Abstract
Interleukin-1β (IL-1β) is a proinflammatory cytokine associated with the pathophysiology of demyelinating disorders such as multiple sclerosis and viral infections of the CNS. However, we demonstrate here that IL-1β appears to promote remyelination in the adult CNS. InIL-1β−/− mice, acute demyelination progressed similarly to wild-type mice and showed parallel mature oligodendrocyte depletion, microglia–macrophage accumulation, and the appearance of oligodendrocyte precursors. In contrast,IL-1β−/− mice failed to remyelinate properly, and this appeared to correlate with a lack of insulin-like growth factor-1 (IGF-1) production by microglia–macrophages and astrocytes and to a profound delay of precursors to differentiate into mature oligodendrocytes. Thus, IL-1β may be crucial to the repair of the CNS, presumably through the induction of astrocyte and microglia–macrophage-derived IGF-1.
Keywords: oligodendrocytes, astrocytes, microglia, remyelination, cytokines, growth factors
Metabolic, toxic, or autoimmune insult of the adolescent and adult CNS may lead to the depletion of mature oligodendrocyte population within the lesion during demyelination (Blakemore, 1973; Yagima and Suzuki, 1979; Yao et al., 1995; Gensert and Goldman, 1997; Mason et al., 2000a). Consequently, an alternative source of oligodendrocytes most likely participates in the remyelination. Several studies have identified differentiating oligodendrocyte progenitors as the cells responsible for remyelinating lesions within the adolescent (Ludwin, 1979) and adult (Gensert and Goldman, 1997; Mason et al., 2000a) CNS. The factor(s) responsible for the recruitment and differentiation of these progenitors in vivo has not been fully delineated.
One factor is a 17 kDa proinflammatory cytokine, interleukin-1β (IL-1β) (Bauer et al., 1993; Sairanen et al., 1997), that is produced primarily by microglia and macrophages (Giulian et al., 1986; Bauer et al., 1993; Sairanen et al., 1997). This cytokine, in turn, induces the production of IL-6 and tumor necrosis factor-α, as well as nitric oxide (for review, see Lee et al., 1995). It also induces the proliferation of macrophages (Feder and Laskin, 1994) and astrocytes (Giulian and Lachman, 1985; Giulian et al., 1988), both in vitro and in vivo, but not oligodendrocyte progenitorsin vitro (Merrill, 1991). In contrast, IL-1β has been shown to be cytotoxic to mature oligodendrocytes in vitro(Merrill, 1991; Brogi et al., 1997). Therefore, IL-1β has been associated predominantly with exacerbating pathology in the CNS.
Repair of insult to the CNS has been increasingly attributed to immune responses (Diemel et al., 1998; Schwartz et al., 1999; Warrington et al., 2000). Along these lines, IL-1β may activate cells within the CNS to produce growth factors known to induce the proliferation and differentiation of oligodendrocyte progenitors in vitro(Araujo and Cotman, 1992; Silberstein et al., 1996; Glazebrook et al., 1998). One of these growth factors, insulin-like growth factor-1 (IGF-1), has been colocalized within demyelinating lesions of the CNS (Komoloy et al., 1992; Liu et al., 1994; Yao et al., 1995) and shown to parallel the accumulation and differentiation of oligodendrocyte progenitors (Mason et al., 2000a). Furthermore, the early expression of IGF-1 before morphologic demyelination protects mature oligodendrocytes from cell death and promotes rapid repair of the lesion (Mason et al. 2000b).
To address the role of IL-1β in the remyelination process, we used a model in which continuous cuprizone (bis-cyclohexanone oxaldihydrazone) intoxication of adult C57BL/6 mice leads to perturbation and death of mature oligodendrocytes. This is followed by massive demyelination in the corpus callosum by 5 weeks (Hiremath et al., 1998; Morell et al., 1998; Mason et al., 2000a). Simultaneously, oligodendrocyte progenitors accumulate within the lesion beginning at 3–4 weeks and results in mature oligodendrocytes repopulation beginning at week 6, with remyelination occurring over the next 4 weeks (Morell et al., 1998; Mason et al., 2000a, 2001). In this study, we noted that IL-1β was upregulated in mice exposed to cuprizone and used IL-1β-deficient mice (Shornick et al., 1996) to examine the functional importance of IL-1β in demyelination–remyelination.
MATERIALS AND METHODS
Induction of demyelination–remyelination. IL-1β−/− mice on the C57BL/6 background were bred in our mouse colony, and C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). At 8 weeks of age, the mice were fed a diet containing 0.2% cuprizone (Sigma, St. Louis, MO) by weight, mixed into ground mouse chow for 6 weeks to induce demyelination (Hiremath et al., 1998). Subsequently, mice were returned to a normal diet for another 6 weeks to allow remyelination to occur. Sham and cuprizone-treated mice were killed weekly. The forebrains from the mice were removed for RNA, protein, and immunohistochemical analysis as described previously (Mason et al., 2000a). All mice were maintained in accordance with guidelines approved by the Institutional Animal Care and Use Committee and the University of North Carolina Division of Laboratory Animal Medicine.
RNA analysis. Total RNA was prepared from half of the forebrain of each mouse, reverse transcribed into cDNA, and then amplified by PCR as described previously (Mason et al., 2000a). Primer sequences used for the PCR reactions have been published previously: IGF-1 (Shinar and McMorris, 1995) and IL-1β and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (Bencsik et al., 1996). Templates were denatured at 95°C for 5 min, followed by 30 cycles of denaturation (95°C for 1 min) primer annealing (62°C for 1.5 min) and extension (72°C for 1 min), with a final extension step at 72°C for 15 min. The amplified cDNA mixture of each sample was separated on a 2.5% agarose gel containing ethidium bromide and photographed. Amplification of the housekeeping gene G3PDH confirmed RNA amplification and equal loading of each sample. The mRNA profiles were semiquantitated using a densitometer to normalize all samples for comparison.
Immunohistochemistry. All comparative analyses were focused in the corpus callosum on either side of midline in sections 220–260 of the mouse brain atlas (Sidman et al., 1971). The frozen sections were stained for IL-1β (Endogen, Woburn, MA), IGF-1 (the rabbit anti-IGF-1 antibody was a gift from Dr. Underwood, Chapel Hill, NC), and NG2 (the rabbit anti-NG2 antibody was a gift from Dr. William Stallcup, San Diego, CA) as described previously (Mason et al., 2000a).
In addition, adjacent sections were double-stained for GFAP, an astrocyte marker (Santa Cruz Biotechnology, Santa Cruz, CA), and either IL-1β or IGF-1, or sections were stained for CD11b (Mac-1), a microglial–macrophage marker (PharMingen, San Diego, CA) and either IL-1β or IGF-1. Tissue sections were incubated with rabbit anti-IGF-1 antibody and either goat anti-GFAP or biotin-conjugated rat anti-CD11b antibody, or rabbit anti-IL-1β antibody and either goat anti-GFAP or biotin-conjugated rat anti-CD11b antibody overnight at 4°C. The GFAP–IGF-1 and GFAP–IL-1β-labeled sections were then incubated with biotin-conjugated horse anti-goat secondary antibody (Vector Laboratories, Burlingame, CA). The GFAP–IGF-1, GFAP–IL-1β, CD11b–IGF-1, and CD11b–IL-1β-labeled sections were incubated with a combination of a rhodamine-conjugated goat anti-rabbit secondary antibody (Vector Laboratories) strepavidin–fluorescein complex (Vector Laboratories) before mounting with a coverslip. No positive cell stain was observed in tissue sections incubated with control isotype-matched antibodies (rabbit IgG, Vector Laboratories; goat IgG, Vector Laboratories; or rat IgG2a, PharMingen) in place of the primary antibodies. Positive-stained cells were quantified only if a nucleus was observed.
Paraffin-embedded sections were labeled for the Pi isoform of glutathione S-transferase (GST-Pi; a mature oligodendrocyte marker) or stained with Ricinus communis agglutin-1 (RCA-1; marks microglia–macrophage) as described previously (Morell et al., 1998; Mason et al., 2000a).
Cell number quantification.IL-1β+, IL-1β+–GFAP+, IL-1β+–Mac-1+, IGF-1+, NG2+, RCA-1+, and GST-Pi+ cells from three to four mice were analyzed using a Nikon (Tokyo, Japan) Optiphot FXA microscope with epifluorescence optics as described previously (Mason et al., 2000a).
Electron microscopy. Tissue samples from the forebrain of 0, 5, and 10 week treated mice (n = 3) were processed for electron microscopic analysis, and the cross-sections of the corpus callosi were analyzed as described previously (Coetzee et al., 1996;Dupree et al., 1998).
Protein analysis. Protein was extracted from half of a frozen forebrain of the wild-type andIL-1β−/− mice. The frozen forebrains were homogenized and boiled in 1% SDS as described previously (Coetzee et al., 1996). Protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard. Quantification of IGF-1 protein levels were determined by radioimmunoassay as described previously (Ye et al., 1996) using 20 μg of total protein.
Statistical analysis. Statistical comparisons were made using a one-factor between-subjects ANOVA. Multiple comparisons among treatment groups were made with Tukey's test.
RESULTS
Expression of IL-1β precedes an increase in IGF-1 expression
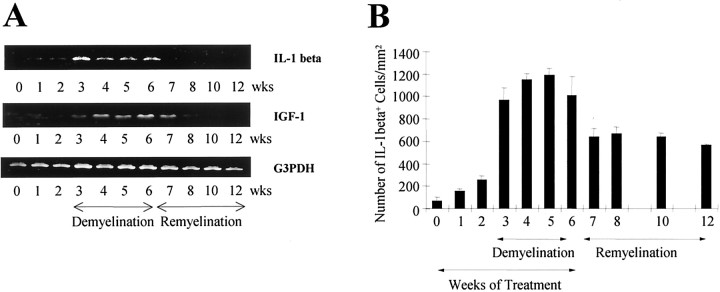
The expression of IL-1β and IGF-1 mRNA during demyelination and remyelination was analyzed from the forebrains of wild-type mice. IL-1β mRNA levels, although undetectable in sham mice, was markedly upregulated during cuprizone exposure (Fig.1A). At week 3, there was a dramatic increase in IL-1β that was sustained up to 6 weeks. Thereafter, when cuprizone was removed from the diet at week 6 and remyelination was allowed to proceed, IL-1β mRNA levels diminished to control levels. The mRNA levels for IGF-1 followed the increased expression of IL-1β (Fig. 1A), in which a low level of IGF-1 was detected at week 3 followed by an increased expression from week 4 through week 7 (1 week into the remyelination phase).
Fig. 1.
Expression of IL-1β during demyelination–remyelination in wild-type mice. A, Reverse transcription-PCR of RNA extracted from brains of mice at weekly intervals was examined for IL-1β and IGF-1. Mice were exposed to cuprizone for 6 weeks and allowed to recover. A representative example of three time course experiments is illustrated.B, The number of IL-1β+ cells in the corpus callosum at the level of the fornix. The mean and SEM bars representing the number of IL-1β+ cells per square millimeter are plotted for the triplicate set of samples.
Microglia–macrophages and some astrocytes produce IL-1β
A few IL-1β+ cells began to accumulate in the corpus callosum at 1 and 2 weeks compared with untreated control mice (Figs. 1B,2A). This number dramatically increased and coincided with the progression of demyelination at 3 weeks in wild-type mice (Figs. 1B,2B). The peak in both the number of IL-1β+ cells and demyelination within the corpus callosum between 4 and 5 weeks (Fig. 1B) is temporally and spatially correlated with the dramatic accumulation of IGF-1+ cells (see Fig.6A) and NG2+oligodendrocyte progenitors (Mason et al., 2000a) within the demyelinating lesion. Thereafter, a large number of IL-1β+ cells remained in the corpus callosum during the remyelination process (Fig. 1B), when mature oligodendrocytes begin to repopulate the lesion area (Mason et al., 2000a).
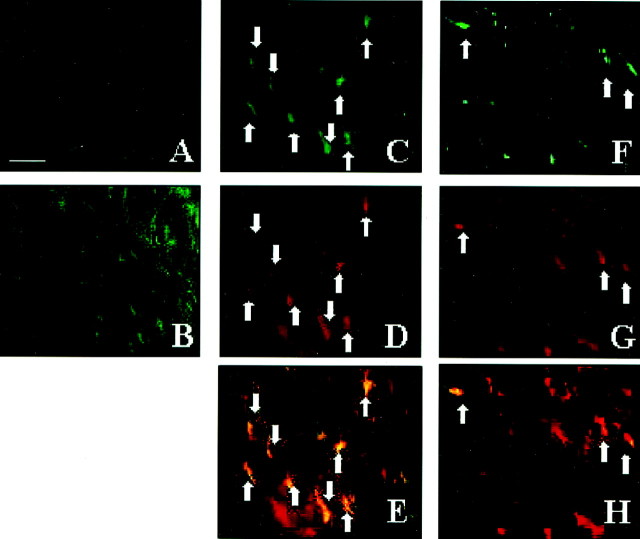
Fig. 2.
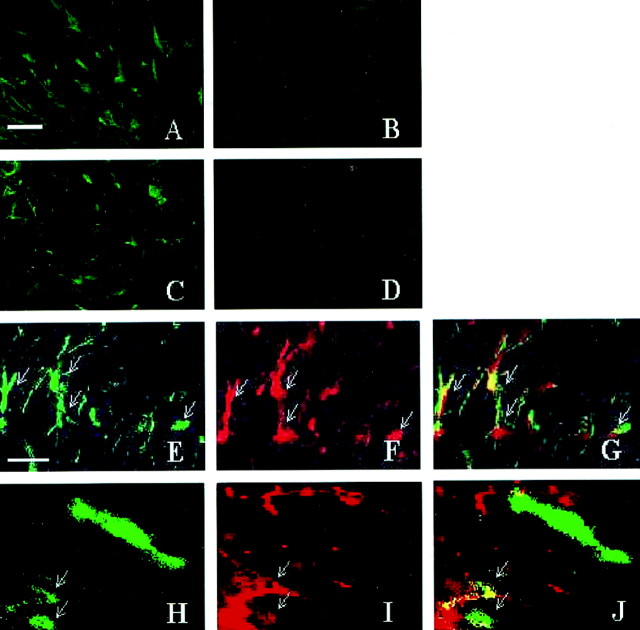
Most microglia–macrophages and some astrocytes express IL-1β during demyelination–remyelination in wild-type mice. A, Few or no IL-1β+ cells are present in the untreated corpus callosum. B, A large accumulation of IL-1β+ cells begins at week 3 in the medial region of the corpus callosum posterior to the fornix.C–E, Representative sections demonstrating the colocalization (arrows) of IL-1β-expressing cells (green-stained cells in C) to nearly all of the Mac-1+ macrophages (red-stained cells in D and overlaid inE) in the corpus callosum at week 4.F–H, The colocalization (arrows) of a few IL-1β-expressing cells (green-stained cells in F) to GFAP+ astrocytes (red-stained cells in G and overlaid inH) in the corpus callosum. Scale bar, 30 μm.
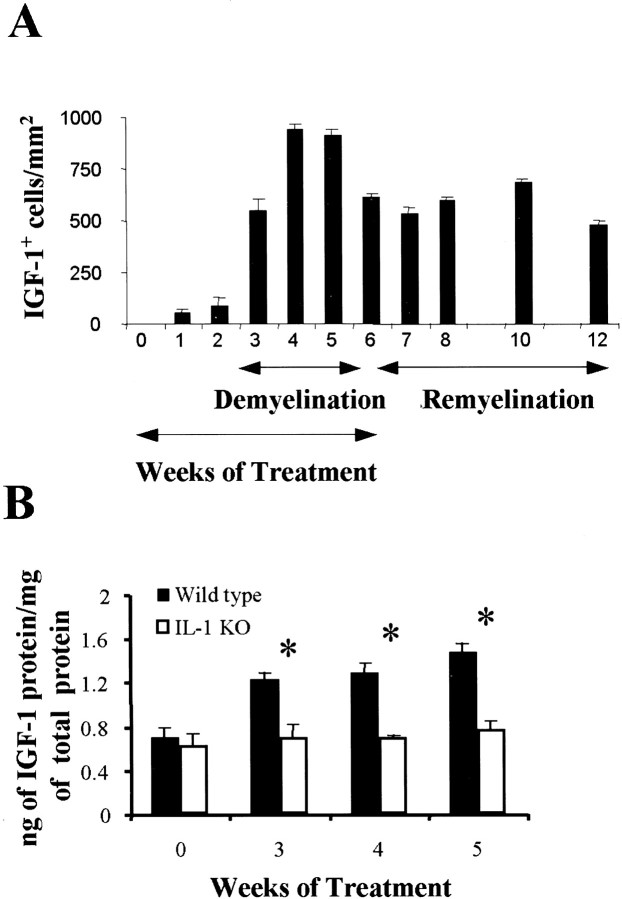
Fig. 6.
The number of IGF-1+ cells and amount of IGF-1 protein increase within a demyelinating corpus callosum in wild-type mice but not in IL-1β−/−mice. A, The mean and SEM bars representing the number of IGF-1+ cells per square millimeter in wild-type mice is plotted for the duplicate set of samples (*p < 0.001). B, The mean and SEM bars representing the amount of IGF-1 protein within the corpus callosum of wild-type and IL-1β−/− mice is plotted for the triplicate set of samples. KO, Knock-out.
The large accumulation of IL-1β-expressing cells in the corpus callosum of wild-type mice detected at week 3 (Fig.2B) closely parallels the increased presence of microglia–macrophages and astrocytes as reported previously (Hiremath et al., 1998; Morell et al., 1998). Immunohistochemistry showed that 81% of the IL-1β+ cells were Mac-1+ microglia–macrophages at week 4, whereas the remainder were mostly astrocytes. Nearly all of Mac-1+ microglia–macrophages (Fig.2C–E) and only 49% of GFAP+astrocytes colocalized with IL-1β expression, as demonstrated in Figure 2F–H. These results suggest that microglia–macrophages are the predominant cell type producing IL-1β during the peak stages of demyelination.
Demyelination and oligodendrocyte depletion progressed similarly in both wild-type and IL-1β−/− mice
A similar number of GST-Pi+ mature oligodendrocytes and myelinated axons (85.6 ± 4.8 vs 93.8 ± 2.3%, respectively) was observed in both untreatedIL-1β−/− and wild-type mice (Figs.3A,B,4A,B,5). During exposure to cuprizone, the axons in the corpus callosum ofIL-1β−/− (4.2 ± 1.8% myelinated) and wild-type (7.6 ± 2.1% myelinated) mice were almost completely demyelinated at 5 weeks (Fig.3C,D). Thus, IL-1β alone does not appear to contribute to demyelination. Coinciding with this demyelination at 5 weeks was the accumulation of RCA-1+microglia–macrophage within the lesion site in bothIL-1β−/− (3165 ± 127 per square millimeter) and wild-type (2949 ± 226 per square millimeter) mice. In addition, GST-Pi+ mature oligodendrocytes were also depleted within the lesion in both wild-type andIL-1β−/− mice at 5 weeks (Figs. 4C,D, 5), as expected from our previous report (Mason et al., 2000). These results suggest that the absence of IL-1β does not have a dramatic effect on the pathological processes induced by cuprizone exposure.
Fig. 3.
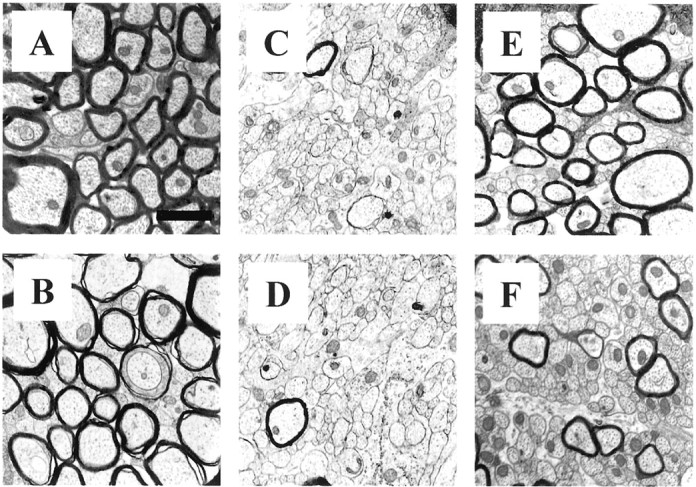
Representative electron micrographs of the myelinated, demyelinated, and remyelinated axons in the corpus callosum of wild-type and IL-1β−/− mice. Almost all axons are myelinated in the corpus callosum of untreated wild-type (A) andIL-1β−/− (B) mice. Negligible number of myelinated axons, corresponding to peak demyelination, are present in the corpus callosum of 5 week treated wild-type (C) andIL-1β−/− (D) mice.E, Wild-type mice show that a large portion of the axons in the corpus callosum have remyelinated, butIL-1β−/− mice show fewer remyelinated axons at week 10 (F), 4 weeks after removal of cuprizone. Scale bar, 1.2 μm.
Fig. 4.
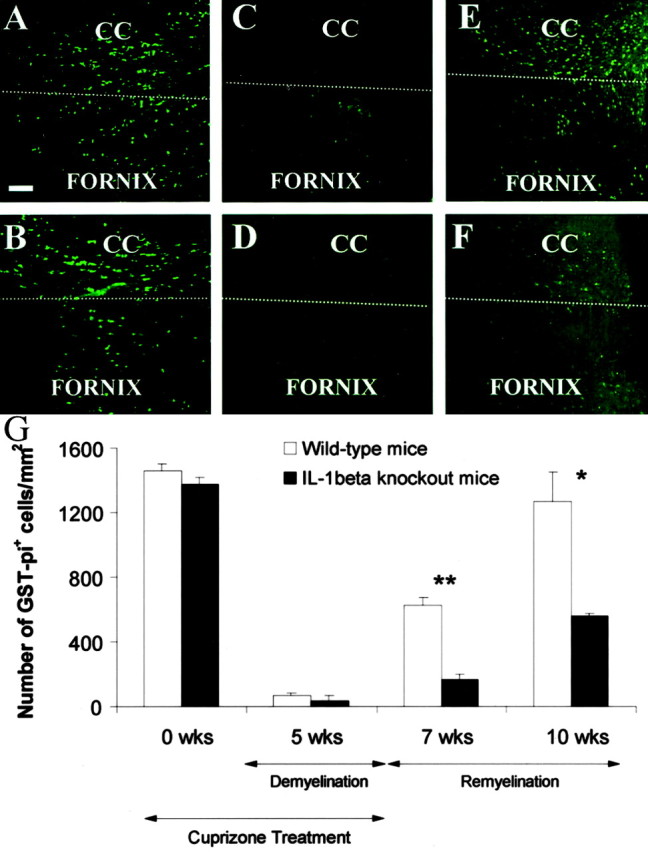
GST-Pi+ mature oligodendrocytes in the corpus callosum of wild-type andIL-1β−/− mice during demyelination and remyelination. GST-Pi+ mature oligodendrocytes in the corpus callosum of untreated wild-type (A) and IL-1β−/− (B) mice at 0 weeks. Mature oligodendrocyte recovery of wild-type (C) and IL-1β−/−(D) mice at 5 weeks of treatment. Recovery of GST-Pi+ cells in the corpus callosum of wild-type (E) and IL-1β−/−(F) mice at 10 weeks. G, The mean and SEM bars representing the number of GST-Pi+cells per square millimeter are plotted for the triplicate set of samples. Scale bar, 50 μm. CC, Corpus callosum. Thewhite dashed line separates the corpus callosum and fornix. *p < 0.005; **p < 0.0005.
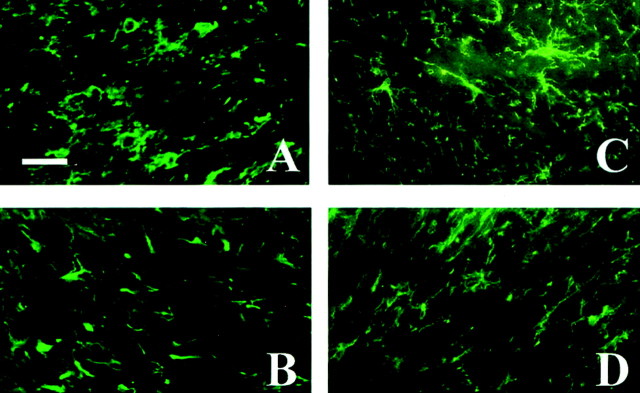
Fig. 5.
The presence of NG2+ cells during demyelination and remyelination inIL-1β−/− mice. NG2+oligodendrocytes accumulate in the corpus callosum of wild-type (A) and IL-1β-deficient (B) mice after 5 weeks of cuprizone treatment. A reduction in the number of NG2+ oligodendrocyte progenitors (450 ± 30 cells/mm2) in the corpus callosum was observed in wild-type mice (C) (only stained cells with nuclei were counted). This was in contrast to the continued presence of progenitors (703 ± 38 cells/mm2) in the corpus callosum ofIL-1β−/− mice after 1 week of recovery after 6 weeks of cuprizone treatment (D). Scale bar, 20 μm.
Remyelination is dramatically reduced in the absence of IL-1β
A large number of axons in wild-type mice showed a substantial recovery (67.1 ± 2.5%) from the demyelinating insult by 10 weeks, 4 weeks after cuprizone was removed from the diet (Fig.3E). In contrast, there was a significant (p < 0.0002) reduction in the number of remyelinated axons (45.1 ± 1.6%) within the corpus callosum ofIL-1β−/− mice at 10 weeks (Fig. 3F). Thus, IL-1β appears to play a prominent role in promoting the remyelination process.
Repopulation of the GST-Pi+ mature oligodendrocytes is dramatically reduced in the absence of IL-1β
We have shown previously that new oligodendrocytes repopulate the corpus callosum and are presumably responsible for remyelinating the demyelinated lesion (Mason et al., 2000a). The lack of proper remyelination in IL-1β−/−mice may be attributable to an inability of these mice to regenerate new mature oligodendrocytes. After 5 weeks of exposure to cuprizone, the GST-Pi+ mature oligodendrocytes began to reappear within the demyelinated corpus callosum of wild-type mice (Fig.4C,E,G). However, in the absence of IL-1β, there was a dramatic reduction in the number of GST-Pi+ oligodendrocytes and a significant delay in their reappearance within the corpus callosum at 7 (p < 0.0005) and 10 (p< 0.005) weeks (Fig. 4F,G). Thus, IL-1β appears to facilitate the regeneration of the mature oligodendrocyte population after a demyelinating insult.
NG2+ oligodendrocyte progenitor accumulation occurs in the absence of IL-1β
Failure of mature oligodendrocytes to adequately repopulate the demyelinated corpus callosum inIL-1β−/− mice warranted examination of oligodendrocyte progenitors. A large number of NG2+ progenitors accumulated within the demyelinated corpus callosum at 5 weeks in both wild-type (734 ± 41 cells/mm2) andIL-1β−/− (710 ± 78 cells/mm2) mice (Fig.5A,B). During remyelination when progenitors presumably differentiate into mature oligodendrocytes, NG2+ progenitor cells were significantly diminished (p < 0.004) in number (450 ± 30 cells/mm2) by week 7 in wild-type mice compared with that observed inIL-1β−/− mice (703 ± 38 cells/mm2) (Fig.5C,D). Thus, although NG2+ cells diminished in numbers presumably by differentiating into mature oligodendrocytes during recovery in wild-type mice (Fig. 4G), they appear to remain as progenitors and are not able to mature in the absence of IL-1β and/or IL-1β-derived factors.
IGF-1+ microglia–macrophages and astrocytes accumulate during demyelination in wild-type mice
Very little IGF-1 protein or IGF-1+cells were present in the corpus callosum during the first 2 weeks of treatment (Fig.6A,B). At 3 weeks, an increase in both IGF-1 protein levels and the number of IGF-1+ cells was observed, and this was consistent with the appearance of IGF-1 mRNA observed in Figure 1. The amount of IGF-1 protein and the number of IGF-1+ cells in the corpus callosum peaked between 4 and 5 weeks, with a large number of IGF-1+ cells remaining in the corpus callosum throughout the remyelination process (Figs.6A,B,7A,C). Thus, elevated numbers of IGF-1+ cells in the corpus callosum correlates with increased levels of IGF-1 mRNA and IGF-1 protein.
Fig. 7.
The absence of IGF-1+astrocytes and microglia–macrophages during demyelination and remyelination in IL-1β−/− mice. IGF-1+ cells appear in the corpus callosum of wild-type mice (A) but not in IL-1β-deficient mice (B) after 5 weeks of treatment. IGF-1+ cells remain in the corpus callosum of wild-type mice (C) undergoing remyelination but are absent in IL-1β-deficient mice (D) after 1 week of recovery after 6 weeks of cuprizone treatment.E–J, Representative sections from wild-type mice demonstrating the colocalization of IGF-1 to GFAP+cells and Mac-1+ cells within the demyelinating corpus callosum at 4 weeks. E–G, The colocalization (arrows) of IGF-1+ cells (green-stained cells in E) to nearly all of the GFAP+ astrocytes (red-stained cells in F and overlaid inG) within the lesion. H–J, The colocalization (arrows) of a few IGF-1+ cells (green-stainedcells in H) to Mac-1+microglia–macrophages (red-stained cells inI and overlaid in J) within the lesion. Scale bars: A–D, 20 μm; E–H, 10 μm.
The accumulation of IGF-1+ cells parallels the accumulation of microglia–macrophages and astrocytes in the demyelinating corpus callosum as reported previously (Hiremath et al., 1998; Morell et al., 1998). Figure7E–J demonstrates colocalization of IGF-1 to nearly all GFAP+ astrocytes and to a subpopulation of Mac-1+ microglia–macrophages. This finding suggests that, not only are astrocytes capable of IGF-1 production, but microglia–macrophages also are responsible for producing IGF-1 within the demyelinating lesion.
Lack of IGF-1-producing cells in the absence of IL-1β
In contrast to wild-type mice, there was no increase in IGF-1 protein and no IGF-1+ cells detected within a demyelinating corpus callosum at 5 weeks (Figs.6B, 7B) or a remyelinating corpus callosum at 10 weeks (Fig. 7D) inIL-1β−/− mice. The lack of IGF-1 expression inIL-1β−/− mice is attributable to a diminished IGF-1 mRNA transcription (J. L. Mason, unpublished observation). The lack of IGF-1 is not attributable to reduced numbers of microglia–macrophages, which accumulate similarly in the corpus callosum of wild-type andIL-1β−/− mice, as described above. Thus, IL-1β appears to be a critical factor in mediating the production of IGF-1 during demyelination and remyelination. The inability of the oligodendrocyte progenitors to differentiate properly may be attributable to this absence of IGF-1.
DISCUSSION
Although the primary insult to oligodendrocytes is toxicity to cuprizone, the proinflammatory role associated with IL-1β suggested that its absence inIL-1β−/− mice during acute demyelination would result in an ameliorated neuropathology. Our report is contrary to the notion that IL-1β would exacerbate demyelinating diseases. We demonstrate a number of molecular and biological events relating IL-1β to the remyelination process. (1) IL-1β expression appears to precede and then parallel the expression of IGF-1 during demyelination and remyelination. (2) In the absence of IL-1β, demyelination, mature oligodendrocyte depletion, and the accumulation of oligodendrocyte progenitors within demyelinating lesions progressed similarly to wild-type mice. (3) IGF-1 protein and mRNA levels are not elevated and IGF-1+ cells are not present within lesions in mice deficient for IL-1β. (4) The lack of IGF-1 within the lesions inIL-1β−/− mice is not attributable to the absence or reduced numbers of microglia–macrophages within the lesion. (5) IL-1β (and perhaps IGF-1) is important for normal regeneration of the mature oligodendrocyte population from a normal pool of progenitors and for remyelination.
The expression of IL-1β in wild-type mice that were exposed to cuprizone correlated with active demyelination and smaller axonal caliber, microglia–macrophage accumulation, IGF-1 expression, and the recruitment oligodendrocyte progenitors (Figs. 1, 2B,6B, 7) (Hiremath et al., 1998; Morell et al., 1998;Mason et al., 2000a, 2001). Our report of microglia–macrophages as the predominant cell type expressing IL-1β is consistent with previous investigations (Giulian et al., 1986; Bauer et al., 1993). We also observed some IL-1β-producing astrocytes, which both supports (Giulian et al., 1986; Sairanen et al., 1997) and contradicts (Bauer et al., 1993) previous observations concerning the ability of astrocytes to produce IL-1β. The data here suggest that microglia–macrophages are the predominant source of IL-1 and they appear to be a strategic cell type in the remyelination process.
Surprisingly, the absence of IL-1β did not prevent the depletion of mature oligodendrocytes, presumably by apoptosis during the first 4 weeks into demyelination (Mason et al., 2000a), or myelin pathology. Although cuprizone toxicity is believed to be the primary insult to oligodendrocytes, it is thought that proinflammatory cytokines such as IL-1 may exacerbate neuropathology. Indeed, demyelination appeared similar in the presence or absence of IL-1β within the scoring area of the corpus callosum; however, in theIL-1β−/− mice, demyelination encompassed other more peripheral areas of the corpus callosum (data not shown). This observation would suggest that IL-1β may be somewhat protective even during demyelination, which is contrary to in vitro work citing that IL-1 is detrimental to oligodendrocytes (Merrill, 1991). Furthermore, the typical accumulation of microglia–macrophages or oligodendrocyte progenitors after exposure to cuprizone, either as the result of progenitor migration from other areas of the brain and/or the proliferation and activation of local progenitors (Mason et al., 2000a), is not dependent on IL-1β (Fig. 5). This is consistent with previous in vitro studies demonstrating the inability of IL-1β to induce the proliferation or differentiation of oligodendrocyte progenitors (Merrill, 1991). However, IGF-1 can induce the differentiation of oligodendrocyte progenitors into mature oligodendrocytes (McMorris et al., 1986; McMorris and Dubois-Dalq, 1988; Mozell and McMorris, 1991). Our data in this report, showing an apparent block in oligodendrocyte differentiation and perhaps mature oligodendrocyte survival during remyelination, is consistent with the lack of IGF-1 production that correlates well with the absence of IL-1β (Mason et al., 2000a).
The absence of IL-1β appeared to have a profound effect on the recovery of mature oligodendrocyte and remyelination in the CNS. There is a reduction in the mature oligodendrocyte population between the first (week 7) (Fig.4C,D,G) and fourth (week 10) (Fig. 4E–G) week into recovery in theIL-1β−/− mice compared with that observed in the wild-type mice. Consequently, there is also a significant reduction in the number of axons that were remyelinated in the IL-1β−/− mice compared with the wild-type mice at 10 weeks (Fig.3E,F). Interestingly, there was a delayed regeneration of a small population of mature oligodendrocytes in theIL-1β−/− mice at 10 weeks (Fig. 4F,G), suggesting that additional factors, whose expression is not dependent on IL-1β, are contributing to the delayed partial remyelination observed in these mice.
Our results are consistent with others in demonstrating the ability of astrocytes to produce IGF-1 within demyelinating lesions (Komoly et al., 1992; Liu et al., 1994; Yao et al., 1995). However, our work showed for the first time that microglia–macrophages within demyelinating lesions also produce IGF-1. Furthermore, it has been shown previously that microglia–macrophages express an array of growth factor mRNAs in vitro (Rappolee et al., 1988; Elkabes et al., 1996), including IGF-1 mRNA (Arkins et al., 1993). This presence of microglia–macrophage is associated with active myelination during development (Hutchins et al., 1992; Ellison and de Vellis, 1995) and with the ability of macrophages to induce myelin gene expression in oligodendroglial cultures (Hamilton and Rome, 1994; Laughlin et al., 1997). Our results fit well with the hypothesis that microglia–macrophage may be acting to promote remyelination. By producing IL-1β, an induction of growth-promoting factors such as IGF-1 may be fostering mature oligodendrocyte repopulation and remyelination during a pathological insult within the CNS.
Footnotes
This work was supported in part by the Howard Hughes Medical Institute (D.D.C.), National Institute of Neurological Disorders and Stroke Grants NS35372 (G.K.M.) and NS24453 (K.S.), and National Multiple Sclerosis Society Grant RG2754B (G.K.M.). We thank Clarita Langerman for the preparation and sectioning of tissue for electron microscopy and Dr. Robert Bagnell and Victoria Maden for their photographic expertise at electron microscopy. We are grateful to Dr. Pierre Morell for his comments regarding this manuscript. IGF-1 measurements were conducted graciously by Drs. P. Ye and J. D'Ercole.
Correspondence should be addressed to G. K. Matsushima, University of North Carolina Neuroscience Center, CB #7250, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599. E-mail:gkmats@med.unc.edu.
J. L. Mason's present address: Columbia University, 630 West 168th Street, Department of Pathology, New York, NY 10032.
D. D. Chaplin's present address: University of Alabama, 845 19th Street South, Department of Microbiology, Birmingham, AL 35294.
REFERENCES
- 1.Araujo DM, Cotman CW. Basic FGF in astroglial, microglial, and neuronal cultures: characterization of binding sites and modulation of release by lymphokines and trophic factors. J Neurosci. 1992;12:1668–1678. doi: 10.1523/JNEUROSCI.12-05-01668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. Murine macrophages express abundant insulin-like growth factor-1 class I Ea and Eb transcripts. Endocrinology. 1993;133:2334–2343. doi: 10.1210/endo.133.5.8404686. [DOI] [PubMed] [Google Scholar]
- 3.Bauer J, Berkenbosch F, Van Dam A-M, Dijkstra CD. Demonstration of interleukin-1β in Lewis rat brain during experimental allergic encephalomyelitis by immunohistochemistry at the light and ultrastructural level. J Neuroimmunol. 1993;48:13–22. doi: 10.1016/0165-5728(93)90053-2. [DOI] [PubMed] [Google Scholar]
- 4.Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M-F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. doi: 10.1016/0165-5728(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- 6.Brogi A, Strazza M, Melli M, Costantino-Ceccarini E. Induction of intracellular ceramide by interleukin-1β in oligodendrocytes. J Cell Biochem. 1997;66:532–541. doi: 10.1002/(sici)1097-4644(19970915)66:4<532::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 8.Diemel LT, Copelman CA, Cuzner ML. Macrophages in CNS remyelination: friend or foe? Neurochem Res. 1998;23:341–347. doi: 10.1023/a:1022405516630. [DOI] [PubMed] [Google Scholar]
- 9.Dupree JL, Coetzee T, Blight A, Suzuki K, Popko B. Myelin galactolipids are essential for proper node of ranvier formation in the CNS. J Neurosci. 1998;18:1642–1649. doi: 10.1523/JNEUROSCI.18-05-01642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkabes S, DiCicco-Bloom E, Black IB. Brain microglia express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison JA, de Vellis J. Amoebid microglia expressing DD3 ganglioside are concentrated in regions of oligodendroglenesis during development of the rat corpus callosum. Glia. 1995;14:123–132. doi: 10.1002/glia.440140207. [DOI] [PubMed] [Google Scholar]
- 12.Feder LS, Laskin DL. Regulation of hepatic endothelial cell and macrophage proliferation and nitric oxide production by GM-CSF, M-CSF and Il-1 beta following acute endotoxemia. J Leukoc Biol. 1994;55:507–513. [PubMed] [Google Scholar]
- 13.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 14.Giulian D, Lachman LB. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985;228:227–228. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- 15.Giulian D, Baker TJ, Shih L-CN, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glazebrook H, Hatch T, Brindle NP. Regulation of insulin-like growth factor-1 expression in vascular endothelial cells by the inflammatory cytokine interleukin-1. J Vasc Res. 1998;35:143–149. doi: 10.1159/000025577. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SP, Rome LH. Stimulation of in vitro myelin synthesis by microglia. Glia. 1994;11:326–335. doi: 10.1002/glia.440110405. [DOI] [PubMed] [Google Scholar]
- 19.Hiremath MM, Saito Y, Knapp GW, Ting JP-Y, Suzuki K, Matsushima GK. Microglia/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 20.Hutchins KD, Dickson DW, Rashbaum WK, Lyman WD. Localization of microglia in the human fetal cervical spinal cord. Dev Brain Res. 1992;66:270–273. doi: 10.1016/0165-3806(92)90091-a. [DOI] [PubMed] [Google Scholar]
- 21.Komoly S, Hudson LD, Webster HD, Bondy CA. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc Natl Acad Sci USA. 1992;89:1894–1898. doi: 10.1073/pnas.89.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughlin AJ, Copelman CA, Hall A, Armer T, Young BC, Landon DN, Cuzner ML. Myelination and remyelination of aggregate rat brain cell cultures enriched with macrophages. J Neurosci Res. 1997;47:384–392. [PubMed] [Google Scholar]
- 23.Lee SC, Dickson DW, Brosnan CF. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav Immunol. 1995;9:345–354. doi: 10.1006/brbi.1995.1032. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Yao D-L, Bondy CA, Brenner M, Hudson LD, Zhou J, Webster HD. Astrocytes express insulin-like growth factor-1 (IGF-1) and it's binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol Cell Neurosci. 1994;5:418–430. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- 25.Ludwin SK. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am J Pathol. 1979;95:683–696. [PMC free article] [PubMed] [Google Scholar]
- 26.Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000a;61:251–262. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000b;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason JL, Suzuki K, Morell P, Matsushima GK. Episodic demyelination and remyelination in the central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001;27:50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- 29.McMorris FA, Dubois-Dalq M. Insulin-like growth factor 1 promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- 30.McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci USA. 1986;83:822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merrill JE. Effects of interleukin-1 and tumor necrosis factor-α on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13:130–137. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- 32.Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci. 1998;12:220–227. doi: 10.1006/mcne.1998.0715. [DOI] [PubMed] [Google Scholar]
- 33.Mozell RL, McMorris FA. Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat aggregate cultures. J Neurosci Res. 1991;30:382–390. doi: 10.1002/jnr.490300214. [DOI] [PubMed] [Google Scholar]
- 34.Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-α and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 35.Sairanen TR, Lindsberg PJ, Brenner M, Sirén A-L. Global forebrain ischemia results in differential cellular expression of interleukin-1β (IL-1β) and its receptor at mRNA and protein level. J Cereb Blood Flow Metab. 1997;17:1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M, Cohen I, Lazarov-Spiegler O, Moalem G, Yoles E. The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J Mol Med. 1999;77:713–717. doi: 10.1007/s001099900047. [DOI] [PubMed] [Google Scholar]
- 37.Shinar Y, McMorris FA. Developing oligodendroglia express mRNA for insulin-like growth factor-1, a regulator of oligodendrocyte development. J Neurosci Res. 1995;42:516–527. doi: 10.1002/jnr.490420410. [DOI] [PubMed] [Google Scholar]
- 38.Shornick LP, De Togni P, Mariathansan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1β manifest impaired contact hypersensitivity to trinitrochlorobenzene. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidman RL, Abervine JB, Pierec ET. Atlas of the mouse brain and spinal cord. Harvard UP; Cambridge, MA: 1971. [Google Scholar]
- 40.Silberstein FC, Simone RD, Levi G, Aloisi F. Cytokine-regulated expression of platelet-derived growth factor gene and protein in cultured human astrocytes. J Neurochem. 1996;66:1409–1417. doi: 10.1046/j.1471-4159.1996.66041409.x. [DOI] [PubMed] [Google Scholar]
- 41.Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, Kyle RA, Pease LR, Rodriguez M. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci USA. 2000;97:6820–6825. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagima K, Suzuki K. Demyelination and remyelination in the rat central nervous system following ethidium bromide injection. Lab Invest. 1979;41:385–392. [PubMed] [Google Scholar]
- 43.Yao D-L, West NR, Bondy CA, Brenner M, Hudson LD, Zhou J, Collins GH, Webster H-d. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor 1 and insulin-like growth factor binding protein 2 during myelin regeneration. J Neurosci Res. 1995;40:647–659. doi: 10.1002/jnr.490400510. [DOI] [PubMed] [Google Scholar]
- 44.Ye P, Xing Y, Dai Z, D'Ercole AJ. In vivo actions of insulin-like growth factor-1 (IGF-1) on cerebellum development in transgenic mice: evidence that IGF-1 increases proliferation of granule cell progenitors. Dev Brain Res. 1996;95:44–54. doi: 10.1016/0165-3806(96)00492-0. [DOI] [PubMed] [Google Scholar]