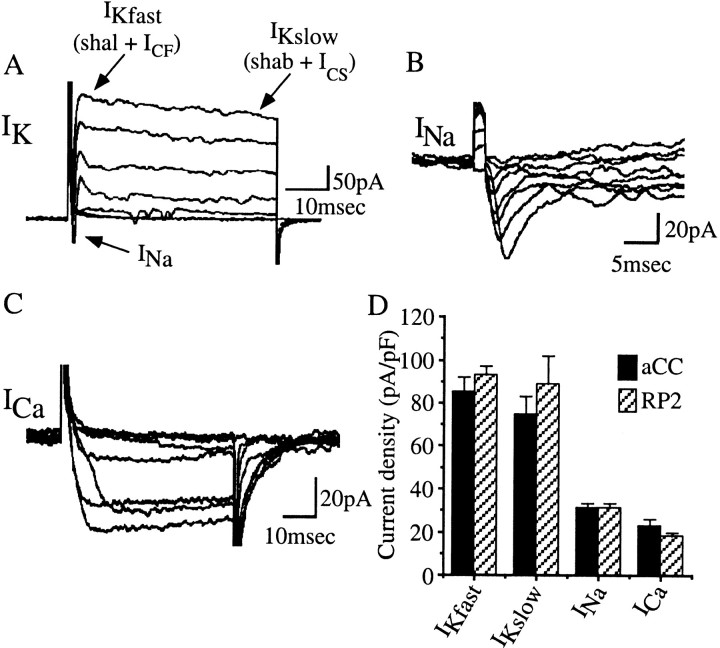
At 20–21 hr AEL, aCC and RP2 express a range of voltage-dependent conductances, including outward K+ and inward Na+ and Ca2+ currents (Fig.3). The voltage-dependent outwardIK of aCC/RP2 is likely to consist of at least five voltage-dependent components, two of which are additionally Ca2+-dependent (Saito and Wu, 1991; Baines and Bate, 1998). The purely voltage-activated currents are a fast IA (encoded byshal) and a slower delayed rectifierIK (shab and to a lesser extent shaw), whereas the two Ca2+-dependent K+ currents are a fastICF (slowpoke,slo) and a slower, and as yet genetically unidentified,ICS (Fig. 3A). The voltage-activated inward currents are anINa (paralytic,para) and an ICa (which is composed of at least two, genetically unidentified, currents; Baines and Bate, 1998) (Fig. 3B,C). For all the currents isolated, the peak current density is identical for aCC and RP2 (Fig.3D), and because of this, data from the two neurons were pooled.
Fig. 3.
Voltage-dependent ion channel characteristics in aCC and RP2. A, Whole-cell voltage clamp of aCC/RP2 reveals the presence of at least two voltage-activated outward K+ macro currents (IKfastand IKslow) and a voltage-activated inward sodium current (INa). These neurons also exhibit voltage-activated inward calcium currents that are masked by IK under these conditions.IKfast and IKsloware composed of at least four individual currents, shal+ ICF and shab +ICS, respectively, see Results for details. B, Blocking both outwardIK and inward ICaisolates the voltage-activated inward INa.C, Isolation of voltage-activatedICa was achieved by blocking voltage-activated IK andINa currents. ICawas measured using barium as the permeant ion (see Baines and Bate, 1998 for discussion of use of this ion). Currents shown are from aCC in embryos at ∼20 hr AEL. Currents were evoked using voltage steps (15 mV increments; range, −60 to +45 mV; 50 msec) applied from a conditioning prepulse of −90 mV (100 msec duration). Traces shown are the average of five trials. D, Peak current density, normalized to membrane capacitance, for the currents isolated in aCC and RP2. No currents are significantly different between aCC and RP2. Values shown are mean ± SE; n ≥ 8.
In the absence of synaptic activity (TeTxLC), aCC and RP2 have abnormal electrical properties. Peak totalIKfast (shal +ICF) is greater than in controls (inactive TeTxLC) where synaptic activity is normal (131 ± 4.8 vs 99.5 ± 5 pA/pF; p ≤ 0.01; Fig.4A). Similarly, totalIKslow (shab,shaw + ICS) is increased in the absence of synaptic activity (125 ± 3.9 vs 91 ± 6 pA/pF; p ≤ 0.01; Fig. 4). BlockingIKCa (zero external Ca2+ and 200 nmcharybdotoxin) shows that the increases in total outward K+ current are attributable, at least in part, to increases in the purely voltage-activated currents,shal and either shab and/or shaw (Fig.4). In addition to increasing outward K+currents, the absence of synaptic activity also leads to a marked increase in INa (39 ± 2.2 vs 30 ± 1.4 pA/pF; p ≤ 0.05; Fig. 4). However, the absence of synaptic activity does not seem to increaseICa in aCC/RP2. On the contrary, a small, although statistically insignificant, decrease is observed (15.4 ± 1.9 vs 18.6 ± 1.4 pA/pF; p > 0.05; Fig. 4A). In all cases membrane capacitance was unaffected by the absence of synaptic transmission (2.5 ± 0.05 vs 2.5 ± 0.06 pF for control vs synaptically deprived aCC/RP2 neurons).
Fig. 4.
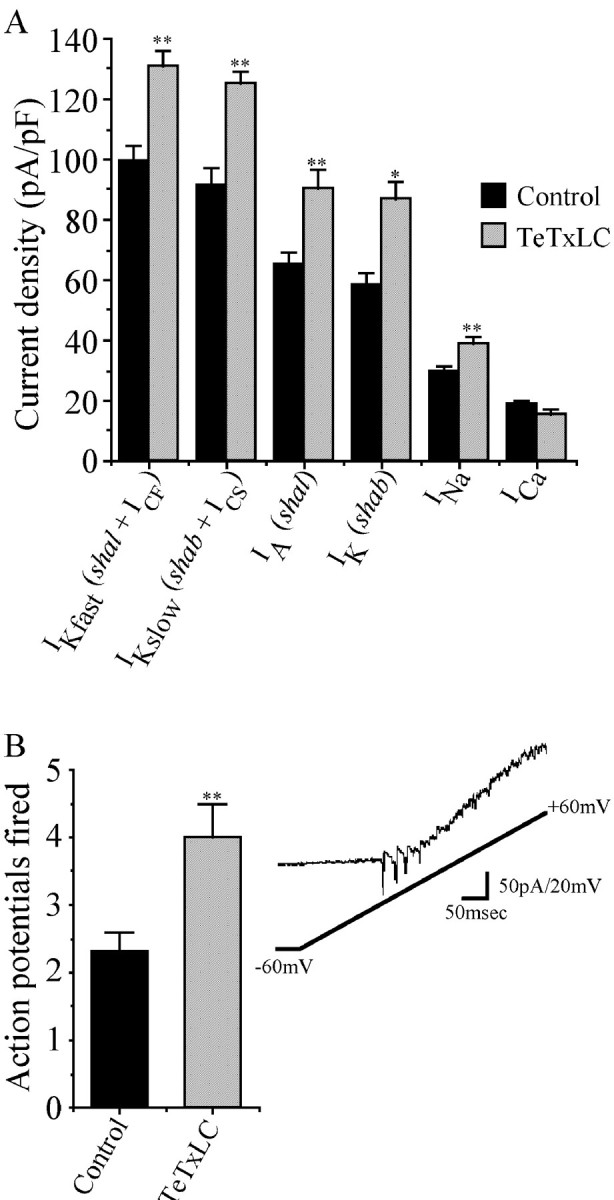
Absence of synaptic input changes aCC/RP2 electrical properties. A, Peak current density for the voltage-activated ion currents isolated in aCC/RP2 in either a TeTxLC-expressing CNS (no synaptic activity) or a control (inactive TeTxLC) CNS in which synaptic activity is normal. Currents have been normalized to membrane capacitance. The absence of synaptic activity results in a significant increase in IKfast(shal + ICF),IKslow (shab +ICS), IA(shal), IK(shab), and INa(para), but not ICa. Values are mean ± SE; n > 8; *p ≤ 0.05; **p ≤ 0.01.B, A ramp depolarization, generated using voltage clamp, fires significantly more action potentials in aCC/RP2 neurons that have been deprived of synaptic input during their development (TeTxLC) compared with controls (inactive TeTxLC). Values are mean ± SE;n = 11; p ≤ 0.01.Inset shows a typical current recording from an aCC neuron in a CNS lacking synaptic activity; four action potentials are fired, which become progressively smaller because of the increasing membrane potential.
To determine how the alterations in conductances influence the functional properties of aCC/RP2, we depolarized the neurons from −60 to +60 mV over 500 msec using voltage clamp. Although current clamp is the preferred method for this analysis, Drosophila embryonic neurons are not suited to this technique (Baines and Bate, 1988). aCC/RP2 respond to depolarization, using voltage clamp, by firing action potentials suggesting that some regions of the neurons are poorly clamped. Depolarization of aCC/RP2 neurons that have been deprived of synaptic excitation (TeTxLC) results in significantly more action potentials fired compared with controls in which synaptic activity was normal (inactive TeTxLC). The number of action potentials fired increases from 2.3 ± 0.3 to 4.0 ± 0.5 (n = 11; p ≤ 0.01; Fig.4B). Synaptic blockade also results in a significant increase in the amplitude of the first action potential fired (subsequent action potentials were not analyzed because their kinetics are more prone to alteration because of increasing membrane potential). Peak inward current of the initial action potential increases from 64 ± 9 to 90 ± 7 pA (n = 11;p ≤ 0.05), whereas the total amount of inward current produced (determined by integrating the area under the action potential waveform) increases from 87 ± 11 pA/msec to 129 ± 17 pA/msec (n = 11; p ≤ 0.05). By contrast, the threshold for action potential firing does not change (−33 ± 1.7 vs −35 ± 1.9 mV; active vs inactive TeTxLC;n = 11; p > 0.05). We tentatively conclude from this analysis that the changes in membrane conductances we observe after synaptic blockade tend to increase the responsiveness of a neurons to a depolarizing stimulus.
Synaptic input regulates electrical development
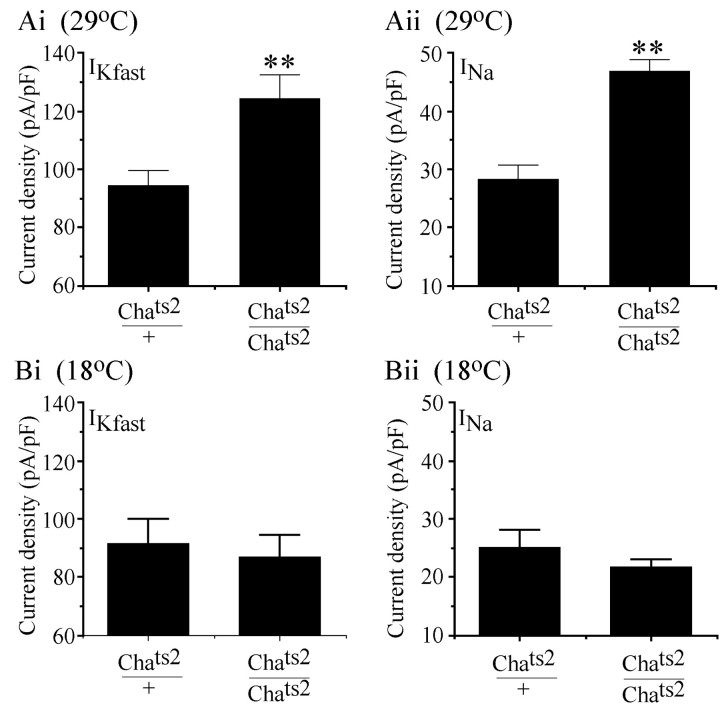
Pan neuronal expression of TeTxLC not only removes all synaptic input from aCC/RP2, but also blocks the release of neurotransmitter from the neurons themselves (data not shown; but see, Baines et al., 1999). Thus, the changes in electrical properties we observe could occur either as a consequence of removing synaptic input from aCC/RP2 or as a result of blocking their synaptic output. To distinguish between these alternatives, we usedChats2 at the restrictive temperature to block synaptic input to aCC/RP2 without affecting their ability to release neurotransmitter. InChats2 raised at 29°C, stimulation of the axons of aCC/RP2 continues to evoke excitatory junctional currents (EJCs) in the target muscles, demonstrating that their ability to release neurotransmitter at the NMJ (l-glutamate; Jan and Jan, 1976) is unaffected (R. A. Baines, unpublished data). However, removing presynaptic input from aCC/RP2 throughout development by raisingChats2 embryos at 29°C increases the peak current density of both totalIKfast (shal +ICF) andINa (other currents were not analyzed; Fig. 5A). At 29°C, peakIKfast is increased to 124 ± 8.5 pA/pF from 94 ± 5.4 pA/pF (n = 9;p ≤ 0.01), and INa is increased to 46.7 ± 2 pA/pF from 28 ± 2.7 pA/pF (n = 10; p ≤ 0.01). The control, in this instance, was an outcross ofChats2 to WT. InChats2 embryos raised at the permissive temperature (18°C), at which synaptic input is normal (Fig. 1), there were no differences in the peak current density for either IKfast orINa (Fig. 5B).
Fig. 5.
Selective blockade of synaptic input mimics TeTxLC expression. A,Chats2embryos raised at 29°C result in a paralytic phenotype caused by a significant reduction of ACh. At this temperature, aCC/RP2 show no synaptic input (Fig. 1C,D), although their capability to release neurotransmitter is not affected. Under these conditions, aCC/RP2 show increased current densities of bothIKfast (shal +ICF) (Ai) andINa (Aii) compared with controls (Chats2/+). Flies were allowed to lay eggs at 18°C for 2 hr periods after which embryos were raised at 29°C. B, At a continuous developmental temperature of 18°C, Chats2 embryos are viable and recordings show that aCC/RP2 receive normal levels of synaptic input (Fig. 1C,D). In addition,IKfast (Bi) andINa (Bii) are not increased above controls (Chats2/+). Values shown represent mean ± SE; n ≥ 8. Values forIKfast and INa at 29°C are significantly different from controls (p ≤ 0.01).
Altered electrical properties are reversible
If changing levels of synaptic input are able to regulate ionic conductances in embryonic neurons, we might predict that any changes induced by blocking synaptic transmission would be reversed by restoring normal synaptic function to the CNS. We took advantage of the temperature sensitivity of Chats2 to test this idea. Neurons in the CNS of the Drosophila embryo begin to develop electrical properties between 13 and 14 hr AEL (25°C), and these are sufficiently mature by 19 hr AEL to support circuit activity underlying coordinated peristaltic movements, a feature that we take to be diagnostic of the presence of functional neural circuits (Baines and Bate, 1998). We shiftedChats2 embryos from 18 to 29°C at early stage 16 (which approximates to 13 hr AEL at 25°C) and maintained this temperature for 5 hr (i.e., until 19 hr AEL at 25°C) before returning the embryos to 18°C (Fig.6Ai). This temperature shift effectively suppresses all excitatory synaptic input to aCC/RP2 during the period when normal electrogenesis occurs (data not shown, but see Fig. 1). Under these conditions there is an increase in the peak current density of IKfast(INa was not measured in this experiment) when recording at 19 hr AEL (122 ± 7.5 vs 97 ± 6 pA/pF; n = 9; p ≤ 0.05; Fig.7Ai). Other embryos, treated identically, were then returned to 18°C and allowed to recover. The temperature shift used in this experiment delayed hatching by 4–6 hr after the embryos were returned to 18°C (equivalent to 2–3 hr at 25°C; Fig. 7Aii). Recordings from these embryos as they hatched showed no significant increase inIKfast compared with age-matched controls (96 ± 5 vs 100 ± 15 pA/pF; control vsChats2; n = 8;p > 0.05; Fig. 6Aii). Thus, the alterations in membrane conductance produced as a result of removing synaptic input are not set but can be reversed after a period in which synaptic function is restored.
Fig. 6.
Alterations in electrical properties produced by the removal of synaptic input are reversible. Ai, By shifting Chats2 embryos from 18°C (permissive temperature) to 29°C (restrictive temperature,black bar) between 13 and 19 hr AEL, it is possible to suppress synaptic input to aCC/RP2 during the period in which electrogenesis occurs in these neurons. After this treatment embryos were returned to 18°C. Recordings at 20 hr AEL show that peakIKfast (shal +ICF) density is significantly increased (p ≤ 0.05). Aii,This temperature shift, however, also delays hatching until ∼23–24 hr AEL. Recordings from aCC/RP2 in embryos on hatching show that peakIKfast density has decreased to control levels. Values shown represent mean ± SE; n≥ 8. All timings shown are normalized to development at 25°C (21 hr development time, 41 hr at 18°C).
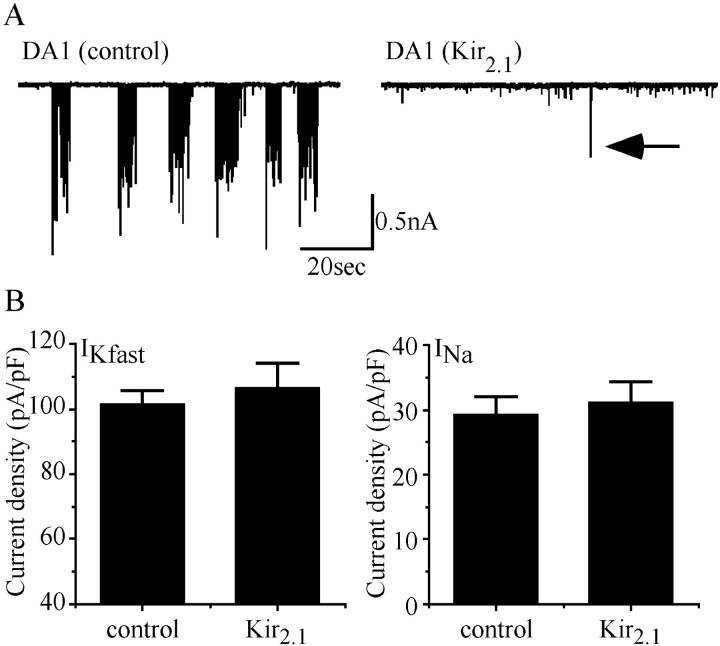
Fig. 7.
Suppression of action potentials does not influence electrical properties. A, Expression of Kir2.1 in aCC using RRC-GAL4 blocks the ability of this neuron to fire action potentials as evidenced by the significant reduction of EJCs recorded in its target muscle (DA1) compared with controls (UAS parental line shown). In the trace shown (right), just one EJC is visible (arrow), although smaller events caused by spontaneous release of neurotransmitter are unaffected. Frequency of synaptic input to aCC is unaffected by expression of Kir2.1 (see Results), although sustained currents fail to trigger action potentials.B, Measurement of peak IKfast(shal + ICF) andINa in aCC in either control (Gal4 and UAS parental lines) or when expressing Kir2.1 shows no significant differences. Values shown represent mean ± SE;n ≥ 8.
Suppression of action potentials does not affect electrical development
Our data suggest that embryonic neurons in Drosophilaare able to sense changes in the level of synaptic excitation they receive and compensate by altering their own intrinsic membrane conductances. The mechanism involved is not yet clear, but one possibility is that developing neurons monitor the number of action potentials they fire, this being a measure of the amount of excitatory synaptic input they receive (Turrigiano and Nelson, 2000). To test this idea in Drosophila, we selectively suppressed action potentials in aCC and monitored its electrical characteristics. We targeted the expression of a human inwardly rectifying K+ channel (Kir2.1) using RRC-GAL4, which is expressed strongly in aCC (and to a lesser extent in RP2, see Baines et al., 1999). Expression of Kir2.1 hyperpolarizes mammalian neurons, thus reducing the probability of action potential firing and suppressing the release of neurotransmitter (Johns et al., 1999). Expression of Kir2.1 in aCC results in the almost total absence of excitatory junctional currents in its target muscle (which result from action potential-mediated release of neurotransmitter; Fig.7A). Spontaneous release of neurotransmitter however, is unaffected (Fig. 7A). Thus, the consequences of expressing Kir2.1 in aCC are consistent with a block to the evoked release of neurotransmitter by a mechanism that suppresses the initiation of action potentials.
Although the expression of Kir2.1 dramatically reduces the ability of aCC to drive its postsynaptic target, recordings from aCC show that it receives a normal level of synaptic input, although, as expected, the firing of action potentials by sustained currents is dramatically reduced (>95%, data not shown). Significantly, a comparison of peak current densities for bothIKfast andINa between control and Kir2.1-expressing aCC neurons shows no differences (Fig. 7B). We conclude that these cells do not use the number of action potentials they fire as a measure of intrinsic activity in regulating their electrical properties.
DISCUSSION
We report the effects of a complete block to evoked excitatory synaptic transmission on the differentiation of identified neurons developing in an otherwise intact nervous system. Our results indicate that whereas the neurons develop apparently normal dendritic arborizations and synaptic connections, their electrical phenotype is disturbed. These findings suggest that synaptic communication between partner cells has a regulatory function during the normal development of excitable properties.
There are two main caveats to the approach that we have adopted that need to be borne in mind when considering the results of these experiments. First, whereas TeTxLC expression blocks evoked release of transmitter, it does not prevent (although it reduces) spontaneous release of synaptic vesicles (Sweeney et al., 1995; Deitcher et al., 1998). Nor is it likely to affect the “leakage” of neurotransmitter from growth cones in the developing nervous system (Haydon et al., 1984; McCobb et al., 1988; Wei-Dong et al., 2000). Such early release events may be developmentally significant given that embryonic neurons, including those of Drosophila, respond to neurotransmitter before the formation of synapses (Baines and Bate, 1998). Second, although aCC/RP2 acquire a normal morphology and apparently normal connectivity in the absence of any evoked synaptic transmission, we cannot conclude that there is no role for synaptic transmission in these developmental processes. In fact, where effects of activity on connectivity have been documented, these commonly involve interactions between competing fibers for the formation of synapses on target cells (Goodman and Shatz, 1993; Hall and Sanes, 1993). In these instances it is the level and timing of synaptic inputs that is crucial to the outcome. The present study does not address the question of whether the balance of synaptic inputs might have important consequences for developing connectivity.
Although the apparent stability of dendritic morphology and synaptic inputs to motorneurons resembles the apparent “hardwiring” of the embryonic NMJ in Drosophila (Sweeney et al., 1995), the similarity could be misleading. The terminal arbors of motor neurons form normally on their target muscles in the Drosophilaembryo in a variety of genetic backgrounds where they will, at later stages, manifest considerable activity-dependent growth and plasticity. For example, where the NCAM FasII is overexpressed on one of two target muscles innervated by a single motor neuron, a normal NMJ forms on both muscles during embryogenesis. At later stages, however, growth of the NMJ is favored on the muscle with high levels of FasII, at the expense of the muscle with proportionately lower levels (Davis et al., 1997;Davis and Goodman, 1998). Similarly, embryonic NMJ development appears normal in hyperactivity mutants, although it is dramatically effected during subsequent larval life (Budnik et al., 1990). It is not yet clear that simple specification of growth cone targeting, termination, and synaptogenesis on the target cell will account for the normal development of central synaptic connections in the embryo. In this case, growth cones seek out their synaptic partners in the relatively complex environment formed by the dendritic arborizations of their targets and other neurons. Our own results indicate that where there is an imbalance in synaptic transmission between presynaptic and postsynaptic partners, there can be effects on connectivity in the embryo. In this instance, if the postsynaptic neuron alone expresses TeTxLC, the formation of presynaptic terminals on its dendritic arbor is drastically reduced (Baines et al., 1999). How this effect is mediated is unclear. It may not be directly related to evoked release of neurotransmitter in the postsynaptic partner. It could be associated with elevated levels of a CAM such as FasII that are known to accompany ectopic expression of TeTxLC (Hiesinger et al., 1999; Baines, unpublished data).
The clearest outcome of blocking excitatory synaptic input in our experiments is the alteration to the electrical phenotype of aCC/RP2. In the absence of evoked release of transmitter onto these neurons, there is a significant increase in the current density of specific ion channels that we detect. Because of the space-clamp limitations associated with the whole-cell patch technique, it could be argued that the effects we see depend solely on a redistribution of ion channels from prospective sites of synaptic input on the dendritic arbor, where they are not fully resolved, to the cell body where they can be detected without impairment (Jackson, 1992). On the other hand, it is equally possible that the increase in ion channel density is the result of increased gene expression of transcripts coding for specific ion channels. Indeed, using RNase protection, we have preliminary evidence to indicate that blockade of synaptic activity in the embryonic CNS results in an upregulation of genes coding for inward (para) and outward (slo) conductances (R. Bohm, N. Atkinson, and R. Baines, unpublished data). Regardless of the precise mechanism, one difficulty we face is in accounting for the fact that both inward and outward currents increase when synaptic input is removed. We suspect that the effects that we describe mask local changes in ion channel concentration and distribution within the cell and its dendritic arbor that will be the actual determinants of its responsiveness to synaptic inputs. Without an analysis of these effects, we cannot predict with any certainty what the outcome of the changes we observe is for the overall excitability of the neuron. We can only speculate at present that, based on the significant increase in inward Na+ current and the ability of neurons to fire an increased number of action potentials, the alteration of membrane conductances in aCC/RP2 promote excitability rather than reduce it. The additional increase in outward K+ current may be a compensatory mechanism triggered by increased inward current. Such a mechanism may serve to maintain action potential kinetics within appropriate limits. Overexpression of Na+ channels inXenopus embryonic skeletal muscle, which has the effect of increasing electrical activity, evokes a similar compensatory increase in K+ channel expression (Lindsell and Moody, 1994).
It seems likely that regulatory effects on the electrical phenotype of differentiating neurons will have features in common with homeostatic mechanisms that control the excitability of more mature neurons. Mechanisms of this kind have been convincingly demonstrated in the stomatogastric (STG) neurons of crustacea and in rat cortical neurons grown in culture (Turrigiano et al., 1994; 1998; Desai et al., 1999). When they are acutely isolated from their synaptic inputs, neurons from the STG that would normally fire in bursts now fire tonically. However, if the isolation is continued for several days in culture, the neurons begin to fire in bursts. This bursting behavior can be reversed by as little as 1 hr of rhythmically patterned stimulation (Turrigiano et al., 1994). Clearly input and the lack of it has the effect of triggering a homeostatic mechanism that restores bursting behavior to the neurons. The underlying mechanism involved in the transition from tonic to bursting activity involves an upregulation of inward Ca2+ conductance and a downregulation of outward K+ conductances (Turrigiano et al., 1995). Cortical neurons also respond to changing levels of synaptic excitation by altering their intrinsic excitability and, additionally, their ability to respond to release of presynaptic neurotransmitter. Intrinsic excitability is altered through modification of membrane conductance, whereas response to neurotransmitter is effected by changes in density of excitatory AMPA receptors at the site of synaptic input (Turrigiano et al., 1998; Desai et al., 1999). Through the action of these mechanisms, cortical neurons respond to weak levels of synaptic input by increasing excitability, although they suppress their excitability when faced with strong synaptic input.
The precise feature of neuronal activity that is being preserved by homeostatic mechanisms is unclear. Possible candidates include average firing rate or intracellular Ca2+concentration (Turrigiano and Nelson, 2000). The possibility that neurons monitor the number of action potentials they fire is not supported experimentally by our results. The more promising candidate is Ca2+, not least because the rate at which it enters into a neuron is well correlated to the level of electrical activity (Ross, 1989). Such a role for Ca2+ is supported experimentally; blockade of its entry into neurons can prevent changes in neuronal excitability observed as a result of activity manipulation (Offord and Catterall, 1989; Desarmenien and Spitzer, 1991; Golowasch et al., 1999). Through the alteration of membrane conductances, neurons appear able to increase or decrease their excitability and, as such, are possibly able to maintain intracellular Ca2+ levels within a predetermined range. We could envisage a similar mechanism at work in aCC/RP2. Loss of synaptic input would be expected to depress intracellular Ca2+ levels, and it could be that it is altered levels of Ca2+ that lead to changes in gene expression that may underlie the alteration in conductances that we observe (Bito, 1998). Certainly the mechanism is a highly dynamic one, with currents returned to their normal levels within a few hours of restoring synaptic input to the cells concerned.
Because synaptic input appears to be required for the normal development of electrical properties, it follows that the development of motor circuits require activity in the final phases of neuronal maturation. It may well be that it is this activity-dependent phase that accounts for the apparently random movements that precede the emergence of more coordinated movement during embryogenesis. The importance of the results presented in this study is that they appear to demonstrate a significant developmental mechanism, namely a role for excitatory synaptic inputs in determining the electrical characteristics of developing motorneurons.
Footnotes
This work was supported by the Wellcome Trust to M.B. (052032). S.T.S. was supported by a Research Fellowship from Darwin College (Cambridge, UK) and the Wellcome Trust (048476/Z/96/Z to C. O'Kane). We thank P. Salvaterra for Chats2 flies, C. O'Kane in whose lab pUAS-EGFP-Kir2.1 was made, D. Johns and E. Marbán for providing the Nhe/Hpal fragment containing EGFP-Kir2.1, C. Goodman for anti-FasII antibody, M. Day for help with electron microscopy, and M. Landgraf, S. Laughlin, R, Schulz, L. Seugnet, and M. Suster for comments.
Correspondence should be addressed to Dr. R. A. Baines, Department of Zoology, University of Cambridge, Cambridge, CB2 3EJ UK. E-mail:rab41@cam.ac.uk.
Dr. Sweeney's present address: Department of Biochemistry and Biophysics, University of California, San Francisco, CA 94143.
REFERENCES
- 1.Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines RA, Robinson SG, Fujioka M, Jaynes JB, Bate M. Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila. Curr Biol. 1999;9:1267–1270. doi: 10.1016/s0960-9822(99)80510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bate M. Development of motor behaviour. Curr Opin Neurobiol. 1999;9:670–675. doi: 10.1016/s0959-4388(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 4.Bito H. The role of calcium in activity-dependent neuronal gene expression. Cell Calcium. 1998;23:143–150. doi: 10.1016/s0143-4160(98)90113-0. [DOI] [PubMed] [Google Scholar]
- 5.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 6.Broadie KS, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;13:144–166. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- 7.Budnik V, Zhing Y, Wu C-F. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows M. The neurobiology of an insect brain, pp 177–180. Oxford UP; Oxford: 1996. [Google Scholar]
- 9.Chase BA, Kankel DR. On the role of normal acetylcholine metabolism in the formation and maintenance of the Drosophila nervous system. Dev Biol. 1988;125:361–380. doi: 10.1016/0012-1606(88)90218-7. [DOI] [PubMed] [Google Scholar]
- 10.Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- 11.Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- 12.Davis GW, Schuster CM, Goodman CS. Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 13.Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai NS, Rutherford L, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- 15.Desarmenien MG, Spitzer NC. Role of calcium and protein kinase C in development of the delayed rectifier potassium current in Xenopus spinal neurons. Neuron. 1991;7:797–805. doi: 10.1016/0896-6273(91)90282-5. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golowasch J, Abbot LF, Marder E. Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab, Cancer borealis. J Neurosci. 1999;19:RC33. doi: 10.1523/JNEUROSCI.19-20-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72/Neuron 10[Suppl]:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan RJ. Mutations of choline acetyltransferase and associated neural defects in Drosophila melanogaster. J Comp Physiol. 1980;137:83–92. [Google Scholar]
- 20.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72/Neuron 10[Suppl]:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 21.Haverkamp LJ. Anatomical and physiological development of the Xenopus embryonic motor system in the absence of neural activity. J Neurosci. 1986;6:1338–1348. doi: 10.1523/JNEUROSCI.06-05-01338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkamp LJ, Oppenheim RW. Behavioral development in the absence of neural activity: effects of chronic immobilization on amphibian embryos. J Neurosci. 1986;6:1332–1337. doi: 10.1523/JNEUROSCI.06-05-01332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haydon PG, McCobb DP, Kater SB. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;226:561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- 24.Hiesinger PR, Reiter C, Schau H, Fischbach K-F. Neuropil pattern formation and regulation of cell adhesion molecules in Drosophila optic lobe development depend on synaptobrevin. J Neurosci. 1999;19:7548–7556. doi: 10.1523/JNEUROSCI.19-17-07548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson MB. Cable analysis with the whole cell patch clamp: theory and experiment. Biophys J. 1992;61:756–766. doi: 10.1016/S0006-3495(92)81880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jan LY, Jan YN. l-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol (Lond) 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns DC, Marx R, Mains RE, O'Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Käethner RJ, Stüermer CA. Dynamics of terminal arbor formation and target approach in retinotectal axons in living Zebrafish embryos: a time-lapse study of single axons. J Neurosci. 1992;12:3257–3271. doi: 10.1523/JNEUROSCI.12-08-03257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 30.Lindsell P, Moody WJ. Na+ channel mis-expression accelerates K+ channel development in embryonic Xenopus laevis skeletal muscle. J Physiol (Lond) 1994;480:405–410. doi: 10.1113/jphysiol.1994.sp020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCobb DP, Haydon PG, Kater SB. Dopamine and serotonin inhibition of neurite elongation of different identified neurons. J Neurosci Res. 1988;19:19–26. doi: 10.1002/jnr.490190104. [DOI] [PubMed] [Google Scholar]
- 32.Misra S, Rio DC. Cytotype control of Drosophila P-element transposition: the 66KD protein is a repressor of transposase activity. Cell. 1990;62:269–284. doi: 10.1016/0092-8674(90)90365-l. [DOI] [PubMed] [Google Scholar]
- 33.Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- 34.Moody WJ. The development of voltage-gated ion channels and its relation to activity-dependent developmental events. Curr Topics Dev Biol. 1998;39:159–185. doi: 10.1016/s0070-2153(08)60455-x. [DOI] [PubMed] [Google Scholar]
- 35.Offord J, Catterall WA. Electrical activity, cAMP and cytosolic calcium regulate mRNA encoding sodium channel α subunits in rat muscle cells. Neuron. 1989;2:1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- 36.Ribera AB, Nüsslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for developmental regulation of sodium current. J Neurosci. 1998;18:9181–9191. doi: 10.1523/JNEUROSCI.18-22-09181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross WM. Changes in intracellular calcium during neuron activity. Annu Rev Physiol. 1989;51:491–506. doi: 10.1146/annurev.ph.51.030189.002423. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Wu C-F. Expression of ion channels and mutational effects in giant Drosophila neurons differentiated from cell division-arrested embryonic neuroblasts. J Neurosci. 1991;11:2135–2150. doi: 10.1523/JNEUROSCI.11-07-02135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvaterra PM, McCaman RE. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci. 1985;5:903–910. doi: 10.1523/JNEUROSCI.05-04-00903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanes DH, Reh TT, Harris WA. Development of the nervous system, pp 408–419. Academic; San Diego: 2000. [Google Scholar]
- 41.Spradling AC. P-element-mediated transformation. In: Roberts DB, editor. Drosophila: a practical approach. IRL; Oxford: 1986. pp. 60–73. [Google Scholar]
- 42.Sun Q, Dale N. Developmental changes in expression of ion currents accompany maturation of locomotor pattern in frog tadpoles. J Physiol (Lond) 1998;507:257–264. doi: 10.1111/j.1469-7793.1998.257bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 44.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 45.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 46.Turrigiano GG, Abbot LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- 47.Turrigiano GG, LeMasson G, Marder E. Selective regulation of current densities underlies spontaneous changes in activity in cultured neurons. J Neurosci. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 49.Wei-Dong Y, Rusch J, Poo M-m, Wu C-F. Spontaneous acetylcholine secretion from developing growth cones of Drosophila central neurons: effects of cAMP-pathway mutations. J Neurosci. 2000;20:2626–2637. doi: 10.1523/JNEUROSCI.20-07-02626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]