Abstract
Autonomous activation of calcium–calmodulin kinase (CaMKII) has been proposed as a molecular mechanism for decoding Ca2+ spike frequencies resulting from action potential firing, but this has not been investigated in intact neurons. This was studied in mouse DRG neurons in culture using confocal measurements of [Ca2+]i and biochemical measurements of CaMKII autophosphorylation and autonomous activity. Using electrical stimulation at different frequencies, we find that CaMKII autonomous activity reached near maximal levels after ∼45 impulses, regardless of firing frequency (1–10 Hz), and autonomous activity declined with prolonged stimulation. Frequency-dependent activation of CaMKII was limited to spike frequencies in the range of 0.1–1 Hz, despite marked increases in [Ca2+]i at higher frequencies (1–30 Hz). The high levels of autonomous activity measured before stimulation and the relatively long duration of Ca2+ spikes induced by action potentials (∼300 msec) are consistent with the lower frequency range of action potential decoding by CaMKII. The high autonomous activity under basal conditions was associated with extracellular [Ca2+], independently from changes in [Ca2+]i, and unrelated to synaptic or spontaneous impulse activity. CaMKII autonomous activity in response to brief bursts of action potentials correlated better with the frequency of Ca2+ transients than with the concentration of [Ca2+]i. In conclusion, CaMKII may decode frequency-modulated responses between 0.1 and 1 Hz in these neurons, but other mechanisms may be required to decode higher frequencies. Alternatively, CaMKII may mediate high-frequency responses in subcellular microdomains in which the enzyme is maintained at a low level of autonomous activity or the Ca2+ transients have faster kinetics.
Keywords: Ca2+–calmodulin-dependent protein kinase II, autophosphorylation, Thr-286, frequency decoding, cytoplasmic calcium, extracellular calcium sensor, DRG neurons, CaMKII, LTP
Action potential firing can regulate many neuronal responses, but it is not understood how the frequency of firing is decoded by intracellular signaling pathways. Theoretical modeling and in vitro simulations provide evidence that calcium–calmodulin kinase (CaMKII) can decode the frequency of Ca2+ spikes into graded amounts of kinase activity (Hanson et al., 1994; Dosemeci and Albers, 1996; De Koninck and Schulman, 1998). This results from autophosphorylation at Thr-286, which promotes calmodulin binding to the enzyme (Meyer et al., 1992;Hanson et al., 1994), and converts the enzyme into a Ca2+-independent (autonomous) state (Miller and Kennedy, 1986; Thiel et al., 1988; Lou and Schulman, 1989;Mukherji and Soderling, 1994). Autonomously active CaMKII remains catalytically active after [Ca2+]i returns to basal level after stimulation. This property could enable the enzyme to sustain cellular responses long after a transient stimulus (Lisman and Goldring, 1988). CaMKII has a critical role in many neuronal responses that exhibit frequency-dependent modulation such as hippocampal long-term potentiation (LTP) and long-term depression (LTD) (Stevens et al., 1994; Lledo et al., 1995; Mayford et al., 1995), and experiments on transgenic mice indicate that Thr-286 is a critical locus for induction of LTP (Giese et al., 1998).
In vitro simulations indicate that the frequency response of CaMKII can be modulated by factors such as the amplitude of the Ca2+ spike, the duration of the Ca2+ spike, the subunit composition of the enzyme, and the previous activation state of the kinase (De Koninck and Schulman, 1998). These forms of regulation are thought to contribute specificity to activation of this multifunctional enzyme, to enable phosphorylation of appropriate substrates in response to distinct cellular stimuli. It is important to determine how these factors affect the frequency-decoding properties of CaMKII in neurons in response to naturally occurring spike-driven Ca2+fluxes. This was investigated using a dorsal root ganglion (DRG) cell culture preparation equipped with stimulating electrodes (Fields et al., 1992).
This preparation offers a number of advantages. DRG neurons do not form synapses in culture, and they are not spontaneously active. This allows good control of the firing frequency and provides a simpler model system than one involving synaptic transmission. DRG neurons have a large spherical cell body, with no dendrites, which allows good correlation between action potential-induced Ca2+ dynamics in the cell body with CaMKII activity measured in lysates of the neurons. Many processes are regulated by action potentials at 0.1–10 Hz in this preparation. This includes gene expression (Sheng et al., 1993; Itoh et al., 1995, 1997;Fields et al., 1997), neurite outgrowth (Fields et al., 1990, 1993), synaptic plasticity, and elimination (Nelson et al., 1989; Fields et al., 1991), calcium channel expression (Li et al., 1996), myelination (Stevens et al., 1998), and Schwann cell proliferation and differentiation (Stevens and Fields, 2000).
These studies are the first test of frequency-dependent decoding by CaMKII in intact neurons. The results indicate that the spike frequency decoding of CaMKII is shifted toward low frequencies of action potential firing, consistent with the high level of autonomous activity before stimulation and the long duration of Ca2+ transients induced by action potentials.
Preliminary results have been reported in Eshete and Fields (1999).
MATERIALS AND METHODS
Materials. Phosphosite-specific antiserum was a gift from Dr. Y. Yamagata (Laboratory of Neurochemistry, Okazaki, Japan). CaMKII antibodies were purchased from Life Technologies (Gaithersburg, MD), Santa Cruz Biotechnology (San Diego, CA), Transduction Laboratories (Lexington, KY), and Zymed (San Francisco, CA). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse Ig, ECL, and ECL Plus substrate were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Anti-goat Ig was from Santa Cruz Biotechnology. The special minimum essential medium (Eagle) with Earle's salts used for culturing neurons and phosphocellulose paper were obtained from Life Technologies. [γ-32ATP] (3000 Ci/mmol) was purchased from DuPont-New England Nuclear (Boston, MA). Polyvinylidene difluoride (PVDF) membrane (Immobilon-P) was purchased from Millipore (Bedford, MA). ImageQuant and the Storm image analysis system were from Molecular Dynamics (Sunnyvale, CA). Autocamtide-2, synthide-2, autocamtide-2 related peptides, PKI 6–22 amide, and PKC19–36 were purchased from Bachem (Torrance, CA). Protease inhibitor cocktail (Complete) was from Boehringer Mannheim, and indo-1/AM, flu-3, and mag-indo-1 were purchased from Molecular Probes (Eugene, OR). All other chemicals were analytical grade and were purchased from Sigma (St. Louis, MO).
Cell culture. Multicompartment chambers were made of Teflon and attached to collagen-coated 35 mm culture dishes as described (Fields et al., 1992). DRG neurons, dissociated from 13.5 d mouse fetuses, were plated at a density of 0.5 × 106 cells into each side compartment in a culture medium containing 5% horse serum and 50 ng/ml nerve growth factor as described previously (Sheng et al., 1993). Non-neuronal cells were prevented from multiplying by adding 13 μg/ml fluoro-2-deoxyuridine 1–2 d after seeding. Cultures were used for experiments 3–4 weeks after plating to allow time for most DRG neurons in the side compartments to extend axons under the barrier between the side and central compartments.
Electrical stimulation of DRG neurons. Axons traversing under the barrier separating the two side compartments from the central compartment of the multicompartment insert were stimulated through platinum electrodes in contact with the culture medium on opposite sides of the barrier. Stimulation parameters and electrophysiological responses to stimulation have been reported previously for DRG neurons in these multicompartment chambers (Fields et al., 1992). DRG neurons in this preparation respond with a single action potential to stimulation with a 1–5 V, 200 μsec biphasic pulse. They follow stimulation reliably and indefinitely at rates up to 3 Hz and for several tens of seconds at 30 Hz (Fields et al., 1990, 1992).
Characterization of CaMKII isozymes. CaMKII isozymes were characterized by 8% SDS-PAGE from DRG and brain lysates in sample buffer (125 mm Tris-HCl, pH 6.8, 4% SDS, 10% β-mercaptoethanol, 20% glycerol, and 0.04% bromphenol blue) and immunoblotting. The PVDF membranes were blocked in 5% milk in TTBS (10 mm Tris-HCl, pH 7.5, 150 mmNaCl, and 0.1% Tween 20) for 2 hr, washed, and incubated in a manifold (Deca-Probe; Hoefer) with polyclonal antibodies against CaMKII-α, -β, -γ, and -δ (Santa Cruz Biotechnology), monoclonal antibodies Cbα-2 (Life Technologies), and CBα-2 (Zymed) at 1:1000 for 2 hr at room temperature. The washed membranes were then reacted with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature and detected by ECL.
Autophosphorylation of CaMKII at Thr-286. CaMKII autophosphorylation at Thr-286 was analyzed by immunoblotting using a phosphosite-specific antibody that recognizes CaMKII only when it is autophosphorylated at Thr-286 (α) or Thr-287 (β, γ, δ) (Yamagata and Obata, 1998). Neurons were washed three times and changed to physiological saline solution containing 50 nmfree Ca2+ (Scholz and Palfrey, 1998). This was obtained by substituting (in mm): 0.74CaCl2, 1.13 MgCl2, and 2 EGTA in the standard PBS solution (PSS). After 1 hr of equilibration, the calcium concentration was raised to 1.2 mm free Ca2+ for 5–45 sec by adding 100 mmCaCl2 to the same solution. Treated neurons were lysed in boiling sample buffer for electrophoresis and immunoblot analysis.
For quantification of Thr-286 autophosphorylation, equal volume of lysates from control and treated neurons were resolved in parallel by SDS-PAGE in duplicate 10% gels and electroblotted to PVDF membranes. Membranes were blocked in 5% milk in TTBS for 2 hr at room temperature, washed, and incubated in antibody that recognized total CaMKII at 1:1500 (monoclonal antibody clone 38; Transduction Laboratories) or phosphosite-specific antibody (1:10,000) overnight at 4°C. Incubated membranes were washed and reacted in horseradish peroxidase-conjugated secondary antibody for 1 hr at room temperature. The immunocomplexes were visualized with ECL Plus substrate and quantified with ImageQuant and Storm image analysis system. The linearity of the immunoreactivity measurement was tested by loading different volumes of lysate from DRG neurons cultured in chambers. The relative immunoreactivity (RFU) against both the autophosphorylated and total enzyme was within the dynamic range. According to the Storm manufacturer (Application note # 60), the dynamic range of measurement is 0–3 × 107 RFU, whereas the readings in this study varied between 0.2 and 1 × 106 RFU. Relative autophosphorylation at Thr-286 was compared by normalization of the RFU obtained with the phosphorylated enzyme to that of the total enzyme in the same sample from parallel experiments.
To determine the [Ca2+] needed to autonomously activate CaMKII in vitro, DRG supernatant from unstimulated neurons was autophosphorylated using an adaptation of the method of Molloy and Kennedy (1991). Briefly, assay tubes containing different concentration of Ca2+ (0.1–1000 μm) and 6 μm CaM were phosphorylated for either 15 or 45 sec with 1 mmMgCl2 and 0.02 mm ATP. After autophosphorylation, autocamtide-2 plus EGTA (2 mm final) was added to each tube, and incubation continued for 5 min. Ca2+-dependent and independent activity was also determined in parallel in supernatants that were not autophosphorylated in vitro.
CaMKII activity assay.Ca2+–CaM-dependent and -independent activity of CaMKII was measured in neuronal homogenates by phosphorylation of autocamtide-2 (KKALRRQETVDAL), a peptide derived from the autophosphorylation site of CaMKII. The kinase assay was linear with respect to time and the amount of lysate used for analysis. Neurons equilibrated in 1.2 mmCa2+-PSS for 1 hr were electrically stimulated at 0.1, 0.3, 0.5, 1, and 10 Hz, delivering 45 pulses at these frequencies for durations of up to 10 min. At the end of stimulation, neurons were harvested in 0.20 ml of ice-cold lysis buffer (50 mm HEPES, pH 7.5, 1 mmEDTA, 5 mm EGTA, 2 mm DTT, 0.1% TX-100, 100 mm β-glycerol phosphate, 10 mm sodium pyrophosphate, 50 mm NaF, protease inhibitor cocktail, and 1 μm Microcystein LR). The harvested neurons were sonicated in an ice bath with a Bronson probe sonicator (two pulses at 40% duty cycle and output of 2) and centrifuged at 15,000 ×g, for 15 min at 4°C. Kinase assays were performed on 8.5 μl aliquots of the supernatant using 20 μmautocamtide-2 in the presence of 1 mmCa2+ and 6 μm CaM (total activity), and in parallel samples the reaction was performed in the absence of Ca2+ with 1 mm EGTA (Ca2+-independent activity). Background activity was determined from supernatant reacted without the substrate. The reaction tubes also contained 5 μm of the protein kinase A inhibitor PKI 6–22 amide and 2 μm of the protein kinase C inhibitor (PKC19–36). Enzyme reactions, in a final volume of 50 μl, were initiated by the addition of Mg2+–ATP cocktail [5 mm magnesium acetate and 0.1 mm ATP (2000–3000 cpm/pmol)]. The reaction was stopped after 5 min with saturated solution of EDTA–EGTA, and 40 μl was spotted onto phosphocellulose paper. The phosphocellulose paper was washed once in water and three times in 75 mmphosphoric acid. The membranes were air-dried and used for radioactivity measurement by liquid scintillation counting. For each sample, the radioactive phosphorous counts from assay tubes in which the reaction mix contained cell lysate in the presence of 1 mm EGTA and no Ca2+were compared with counts from tubes in which an equal amount of the cell lysate was reacted in the presence of 1 mmCa2+ and 6 μm CaM. The autonomous activity of CaMKII in these cell lysates was determined by the ratio of the calcium-independent and calcium-dependent activities.
Intracellular calcium measurements. Electrically or chemically evoked calcium transients in DRG neurons were measured using a Bio-Rad (Hercules, CA) 1024 visible/UV confocal microscope and a Nikon 40× 1.3 numerical aperture oil immersion objective on a Nikon inverted microscope. Quantitative calcium measurements were made using ratiometric measurements of fluorescence intensity at 460 and 405 nm emission from DRG neurons loaded by incubation in 7.5 μm indo-1/AM or mag indo-1/AM and excited by an argon–ion laser at 350 nm (Fields and O'Donovan, 1997). Measurements were performed at room temperature in HEPES-buffered balanced salt solution, pH 7.2. In-cell calibration, as described in Fields et al. (1993), was used to provide an estimate of the [Ca2+]i associated with the fluorescence ratios. Briefly,Rmin andRmax were determined in neurons permeabilized by 10 μm ionomycin, in solutions containing 1.8 mmCa2+ and 0 mmCa2+/10 mm EGTA, under the same intensifier gains and pinhole settings that were used during the experiments. Measurements of [Ca2+]i were made within an optical plane passing through the center of the nucleus in the area of the cytoplasm midway between cell membrane and the nucleus. The area of measurement comprised ∼1/8 of the area of cytoplasm in the plane of section. The measured responses were uniform within different regions of the cell on the time scale reported in these experiments. An electromagnetic valve-controlled perfusion device (Warner Instruments, Hamlin, CT) was used to rapidly change the concentration of extracellular calcium in the bathing solution.
Single line-scan mode confocal microscopy was used for high-speed acquisition of changes in [Ca2+]i in response to action potentials of 0.1–30 Hz using the ratiometric calcium indicator indo-1 and the fluorescent intensity indicator fluo-3. Data were acquired at a rate of 8 msec/line scan for 2 sec (250 line scans) for both ratiometric and nonratiometric indicators. In individual neurons, quantitative measurements were taken at four points along a line bisecting the neuron within an optical section passing through the center of the nucleus: (1) in the submembrane region, (2) in the cytoplasm midway between the membrane and the nucleus, (3) in the center of the nucleus, and (4) at the midpoint in the cytoplasm on the opposite side of the nucleus.
RESULTS
Cytoplasmic Ca2+ transients induced by different frequencies of action potentials
Intracellular calcium imaging was used to determine the spatial and temporal characteristics of intracellular calcium responses to action potentials delivered at different frequencies in DRG neurons. Changes in [Ca2+]iwere measured in the cell body using confocal microscopy in single line-scan and x–y scanning modes with fluo-3, indo-1, and mag-indo fluorescent calcium indicators. Several calcium indicators were used to explore possible effects of the calcium dissociation constant of the indicator on the measurements and to evaluate potential differences caused by ratiometric and nonratiometric measurement methods.
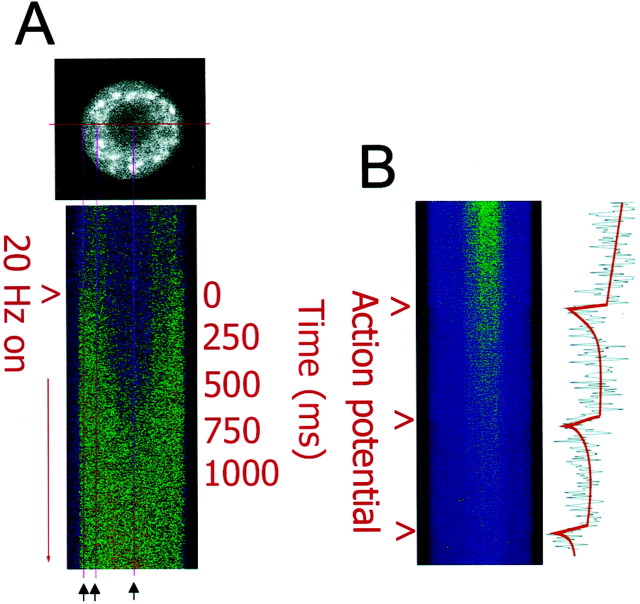
Responses to individual action potentials could be resolved with high-speed calcium imaging. In Figure1A, an optical section of a single mouse DRG neuron is shown loaded with the calcium indicator fluo-3. The nucleus can be discerned by differential fluorescence of the calcium indicator in the nucleus and cytoplasm; this is not caused by differences in free calcium concentration between the nucleus and the cytosol in the resting state (Gillot and Whitaker, 1993, 1994;O'Malley 1994; Meyer et al., 1995). To obtain high-speed measurements, the excitation laser was scanned repeatedly through the center of the cell, and data were acquired at a rate of 8 msec/scan. Fluorescence intensity changes shown in Figure 1B are from a DRG neuron filled with the calcium indicator indo-1, which exhibits a decrease in fluorescence intensity at 460 nm emission with increasing calcium concentration. A single action potential induced an increase in intracellular calcium with a magnitude of ∼20 nm and a duration of ∼300 msec (Fig.1B). The calcium increase was much faster than the kinetics of recovery to resting calcium concentration.
Fig. 1.
High-speed measurements of action potential-induced changes in intracellular calcium in DRG neurons using confocal microscopy in single line-scan mode. A, An optical section of a single mouse DRG neuron is shown loaded with the calcium indicator fluo-3 (black and white image). The excitation laser was scanned repeatedly through the center of the cell, and data were acquired at a rate of 8 msec/scan and displayed as a stack of sequential scans. Changes in intracellular calcium induced by action potential stimulation are shown for 250 sequential samples (2 sec total). Thus, the vertical dimension displays changes in calcium concentration with time, and the horizontal dimension displays one-dimensional spatial information on the calcium concentration along a transect across the center of the neuronal cell body. Measurements were taken in response to 0.1–30 Hz stimulation; the response to 20 Hz stimulation is shown here. Electrical stimulation caused an influx of calcium from the cell membrane and the increase in intracellular calcium propagated to the nucleus within ∼200 msec. Intracellular calcium concentration reached similar peak levels in the cytoplasm and nucleus. The three vertical magenta linesindicate the regions used for quantitative comparisons (black arrows). B, The increase in intracellular calcium concentration induced by single action potentials could be resolved with single line-scan measurements. Fluorescence intensity at 460 nm emission is shown from a DRG neuron filled with the calcium indicator indo-1. This indicator exhibits a decrease in fluorescence intensity with increasing calcium concentration at this emission wavelength. The ratio of such images at 460 and 405 nm emission wavelengths was used for quantitative analysis, as shown in Figure2G. The increase in intracellular calcium induced by an action potential lasted ∼300 msec after each action potential, and the kinetics of increase were much faster than the recovery to resting calcium levels. Scale bar, 10 μm.
After delivering trains of action potentials, a rapid increase in intracellular calcium could be resolved with single line-scan calcium imaging spreading from the membrane to the nucleus within ∼200 msec (Fig. 1A) (see also Hernandez-Cruz et al., 1990). The peak concentration of calcium was quantified adjacent to the plasma membrane, in the cytoplasm midway between the cell membrane and nucleus, and in the center of the nucleus (Fig. 1A, three vertical magenta lines). Intracellular calcium concentration quickly reached similar peak levels in all three regions of the cell after electrical stimulation, with a phase lag of ∼300 msec from the cell membrane to the center of the nucleus.
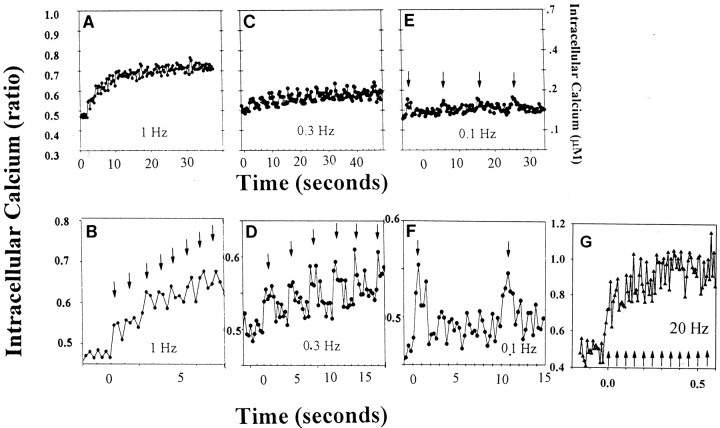
The relation between action potential firing frequency and cytoplasmic calcium dynamics in DRG neurons was determined in response to stimulus trains of 15–30 sec, using confocal calcium imaging in x–yscanning mode at acquisition rates of 3 images/sec. During low-frequency stimulus trains (0.1–1 Hz), individual spikes in [Ca2+]i could be resolved in response to individual action potentials (Fig.2B, D–F). Trains of stimulation at and above frequencies of 0.3 Hz resulted in temporal summation of calcium spikes and calcium accumulation (Fig.2A–D). In response to high-frequency stimulation (20 Hz), individual calcium spikes could only be resolved with high-speed single line-scan confocal microscopy (Fig. 2G). At these higher frequencies, temporal summation of calcium spikes resulted in a rapid and large increase in intracellular calcium that was maintained for the duration of the stimulus burst (Fig. 2G). As with the single-line scan measurements, and as previously reported byO'Malley (1994) and Fields et al. (1997), peak calcium responses were similar in the submembrane region, central cytoplasm, and nucleus.
Fig. 2.
Intracellular calcium transients induced by action potential stimulation of different frequencies were measured in the cell body of DRG neurons with confocal microscopy and the ratiometric calcium indicator indo-1. Individual cytoplasmic calcium spikes (B, D–F) can be seen in response to individual action potentials (arrows) elicited by electrical stimulation at different frequencies. Stimulation at higher frequencies resulted in temporal summation and an elevation in intracellular calcium level that was positively correlated with the stimulus frequency and sustained for the period of the stimulus train (A–D). In response to higher frequency stimulation, individual calcium spikes (arrows) could be resolved using single line-scan mode confocal microscopy on top of a large increase in intracellular calcium concentration (G). Note the difference in axis scaling for each graph.
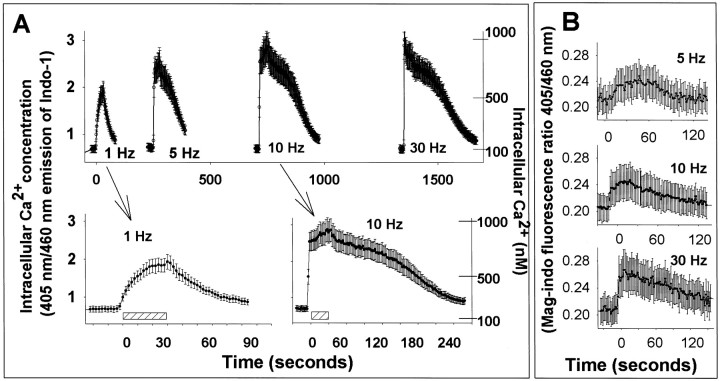
The increase in peak [Ca2+]i and the rise time were positively correlated with the frequency of stimulation (Fig. 3A). After stopping stimulus trains, calcium levels recovered at a slower rate; with higher frequency stimulation causing more sustained elevations in calcium concentration than lower frequency stimulation (Fig. 3A, insets). Similar calcium dynamics were seen using the calcium indicator indo-1, a ratiometric indicator that is not subject to artifacts associated with fluorescence intensity calcium indicators (e.g., fura-2 and fluo-3), and using the very low-affinity calcium indicator, mag-indo-1 (Kd = 35,000 nm as compared with 230 nmfor indo-1) (Fig. 3B).
Fig. 3.
The concentration dynamics of electrically evoked intracellular Ca2+ concentration varied directly with the stimulus frequency. A, Changes in cytoplasmic calcium were measured by confocal microscopy using the ratiometric fluorescent calcium indicator indo-1/AM. The rate of rise, peak, concentration, and duration of Ca2+ increase were positively correlated with stimulus frequency between 1 and 30 Hz. Data for 1 and 10 Hz are shown as insets on an expanded time scale. Results shown are mean ± SEM (n = 12 neurons). Scale bar, 30 sec stimulation. B, Similar responses are observed using the low-affinity indicator mag-indo-1, which is less sensitive to small changes in intracellular calcium.
The results of these calcium imaging studies show that action potentials induced by electrical stimulation of different frequencies are effective in causing large changes in [Ca2+]i that are positively correlated with the frequency of stimulation. However, the action potential-induced intracellular Ca2+ fluxes are much slower and more persistent than the spikes used in some theoretical models (Hanson et al., 1994; Okamoto and Ishikawa 2000) and in vitrosimulations giving sensitive frequency decoding (De Koninck and Schulman, 1998).
Within the temporal and spatial limits defined by these measurements, microdomains of [Ca2+]i were not apparent in response to membrane depolarization from trains of action potentials lasting several seconds. This allows a reasonably good correlation between action potential-induced calcium dynamics measured in the cell body after several seconds of action potential stimulation, with CaMKII autonomous activity measured from lysates of neurons in the side compartments of culture chambers that contained the cell bodies of DRG neurons.
Autonomous activity is the ratio of catalytic activity of CaMKII in the absence of Ca2+ to the activity in the presence of Ca2+. This is of interest because autonomous activity of CaMKII allows the enzyme to sustain a response long after the neuron experiences a burst of action potentials and intracellular calcium returns to basal levels. Autonomous activity derives from phosphorylation of Thr-286, which we assessed by an immunoblot using a phosphosite-specific antibody (Yamagata and Obata, 1998).
In vitro measurements using CaMKII-α immobilized in PVC tubing indicate that varying the duration of individual calcium pulses shifted the frequency response of CaMKII autonomous activation and changed its steepness (De Koninck and Schulman, 1998). The frequency response of CaMKII to calcium pulses with a duration of 300 msec or longer would be relatively shallow and limited to frequencies of less than ∼1 Hz (De Koninck and Schulman, 1998). Therefore, we tested the autonomous activation of CaMKII in response to different frequencies of action potential stimulation in DRG neurons. Before measuring the basal and stimulus-induced autophosphorylation of CaMKII at Thr-286, we examined the subunit composition of CaMKII in DRG neurons.
Characterization of CaMKII in DRG neurons
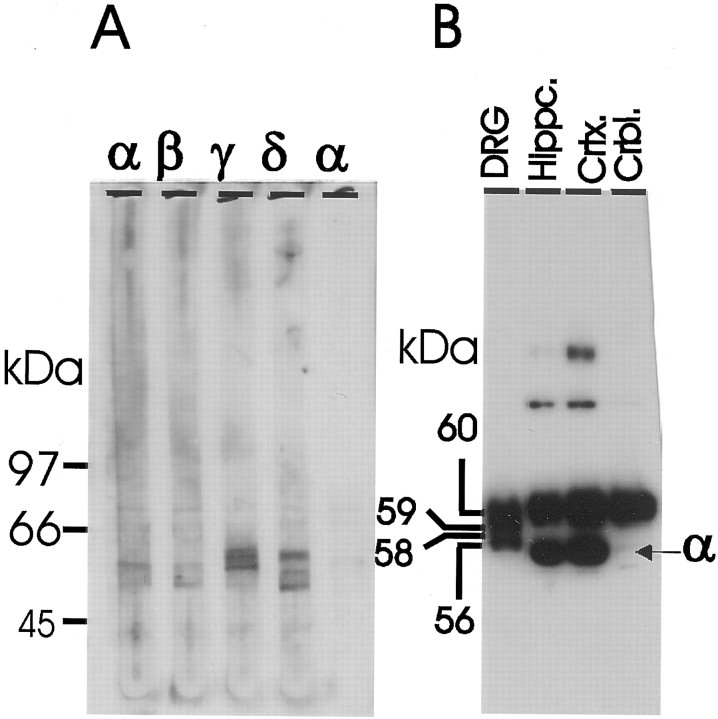
Immunohistochemical staining shows a ubiquitous distribution of CaMKII throughout the neuron, and the enzyme represented ∼70–80% of the total Ca2+–CaM-dependent kinase activity in cultured DRG neurons as determined by inhibition of phosphorylation of the glycogen synthase peptide (syntide-2) with a specific inhibitory peptide related to autocamtide-2 (F. Eshete and R. D. Fields, unpublished data). Immunoblotting studies revealed that the γ (∼58–60 kDa) and δ (∼60, 58, 56 kDa) isozymes are the predominant CaMKII isozymes in DRG neurons, whereas the α and β isozymes are either expressed in very low amounts or absent from these neurons (Fig. 4A,B). These observations are consistent with the recent report on the developmental expression of CaMKII isozymes in the mouse nervous system (Bayer et al., 1999).
Fig. 4.
δ and γ are the two major CaMKII isozymes expressed in cultured DRG neurons. A, Lysate from cultured neurons was resolved in 8% single well gel and immunoblotted in a manifold with polyclonal antibodies that reacted with the α, β, γ, and δ isozymes (Santa Cruz Biotechnology) and a monoclonal antibody specific to CaMKII-α (Life Technologies). The anti-CaMKII-γ antibody reacted with bands (58–60 kDa) representing the γ isoforms, whereas the antibody against CaMKII-δ recognized bands with apparent molecular mass of ∼56, 58, and 60 kDa.B, Lysates from DRG neurons, mouse hippocampus, cortex, and cerebellum were immunoblotted with antibody specific to CaMKII phosphorylated at Thr-286. In DRG lysate the phospho-specific antibody mainly reacted with bands of ∼56, 58, 59, and 60 kDa. Note the relative absence of the CaMKII-α band in both DRG neurons and mouse cerebellum.
The CaMKII enzymes present in DRG neurons would be expected to exhibit similar frequency responses to the isotypes examined in vitro (De Koninck and Schulman, 1998). The CaM affinity of the γ and δ isoforms of CaMKII is similar to that of CaMKII-α isozymes (Edman and Schulman, 1994). All CaMKII isozymes contain a MHRQETV motif that is responsible for autonomous activation of the enzyme through phosphorylation of Thr 286. These isoforms differ in the variable domain of the protein (Brocke et al., 1999). CaMKII measured in brain homogenates and the δ isozymes (Edman and Schulman, 1994) both exhibit Ca2+-independent activation after autophosphorylation of Thr-286 (Kwiatkowski and McGill, 2000).
CaMKII autophosphorylated at Thr-286 was detected by immunoblotting with a phosphosite-specific CaMKII antibody (Fig.4B). All isoforms of CaMKII in DRG neurons, freshly dissected hippocampus, cortex, and cerebellum showed immunoreactivity to CaMKII phosphorylated at Thr-286 (Fig. 4B). Note the low levels of the α-CaMKII isoform in DRG neurons and cerebellum with the phosphosite-specific antibody.
Action potential frequency dependence of CaMKII autonomous activity
CaMKII autonomous activity was found to vary as a function of action potential firing frequency and the duration of action potential firing (p < 0.0001, ANOVA) (Fig.5A, Table1). The observed response to constant frequency action potential stimulation was biphasic, with a rapid increase to peak CaMKII autonomous activation followed by a subsequent decline in autonomous activity with more prolonged stimulation. The frequency of action potentials in the stimulus train affected both the rising and declining phase of CaMKII autonomy with respect to stimulus time. Higher frequency stimulation promoted both the rate of increase and the rate of decline in the autonomous activity of the enzyme. CaMKII autonomous activity was increased significantly (p < 0.01) after stimulation at 1, 3, and 10 Hz stimulation for 15 sec, but the differences among these stimulus frequencies were not statistically significant at 15 and 45 sec.
Fig. 5.
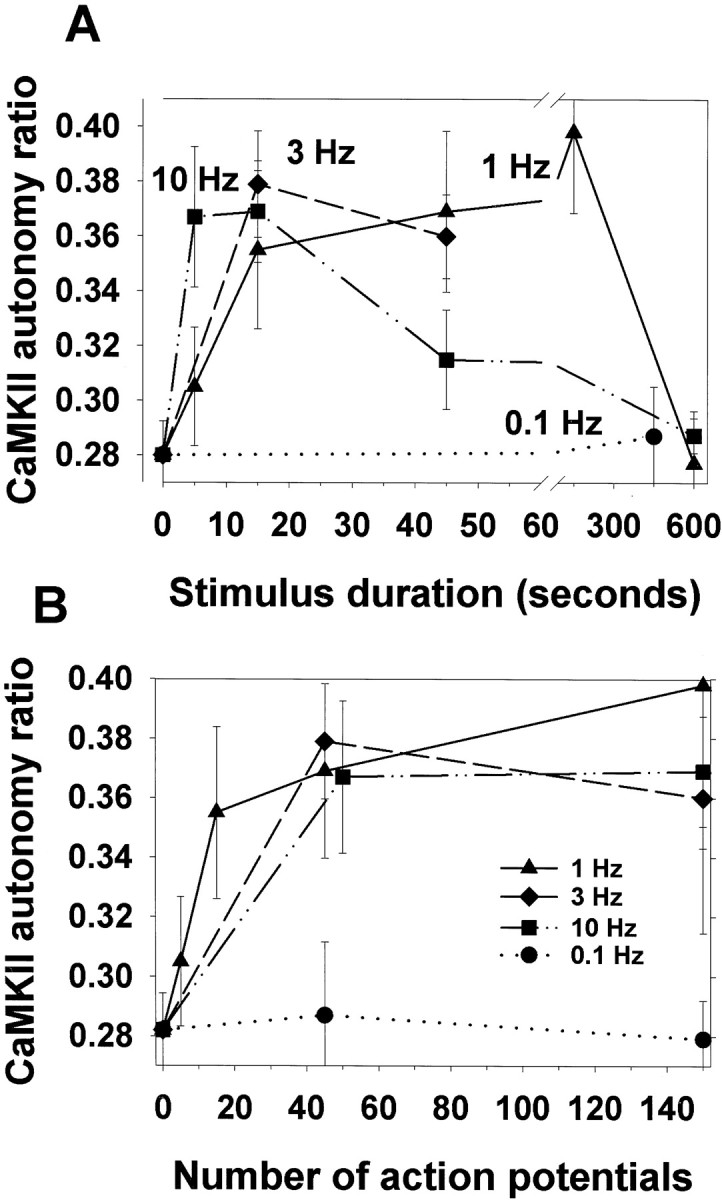
The effect of action potential frequency and stimulus duration on autonomous activation of CaMKII. CaMKII autonomy ratio is the ratio of Ca2+-independent activity/total activity. Autonomous activity of CaMKII was measured byin vitro phosphorylation assay in homogenates of neurons electrically stimulated for 5, 15, 45, and 600 sec at 0.1, 1, 3, and 10 Hz. A, Stimulation at 1, 3, and 10 Hz for 15 sec induced statistically significant Ca2+-independent activation of CaMKII compared with unstimulated controls (p < 0.01; t test;n = 24 dishes), but the differences among responses to 1, 3, and 10 Hz were not statistically significant at 15 and 45 sec. The results shown (mean ± SEM) are from five independent experiments (ANOVA; p = 0.02; n= 150 dishes). Inactivation of the enzyme was promoted by long-duration high-frequency stimulation. The duration of stimulation required to reach maximal levels of CaMKII autonomy varied inversely with the frequency of stimulation. Stimulation at very low frequency (0.1 Hz) failed to increase CaMKII autonomous activity significantly.B, Maximal autonomous activation of CaMKII correlated with the number of action potentials delivered at these different frequencies. Within the range of 1–10 Hz, near-maximal autonomous activity was reached after ∼45 action potentials, regardless of stimulus frequency. Statistical analysis by ANOVA (p < 0.0001; n = 178), followed by Fisher's LSD multiple comparison procedure (Table 1) indicates significant increases in CaMKII autonomous activation after 45 action potentials are delivered at all frequencies tested within the range of 0.5–10 Hz, despite large differences in the stimulus duration required to reach 45 action potentials at these different frequencies (i.e., 4.5–90 sec).
Table 1.
Pairwise multiple comparison test using Fisher's LSD procedure for differences in CaMKII autonomous activation after electrical stimulation, varying action potential frequency, number, and stimulus duration
| Hz | 0 | 1 | 1 | 0.1 | 0.3 | 0.5 | 1 | 3 | 10 | 1 | 3 | 10 | 10 | 1 | 10 | ||||
| AP | 0 | 5 | 15 | 45 | 45 | 45 | 45 | 45 | 45 | 150 | 150 | 150 | 450 | 600 | 6000 | ||||
| sec | 0 | 5 | 15 | 450 | 150 | 90 | 45 | 15 | 4.5 | 150 | 50 | 15 | 45 | 600 | 600 | ||||
| n | 32 | 9 | 15 | 13 | 9 | 9 | 21 | 10 | 15 | 3 | 3 | 15 | 15 | 6 | 6 | ||||
| % | 100 | 106 | 147 | 93 | 114 | 131 | 127 | 126 | 128 | 142 | 128 | 164 | 124 | 116 | 104 | ||||
| AP | Hz | sec | n | % | |||||||||||||||
| 0 | 0 | 0 | 32 | 100 | x | ||||||||||||||
| 5 | 1 | 5 | 9 | 106 | NS | x | |||||||||||||
| 15 | 1 | 15 | 15 | 147 | * | * | x | ||||||||||||
| 45 | 0.1 | 450 | 13 | 93 | NS | NS | * | x | |||||||||||
| 45 | 0.3 | 150 | 9 | 114 | NS | NS | * | NS | x | ||||||||||
| 45 | 0.5 | 90 | 9 | 131 | * | NS | NS | * | NS | x | |||||||||
| 45 | 1 | 45 | 21 | 127 | * | NS | NS | * | NS | NS | x | ||||||||
| 45 | 3 | 15 | 10 | 126 | * | NS | NS | * | NS | NS | NS | x | |||||||
| 45 | 10 | 4.5 | 15 | 128 | * | NS | NS | * | NS | NS | NS | NS | x | ||||||
| 150 | 1 | 150 | 3 | 142 | * | NS | NS | * | NS | NS | NS | NS | NS | x | |||||
| 150 | 3 | 50 | 3 | 128 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | x | ||||
| 150 | 10 | 15 | 15 | 164 | * | * | NS | * | * | * | * | * | * | NS | NS | x | |||
| 450 | 10 | 45 | 15 | 124 | * | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | * | x | ||
| 600 | 1 | 600 | 6 | 116 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | x | |
| 6000 | 10 | 600 | 6 | 104 | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | x |
A subset of these data are plotted against stimulus time in Figure 5A and action potential number in Figure5B; p < 0.0001 by one-way ANOVA;n = 178. %, Autonomous CaMKII activity relative to unstimulated control; Hz, stimulus frequency (action potentials/sec); AP, number of action potentials in stimulus; sec, stimulus duration in seconds; n number of samples (cultures);
p < 0.05, pairwise comparison; NS,p > 0.05, pairwise comparison.
Similar peak levels of CaMKII autonomous activity were reached with stimulus trains of 1–10 Hz, but the optimal duration of constant-frequency action potential firing was different for each stimulus frequency and varied inversely with the action potential firing frequency. Near maximal levels were reached after only 4.5 sec stimulation at 10 Hz, in contrast to 15–150 sec for stimulation at 1 Hz. The slope of the function after 15 sec stimulation at 1 Hz was positive (p < 0.0001), but after 45 action potentials, the slope could not be distinguished statistically from zero, indicating a near maximal response had been reached. Low-frequency stimulation (0.1 Hz) did not alter CaMKII autonomous activity significantly, even after prolonged stimulation (7.5 min).
The optimal duration of stimulation and inverse relation to the stimulus frequency implies that maximal CaMKII autonomous activity may be dependent on the number of action potentials delivered over this range of stimulus frequencies. Replotting the data in Figure5A as a function of action potential number, rather than stimulus duration shows that near maximal CaMKII autonomous activation was attained by stimulation with 45 action potentials at 1 Hz, and by 45 action potentials (the shortest duration assayed) at 3 and 10 Hz (Fig. 5B). Analysis by one-way ANOVA, followed by Fisher's LSD multiple comparison test showed statistically significant increases in autonomous activation after 45 action potentials delivered at 0.5–10 Hz, despite marked differences in the duration of stimulation required to deliver 45 action potentials at these different frequencies (Table 1).
The ratio of calcium-independent to calcium-dependent CaMKII activity was high in DRG neurons under basal conditions (0.28 ± 0.068;n = 36 dishes). Previous studies have also shown high autonomous CaMKII activity in freshly dissociated brain tissue and unstimulated hippocampal slice in the range of 0.15–0.20 (Molloy and Kennedy, 1991; Ocorr and Schulman, 1991).
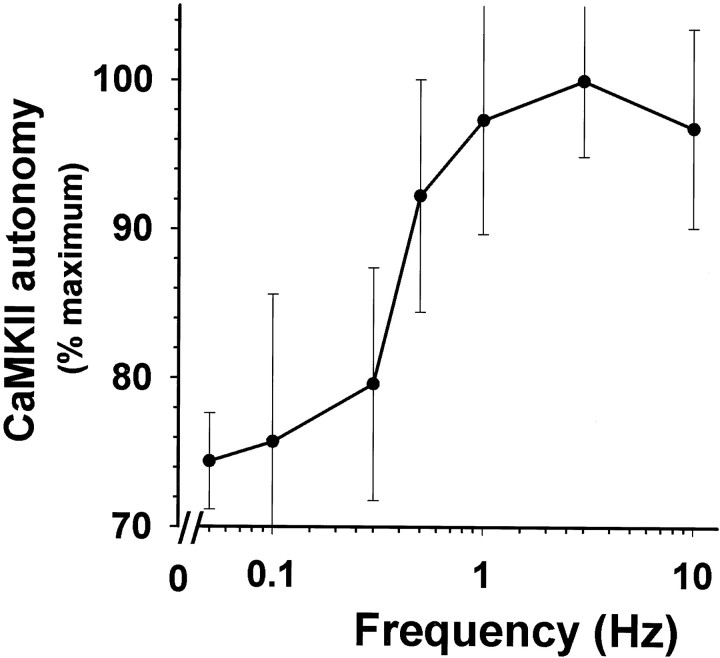
A frequency response curve for CaMKII autonomous activation was derived by delivering equal numbers of action potentials (45) at different frequencies within the range of 0.1–10 Hz (Fig.6). CaMKII exhibited frequency-dependent activation in DRG neurons in response to action potentials between 0.1 and 1 Hz (p < 0.001; slope of linear regression = +30.2 ± 9.01; n = 84), but the response plateaued at higher frequencies (1–10 Hz) (slope not significantly different from 0; p = 0.89;n = 46), despite marked differences in [Ca2+]i in response to these frequencies of stimulation (Fig. 3). Spike frequency decoding by CaMKII has not been demonstrated in intact neurons, but the behavior of CaMKII-α in vitro would predict that the high level of CaMKII autophosphorylation in unstimulated DRG neurons and the persistence of [Ca2+]i after action potentials should shift the spike frequency decoding to lower frequencies of calcium pulses, which is consistent with the present results.
Fig. 6.
Spike frequency decoding by CaMKII in DRG neurons. The frequency response curve was derived by delivering 45 action potentials at frequencies of 0.1, 0.3, 1, 3, and 10 Hz. Results are plotted as percentage of maximum stimulus-induced increase in CaMKII autonomy ratio (mean ± SEM; n = 111 dishes). CaMKII in DRG neurons showed sensitivity to low frequencies of action potentials <1 Hz, but higher frequency stimulation produced similar levels of CaMKII autonomous activation, despite marked differences in intracellular Ca2+ (compare Fig. 3). The mean ratio of Ca2+-independent activity/total activity in unstimulated neurons (0 Hz) was 0.28 ± 0.068 (n = 36 dishes).
Relation between CaMKII autonomy and intracellular calcium dynamics
A useful simplifying assumption in mathematical modeling andin vitro simulations of CaMKII activity has been to model action potential-induced calcium transients as fixed-amplitude rectangular pulses of defined pulse width that returned to baseline during the interpulse interval (Hanson et al., 1994; DeKoninck and Schulman 1998; Okamoto and Ishikawa, 2000.) The actual [Ca2+] dynamics induced by action potentials are somewhat more complicated. Notably, higher frequency stimulation leads to temporal summation of intracellular calcium concentration because the rate of restoring intracellular Ca2+ to basal levels, via pumps and exchangers, is much slower than the rate of Ca2+ accumulation in the cytoplasm via influx through Ca2+ channels in the plasma membrane and intracellular organelles. The accumulation of “residual” Ca2+ accompanying higher frequency action potential stimulation has important physiological implications, for example in paired-pulse facilitation, and this behavior is shown clearly by Ca2+ imaging in DRG neurons (Fig. 1A–D). Changes in calcium buffering mechanisms after trains of stimulation may also increase calcium accumulation and raise the steady-state calcium set-point (Colegrove et al., 2000). These Ca2+measurements show that accumulation of residual Ca2+ and peak Ca2+ levels are positively correlated with action potential frequency (Fig. 3A). Thus, it is necessary to examine the extent to which the action potential decoding of CaMKII is dependent on the temporal dynamics of intracellular Ca2+ spikes or the associated concentration of cytoplasmic Ca2+.
Several lines of evidence suggest that the Ca2+ spike frequency can be more important than the [Ca2+]iin autonomous activation of CaMKII. Over the range of action potential frequencies in which CaMKII decodes action potential frequency (0.1–1 Hz), the increase in residual Ca2+ is comparatively small (100–300 nm) (Fig. 1), relative to the concentration of Ca2+ necessary for autophosphorylation of the enzyme in vitro. Consistent with studies from several laboratories (Katoh and Fujisawa, 1991; Rich and Schulman, 1998), our measurements of CaMKII autophosphorylation in lysates of DRG neurons in vitro show a dependence on steady-state Ca2+ concentration >1 μm, which is much greater than the residual [Ca2+]i induced by 0.1–1 Hz stimulation. Ca2+-independent activity (autonomy ratio) was measured in lysates of unstimulated neurons after autophosphorylation for 15 and 45 sec in the presence of 0.1, 0.2, 0.3, and 1 μm and 1 mm Ca2+in vitro. The autonomy ratios after autophosphorylation at 0.1–1 μm Ca2+ were not significantly different from controls, but increased significantly to 0.72 ± 0.09 after 15 sec autophosphorylation with 1 mm Ca2+ (ANOVA with Fisher's multiple comparison test; n = 21).
Second, there was not a good correlation between residual or peak calcium levels and CaMKII autonomous activity. Similar levels of CaMKII autonomous activity were reached in response to trains of action potentials of 1–10 Hz (Fig. 5A), but the residual or peak increase in [Ca2+]i were markedly different (Fig. 3A). A 10 Hz stimulus raised average [Ca2+]i to a peak level of 780 nm after 15 sec of stimulation compared with 370 nm peak response to 1 Hz stimulation. Stimulation of ∼2 sec was required to achieve 50% of the maximum cytoplasmic calcium amplitude with 10 Hz stimulus frequency (Fig. 3C) compared with 10 sec for 1 Hz stimulation (Fig. 3B). The increase in [Ca2+]i was larger and remained elevated for a longer time after stimulation regardless of whether a high [Ca2+] affinity (indo-1) or low Ca2+ affinity (mag-indo-1) Ca2+ indicator was used. The sustained large increase in [Ca2+]i argues against ineffective stimulation as an explanation for the similar stimulus-induced increase in CaMKII autonomy in response to action potential firing at 1–10 Hz.
To further explore this issue, bursts of action potentials were used to vary the duration of cytoplasmic calcium transients, their amplitude, and the level of cytoplasmic calcium accumulation underlying the transients. The change in [Ca2+]i in response to a single action potential was resolved in these experiments (Fig. 2F) and in previous studies (Fields et al., 1997) and was estimated to reach a peak of ∼20 nm above resting levels. To distinguish whether the enzyme failed to respond to 0.1 Hz stimulation because of Ca2+ pulse frequency decoding of the enzyme or its sensitivity to Ca2+concentration, short bursts of 18 action potentials (at 10 Hz) were delivered repeatedly at intervals of 10 sec (a frequency of 0.1 Hz). Intracellular calcium measurements in these neurons show that a single burst of this type produces a peak concentration of ∼500 nm (Fields et al., 1997), and trains of such bursts repeated at 0.1 Hz result in a large increase in [Ca2+]i (Fig.7Aa). When 45 bursts of this type were repeated at a frequency of 0.1 Hz, CaMKII autonomous activity was not significantly different from unstimulated neurons (Fig.7B, bar a). Although this pulsed stimulus delivered a total of 810 individual action potentials and raised [Ca2+]i to high levels, the low-frequency pulse-train of action potential bursts was not effective in increasing the autonomous activity of the enzyme, whereas only 15 individual action potentials at 1 Hz was highly effective (Fig. 5A).
Fig. 7.
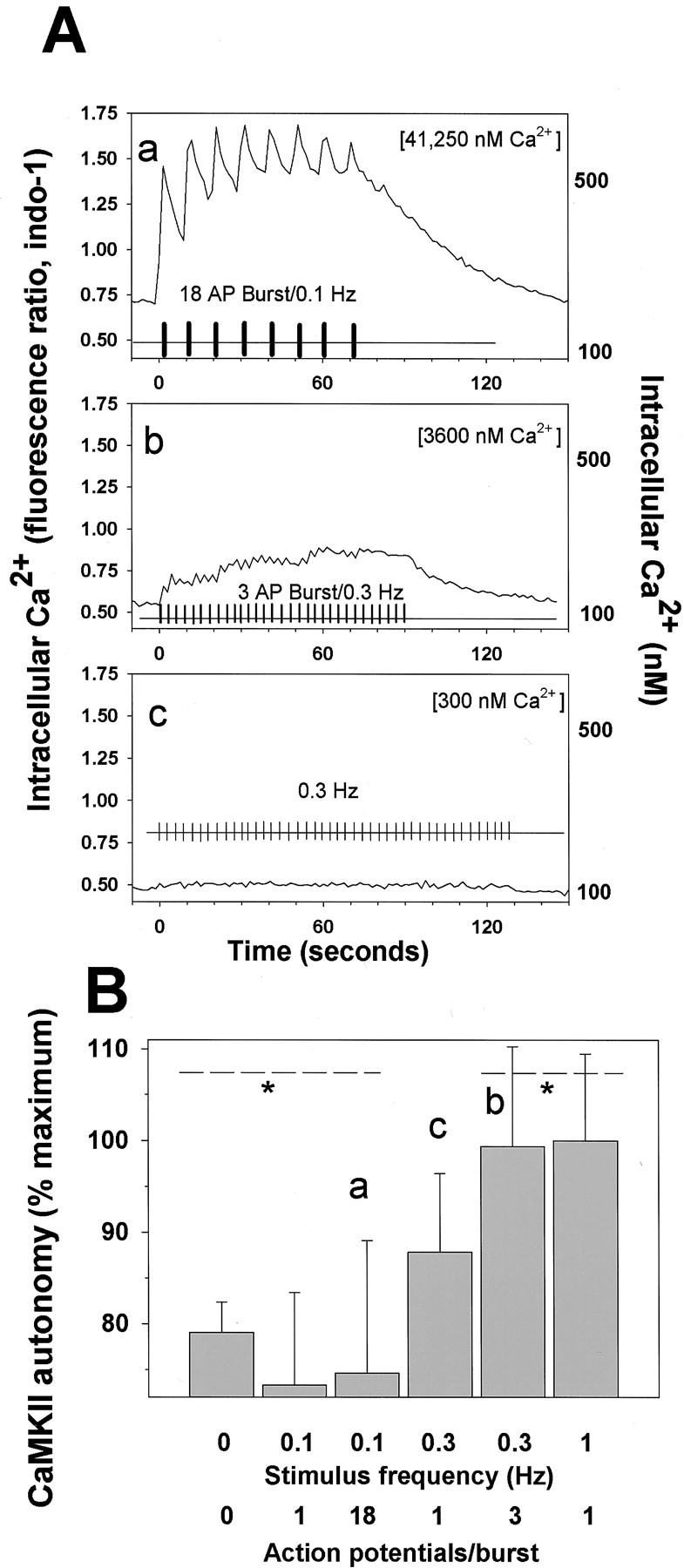
CaMKII autonomous activity in response to periodic bursts of action potentials correlates with the frequency of calcium pulses rather than concentration of intracellular calcium.A, Intracellular calcium transients were measured by confocal microscopy in neurons stimulated in three patterns: 18 action potential bursts (at 10 Hz) repeated at 10 sec intervals (0.1 Hz) (a); three action potential bursts (at 10 Hz) repeated at 3 sec intervals (0.3 Hz) (b); and single action potentials delivered at 3 sec intervals (0.3 Hz) (c). The net increase in [Ca2+]i during the entire stimulus period with each stimulus pattern is given in brackets in the top right of each plot (calcium concentration time integral, nanomolar con- centration). The average calcium response of the same five neurons is plotted in response to each stimulus.B, Comparing CaMKII autonomous activity in response to single action potentials and repetitive bursts of action potentials at different frequencies. Stimulation at low frequency failed to increase CaMKII autonomous activity regardless of whether single action potentials or bursts of 18 action potentials were delivered. The increase in [Ca2+]i produced by these different stimuli (A, a–c) did not correlate with CaMKII autonomous activation, suggesting that the frequency of calcium transients was more critical than the calcium levels. CaMKII autonomy is plotted as the percentage of maximal stimulus-induced increase in ratio of calcium-independent–calcium-dependent activity, which averages 0.28 ± 0.068 in unstimulated neurons; p < 0.01; ANOVA; n = 96; *values outside the indicated range are significantly different by Fisher's LSD multiple comparison test.
In addition to peak and residual [Ca2+]i, the time-integrated increases in [Ca2+]i produced during the stimuli were not well correlated with the autonomous activity of CaMKII (Fig. 7B vs the net increase in Ca2+ shown in brackets in Fig.7A). In contrast to the long-duration bursts of 18 action potentials, shorter bursts (3 action potentials at 10 Hz) that were repeated at more frequent intervals of 3 sec (0.3 Hz) did increase CaMKII autonomous activity to near maximal levels (Fig. 7B, bar b). The long-duration pulses of 18 action potentials repeated at 0.1 Hz (Fig. 7Aa), caused ∼11.4 times greater time-integrated [Ca2+]i increase than the short-duration pulses (3 action potentials repeated at 0.3 Hz) (Fig. 7Ab), and ∼137 times more than constant-frequency stimulation at 0.3 Hz (Fig. 7Ac), yet this stimulus pattern was less effective than either pulsed or single action potential stimulation at 0.3 Hz in increasing autonomous activity of CaMKII (Fig.7B, bars b,c). As with constant frequency stimulation, the best correlation with CaMKII autonomous activity after bursts of action potentials was with the frequency of Ca2+transients rather than with the [Ca2+]i.
Extracellular calcium affects CaMKII autophosphorylation and autonomy
The high levels of autonomously active CaMKII in unstimulated neurons has significant functional effects on the frequency response of the enzyme, making it important to better understand the mechanisms regulating the level of CaMKII autonomy in unstimulated conditions. Previous studies in hippocampal slice (Molloy and Kennedy, 1991) and hippocampal neurons in culture (Scholz and Palfrey, 1998) also report high levels of CaMKII autonomous activity under basal conditions. Interestingly, those studies reported that the level of CaMKII autonomy could be reduced by lowering the concentration of extracellular calcium ([Ca2+]o) (Molloy and Kennedy, 1991; Scholz and Palfrey, 1998). It has been hypothesized that the high levels of autophosphorylation of CaMKII in unstimulated neurons is a result of sustained kinase activity associated with spontaneous synaptic activity in the cultures or brain slice, which is suppressed by lowering the [Ca2+]o. De Koninck and Schulman (1998) suggest that this relatively high level of autonomously active CaMKII in unstimulated conditions could allow CaMKII to act as a tag of the history of activity at a synapse, because partial autophosphorylation of CaMKII under basal conditions would shift the frequency response curve toward lower frequencies of Ca2+ spikes. This property of the enzyme would permit low-frequency, subthreshold activation to maintain the phosphorylated state of the enzyme at the tagged synapse.
Alternatively, changes in [Ca2+]i secondary to changes in [Ca2+]o could affect autophosphorylation and autonomy of CaMKII (Scholz and Palfrey, 1998). It has also been proposed that the high levels of CaMKII autonomy under basal conditions and the reduction after lowering [Ca2+]o, could result from phosphorylation of the enzyme that is dependent on a specialized synaptic extracellular Ca2+sensor (Scholz and Palfrey, 1998).
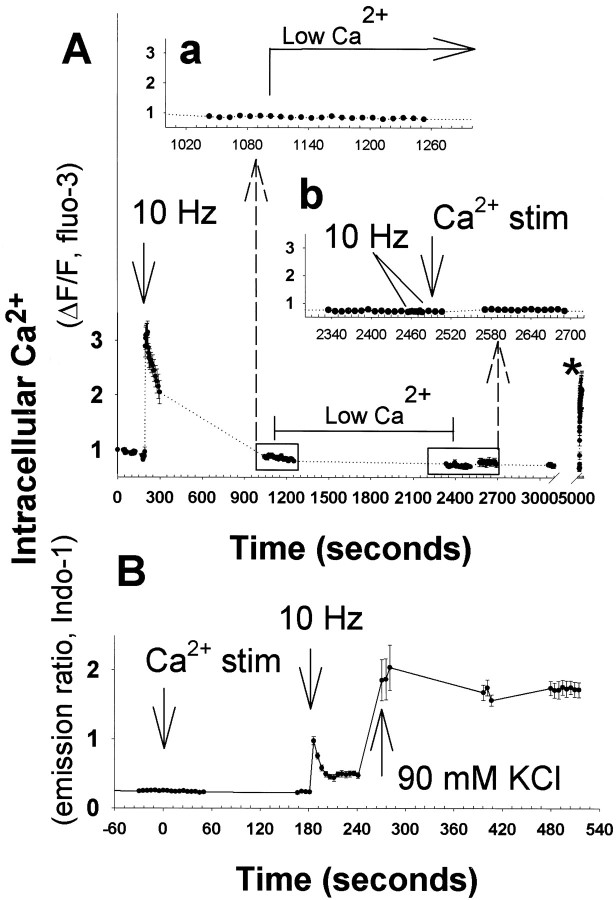
Studies in DRG neurons could be helpful in distinguishing between these alternatives, because DRG neurons in culture lack synapses and spontaneous impulse activity. The results showed that incubating DRG neurons in low Ca2+ solution (50 nm) for 1 hr induced a dramatic reduction in the autophosphorylation (Fig.8A,B) and the autonomous activity of CaMKII (Fig. 8C). When neurons preincubated in low [Ca2+]o (50 nm Ca2+-PSS for 60 min) were switched to physiological calcium concentration of 1.2 mm, a large and significant increase (p = 0.0004; t test;n = 8 dishes) in autophosphorylation of CaMKII at Thr-286 was observed (Fig. 8B). Lowering [Ca2+]o also induced a decline in CaMKII autonomous activity measured in thein vitro kinase assay. This decline in enzyme activity caused by preincubation in low [Ca2+]o (50 nm Ca2+-PSS) was reversed significantly within 5 sec of switching to 1.2 mm Ca2+(p < 0.001; ANOVA; n = 36 dishes) (Fig. 8C). Moreover, the enzyme maintained a prolonged autonomous activity at 1.2 mm[Ca2+]o for up to 30 min. This is consistent with the high level of autonomous activity observed in unstimulated neurons in culture.
Fig. 8.
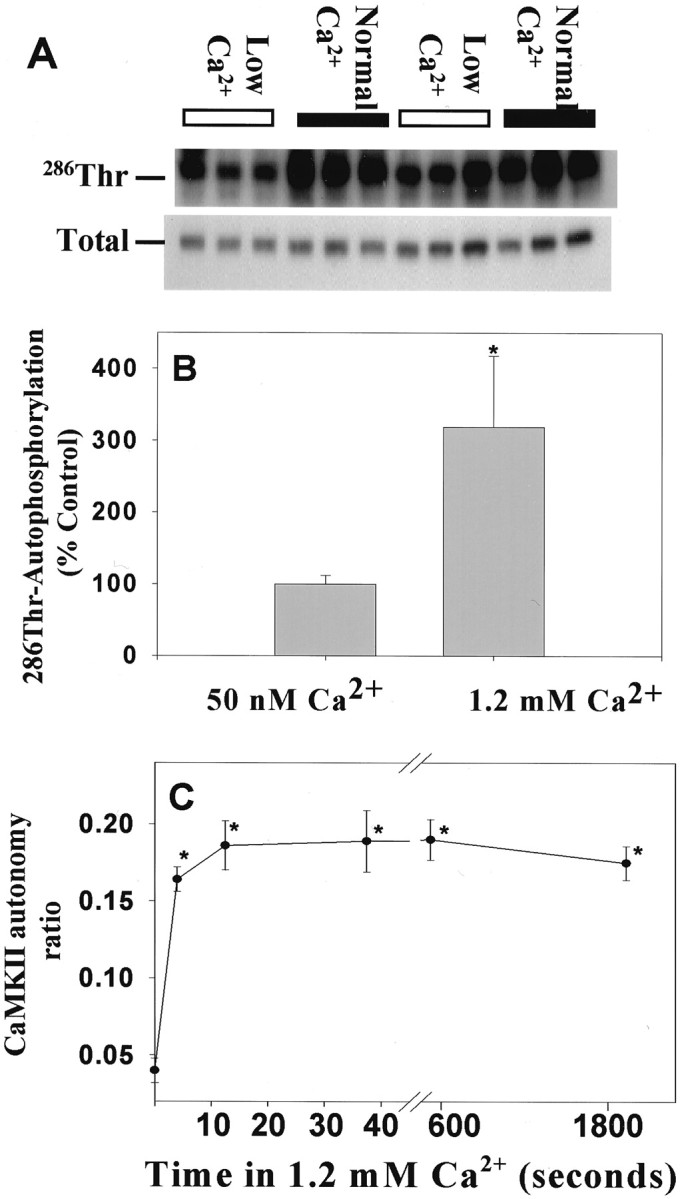
Autophosphorylation of CaMKII at Thr-286 was reduced in DRG neurons in low extracellular concentration of Ca2+. A, DRG neurons were changed to low calcium (50 nm Ca2+-PSS) for 1 hr before switching to normal calcium concentration (1.2 mmCa2+-PSS) for 45 sec. Lysates were analyzed by immunoblotting with an antibody recognizing the phospho-Thr-286 CaMKII. Each lane represents data from a single neuronal culture. Two replicate experiments of three cultures per treatment (12 cultures total) are shown, in which neurons were either incubated in 50 nm Ca2+ for 1 hr (Low Ca2+) or switched from this low-Ca2+ solution to 1.2 mmCa2+ (Normal Ca2+) for 45 sec. The same volume of lysate was analyzed for both phospho- and total-CaMKII immunoreactivity. B shows the normalized relative immunoreactivity of Thr-286 CaMKII in low and normal [Ca2+]. Data are mean ± SEM (n = 8 dishes). *Significantly different from neurons equilibrated at low [Ca2+]o(p = 0.0004; t test).C, The autonomous activation of CaMKII is rapid and persistent in DRG neurons equilibrated in low [Ca2+]o and switched into 1.2 mm Ca2+. Cultured neurons exposed to 50 nm Ca2+ for 1 hr were switched to 1.2 mm Ca2+-PSS for 5, 15, and 45 sec and 10 and 30 min before lysis. Data are mean ± SEM from two separate experiments of equal sample size. Autonomous activity ratio (Ca2+-independent/total) was calculated for each dish. *The level of activation is significantly different from neurons equilibrated in 50 nmCa2+-PSS (p = 0.001; ANOVA; n = 36 dishes). Note that changes in extracellular calcium concentration did not alter intracellular calcium levels measurably (Fig. 9).
Calcium imaging was used to determine if these changes in [Ca2+]o altered the level of free calcium in the cell body. No measurable alterations in [Ca2+]i were detected when the [Ca2+]o was either lowered to 50 nm from 1.2 mm or raised to 1.2 mm from 50 nm (Fig.9). In contrast, brief electrical stimulation at 10 Hz, depolarization with 90 mm KCl, or calcium ionophore caused large increases in [Ca2+]i in these same neurons, validating the measurement method (Fig. 9). This would be consistent with minimal transmembrane Ca2+leak currents in unstimulated DRG neurons and a lack of spontaneous synaptic activity.
Fig. 9.
Changes in concentration of extracellular Ca2+ that affect CaMKII autophosphorylation produced no detectable change in intracellular concentration in DRG neurons.A, Action potentials induced by brief electrical stimulation (10 Hz) caused a large increase in intracellular calcium as measured with fluo-3 fluorescence in time-lapse confocal microscopy. After intracellular calcium concentration recovered to basal levels, the extracellular calcium concentration was lowered from 1.2 mm Ca2+ to 50 nmCa2+ using a rapid bath perfusion system (Low Ca2+). The intracellular calcium concentration was not altered significantly (a), and remained constant over the next 25 min (b). Electrical stimulation produced no change in intracellular calcium in low calcium conditions (b, inset, 10 Hz), indicating that the change to low extracellular calcium concentration by perfusion of 50 nm Ca2+ solution had been effective. After then returning extracellular calcium concentration to 1.2 mm (Ca2+stim), intracellular calcium concentration remained constant for the next 40 min (b, inset). Calcium ionophore A23187 applied at the end of the experiment (*) caused a large increase in intracellular calcium, demonstrating the efficacy of the measurement technique. B, Similar results were obtained using the ratiometric calcium indicator indo-1, to correct for decreasing fluorescence caused by photobleaching in prolonged recordings and to obtain a quantitative estimate of changes in intracellular calcium. Brief 10 Hz stimulation or depolarization with 90 mm KCl caused large increases in intracellular Ca2+ concentration, but intracellular Ca2+ levels remained constant after lowering extracellular Ca2+ concentration from 1.2 mm to 50 nm or increasing extracellular Ca2+ from 50 nm to 1.2 mm (Ca2+stim). Results shown are mean ± SEM;n = 8 neurons in A and 13 neurons inB. Although extracellular Ca2+stimulation did not alter intracellular calcium measurably, the changes in extracellular calcium caused large changes in autonomous activity of CaMKII (Fig. 8).
The results indicate that mechanisms linked to the [Ca2+]o in nonsynaptic regions of the neuron are responsible for the high level of CaMKII autonomy and autophosphorylation under basal conditions, rather than effects dependent on synaptic activity or changes in [Ca2+]i. This is further supported by experiments showing that CaMKII autonomous activity was not different in cultures exposed to 1 μmTTX for 1 hr (0.26 ± 0.013; n = 8 dishes; mean ± SD) versus unstimulated cultures (0.28 ± 0.068;n = 36 dishes).
DISCUSSION
These are the first measurements of the frequency response of CaMKII decoding in neurons. The autonomous activity of the enzyme has been tested in response to a natural stimulus generating intracellular calcium fluxes. This approach allows examination of the enzyme under conditions that simulate the natural environment, a necessary step in understanding the frequency decoding properties of CaMKII. The results indicate that the spike frequency decoding by CaMKII autonomous activation is limited to action potential frequencies of <1 Hz and >0.1 Hz. These findings are consistent with the behavior of the enzymein vitro to Ca2+ pulses of different frequencies (De Koninck and Schulman, 1998). The in vitro studies indicated that a sharper threshold for frequency decoding by CaMKII is promoted by shorter duration Ca2+ pulses, lower free CaM concentration, and isozymes with higher affinity for CaM (De Koninck and Schulman, 1998). The physiological conditions in DRG neurons would modulate the frequency response of CaMKII toward higher levels of activation in response to low-frequency stimulation. Frequency decoding of CaMKII-α is reported to be <1–3Hz for Ca2+ pulses >300 msec in duration (approximately the duration of a calcium transient induced by an action potential in DRG neurons); and partial autophosphorylation of the kinase shifts the level of autonomy to near maximal levels in response to low-frequency stimulation. CaMKII was highly autophosphorylated in unstimulated DRG neurons.
The high levels of autonomous activity of CaMKII in DRG neurons in the basal state are consistent with previous studies on other preparations (Molloy and Kennedy, 1991). This was attributed to low-frequency spontaneous synaptic activity, but the present studies show that synaptic activity is not essential for maintaining the highly autophosphorylated state of CaMKII in basal conditions, because this preparation lacks synapses. Spontaneous action potential activity also does not appear to be responsible for the high autophosphorylated state in unstimulated conditions, because the DRG neurons are not spontaneously active in vitro, and treatment with TTX did not lower the CaMKII autonomous activity. However, the [Ca2+]o strongly modulated this critical property of the enzyme.
The mechanism for the link between extracellular calcium and high levels of CaMKII autophosphorylation is unknown, but [Ca2+]i imaging shows that it is not associated with measurable changes in [Ca2+]i. Regulation of phosphatases, calmodulin-binding proteins, and kinase activity linked to the [Ca2+]o are reasonable mechanisms. These studies indicate that the processes responsible for the effect of extracellular Ca2+ on CaMKII autonomy are not limited to specialized subsynaptic compartments, as had been assumed, because this preparation lacks synapses.
Conditions may exist in specialized cellular compartments to maintain the enzyme at a lower level of autophosphorylation, which would permit the enzyme to decode higher frequencies of stimulation. It is possible that the kinetics of Ca2+ transients are more rapid in microdomains beyond the resolution of confocal measurements. Shorter duration Ca2+ pulses would permit the kinase to exhibit a sharper frequency-dependent activation in association with activity-dependent functions, such as synaptic plasticity. Greater spatial heterogeneity and compartmentalization would be provided by neurons that are morphologically more complex than DRG neurons, which lack dendrites and dendritic spines. In this regard, local phosphatase activity could have a significant influence on the frequency-response of CaMKII.
CaMKII autonomous activity declined to near baseline with longer stimulation (>1 min) at higher frequencies. This could result from stimulus failure, loss of total CaMKII activity, phosphorylation of an inhibitory site, e.g., Thr-305/306 (Patton et al., 1990; Dosemeci and Albers, 1993; Coomber, 1998), or increased phosphatase activity. There is no evidence of stimulus failure, because [Ca2+]i remained elevated throughout the stimulus period, and another kinase (extracellular signal regulated kinase) exhibited increased phosphorylation under these conditions (Eshete and Fields, unpublished data). No decrease in total CaMKII activity was observed on immunoblots or activity assays in lysates from neurons stimulated 10 min at 10 Hz or (data not shown). This suggests that phosphatases may be activated by prolonged stimulation. Previous studies have shown that electroconvulsive treatment causes dephosphorylation of CaMKII at Thr-286 in rat hippocampus and cortex (Yamagata and Obata 1998). The spike frequency decoding by CaMKII could be modulated by regulation of phosphatases in response to longer duration action potential firing. The effects of phosphatase activity on spike frequency decoding by CaMKII autonomous activity has been incorporated in some mathematical models (Dupont and Goldbetter, 1992; Matsushita et al., 1995, Coomber, 1998; Holmes, 2000).
An important finding of the present study is that the kinetics of Ca2+ transients in these neurons induced by trains of action potentials were markedly different from the Ca2+ pulses that exhibited strong frequency decoding in experiments using pulsed perfusion of calcium solutions in vitro (De Koninck and Schulman, 1988). The cytoplasmic Ca2+ transient in these neurons lasts ∼300 msec or longer after single action potentials. This makes the amplitude and duration of the calcium response highly dependent on action potential firing frequency. The Ca2+ transient measured in the cell body of DRG neurons evoked by electrically elicited action potentials persisted well beyond the stimulus period; ∼1 min after 15 sec of 1 Hz stimulation and 4 min after 15 sec of 10 Hz stimulation (Fig.3B,C). These sustained increases in intracellular calcium may influence other calcium-dependent processes, possibly including phosphatase activity, that might interact with phosphorylation of certain CaMKII substrates. If there are substrates of CaMKII that are selectively regulated by higher frequencies of action potentials, the present findings suggest that this would occur only in local submembrane regions surrounding Ca2+-permeable channels or in specialized compartments, such as dendritic spines, because the Ca2+ transients induced by action potentials may only have temporal dynamics that are appropriate for high frequency-dependent decoding by CaMKII autonomy in microscopic domains beyond the limits of current measurements.
The correlation with Ca2+ pulse frequency, not simply the [Ca2+]i suggests that time-dependent processes, such as the kinetics of CaM dissociation, phosphorylation of regulatory and inhibitory sites on the enzyme, and activation of phosphatases cooperate in regulating the level of autonomous activation induced by action potential firing patterns. Such time-dependent effects on CaMKII regulation would appear to allow more robust responses to dynamic stimulation than would be afforded by simple dependence on calcium concentration in equilibrium reactions. In combination with concentration-dependent forms of regulation, these time-dependent regulatory effects may be essential in transducing the information contained in the temporal pattern of neuronal membrane depolarization into intracellular signaling reactions that control the appropriate neuronal response.
Thus, CaMKII could participate in decoding action potentials at a frequency <1 and >0.1 Hz in these neurons. This may be important during development when neurons generate low-frequency bursts of action potentials spontaneously (Fields, 1998). This is not a favorable mechanism for regulating activity-dependent responses in these neurons in the range of 1–10 Hz. The specific activation of genes and other functional responses of DRG neurons that have been observed in response to appropriate frequencies of action potentials at frequencies >1 Hz are either dependent on localized responses of CaMKII beyond the limits of the present measurement techniques or they may involve other frequency-decoding intracellular signaling mechanisms, such as temporal integration within intracellular signaling networks (Fields, 1996;Fields et al., 1997, 2001).
Footnotes
This work was supported by the National Institute of Child Health and Human Development. We thank B. Stevens for providing the cultures of DRG neurons and Dr. Yoko Yamagata for the phosphosite-specific antibodies used in the study.
Correspondence should be addressed to Dr. R. Douglas Fields, Head, Neurocytology and Physiology Unit, National Institutes of Health, National Institute of Child Health and Human Development, Laboratory of Cellular and Synaptic Neurophysiology, 49 Convent Drive, Building 49, Room 5A78, Bethesda, MD 20892-4480. E-mail: fields@helix.nih.gov.
REFERENCES
- 1.Bayer K-U, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous γ- and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 2.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM Kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 3.Colegrove SL, Albrecth MA, Friel DD. Quantitative analysis of mitochondrial C12+ uptake and release pathways in sympathetic neurons. Reconstruction of the recovery after depolarization-evoked [Ca2+]i elevations. J Gen Physiol. 2000;115:347–350. doi: 10.1085/jgp.115.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coomber CJ. Site-selective autophosphorylation of Ca2+/calmodulin-dependence protein kinase II as a synaptic encoding mechanism. Neural Comput. 1998;10:1653–1678. doi: 10.1162/089976698300017070. [DOI] [PubMed] [Google Scholar]
- 5.De Koninck P, Schulman H. Sensitivity of CaMKII to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 6.Dosemeci A, Albers RW. A mechanism for synaptic frequency detection through autophosphorylation of CaMKII. Biophys J. 1996;70:2493–2501. doi: 10.1016/S0006-3495(96)79821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont G, Goldbeter A. Protein phosphorylation driven by intracellular calcium oscillations: a kinetic analysis. Biophys Chem. 1992;42:257–270. doi: 10.1016/0301-4622(92)80018-z. [DOI] [PubMed] [Google Scholar]
- 8.Edman CF, Schulman H. Identification and characterization of δB-CaM kinase and δC-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta. 1994;1221:89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 9.Eshete F, Fields RD. Spike frequency decoding and autonomous activation of CaM kinase II in DRG neurons. Soc Neurosci Abstr. 1999;25:1192. doi: 10.1523/JNEUROSCI.21-17-06694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields RD. Signaling from neural impulses to genes. The Neuroscientist. 1996;2:315–325. [PMC free article] [PubMed] [Google Scholar]
- 11.Fields RD. Effects of ion channel activity on development of dorsal root ganglion neurons. J Neurobiol. 1998;37:158–170. doi: 10.1002/(sici)1097-4695(199810)37:1<158::aid-neu12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Fields RD, O'Donovan MJ. Imaging nervous system activity. In: Cranley JN, Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current protocols in neuroscience. Wiley; New York: 1997. pp. 2.3.1–2.3.12. [Google Scholar]
- 13.Fields RD, Neale EA, Nelson PG. Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. J Neurosci. 1990;10:2950–2964. doi: 10.1523/JNEUROSCI.10-09-02950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields RD, Yu C, Nelson PG. Calclium, network activity, and the role of NMDA channels in synaptic plasticity in vitro. J Neurosci. 1991;10:134–146. doi: 10.1523/JNEUROSCI.11-01-00134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields RD, Yu C, Neale EA, Nelson PG. Recording chambers in cell culture. In: Kettenmann H, Grantyn R, editors. Electrophysiological methods for in vitro studies in vertebrate neurobiology. Liss; New York: 1992. pp. 67–76. [Google Scholar]
- 16.Fields RD, Guthrie PB, Russell JT, Kater ST, Malhotra BS, Nelson PG. Accommodation of mouse DRG growth cones to electrically induced collapse: kinetic analysis of calcium transients and set-point theory. J Neurobiol. 1993;24:1080–1098. doi: 10.1002/neu.480240807. [DOI] [PubMed] [Google Scholar]
- 17.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fields RD, Eshete F, Dudek S, Ozsarac N, Stevens B. Regulation of gene expression by action potentials: dependence on complexity in cellular information processing. Novartis Found Symp. 2001;239:160–176. doi: 10.1002/0470846674.ch13. [DOI] [PubMed] [Google Scholar]
- 19.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr-286 of the calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 20.Gillot I, Whitaker M. Imaging calcium waves in eggs and embryos. J Exp Biol. 1993;184:213–219. [Google Scholar]
- 21.Gillot I, Whitaker M. Calcium signals in and around the nucleus in sea urchin eggs. Cell Calcium. 1994;16:269–278. doi: 10.1016/0143-4160(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Hanson PI, Meyer T, Stryer L. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Cruz A, Sala F, Adams PR. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990;16:858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- 24.Holmes WR. Models of calmodulin trapping and CaM kinase II activation in a dendritic spine. J Comput Neurosci. 2000;8:65–85. doi: 10.1023/a:1008969032563. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Stevens B, Schachner M, Fields RD. Regulation of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Katoh T, Fujisawa H. Autoactivation of calmodulin-dependent protein kinase II by autophosphorylation. J Biol Chem. 1991;266:3039–3044. [PubMed] [Google Scholar]
- 28.Kwiatkowski AP, McGill JM. Alternative splice variant of γ-calmodulin-dependent protein kinase II alters activation by calmodulin. Arch Biochem Biophys. 2000;378:377–383. doi: 10.1006/abbi.2000.1846. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Jia M, Fields RD, Nelson PG. Modulation of calcium currents by electrical activity. J Neurophysiol. 1996;76:2595–2607. doi: 10.1152/jn.1996.76.4.2595. [DOI] [PubMed] [Google Scholar]
- 30.Lisman J, Goldring M. Evaluation of a model of long-term memory based on the properties of the Ca2+/calmodulin-dependent protein kinase. J Physiol (Paris) 1988;83:187–197. [PubMed] [Google Scholar]
- 31.Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lou LL, Schulman H. Distinct autophosphorylation sites sequentially produce autonomy and inhibition of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1989;9:2020–2032. doi: 10.1523/JNEUROSCI.09-06-02020.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita T, Moriyama S, Fukai T. Switching dynamics and the transient memory storage in a model enzyme network involving Ca2+/calmodulin-dependent protein kinase II in synapses. Biol Cybern. 1995;72:497–509. doi: 10.1007/BF00199892. [DOI] [PubMed] [Google Scholar]
- 34.Mayford M, Wang J, Kandel ER, O'Dell TJ. CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995;81:891–904. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 35.Meyer T, Hanson P, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 36.Meyer T, Allbritton NL, Oancea E. Regulation of nuclear calcium concentration. In Calcium waves, gradients and oscillations. Ciba Found Symp. 1995;188:252–266. doi: 10.1002/9780470514696.ch14. [DOI] [PubMed] [Google Scholar]
- 37.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 38.Molloy SS, Kennedy MB. Autophosphorylation of type II Ca2+/calmodulin-dependent kinase in cultures of postnatal rat hippocampal slices. Proc Natl Acad Sci USA. 1991;88:4756–4760. doi: 10.1073/pnas.88.11.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherji S, Soderling TR. Regulation of Ca2+/calmodulin-dependent protein kinase II by inter- and intrasubunit-catalyzed autophosphorylations. J Biol Chem. 1994;269:13744–13747. [PubMed] [Google Scholar]
- 40.Nelson PG, Yu C, Fields RD, Neale EA. Competition between convergent inputs in vitro: Electrical activity modulates efficacy and number of synapses. Science. 1989;244:585–587. doi: 10.1126/science.2717942. [DOI] [PubMed] [Google Scholar]
- 41.Ocorr KA, Schulman H. Activation of multifunctional Ca2+/calmodulin-dependent kinase in intact hippocampal slices. Neuron. 1991;6:907–914. doi: 10.1016/0896-6273(91)90231-n. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto H, Ishikawa K. Switching characteristics of a model for biochemical-reaction networks describing autophosphorylation versus dephosphorylation of Ca2+/calmodulin-dependent protein kinase II. Biol Cybern. 2000;82:35–47. doi: 10.1007/PL00007960. [DOI] [PubMed] [Google Scholar]
- 43.O'Malley DM. Calcium permeability of the neuronal nuclear envelope: evaluation using confocal volumes and intracellular perfusion. J Neurosci. 1994;14:5741–5758. doi: 10.1523/JNEUROSCI.14-10-05741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by calcium/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- 45.Rich RC, Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+calmodulin-dependnet protein kinase II. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- 46.Scholz WK, Palfrey HC. Activation of Ca2+/calmodulin-depenedent protein kinase II by extracellular calcium in cultured hippocampal pyramidal neurons. J Neurochem. 1998;71:580–591. doi: 10.1046/j.1471-4159.1998.71020580.x. [DOI] [PubMed] [Google Scholar]
- 47.Sheng HZ, Fields RD, Nelson PG. Specific regulation of immediate early genes by patterned neuronal activity. J Neurosci Res. 1993;35:459–467. doi: 10.1002/jnr.490350502. [DOI] [PubMed] [Google Scholar]
- 48.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 49.Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;15:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens CF, Tonegawa S, Wang Y. The role of calcium-calmodulin kinase II in three forms of synaptic plasticity. Curr Biol. 1994;4:687–693. doi: 10.1016/s0960-9822(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 51.Thiel G, Czernik AJ, Gorelick F, Nairn AC, Greengard P. Ca2+/calmodulin-dependent protein kinase II: Identification of threonine-286 as the autophosphorylation site in the α-subunit associated with the generation of Ca2+-independent activity. Proc Natl Acad Sci USA. 1988;85:63377–63341. doi: 10.1073/pnas.85.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamagata Y, Obata K. Dynamic regulation of the activated, autophosphorylated state of Ca2+/calmodulin-dependent protein kinase II by acute neuronal excitation in vivo. J Neurochem. 1998;71:427–439. doi: 10.1046/j.1471-4159.1998.71010427.x. [DOI] [PubMed] [Google Scholar]