Abstract
To investigate the cortical information processing during the preparation of vocalization, we performed transcranial magnetic stimulation (TMS) over the cortex while the subjects prepared to produce voice in response to a visual cue. The control reaction time (RT) of vocalization without TMS was 250–350 msec. TMS prolonged RT when it was delivered up to 150–200 msec before the expected onset of voice (EOV). The largest delay of RT was induced bilaterally over points 6 cm to the left and right of the vertex (the left and right motor areas), resulting in 10–20% prolongation of RT. During the early phase of prevocalization period (50–100 msec before EOV), the delay induced over the left motor area was slightly larger than that induced over the right motor area, whereas, during the late phase (0–50 msec before EOV), it was significantly larger over the right motor area. Bilateral and simultaneous TMS of the left and right motor areas induced delays not significantly different from that induced by unilateral TMS during the early phase, but induced a large delay well in excess of the latter during the late phase. Thus, during the cortical preparation for human vocalization, alternation of hemispheric lateralization takes place between the bilateral motor cortices near the facial motor representations, with mild left hemispheric predominance at the early phase switching over to robust right hemispheric predominance during the late phase. Our results also suggested involvement of the motor representation of respiratory muscles and also of supplementary motor cortex.
Keywords: vocalization, transcranial magnetic stimulation, motor area, supplementary motor area, hemispheric lateralization, reaction time
Anatomically, two predominant pathways from the cortex have been described for vocalization in primates: one descending from the limbic system to the periaqueductal gray responsible for nonverbal emotional vocal utterances, and another descending from the neocortex, more specifically from the facial and laryngeal areas of the motor cortex, which is responsible for the production of learned vocal patterns (Jürgens and Ploog, 1970,1976; Jürgens and Zwirner, 1996). The relative importance of the latter pathway becomes greater in animals higher in the phylogenetic tree and culminates in human voice production in which highly learned vocal patterns apart from emotional contents are required for speech. In primates, cortical potentials preceding vocalization have been recorded over the motor cortex in the posterior bank of the inferior limb of the arcuate sulcus (Gemba et al., 1995, 1997). However, it is only in humans that electrical stimulation or repetitive transcranial magnetic stimulation of the cerebral cortex can produce vocalization or its arrest (Penfield and Rasmussen, 1949; Penfield and Roberts, 1959; Hast et al., 1974; Pascual-Leone et al., 1991). Therefore monkeys do not provide appropriate models for the learned type of vocalization, leaving the cortical control of vocalization as an issue that can be only studied in humans.
Many positron emission tomography (PET) studies hitherto have shown that vocalization in humans involves various regions of the brain such as the facial representations of bilateral sensorimotor cortices, supplementary motor area, and the cerebellum (Petersen and Fiez, 1993;Herholz et al., 1994; Wildgruber et al., 1996; Yonekura et al., 1997). However, little is known about the temporal evolution of activities in these cortical regions.
Here we studied the cortical motor preparation for vocalization by using transcranial magnetic stimulation (TMS) to suppress cortical functions temporarily and focally in a manner analogous to that used in animal studies, producing as it were a “virtual lesion” (Day et al., 1989; Priori et al., 1993; Terao et al., 1998). If the onset of voice is delayed by TMS delivered at a certain time, the focal cortical area just underneath the coil should then be active and necessary. Investigating where the maximal suppression occurs by delivering TMS at various time intervals and locations would reveal not only which cortical regions are active during task performance, but also when they are active and necessary.
The laryngeal and facial representations of the motor cortex, considered responsible for vocalization and speech, send bilateral projections to the nuclei of cranial nerves and other brainstem structures. Goodale (1988) described right-sided asymmetries (left hemispheric predominance) in movements of a mouth during verbal and nonverbal tasks. A recent study also suggested that left hemispheric dominance for speech production includes the primary motor cortex even for simple verbal tasks such as automatic speech lacking prosodic component (Wildgruber et al., 1996). What does “hemispheric” dominance imply in the presence of such bilateral projections? The present study also addressed this issue and provides the first evidence of hemispheric lateralization and also of its alternation, for the cortical preparation of vocalization.
MATERIALS AND METHODS
The following experiments were done with the approval of the Ethics Committee of the University of Tokyo. Thirteen normal subjects altogether (nine males, four females, age 28–45) took part in the experiments who all gave written informed consent to participate before the experiments. All the subjects were right-handed, and the Edinburgh inventory score ranged from 60 to 100, with an average of 95.5 ± 3.65 (mean ± SE) (Oldfield, 1971).
Experiment 1. Subjects were required to produce a short sound [a] (one of the five elementary Japanese vowel sounds) quickly in response to the presentation of a visual cue, i.e., flash of a light-emitting diode (LED) placed 50 cm in front of the face, 2.5 mm in diameter. The light cues were presented at 4–6 sec intervals. To prevent the reaction time (RT) of voice from being affected by the respiratory phase in which vocalization is made (during inspiration or expiration), the subjects were asked to wait in full inspiration for the coming cue and not to take a breath just before voice production. The subjects were encouraged to produce a voice of the same pitch, volume, and duration throughout the experiment. The intensity of voice was slightly above the level that the subjects would use for natural conversation, so that they would endure the long recording sessions. Voice was picked up by a microphone (Dynamic microphone, F-V20II; Sony, Tokyo, Japan), and the signals were amplified through filters set at 100 Hz and 3 kHz, which were then full wave-rectified (DP-1200; NEC Medical Systems, Tokyo, Japan). Because the recordings were inevitably contaminated by the click sounds accompanying the magnetic pulse, we also recorded the click sounds in a separate session in which the subjects did not perform the task, but when the magnetic coil was placed at the same position over the subject's head. These recordings were compared with the recordings of each single trial when the subjects phonated, so that the onset and offset of voice were respectively defined as the times when the record for the latter condition deviated from and returned to the former by a difference of more than two times the level of the ambient noise recorded by the same microphone.
For TMS, we used a circular coil (inner diameter 8 cm) connected to a magnetic stimulator (Magstim 200; Magstim, Welwyn Garden City, UK). The coil was centered over the vertex either with side A upward, which induced counterclockwise current in the coil as viewed from above, or with side B upward, which induced current in the opposite direction (clockwise current). The former coil placement is known to be optimal for stimulating the hand motor area of the left hemisphere and eliciting motor-evoked potentials (MEPs) in right-hand muscles, whereas the latter is optimal for stimulating the hand motor area of the right hemisphere and eliciting MEPs in the left-hand muscles. Postulating that preferential activation of the left and right hemispheres holds true also for the cortical regions implicated in vocalization, we would be able to investigate the relative contribution of the two hemispheres to the preparation of vocalization by experimenting with these two current directions; if counterclockwise current direction effective for the left hemisphere induces a greater delay in the onset of vocalization, it would mean that the left hemisphere is more involved in the preparation of vocalization, whereas the right hemisphere should be more involved if clockwise current direction has a greater effect. In this part of experiment, we used a relatively strong intensity, i.e., 80–100% of the maximal output of the stimulator, as has been used for similar experiments that investigated the hand (Day et al., 1989) or saccade reaction time (Priori et al., 1993).
RT in each trial was defined as the time between cue presentation and the onset of voice measured on each recording. The amount of voice was defined as the area under the rectified waveform of the recorded voice. The duration of voice was defined as the time interval between the onset and offset of voice.
Before test sessions, at least 20 practice trials without TMS were given until the RT became stable. The stable mean value of RT in these preliminary trials was used to define the expected onset of vocalization (EOVp), which ranged from ∼250–350 msec depending on the subject. Subsequently, the subjects went through the test sessions, in which TMS was given 0, 50, 100, 150, 200, 250, and 300 msec before EOVp. In some subjects, 350 msec before EOVp was also studied. However, the RT, hence the expected onset of voice, varied slightly with sessions. Therefore, the time of TMS was redefined as the time interval between TMS delivery and the expected time of voice in each session (EOVt), i.e., how long it preceded EOVt. Test (with TMS delivered at the above time intervals) and control trials (click sounds were given at the corresponding time intervals, but the magnetic coil was delivered off the scalp) were intermixed in a randomized order. Each session included trials for two to three TMS intervals (10–15 trials for each TMS interval) as well as 10–15 control trials. In addition, catch trials were included in which TMS was given but no visual cue was presented, so that the subject had to withhold a response. These trials comprised 10–15% of the total trial number in each session and ensured that the subjects reacted in response to the visual cue, but not to TMS. If the subject inadvertently responded in any of the catch trials, all the responses in that session were discarded.
Experiment 2. Experiment 1 was mainly aimed at determining the time interval for delivering TMS that was most effective either in delaying the onset latency or increasing the amount of voice. In experiment 2, the basic experimental setup was similar to that described for experiment 1, except that a figure eight coil (inner diameter 8 cm, outer diameter 11.5 cm) was used, which enabled localized stimulation of the brain to study the topography of effective regions, i.e., the most effective site to delay the onset of vocalization and whether the topography of these active regions changed with the time of TMS. TMS was delivered at the effective time intervals as revealed in experiment 1, i.e., ∼0, 50, 100, 150, and 200 msec before EOVt. Additionally, in experiments 2 and 3, 6 of 11 subjects were selected to study the time courses in more detail, with time intervals of TMS varied in 10–20 msec steps. The data for these six subjects were used for statistical evaluation, as will be described later.
According to the results of recent functional magnetic resonance imaging studies (Hikosaka et al., 1996; Lee et al., 1999), we focused our study on scalp sites overlying the motor strip and the supplementary motor area proper (SMA proper). The motor strip was considered to extend along lines drawn from Cz in the 10–20 international electrode system toward the tragi of both ears. Thus seven points of interest were selected over the scalp to cover evenly over the motor strip (Fig.1B). These points were the vertex (Cz in the international 10–20 electrode system, point D in Fig. 1), points 3 cm to the left and right (points C and E), points 6 cm to the left and right (points B and F), and points 9 cm to the left and right (points A and G) of Cz. These points should span respectively the medial, middle, and lateral portions of the motor strip. The facial motor representations as studied by TMS have been located at ∼6–8 cm lateral to Cz over the scalp (Meyer et al., 1994). The coil was positioned flat and tangential to the scalp surface over each of these points such that the induced current in the brain flowed in the posterior-to-anterior direction.
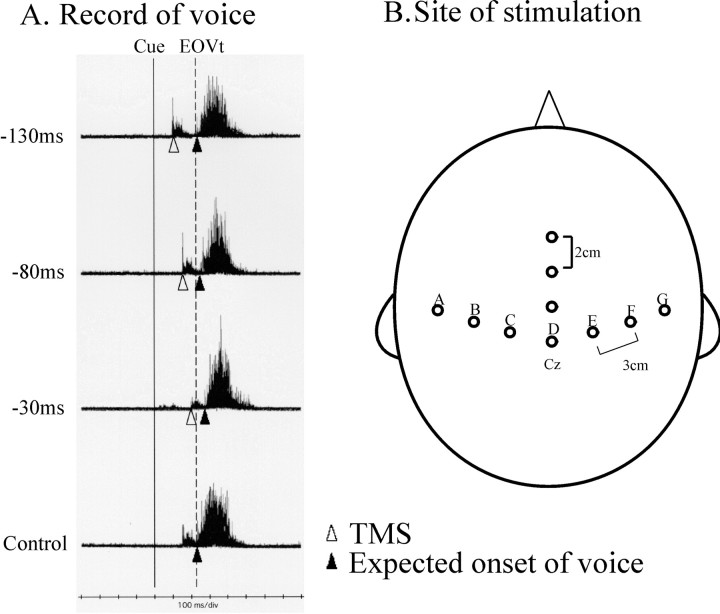
Fig. 1.
An example of voice recordings (A) and sites of TMS over the scalp (B). A, Delay in the RT of voice induced by TMS given at various time intervals. Here the round coil was centered over the vertex (counterclockwise current in the coil). Each trace gives the superimposition of voice recordings for 10 trials. Thebottom trace is the recording when the magnetic coil was delivered off the scalp, but the subject heard the click sound accompanying the magnetic pulse. The time of visual cue presentation is indicated by the vertical solid line, and the control reaction time is marked by the vertical dashed line. The time of TMS delivery is indicated by the white triangles. In the top three traces, TMS was applied ∼130, 80, and 30 msec before EOVt [Actually, TMS was delivered 50, 100, and 150 msec before EOVp. There was a correction of 20 msec because the onset of voice (EOVt) was 20 msec shorter than EOVp in this session]. Note that the onsets of voice (marked byblack triangles) are progressively more delayed in comparison with the control reaction time when TMS is applied at a later interval. The magnetic pulses are accompanied by click sounds, which give rise to the artifacts preceding the sound records for vocalization. B, The figure eight coil was placed over points spanning the motor strip and SMA proper. To cover the motor strip, seven points were selected over the lines drawn from Cz toward the tragi of both ears. The points were the vertex (Cz, pointD), points 3 cm to the left and right (pointsC and E), points 6 cm to the left and right (points B and F), and points 9 cm to the left and right (points A andG) of Cz. Three other points, 2, 4, and 6 cm anterior to Cz, were selected over the midline to cover the presumed location of SMA proper. According to preceding studies, the SMA proper (hand area) is considered to lie beneath a scalp point 2–3 cm anterior and 1 cm lateral to Cz.
Electrical stimulation of SMA proper can elicit vocalization in primates (Jürgens and Ploog, 1970), and cortical potentials related to vocalization have been recorded over SMA in humans (Ikeda et al., 1992). Thus, to investigate the possible effect of TMS over SMA proper, we also investigated the midline regions in 6 of 11 subjects. According to preceding TMS (Müri et al., 1995; Gerloff et al., 1999) and neuroimaging studies (Hikosaka et al., 1996; Lee et al., 1999), the hand area of SMA proper has been located beneath a point over the scalp 2–3 cm anterior and 1 cm lateral to Cz where the midline of scalp crosses the motor strip and the pre-SMA further anterior. We have also located the pre-SMA underneath a scalp point ∼6 cm anterior to Cz (our unpublished data). Thus, the center of the coil was placed either over Cz or over points 2, 4, and 6 cm anterior to Cz to test the effect of TMS over the midline region. A graph was constructed plotting the delay in RT (ordinate) against the site of stimulation (abscissa) at each of the time intervals.
In this part of experiment, special care was taken as to the intensity of TMS because it was shown in experiment 1 that the RT of vocalization is remarkably susceptible to intersensory facilitation, a phenomenon whereby RT is shortened when a sensory stimulus of various modalities accompanies the cue signal (in this case, the visual cue for triggering vocalization). With TMS of strong intensity, RT could be shortened because of accessory sensory inputs accompanying TMS, such as the click sound, slight percussion onto the head, current induced in the scalp, and the resulting contraction of the muscles. In preliminary sessions, we investigated the optimal intensity for obtaining a maximal delay, namely, an intensity powerful enough to physically stimulate the cortical region just underneath the coil and to induce a delay in RT, but not too strong to induce an intersensory facilitation as large as to override the induced delay, which would result in the acceleration of RT. We found that the intensity 5–10% above the active motor threshold of the hand motor area of the left hemisphere was most optimal for this purpose. This intensity was first determined for each subject and was used in the subsequent sessions.
Based on the results, we constructed plots describing the time course of induced delay against the site of stimulation over the motor strip or the midline regions (see Fig. 3A–C). In addition, we also plotted the time course of induced delay, amount, and duration of the voice as a function of the time of TMS. As will be described in Results, the induced delay was most robust when TMS was delivered over points 6 cm to the left and right of Cz and points 2–4 cm anterior to Cz. These regions will be termed the left and right motor areas and the SMA proper region in the following. The detailed time courses for the left and right motor areas and the SMA proper region were given as separate plots for six subjects whose time courses were studied in detail (see Fig. 4A).
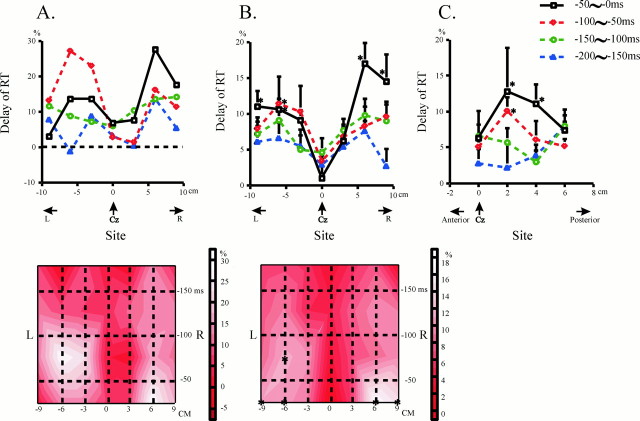
Fig. 3.
The effect of TMS over the motor strip (A, B) and midline region (C) in relation to the site of TMS (experiment 2). A, In one subject, the delay of RT was plotted as a function of the site where focal TMS was delivered over the motor strip. The right side of the figure (positive values on the abscissa) corresponds to the right of the head, and the left side (negative values on the abscissa) to the left. The four curves in the top figure each depict the delay when TMS was applied 0–50, 50–100, 100–150, and 150–200 msec before EOVt. In this and the following figures, the delay is expressed as its percentage to the control reaction time in the same session. As shown above, the delay increased as TMS was applied at a later time interval. The most prominent delay was induced over points 6 cm to the right and left of Cz (right and left motor areas). The delay was greater over the left motor area than over the right motor area 50–100 msec before EOVt, whereas the latter was greater than the former 0–50 msec before EOVt. The accompanying diagram beneath shows the same data as a contour plot in the spatiotemporal domain. The abscissa shows scalp regions over the motor strip, ranging from 9 cm to the left and to the right of Cz (left and right motor areas), and the ordinate gives the time when TMS was delivered. As shown in the bars on the right, regions in lighter tints depict the regions where the delay induced by TMS was large, whereas those with darker tints correspond to regions where the effect of TMS was small. Note again that the delay induced by TMS was most prominent over the left and right motor areas and that the “activity” of the left motor area preceded that of the right motor area. B, A similar trend was noted in all the subjects. These plots were constructed for the delays averaged across all the subjects. Conventions as in A, except that error bars give the SEs, and asterisks indicate significant delay compared with control RT in the same session (paired t test,p < 0.05). Here again, the delay increased with time and was maximal over the left and right motor areas. Note also that mild left hemispheric predominance 50–100 msec before EOVt switched over to robust right hemispheric predominance 0–50 msec before EOVt. The bottom figure illustrates the same data as a plot in the spatiotemporal domain. C, A similar plot when the magnetic coil was placed over the midline region, 0, 2, 4, and 6 cm anterior to Cz. This graph also plots the delays averaged across all the subjects. Error bars indicate SEs.Asterisks indicate significant delay compared with control reaction times (paired t test,p < 0.05). Significant delay was noted over points 2–4 cm anterior to Cz at time intervals of 0–50 and 50–100 msec before EOVt.
Fig. 4.
Time courses for delay and voice volume with TMS over the motor strip and midline region as a function of the time of TMS (experiment 2). A, In this plot, we compare the time courses of delay induced over points 6 cm to the left and right of Cz with that induced over the presumed location of SMA proper (2–4 cm anterior to Cz). Conventions as in Figure 2A. During the period preceding EOVt by 50–150 msec, the delay induced over the motor areas was larger than that induced over the SMA proper region. B, Change in voice volume induced over the motor area (top) and SMA proper (bottom). Conventions as in Figure 2B. No significant change in voice ratio was induced by unilateral TMS over the motor area or over the presumed location of SMA proper at any of the time intervals.
Experiment 3. In this part of experiment, the effect of bilateral TMS was compared with that of unilateral TMS. To study the precise time course of the effect of TMS, we placed two figure eight coils over two sites where the effect of focal TMS was maximal, namely over the left and right motor areas. Trials in which TMS was delivered bilaterally and simultaneously over these two points (bilateral TMS) were randomly intermixed with those in which TMS was given unilaterally at either of the points (unilateral TMS). The intensity used for stimulation was the same as in experiment 2.
Again, the time course of induced delay, amount, and duration of the voice were plotted as a function of the time of TMS (see Fig. 5).
Fig. 5.
Comparison of the effects of unilateral and bilateral TMS (experiment 3). A, This plot compares the delay induced by unilateral TMS over either of the motor areas with that induced by bilateral TMS. Dots represent unilateral TMS, whereas circles stand for bilateral TMS. Because the time courses for unilateral TMS over the left and right motor areas were similar in basic features, the data were combined to produce a single plot for both motor areas. The abscissa gives the time of TMS relative to EOVt, and the ordinate gives the delay induced by TMS. During the period preceding EOVt by 50–200 msec, the delay induced by unilateral and bilateral TMS was comparable. During a later period (0–50 msec preceding EOVt), the delay induced by bilateral TMS was much greater than that by unilateral TMS. Plots at the time bin 0–25 msec before EOVt are not shown for bilateral TMS because we were not able to collect enough data for this time interval. The same applies to B. B, This plot illustrates the time course of voice volume, i.e., the ratio of conditioned to control trials (ordinate), as a function of the time of TMS (abscissa). The dots stand for unilateral TMS, and thecircles denote bilateral TMS. Error bars indicate SEs. The volume of voice slightly increased when bilateral TMS is applied during the late stage.
Data processing and statistical analysis. The onset time of voice relative to the time of visual cue (i.e., RT), its volume, and duration were collected for each trial. RT was averaged at each TMS interval (with four time bins of 0–50, 50–100, 100–150, and 150–200 msec before EOVt) and for control trials. The TMS-induced delay in RT was calculated by subtracting the mean RT of control trials from that of test trials at each TMS interval. In experiments 2 and 3, the delay of RT was expressed as its percentage to the mean control RT in the same session. The average amount of voice at each TMS interval was expressed as its ratio to the average amount of voice in control trials in the same session (voice ratio). Similarly, the duration of voice was expressed as its ratio to the average duration of voice in control trials. Thereafter RT and the volume and duration of voice in test and control trials under each stimulus condition were compared statistically using the paired Student's t test (p < 0.05) to see whether there was a significant delay of RT at each time interval of TMS.
To compare the time courses of these measures under different stimulus conditions (TMS over the motor areas or that over the SMA proper region in experiment 2, unilateral or bilateral TMS in experiment 3), statistical assessment was performed with data collected from six subjects in whom the time courses were studied in detail. Three measures of voice (delay of RT, volume, and duration) were considered to be functions of three independent factors, i.e., subject, time interval of TMS (0–50, 50–100, 100–150, and 150–200 msec before EOVt), and the type of stimulation (TMS over the left or right motor area, or over SMA proper in experiment 2, unilateral and bilateral TMS in experiment 3) and were subjected to statistical analysis using ANOVA. The factor subject exhibited no significant effect on any of these measures alone nor any significant interaction between the other two factors, and therefore this factor was excluded from the independent factors. Because the time courses for unilateral TMS over the left and right motor areas were quite similar in basic features, the data were pooled for these areas and will be described simply as data for the motor areas. Consequently, for experiment 2, ANOVA was performed with two factors, time interval of TMS, and the type of stimulation (TMS over the motor areas or over SMA proper). Similarly, for experiment 3, ANOVA was performed with two factors, the time interval of TMS and stimulus condition [unilateral (left or right) and bilateral TMS]. Post hoc analysis was submitted to Bonferroni's correction to reveal what differences contributed to the significant effects or interactions detected by ANOVA.
RESULTS
Experiment 1
Delay in the onset of voice induced by TMS
The mean control RT in the subjects was 303.0 ± 13.75 (mean ± SE) msec. Using a strong intensity up to 90–100% of the maximal output of the stimulator with a round coil, we could delay the onset of vocalization by TMS in five of nine subjects recruited in this study. When the delay was plotted as a function of the time of TMS, a small delay was noted when TMS was delivered between 0 and 200 msec before EOVt (Fig. 2A) and the maximal value of delay ranged from 15–40 msec in different subjects. The amount of delay did not differ significantly whether TMS was placed with side A or B upward (data not shown). However, this may have been attributable to the small delay induced in each of these subjects.
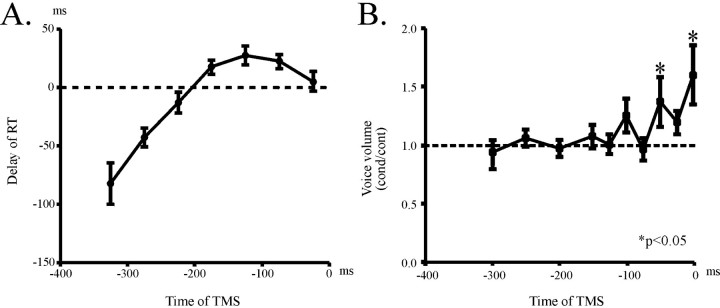
Fig. 2.
Delay of vocalization induced by TMS using a round coil (experiment 1). A, A graph plotting the delay of RT (ordinate) as a function of the time interval (abscissa) of TMS delivered with a round coil centered over the vertex. Error bars indicate SEs. The time interval of TMS was expressed as how long it preceded EOVt (expected onset of voice in test sessions, or control reaction time of vocalization). The delay emerged at ∼200 msec before EOVt and gradually increased with the time interval of TMS, culminating at ∼100 msec before EOVt. At even shorter time intervals of TMS, shortening of RT was noted, which was considered to be attributable to intersensory facilitation caused by accessory sensory inputs accompanying the magnetic pulse. B, A graph plotting the voice ratio as a function of the time interval of TMS. Abscissa gives the time of TMS delivery preceding EOVt. The amount of voice (ordinate) was expressed as its ratio to the amount of voice in control trials in the same session and was plotted as a function of the time of TMS (abscissa). The volume of voice began to increase as early as ∼100 msec before EOVt and showed a marked increase at 0–50 msec before EOVt. Asterisks indicate significant increase in voice volume as compared with control (pairedt test, p < 0.05).
On the other hand, an obvious shortening of RT was noted when TMS was delivered 200–350 msec before EOVt, which was ascribed to intersensory facilitation (Terao et al., 1997). The amount of shortening reached as large as 100 msec, which was much greater in magnitude in comparison with the delay. Furthermore, TMS produced no delay in four subjects. Control RT of these subjects was 347.1 ± 10.9 msec. In two of them, even an acceleration of RT was noted at all the TMS intervals studied, again presumably because of intersensory facilitation. In these four cases, we may be looking at the effect of TMS blocking the cortical information process and inducing a delay in RT, combined with that of accessory sensory inputs eliciting intersensory facilitation and the shortening of RT. Because we used a relatively high intensity for TMS, a strong intersensory facilitation was induced, whereas the effect of blocking the cortical processing was relatively small in some subjects. Hence, the net result was a slight acceleration of the RT of voice. The four subjects may presumably have had a “high threshold” in terms of the blocking effect of TMS, although this “threshold” did not correlate with the motor threshold in the same subjects.
In summary, we could induce a small delay in most of the subjects with TMS delivered between 0 and 200 msec before EOVt. However, this small delay was greatly obscured by the contamination of a much larger intersensory facilitation induced by the high intensity of TMS we used in this part of experiment. Indeed, the former may have been entirely masked by the latter in four subjects. Thus we studied the effect of TMS using a much lower stimulus intensity in experiment 2.
Increase in the volume of voice induced by TMS
We plotted the voice ratio (ordinate) as a function of the time interval of TMS (abscissa; Fig. 2B). The amount of voice increased significantly when TMS was applied 0–50 msec before EOVt (paired Student's t test p < 0.05). The mean voice volume when TMS was delivered just before EOVt amounted to as much as 1.5 times the voice volume of control trials. The increase in the amount of voice was noted even in the four subjects in whom the delay in RT was not apparent. This increase in voice volume was not observed when the coil was delivered off the scalp or when current was induced in the scalp by a peripheral electrical stimulator at the same time interval. There was also a slight increase in the pitch of voice, although this was not measured in the present study.
Experiment 2
The effect of TMS over the motor strip
The second part of the experiment was performed to investigate where the effect of TMS was largest over the motor strip and midline regions. Nine subjects altogether were recruited for this experiment, seven of which were the same subjects as those recruited in experiment 1. The control RT in these subjects was 275.3 ± 9.2 msec. TMS was delivered ∼50, 100, 150, and 200 msec before the expected onset of voice, because it was shown in experiment 1 that TMS delivered between 0 and 200 msec before EOVt was effective for delaying the RT of vocalization (see Materials and Methods). Because of a slight jitter of RT among sessions, the time intervals of TMS could not be adjusted exactly to 50, 100, 150, and 200 msec before EOVt. Therefore, the overall time intervals during which TMS was delivered relative to EOVt were divided into time bins of 50 msec (0–50, 50–100, 100–150, and 150–200 msec preceding EOVt), according to which the data of single trials were sorted out. The data subsumed within each bin were averaged together, and their SEs were calculated as well to generate plots of time courses as described above.
Figure 3A (top) plots the delay of RT induced over the motor strip in one of the typical subjects when TMS was delivered at various time intervals. The delay increased as TMS was given at a later time. TMS delayed the onset of voice maximally when the coil was placed 6 cm lateral to the left or right of Cz over either hemisphere (left and right motor areas).
Interestingly, the time when the maximal delay occurred over the left and right motor points was somewhat different. Over the left motor area, the delay was maximal with TMS delivered 50–100 msec before EOVt. In contrast, the delay induced over the right motor area became largest when TMS was applied just before the onset of voice (0–50 msec before EOVt). At this late interval, the delay induced over points 9 cm to the left and right of Cz was also significant (as well as over the motor areas). The effective zones for inducing the largest delay (6 cm to the right and left of Cz) roughly corresponds to the locations of the facial representations of the motor strip, which has been implicated in the preparation of vocalization (see Discussion). Therefore the effective regions for inducing delay were taken to represent the regions “active” during the preparation of vocalization. The “activity” of left motor area to precede that of right motor area is also apparent in Figure 3B(bottom), where the same data are depicted as a spatiotemporal contour plot with the site of TMS on the abscissa and the time of TMS on the ordinate.
All the subjects exhibited a similar trend. The plot of grand average is given in Figure 3B (top). There was always a bilateral effect with the delay being most prominent over the left and right motor areas. Coexcitation of the contralateral hemisphere by current spread could be ruled out, because no significant delay was evoked when TMS was delivered over Cz (Fig. 3A,B). The amount of delay induced over these two regions was similar at intervals of 100–150 and 150–200 msec before EOVt, when the “activities” of these regions have just begun to take place. However, the delay induced over the left motor area came to predominate 50–100 msec before EOVt, whereas, at later intervals (0–50 msec before EOVt), the delay induced over the right motor area became more prominent. Overall, the induced delay grew with the time of TMS and the maximal delay of RT attained in each subject reached 29.7–70.0 msec, corresponding to ∼10–20% prolongation of the control RT. Again, the delay over the left motor area was mildly but significantly larger than that induced over the right motor area during the period 50–100 msec before EOVt (pairedt test, p = 0.0276). In contrast, the delay over the right motor area was significantly larger than that induced over the left motor area during the period 0–50 msec before EOVt (paired t test, p = 0.00231). Thus, a mild left hemispheric predominance of activity at early time intervals (50–100 msec before EOVt) switched over to a robust right hemispheric predominance at later intervals (0–50 msec before EOVt). The initial left over right predominance was very mild, whereas the later right over left predominance was robust. This may be ascribed to the fact that the maximal delay over the right motor area always occurred just before the onset of voice (0–50 msec before EOVt), whereas the delay over the left motor area peaked at various latencies relative to EOVt in different subjects, so that in the average plot, the effect over the left motor area was largely smoothed out, whereas that for the right motor area was not. The delay induced at Cz or regions nearby (3 cm to the left and right of Cz) was invariably small. The entire trend described was apparent in the spatiotemporal contour plot in Figure3B (bottom).
The effect of TMS over the midline region
A small delay in RT was also induced when TMS was applied over the midline region (Fig. 3C). Here again, the amount of delay over the midline region increased as TMS was applied at a later time interval. The maximal delay of RT in each subject reached 21.5–51.7 msec at the maximum, corresponding to 8–17% prolongation of the control RT. The most prominent delay for the midline region was noted over a point 2 cm anterior to Cz (significant delay noted at 0–50 and 50–100 msec before EOVt), and a smaller but still significant delay was also induced over a point 4 cm anterior to Cz (at 0–50 msec before EOVt). Conversely, neither the delay induced over Cz nor over a point 6 cm anterior was significant at any of the TMS intervals. Thus, there was a focal region 2–4 cm anterior to Cz at which TMS could induce a significant delay in the onset of voice. These scalp sites lie over the presumed location of SMA proper and were taken to reflect its cortical activity during the late prevocalization period. Meanwhile, no significant delay was induced at a point 6 cm anterior to Cz where the pre-SMA must be located. Thus, pre-SMA does not appear to be involved in the preparation of vocalization as studied by the present experimental paradigm.
Time courses of delay induced over the left and right motor areas and the SMA proper region
So far we have identified activities in three different cortical regions (the right and left motor areas and the SMA proper region) that were considered to be involved in the preparation of vocalization. Figure 4A shows how the relative magnitude of the induced delay changed with time. Here we focused on the three regions and investigated the time courses of the effect of TMS with a small time bin (25 msec) in six subjects.
As noted in experiment 1, TMS was effective in inducing a delay in RT as early as 150–200 msec before the expected onset of voice at all of the three regions. However, the time course of delay differed among regions. As noted in experiment 2, the delay induced over the left motor area was mildly larger than that over the right motor area during the period 50–100 msec before EOVt, whereas a larger delay was noted over the right hemisphere during a later period (0–50 msec before EOVt).
Apart from this difference, the time courses for the motor areas exhibited basically similar features. In contrast, the time course for SMA proper was much different from these. The delay induced over SMA proper was relatively small throughout most of the time period. With TMS applied during a later period (0–50 msec before EOVt), however, the induced delay was comparable for the motor areas and the SMA proper.
To compare the time courses between the motor areas and SMA proper, we first collapsed the data for the left and right motor areas and then subjected them to ANOVA with two factors, the time of TMS, and the site of stimulation (TMS over the motor areas, or TMS over SMA proper). This demonstrated a significant main effect for the time of TMS (F = 11.945; p < 0.0001) as well as a significant interaction between the two factors (F = 3.529; p = 0.0164). The effect of the site of stimulation did not reach significance (F = 1.504;p = 0.2219). Post hoc analysis revealed that this interaction was partly because of significant differences in the delay induced over the motor areas and over the SMA proper region at the time bin 50–100 msec before EOVt, where the former was significantly greater than the latter (p = 0.0004). The difference at the time bin 100–150 msec before EOVt also approached significance (delay over the motor areas greater than that over the SMA proper; p = 0.0573). On the other hand, there was no significant differences between these delays at time bins of 0–50 and 150–200 msec before EOVt (p = 0.3465 and 0.1438, respectively).
Therefore, in this plot we were able to discriminate two distinct phases in the prevocalization period; during the early phase (50–100 msec before EOVt), the delay induced over the motor areas was significantly larger than that induced over the SMA proper region. During the late phase (0–50 msec before EOVt), on the other hand, the delay induced over these two regions was approximately in the same range.
The effect of focal TMS on the volume and duration of voice
TMS using a figure eight coil did not change the volume of voice significantly. ANOVA did not indicate any significant effect for either the time of TMS or the site of stimulation, nor for their interaction (Fig. 4B; ANOVA, p > 0.2). This result, not in keeping with that obtained in experiment 1, was presumably because of the low intensity of the stimuli used in this part of experiment.
Nor did focal TMS elicit any significant change in the duration of voice. ANOVA performed for the duration of voice did not point to any significant effect either for the time of TMS or for the type of stimulation, nor for their interaction (p > 0.3).
Experiment 3
Comparison of the effect of unilateral and bilateral TMS
The preceding experiments showed that both the left and right motor areas are involved in the preparation of vocalization, pointing to the bilateral nature of the motor preparation for vocalization. In this part of experiment, we compared the effect of unilateral versus bilateral TMS delivered over the left and right motor areas. During the period preceding EOVt by 50–150 msec, the delay induced by unilateral TMS (the data were collapsed for both the left or right motor areas) and that induced by bilateral TMS exhibited an almost identical time course (Fig. 5A; in this figure, data for unilateral left and right motor areas were pooled together into a single plot). Indeed, the delay was somewhat smaller for bilateral TMS during this period. This may have been caused by intersensory facilitation; accessory sensory inputs accompanying bilateral TMS were probably greater than those accompanying unilateral TMS and therefore may have caused a larger intersensory facilitation (Terao et al., 1997).
In comparison, during the period 0–50 msec before EOVt, bilateral TMS induced a significantly larger delay than unilateral TMS. ANOVA demonstrated a significant effect for the time of TMS (F = 11.945; p < 0.001), but not for the type of stimulation (unilateral or bilateral TMS, F= 1.504; p = 0.2219). A significant interaction was noted between the two factors (F = 2.119;p = 0.0164). The interaction was ascribed to the significantly larger delay induced by bilateral TMS than by unilateral TMS during the late phase (p = 0.0079), whereas there was no significant difference in delay during the early phase (p > 0.2).
The effect of bilateral TMS on voice volume
Bilateral TMS slightly increased the amount of voice when TMS was applied 0–100 msec before EOVt (Fig. 5B). ANOVA indicated a significant effect for the type of stimulation (unilateral versus bilateral TMS; F(1,5) = 9.872,p = 0.0020), but not for the time of TMS (F = 1.974; p = 0.1202). Although the interaction between these two factors was not significant (F = 1.513; p = 0.2681), the volume of voice tended to increase when bilateral TMS was applied at the late phase (0–50 and 50–100 msec before EOVt). This was reflected in the mild trend toward an increase in voice volume with bilateral TMS at time bins of 0–50 msec (p = 0.0194) and 50–100 msec (p = 0.0210) in the post hocanalysis.
To compare this with the results of experiments 1 and 2, the increase of voice volume was noted only with TMS of high intensity (experiment 1) or bilateral focal TMS (experiment 3), but not with unilateral TMS (experiment 2). This was considered because of the relatively “high threshold” for stimulating the motor representations of expiratory muscles (Gandevia and Rothwell, 1987; Maskill et al., 1991).
DISCUSSION
Time course and topography of the effect of TMS
TMS delivered up to 150–200 msec before the expected onset of voice delays the RT of vocalization. This suggested that the cortical preparation for vocalization starts as early as or earlier than 150–200 msec before the onset of voice. Because RT of voice ranged from 250–300 msec after the visual cue, the onset of this cortical process may be initiated slightly earlier than 100–150 msec after the visual cue. The delay persisted until TMS was applied just before the onset of voice, i.e., the process continued until the onset of voice.
The effect of TMS was largest when delivered 6 cm lateral to Cz over each hemisphere. These regions correspond to the presumed locations of facial/laryngeal motor areas, in which Penfield et al. (1949, 1959) could arrest vocalization most effectively by electrical stimulation in humans.
Recent neuroimaging studies during vocalization have found activities also in the motor representations of truncal/respiratory muscles, in accordance with their known role in phonation (Price et al., 1996;Hirano et al., 1997; Gunji et al., 2000). However, TMS induced only a small delay over these cortical regions (Fig. 1A,sites C and E). In our experimental paradigm, the subjects may have produced small pulses of breath to respond quickly to the cues mainly by contracting small muscles such as the internal intercostal muscles, without recruiting larger truncal muscles. Thus, activity in the motor representation of truncal/respiratory muscles may have remained lower than expected, which may explain the small effect of TMS.
Based on this and other preceding studies (Amassian et al., 1989;Maccabee et al., 1991), the time course from the presentation of the visual cue to the onset of voice may be described as follows (Fig.6). Visual input to the retina is transferred to the calcarine cortex within 40–60 msec, is processed in the visual cortical areas, and travels through them in 120 msec. Our study along with that of Cracco et al. (1996) showed that the cortical preparation for vocalization had already begun by 100–150 msec (120–140 msec in Cracco et al., 1996) after the visual cue.
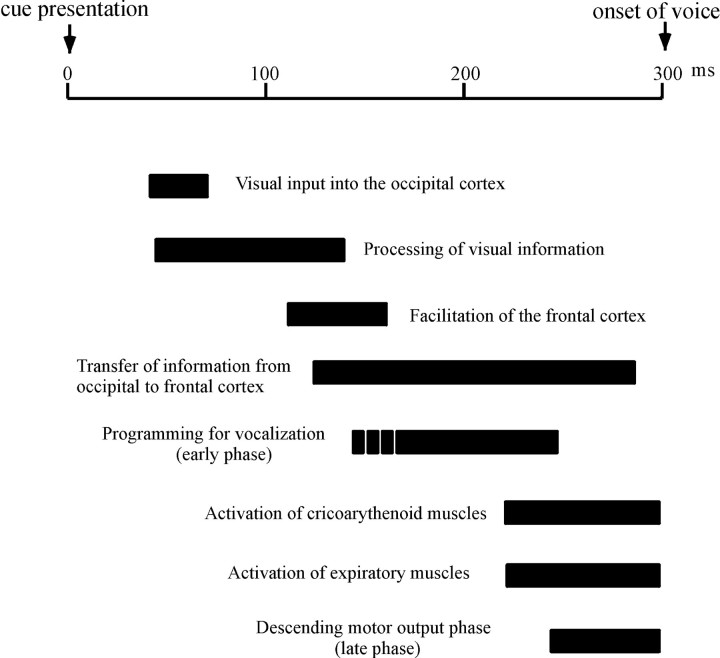
Fig. 6.
Schematic diagram of the cortical preparation for human vocalization. A diagram illustrating the time course of the cortical preparation for vocalization based on the results of the present and preceding studies. The entire duration of the prevocalization period corresponds to the reaction time of voice measured from the time of visual cue to the onset of voice, which is ∼350 msec in Cracco's study and 250–300 msec in ours. To accommodate all the results from preceding and present studies into a single diagram, the time course was scaled such that the total duration was 300 msec. Visual input to the retina is transferred to the visual cortex in 40–60 msec, which is processed in and travels out of the striate and extrastriate visual cortices by 120 msec (Amassian et al., 1989). Activation of the frontal cortex is apparent at ∼120 msec (Cracco et al., 1996). The early phase of cortical preparation starts as early as 150–200 msec before the expected onset of voice (i.e., 100–150 msec after visual cue presentation), which is followed by a distinct later phase 0–50 msec before EOVt. During the early phase (from 50 to 100 msec before the onset of voice), a mild left hemispheric predominance for inducing the delay is noted. During the later phase, this switches over to robust right hemispheric predominance. During the same period, the volume of voice increases by TMS of high intensity or by bilateral TMS. These almost coincide in time with the muscle contraction of the lateral cricoarythenoid muscle, beginning 80–100 msec before the onset of voice (Hirose and Gay, 1972) and the activation of laryngeal EMG, preceding the onset of voice by 80 msec (Cracco et al., 1996). Activation of the SMA proper region was also apparent during this late phase. The effect of bilateral TMS is greater than that of unilateral TMS during this late period. These results indicate that multiple cortical areas are active during the late phase.
The delay induced by TMS over the SMA proper region was small during the early phase (50–100 msec before EOVt) but became comparable with those induced over the motor areas during the late phase of the prevocalization period (0–50 msec before EOVt; Fig.4A). Thus, SMA proper may play only a minor role during the early phase, but become much more involved during the late phase. Although SMA proper has been implicated in the programming of voluntary movements (Cheyne and Weinberg, 1989) and speech (Grözinger et al., 1980), Dum and Strick (1996) showed that SMA proper sends direct projections to the spinal cord and may be more directly involved in motor execution.
Changes in the voice volume induced by TMS
TMS delivered during the late phase increased the volume of voice. Vocalization concludes with the expiration of air through vocal folds when subglottic air pressure overcomes vocal fold resistance. Expiratory muscles are thus presumably active during this late phase. In limb muscles, voluntary muscle contraction raises the excitability of motor neuron pool innervating the activated muscles and makes them more ready to discharge by other descending commands, enhancing the amplitude of MEPs elicited by TMS. Likewise, TMS of the same intensity would evoke a larger contraction in expiratory muscles during activation than at rest, resulting in an increase in subglottic pressure and airflow through the vocal cord.
Vocal intensity increases with subglottic pressure, and if this pressure increases without muscular adjustments of the vocal folds, the fundamental frequency of voice will increase as well as its intensity (Borden et al., 1994). Thus, when one is struck in the stomach while phonating a steady tone or just as one is going to produce a voice, the tone not only gets louder, but increases in pitch. A similar mechanism may explain the increase in vocal intensity and pitch induced by TMS during the late phase.
Bilateral motor control for vocalization
Activation of bilateral hemispheres during vocalization as demonstrated in PET studies may reflect either the activities of both hemispheres working in concert or the activity of one active hemisphere inhibiting that of the other. The former is more likely because, if either of the two hemispheres were capable of producing vocalization alone, the delay would not have been evoked by unilateral TMS.
In primates, the periaqueductal gray (PAG) serves as a bottleneck region for vocalization that receives all the descending inputs from supraspinal centers, including the cerebral cortex, and relays these to the phonatory motoneuron pools located in the medulla and the spinal cord (Jürgens and Zwirner, 1996; Jürgens, 1998). If we postulate a similar pathway for humans, the cortical preparation for vocalization may be considered as a process through which motor buffer is formed within bilateral motor cortices and released to relevant brainstem centers.
Cortical processing during the early and late phase
Given the bilateral motor control for vocalization, what would happen if both hemispheres were “blocked” at the same time? If TMS mainly interfered with the formation of motor buffer, the cortical process for this formation may be slowed, but the buffer itself would not disappear. Once formed and ready in both hemispheres, the motor buffer is released to relevant brainstem centers, so that the effect of TMS cannot be profound even if it is delivered bilaterally. If bilateral TMS delays buffer formation in both hemispheres by the same amount of time Δt, RT would also be delayed by Δt, because in both hemispheres, the buffer formation is completed with a delay of Δt. Unilateral TMS may delay buffer formation in the stimulated hemisphere by time Δt, but not in the unstimulated hemisphere. Here again, RT may be delayed by the same amount of time Δt, if we postulate that the motor buffers in both hemispheres should be completed for them to be released for motor output. TMS during the early phase may have interfered mainly with the buffer formation, because the induced delay was nearly identical with unilateral or bilateral TMS.
On the other hand, if TMS blocked the release of motor buffer into relevant brainstem centers, bilateral TMS would induce a delay of RT well in excess of that induced by unilateral TMS. This is because bilateral TMS would abolish the descending commands from both hemispheres, greatly reducing the motor output, whereas unilateral TMS would spare at least the motor output from the unstimulated hemisphere. Because bilateral TMS induced a much larger delay than unilateral TMS during the late phase, this phase should be mainly dedicated to the release of motor commands into relevant brainstem centers.
Hemispheric lateralization in the motor preparation for vocalization
Concurrent activation of both hemispheres to achieve a motor task is not unique to vocalization and has also been described for oculomotor tasks (Terao et al., 1998), in which the time courses of activities in homologous regions of both hemispheres were almost identical. By contrast, bilateral motor areas exhibited activities with slightly different time courses for vocalization. A mild predominance of left motor area activity was apparent during the early phase, which switched over to robust right hemispheric predominance during the late phase. Thus, activation of the left motor area preceded the right motor activity by 50–100 msec, and the entire process may be looked on as an alternation of hemispheric lateralization (from right to left) as the cortical preparation of vocalization proceeded from the early to late phase.
The left predominance during the early phase, i.e., during the programming of motor buffer, is consistent with some recent PET studies (Wildgruber et al., 1996). Meanwhile, active expiration takes place during the late phase of vocalization. PET studies during expiration have revealed blood flow increases bilaterally in the primary motor cortex, the right premotor cortex, the SMA, and the cerebellum (Colebatch et al., 1991; Ramsay et al., 1993). The cortical regions activated on the right lateral convexity was more prominent than on the left (the premotor cortex was activated only on the right side), which is congruent with the demonstrated right hemispheric predominance during the late phase.
Recently, Jürgens and Zwirner (2000) implanted electrodes into the facial motor cortices bilaterally at sites where electrical stimulation evoked vocal fold adduction and also at PAG sites producing vocalization. Motor cortical stimulation blocked vocalization elicited by the stimulation of PAG. This was more evident with left-sided ipsilateral motor cortex/PAG stimulation than with right-sided motor cortex/PAG stimulation in half of the animals. The reverse was true for the rest of the animals. Therefore, the majority of monkeys exhibited hemispheric asymmetry in vocal fold control, whether right- or left-dominant. If the motor cortex/PAG connection was right-predominant in our subjects, it would be plausible that the right motor area is active predominantly during the late phase of prevocalization period serving as its motor output phase. PET studies, however, have failed to demonstrate hemispheric predominance probably because the right predominance was noted phasically only during the late phase.
Footnotes
This work has been supported in part by the Sankyo Foundation of Life Science and Research Project Grant-in-aid for Scientific Research number 12680768 from the Ministry of Education, Sciences, Sports, and Culture of Japan.
Correspondence should be addressed to Yoshikazu Ugawa, Department of Neurology, Division of Neuroscience, Graduate School of Medicine, University of Tokyo 7-3-1 Hongo, Bunkyo-ku, Tokyo 113–8655, Japan.E-mailugawa-tky@umin.ac.jp.
REFERENCES
- 1.Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell AP, Eberle L. Suppression of visual perception by magnetic coil of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Borden GJ, Harris KS, Raphael LJ. Speech science primer. Williams & Wilkins; Baltimore: 1994. [Google Scholar]
- 3.Cheyne D, Weinberg H. Neuromagnetic fields accompanying unilateral finger movements: premovement and movement-evoked fields. Exp Brain Res. 1989;78:604–612. doi: 10.1007/BF00230248. [DOI] [PubMed] [Google Scholar]
- 4.Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol (Lond) 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Flow of symbolic visual information from retina to calcarine cortex to frontal lobe to vocalization. Electroencephalogr Clin Neurophysiol. 1996;103:13. [Google Scholar]
- 6.Day BL, Rothwell JC, Thompson PD, Maertens de Noorthout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- 7.Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol (Lond) 1987;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemba H, Miki N, Sasaki K. Cortical field potentials preceding vocalization and influences of cerebellar hemispherectomy upon them in monkeys. Brain Res. 1995;697:143–151. doi: 10.1016/0006-8993(95)00797-t. [DOI] [PubMed] [Google Scholar]
- 10.Gemba H, Miki N, Sasaki K. Cortical field potentials preceding vocalization and in monkeys. Acta Otolaryngol (Stockh) 1997;[Suppl ] 532:96–98. doi: 10.3109/00016489709126151. [DOI] [PubMed] [Google Scholar]
- 11.Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation of the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1999;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- 12.Goodale MA. Hemispheric differences in motor control. Behav Brain Res. 1988;30:203–214. doi: 10.1016/0166-4328(88)90149-0. [DOI] [PubMed] [Google Scholar]
- 13.Grözinger B, Kornhuber HH, Kriebel J, Szirtes J, Westphal KTP. Participation of medial cortex in speech: evidence from cerebral potentials preceding speech production in man. Exp Brain Res. 1980;[Suppl ] 2:189–192. [Google Scholar]
- 14.Gunji A, Kakigi R, Hoshiyama M. Spatiotemporal source analysis of vocalization-associated magnetic fields. Cogn Brain Res. 2000;9:157–163. doi: 10.1016/s0926-6410(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 15.Hast MH, Fischer JM, Wetzel AB, Thompson VE. Cortical motor representation of the laryngeal muscles in Macaca mulatta. Brain Res. 1974;73:229–240. doi: 10.1016/0006-8993(74)91046-4. [DOI] [PubMed] [Google Scholar]
- 16.Herholz K, Pietrzk U, Karbe H, Würker M, Wienhard K, Heiss W-D. Individual metabolic anatomy of repeating words demonstrated by MRI-guided positron emission tomography. Neurosci Lett. 1994;182:47–50. doi: 10.1016/0304-3940(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 17.Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Pütz B. Activation of human presupplementary area in learning of sequential procedures: a functional MRI study. J Neurophysiol. 1996;76:617–621. doi: 10.1152/jn.1996.76.1.617. [DOI] [PubMed] [Google Scholar]
- 18.Hirano S, Kojima H, Naito Y, Honjo I, Kamoto Y, Okazawa H, Ishizu K, Yonekura Y, Nagahama Y, Fukuyama H, Konishi J. Cortical processing mechanism for vocalization with auditory verbal feedback. NeuroReport. 1997;8:2379–2382. doi: 10.1097/00001756-199707070-00055. [DOI] [PubMed] [Google Scholar]
- 19.Hirose H, Gay T. The activity of the intrinsic laryngeal muscles in voicing control. Phonetica. 1972;25:140–164. doi: 10.1159/000259378. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda A, Lüders HO, Burgess RC, Shibasaki H. Movement-related potentials recorded from the supplementary motor area and primary motor area. Brain. 1992;115:1017–1043. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- 21.Jürgens U. Neuronal control of mammalian vocalization, with special reference to the squirrel monkey. Naturwissenschaft. 1998;85:376–388. doi: 10.1007/s001140050519. [DOI] [PubMed] [Google Scholar]
- 22.Jürgens U, Ploog D. Cerebral representations of vocalization in the squirrel monkey. Exp Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- 23.Jürgens U, Ploog D. Zur Evolution der Stimme. Arch Psychiatr Nervenkr. 1976;222:117–137. doi: 10.1007/BF02206613. [DOI] [PubMed] [Google Scholar]
- 24.Jürgens U, Zwirner P. The role of the periaqueductal gray in limbic and neocortical vocal fold control. NeuroReport. 1996;7:2921–2923. doi: 10.1097/00001756-199611250-00023. [DOI] [PubMed] [Google Scholar]
- 25.Jürgens U, Zwirner P. Individual hemispheric asymmetry in vocal fold control of the squirrel monkey. Behav Brain Res. 2000;109:213–217. doi: 10.1016/s0166-4328(99)00176-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee KM, Chang KH, Roh JK. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. NeuroImage. 1999;9:117–123. doi: 10.1006/nimg.1998.0393. [DOI] [PubMed] [Google Scholar]
- 27.Maccabee PJ, Amassian VE, Cracco RQ, Cracco JB, Rudell AP, Eberle LP, Zemon V. Magnetic coil stimulation of human visual cortex: studies of perception. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:111–120. [PubMed] [Google Scholar]
- 28.Maskill D, Murphy K, Mier A, Guz A. Motor cortical representation of the diaphragm in man. J Physiol (Lond) 1991;443:105–201. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer BU, Werhahn K, Rothwell JC, Roericht S, Fauth C. Functional organisation of corticonuclear pathways to motoneurones of lower facial muscles in man. Exp Brain Res. 1994;101:465–472. doi: 10.1007/BF00227339. [DOI] [PubMed] [Google Scholar]
- 30.Müri RM, Rösler KM, Hess CW. Influence of transcranial magnetic stimulation on the execution of memorized sequence of saccades in man. Exp Brain Res. 1995;101:163–166. doi: 10.1007/BF00227345. [DOI] [PubMed] [Google Scholar]
- 31.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- 33.Penfield W, Rasmussen T. Vocalization and arrest of speech. Arch Neurol Psychiatr. 1949;61:21–27. doi: 10.1001/archneurpsyc.1949.02310070027002. [DOI] [PubMed] [Google Scholar]
- 34.Penfield W, Roberts L. Speech and brain mechanism. Princeton UP; Princeton: 1959. [Google Scholar]
- 35.Petersen SE, Fiez JA. The processing of single words studied with positron emission tomography. Annu Rev Neurosci. 1993;16:509–520. doi: 10.1146/annurev.ne.16.030193.002453. [DOI] [PubMed] [Google Scholar]
- 36.Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 37.Priori A, Bertolasi L, Rothwell JC, Day BL, Marsden CD. Some saccadic eye movements can be delayed by transcranial magnetic stimulation. Brain. 1993;116:355–367. doi: 10.1093/brain/116.2.355. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Baily DL, Frackowiak RSJ, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol (Lond) 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terao Y, Ugawa Y, Suzuki M, Sakai K, Gemba-Shimizu K, Kanazawa I. Shortening of the simple reaction time by peripheral electrical and sub-motor-threshold magnetic cortical stimulation. Exp Brain Res. 1997;115:541–545. doi: 10.1007/pl00005724. [DOI] [PubMed] [Google Scholar]
- 40.Terao Y, Fukuda H, Ugawa Y, Hikosaka O, Furubayashi T, Hanajima R, Sakai K, Kanazawa I. Visualization of the information through human oculomotor cortical regions by transcranial magnetic stimulation. J Neurophysiol. 1998;80:936–946. doi: 10.1152/jn.1998.80.2.936. [DOI] [PubMed] [Google Scholar]
- 41.Wildgruber D, Ackermann H, Klose U, Kardatzki B, Grodd W. Functional lateralization of speech production at primary motor cortex: a fMRI study. NeuroReport. 1996;7:2791–2795. doi: 10.1097/00001756-199611040-00077. [DOI] [PubMed] [Google Scholar]
- 42.Yonekura Y, Nagahama Y, Fukuyama H, Konishi J. Cortical processing mechanism for vocalization with auditory verbal feedback. NeuroReport. 1997;8:2379–2382. doi: 10.1097/00001756-199707070-00055. [DOI] [PubMed] [Google Scholar]