Abstract
Recent studies have shown that neurogenesis is enhanced after hypoxia and that erythropoietin (EPO), an inducible cytokine, is produced in the brain as part of the intrinsic hypoxia response. Thus, we asked whether EPO might regulate neurogenesis by forebrain neural stem cells (NSCs). We found that EPO receptors are expressed in the embryonic germinal zone during neurogenesis as well as in the adult subventricular zone, which continues to generate neurons throughout adulthood. Cultured NSCs exposed to a modest hypoxia produced two- to threefold more neurons, which was associated with an elevation in EPO gene expression. The enhanced neuron production attributable to hypoxia was mimicked by EPO and blocked by coadministration of an EPO neutralizing antibody. EPO appears to act directly on NSCs, promoting the production of neuronal progenitors at the expense of multipotent progenitors. EPO infusion into the adult lateral ventricles resulted in a decrease in the numbers of NSCs in the subventricular zone, an increase in newly generated cells migrating to the olfactory bulb, and an increase in new olfactory bulb interneurons. Infusion of anti-EPO antibodies had the opposite effect: an increase in the number of NSCs in the subventricular zone and a decrease in the number of newly generated cells migrating to the bulb. These findings suggest that EPO is an autocrine–paracrine factor, capable of regulating the production of neuronal progenitor cells by forebrain NSCs.
Keywords: neural stem cells, erythropoietin, neuronal progenitors, neurogenesis, differentiation, Mash1, NF-κB
Oxygen deficiency, which results from hypoxic insults, triggers a host of intrinsic adaptive processes designed to promote tissue protection and regeneration (Bunn and Poyton, 1996). Perhaps the best example of this process is the hypoxia-induced expression of erythropoietin (EPO), which acts at the EPO receptor to promote proliferation and differentiation of erythroid progenitors and the survival of maturing erythroid cells (Youssoufian et al., 1993). The expression of the EPO receptor in the developing mouse and human CNSs (Liu et al., 1994, 1997;Juul et al., 1998b, 1999) supports a possible role for EPO in CNS development. Furthermore, persistent expression of EPO and EPO receptors in the adult CNS and the upregulation of EPO in the CNS after hypoxia (Digicaylioglu et al., 1995; Marti et al., 1996; Morishita et al., 1997; Chikuma et al., 2000), support a role for EPO in the brain's response to injury. In line with this hypothesis, previous studies provide evidence for EPO as a neuroprotectant in the CNS.In vitro studies of cultured CNS neurons have shown that EPO protects against cell death induced by hypoxia or glutamate (Morishita et al., 1997; Juul et al., 1998a). As well, embryonic precursors from both the peripheral nervous system and CNS showed enhanced neuronal proliferation and differentiation in response to lowered oxygen (Morrison et al., 2000; Studer et al., 2000). Although the mechanism of such enhanced neurogenesis was not determined, the significant increase in EPO expression and function, as described above, suggests that this cytokine is a candidate for mediating enhanced neuronal production after hypoxia.
We have previously identified epidermal growth factor (EGF)-responsive neural stem cells (NSCs) in the forebrain embryonic germinal zone (Reynolds et al., 1992; Reynolds and Weiss, 1996) and adult subventricular zone (Reynolds and Weiss, 1992; Morshead et al., 1994). In culture, these NSCs proliferate to form spheres of undifferentiated cells that produce neurons, astrocytes, and oligodendrocytes, as well as precursors to secondary spheres (self-renewal) (Reynolds and Weiss, 1996). In the adult, these NSCs participate in the repopulation of the subventricular zone (Morshead et al., 1994) and appear to be the source of new neurons that replenish the olfactory bulb (Shimazaki et al., 2001). Given increased neurogenesis and expression of EPO after hypoxia, we asked whether EPO might act to regulate neuronal production by forebrain NSCs. Our results suggest that EPO is an intrinsic, hypoxia-regulated factor, capable of regulating the production of neuronal progenitors by NSCs, both in cell culture and in situ in the adult CNS.
MATERIALS AND METHODS
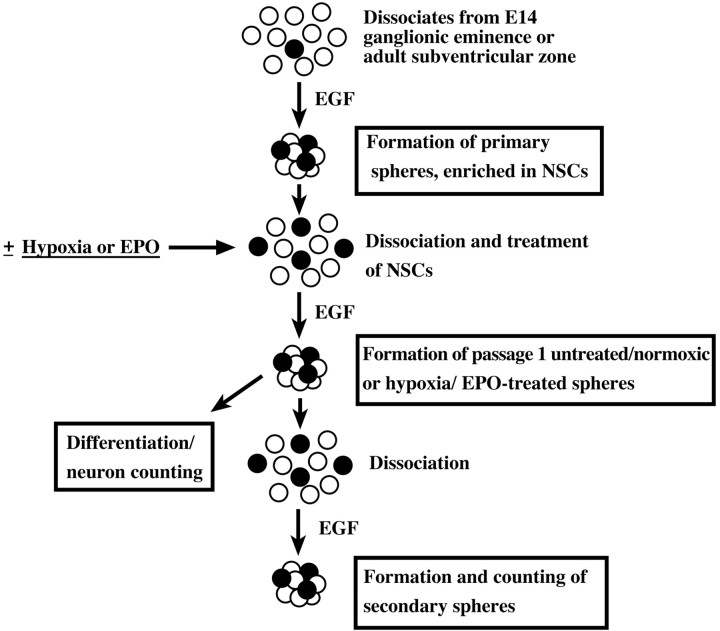
Neural stem cell culture and analysis (see Fig. 1)
Fig. 1.
The basic experimental protocol for the in vitro assessment of neural stem cell activity in this study. A neural stem cell (●) can be expanded by the formation of a clonally derived cell cluster, termed a sphere, in EGF-containing growth medium. Neural stem cells are thus enriched by generation of primary spheres from dissociates of the E14 ganglionic eminence or adult subventricular zone. These primary spheres containing a number of neural stem cells (∼20%) (Reynolds and Weiss, 1996) are dissociated and cultured in populations (5 × 104 cells per milliliter) for 7 d or clonally (150 cells per milliliter per 9.6 cm2) for 12–13 d, in EGF alone or after being made hypoxic or having EPO added to the medium. This results in the generation of passage 1 spheres, which are then assessed for (1) neuron number by differentiation and immunocytochemistry or (2) renewal–expansion by dissociation, generation, and counting of secondary spheres.
Primary cell culture. The procedures used to generate neurospheres from the embryonic and adult forebrain were adopted as described previously, with minor modifications (Reynolds and Weiss, 1992, 1996). Briefly, the ganglionic eminences were dissected from CD1 mouse embryos at embryonic day (E) 14 in Dulbecco's PBS (Life Technologies, Gaithersburg, MD) containing 0.6% glucose, penicillin (50 U/ml), and streptomycin (50 U/ml) (both from Life Technologies) and then transferred to defined media (MHM) composed of DMEM–F12 (1:1), glucose (0.6%), glutamine (2 mm), sodium bicarbonate (3 mm), HEPES (5 mm), insulin (25 μg/ml), transferrin (100 μg/ml), progesterone (20 nm), putrescine (60 μm), and selenium chloride (30 nm) [all from Sigma (St. Louis, MO), except glutamine from Life Technologies]. The tissues were mechanically triturated into single cells with a fire-polished pipette. Cells were cultured for 7 d in vitro (DIV) at a density of 100 cells per microliter in 20 ng/ml EGF (human recombinant; PeproTech, Rocky Hill, NJ)-containing media. For adult neural stem cell cultures, the defined tissue surrounding the lateral ventricles from the rostral tip to the crossing point of both ventricles (without contamination of the cortex and hippocampus) was dissected, transferred into MHM containing 1.33 mg/ml trypsin, 0.67 mg/ml hyaluronidase, and 0.2 mg/ml kynurenic acid (all from Sigma), and then incubated for 20 min at 37°C. After complete trituration with a micropipette, the suspension was transferred into the same volume of media containing 0.7 mg/ml trypsin inhibitor (Roche Diagnostics, Laval, Quebec, Canada). This suspension was spun down at 600 rpm for 5 min, resuspended, and then plated at a density of 1000–2000 cells per milliliter in a six-well plate in the EGF-containing media. The generated neurospheres (primary spheres) were passaged by mechanical dissociation and reseeded as single cells at a density of 50 cells per microliter in embryos and at a density of 20 cells per microliter in adult in EGF-containing media (passage 1 cells). Passage 1 cells were processed for various experiments as described below and illustrated in Figure1. All mice were killed by cervical dislocation for culture experiments.
Hypoxia and EPO administration. Passage 1 cells (in flasks) were made hypoxic by placing them in a modular incubator chamber (Billups-Rothenberg) and flushing it with 95% N2/5% CO2 for 10 min, decreasing the PO2 in the atmosphere to 0 mmHg. The flasks were left in the chamber at 37°C for up to 12 hr (PO2 in media decreased to 30 mmHg vs 135 mmHg in control), after which time they were placed back into the standard incubator conditions of 95% air/5% CO2. For the EPO experiments, EPO (R&D Systems, Minneapolis, MN or Janssen-Ortho, Toronto, Canada) was added at various concentrations at the indicated times in culture. For the washout experiments, media was removed and replaced with fresh media at various times after hypoxia or EPO treatment. For EPO neutralization experiments, anti-EPO neutralizing antibodies and control rabbit IgGs (both from R&D Systems) were added at a concentration of 3 μg/ml.
Counting of neuron numbers/sphere (neurogenesis) and secondary spheres/sphere (expansion–renewal). Passage 1 spheres generated after hypoxia or EPO administration (or control–normoxic counterparts) were either differentiated to assess neuronal production or dissociated and replated in EGF to assess renewal–expansion. To observe neuronal differentiation in vitro, two methodologies were performed (Reynolds and Weiss, 1996): (1) each sphere (200 μm diameter) was transferred onto a poly-l-ornithine-coated coverslip in MHM, or (2) dissociated spheres were plated onto the coverslip at a density of 100,000–500,000 cells per milliliter per well in 24-well plates. After various periods of differentiation, coverslips were fixed in 4% paraformaldehyde in PBS and incubated with mouse anti-β-tubulin III antibody (1:1000; Sigma) in 0.3% Triton X-100–PBS containing 10% normal goat serum (NGS). After washing with PBS, cells were reacted with rhodamine-conjugated anti-mouse antibody (1:200; Jackson ImmunoResearch, West Grove, PA). Labeled cells were counterstained with Hoechst 33258 (0.015 mg/ml stock solution diluted to 0.001 mg/ml; Sigma) and mounted on glass slides with Fluorsave (Calbiochem, San Diego, CA). For renewal–expansion assays (counting of secondary spheres) (Reynolds and Weiss, 1996), passage 1 spheres (200 μm diameter) were individually transferred into the wells of a 96-well plate in 200 μl of EGF-containing media and triturated to single cells with micropipettes. After 7–10 DIV, the number of secondary spheres/single sphere were counted.
NSC sphere-forming assay at clonal density
To preclude indirect actions of trophic molecule administration, where indicated some experiments were performed at clonal density. Passage 1 cells (embryo or adult) were plated in six-well plates at a density of 150 cells per milliliter per well (35 mm2) in the presence of EGF. After 6 d, 1 ml of EGF-containing media was added to each well, or in the case of the EPO treatment study, EPO was added for various time periods as described. Virtually all spheres are clonally derived at this density (Tropepe et al., 1999).
EPO RT-PCR and RT-PCR–Southern blots
Total RNA was isolated from EGF-generated stem cells at varying times after 4 hr of hypoxia by using Trizol reagent (Life Technologies). First-strand cDNA was synthesized using Superscript (Life Technologies) and then amplified using Taq-DNA polymerase (Life Technologies) with 30 cycles of denaturation (94°C, 45 sec), primer annealing (58°C, 45 sec), and extension (72°C, 45 sec) in the presence of 0–5% DMSO. Primers were: EPO, 5′-ACTCCGAACACTCACAGTGGATAC-3′ and downstream 5′-GATTCTGAGGCTCTTCTTCTCTGG-3′, and ACTIN, upstream position 182–202 and downstream 424–404 (Tokunaga et al., 1986) (GenBank accession number X03672). PCR products were run on 1.5–2% agarose gels. For RT-PCR–Southern blots for EPO, the products were blotted onto positively charged nylon membranes (Amersham Pharmacia Biotech, Quebec, Canada) and hybridized with fluorescein-labeled cDNA probes (sizes as above, prepared from PCR-based direct cloning) for EPO prepared by using Gene Image Labeling Kit (Amersham Pharmacia Biotech). Thirty cycles of each PCR reaction described above was performed, and then the products were purified using Geneclean II kit (Bio 101, La Jolla, CA) and ligated into pGEM-T vector plasmids (Promega, Madison, WI). Correct plasmid clones were identified by sequencing.
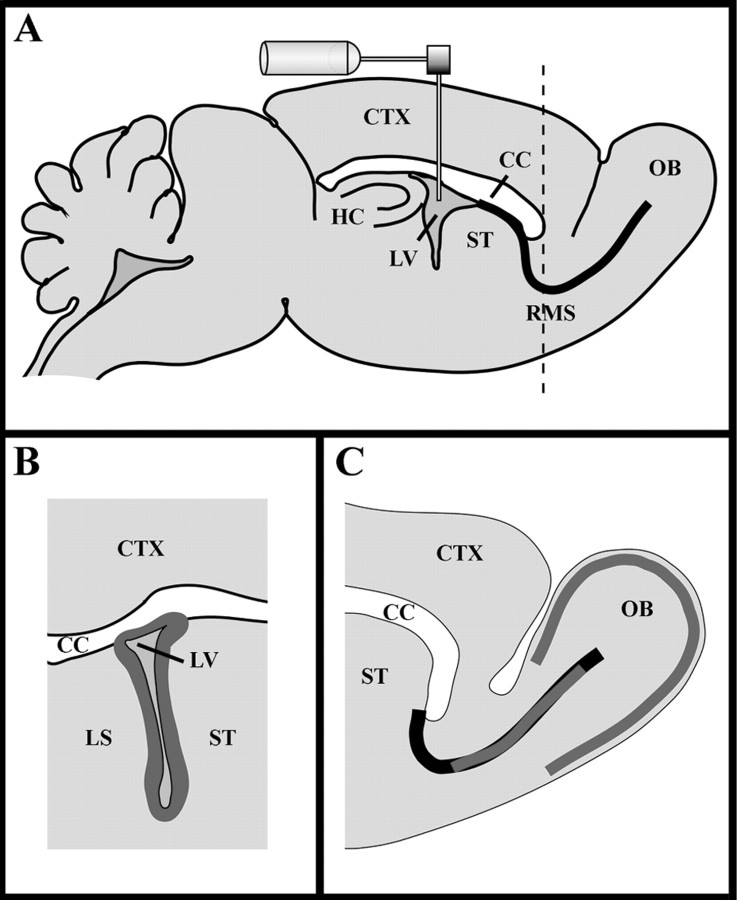
Implantation of the osmotic pumps and growth factor infusion (see Fig. 5)
Fig. 5.
Schematic drawing of the subventricular zone (SVZ), rostral migratory stream (RMS), and olfactory bulb (OB). A, Sagittal view of the adult mouse forebrain, which demonstrates the migratory pathway from the SVZ to the OB along the RMS, as well as the implantation site of the cannulas into the lateral ventricle (LV).Broken line indicates the division between sagittally (rostral) and coronally (caudal) sectioned regions. B, Coronal section of the LV with corpus callosum (CC), lateral septum (LS), and striatum (ST). The SVZ region surrounding the LV, indicated in dark gray, was counted for BrdU- or Mash1-positive cells. C, Sagittal section through the RMS and OB. Areas of the RMS or periglomerular layer in the OB analyzed for BrdU-positive cells are indicated with thick dark gray lines.
Two-month-old CD-1 mice (Charles-River, Laval, Quebec, Canada) were anesthetized with sodium pentobarbital (120 mg/kg, i.p.) and implanted with an osmotic pump (Alzet 1007D; AlzaCorporation, Palo Alto, CA). The cannula was located in the right lateral ventricle (anteroposterior +0.2 mm, lateral +0.8 mm to bregma, and dorsoventral −2.5 mm below dura with the skull leveled between lambda and bregma). Human recombinant EPO (1000 IU/ml), rabbit anti-EPO neutralizing antibody (100 μg/ml), or rabbit IgG (100 μg/ml) was dissolved in 0.9% saline containing 1 mg/ml mouse serum albumin (Sigma). Each animal was infused for 6 consecutive days with EPO (n = 20), vehicle (n = 20), anti-EPO (n = 10), or IgG (n = 10) at a flow rate of 0.5 μl/hr, resulting in a delivery of ∼25 IU of EPO per day or 3 μg of anti-EPO per day of each factor.
Adult neural stem cell culture after infusion
After 6 d of infusion, mice were killed by cervical dislocation, and the brains were excised. Adult neural stem cell culture was performed as described above. After 10–12 DIV, the total number of spheres was counted.
Bromodeoxyuridine labeling and detection
After 6 d of intraventricular infusion, mice were injected with bromodeoxyuridine (BrdU) (Sigma) (120 mg/kg, i.p., dissolved in 0.007% NaOH in phosphate buffer) every 2 hr for 10 hr and killed 0.5 hr (or longer) after the last injection. Animals were killed, and brains were processed for immunohistochemistry as described below. Rat monoclonal anti-BrdU (1:50; Harlan Seralab, Loughborough, UK) and biotinylated donkey anti-rat (1:200, Jackson ImmunoResearch) with streptavidin-Cy3 (1:2000; Jackson ImmunoResearch) were used for BrdU detection. To detect the BrdU-positive cells in the olfactory bulb 6 weeks after EPO (n = 5) or vehicle (n = 5) infusion, pumps were removed 1 d after BrdU injections. Animals were then maintained on a 12 hr light/dark cycle with food and waterad libitum, allowed to survive for 6 weeks, and then killed; the tissue was processed for immunohistochemistry as described below.
Immunohistochemistry
Animals were killed by anesthetic overdose and perfused transcardially with 4% paraformaldehyde in PBS, pH 7.2. Brains were post-fixed in the perfusing solution overnight at 4°C, and then cryoprotected for at least 24 hr in 20% sucrose in PBS. The brains were coronally cut on the anterior tip of the corpus callosum to provide for sagittal sections of the olfactory bulb (OB) and rostral migratory stream (RMS) and coronal sections of the subventricular zone, and then embedded in Tissue Tek O.C.T. compound (Sakura Finetek, Torrance, CA) before they were cryosectioned at 14 μm. The following primary antibodies (final dilution and source) were used for tissue staining: sheep anti-EGF receptor (1:50; BioDesign, Saco, ME), mouse or rabbit anti-EPO receptor (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-nestin (1:1; Rat 401 from Developmental Studies Hybridoma Bank), rabbit anti-tyrosine hydroxylase (1:200; Pel-Freez, Rogers, AR), mouse anti-Mash1 (1:5; provided by D. Anderson, Caltech) (Lo et al., 1991), and mouse anti-stat5 (1:500; Santa Cruz Biotechnology). Before immunohistochemistry, sections were post-fixed with acetone for 30 sec at room temperature, then washed with PBS. For BrdU staining, tissues were treated with 1m HCl for 30 min at 60°C to denature cellular DNA. Sections were incubated for 24 hr at 4°C in primary antibody diluted in 0.3% Triton X-100–PBS containing NGS, washed with PBS, and then incubated with regular secondary antibodies conjugated to FITC, rhodamine, or biotinylated secondary antibodies for 1 hr at room temperature followed by incubation with streptavidin-Cy3 for 1 hr at room temperature, together with Hoechst 33258. After rinsing with water, sections were mounted with Fluorosave and viewed or photographed with a Zeiss Axiophot fluorescence microscope.
TUNEL staining
To detect cells undergoing apoptosis, an In Situ Detection kit (Roche Diagnostics) was used. In brief, EPO-treated or untreated neurospheres (200 μm in diameter) were plated onto poly-l-ornithine-coated coverslips after 10–12 DIV for 1 hr and fixed with ice-cold 4% paraformaldehyde. Spheres were permeabilized with 0.3% Triton X-100 in PBS for 10 min at room temperature and then incubated with fluorescein-labeled TUNEL reaction mixture for 30 min at 37°C. Labeled cells were counterstained with Hoechst 33258 and mounted with Fluorosave.
Quantification (see Fig. 5): subventricular zone
A one-in-seven series of coronal sections (14 μm) from the rostral tip of lateral ventricle to 980 μm caudal of the ventricles (total 10 sections) was performed. As illustrated in Figure5B, BrdU or Mash1-positive cells were counted in the defined ependymal–subependymal layer, which could be visualized by Hoechst staining.
RMS and OB. A one-in-seven series of sagittal sections (14 μm) from medial side of OB to 1176 μm lateral (total 12 sections) was performed. As illustrated in Figure 5C, BrdU-positive cells were counted in the RMS between rostral tip of the RMS in the OB and rostral tip of the corpus callosum, and in the periglomerular layer [tyrosine hydroxylase (TH)-positive layer] of the OB. Analysis of significant differences was performed using ANOVA followed by paired t test to compare values within experiments. Values expressed as percentage (e.g., number of neurons) were analyzed using χ2test.
Preparation of whole-cell protein lysates or nuclear extracts
For whole-cell lysates, the spheres were harvested, washed twice with cold PBS, and lysed in RIPA buffer [1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 mm sodium orthovanadate, and protease inhibitor mix (Roche)]. These protein extracts were used for immunoprecipitation or Mash1 Western blots. For nuclear extract preparation, Nu-CLEAR Extraction kit (Sigma) was used. In brief, cultures of spheres were lysed in isotonic buffer (10 mm Tris-HCl, pH 7.5, 2 mmMgCl2, 3 mmCaCl2, 0.5 m sucrose, 1 mm DTT, and protease inhibitor mixture) on ice for 20 min, and 0.6% IGEPAL CA-630 solution was added followed by centrifugation at 2000 rpm for 1 min. The nuclear pellet was resuspended in extraction buffer (20 mm HEPES, pH 7.9, 1.5 mm MgCl2, 0.42 M NaCl, 0.2 mm EDTA, 25% glycerol, 1 mm DTT, and protease inhibitor mixture) on a vortex mixer for 30 min at 4°C and centrifuged at 15,000 rpm for 20 min. The supernatants were used as nuclear extracts for NF-κB blots.
Immunoprecipitation of STAT5
For immunoprecipitation analysis of STAT5, 500 μg of whole-cell extracts were incubated with agarose-conjugated STAT5 antibody (Santa Cruz Biotechnology) overnight at 4°C and washed with RIPA buffer. The pellets were resuspended in electrophoresis sample buffer (62.5 mm Tris-HCl, pH 6.7, 5% glycerol, 1% 2-mercaptoethanol, and bromophenol) and boiled for 3 min. The supernatants were applied as described below. Primary antibody was mouse anti-phosphotyrosine (1:1000; BD Transduction Labs, Mississauga, Ontario, Canada). After developing, blots were reprobed with rabbit anti-STAT5 (Santa Cruz Biotechnology) to confirm that samples contained equal amounts of protein.
Western blot analysis of NF-κB and Mash1
Immunoprecipitates or 20 μg of whole-cell or nuclear extracts were fractionated by 10% SDS-polyacrylamide electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked in blocking buffer (25 mm Tris-HCl, pH 7.5, 0.5m NaCl, 0.3% Tween 20, and 5% non-fat skim milk) and incubated with primary antibodies rabbit anti-NF-κB p50, p52, p65 (1:500; Santa Cruz Biotechnology) or mouse anti-mash1 (1:10) in the blocking buffer overnight at 4°C. The blots were washed and then incubated with the various peroxidase-conjugated secondary antibodies (1:5000; Jackson ImmunoResearch). Immunoreactivity was developed by enhanced chemoluminescence (Amersham Pharmacia Biotech).
Inhibition of activated NF-κB
For inhibition of the nuclear translocation of activated NF-κB, SN50 (a cell-permeable peptide inhibitor of NF-κB from BIOMOL) or mutant SN50 (SN50M from BIOMOL) were used. However, passage 1 cells could not generate floating neurospheres in the presence of >10 μg/ml SN50 (data not shown). Therefore, 1 hr before EPO stimulation, 6 DIV passage 1 cells (clonal density) were treated in 10 μg/ml of SN50 or SN50M and after 24 hr were then changed to new EGF-containing media. Seven days after treatment, the spheres were dissociated for sphere-forming assays or processed to assess neuron number as described.
RESULTS
Hypoxia-enhanced neurogenesis by NSCs is mediated by EPO
Before beginning studies aimed at examining roles for EPO in regulating forebrain NSC neurogenesis, we first asked whether the EPO receptor was expressed in vivo in a manner that would support its putative interaction with EGF-responsive NSCs. We first examined EPO receptor expression in the ganglionic eminence of the E14 mouse forebrain. In a previous study, we showed that the germinal zone of the E14 ganglionic eminence, the putative location of EGF-responsive NSCs, is characterized by the expression of the neuroepithelial intermediate filament nestin (Shimazaki et al., 1999). Immediately adjacent to the germinal zone is the location of more restricted, migrating neuronal progenitors that express the transcription factor Brn-4. Double labeling for the EPO receptor (Fig.2A) and nestin (Fig.2B) showed that the two antigens were very closely colocalized (Fig. 2C), confirming the germinal zone localization of the EPO receptor. These findings supported our further testing of the hypothesis that EPO receptor activation on EGF-responsive NSCs could influence the production of neuronal progenitors.
Fig. 2.
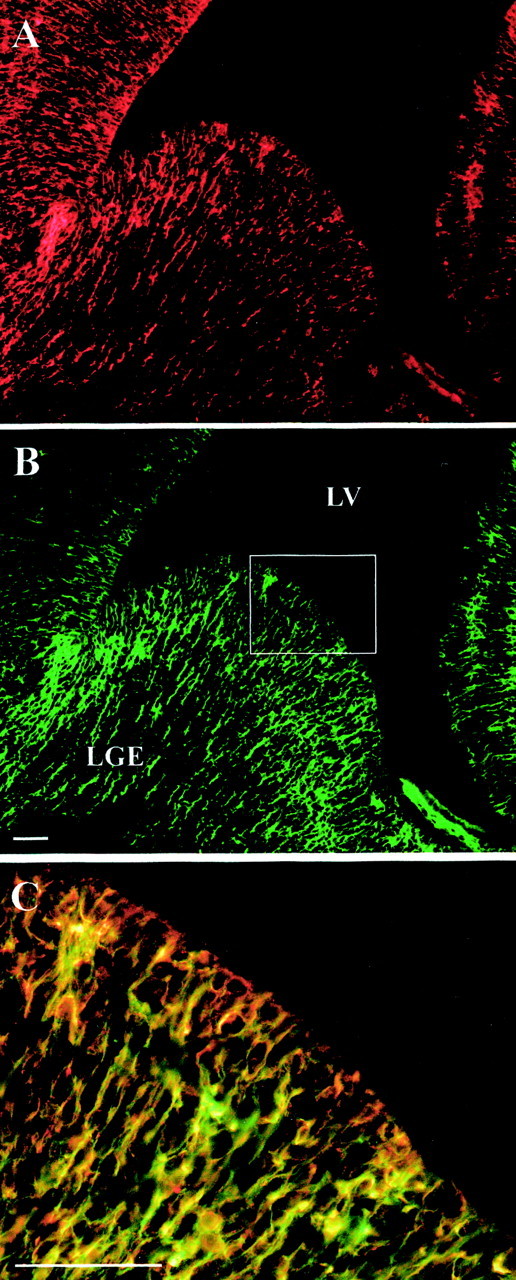
EPO receptors are coexpressed with nestin in the basal forebrain germinal zone. Indirect immunocytochemistry of the E14 lateral ganglionic eminence (LGE). Dual-labeling of EPO receptors (EPO-Rs) (A) and nestin (B) in the ventricular zone shows very close colocalization (orange–yellow inC; the merged image). The enlarged area inC is that indicated by the rectangle inB. Scale bars, 50 μm. LV, Lateral ventricle.
In the first series of in vitro experiments, we asked whether a modest hypoxia (for protocol details, see Materials and Methods and Fig. 1) would influence neurogenesis by NSCs, and if so, whether EPO might be involved. The results are given in Table1. During the early growth of NSC-generated spheres, at 3 DIV, only 13 ± 2% of spheres contain neurons, with approximately one to two neurons/sphere. However, a 4 or 8 hr hypoxia resulted in 31 ± 1 and 42 ± 9% of the spheres (a three- to fourfold increase) containing neurons at 3 DIV, respectively, without changing the numbers of neurons/sphere. After 6 DIV, when virtually all spheres contain neurons (whether originating from hypoxic or normoxic NSCs), a two- to threefold increase in the numbers of neurons/sphere was observed in spheres resulting from NSCs that had been made hypoxic. These findings suggest that hypoxia enhances both the onset and extent of neurogenesis by NSCs.
Table 1.
Hypoxia enhances the numbers of neuron-containing EGF-generated spheres and the number of neurons/sphere
| Treatment | Frequency of neuronal spheres | Neuron number | ||
|---|---|---|---|---|
| 3 DIV | 6 DIV | 3 DIV | 6 DIV | |
| % of total spheres | Neurons/sphere | |||
| Control | 13 ± 2 | 86 ± 6 | 1.6 ± 0.1 | 7.1 ± 0.2 |
| 4 hr hypoxia | 31 ± 1* | 83 ± 9 | 2.5 ± 0.4 | 11.5 ± 1.01-160 |
| 8 hr hypoxia | 42 ± 9* | 92 ± 3 | 2.4 ± 0.6 | 15.4 ± 1.01-160 |
Three and 6 DIV after a modest hypoxia, single EGF-generated spheres were plated on coverslips in defined media for 7 DIV. The numbers of neuron-containing spheres and neurons/sphere were counted in three independent cultures.
p < 0.05 versus control;
F1-160: p < 0.01 versus control.
EPO is produced and released by erythroid progenitors in response to hypoxia and acts in turn to enhance erythropoiesis. We asked whether the hypoxia-induced increase in neurogenesis might be caused, at least in part, by the production of EPO by NSCs or NSC progeny. We used PCR–Southern blot analysis to quantitate EPO mRNA in NSC cultures. Immediately after a 4 hr hypoxia, the production of EPO mRNA in NSC cultures was increased significantly (Fig.3A). The increased EPO expression peaked at 2 hr after the 4 hr hypoxia and was sustained for up to 8 hr. The production of EPO by NSC cultures prompted us to ask whether EPO release and the action on NSCs might be responsible for enhanced neurogenesis. To test this, we used an anti-EPO neutralizing antibody, with normal rabbit IgGs serving as controls. With or without a 4 hr hypoxia, NSC cultures were cultured in the absence or presence of anti-EPO antibodies for the remainder of the culture period. After 7 DIV, spheres were dissociated and plated in differentiating conditions for a further 7 DIV, at which point neuron numbers relative to total cells were counted. Under control conditions, ∼4–5% of total cells were neurons, and this was not significantly altered in the presence of anti-EPO antibodies or rabbit IgGs (Fig. 3B). NSC cultures that had experienced a 4 hr hypoxia contained 12–14% neurons, whereas the increase in neuron numbers caused by the 4 hr hypoxia was completely blocked by coincubation of cultures with anti-EPO antibodies. Control IgGs did not reduce the hypoxia-induced increase in NSC neurogenesis. Taken together, these findings suggest that the hypoxia-induced increase in neurogenesis by NSCs is caused by the release and autocrine–paracrine action of EPO.
Fig. 3.
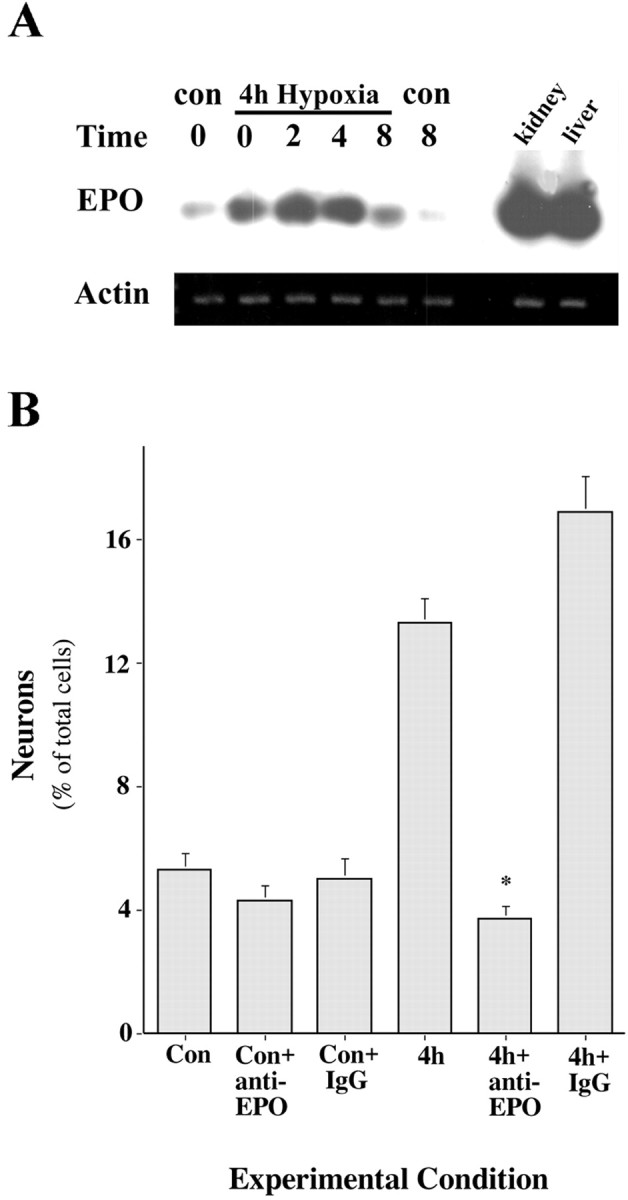
Evidence for endogenous EPO as a hypoxia-induced factor that regulates NSC neurogenesis.A, Hypoxia induces the expression of EPO in NSCs and progeny. RT-PCR–Southern blot analysis demonstrates a significant increase in EPO gene expression in NSCs immediately after a 4 hr hypoxic insult and up to 8 hr afterward. B, Hypoxia-induced neurogenesis by neural stem cells is blocked by an anti-EPO antibody. Anti-EPO antibody was introduced immediately before a 4 hr modest hypoxic insult and remained present during the 7 d of EGF-generated sphere formation. The presence of the anti-EPO antibody, but not a nonspecific IgG, blocked hypoxia-induced neurogenesis (*p < 0.001 vs 4 hr;n = 3).
EPO directs the enhanced NSC production of neuronal progenitors relative to multipotent precursors
In the next series of experiments, we sought to determine whether EPO acts directly on NSCs to instruct them to produce more neuronal progenitors. We hypothesized that if EPO acted directly on NSCs (rather than on more restricted progenitor cells within spheres), then a 24 hr exposure, before the first cell division and the subsequent generation of spheres (Reynolds et al., 1992; Reynolds and Weiss, 1996), should be sufficient to enhance neurogenesis. Cultures of NSCs were made hypoxic for 4 hr or exposed to increasing concentrations of EPO, and after 7 DIV, the spheres were differentiated and examined for neuronal production. In sister cultures, the media was replaced (24 hr wash) after 1 DIV with EGF alone and cultured until 7 DIV. The results are presented in Table 2. The two- to threefold increase in neuronal production caused by a 4 hr hypoxia was mimicked by EPO in a dose-dependent manner. Maximal actions of EPO at 10 IU/ml resulted in a 2.2-fold increase in neuronal production. Greater concentrations of EPO did not further enhance neuronal production (data not shown). Washout after 24 hr, either after hypoxia or with exposure to EPO, did not significantly reduce the enhanced neurogenesis by EGF-responsive NSCs. Thus, these results support the contention that EPO (released by hypoxia or added exogenously) acts directly on EGF-responsive NSCs to enhance the production of neuronal progenitors.
Table 2.
Persistent actions of a 24 hr hypoxia or EPO treatment in enhancing neurogenesis after washout suggests a direct action on embryonic neural stem cells
| Hypoxia (hr) | Erythropoietin (IU/ml) | |||||
|---|---|---|---|---|---|---|
| 0 | 4 | 0 | 0.1 | 1 | 10 | |
| Neurons (% of total cells) | ||||||
| Control | 4.9 ± 0.6 | 11.3 ± 0.8* | 4.3 ± 0.4 | 3.9 ± 0.4 | 7.6 ± 0.6* | 9.1 ± 0.7* |
| 24 hr wash | 4.0 ± 0.6 | 7.7 ± 0.8* | 3.7 ± 0.8 | 3.0 ± 0.5 | 6.5 ± 0.5* | 8.2 ± 0.5* |
Control, hypoxic, or EPO-stimulated neural stem cells were washed after 24 hr and replaced with media containing EGF only. After 7 DIV, resultant spheres were dissociated and plated for a further 7 DIV and then examined for neuron number as a percentage of total cells.
p < 0.01 versus control.
EGF-responsive NSCs produce spheres that contain precursors to neurons, astrocytes, and oligodendrocytes as well as secondary NSCs that themselves produce spheres (Reynolds and Weiss, 1996). In preliminary experiments, we found that total cell number and astroglial cell number were not different in control versus EPO-treated spheres (n = 3; data not shown). We then asked whether EPO might regulate the production of neuronal progenitors relative to multipotent progenitors, a process that occurs during the transition from ventricular zone expansion to neurogenesis and possibly (we hypothesize) in response to stress-induced alterations in cell requirements. Thus, we cultured NSCs at clonal density and examined the relative production of neurons and secondary NSCs. Single spheres, cultured in the absence of EPO, or after a 6 hr, 24 hr, or 7 d exposure to EPO, were examined for neuron number and the numbers of secondary spheres they formed when dissociated and replated in EGF. The results are illustrated in Table 3. Whether exposed to EPO for 7 d or 24 hr, spheres generated in the presence of EPO showed an increase in the numbers of neurons and a corresponding decrease in secondary spheres. Exposure for a shorter 6 hr period was not sufficient to either increase neuron number or decrease secondary NSCs. Whether exposed to EPO for 24 hr or 6 d, resultant spheres showed no difference in the number of TUNEL-labeled cells (EGF alone, 158 ± 18 cells; EGF + EPO (24 hr), 144 ± 12 cells; EGF + EPO (6 d), 158 ± 14 cells), suggesting that EPO does not prevent the death of neuronal precursors. Thus, EPO apparently increases the numbers of neuronal precursors at the expense of multipotent sphere-forming cells.
Table 3.
EPO increases neuronal production by embryonic EGF-responsive neural stem cells while decreasing the production of secondary neural stem cells
| Treatment | Neurogenesis: neurons/sphere | NSC expansion: secondary spheres/sphere |
|---|---|---|
| Control | 16 ± 5 | 292 ± 9 |
| EPO (6 hr) | 17 ± 3 | 290 ± 9 |
| EPO (24 hr) | 27 ± 33-160 | 247 ± 103-150 |
| EPO (7 d) | 25 ± 23-160 | 242 ± 123-150 |
Passage 1 cells were plated at a clonal density of 150 cells per milliliter per well (35 mm2) in the presence of EGF. After 6 d, 10 IU/ml of EPO was added to each well, or 1 ml of EGF media was added to controls. This media was then washed out after 6 or 24 hr and replaced with media containing EGF only. After a further 7 DIV (13 DIV in total), spheres (200 μm diameter) were examined for numbers of neurons and expansion as described in Materials and Methods. Counts represent the mean ± SEM number of neurons (144 spheres were assayed per experiment) or spheres generated (72 spheres were assayed per experiment) in three independent experiments.
F3-150: p < 0.05 versus control;
F3-160: p < 0.01 versus control.
EPO regulates neurogenesis by adult NSCs in vitroand in vivo
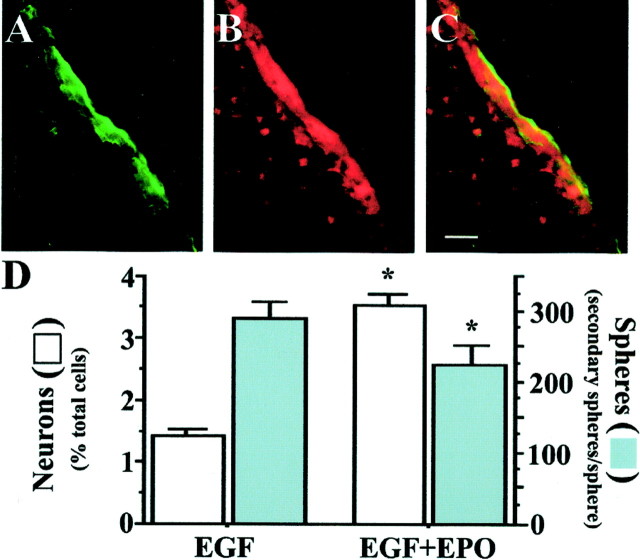
The ability of EPO to regulate the production of neurons by embryonic EGF-responsive NSCs prompted us to examine whether this regulation continues to adult NSCs and could play a role in vivo. We first sought to establish that EPO receptors were expressed in the adult subventricular zone, where EGF-responsive NSCs are localized. Indirect immunocytochemistry showed a colocalization of EGF and EPO receptors in the adult subventricular zone (Fig.4A–C). We then examined whether EPO would influence adult EGF-responsive NSCs in a manner similar to that seen with their embryonic counterparts. Adult EGF-responsive NSCs were cultured at clonal density in the absence or presence of 10 IU/ml of EPO. After 10 DIV, single spheres were examined for neuron numbers and secondary sphere formation (Fig.4D). As seen with embryonic EGF-responsive NSCs, the adult NSCs that had been exposed to EPO produced two- to threefold more neurons and 15–20% fewer secondary NSCs. These findings demonstrate that EPO receptors on adult NSCs influence the generation of neuronal progenitors and suggest that this process may be important in regulating intrinsic adult NSC function.
Fig. 4.
EPO regulates the relative production of neuronal progenitors and secondary neurospheres by adult NSCs.A–C, EGF-R (green) (A) and EPO-R (red) (B) were coexpressed in the subventricular zone of the adult forebrain, as shown by theorange–yellow staining in the merged image (C). Note the presence of EPO-R-positive cells in the striatum (B) that are not EGF-R positive (A), suggesting that colocalization is apparent only in the subventricular zone. Scale bar, 10 μm.D, EPO increased neuron production by adult EGF-responsive NSCs and decreased the production of secondary neurospheres. Adult neurospheres from the isolated and dissociated mouse lateral ventricles were generated at clonal density in EGF in the absence or presence of 10 IU/ml EPO. Individual spheres either were dissociated and the number of secondary spheres generated per sphere was assessed or plated on coverslips and the neuron number was assessed by β-tubulin staining. *p < 0.05 versus EGF alone.
To test the hypothesis that EPO may play a role in adult neurogenesis, we used intraventricular infusion of EPO and anti-EPO antibodies into the lateral ventricles of adult mice, followed by examination of NSC number and levels of neurogenesis (Fig. 5) (see Materials and Methods for details). The results are illustrated in Figure 6 and summarized in Table 4. A 6 d infusion of EPO dramatically influenced adult forebrain neurogenesis. When examined immediately or 24 hr after removal of the pumps, EPO infusion resulted in a 48% reduction in the total number of subsequently derived adult EGF-responsive NSCs and a 22% reduction in BrdU-labeled cells within the subventricular zone (Table 4). Intraventricular infusions and inherent damage at the infusion sites reduced NSC recovery to ∼75–80% of that obtained from uninfused animals; however, this would be the same for both control and EPO-infused animals. At the same time, EPO infusion induced a 77% increase in the numbers of Mash1-immunoreactive cells within the dorsolateral corner of the subventricular zone (Fig.6A,B; Table 4), indicative of enhanced neuronal precursors destined for the olfactory bulb. This was further confirmed by a 30% increase in BrdU-labeled cells in the rostral migratory stream (Fig. 6C,D; Table 4). To definitively determine whether EPO influences adult neurogenesis, we waited a further 6 weeks after pump removal and BrdU injection and then examined the numbers of newly generated TH-immunoreactive neurons in the periglomerular layer of the olfactory bulb. Animals that had received EPO infusions had a 30% greater number of newly generated interneurons than vehicle-treated animals (Fig.6E,F; Table 4). In a parallel series, we compared the effect of 6 d infusions of anti-EPO antibodies versus normal rabbit IgGs on NSC number and function (Table4). The infusion of anti-EPO antibodies resulted in a 22% increase in the number of adult EGF-responsive NSCs. Small increases in the numbers of BrdU-labeled cells in the subventricular zone were observed, but these did not reach statistical significance. Although no decrease in Mash1-labeled cells was observed in the dorsolateral corner of the subventricular zone, there was a 17% reduction in BrdU-labeled cells in the rostral migratory stream. These findings suggest that both exogenous and endogenous EPO regulates adult forebrain neurogenesisin vivo.
Fig. 6.
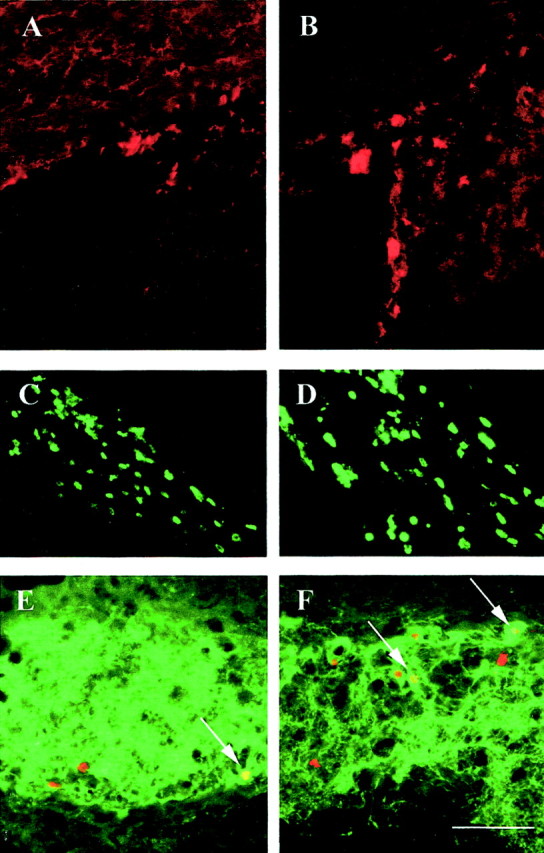
Characterization of newly generated cells after a 6 d EPO infusion. Representative photographs of tissue sections from the SVZ (A, B), RMS (C, D), or OB (E,F) from animals treated with vehicle (A, C, E) or EPO (B, D, F) are shown. EPO infusion increased the number of Mash1 (red)-expressing cells present in the SVZ (B) compared with vehicle controls (A). Also, an increase in the number of BrdU-positive cells was observed in the RMS of EPO-infused animals immediately after a 6 d infusion (D), when compared with vehicle control (C). Six weeks after the infusions, EPO induced an increased number of BrdU-labeled nuclei (red) in the TH-immunoreactive (green) periglomerular layer (F), in comparison with vehicle controls (E). Arrows indicate examples of double-labeled BrdU–TH neurons. Scale bar (shown inF): 50 μm.
Table 4.
Actions of exogenous EPO and anti-EPO antibodies on adult forebrain neural stem cell function in vivo
| Intraventricular infusion | ||||
|---|---|---|---|---|
| Vehicle | EPO | IgG | Anti-EPO | |
| Total forebrain spheres (NSCs) | 663 ± 96 | 340 ± 604-160 | 515 ± 29 | 630 ± 164-160 |
| BrdU cells in SVZ | 669 ± 30 | 524 ± 344-150 | 603 ± 39 | 699 ± 47 |
| Mash1 cells in SVZ | 66 ± 11 | 117 ± 124-150 | 77 ± 6 | 71 ± 6 |
| BrdU cells in RMS | 248 ± 25 | 321 ± 224-150 | 301 ± 29 | 249 ± 194-150 |
| TH/BrdU cells in OB | 319 ± 16 | 414 ± 124-150 | ND | ND |
Adult mice were infused with EPO (25 IU/d; n = 20), vehicle (n = 20), anti-EPO (3 μg/d;n = 10), or IgG (3 μg/d; n = 10) for 6 d as described in Materials and Methods. Data presented represent the mean ± SEM.
F4-150: p < 0.05 versus vehicle or IgG;
F4-160: p < 0.01 versus vehicle or IgG. SVZ, Subventricular zone; RMS, rostral migratory stream; OB, olfactory bulb. Abbreviations refer to regions counted as illustrated in Figure 5.
EPO actions on NSC-enhanced production of neuronal progenitors is mediated by NF-κB
Little is known about intracellular mechanisms that regulate the production of neuronal progenitors by multipotent NSCs. The ability of EPO to stimulate the production of neuronal progenitors by NSCs, and for this action to persist after a short application of the molecule, suggests the triggering of a novel neural genetic program. Although one might predict that the ultimate result of EPO actions involves the stimulation of pro-neural genes such as Mash1, an understanding of the manner in which these genes might be activated would be highly instructive. Guided by mechanistic studies of the sequelae of signals that direct enhanced erythropoiesis, we examined the putative signaling systems that might be used by EPO in regulating the production of neuronal progenitors. The signal transducer and transcriptional activator STAT5 is expressed in the developing forebrain (De-Fraja et al., 1998) and is phosphorylated in erythroid precursors in response to EPO (Penta and Sawyer, 1995). Thus, we examined STAT5 expression in the E14 germinal zone and its possible in vitro phosphorylation by EPO in cultures of NSCs. Dual labeling for the EPO receptor and STAT5 showed that both were expressed in the germinal zone of the E14 ganglionic eminence (Fig.7A–C). When cultured NSCs were exposed to EPO, STAT5 was phosphorylated within 5 min of stimulation (Fig. 7D). In parallel experiments, we found that STAT3 phosphorylation in spheres was unchanged after EPO stimulation (data not shown). Thus its expression pattern and in vitro phosphorylation support a role for STAT5 in the early transduction of EPO signals in NSCs.
Fig. 7.
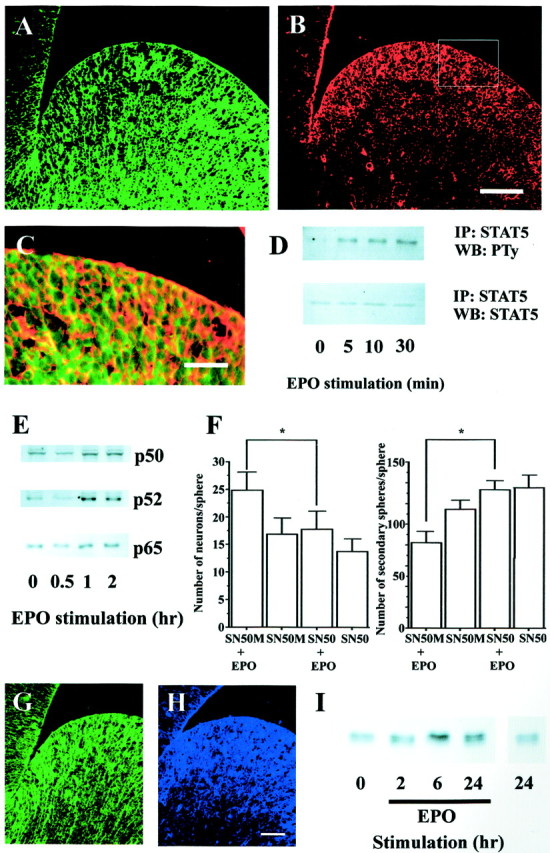
Putative signaling mechanisms for EPO actions on NSCs. A, B, EPO-R (A,red) and STAT5 (B, green) were coexpressed in the progenitor cell population of the E14 mouse ganglionic eminence, as shown by theorange–yellow staining in the merged image (C). The enlarged area in Cis that indicated by the rectangle in B. Scale bars: (shown in B for A,B), 100 μm; C, 50 μm. D, Passage 1 neurospheres from the E14 mouse ganglionic eminence were stimulated with 10 IU/ml EPO for the time periods indicated. Cellular extracts were immunoprecipitated (IP) with anti-STAT5, and immunoprecipitates were analyzed by Western blot (WB) with antiphosphotyrosine (PTy). Equalized protein loading was controlled for with anti-STAT5.E, Passage 1 neurospheres generated from the E14 mouse ganglionic eminence were stimulated with 10 IU/ml EPO for the time periods indicated. Nuclear extracts were analyzed by Western blotting with anti-NF-κB p50, p52, and p65 antibodies. F, Before EPO stimulation, passage 1 cells (clonal density) were treated with 10 μg/ml of SN50 or SN50M for 24 hr, and the media was then changed to EGF (with or without EPO)-containing media. Seven days after treatment, the spheres were dissociated for sphere-forming assays or processed to assess neuron number (*p < 0.05).G, H, EPO-R (G,green) and Mash1 (H, blue) were coexpressed in the progenitor cell population of the E14 mouse ganglionic eminence. Scale bar, 50 μm. I, Passage 1 neurospheres from the E14 mouse ganglionic eminence were stimulated with 10 IU/ml EPO for the time periods indicated, and WBs were used to analyze cellular extracts for Mash1 expression.
The transcription factors in the NF-κB family have been shown to be involved in proliferation, differentiation, and survival of many diverse cell types (Siebenlist et al., 1994; Baldwin, 1996; Ghosh et al., 1998). In erythropoiesis, NF-κB translocation to the nuclei of erythroid progenitors appears to play a role in supporting proliferation (Zhang et al., 1998). Thus, we asked whether NF-κB may be translocated to the nuclei of NSCs and whether this action could mediate EPO actions on the relative production of neuronal progenitors and secondary NSCs. NSC cultures were exposed to EPO for increased periods of time (30 min, 1 hr, and 2 hr), after which the cultures were harvested and the nuclei isolated and examined for expression of NF-κBs using Western blots (Fig. 7E). After 30 min of stimulation there was no significant increase in NF-κB nuclear localization. However, after 1 and 2 hr exposures to EPO, all three NF-κB isoforms examined (p50, p52, and p65) showed significant nuclear translocation, suggesting that these factors may play important roles in the upregulation of NSC neurogenesis and downregulation of secondary NSC production. To test this hypothesis, we examined the actions of a blocker of NF-κB translocation, the molecule SN50 (Lin et al., 1995) and its inactive congener SN50M, in EPO-stimulated NSC cultures. NSCs cultured at clonal density were exposed to EPO in the absence or presence of SN50 or SN50M for the 7 DIV growth of spheres. Resultant spheres were then examined for neuron number and the numbers of secondary spheres that they could generate. The results are shown in Figure 7F. Exposure of control (no EPO) growing spheres to either SN50 or SN50M did not change neuron number or the numbers of secondary spheres arising from individual primary spheres. However, the increase in neuron number and the decrease in secondary spheres in spheres that had been exposed to EPO were blocked by SN50 but not by SN50M. Thus, the translocation of NF-κB transcription factors by EPO is necessary for its action in regulating the relative production of neurons and secondary spheres by EGF-responsive NSCs.
Both in vitro and in vivo studies have implicated the bHLH transcription factor Mash1 in the regulation of neurogenesis, and the relative numbers of multipotent and restricted progenitors, in the developing ventral forebrain (Casarosa et al., 1999; Torii et al., 1999). The increase in Mash1-immunopositive progenitors in the dorsolateral corner of the adult subventricular zone (Fig.6A,B; Table 4), in response to EPO, further supports a role for this transcription factor in the production of neuronal progenitors. In the final series of experiments, we sought to directly demonstrate a role for EPO in regulating Mash1 expression. First, we confirmed the previous observation that Mash1 is localized to the embryonic germinal zone and found that this expression pattern is closely aligned with that of the EPO receptor (Fig.7G,H). We then asked whether Mash1 expression might be regulated by EPO. Cultures of NSCs were exposed to EPO for increasing periods of time and then harvested for Mash1 expression analysis using Western blot. EPO stimulation induced a time-dependent increase in Mash1 expression that was apparent at 2 hr and peaked after 6 or 24 hr of EPO exposure (Fig.7I). No increase in Mash1 expression was observed after 24 hr in the absence of EPO. These results suggest that EPO regulation of neurogenesis may be associated, in part, with the regulation of Mash1 expression in NSCs.
DISCUSSION
The results of this study suggest that EPO can function to regulate neurogenesis by multipotent NSCs and that it mediates thein vitro enhancement of neurogenesis resulting from hypoxia. Evidence for its endogenous release and action in the adult forebrain in vivo suggests that EPO serves in the regulation of neurogenesis in response to homeostatic requirements. The in vitro and in vivo regulation of neurogenesis by EPO suggests a potentially novel mechanism: the directed restriction of multipotent NSCs to become neuronal progenitors, mediated by the nuclear translocation of NF-κB and the upregulation of Mash1.
EPO regulates Mash1 expression and the number of neuronal progenitors generated by forebrain NSCs
EGF, fibroblast growth factor-2 (FGF-2), and insulin-like growth factor-1 (IGF-1) all appear to play critical roles in supporting NSC proliferation, whereas ciliary neurotrophic factor, bone morphogenetic protein-2, and platelet-derived neurotrophic factor appear to influence the relative production of neurons and astrocytes by NSCs and more restricted progenitors (for review, see Gage, 2000). We have previously found that FGF-2 (Vescovi et al., 1993) or retinoic acid (Wohl and Weiss, 1998) can enhance neurogenesis by restricted neuronal progenitors derived from EGF-responsive NSCs. In turn, we found that brain-derived neurotrophic factor and IGF-1 are able to promote the terminal differentiation of these newly generated, committed neuroblasts (Ahmed et al., 1995; Arsenijevic and Weiss, 1998; Shimazaki et al., 1999). On the other hand, virtually nothing is known about factors or mechanisms that directly regulate the production of neuronal progenitors by NSCs. Moreover, there are no reports of factors that regulate the relative generation of multipotent versus neuronal progenitors.
A principal finding of this study is that EPO induces a two-to threefold increase in neuronal output by EGF-responsive NSCs, and this correlated with a reduced number of secondary multipotent NSCs. Basic helix-loop-helix genes regulate the production of neurons in the ventral forebrain. Studies of null mutant mice demonstrate that in the ventral telencephalon, neuronal progenitor production appears to require the neurogenic gene Mash1 (Casarosa et al., 1999; Torii et al., 1999), which is repressed by the notch-regulated transcription factor Hes-1 (Nakamura et al., 2000). Those studies also suggest that this production of neuronal progenitors may involve the commitment or restriction of multipotent precursors to the neuronal lineage. However, the extracellular signaling molecules that may trigger this process have yet to be identified. The results of this study suggest that EPO may function to regulate the production of neuronal progenitors. In vitro, EPO instructs both embryonic and adult EGF-responsive NSCs to produce more neuronal progenitors while decreasing the numbers of secondary multipotent NSCs. This process is associated with an elevation in Mash1 protein expression in NSC cultures. In vivo, EPO receptors are expressed in the E14 ventricular zone, coincident with nestin and closely aligned with Mash1. When infused into the adult lateral ventricles, where EPO receptor expression is coincident with EGF receptor expression in cells of the subventricular zone, EPO decreases the numbers of NSCs and increases the numbers of Mash1-immunopositive neuronal progenitors, ultimately resulting in an increased number of neurons in the target structure, the olfactory bulb. Thus, it seems plausible to conclude that EPO receptor activation and increased Mash1 production induce the commitment or restriction of multipotent precursors to the neuronal lineage (discussed further below), and this process operates during EPO-enhanced neurogenesis in the adult forebrain.
EPO may be a homeostatic autocrine–paracrine signaling molecule for NSC neurogenesis with actions that are mediated by nuclear translocation of NF-κB
Recent evidence suggests that some autocrine–paracrine factors may also be important for neural stem cell proliferation. For example, the glycosylated form of cystatin C has been demonstrated to be an autocrine–paracrine cofactor for FGF-2 regulation of hippocampal NSC proliferation (Taupin et al., 2000). We have recently found that IGF-1 is an autocrine–paracrine factor for the regulation of EGF-responsive NSC proliferation (Arsenijevic et al., 2001), as it is for the differentiation of neuronal precursors (Arsenijevic and Weiss, 1998; Shimazaki et al., 1999). Although retinoic acid has been suggested to function as an autocrine–paracrine factor for early events in nervous system development (for review, seeMcCaffery and Drager, 2000), little is known about autocrine–paracrine factors that specifically regulate neurogenesis. The lowered O2-enhanced differentiation of dopaminergic neurons, from FGF-2-expanded neural precursors, was partially mimicked by EPO and partially reduced by an EPO antibody (Studer et al., 2000). That study did not determine whether EPO was specifically regulating proliferation or differentiation, or both. In the present study, in vitro and in vivo data support a role for EPO as an autocrine–paracrine regulator of NSC neurogenesis. Hypoxia-enhanced neurogenesis of embryonic NSCs was exactly mimicked by EPO and completely inhibited by an EPO-neutralizing antibody. Moreover, infusion of EPO-neutralizing antibodies in the lateral ventricles altered the relative numbers of NSCs and neuronal precursors in the rostral migratory stream, suggesting an autocrine–paracrine role for endogenous EPO in NSC adult neurogenesis in vivo. Taken together with recent studies that revealed active ephrin (Conover et al., 2000) and bone morphogenetic protein (Lim et al., 2000) signaling in the adult subventricular zone, our findings further support the role of endogenous local signals, both fixed and diffusible, in regulating adult NSC neurogenesis.
The signals and associated transduction mechanisms for the regulation of neuronal progenitor numbers have not been elucidated. EPO actions appear to involve specific signaling events. We found that EPO stimulated the rapid phosphorylation of STAT5 in NSCs. The coexpression of STAT5 with the EPO receptor, in the E14 basal forebrain germinal zone, coupled with reports of STAT5 mediation of interleukin-3-stimulated proliferation of ST14A (immortalized striatal precursors) cells (Cattaneo et al., 1996), supports a putative role for STAT5 in neurogenesis during development (De-Fraja et al., 1998;Cattaneo et al., 1999). STAT5 has also been found to mediate the cytokine-induced proliferation of microglia (Liva et al., 1999) and thus may serve as a general mediator of cytokine proliferation of neural cells. The results of this study found that EPO actions were mediated by the nuclear translocation of NF-κB, a well established regulator of immune and inflammatory responses (Ghosh et al., 1998), and implicated in anti-apoptotic and pro-proliferative aspects of oncogenesis (Baldwin, 2001). During development, NF-κB may mediate cytokine-induced neuronal survival (Middleton et al., 2000); however, its role in neurogenesis has not been examined. In addition to the finding that EPO activates the nuclear translocation of NF-κB, blockade of this process with SN50 prevents EPO-induced increased production of neurons and decreased production of secondary NSCs. By counting the numbers of TUNEL-labeled cells and finding no difference in control and EPO-treated spheres, we believe that the actions of EPO and NF-κB do not involve the regulation of neuronal progenitor survival. It is tempting to speculate that EPO stimulation of STAT5 phosphorylation, NF-κB translocation, and Mash1 gene expression are linked, given that their time courses are sequential. However, such links will require investigating whether STAT5 phosphorylation influences IκB proteins, the intrinsic suppressors of NF-κB translocation (Baldwin, 1996), and whether NF-κB directly regulates Mash1 transcription.
Does the apparent restriction by EPO of adult multipotent NSCs to a neuronal progenitor fate suggest that adult NSCs turn over?
Adult forebrain NSC neurogenesis in rodents appears to be primarily restricted to replenishing neurons of the olfactory bulb and dentate gyrus of the hippocampus, although recent evidence suggests that this replenishment may include other regions of the hippocampus and cerebral cortex (Magavi et al., 2000; Rietze et al., 2000). Although ongoing replacement of neurons that originate from the subventricular zone may occur continually in the adult primate cerebral cortex (Gould et al., 1999), it was only apparent in the adult rodent cerebral cortex after a discrete lesion (Magavi et al., 2000). Although various pathologic and physiologic stimuli appear to regulate neurogenesis in the adult dentate gyrus (for review, see McEwen, 1999;Gross, 2000; Kempermann and Gage, 2000), much less is known about the NSC lineage and its role in olfactory neurogenesis. EPO regulation of NSC-dependent neurogenesis to the olfactory bulb appears not to simply regulate neuron output; rather, it appears to regulate the relative numbers of NSCs and neuronal progenitors. In fact, taken together with our in vitro data, our findings suggest that EPO regulates the restriction of multipotent NSCs to the neuronal lineage. This is not unlike the presumed restriction of ventricular zone cells to neuronal precursors, which occurs during their ventral migration along radial glia during basal forebrain development. Thus our findings suggest that long distance neuronal production and migration to the olfactory bulb may use the same primary neurogenic processes of the embryonic basal forebrain. These parallels may not be so surprising, given that in the latter case some of the neuronal precursors that arise from the ganglionic eminences migrate a long distance dorsally to integrate into the developing cerebral cortex (Anderson et al., 1997; Tamamaki et al., 1997). What is surprising, however, is the implication that adult forebrain NSC numbers may be more plastic and variable than previously thought. This is further supported by our recent findings whereby a 6 d infusion of CNTF into the lateral ventricles induced an increase in NSC number, in parallel with the in vitro ability of CNTF to increase NSC numbers by preventing their differentiation toward a glial restricted lineage (Shimazaki et al., 2001). Although forebrain NSC numbers have been reported to be static throughout adulthood (Tropepe et al., 1997; Morshead et al., 1998), our findings suggest that this static nature may result, in fact, from a replenishment of NSC numbers. Such replenishment may occur through either symmetric division of EGF-responsive NSCs or, alternatively, their production from a more primitive NSC as proposed by Johansson et al. (1999). Ongoing NSC production and the restriction of NSCs to neuronal progenitors may be part of the natural process that underlies continued neurogenesis in the adult forebrain, and factors such as endogenous EPO may serve to regulate this process. Further understanding of this process may permit its utilization as a component of strategies for self-repair of the CNS after injury or disease.
Footnotes
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation of Canada. T.S. is the recipient of a Huntington's Society of Canada/CIHR Fellowship. S.T.S. was the recipient of a Clinical Fellowship from the Alberta Heritage Foundation for Medical Research (AHFMR). S.W. is an AHFMR Scientist. We thank Dorothea Livingstone and Joy Goldberg for excellent technical assistance, and Dr. Derek van der Kooy and members of the Weiss lab for critical review of an earlier version of this manuscript.
Correspondence should be addressed to Samuel Weiss, Genes & Development Research Group, Department of Cell Biology and Anatomy, University of Calgary, Faculty of Medicine, Calgary, Alberta, Canada T2N 4N1. E-mail: weiss@ucalgary.ca.
T. Shimazaki's present address: Department of Physiology, Keio University School of Medicine, 35 Shinanomachi, Shinjyuku-ku Tokyo, 160-8582 Japan.
REFERENCES
- 1.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 3.Arsenijevic Y, Weiss S. Insulin growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arsenijevic Y, Weiss S, Schneider B, Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci. 2001;21:7194–7202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin AS. The NFκB and IκB proteins: new discoveries and insight. Annu Rev Immunol. 1996;12:141–179. [Google Scholar]
- 6.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 8.Casarosa S, Fode C, Guillemot F. Mash-1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo E, De Fraja C, Conti L, Reinach B, Bolis L, Govoni S, Liboi E. Activation of the JAK/STAT pathway leads to proliferation of ST14A central nervous system progenitor cells. J Biol Chem. 1996;271:23374–23379. doi: 10.1074/jbc.271.38.23374. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo E, Conti L, De-Fraja C. Signaling through the JAK-STAT pathway in the developing brain. Trends Neurosci. 1999;22:365–369. doi: 10.1016/s0166-2236(98)01378-2. [DOI] [PubMed] [Google Scholar]
- 11.Chikuma M, Masuda S, Kobayashi T, Nagao M, Sasaki R. Tissue-specific regulation of erythropoietin production in the murine kidney, brain and uterus. Am J Physiol Endocrinol Metab. 2000;279:E1242–E1248. doi: 10.1152/ajpendo.2000.279.6.E1242. [DOI] [PubMed] [Google Scholar]
- 12.Conover JC, Doetsch F, Garcia-Verdugo J-M, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 13.De-Fraja C, Conti L, Magrassi L, Govoni S, Cattaneo E. Members of the JAK/STAT proteins are expressed and regulated during development in the mammalian forebrain. J Neurosci Res. 1998;54:320–330. doi: 10.1002/(SICI)1097-4547(19981101)54:3<320::AID-JNR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Digicaylioglu M, Bichet S, Marti HH, Wenger FH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, May M, Kopp E. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Reeves AJ, Graziano MSA, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 18.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 19.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 20.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998a;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998b;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 22.Juul SE, Yachnis AT, Rojiani AM, Christensen RD. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol. 1999;2:148–158. doi: 10.1007/s100249900103. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. [PubMed] [Google Scholar]
- 24.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 25.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Shen K, Liu Z, Noguchi CT. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–32400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZY, Chin K, Noguchi CT. Tissue specific expression of human erythropoietin receptor in transgenic mice. Dev Biol. 1994;166:159–169. doi: 10.1006/dbio.1994.1304. [DOI] [PubMed] [Google Scholar]
- 28.Liva SM, Kahn MA, Dopp JM, de Vellis J. Signal transduction pathways induced by GM-CSF in microglia: significance in the control of proliferation. Glia. 1999;26:344–352. doi: 10.1002/(sici)1098-1136(199906)26:4<344::aid-glia8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 30.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 31.Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 32.McCaffery P, Drager UC. Regulation of retinoic acid signaling in the embryonic nervous system: a master differentiation factor. Cytokine Growth Factor Rev. 2000;11:233–249. doi: 10.1016/s1359-6101(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 34.Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffray E, Hay RT, Davies AM. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J Cell Biol. 2000;148:325–332. doi: 10.1083/jcb.148.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 36.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 38.Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H. The bHLH gene Hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penta K, Sawyer ST. Erythropoietin induces the tyrosine phosphorylation, nuclear translocation, and DNA binding of STAT1 and STAT5 in erythroid cells. J Biol Chem. 1995;270:31282–31287. doi: 10.1074/jbc.270.52.31282. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rietze R, Poulin P, Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol. 2000;424:397–408. [PubMed] [Google Scholar]
- 45.Shimazaki T, Arsenijevic Y, Ryan AK, Rosenfeld MGR, Weiss S. A role for the POU-III transcription factor Brn-4 in the regulation of striatal neuron precursor differentiation. EMBO J. 1999;18:444–456. doi: 10.1093/emboj/18.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimazaki T, Shingo T, Weiss S. The CNTF/LIF/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 48.Studer L, Csete M, Lee S-H, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taupin P, Ray J, Fischer WH, Suhr ST, Hakansson K, Grubb A, Gage FH. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 51.Tokunaga K, Taniguchi H, Yoda K, Shimizu M, Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986;14:2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- 53.Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-a null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 55.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 56.Wohl CA, Weiss S. Retinoic acid enhances neuronal proliferation and astroglial differentiation in cultures of CNS stem cell-derived precursors. J Neurobiol. 1998;37:281–290. doi: 10.1002/(sici)1097-4695(19981105)37:2<281::aid-neu7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Youssoufian H, Longmore G, Neumann D, Yoshimura A, Lodish HF. Structure, function, and activation of the erythropoietin receptor. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 58.Zhang M-Y, Sun S-C, Bell L, Miller BA. NF-κB transcription factors are involved in normal erythropoiesis. Blood. 1998;91:4136–4144. [PubMed] [Google Scholar]