Abstract
Neurophysiological and animal ablation studies concur that primary auditory cortex is necessary for computation of the spatial coordinates of a sound source. Human studies have reported conflicting findings but have often suffered from inadequate psychophysical measures and/or poor lesion localization. We tested patients with unilateral temporal lobe excisions either encroaching on or sparing Heschl's gyrus (HG), quantifying lesion extent using anatomical magnetic resonance imaging measures. Subjects performed two tasks. In the localization task, they heard single clicks in a free-field spatial array subtending 180° of azimuth and indicated the perceived location with a laser pointer. In the discrimination task, two clicks were presented, and subjects indicated if they were in the same or different position. As a group, patients with right temporal excision, either encroaching onto HG or not, were significantly impaired in both hemifields in both tasks, although this was not true for all individuals. Patients with left temporal resections generally performed normally, although some of the patients with left HG excision showed impaired performance bilaterally, especially in the discrimination task. This pattern stands in marked contrast to previous studies showing significant preservation of localization in hemispherectomized patients. We conclude that (1) contrary to hypotheses derived from animal studies, human auditory spatial processes are dependent primarily on cortical areas within right superior temporal cortex, which encompass both spatial hemifields; (2) functional reorganization may not take place after restricted focal damage but only after more extensive early damage; and (3) the existence of individual differences likely illustrates differential patterns of functional lateralization and/or recovery.
Keywords: auditory cortex, Heschl's gyrus, auditory localization, functional reorganization, hemispheric specialization, spatial discrimination
The spatial position of a sound is computed by the auditory nervous system based on interaural differences in intensity and time of arrival, as well as monaural cues (Middlebrooks and Green, 1991). Although binaural information is first processed in the olivary nuclei, several sources of evidence implicate cortical mechanisms in auditory localization. Neurophysiological recordings from area A1, the primary auditory cortex (PAC), in several species indicate sensitivity to interaural cues for azimuth position, especially for contralateral sound sources (Phillips and Brugge, 1985). It is also well established that unilateral PAC lesions in cats (Jenkins and Masterton, 1982), ferrets (Kavanagh and Kelly, 1987) and monkeys (Heffner and Masterton, 1975; Heffner and Heffner, 1990) produce severe impairments in localization of sound in the contralateral hemifield but leave ipsilateral localization primarily intact. Moreover, Jenkins and Merzenich (1984) suggest that A1 is both necessary and sufficient for contralateral localization.
The findings from animal studies suggest that each hemifield is represented in the auditory cortices of the opposite hemisphere, leading to the expectation that a similar organization exists in humans. However, a variety of deficits of spatial localization have been observed after unilateral lesions in humans. These disorders have been described (1) only in the contralateral hemifield after damage to either temporal lobe (Efron et al., 1983); (2) in both hemifields, especially after left hemisphere damage (Pinek et al., 1989); (3) in both hemifields primarily after right hemisphere damage (Ruff et al., 1981); and (4) in both hemifields after right hemisphere damage but only when accompanied by visual field disturbance (Bisiach et al., 1984).
These discrepancies may be attributed to a number of variables that have not always been appropriately controlled. One issue is that most of the previous studies did not provide clear evidence for the role of any particular cortical field, because lesions were generally diffuse and not well documented or quantified. In particular, it has not been possible to determine the specific role of primary as opposed to other auditory cortical areas, nor has it always been possible to dissociate possible global spatial impairments attributable to parietal lobe damage in the studied populations as opposed to specifically auditory disturbances.
A third factor is that previous studies have generally not distinguished between tasks that require a spatial response as opposed to those that involve interaural cues but do not require a true spatial response. Heffner and Masterton (1975) showed that unilateral PAC lesions that prevented a monkey from approaching a contralateral sound source did not affect performance in a right–left discrimination task. This dissociation suggests that auditory cortex may be important for spatial representations but is not necessarily critical for using interaural cues in a nonspatial context (Whitfield, 1985). Finally, previous studies have generally not eliminated monaural intensity cues to localization.
In the present study, we sought to clarify the role of superior temporal auditory cortical regions in human sound localization by testing subjects with well documented and quantified damage to these areas using a roving-intensity paradigm. We predicted that lesions encroaching onto Heschl's gyrus (HG), the medial portion of which contains PAC (Liégeois-Chauvel et al., 1991; Rademacher et al., 1993), would lead to deficits in localization of sounds in the contralateral hemifield but not in a discrimination task that did not require explicit spatial responses.
MATERIALS AND METHODS
Subjects
Each of the patients who participated in this experiment had undergone surgical removal at the Montreal Neurological Hospital to relieve pharmacologically intractable seizures. In the majority of cases, the cause of the seizures was focal cerebral atrophy dating from birth or early life. Average age at surgery was 27.9 years; average time elapsed between surgery and testing was 11.5 years. Patients were excluded from the study if they presented atypical speech representation (as determined via intracarotid sodium Amytal testing;Branch et al., 1964), known damage outside the region of surgical excision, EEG abnormality contralateral to the epileptogenic focus, a malignant tumor, full-scale IQ [Wechsler Adult Intelligence Scale (revised)] score under 75, or evidence of significant hearing loss or impairment on standard audiometric assessment. The Ethics Committee of the Montreal Neurological Institute approved the experimental protocol, and written informed consent was obtained from all subjects before testing.
Lesion site and extent for the patients with excision within HG was documented and quantified on postoperative magnetic resonance imaging (MRI) scans. MRI scans were obtained on a Philips Gyroscan system with a 1.5 T superconducting magnet using a three-dimensional FFE acquisition sequence to collect 160 contiguous 1 mm T1-weighted images in the sagittal plane (repetition time, 18 msec; echo time, 10 msec). The resection in all patients consisted of a unilateral subtotal anterior temporal corticectomy, starting at the anterior pole and proceeding posteriorly to varying extents. In the medial aspect, the lesions included varying amounts of the amygdala and the uncus; the extent of the resection along the hippocampus and parahippocampal gyrus also varied from patient to patient. Of greater importance for the present study, the extent of the lateral neocortical excision also varied and included portions of HG in some cases. The extent of excision was dictated primarily by the location and degree of epileptogenic abnormality and was unrelated to age or years of seizure disturbance.
Determination of the precise area of damage is often rendered difficult because of individual differences in brain anatomy and uncertainty regarding the location of excised tissue with respect to anatomical landmarks that are destroyed or altered by the surgical intervention. To further complicate matters, the MRI scans were only available postoperatively. To address these issues, we used a method based on an anatomical map of HG in stereotaxic space derived from normal subjects (Penhune et al., 1996) to help identify the location of any remaining HG tissue in the MRI scans of patients. The location and extent of the lesions in patients with HG excision was then determined in the following manner. MR scans were linearly transformed into stereotaxic space (Talairach and Tournoux, 1988), using an automatized feature-matching algorithm (Collins et al., 1994) and viewed using an interactive three-dimensional imaging software package that allowed simultaneous inspection in the coronal, horizontal, and sagittal planes of section. The patients' scans were then coregistered with the probabilistic anatomical map of HG (Fig.1), which helps to disambiguate whether the gyri remaining correspond to HG or not.
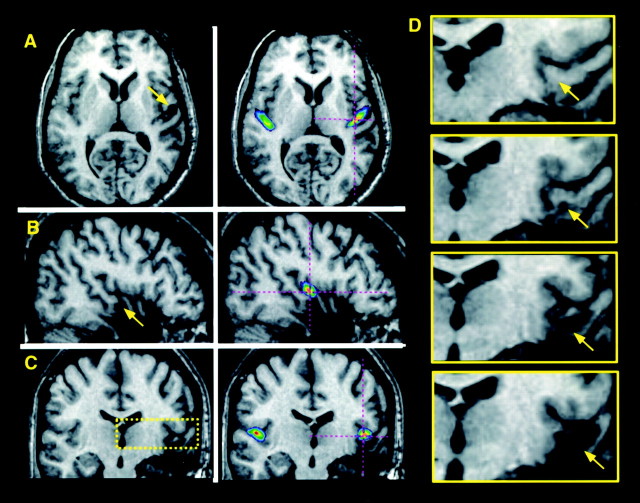
Fig. 1.
MRI scan of a patient (RTA3 in Table 1) with a removal in the right HG in whom the excision includes the anterolateral 50–60% and the undercutting extends to 60–70%. The MRI scan is transformed into standardized stereotaxic space; illustrated are planes of section oriented horizontally (A; z = 4), sagittally (B; x = 46), and coronally (C; y = −17). The left panels of A–C show the patient's scan alone, with an arrow indicating the region of excision–undercutting. The right panels ofA–C show the patient's scan coregistered with an anatomical probabilistic map of HG derived from normal individuals (Penhune et al., 1996); the map is scaled to show voxels that have a 25% or greater probability of lying within HG. Thecrosshairs indicate the same position in standardized space as the arrow. Note the correspondence between the position of HG as determined from the map and the patient's partially excised HG region. The yellow box in Cindicates the region of the removal pictured in close-up inD, which illustrates the transition from intact, to undercut, to fully excised tissue (coronal sections taken at 3 mm intervals; posterior to anterior; from y = −23 to −14). Arrows again correspond to thecrosshairs in the other panels and indicate the location of HG region.
Estimation of the extent of both excision and undercutting was made by finding the most anterior plane of section in each scan in which HG had been removed or undercut, identifying these locations in stereotaxic space, and comparing these planes with the map. The probability maps in each hemisphere were scaled to show the region of 25–100% probability and were divided anteroposteriorly into 10 equal-length segments, corresponding to a 0–10% resection, a 10–20% resection, etc. The posterior limit of resection and of undercutting was located within one of these intervals for each patient. Table1 gives the estimates of extent of the lesion in HG. For additional details of the lesion quantification procedure, see Penhune et al. (1999). Extent of excision was not quantified in those patients who had not received any HG excision.
Table 1.
Details of amount of HG excised or undercut in patients with excision extending into HG (LTA and RTA groups) and total amount of HG destruction or disconnection, as determined by comparison of MRIs to anatomical probabilistic map (see Materials and Methods for details)
| Patient | % HG excised | % HG undercut | % Total HG |
|---|---|---|---|
| LTA4 | 0 | 10–20 | 10–20 |
| LTA7 | 0 | 10–20 | 10–20 |
| LTA6 | 0 | 40–50 | 40–50 |
| LTA1 | 20–30 | 10–20 | 30–40 |
| LTA2 | 40–50 | 10–20 | 50–60 |
| RTA8 | 0 | 20–30 | 20–30 |
| RTA1 | 0 | 40–50 | 40–50 |
| RTA2 | 0 | 30–40 | 30–40 |
| RTA5 | 20–30 | 10–20 | 40–50 |
| RTA6 | 20–30 | 10–20 | 40–50 |
| RTA4 | 30–40 | 30–40 | 60–70 |
| RTA3 | 50–60 | 10–20 | 60–70 |
| RTA9 | 80–90 | 10–20 | 90–100 |
Patients were subsequently assigned to four different groups based on the side of their excision (left, LT; or right, RT) and the degree to which HG was included in the removal. If the first transverse gyrus of Heschl was encroached on (either undercut or excised) to any degree, the patient was classified as having a removal from HG (denoted by LTA or RTA; n = 5 and 8, respectively). If the resection stopped anterior to the later almost aspect of HG, the patient was classified as sparing HG (denoted by LTa or RTa; n = 19 and 10, respectively). Two LT patients whose surgical report had indicated encroachment onto HG were found to have complete sparing of HG during MRI analysis; these two patients were thus assigned to the LTa group. Eleven neurologically normal control participants, matched to the patients with respect to age and level of education, were also tested. See Figure 2 for MRI scans of representative excisions.
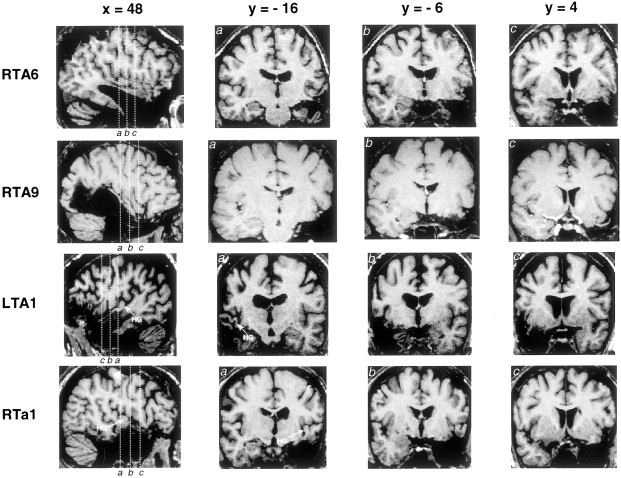
Fig. 2.
Magnetic resonance imaging scans of representative individual patients with varying amounts of resection from the superior temporal gyrus. Scans were converted into the standardized stereotaxic space of Talairach and Tournoux (1988). Each horizontal row corresponds to a single individual. The first panel in each row is a sagittal section taken at 48 mm lateral to midline, and the three subsequent panels are coronal sections taken at positions indicated by thevertical lines in the sagittal section. Heschl's gyrus is not visible in patients RTA6 and RTA9, because most of it had been excised. The position of remaining Heschl's gyrus tissue is indicated in patient LTA1, who had significant undercutting of this area (visible in coronal section marked a; b andc are taken anterior to Heschl's gyrus). The lesion in patient RTa1 included anterior superior temporal cortex but did not extend into Heschl's gyrus, which is intact and may be seen ina.
Human PAC is likely found within the medial portion of HG based on the fact that cytoarchitectonic criteria have identified that the characteristic granular koniocortex always covers portions of HG (Rademacher et al., 1993, 2001); in particular, the koniocortical field is found within the more medial aspect of the first HG (if there are more than one in a hemisphere), does not generally extend anteriorly to the sulcus defining the anterior border of HG, and is never found within the anterior superior temporal gyrus (STG), or planum polare. Thus, patients in the LTa or RTa groups in the present study, whose lesions all stopped well short of HG, would be very unlikely to have any damage to PAC. Although the precise relationship between koniocortex and physiologically defined primary fields (A1) is currently unknown, electrophysiological recordings suggest multiple fields within HG (Liégeois-Chauvel et al., 1991, 1994). If the human PAC follows an organization analogous to that of the macaque (Kaas et al., 1999), then at least three fields may exist within HG, which is also consistent with cytoarchitectonic analyses (Morosan et al., 2001). Because the excisions in the RTA and LTA patients proceeded from anterior to posterior and lateral to medial along HG, all of these patients would have had some damage to the more anterolateral locations, possibly corresponding to field Te1.2 of Morosan et al. (2001), whereas only those in whom the excision or undercutting exceeded ∼50% would be likely to have had damage within the PAC proper.
Stimulus
A 10 msec square-wave pulse, perceived as a click, was used for all tasks. Intensity was randomly varied in three 5 dB steps across trials between 51.5 and 61.5 dB sound pressure level (A-weighted), to avoid the possible use of monaural intensity cues. Calibration was accomplished by placing a GenRad sound pressure meter at the position of the subject's head and measuring the levels.
Apparatus
A horizontal semicircular array (diameter of 2 m) was used, with 13 enclosed speakers (model AR-410W8, diameter of 10 cm; Accusonic Corp., Toronto, Canada) hidden from view and positioned every 15° from −90° to +90° in the frontal azimuth plane (here, and throughout, negative and positive refer to left and right sides, respectively). The subject sat in a chair positioned in the center of the array, with the height adjusted so that the ears would be on the same plane as the speakers. The chair was provided with a head holder that allowed the subject to position the head consistently facing forward on each trial, but it did not impede movement. The walls on both sides and behind the subject were covered with Sonex sound-absorbing foam material; the floor was carpeted, and the ceiling consisted of acoustic tile.
Procedure
Spatial localization. A single click was presented randomly at one of the 13 positions on each trial. Each position was sampled nine times within a run (three times at each of the three intensities used) for a total of 117 trials. Subjects were instructed to face forward before the start of each trial and to point to the perceived location of the click on a strip of paper located just below the speaker array using a laser pointer; they were allowed to move their head after each stimulus presentation so that they could see clearly where they were pointing and were told to ignore the intensity differences in the stimuli. The instructions emphasized that sounds could come from anywhere along the semicircular array, and subjects were encouraged to use the entire range of responses available. Responses were recorded to the nearest degree by the experimenter who observed the position of the pointer in relation to marks made every 3°. Trials were self-paced, so that the next stimulus was not presented until after a response had been given. Practice trials were given before starting to familiarize subjects with the procedure, but no feedback was given other than to confirm that stimuli always came from the front azimuthal plane. All control, LTA, and RTA patients were tested on this task, but because of time constraints, only 11 LTa and 8 RTa patients participated.
Discrimination. A pair of clicks (interclick interval of 500 msec), either at the same location or separated by 30°, was presented on each trial. The absolute location of the stimulus pair varied across trials randomly; the locations used were varied in 15° steps, so that on “different” trials the stimuli would be presented at −90°/−60°, −75°/−45°, −60°/−30°, and so on, whereas on “same” trials, the pair of clicks was presented in the same location (at −90°, −75°, −60°, etc). The two stimuli on each trial always differed in intensity by ±5 or 10 dB, thus eliminating any intensity cues that might have contributed to discrimination performance. Each location or pair of locations was sampled six times, with an equal number of same and different items, for a total of 88 trials. Subjects were instructed to ignore intensity differences and respond verbally “same” or “different” depending on whether the click was in the same position or not, regardless of absolute spatial location. Practice trials were given with feedback before testing for familiarization. All patients and controls participated in the discrimination testing, which was always administered after the localization task when both were given.
RESULTS
Localization task
Performance accuracy overall was much better toward the center than at more eccentric locations (Fig.3). The majority of subjects showed a tendency to overshoot the target position (with the exception of the two extreme positions, where pointing responses were constrained by the end of the array). Performance of the two subject groups with right temporal lobe lesions appeared to be most impaired compared with normal (Figs. 3, 4). Several statistical analyses were conducted to confirm this observation.
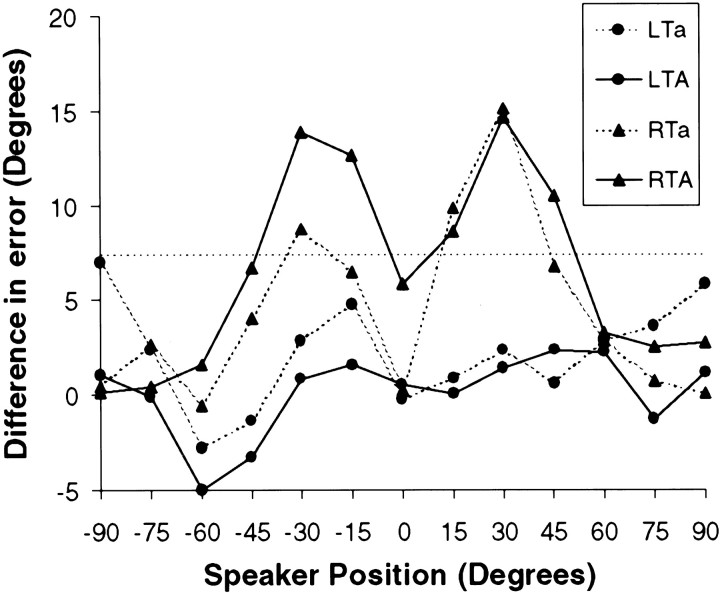
Fig. 3.
Performance on auditory localization task (pointing to perceived position of target) in four groups of patients tested (LTa, LTA, RTa, and RTA). The measure used is mean absolute error (absolute value of difference between correct position and response given); the ordinate plots the difference between performance of each group relative to that of control subjects as a function of azimuthal position (positive numbers indicate higher absolute error than normal). Thedotted line at 7.4 indicates the cutoff for significant impairment based on planned comparisons from the ANOVA (see Results). Note significantly elevated error rate for the RTA and RTa groups in the middle range of both spatial hemifields.
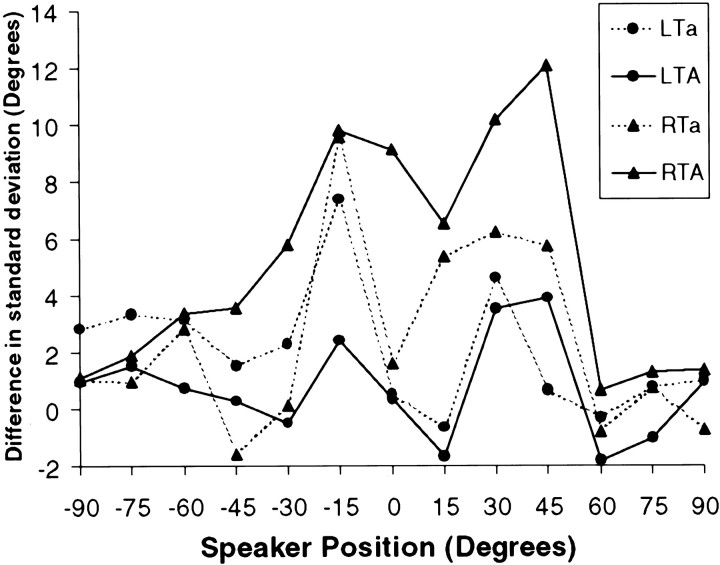
Fig. 4.
SD of responses in the localization task as a function of position. The ordinate plots the difference in SD between the control group and the four lesion groups (positive numbers indicate greater dispersion in the responses than normal). Only the RTA group demonstrated significantly elevated SD (see Results).
Three indices of localization performance were computed. The signed error score was defined as the difference between the correct position and the position pointed to by the subject, in which a positive number indicates a more eccentric response than the true value (overshooting), whereas a negative number indicates a response closer to the midline than the correct value (undershooting). The signed error score is able to describe errors with respect to their direction (i.e., it is sensitive to directional biases) but is not sensitive to errors whose average is close to or at the target position (because positive and negative errors would cancel each other). The absolute error score consisted of the absolute value of the difference score between the response and the true position. Thus, this measure is insensitive to directional biases (overall tendencies to respond more to the right or to the left) but is a better measure of accuracy around the target position. As a measure of dispersion, an SD score on the signed errors was also computed. This measure indicates how variable the responses were; thus, high values indicate inconsistent responses. The values from each of these three indices were averaged across trials for each position and for each subject and entered into separate ANOVAs with two factors: lesion group and spatial position.
For the signed error score, there was a highly significant position effect (F(12,456) = 37.0;p < 0.0001), indicating that pointing responses were more accurate toward the midline than at the more lateral positions, but no significant main effect of group was obtained, nor was there an interaction effect. In contrast, for the absolute error score, there was both a position effect (F(12,456)= 12.36; p < 0.0001) and a significant position by group interaction (F(48,456) = 1.43;p=0.036), indicating that performance across groups was different at certain positions (Fig. 3). To determine whether this effect could be ascribed to differences in accuracy between the left and right hemifields, the data were retabulated so that the six positions on each side were considered as belonging to two factors: side (left or right) and eccentricity (six positions, 15° through 90°). The center position was not used for this analysis. The results mirrored the previous analysis in that there was a group by eccentricity interaction (F(20,190) = 1.67; p=0.042), but, notably, there was no main effect of side, nor were there any interactions involving this variable. Thus, any deficits in performance were not confined to one or the other hemifield, nor were deficits in any group present in only one hemifield.
To determine which groups showed significant impairments, and at which positions, the differences were computed between the error scores of the normal control group at each position and the performance of each lesion group. These differences were compared with a critical value for planned comparisons computed from the mean square error term of the group by position interaction effect from the ANOVA (Winer, 1971). Significantly impaired performance was observed only for some positions of the two groups with right temporal lobe damage, the RTa and RTA groups. For the RTA group, significant deficits were found at two locations on the left side (− 15° and −30°) and three locations on the right (15°, 30°, and 45°). For the RTa group the significant deficits were confined to −30°, 15°, and 30° (Fig. 3).
The result of the analysis on the SDs (Fig. 4) yielded a significant position effect (F(12,456) = 5.02;p < 0.001), which is attributable to the fact that performance was much less variable in the center and at the extremes than at intermediate lateral positions, consistent with the signed and absolute error scores. Furthermore, there was an overall group effect (F(4,38) = 3.16; p < 0.03) but no significant interaction (p = 0.10) between group and position. Planned comparisons were once again applied but only to the mean SD across positions, given the lack of interaction with the latter variable. Only the RTA group (mean SD of 13.4) was found to be impaired by this comparison relative to the control group (mean SD of 7.9). Although the mean SD of the RTa group was also somewhat elevated (10.6), it did not reach the nominal level of significance.
Individual differences
The analysis of the group data indicate the general trends in the findings but do not capture some of the large individual differences that were noted across the subject samples. In particular, it is of interest to note the pattern of preserved versus impaired performance in patients with similar lesion extent and location.
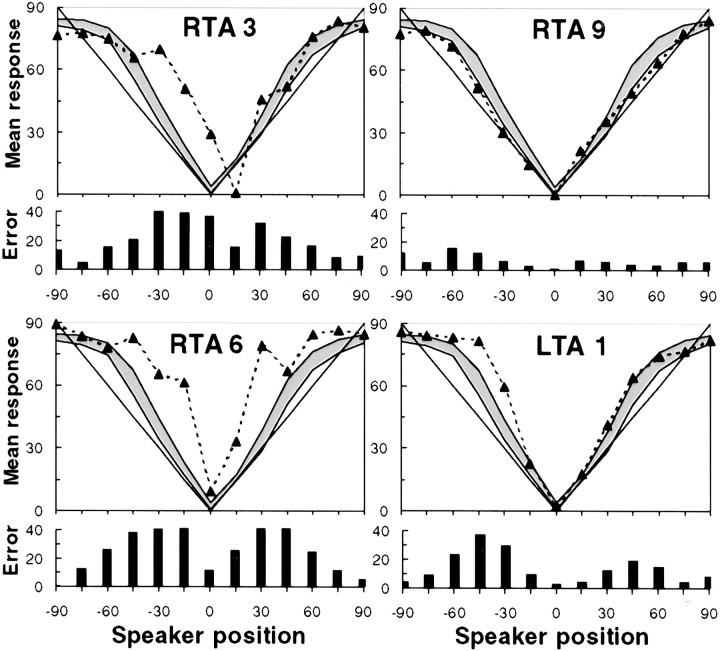
Within the RTA sample, for example, whose localization performance was clearly impaired as a group, wide variability was seen: five of eight patients showed impaired performance, whereas the others showed relative preservation of function. A deficit was considered to exist if the average response at a given location was outside the range of two SEs to the left or right of the normal control response distribution. There was no consistent relationship between the amount of excision (Table 1) and performance, because the impairments were observed in subjects with more restricted damage (RTA1 and RTA2), as well as in those with more extensive removal (RTA3, RTA5, and RTA6). Illustrative examples are shown in Figure 5, which plots the data for two patients (RTA3 and RTA6) with poor performance (in both hemifields), together with that of another individual (RTA9) in the same lesion group whose localization ability was essentially preserved. It is notable that the latter patient had the most extensive encroachment within HG (Table 1, Fig. 2), as measured by the probabilistic mapping technique discussed in Materials and Methods. Conversely, although as a group the LTA subjects did not demonstrate impairment, some individuals within this group performed well outside the normal range, as shown by one example in Figure 5 (patient LTA1). However, no individual patient within the LTa group demonstrated any impairment as defined by these criteria.
Fig. 5.
Individual localization performance on pointing task for four selected patients. The top of eachpanel shows the subject's mean localization responses (dotted line), as well as the range of responses from the normal control sample (shaded area indicates mean ± 2 SEs), as a function of azimuth position; solid line represents perfect responses. The bottom of each panel shows the mean absolute error. Note the severe localization errors made by patients RTA3 and RTA6 in both hemifields, whereas patient RTA9 was unimpaired. Patient LTA1 showed significant disturbance primarily in the left hemifield.
Finally, it is pertinent to mention some qualitative aspects of the data that have not been addressed in the foregoing analyses. The most salient aspect is that the patients in the RTA group, although not statistically different from those in the RTa group in terms of performance on the absolute error score, nonetheless demonstrated certain particularly severe localization disturbances. A good example is provided by the presence of right–left confusions (i.e., trials in which the pointing response is given in the field opposite to the position of the sound). Whereas not a single patient in the RTa group (or any of the others) showed such behavior, this phenomenon was observed on at least a few trials in three individuals within the RTA group. Similarly, it was noted that several patients in the RTA group complained during familiarization trials that the sounds were coming from behind or from above and were therefore difficult to point to in the frontal plane. Although all eventually were able to give a response within the frontal plane to each sound, their report indicates a more severe perceptual disturbance than is captured by the error scores used here.
Discrimination task
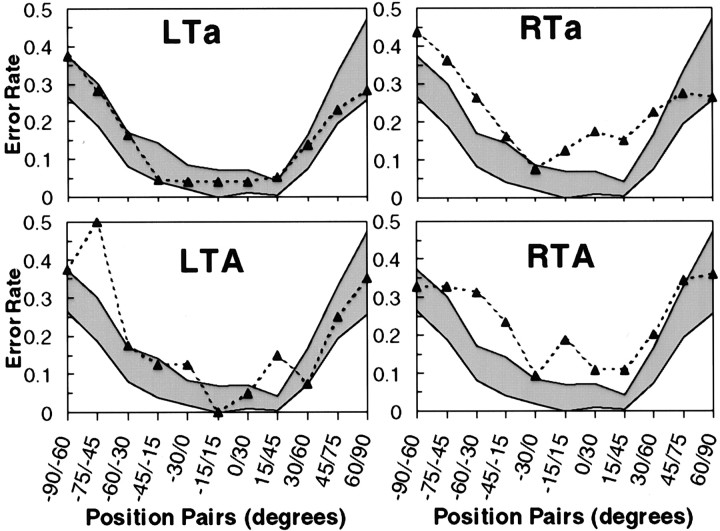
The dependent variable for the discrimination task was percentage of error at each of the positions defined by the pair of sounds presented. Performance across all groups tended to deteriorate as a function of increasing eccentricity of the discrimination pairs (Fig.6), as expected based on psychophysical data (Perrott et al., 1993; Recanzone et al., 1998). Inspection of the data once again suggests worst performance by the RTa and RTA groups (Fig. 6), but some locations appeared to elicit high error rates in the LTA group as well. ANOVA was performed following the same logic as for the localization task to verify these observations.
Fig. 6.
Performance of four patient groups on spatial discrimination task. Error rate is plotted as a function of azimuth position of stimulus pairs. Range of control performance (mean ± 2 SEs) is shown by shaded area. Only group LTa demonstrated unimpaired discrimination.
The main effect of position was highly significant (F(10,480) = 42.29; p< 0.0001), confirming that discrimination is much better toward the center. Most importantly, there was a significant interaction of group by position (F(40,480) = 1.54;p=0.02). Once again, planned comparisons were computed at each position to determine the positions at which impaired performance could be demonstrated. This analysis showed that performance was again impaired in the RTa and RTA groups (Fig. 6). The RTa group was impaired at three positions in the left hemifield (−90°/−60°, −75°/−45°, and −60°/−30°) and two in the right (+0°/+30°, and +15°/+45°); the RTA group was impaired at two left positions (−60°/−30° and −45°/−15°) and on the pair straddling the midline (−15°/+15°). In addition, and in contrast to the localization data, the LTA group also showed impaired performance at two locations (−75°/−45° and +15°/+45°).
DISCUSSION
The results were surprising, because the predictions predicated on the animal literature were not substantiated. First, we observed bilateral localization deficits primarily after damage to the right temporal lobe rather than the expected localization deficits contralateral to a lesion in either hemisphere. Second, anterior STG damage that did not encroach onto PAC was sufficient to produce a deficit in both hemifields. Third, the prediction that same–different discrimination would be preserved after temporal cortex lesions was also not upheld. Finally, the individual differences observed were not entirely expected.
Hemispheric specialization in auditory spatial processing
Perhaps the most salient result to emerge from this study is that damage to the right temporal neocortex produces localization disturbances in both spatial hemifields, whereas similar damage on the left generally results in little or no disturbance. Although this result is not predictable based on studies in other species, the finding does fit with considerable neuropsychological evidence linking the human right cerebral hemisphere to various types of spatial tasks (Benton and Tranel, 1993; Mesulam, 1999). Much of that literature, however, deals with global spatial processing deficits, often resulting from parietal lobe damage. Similarly, several functional imaging studies have shown preponderant right parietal lobe activity with auditory spatial tasks (Griffiths et al., 1998; Bushara et al., 1999;Zatorre et al., 1999), although it not yet clear to what extent this activity may represent integration of auditory cues with sensorimotor representations of space.
What is notable in the present data are that restricted lesions of the anterior temporal neocortex, not close to the parietal lobe, are sufficient to produce disturbed auditory localization, without any associated global spatial disorder such as hemineglect. The relative specialization of the right cerebral hemisphere for auditory spatial processing thus appears to encompass processes within unimodal auditory cortex. It is also of interest that right temporal lobe damage resulted in relatively similar impairments in the two hemifields, indicating that all of auditory space is represented within right auditory cortical regions. These conclusions are supported by recent functional MRI and magnetoencephalogram studies (Baumgart et al., 1999; Kaiser et al., 2000), which find evidence for stronger responses in right auditory cortex to auditory spatial stimuli. The right hemisphere specialization is not absolute, however, because the LTA group was impaired in the discrimination task and because at least one LTA patient was significantly impaired in the localization task (Fig. 5), a finding we shall return to below. As noted above, previous human studies have also reported spatial deficits after left hemisphere lesions (Clarke et al., 2000)
Role of HG and anterior STG cortices
Another expectation not met by the data concerns the role of cortex within HG. Behavioral lesion studies in various species generally indicate that damage to PAC is necessary for behavioral impairments in localization tasks; in fact, sparing of even small portions of PAC results in sparing of function (Jenkins and Merzenich, 1984). The medial aspect of the first HG likely corresponds to human histologically defined PAC (Rademacher et al., 1993); PAC is never found anterior to HG. Furthermore, both depth-electrode recordings (Liégeois-Chauvel et al., 1991, 1994) and functional imaging data (Zatorre et al., 1992) suggest that responses from the medial portion of HG are likely to represent PAC. Belt and parabelt regions are thought to surround this region (Galaburda and Sanides, 1980; Kaas et al., 1999). However, we observed comparable deficits in the RTa and RTA groups in both tasks, except for some qualitative differences. These results therefore implicate STG neurons anterior to PAC, in auditory parabelt areas, in auditory localization. The qualitative differences between RTA and RTa groups (for example, right–left errors) does suggest, however, that cortex within HG may play a more important role in computing spatial position than areas anterior to this location. This conclusion is strengthened by the fact that only the RTA group showed a statistically significant increase in the SD of responses (Fig. 4). A role for regions within left HG may also be gleaned from the finding that only the LTA and not LTa groups showed significant impairment in the discrimination task (Fig. 6).
The finding that STG belt or parabelt areas anterior to PAC participate to an important degree in spatial processing would not be entirely compatible with the hypothesis that posterior–dorsal areas are concerned with auditory spatial processes (Rauschecker, 1998). At a minimum, the findings from this study suggest that anterior regions play some necessary role for normal spatial function. It is possible, however, that regions posterior to HG might be more important than these anterior regions for spatial tasks (Recanzone et al., 2000; Tian et al., 2001) and that damage to such areas might result in more severe deficits than those observed here (Clarke et al., 2000). An alternative interpretation is that spatial encoding is accomplished on a population basis (Middlebrooks et al., 1994; Furukawa et al., 2000) by neurons distributed throughout the auditory cortices. According to this view, damage anywhere within the STG might lead to some degree of impairment, although the function would not necessarily be abolished.
Discrimination versus localization tasks
The discrimination task was designed based on similar tasks that have shown behavioral sparing in monkeys with auditory cortex lesions (Heffner and Masterton, 1975). In principle, the task should not require access to spatial representations per se, because all that is necessary is that a difference be registered, not that a position be computed (Whitfield, 1985). However, three of the four patient groups were significantly impaired on this task, even across the midline for the RTA group. One hypothesis that may explain this finding is that, despite the fact that the task may be accomplished without reference to spatial position, this does not guarantee that the spatial percept associated with the stimulus is necessarily ignored. Indeed, it may be that the spatial percept, although presumably disturbed in the patients, is quite salient and predominates in the judgment, although the task could have been solved on a nonspatial basis (Hartmann and Rakerd, 1989).
In this respect, it is important to point out an important difference between behavioral techniques used in animal studies and those applicable to human research: whereas animals are trained by operant techniques to respond on the basis of reinforcement with feedback on each trial, humans are merely given verbal instructions without reinforcement training, so that there is little or no opportunity for learning to take place. We speculate, therefore, that the subjects in the present experiment might have learned to perform better with appropriate feedback. It should also be pointed out, however, that some animal studies have also demonstrated poor performance in discrimination tasks after auditory cortex lesions (Heffner and Heffner, 1990).
Functional reorganization and individual differences
Using stimuli and tasks identical to those used here, Zatorre et al. (1995) (see also Poirier et al., 1994) found considerable sparing of function in five right and one left hemispherectomy patients. This spared localization ability contrasts strikingly with the data from the present patient groups who showed much worse localization ability, despite the fact that hemispherectomy subjects have complete removal of all auditory cortices. One explanation for these contrasting data are that, in the hemispherectomy cases, a great deal of functional reorganization has taken place. Reorganization may be facilitated by the fact that that the damage in these patients occurs relatively early in life, but it may also be critical that dysfunctional cortex is completely excised. Most of the patients in the present study also had some early damage, but the excisions were all subtotal; we speculate that reorganization (presumably within the remaining hemisphere) only takes place to a substantial degree when the damage is so complete that no cortical remnants exist in one hemisphere.
The important individual differences observed in the present sample (Fig. 5) also suggest that various idiosyncratic factors may be important in determining the degree of functional recovery. There was no obvious relationship between size of resection and performance, but it is interesting to note that patient RTA9, with the largest excision (Fig. 2), was also among the best on the localization task, perhaps because the near-complete excision of auditory cortex allows for greater reorganization in the remaining hemisphere, as in the hemispherectomy cases. Conversely, at least one LTA patient was significantly impaired in localization (Fig. 5). Thus, the data indicate that, at an individual level, several factors may interact in determining the effect of a cortical lesion; there may be exceptions to the general rule that right auditory cortices are most important for auditory spatial processing, just as there are exceptions to the typical pattern of left lateralization of language processes, even among right-handed persons (Branch et al., 1964). Furthermore, the degree of functional recovery may depend on the initial degree of functional lateralization, extent of tissue damaged, age at which the damage occurred, and the time elapsed since. Finally, it is also relevant to mention that functional recovery has not been well explored in animal studies because only the acute state is typically explored (but see Heffner and Heffner, 1990). In the human subjects tested here, in contrast, ample opportunity for relearning is provided by the many years that have elapsed between surgery and testing.
Conclusion
The present data indicate that lesions of right auditory cortex anterior to or including portions of PAC disturb localization performance on both sides of space. We conclude therefore that a relative functional asymmetry exists in the representation of auditory space, which arises at early levels of cortical processing, but not exclusively within PAC. Individual differences in patterns of lateralization and degree of impairment suggest that several factors may interact in determining the extent of functional reorganization that occurs after damage to auditory cortex. These factors, which require additional systematic study, likely include extent and location of cortical damage, as well as age and time elapsed since the lesion.
Footnotes
This work was supported by Grant MT11541 from the Canadian Institutes for Health Research and an award from the McDonnell-Pew Cognitive Neuroscience Program. We thank Drs. W. Feindel and A. Olivier for access to their patients, P. Bermudez, M. Bouffard, R. Dorsaint-Pierre, and I. Loy for assistance in testing and analysis, and the patients studied for their cooperation.
Correspondence should be addressed to Robert J. Zatorre, 3801 University Street, Montreal, Quebec, Canada H3A 2B4. E-mail:robert.zatorre@mcgill.ca.
REFERENCES
- 1.Baumgart F, Gaschler-Markefski B, Woldorff M, Heinze H-J, Scheich H. A movement-sensitive area in auditory cortex. Nature. 1999;400:724–725. doi: 10.1038/23390. [DOI] [PubMed] [Google Scholar]
- 2.Benton A, Tranel D. Visuoperceptual, visuospatial, and visuoconstructive disorders. In: Heilman KN, Valenstein E, editors. Clinical neuropsychology, Ed 3. Oxford UP; New York: 1993. pp. 165–214. [Google Scholar]
- 3.Bisiach E, Cornacchia L, Sterzi R, Vallar G. Disorders of perceived auditory lateralization after lesions of the right hemisphere. Brain. 1984;107:37–52. doi: 10.1093/brain/107.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Branch C, Milner B, Rasmussen T. Intracarotid sodium Amytal for the lateralization of cerebral speech dominance: observations in 123 patients. J Neurosurg. 1964;21:399–405. doi: 10.3171/jns.1964.21.5.0399. [DOI] [PubMed] [Google Scholar]
- 5.Bushara K, Weeks R, Ishii K, Catalan M, Tian B, Rauschecker J, Hallett M. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci. 1999;2:759–766. doi: 10.1038/11239. [DOI] [PubMed] [Google Scholar]
- 6.Clarke S, Bellman A, Meuli R, Assal G, Steck A. Auditory agnosia and auditory spatial deficits following left hemispheric lesions: evidence for distinct processing pathways. Neuropsychologia. 2000;38:797–807. doi: 10.1016/s0028-3932(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 7.Collins D, Neelin P, Peters T, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 8.Efron R, Crandall P, Koss B, Divenyi P, Yund E. Central auditory processing. III. The “cocktail party” effect and anterior temporal lobectomy. Brain Lang. 1983;19:254–263. doi: 10.1016/0093-934x(83)90069-x. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa S, Xu L, Middlebrooks JC. Coding of sound-source location by ensembles of cortical neurons. J Neurosci. 2000;20:1216–1228. doi: 10.1523/JNEUROSCI.20-03-01216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths TD, Rees G, Rees A, Green GGR, Witton C, Rowe D, Büchel C, Turner R, Frackowiak RSJ. Right parietal cortex is involved in the perception of sound movement in humans. Nat Neurosci. 1998;1:74–79. doi: 10.1038/276. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann W, Rakerd B. On the minimum audible angle: a decision theory approach. J Acoust Soc Am. 1989;85:2031–2041. doi: 10.1121/1.397855. [DOI] [PubMed] [Google Scholar]
- 13.Heffner H, Heffner R. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- 14.Heffner HE, Masterton B. Contribution of auditory cortex to hearing in the monkey (Macaca mulatta). J Neurophysiol. 1975;38:1340–1358. doi: 10.1152/jn.1975.38.6.1340. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins W, Masterton R. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins W, Merzenich M. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- 17.Kaas JH, Hackett TA, Tramo MJ. Auditory processing in primate cerebral cortex. Curr Opin Neurobiol. 1999;9:164–170. doi: 10.1016/s0959-4388(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser J, Lutzenberger W, Preissl H, Akermann H, Birbaumer N. Right-hemisphere dominance for the processing of sound-source lateralization. J Neurosci. 2000;20:6631–6639. doi: 10.1523/JNEUROSCI.20-17-06631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization in the ferret (Mustela putorius). J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- 20.Liégeois-Chauvel C, Musolino A, Chauvel P. Localization of the primary auditory area in man. Brain. 1991;114:139–153. [PubMed] [Google Scholar]
- 21.Liégeois-Chauvel C, Musolino A, Badier J, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. J Electroencephalagr Clin Neurophysiol. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 22.Mesulam M. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Phil Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middlebrooks J, Green D. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- 24.Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science. 1994;264:842–844. doi: 10.1126/science.8171339. [DOI] [PubMed] [Google Scholar]
- 25.Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 26.Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from MR scans. Cereb Cortex. 1996;6:661–672. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- 27.Penhune VB, Zatorre RJ, Feindel W. The role of auditory cortex in retention of rhythmic patterns in patients with temporal lobe removals including Heschl's gyrus. Neuropsychologia. 1999;37:315–331. doi: 10.1016/s0028-3932(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 28.Perrott D, Constantino B, Cisneros J. Auditory and visual localization performance in a sequential discrimination task. J Acoust Soc Am. 1993;93:2134–2138. doi: 10.1121/1.406675. [DOI] [PubMed] [Google Scholar]
- 29.Phillips D, Brugge J. Progress in neurophysiology of sound localization. Annu Rev Psychol. 1985;36:245–274. doi: 10.1146/annurev.ps.36.020185.001333. [DOI] [PubMed] [Google Scholar]
- 30.Pinek B, Duhamel J-R, Cavé C, Brouchon M. Audio-spatial deficits in humans: differential effects associated with left versus right hemisphere partial damage. Cortex. 1989;25:175–186. doi: 10.1016/s0010-9452(89)80035-8. [DOI] [PubMed] [Google Scholar]
- 31.Poirier P, Lassonde M, Villemure J, Geoffroy G, Lepore F. Sound localization in hemispherectomized patients. Neuropsychologia. 1994;32:541–553. doi: 10.1016/0028-3932(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 32.Rademacher J, Caviness VS, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 33.Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund H-J, Zilles K. Probabilistic mapping and volume measurement of human primary auditory cortex. NeuroImage. 2001;13:669–683. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- 34.Rauschecker J. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8:516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- 35.Recanzone G, Makhamra S, Guard D. Comparison of relative and absolute sound localization ability in humans. J Acoust Soc Am. 1998;103:1085–1097. doi: 10.1121/1.421222. [DOI] [PubMed] [Google Scholar]
- 36.Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- 37.Ruff RM, Hersh NA, Pribram KH. Auditory spatial deficits in the personal and extrapersonal frames of reference due to cortical lesions. Neuropsychologia. 1981;19:435–443. doi: 10.1016/0028-3932(81)90073-7. [DOI] [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system. An approach to cerebral imaging. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- 39.Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- 40.Whitfield IC. The role of auditory cortex in behavior. In: Peters A, Jones EG, editors. Cereb cortex. Plenum; New York: 1985. pp. 329–349. [Google Scholar]
- 41.Winer BJ. Statistical principles in experimental design. McGraw-Hill; New York: 1971. [Google Scholar]
- 42.Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch processing in speech perception. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- 43.Zatorre RJ, Ptito A, Villemure J-G. Preserved auditory spatial localization following cerebral hemispherectomy. Brain. 1995;118:879–889. doi: 10.1093/brain/118.4.879. [DOI] [PubMed] [Google Scholar]
- 44.Zatorre RJ, Mondor T, Evans AC. Functional activation of right parietal and frontal cortex during auditory attention to space and frequency. NeuroImage. 1999;10:544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]