Abstract
The Unc-33-like phosphoprotein/collapsin response mediator protein (Ulip/CRMP) family consists of four homologous phosphoproteins considered crucial for brain development. Autoantibodies produced against member(s) of this family by patients with paraneoplastic neurological diseases have made it possible to clone a fifth human Ulip/CRMP and characterize its cellular and anatomical distribution in developing brain. This protein, referred to as Ulip6/CRMP5, is highly expressed during rat brain development in postmitotic neural precursors and in the fasciculi of fibers, suggesting its involvement in neuronal migration/differentiation and axonal growth. In the adult, Ulip6/CRMP5 is still expressed in some neurons, namely in areas that retain neurogenesis and in oligodendrocytes in the midbrain, hindbrain, and spinal cord. Ulip2/CRMP2 and Ulip6/CRMP5 are coexpressed in postmitotic neural precursors at certain times during development and in oligodendrocytes in the adult. Because Ulip2/CRMP2 has been reported to mediate semaphorin-3A (Sema3A) signal in developing neurons, in studies to understand the function of Ulip6/CRMP5 and Ulip2/CRMP2 in the adult, purified adult rat brain oligodendrocytes were cultured in a Sema3A-conditioned medium. Oligodendrocytes were found to have Sema3A binding sites and to express neuropilin-1, the major Sema3A receptor component. In the presence of Sema3A, these oligodendrocytes displayed a dramatic reduction in process extension, which was reversed by removal of Sema3A and prevented by anti-neuropilin-1, anti-Ulip6/CRMP5, anti-Ulip2/CRMP2 antibodies, or VEGF-165, another neuropilin-1 ligand. These results indicate the existence in the adult brain of a Sema3A signaling pathway that modulates oligodendrocyte process extension mediated by neuropilin-1, Ulip6/CRMP5, and Ulip2/CRMP2, and they open new fields of investigation of neuron/oligodendrocyte interactions in the normal and pathological brain.
Keywords: Ulip/CRMP, oligodendrocyte, Sema3A, process extension, anatomical expression, neurodegenerative disorders
The Unc-33-like phosphoprotein/collapsin response mediator protein (Ulip/CRMP) family consists of four homologous cytosolic phosphoproteins (Minturn et al., 1995; Byk et al., 1996; Hamajima et al., 1996; Wang and Strittmatter, 1996; Quach et al., 1997) that are highly expressed in the developing brain and have unique and as yet poorly understood molecular mechanisms of action. Ulip2/CRMP2, the most widely studied member, is reported to mediate semaphorin-3A (Sema3A)-induced growth cone collapse through a signal transduction cascade involving heterotrimeric G-proteins (Goshima et al., 1995), growth cone collapse induced by lysophosphatidic acid acting via Rho-associated protein kinase (Arimura et al., 2000), and neuronal apoptotic death (Shirvan et al., 1999). Although dramatically downregulated in the adult, Ulip/CRMPs are still expressed in structures that retain neurogenesis (Wang and Strittmatter, 1996;Kamata et al., 1998; Pasterkamp et al., 1998a; Nacher et al., 2000). Interestingly, members of the Ulip/CRMP family have been implicated in human neurodegenerative disorders. In Alzheimer's disease, increased levels of highly phosphorylated Ulip2/CRMP2 are associated with neurofibrillary tangles (Yoshida et al., 1998; Gu et al., 2000). In paraneoplastic neurological diseases (PNDs), autoimmune neurodegenerative disorders involving the cerebellum and dentate gyrus, some patients develop autoantibodies (anti-CV2 antibodies) that recognize Ulip/CRMP proteins (Honnorat et al., 1999). Intriguingly, although all anti-CV2 sera tested recognized the same protein (Honnorat et al., 1996) and immunolabeled the same postmitotic neural precursors in the developing brain and the same population of oligodendrocytes (Honnorat et al., 1998), a few failed to recognize any of the four known Ulip/CRMPs, suggesting the existence of another member that was the main target for these antibodies. In the present study, we used one of these anti-CV2 sera to clone a fifth human Ulip/CRMP member, referred to as Ulip6/CRMP5. This protein displays 50% homology with the other human Ulip/CRMPs and is the human equivalent of the recently identified CRAM and CRMP5 proteins, respectively, in the rat and mouse (Fukada et al., 2000; Inatome et al., 2000). The distribution pattern of Ulip6/CRMP5 mRNA and protein was studied in the developing and adult rat brain. Because we observed an expression in adult oligodendrocytes as described for Ulip2/CRMP2 (Ricard et al., 2000), we compared the distribution of these two proteins and found that they were coexpressed at certain times during development and in oligodendrocytes. In studies to understand the function of Ulip6/CRMP5 and Ulip2/CRMP2 in adult, purified adult rat brain oligodendrocytes were submitted to Sema3A, a semaphorin known for its attractive/repulsive properties on growing axons (Püschel, 1999; Bagnard et al., 1998, 2000). These oligodendrocytes were found to have Sema3A binding sites and to express neuropilin-1, the major component of the Sema3A receptor complex (He and Tessier-Lavigne, 1997; Kolodkin et al., 1997). In the presence of Sema3A, the oligodendrocyte process extensions displayed a dramatic decrease that was reversed by removing the Sema3A or prevented by anti-neuropilin-1, anti-Ulip6/CRMP5, or anti-Ulip2/CRMP2 antibodies or VEGF-165, another ligand for neuropilin-1 (Miao et al., 1999). These results indicate the existence of a Sema3A signaling pathway controlling oligodendrocyte process extension in adult brain via neuropilin-1, Ulip6/CRMP5, or Ulip2/CRMP2.
MATERIALS AND METHODS
Reagents. Unless specified otherwise, all reagents were purchased from Sigma (L'Isle d'Abeau, France).
Production of recombinant proteins. cDNAs coding for mouse Ulip1/CRMP4 (GenBank accession number X87817), Ulip2/CRMP2 (GenBank accession number Y10339), Ulip3/CRMP1 (GenBank accession number Y09080), and Ulip4/CRMP3 (GenBank accession number Y09079), kindly provided by A. Sobel (Institut National de la Santé et de la Recherche Médicale U440, Paris), were cloned in-frame with a flag sequence (Sigma) in the pSG5 vector (Stratagene, Amsterdam, The Netherlands) and used to produce recombinant proteins in HeLa cells as described previously (Ricard et al., 2000). Human Ulip6/CRMP5 cDNA (GenBank accession number AF264015), cloned in-frame with theLac-Z gene in pBluescript KS, was used to produce bacterial recombinant protein. Briefly, Escherichia coli cells were grown for 1 hr at 37°C, then Ulip6/CRMP5 expression was induced with isopropyl-1-thio-β-d-galactopyranoside (0.1 mm). After 3 hr at 37°C, the cells were lysed by sonication, and the soluble extract containing the Ulip6/CRMP5 recombinant protein was obtained by centrifugation for 10 min at 2000 × g.
Antibodies. The peptides chosen to generate specific antisera were KEMGTPLADTPTRPVTRHGG (amino acids 505–524) for anti-Ulip6/CRMP5, LEDGTLHVTEGS and ITGPEGHVLSRPEEVE (amino acids 454–465 and 217–232, respectively) for anti-Ulip2/CRMP2, LTSFEKWHEAADTKS (amino acids 117–131) for anti-Ulip3/CRMP1, and EHDSHAQLRWRVL (amino acids 664–676) for anti-neuropilin-1. The synthetic peptides were conjugated to keyhole limpet hemocyanin and used to immunize rabbits or rats as described previously (Honnorat et al., 1999). The antibodies were purified from anti-Ulip6/CRMP5, anti-Ulip3/CRMP1, and anti-neuropilin-1 antisera using the corresponding immobilized peptide.
Protein samples. Male rats (OFA; Iffa-Credo, L'Arbresle, France) were anesthetized with pentobarbital. Tissues were sonicated in 10 mm Tris-HCl, pH 7.4, 0.02% sodium azide, 1 mm EDTA, 0.2% Triton X-100, 10 μg/ml of leupeptin, 5 μg/ml of pepstatin, and 10 μg/ml of aprotinin, then centrifuged for 10 min at 2000 × g at 4°C. The proteins in the supernatant were quantified (Coomassie Plus Protein Assay Reagent, Pierce, Interbiotech, Montluçon, France), diluted in the homogenization buffer to a concentration of 2 mg/ml for neural tissues or 4 mg/ml for non-neural tissues, and stored at −20°C until required.
Purified oligodendrocyte cultures. Oligodendrocytes were isolated from six 4-week-old Sprague Dawley male rats (Iffa-Credo) using the procedure of Lisak et al. (1981), as modified by Lubetzki et al. (1988). Freshly isolated cells were plated on poly-l-lysine-coated glass coverslips (OSI, Maurepas, France) in 24-well plates (Costar Corporation, Cambridge, MA) at a density of 5 × 104 cells per well, initially for 1 hr in DMEM (Life Technologies, Cergy-Pontoise, France) containing 10% fetal calf serum (FCS; Eurobio, Les Ulis, France) to facilitate attachment, and then in standard culture medium consisting of Bottenstein and Sato medium (BS) (Bottenstein and Sato, 1979) supplemented with 5 U/ml of penicillin and 5 μg/ml of streptomycin (Life Technologies).
cDNA cloning. The cDNA library used in this study was a human spinal cord cDNA library in λ gt11 phage (Clontech, Palo Alto, CA). Recombinant phages were screened at a density of 2 × 104 PFU per 150 mm plate of E. coli Y1090r−. The library was first screened using serum from a patient with anti-CV2 antibodies (number 94–799) (Rogemond and Honnorat, 2000); primary antibody binding was visualized using peroxidase-labeled anti-human IgG antibody and colorimetric detection with diaminobenzidine. Positive clones were purified by several rounds of antibody screening until 100% of the plaques gave positive signals. Four positive clones were obtained, PCR-amplified, and sequenced. The longest (C97, 1.6 kb) was subcloned into the EcoRI sites of pBluescript KS (Stratagene) and resequenced. To isolate the full-length cDNA, the human spinal cord cDNA library was screened using a32P-labeled 270 bp fragment of clone C97, obtained by PCR using primers chosen on the basis of the sequence of the partial cDNA clone (C97). Hybridization was performed using ExpressHyb hybridization solution (Clontech), and positive clones were purified and sequenced. One of these, containing the complete coding region, was subcloned into pBluescript KS (Stratagene) and resequenced.
Northern blot analysis. Northern blot analysis of Ulip6/CRMP5 expression was performed on a human adult multiple-tissue RNA blot (MTN, Clontech) containing 2 μg of purified poly(A+) RNA using the full-length cDNA (2 kb) labeled with α32P-dCTP by random priming (Life Technologies). Hybridization was performed in ExpressHyb hybridization solution (Clontech) following the manufacturer's instructions, and the blot was exposed to x-ray film at −80°C.
Western blot analysis. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, St. Quentin-en-Yvelines, France) using a semidry electroblotting system with a continuous buffer (Tris 25 mm, glycine 192 mm, methanol 20%, pH 8.5). The membranes were saturated with 2% nonfat dry milk in PBS, then probed with primary antibodies. Bound antibodies were detected using peroxidase-coupled anti-IgG antibodies and diaminobenzidine oxidation.
Immunohistochemistry. Four adult male, four 2-week-old [postnatal day 15 (P15)], four 5-d-old (P5), and four pregnant female rats (OFA; Iffa-Credo) were used. The adult male and P15 rats were anesthetized with pentobarbital and perfused intracardially with 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4, then the brains were removed and post-fixed in 4% paraformaldehyde for 12 hr. The brains of anesthetized P5 rats were fixed by immersion for 12 hr in 4% paraformaldehyde in PB. After three rinses and overnight incubation in PB/20% sucrose, the brains were frozen at −60°C in methyl-butane. Embryos [embryonic day 16 (E16) and E19, respectively] were removed from the anesthetized pregnant females and fixed by immersion for 12 hr in 4% paraformaldehyde, then treated in the same way as the adult tissues. Sagittal cryostat sections (14 μm thick) were collected on Superfrost Plus slides (Polylabo, Strasbourg, France) and stored at −20°C until required. Immunohistochemistry was performed as described previously (Honnorat et al., 1998). Briefly, the tissue sections were incubated overnight at room temperature with anti-Ulip6/CRMP5 antibodies (1:100 dilution) or anti-Ulip2/CRMP2 antibodies (1:50 dilution), and bound antibodies were detected using fluorescein-conjugated anti-rabbit IgG antibodies.
In situ hybridization. Sense or antisense digoxigenin-labeled riboprobes were generated by transcription of mouse Ulip2/CRMP2 cDNA (GenBank accession number Y10339; generously provided by Dr. A. Sobel) and human Ulip6/CRMP5 cDNA (GenBank accession numberAF264015) in pBluescript SK, using the T3 or T7 promoters and labeling with digoxigenin-UTP (Roche, Meylan, France), following the manufacturer's instructions. The human Ulip6/CRMP5 cDNA-derived riboprobe was suitable for hybridization with rat tissue sections because the sequence of this human riboprobe displays >90% homology with the corresponding rat sequence. Tissue sections were prepared as described above for immunohistochemistry, then treated with the sense and antisense riboprobes as described previously (Ricard et al., 2000). For neuropilin-1, after 48 hr of culture, purified oligodendrocytes were fixed in 4% paraformaldehyde, then subjected to in situ hybridization with digoxigenin-labeled oligonucleotide probes (antisense: CAGACATGTGATACCAGAAGGTCATGCAGT from the neuropilin-1 sequence; GenBank accession number D50086) as described previously (Bagnard et al.,2001).
Receptor affinity probes. Alkaline phosphatase (AP) was fused to the amino terminus of Sema3A as described previously (Bagnard et al., 1998). To characterize Sema3A binding sites in highly purified oligodendrocytes in culture, the cells were incubated for 90 min with the AP-Sema3A recombinant protein in HBSS supplemented with 20% FCS, washed three times in PBS, then fixed for 1 hr in 4% paraformaldehyde. After one wash in PBS, endogenous phosphatases were heat-inactivated at 65°C for 50 min, then the preparations were equilibrated for 20 min with AP buffer (100 mm Tris, 100 mm NaCl, and 5 mm MgCl2, pH 9.5), and the bound AP-Sema3A was visualized using a staining solution containing 34 mg/ml of Nitro-blue-tetrazolium and 18 mg/ml of 5-bromo-4-chloro-3-indolylphosphate (Roche) in AP buffer. Immunostaining with monoclonal Rip antibody, an oligodendrocyte marker (Friedman et al., 1989), was then used to visualize oligodendrocytes. The controls performed consisted of oligodendrocytes incubated in culture medium without recombinant protein or in the presence of an excess of untagged Sema3A.
Oligodendrocyte process extension assay. Highly purified mature oligodendrocytes were obtained and grown for 48 hr in BS medium (see above); then the BS medium was replaced with either Sema3A-conditioned medium (Sema3A medium) obtained from human embryonic kidney (HEK 293) cells transfected with Sema3A expression vector, as described previously (Bagnard et al., 1998), or control medium from untransfected HEK 293 cells. Purified oligodendrocytes were also incubated for 48 hr in Sema3A medium containing either 50 ng/ml of VEGF-165 (Miao et al., 1999) or various concentrations of antibodies (2, 4, or 8 μg/ml of immunopurified anti-neuropilin-1, anti-Ulip6/CRMP5, or anti-Ulip3/CRMP1 antibodies or 4, 8, or 20 μg/ml of IgG purified from anti-Ulip2/CRMP2 antisera and preimmune sera). Incubation with the different anti-Ulip/CRMP antibodies at 8 μg/ml was also performed without Sema3A. We added the antibodies to the culture medium because the oligodendrocytes internalize IgG (see below), as described for other neural cells (Fishman et al., 1990,1991; Greenlee et al., 1993). The cultures were then fixed in 4% paraformaldehyde and analyzed. They were first immunostained using the Rip monoclonal antibody and microphotographed using a 40× objective (Zeiss). Processes were quantified on the photographs using a grid composed of concentric circles separated by 10 μm and centered on the cell body (see Fig. 9). The number of intersections between the circles and processes was counted for each cell, defining a branching index (BI); 20 cells were counted in each test sample to determine the mean BI. The results were confirmed in at least two independent experiments. Effects of treatments were quantified using the percentage extension compared with that under control conditions calculated as [(BI in control medium − BI in Sema3A medium)/BI in control medium] × 100. The statistical significance of the results was evaluated using the unpaired Student's t test.
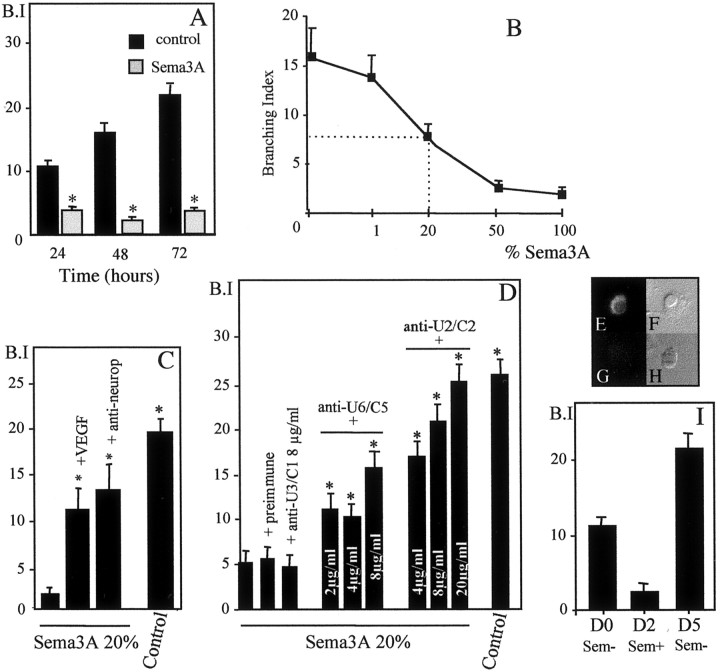
Fig. 9.
Quantitative evaluation of oligodendrocyte process extension. Concentric circles separated by 10 μm were drawn around the cell bodies of the microphotographed oligodendrocytes. Intersections of the oligodendrocyte processes with the concentric circles were counted to define a BI.
Oligodendrocyte viability assay. Viability of the oligodendrocytes cultured for 48 hr with or without Sema3A was estimated by propidium iodide and trypan blue staining. Cells adhering on the glass coverslides or recovered in the culture medium were quantified.
Antibody penetration in living oligodendrocytes. Highly purified oligodendrocytes, grown for 48 hr in BS medium, were incubated for 1 hr with rabbit anti-Ulip2/CRMP2 or anti-Ulip6/CRMP5 IgG (30 μg/ml) either at 37°C or at 4°C for control. Cells were then washed twice in PBS and fixed for 20 min in 4% paraformaldehyde. After two washes in PBS, cells were incubated for 10 min in PBS containing 0.2% gelatin and 0.1% Triton X-100 and then with Alexa 488-conjugated anti-rabbit IgG (Molecular Probes, Interchim, Montluçon, France) for 45 min. Rabbit IgGs were clearly detected in the cytoplasm of 70% of the oligodendocytes when antibody incubation was performed at 37°C (see Fig. 10E). No labeling was observed when antibodies were incubated at 4°C (see Fig. 10G), indicating that IgG penetration in living oligodendrocytes is a physiologic mechanism.
Fig. 10.
Quantitative effect of Sema3A on purified adult rat brain oligodendrocytes. A, Time course of the Sema3A effect on the oligodendrocyte branching index(B.I). The cells were incubated for 24, 48, and 72 hr with Sema3A-conditioned medium (Sema3A) or control medium (control), and the branching index was compared (*p < 0.0001). B, Dose–response curve for the effect of Sema3A on the branching index. Oligodendrocytes were cultured for 48 hr in control medium (0) or different dilutions of Sema3A-conditioned medium in control medium (100 and 1% represent, respectively, undiluted and a 1:100 dilution of Sema3A-conditioned medium).C, Effect of VEGF-165 or anti-neuropilin-1 antibodies on the branching index (B.I) of purified oligodendrocytes cultured in the presence of Sema3A. Cells were incubated with a 1:5 dilution of Sema3A-conditioned medium in control medium in the presence of VEGF-165 (+VEGF) or anti-neuropilin-1 antibodies(+anti-neurop) and with control medium(control) (*p < 0.001).D, Effect of anti-Ulip2/CRMP2 and anti-Ulip6/CRMP5 antibodies on the branching index (B.I) of purified oligodendrocytes cultured in the presence of Sema3A. Purified oligodendrocytes were cultured in Sema3A-conditioned medium in the presence of anti-Ulip2/CRMP2 (anti-U2/C2,4, 8, or 20μg/ml), anti-Ulip6/CRMP5 (anti-U6/C5,2, 4, or 8μg/ml), or anti-Ulip3/CRMP1(anti-U3/C1, 8 μg/ml) antibodies or preimmune IgG (8 μg/ml) to block the Sema3A effect. Penetration of antibodies in living oligodendrocytes was illustrated for anti-Ulip2/CRMP2, after 1 hr incubation with the antibodies at 37°C (E) or at 4°C (G) (F and H show the oligodendrocytes in Nomarski microscope). The antibodies were revealed after fixation by immunofluorescent anti-rabbit IgG. I, Effect of removal of Sema3A-conditioned medium on the branching index(B.I) of purified oligodendrocytes cultured 48 hr in Sema3A. Purified oligodendrocytes were cultured 24 hr in BS (Sem-, D0) and were put into presence of undiluted Sema3A-conditioned medium (Sem+) during 48 hr (D2). The Sema3A-conditioned medium was then removed, and oligodendrocytes were cultured for 72 hr in control medium (Sem-, D5). The data are the mean ± SD (bars) values for 20 cells in each case. The branching index for each condition was compared with the branching index obtained in the presence of Sema3A alone (*p< 0.001).
All animal experiments were performed in accordance with French legal requirements (decree 87–848) and with the European Community Council Directive of November 24, 1986 (86/609/EEC).
RESULTS
Molecular characterization and tissue distribution of human Ulip6/CRMP5
A human spinal cord cDNA library was screened using an anti-CV2 serum from a patient with PND and small-cell lung carcinoma that recognized a 66 kDa protein on Western blots of newborn rat brain protein extracts, but did not recognize any of the four previously known Ulip/CRMP recombinant proteins. This led to the identification of one partial-length clone (C97) containing a 1.6 kb cDNA insert yielding a 90 amino acid open reading frame that showed 35% homology with the C-terminal region of the four known human Ulip/CRMP proteins. The cDNA containing the full-length coding region was obtained by screening the same library with a radioactive probe corresponding to the coding region of C97 (270 bp). A 2 kb cDNA, referred to as Ulip6/CRMP5, which contains an open reading frame coding for 564 amino acids, was isolated. The C-terminal region of this protein was identical to the 90 amino acids encoded by C97. On Western blots, the Ulip6/CRMP5 recombinant protein was recognized by all 20 anti-CV2 sera tested (Fig.1C) but not by 100 sera from patients without PND (half of them having small-cell lung carcinoma), suggesting that Ulip6/CRMP5 was the major antigen recognized by anti-CV2 antibodies. The overall sequence of the Ulip6/CRMP5 cDNA (GenBank accession number AF 264015) consists of 3074 bp made up of a 162 bp 5′-noncoding region, a 1692 bp protein coding region, and a 1220 bp 3′-noncoding region. The initiation codon was assigned to the Met codon at position 163–165. The deduced protein sequence predicted a protein with a molecular mass of 61.424 kDa and an isoelectric point of 7.46.
Fig. 1.
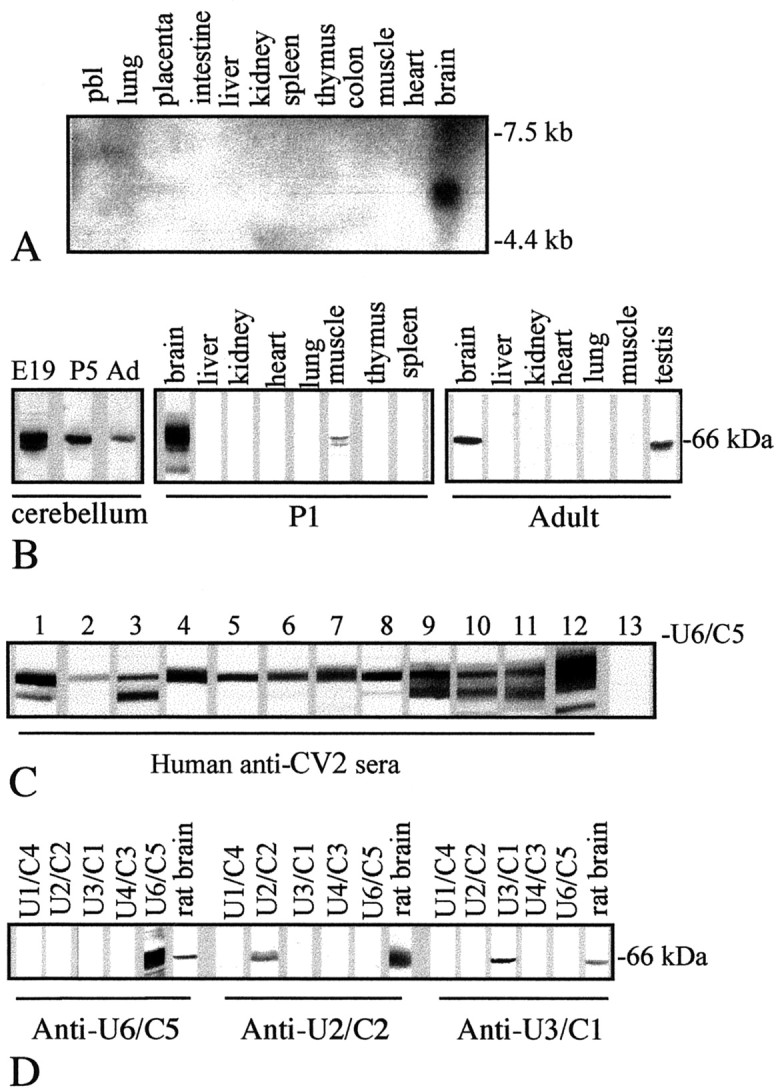
Ulip6/CRMP5 mRNA and protein expression and specificities of anti-CV2, anti-Ulip6/CRMP5, anti-Ulip2/CRMP2, and anti-Ulip3/CRMP1 antibodies. A, Northern blot of adult human tissue showing specific expression of Ulip6/CRMP5 mRNA in the brain (5.5 kb) (pbl, peripheral blood leukocytes). B, Western blot showing Ulip6/CRMP5 protein expression in cerebellum extracts (cerebellum) with a maximal expression at E19, lower at P5, and weak in the adult (Ad). In 1-d-old rat tissue extract (P1), Ulip6/CRMP5 protein is expressed at a high level in brain and at a lower level in muscle. In adult rat tissue extracts(Adult), Ulip6/CRMP5 is found in brain and testis.C, Western blot with Ulip6/CRMP5 recombinant protein showing that anti-CV2 sera from 12 PND patients recognized this protein (lanes 1–12). Lane 13 shows the lack of binding of a representative control serum. D, Western blot showing specific binding of anti-Ulip6/CRMP5, anti-Ulip2/CRMP2, or anti-Ulip3/CRMP1 antibodies to the corresponding Ulip/CRMP recombinant protein. Rat brain extract (rat brain) was used as a positive control for Ulip2/CRMP2, Ulip6/CRMP5, and Ulip3/CRMP1 expression in brain. U1/C4, Ulip1/CRMP4;U2/C2, Ulip2/CRMP2; U3/C1, Ulip3/CRMP1;U4/C3, Ulip4/CRMP3; U6/C5, Ulip6/CRMP5.
Alignment of the sequence of the Ulip6/CRMP5 protein with those for the four known human Ulip/CRMP proteins showed 48–50% identity. Ulip6/CRMP5 and the other members of the family share the same degree of identity (∼33%) with the Caenorhabditis elegans gene product, unc-33 (Byk et al., 1998), a gene required for neurite outgrowth and axonal guidance (Li et al., 1992). The Ulip6/CRMP5 sequence contains consensus sites for several protein kinases, such as casein kinase II (eight sites), tyrosine kinase (two sites), protein kinase A (one site), and protein kinase C (eight sites). Alignment of the sequence of the human Ulip6/CRMP5 protein with those of rat CRAM (GenBank accession number AB029432) and mouse CRMP5 (GenBank accession number AF249295) showed 97% identity, and comparison of the cDNA sequences showed >80% identity.
Northern blot analysis using a Ulip6/CRMP5 RNA probe identified a 5.5 kb band in human brain mRNA, whereas mRNAs prepared from various adult human peripheral tissues gave no hybridization signal (Fig.1A), indicating preferential expression of Ulip6/CRMP5 mRNA in neural tissue. Expression of Ulip6/CRMP5 protein was analyzed by Western blotting using a rabbit polyclonal antiserum that, as shown in Figure 1D, recognized the Ulip6/CRMP5 recombinant protein but not the other four Ulip/CRMPs. As for the other Ulip/CRMPs (Hamajima et al., 1996; Wang and Strittmatter, 1996; Byk et al., 1998), Ulip6/CRMP5 protein was highly expressed in the embryonic brain and showed a dramatic downregulation during ontogenesis, as illustrated in the cerebellum (Fig.1B). During development, Ulip6/CRMP5 was detected mainly in brain and lightly in muscle (Fig. 1B, P1). In adult rat tissue extracts, expression of Ulip6/CRMP5 was seen in brain and, at a lower level, in testis but not in muscle (Fig.1B).
Distribution of Ulip6/CRMP5 in the developing and adult rat brain
To investigate the function of Ulip6/CRMP5, we determined the distribution pattern of the mRNA and protein using in situhybridization or immunohistochemistry, respectively, on sections of E16 and E19 rat embryo and postnatal rat brain (P5, P15, and adult). Sense probes and preimmune serum, used as controls, gave no signal (data not shown). Ulip6/CRMP5 mRNA and protein were found to be highly expressed in the embryonic (E16 and E19) and postnatal (P5 and P15) brain and downregulated in the adult. The distribution of the protein was studied using anti-Ulip6/CRMP5 antibodies, which specifically recognized recombinant Ulip6/CRMP5 protein (Fig. 1D). The results are summarized in Table 1 and described in detail below. The observed distribution was identical to that described previously with anti-CV2 sera (Honnorat et al., 1996,1998, 1999). In addition, the distribution of Ulip6/CRMP5 mRNA and protein in the adult brain was similar to that described for Ulip2/CRMP2 (Ricard et al., 2000), so Ulip6/CRMP5 and Ulip2/CRMP2 expression patterns were compared in detail in embryonic and postnatal rat brain.
Table 1.
Immunohistochemical study of Ulip6/CRMP5 protein expression
| E16 | |
| Retina | + |
| Cortex | + |
| Globus pallidus | − |
| Thalamus | + |
| Hypothalamus | + |
| Midbrain | + |
| Cerebellum | + |
| Pons | + |
| Spinal cord | + |
| Dorsal root ganglia | + |
| P5 | |
| Olfactory bulb | + |
| Retina | + |
| Cortex | + |
| Dentate gyrus | ++ |
| Amygdala | ++ |
| Thalamus | +/− |
| Hypothalamus | +/− |
| Cerebellum | |
| External part of EGL | − |
| Internal part of EGL | + |
| Internal granular layer | + |
| Spinal cord | ++ |
| Adult | |
| Olfactory epithelium | ++ |
| Olfactory bulb | + |
| Retina | + |
| Optic nerve | + |
| Cortex | +/− |
| Corpus callosum | − |
| Dentate gyrus | + |
| Fimbria of the hippocampus | − |
| Amygdala | + |
| Basal ganglia | − |
| Thalamus | − |
| Hypothalamus | − |
| Stria medullari | − |
| White matter of the cerebellum | + |
| Midbrain | |
| Medial longitudinal fasciculus | + |
| Posterior commissure | − |
| Tract spinocerebellar | ++ |
| Tract tectospinal | ++ |
| Cerebellar peduncles | +++ |
| Trigeminal nerve | ++ |
| Facial nerve | + |
| Trapezoid body | ++ |
| Spinal cord | |
| White matter | ++ |
| Cuneiforme fascicule | − |
| Dorsal root ganglia | − |
+++, Strong signal; ++, moderate signal; +, weak signal; +/−, very weak signal; −, undetectable signal.
Distribution of Ulip6/CRMP5 mRNA and protein in the developing brain
In the embryo and during the first postnatal days (P5), immunolabeling and in situ hybridization gave globally similar results (Fig. 2), indicating expression of Ulip6/CRMP5 protein in cells expressing mRNAs. All ventricular regions, such as in the cortex (Fig.2A,B) and spinal cord (Fig.2C,D), in which mitosis occurs were always negative, suggesting that expression of Ulip6/CRMP5 mRNA and protein was restricted to postmitotic neural cells. At E16, E19, P5, and P15, Ulip6/CRMP5 expression was prominent in the neocortex, hippocampus, and spinal cord (Fig. 2, Table 1) and was also seen in the retina, hypothalamus, thalamus, midbrain, cerebellum, olfactory epithelium, olfactory bulb, and dorsal root ganglia (Table 1). Several neuronal fibers, such as those in the fimbria (Fig. 2B), spinal tracts, or peripheral nerves (Fig. 2D) were also immunostained. The intensity of labeling of cell bodies and fibers decreased during the first 2 weeks after birth.
Fig. 2.

Expression of Ulip6/CRMP5 mRNA and protein in the embryonic rat brain. Sagittal sections (14 μm) of E19 dorsal telencephalon (A, B) or frontal sections (14 μm) of E16 spinal cord (C, D) were hybridized with the Ulip6/CRMP5 ripobrobe (A,C) or immunolabeled with anti-Ulip6/CRMP5 antibodies (B, D). Expression of Ulip6/CRMP5 mRNA (A, C) or protein (B,D) was never detected in the neuroepithelium zone of the cerebral neocortex and spinal cord (large asterisk). Ulip6/CRMP5 mRNA and protein were highly expressed in the differentiating field of the neocortex (nc), hippocampus (hc) (A,B), spinal cord (sc), and dorsal root ganglia (drg) (C, D). Ulip6/CRMP5 protein was especially strongly expressed in the hippocampal fimbria (arrowhead) (B), spinal tracts, and peripheral nerves (arrows) (D). No Ulip6/CRMP5 mRNA or protein was detected in the basal ganglia (bg) (A, B). Scale bar, 330 μm.
Temporal expression of Ulip6/CRMP5 and Ulip2/CRMP2 was compared in the developing cerebellum, chosen as a model structure characterized by postnatal directional migration, differentiation, and synaptogenesis with precise spatiotemporal order of positioning (Altman, 1972a,b,c). At E19, Ulip6/CRMP5 mRNA and protein were expressed in all cerebellar layers, except the external granular layer (EGL) in which mitosis occurs (Figs. 3A,4A), whereas Ulip2/CRMP2 mRNA and protein were highly expressed in the EGL and to a lesser extent in the inner part of the cerebellum (Figs. 3D, 4B). At P5 and P15, Ulip6/CRMP5 was not expressed in the external part of the EGL but was expressed in the internal part (Figs. 3B, 4C) in which future granular neurons start migrating toward the internal granular layer (IGL), suggesting that Ulip6/CRMP5 is expressed by postmitotic granular neurons that are starting to migrate. At these stages, the neural progenitors in the external part of the EGL expressed high levels of Ulip2/CRMP2 but not Ulip6/CRMP5 (Figs.3E, 4D). Double-labeling showed that in the internal part of the EGL, Ulip2/CRMP2 and Ulip6/CRMP5 proteins were coexpressed in granular neurons (Fig.4C,D,insets). At P15, Ulip6/CRMP5 mRNA and protein were also expressed by granular neurons in the IGL, but not by Purkinje cells (Figs. 3B, 4C), whereas Ulip2/CRMP2 mRNA and protein were highly expressed in Purkinje cells, but only weakly detectable in the granular neurons of the IGL (Figs. 3E,4D). At P15, Ulip2/CRMP2 and Ulip6/CRMP5 proteins were both highly expressed in growing fibers of the molecular layer and white matter (Fig. 4C,D).
Fig. 3.
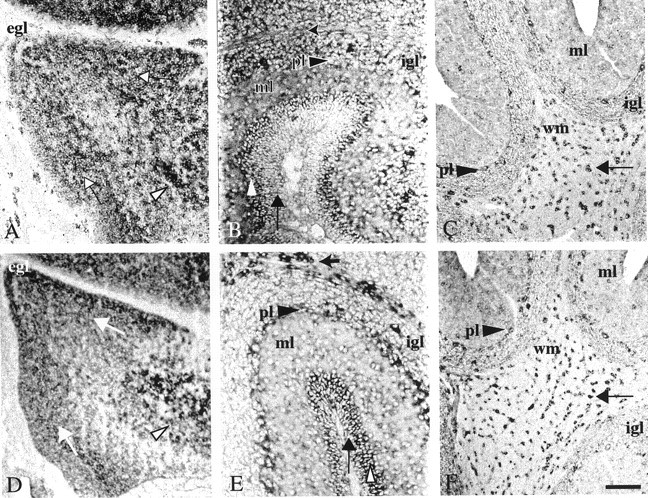
Expression of Ulip6/CRMP5 and Ulip2/CRMP2 mRNAs in the developing rat cerebellum. Sagittal sections (14 μm) of E19 (A, D), P15 (B,E), and adult (C,F) rat cerebellum were hybridized with the Ulip6/CRMP5 (A–C) or Ulip2/CRMP2 (D–F) riboprobes. At E19, Ulip6/CRMP5 (A) and Ulip2/CRMP2 (D) mRNAs were detected in the migrating cells under the EGL (white arrows) and in the deep nuclei(white arrowhead). Only Ulip2/CRMP2 mRNA (D) was expressed in the EGL(egl). At P15, both Ulip6/CRMP5 (B) and Ulip2/CRMP2 (E) mRNAs were expressed in the internal part of the EGL (white arrowhead). Expression of Ulip6/CRMP5 mRNA and to a lesser extent Ulip2/CRMP2 mRNA was seen in the molecular layer (ml) and IGL (igl). Only Ulip2/CRMP2 mRNA was detected in the external part of the EGL(thin black arrow), the Purkinje cells layer (pl), and oligodendrocytes of the white matter (thick black arrow). In the adult cerebellum, expression of Ulip6/CRMP5 mRNA (C) was detected in the Purkinje cells layer (pl), oligodendrocytes of the white matter (wm, black arrow), and to a lesser extent in the molecular layer (ml) and internal granular layer (igl). Ulip2/CRMP2 mRNA (F) was still expressed in the Purkinje cell layer (pl), oligodendrocytes of the white matter (wm, black arrow), and to a lesser extent in the molecular layer (ml) and internal granular layer (igl). Scale bar: A,D, C, F, 120 μm;B, E, 90 μm.
Fig. 4.

Expression of Ulip6/CRMP5 and Ulip2/CRMP2 proteins in the developing rat cerebellum. Sagittal sections (14 μm) of E19 (A, B), P15 (C,D), or adult (E, F) rat cerebellum were immunolabeled with anti-Ulip6/CRMP5 (A, C, E) or anti-Ulip2/CRMP2 (B, D,F) antibodies. At E19, Ulip6/CRMP5 protein (A) was expressed in all layers of the cerebellum except the EGL (egl), whereas Ulip2/CRMP2 protein (B) was detected in the EGL (egl) and to a lesser extent in the region under the EGL (arrows). At P15, only Ulip2/CRMP2 protein (D) was detected in the external part of the EGL (thin arrow) and in the Purkinje cell layer (pl), whereas both Ulip6/CRMP5 (C) and Ulip2/CRMP2 (D) proteins were expressed in the internal part of the EGL (arrowhead) and in the molecular layer (ml). Double labeling showed coexpression of Ulip6/CRMP5 (C) and Ulip2/CRMP2 (D) in neural precursors of the internal EGL (inset, arrow). Ulip6/CRMP5 protein (C) and to a lesser extent Ulip2/CRMP2 protein (D) were detected in the IGL (igl) and the white matter (thick arrow). In the adult cerebellum, expression of Ulip6/CRMP5 (E) and Ulip2/CRMP2 (F) proteins was detected only in the oligodendrocytes of the white matter (wm, arrow). Scale bar: A,B, 180 μm; C, D, 90 μm; C, D, inset, 15 μm; E, F, 40 μm.
Distribution of Ulip6/CRMP5 mRNA and protein in the adult brain
Between P20 and the adult, the pattern of expression of Ulip6/CRMP5 was constant. In the adult brain, neurons expressing Ulip6/CRMP5 were identified by their anatomical localization, size, and shape. Ulip6/CRMP5 mRNA and protein were expressed in migrating neurons in the rostral migratory stream of the olfactive bulb, scarce neurons throughout the neocortex (Fig.5A,B), and granular neurons in the juxta-hilar portion of the granular cell layer of the hippocampus (Fig. 5C,D). Moreover, low expression of Ulip6/CRMP5 mRNA in the absence of detectable protein was seen in a few neurons, namely the molecular and granular neurons of the IGL and a few Purkinje cells in the cerebellum (Figs.3C, 4E). Similarly, Ulip2/CRMP2 mRNA was expressed in Purkinje cells and to a lesser extent in molecular and granular neurons of the IGL (Fig. 3F), despite the absence of detectable Ulip2/CRMP2 protein in these neurons (Fig.4F). The presence of Ulip6/CRMP5 and/or Ulip2/CRMP2 mRNAs in some neurons in the absence of detectable protein indicates either rapid turnover of the protein or translational or post-translational regulation of the protein. Phosphorylation, glycosylation, or association of Ulip6/CRMP5 and Ulip2/CRMP2 with other proteins (Bulliard et al., 1997; Wang and Strittmatter, 1997; Inatome et al., 2000) could limit the recognition of the protein by the antibodies.
Fig. 5.

Expression of Ulip6/CRMP5 mRNA and protein in adult rat brain. Sagittal sections (14 μm) of the frontal cortex (A, B), hippocampus (C,D), or spinal cord (E,F) were hybridized with the Ulip6/CRMP5 riboprobe (A, C, E) or immunolabeled with anti-Ulip6/CRMP5 antibodies (B, D,F). Both mRNA (A,C) and protein (B, D) were expressed in some neurons of the frontal cortex (A,B) and hippocampus (C, D), especially in the infragranular layer (arrow). Both mRNA (E) and protein (F) were also expressed in oligodendrocytes of the spinal cord (arrowhead). Scale bar: A, 60 μm; B, 30 μm; C, 310 μm;D, 50 μm; E, 40 μm; F, 25 μm.
In the adult brain, the strongest Ulip6/CRMP5 mRNA and protein expression was seen in oligodendrocytes of the myelinated tracts of the spinal cord, hindbrain, midbrain, and cerebellum (Figs. 3C,4E, 5E,F). Ulip6/CRMP5 mRNA and protein were detected in small cells distributed in rows in the myelinated tracts and double labeled with the oligodendrocyte-specific Rip monoclonal antibody (data not shown), as described previously using anti-CV2 sera (Honnorat et al., 1996, 1998). Ulip6/CRMP5-expressing oligodendrocytes were detected according to an increasing rostral to caudal gradient, starting in the anterior part of the basal cerebral peduncle. In the brainstem, the highest number of Ulip6/CRMP5-positive oligodendrocytes was found in the cerebellar peduncles (Fig. 6A), the spinal tract of the trigeminal nerve, the tractus pyramidalis, and the ventrospinocerebellar tract. Within the nerve tracts, immunostained cells were widespread and bore thin stained processes clinging to the myelin sheath (Figs. 5F, 6A). The spinal cord contained the greatest number of immunostained cells (Fig.5F). All along the spinal cord, many Ulip6/CRMP5-positive oligodendrocytes were seen in all the tracts of the white matter, except in the ventral part of the dorsal corticospinal tract (Fig. 6C), whereas no labeling was seen in the gray matter. These immunostained cells defined a subset of oligodendrocytes that are estimated, using anti-CV2 sera, to account for one-third of spinal cord oligodendrocytes, with a rostrocaudal gradient (Honnorat et al., 1998). Ulip6/CRMP5-positive oligodendrocytes were rarely found in the forebrain: the gray matter or myelinated fiber tracts, such as the corpus callosum or anterior commissure. Similarly, Ulip2/CRMP2 has been shown to be expressed by a subpopulation of oligodendrocytes in adult brain (Ricard et al., 2000). In spinal cord and hindbrain and midbrain white matter, all oligodendrocytes stained by anti-Ulip6/CRMP5 antibodies were double stained by anti-Ulip2/CRMP2 antibodies, demonstrating that these two Ulip/CRMP proteins were coexpressed by certain oligodendrocytes (Fig.6A,B). Interestingly, some Ulip2/CRMP2-expressing oligodendrocytes in the midbrain (Fig.6B) and spinal cord, i.e., the ventral part of the dorsal corticospinal tracts (Fig. 6D), did not express Ulip6/CRMP5 (Fig. 6A,C). Because Ulip2/CRMP2 protein is expressed by only 40% of spinal cord oligodendrocytes (Ricard et al., 2000), three different subsets of oligodendrocytes can be distinguished in the spinal cord: one expressing both Ulip6/CRMP5 and Ulip2/CRMP2, another expressing only Ulip2/CRMP2, and a third expressing neither. On the other hand, it is noteworthy that, during ontogenesis, Ulip2/CRMP2 was detectable in oligodendrocytes at P15, whereas the earliest Ulip6/CRMP5-expressing oligodendrocytes appeared at P18 (Fig.3B,E).
Fig. 6.

Expression of Ulip6/CRMP5 and Ulip2/CRMP2 mRNAs and proteins in oligodendrocytes. Sections (14 μm) of adult rat cerebellar peduncles were immunolabeled with both rabbit anti-Ulip6/CRMP5 antibodies (A) and rat anti-Ulip2/CRMP2 antibodies (B). All oligodendrocytes labeled by anti-Ulip6/CRMP5 antibodies expressed Ulip2/CRMP2 protein (arrow). A few oligodendrocytes expressing Ulip2/CRMP2 protein were negative for Ulip6/CRMP5 protein (arrowhead). Frontal sections (14 μm) of adult rat spinal cord were hybridized with the Ulip6/CRMP5 (C) or Ulip2/CRMP2 (D) riboprobes. Oligodendrocytes of the internal part of corticospinal tract expressing Ulip2/CRMP2 mRNA were negative for Ulip6/CRMP5 mRNA (arrows). Scale bar: A, B, 30 μm; C, D, 200 μm.
Inhibition of oligodendrocyte process extension by Sema3A: involvement of Ulip6/CRMP5 and Ulip2/CRMP2
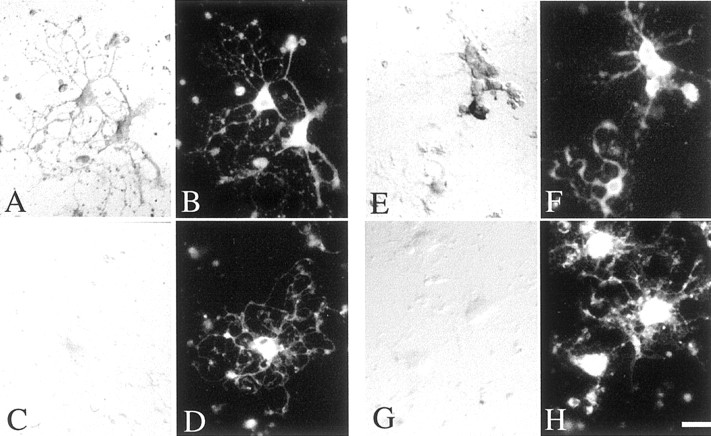
To investigate the role of Ulip6/CRMP5 and Ulip2/CRMP2 in oligodendrocytes, we used highly purified adult rat brain oligodendrocytes, previously shown to express Ulip2/CRMP2 protein (Ricard et al., 2000) and shown, in the present study, to express Ulip6/CRMP5 protein (see Fig.8A,B).
Fig. 8.
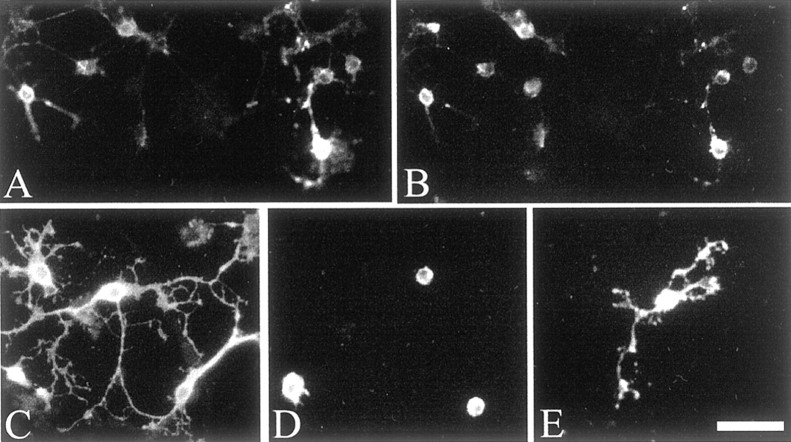
Sema3A inhibition of process extension by Ulip6/CRMP5-expressing adult rat brain oligodendrocytes.A, B, Immunolabeling of Ulip6/CRMP5 protein on oligodendrocytes (A) double labeled with Rip antibody (B). C, Purified oligodendrocytes grown 48 hr in control medium, showing process extension, immunolabeled with Rip antibody. D, Oligodendrocytes cultured 48 hr in a Sema3A-conditioned medium, showing a decrease of process extension, immunolabeled with Rip antibody.E, Oligodendrocytes immunolabeled with Rip antibody treated with Sema3A-conditioned medium as in D,followed by removal of the Sema3A-conditioned medium and incubation for 72 hr in control medium showing restoration of process extension. Scale bar, A, B, 40 μm; C–E, 30 μm.
Because the cultured oligodendrocytes had been shown to have Sema3A binding sites (Fig.7A–D) and to express the neuropilin-1 receptor sites (Fig.7E–H), we examined their response to soluble Sema3A by incubating them for 24, 48, or 72 hr with or without Sema3A-conditioned medium. When cultured in the control medium, the cells displayed the morphological characteristics of oligodendrocytes, having round or ovoid cell bodies with a radiating array of thin tapering and branching processes, and expressing the oligodendrocyte marker, Rip (Fig. 8C); under these conditions, the oligodendrocytes could survive up to 20 d in culture. After 48 hr incubation in a Sema3A-conditioned medium, the oligodendrocytes showed significant loss of processes (Fig.8D) compared with controls. Removal of Sema3A-conditioned medium led to oligodendrocyte process regrowth (Fig.8E). To quantify oligodendrocyte arborization, we used a grid of concentric circles (Fig.9) to define a BI (see Material and Methods). Freshly isolated purified oligodendrocytes initially had a mean BI close to zero (data not shown), then started to spontaneously send out processes with the time course shown in Figure10A(control), with a maximal mean BI of 21.5 at 72 hr of culture. In Sema3A-conditioned medium, the BI decreased by 72% at 24 hr, 81% at 48 hr, and 88% at 72 hr compared with controls (p < 0.0001) (Fig. 10A). The Sema3A dose–response curve, determined using a range of dilutions of Sema3A-conditioned medium (undiluted to 1:100) diluted in control medium (Fig. 10B), showed a sigmoid shape consistent with a specific biologic effect. The half-effect, corresponding to a BI reduction of 50% (p < 0.005), was obtained at a 1:20 dilution (25 ng/ml of Sema3A) (Bagnard et al., 1998). When Sema3A-conditioned medium was added to oligodendrocytes grown 24 hr in control medium and displaying processes (mean BI of 12.2), no process alteration was observed at 2, 4, or 6 hr, but retraction of process extension was seen after 24 hr and was still observed after 48 hr (mean BI of 3.8) (Fig. 10I). The Sema3A effect was totally reversed after removal of the Sema3A-conditioned medium and 72 hr incubation in control medium, the mean BI increasing to 20.8 (Fig.10I). It is noteworthy that oligodendrocytes cultured in Sema3A-conditioned medium expressed Rip, a marker of late stages of oligodendrocytic differentiation (Friedman et al., 1989) (Fig.8D).
Fig. 7.
Sema3A binding and neuropilin-1 mRNA expression in purified adult rat brain oligodendrocytes. A,B, AP-Sema3A binding sites visualized on oligodendrocytes using AP staining solution (A) and labeling with Rip antibody (B).C, D, AP-Sema3A binding was blocked by an excess of Sema3A on purified oligodendrocytes (C) immunolabeled with Rip antibody (D).E, F, Expression of neuropilin-1 mRNA on oligodendrocytes by in situ hybridization with antisense probe (E) and labeled with Rip antibody (F). G, H,In situ hybridization using the neuropilin-1 sense probe showed absence of signal (G) on oligodendrocytes immunolabeled with Rip antibody (H). Scale bar, 24 μm.
Because it has been shown that Sema3A can induce apoptosis on developing or mature neurons (Shirvan et al., 1999; Bagnard et al., 2001), oligodendrocyte apoptosis was studied using propidium iodide staining. By this method, we failed to detect any apoptotic cell. Because oligodendrocytes seemed less numerous when cultured in Sema3A-conditioned medium than in control medium, we quantified both adherent and nonadherent cells. In Sema3A-conditioned medium, 22% of the cells were recovered in the culture medium, versus 6% in control medium (p < 0.01). By trypan blue staining, we noted no significant difference in cell death among these detached cells in Sema3A-conditioned medium (61%) versus control medium (44%). This decrease of adherent cells could be attributable to poorer adhesion of the Sema3A-treated oligodendrocytes caused by the absence of process arborization.
The effect of Sema3A signal on oligodendrocyte process extension was further investigated by blocking neuropilin-1 using antibodies directed against the MAM part of the receptor (Chen et al., 1998), which have been used successfully to block the effect of Sema3A on neurons (Bagnard et al., 2001). After 48 hr incubation in Sema3A-conditioned medium in the presence of anti-neuropilin-1 antibodies (4 μg/ml), the oligodendrocytes displayed a BI reduction of 25% compared with a reduction of 81% in the absence of antibodies (p < 0.001) (Fig. 10C). Furthermore, when VEGF-165, which has been proposed to antagonize Sema3A binding to neuropilin-1 (Miao et al., 1999), was added to Sema3A-conditioned medium at a concentration of 50 ng/ml, the BI was reduced by only 40% compared with 81% in the absence of VEGF-165 (p< 0.001) (Fig. 10C). These results indicated that the effect of Sema3A on oligodendrocytes was mediated by neuropilin-1.
To assess the role of Ulip2/CRMP2 and Ulip6/CRMP5 in transducing the Sema3A-induced inhibition of oligodendrocyte process extension, we used anti-Ulip2/CRMP2 antibodies to block Ulip2/CRMP2, as described byGoshima et al. (1995), and anti-Ulip6/CRMP5 or anti-CV2 antibodies to block Ulip6/CRMP5. After 48 hr incubation in Sema3A medium containing anti-Ulip2/CRMP2 antibodies at different concentrations (4, 8, and 20 μg/ml), a dose-dependent increase in the mean BI (BI = 21.2 at 8 μg/ml) was seen compared with oligodendrocytes grown in Sema3A-conditioned medium in the absence of antibodies (BI = 5;p < 0.001) (Fig. 10D). A significant block of the Sema3A effect on oligodendrocyte process extension was also seen using anti-Ulip6/CRMP5 antibodies (2, 4, and 8 μg/ml) (Fig.10D) and anti-CV2 antibodies (data not shown). In contrast, anti-Ulip3/CRMP1 antibodies, recognizing specifically the Ulip3/CRMP1 protein (Fig. 1D), which is not expressed by oligodendrocytes, failed to block the Sema3A effect on oligodendrocyte process extension (Fig. 10D). In addition, oligodendrocytes cultured for 48 hr with anti-Ulip2/CRMP2 or anti-Ulip6/CRMP5 or anti-Ulip3/CRMP1 antibodies in control medium without Sema3A showed no morphological change. These results indicated that Ulip2/CRMP2 and Ulip6/CRMP5 mediate the Sema3A effect on oligodendrocyte process extension.
DISCUSSION
The four previously described Ulip/CRMPs are highly expressed in the developing brain and are still expressed in the adult in some neurons (Minturn et al., 1995; Hamajima et al., 1996; Wang and Strittmatter, 1996; Byk et al., 1998) and oligodendrocytes (Kamata et al., 1998; Nacher et al., 2000; Ricard et al., 2000). During the course of this manuscript preparation, a fifth member with 50% homology with other Ulip/CRMPs was described in rat (CRAM) (Inatome et al., 2000) and mouse (CRMP5) (Fukada et al., 2000) brain. CRAM was discovered when antibodies against Zap-70, a Syk tyrosine kinase essential for T lymphocyte function, were found to cross-react with a protein kinase interacting with CRAM, and CRMP5 was discovered while studying molecules involved in regional specificity of the retina. Identifying an antigenic target in PND, we have cloned the fifth human Ulip/CRMP, characterized its cellular and anatomical distribution compared with Ulip2/CRMP2, and demonstrated its potential role in mediating the inhibitory effect of Sema3A on process extension of adult brain oligodendrocytes.
Molecular and cellular characterization of Ulip6/CRMP5
PNDs are characterized by autoimmune neuronal degeneration developing in patients with systemic cancer (Posner, 1997). Several autoantibodies associated with PND are directed against various nervous system antigens, including a group of proteins with potential roles in the signal transduction pathway (Darnell, 1996). The discovery of anti-CV2 autoantibodies allowed us to identify Ulip/CRMP proteins as potential targets for these autoantibodies (Honnorat et al., 1999). Serum from one patient with anti-CV2 autoantibodies that did not recognize the already known 4 Ulip/CRMP proteins allowed us to clone the fifth human Ulip/CRMP, referred to as Ulip6/CRMP5. The recombinant Ulip6/CRMP5 protein was recognized by all anti-CV2 sera tested, and the distribution of Ulip6/CRMP5 in the developing and adult brain, demonstrated using specific antibodies, was identical to that seen using anti-CV2 sera (Honnorat et al., 1998), indicating that this protein is probably the main antigen for these autoantibodies. Ulip6/CRMP5 is 97% homologous to CRAM and CRMP5 and represents the human counterpart of these rat and mouse proteins.
Ulip6/CRMP5 mRNA was found exclusively in brain, but the protein was also transiently detected in neonatal muscle, suggesting that it may be transiently expressed during onset of innervation at the neuromuscular junction, as described for Ulip1/CRMP4 (Byk et al., 1996). Expression of this protein in adult testis might also be related to the detection of Ulip1/CRMP4 in postmeiotic germ cells (Taketo et al., 1997; Kato et al., 1998).
In the brain, Ulip6/CRMP5 was highly expressed during development by almost all postmitotic neural precursors and by fasciculi of fibers of the white matter, suggesting a role in neuronal migration and axonal growth. The continued expression of Ulip6/CRMP5 in the adult brain, as seen with the other Ulip/CRMPs, in areas that retain postnatal neurogenesis (dentate granular layer, olfactory bulb, and rostral migratory stream), confirms the role of this member in neuronal migration/differentiation. Ulip6/CRMP5 mRNA was also detected in neurons of the hypothalamus, thalamus, cortex, amygdala, brainstem, and cerebellum. Because the protein was detected only in a few neurons in the cortex and amygdala, the neuronal expression of Ulip6/CRMP5 protein might be transient and required for synaptic plasticity.
In the adult brain, the most intense Ulip6/CRMP5 mRNA and protein expression was seen in oligodendrocytes in the pons, cerebellum, and spinal cord, a distribution similar to that seen for Ulip2/CRMP2 (Ricard et al., 2000), suggesting the coexpression of these proteins. Our demonstration, by double labeling, of the coexpression of these two Ulip/CRMPs in a subpopulation of oligodendrocytes corroborates previous reports of specific interactions between Ulip/CRMP isoforms (Wang and Strittmatter, 1997; Fukada et al., 2000; Inatome et al., 2000). Expression of Ulip1/CRMP4 protein has also been described in adult brain oligodendrocytes (Nacher et al., 2000). However, we have been unable to detect oligodendrocytic expression of this member by eitherin situ hybridization (Ricard et al., 2000) or immunohistochemistry with antibodies recognizing specifically recombinant Ulip1/CRMP4 (E. Charrier, unpublished data). Interestingly, some Ulip2/CRMP2-positive oligodendrocytes, namely in the corticospinal tracts, did not express detectable levels of Ulip6/CRMP5, demonstrating the presence of subsets of oligodendrocytes differing in terms of Ulip/CRMP expressions. The presence of Ulip6/CRMP5and Ulip2/CRMP2 in oligodendrocytes might be related to different functions of these cells, depending on their localization or degree of maturation. In fact, some Ulip/CRMP-expressing cells might be oligodendrocyte progenitors, retaining the capacity of migration/differentiation in adult brain (Zhang et al., 1999). However, differentiated oligodendrocytes expressed Ulip6/CRMP5, because loops around axons belonging to myelinating oligodendrocytes were labeled by anti-Ulip6/CRMP5 antibodies.
Interestingly, similar coexpression or lack of coexpression of Ulip2/CRMP2 and Ulip6/CRMP5 was seen during development. In the cerebellum, only Ulip2/CRMP2 was highly expressed in the external part of EGL containing the mitotic neural precursors, whereas both Ulip2/CRMP2 and Ulip6/CRMP5 were expressed in the internal part of the EGL, which contains the postmitotic migrating neuronal precursors. After migration, neuronal precursors in the IGL showed high expression of Ulip6/CRMP5 but low expression of Ulip2/CRMP2. In addition, during brain development, Ulip2/CRMP2 was expressed before Ulip6/CRMP5 in oligodendrocytes. Taken together, these results indicate that Ulip2/CRMP2 and Ulip6/CRMP5 either may have different roles in the intracellular signal cascade pathway in response to the same signal or may mediate different signals involved in the balance of positive and negative growth cues required in the regulation of neuronal migration/axonal growth and oligodendrocyte migration/process extension.
Effect of Sema3A on oligodendrocytes: involvement of Ulip2/CRMP2 and Ulip6/CRMP5
Ulip2/CRMP2 is reported to mediate Sema3A signaling axon guidance and collapse during development (Goshima et al., 1995; Pasterkamp et al., 1998a). Several lines of evidence suggest that Sema3A can act on oligodendrocytes in the adult brain: (1) neuropilin-1, a component of the Sema3A receptor complex (He and Tessier-Lavigne, 1997; Kolodkin et al., 1997), was shown to be expressed by oligodendrocytes, (2) addition of Sema3A to the culture medium dramatically reduced oligodendrocyte process extension, (3) removal of Sema3A resulted in restoration of process extension, and (4) the effect of Sema3A was blocked by anti-neuropilin-1 antibodies or VEGF-165, another neuropilin-1 ligand (Miao et al., 1999). The blocking of the Sema3A effect by specific anti-Ulip2/CRMP2 or anti-Ulip6/CRMP5 antibodies, as described for Ulip2/CRMP2 in developing neurons (Goshima et al., 1995), and the lack of effect of anti-Ulip3/CRMP1 antibodies and preimmune sera indicate that Ulip2/CRMP2 and Ulip6/CRMP5 are involved in mediating the Sema3A signal. Interestingly, the time course of the Sema3A effect on oligodendrocytes differs from that on neuronal growth. Although growth cone collapse is seen 10 min to 6 hr after Sema3A contact with neurons (Bagnard et al., 1998), in oligodendrocytes the Sema3A effect was seen after 24 hr. This time course is similar to that for the effect of phorbol ester on oligodendrocyte process extension (Stariha et al., 1997). Sema3A, via Ulip2/CRMP2 or Ulip6/CRMP5, may act on the dynamics of microtubules, leading to the decrease in process extension. Several results support this hypothesis: (1) microtubules are present in oligodendrocytes and reflect oligodendrocyte function (Lunn et al., 1997), (2) Ulip2/CRMP2 mediates the dynamics of microtubules (Gu and Ihara, 2000), and (3) protein kinase, which could phosphorylate the numerous phosphorylation sites of the Ulip/CRMPs, regulates oligodendrocyte process extension (Stariha et al., 1997) and can affect microtubule dynamics (Gotoh et al., 1991). Although these results show that oligodendrocytes are sensitive to Sema3A, other signals might also be mediated by Ulip/CRMPs (Wang and Strittmatter, 1997).
Functional significance of Ulip6/CRMP5 and Ulip2/CRMP2 expression in the adult brain
Oligodendrocytic expression of Ulip6/CRMP5 and Ulip2/CRMP2 could be crucial in mediating the signals involved in myelination, demyelination, and remyelination (at least in the pons, cerebellum, and spinal cord) in the normal and pathological brain. In demyelinating disorders, such as multiple sclerosis, before oligodendrocytes can remyelinate, they must extend and contact the demyelinated axons. The role of Ulip2/CRMP2 and Ulip6/CRMP5 in the response to signals, such as Sema3A, could be crucial in the reinitiation and regulation of process extension by surviving oligodendrocytes. High levels of Sema3A are expressed namely by fibroblasts in adult CNS scar tissue (Pasterkamp et al., 1998a, 1999), and Sema3A and Ulip2/CRMP2 expression has been shown to be upregulated in injured axons (Minturn et al., 1995; Pasterkamp et al., 1998b). Scar-derived Sema3A could thus affect both oligodendrocytes process extension and migration and axonal regrowth around the lesion in the injured brain. A physiologic role of Sema3A on oligodendrocytes should be also consider and could be assessed in Sema3A-deficient mice that are viable after birth (Taniguchi et al., 1997). In these mice, it would be interesting to study neuronal regeneration and myelination after brain injury.
The fact that neurons and oligodendrocytes may respond to similar signals, mediated by Ulip/CRMPs, opens new fields of investigation into the role of the neuron/oligodendrocyte interaction in axonal growth, in addition to the myelin-derived axonal growth inhibitors, MAG and NI35/250 (Shibata et al., 1998; Buffo et al., 2000; GrandPre et al., 2000). On the other hand, the specific increase of Ulip2/CRMP2 in Alzheimer's disease (Gu et al., 2000) and the involvement of Ulip6/CRMP5 in PND patients emphasize the role of these proteins in neurological diseases. The tightly regulated spatial and temporal expression of the five Ulip/CRMPs is probably crucial in modulating the signal cascade pathways of positive/negative cues in both the developing and adult brain.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale and grants from the Ligue contre le Cancer du Rhône, the Association pour la Recherche contre le Cancer, and the European Leucodystrophy Association. We thank A. Sobel and T. Byk for providing the mouse Ulip/CRMP cDNAs, A. W. Püschel for providing stable cell lines expressing Sema3A, and C. A. Vergoin for technical assistance.
D.R., V.R., and E.C. contributed equally to this work.
Correspondence should be addressed to Dr. J. Honnorat, Neurologie B, Hôpital Neurologique, 59 Boulevard Pinel, BP Lyon Montchat, 69394 Lyon Cedex 03, France. E-mail:honnorat@cismsun.univ-lyon1.fr.
REFERENCES
- 1.Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972a;145:353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972b;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 3.Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J Comp Neurol. 1972c;145:465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- 4.Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275:23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- 5.Bagnard D, Lohrum M, Uziel D, Püschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- 6.Bagnard D, Thomasset N, Lohrum M, Püschel AW, Bolz J. Spatial distributions of guidance molecules regulate chemorepulsion and chemoattraction of growth cones. J Neurosci. 2000;20:1030–1035. doi: 10.1523/JNEUROSCI.20-03-01030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Püschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured Purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulliard C, Zurbriggen R, Tornare J, Faty M, Dastoor Z, Dreyer JL. Purification of a dichlorophenol-indophenol oxidoreductase from rat and bovine synaptic membranes: tight complex association of a glyceraldehyde-3-phosphate dehydrogenase isoform, TOAD64, enolase-gamma and aldolase C. Biochem J. 1997;324:555–563. doi: 10.1042/bj3240555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byk T, Dobransky T, Cifuentes-Diaz C, Sobel A. Identification and molecular characterization of Unc-33-like phosphoprotein (Ulip), a putative mammalian homolog of the axonal guidance associated unc-33 gene product. J Neurosci. 1996;16:668–701. doi: 10.1523/JNEUROSCI.16-02-00688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byk T, Ozon S, Sobel A. The Ulip family of phosphoproteins. Eur J Biochem. 1998;254:14–24. doi: 10.1046/j.1432-1327.1998.2540014.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 14.Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishman PS, Farrand DA, Kristt DA. Internalization of plasma proteins by cerebellar Purkinje cells. J Neurol Sci. 1990;100:43–49. doi: 10.1016/0022-510x(90)90011-b. [DOI] [PubMed] [Google Scholar]
- 16.Fishman PS, Farrand DA, Kristt DA. Penetration and internalization of plasma proteins in the human spinal cord. J Neurol Sci. 1991;104:166–175. doi: 10.1016/0022-510x(91)90306-r. [DOI] [PubMed] [Google Scholar]
- 17.Friedman B, Hockfield S, Black JA, Woodruff KA, Waxman SG. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2:380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- 18.Fukada M, Watakabe I, Yuasa-Kawada J, Kawachi H, Kuroiwa A, Matsuda Y, Noda M. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem. 2000;275:37957–37965. doi: 10.1074/jbc.M003277200. [DOI] [PubMed] [Google Scholar]
- 19.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 20.Gotoh Y, Nishida E, Matsuda S, Shiina N, Kosako H, Shiokawa K, Akiyama T, Otha K, Sakai H. In vitro effect on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991;349:251–259. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- 21.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 22.Greenlee JE, Parks TN, Jaeckle KA. Type IIa (“anti-Hu”) anti-neuronal antibodies produce destruction of rat cerebellar granule neurons in vitro. Neurology. 1993;43:2049–2054. doi: 10.1212/wnl.43.10.2049. [DOI] [PubMed] [Google Scholar]
- 23.Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem. 2000;275:17917–17920. doi: 10.1074/jbc.C000179200. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Hamajima N, Ihara Y. Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry. 2000;39:4267–4275. doi: 10.1021/bi992323h. [DOI] [PubMed] [Google Scholar]
- 25.Hamajima N, Matsuda K, Sakata S, Tamaki M, Nonaka M. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene. 1996;180:157–163. doi: 10.1016/s0378-1119(96)00445-3. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 27.Honnorat J, Antoine JC, Derrington EA, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kD developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 1996;61:270–278. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honnorat J, Aguera M, Zalc B, Goujet C, Quach T, Antoine JC, Belin MF. POP66, a paraneoplastic encephalomyelitis-related antigen, is a marker of adult oligodendrocytes. J Neuropathol Exp Neurol. 1998;57:311–322. doi: 10.1097/00005072-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Honnorat J, Byk T, Kuster I, Aguera M, Ricard D, Rogemond V, Quach T, Aunis D, Sobel A, Mattei MG, Kolattukudy P, Belin MF, Antoine JC. Ulip/CRMP proteins are recognized by autoantibodies in paraneoplastic neurological syndromes. Eur J Neurosci. 1999;11:4226–4232. doi: 10.1046/j.1460-9568.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 30.Inatome R, Tsujimura T, Hitomi T, Mitsui N, Hermann P, Kuroda S, Yamamura H, Yanagi S. Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J Biol Chem. 2000;275:27291–27302. doi: 10.1074/jbc.M910126199. [DOI] [PubMed] [Google Scholar]
- 31.Kamata T, Subleski M, Hara Y, Yuhki N, Kung H, Copeland NG, Jenkins NA, Yoshimura T, Modi W, Copeland TD. Isolation and characterization of a bovine neural specific protein (CRMP-2) cDNA homologous to unc-33, a C. elegans gene implicated in axonal outgrowth and guidance. Mol Brain Res. 1998;54:219–236. doi: 10.1016/s0169-328x(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 32.Kato Y, Hamajima N, Inagaki H, Okumura N, Koji T, Sasaki M, Nonaka M. Post-meiotic expression of the mouse dihydropyrimidinase related protein 3 gene during spermiogenesis. Mol Reprod Dev. 1998;51:105–111. doi: 10.1002/(SICI)1098-2795(199809)51:1<105::AID-MRD13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Herman RK, Shaw E. Analysis of the Caenorhabditis elegans axonal guidance and outgrowth gene unc-33. Genetics. 1992;132:675–689. doi: 10.1093/genetics/132.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisak RP, Pleasure DE, Silberberg DH, Manning MC, Saida T. Long term culture of bovine oligodendroglia isolated with a Percoll gradient. Brain Res. 1981;223:107–122. doi: 10.1016/0006-8993(81)90809-x. [DOI] [PubMed] [Google Scholar]
- 36.Lubetzki C, Gansmuller A, Lachapelle F, Lombrail P, Gumpel M. Myelination by oligodendrocytes isolated from 4–6-week-old rat central nervous system and transplanted into new-born shivered brain. J Neurol Sci. 1988;88:161–175. doi: 10.1016/0022-510X(88)90214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lunn KF, Baas PW, Duncan ID. Microtubule organization and stability in the oligodendrocyte. J Neurosci. 1997;17:4921–4932. doi: 10.1523/JNEUROSCI.17-13-04921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minturn JE, Fryer HJL, Geschwind DH, Hockfield S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J Neurosci. 1995;15:6757–6766. doi: 10.1523/JNEUROSCI.15-10-06757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacher J, Rosell DR, McEwen BS. Widespread expression of rat collapsin response-mediated protein 4 in the telencephalon and other areas of the adult rat central nervous system. J Comp Neurol. 2000;424:628–639. doi: 10.1002/1096-9861(20000904)424:4<628::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Pasterkamp RJ, De Winter F, Holtmaat AJGD, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J Neurosci. 1998a;23:9962–9976. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasterkamp RJ, Giger RJ, Verhaagen J. Regulation of semaphorin III/collapsin-1 gene expression during peripheral nerve regeneration. Exp Neurol. 1998b;153:313–327. doi: 10.1006/exnr.1998.6886. [DOI] [PubMed] [Google Scholar]
- 43.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed after injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 44.Posner JB. Paraneoplastic syndromes: a brief review. Ann NY Acad Sci. 1997;835:83–90. doi: 10.1111/j.1749-6632.1997.tb48620.x. [DOI] [PubMed] [Google Scholar]
- 45.Püschel AW. Semaphorins: repulsive guidance molecules show their attractive side. Nat Neurosci. 1999;2:777–778. doi: 10.1038/12140. [DOI] [PubMed] [Google Scholar]
- 46.Quach TT, Rong Y, Belin MF, Duchemin AM, Akakoa H, Ding S, Baudry M, Kollattukudy PE, Honnorat J. Molecular cloning and expression of a new unc-33 like cDNA from rat brain and its relation to paraneoplastic neurological syndromes. Mol Brain Res. 1997;46:329–332. doi: 10.1016/s0169-328x(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 47.Ricard D, Stankoff B, Bagnard D, Aguera M, Rogemond V, Antoine JC, Spassky N, Zalc B, Lubetzki C, Belin MF, Honnorat J. Differential expression of collapsin response mediator proteins (CRMP/ULIP) in subsets of oligodendrocytes in the post-natal rodent brain. Mol Cell Neurosci. 2000;16:324–337. doi: 10.1006/mcne.2000.0888. [DOI] [PubMed] [Google Scholar]
- 48.Rogemond V, Honnorat J. Anti-CV2 autoantibodies and paraneoplastic neurological syndromes. Clin Rev Allergy Immunol. 2000;19:48–56. doi: 10.1385/CRIAI:19:1:51. [DOI] [PubMed] [Google Scholar]
- 49.Shibata A, Wright MV, David S, McKerracher L, Braun PE, Kater SB. Unique responses of differentiating neuronal growth cones to inhibitory cues presented by oligodendrocytes. J Cell Biol. 1998;142:191–202. doi: 10.1083/jcb.142.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirvan A, Ziv I, Flemminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A. Semaphorins as mediators of neuronal apoptosis. J Neurochem. 1999;73:961–971. doi: 10.1046/j.1471-4159.1999.0730961.x. [DOI] [PubMed] [Google Scholar]
- 51.Stariha RL, Kikuchi S, Siow YL, Pelech SL, Kim M, Kim SU. Role of extracellular signal-regulated protein kinases 1 and 2 in oligodendroglial process extension. J Neurochem. 1997;68:945–953. doi: 10.1046/j.1471-4159.1997.68030945.x. [DOI] [PubMed] [Google Scholar]
- 52.Taketo MM, Araki Y, Matsunaga A, Yokoi A, Tsuchida J, Nishina Y, Nosaki M, Tanaka H, Koga M, Uchida K, Matsumiya K, Okuyama A, Rochelle JM, Nishimune Y, Matsui M, Seldin MF. Mapping of eight testis specific genes to mouse chromosomes. Genomics. 1997;46:138–142. doi: 10.1006/geno.1997.5014. [DOI] [PubMed] [Google Scholar]
- 53.Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Takeshi Y. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang LH, Strittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci. 1996;16:6197–6207. doi: 10.1523/JNEUROSCI.16-19-06197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang LH, Strittmatter SM. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J Neurochem. 1997;69:2261–2269. doi: 10.1046/j.1471-4159.1997.69062261.x. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H, Watanabe A, Ihara Y. CRMP-2 is associated with neurofibrillary tangles in Alzheimer's disease. J Biol Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- 57.Zhang SC, Ge B, Duncan ID. Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci USA. 1999;9:4089–4094. doi: 10.1073/pnas.96.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]