Abstract
Visual pathways to the amygdala, a brain structure critical for classical fear conditioning, were investigated. Conditioned fear was measured in rats as increased acoustic startle amplitude in the presence versus absence of a light or an odor paired previously with foot shock (fear-potentiated startle). Post-training lesions of both the lateral geniculate body (LG) and lateral posterior nucleus (LP) of the thalamus together, but not lesions of LG or LP alone, completely blocked the expression of fear-potentiated startle to a visual conditioned stimulus (CS) but not to an olfactory CS. These lesions also did not block contextual fear conditioning using startle or freezing as measures. Local infusion of 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f] quinoxaline-7-sulfonamide disodium, an AMPA antagonist, into the visual thalamus immediately before testing also blocked fear-potentiated startle to a visual CS, suggesting that the lesion effects were not attributable to damage of fibers of passage. Iontophoretic injections into the LP of the anterograde tracer biotinylated dextran amine resulted in heavy anterograde labeling in two amygdala–fugal cortical areas: area TE2 and dorsal perirhinal cortex (PR), and moderate labeling in the lateral amygdaloid nucleus (L). These results suggest that, during classical fear conditioning, a visual stimulus can be transmitted to the amygdala via either lemniscal (i.e., LG → V1, V2 → TE2/PR) or non-lemniscal (i.e., LP → V2, TE2/PR) thalamo-cortico-amygdala pathways, or direct thalamo-amygdala (i.e., LP → L) projections.
Keywords: fear, conditioning, amygdala, visual thalamus, perirhinal cortex, startle
Acquisition of conditioned fear depends on the association of a conditioned stimulus (CS) and an unconditioned stimulus (US). It is widely believed that CS and US information converges in the basolateral amygdala in which the association and possibly plasticity occurs (LeDoux et al., 1990;LeDoux, 1992, 2000; Romanski et al., 1993; Davis et al., 1994; Shi and Davis, 1999). The pathways through which acoustic CS information is transmitted to the amygdala have been studied extensively. Auditory inputs to the amygdala arise from both auditory thalamus and the auditory association cortex and terminate exclusively in the lateral amygdaloid nucleus (L) (LeDoux et al., 1990; Mascagni et al., 1993;Romanski and LeDoux, 1993b; Shi and Cassell, 1997; McDonald, 1998;Doron and LeDoux, 1999). Fear conditioning to a simple auditory CS can be mediated by either of these thalamo-amygdala or thalamo-cortico-amygdala pathways (Romanski and LeDoux, 1992b; Campeau and Davis, 1995b). In parallel, recent behavioral studies from our laboratory suggested that foot shock US information can be transmitted to the amygdala during fear conditioning via either thalamic or cortico-amygdala pathways (Shi and Davis, 1999). Although the amygdala is also critical for conditioned fear to a visual CS (LeDoux et al., 1989; Sananes and Davis, 1992; Campeau and Davis, 1995a), the brain structures and pathways by which a visual CS accesses the amygdala remain unknown.
In the rat, the superficial layers of superior colliculus (SC) are the major targets of retinal inputs (Linden and Perry, 1983), and these, in turn, project to the visual thalamus. However, studies from our laboratory found that lesions or chemical inactivation of the superficial layers of the SC, in which the retina projects, did not disrupt the expression of fear-potentiated startle to a visual CS (Tischler and Davis, 1983; Meloni and Davis, 1999). The dorsal lateral geniculate nucleus (LGD) and the lateral posterior nucleus (LP) of thalamus receive direct projections from the retina as well as from the SC, and these structures in turn project to retinotopically organized areas within the occipital cortex. However, post-training lesions of the occipital cortex did not prevent either the expression or extinction of conditioned fear responses using a visual CS (Tischler and Davis, 1983; LeDoux et al., 1989; Rosen et al., 1992; Falls and Davis, 1994), suggesting that subcortical and/or additional thalamo-cortical pathways must contribute visual information to the amygdala. Anatomic tract tracing studies in our laboratory (Shi and Davis, 1999) suggested that the LP might project directly and/or indirectly via the perirhinal cortex (PR) and caudal temporal cortex to the amygdala in rat. Thus, these thalamo-amygdala and thalamo-cortico-amygdaloid pathways could allow a visual CS to activate the amygdala during visual fear conditioning.
The present experiments were designed to assess whether the visual thalamus, in particular the LP, is crucial in fear conditioning using a visual CS and how this visual information is transmitted from LP to the amygdala. Rats were first trained to fear a light by pairing it with foot shock. Electrolytic lesions restricted to the LP, the dorsal lateral geniculate body (LG), or both structures together were performed after fear conditioning. Conditioned fear was measured using fear-potentiated startle (increased acoustic startle amplitude in the presence vs absence of the visual CS) (Brown et al., 1951; Davis and Astrachan, 1978). To evaluate modality specificity, the effects of these lesions on fear-potentiated startle using an odor CS was performed. To evaluate the possible contribution of damage to fibers of passage, the effects on fear-potentiated startle of reversible inactivation of the visual thalamus using pretest infusions of the AMPA receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX) were also assessed. Anterograde tract tracing with biotinylated dextran amine (BDA) was then used to identify the potential thalamo-cortical and/or thalamo-amygdala connections that may relay visual information to the amygdala.
MATERIALS AND METHODS
Subjects
Adult male albino Sprague Dawley rats (Charles River Laboratories, Portage, MI) weighing 350–400 gm were used. Animals for lesion or tract tracing studies were housed in groups of two or three in plastic cages (20 × 26 × 48 cm), and those for infusion studies were housed individually in wire cases (17 × 15 × 15 cm) with water and laboratory chow available ad libitum. They were maintained on a 12 hr light/dark cycle (lights on at 7:00 A.M.). Behavioral procedures occurred during the light period. Rats were acclimated to the colony rooms for 3 weeks before behavioral experiments.
Apparatus
Animals were trained and tested in stabilimeter devices that have been described previously (Cassella and Davis, 1986). Briefly, each stabilimeter consisted of an 8 × 15 × 15 cm Plexiglas and wire mesh cage suspended within a steel frame. The floor of each stabilimeter consisted of four 6.0-mm-diameter stainless steel bars spaced 18 mm apart through which shock could be delivered. Within the steel frame, the cage was compressed between four springs above and a 5 × 5 cm rubber cylinder below, with an accelerometer (model 2217E; PCB Piezotronics, Depew, NY) located between the cage and the rubber cylinder. Cage movement resulted in displacement of an accelerometer in which the resultant voltage was proportional to the velocity of the cage displacement. Startle amplitude was defined as the maximum accelerometer voltage that occurred during the first 0.2 sec after the startle stimulus was delivered. The analog output of the accelerometer was amplified (model 104; Endevco), digitized on a scale of 0–4096 units by a MacADIOS II board (GW Instruments, Somerville, MA) and stored on a Macintosh II microcomputer.
In the visual CS chamber, each of five stabilimeters was enclosed in a ventilated, light- and sound-attenuating box (68.5 × 35.5 × 42 cm). This inner isolation box was located within an additional outer ventilated plywood isolated box (76 × 47 × 51 cm). The five wooden boxes, in turn, were housed in a larger ventilated, light- and sound-attenuating chamber (2.5 × 2.5 × 2.0 m; Industrial Acoustic Co., Bronx, NY). A television camera (Ikegami, Maywood, NJ) for observing the behavior of the rats during the experiment was positioned behind the stabilimeter within the inner isolation box and was connected to a television monitor located outside of the chamber. A red light bulb (7.5 W) was located on the floor of the inner isolation box to provide illumination for the cameras in the otherwise dark box.
In the odor conditioning chamber, all training and testing occurred in two identical stabilimeter cages located within a sound attenuating chamber (inside dimensions of 56 × 56 × 81 cm; Industrial Acoustics Co.). The Industrial Acoustics box was modified so that the top and inner walls were lined with 6.3 mm Plexiglas. In addition, the fiberglass material in the ventilation unit was removed to avoid absorption of odors. The odor CS was delivered through an olfactometer (model E15-03; Coulbourne Instruments, Allentown, PA) mounted outside of the sound-attenuating chamber. It consisted of three solenoid valves with three independent input ports mixing to a common output port. The output was routed into the chamber with PharMed (Miami, FL) Tygon tubing (3.2 mm inner diameter × 6.3 mm outer diameter; Norton Plastics, Fisher Scientific, Houston, TX). Inside the chamber, the tubing was split with a “y” connector, and each end was attached to a 15 cm length of tubing that fit tightly onto a tube that protruded through an opening in the top of each cage. To deflect any direct airflow onto the animal's back, a 2.54 cm square metal shield bent in the form of a “V” was placed underneath the tube that delivered the air stream.
A compressed air tank (10 psi) was connected to a flowmeter (Fraser Harlake, Orchard Park, NY). From the flowmeter, the tubing was split with a “y” connector to two separate check values to prevent back flow. Tubing from each check valve was connected to activated carbon filter devices (Whatman Carbon-Cap 150; Fisher Scientific). Tubing from one filter was attached directly to one of the input ports of the olfactometer (“air” solenoid). Tubing from the second filter was fitted with a check valve, and tubing from the valve was attached to a brass inlet connector in the lid of the jar containing 20 ml of the olfactant (I-CHEM100, 100 ml glass jars with Teflon-lined polypropylene lids; VWR Scientific, West Chester, PA). The outlet connector in the lid was connected to a second flowmeter, which connected to a second input port of the olfactometer (“odor 1” solenoid). The inlet and outlet connectors were sealed in the lid with 100% silicone adhesive (Dow Corning, Midland, MI) to avoid any leakage.
After the rats were placed in the test cages, the air solenoid valve was opened to provide a clean air stream continuously during all training and testing sessions at a rate of 2.00 l/min to clear the tubing both before and after odor presentations. Between individual training–testing sessions, once the animals were removed from the cages, clean air was delivered through the tubing for an additional 5 min to further clear any residual odor. For the odor cue presentations, the odor 1 solenoid valve containing the olfactant was opened for 4 sec and blended into the air stream at a flow rate of 0.75 l/min. When the odor solenoid was opened, the clean airflow rate dropped to 1.25 l/min, making the final stimulus flow rate 2.00 l/min. Hence, the introduction of the odor did not lead to any net change in air flow.
A blower fan (McMaster-Carr, Atlanta, GA) provided a continuous influx of clean air into the chamber, and an exhaust fan (McMaster-Carr) continually exhausted air from the chamber to minimize any odor lingering between CS presentations. Air was exhausted through a 7.62-cm-diameter opening at a rate of 10.16 cubic feet per second as measured by a Kestrel 1000 wind meter (Nielsen-Kellerman, Chester, PA). The stabilimeter cages and chamber were cleaned daily after training–testing sessions with warm tap water and 95% alcohol and were air dried for at least 8 hr.
Stimuli
All sound level measurements were made with a Precision Sound Level Meter (A scale, model 2235; Brüel & Kjær, Norcross, GA). Background noise (0–20 kHz, 55 dB) was produced by a white-noise generator (model 15800; Lafayette, Lafayette, ID) and delivered through high-frequency speakers (Radio Shack Supertweeters; range, 5–40 kHz) located 2 cm from the front of each stabilimeter. This plus the noise of the ventilating fan attached to the sidewall of each wooden box produced an overall background noise level of 64 dB. The startle stimulus was a 50 msec burst of white noise (5 msec rise–decay time) of various intensities generated by a white-noise generator (Model 15800; Lafayette) and delivered through the same speakers as the background noise.
The visual CS was a 3.7 sec light produced by an 8 W fluorescent bulb (100 μsec rise time, 700 feet lamberts) attached to the back of each stabilimeter. The US was a 0.4–0.6 mA foot shock with a duration of 0.5 sec, generated by five LeHigh Valley (Beltsville, MD) constant-current shockers (model SGS-004) located outside of the chamber. Shock intensity was measured with a 1 kΩ resistor across a differential channel of an oscilloscope in series with a 100 kΩ resistor connected between adjacent floor bars within each stabilimeter. Current was defined as the root mean square voltage across the 1 kΩ resistor, in which milliamperes is 0.707 × 0.5 × peak-to-peak voltage.
The odor CS was created by the air stream passing through a 5% solution of amyl acetate (Sigma, St. Louis, MO) diluted in propylene glycol. The odor CS was freshly prepared each day before training–testing sessions. The presentation and sequencing of all stimuli were under the control of the Macintosh computer.
Behavioral procedures
Matching. On the first 2 d of all experiments, rats were placed in the startle cages, and baseline activity was sampled once every 10 sec for the first 5 min. An activity sample was defined as the peak accelerometer voltage that occurred during a 500 msec sampling period. Then, animals were presented with 30 startle stimuli at a 30 sec interstimulus interval (ISI). Intensities of 90, 95, and 105 dB were used with 10 startle stimuli at each intensity. Startle stimuli were presented in a balanced, irregular sequence with the restriction that each of the three intensities had to occur once in each trial block. The mean startle amplitude across the 30 startle stimuli on the last matching day was used to assign rats into sham or lesion groups, such that the mean startle level for each group before training was comparable.
Training. Rats were placed in the stabilimeter cages and, 5 min later, were presented with the first of 10 visual or five odor CS–shock pairings. The shock was delivered during the last 0.5 sec of the CS at an average intertrial interval of 4 min (range, 3–5 min).
Testing. Rats were placed in the same startle cages in which they were trained, and baseline activity was sampled once every 10 sec for the first 5 min. Then, for the visual CS, animals were presented with 18 startle-eliciting stimuli in the dark (six at each of three intensities: 90, 95, or 105 dB). These initial startle stimuli (hereafter called “leaders”) were used to evaluate fear-potentiated startle to the context compared with baseline startle during second day matching. Thirty seconds after the last leader stimulus, each animal received 60 startle stimuli (20 at each of three intensities: 90, 95, or 105 dB), with half of the stimuli presented alone (noise-alone trials) and the other half presented 3.2 sec after the onset of the 3.7 sec CS (light–noise trials). The six trial types were presented in a computer-generated randomized order with the restriction that each trial type had to occur once within each block of six trials. All startle stimuli were presented at a 30 sec ISI.
For testing with the odor CS, the animals were placed individually into the two test cages within the olfactory chamber. After a 5 min acclimation period, animals were presented with 30 95 dB startle stimuli at a 30 sec interstimulus interval. Thirty seconds after the last leader trial, the animals were presented with the first of 10 odor plus startle stimulus trials (odor–noise trials). On these test trials, the odor was delivered for 4 sec, and then the 50 msec startle stimulus was delivered 3.5 sec after the onset of the CS. After each odor–noise trial, three noise-alone trials were presented at a 30 sec intertrial interval so that noise-alone trials occurred 30, 60, and 90 sec after each odor.
Statistical analyses
Freezing to the context was defined as the mean activity before training minus the mean activity after training. Fear-potentiated startle to the context was defined as the mean startle amplitude of leaders during testing minus the mean startle amplitude (across the first 18 trials) during the second day of matching. Fear-potentiated startle to an explicit CS was defined as the difference in startle amplitude on the light–noise or odor–noise minus the noise-alone trials. ANOVAs were conducted using trial type (light–noise or odor–noise versus noise-alone) as a within-subjects factor and lesion or drug infusion as between-subjects factors. These analyses were complemented, when required, by t tests.
Surgery
Stereotaxic surgical procedures were performed under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). All coordinates were based on the rat atlas of Paxinos and Watson (1997).
Electrolytic lesions. Lesions were made with stainless steel electrodes (0.25 mm in diameter) insulated except for the 0.5 mm at the tip. A constant-current source generated DC anodal current for all electrolytic lesions at an intensity of 1.0 mA. Lesions of LG or LP were performed by making two lesions at different anteroposterior coordinates on each side of the brain. Combined lesions of LG and LP were produced by making four lesions at different anteroposterior coordinates on each side of the brain. Detailed coordinates for each lesion are shown in Table 1. The anteroposterior (AP) and lateromedial (LM) coordinates are relative to bregma; the dorsoventral (DV) coordinates are relative to the surface of the brain above the targets. For sham lesions, the electrode was lowered 1.0 mm above the dorsoventral lesion coordinate without passing current.
Table 1.
Coordinates of electrode placements for electrolytic lesions
| Lesion area | AP (mm) | LM (mm) | DV (mm) | Time (sec) |
|---|---|---|---|---|
| LG | −4.1 | ±4.0 | −4.5 | 10 |
| −4.8 | ±4.0 | −5.0 | 10 | |
| −3.8 | ±2.0 | −4.5 | 7 | |
| LP | −5.3 | ±2.8 | −4.5 | 7 |
| −3.8 | ±2.2 | −4.2 | 10 | |
| 3.8 | ±3.4 | −4.2 | 10 | |
| −4.8 | ±3.2 | −4.2 | 10 | |
| LG–LP | −4.8 | ±4.0 | −4.5 | 10 |
The AP and LM coordinates refer to bregma, and DV refers to the surface of the brain above the targets.
Cannulations. Rats were stereotaxically implanted with guide cannulas (22 gauge, 11 mm length; Plastics One Inc., Roanoke, VA) aimed bilaterally at the visual thalamus at the following coordinates: AP, −4.6 mm; ML, ±3.0 mm; and DV, −4.6 mm (to dura). The implanted cannulas were held in place using jewelers screws attached to the skull and a crown of dental acrylic. Cannula patency was maintained using internal stylets that protruded 1 mm beyond cannula tips.
Tract tracing. The anterograde tracer BDA (10,000 molecular weight; 10% in 0.1 m PBS; Molecular Probes, Eugene, OR) was iontophoretically injected via a glass micropipette (tip diameter of 20–35 μm) at various locations of the target areas. After placement of the micropipette, a constant positive current of 4 μA was applied for 5–10 min using a constant-current generator. The micropipette was then slowly removed, and the scalp incision was closed. Animals were allowed to survive for 4–7 d before perfusion.
Histology
For perfusions, animals were given an excessive dose of chloral hydrate and then exsanguinated with 100 ml of physiological saline and perfused through the ascending aorta with 500 ml of fixative containing 4% paraformaldehyde in 0.1 m PBS, pH 7.4, for 30 min. Brains were removed and stored in a solution of 30% sucrose in formalin for at least 2 d. Sections (50-μm-thick) were cut through lesion or cannulation sites on a frozen microtome and mounted on gelatin-coated slides. After drying, the slides were stained with cresyl violet, and the extent of the lesion or location of the cannula tip was evaluated under a microscope.
The brains with BDA injections were sectioned into 30-μm-thick coronal sections using a freezing microtome. Serial sections were collected and stored in cold PBS. BDA was visualized by incubating sections for 2 hr at room temperature in ABC reagent (Vector Laboratories, Burlingame CA) diluted in 0.1 m PBS (1:100) containing 0.3% Triton X-100, followed by a second incubation in biotinylated goat anti-avidin (Vector Laboratories) diluted in 0.1m PBS (1:200) containing 0.3% Triton X-100 for 2 hr. Sections were then reincubated in ABC for 1 hr. Between each incubation, sections were washed for 15 min in three changes of PBS. Peroxidase histochemistry was performed by reacting the sections for 10 min in a solution containing 0.05% diaminobenzidine and 0.01% hydrogen peroxide in 0.1 m phosphate buffer. Sections were mounted on chrome alum–gelatin-coated slides and mounted in Eukitt after dehydration and clearing. Alternate sections were counterstained with cresyl violet. The distribution of anterograde labeling in the cortex was analyzed using dark-field illumination. The position and density of labeled fibers and terminals were imaged with a digital camera (model DXC5000; Sony, Tokyo, Japan) of representative sections.
RESULTS
Effects of post-training lesions of LG and/or LP on expression of fear-potentiated startle to a visual CS
This experiment evaluated the effects of lesions of dorsal LG and/or LP on expression of fear-potentiated startle in which animals were lesioned after training. A total of 52 rats were matched into six groups of 8–10 rats each. On the following 2 d, all animals were conditioned by pairing the light with foot shock. One or 2 d after training, rats received electrolytic or sham lesions aimed at the LG alone, the LP alone, or both. One week after surgery, animals were tested for fear-potentiated startle.
Histology
Figure 1 shows histological reconstructions of representative cases with the LP or LG nucleus lesions. Figure 2 shows histological reconstructions depicting the smallest and largest LP–LG lesions included in the analysis. From the 10 rats that received bilateral electrolytic lesions aimed at LP, two were excluded because of incomplete damage to the LP. Because the dorsal part of the suprageniculate thalamic nucleus (SG) shares similar connectivity as mediocaudal LP (see Discussion), we considered this area as a part of LP and included it in the lesion sites. In the other rats (n = 8), there was also inconsistent damage to the adjacent posterior nucleus of thalamus, anterior pretectal nucleus, and dorsal portion of the medial geniculate body. However, no case had significant damage to LG. In the nine animals with intended LG lesions, six had a complete lesion of the LGD without significant damage to the adjacent LP and were included in the analyses. Most of these cases also had complete bilateral damage to the ventral lateral geniculate nucleus (LGV). In the combined lesion group, three animals had incomplete damage to the LGD and/or LP, and their data were thus excluded from the statistical analyses. In the animals with complete LG–LP damage (n = 6), there was also variable damage to the LGV, lateral dorsal, posterior, ventroposterior, peripeduncular, and anterior pretectal thalamic nuclei, as well as to the medial geniculate body.
Table 2.
Abbreviations list
| 3V | 3rd ventricle | LGD | dorsal lateral geniculate nucleus |
| APT | anterior pretectal nucleus | LGV | ventral lateral geniculate nucleus |
| ar | acoustic radiation | LP | lateral posterior nucleus |
| Astr | amygdalostriatal transition zone | LPLC | lateral posterior nucleus, laterocaudal subdivision |
| BL | basolateral amygdaloid nucleus | LPLR | lateral posterior nucleus, |
| BLA | basolateral amygdala complex | laterorostral subdivision | |
| bsc | brachium superior colliculus | LPMC | lateral posterior nucleus, |
| Ce | central amygdaloid nucleus | mediocaudal subdivision | |
| CM | central medial thalamic nucleus | LPMR | lateral posterior nucleus, |
| CP | caudate–putamen | mediorostral subdivision | |
| CRN | cochlea root neurons | MGD | dorsal medial geniculate nucleus |
| DpG | deep gray layer superior | MGM | medial geniculate nucleus |
| colliculus | MGV | ventral medial geniculate nucleus | |
| ER | entorhinal cortex | ml | medial leminscus |
| fr | fornix | opt | optic tract |
| Hb | habenular nucleus | OT | nucleus of optic tract |
| ic | internal capsule | PAG | periaqueductal gray |
| IGL | intergeniculate leaf | PF | parafascicular nucleus |
| InG | intermediate gray superior | PIL | posterior intralaminar thalamic |
| colliculus | nucleus | ||
| L | lateral amygdaloid nucleus | PLi | posterior limitans thalamic nucleus |
| LD | laterodorsal nucleus | PnC | pontine reticular nucleus, caudal |
| LG | lateral geniculate body | Po | posterior thalamic nucleus |
| PoT | posterior nucleus, triangular | SuG | superior colliculus, superficial |
| PP | peripeduncular nucleus | gray | |
| PR | perirhinal cortex | TE2/3 | temporal cortical areas |
| SC | superior colliculus | V1, V2 | primary and secondary visual |
| scp | superior cerebellar peduncle | cortices | |
| SG | suprageniculate nucleus | V2L | 2nd visual cortex, lateral |
| st | stria terminalis | V2M | 2nd visual cortex, medial |
| str | superior thalamic radiation | VPM | ventral posteromedial thalamic |
| SubG | subgeniculate nucleus | nucleus | |
| ZI | zona incerta |
Fig. 1.
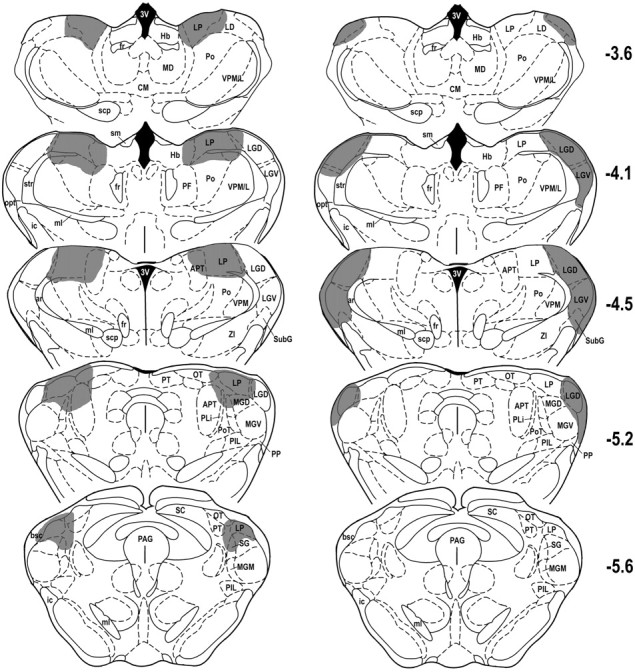
Histological reconstructions of representative cases with post-training lesions of lateral posterior (left column) and lateral geniculate (right column) thalamic nuclei on coronal plates from the atlas ofPaxinos and Watson (1997). Thenumbers to the right indicate rostrocaudal levels relative to bregma. Shaded areasindicate lesion sites. See Table 2 for abbreviations.
Fig. 2.
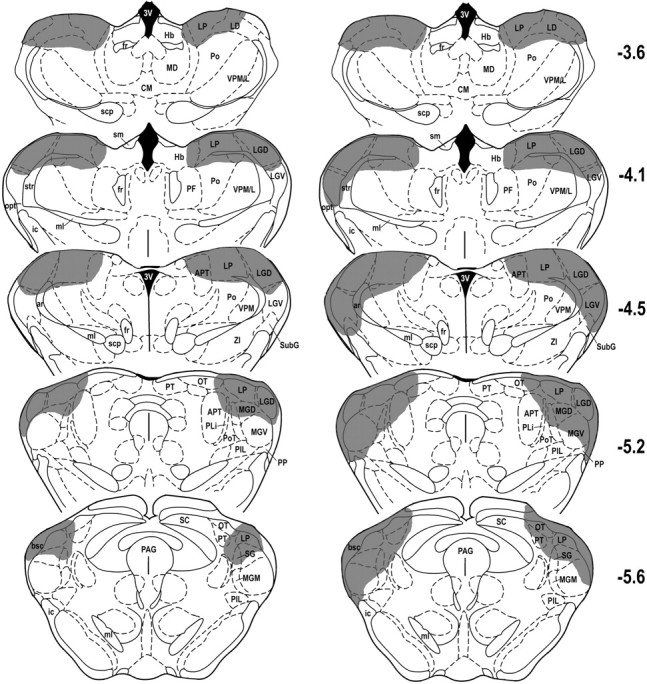
Histological reconstructions of the smallest (left column) and largest (right column) combined lesions of lateral geniculate and lateral posterior nuclei of thalamus transcribed on coronal plates from the atlas of Paxinos and Watson (1997). The numbers to the rightindicate rostrocaudal levels relative to bregma. Shaded areas indicate area lesion extent. See Table 2 for abbreviations.
Fear-potentiated startle
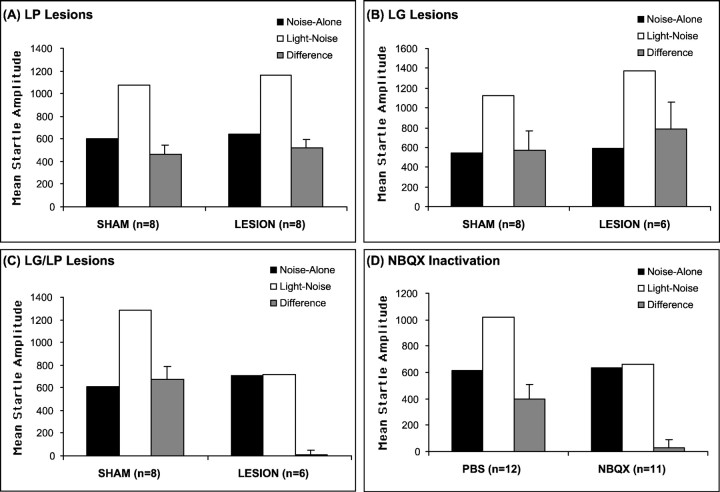
Figure 3A–C shows the mean startle amplitude on noise-alone and light–noise trials and the difference scores between these two trial types for animals with sham, LP, LG, or LG–LP lesions. Figure 3A shows that startle amplitudes during light–noise trials were significantly greater than startle amplitudes during noisealone trials in both sham (t(7) = 6.16; p < 0.01) and LP-lesioned (t(7) = 7.39;p < 0.01) animals, indicative of fear-potentiated startle to visual CS in both groups. A repeated-measures ANOVA found no treatment × trial type interaction (F(1,14) = 0.24; p > 0.05), indicating that the LP lesion did not significantly alter the magnitude of fear-potentiated startle. Figure 3B shows that the LG-lesioned groups also exhibited significant fear-potentiated startle to a visual CS (t(5) = 2.87;p < 0.05), as did the sham animals (t(7) = 3.24; p < 0.05), indicative of conditioned fear. There was no significant treatment × trial type interaction (F(1,12) = 0.38; p > 0.05), indicating equivalent levels of fear-potentiated startle in the sham versus lesioned groups.
Fig. 3.
Mean amplitude startle response on noise-alone trials (black bars) and light–noise trials (white bars) and the difference (+SEM) between these two trial types (gray bars) in sham-operated and lesioned animals (A–C) and in rats infused with PBS or NBQX in the chemical inactivation experiment (D). A, LP lesions;B, LG lesions; C, combined LG and LP lesions; D, chemical inactivation of visual thalamus with NBQX. The post-training lesions of LP or LG alone had no significant effect on fear-potentiated startle, but either combined post-training lesions of LG and LP or pretesting chemical inactivation of the visual thalamus completely blocked expression of fear-potentiated startle to a visual CS.
Figure 3C shows that, in the combined LG–LP-lesioned group, there was no increase in startle amplitude on the light–noise versus noise-alone trials (t(5) = 0.11;p > 0.05). In contrast, sham animals exhibited a significant increase in startle amplitude on the light–noise versus noise-alone trials (t(7) = 6.13;p < 0.01). A repeated-measures ANOVA showed a significant treatment condition × trial type interaction (F(1,12) = 25.42; p < 0.01) between the two groups. An ANOVA on the noise-alone scores showed no significant differences in startle amplitude (F(1,12) = 0.67; p > 0.05). These data indicate that post-training electrolytic lesions of the LG or LP alone had no effect on the expression of fear-potentiated startle, but combined lesions of both completely blocked the expression of fear-potentiated startle to a visual CS
Lesions of visual thalamus did not interrupt expression of fear-potentiated startle to either an olfactory or a context cue
To test that the above lesion effects were specifically interrupting transmission of visual information to the amygdala rather than a general failure of memory retrieval or failure of enhancement of startle, this experiment evaluated the expression of fear-potentiated startle using an olfactory cue as a CS. Conditioned fear to the context was also measured in the test chamber in which the animals trained and tested with the visual CS. A total of 16 rats were matched into two groups of eight rats each. After matching tests, animals were first conditioned by pairing the light with foot shock for 2 d. On the following day, all animals were conditioned by pairing an odor with foot shock in a novel chamber. One or 2 d after training, rats received electrolytic or sham lesions of visual thalamus. After recovery, animals were first tested for freezing to the context and fear-potentiated to the context as well fear-potentiated startle to the visual CS in the same chamber used for light–shock pairing training (see Materials and Methods). On the second day, animals were retested for fear-potentiated startle to the odor CS in the odor conditioning chamber.
Histology
From the eight rats that received bilateral electrolytic lesions aimed at LP and LG, three were excluded because of incomplete damage to the targets. In the other rats (n = 5), the lesion extent was similar to the combined lesion group described above. One of eight animals in the sham-lesioned group exhibited extremely low baseline startle after surgery, and its data were excluded from the statistical analyses.
Fear-potentiated startle
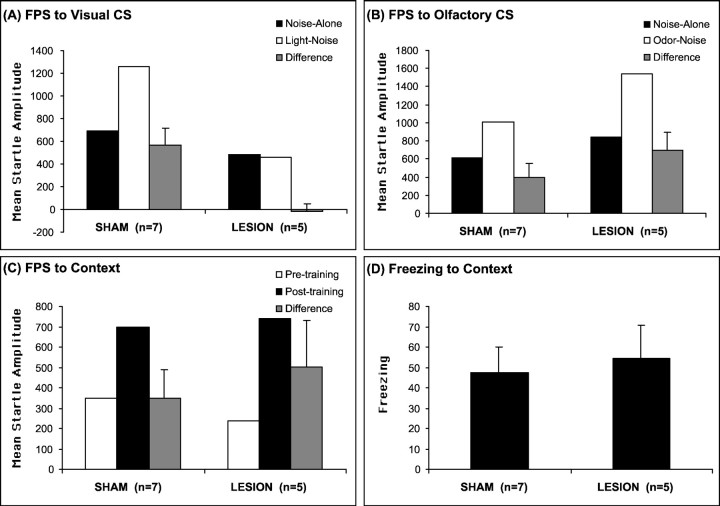
Lesions of visual thalamus totally blocked fear-potentiated startle to visual CS but not to the odor CS. Figure4, A and B, shows the mean startle amplitude on noise-alone and light–noise or odor–noise trials and the difference scores between these two trial types for animals with sham or LG–LP lesions. Consistent with the previous results, the visual thalamus-lesioned animals did not exhibit any increase in startle amplitude on the light–noise versus noise-alone trials (t(4) = 0.335;p > 0.05), whereas sham animals exhibited a significant increase in startle amplitude on the light–noise versus noise-alone trials (t(6) = 4.153;p < 0.01). A repeated-measures ANOVA showed a significant treatment condition × trial type interaction [F(1,10) = 11.782; p< 0.01) between the two groups. In contrast, both sham (t(6) = 2.634; p < 0.05) and lesion (t(4) = 2.527;p < 0.05) animals exhibited significant increases in startle amplitude on the odor–noise versus noise-alone trials. A repeated-measures ANOVA showed no significant treatment condition × trial type interaction (F(1,10) = 1.023; p > 0.05) between the two groups.
Fig. 4.
Fear-potentiated startle responses (FPS) to the visual (A), olfactory (B), and contextual (C) CSs, and freezing responses to the context (D) in sham-operated and visual thalamus-lesioned animals. Combined lesions of the LG and LP specifically blocked expression of fear-potentiated startle to the visual CS but had no effect on fear-potentiated startle to the odor CS, as well as startle or freezing to the context.
Furthermore, post-training lesions of visual thalamus had no effect on expression of conditioned fear responses to the training context using both fear-potentiated startle (Fig. 4C) and freezing (Fig.4D) as measures compared with shams. A repeated-measures ANOVA showed a significant overall difference of activity (F(1,10) = 31.43;p < 0.01) and startle amplitude (F(1,10) = 13.982; p< 0.01) between the pretraining and post-training trials, indicative of significant freezing and fear-potentiated startle to context CS. However, there was no treatment × trial type interaction (F(1,10) = 0.147 for activity;F(1,10) = 0.455 for startle;p > 0.05). These data indicate that post-training electrolytic lesions of the LG and LP had no effect on the expression of conditioned fear to either the olfactory cue or context CS, although it completely blocked the expression of fear-potentiated startle to a visual CS.
Pretesting infusion of NBQX into visual thalamus blocks expression of fear-potentiated startle to a visual cue
Because electrolytic lesions damage both neurons and passing fibers, this experiment reevaluated the role of dorsal lateral geniculate and lateral posterior nuclei on expression of fear-potentiated startle using local infusion of NBQX, an AMPA receptor antagonist, to temporarily block local glutamate transmission at AMPA receptors within these structures.
After cannula implantation, animals recovered for 1 week before being matched into two groups. On the next 2 d, the rats were given fear conditioning and tested for potentiated startle the next day. Immediately before behavioral testing, rats were infused with 6 μg of NBQX (dissolved in a 1 μl volume of PBS; Research Biochemicals, Natick, MA) or with PBS alone. Infusions (0.25 μl/min) were made through 28 gauge injection cannulas attached by polyethylene tubing to a Hamilton microsyringe. After the infusion was completed, the injection cannulas were left in place for 2 min before being withdrawn.
Histology
Ten of the 33 animals did not have bilateral cannulation of the visual thalamus or lost their head caps before testing, and their data were excluded from additional analyses.
Fear-potentiated startle
As shown in Figure 3D, startle amplitude during light–noise trials was significantly greater than startle amplitude during noise-alone trials for PBS-infused rats (t(11) = 3.86; p < 0.01). Infusion of NBQX into the visual thalamus had no effect on baseline startle (i.e., noise-alone trials) (F(1,21) = 0.02; p > 0.05) compared with PBS-infused rats but completely blocked fear-potentiated startle to a visual CS, as shown by the lack of difference between startle amplitude on the light–noise versus noise-alone trials after infusion of NBQX (t(10) = 0.47; p > 0.05), as well as a significant difference in fear-potentiated startle (i.e., difference scores) for PBS- versus NBQX-infused rats (t(21) = 3.04; p < 0.01). These data indicate that glutamate transmission via AMPA receptors in the visual thalamus is crucial for the expression of fear conditioning using a visual CS. Furthermore, these data suggest that the blockade of fear-potentiated startle by electrolytic lesions of the visual thalamus was not attributable to damage of the optic track and/or brachium of superior colliculus, which carry efferents from retina and SC to visual thalamus and the pretectum. Finally, these data strongly suggest that visual transmission within the visual thalamus is mediated by glutamate acting on AMPA receptors.
Anterograde tract tracing with BDA from LP
Because the LP proved to be critical for the expression of fear-potentiated startle using a visual CS and because the amygdala also is critical in this test (LeDoux et al., 1989; Sananes and Davis, 1992; Campeau and Davis, 1995a; Lee et al., 1996), we used the anterograde tracer BDA to map the pathways that connect the LP to the amygdala. Because the caudal boundary of LP with medial geniculate body is very difficult to determine on Nissl-stained coronal sections, we used the termination of superior colliculus afferents to define the extent of LP based on BDA injections into the superficial layers of superior colliculus in several additional animals.
Figure 5 shows a representative BDA injection (A) into the superior colliculus and the termination pattern in the visual thalamus (B–D). The injection was centered in the superficial layers in which retinal inputs are distributed. Heavy anterograde labeling was present in the midcaudal two-thirds of the LP, covering the most caudal part of laterorostral LP and the whole extent of its laterocaudal (LPLC) and mediocaudal (LPMC) subdivisions. In contrast, the most rostral LP exhibited only scattered labeled axon fibers and terminals. As shown in Figure 5D, very dense labeling in caudal LP extended ventromedially into the dorsal half of the SG, which is generally included in the auditory thalamus. Thus, at least this dorsal portion of SG, which was targeted by visual inputs from superior colliculus like the rest of the caudal LP, should be considered as a subdivision of the visual thalamus (LPSG). Finally, as expected, injections into superficial layers of the SC produced a dense and topographically organized distribution of anterograde labeling in LGD and LGV.
Fig. 5.
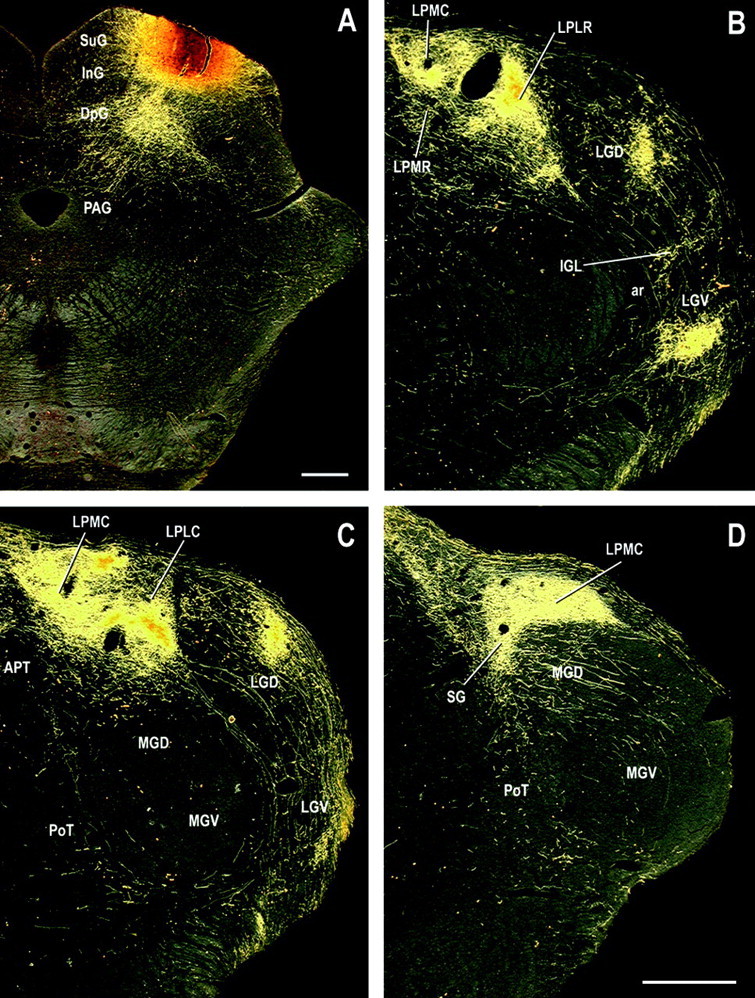
Digital dark-field photomicrographs of representative coronal sections through midcaudal levels of the visual thalamus showing the distribution of labeled tecto-thalamic fibers and terminals (B–D) after a BDA injection into the superficial layers of the superior colliculus (A). Note the continuity of labeling between the dorsal SG and LPMC in D. Scale bars, 200 μm. See Table 2 for abbreviations.
Injections of BDA into different subdivisions of LP produced topographically organized anterograde labeling in association with visual cortex and medial and lateral V2. Because the details of this visual cortical projection are not the focus of this study, they will be presented elsewhere. The animals with injections into medial and caudal LP exhibited heavy anterograde labeling in temporal cortical area TE2, dorsal PR, and the lateral nucleus of the amygdala. Two representative cases (3 and 47) are illustrated and described here.
In case 47, the injection site was located in the most caudomedial part of LP, involving the medial part of LPMC and adjacent LPSG (Fig.6A). This injection produced heavy anterograde labeling in dorsal PR (Fig.6B) and moderate labeling in lateral amygdaloid nucleus, amygdalostriatal transition zone, and posterior striatum (Fig.6C,D). The labeling in PR extended rostrally from ∼3.8 mm behind bregma to the caudal end of the rhinal sulcus, with a predominance in its rostral half. In coronal sections, terminal labeling in PR was densest in the middle layers (III and IV) and layer I. The layer I labeling of PR extended dorsally into TE3 but with much lower density and was restricted to the superficial portion. There was no labeling in the middle layers of TE3, indicating that the effective injection site did not invade dorsal medial geniculate body, which projects mainly to the middle laminas of TE3 (our unpublished observation). Subcortically, anterograde labeling was present in the L, the amygdalostriatal transition zone, and the caudal caudate–putamen. Labeling in the L was moderate in density and extended to its midcaudal two-thirds. In coronal sections, labeling was mostly distributed in the dorsolateral subdivision, with highest density in the ventromedial portion, similar to the projection pattern of visual association cortex area TE2. This contrasts with the termination pattern of acoustic inputs from auditory thalamus and cortex (area TE3), which mainly targets the most dorsal portion of the lateral nucleus (Romanski and LeDoux, 1993b; Shi and Cassell, 1997; Linke et al., 2000). Dense anterograde labeling was also present in area TE2, but no labeling was observed in the primary and secondary visual cortices V1 and V2.
Fig. 6.
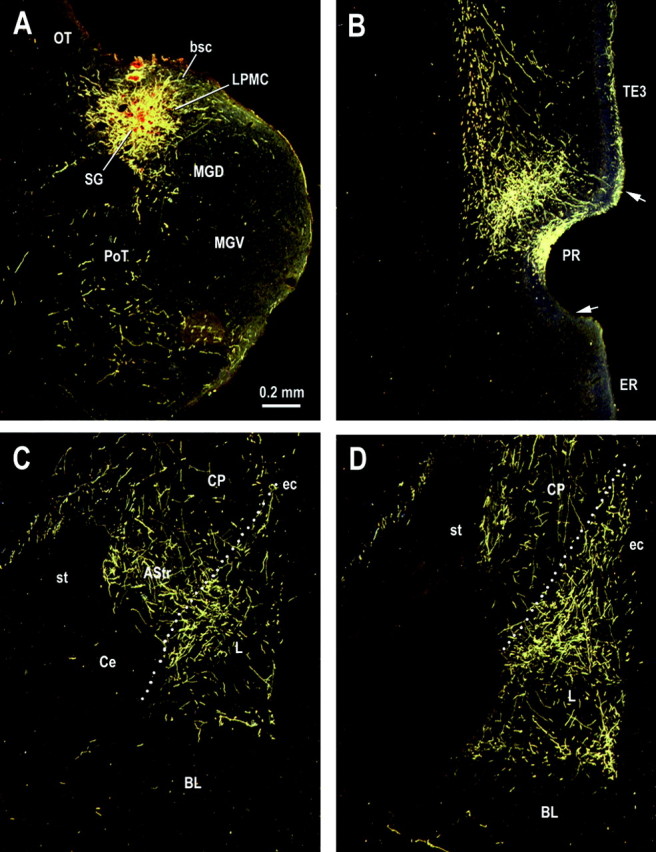
Digital photomicrographs showing the distribution of labeled axon fibers and terminals in representative coronal sections through rostral levels of the perirhinal cortex (B) and midcaudal levels (C,D) of the amygdaloid complex after a BDA injection into the mediocaudal part of LP (A). See Table 2 for abbreviations.
The other animal (case 3) had an injection also in the LPMC (Fig.7A) but relatively rostral. In contrast to the most caudal injections, this injection produced heavy anterograde labeling in the caudal part of lateral association visual cortex and area TE2 (Fig. 7C,D). The cortical labeling was primarily distributed in the middle lamina and superficial portions of the molecular layer. The dorsal PR (Fig. 7B–D) and the L exhibited light to moderate density of labeling, with a similar pattern as that of the last animal.
Fig. 7.
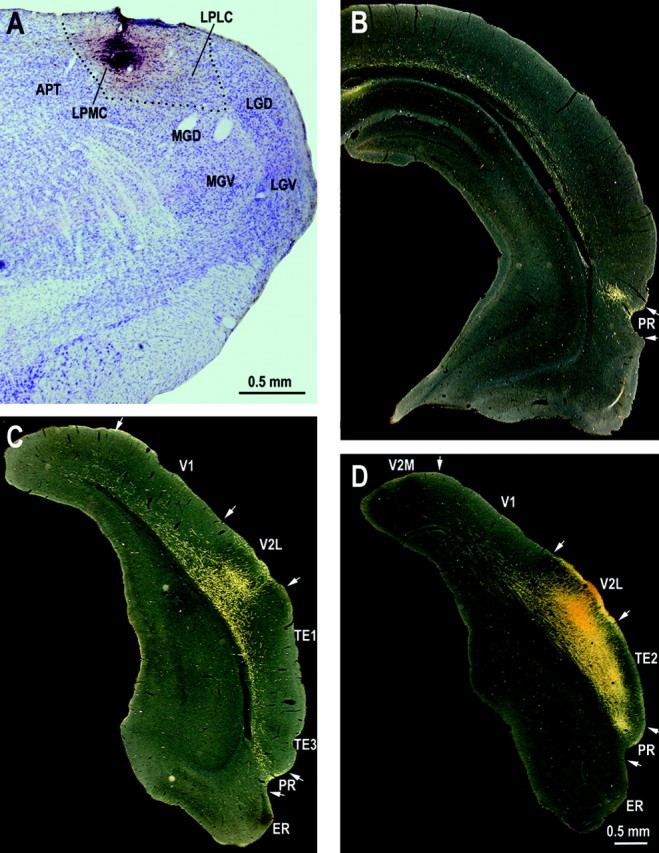
Digital bright-field photomicrograph showing a BDA injection site in the rostral part of LPMC (A) and dark-field photomicrographs showing distribution of labeled axon fibers and terminals in three representative levels of the temporal cortex. See Table 2 for abbreviations.
Thus, consistent with previous retrograde data, the present anterograde tracing data clearly show that the LP provides direct projections to the amygdala. This subcortical visual pathway exclusively targets the L. The results also show that the LP provides heavy thalamo-cortical projections to area TE2 and dorsal PR, both of which give rise to direct projections to the amygdala, specifically, the lateral amygdaloid nucleus.
DISCUSSION
The present studies investigated visual CS pathways to the amygdala during the expression of conditioned fear. The lesion experiments demonstrated that bilateral electrolytic lesions of both LGD and LP of the thalamus applied after training completely blocked the expression of fear-potentiated startle to a visual CS but not to an olfactory CS or to context cues. In contrast, bilateral lesions of either structure alone had no effect on the expression of fear-potentiated startle. Furthermore, local infusion of NBQX into visual thalamus immediately before testing prevented expression of conditioned fear to a visual CS. Discrete injections of the anterograde tracer BDA into the LP revealed direct thalamo-amygdala pathway and indirect thalamo-cortical connections via dorsal PR and caudal temporal cortex area TE2. Considered with previous studies, the present results suggest that the LP transmits visual CS to the basolateral amygdala via both cortical (LP–TE2/PR–amygdala) and subcortical (LP–amygdala) pathways.
Direct and indirect visual thalamo-amygdala connectivity
As in other sensory modalities, there is parallel processing of information reaching the rat's visual cortex. One main (lemniscal) pathway is via the LGD to the V1 and then from V1 to the V2, whereas the other (non-lemniscal) pathway is via the LP to the association visual cortex. In rodents, V1 and V2 provide no direct projections to the amygdala. However, area TE2, which receives afferents from both V1 and V2 (Miller and Vogt, 1984; Vaudano et al., 1991; Coogan and Burkhalter, 1993; McDonald, 1998), provides substantial innervation to the amygdala (Mascagni et al., 1993; Shi and Cassell, 1997). Besides connecting with V1 and V2, TE2 also has direct reciprocal connections with the LP. Early anatomic studies found that infusion of retrograde tracers into an area lateral to caudal peristriate cortex retrogradely labeled a group of neurons in the caudal portion of LP (Coleman and Clerici, 1980; Mason and Gross, 1981; Scheel, 1988; Vaudano et al., 1991). In the present study, injections of the anterograde tracer BDA into the caudal LP, the tecto-recipient zone that receives bilateral superior collicular projections (Mason and Gross, 1981; present study), led to heavy axon and terminal labeling in area TE2. Thus, visual information can be transmitted to the basolateral amygdala via either LP–TE2 or LGD/LP–V1/V2–TE2 pathways.
The PR is generally considered as a multimodal cortex, in which different modalities of sensory information converge via cortico-cortical connections, which provide major projections to the amygdala and hippocampus (Deacon et al., 1983; Mascagni et al., 1993;Romanski and LeDoux, 1993b; Shi and Cassell, 1997, 1999). Recent anatomic studies show that injections of retrograde tracers into ventral temporal cortex, including the PR, consistently labeled a group of cells in LP, in addition to labeling in auditory thalamus (Namura et al., 1997; Doron and LeDoux, 1999). Here we demonstrated that the cortical projections of LP go specifically to the dorsal part of perirhinal cortex, but very sparse or no projections go to adjacent auditory cortex or the ventral bank of the rhinal sulcus. Consistent with retrograde tracing findings (Doron and LeDoux, 1999), anterograde labeling was seen in the PR after injections into medial and caudal LP but not in cases with injections into rostrolateral part of LP. The heaviest labeling was seen in cases with injections into caudomedial LP, the area that also sends efferents to the amygdala (see below). Therefore, by receiving visual inputs from both visual thalamus (LP) and cortex (V2 and TE2), the PR is another potential visual transmission route to amygdala.
The present anatomic data also confirmed a previous conclusion from retrograde tract tracing studies that there might be a direct visual thalamo-amygdala pathway (LeDoux et al., 1990a; Shi and Davis, 1996;Doron and LeDoux, 1999; Linke et al., 1999). Injections of BDA into caudal and medial LP produced a moderate density of axon and terminal labeling in the L and amygdalostriatal transition zone, in addition to cortical labeling in TE2 and PR. Besides LP, the dorsal part of SG is the other extrageniculate target of tecto-thalamic pathways and also projects to the amygdala (Doron and LeDoux, 1999; Linke et al., 1999;Linke et al., 2000). As shown in Figure 5D, the dense labeling from superficial layers of superior colliculus in dorsal SG is inseparable with that in LP, indicating homogeneity of the two areas. Electrophysiological recording studies have also found that neurons in SG and LP have similar properties. Both have large receptive fields and respond very reliably to fast moving visual stimuli (Hicks et al., 1984; Hutchins and Updyke, 1989; Casanova and Molotchnikoff, 1990). Furthermore, the present data show that the dorsal SG provides projections to the PR and the L, as does the adjacent LP. Thus, we suggest that this dorsal part of SG should be considered as a part of LP (SG subdivision of LP), in contrast to the rest of SG, which is a part of auditory thalamus.
Together, the suprageniculate and mediocaudal subdivisions of LP provide a subcortical visual pathway to the basolateral amygdala, in parallel with cortical visual pathways arising from the area TE2 and dorsal PR.
Cortical versus subcortical visual CS pathways in fear conditioning
Previous anatomic and behavior studies have suggested that a foot shock US and an auditory CS can be relayed to the basolateral amygdala via both subcortical and cortical pathways (see introductory remarks). The present anatomic data indicate that this scheme of parallel subcortical and cortical pathways seems also to apply to visual input to the amygdala. Thus, visual information may be relayed to the basolateral amygdala via either thalamo-amygdala or thalamo-cortico-amygdala pathways (see above).
However, conclusions about the participation or nonparticipation of cortical versus subcortical routes may depend on how the experiments are performed. We believe that a disruption of conditioned fear produced by post-training lesions of a sensory pathway(s) indicates that this pathway(s) is normally used to mediate conditioned fear responses. However, if this pathway is damaged (e.g., by pretraining lesions), then other pathways may take over (Maren et al., 1996, 1997; Maren, 1999). Thus, the use of pretraining lesions or post-training lesions followed by retraining may identify pathways that can mediate fear conditioning, even if these pathways are not normally used.
In the present study, post-training lesions restricted to the LP, which gives rise to subcortical visual inputs to the amygdala, did not block the expression of fear conditioning using a visual CS. In addition, post-training lesions of LG, which projects to both primary and secondary visual cortices, did not block fear-potentiated startle using a visual CS. Because LP also projects to visual association cortices, this thalamo-cortical route might be expected to mediate fear conditioning using a visual CS. However, previous studies showed that extensive post-training visual cortical lesions also failed to block fear conditioning using a visual CS (Rosen et al., 1992; Falls and Davis, 1994). Thus, these data suggest that neither the direct subcortical thalamo-amygdala pathway from LP nor the LG and/or the LP to V1/V2 pathways appear to be critical in mediating fear conditioning using a visual CS in intact animals.
However, both TE2 and PR receive visual inputs from LP (present study) and visual cortices and in turn project to the amygdala. Thus, PR, together with the dorsally adjacent area TE2, represent an area of convergence of thalamo-cortical and cortico-cortical visual inputs that may provide information of visual CS to the amygdala during classical fear conditioning. In fact, previous studies have shown that post-training chemical lesions of the temporal cortex, including PR and area TE2 and TE3, totally blocked the expression of conditioned fear using a visual CS (Campeau and Davis, 1995a). Consistent with this, the present study showed that combined lesions of both LG and LP, which would cutoff both thalamic and cortical routes to TE2 and PR, also totally blocked the expression of conditioned fear using a visual CS. Local infusion of the glutamate antagonist NBQX had the same effect, suggesting that the lesion effect did not result from damage to fibers of passage. Furthermore, the lesioned animals exhibited a deficit of fear-potentiated startle specifically to the visual CS but not to an olfactory or context CS, indicating that this lesion effect was not attributed to a general failure of memory retrieval or to a deficit on enhancement of startle but by interrupting sensory transmission of visual information to the amygdala. Based on these and other results, we conclude that visual input carried by projections from LG and LP via connections through TE2 and PR to the amygdala normally are used in conditioned fear using a visual CS.
In parallel, the PR also receives potential auditory inputs from extraleminscal thalamic auditory areas (dorsal medial and medial medial geniculate nuclei) (Namura et al., 1997; Doron and LeDoux, 1999) and from the dorsally located temporal neocortical area (TE3) (Deacon et al., 1983; Mascagni et al., 1993; Romanski and LeDoux, 1993b; Shi and Cassell, 1997). Moreover, post-training lesions of PR and TE3 also block the expression of conditioned fear using an auditory CS (Campeau and Davis, 1995b). Together, these data suggest that thalamo-cortical and cortico-cortical pathways to TE2/TE3 and/or PR are the primary visual and auditory transmission routes to amygdala in emotional learning. However, at present, it cannot be determined whether both TE2/TE3 and PR or PR alone is critically involved.
The alternate interpretation of the present data are that subcortical and cortical pathways are equivalently involved in transmitting a simple light CS to the amygdala, as suggested by others (Romanski and LeDoux, 1992b; Campeau and Davis, 1995b), and the blockade of conditioned fear by post-training lesion of temporal cortex (i.e., TE2, TE3, and PR) was attributable to a failure of memory retrieval. However, the later hypothesis is not supported by available data. First, the lack of an effect of pretraining lesions of the same cortical area in previous studies is not consistent with a primary role of the PR in memory retrieval (Campeau and Davis, 1995b), although admittedly other areas might subsume this function after damage to PR. Second, the post-training lesions of PR interrupt conditioned fear only to visual and auditory CSs but not to contextual cues (Phillips and LeDoux, 1995), suggesting a specific involvement in sensory transmission rather than a general effect on learning and memory.
Previous studies by Rosen et al. (1992) found that electrolytic lesions of so-called “rostral perirhinal cortex” (from ∼1.8 to 3.8 mm posterior to bregma) totally blocked fear-potentiated startle to a visual CS, suggesting that it was part of a visual CS pathway. However, recent anatomical studies found that this portion of rhinal cortex has reciprocal connections with somatosensory cortex and thalamus rather than visual or auditory system and redefined it as a part of insular cortex, the so-called “parietal insula” (Shi and Cassell, 1997,1998a,b). Our behavioral studies indicated that the parietal insular cortex appears to be part of a foot shock US pathway rather than a visual or auditory CS pathway (Shi and Davis, 1999). Consistent with this, the present tracing data also show that the visual thalamus, specifically LP, does not project to this part of insular cortex but to the dorsal PR caudal to it. Interestingly, post-training lesions of parietal insular cortex [referred as the rostral perirhinal cortex byRosen et al. (1992)] appear to disrupt fear conditioning not only to a visual stimulus but to auditory (Campeau and Davis, 1995b) and contextual (Corodimas and LeDoux, 1995) cues as well. So it has been suggested that this parietal insula may be involved in some general learning process, such as memory storage or retrieval (Campeau and Davis, 1995b; Corodimas and LeDoux, 1995; Gewirtz and Davis, 2000).
The fact that unit conditioned responses to an auditory CS in the lateral nucleus of the amygdala occur with very short latencies in normal, unlesioned rats (e.g., <15 msec) has been taken as evidence that the direct thalamo-amygdala connection must normally mediate conditioned fear (Quirk et al., 1995). Once again, it is difficult to reconcile this interpretation with the fact that post-training lesions of direct thalamo-amygdala pathways fail to block the expression of conditioned fear. However, direct thalamo–PR–amygdala transmission, bypassing the primary auditory cortex, might well support these very short latency responses recorded in the amygdala, so that these unit data may be fully compatible with the lesion data.
Although we believe that the subcortical visual and auditory pathways are not primary routes whereby CS information is transmitted to the amygdala during fear conditioning, it is clear they can be recruited when the cortical CS pathways are not available during training. Thus, pretraining lesions of cortical auditory and visual pathways did not prevent the development of conditioned fear to an auditory CS (DiCara et al., 1970; Romanski and LeDoux, 1992a,b; Campeau and Davis, 1995b), and animals could relearn conditioned fear using either an auditory or visual CS after cortical lesions (Campeau and Davis, 1995b). Both sets of data indicate that the subcortical pathways are sufficient for transmitting CS information to the amygdala after damage to the cortical pathways. On the other hand, combined lesions of cortical and subcortical auditory pathways, made before training, totally block fear conditioning to an auditory CS (Romanski and LeDoux, 1992b).
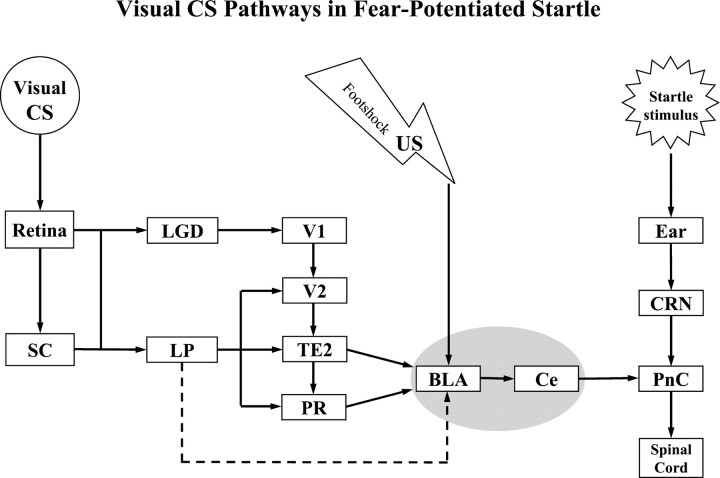
Overall, the present studies suggest that visual information necessary for the expression of conditioned fear can be transmitted to the amygdala via either lemniscal (i.e., LG → V1/V2 → TE2/PR) or non-lemniscal (i.e., LP → V2, TE2/PR) thalamo-cortico-amygdala pathways or direct thalamo-amygdala (i.e., LP → L) projections during classic fear conditioning (Fig. 8). In normal, unlesioned animals, the cortico-amygdala pathways may normally relay visual CS information to the amygdala. However, subcortical pathways may be important when these cortical pathways are damaged.
Fig. 8.
Schematic diagram summarizing thalamo-cortico-amygdala and thalamo-amygdala visual pathways involved in fear-potentiated startle. The pathway indicated by the dashed line may not be critical in normal visual fear conditioning paradigm. See Table 2 for abbreviations.
Footnotes
This work was supported by National Institute of Mental Health Grants MH-57250, MH-58922, MH-52384, MH 59906, and MH-47840, the Woodruff Foundation, and The Center for Behavioral Neuroscience of the National Science Foundation under Agreement Number IBN-9876754. We are grateful to Dr. David Walker for reading and commenting on this manuscript and Dr. Gayla Paschall for her significant contribution involving running the odor conditioning and testing experiment.
Correspondence should be addressed to Dr. Changjun Shi, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 1636 Pierce Drive, Suite 4000, Atlanta, GA 30322. E-mail:cshi@emory.edu.
REFERENCES
- 1.Brown JS, Kalish HI, Farber IE. Conditional fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol. 1951;41:317–328. doi: 10.1037/h0060166. [DOI] [PubMed] [Google Scholar]
- 2.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995a;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995b;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casanova C, Molotchnikoff S. Influence of the superior colliculus on visual responses of cells in the rabbit's lateral posterior nucleus. Exp Brain Res. 1990;80:387–396. doi: 10.1007/BF00228166. [DOI] [PubMed] [Google Scholar]
- 5.Cassella JV, Davis M. The design and calibration of a startle measurement system. Physiol Behav. 1986;36:377–383. doi: 10.1016/0031-9384(86)90032-6. [DOI] [PubMed] [Google Scholar]
- 6.Coleman J, Clerici WJ. Extrastriate projections from thalamus to posterior occipital-temporal cortex in the rat. Brain Res. 1980;194:205–209. doi: 10.1016/0006-8993(80)91329-3. [DOI] [PubMed] [Google Scholar]
- 7.Coogan TA, Burkhalter A. Hierarchical organization of areas in rat visual cortex. J Neurosci. 1993;13:3749–3772. doi: 10.1523/JNEUROSCI.13-09-03749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corodimas K, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behav Neurosci. 1995;109:613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- 9.Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- 10.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 11.Deacon TW, Eichenbaum H, Rosenberg P, Ecknamm KW. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983;229:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- 12.DiCara LV, Braun JJ, Pappas BA. Classical conditioning and instrumental learning of cardiac and gastrointestinal responses following removal of neocortex in the rat. J Comp Physiol Psychol. 1970;73:208–216. doi: 10.1037/h0030200. [DOI] [PubMed] [Google Scholar]
- 13. Doron NN, LeDoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol 412 1999. 383 409[Erratum (2000) 417:385–386]. [PubMed] [Google Scholar]
- 14.Falls WF, Davis M. Visual cortex ablations do not prevent extinction of fear-potentiated startle using a visual conditioned stimulus. Behav Neural Biol. 1994;60:259–270. doi: 10.1016/0163-1047(93)90504-b. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz JC, Davis M. Using pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn Mem. 2000;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- 16.Hicks TP, Watanabe S, Miyake A, Shoumura K. Organization and properties of visually responsive neurons in the suprageniculate nucleus of the cat. Exp Brain Res. 1984;55:359–367. doi: 10.1007/BF00237286. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins B, Updyke BV. Retinotopic organization within the lateral posterior complex of the cat. J Comp Neurol. 1989;285:350–398. doi: 10.1002/cne.902850306. [DOI] [PubMed] [Google Scholar]
- 18.LeDoux J. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 19.LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, editor. The amygdala: a functional analysis, Ed 2. Oxford UP; New York: 2000. pp. 289–310. [Google Scholar]
- 20.LeDoux JE, Romanski L, Xagoraris A. Indelibility of subcortical memories. J Cognit Neurosci. 1989;1:238–243. doi: 10.1162/jocn.1989.1.3.238. [DOI] [PubMed] [Google Scholar]
- 21.LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990a;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990b;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y, Walker D, Davis M. Lack of a temporal gradient of retrograde amnesia following NMDA-induced lesions of the basolateral amygdala assessed with the fear-potentiated paradigm. Behav Neurosci. 1996;110:836–839. doi: 10.1037//0735-7044.110.4.836. [DOI] [PubMed] [Google Scholar]
- 24.Linden R, Perry VH. Retrograde and anterograde-transneuronal degeneration in the parabigeminal nucleus following tectal lesions in developing rats. J Comp Neurol. 1983;218:270–281. doi: 10.1002/cne.902180304. [DOI] [PubMed] [Google Scholar]
- 25.Linke R, De Lima AD, Schwegler H, Pape HC. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 1999;403:158–170. [PubMed] [Google Scholar]
- 26.Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res. 2000;134:520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- 27.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditioned fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 29.Maren S, Aharanov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 30.Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 31.Mason R, Gross GA. Cortico-recipient and tecto-recipient visual zones in the rat's lateral posterior (pulvinar) nucleus: an anatomical study. Neurosci Lett. 1981;25:107–122. doi: 10.1016/0304-3940(81)90316-5. [DOI] [PubMed] [Google Scholar]
- 32.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 33.Meloni EG, Davis M. Muscimol in the deep layers of the superior colliculus/mesencephalic reticular formation blocks expression but not acquisition of fear-potentiated startle in rats. Behav Neurosci. 1999;113:1152–1160. doi: 10.1037//0735-7044.113.6.1152. [DOI] [PubMed] [Google Scholar]
- 34.Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 1984;226:184–202. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- 35.Namura S, Takada M, Kikuchi H, Mizuno N. Collateral projections of single neurons in the posterior thalamic region to both the temporal cortex and the amygdala: a fluorescent retrograde double-labeling study in the rat. J Comp Neurol. 1997;384:59–70. [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 3. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 37.Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 39.Romanski LM, LeDoux JE. Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neurosci Lett. 1992a;142:228–232. doi: 10.1016/0304-3940(92)90379-l. [DOI] [PubMed] [Google Scholar]
- 40.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico amygdala circuits in auditory fear conditioning. J Neurosci. 1992b;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:525–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 42.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 43.Rosen JB, Hitchcock JM, Miserendino MJD, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sananes CB, Davis M. N-Methyl-d-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- 45.Scheel M. Topographic organization of the auditory thalamocortical system in the albino rat. Anat Embryol. 1988;179:181–190. doi: 10.1007/BF00304700. [DOI] [PubMed] [Google Scholar]
- 46.Shi C, Davis M. Anatomical tracing and lesion studies of visual pathways involved in fear conditioning measured with fear potentiated startle. Soc Neurosci Abstr. 1996;22:1115. [Google Scholar]
- 47.Shi C-J, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J Comp Neurol. 1997;381:1–23. [PubMed] [Google Scholar]
- 48.Shi C-J, Cassell MD. Cortical, thalamic and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998b;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Shi C-J, Cassell MD. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the posterior parietal insular cortex. J Comp Neurol. 1998a;399:469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<469::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Shi C-J, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Shi C-J, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tischler MD, Davis M. A visual pathway that mediated fear-conditioned enhancement of acoustic startle. Brain Res. 1983;276:55–71. doi: 10.1016/0006-8993(83)90548-6. [DOI] [PubMed] [Google Scholar]
- 53.Vaudano E, Legg CR, Glickstein M. Afferent and efferent connections of temporal association cortex in the rat: a horseradish peroxidase study. Eur J Neurosci. 1991;3:317–330. doi: 10.1111/j.1460-9568.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]