Abstract
Serotonergic neurotransmission in prefrontal cortex (PFC) has long been known to play a key role in regulating emotion and cognition under normal and pathological conditions. However, the cellular mechanisms by which this regulation occurs are unclear. In this study, we examined the impact of serotonin on GABAA receptor channels in PFC pyramidal neurons using combined patch-clamp recording, biochemical, and molecular approaches. Application of serotonin produced a reduction of postsynaptic GABAA receptor currents. Although multiple 5-HT receptors were coexpressed in PFC pyramidal neurons, the serotonergic modulation of GABA-evoked currents was mimicked by the 5-HT2-class agonist (−)-2,5-dimethoxy-4-iodoamphetamine and blocked by 5-HT2 antagonists risperidone and ketanserin, indicating the mediation by 5-HT2 receptors. Inhibiting phospholipase C blocked the 5-HT2 inhibition of GABAA currents, as did dialysis with protein kinase C (PKC) inhibitory peptide. Moreover, activation of 5-HT2 receptors in PFC slices increased the in vitro kinase activity of PKC toward GABAA receptor γ2 subunits. Disrupting the interaction of PKC with its anchoring protein RACK1 (receptor for activated C kinase) eliminated the 5-HT2 modulation of GABAA currents, suggesting that RACK1-mediated targeting of PKC to the vicinity of GABAA receptors is required for the serotonergic signaling. Together, our results show that activation of 5-HT2 receptors in PFC pyramidal neurons inhibits GABAA currents through phosphorylation of GABAAreceptors by the activation of anchored PKC. The suppression of GABAergic signaling provides a novel mechanism for serotonergic modulation of PFC neuronal activity, which may underlie the actions of many antidepressant drugs.
Keywords: prefrontal cortex, serotonin receptors, GABAAreceptors, phosphorylation, anchoring proteins, RACK1, patch-clamp, single-cell mRNA profiling
Serotonin is a powerful modulator of emotional processes in the CNS. Dysfunction of serotonergic neurotransmission has long been implicated in the pathogenesis of neuropsychiatric disorders, including schizophrenia, depression, and anxiety (Breier, 1995; Dubovsky and Thomas, 1995; Abi-Dargham et al., 1997; Stockmeier, 1997). Many effective drugs for these disorders act primarily on the serotonin system (Fuxe et al., 1983; Deakin, 1988;Griebel, 1995; Meltzer, 1995; Kapur and Remington, 1996; Busatto and Kerwin, 1997; Lieberman et al., 1998). One of the main target structures of the serotonergic system is prefrontal cortex (PFC), a brain region associated with high-level, “executive” processes needed for complicated goal-directed behavior (Goldman-Rakic, 1995;Miller, 1999). PFC is composed of two major neuronal populations: glutamatergic pyramidal principal neurons and GABAergic interneurons. The axon terminals of local GABAergic neurons form numerous synapses with pyramidal projection neurons (Somogyi et al., 1983), exerting powerful inhibitory control over the excitatory output of PFC. Serotonergic projections target both types of PFC neurons in a synaptic and nonsynaptic manner (Smiley and Goldman-Rakic, 1996). Specific changes of the PFC serotonin system and PFC neuronal activity that are found in patients with neuropsychiatric disorders (Breier, 1995;Dubovsky and Thomas, 1995; Sumiyoshi et al., 1996; Abi-Dargham et al., 1997; Stockmeier, 1997; Dean et al., 1999; Meyer et al., 1999) suggest that serotonin plays a crucial and unique role in PFC.
Molecular cloning experiments have identified at least 13 G-protein-coupled serotonin receptor subtypes, which can be grouped into several classes based on their distinct downstream signal transduction pathways. Serotonin can have both inhibitory and excitatory functions in neuronal networks through the coupling of different 5-HT receptors to distinct ion channels (for review, seeAndrade, 1998). Mice lacking serotonin receptors show phenotypes ranging from epilepsy syndrome (Tecott et al., 1995) to increased impulsive aggression (Saudou et al., 1994) to elevated anxiety and antidepressant-like response (Heisler et al., 1998). Because GABAA receptor-mediated inhibitory synaptic transmission is highly involved in epilepsy, anxiety, and depression (Macdonald and Olsen, 1994), it suggests that the phenotypes in 5-HT receptor knock-out mice may be correlated with changes in GABAergic transmission of PFC neurons. Furthermore, selective alterations in GABAA receptors, GABA content, and GABAergic local circuit neurons have been discovered in PFC of patients with mental disorders (Benes et al., 1996; Dean et al., 1999; Ohnuma et al., 1999; Lewis, 2000). These findings led us to speculate that one potential cellular substrate of serotonin in PFC is the GABAA receptor, and dysregulation of GABAergic transmission by serotonin in PFC is an etiological factor in neuropsychiatric diseases. To test this hypothesis, we examined the impact of serotonin on postsynaptic GABAAreceptor-mediated currents in dissociated PFC pyramidal neurons. Our results may provide the molecular and cellular mechanisms for serotonergic regulation of inhibitory synaptic transmission in PFC slices, as well as a framework within which the role of serotonin in normal mental functions and neuropsychiatric disorders can be better understood.
MATERIALS AND METHODS
Acute-dissociation procedure. PFC neurons from young adult (3–5 weeks postnatal) rats were acutely dissociated using procedures similar to those described previously (Yan and Surmeier, 1996). In brief, rats were anesthetized and decapitated; brains were quickly removed, iced, and then blocked for slicing. The blocked tissue was cut in 400 μm slices with a vibratome while bathed in a low Ca2+ (100 μm), HEPES- buffered salt solution [in mm: 140 Na isethionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 23 glucose, and 15 HEPES, pH 7.4 (300–305 mOsm/l)]. Slices were then incubated for 1–6 hr at room temperature (20–22°C) in NaHCO3 buffered saline bubbled with 95% O2, 5% CO2 (in mm): 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 1 pyruvic acid, and 10 glucose, pH 7.4 with NaOH (300–305 mOsm/l). All reagents were purchased from Sigma (St. Louis, MO).
Slices were then removed into the low Ca2+buffer, and regions of the PFC were dissected and placed in an oxygenated Cell-Stir chamber (Wheaton Inc., Millville, NJ) containing pronase (1–3 mg/ml) in HEPES-buffered HBSS (Sigma) at 35°C. After 20–30 min of enzyme digestion, tissue was rinsed three times in the low Ca2+, HEPES-buffered saline and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was then plated into a 35 mm Lux Petri dish, which was then placed on the stage of an inverted microscope.
Whole-cell recordings. Whole-cell recordings of GABA-activated currents used standard techniques (Surmeier et al., 1995; Yan and Surmeier, 1997). Electrodes were pulled from Corning 7052 glass (A-M Systems Inc., Carlsberg, WA) and fire-polished before use. The internal solution consisted of (in mm): 180N-methyl-d-glucamine, 40 HEPES, 4 MgCl2, 0.5 BAPTA, 12 phosphocreatine, 2 Na2ATP, 0.2 Na3GTP, and 0.1 leupeptin, pH 7.2–3 with H2SO4 (265–270 mOsm/l). The external solution consisted of (in mm): 135 NaCl, 20 CsCl, 1 MgCl2, 10 HEPES, 0.001 TTX, 5 BaCl2, and 10 glucose, pH 7.3 with NaOH (300–305 mOsm/l).
Recordings were obtained with an Axon Instruments 200 patch-clamp amplifier that was controlled and monitored with a IBM personal computer running pClamp (version 8.1) with a DigiData 1320 series interface (Axon instruments, Foster City, CA). Electrode resistances were typically 2–4 MΩ in the bath. After seal rupture, series resistance (4–10 MΩ) was compensated (70–90%) and periodically monitored. The cell membrane potential was held at 0 mV. GABA (100 μm) was applied briefly (2–3 sec) every minute. Drugs were applied with a gravity-fed “sewer pipe” system. The array of application capillaries (∼150 μm inner diameter) was positioned a few hundred micrometers from the cell under study. Solution changes were effected by altering the position of the array with a DC drive system controlled by a microprocessor-based controller (Newport, Irvine, CA).
Serotonin receptor ligands [serotonin, (−)-2,5-dimethoxy-4-iodoamphetamine (R(−)-DOI), 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), methysergide, risperidone, ketanserin, and cyanopindolol (Sigma)] were made freshly on the day of experiments. Second messenger reagentsU73122, U74133 (Calbiochem, San Diego, CA), heparin (Sigma), phorbol-12-myristate-13-acetate (PMA), and 4α-phorbol, PKC19–31 (Alexis Biochemical Co., San Diego, CA) were made up as concentrated stocks in water or DMSO and stored at −20°C. Stocks were thawed and diluted immediately before use. The amino acid (aa) sequence for the PKC anchoring inhibitory peptide RACK1 (receptor for activated C kinase)-rVI is DGGDIINALCFSPNR. The amino acid sequence for the scrambled peptide sRACK1-rVI is FDSRGIGPDINCANL. The amino acid sequence for the PKC anchoring inhibitory peptide AKAP[31–52] is KASMLCFKRRKKAAKLAKPKAG.
Data analyses were performed with AxoGraph (version 3.0; Axon Instruments), Kaleidagraph (version 3.0.4; Albeck Software, Reading, PA), and Statview (version 4.5; Abacus Concepts, Calabasas, CA). Box plots were used for graphic presentation of the data because of the small sample sizes (Tukey, 1977). The box plot represents the distribution as a box with the median as a central line and the hinges as the edges of the box. The inner fences run to the limits of the distribution excluding outliers. For analysis of statistical significance, paired t tests were performed to compare the differential degrees of current inhibition between groups subjected to different treatment.
Single-neuron mRNA profiling. For the detection of serotonin receptor mRNAs in PFC pyramidal neurons, we used the single-cell reverse transcription (RT)-PCR technique similar to those described previously (Surmeier et al., 1996; Yan and Surmeier, 1996, 1997). A patch electrode was used to lift a dissociated neuron into a stream of control solution, and then the neuron was aspirated into the electrode by applying negative pressure. After aspiration, the electrode was broken, and the contents were ejected into a 0.5 ml Eppendorf tube containing 5 μl of diethylpyrocarbonate-treated water, 0.5 μl of RNAsin (28 U/μl), 0.5 μl of dithiothreitol (DTT) (0.1m), and 1 μl of oligo-dT primer (0.5 μg/μl). The mixture was heated to 70°C for 10 min and then incubated on ice for >1 min. Single-strand cDNA was synthesized from the cellular mRNA by adding SuperScript II reverse transcriptase (1 μl, 200 U/μl) and buffer [4 μl, 5× first-strand buffer (in mm): 250 Tris-HCl, 375 KCl, and 15 MgCl2], RNAsin (0.5 μl, 28 U/μl), DTT (1.5 μl, 0.1 m), and mixed dNTPs (1 μl, 10 mm each). The reaction mixture (20 μl) was incubated at 42°C for 50 min. The reaction was terminated by heating the mixture to 70°C for 15 min and then icing. The RNA strand in the RNA–DNA hybrid was then removed by adding 1 μl of RNase H (2 U/μl) and incubating for 20 min at 37°C. All reagents were obtained from Life Technologies (Grand Island, NY).
The cDNA from the RT of RNA in single PFC neurons was amplified with the PCR, which was performed with a thermal cycler (MJ Research Inc., Watertown, MA) in thin-walled plastic tubes. Reaction mixtures contained 2–2.5 mm MgCl2, 0.5 mm each of the dNTPs, 0.8–1 μm primers, 2.5 U Taq DNA polymerase (Promega, Madison, WI), 5 μl of 10× buffer (Promega), and one-fourth (5 μl) of the cDNA template made from the single cell RT reaction. The thermal cycling program for the first-round amplification was as follows: 94°C for 1 min, 52°C for 1 min, and 72°C for 1.5 min for 30 cycles. Two microliters of the first-round PCR product was used as the cDNA template for the second-round amplification: 94°C for 1 min, 56°C for 1 min, and 72°C for 1.5 min for 45 cycles. Ten microliters of the second-round PCR product was separated by electrophoresis in ethidium bromide-stained 1.5% agarose gels. Negative controls for contamination from extraneous and genomic DNA were run for every batch of neurons. The tissue RT-PCR procedure (Surmeier et al., 1996) was used to detect mRNAs in PFC.
PCR primers were designed based on GenBank sequences for serotonin receptors. The primers used to amplify GAD67 (GABA synthesizing enzyme glutamic acid decarboxylase) mRNA were as follows: 5′-CAGACAAGCAGTATGACGTCTCCT and 5′-AGGAAATCGATGTCAGACTGGGTG. The size of GAD67 amplicon was 435 bp. One round (45 cycle) of PCR amplification was performed for the detection of GAD mRNA. Two rounds of nested PCR amplification were performed for the detection of 5-HT receptor mRNAs. The outer primers used to amplify 5-HT1A/1D and 5-HT1Breceptors were as follows: 5′-AACTATCTCATYGGCTCC; 5′- CAGCCAGCAGAKGATRAA; 5′-TAACTACCTGATCGCCTC; and 5′-GAGCCAGCACACAATAAA. The outer primers used to amplify 5-HT2A/2Creceptors were 5′-GCCATWGCTGATATGCTG and 5′-CCASACAAACACATTGAG. The outer primers used to amplify 5-HT4 receptors were 5′-ACAAGAT GACCCCTCTAC and 5′-TAGCGCTCATCATCACAG. The outer primers used to amplify 5-HT6 and 5-HT7 receptors were as follows: 5′- CTTCACGTCGGACTTGAT; 5′-TGTGAGGACATCGAAGAG; 5′-CGGTCATGCCTTTCGTTA; and 5′-ATATTCCGGTACTGGCAC.
The specific inner primers for 5-HT1A were 5′-TCTGTACCAGGTGCTCAACAAG and 5′-AGAGGAAGGTGCTCTTTGGAGT. The size of 5-HT1A amplicon was 638 bp. The specific inner primers for 5-HT1B were 5′-ATCAGCACCATGTACACGGTCA and 5′-GACTTGGTTCACGTACACAGGA. The size of 5-HT1B amplicon was 557 bp. The specific inner primers for 5-HT1D were 5′-AGATGTCTGACTGCCTGGTGAA and 5′-TGCGTTCTAAGATGCTATCAGC. The size of 5-HT1D amplicon was 316 bp. The specific inner primers for 5-HT2A were 5′-ATTGCCGTGTGGACCATATCTG and 5′-GCAGGATTCTTTGCAGATGACG. The size of 5-HT2A amplicon was 460 bp. The specific inner primers for 5-HT2C were 5′-GCCATCATGAAGATTGCCATCG and 5′-CGACGTGGTTTCTGATCTGGAT. The size of 5-HT2C amplicon was 358 bp. The specific inner primers for 5-HT4 were 5′-CCCATAATGCAAGGCTGGAACA and 5′-GGAAGGCACGTCTGAAAGACTT. The size of 5-HT4 amplicon was 504 bp. The specific inner primers for 5-HT6 were 5′-AGCCATGCTGAACGCGCTGTAT and 5′-CAAGGCCTTCCTGCTATGCTTG. The size of 5-HT6 amplicon was 546 bp. The specific inner primers for 5-HT7 were 5′-CCACTTCTTCTGCAACGTCTTC and 5′-GTGTTTGAGCAGTCTCGAAAGG. The size of 5-HT7 amplicon was 480 bp.
Protein kinase assay. Brain slices were incubated with the 5-HT2 receptor agonist DOI or PMA for 20 min and then lysed in cold lysis buffer (0.5% Nonidet P-40, 0.1 mm EDTA, 50 mm Tris-HCl, 125 mm NaCl, 0.1 mmNa3VO4, 50 mm NaF, and 1 mmphenylmethylsulfonyl fluoride) on ice for 30 min. Brain lysates were centrifuged and ultracentrifuged, and PKC was immunoprecipated with rabbit polyclonal anti-PKCαβγ (Life Technologies) for 1 hr, followed by the addition of 50 μl of protein A Sepharose beads and incubation for 1 hr at 4°C. The beads were pelleted by centrifugation and washed three times with lysis buffer and three times with kinase buffer (50 mm Tris-HCl pH 7.5, and 5 mm MgCl2) and then resuspended in 30 μl of kinase buffer. In vitro kinase activity was measured in the PKC immunoprecipitates using peptides derived from GABAA receptors as substrates. The intracellular regions between the third and the fourth transmembrane domain of GABAA receptor β2 and γ2 subunits contain identified PKC phosphorylation sites. The sequences for the two peptides against these regions are as follows: GABAA-β2, KSRLRRRASQLKITI (amino acids 402–416 in mature β2 protein without the 24 aa signal peptide); and GABAA-γ2, SNRKPSKDKDKKKKNPAPT (amino acids 322–340 in mature γ2 protein without the 38 aa signal peptide). The assay was initiated by the addition of 5 μl of [γ-32P]ATP (10 mCi/ml) and 1 μl of peptide (10 mg/ml), continued for 20 min at room temperature, and stopped by boiling samples in SDS-PAGE sample buffer. In some cases, 1 μl of the myristoylated PKC inhibitory peptide PKC19–31 (10 mg/ml; Promega) was added to the reaction mixture. Samples were loaded onto a 20% polyacrylamide gel and subjected to electrophoresis. The gels were vacuum dried and exposed to Biomax film (Eastman Kodak, Rochester, NY). Kinase activity was quantified by an phosphoimager.
RESULTS
Serotonin reduces GABA-activated currents in PFC pyramidal neurons
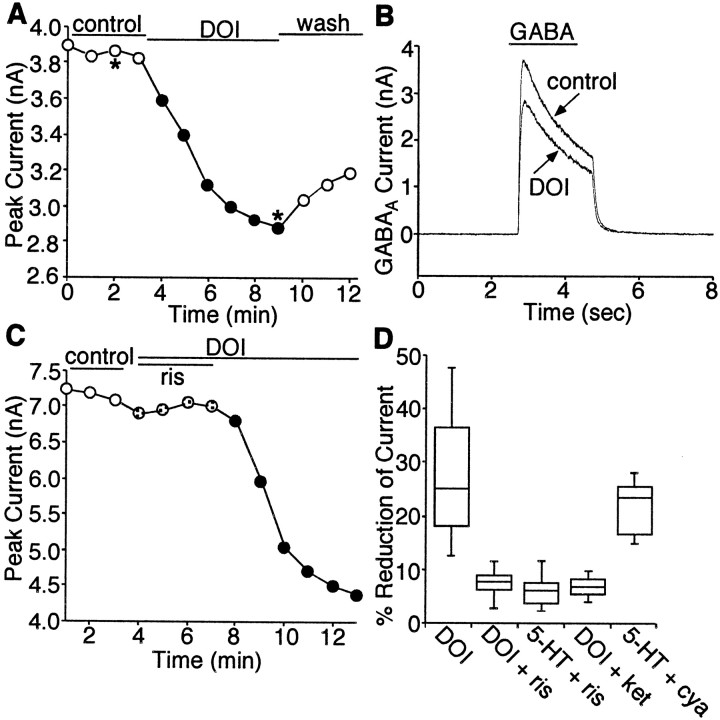
In rats, the prelimbic, infralimbic, and ventral anterior cingulate cortex represent the major subdivisions of PFC (Groenewegen, 1988). Based on their patterns of neural connectivity, these regions are thought to be functionally related to PFC in the primate (Kolb, 1984; Uylings and van Eden, 1990; Conde et al., 1995). To test the potential impact of serotonin on GABAergic signaling, we examined the effect of serotonin on GABAA receptor-mediated currents in pyramidal neurons located in the intermediate and deep layers (III–VI) of the rat PFC. GABA was applied to these neurons that were voltage clamped using whole-cell techniques. The application of GABA (100 μm) evoked a partially desensitizing outward current with the decay rate fitted by a single or double exponential. This current was completely blocked by the GABAAreceptor antagonist bicuculline (30 μm, n= 3; data not shown), confirming mediation by the GABAA receptor. Application of serotonin (20 μm) caused a reduction in the amplitudes of GABAA currents in ∼90% of PFC pyramidal neurons tested (n = 21). Shown in Figure1A is a plot of peak currents evoked by repeated application of GABA (100 μm) as a function of time. GABA was applied once per minute for 2–3 sec to minimize desensitization-induced decrease of current amplitude. The serotonergic reduction of GABAA currents had slow onset kinetics, taking 3–4 min to stabilize. The modulation was not accompanied by changes in current decay kinetics. The median reduction of peak GABAA currents by serotonin was 25.4% (n = 21) (Fig. 1B). This serotonin-mediated inhibition of GABAA currents did not result from an agonist-independent run-down of the current, because no significant decrease of the current was observed in the absence of serotonin (Fig. 1A). Application of the nonselective 5-HT receptor antagonist methysergide (10 μm) primarily abolished the inhibition, and washing off the antagonist led to recovery of 5-HT effect on GABAA currents (Fig. 1C,D). As shown in Figure 1C, inset, 70–90% of serotonergic inhibition of GABAA currents was blocked by methysergide (n = 8), confirming that this effect is mediated by 5-HT receptors.
Fig. 1.
Application of serotonin causes a reduction of GABAA receptor currents in PFC pyramidal neurons.A, Time course of peak current evoked by GABA (100 μm) in the absence (control) or presence of serotonin (20 μm). The starting point for continuous application of serotonin is marked by the arrow. Application of serotonin caused an inhibition of GABAA receptor currents, whereas repeated application of GABA alone evoked a current that was stable during the whole-cell recording. B, Box plot summary of the percentage of reduction of peak GABAAcurrents produced by serotonin in a sample of 21 PFC pyramidal neurons.C, Plot of peak GABAA current as a function of time and ligand application. In the presence of the nonselective 5-HT receptor antagonist methysergide (meth; 10 μm), serotonin had little effect on GABAAcurrents; washing off the antagonist led to recovery of the serotonin inhibition (wash). The inset is a box plot summary showing the percentage of serotonin effect blocked by methysergide (n = 8). D, Representative current traces taken from the records used to constructC (at time points denoted by *).
PFC pyramidal neurons express multiple 5-HT receptor mRNAs
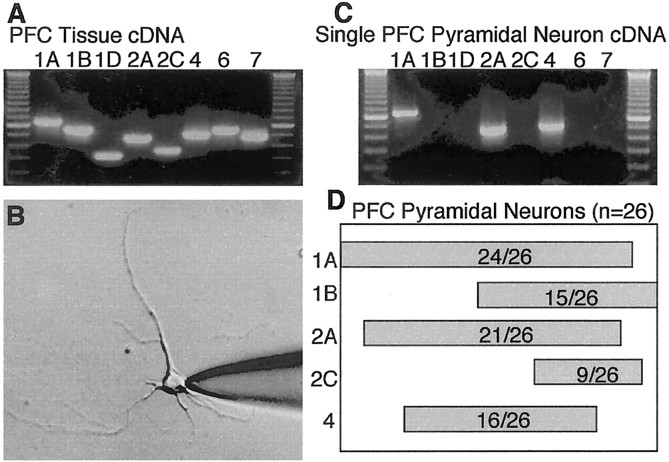
To test which 5-HT receptor may mediate the serotonergic inhibition of GABAA currents, we first examined the expression of 5-HT receptor mRNAs in PFC neurons. The mRNAs for 5-HT1 (5-HT1A, 1B, 1D), 5-HT2 (5-HT2A, 2C), 5-HT4, 5-HT6, and 5-HT7 receptors were first detected with cDNA obtained from the whole PFC tissue. As shown in Figure2A, all of these receptor subtypes are present in this brain region. Because PFC is composed of heterogeneous neuronal populations, it is important to determine how serotonin receptors are coordinately expressed in individual PFC pyramidal neurons. Cells were harvested after recording and analyzed using the single-cell RT-PCR technique (Yan and Surmeier, 1996, 1997). Acutely isolated PFC pyramidal neurons were readily distinguished from GABAergic interneurons by their distinct morphological features: a pyramidal-shaped soma and a prominent apical dendrite. The expression of GAD mRNA was consistently negative in the harvested neurons (data not shown), confirming that they are not GABAergic interneurons. For the detection of 5-HT receptor subtypes, a two-stage PCR amplification protocol was used to minimize problems associated with template abundance. In the first round of amplification, each family of subunits was amplified using degenerate primer sets. In the second round, subunit-specific “nested” primers were used.
Fig. 2.
Multiple 5-HT receptor mRNAs are coexpressed in single PFC pyramidal neurons. A, Photomicrograph of an ethidium bromide-stained gel showing the expression profile of 5-HT receptor mRNAs in PFC tissue by RT-PCR. B, Photomicrograph of an acutely isolated PFC pyramidal neuron. After recording, the cell was harvested by the patch electrode and subject to mRNA profiling. C, Expression profile of 5-HT receptor mRNAs in this PFC pyramidal neuron showing the coexpression of 5-HT1A, 5-HT2A, and 5-HT4 receptor mRNAs. C, Bar plot showing the coordinated expression of major 5-HT receptor mRNAs in a sample of 26 PFC pyramidal neurons. The extent of coexpression is indicated by the overlap of the bars.
A representative picture of an acutely dissociated PFC pyramidal neuron is shown in Figure 2B. The mRNA profile for 5-HT receptor subtypes of this cell is shown in Figure 2C. The 5-HT1A, 5-HT2A, and 5-HT4 receptor mRNAs were coexpressed in this neuron. In 26 individual PFC pyramidal neurons tested, nearly all of them expressed the 5-HT1A receptor mRNA (24 of 26). Approximately 60% of these cells expressed the 5-HT1B receptor mRNA (15 of 26). A large portion of these cells expressed the 5-HT2A receptor mRNA (21 of 26), whereas the 5-HT2C receptor mRNA was detected in only one-third of these cells (9 of 26). The 5-HT4 receptor mRNA was found in ∼60% of these cells (16 of 26). Other 5-HT receptor subtype mRNAs (e.g., 5-HT1D, 5-HT6, and 5-HT7) were rarely detected (<10%). The coordinated expression of major 5-HT receptor mRNAs in the sample of 26 PFC pyramidal neurons is summarized in Figure 2D, from which several coexpression patterns of 5-HT receptors detectable in individual cells could be seen. For example, the simultaneous mRNA expression of all five subtypes (5-HT1A,1B, 2A,2C, 4) was detected in 5 of 26 neurons, and coexpression of four subtypes (5-HT1A, 1B,2A, 4) was detected in 4 of 26 neurons. A subset of neurons (7 of 26) had detectable mRNA levels of three subtypes (5-HT1A, 2A,4), and other neurons (4 of 26) had only two subtypes (5-HT1A, 2A). The coordinated expression of mRNAs encoding multiple 5-HT receptor subtypes suggests that 5-HT could regulate PFC functions by simultaneously activating distinct signaling cascades mediated by these receptors.
Serotonergic modulation of GABA-activated currents is mediated by 5-HT2 receptors
Because multiple 5-HT receptors are simultaneously expressed in individual PFC pyramidal neurons, we next used subtype-specific agonists and antagonists to examine which 5-HT receptor was involved in the modulation of GABAA currents. 5-HT1A and 5-HT2A are the most prominent serotonin receptor subtypes expressed in PFC pyramidal neurons, so we first examined the potential role of these receptors in the modulation of GABAA currents. In eight PFC pyramidal neurons tested, GABAA currents were not affected by the 5-HT1A receptor agonist 8-OH-DPAT (20 μm; data not shown), suggesting that the serotonergic effect on GABAA channels was not mediated by the 5-HT1A receptor. On the other hand, application of the 5-HT2A/C receptor agonist DOI (10 μm) caused a reduction (27.1 ± 10.6%, mean ± SD; n = 24) in the amplitudes of GABAA currents without affecting the time constants for current decay in most of the PFC pyramidal neurons tested (87.5%) (Fig.3A,B), mimicking the inhibitory effect of 5-HT. Washing off DOI led to a partial or complete recovery of GABAA currents. To verify that 5-HT2A/C receptors were mediating the modulation seen with DOI or 5-HT, the ability of 5-HT2-class antagonist risperidone (Baxter et al., 1995) to prevent the action of DOI or 5-HT was examined. As shown in Figure 3C, risperidone (10 μm) almost completely eliminated the effects of DOI (10 μm). Removing the antagonist restored the ability of DOI to modulate GABAA currents. The median reduction of peak GABAA currents by DOI was 25.1% (n = 24) (Fig. 3D), similar to the 5-HT effect (median reduction of 25.4%; n = 21) (Fig. 1B). In the presence of risperidone, the inhibition of GABAA currents by DOI (n = 10) or 5-HT (n = 6) was both significantly blocked (median reduction of 7.8 and 5.8%, respectively) (Fig. 3D). Because risperidone also blocks D2 dopamine receptors, we further tested the DOI effect in the presence of the selective D2antagonist sulpiride. Application of sulpiride (10 μm) did not block the inhibitory effect of DOI on GABAA currents (data not shown), suggesting that D2 receptors were not involved in the action of DOI. Moreover, the DOI or 5-HT effect was greatly attenuated by another 5-HT2-class antagonist, ketanserin (median reduction of 6.4%; n = 5) (Fig. 3D) but was not altered by a 5-HT1-class antagonist, cyanopindolol (median reduction of 22.3%; n = 5) (Fig.3D). These results suggest that the serotonergic effect on GABAA channels is mediated by 5-HT2A/C receptors. Our single-cell mRNA profiling experiments have shown that 5-HT2Areceptors are expressed in the majority of PFC pyramidal neurons (>80%), whereas 5-HT2C receptors are detected in only one-third of these cells, suggesting that the effects of DOI on GABAA currents found in most PFC pyramidal neurons can be primarily attributed to 5-HT2Areceptors.
Fig. 3.
Serotonergic modulation of GABAAcurrents is mediated by 5-HT2 receptors. A, Plot of peak GABAA current as a function of time and agonist application. The 5-HT2A/C agonist DOI (10 μm) caused an inhibition of GABAA currents.B, Representative current traces taken from the records used to construct A (at time points denoted by *).C, Plot of peak GABAA current as a function of time and ligand application. The 5-HT2 receptor antagonist risperidone (ris; 10 μm) blocked the effect of DOI on GABAA currents.D, Box plot summary of the percentage of reduction of peak GABAA currents produced by DOI or 5-HT in the absence and presence of different antagonists. The 5-HT2-class antagonist risperidone (ris; 10 μm) and ketanserin (ket; 20 μm) blocked the effect of DOI and 5-HT, whereas the 5-HT1-class antagonist cyanopindolol (cya; 20 μm) was ineffective.
5-HT2 receptors modulate GABAAcurrents via the phospholipase C β isoform-mediated pathway
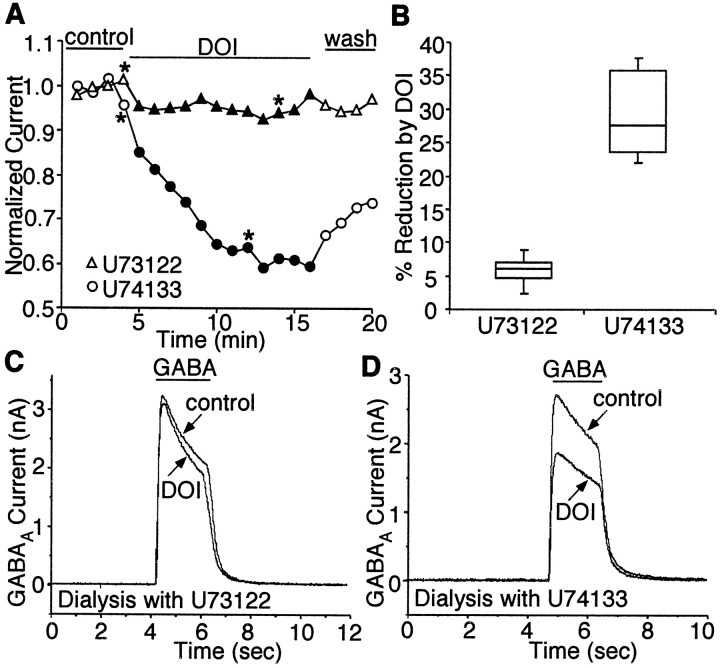
We next examined the signal transduction pathways mediating the modulation of GABAA currents by 5-HT2 receptors. In studies using cell lines, it has been found that activation of 5-HT2 receptors stimulates phospholipase C β isoform (PLCβ), leading to the release of ionsitol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) through the hydrolysis of membrane phosphoinositol lipids. To test whether the modulation of GABAAcurrents by 5-HT2 receptors is through the PLCβ-mediated pathway, we dialyzed the neuron with the selective PLCβ inhibitor U73122 and examined the 5-HT2effect on GABAA currents under this condition. If the 5-HT2 agonist was exerting its effect through the phospholipid cascade, then inhibiting PLCβ should block the effect of receptor activation. In agreement with this model, dialysis with the PLCβ inhibitor U73122 (4 μm) significantly reduced the ability of DOI to inhibit GABA-evoked currents, whereas the inactive analog U74133 (4 μm) had no effect. A representative experiment is shown in Figure4. The median reduction of peak GABAA currents by DOI was 6.2% in the presence of U73122 (n = 5) (Fig. 4B) and 27.5% in the presence of U74133 (n = 5;p < 0.005; paired t test) (Fig.4B). These results indicate that 5-HT2 receptors modulate GABAA currents through the PLCβ-mediated pathway.
Fig. 4.
5-HT2 modulation of GABAAcurrents is blocked by inhibition of PLCβ. A, Plot of peak GABAA current as a function of time and agonist application with U73122 (4 μm) or U74133 (4 μm) in the recording pipette. Dialysis with the PLCβ inhibitor U73122, but not its inactive analog U74133, blocked the DOI effect on GABAA currents. B, Box plot summary of the modulation of GABAA currents by DOI in the presence of U73122 (n = 5) or U74133(n = 5). C, D, Representative current traces taken from the records used to constructA (at time points denoted by *).
The 5-HT2 modulation of GABAAcurrents is dependent on PKC activation
GABAA channels are thought to be heteropentameric structures, composed of different subunits (Macdonald and Olsen, 1994). Protein phosphorylation exerts a powerful impact on the regulation of GABA-activated currents in recombinant and native GABAA channels (Porter et al., 1990; Kellenberger et al., 1992; Krishek et al., 1994). Because 5-HT2 receptors couple to the PLCβ–IP3–DAG pathway, which could lead to the activation of PKC, we tested whether the 5-HT2-induced reduction of GABAA currents in PFC neurons was mediated by activated PKC.
Application of the PKC activator PMA (1 μm), but not its inactive analog 4α-phorbol (1 μm), mimicked the serotonergic effect on GABAA channels, causing an irreversible decrease of the amplitude of GABAAcurrents (Fig. 5A). The median inhibition was 37.3% by PMA (n = 5) (Fig.5B) and was only 5.6% by 4α-phorbol (n = 5; p < 0.002; paired t test) (Fig.5B). If 5-HT2 receptors were exerting its effect through PKC, then inhibiting the activation of PKC should eliminate the effect of 5-HT2 on GABAA currents. Because conventional PKC isoforms (PKCα, PKCβ, and PKCγ) depend on Ca2+ for their activation (Tanaka and Nishizuka, 1994), we used the IP3 receptor antagonist heparin (10 U/ml) to block the elevation of intracellular free Ca2+ (Finch et al., 1991; Finch and Augustine, 1998) and examined 5-HT2 modulation of GABAA currents under this condition. As shown in Figure 5C, dialysis with heparin significantly attenuated the effect of DOI on GABAA currents. The median reduction of peak GABAA currents by DOI was 9.1% in the presence of heparin (n = 5) (Fig.5D), whereas in control cells DOI reduced GABAA currents by 23.5% (n = 6;p < 0.005; paired t test) (Fig.5D). To further test the involvement of intracellular Ca2+ in 5-HT2modulation of GABAA currents, we also dialyzed neurons with a high concentration (10 mm) of BAPTA, a potent and rapid Ca2+ chelator. As shown in Figure 5D, the median reduction of peak GABAA currents by DOI was only 3.8% in the presence of high BAPTA (n = 5), which was significantly smaller than the DOI effect in control cells (n = 6;p < 0.005; paired t test). These results suggest that 5-HT2 modulation of GABAA channels requires intracellular Ca2+. To provide additional evidence for the involvement of PKC, we dialyzed neurons with the PKC inhibitory peptide PKC19–31 (20 μm). As shown in Figure 5E, PKC19–31 blocked the effect of DOI on GABAA currents. The median reduction of peak GABAA currents by DOI was 1.5% in the presence of PKC19–31 (n = 6) (Fig.5F), which was significantly smaller than the DOI effect in control cells loaded with peptide-free internal solutions (median reduction of 30.5%; n = 5; p< 0.005; paired t test) (Fig. 5F). Together, these data show that 5-HT2receptor-mediated inhibition of GABAA currents is dependent on PKC activation.
Fig. 5.
5-HT2 modulation of GABAAcurrents is dependent on activation of PKC. A, Time course of peak GABAA current in the presence of PMA (1 μm) or 4α-phorbol (1 μm). The starting point for continuous application of PMA or 4α-phorbol is marked by the arrow. The PKC activator PMA caused an irreversible reduction of GABAA receptor currents, whereas the inactive analog 4α-phorbol had little effect on GABAA currents.B, Box plot summary of the modulation of GABAA currents by PMA (n = 5) or 4α-phorbol (n = 5). C, Plot of peak GABAA current as a function of time and agonist application with or without heparin (10 U/ml) in the recording pipette. Blocking IP3-mediated Ca2+ release with heparin significantly attenuated the effect of DOI on GABAAcurrents. D, Box plot summary of the modulation of GABAA currents by DOI in the absence (control;n = 6) and presence of heparin (n = 5) or the high concentration (10 mm) of BAPTA (n = 5). E, Plot of peak GABAA current as a function of time and agonist application with or without PKC19–31 (20 μm) in the recording pipette. Dialysis with the PKC inhibitory peptide PKC19–31 blocked the effect of DOI on GABAA currents. F, Box plot summary of the modulation of GABAA currents by DOI in the absence (control; n = 5) and presence of PKC19–31 (n = 6).
Activation of 5-HT2 receptors increases PKC phosphorylation of GABAA receptor γ2 subunits
Because our electrophysiological experiments have provided strong evidence for the involvement of PKC in 5-HT2modulation of GABAA currents, we hypothesize that phosphorylation of GABAA receptors by activated PKC is the underlying molecular mechanism for 5-HT2 inhibition of GABAAcurrents. To test this hypothesis, we used biochemical methods to examine whether the activation of 5-HT2 receptors could enhance PKC phosphorylation of GABAAreceptor subunits in PFC networks.
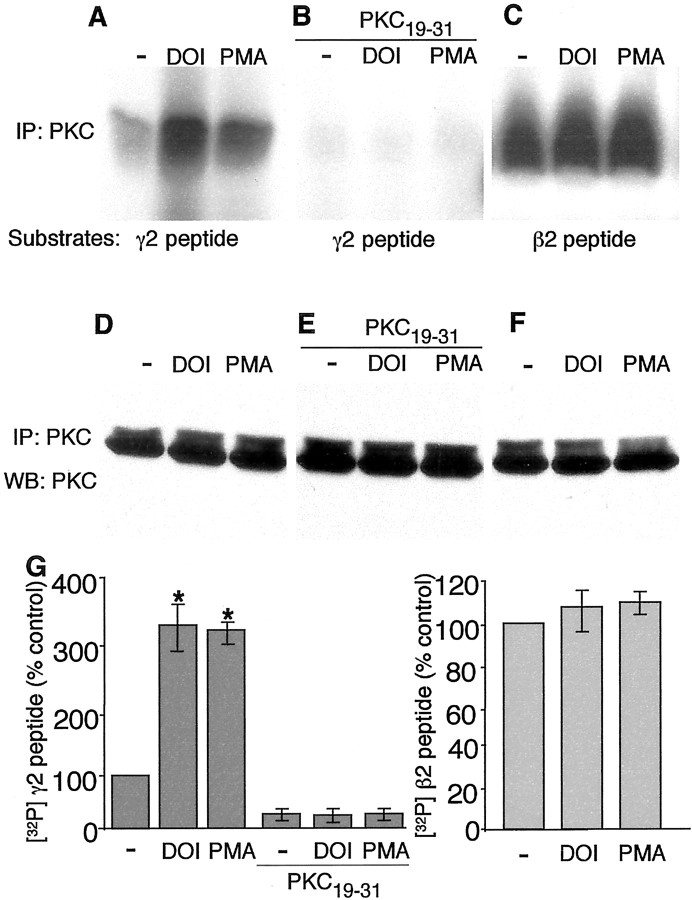
Multiple PKC phosphorylation sites have been identified in GABAA receptor γ subunits and β subunits (Macdonald and Olsen, 1994). Peptides derived from the intracellular regions between the third and the fourth transmembrane domain of GABAA receptor β2 and γ2 subunits, which contain PKC phosphorylation sites that have been characterized previously (Moss et al., 1992), were used as substrates in in vitro kinase assays. Brain slices containing PFC were treated with or without the 5-HT2 agonist DOI or the PKC activator PMA, and PKC phosphorylation of these peptides were compared in these slices. As shown in Figure 6, PKC phosphorylation of the peptide derived from GABAA receptor γ2 subunit was significantly increased in slices treated with DOI or PMA (Fig.6A). These effects were completely eliminated by including the PKC inhibitory peptide PKC19–31 in the in vitro kinase reactions (Fig. 6B). In contrast, PKC phosphorylation of the GABAAreceptor β2 peptide was not significantly altered by DOI or PMA treatment (Fig. 6C). Equal loading of PKC in the in vitro kinase assay was shown by Western blotting of the PKC immunoprecipitates with an antibody against PKC (Fig.6D–F). Quantification of three similar experiments showed that DOI increased PKC phosphorylation of the γ2 peptide by threefold to fourfold, similar to the PMA effect, whereas DOI or PMA treatment did not significantly increase PKC phosphorylation of the β2 peptide over the high basal level (Fig. 6G). These results suggest that activation of 5-HT2receptors enhances the kinase activity of PKC, causing increased phosphorylation of GABAA receptor γ2 subunits.
Fig. 6.
5-HT2 receptor activation increases PKC phosphorylation of GABAA receptor subunits.A, In vitro kinase activity of PKC immunoprecipitates toward a peptide derived from GABAAreceptor γ2 subunit. Brain slices containing PFC were incubated for 15 min in the absence (−) or presence of either DOI (20 μm) or PMA (1 μm). Lysates of these slices were used for immunoprecipitation with antibodies to PKCαβγ. PKC kinase activity of the immune complex was measured using a peptide derived from GABAA γ2 subunit as the substrate. Application of DOI or PMA enhanced PKC phosphorylation of the GABAA γ2 peptide. B, Including 1 μl of the PKC inhibitory peptide PKC19–31 (10 mg/ml) in thein vitro kinase reactions blocked PKC phosphorylation of the GABAA γ2 peptide induced by DOI or PMA.C, In vitro kinase activity of PKC immunoprecipitates toward a peptide derived from GABAAreceptor β2 subunit. Lysates of brain slices treated with or without DOI (20 μm) or PMA (1 μm) were immunoprecipitated with PKCαβγ antibodies, and PKC activity of the immune complex was measured using a GABAA β2 subunit peptide as the substrate. Application of DOI or PMA did not significantly alter PKC phosphorylation of the GABAA β2 peptide over the high basal level. D–F, Equal loading of PKC in the in vitro kinase assay. Half of the brain lysates used for in vitro kinase assay was immunoprecipitated with PKCαβγ antibodies and Western blotted with PKCαβγ antibodies. G, Histogram summary of the phosphorylation of GABAA receptor γ2 subunit-derived peptide and β2 subunit-derived peptide in PFC slices. Treatment with DOI or PMA significantly increased the phosphorylation of GABAA receptor γ2 subunit, which was blocked by the PKC inhibitory peptide PKC19–31 (left panel;n = 3; *p < 0.01), but did not significantly enhance the phosphorylation of GABAA receptor β2 subunit (right panel; n = 3).
The 5-HT2 modulation of GABAA currents requires the anchoring of activated PKC by RACK1
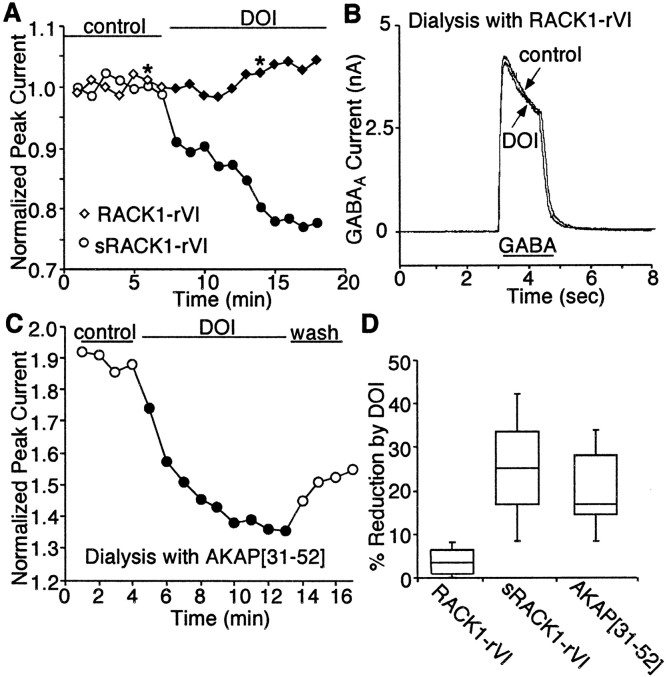
In CNS neurons, ion channels and signaling enzymes are not diffusely located but are compartmentalized to particular subcellular regions by association with various anchoring proteins (Pawson and Scott, 1997). PKC, like other kinases with broad substrate selectivity, achieves the efficacy and specificity of signal transduction through anchoring protein-mediated subcellular targeting to its substrates (Mochly-Rosen, 1995). PKC has multiple binding proteins (Mochly-Rosen and Gordon, 1998). One class of proteins that binds only activated PKC isoforms in a selective manner is called RACKs (Mochly-Rosen et al., 1991). One member of this family of proteins, RACK1, binds to the GABAA receptor intracellular domain (Brandon et al., 1999). This evidence suggests that RACK1 may be responsible for targeting PKC to GABAA receptor channels and allowing PKC to effectively phosphorylate these receptors. We hypothesize that blocking PKC–RACK1 interaction may lead to the removal of PKC from the vicinity of GABAAreceptors, thereby attenuating PKC regulation of these channels. To test this hypothesis, we dialyzed neurons with a PKC anchoring inhibitory peptide. RACK1 contains seven WD40 motifs, an internally repeating element that is thought to be involved in protein–protein interactions. A peptide derived from the sequence within the sixth WD40 repeats of RACK1 has been found to inhibit PKC binding to RACK1in vitro (Ron et al., 1994). Based on this result, we synthesized the peptide RACK1-rVI using the sequence from the sixth WD40 repeats of RACK1 and dialyzed the cell with this peptide. As shown in Figure 7, A andB, dialysis with the PKC anchoring inhibitory peptide RACK1-rVI eliminated the ability of DOI to inhibit GABAA currents, whereas the control peptide with scrambled amino acid sequence had no effect on 5-HT2 reduction of GABAAcurrents.
Fig. 7.
5-HT2 modulation of GABAAreceptor function requires anchoring of activated PKC to the channel by RACK1. A, Plot of peak GABAA current as a function of time and agonist application with RACK1-rVI peptide (40 μm) or the scrambled peptide sRACK1-rVI (40 μm) in the recording pipette. Disruption of the interaction between PKC and RACK1 with RACK1-rVI peptide, but not the scrambled peptide sRACK1-rVI, blocked the DOI effect on GABAA currents. B, Representative current traces taken from the records used to construct A (at time points denoted by *). C, Plot of peak GABAA current as a function of time and agonist application with AKAP[31–52] peptide (10 μm) in the recording pipette. D, Box plot summary of the modulation of GABAA currents by DOI in the presence of RACK1-rVI peptide (n = 11) or the scrambled peptide sRACK1-rVI (n = 5), or the AKAP[31–52] peptide (n = 5).
To determine the specific involvement of RACK1 in 5-HT2 modulation of GABAAcurrents, we also tested the role of another PKC anchoring protein AKAP79 (Klauck et al., 1996) in this process. AKAP79 is a member of the “PKC substrate-binding proteins” that could be anchoring proteins for inactive PKC (Mochly-Rosen and Gordon, 1998). Biochemical studies have shown that residues 31–52 of AKAP79 are the putative PKC binding site (Klauck et al., 1996). A peptide encompassing this region of AKAP79 specifically blocked the interaction of AKAP79 with PKC in the overlay assay (Klauck et al., 1996). This peptide, named AKAP[31–52], was dialyzed to PFC pyramidal neurons, and DOI effects on GABAA currents in these cells were measured. As shown in Figure 7C, in the presence of peptide AKAP[31–52], DOI still reduced GABAA currents, which was similar to the DOI effect in control cells with no peptide dialyzed (Fig. 3A). As summarized in Figure 7D, the DOI effect on GABAA currents was significantly smaller in neurons dialyzed with the peptide RACK1-rVI (median reduction of 2.9%; n = 11) compared with neurons dialyzed with the scrambled peptide sRACK1-rVI (median reduction of 25.5%; n = 5; p < 0.01; paired t test). However, the DOI effect on GABAA currents in neurons dialyzed with the peptide AKAP[31–52] was not significantly different from that in control cells (median reduction of 17.6%; n = 5;p > 0.05; paired t test). These results suggest that, in PFC pyramidal neurons, serotonergic modulation of GABAA receptors requires the anchoring of activated PKC to the vicinity of the channel by RACK1.
DISCUSSION
Because dysfunction of both serotonin and GABA neurotransmission in PFC has been implicated in mental disorders (Manji et al., 2001) and the link between them was unclear, we examined the impact of serotonin on postsynaptic GABAA receptor functions in PFC pyramidal neurons. Our results show that application of serotonin leads to a reduction of GABAA receptor currents, suggesting that activation of serotonin signaling can suppress the GABAergic inhibition in PFC circuits. Indeed, recordings of pyramidal neurons in frontal cortical slices have shown that 5-HT decreases the amplitude of evoked IPSCs by 20% (Zhou and Hablitz, 1999). Although 5-HT could increase the excitability of PFC GABAergic interneurons, leading to increased presynaptic release of GABA (Zhou and Hablitz, 1999), our current study provides evidence showing that 5-HT could also decrease the postsynaptic response to GABA in pyramidal neurons through an intracellular signaling cascade. This suppression of GABAergic inhibition may provide a negative feedback mechanism for serotonergic regulation of the activity of PFC circuits.
To determine which 5-HT receptor(s) may mediate the serotonergic modulation of GABAA receptor currents, we first need to know what 5-HT receptors are expressed in PFC neurons. With the single-cell RT-PCR technique (Lambolez et al., 1992; Monyer and Lambolez, 1995; Surmeier et al., 1996, 1997; Yan and Surmeier, 1996), mRNAs for multiple 5-HT receptors are found in individual PFC pyramidal neurons. Particularly, 5-HT1A and 5-HT2A receptor mRNAs are most frequently detected in these cells, and 5-HT1B, 5-HT4, and 5-HT2C receptor mRNAs are also present in a subset of them. These results provide a comprehensive “blueprint” for the potential coexpression of 5-HT receptor subtypes in PFC pyramidal neurons. Moreover, our results are consistent with previous anatomical and physiological studies suggesting the possible colocalization of 5-HT1and 5-HT2 receptors in PFC neurons (Goldman-Rakic et al., 1990; Araneda and Andrade, 1991). The coexpression of multiple 5-HT receptors in the same neuron provides a flexible mechanism by which serotonin may modify the function of PFC in a manner that is both selective and precise (Andrade, 1998).
With the possible coexpression of multiple 5-HT receptors in PFC pyramidal neurons, selective pharmacological tools have been used to determine the 5-HT receptor(s) involved in serotonergic modulation of GABAA receptors. The serotonin effect on GABAA currents can be mimicked by a 5-HT2A/2C agonist and blocked by 5-HT2A/2C antagonists, suggesting that 5-HT2A/2C receptors play a predominant role in the serotonergic modulation of GABAA channels. Postsynaptic 5-HT2A receptors are enriched in apical dendrites proximal to the soma in PFC pyramidal neurons (Jakab and Goldman-Rakic, 1998), whereas GABAA receptors exhibit a compartmentalized distribution on postsynaptic domains of GABAergic synapses on the soma and proximal dendrites (Nusser et al., 1996), suggesting that 5-HT2A receptors may be localized in the vicinity of GABAA receptors in PFC pyramidal neurons. This synaptic organization would enable direct serotonergic modulation of local responses to inhibitory input, thereby regulating the integration of the neuron of its myriad inputs and ultimately affecting its output via axonal projections. Intracellular recordings of PFC slices show that activation of 5-HT2 receptors induces membrane depolarization and a burst of spikes (Araneda and Andrade, 1991; Tanaka and North, 1993). The 5-HT2 receptor-mediated reduction of GABAergic inhibition could be one of the potential mechanisms for the excitatory actions of 5-HT2 receptors in PFC circuits.
Several lines of evidence in the present study show that 5-HT2 modulation of GABAAreceptor currents is through a PKC-mediated pathway. First, inhibition of the upstream signaling component PLCβ, which should block the activation of PKC, eliminated 5-HT2 receptor modulation of GABAA currents. Second, the PKC activator PMA mimicked the 5-HT2 effect on GABAA currents. Third, the IP3 receptor antagonist and high BAPTA, which should block the elevation of intracellular Ca2+ and the activation of conventional PKC isoforms, greatly attenuated the ability of 5-HT2 receptors to modulate GABAA currents. Fourth, the PKC inhibitory peptide, which inhibits both autophosphorylation and substrate phosphorylation, blocked the 5-HT2 effect on GABAA currents. These physiological results suggest that PKC activation is required for 5-HT2modulation of GABAA channels. Because membrane-translocated PKC can be converted into an effector-independent form for sustained activation (Huang, 1989) once the C-terminal sites have been phosphorylated after the activation loop phosphorylation (Newton, 1997; Dutil et al., 1998; Le Good et al., 1998), 5-HT2 receptors can elicit physiological effects that are long lasting.
The involvement of PKC led us to speculate that PKC-induced phosphorylation of GABAA receptor channels may be the underlying molecular mechanism for 5-HT2modulation of GABAA currents. To test this, we used in vitro kinase assay to examine whether 5-HT2 receptors can increase PKC phosphorylation of GABAA receptor subunits. Treatment of PFC slices with a 5-HT2 receptor agonist significantly increased the in vitro kinase activity of PKC toward the peptide derived from GABAA receptor γ2 subunit, but not the peptide derived from GABAA receptor β2 subunit. These results suggest that activation of 5-HT2 receptors in PFC can enhance the PKC catalytic activity and potentiate PKC phosphorylation of GABAA receptors in a subunit-specific manner. In heterologous expression systems, phosphorylation of Ser-410 in β2, Ser-327 in γ2, and Ser-343 in γ2L is crucial for PKC-mediated downregulation of GABA currents (Kellenberger et al., 1992; Krishek et al., 1994), and PKC is much more effective with γ2-containing GABAA receptors (Krishek et al., 1994). Consistent with this, our data reveal that increased PKC phosphorylation of Ser-327 in γ2 subunit by 5-HT2 signaling may play a major role in mediating the serotonergic modulation of GABAAcurrents in PFC neurons. Although the β2 peptide is strongly phosphorylated in both control (−) and DOI-treated PFC slices, the lack of additional enhancement of its phosphorylation after 5-HT2 receptor activation could be attributable to its high basal phosphorylation by endogenous constitutively active PKC.
Given the broad substrate selectivity of PKC, the control of specificity becomes a crucial issue in signal transduction. Subcellular targeting through association with anchoring proteins has emerged as an important mechanism by which signaling enzymes achieve precise substrate recognition and enhanced efficacy of signal transduction (Rosenmund et al., 1994; Gao et al., 1997; Pawson and Scott, 1997; Yan et al., 1999; Feng et al., 2000). The PKC family is composed of at least 10 isoforms, which are localized in different subcellular compartments to control different functions (Tanaka and Nishizuka, 1994). Recent studies have revealed the key role played by anchoring proteins in mediating PKC compartmentalization and functions (Mochly-Rosen, 1995; Mochly-Rosen and Gordon, 1998). RACK1, one member of the RACK family proteins, binds only activated PKC isoforms in a selective manner (Mochly-Rosen et al., 1991) and is strongly associated with neuronal GABAA receptors in in vitro assays (Brandon et al., 1999). Binding to RACK1 results in augmentation of substrate phosphorylation by PKC, and blocking PKC–RACK1 interaction leads to inhibition of PKC-mediated function (Ron et al., 1994, 1995). In aged rat brain cortex, the expression of RACK1 is significantly reduced, and the RACK1-interacting PKC isoenymes do not translocate from soluble to membrane during stimulation (Battaini et al., 1997), suggesting that RACK1 is particularly important in cortical functions. To test whether the targeting of activated PKC to GABAA receptors via RACK1 may endow the enzyme to effectively phosphorylate these receptors in vivo, we examined the involvement of RACK1 in serotonergic modulation of GABAA currents in PFC neurons. Dialysis with a RACK1-derived peptide that can specifically disrupt the interaction between RACK1 and PKC (Ron et al., 1994) eliminated the serotonergic modulation of GABAA currents, whereas a peptide that can disrupt the AKAP79–PKC complex (Klauck et al., 1996) had no effect on serotonergic modulation of GABAA currents. These results suggest that targeting of activated PKC to GABAA receptors via RACK1 is crucial for the regulation of GABAAcurrents by the 5HT2–PKC signaling pathway.
Together, our results show that activation of 5-HT2 receptors decreased GABAA receptor currents in PFC pyramidal neurons, which may provide a postsynaptic mechanism for an excitatory role of serotonin in PFC circuits. In serotonin deficit diseases (e.g., depression), the GABAergic inhibition in PFC may be over potent, leading to the decreased activity (hypoactivity). On the other hand, in serotonin excess diseases (e.g., anxiety), the GABAergic inhibition in PFC may be over suppressed, leading to the increased activity (hyperactivity). The serotonergic modulation of GABAA receptor functions is dependent on activation of the RACK1-anchored PKC. Elucidation of the signal transduction pathway engaged in serotonergic modulation of GABAA receptors raises the possibility that intracellular signaling components could be potential targets for novel pharmacological agents with greater therapeutic potential and fewer side effects in the treatment of neuropsychiatric disorders.
Footnotes
This work was supported by start-up packages from State University of New York at Buffalo (J.F. and Z.Y.), a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (Z.Y.), and National Institutes of Health Grant NS41722 (J.F.).
Correspondence should be addressed to Dr. Zhen Yan, Department of Physiology and Biophysics, State University of New York at Buffalo, 124 Sherman Hall, Buffalo, NY, 14214. E-mail: zhenyan@buffalo.edu.
REFERENCES
- 1.Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann NY Acad Sci. 1998;861:190–203. doi: 10.1111/j.1749-6632.1998.tb10191.x. [DOI] [PubMed] [Google Scholar]
- 3.Araneda R, Andrade RA. 5-HT2 and 5-HT1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 4.Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the aging rat brain. Trends Neurosci. 1997;20:410–415. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- 5.Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: a family re-united? Trends Pharmacol Sci. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- 6.Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 7.Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breier A. Serotonin, schizophrenia and antipsychotic drug action. Schizophr Res. 1995;14:187–202. doi: 10.1016/0920-9964(94)00043-8. [DOI] [PubMed] [Google Scholar]
- 9.Busatto GF, Kerwin RW. Perspectives on the role of serotonergic mechanisms in the pharmacology of schizophrenia. J Psychopharmacol. 1997;11:3–12. doi: 10.1177/026988119701100102. [DOI] [PubMed] [Google Scholar]
- 10.Conde F, Marie-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 11.Deakin JF. 5HT2 receptors, depression and anxiety. Pharmacol Biochem Behav. 1988;29:819–820. doi: 10.1016/0091-3057(88)90215-8. [DOI] [PubMed] [Google Scholar]
- 12.Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, Opeskin K, Copolov DL. Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem. 1999;72:1593–1599. doi: 10.1046/j.1471-4159.1999.721593.x. [DOI] [PubMed] [Google Scholar]
- 13.Dubovsky SL, Thomas M. Serotonergic mechanisms and current and future psychiatric practice. J Clin Psychiatry. 1995;2 [Suppl 56]:38–48. [PubMed] [Google Scholar]
- 14.Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr Biol. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Yan Z, Ferreira AB, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Regulation of the formation and function of dendritic spines by spinophilin. Proc Natl Acad Sci USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 17.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 18.Fuxe K, Ogren SO, Agnati LF, Benfenati F, Fredholm B, Andersson K, Zini I, Eneroth P. Chronic antidepressant treatment and central 5-HT synapses. Neuropharmacology. 1983;22:389–400. doi: 10.1016/0028-3908(83)90188-0. [DOI] [PubMed] [Google Scholar]
- 19.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 20.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 21.Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- 23.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 24.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. levated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor utant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang KP. The mechanism of protein kinase C activation. Trends Neurosci. 1989;12:425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- 26.Jakab RL, Goldman-Rakic PS. 5-hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 28.Kellenberger S, Malherbe P, Sigel E. Function of the alpha 1 beta 2 gamma 2S gamma-aminobutyric acid type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- 29.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 30.Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res Rev. 1984;8:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 31.Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 32.Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 33.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 34.Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, Kraus JE. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998;44:1099–1117. doi: 10.1016/s0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 37.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 38.Meltzer HY. Role of serotonin in the action of atypical antipsychotic drugs. Clin Neurosci. 1995;3:64–75. [PubMed] [Google Scholar]
- 39.Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, Wilson AA, Kennedy SH. Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry. 1999;156:1029–1034. doi: 10.1176/ajp.156.7.1029. [DOI] [PubMed] [Google Scholar]
- 40.Miller EK. The prefrontal cortex: complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- 41.Mochly-Rosen D. Compartmentalization of protein kinases by intracellular receptor proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 42.Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- 43.Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monyer H, Lambolez B. Molecular biology and physiology at the single-cell level. Curr Opin Neurobiol. 1995;5:382–387. doi: 10.1016/0959-4388(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 45.Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- 46.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 47.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 49.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 50.Porter NM, Twyman RE, Uhler MD, Macdonald RL. Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron. 1990;5:789–796. doi: 10.1016/0896-6273(90)90338-g. [DOI] [PubMed] [Google Scholar]
- 51.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of beta protein kinase C in vivo. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- 53.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 54.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 55.Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Somogyi P, Kisvardy ZF, Martin KAC, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized basket cells in the striate cortex of the cat. Neuroscience. 1983;10:261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- 57.Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann NY Acad Sci. 1997;836:220–232. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- 58.Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY. Serotonin1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res. 1996;708:209–214. doi: 10.1016/0006-8993(95)01361-x. [DOI] [PubMed] [Google Scholar]
- 59.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 60.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- 63.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 64.Tukey JW. Exploratory data analysis. Addison-Weley; Menlo Park, CA: 1977. [Google Scholar]
- 65.Uylings HBM, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–61. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 66.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 68.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 69.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]