Abstract
While scanning a textured surface with fingers, tactile information is encoded both spatially, by differential activation of adjacent receptors, and temporally, by changes in receptor activation during movements of the fingers across the surface. We used a tactile discrimination task to examine the dependence of human tactile perception on the availability of spatial and temporal cues. Subjects discriminated between spatial frequencies of metal gratings presented simultaneously to both hands. Tactile temporal cues were eliminated by preventing lateral hand movements; tactile spatial cues were eliminated by using gloves with an attached rubber pin. Analysis revealed separation of the subjects into two groups: “spatiotemporal” (ST) and “latent-temporal” (LT). Under normal conditions, the performance of ST subjects was significantly better than that of the LT subjects. Prevention of lateral movements impaired performance of both ST and LT subjects. However, when only temporal cues were available, the performance of ST subjects was significantly impaired, whereas that of the LT subjects either improved or did not change. Under the latter condition, LT subjects changed strategy to scanning with alternating hands, at velocities similar to the velocities normally used by ST subjects. These velocities generated temporal frequencies between 15 and 30 Hz. The LT subjects were unaware of their improved performance. Nine of ten LT subjects significantly improved their performance under normal conditions when trained to scan gratings using alternating hands and velocities similar to those used by ST subjects. We conclude that (1) temporal cues are essential for spatial-frequency discrimination, (2) human subjects vary in the tactile strategies they use for texture exploration, and (3) poor tactile performers can significantly improve by using strategies that emphasize temporal cues.
Keywords: humans, learning, neural code, psychophysics, spatial coding, tactile discrimination, temporal coding
Finger movement is essential for tactile perception—without it, object identification becomes difficult, even impossible (Katz, 1989; Morley et al., 1983; Phillips et al., 1983; Srinivasan et al., 1990; Hollins and Risner, 2000). Initially, this was thought to reflect the brain's need to use temporal cues generated by the movements (Katz, 1989; Gibson, 1962). During finger movement, spatial intervals were assumed to be encoded by temporal intervals of receptor activation, with a spatial intervaldx being encoded by a temporal interval dt, wheredt = dx/v, and v is the finger velocity (Darian-Smith and Oke, 1980; Morley and Goodwin, 1987). This interpretation was challenged by Lederman (1974), who observed that the scanning velocity is not important for the estimation of roughness and concluded that roughness estimation can be obtained without temporal cues. Consistent with this, roughness estimation was found to be best correlated with the spatial variations across the fingertip (Connor et al., 1990; Connor and Johnson, 1992;Johnson and Hsiao, 1994). According to this view, finger movement is required only for the prevention of receptor adaptation (Taylor and Lederman, 1975) or the enhancement of spatial variations (Johnson and Lamb, 1981; Phillips et al., 1983) but provides no sensory information by itself.
However, not all results are consistent with this view. Lamb (1983)observed that tactile discrimination of dot spacing is better along the track of finger movement than perpendicular to it. Ahissar and Gamzu (1995) observed that discrimination of spatial frequencies by naive subjects does depend on finger velocity. These observations suggested that temporal cues, determined by the finger velocity along the scanning direction, might be used for texture analysis. Indeed, it has recently been shown that roughness can be perceived when spatial tactile cues are eliminated (Klatzky and Lederman, 1999). Furthermore, the availability of temporal cues seems to be crucial for roughness perception within certain ranges of spatial frequencies (Hollins and Risner, 2000) or stimulus parameters (Cascio and Sathian, 2001). These studies indicate that temporal cues carry significant tactile information. However, what is the relative contribution of spatial and temporal cues, what are the scanning strategies involved, and what are the differences between individual subjects, is still not known.
In this paper we examined to what extent spatial and temporal cues are used by individual subjects during tactile discrimination of gratings and how scanning strategy depends on cue availability. We addressed these questions by eliminating either spatial or temporal tactile cues in a spatial-frequency discrimination task and by monitoring finger movement. We define tactile spatial cues as those conveyed by the differential activation of adjacent receptors at a given time. We define temporal cues as those conveyed by the temporal profile of the activity of single receptors. The spatial information, encoded during finger movement by both spatial and temporal cues, can be decoded by a variety of neuronal mechanisms, using tactile, proprioceptive and motor signals. Here, we do not ask how this information is decoded, but rather which tactile signals are essential for decoding. We show that temporal cues are essential for tactile discrimination, and that, in approximately half of the subjects, their utilization is impaired by the availability of spatial cues. This can be overcome by guided training, which entails change of scanning strategy.
Preliminary reports of our findings have been presented (Gamzu and Ahissar, 1998; Gamzu et al., 2000).
MATERIALS AND METHODS
Subjects. Thirty right-handed subjects (13 male and 17 female students, aged 21–35 years) were recruited and financially compensated for their participation. None of the subjects had a developmental or neurological disorder or a history of trauma affecting the hands. None of the subjects had any previous experience with the stimuli or tasks used in this study.
Tactile stimulation. Gratings were prepared as printed circuits, in which gold-plated copper bars were fixed on a firm plastic plate. The spatial frequencies (SF) of the gratings were from 219 to 800 bars/m, with the spaces between the bars of each grating constant. To test the effect of the range of the available SFs on performance and scanning strategies, the gratings were divided into two sets, each consisting of eight gratings (Table 1). In the first set, termed “24”, the frequencies of the bars ranged from 219 to 400 bars/m. In the second, termed “48”, the frequencies of the bars ranged from 438 to 800 bars/m. Within each set, the ratio between two successive SFs was 1.09. The heights of the bars were either 30 μm (“low-amplitude”) or 100 μm (“high-amplitude”), and the widths (“ridge width”) were 300 μm. The distance between two adjacent bars (“groove width”) varied from grating to grating, according to the SF (Table 1). Each printed circuit (“gratings surface”) contained eight gratings of the same height of either the 24 or the 48 set.
Table 1.
Spatial dimensions of gratings
| SET | SF (bars/m) | Period (mm) | Groove width (mm) |
|---|---|---|---|
| 24 | 219 | 4.57 | 4.27 |
| 238 | 4.20 | 3.90 | |
| 260 | 3.85 | 3.55 | |
| 283 | 3.53 | 3.23 | |
| 309 | 3.24 | 2.94 | |
| 337 | 2.97 | 2.67 | |
| 367 | 2.72 | 2.42 | |
| 400 | 2.50 | 2.20 | |
| 48 | 438 | 2.28 | 1.98 |
| 477 | 2.10 | 1.80 | |
| 520 | 1.92 | 1.62 | |
| 567 | 1.76 | 1.46 | |
| 618 | 1.62 | 1.32 | |
| 673 | 1.49 | 1.19 | |
| 734 | 1.36 | 1.06 | |
| 800 | 1.25 | 0.95 |
Each set (“24” and “48”) contained eight gratings with various spatial frequencies (SF), spatial periods (i.e., the distance between two adjacent ridge onsets), and groove widths (i.e., the distance between ridge offset to ridge onset).
Testing apparatus. For performance of the perceptual task, each subject sat in front of a wooden frame that was constructed such that the two grating surfaces could be introduced to the subjects while hidden from their sight (Fig.1A). The grating surfaces were inserted underneath a Plexiglas board (45 × 32 × 0.5 cm) with two rectangular windows (10 × 2.5 cm each) that permitted exposure of the gratings to the fingertips of the subjects. The distance between the centers of the windows was 22 cm. Before each trial, one grating of each surface was manually positioned by the experimenter underneath each window. The order of grating presentations was determined by a computer, according to a random selection procedure, with a different seed for each session. During each trial, the location of the scanning fingers of each hand was sampled (temporal resolution of 40 msec and spatial resolution of 0.1 mm) by an infrared, ultrasonic, location detector (V-Scope LVS-11-pro; Litek, Tel-Aviv, Israel).
Fig. 1.
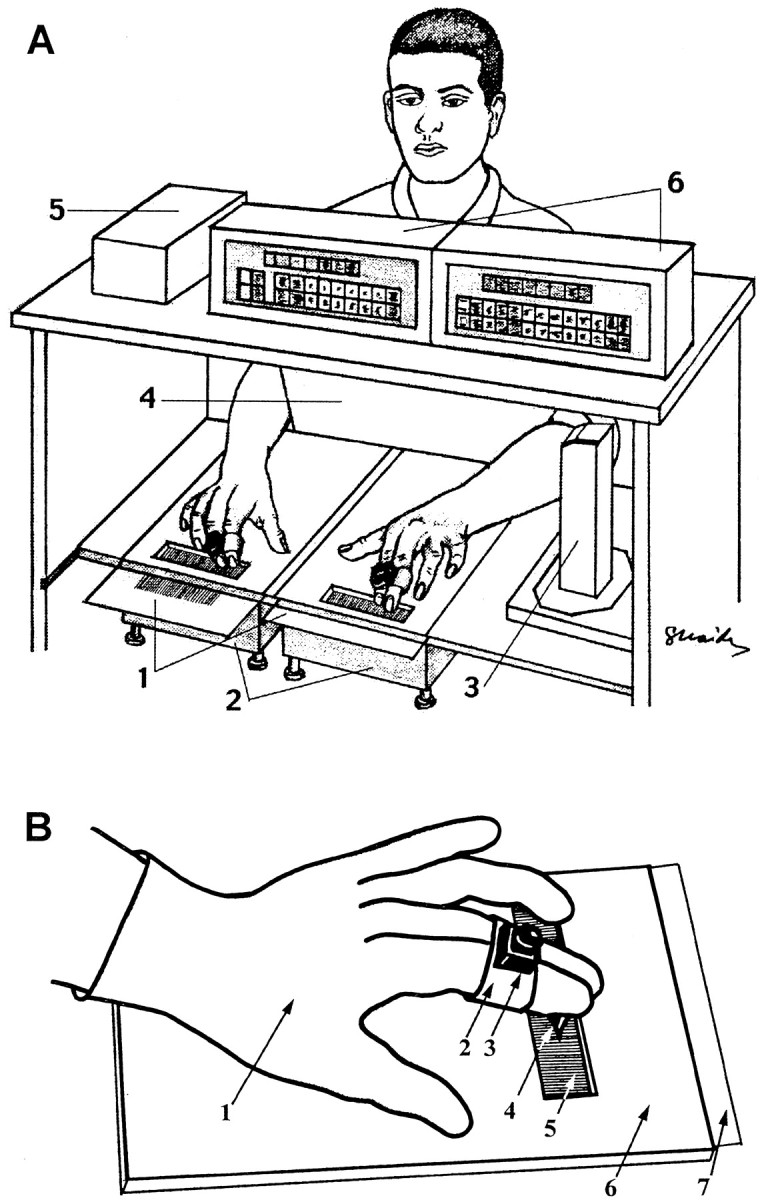
Experimental apparatus. A, General scheme. 1, Grating surfaces; 2,electronic scales; 3, component of the location detector containing infrared transmitter and ultrasonic receiver;4, screen; 5, interface box of the location detector; and 6, monitors and computer interface of electronic scales. B, Scanning with gloves.1, Latex glove; 2, Velcro band holding the sensor of the location detector; 3, sensor of the location detector; 4, rubber pin; 5,grating; 6, Plexiglas board; and 7,grating surface (inserted underneath the Plexiglas board).
Testing procedure. The testing phase consisted of several sessions, each of which included 128 trials. In each trial, the subject performed a two-alternative forced-choice discrimination task, in which he or she had to scan two gratings, one with each hand, and to determine “which hand was presented with the denser grating.” The subjects also had to state the confidence level of their answer on a scale of 0 (guessing) to 10 (absolutely confident). The difference between the SFs of the gratings presented to the two hands in each trial was expressed in log units [dSF = log1.09 (SFright/SFleft)] and was limited to 2 ≤ abs(dSF) ≤ 5. Within a session, each grating was presented an equal number of times. Subjects were instructed to scan the gratings with both their index and middle fingers, which were held together by the sensor of the location detector (Fig.1B).
Trial time was limited to 4 sec, and no feedback was provided. The subjects were instructed not to use extensive finger force during scanning, but no restraints were put on the amount used, i.e., they were free to choose the force they desired. The finger force used by the subjects was monitored by two electronic digital scales (MD-901; Bolet, Rosh-Ha'ayin, Israel) and was < 200 gm wt for all subjects and sessions. Normal, vertical, and glove scanning methods were used. For normal scanning, subjects were instructed to scan laterally, across the grating bars, and not to remove their fingers from the surface during a trial; the subjects could choose the scanning velocity and profile. For vertical scanning, the subjects were instructed not to move their fingers laterally across the grating bars; the subjects could use vertical scanning in both planes (along the grating bars, or up and down). During “glove sessions,” subjects wore disposable latex gloves of appropriate sizes on both hands (Fig.1B). Rubber tips (obtained from the ends of toothbrushes; model number 407; Butler USA) were trimmed at the base and glued (with super glue) to the index fingers of the gloves while on the subject, and then the rubber tip was cut diagonally ∼2 mm from the end, to remove the flexible edge. The diameter of the tip at the base of the diagonal cut was between 1 and 1.2 mm. The subjects were allowed to scan laterally, only using that tip, but could choose the scanning velocity and profile. Earphones were used to prevent possible auditory cues produced by friction.
Because one goal of this study was to determine whether subjects adjust their finger velocity according to the context of the stimulus and scanning type, a block design (consisting of 12 sessions) was used in which, during all 128 trials of a single session, the same experimental conditions were used. The order of the sessions was 24 high normal, 48 high normal, 24 low normal, 48 low normal, 24 high vertical, 48 high vertical, 24 low vertical, 48 low vertical, 24 high glove, 48 high glove, 24 low glove, and 48 low glove; where 24 and 48 are the sets of SFs; high and low refer to the amplitude (height) of the bars; and normal, vertical and glove refer to the scanning type. During the testing phase, 10 subjects performed all 12 sessions. Three of these subjects performed additional four normal sessions (24 high, 48 high, 24 low, 48 low) at the end of the block. The other 20 subjects performed only the four sessions in which the low-amplitude gratings of the 48 set were used (i.e., normal, vertical, glove, normal).
Training. Ten subjects, which were defined as latent-temporal following the above testing procedure (see Results), were trained on low-amplitude gratings of the 48 set using normal scanning. Five of these ten were trained with guidance, and the other five were trained first without and then with guidance. Guidance consisted of instructions regarding scanning velocity and profile. To ease the training process on the subjects and to try to assess the contribution of each of the scanning factors, we guided the subjects to change only one factor at a time. In addition, the order of guiding was changed between the two groups. Because changing velocity was more difficult for the subjects, two sessions were dedicated for it: the first was with 8-sec-long trials, and the second was with the usual trial duration of 4 sec. The first five subjects were first instructed to scan freely (control, one session), then to use alternating hands at any velocity (1 session), then both hands simultaneously at low velocity (two sessions), then alternating hands at low velocity (one session), and then free style (control, one session). The other five subjects, after completing the nonguided training (five sessions), were first instructed to use low scanning velocity (two sessions), then alternating hands at any velocity (one session), then alternating hands at low velocity (two sessions), and then free style (control, one session).
Data analysis. For each trial, session, subject, and group of subjects, the performance levels, confidence levels, and finger velocities were analyzed. For the basic velocity measure (V), the root mean square of the velocity along each trial was used. The average temporal frequency (f) for each hand during each trial was estimated to be V * SF.
Motion profiles were analyzed in a sample of 40 trials for each subject and each session. In each sample, all SF combinations were represented. The motion profiles for both hands along the entire trials were plotted, and the types of profiles were classified (see Results and Fig. 3). For each session, a sample was defined as homogeneous if >95% (38 trials) of the motion profiles were of the same type.
Fig. 3.
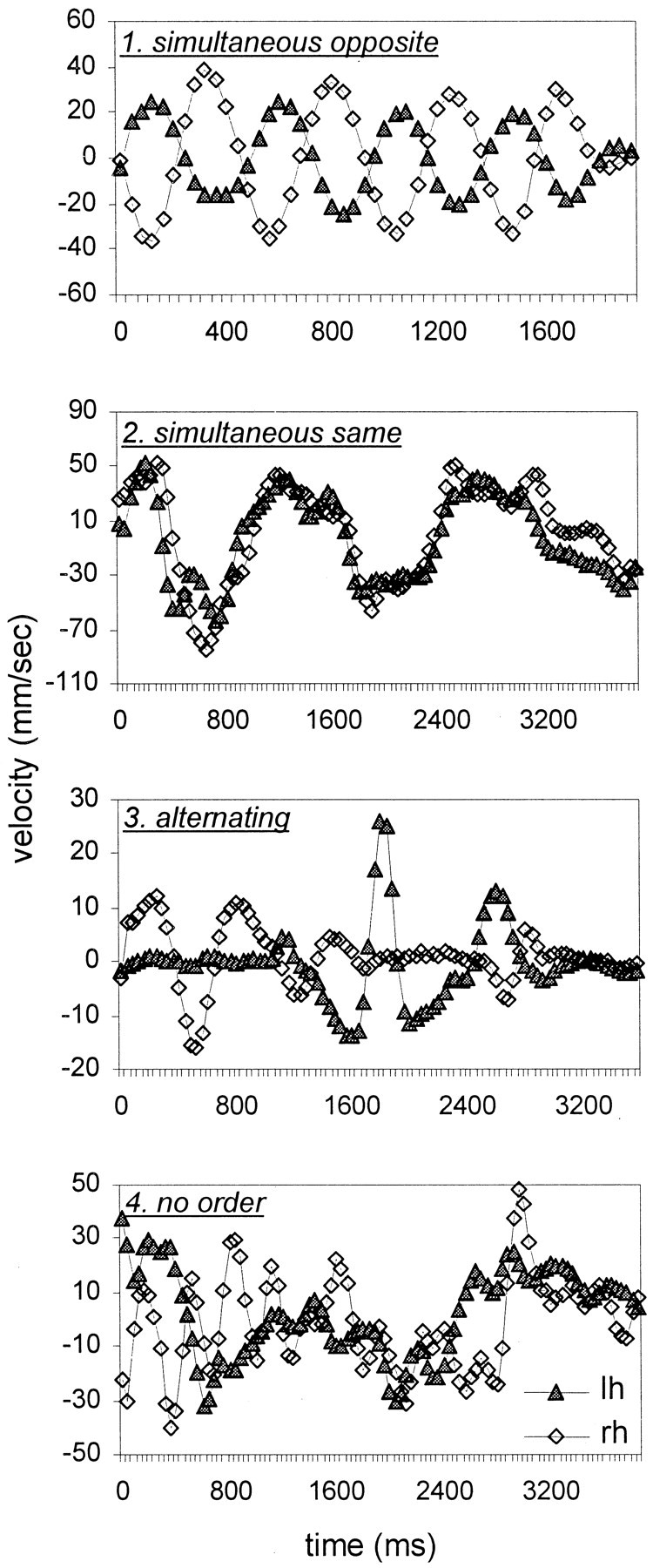
Motion profiles during single trials.1. Simultaneous opposite, Simultaneous scanning, opposite directions. 2. Simultaneous same, Simultaneous scanning, same direction. 3.Alternating, Scanning with alternating hands.4. No order. lh, Left hand; rh, right hand. Curves were smoothed by a convolution with a triangular of area 1 and a base of ± two samples.
All subjects, except for one, used the entire range (0–10) of confidence levels, with the levels used for the one exception being 0–8. The confidence evaluations of this subject were normalized to span the range 0–10.
One-, two- and three-way ANOVAs with repeated measures were performed using SAS software, version 6.12 for windows. When the dependent variable was the success rate, ANOVA was performed on the arcsine of the square root of the success rate, to correct for the non-Gaussian distribution. Significant ANOVA results were followed by multiple comparisons, using Fisher's Protected Least Significant Difference (LSD) procedure with α = 0.05, to define homogeneous subgroups. For comparison of distributions we used Kolmogorov–Smirnov (KS) test. t test and Wilcoxon Rank Sum (WRS) test were used for comparing averages of normal distributions and medians of non-normal distributions, respectively. All correlation coefficients (r) are Pearson's r.
RESULTS
Each of the first 10 subjects performed 12 types of discrimination task sessions: using 24 and 48 sets of gratings, low- and high-amplitude sets of gratings, and “normal”, “vertical” and “glove” types of scanning. Psychometric curves were determined for each subject and each session. The average psychometric curves of all 10 subjects, resulting from all sessions of low-amplitude gratings, are depicted in Figure 2, A andB. The average performance of these subjects was better with gratings whose SF was 219–400 bars/m (24) than those whose SF was 438–800 bars/m (48) (ANOVA; p < 0.001) and improved with larger dSFs (p < 0.001). For both the 24 and 48 sets and high- and low-amplitude gratings, the performance of the subjects was best with normal scanning, worse during glove scanning, and worst during vertical (no lateral movement) scanning (ANOVA; p < 0.001). In fact, the elimination of lateral movements abrogated the ability to discriminate between gratings with dSF < 4 (performance did not differ from chance level with these dSFs in both 24 and 48 sets; ANOVA; p> 0.05), while allowing only marginal discrimination with dSFs of 4 and 5 (p < 0.01) (Fig. 2A,B). The differences in performance level between scanning of the high-amplitude (100 μm height) and low-amplitude (30 μm height) gratings were not significant (ANOVA followed by LSD; α = 0.05), except for normal scanning of the 24 set, in which low-amplitude gratings yielded slightly better performance (87 vs 83% on average).
Fig. 2.
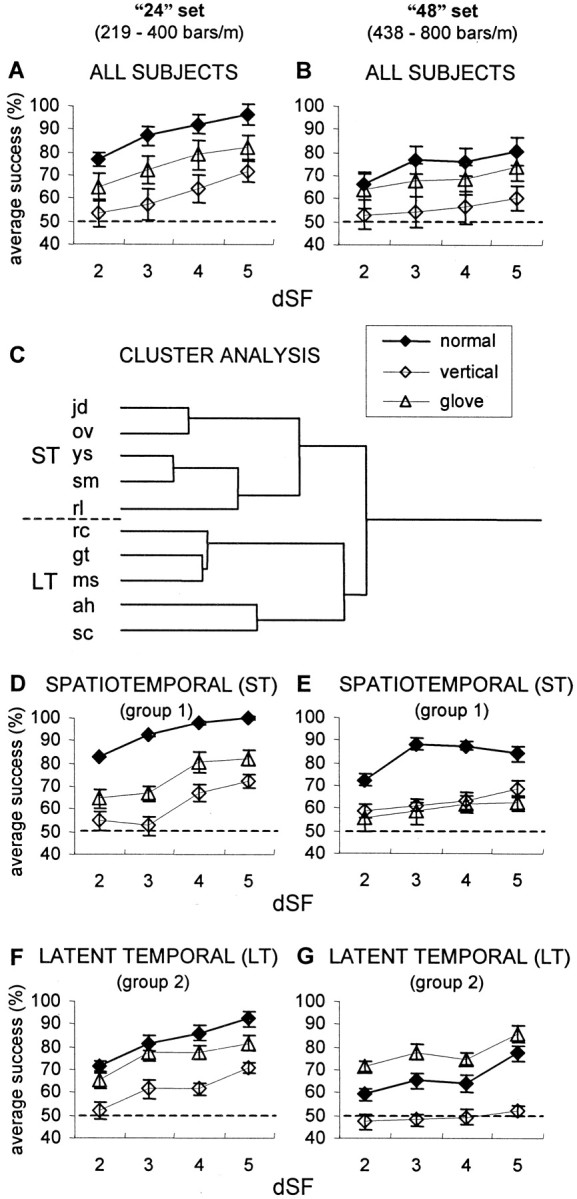
Effect of scanning type on average psychometric curves. A, Average performance of the initial 10 subjects on the 24 low-amplitude sets of gratings. For each subject, the success rate was the percentage of successful trials of the total number of trials. dSF is the difference, in log units, between the SFs presented to the two hands. Error bars indicate SEM.Dashed line represents chance level. B,Average performance of the initial 10 subjects on the 48 low-amplitude sets of gratings. C, Pearson complete cluster analysis of data in A and B (see Results). Group 1 (ST), subjects jd, ov,ys, sm, and rl; group 2 (LT), subjects rc, gt,ms, ah, and sc. The lengths of the branches represent the distances Dij between the subjects: Dij = 1 −Pij, wherePij is the Pearson product moment correlation between subjects i and j.D–G, Psychometric curves of ST group (n = 5) with 24 (D) and 48 (E) sets of gratings, and LT group (n = 5) with 24 (F) and 48 (G) sets of gratings. Results were averaged for each group.
In addition to this common pattern, the performance of individual subjects differed significantly (ANOVA; p < 0.0001). Subjects also differed in their scanning patterns and velocities and were affected differently by the elimination of spatial and temporal cues. For each subject and each condition (24, 48, high-amplitude, low-amplitude), correlation coefficients of the success rates in normal sessions versus (1) vertical scanning sessions and (2) glove sessions, were calculated. Pearson complete cluster analysis of these correlation coefficients revealed two separate groups, each containing five subjects (Fig. 2C). When the performance of these two groups was compared by their averaged psychometric curves (Fig.2D–G), it was found that one group (group 1) performed best during normal scanning of both the 24 and the 48 sets (ANOVA; p < 0.0001, followed by LSD; α = 0.05), whereas the other (group 2) performed best with glove scanning of the 48 set (p < 0.0001) and equally good with glove and normal scanning of the 24 set (p = 0.134 and 0.94 for low- and high-amplitude gratings, respectively). Under normal conditions, the performance of group 1 was better than that of group 2 with both the 24 and 48 sets (ANOVA; p < 0.003). However with glove scanning, the performance of group 2 was either similar to (24 set; p = 0.58) or better than (48 set;p < 0.0001) the performance of group 1. In fact, with the 48 set, the performance of group 2 with gloves was similar to the performance of group 1 under normal conditions (ANOVA;p > 0.3). With glove scanning, the performance of both groups with both 24 and 48 sets was better than chance (ANOVA;p < 0.05). With vertical scanning, the performance was also better than chance (p < 0.05), except for group 2 with the 48 set (p = 0.67).
Thus, elimination of sensory cues affected different subjects differently. For group 1, referred to as spatiotemporal (ST), both spatial and temporal sensory cues appeared to be essential for the tactile discrimination task. For group 2, referred to as latent-temporal (LT), elimination of tactile spatial cues and utilization of only temporal cues either improved performance (with the 48 set) or did not affect performance (with the 24 set). With these subjects, thus, the availability of spatial cues appeared to interfere with tactile perception of SFs between 400 and 800 bars/m. For both the ST and LT groups, no significant differences in performance were observed between the scanning of high-amplitude and low-amplitude gratings.
Klatzky and Lederman (1999) showed that with roughness discrimination performance depends also on the absolute value of SF and not only on the differential value. In our experiments, the dependency of success rate on the absolute value of SF (defined as the averaged SF presented to the two hands, aSF) is most clearly demonstrated by the reduction in performance when moving from the 24 set to the 48 set (Fig. 2,left vs right columns). However, within each set and each group the effect of aSF on performance was much less pronounced and usually did not reach significance level (Table2). In general, the dependency of performance on aSF was much less significant than the dependency on dSF.
Table 2.
Dependency of success rate on aSF and dSF and their interaction
| Session | aSF | dSF | aSF * dSF |
|---|---|---|---|
| All | +++ | +++ | + |
| 24 | +++ | +++ | +++ |
| 48 | − | +++ | − |
| HT 24 | + | +++ | + |
| HT 48 | − | +++ | − |
| ST 24 | +++ | +++ | + |
| ST 48 | − | +++ | − |
| HT 24 normal | + | +++ | − |
| HT 24 glove | − | ++ | + |
| HT 24 vertical | − | + | − |
| ST 24 normal | + | +++ | − |
| ST 24 glove | +++ | +++ | − |
| ST 24 vertical | − | +++ | − |
| HT 48 normal | − | + | − |
| HT 48 glove | − | +++ | − |
| HT 48 vertical | − | − | − |
| ST 48 normal | − | +++ | − |
| ST 48 glove | − | − | − |
| ST 48 vertical | − | − | − |
Results of univariate ANOVA tests for the different categories are presented. aSF = (SFr +SFI)/2; dSF = log1.09(SFR) − log1.09(SFI), where r andl indicate right and left hands, respectively. −,P > 0.05; +, P < 0.05; ++, P < 0.01; +++,P < 0.001.
Profiles of scanning motion
Profiles of scanning motion describe the velocity of movement of the hands as a function of time during single trials. Four types of scanning profiles were observed (Fig. 3): (1) simultaneous opposite: both hands scanning simultaneously in opposite directions; (2) simultaneous same, both hands scanning simultaneously in the same direction; (3) alternating, while one hand scanning, the other was stationary; and (4) no order. For each subject and session, the motion profile was usually consistent for at least 95% of the trials.
With few exceptions, all LT subjects switched from simultaneous opposite scanning (type 1) during normal scanning sessions to alternating scanning (type 3) during glove sessions (Table3). ST subjects did not exhibit such a stereotypical behavior. Yet, similarly to the LT subjects, ST subjects usually exhibited alternating scanning during the glove sessions.
Table 3.
Scanning profiles
| Group | Subject | 24 high | 24 low | 48 high | 48 low |
|---|---|---|---|---|---|
| LT | ah | 1 → 3 | 1 → 3 | 1 → 4 | 1 → 4 |
| gt | 1 → 3 | 1 → 3 | 1 → 3 | 1 → 3 | |
| rc | 1 → 3 | 1 → 3 | 1 → 3 | 1 → 3 | |
| ms | 1 → 3 | 1 → 3 | 1 → 3 | 1 → 3 | |
| sc | 3 → 3 | 1 → 3 | 1 → 3 | 1 → 3 | |
| ST | ys | 2 → 1 | 2 → 1 | 2 → 1 | 2 → 1 |
| sm | 1 → 2 | 1 → 2 | 4 → 3 | 2 → 3 | |
| jd | 3 → 3 | 3 → 3 | 3 → 3 | 3 → 3 | |
| ov | 3 → 3 | 3 → 3 | 1 → 3 | 1 → 3 | |
| rl | 3 → 3 | 1 → 3 | 1 → 3 | 1 → 3 |
For each subject, the scanning types (Fig. 3) during normal (left-hand side of the arrow) and glove sessions are depicted. “High” and “low” refer to the amplitude of gratings.
Tuning of scanning velocities
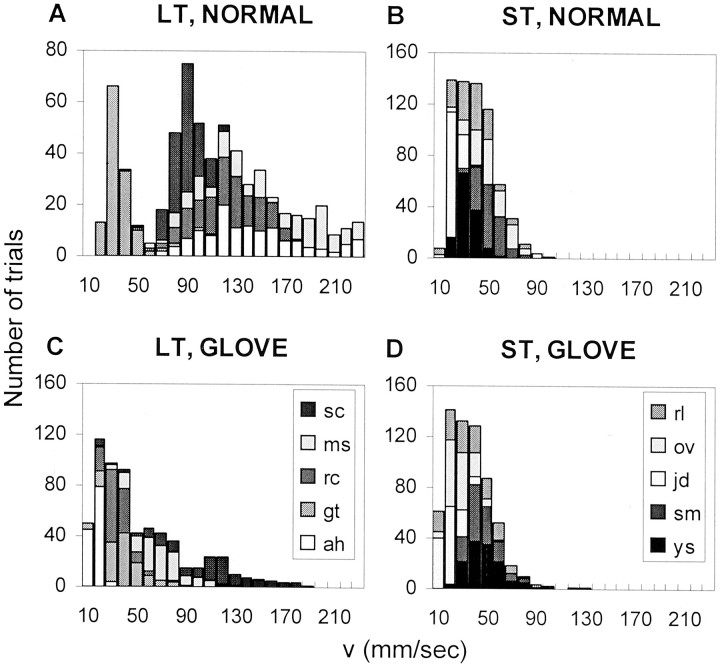
The average scanning velocities varied in different experimental conditions. This “coarse tuning” of the scanning velocity is demonstrated by the distributions of average trial velocities of the two groups of subjects under the different conditions. LT subjects used a wider range of velocities than ST subjects, with both normal and glove scanning types (KS, p < 0.0001; WRS,p < 0.0001). The distributions of average scanning velocities of the left-hand with low-amplitude 48 gratings are depicted in Figure 4; the scanning velocity distributions observed with the other conditions of the 48 set (right-hand low-amplitude, right-hand high-amplitude, and left-hand high-amplitude) were similar (data not shown). Similar differences between ST and LT subjects were observed with the 24 set. When the ST subjects switched from normal to glove scanning, their scanning velocities did not change (Table 4) (WRS;p > 0.1 for both hands). When the LT subjects switched from normal to glove scanning, their scanning velocities changed significantly (KS, p < 0.0001; WRS, p< 0.0001). The resulting distribution of LT velocities was closer to the distributions of ST velocities (Fig. 4, compare C withB and D; Table 4). The average temporal frequency (f) generated for every trial is represented by f = V * SF, thus changes in scanning velocities induced changes in the temporal frequencies. On average, the temporal frequencies generated by LT subjects during sessions with glove scanning were close to those generated by ST subjects under both normal and glove conditions, and were usually between 15 and 30 Hz (Table 4). Keeping f within this range was accompanied by a reduction of the scanning velocities when moving from the 24 to the 48 set (Table 4).
Fig. 4.
Distribution of the trial velocities(V) of the left hand during the scanning of the 48 low-amplitude sets of gratings. Each gray levelrepresents a different subject. A, LT subjects and normal scanning. B, ST subjects and normal scanning.C, LT subjects and glove scanning. D, ST subjects and glove scanning.
Table 4.
Means (± SEM) and medians of velocities and temporal frequencies during normal and glove scanning of “24” and “48” low-amplitude gratings
| Set | Group session | LT | ST | ||
|---|---|---|---|---|---|
| Normal | Glove | Normal | Glove | ||
| 24 | V left, (mm/sec) | 102 ± 2.2 (85) | 55 ± 1.2 (48) | 47 ± 1 (41) | 43 ± 0.8 (39) |
| V right, (mm/sec) | 124 ± 2.4 (107) | 50 ± 0.9 (50) | 46 ± 1.3 (36) | 50 ± 1.1 (43) | |
| V (avg), (mm/sec) | 113 ± 2.2 (97) | 53 ± 0.9 (50) | 46 ± 1 (39) | 46 ± 0.8 (41) | |
| f left, (Hz) | 31 ± 0.7 (26) | 17 ± 0.4 (14) | 14 ± 0.3 (13) | 13 ± 0.3 (11) | |
| f right, (Hz) | 38 ± 0.9 (31) | 15 ± 0.3 (14) | 14 ± 0.4 (11) | 15 ± 0.4 (13) | |
| f (avg), (Hz) | 34 ± 0.7 (29) | 16 ± 0.3 (15) | 14 ± 0.3 (12) | 14 ± 0.3 (13) | |
| 48 | V left, (mm/sec) | 105 ± 2.2 (100) | 49 ± 1.5 (35) | 35 ± 0.6 (33) | 32 ± 0.7 (30) |
| V right, (mm/sec) | 133 ± 2.9 (124) | 45 ± 0.9 (42) | 36 ± 0.8 (33) | 38 ± 0.6 (37) | |
| V (avg), (mm/sec) | 119 ± 2.4 (113) | 47 ± 1 (43) | 35 ± 0.7 (34) | 35 ± 0.5 (34) | |
| f left, (Hz) | 63 ± 1.4 (60) | 30 ± 1 (22) | 21 ± 0.4 (19) | 19 ± 0.4 (17) | |
| f right, (Hz) | 81 ± 1.9 (72) | 27 ± 0.6 (24) | 22 ± 0.5 (19) | 23 ± 0.4 (22) | |
| f (avg), (Hz) | 72 ± 1.5 (67) | 28 ± 0.6 (26) | 21 ± 0.4 (20) | 21 ± 0.3 (20) | |
Medians in parentheses. V, Velocity; f, temporal frequency; Hz, bars/sec; left, left hand; right, right hand; avg, average across hands.
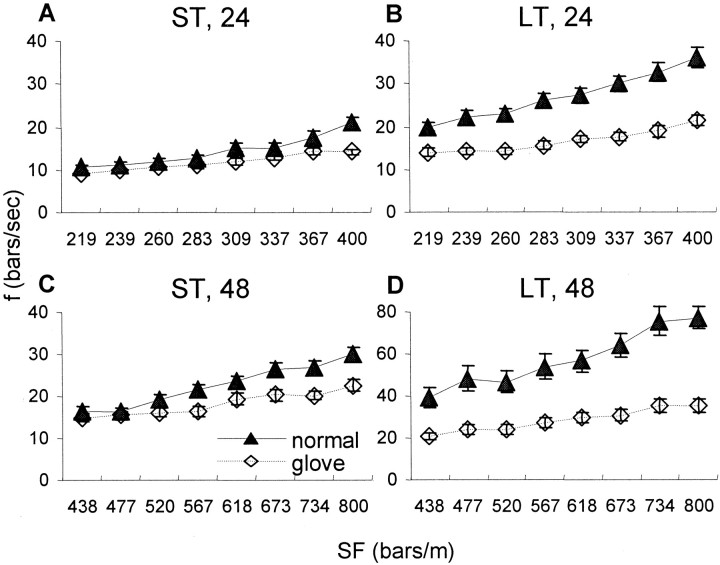
Fine adjustments in scanning velocities (“fine tuning”), i.e., subjects tuning their finger velocities during a trial according to the SF of the gratings presented in that trial, was revealed by averaging the scanning velocities of trials having the same SF for the five subjects of each group (LT and ST) and each experimental condition. For both the LT and ST subjects, the finger velocities during normal scanning usually did not depend on the SF (Table5), and therefore, the temporal frequency increased in proportion with increasing SFs (Fig.5, filled symbols). In contrast, the finger velocities during glove scanning usually correlated negatively with SF (Table 5), and thus, the temporal frequencies were confined to a narrower range during glove than normal scanning sessions, with both the LT and ST subjects (Fig. 5, open symbols). This difference in the dependence of f on SF between normal and glove sessions was statistically significant for all conditions (ANOVA interactions; p < 0.05), except the 24 left-hand of LT subjects.
Table 5.
Linear regressions of V and SF within sessions
| Session | Normal | Glove | ||||
|---|---|---|---|---|---|---|
| Hand | Group | Set | r | p | r | p |
| Left | LT | 24 | −0.39 | 0.34 | −0.81 | 0.0145-150 |
| 48 | 0.4 | 0.32 | −0.59 | 0.13 | ||
| ST | 24 | 0.5 | 0.21 | −0.93 | 0.00085-150 | |
| 48 | 0.31 | 0.46 | −0.87 | 0.0055-150 | ||
| Right | LT | 24 | 0.49 | 0.22 | −0.82 | 0.0135-150 |
| 48 | 0.07 | 0.87 | −0.45 | 0.27 | ||
| ST | 24 | 0.5 | 0.2 | −0.83 | 0.00115-150 | |
| 48 | 0.82 | 0.0125-150 | −0.83 | 0.015-150 | ||
Velocities with high and low-amplitude gratings were averaged.
F5-150: , Significant.
Fig. 5.
Dependency of the temporal frequency (f) of scanning on the SF of the gratings during normal (filled symbols) and glove (open symbols) sessions. Temporal frequencies generated while scanning the high- and low-amplitude sets of gratings with the left hand were averaged for each SF (mean ± SEM across subjects are depicted). A, ST subjects with 24 sets of gratings;B, LT subjects with 24 set; C, ST subjects with 48 set; and D, LT subjects with 48 set. Note the different scale for D.
Average trial velocities and temporal frequencies were computed for bins of eight trials in each session. ST subjects exhibited more-or-less constant scanning velocities within each testing session, across sessions, and across experimental conditions (48 and 24, low- and high-amplitude gratings). This is demonstrated by the temporal frequencies computed for normal and glove sessions of all grating sets (Fig. 6, left column). During both types of scanning sessions, which were separated by a few days, the average scanning velocity, and thus, the average temporal frequency, was more-or-less constant. In contrast, LT subjects exhibited larger variability in scanning velocity during normal than glove scanning and a sharp transition in scanning velocity when switching from normal to glove scanning sessions, in all experimental conditions (Fig. 6, right column). With LT subjects, the average scanning velocity was reduced during the first eight trials (first bin) of the glove session and stabilized on values that produced temporal frequencies of 15–30 Hz (Table 4). The average temporal frequency generated by the LT subjects during glove scanning was similar to that of the ST subjects during both normal and glove sessions. Another change induced by the transition to glove scanning was that the consistent and significant (two-tailed t test;p < 0.0001) difference between the frequencies generated by the right and left hands of LT subjects during normal scanning was reduced and became insignificant.
Fig. 6.
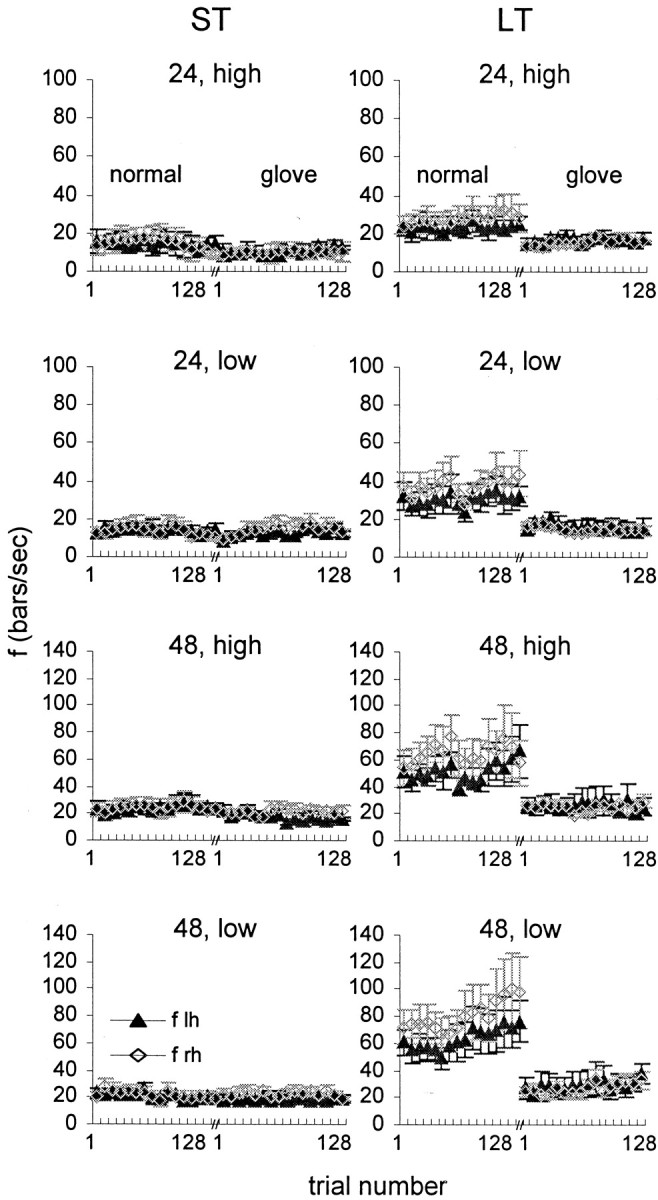
Temporal frequencies as a function of trial number during normal and glove sessions. Mean ± SEM (across subjects) of ST subjects (left column) and LT subjects (right column) are depicted for left (lh, filled symbols) and right (rh, open symbols) hands. Bin, Eight trials. For each subject, normal and glove sessions were conducted on different days.
For the ST subjects, the temporal frequency barely changed from session to session, and thus, did not correlate with the success rate, which changed considerably (r = −0.07, p = 0.67; temporal frequency averaged across the two hands); However, for the LT subjects, the temporal frequency correlated negatively and significantly with the level of performance (r = −0.43; p = 0.006).
Performance and self confidence
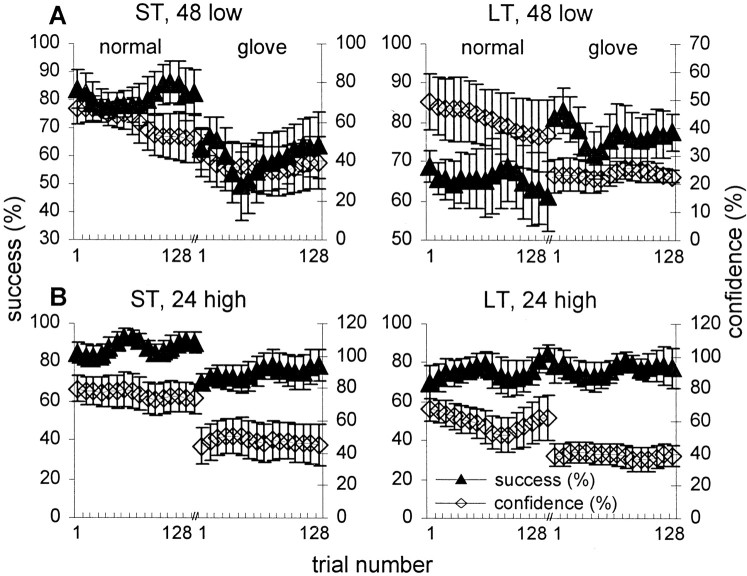
Figure 7 describes the correlation between confidence and performance levels during normal and glove scanning (data from 48 low-amplitude and 24 high-amplitude gratings are presented; similar results had been obtained with the 48 high-amplitude and 24 low-amplitude gratings). Interestingly, although the confidence levels of the ST subjects were, in general, correlated with their performance levels (r > 0.7, p < 0.0001 for all four conditions), those of the LT subjects were not (r < 0.3 and p > 0.5 for all conditions except for 24 low-amplitude in which r = 0.4, p < 0.03). In fact, LT subjects exhibited less confidence during the glove scanning session, even though they actually performed better (48 set) or equally well (24 set) with this mode of scanning (Fig. 7A,B). This surprising behavior was evident for all five LT subjects. The correlation between confidence and performance across all 12 sessions was also computed for each subject. Individual ST subjects displayed high correlations (r = 0.71 ± 0.07; mean ± SEM across all ST subjects), whereas individual LT subjects displayed significantly weaker correlations (r = 0.44 ± 0.1; one-sided WRS, p= 0.028).
Fig. 7.
Performance and confidence. A,B, Success rate (filled symbols) and confidence level (open symbols) as a function of trial number during normal and glove 48 low-amplitude (A) and 24 high-amplitude (B) sessions. Bin, Eight trials; mean ± SEM across subjects are depicted; within each session, curves were smoothed by a convolution with a triangular of area 1 and a base of ± 2 bins.
Guided training
Results obtained with the initial 10 subjects (described above) indicated that the level of performance of LT subjects improved during scanning with the gloves, when tactile spatial cues were eliminated and tactile information was carried only by temporal cues. This improvement might suggest that under normal conditions the temporally encoded information is masked by the spatially encoded information. Alternatively, the improvement might be the result of the change in scanning strategy observed during those trials: the LT subjects usually reduced their scanning velocities and switched to alternating-hand scanning. To test whether the change of strategy can lead to improved performance also during normal scanning, i.e., when spatial cues are available, we guided LT subjects to use low velocity and alternating-hand scanning during normal sessions. This guidance was performed during several training sessions (see Materials and Methods) and, as before, without feedback.
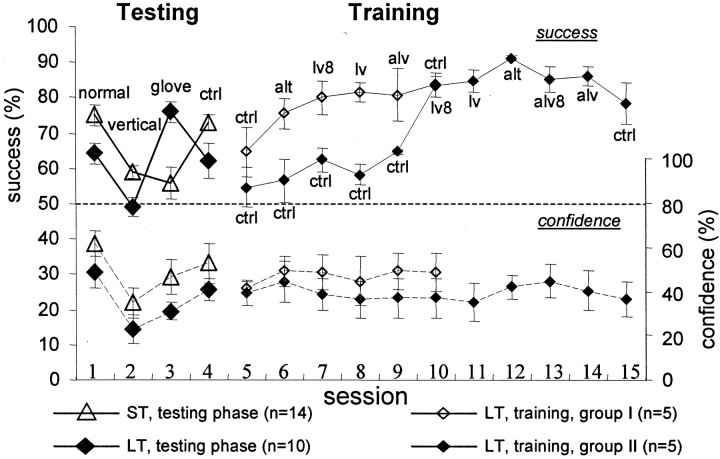
Additional 20 subjects were tested with the low-amplitude 48 gratings. Eleven subjects of the 20 were identified as LT, i.e., subjects whose level of performance when scanning with gloves was better than under normal conditions. With 10 of these 11 subjects, performance with gloves was >15% better than normal performance; the performance with gloves of all other nine subjects was worse than normal performance by >10%. Of the 16 LT subjects identified (the latter 11 + the initial 5), 10 were trained with normal scanning as follows. Half (n = 5) were guided to scan with low velocities (between 20 and 40 mm/sec) and alternating scanning. The interval between testing and training phases was 2 years for four subjects and 1 week for one subject. After one nonguided (control) session, the subjects were guided to use alternating hand scanning at their default velocities, (one session), then they were guided to scan with low velocities (two sessions), then they were guided to use alternating hand scanning at low velocity (one session), and finally they were asked to restore their default scanning patterns and velocities (one session, control). The average performance of these subjects increased during the first guided session (ANOVA; p < 0.0001; followed by LSD; α = 0.05; n = 5) as well as during the following sessions, until stabilization at ∼80% correct (Fig. 8, solid curve andsmall open diamonds). This improvement was preserved during the final nonguided (control) session. Of these five subjects, one did not improve during the training. However, the performance of this subject improved significantly between the last control session in the testing phase and the first control session in the training phase (sessions that with this subject were separated by 2 years).
Fig. 8.
Effect of training and guidance on success rates and confidence levels during the scanning of the low-amplitude 48 sets of gratings. Mean ± SEM of success rates (solid lines) and confidence levels (dashed lines) are depicted. Trials were 4 sec long, unless mentioned otherwise.Filled diamonds, LT subjects, testing phase (n = 10 for sessions 1–3; n = 7 for session 4). Open triangles, ST subjects, testing phase (n = 14 for sessions 1–3;n = 10 for session 4). Open diamonds, LT subjects (n = 5), training phase; these subjects performed a normal control session (session 5) and then were trained with guidance in the following sequence of sessions: 6, alternating scanning (alt); 7, low velocity (8 sec trials; lv8); 8, low velocity (lv); 9, alternating scanning with low velocity (alv); and 10, a normal control (ctrl). Filled diamonds, LT subjects (n = 5), training phase; these subjects performed five normal sessions without guidance after completing the testing phase (sessions 5–9; ctrl) and then were trained with guidance for additional five sessions: 10, low velocity (8 sec trials; lv8); 11, low velocity (lv); 12, alternating scanning (alt); 13, alternating scanning with low velocity (8 sec trials; alv8); 14, alternating scanning with low velocity (alv); and 15, a normal control (ctrl). One of the subjects in the latter group (ga) had success rates of <30% in the first guided session, which indicated a possible systematic reversal of his reports during that session. This session was excluded from the data. Exclusion of the entire data of that subject did not significantly change the average curve depicted in the figure.
During guidance, as during previous testing, these LT subjects were not aware of an improvement in their performance: no correlation was observed between the success rate and the confidence level of these subjects (r = 0.21; p = 0.28). However, during the training period, a negative correlation was observed between the finger scanning velocity (averaged for both hands) and the success rate (r = −0.425; p = 0.02) of the LT subjects, and, as a result, between the temporal frequency (averaged for both hands) and the success rate (r = −0.428;p = 0.02). During the final scanning session, LT subjects (four of five subjects were tested) were asked to scan the gratings using simultaneous scanning and high velocities (>50 mm/sec). For three of the four subjects this scanning was similar to their pretraining scanning while for the other one the velocity was significantly higher than his pretraining velocity. Interestingly, all subjects found it difficult to implement the instructions and used a mixture of the pretraining and guided scanning strategies.
The other group of five LT subjects began the training phase 2 d after completing the testing phase. They were first allowed to practice on normal scanning, with no guidance, for five sessions, which did not improve their performance, and then guided training commenced (Fig. 8,solid curve and small filled diamonds). These subjects were first guided to use low scanning velocity with their default scanning pattern (two sessions), then they were guided to use alternating hand scanning at their default velocities (one session), then they were guided to employ alternating hand scanning at low velocity (two sessions), and finally they were instructed to restore their default scanning pattern and velocity. With this group also, improvement in performance was expressed immediately after starting guidance (ANOVA; p < 0.0001; followed by LSD; α = 0.05), and was usually (three of five subjects) preserved during the final control session. Furthermore, similar to the other LT group, no correlation was observed between the success rate and the confidence level of these subjects (r = 0.13; p = 0.33), whereas a negative correlation was observed between the success rate and the finger scanning velocity (r = −0.29;p = 0.03) and the temporal frequency (r= −0.29; p = 0.02).
DISCUSSION
Utilization of spatial and temporal cues by humans and the role of lateral finger movements
Tactile processing has to deal with diverse stimuli and different kinds of perceptual tasks. Does the brain use a single strategy to process all types of textural stimuli and to solve all sorts of tactile tasks or does the brain change its strategy depending on stimulus parameters (e.g., spatial and temporal frequencies) and the task in hand (e.g., roughness estimation versus spatial-frequency discrimination)?
Until now, this issue had been addressed almost exclusively with roughness estimation. The psychophysical results of Lederman (1974),(1981), and (1982) and Lederman and Taylor (1972) suggested that a single strategy based on spatial cues is used to estimate roughness of textures, where only the groove-width changes while the ridge-width is constant. Johnson and colleagues had identified the neuronal mechanism underlying roughness estimation of spatial frequencies (SFs) between 250 and 666 dots/m, as a one computing the spatial variations across adjacent slowly adapting type I (SAI) mechanoreceptors (Connor et al., 1990; Connor and Johnson, 1992; Johnson and Hsiao, 1994).Meftah et al. (2000) suggested that such a computation might be based on a “simple intensive code,” perhaps used to assess the average densities and heights of texture elements. However, it has been recently shown that roughness can be perceived without spatial tactile cues, albeit with a lower precision (Klatzky and Lederman, 1999), suggesting that alternative coding schemes can be used when spatial cues are not available. Furthermore, with different (higher) ranges of spatial frequencies (Hollins and Risner, 2000) or when roughness is changed by changing the ridge-width instead of the groove-width (Cascio and Sathian, 2001), subjects probably adopt strategies that use the temporal cues generated by the finger movements even when spatial cues are available.
In our study, we asked what is the importance of temporal and spatial cues not for roughness estimation, but for spatial-frequency discrimination. Our results suggest that temporal cues are essential for the latter, at least in the range of 200–800 bars/m. When temporal cues were eliminated, during vertical scanning, performance in the spatial-frequency discrimination task was significantly impaired; in fact it dropped near the chance level. In contrast, elimination of tactile spatial cues, during glove sessions, did not yield homogeneous results; whereas the performance of approximately half the subjects (the ST subjects) was significantly impaired, the performance of the other half (the LT subjects) was either better than (with higher SFs), or similar to (with lower SFs), normal performance.
The primary indication for the importance of temporal cues comes from the impaired performance during vertical scanning, an impairment that was observed previously with other tasks (Morley et al., 1983; Phillips et al., 1983; Katz, 1989; Srinivasan et al., 1990; Hollins and Risner, 2000). However, this impairment could also be caused by the elimination of the enhancement of spatial cues, an enhancement that is obtained by lateral movements (Johnson and Lamb, 1981; Phillips et al., 1983). Although it was not possible to directly discriminate between these two factors in our experiments, our data suggest that in the case of LT subjects the elimination of temporal cues was more crucial. This is because the elimination of tactile spatial cues (during lateral movements with gloves) improved their performance. This is not necessarily true for ST subjects, for whom elimination of spatial cues was detrimental. However, even ST subjects could obtain significant amount of textural information in the absence of spatial cues, especially with low spatial frequencies (Fig. 2D, glove session). These results are consistent with those of Lamb (1983), who observed that tactile discrimination of dot spacing is better when it varies along rather than across scanning direction; this difference should be attributable to differences in the availability of temporal cues because spatial-cue enhancement should be similar for both axes.
Elimination of spatial cues has been much less studied. Katz reported that subjects can discriminate between various papers by writing on them with a rigid pen (Krueger, 1970) and LaMotte (2000) showed that subjects can discriminate softness as well by means of a stylus as by contacting the objects directly with the fingerpad. As mentioned above,Klatzky and Lederman (1999) have recently found that, on the average, even roughness perception is only slightly impaired when spatial tactile cues are eliminated. However, we showed here that the effect of such elimination is not homogeneous across subjects. Although ST subjects were significantly impaired, LT subjects significantly improved when spatial cues were eliminated. This observation, together with the stereotypic scanning patterns in each group, and with the effect of guided training, strongly suggests that the effect of elimination depends crucially on the strategy of sensory acquisition, which varies across subjects.
Elimination of tactile spatial cues necessarily makes the sensory input more similar to that obtained with vibrotactile stimuli, because in both cases only the temporal cues are available. To achieve good performance with the gloves, and later on during training in normal conditions, our LT subjects reduced their scanning velocity such that the temporal frequency was between 15 and 30 Hz (Fig. 6). This range, in which the sensitivity of RA fibers is the highest (Talbot et al., 1968; Johansson et al., 1982), and vibrotactile frequencies can be discriminated with fairly high resolution (LaMotte and Mountcastle, 1975), is probably a “proper” range for solving the task based on vibrotactile cues. Yet, while having the temporal frequencies within the proper range seems to be a necessary condition, it is not sufficient: ST subjects performed poorly with the gloves even though their temporal frequencies were in the proper range (Fig. 6).
Taken together with the aforementioned previous results [mainly those of Klatzky and Lederman (1999), Hollins and Risner (2000), and Cascio and Sathian (2001)], our results suggest that individual subjects use different strategies depending on the stimulus and task. Moreover, our training results indicate that these strategies can be modified by proper guidance, suggesting that intersubject strategy differences might emerge from differences in idiosyncratic history of tactile experience.
Spatiotemporal interference: masking or choice of strategy?
The poor performance of LT subjects in normal scanning tasks can be explained in two ways, which are not mutually exclusive. Either the processing of the spatially encoded information may mask the processing of the temporally encoded information (a “bottom-up” explanation), or the availability of spatial cues may lead LT subjects to choose a nonefficient scanning strategy (a “top-down” explanation). If the former holds, LT subjects should perform better, when eliminating the spatial cues, without changing strategies. In the latter case, LT subjects should change their strategy once they are left only with the temporal cues, cues that could not be properly used with their previous strategy.
Because the LT subjects changed their scanning strategy immediately after starting the glove sessions, the “strategy hypothesis” seems more likely. This hypothesis is further supported by the finding that the LT subjects modified their scanning velocities to values similar to those normally used by ST subjects (Fig. 6), velocities with which ST subjects achieved good performance (Fig. 2). According to this interpretation, normal utilization of temporal cues by LT subjects is not efficient, probably because of the nonoptimal velocities they use. Only when LT subjects are forced to use temporal cues alone, do they use a scanning strategy that allows efficient processing of these cues.
To see whether choosing a particular scanning strategy can indeed improve performance, we trained LT subjects to use both optimal scanning velocities and alternating hand scanning during normal scanning sessions. After training, the LT subjects performed virtually as good as the ST subjects (Fig. 8). Only short training periods were necessary to improve performance of the LT subjects. Improvement was observed already after the first session of guided training and was clearly the result of guidance, because mere practice did not result in improved performance (Fig. 8). The fact that the same behavior was observed with intervals of a few days and of two years between testing and training is consistent with the notion that tactile strategies in the adult are, to a large degree, fixed.
During this study, no feedback was provided; the LT subjects were not aware of improvement in their performance, neither during the scanning sessions with gloves nor during guided training. This training procedure is different from classical procedural learning, which is based on persistent practice with feedback. Our procedure appears to evoke a “eureka” effect, in which subjects are guided to use an effective learning track (Ahissar and Hochstein, 1997). Our findings indicating that the LT subjects did not learn how to efficiently scan surfaces during their life-span, and their self-estimation of performance was not a reliable indicator of their actual performance, support the possibility that natural learning led these subjects into a wrong learning track, from which they could recover by guidance (Ahissar and Hochstein, 1997).
Implications for neuronal mechanisms involved in tactile texture discrimination
An important issue regarding tactile neuronal processing relates to the question of which type of the peripheral mechanoreceptive fibers carry the relevant information (Sathian, 1989). As indicated by previous studies, the answer to this question probably depends on the type of task and the parameters of the stimuli. For example, with roughness estimation, and spatial frequencies between 250 and 666 dots/m, the most relevant mechanoreceptive fibers are probably the SAI fibers (Connor and Johnson, 1992). In contrast, for purely temporal tasks, such as classification or discrimination of the frequency of vibrotactile stimuli using light touch, rapidly adapting (RA) and Pacinian fibers probably carry most of the relevant information (Hyvarinen et al., 1968; Talbot et al., 1968; Darian-Smith and Oke, 1980). Because both spatial and temporal cues were involved in the discrimination task given to our subjects, it was possible that both SAI and RA fibers were involved in the neuronal processing (because of the relatively high spatial frequencies and low temporal frequencies, Pacinian fibers probably contribute little to this particular discrimination task). However, in our experiments, the information provided by the RA fibers appeared to be more important. When subjects (both ST and LT) performed well, they used scanning velocities that induced temporal frequencies between 15 and 30 Hz, frequencies that are best conveyed by the RA fibers (Talbot et al., 1968; Freeman and Johnson, 1982; Johansson et al., 1982; Goodwin et al., 1989). Furthermore, subjects did not exhibit significant differences in discriminating low-amplitude (30 μm) and high-amplitude (100 μm) gratings. With frequencies between 15 and 30 Hz, RA fibers convey a significant amount of information already with indentations of 30 μm, whereas SAI fibers are hardly activated by such indentations (Talbot et al., 1968; Johansson et al., 1982). The similar levels of task performance observed when our subjects scanned gratings with low and high amplitudes suggest that SAI activation is not crucial for performing these tasks.
Johnson and Phillips (1984) have suggested that both “spatial” (involving the SAI system) and “nonspatial” (involving the RA system) mechanisms underlie texture perception; the RA system probably encodes the microscopic, and the SAI the macroscopic, dimensions of a texture. The distinction between spatial and nonspatial information raises the possibility that, like in other systems (Carr, 1993), these two kinds of information are processed separately in the brain, at least up to a certain level. Recent evidence from our laboratory indicates that this is indeed the case in rodents. Our recordings along the afferent tactile pathways of anesthetized rats revealed two different schemes of sensory representations. The input temporal frequency is represented primarily by time in the paralemniscal system and by amplitude in the lemniscal system (Ahissar et al., 2000, 2001;Sosnik et al., 2001). These observations, taken together with the spatial resolution of these systems, are consistent with temporal and spatial cues being processed by the paralemniscal and lemniscal systems, respectively (Ahissar and Zacksenhouse, 2001). If a similar separation exists in primates, spatial and temporal cues would be expected to be processed separately, and in parallel, along different anatomical pathways that lead to the cortex. According to this scheme, the temporal encoding–decoding scheme consists of first encoding spatial intervals by temporal intervals (Darian-Smith and Oke, 1980) and then recoding them with spike counts (rate coding) (Ahissar and Vaadia, 1990; Ahissar, 1998; Ahissar and Zacksenhouse, 2001). The temporally encoded information is valid up to and including the thalamic and primary cortical levels, but not at higher levels (Ahissar, 1998). Consistent with this temporal-to-rate transformation scheme are the results of Salinas et al. (2000), whereas both temporally encoded information and its related rate-coded information are valid in SI of monkeys performing a temporal-frequency discrimination task, only the rate-code is valid in SII. This scheme for separate, parallel processing of spatial and temporal cues suggests that the main reason of the recoding from temporal to rate code is to allow the two streams of information to integrate their outputs using a common code. Such a common rate code could be, for example, the code used by SI and SII “graded neurons” to grade different spatial frequencies (Sinclair and Burton, 1991; Jiang et al., 1997; Pruett et al., 2000).
Footnotes
This work was supported by the United States–Israel Binational Science Foundation (Israel) and the Abramson Family Foundation (United States).We thank Sebastian Haidarliu for the preparation of Figure 1 and for his help during this study, Merav Ahissar and Marcin Szwed for helpful comments on this manuscript, Edna Schechtman for statistical assistance, and Barbara Schick for reviewing this manuscript.
Correspondence should be addressed to Dr. Ehud Ahissar, Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel. E-mail: Ehud.Ahissar@weizmann.ac.il.
REFERENCES
- 1.Ahissar E. Temporal-code to rate-code conversion by neuronal phase-locked loops. Neural Comput. 1998;10:597–650. doi: 10.1162/089976698300017683. [DOI] [PubMed] [Google Scholar]
- 2.Ahissar E, Gamzu E. Utilization of temporally-encoded versus spatially-encoded information during the performance of a tactile discrimination task. Soc Neurosci Abstr. 1995;21:1018. [Google Scholar]
- 3.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 4.Ahissar E, Vaadia E. Oscillatory activity of single units in a somatosensory cortex of an awake monkey and their possible role in texture analysis. Proc Natl Acad Sci USA. 1990;87:8935–8939. doi: 10.1073/pnas.87.22.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahissar E, Zacksenhouse M. Temporal and spatial coding in the rat vibrissal system. Prog Brain Res. 2001;130:75–88. doi: 10.1016/s0079-6123(01)30007-9. [DOI] [PubMed] [Google Scholar]
- 6.Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. doi: 10.1038/35018568. [DOI] [PubMed] [Google Scholar]
- 7.Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol. 2001;86:354–367. doi: 10.1152/jn.2001.86.1.354. [DOI] [PubMed] [Google Scholar]
- 8.Carr CE. Processing of temporal information in the brain. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- 9.Cascio C, Sathian K. Temporal cues contribute to tactile perception of roughness. J Neurosci. 2001;21:5289–5296. doi: 10.1523/JNEUROSCI.21-14-05289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor CE, Johnson KO. Neural coding of tactile texture: comparison of spatial and temporal mechanisms for roughness perception. J Neurosci. 1992;12:3414–3426. doi: 10.1523/JNEUROSCI.12-09-03414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor CE, Hsiao SS, Phillips JR, Johnson KO. Tactile roughness: Neural codes that account for psychophysical magnitude estimates. J Neurosci. 1990;10:3823–3836. doi: 10.1523/JNEUROSCI.10-12-03823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darian-Smith I, Oke LE. Peripheral neural representation of the spatial frequency of a grating moving at different velocities across the monkey's finger pad. J Physiol (Lond) 1980;309:117–133. doi: 10.1113/jphysiol.1980.sp013498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman AW, Johnson KO. Cutaneous mechanoreceptors in macaque monkey: Temporal discharge patterns evoked by vibration, and a receptor model. J Physiol (Lond) 1982;323:21–41. doi: 10.1113/jphysiol.1982.sp014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamzu E, Ahissar E. Temporal and spatial processing in the human somatosensory system. Neurosci Lett [Suppl] 1998;51:S12. [Google Scholar]
- 15.Gamzu E, Barash S, Ahissar E. Improving tactile discrimination by eliminating spatial cues. Soc Neurosci Abstr. 2000;26:1465. [Google Scholar]
- 16.Gibson JJ. Observations on active touch. Psychol Rev. 1962;69:477–491. doi: 10.1037/h0046962. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin AW, John KT, Sathian K, Darian-Smith I. Spatial and temporal factors determining afferent fibre responses to a grating moving sinusoidally over the monkey's fingerpad. J Neurosci. 1989;9:1280–1293. doi: 10.1523/JNEUROSCI.09-04-01280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollins M, Risner SR. Evidence for the duplex theory of tactile texture perception. Percept Psychophys. 2000;62:695–705. doi: 10.3758/bf03206916. [DOI] [PubMed] [Google Scholar]
- 19.Hyvarinen J, Sakata H, Talbot WH, Mountcastle VB. Neuronal coding by cortical cells of the frequency of oscillating peripheral stimuli. Science. 1968;162:1130–1132. doi: 10.1126/science.162.3858.1130. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Tremblay F, Chapman CE. Neuronal encoding of texture changes in the primary and the secondary somatosensory cortical areas of monkeys during passive texture discrimination. J Neurophysiol. 1997;77:1656–1662. doi: 10.1152/jn.1997.77.3.1656. [DOI] [PubMed] [Google Scholar]
- 21.Johansson RS, Landstrom U, Lundstrom R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res. 1982;244:17–25. doi: 10.1016/0006-8993(82)90899-x. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KO, Hsiao SS. Evaluation of the relative roles of slowly and rapidly adapting afferent fibers in roughness perception. Can J Physiol Pharmacol. 1994;72:488–497. doi: 10.1139/y94-072. [DOI] [PubMed] [Google Scholar]
- 23.Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J Physiol (Lond) 1981;310:117–144. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KO, Phillips JR. Spatial and nonspatial neural mechanisms underlying tactile spatial discrimination. In: von Euler C, Franzen O, Lindblom V, Ottoson D, editors. Wenner-Gren international symposium series, Vol 41, Somatosensory mechanisms. Macmillan; London: 1984. pp. 237–248. [Google Scholar]
- 25.Katz D. The world of touch (Krueger LE, translator) (original work published in 1925). Erlbaum; Hillsdale, NJ: 1989. [Google Scholar]
- 26.Klatzky RL, Lederman SJ. Tactile roughness perception with a rigid link interposed between skin and surface. Percept Psychophys. 1999;61:591–607. doi: 10.3758/bf03205532. [DOI] [PubMed] [Google Scholar]
- 27.Krueger L. David Katz's Der Aufbau der Tastwelt (the world of touch): a synopsis. Percept Psychophys. 1970;7:337–341. [Google Scholar]
- 28.Lamb GD. Tactile discrimination of textured surfaces: Psychophysical performance measurements in humans. J Physiol (Lond) 1983;338:551–565. doi: 10.1113/jphysiol.1983.sp014689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaMotte RH. Softness discrimination with a tool. J Neurophysiol. 2000;83:1777–1786. doi: 10.1152/jn.2000.83.4.1777. [DOI] [PubMed] [Google Scholar]
- 30.LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975;38:539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- 31.Lederman SJ. Tactile roughness of grooved surfaces: The touching process and effects of macro- and microsurface structure. Percept Psychophys. 1974;16:385–395. [Google Scholar]
- 32.Lederman SJ. The perception of surface roughness by active and passive touch. Psychonomic. 1981;18:253–255. [Google Scholar]
- 33.Lederman SJ. The perception of texture by touch. In: Schiff W, Foulke E, editors. Tactual perception: a sourcebook. Cambridge, UK; 1982. pp. 130–167. [Google Scholar]
- 34.Lederman SJ, Taylor MM. Fingertip force, surface geometry, and the perception of roughness by active touch. Percept Psychophys. 1972;12:401–408. [Google Scholar]
- 35.Meftah E-M, Belingard L, Chapman CE. Relative effects of the spatial and temporal characteristics of scanned surfaces on human perception of tactile roughness using passive touch. Exp Brain Res. 2000;132:351–361. doi: 10.1007/s002210000348. [DOI] [PubMed] [Google Scholar]
- 36.Morley JW, Goodwin AW. Sinusoidal movement of a grating across the monkey's fingerpad: temporal patterns of afferent fiber responses. J Neurosci. 1987;7:2181–2191. doi: 10.1523/JNEUROSCI.07-07-02181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JW, Goodwin AW, Darian-Smith I. Tactile discrimination of gratings. Exp Brain Res. 1983;49:291–299. doi: 10.1007/BF00238588. [DOI] [PubMed] [Google Scholar]
- 38.Phillips JR, Johnson KO, Browne HM. A comparison of visual and two modes of tactual letter resolution. Percept Psychophys. 1983;34:243–249. doi: 10.3758/bf03202952. [DOI] [PubMed] [Google Scholar]
- 39.Pruett JR, Jr, Sinclair RJ, Burton H. Response patterns in second somatosensory cortex (SII) of awake monkeys to passively applied tactile gratings. J Neurophysiol. 2000;84:780–797. doi: 10.1152/jn.2000.84.2.780. [DOI] [PubMed] [Google Scholar]
- 40.Salinas E, Hernandez A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathian K. Tactile sensing of surface features. Trends Neurosci. 1989;12:513–519. doi: 10.1016/0166-2236(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair RJ, Burton H. Neuronal activity in the primary somatosensory cortex in monkeys (Macaca mulatta) during active touch of textured surface gratings: responses to groove width, applied force, and velocity of motion. J Neurophysiol. 1991;66:153–169. doi: 10.1152/jn.1991.66.1.153. [DOI] [PubMed] [Google Scholar]
- 43.Sosnik R, Haidarliu S, Ahissar E. Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. J Neurophysiol. 2001;86:339–353. doi: 10.1152/jn.2001.86.1.339. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan MA, Whitehouse JM, LaMotte RH. Tactile detection of slip: surface microgeometry and peripheral neural codes. J Neurophysiol. 1990;63:1323–1332. doi: 10.1152/jn.1990.63.6.1323. [DOI] [PubMed] [Google Scholar]
- 45.Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 46.Taylor MM, Lederman SJ. Tactile roughness of grooved surfaces: a model and the effect of friction. Percept Psychophys. 1975;17:23–36. [Google Scholar]