Abstract
In peripheral nerves, Schwann cells (SCs) form contacts with axons, other SCs, and extracellular matrix components that are critical for their migration, differentiation, and response to injury. Here, we report that lysophosphatidic acid (LPA), an extracellular signaling phospholipid, regulates the morphology and adhesion of cultured SCs. Treatment with LPA induces f-actin rearrangements resulting in a “wreath”-like structure, with actin loops bundled peripherally by short orthogonal filaments. The latter appear to anchor the SC to a laminin substrate, because they colocalize with the focal adhesion proteins, paxillin and vinculin. SCs also respond to LPA treatment by forming extensive cell–cell junctions containingN-cadherin, resulting in cell clustering. Pharmacological blocking experiments indicate that LPA-induced actin rearrangements and focal adhesion assembly involve Rho pathway activation via a pertussis toxin-insensitive G-protein. The transcript encoding LPA1, the canonical G-protein-coupled receptor for LPA, is upregulated after sciatic nerve transection, and SCs cultured from lpA1-null mice exhibit greatly diminished morphological responses to LPA. Cultured SCs can release an LPA-like factor implicating SCs as a potential source of endogenous, signaling LPA. These data, together with the previous demonstration of LPA-mediated SC survival, implicate endogenous receptor-mediated LPA signaling in the control of SC development and function.
Keywords: LPA, N-cadherin, focal adhesion, actin, edg2, G-protein-coupled receptor
Schwann cells (SCs), the myelinating glia of the peripheral nervous system, are derived from the embryonic neural crest. SC precursors migrate into developing nerves, forming loose contacts with multiple axons. As SCs differentiate, they lose contact with all but a single axon segment, which they subsequently ensheathe with cytoplasmic processes or with a myelin internode (Zorick and Lemke, 1996). Axonal contacts with developing SCs are essential for myelination to proceed, with influences on the expression of myelin genes and assembly of the SC extracellular matrix (ECM) (Bunge et al., 1989; Scherer et al., 1994; Scherer, 1997). In addition, SC–axon and SC–SC interactions are essential for regeneration after nerve injury: SCs form elongated “bands of Bungner” that bridge the injury site and provide an adhesive substrate for regrowing axons (Cajal, 1928; Fu and Gordon, 1997).
Some of the molecules underlying these interactions have been identified. SCs produce basement membrane components including laminin, type IV collagen, entactin, and fibronectin, to which they adhere via integrin receptors, dystroglycan and N-syndecan (Bunge et al., 1989; Mirsky and Jessen, 1996; Scherer, 1997). Interactions with ECM components such as laminin may be important for both SC migration (Milner et al., 1997) and myelination (Fernandez-Valle et al., 1994;Chen et al., 2000). The calcium-dependent, homophilic adhesion moleculeN-cadherin is expressed by SCs in vivo(Cifuentez-Diaz et al., 1994). It is important for SC–axon and SC–SC interactions in vitro (Letourneau et al., 1991), and is present at sites of SC–axon contact during regeneration (Shibuya et al., 1995). In addition, actin cytoskeleton-based morphological rearrangements are critical for SC differentiation and myelination (Fernandez-Valle et al., 1997), and may be modulated both by integrins, through the assembly of focal adhesions (Fernandez-Valle et al., 1998;Longhurst and Jennings, 1998; Chen et al., 2000), and byN-cadherin, through its intracellular catenin signaling partners (Yap et al., 1997).
Comparatively less is known about the signaling pathways that regulate SC adhesion and morphology. One attractive candidate is the growth factor-like serum phospholipid lysophosphatidic acid (LPA). LPA signals through G-protein-coupled receptors (GPCRs) to induce diverse cellular effects, including actin rearrangements and focal adhesion assembly via the small GTPase, Rho, and its downstream effectors (Moolenaar et al., 1997; Fukushima et al., 1998; Chun et al., 1999). Recent studies have identified three genes encoding GPCRs for LPA:lpA1/Edg2,lpA2/Edg4, andlpA3/Edg7, which together encode the LPA receptor family (Hecht et al., 1996; Fukushima et al., 1998, 2001; Chun et al., 1999; Contos et al., 2000a). We demonstrated previously thatlpA1 is expressed by postnatal SCsin vivo and in vitro and that LPA promotes SC survival via activation of LPA1 and a downstream pathway including Gi, phosphoinositide-3-kinase (PI3K), and Akt (Weiner and Chun, 1999). Here, we examine the cytoskeletal and ECM effects of LPA1-mediated LPA signaling in primary SCs cultures, including cells derived from mice lacking lpA1, and assess possible sources of endogenous LPA.
MATERIALS AND METHODS
Reagents and pharmacological treatments. Lyophilized LPA (Avanti Polar Lipids) was resuspended and diluted in H2O. Lysophosphatidyl choline, lysophosphatidyl ethanolamine, lysophosphatidyl glycerol, and phosphatidic acid (all from Avanti Polar Lipids) were resuspended in H2O. Sphingosine 1-phosphate (S1P) (Biomol, Plymouth Meeting, PA) was prepared in 0.01% fatty acid-free BSA. C3 exoenzyme was prepared, and its efficacy was confirmed, as described (Fukushima et al., 1998). Cultures were treated with 30 μg/ml C3 for ∼18 hr before LPA treatment. Y-27632 was added to cultures at 2 μm 10 min before LPA treatment. Pertussis toxin (PTX; Calbiochem, La Jolla, CA) was added at 200 ng/ml to cultures ∼18 hr before, and again at the time of, LPA treatment. Efficacy of PTX was confirmed in an in vitroADP-ribosylation assay using SC membranes (data not shown).
Primary SC culture. Sciatic nerves were excised from rats at postnatal day 2 or 3 or from wild-type orlpA1(−/−) mice (Contos et al., 2000b) at postnatal day 3 or 4, and SCs were purified essentially as described previously (Brockes et al., 1979). Cells were passaged routinely on poly-l-lysine (0.1 mg/ml)-coated dishes and plated for experiments on poly-l-lysine and laminin (10 μg/ml)-coated glass coverslips. Growth medium was DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS, 20 μg/ml pituitary extract (Sigma), 2 μm forskolin, and penicillin–streptomycin. For serum-free experimental conditions, a modified “Sato” medium (Milner et al., 1997) was used, consisting of DMEM with 1× N2 supplements (Life Technologies), 20 μg/ml pituitary extract, 0.1 mg/ml fatty acid-free BSA (Sigma), 400 ng/ml each of T3 and T4 (Sigma), 4 μm forskolin, and penicillin–streptomycin. In experiments in which Ca2+ concentration was manipulated, Sato medium was prepared using CaCl2-free DMEM (Life Technologies), and CaCl2 was then added to 0.2 or 2 mm. Cultures were serum-starved in Sato medium overnight (12–18 hr) before LPA treatments. SC cultures were >98% pure, as assessed by anti-P0 and anti-S100 (Dako, Carpinteria, CA) immunofluorescence. For conditioned medium (CM) production, near-confluent SC cultures were maintained in Sato medium for 72 hr. CM was collected, filtered (0.2 μm), and applied to assay cultures immediately. In some cases, early passage SCs were cryoprotected in DMEM with 20% FCS and 10% DMSO and stored in vials under liquid N2. Once thawed, SCs were used for no more than three passages before being discarded, and maintenance of cellular phenotype was routinely monitored by immunofluorescence.
Immunofluorescence and actin staining. SC cultures were fixed for 15 min with 4% paraformaldehyde and rinsed several times with PBS. Coverslips were incubated in blocking solution containing 2% BSA and 0.1% Triton X-100 in PBS for 1 hr, followed by overnight incubation with the following monoclonal antibodies (all from Transduction Laboratories, Lexington, KY): anti-vinculin (5 μg/ml), anti-paxillin (0.5 μg/ml), anti-N-cadherin (5 μg/ml), and anti-β-catenin (1.5 μg/ml). Bound antibodies were visualized with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA; 1:200), followed by Alexa488-streptavidin (Molecular Probes, Eugene, OR; 2 μg/ml). For visualization of f-actin, cells were incubated with TRITC-phalloidin (0.1 μg/ml). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO; 10 min at 0.35 μg/ml).
Reverse transcription-PCR. Reverse transcription-PCR (RT-PCR) was performed as described previously (Contos and Chun, 1998). cDNAs were prepared from mouse sciatic nerve at embryonic day 12.5 (E12.5), E13.5, E15.5, and E17.5 (a kind gift from Drs. David Parkinson and Kristjàn Jessen, University College, London, UK) or from cultured SCs or neonatal sciatic nerves oflpA1(+/+) andlpA1(−/−) mice, using standard protocols. Primers used for PCR were the following:lpA1: 513C/513T;lpA2: edg6f/edg6e3c′ (Contos and Chun, 2000); lpB1: edg1p (5′-CCGTCAGTCGCCGACAACAA-3′)/edg1b (5′-GTAGAGGATGGCGATGGAAA-3′);lpB2: edg4SP12 (5′-AGC CAA CAG TCT CCA AAA CCA-3′)/edg4b (5′-ACG ATG GTG ACC GTC TTG AGC A-3′);lpB3: edg3SP6 (5′-AGG GGC AGG CGA CAA GGT-3′)/edg3c (5′-GGG TTC ATG GCG GAG TTG AG-3′). To prevent any potential contaminating signal from genomic DNA, all primer pairs were designed so that the amplified product would cross an intron–exon boundary. Control reactions using genomic DNA as a template were negative in all cases (see Fig. 6f). Southern blot analysis for PCR products was performed using standard protocols (Ausubel et al., 1994) with a 32P-labeled fragment of the lpA1 open reading frame probe.
Fig. 6.
LPA responsiveness is greatly reduced inlpA1(−/−)Schwann cells. a–d, TRITC–phalloidin staining of SCs from neonatallpA1(+/+) andlpA1(−/−)mice.lpA1(+/+) SCs respond to 100 nm LPA with the formation of actin wreath-like structures, whereaslpA1(−/−)SCs respond with only slight actin rearrangements. Scale bars, 40 μm.e, Dose–response relationship for LPA-induced actin wreath formation inlpA1(+/+) andlpA1(−/−)SCs. Values represent mean ± SEM (n = 6). *p < 0.03 (−/− vs +/+ at each dose; ANOVA with Fisher's post hoc test). LPA-induced actin wreath formation is minimal inlpA1(−/−)SCs, yet they respond to S1P with actin rearrangements as robust as those of wild-type cells. f, RT-PCR detection oflpA1,lpA2,lpA3,lpB1,lpB2, andlpB3, inlpA1(+/+) andlpA1(−/−)cultured SC and intact sciatic nerve (postnatal day 7) cDNAs. This analysis confirms the lack of expression oflpA1 inlpA1(−/−)SCs and nerves and suggests that the small residual LPA response may be mediated by LPA2. All primer pairs crossed intron–exon boundaries; therefore, lack of a product from PCR performed on genomic DNA (gDNA) is shown as a negative control. Lung cDNA is shown as a positive control, and PCR using actin primers is shown to indicate equivalent cDNA input.
Northern blot of transected sciatic nerve RNA. Adult rat sciatic nerve transections were performed essentially as described previously (Scherer et al., 1994). Briefly, the sciatic nerve was exposed at the sciatic notch. The nerve was double ligated and transected with fine scissors, and the two nerve stumps were sutured at least 1 cm apart; this technique prevents axonal regeneration to the distal nerve stump for at least 2 months. At various times after transection, the animals were killed, the entire distal nerve stumps (from just below the lesion to the ankle) were removed, and RNA extracted by standard methods (Ausubel et al., 1994). Northern blots of 10 μg each RNA were made and probed with the following32P-labeled (1 × 106 cpm/ml) cDNA fragments: a full-lengthlpA1 cDNA insert (Hecht et al., 1996); a 0.7 kb BamHI fragment of p75 (the low-affinity nerve growth factor receptor; Radeke et al., 1987); a full-length cDNA of rat P0 (Lemke and Axel, 1985); and a full-length cDNA of rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Fort et al., 1985).
Determination of LPA-like activity in SC CM. LPA-like activity was assayed by measuring stress fiber-forming activity in RH7777 cells expressing lpA1(RH/LPA1 cells), and S1P-like activity was assayed by measuring cell rounding activity in B103 neuroblastoma cells (Fukushima et al., 1998). Native RH7777 cells (which express no known LPA receptors and exhibit no LPA responsivity) were infected with retroviruses expressing FLAG-tagged lpA1for 18 hr and further cultured in 300 μl of serum-free medium for 1 d. SC-CM was added for 30 min, and cells were fixed and double-stained for FLAG and f-actin (Fukushima et al., 1998, 2000). FLAG-positive cells with stress fibers were counted, and the concentration of LPA-like activity in CM was estimated by comparison to a standard LPA dose–response curve (0.3–10 nm). B103 cells were serum-starved overnight and treated with SC-CM for 30 min. Cells were fixed and rounded cells were counted under phase-contrast optics.
Figure preparation. Micrographs and gel photographs were scanned into Adobe Photoshop 4.0, which was used to compile the composite figures, to equalize brightness, contrast, and color balance, and to add text.
RESULTS
LPA induces actin cytoskeleton-based morphological changes in cultured SCs
During previous studies of the effects of LPA on SC survival (Weiner and Chun, 1999), we noted that LPA treatment appeared to produce concomitant changes in cell morphology. This effect was examined further in cultured neonatal rat SCs. Control SCs grown on laminin in a defined, serum-free medium exhibited a stereotypical bipolar morphology with elongated processes (Fig.1a), whereas SCs treated with LPA lost their processes and adopted a flattened morphology accompanied by cell spreading (Fig. 1b). Because LPA is known to be a potent effector of actin cytoskeletal rearrangements in numerous cell types (Jalink et al., 1993; Moolenaar et al., 1997; Fukushima et al., 1998), we examined SCs for changes in actin structure after LPA treatment.
Fig. 1.

LPA induces marked, actin cytoskeleton-based morphological changes in Schwann cells.a–d, Phase contrast microscopy (a, b) and TRITC—phalloidin-stained f-actin images (c, d) of control and LPA-treated SCs. SCs in control cultures (a, c) exhibit bipolar morphologies with elongated processes containing a small number of thick actin bundles. Treatment with 1 μm LPA (3 hr) results in process retraction, cell flattening, and spreading, and the formation of a polymerized actin wreath-like structure (b, d). Scale bars:a, b, 100 μm; c,d, 50 μm. e, Higher magnification view of an LPA-treated SC stained with TRITC–phalloidin. The actin wreath can be seen to consist of many loops of f-actin apparently bundled by short orthogonal filaments (arrowheads). Scale bar, 10 μm. f, Dose–response relationship of LPA-induced actin wreath formation. *p < 0.003 (vs control; ANOVA with Fisher's post hoc test). Values represent means ± SEM (n = 6). g, The effects of various lysophospholipids on actin wreath formation.PA, Phosphatidic acid; LPC, lysophosphatidyl choline; LPE, lysophosphatidyl ethanolamine; LPG, lysophosphatidyl glycerol; S1P, sphingosine 1-phosphate. *p < 0.0001 (vs control; ANOVA with Fisher'spost hoc test). Values represent means ± SEM (n = 6).
In control SC cultures, f-actin visualized with TRITC–phalloidin was organized into several bright bundles oriented along the cell axis (Fig. 1c). SCs treated with LPA (1 μm, 3 hr) exhibited a dramatic reorganization of the cytoskeleton, forming compact, circular bundles of actin filaments resembling a wreath (Fig. 1d). At higher magnification, this actin structure could be seen to consist of fine loops of f-actin, apparently interconnected by several short, orthogonal actin filaments that extended centripetally toward other SCs or the laminin substrate (Fig. 1e). The reorganized actin was neither strictly cortical nor perikaryal, because it was excluded from areas directly abutting the nucleus and plasma membrane (see Fig.4g).
Fig. 4.
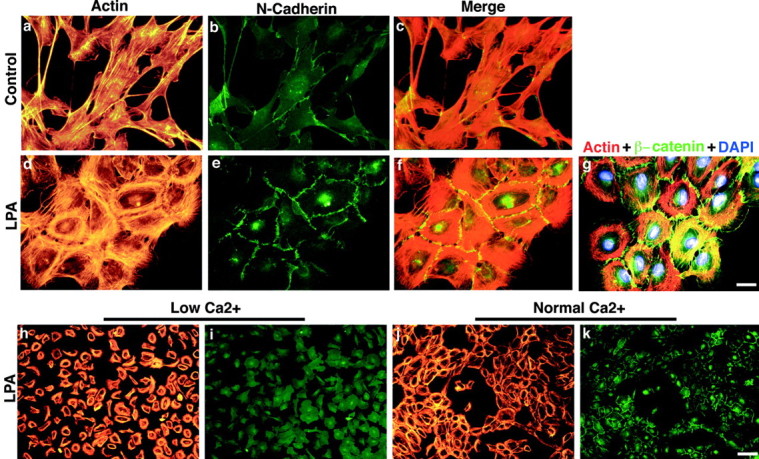
LPA induces N-cadherin-based cell–cell adhesion in cultured Schwann cells. a–f,Control and LPA-treated SCs stained with TRITC–phalloidin (red) and an antibody to N-cadherin (green). g, Triple staining of LPA-treated SCs with TRITC–phalloidin (red), an antibody to β-catenin (green), and the nuclear stain DAPI (blue). Control SCs have few brightN-cadherin-positive contacts. In contrast, LPA-treated SCs have many bright N-cadherin- or β-catenin-positive contacts that cover the entire cell–cell surface. Merged or triple-exposure images indicate the expected colocalization ofN-cadherin and β-catenin with actin, to which the catenin complex binds. h–k, The effects of low Ca2+ concentration on LPA-induced actin wreath formation and N-cadherin-based clustering. SCs were treated with LPA (1 μm, 3 hr) in normal medium (j, k) or in low (0.2 mm) calcium medium (h, i) known to disrupt calcium-dependent cadherin binding (Letourneau et al., 1991). Low calcium did not prevent LPA-induced wreath formation (h) but did disruptN-cadherin-mediated contacts (i), resulting in single, dissociated wreath-containing SCs. Scale bars:a, g, 25 μm; h–k, 100 μm.
LPA induction of actin rearrangements was dose-dependent and was maximal at 1 μm, with an estimated EC50 of 50 nm (Fig.1f). Although complete wreath formation was useful as an unambiguous criterion for quantitation, this tended to underestimate the potency of LPA because low nanomolar doses induced substantial, but incomplete, actin rearrangements in most SCs (data not shown). Similarly, all SCs responded to 1 μmLPA with profound actin rearrangements, although only 75–80% assembled a clearly complete actin wreath (Fig. 1f). The response was specific for LPA, because the structurally related lipids, phosphatidic acid, lysophosphatidyl choline, lysophosphatidyl ethanolamine, and lysophosphatidyl glycerol failed to induce any actin rearrangements (Fig. 1g). Another type of lysophospholipid, S1P, which also induces cytoskeletal effects via cognate GPCRs homologous to the LPA family (the LPB receptor family; Postma et al., 1996; Lee et al., 1998; Chun et al., 1999; van Brocklyn et al., 1999; Zhang et al., 1999; Fukushima et al., 2001), did produce significant actin reorganization, but with much less efficacy than LPA in this assay (Fig. 1g).
To examine how these actin rearrangements progressed over time, SCs were exposed to LPA, fixed, and stained with TRITC–phalloidin at various time points. As early as 15 min after LPA exposure, all SCs examined exhibited the onset of actin reorganization, accompanied by changes in cell shape (Fig. 2, comparea, b). Fine actin filaments appeared to bundle and thicken with time, with the subsequent formation of orthogonal actin filaments (Fig. 2c–e). Although maximal actin wreath formation was observed 1–3 hr after LPA exposure (Fig.2f), some SCs had completed actin reorganization by 15–30 min (Fig. 2b, arrowhead).
Fig. 2.
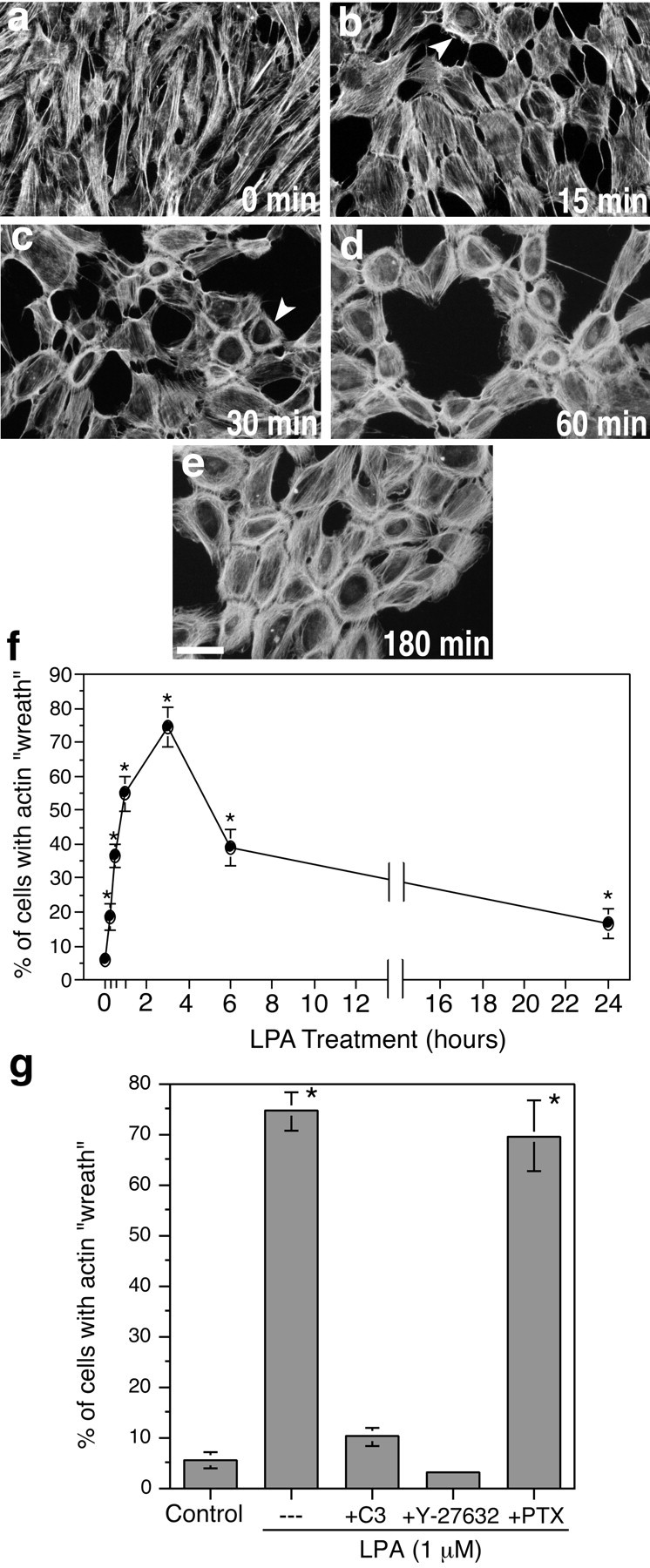
LPA-induced actin rearrangements in Schwann cells are initiated rapidly and depend on Rho activation.a–e, TRITC–phalloidin staining of SCs treated with 1 μm LPA for the indicated times. f,Quantitated time course of LPA-induced actin wreath formation. Actin rearrangement is initiated rapidly, with some SCs already exhibiting mature wreath structures by 15–30 min (arrowheads inb, c), and is half-maximal by 30 min (f). Wreath formation is maximal between 1 and 3 hr, after which the structures are gradually lost. g,Quantitation of the effects of pharmacological inhibitors on LPA-induced actin wreath formation. LPA-induced actin reorganization is completely blocked by pretreatment with C3 exoenzyme (30 μg/ml, 18 hr) or with Y-27632 (2 μm, 10 min), but not with PTX (200 ng/ml, 18 hr). Scale bar, 50 μm. *p < 0.0005 (vs control; ANOVA with Fisher's post hoc test). Values represent means ± SEM (n = 6).
Extracellular LPA activates multiple G-protein signaling pathways, including a PTX-sensitive Gi/PI3K pathway important for SC survival (Weiner and Chun, 1999) and a PTX-insensitive Rho pathway leading to cytoskeletal rearrangements in many cell types (Moolenaar et al., 1997; Fukushima et al., 1998; Gohla et al., 1998). Consistent with this, SC actin rearrangements were blocked completely both by C3 exoenzyme, which ADP-ribosylates and inactivates Rho (Jalink et al., 1994), and by Y-27632, a specific inhibitor of p160ROCK, an effector kinase downstream of Rho (Uehata et al., 1997) (Fig.2g). In contrast, PTX treatment did not inhibit LPA-induced actin rearrangements (Fig. 2g).
LPA induces SC focal adhesion assembly
SC function depends critically on adhesive interactions with ECM components such as laminin (Bunge et al., 1989), which are mediated in part by the focal adhesion complexes that link integrins to the actin cytoskeleton (Fernandez-Valle et al., 1994, 1998; Milner et al., 1997;Chen et al., 2000). To determine whether LPA-induced actin rearrangement was accompanied by focal adhesion assembly, SCs were treated with LPA and subsequently double stained with TRITC–phalloidin and antibodies against paxillin or vinculin, two protein components of focal adhesion complexes (Longhurst and Jennings, 1998). Control SCs had relatively few paxillin- or vinculin-positive focal adhesions where they contacted the laminin substrate (Fig.3a–c). After LPA treatment, SCs exhibited many bright paxillin- and vinculin-positive focal adhesions at the outer edge of the actin structure, where they contacted the substrate (Fig. 3d–f, i). Merged images (Fig.3f,i) illustrated colocalization of f-actin with focal adhesion proteins near the cell surface. LPA-induced assembly of focal adhesions was blocked by pretreatment with Y-27632 (Fig.3g,h) but not by PTX (data not shown), indicating involvement of the Rho/ROCK pathway, consistent with previous reports (Ridley and Hall, 1992; Uehata et al., 1997). Focal adhesion assembly was observed as early as 15 min after LPA exposure (Fig.3g), although maximal effects were seen at 1–3 hr, concomitantly with the completion of actin reorganization. Both LPA-induced actin rearrangements and focal adhesion assembly were weak or nonexistent when SCs were grown on a substrate of poly-l-lysine alone (data not shown). These data, taken together, suggest that LPA signaling can increase SC adhesion to ECM components through focal adhesion complex assembly.
Fig. 3.

LPA induces focal adhesion assembly in Schwann cells. a–f, Control and LPA-treated SCs stained with TRITC–phalloidin (red) and an antibody to the focal adhesion protein paxillin (green). Control SCs have relatively few focal adhesions. In contrast, LPA-treated SCs have many bright paxillin-positive (e) or vinculin-positive (i) “spikes” near the cell periphery (arrowheads), indicating increased focal adhesion assembly. Insets in b ande are 3× magnifications of a portion of the field showing the cell periphery, with the plasma membrane indicated witharrowheads. Merged or triple-exposure images (including nuclei stained with DAPI; blue) indicate the expected colocalization of actin with focal adhesion proteins (seen asyellow). g, h, Effect of Y-27632 on LPA-induced focal adhesion assembly. When SCs were treated with Y-27632 before 15 min LPA treatment, focal adhesion assembly was blocked. Although the maximal effect was seen in longer LPA treatments (e), note that initial focal adhesion assembly was observed as early as 15 min after LPA exposure (g). Scale bar, 25 μm.
LPA induces N-cadherin-mediated cell–cell contacts in SCs
Compared with control cells, LPA-treated SCs appeared to cluster together as they assembled actin wreath structures (Fig.2a–e). Because SC–SC and SC–neurite contacts have been shown to depend on the homophilic calcium-dependent cell adhesion molecule N-cadherin (Letourneau et al., 1990, 1991; Shibuya et al., 1995), we examined whether LPA increasedN-cadherin-mediated cell contacts. Control and LPA-treated cultures were double stained with TRITC–phalloidin and an antibody against N-cadherin. SCs in control cultures exhibited fewN-cadherin-positive contacts, and these generally covered a small area (Fig. 4a–c). In LPA-treated cultures, SCs with actin wreaths appeared closely linked to one another, with large, bright N-cadherin-positive junctions that spanned the entire cell–cell border (Fig.4d–f). A similar pattern was also obtained using an antibody against β-catenin, an intracellular signaling molecule associated with N-cadherin (Fig. 4g).
Growing SCs in medium containing a low concentration (0.2 mm) of CaCl2, demonstrated previously to disrupt calcium-dependent cadherin binding (Letourneau et al., 1991), did not affect LPA-induced actin rearrangements (Fig. 4, compareh, j). Cell–cell adhesion, however, was significantly disrupted, resulting in single, dissociated wreath-containing SCs that completely lacked N-cadherin-positive contacts (Fig. 4, compare i, k). These results confirmed that LPA-induced cell–cell contacts were cadherin-based and further demonstrated that these contacts were not simply a consequence of actin reorganization.
The LPA1 receptor gene is expressed throughout sciatic nerve development and is upregulated after injury
Aspects of SC morphology and adhesion modulated by LPA in vitro have been suggested to be important for migration, myelination, and response to nerve injury in vivo (Bunge et al., 1989; Letourneau et al., 1991; Fernandez-Valle et al., 1994, 1997,1998; Fu and Gordon, 1997; Milner et al., 1997). To determine whether LPA could regulate such processes in vivo, we examined the expression of lpA1, the major LPA receptor gene in the postnatal nerve (Weiner and Chun, 1999), during embryonic nerve development and after adult nerve transection. First, we performed RT-PCR with primers specific for thelpA1 gene on cDNAs derived from embryonic and neonatal mouse sciatic nerves. ThelpA1 transcript was detected at all ages examined, including the period (∼E12–E15) when SC precursors migrate along the developing nerve (Fig.5a) (Jessen et al., 1994;Mirsky and Jessen, 1996; Scherer, 1997). The fragment amplified by RT-PCR was confirmed to represent lpA1 by Southern blot analysis using an lpA1 probe devoid of employed primer sequence (Fig. 5a). We next analyzed, by Northern blot, lpA1expression in the distal nerve stumps of adult rat sciatic nerves after transection. SCs modify their gene expression after transection, downregulating genes indicative of mature, myelinating phenotype (P0) while upregulating genes indicative of an immature, regeneration-supporting phenotype (GAP43 and the p75 nerve growth factor receptor) (Fig. 5b) (Fu and Gordon, 1997;Scherer, 1997). Expression of the lpA1transcript increased in distal nerve stumps ∼1 week after transection, and this increase was maintained for at least 8 weeks, similar to that of GAP-43 (Fig. 5b). This time course further parallels the upregulation of ECM components and cell adhesion molecules, including N-cadherin, which has been reported previously (Bunge et al., 1989; Cifuentez-Diaz et al., 1994; Fu and Gordon, 1997).
Fig. 5.
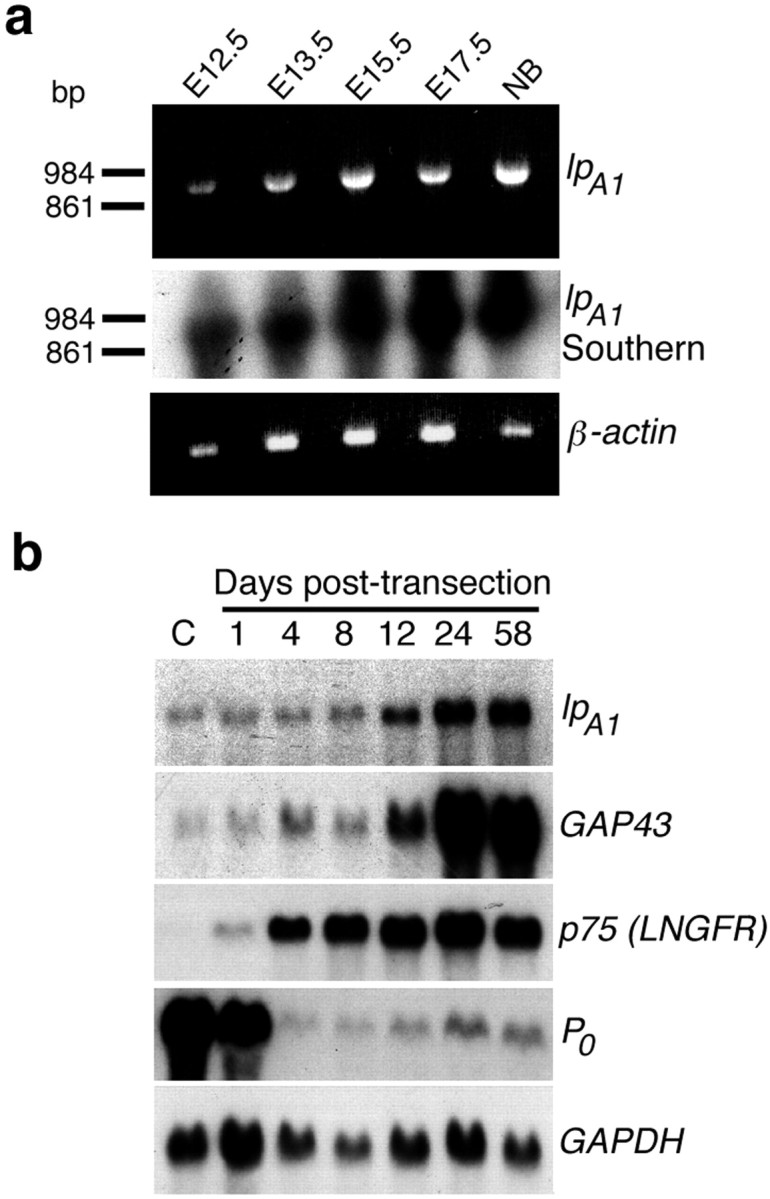
The lpA1transcript is expressed in the embryonic sciatic nerve and is upregulated after adult sciatic nerve transection. a,RT-PCR analysis of embryonic (E12.5–E17.5) and newborn (NB) mouse sciatic nerve cDNA using a primer pair crossing an intron–exon boundary (intron > 20 kb). ThelpA1 transcript is detected at all ages examined, including those (E12.5-E15.5) encompassing the period of SC migration (top panel). PCR of genomic DNA with the same primers gave no product (data not shown). A Southern blot of the PCR gel probed with a lpA1fragment confirms the identity of the product (middle panel). A β-actin PCR (bottom panel) is shown as a loading control.b, Northern blot analysis of RNA derived from adult rat sciatic nerves before (C, control), and at subsequent days after transection (RNA is from the distal stumps). ThelpA1 transcript is upregulated ∼1 week after transection, and levels remain elevated for at least 8 weeks. This parallels similar rises in expression of markers of immature SCs, GAP43, and the p75 low-affinity nerve growth factor receptor (LNGFR), and contrasts with the abrupt downregulation of the gene encoding the myelin protein P0. A re-probing for the GAPDH gene is shown as a loading and transfer control. Blot exposure times: lpA1, 14 d; GAP43, 7 d; LNGFR, 3 d; GAPDH, 3 hr; P0, 16 hr.
LPA-induced actin rearrangement is decreased in SCs lackinglpA1 expression
LPA1 is the major LPA receptor in the postnatal mouse sciatic nerve (Weiner and Chun, 1999), and therefore the role of this receptor in the morphological effects demonstrated here was examined. Neonatal SC cultures were prepared from wild-type (lpA1(+/+)) mice and from mice (lpA1(−/−)) in which the gene encoding LPA1 was disrupted, resulting in a null genotype (Contos et al., 2000b). Approximately 50% of lpA1(−/−)mice die between the perinatal period and weaning; thus, examined nerves were by necessity biased toward animals that could survive to 3–4 d postnatal, at which time nerves were isolated. Wild-type mouse SCs responded to LPA with dose-dependent actin rearrangements similar to those observed in rat SCs (Fig.6a,c), albeit with a somewhat reduced maximal effect (Fig. 6e). In contrast, SCs cultured from lpA1(−/−)mice exhibited a severe reduction in the LPA response, with most cells failing to respond at all (Fig. 6b,d,e). SCs fromlpA1(+/+) andlpA1(−/−) mice responded equally well to S1P exposure (Fig. 6e) (note that the S1P response is stronger in mouse SCs than in rat SCs), indicating that the reduced effect did not result from a general cytoskeletal defect. Furthermore, these results demonstrate that LPA1 is not required for S1P-dependent responses. RT-PCR analyses (Fig. 6f) confirmed thatlpA1(−/−) nerves and SC cultures lacked lpA1 expression, but continued expression of another LPA receptor gene,lpA2 (which may account for the residual LPA response), as well as multiple members of thelpB S1P receptor gene family.
SCs can release signaling LPA
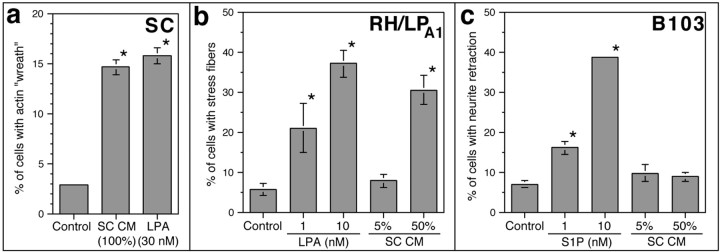
Actin wreaths in untreated SC cultures, essentially absent under the conditions of this study, could be produced by overgrowth of SCs (data not shown). This result suggested the possibility that SCs themselves might be capable of producing signaling LPA. To address this issue, culture medium conditioned for 72 hr by confluent rat SCs was added to fresh SC cultures and assayed for actin reorganizing activity. Undiluted SC-CM, but not control medium, had an activity comparable with 30 nm LPA in this assay (Fig.7a). To confirm that this activity was in fact LPA, we tested Schwann cell-conditioned medium (SC–CM) in two sensitive and specific bioassays using cell lines expressing different complements of LP GPCRs (Fukushima et al., 1998, 2000): (1) RH7777 hepatoma cells, which normally lack anylpA or lpBreceptor gene expression and thus respond to neither LPA nor S1P (Zhang et al., 1999), infected with a retrovirus encoding LPA1. These RH/LPA1 cells respond specifically to nanomolar LPA by forming stress fibers (Fukushima et al., 1998); and (2) B103 neuroblastoma cells, which lack any LPA receptors and responses, but express S1P receptors and respond specifically to S1P with neurite retraction and cell rounding (Fukushima et al., 1998; Chun et al., 1999; Ishii et al., 2000). Application of SC-CM resulted in significant stress fiber formation in RH/LPA1 cells (Fig. 7b), but had no effect on neurite retraction in B103 cells (indicating that no significant S1P-like activity was present) (Fig. 7c). When normalized to cellular protein from conditioning cultures, the specific LPA-like activity present in SC-CM was ∼0.99 ± 0.35 nmol/mg. Together, these analyses demonstrated that SCs can release active signaling LPA, providing at least one potential source in the developing nerve.
Fig. 7.
Schwann cell-conditioned medium contains an LPA-like activity. a, The effects of SC-CM on actin wreath formation in SCs. Medium conditioned by confluent SC cultures for 72 hr induces wreath formation in fresh SC cultures with an activity equivalent to 30 nm LPA. b, The effects of SC-CM on stress fiber formation in RH7777 hepatoma cells expressing lpA1(RH/LPA1). c, The effects of SC-CM on neurite retraction in B103 cells. RH/LPA1 cells respond to SC-CM [added at 5 or 50% (v/v)] with stress fiber formation, whereas B103 cells do not respond to SC-CM with neurite retraction. These two sensitive and specific bioassays (Fukushima et al., 1998, 2000) suggest that the activity in SC-CM is LPA. *p < 0.01 (vs control); values represent means ± SEM (n = 6).
DISCUSSION
SC development depends critically on the regulation of adhesion both to ECM components of the basal lamina (Bunge et al., 1989;Scherer, 1997) and to axons and other SCs (Letourneau et al., 1990, 1991; Lemke, 1993; Cifuentez-Diaz et al., 1994; Scherer et al., 1994). SCs undergo changes in morphology and adhesion as they migrate, differentiate, and form the mature axonal contacts that lead to elaboration of the myelin sheath. Modulations of morphology and adhesion are similarly important after nerve injury, when SCs and their basal lamina- and cell adhesion-associated molecules form an adhesive substrate that promotes nerve regeneration (Shibuya et al., 1995; Fu and Gordon, 1997; Scherer, 1997). Several factors with the potential to affect SC adhesion have been identified. For example, NGF upregulates expression of the L1 adhesion molecule (Seilheimer and Schachner, 1987), both NGF and glial growth factor (neuregulin) promote SC migration in vitro (Anton et al., 1994; Mahanthappa et al., 1996), and transforming growth factor-β stimulates expression of the gene encoding type IV collagen, a basal lamina component (Rogister et al., 1993). However, no factor has been reported to induce major changes in SC actin structure, cell–substrate adhesion, and cell–cell contacts.
The serum phospholipid LPA, via activation of the G12/13 family, is a prototypical regulator of Rho-dependent actin cytoskeletal reorganizations in several cell types (Ridley and Hall, 1992; Moolenaar, 1995; Gohla et al., 1998; Sah et al., 2000). The identification of the LPA receptor genelpA1 (Hecht et al., 1996; Fukushima et al., 1998), and the demonstration of its expression by SCs (Weiner and Chun, 1999) and oligodendrocytes (Weiner et al., 1998), suggested roles for LPA signaling in the regulation of myelinating cell adhesion and morphology. The data presented here implicate LPA1-mediated LPA signaling as an endogenous regulatory system for SC morphology and adhesion.
LPA treatment of SCs led to actin rearrangements, focal adhesion assembly, and N-cadherin-mediated cell clustering. Although the significance of LPA-induced focal adhesion assembly to SC biologyin vivo is not yet clear, increased adhesion to ECM components, accompanied by a transition from an elongated, bipolar morphology to a flatter shape, is reminiscent of changes that occur as SCs stop migrating and begin elaborating the myelin sheath. Consistent with this are reports that laminin–integrin interactions enhance myelin formation in cultured SCs and oligodendrocytes (Fernandez-Valle et al., 1994; Buttery and ffrench-Constant, 1999). The assembly ofN-cadherin junctions and the resulting cell clustering appear to represent a distinct response to LPA, because blocking cadherin function had no apparent effect on actin reorganization (Fig.4h–k). The regulation of cadherin junctions by LPA may have important consequences for SC development. Cadherin cell junctions are observed between migrating SCs and neurites in vitro(Letourneau et al., 1991) and between individual layers of the myelin sheath in vivo (Fannon et al., 1995). Furthermore, SC myelination of axons in vitro was found to be aberrant in low calcium medium (Blank et al., 1974), likely reflecting disrupted cadherin function. Initial coculture experiments have suggested that LPA also increases N-cadherin-containing contacts between SCs and dorsal root ganglion neurites (N. Fukushima and J. Chun, unpublished data). LPA may thus regulate cell–cell contacts in vivo not only between SCs but also between SCs and axons.
SCs isolated fromlpA1(−/−) mice exhibited greatly decreased LPA responsiveness. Although previous Northern blot analysis of rat SCs did not detect expression of the gene encoding LPA2, a second, related GPCR for LPA (Weiner and Chun, 1999), mouse SCs do express this gene at levels detectable by RT-PCR. The residual actin rearrangements observed inlpA1(−/−) SCs may thus be explained by action through LPA2, which mediates morphological responses to LPA with somewhat less efficacy than LPA1 (Ishii et al., 2000). Although SC apoptosis is significantly increased in the sciatic nerves of adultlpA1(−/−) mice (Contos et al., 2000b), myelination and sciatic nerve structure appear grossly normal (J. A. Weiner and J. Chun, unpublished data). This may reflect compensation by several signaling pathways: LPA signaling through other LPA receptors; S1P signaling, which induced similar actin reorganization in both wild-type andlpA1(−/−) mouse SCs; and/or the action of peptide growth factors (e.g., neuregulins) on their cognate receptors.
LPA-induced cytoskeletal signaling mechanisms could conceivably contribute not only to the control of myelination (Fernandez-Valle et al., 1994, 1997; Chen et al., 2000) but also to SC survival. The major survival-promoting response to LPA in neonatal SCs is mediated through a Gi–PI3K–Akt pathway (Weiner and Chun, 1999), but Rho-dependent cytoskeletal signaling mechanisms could feed into this pathway. Loss of contact with the ECM can induce a form of apoptosis called “anoikis” in several cell types, and both integrin signaling and the actin binding protein ezrin have been shown to activate the PI3K–Akt survival pathway (Frisch and Ruoslahti, 1997;Khwaja et al., 1997; King et al., 1997; Longhurst and Jennings, 1998; Gautreau et al., 1999). This is consistent with our observation that the ability of LPA to promote SC survival is enhanced when cells are grown on laminin compared with poly-l-lysine alone (J. A. Weiner and J. Chun, unpublished data). The increased apoptosis observed in the sciatic nerve of adultlpA1(−/−) mice (Contos et al., 2000b) may thus indicate that LPA signaling helps maintain the stability of SC–axon or SC–matrix interactions in the mature nerve. A relationship between the organization of the actin cytoskeleton and the growth and survival of SCs would not be surprising in view of the fact that loss-of-function mutations in merlin, an actin-binding protein of the ezrin-radixin-moesin family (Tsukita and Yonemura, 1999), were identified as the cause of neurofibromatosis type 2, an inherited human disorder characterized by Schwannoma formation (Rouleau et al., 1993; Scherer and Gutmann, 1996). Cultured human Schwannoma cells exhibit a markedly aberrant actin organization in culture attributable in part to abnormal Rho activation (Pelton et al., 1998).
SC survival is dependent on autocrine and/or paracrine signaling loops, in which insulin-like growth factor, neurotrophin-3, and neuregulin have been implicated (Mirsky and Jessen, 1996; Cheng et al., 1998; Meier et al., 1999). Thus, the ability of LPA to promote SC survival (Weiner and Chun, 1999) is particularly interesting in light of the present demonstration of LPA activity in SC CM. Because SCs are also dependent on axon-derived signals for survival, neurons are another potential source of endogenous LPA. Detection of LPA release by dorsal root ganglion or motor neurons in vitrohas been hampered by the need for high-purity, high-cell density, extended culture times, and serum-free (i.e., exogenous LPA-free) conditions. However, we have recently identified LPA activity in medium conditioned by postmitotic cortical neurons (but not by mitotic neuroblasts) (Fukushima et al., 2000), and thus it is probable that both axons and SCs can release signaling LPA in peripheral nerves.
Collectively, the data presented here and previously (Weiner and Chun, 1999) demonstrate multiple effects of LP receptor-mediated LPA signaling on SC biology and suggest in vivo roles in peripheral nerve development and regeneration. The production of mice lacking multiple lysophospholipid receptor genes, as well as other methods of manipulating LPA signaling in vivo, are being pursued to test this prediction. Such studies, as well as further dissection of the signaling pathways activated by LPA in SCs, may in the future lead to novel therapies for disorders of myelination and nerve function.
Footnotes
This work was funded by grants from the National Institute of Mental Health (NIMH) (J.C.), National Research Service Award predoctoral fellowships from the NIMH (J.W., J.J.A.C.), and the Uehara Foundation (N.F.). We gratefully thank Drs. Kristjàn Jessen and David Parkinson (University College, London, UK) for the generous gift of embryonic mouse nerve cDNA. Y-27632 was the kind gift of Masayoshi Uehata (WelFide Corporation, Saitama, Japan). We thank Dr. Don Cleveland for critically reading this manuscript and Drs. Valerie Sah, Paul Martin, and Greg Lemke for helpful comments.
J.W. and N.F. contributed equally to this work.
Correspondence should be addressed to Jerold Chun at his present address: Merck Research Laboratories, San Diego, 3535 General Atomics Court, San Diego, CA 92121. E-mail: jerold_chun@merck.com.
J.A. Weiner's present address: Department of Anatomy and Neurobiology, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110.
REFERENCES
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley; New York: 1994. [Google Scholar]
- 4.Blank WF, Bunge MB, Bunge RP. The sensitivity of the myelin sheath, particularly the Schwann cell-axolemma junction, to lowered calcium levels in cultured sensory ganglia. Brain Res. 1974;67:503–518. doi: 10.1016/0006-8993(74)90498-3. [DOI] [PubMed] [Google Scholar]
- 5.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 6.Bunge MB, Bunge RP, Kleitman N, Dean AC. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev Neurosci. 1989;11:348–360. doi: 10.1159/000111911. [DOI] [PubMed] [Google Scholar]
- 7.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 8.Chen L-M, Bailey D, Fernandez-Valle C. Association of β1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L, Esch FS, Marchionni MA, Mudge AW. Control of Schwann cell survival and proliferation: autocrine factors and neuregulins. Mol Cell Neurosci. 1998;12:141–156. doi: 10.1006/mcne.1998.0706. [DOI] [PubMed] [Google Scholar]
- 10.Chun J, Contos JJA, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs). Cell Biochem Biophys. 1999;30:213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- 11.Cifuentez-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult, and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development. 1994;120:1–11. doi: 10.1242/dev.120.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Contos JJ, Chun J. Complete cDNA sequence, genomic structure, and chromosomal localization of the LPA receptor gene, lpA1/vzg-1/Gpcr26. Genomics. 1998;51:364–378. doi: 10.1006/geno.1998.5400. [DOI] [PubMed] [Google Scholar]
- 13.Contos JJA, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lpA2/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64:155–169. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- 14.Contos JJA, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000a;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 15.Contos JJA, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000b;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL, Jr, Colman D. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol. 1995;129:189–202. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-beta1 integrin antibody inhibits Schwann cell myelination. J Neurobiol. 1994;25:1207–1226. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Valle C, Wood PM, Bunge MB. Localization of focal adhesion kinase in differentiating Schwann cell/neuron cultures. Microsc Res Tech. 1998;41:416–430. doi: 10.1002/(SICI)1097-0029(19980601)41:5<416::AID-JEMT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Fort P, Marty L, Piechaczyk M, Sabrouty SE, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde 3-phosphate dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 22.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima N, Kimura Y, Chun J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G-proteins and mediates multiple cellular responses to lysophosphatidic acid (LPA). Proc Natl Acad Sci USA. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima N, Weiner JA, Chun J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol. 2000;228:6–18. doi: 10.1006/dbio.2000.9930. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima N, Ishii I, Contos JJA, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- 26.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem. 1998;273:4653–4659. doi: 10.1074/jbc.273.8.4653. [DOI] [PubMed] [Google Scholar]
- 28.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii I, Contos JJA, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LPA1/VZG-1/EDG-2, LPA2/EDG-4, and LPA3/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol. 2000;58:895–902. doi: 10.1124/mol.58.5.895. [DOI] [PubMed] [Google Scholar]
- 30.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 31.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 33.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee M-J, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 36.Lemke G. The molecular genetics of myelination: an update. Glia. 1993;7:263–271. doi: 10.1002/glia.440070402. [DOI] [PubMed] [Google Scholar]
- 37.Lemke G, Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985;40:501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- 38.Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V. Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol. 1990;138:430–442. doi: 10.1016/0012-1606(90)90209-2. [DOI] [PubMed] [Google Scholar]
- 39.Letourneau PC, Roche FK, Shattuck TA, Lemmon V, Takeichi M. Interactions of Schwann cells with neurites and with other Schwann cells involve the calcium-dependent adhesion molecule, N-cadherin. J Neurobiol. 1991;22:707–720. doi: 10.1002/neu.480220706. [DOI] [PubMed] [Google Scholar]
- 40.Longhurst CM, Jennings LK. Integrin-mediated signal transduction. Cell Mol Life Sci. 1998;54:514–526. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahanthappa NK, Anton ES, Matthew WD. Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J Neurosci. 1996;16:4673–4683. doi: 10.1523/JNEUROSCI.16-15-04673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, Streuli C, Pytela R, ffrench-Constant C. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol. 1997;185:215–228. doi: 10.1006/dbio.1997.8547. [DOI] [PubMed] [Google Scholar]
- 44.Mirsky R, Jessen KR. Schwann cell development, differentiation, and myelination. Curr Opin Neurobiol. 1996;6:89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 45.Moolenaar WH. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 46.Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 47.Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, Ratner N. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 1998;17:2195–2209. doi: 10.1038/sj.onc.1202141. [DOI] [PubMed] [Google Scholar]
- 48.Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- 49.Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- 50.Ramon y Cajal S. Degeneration and regeneration of the nervous system. Oxford UP; New York: 1928. [Google Scholar]
- 51.Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 52.Rogister B, Delree P, Leprince P, Martin D, Sadzot C, Malgrange B, Munaut C, Rigo JM, Lefebvre PP, Octave J-N, Schoenen J, Moonen G. Transforming growth factor beta as a neuronoglial signal during peripheral nervous system response to injury. J Neurosci Res. 1993;34:32–43. doi: 10.1002/jnr.490340105. [DOI] [PubMed] [Google Scholar]
- 53.Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk M, Desmaze C, Plougastel B, Pulst SM, Lenoir G, Bijisma E, Fashold R, Dumanski J, de Jong P, Parry D, Eldrige R, Aurias A, Delattre O, Thomas G. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 54.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 55.Scherer SS. The biology and pathobiology of Schwann cells. Curr Opin Neurol. 1997;10:386–397. doi: 10.1097/00019052-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Scherer SS, Gutmann DH. Expression of the neurofibromatosis 2 tumor suppressor gene product, merlin, in Schwann cells. J Neurosci Res. 1996;46:595–605. doi: 10.1002/(SICI)1097-4547(19961201)46:5<595::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 57.Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. J Neurosci. 1994;14:1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seilheimer B, Schachner M. Regulation of neural cell adhesion molecule expression on cultured mouse Schwann cells by nerve growth factor. EMBO J. 1987;6:1611–1616. doi: 10.1002/j.1460-2075.1987.tb02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibuya Y, Mizoguchi A, Takeichi M, Shimada K, Ide C. Localization of N-cadherin in the normal and regenerating nerve fibers of the chicken peripheral nervous system. Neuroscience. 1995;67:253–261. doi: 10.1016/0306-4522(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 60.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (Ezrin/Radixin/Moesin) proteins. J Biol Chem. 1999;274:34507–34510. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 61.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–997. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 62.van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 63.Weiner JA, Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci USA. 1999;96:5233–5238. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- 65.Yap AS, Brieher WM, Gumbiner M. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 66.Zhang G, Contos JJA, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- 67.Zorick TS, Lemke G. Schwann cell differentiation. Curr Opin Cell Biol. 1996;8:870–876. doi: 10.1016/s0955-0674(96)80090-1. [DOI] [PubMed] [Google Scholar]