Abstract
The cerebral cortex provides a major source of inputs to the basal ganglia. As has been well documented, the topography of corticostriatal projections subdivides the striatum into a mosaic of functionally distinct sectors. How information flow from these striatal sectors remains segregated or not within basal ganglia output nuclei has to be established.
Electrophysiologically identified neurons of the rat substantia nigra pars reticulata were labeled by juxtacellular injection of Neurobiotin, and the spatial organization of their dendritic arborizations was analyzed in relation to the projection fields of individual striatal sectors. Thirty-nine nigral neurons located in the projection territory of the distinct striatal sensorimotor sectors were reconstructed. The data show that the dendritic arborizations of nigral neurons conform to the geometry of striato-nigral projections. Like striatal projections, the arborizations formed a series of curved laminas enveloping a dorsolaterally located core. Although dendritic fields of the neurons lying in the laminae were flat, those located in the core were spherical or cylindrical, thereby conforming to the shape of the striatal projection fields. This remarkable alignment between the dendritic arborizations of nigral neurons and the projection fields from individual striatal districts supports the concept of a parallel architecture of the striato-nigral circuits. However, pars reticulata neurons usually extend part of their dendrites within adjacent striatal projection fields, thereby ensuring a continuum between channels. The extension of the dendritic arborizations within the striatal projection fields suggests that nigral neurons integrate the information that is relevant for the completion of the specific motor behavior they control.
Keywords: substantia nigra pars reticulata, dendritic fields, juxtacellular injection, functional compartmentation of the basal ganglia, three-dimensional reconstruction, striato-nigral projections
The basal ganglia provide a major integrative system of the forebrain that is involved in adaptive control of behavior (Graybiel, 1998). To achieve this function, basal ganglia process information from the entire cerebral cortex and redistribute these integrated signals toward various thalamic and brainstem nuclei related to motor, premotor, prefrontal, and limbic cortical areas (Parent, 1990).
How the basal ganglia integrate the diversity of cortical inputs remains controversial. According to current working models, a parallel modular architecture allows basal ganglia to segregate information originating from functionally different cortical areas (Alexander et al., 1986; Groenewegen and Berendse, 1994; Deniau and Thierry, 1997). In fact, the striatum, the input stage of basal ganglia, contains an ordered representation of the cerebral cortex (Webster, 1961; Yeterian and Van Hoesen, 1978; Veening et al., 1980; McGeorge and Faull, 1989;Alexander and Crutcher, 1990; Berendse et al., 1992; Kincaid and Wilson, 1996; Deniau and Thierry, 1997; Brown et al., 1998). This cortical representation subdivides the striatum into a mosaic of functionally distinct sectors. Because of the topographic organization of striatal projections, the segregation of cortical information appears to be further maintained in the subsequent stages of the basal ganglia system (Alexander et al., 1986; Hedreen and DeLong, 1991;Kitano et al., 1998). Supporting this statement, we have shown in the rat that the various sectors of the striatal mosaic are mapped in an orderly manner onto the substantia nigra pars reticulata (SNR) in the form of spatially segregated laminas arranged in an onion-like manner (Deniau et al., 1996). Because the different populations of nigral output neurons innervating distinct thalamic and brainstem targets are also spatially segregated with the same onion-like pattern (Deniau and Chevalier, 1992), we have proposed that such an anatomical arrangement provides a channeling mechanism by which inputs arising from functionally distinct striatal sectors are further maintained in separate output circuits of the basal ganglia. However, the important reduction in the number of neurons when passing from the input to the output nuclei of the basal ganglia indicates that a high degree of convergence must occur in these circuits (Percheron et al., 1987). Moreover, the large dimension and spatial arrangement of the dendrites of SNR neurons might provide a substrate for synaptic integration of information originating from functionally distinct striatal sectors (Grofova et al., 1982; François et al., 1987).
Therefore, to further understand the mode of information processing in basal ganglia, we have studied in the rat the dendritic organizations of SNR neurons and examined their relations to the topography of striato-nigral projections. Only cells of the lateral SNR where projections from the sensorimotor sectors of striatum terminate were investigated. For this purpose, neurons from the different regions of the SNR previously described (Deniau et al., 1996) and electrophysiologically characterized by their response to cortical stimulation were labeled with Neurobiotin using the juxtacellular injection method (Pinault, 1994). Three-dimensional (3D) reconstructions of dendritic arborizations were performed and compared with the lamellar organization of the striato-nigral projections.
MATERIALS AND METHODS
Animal preparation
Experiments were performed on 55 adult male Sprague Dawley rats (weight 250–350 gm; Charles River, St. Aubin les Elbeuf, France). Surgical procedures were applied in strict accordance with the European Communities Council directive 86/609/EEC, 1986. Animals were initially anesthetized by an injection of pentobarbital (40 mg/kg, i.p.; Sanofi, Libourne, France) and fixed in a conventional stereotaxic apparatus (Unimécanique, Epinay sur Seine, France). Anesthesia was maintained throughout the experiment by additional doses of pentobarbital (20 mg/kg, i.p.) or ketamine (50 mg/kg, i.m.; Imalgène 500, Rhone-Mérieux, France). In addition, incision and pressure points were infused with lidocaine. Heart beat and pedal withdrawal reflex were monitored throughout the experiment to assess the depth of anesthesia. Body temperature was maintained between 37 and 38°C by the use of a homeothermic blanket.
Electrophysiological characterization of the nigral neurons
Extracellular single unit recordings (Fig.1A) were made in the SNR using glass pipettes (15–20 MΩ) containing 1.5% Neurobiotin (Vector, Burlingame, CA) in 0.5 m NaCl. Action potentials of single neurons were recorded using the active bridge mode of an Axoclamp 2 B amplifier (Axon Instruments, Foster City, CA), amplified (1000×), filtered (0.3–3 kHz) with an AC/DC amplifier (DAM 50, World Precision Instruments, Hertfordshire, UK), and viewed on a memory oscilloscope (Tektronix, Courtaboeuf, France). GABAergic neurons of the SNR were unambiguously distinguished from the dopaminergic neurons of the substantia nigra pars compacta using their classical electrophysiological characteristics: short duration spikes (total duration <2 msec) and ability to generate high-frequency action potential discharges (>10 Hz) without a decrease in spike amplitude (Bunney et al., 1973; Deniau et al., 1978; Guyenet and Aghajanian, 1978). During these experiments, the electrical activity of SNR cells was examined on-line and stored with a Digital Tape Recorder (DTR-1404, Biologic, Claix, France) for off-line analysis. Spikes were detected from instrumental noise using a window discriminator (World Precision Instruments) and sampled by a computer connected to a laboratory interface (CED 1401Plus, Cambridge Electronic Design, Cambridge, UK). Peristimulus time histograms (1 msec bins) were generated from 50–100 stimulation trials with the Spike 2 data acquisition and analysis program (Cambridge Electronic Design). The criterion used to establish the existence of an excitatory or inhibitory response was a change >50% in the number of spikes as compared with the prestimulus period for at least three consecutive bins.
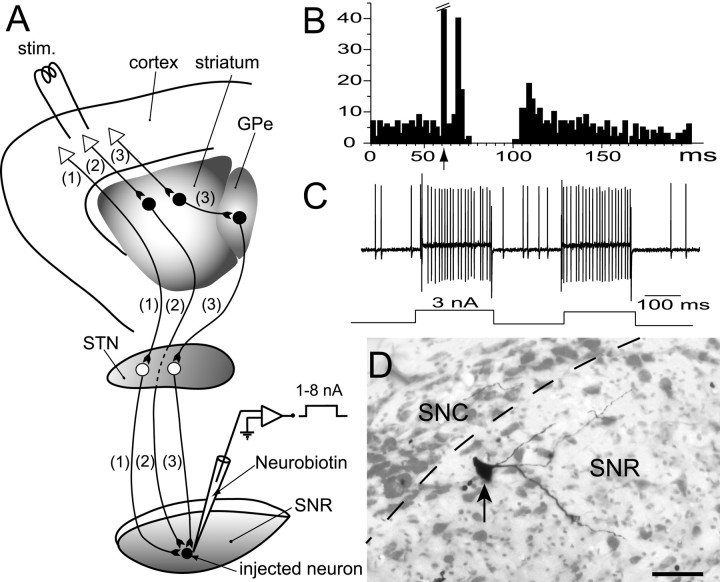
Fig. 1.
Experimental protocol for labeling neurons in the SNR using juxtacellular application of Neurobiotin.A, Experimental design. Neurons of the SNR were extracellularly recorded and characterized on the basis of their spontaneous spike discharges (see Materials and Methods for details) and their response to cortical stimulation (stim.). A cortical stimulation can influence the activity of SNR cells through three main circuits: a fast excitatory cortico-subthalamo-nigral circuit [(1)], an inhibitory cortico-striato-nigral circuit [(2)], and a slow excitatory cortico-striato-pallido-subthalamo-nigral circuit [(3)]. In this diagram, inhibitory neurons are represented as filled circles, and excitatory neurons are represented as open circles andopen triangles. B, Peristimulus time histogram illustrating the typical triphasic excitatory–inhibitory–excitatory sequence evoked by a cortical stimulation in a neuron of the SNR receiving a convergent influence from the three circuits mentioned above. Arrow indicates the time of stimulation. C, Neuronal activity recorded during the juxtacellular application of Neurobiotin with positive current pulses of 200 msec duration and 3 nA intensity (bottom trace: current monitor). D, Photomicrograph of the labeled neuron (arrow points to the cell body). Note that only one cell was labeled. Scale bar, 50 μm. GPe, External globus pallidus; SNC, substantia nigra pars compacta.
To label nigral neurons within the projection fields of the different functional sectors of the sensorimotor striatum, electrophysiological responses of SNR cells evoked by electrical stimulation of various sensorimotor cortical areas were used as a guide for stereotaxic placement of recordings within SNR [stereotaxic coordinates: anterior (A), 3–4 mm; lateral (L), 2–3. Stimulations (200 μsec duration, 20–100 μA intensity) were applied to either the orofacial motor cortex (A, 12–11.5; L, 4.5–4), the foreleg motor cortex (A, 11.2; L, 3.4), the oculomotor area (A, 11.7; L, 1.5) or the prelimbic area of the prefrontal cortex (A, 11.2; L, 0.7, at a depth of 3 mm from the surface] ipsilateral to the recorded SNR. Stereotaxic coordinates of recording and stimulating sites were determined using the atlas of Paxinos and Watson (1986) and the functional map of the sensorimotor cortex established by Neafsey et al. (1986). Electrical stimulation of the motor cortex was achieved through a bipolar stimulating electrode (1 mm tip separation) inserted orthogonally to the cortical surface at a depth of 1.5 mm. The prelimbic cortex was stimulated using a coaxial stainless steel electrode (diameter, 400 μm; tip-barrel distance, 300 μm). As classically established in previous electrophysiological analyses (Ryan and Clark, 1991; Kita, 1994; Maurice et al., 1999), a cortical stimulation evokes three main types of events in SNR cells (Fig. 1B): a short-latency excitation resulting from the activation of the fast cortico-subthalamo-nigral circuit, an inhibition of longer latency caused by the activation of the cortico-striato-nigral circuit, and a late excitation triggered by the activation of the polysynaptic cortico-striato-pallido-subthalamo-nigral circuit.
Labeling and visualization of the nigral neurons
Recorded neurons were labeled using juxtacellular injection of Neurobiotin (Pinault, 1994). Briefly, positive pulses of current (1–8 nA, 200 msec duration) were applied at a frequency of 2.5 Hz through the bridge circuit of the amplifier. The current was slowly increased, and the electrode was advanced by steps of 1 μm (LSS-1000 Inchworm Motor Positioning System, Burleigh Instruments, Fishers, NY) onto the neuron until the cell discharge was driven by the injected current (Fig. 1C). Current pulses were applied for a 10–30 min period to obtain a reliable labeling of neuronal processes (Fig.1D).
Two to five hours after the end of the injection, the animal received a lethal dose of pentobarbital and was perfused via the ascending aorta with 200 ml of saline followed by 500 ml of 0.3% glutaraldehyde and 4% paraformaldehyde in phosphate buffer (PB), 0.1 m, pH 7.4. In all experiments, brains were post-fixed for 2 hr in the same fixative solution without glutaraldehyde and then immersed in 20% sucrose PB at 4°C until sectioned. Before sectioning, brains were cut dorsoventrally at the level of the cerebellum along a vertical plane tilted 18° toward the rostral part of the brain. Frozen sections parallel to this plane were cut at 50–70 μm and serially collected in PB (0.1 m; pH 7.4). After several rinses in PB, Neurobiotin was revealed by incubation of the sections in the avidin–biotin peroxidase complex (Vector Labs; 1:100) in PB containing 0.3% Triton X-100 for at least 12 hr at 4°C. Incubated sections were washed in PB (2 × 10 min) before immersion in a solution containing 0.05% 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO), 0.4% nickel-ammonium sulfate, and 0.0006% H2O2. After several washes in PB, sections were mounted on gelatin-coated slides, counterstained with Safranine, and dehydrated through alcohol to xylene for light microscopic examination.
Anatomical analysis
Neuronal reconstruction. Labeled neurons and boundaries of the SNR were traced and reconstructed from successive serial sections. Two-dimensional (2D) reconstructions were made from drawings performed under 10–40× objectives using a drawing tube attached to a light microscope (Laborlux S, Leitz, Rueil-Malmaison, France). To achieve the 3D reconstructions, cell bodies, dendritic arborizations, and boundaries of the SNR were precisely drawn under 25–63× oil immersion objectives and plotted in 3D using the video computer Neurolucida system (MicroBrightField, Inc.). Three-dimensional models of neurons were visualized using the Lightwave software (Newtek Inc., San Antonio, TX). For this purpose a Perl script that reads a Neurolucida data file and converts it into a Lightwave script in which the X, Y, and Z polygon coordinates corresponding to each structure (i.e., cell body, dendrites, and SNR boundaries) are described as tri-dimensional objects was developed. Models were then processed for solid surface rendering using the Lightwave software. Three-dimensional reconstructed models of neurons could be rotated around any of the x-, y-, and z-axes. In addition, light sources and camera could be adjusted to enhance the 3D appearance of reconstructed neurons on 2D pictures.
Reconstruction of striatal projection fields in the SNR.Three-dimensional reconstructions of striato-nigral projections were made using data from previous experimental studies (Deniau et al., 1996) in which the anterograde tracer wheat germ agglutinin conjugated to horseradish peroxidase was injected into distinct functional sectors of the dorsal striatum. Contours of labeled striatal projection fields within the SNR and cytoarchitectonic boundaries of the SNR were drawn under 10–40× objectives and plotted in 3D using the method described above.
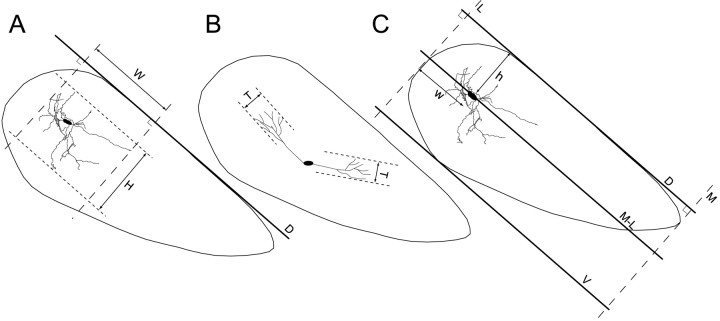
Topological parameters of labeled neurons and striatal projection fields in the SNR. Several topological parameters of the dendritic field of each labeled neuron were measured. The dimension of the dendritic arborization along the rostrocaudal axis was determined on the basis of the number of coronal sections occupied by the dendritic tree and the thickness of brain sections. The shrinkage that occurs in the z-axis of the brain sections during dehydration was measured for each section and corrected for with the software. The mediolateral extension of the dendritic field (Fig.2A,W) was determined on coronal views of the reconstructed neurons by measuring the distance between the extremities of the dendritic field along an axis parallel to the dorsal edge of the SNR (Fig. 2A, axis D). The dorsoventral extension of the dendritic field (Fig. 2A,H) was determined on coronal views of the reconstructed neuron by measuring the length of the dendritic field along an axis orthogonal to the above-defined mediolateral D axis. In neurons characterized by a flat disc shape, an additional parameter of thickness was measured. The thickness (T) of the dendritic arborization was determined at different levels of the dendritic tree by measuring the dendritic arborization along an axis orthogonal to a plane tangential to the surface of the disc (Fig.2B).
Fig. 2.
Coronal sections of the left SNR illustrating the axis of reference used to measure the length of the dendritic arborizations and to determine the coordinates of the somata of the labeled neurons within the SNR. A, The mediolateral (W) and dorsoventral (H) extent of the dendritic fields were measured, relative to the D axis passing along the dorsal edge of the SNR. This axis takes into account the inclination of the SNR into the brain and allows the definition of the largest mediolateral axis of the nucleus. B, Illustration of the method used to measure the thickness (T) of neurons presenting a discoid dendritic field (see Materials and Methods for details). C, The dorsoventral and mediolateral coordinates of the neuronal somata within the SNR were determined using the axes D, M-L, V,M, and L. These axis were traced on the coronal section of the SNR that contains the soma of the neuron studied. The axis D passes along the dorsal surface of the SNR and defines the mediolateral axis of the nucleus. The axisM-L parallels the axis D and passes through the soma of the neuron. The axis V parallels the axis D and passes tangentially to the ventral surface of the SNR. The axis L is orthogonal to the axisD and passes tangentially to the lateral edge of the SNR. Finally, the axis M is orthogonal to the axisD and passes tangentially to the medial edge of the SNR. The dorsoventral coordinate of the neuron (h) was determined by measuring the distance between the axisM-L and the axis D. To compare the position of neurons from different animals, this value was normalized relative to the maximal thickness of the SNR measured as the distance between the axis D and the axis V. The mediolateral coordinate (w) was determined by measuring the distance between the soma of the neuron and the axisL. This measure was normalized relative to the maximal extension of the nucleus along its mediolateral axis. This extension was determined as the distance separating axes L andM.
Similar procedures were used to measure the striatal projection fields in the SNR and to compare the geometry of striatal projections with the dendritic arborizations of nigral neurons. To facilitate this comparison, 3D composite models of the SNR were generated in which reconstructed nigral neurons were incorporated within 3D models of the striatal projection fields. To generate these composite models, the position of neurons within the parent SNR was precisely determined. This was achieved by measuring the distance separating the soma from the boundaries of the nucleus along the rostrocaudal, mediolateral, and dorsoventral axes (Fig. 2C). These values, normalized to the size of the parent SNR along the three x-, y-, and z-axes, were used to reposition the neurons within the 3D models of the striatal projection fields. A similar procedure was used to generate 3D models of SNR incorporating several neurons reconstructed in 3D. To take into account differences in the size and shape of the SNR from different animals, the dendritic lengths of reconstructed neurons were corrected along the x-,y- and z-axes by a factor corresponding to the ratio of length, along the three axes, of the parent SNR of the neurons and the target SNR in which the neurons were repositioned. Numerical values are given as mean ± SD.
RESULTS
General somatodendritic features of the labeled neurons
Thirty-nine neurons were stained in the sensorimotor district of the SNR. The somata of these cells were distributed throughout the lateral region of the SNR where the projections of the sensorimotor sectors of the striatum terminate. Labeled cells were characterized by a soma of variable shape: fusiform, triangular, or polygonal. Cell bodies had a size of 17–60 μm (31.7 ± 9.3 μm;n = 39) along their major axis and 9–25 μm along their minor axis (15.92 ± 3.4 μm; n = 39) with a mean cross-sectional area of 168–645 μm2 (349.2 ± 118.9 μm2; n = 39). Two to five primary dendrites arose from the soma. These dendrites were relatively unbranched, with 18–52 (34.8 ± 10.5;n = 19) bifurcations. The total dendritic length ranged from 5,070 to 14,800 μm (8550 ± 2600 μm; n = 19). Dendrites extended from 350 to 1233 μm (724.1 ± 194.8 μm; n = 39) in the anteroposterior direction, 140–1488 μm (756.8 ± 280.5 μm; n = 39) mediolaterally, and 264–1170 μm (480.2 ± 162.5 μm;n = 39) dorsoventrally.
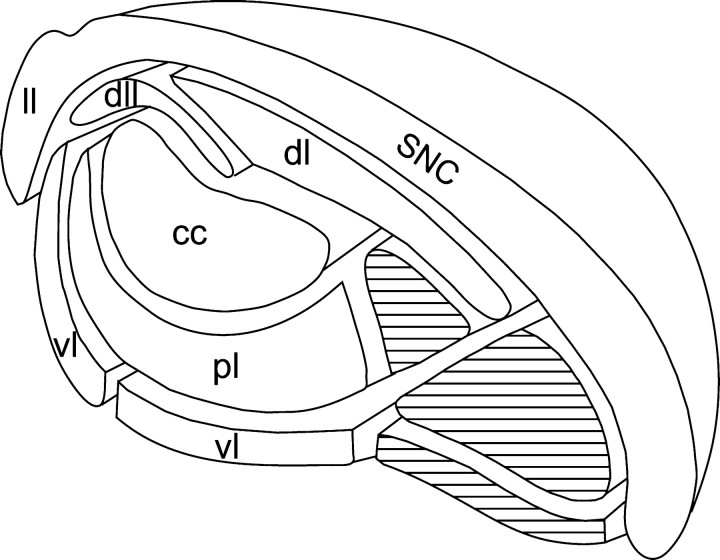
For descriptive purposes and to compare the dendritic arborizations of nigral cells with the striato-nigral projections, the SNR was subdivided into five main regions on the basis of the previously described compartmentalization of striato-nigral projections (Deniau et al., 1996). These subdivisions are illustrated in Figure3. They comprise a central core enveloped by a series of curved lamina organized in an onion-like manner. The central core is located in the dorsolateral part of the SNR and corresponds to the projection field of the orofacial striatal sector. This “core” is overlaid dorsally by a lamina that receives projections of the striatal sectors related to the insular, gustatory, and perirhinal cortices. The lateral extension of this lamina was further individualized because this region does not receive afferents from the perirhinal striatal sector. The central core is enveloped ventrally by the pericore lamina, a subdivision that receives inputs from striatal sectors affiliated with the facial, oculomotor, and limb areas of the sensorimotor cortex. This pericore lamina is edged laterally and ventrally by the lateral and ventral laminae. The lateral lamina receives projections from the auditory striatal sector, and the ventral lamina is innervated by striatal sectors related to visual and oculomotor cortical areas.
Fig. 3.
Schematic representation of the onion-like compartmentalization of the SNR as defined on the basis of the organization of striato-nigral projections. The subdivisions receiving the projections of the sensorimotor districts of the striatum are represented in white and are named by theirinitials. The hatched compartmentsindicate subdivisions related to the limbic striatum. These subdivisions are not considered in the present study.cc, Central core region innervated by the striatal sector related to orofacial sensorimotor cortical areas;dl, dorsal lamina corresponding to the projection field of the striatal sector related to insular, gustatory, and perirhinal cortical areas; dll, dorsolateral lamina corresponding to the projection field of the striatal sector related to insular and gustatory cortical areas; pl, pericore lamina receiving the projections of the striatal sectors affiliated with facial, oculomotor, and limb areas of the sensorimotor cortex,ll, lateral lamina receiving the projections of the auditory striatal sector; vl, ventral lamina innervated by the striatal sectors related to visual and motor cortical areas.
Morphometric measurements and reference numbers of labeled neurons in each of these subdivisions of the SNR are summarized in Table1.
Table 1.
Reference number (#), position of the soma within the SNR, and extension of the dendritic arborizations of the neurons labeled in the various sensorimotor subdivisions of the rat SNR
| Subdivision | Neurons | |||||||
|---|---|---|---|---|---|---|---|---|
| # | Soma position (%) | Extension of dendritic field (in μm) | ||||||
| R-C | L-M | D-V | R-C | M-L | D-V | Thickness | ||
| Dorsal lamina | 4 | 31 | 25 | 26 | 720 | 696 | 336 | 336 |
| 12 | 30 | 36 | 7 | 600 | 672 | 330 | 330 | |
| Dorsolateral lamina | 8g | 53 | 25 | 23 | 909 | 852 | 600 | 153 /255 |
| 35d | 65 | 10 | 28 | 720 | 252 | 564 | 252 | |
| Central core | 6 | 40 | 27 | 25 | 584 | 684 | 456 | |
| 8d | 53 | 24 | 25 | 800 | 408 | 360 | ||
| 11d | 79 | 21 | 27 | 450 | 492 | 408 | ||
| 13g | 54 | 27 | 42 | 415 | 714 | 564 | ||
| 14g | 64 | 27 | 26 | 550 | 504 | 510 | ||
| 14d | 75 | 23 | 25 | 500 | 576 | 564 | ||
| 15d | 61 | 25 | 43 | 960 | 672 | 566 | ||
| 28 | 60 | 25 | 43 | 733 | 720 | 560 | ||
| 35g | 53 | 16 | 43 | 550 | 600 | 384 | ||
| Pericore lamina (facial sector) | 5d | 25 | 37 | 51 | 450 | 1116 | 492 | 115 /384 |
| 9 | 13 | 40 | 65 | 489 | 1416 | 336 | 163 /250 | |
| 13d | 18 | 31 | 65 | 900 | 852 | 588 | 125 /588 | |
| 15g | 61 | 43 | 67 | 840 | 552 | 552 | 105 /221 | |
| 24d | 43 | 41 | 68 | 800 | 768 | 480 | 115 /192 | |
| 26 | 32 | 20 | 75 | 576 | 1056 | 528 | 134 /288 | |
| 27 | 37 | 15 | 66 | 583 | 1002 | 528 | 67 /240 | |
| 30 | 44 | 39 | 63 | 900 | 1032 | 492 | 173 /355 | |
| 39 | 51 | 31 | 80 | 1050 | 678 | 276 | 96 /211 | |
| Pericore lamina (limb sector) | 11g | 55 | 53 | 67 | 650 | 972 | 264 | 67 /115 |
| 18 | 64 | 52 | 60 | 600 | 720 | 390 | 125 /390 | |
| 19 | 26 | 54 | 73 | 850 | 648 | 408 | 192 /408 | |
| 20 | 35 | 53 | 70 | 841 | 948 | 672 | 144 /288 | |
| 33 | 65 | 40 | 76 | 660 | 552 | 408 | 96 /408 | |
| 50 | 23 | 57 | 80 | 600 | 888 | 444 | 125 /444 | |
| Pericore lamina (oculomotor sector) | 10 | 35 | 59 | 66 | 1233 | 1488 | 1170 | 180 /360 |
| 21 | 62 | 70 | 70 | 1010 | 1212 | 780 | 221 /780 | |
| 22g | 31 | 43 | 46 | 350 | 948 | 390 | 96 /180 | |
| Lateral lamina | 23 | 30 | −14 | 23 | 960 | 500 | 417 | 348 /500 |
| 36 | 52 | 5 | 51 | 840 | 648 | 645 | 120 /432 | |
| 37n1 | 28 | −12 | 40 | 800 | 481 | 224 | 388 /555 | |
| 37n2 | 36 | 6 | 41 | 830 | 432 | 429 | 159 /600 | |
| Ventral lamina | 5g | 55 | 50 | 95 | 634 | 1140 | 456 | 101 /144 |
| 22d | 33 | 16 | 88 | 614 | 924 | 270 | 96 /163 | |
| 24g | 35 | 12 | 74 | 950 | 925 | 444 | 96 /228 | |
| 34 | 79 | 21 | 80 | 741 | 926 | 456 | 96 /150 | |
The position occupied by the somata of the labeled neurons is indicated as a percentage of the length of the nucleus along the rostrocaudal (R-C), lateromedial (L-M), and dorsoventral (D-V) axes. A minus sign along the lateromedial axis (neurons 23 and 37n1, lateral lamina) indicates that the neurons were lying beyond the lateral border of the SNR, within the pars lateralis. The thickness of the dendritic arborization was measured on neurons presenting a flat discoid or elliptic shape (see Materials and Methods). Two values are given for neurons with thickness that differed markedly in opposite regions of the dendritic field.
Neurons located in the central core subdivision
The central core subdivision is delineated by the projections of the striatal sector related to orofacial sensorimotor cortical areas. They form a longitudinal cylinder extending throughout the entire rostrocaudal extent of the nucleus (Fig.4A,B). This cylinder is relatively narrow (208 μm mediolateral and 200 μm dorsoventral) in the rostral part of the SNR and progressively expands in the mid rostrocaudal part of the nucleus to reach a diameter of 738 μm along the mediolateral axis and 331 μm along the dorsoventral axis.
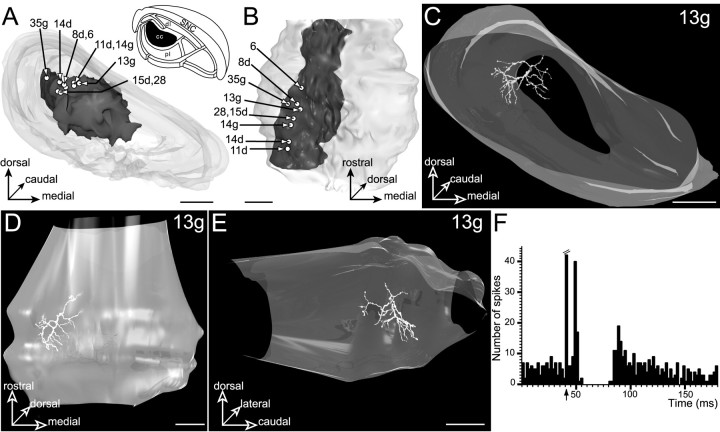
Fig. 4.
Neurons located in the central core subdivision (cc, filled area, inset inA) innervated by the striatal sector related to the orofacial sensorimotor cortical areas. A,B, Three-dimensional reconstruction of SNR illustrating the position of the labeled neurons with respect to the striatal projection field. In this and the following figures of the same type, the boundary of the SNR is represented in light gray, the striatal projection field is represented in dark gray, and the labeled neurons are represented as open circles. The SNR is shown from a rostral view inA and a ventral view in B.C–E, Three-dimensional reconstruction of neuron13g. The neuron is examined from a rostral view inC, a ventral view in D, and a medial view in E. Note that because of the lights placed around and within the SNR, a shadow of the dendritic arborization appears at the surface of the SNR. The reference number of the injected neuron is indicated on the top right corner of each panel. Scale bars, 350 μm. F, Peristimulus time histogram illustrating the electrophysiological response of neuron 13g to stimulation of the orofacial motor cortex (arrow: stimulation artifact). Note the clear-cut inhibitory period induced by the cortical stimulation.
Nine neurons (6, 8d, 11d,13g, 14g, 14d, 15d,28, 35g) were labeled in this nigral subdivision (Fig. 4A,B). In accordance with their anatomical localization within the central core, these cells exhibited a clear-cut inhibition [latency (L), 14.25 msec ± 3.69; duration (D), 27.66 msec ± 5.93] in response to stimulation of the orofacial motor cortex (Fig.4F). As documented previously (Maurice et al., 1999), this type of cortically evoked inhibitory response results from the activation of the direct striato-nigral circuit (Fig.1A).
Three representative neurons (13g, 6, and28) are illustrated in Figures4, 5, and 6. These core neurons presented a spherical or cylindrical dendritic arborization conforming to the shape of the striatal projection field. The length of the dendritic field ranged from 450 to 960 μm (615 ± 168 μm) along the anteroposterior axis, from 408 to 720 μm (596 ± 104 μm) along the mediolateral axis, and from 360 to 564 μm (485 ± 80 μm) along the dorsoventral axis. In all cases, dendritic fields were included almost entirely within the core subdivision (77–98.8% of the total dendritic length). A few dendritic branches extended within only the dorsally and ventrally adjacent subdivisions corresponding to the dorsal and pericore laminas, respectively.
Fig. 5.
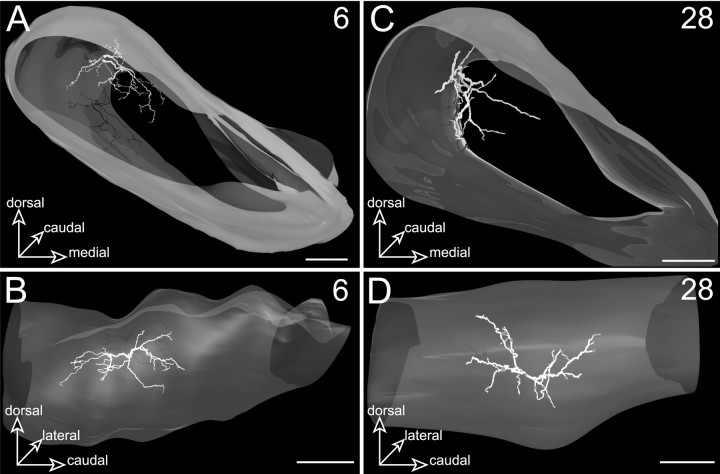
Examples of labeled neurons (6 and 28) located in the central core subdivision of SNR. The 3D reconstructed neurons are shown from a rostral view in A and C and from a medial view in B and D. Note that the dendritic fields of the neurons are essentially directed toward the inner part of the central core subdivision. Although neuron 6, the soma of which lies dorsally, has a dendritic field directed ventrally, neuron 28, the soma of which lies more ventrally, has its dendrites oriented dorsally. Scale bars, 350 μm.
Fig. 6.
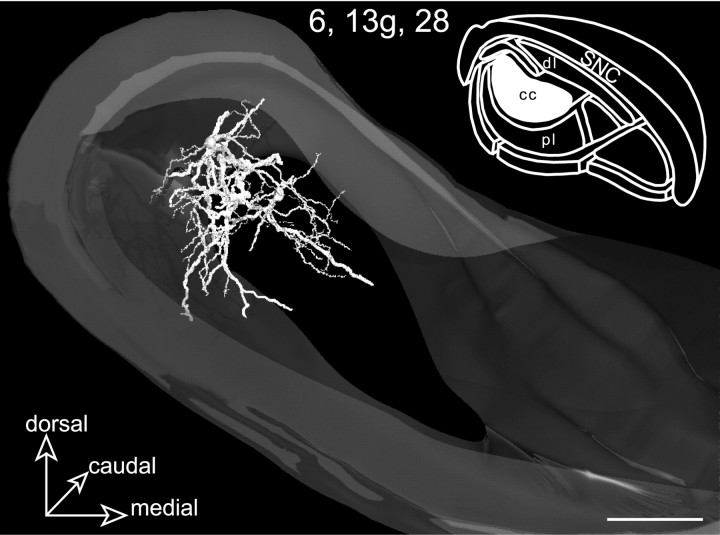
Rostral view of a 3D reconstructed model of the SNR incorporating the neurons 6, 13g, and28 labeled in the central core (cc). Remarkably, the overall dendritic arborizations formed a core structure that perfectly matched the projection field of the orofacial striatal sector (inset, filled area). Scale bars, 350 μm.
Neuron 13g, shown in Figure 4C–E, obeyed these rules of organization. Its soma occupied a dorsal position in the core subdivision. Most dendrites were directed ventrally and arborized extensively within the inner part of this subdivision. Because of its spherical shape, the dendritic field of the neuron perfectly conformed to the geometry of the striatal projection field. Moreover, the comparison of D and B in Figure 4 indicated that the rostrocaudal orientation of dendrites follows the oblique (rostromedial to caudolateral) orientation of the striatal projections. As determined on composite 3D models incorporating neurons and striatal projections, only a few distal dendritic segments (representing <0.5% of the total dendritic length) left the parent core subdivision. These dendritic segments extended ventrally within the pericore lamina.
Neuron 6 (Fig. 5A,B) provides another example of a cell lying dorsally in the central core of the SNR. As for neuron 13g, its dendritic arborization was mainly oriented ventrally to occupy the inner part of the parent core subdivision. A few dendritic branches representing 0.4% of the total dendritic length of the neuron extended ventrally within the pericore lamina.
Neuron 28, illustrated in Figure 5, C and D, provides an example of a neuron localized ventrally in the central core subdivision. Remarkably, its dendritic field adopted a curved shape that conformed to the curvature of the striatal projections. In addition, most dendritic branches were directed medially and dorsally to arborize within the inner part of the central core. However, part of the dendritic field representing 23% of the total dendritic length of the neuron extended laterally and rostrally within the adjacent pericore lamina.
When neurons 6, 13g, and 28 were incorporated within a single 3D model of the SNR (Fig. 6), the overall dendritic arborizations reconstituted a core-like structure with roughly the same shape and dimensions as the striatonigral projection field from the orofacial striatal sector.
Neurons located in the dorsal lamina
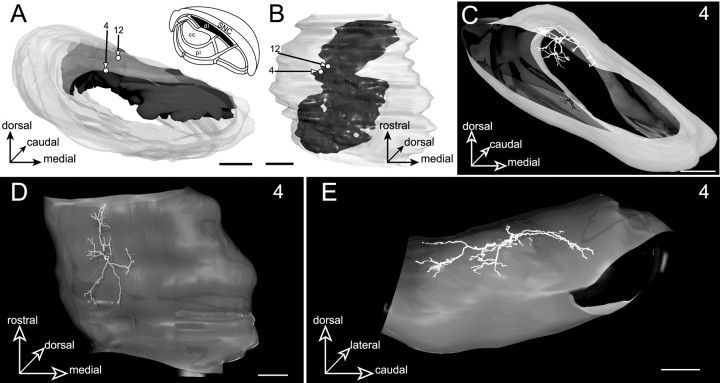
The projection field of the striatal sector related to the insular, gustatory, and perirhinal cortical areas forms a lamina of 227 μm maximal thickness and 858 μm maximal width occupying the dorsal edge of the SNR throughout the entire rostrocaudal extent of the nucleus (Fig.7A,B). Two neurons (4 and 12) were labeled in this dorsal subdivision. The somata of these cells were lying in a nearby position within the lateral part of the striatal projection field, neuron 4 being more ventral than neuron 12 (Fig.7A,B).
Fig. 7.
Neurons located in the dorsal lamina (dl, filled area, inset inA) innervated by the striatal sector related to insular, gustatory, and perirhinal cortical areas. A,B, Three-dimensional reconstruction of the SNR illustrating the position occupied by the somata of the labeled neurons (4 and 12) with respect to the striatal projection field. The SNR is examined from a rostral view inA and from a ventral view in B.C–E, The 3D reconstructed dendritic arborization of neuron 4. The labeled neuron is examined from a rostral view inC, a ventral view in D, and a medial view in E. Note the similitude between the geometry of the dendritic arborization and the spatial organization of the striatal projections. Scale bars, 350 μm.
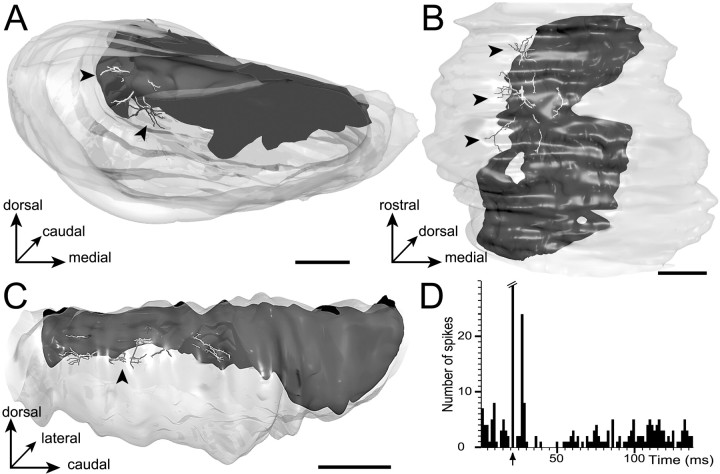
The dendritic arborization of neuron 4 is illustrated in Figure7C–E. The dendritic field had the overall shape of a flat ellipse oriented rostrocaudally. As shown on the medial view (Fig. 7E), dendrites were spreading out along the dorsal surface of the SNR conforming to the curvature of the nucleus. None of the dendrites were seen to enter dorsally in the adjacent pars compacta of the substantia nigra. Although the geometry of the dendritic field matched the lamellar shape of the striatal projection field, dendritic arborizations were not strictly included in this striatal projection field. As evidenced from topological measurements and through the observation of composite 3D models incorporating neurons and striatal projection fields (Fig.8), dendrites were found to cross the border of laterally and ventrally adjacent functional subdivisions, namely the dorsolateral lamina and the central core. The length of dendrites infiltrating these two subdivisions represented 6 and 52%, respectively, of the total neuron dendritic length. The dendritic arborization of neuron 12 (data not shown) shared the basic features of neuron 4 except that its extension was shorter along the rostrocaudal axis.
Fig. 8.
A–C, Composite 3D model of the SNR incorporating labeled neuron 4 (shown in Fig. 7) within the projection field of the striatal sector related to the insular, gustatory, and perirhinal cortical areas that forms the dorsal lamina. Because the striatal projections are made opaque, the only portions of dendrites visible are those extending outside of the striatal projection field. The model is examined from a rostral view inA, a ventral view in B, and a lateral view in C. Note that some terminal dendritic branches extend in adjacent subdivisions that lie ventrally and laterally to the dorsal lamina (see arrowheads). Scale bars, 350 μm.D, Peristimulus time histogram illustrating the electrophysiological response of neuron 4 to stimulation of the orofacial motor cortex (arrow indicates stimulation). The response consisted of a short latency excitation followed by a period of decreased activity.
The extension of the dendritic arborizations of neurons 4 and 12 within the central core subdivision suggested that, albeit located in the dorsal lamina, these cells receive a synaptic influence from the orofacial striatal sector. Accordingly, these two neurons responded to stimulation of the orofacial motor cortex by a brief period of decreased activity (L, 12–14 msec; D, 16–25 msec), consistent with the activation of the direct striato-nigral circuit (Maurice et al., 1999). In the case of neuron 4, this inhibition was preceded by a short latency excitation (Fig.8D).
Neurons located in the dorsolateral lamina
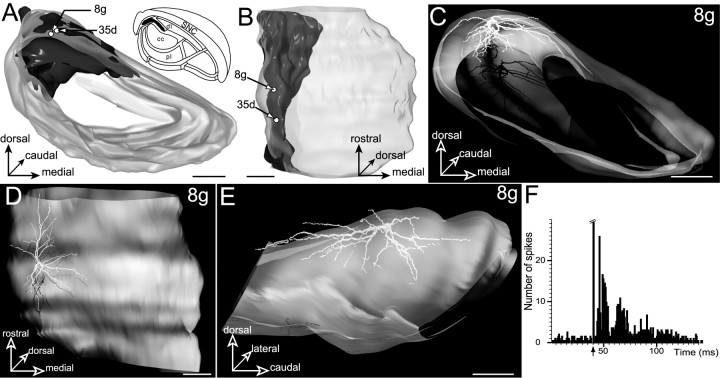
This subdivision corresponds to a lateral extension of the dorsal lamina with which it shares afferents from striatal sectors related to the insular and gustatory cortical areas. Projections of these striatal sectors form a lamina of 74–289 μm thickness occupying the dorsolateral part of the SNR (Fig.9A,B). The mediolateral position occupied by the striatal projection field within the SNR changes along the rostrocaudal axis of the nucleus. Rostrally, it occupies a lamina of 768 μm maximal width that edges the dorsal part of SNR, and caudally its position shifts progressively toward the lateral edge of the nucleus (Fig.9A,B). At this caudal level, striatal projections form a dorsoventrally oriented lamina of 464 μm height.
Fig. 9.
Neurons located in the dorsolateral lamina (filled area, inset inA,) innervated by the striatal sector related to insular and gustatory cortical areas. A, B, Three-dimensional reconstruction of the SNR illustrating the distribution of the labeled neurons (open circles) within the striatal projection field. The SNR is shown from a rostral view in A and a ventral view in B.C–E, Three-dimensional reconstruction of the dendritic arborization of neuron 8g. The neuron is examined from a rostral view in C, a ventral view in D, and a medial view in E. Note that the dendritic arborization of the neuron largely conforms to the spatial organization of the striatal projection field. Scale bars, 350 μm.F, Peristimulus time histogram illustrating the electrophysiological response of neuron 8g to stimulation of the orofacial motor cortex (arrow indicates stimulation). Note the lack of inhibitory component in the evoked response.
Two neurons (8g and 35d) were labeled in this dorsolateral subdivision of the SNR. Neuron 8g was located in the mid rostrocaudal extent of the nucleus, and neuron 35d was located in a more caudal region, where the lamina of striatal projections edges the lateral margin of the nucleus (Fig. 9B).
The dendritic arborization of neuron 8g is illustrated in Figure9C–E. This cell was characterized by a flat dendritic arborization forming a curved disk conforming to the geometry of the striatal projection field. Like striatal projections, the main dendritic trunks spread out rostrocaudally and mediolaterally and curved along the dorsolateral edge of the SNR (Fig.9C,D). With the exception of a few dendritic branches that crossed the boundaries of the medially adjacent dorsal lamina, most of the dendritic arborization (77% of the total dendritic length) was confined to the parent functional subdivision. Contrasting with the neurons of the dorsal lamina described above, no dendritic profile extended within the central core subdivision innervated by the orofacial striatal sector. Accordingly, the response of this neuron to stimulation of the orofacial motor cortex lacked the inhibitory component characteristic of the response induced by the activation of the direct cortico-striato-nigral circuit (Fig.9F).
Like neuron 8g, neuron 35d had a flat dendritic arborization, but it differed by its orientation within the SNR. Conforming to the dorsoventral orientation taken by the striatal projection field in the caudal SNR, the dendritic arborization of this neuron had the shape of a flat ellipse positioned vertically. No dendrite extended within the orofacial core region of the SNR. Accordingly, the stimulation of the orofacial motor cortex failed to evoke an inhibitory response in this neuron.
Neurons located in the pericore lamina
Projections originating from striatal sectors related to the facial, limb, and oculomotor areas of the sensorimotor cortex form a semicircular lamina that envelops ventrally and medially the central core subdivision. Although the projection fields of these three striatal sectors present large areas of overlaps, each of them occupies a defined position within the pericore lamina. Thus they will be considered separately.
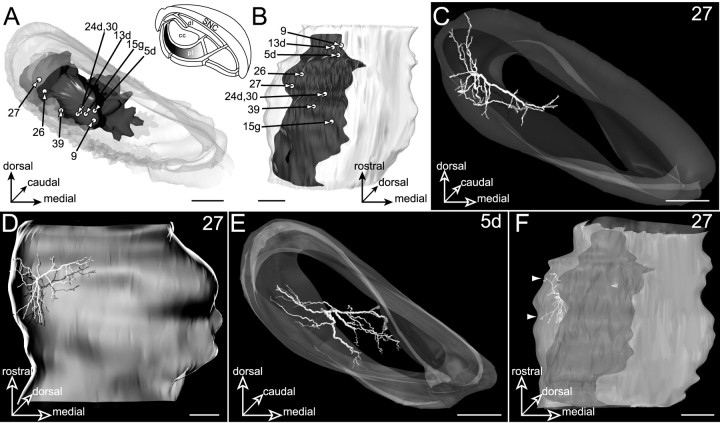
The facial striatal sector
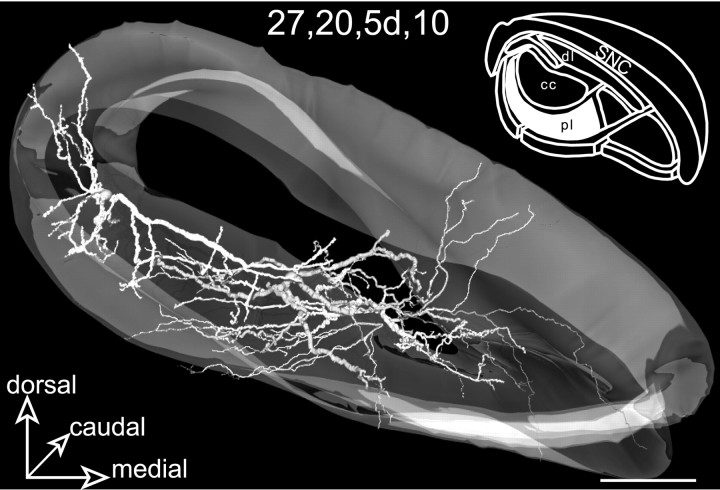
The projection field of the facial striatal sector is illustrated in Figure 10, A andB. It occupies the lateral two-thirds of the pericore lamina (mediolateral extension, 752 μm) throughout the rostrocaudal extent of the SNR, its maximal dorsoventral extension being 239 μm. A total of nine neurons (5d, 9, 13d,15g, 24d, 26, 27,30, and 39) were labeled in this SNR subdivision. These cells, lying in a region corresponding to the ventral part of the pericore lamina (Fig. 10A), were distributed throughout most of the rostrocaudal extent of the SNR (Fig.10B). Six of these neurons (9,13d, 15g, 24d, 26, and27) were functionally identified by their typical inhibitory response to stimulation of the facial motor cortex (L, 12.5 ± 2.5 msec; D, 23 ± 8 msec). Remaining neurons (5d, 30, and 39) were injected in three other experimental cases in which the cortical stimulation was applied in the orofacial motor cortex. In accordance with their location within the SNR, these neurons did not respond to stimulation of the orofacial motor cortex and were positioned ventrally to neurons presenting a typical inhibitory response to stimulation of this cortical area.
Fig. 10.
Neurons located in the lateral part of the pericore lamina (pl, filled area,inset in A) innervated by the striatal sector related to the facial area of the sensorimotor cortex. The SNR is shown from a rostral view in A and a ventral view inB. The labeled neurons, 27 and5d, are examined from a rostral view in Cand E. Note the curved shape of the dendritic arborization that envelops ventrally the core region of SNR. Neuron27 is examined from a ventral view in Dand F. In F the neuron is incorporated within its corresponding striatal projection field. The only visible dendrites (arrowheads) are those extending outside the striatal projection field (dark gray). Scale bars, 350 μm.
Two representative examples of labeled neurons (27,5d) are shown in Figure 10. Typically, the dendritic arborizations of these neurons had the shape of a curved disk conforming to the geometry of the striatal projections. For example, neuron 27, which occupied a lateral position (Fig.10C–F), had a flat and curved dendritic arborization enveloping ventrally the central core subdivision. Interestingly, with the exception of the medial and lateral extremities of the dendritic field, where dendrites envelop the lateral and medial edges of the central core, none of the dendritic branches was directed dorsally toward this subdivision. Neuron 5d, illustrated in Figure10E, presented similar characteristics, its flat dendritic arborization enveloping ventrally and medially the central core subdivision.
The dendritic arborizations of all the labeled neurons largely conformed to the spatial organization of the striatal projection field but also extended within the most ventral nigral subdivision innervated by the visual striatal sector (Fig. 10C,D). In addition, dendrites often extended within another component of the pericore lamina, i.e., the projection fields from the limb and oculomotor striatal sectors. Altogether, the length of dendrites lying outside the facial region of the pericore lamina ranged from 15 to 43% of the total dendritic length of the neurons.
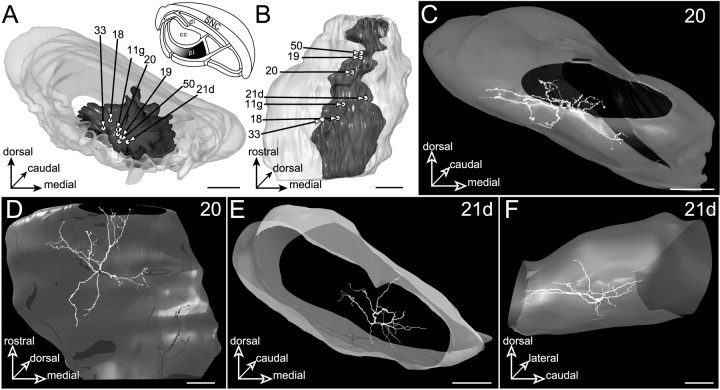
The limb striatal sector
The projection field of the limb striatal sector (Fig.11A,B) extends medially to the projections of the facial striatal sector. The maximal extension of the striatal projection field is 877 μm mediolaterally and 281 μm dorsoventrally. Seven neurons (11g, 18, 19, 20,21d, 33, and 50) were labeled in this SNR region (Fig. 11A,B). Neurons 18, 19, and 20 were functionally identified by their clear-cut inhibitory response (L, 12–16 msec; D, 12–20 msec) to stimulation of the forelimb sensorimotor cortical area. Neuron 11g was injected in another experiment in which the cortical stimulation was placed in the oculomotor cortex. This neuron responded with a typical inhibition to the stimulation of this cortical area. Neurons 21d, 33, and 50 were injected in three other sets of experiments in which the cortical stimulation was applied in the prelimbic area of the prefrontal cortex. These neurons were unaffected by stimulation of this cortical region.
Fig. 11.
Neurons located in the central part of the pericore lamina (pl, filled area,inset in A) innervated by the striatal sector related to the limb areas of the sensorimotor cortex. The SNR is shown from a rostral view in A and a ventral view inB. C–F, The dendritic field of neurons20 and 21d. Neuron 20 is examined from a rostral view in C and a ventral view inD. Neuron 21d is examined from a medial view in F. Scale bars, 350 μm.
Dendritic fields of all investigated neurons extended along both horizontal and vertical planes. Along the horizontal plane, dendrites coursed for long distances and formed ellipses or disks. As indicated by measurements of dendritic lengths (Table 1) and by 3D composite models, these horizontally oriented dendrites extended beyond the projection field of the limb striatal sector within the laterally adjacent portion of the pericore lamina innervated by the facial striatal sector. In the case of neuron 20 (Fig.11C,D), the part of the dendritic field extending within the facial component of the pericore lamina represented ∼10% of the total dendritic length. As shown in Figure11C–F, these neurons also emitted vertically oriented dendrites coursing in dorsal or ventral directions, or both. Ventrally oriented dendrites crossed the boundaries of the pericore lamina to terminate in the ventral lamina innervated by the visual and oculomotor striatal sectors. In the case of neuron 20, the portion of the dendritic tree lying in this ventral SNR subdivision represented ∼20% of the total dendritic length. Dorsally oriented dendrites conformed to the geometry of the pericore lamina, and like striatal projections, they enveloped medially the central core subdivision.
For all studied neurons, the overall proportion of dendrites remaining in the portion of the pericore lamina innervated by the limb striatal sector ranged from 69 to 90%. None of these neurons was found to emit dendritic branches within the central core subdivision or within the medial lamina innervated by the striatal sector related to the prelimbic cortex (Fig. 3).
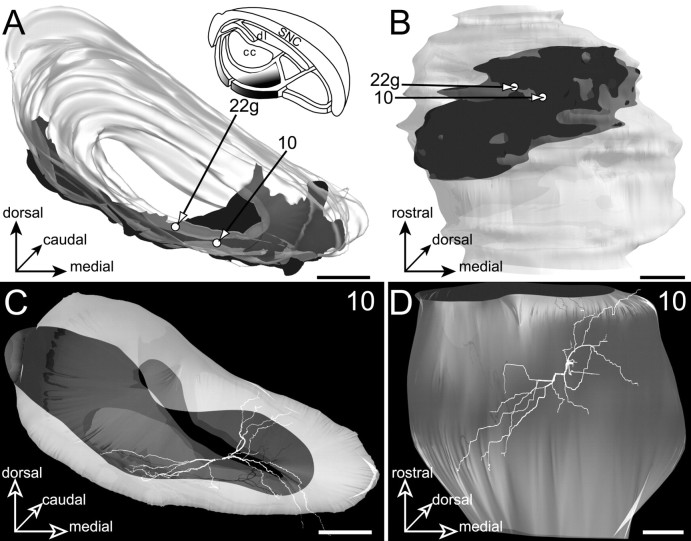
The oculomotor striatal sector
Projections of the oculomotor striatal sector (Fig.12A,B) form a semicircular lamina ranging from 64 to 494 μm thickness that crosses the entire dorsoventral extent of the SNR. This lamina envelops medially and ventrally the projection field of the facial striatal sector. When examined from a horizontal view (Fig.12B), these striatal projections display a remarkable diagonal orientation. Interestingly, in the lateral and caudal aspects of the SNR, the projections of the oculomotor striatal sector that occupy the most ventral part of the nucleus overlap the projection field of the visual striatal sector.
Fig. 12.
Neurons located in the medial part of the pericore lamina (filled area,inset in A) innervated by the striatal sector related to the oculomotor cortex. The SNR is shown from a rostral view in A and a ventral view in B.C, D, The 3D reconstruction of neuron10 is examined from a rostral view in Cand a ventral view in D. Note that the dendritic field of the neuron strikingly parallels the spatial organization of the striatal projections. Scale bars, 350 μm.
Two neurons (10 and 22g) were labeled in the region of SNR corresponding to the projection field of the oculomotor striatal sector. These neurons were functionally characterized by a typical inhibitory response (L, 14 msec; D, 12 msec; andL, 18 msec; D, 20 msec, respectively) to stimulation of the precentral medial area of the sensorimotor cortex. Their dendritic field was characterized by a large dorsoventral extension spanning the entire dorsoventral extent of the SNR, from the pars compacta to the cerebral peduncle (Fig. 12C). Like the striatal projections, these dendritic fields adopted curved shapes that enveloped medially the projections of the facial striatal sector. Moreover, these dendritic fields displayed the characteristic rostromedial to caudolateral diagonal orientation of the striatal projections (Fig. 12, compare B, D).
Altogether, the 3D organization of labeled neurons in the different regions of the pericore lamina indicates that in this nigral subdivision, the geometry of the dendritic fields conforms to the spatial organization of the striatal projections. Indeed, as illustrated in Figure 13, in which four neurons of the pericore lamina were incorporated in a single 3D model of SNR, the dendritic arborizations of these neurons formed a lamellar structure equivalent to the pericore lamina of the striatal projections.
Fig. 13.
Rostral view of a 3D reconstructed model of SNR incorporating the neurons 27, 20,5d, and 10 injected within the pericore lamina. The dendrites of these neurons formed a lamina similar to the pericore lamina occupied by the projections of the striatal sectors related to facial, limb, and oculomotor areas of the sensorimotor cortex (inset, filled area). Scale bars, 350 μm.
Neurons located in the lateral lamina
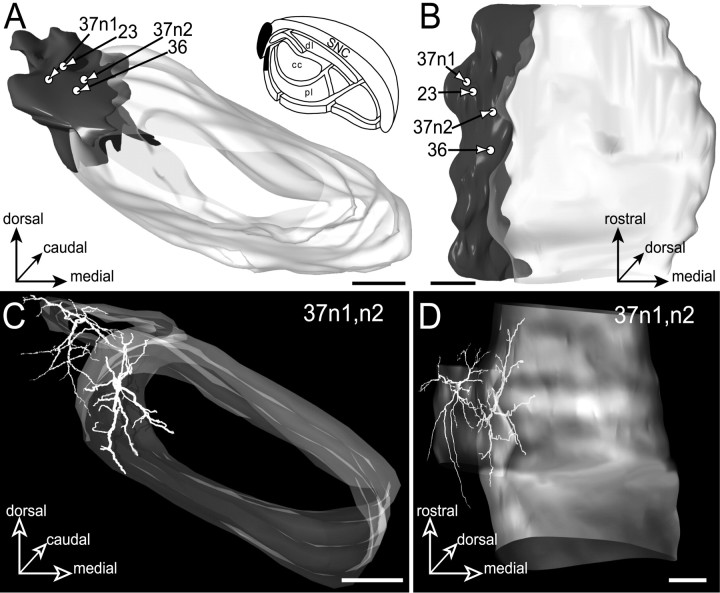
The projections of the striatal sector related to the auditory cortex form a lamina that caps laterally the SN (Fig.14A,B). This lamina, which occupies the pars lateralis and the adjacent lateral part of the SNR, has a maximal dimension of 468 μm mediolaterally and 372 μm dorsoventrally.
Fig. 14.
Neurons located in the lateral lamina (filled area, inset inA) innervated by the striatal sector related to the auditory cortical areas. The SNR is shown from a rostral view inA and a ventral view in B.C and D illustrate the dendritic arborizations of neurons 37n1 (located in the pars lateralis) and 37n2 (located in the lateral SNR) that were injected in the same animal. The neurons are examined from a rostral view in C and a ventral view inD. Scale bars, 350 μm.
Four neurons (23, 36, 37n1, and37n2) were labeled in this lateral subdivision of the SN (Fig. 12A,B). Two cells (23 and 37n1) were located in the pars lateralis, and two others (36 and 37n2) were located more ventrally and medially in the lateral part of SNR. These four neurons shared a similar spatial organization of their dendritic field. As illustrated in Figure 14, C and D(37n1,n2), dendrites were radiating along the rostrocaudal, mediolateral, and dorsoventral axis forming a curved lamina enveloping laterally the SNR and largely comparable to the geometry of the striatal projections. As observed by comparingA and C in Figure 14, both the dendritic fields and the striatal projection field presented a large mediolateral extension in the dorsal part of the SN and gradually tapered toward the cerebral peduncle.
The dendritic arborization of these neurons was not strictly confined to the striatal projection field. The four labeled neurons had dendrites extending ventrally within the ventral lamina subdivision innervated by the visual striatal sector. As measured using 3D composite models, 10–50% of the total dendritic length of these cells was lying in this ventral subdivision. Interestingly, no dendrite extended within the central core subdivision. Accordingly, these neurons did not present an inhibitory response to the stimulation of the orofacial motor cortex.
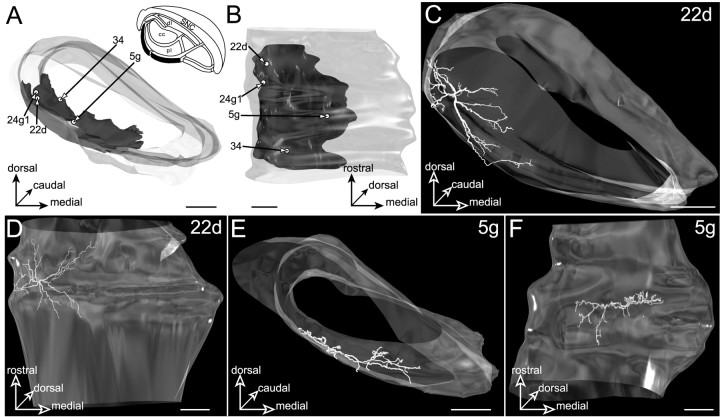
Neurons located in the ventral lamina
Projections originating from the visual striatal sector form a thin lamina of 52–338 μm thickness along the fibers of the cerebral peduncle (Fig.15A,B). This lamina, which occupies nearly the entire rostrocaudal length of the SNR and extends laterally over 1175 μm, constitutes a ventral and medial extension to the projection field of the auditory striatal sector described above.
Fig. 15.
Neurons located in the ventral lamina (filled area, inset inA) innervated by the striatal sector related to visual cortical areas. The SNR is shown from a rostral view inA and a ventral view in B.C–F, The dendritic arborizations of neurons22d and 5g. The neurons are examined from a rostral view in C and E and a ventral view in D and F. Note that the neurons display flat dendritic fields spreading along the ventral border of the SNR. Scale bars, 350 μm.
Four neurons (5g, 22d, 24g1, and34) were labeled in this nigral subdivision (Fig.15A,B). These cells were characterized by dendritic arborizations spreading along the ventral edge of the SNR and forming discs (Fig. 15C,D) or strips (Fig. 15E,F) that paralleled the cerebral peduncle. Although the geometry of the dendritic field of labeled neurons conformed to the lamellar shape of the striatal projection field, part of the dendrite extended within the laterally and dorsally adjacent subdivisions. As measured on composite 3D models, 8% of the total dendritic length of neuron 22d extended within the lateral lamina innervated by the auditory striatal sector, and 25% of the dendrites of neuron 34 extended within the pericore lamina. None of the labeled neurons presented dendrites reaching the central core subdivision, and accordingly, no inhibitory response was recorded in these neurons after stimulation of the orofacial motor cortex.
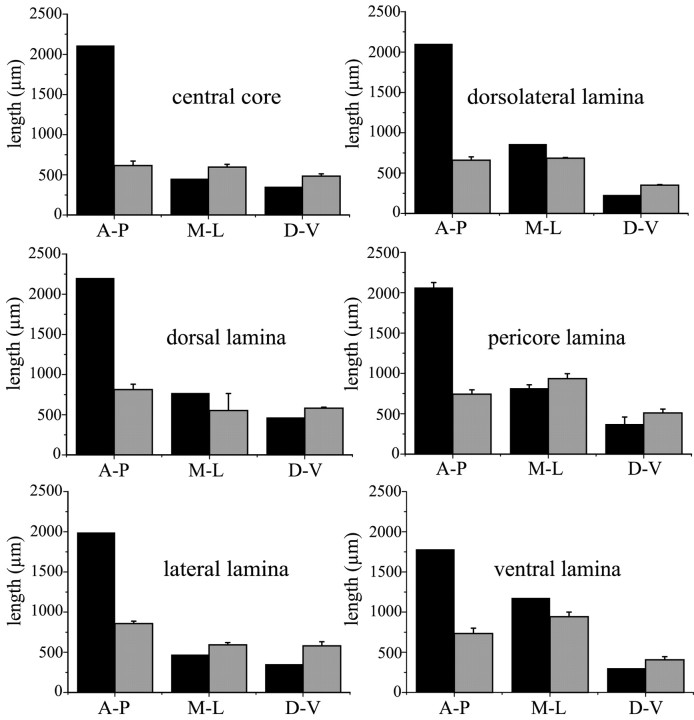
Comparison between the extension of the dendritic fields of nigral neurons and the projection fields of individual striatal sectors
Measurement of the extension of the dendritic fields of labeled neurons in the anteroposterior, mediolateral, and dorsoventral axis were made for comparison with the extension of striatal projection fields. As shown in Figure 16, along the dorsoventral and mediolateral axis, a consistent relationship was found between the dimension of the dendritic fields of nigral cells and the dimension of their corresponding striatal projection fields. Conversely, along the rostrocaudal axis, the size of the dendritic fields was systematically smaller than that of corresponding striatal projection fields. Dendritic fields occupied only 30–40% of the striatal projection fields.
Fig. 16.
Comparison between the extension of the dendritic arborizations of nigral neurons (gray columns) and the projection fields of the related striatal districts (black columns). A-P, Anteroposterior axis; M-L, mediolateral axis; D-V, dorsoventral axis.
DISCUSSION
This study provides the first systematic analysis of the spatial organization of the dendritic arborizations from neurons of the sensorimotor subdivision of the rat SNR. The data show that the dendrites of these neurons conform to the onion-like arrangement of striato-nigral projections. Like striatal afferents, these dendrites extend along longitudinal and curved laminae enveloping a core region occupied by neurons with spherical or cylindrical dendritic fields. Such a remarkable alignment between the dendrites of nigral cells and the projection fields of individual functional districts of the striatum supports the concept that the rat striato-nigral circuits are organized in specific channels of processing. These channels, however, are not strictly segregated from one another. The dendritic arborization of a single neuron usually extends within an adjacent territory, thereby establishing a continuity between neighboring channels.
Typology of labeled neurons
The neurons labeled in the present study presented the morphological features of SNR Golgi type I neurons, and accordingly their axon was leaving the substantia nigra. As reported previously, their cell body was fusiform, pyramidal, or polygonal and ranged from a medium to a large size (Gulley and Wood, 1971; Juraska et al., 1977;François et al., 1979; Grofova et al., 1982; Yelnik et al., 1987). In addition, dendritic fields presented different shapes, including spheres, cylinders, flat ellipses, or discs, and were oriented rostrocaudally, mediolaterally, or dorsoventrally. The morphological heterogeneity of labeled neurons indicates that the present study likely provides a representative picture of the population of output neurons located in the sensorimotor region of the rat SNR.
Spatial organization of dendritic fields in relation to the geometry of striato-nigral projections
The topographical organization of striatal projections is a main characteristic of the mammalian basal ganglia (Alexander et al., 1986;Groenewegen and Berendse, 1994; Deniau and Thierry, 1997). The principle of this topographical representation has been well defined in the rat SNR. The various districts of the functional striatal mosaic are mapped in an orderly manner along a series of longitudinal and curved laminae enveloping a central core occupying the SNR dorsolateral part (Deniau et al., 1996). The topographical distribution of nigral output neurons is ruled by a similar onion-like arrangement (Deniau and Chevalier, 1992). These observations led us to suggest that the lamellar architecture of the SNR underlies the formation of specific input–output assemblies connecting individual components of the striatal mosaic with regionally segregated populations of nigral output neurons. However, this view is challenged by morphological analysis of nigral neurons. In the monkey, 3D reconstructions of Golgi-impregnated neurons led to the suggestion that the dendrites of SNR cells are oriented in such a way that these cells integrate information from the widest possible striatal regions (François et al., 1987). In the rat SNR, although the dendritic arborizations display a specific dorsoventral organization, a single neuron can cover the whole thickness of the SNR, suggesting that in rodents neurons might also integrate information from many diverse striatal sectors (Juraska et al., 1977; Grofova et al., 1982).
The present study provides clear evidence that the dendritic arborization of nigral neurons respects the geometry of striatal projections. Indeed, in the core subdivision of the SNR innervated by the orofacial striatal sector, labeled neurons displayed spherical or cylindrical dendritic fields that complied perfectly with the projections of this striatal sector. Moreover, in regions of the SNR surrounding the core, the neurons exhibited flat and curved dendritic arborizations arranged, like their related striatal projections, into concentric laminae. Such a consistent relationship between the geometry of the dendritic arborizations of nigral neurons and the projection fields of individual striatal sectors suggests the possible existence of a strict interaction between the nigral cells and their striatal afferent. Functionally, such a remarkable alignment strengthens the notion that the lamellar architecture of the rat SNR allows a parallel processing of cortical information in the striato-nigral pathway. Indeed, thanks to this anatomical arrangement, the cortical information processed in a given functional sector of the striatum must be routed specifically to a particular subpopulation of nigral output neurons. This is supported by the existence of a regional specificity in the response of SNR cells to cortical stimulation. Although neurons located in the core region of SNR presented a marked inhibition after stimulation of the orofacial motor cortex, those located laterally in the projection territory of the auditory striatal sector or ventrally in the projection territory of the visual striatal sector did not receive a synaptic input from the orofacial motor cortex via the striatum. Taken together, these data further indicate that basal ganglia output nuclei largely maintain in their outflow the specificity of cortico-striatal afferents as proposed previously in the monkey (Alexander et al., 1986; Kitano et al., 1998).
Segregation and convergence of information in the striato-nigral circuits
Although the coherence between the geometry of the dendritic orientation of the nigral neurons and the topography of the striatal projections supports the existence of parallel channels in the striato-nigral circuits, the considerable extension of the dendritic fields of nigral cells clearly indicates that the connectivity within these channels cannot be limited to a cable-like transmission. Indeed, as indicated from measurements of the length of the dendritic fields of nigral neurons, only three nigral neurons can occupy the projection field of any given striatal district. Therefore, convergence and topography are two major attributes of the basal ganglia circuits that should not be opposed. In fact, the parallel channels of the basal ganglia should be viewed as integrative modules receiving convergent information from specific sets of cortico-striatal inputs.
In their seminal paper on the parallel architecture of the monkey basal ganglia, Alexander et al. (1986) proposed a rather strict compartmentalization into anatomically and functionally segregated channels. Although the spatial arrangement of nigral neurons contributes to the formation of parallel channels in the striato-nigral pathway, the present data indicate that these channels are not strictly segregated from one another. Indeed, in most cases, the dendritic fields of nigral neurons were found to exceed the extension of projection fields from individual striatal sectors. As a consequence, the neurons located within the projection field of a given striatal district shares partly the inputs of neurons located in an adjacent one, thereby establishing an anatomical and functional continuity between neighboring channels. For example, the neurons located in the dorsal lamina of SNR innervated by the striatal sector related to the insular, gustatory, and perirhinal cortices had dendrites extending into the ventrally adjacent region innervated by the orofacial striatal sector, and they presented an inhibitory response to stimulation of the orofacial motor cortex. Respecting the same rule of organization, neurons located in the pericore lamina innervated by somatic sensorimotor sectors of the striatum or neurons located in the lateral lamina innervated by the auditory striatal sector had dendrites extending within the ventrally adjacent region innervated by the visual striatal sector.
What principles underlie the convergence of cortical information in the rodent striato-nigral circuits?
The SNR provides a major output station of the basal ganglia through which the striatum exerts a disinhibitory influence on brainstem and thalamic premotor nuclei (Chevalier and Deniau, 1990;Hikosaka et al., 2000). On the basis of topographical analysis of connections along the cortico-striato-nigro-thalamic and nigro-collicular pathways (Flaherty and Graybiel, 1991; Deniau and Chevalier, 1992; Deniau et al., 1996), we have previously proposed that the basal ganglia channels integrate different information associated with the completion of particular motor behaviors and they activate, via their efferent projections, the executive networks supporting these behaviors (Deniau and Thierry, 1997). The present data on the spatial organization of nigral neurons provide additional support in favor of this proposal. Indeed, neurons extend within the projection fields of several given striatal districts that process information relevant to the motor behavior that they control through their efferent circuitry. Taking for example the neurons of the central core, these cells are part of a sensorimotor channel that controls thalamic and collicular circuits for head positioning and orofacial movements (Redgrave et al., 1992, Deniau and Thierry, 1997). These neurons extend partly within the projection fields of the striatal sectors innervated by gustatory, orofacial, and facial sensorimotor cortices. The information processed in these cortical areas are normally associated with feeding behavior and are relevant to the regulation of cephalic and orofacial movements that support this particular motor behavior. Similarly, neurons located in the pericore lamina, which are part of motor channels implicated in movements of eye/head and parts of the body visible for the animal, have dendrites extending within the projection field of the visual striatal sector (ventral lamina). Conversely, the dendritic arborizations of the neurons located in the projection fields of striatal sectors processing information normally used by the rat in distinct behaviors (such as auditory/visual versus orofacial/gustatory) remained strictly segregated. If the spatial organization of nigral neurons obeys these functional rules, a species specificity might be observed, because across species, similar sensory modalities are likely used in the context of different classes of behavior.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale Grant MILDT98D09. We thank S. Slaght for critical reading of this manuscript and P. Nguyen for his technical assistance.
Correspondence should be addressed to Jean-Michel Deniau, Institut National de la Santé et de la Recherche Médicale U 114, Chaire de Neuropharmacologie, Collège de France, 11 Place M. Berthelot, 75231 Paris Cedex 05, France. E-mail:jean-michel.deniau@college-de-france.fr.
REFERENCES
- 1.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 3.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 4.Brown LL, Smith DM, Goldbloom LM. Organizing principles of cortical integration in the rat neostriatum: corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J Comp Neurol. 1998;392:468–488. [PubMed] [Google Scholar]
- 5.Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- 6.Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- 7.Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience. 1992;46:361–377. doi: 10.1016/0306-4522(92)90058-a. [DOI] [PubMed] [Google Scholar]
- 8.Deniau JM, Thierry AM. Anatomical segregation of information processing in the rat substantia nigra pars reticulata. Adv Neurol. 1997;74:83–96. [PubMed] [Google Scholar]
- 9.Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- 10.Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73:761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- 12.François C, Percheron G, Yelnik J, Heyner S. Demonstration of the existence of small local circuit neurons in the Golgi-stained primate substantia nigra. Brain Res. 1979;172:160–164. doi: 10.1016/0006-8993(79)90905-3. [DOI] [PubMed] [Google Scholar]
- 13.François C, Yelnik J, Percheron G. Golgi study of the primate substantia nigra. II. Spatial organization of dendritic arborizations in relation to the cytoarchitectonic boundaries and to the striatonigral bundle. J Comp Neurol. 1987;265:473–493. doi: 10.1002/cne.902650403. [DOI] [PubMed] [Google Scholar]
- 14.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 15.Groenewegen HJ, Berendse HW. Anatomical relationships between the prefrontal cortex and the basal ganglia in the rat. In: Thierry AM, Glowinski J, Goldman-Rakic P, Christen Y, editors. Motor and cognitive functions of the prefrontal cortex. Springer; Berlin: 1994. pp. 51–77. [Google Scholar]
- 16.Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- 17.Gulley RL, Wood RL. The fine structure of the neurons in the rat substantia nigra. Tissue Cell. 1971;3:675–690. doi: 10.1016/s0040-8166(71)80013-7. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- 19.Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol. 1991;304:569–595. doi: 10.1002/cne.903040406. [DOI] [PubMed] [Google Scholar]
- 20.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 21.Juraska JM, Wilson CJ, Groves PM. The substantia nigra of the rat: a Golgi study. J Comp Neurol. 1977;172:585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- 22.Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Kita H. Physiology of two disynaptic pathways from the sensorimotor cortex to the basal ganglia output nuclei. In: Percheron G, McKenzie JS, Feger J, editors. The basal ganglia IV. Plenum; New York: 1994. pp. 263–276. [Google Scholar]
- 24.Kitano H, Tanibuchi I, Jinnai K. The distribution of neurons in the substantia nigra pars reticulata with input from the motor, premotor and prefrontal areas of the cerebral cortex in monkeys. Brain Res. 1998;784:228–238. doi: 10.1016/s0006-8993(97)01332-2. [DOI] [PubMed] [Google Scholar]
- 25.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the cortico-nigral circuits. J Neurosci. 1999;19:4674–4681. doi: 10.1523/JNEUROSCI.19-11-04674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 27.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 28.Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 30.Percheron G, François C, Yelnik J. Spatial organization and information processing in the core of the basal ganglia. In: Carpenter MB, Jarayaman A, editors. The basal ganglia II. Plenum; New York: 1987. pp. 205–226. [Google Scholar]
- 31.Pinault D. Golgi-like labeling of a single neuron recorded extracellularly. Neurosci Lett. 1994;170:255–260. doi: 10.1016/0304-3940(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 32.Redgrave P, Marrow L, Dean P. Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience. 1992;80:571–595. doi: 10.1016/0306-4522(92)90448-b. [DOI] [PubMed] [Google Scholar]
- 33.Ryan LJ, Clark KB. The role of the subthalamic nucleus in the response of globus pallidus neurons to stimulation of the prelimbic and agranular frontal cortices in rats. Exp Brain Res. 1991;86:641–651. doi: 10.1007/BF00230538. [DOI] [PubMed] [Google Scholar]
- 34.Veening JG, Cornelissen FM, Lieven PA. The topical organization of the afferents to the caudatoputamen of the rat. A horseradish peroxidase study. Neuroscience. 1980;5:1253–1268. doi: 10.1016/0306-4522(80)90198-0. [DOI] [PubMed] [Google Scholar]
- 35.Webster KE. Cortico-striate interrelations in the albino rat. J Anat. 1961;95:532–544. [PMC free article] [PubMed] [Google Scholar]
- 36.Yelnik J, Francois C, Percheron G, Heyner S. Golgi study of the primate substantia nigra. I. Quantitative morphology and typology of nigral neurons. J Comp Neurol. 1987;265:455–472. doi: 10.1002/cne.902650402. [DOI] [PubMed] [Google Scholar]
- 37.Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]