Abstract
The bioactivity of neuropeptides can be regulated by a variety of post-translational modifications, including proteolytic processing. Here, gene-targeted mice producing defective prohormone convertase 2 (PC2) were used to examine the post-translational processing of two neuroendocrine prohormones, pro-opiomelanocortin (POMC) and pro-orphanin FQ (pOFQ)/nociceptin (N), in the brain. Reversed-phase HPLC and gel-exclusion chromatography were combined with specific radioimmunoassays to analyze the processing patterns of these two prohormones in the hypothalamus and the amygdala. In the case of POMC, the lack of PC2 activity completely prevented carboxy-shortening of β-endorphins and greatly diminished conversion of β-lipotropin to γ-lipotropin and β-endorphin. Although conversion of β-lipotropin to β-endorphin decreased, the lack of PC2 activity caused an increase in β-lipotropin and β-endorphin levels in the mutant animals, but no increases in POMC or biosynthetic intermediates were seen. The extent of OFQ/N production was significantly lower in PC2-deficient mice and there was an accumulation of relatively large amounts of pOFQ/N and biosynthetic intermediates. These results demonstrate that PC2 is directly involved in the biogenesis of two brain neuropeptides in vivo and suggest that the specific prohormone and cellular context influences neuropeptide processing by PCs.
Keywords: neuropeptide, proteolytic processing, pro-orphanin FQ/nociceptin, POMC, PC2, gene-targeting
Neuropeptides are generated from inactive precursors by proteolytic processing and concentrated into large, dense core vesicles for release (Sossin et al., 1989). Two members of the subtilisin-like prohormone convertase (PC) family, PC2 and PC1/PC3 (PC1), play major roles in neuroendocrine precursor processing and appear to provide the basis for the tissue-specific aspects of proteolytic peptide processing (Rouille et al., 1995). PC1 and PC2 are expressed exclusively in the brain and neuroendocrine systems, whereas other members of this family are expressed more ubiquitously (Rouille et al., 1995; Zhou et al., 1999).
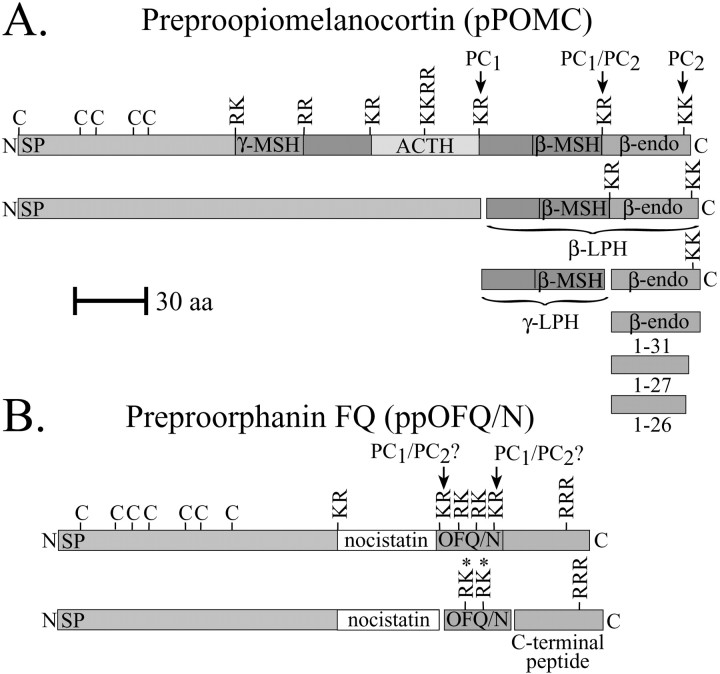
Over the last two decades, the tissue-specific, proteolytic processing of the neuroendocrine prohormone pro-opiomelanocortin (POMC) has been studied in great detail, particularly in the pituitary gland, and has been found to be correlated with PC expression (Mains et al., 1977; Eipper and Mains, 1980; Mains and Eipper, 1981;Rouille et al., 1995). These studies have shown that PC1 is expressed at high levels in the anterior lobe of the pituitary gland, whereas both PC2 and PC1 are expressed in the intermediate lobe (Day et al., 1992; Seidah and Chretien, 1992). PC2 is believed to be responsible for the additional cleavages of POMC-derived peptides found in the intermediate pituitary (Rouille et al., 1995). POMC processing has also been studied in the rat hypothalamus, and the extent of proteolytic processing is similar to the POMC peptide processing patterns found in the neurointermediate pituitary (Emeson and Eipper, 1986). The hallmarks of this proteolytic processing are the essentially complete conversion of β-lipotropin (β-LPH) to γ-LPH and β-endorphin and the subsequent carboxy-shortening of approximately one-third of the β-endorphin 1–31 molecules to β-endorphin 1–27 and 1–26. Figure1A summarizes the general proteolytic processing patterns of the β-LPH–β-endorphin domain in the rat hypothalamus.
Fig. 1.
A, C-terminal proteolytic processing of pre-POMC (pPOMC). A schematic representation of the β-LPH–β-endorphin processing pathway in the rodent hypothalamus. B, A schematic representation of ppOFQ/N processing in the rodent hypothalamus. Predicted and potential cleavages by PC1and PC2 based on pituitary POMC processing are indicated. The processing patterns shown are thought to occur in mice expressing WT PC2. K, Lysine;R, arginine. Asterisks denote potential paired basic cleavages in OFQ/N. Arrows denote PC cleavage sites.
Prepro-orphanin FQ (ppOFQ)/nociceptin (N) is a prohormone encoding the neuropeptide OFQ/N, as well as other potential peptides (Bunzow et al., 1994; Meunier et al., 1995; Reinscheid et al., 1995). OFQ/N and its receptor (ORL1) are expressed abundantly in the brain and spinal cord (Houtani et al., 2000) and have been implicated in a variety of physiological processes, such as cell excitability (Vaughan et al., 1997; Yu et al., 1997; Pan et al., 2000) and cAMP production (Reinscheid et al., 1995); they also possess nociceptive and anxiolytic properties (Jenck et al., 1997; Koster et al., 1999). Relatively little is known about pOFQ/N processing or the role of the PCs in the generation of individual neuropeptides from the proprotein, although we have shown previously that the major OFQ/N-containing peptide produced in the rat, mouse, and monkey hypothalamus is OFQ/N1–17 (Quigley et al., 1998). Figure1B summarizes what is known about pOFQ/N proteolytic processing.
Mice lacking an active PC2 exhibit defective hormone processing in the proinsulin, proglucagon, and prosomatostatin systems of the pancreas (Furuta et al., 1997). In the present study, we have used these mice to examine the effects of PC2 deficiency on the processing of two neuroendocrine precursors in specific brain tissues (Fig. 1). Here we use radioimmunoassays (RIAs) for POMC-derived peptides [β-endorphin and β-melanocyte-stimulating hormone (β-MSH)] and for OFQ/N, combined with biochemical fractionation methods, to show that lack of a functional PC2 affects both rate and site-specific proteolytic cleavages of pOFQ/nociceptin and POMC-derived peptides in a proprotein-specific manner.
MATERIALS AND METHODS
Animals. All +/+ and PC2-deficient −/− mice bred were littermates obtained from the mating of PC2 +/− mice initially provided by Dr. D. Steiner (Howard Hughes Medical Institute, University of Chicago, Chicago, IL). The PC2-deficient mice contain an insertion of a neomycin cassette into exon 3 of the PC2 gene (Furuta et al., 1997). This insertion alters transcript splicing and prevents activation of the mutated PC2 precursor. Homozygous −/− mice thus lack any PC2 activity. All animals were housed and used according to institutional guidelines prescribed by the National Institutes of Health.
Tissue isolation and sample preparation. Individual hypothalamic and amygdala tissues from wild-type (WT), heterozygous, and homozygous mutant mice were extracted in 0.5 ml of 10% acetic acid containing 0.5 mg/ml bovine serum albumin (BSA) and protease inhibitors (1 μl/ml protease inhibitor cocktail; Sigma, St. Louis, MO). Samples were homogenized manually and then frozen and thawed three times. Tissue extracts were centrifuged, and the supernatants were lyophilized for peptide analyses.
Reversed-phase HPLC. After lyophilization, tissue extracts were resuspended in 500 μl of trifluoroacetic acid (TFA) and injected onto a Vydac (214TP54) RP-HPLC column (C4, 300 Å pore size; The Separations Group, Hesperia, CA). The extracts were fractionated using a Waters (Milford, NJ) reversed phase (RP)-HPLC system with linear gradients of acetonitrile in 0.1% TFA and a flow rate of 1 ml/min. Generally, 80 1-min fractions were collected using a gradient of 8–36% acetonitrile. An additional gradient starting with 13.6% acetonitrile for 3 min, a step-up to 17.6% acetonitrile in 2 min, and then a linear gradient to 36% acetonitrile at 80 min was used for some POMC peptide fractionations. Aliquots were then vacuum dried before RIA. In some cases, an additional isocratic elution at 36% acetonitrile, after the linear gradient, was used to elute POMC.
Gel-exclusion chromatography. Lyophilized extracts were resuspended in 0.5 ml of 3 m acetic acid and injected onto a 1 × 35 cm column (Bio-Rad, Richmond, CA) of Sephadex G-50 (Pharmacia, Piscataway, NJ) using 3m acetic acid containing 10 μg/mg BSA to elute the peptides by FPLC (Pharmacia). The flow rate was 1 ml/min, and 1 min fractions were collected. Molecular weight markers are shown in the figures.
RIA. The OFQ/N antiserum is specific to the C terminus of OFQ/N1–17 and does not cross-react with β-endorphin or dynorphin (DYN) A. Tyr14 OFQ/nociceptin was iodinated using the chloramine-T method (Quigley et al., 1998). The β-endorphin and β-MSH RIAs have been described previously (Hatfield et al., 1988). The β-endorphin antiserum is specific to the mid-portion of β-endorphin 1–31 and cross-reacts 1:1 with all β-endorphins and β-LPH (Hatfield et al., 1988). The β-MSH antiserum is specific to the mid-portion of β-MSH and cross-reacts with the C terminus of γ-LPH (Hatfield et al., 1988; Thorne and Thomas, 1990). An antiserum specific for mouse (m)ppOFQ/N160–187 was generated in rabbits against the synthesized peptide after conjugation by Covance (Denver, PA). This antiserum (VO61) was used at a final dilution of 1:3000 and does not cross-react with OFQ/N, mppOFQ/N160–176, or dynorphin A1–13. It also shows minimal cross-reactivity with mppOFQ/N181–187. Thus, the potential internal RRR cleavage site in pOFQ/N160–187 must be intact for full reactivity (Mathis et al., 2001). Vacuum-dried samples were resuspended in phosphate buffer containing β-mercaptoethanol and BSA for RIA. All of the RIA procedures were performed as described previously (Quigley et al., 1998). The RIAs have a sensitivity of ∼1.8 × 10−3 pmol/tube. Recovery of input immunoreactivity of single or pooled brain tissues was ∼85–90%. All peptide nomenclature is based on the mouse primary amino acid sequences of pOFQ/N and POMC.
RESULTS
Lack of a functional PC2 decreases β-LPH conversion to γ-LPH and β-endorphin and abolishes carboxy-shortening of β-endorphin 1–31 in vivo
We fractionated WT and mutant hypothalamic extracts by RP-HPLC and assayed the fractions for β-endorphin and β-MSH immunoreactivity. In the WT hypothalamus, almost all of the β-MSH immunoreactivity is present as γ-LPH (Fig.2A). However, in the PC2-deficient mice, only approximately one-third of the β-LPH was converted to γ-LPH and β-endorphin. This result suggests that PC2 is required to complete the in vivo conversion of β-LPH to γ-LPH and β-endorphin 1–31. Figure 2B confirms that the major peak of immunoreactive material at 76 min is β-LPH, as demonstrated by a single, large peak containing essentially equal amounts of β-endorphin and β-MSH immunoreactivity. An additional peak of β-MSH immunoreactivity at 43 min in the WT animals is absent in the mutants. This peptide is a C-terminal fragment of γ-LPH derived from a cleavage at leu10-glu11 of γ-LPH (Thorne and Thomas, 1990).
Fig. 2.
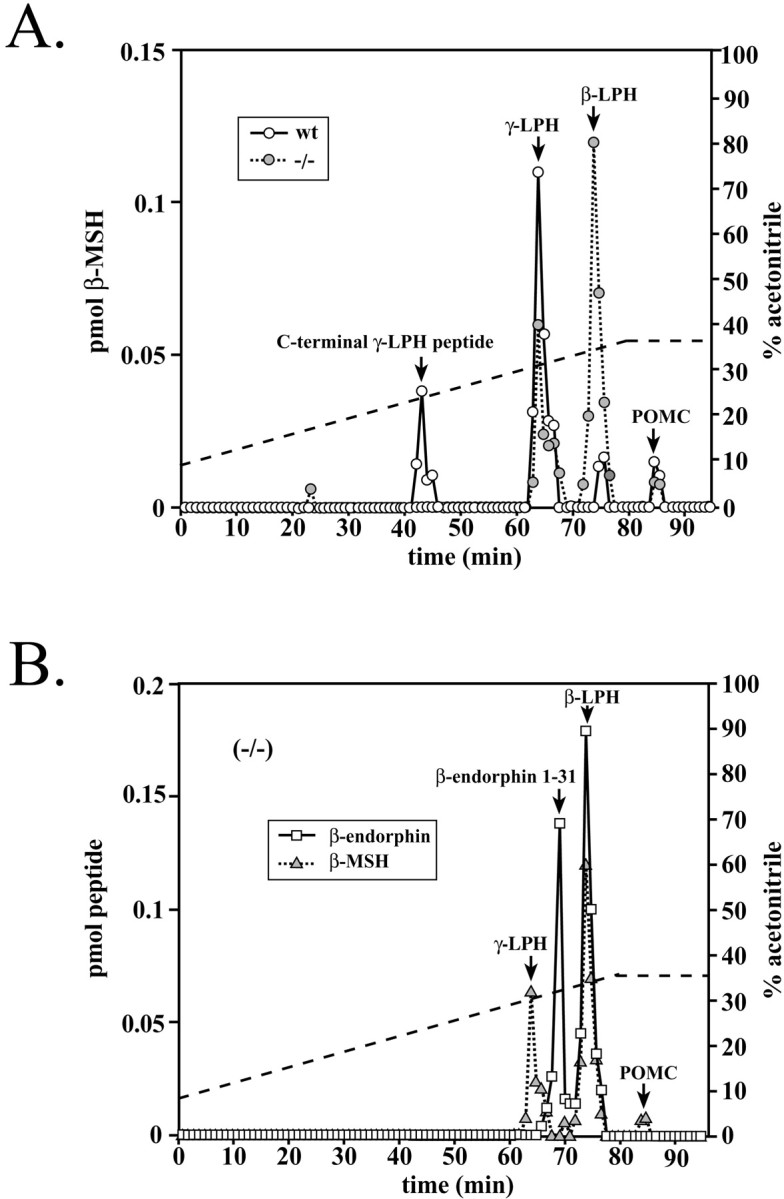
β-LPH domain processing in WT and PC2 mutant mouse hypothalamic extracts. RP-HPLC fractions were assayed for β-endorphin and β-MSH immunoreactivity. The dashed lineindicates the acetonitrile gradient. A, WT β-MSH and mutant animals. B, Mutant PC2. Results shown are representative of four separate determinations.
We subsequently determined the relative sizes of the β-endorphin-immunoreactive material by gel-exclusion chromatography of WT and PC2-deficient hypothalamic extracts. Figure3 shows that essentially all of the β-LPH has been converted to β-endorphin-sized material in the WT hypothalamus, as was found in the RP-HPLC analyses, whereas in the PC2-deficient animals, only approximately one-third of the β-LPH was converted to β-endorphin. The inset in Figure 3 shows that only ∼34% of β-LPH was cleaved to β-endorphin, whereas in the WT animals, ∼96% of the β-LPH was converted to β-endorphin-sized material.
Fig. 3.
Sephadex G-50 gel-exclusion chromatography of WT and mutant PC2 hypothalamic extracts. The fractions were assayed for β-endorphin immunoreactivity. The arrow indicates the elution position of 125I-acetyl-β-endorphin 1–27.V0, Exclusion volume;Vt, total column volume. Theinset shows the percentage of conversion of β-LPH to β-endorphin in a WT compared with a PC2 mutant mouse hypothalamus. The percentage of conversion is the ratio of the immunoreactivity eluting 3–3.5 kDa β-endorphin to the total β-endorphin immunoreactivity. Several RP-HPLC analyses were used for this calculation. n = 3; p = 0.0063; unpaired t test using StatView (Abacus Concepts, Calabasas, CA).
PC2 has been proposed to convert β-endorphin 1–31 to its carboxy-shortened form, β-endorphin 1–27 (Zhou and Mains, 1994). To determine whether mouse hypothalamic β-endorphin 1–31 is carboxy-shortened, we fractionated extracts by gel filtration as in Figure 3, pooled the β-endorphin-sized material, and fractionated the pooled fractions using a gradient that was slightly different from that shown in Figure 2. Figure4A shows that in the WT hypothalamus, some of the β-endorphin 1–31 (∼40%) has been converted to β-endorphin 1–27 and 1–26. Figure 4Bshows that the β-endorphin 1–31 was not processed to carboxy-shortened peptides to any appreciable extent in the mutant hypothalamus. The small amount of immunoreactive material eluting at 62–64 min is β-LPH carryover from the pooled fractions.
Fig. 4.
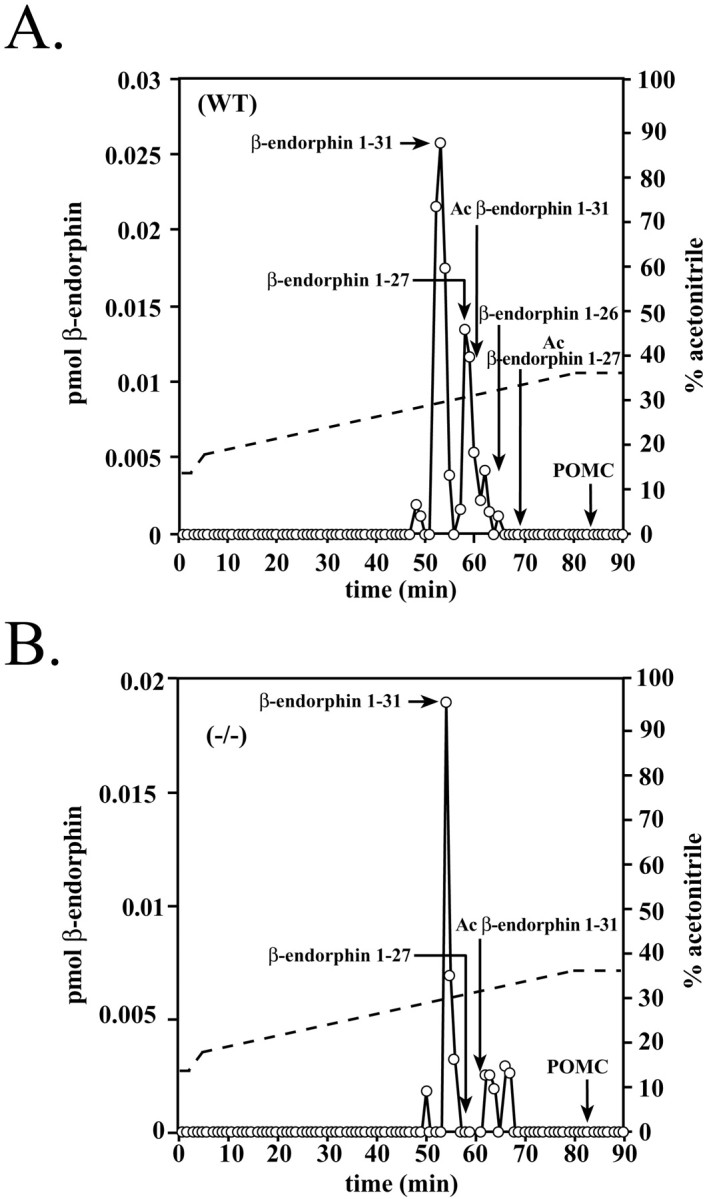
RP-HPLC fractionation of WT and PC2-deficient mouse hypothalamic extracts after gel exclusion, as in Figure 3. The pooled extracts of β-endorphin-sized material were fractionated on the gradient indicated, and the fractions were assayed for β-endorphin immunoreactivity. The arrows indicate the elution times of authentic β-endorphin peptides. The dashed line indicates the acetonitrile gradient. Similar results were seen in two experiments. Ac, Acetyl.
Lack of a functional PC2 causes incomplete conversion of pOFQ/N to OFQ/N in vivo
We then examined the extent of ppOFQ/N processing in PC2-deficient mice using an antibody to OFQ (1–17) that can recognize biosynthetic intermediates (BSIs), as well as the proprotein. Figure5A shows that in the WT hypothalamus virtually all of the OFQ/N immunoreactivity elutes at 31 min. In contrast, Figure 5B shows that although some OFQ/N is produced and elutes at 30–31 min, relatively large amounts of a pOFQ/N-like molecule elute at 70 min. Potential BSIs containing the OFQ/N epitope (66–67 min) were also detected in hypothalamic extracts obtained from PC2 mutant mice. Figure 5C shows that proteolytic processing in the heterozygote is very similar to the processing observed in the WT, indicating that one-half of the PC2 enzymatic activity expressed in the heterozygotes is sufficient to produce WT proteolytic-processing patterns. We also noted a reduction in the total amount of OFQ/N-immunoreactive material contained in extracts from PC2 mutant animals. These results indicate that PC2 is required for complete processing of the OFQ/N precursor and OFQ/N-containing intermediates; its absence causes a substantial reduction in OFQ/N immunoreactivity.
Fig. 5.
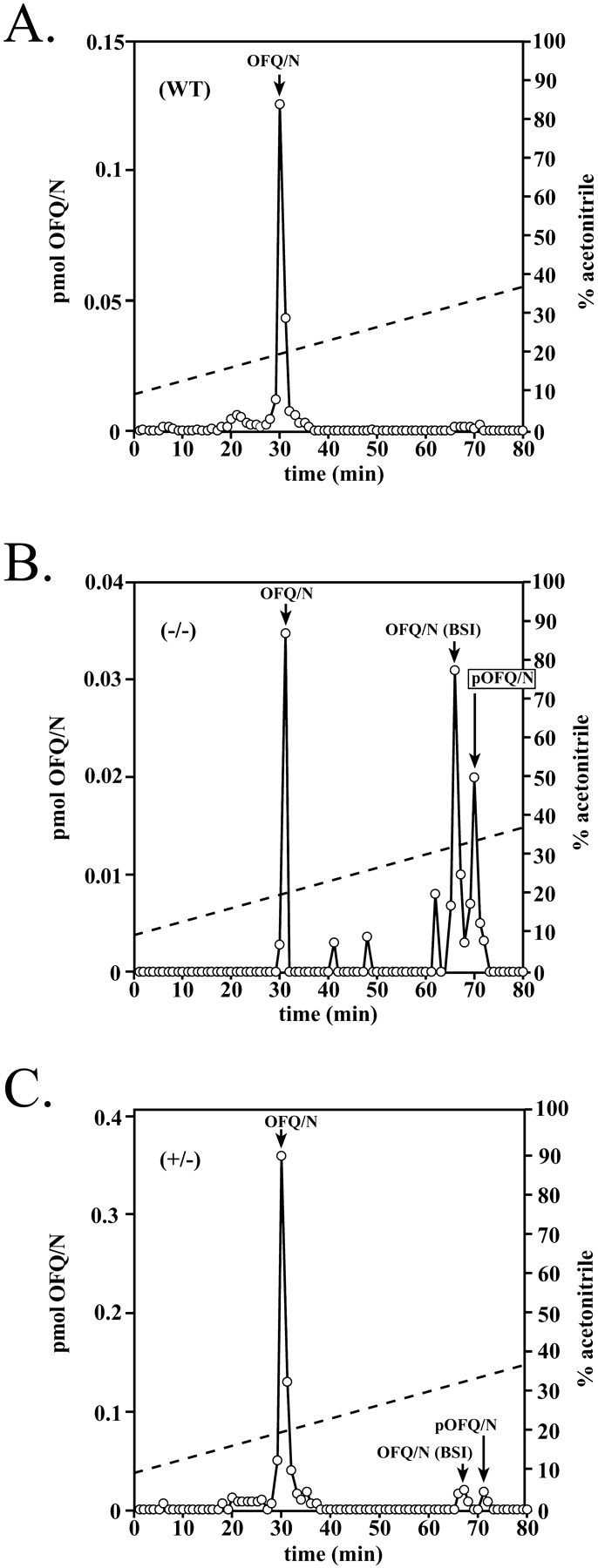
RP-HPLC fractionation of (WT) and PC2 mutant mouse hypothalamic extracts (one mouse hypothalamic equivalent). Extracts from WT (A), PC2-deficient (B), and heterozygous (C) mice were fractionated using a linear gradient of 8–36% acetonitrile (dashed line). The fractions were assayed for OFQ/N immunoreactivity. The arrow indicates the elution position of synthetic OFQ/N. The profile shown is representative of four separate determinations.
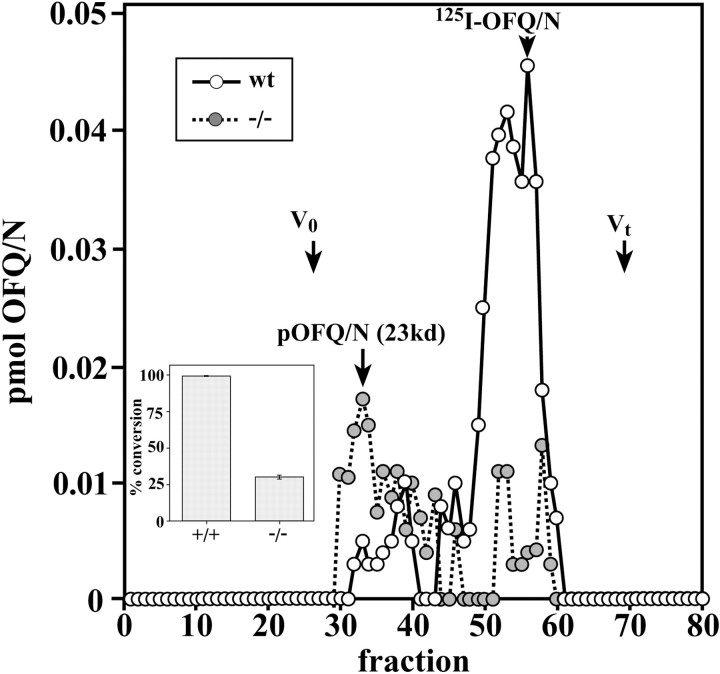
To more fully characterize the effects on OFQ/N processing, we fractionated hypothalamic extracts by gel-exclusion chromatography. Theopen circles in Figure 6indicate that virtually all of the OFQ/N immunoreactivity co-elutes with 125I-OFQ/N in WT animals, whereas larger relative amounts of pOFQ/N-sized material were detected in the PC2 mutant animals. As with the HPLC fractionations, much smaller amounts of OFQ/N immunoreactivity relative to the proprotein were detected in the PC2-defective animals. The inset in Figure 6shows that using the HPLC fractionations, only ∼30% of the total immunoreactivity is converted to OFQ/N in the mutants, compared with ∼98% in the WT animals. The total amount of OFQ/N immunoreactivity found in the HPLC fractionations from mutant mice ranged from 10 to 40% of WT values.
Fig. 6.
Sephadex G-50 chromatography of WT and PC2 mutant mouse hypothalamic extracts. Gel filtration was performed as described in Materials and Methods. Portions of the fractions were vacuum dried and assayed for OFQ/N immunoreactivity. 125I-OFQ/N elutes at fraction 55. Cytochrome C (molecular mass, 22.5 kDa) elutes at fraction 33–34. V0, Exclusion volume; Vt, total column volume. Conversion of total OFQ/N immunoreactivity to mature peptide in WT and PC2 mutant mouse hypothalamus is shown in the inset. The percentage of conversion is the ratio of the immunoreactivity co-eluting with synthetic OFQ/N at 31 min to the total OFQ/N immunoreactivity. We used RP-HPLC analyses for this calculation.n = 3; p = 0.002; unpairedt test using StatView.
We also examined proteolytic processing of OFQ/N in the amygdala. In the WT amygdala, virtually all of the immunoreactive OFQ/N eluted at 31–32 min (Fig. 7), similar to our previous findings analyzing hypothalamic extracts. In the PC2-deficient mice, the peak of OFQ/nociceptin was greatly reduced, and new peaks of immunoreactivity were detected at 71 min (pOFQ/N) and 66–67 min (Fig.7, filled circles). Thus, in both the amygdala and the hypothalamus of the PC2 mutants, small amounts of OFQ/N were produced; however, processing does not proceed to completion, because unprocessed and partially processed immunoreactive material containing the OFQ/N epitope were detected. We also found that in the amygdala, as in the hypothalamus, the level of mature OFQ/N immunoreactivity was dramatically reduced in the mutants. This result again supports the notion that PC2 is required for complete conversion of pOFQ/N to the 17 amino acid OFQ/N peptide in the brain.
Fig. 7.
RP-HPLC fractionation of WT and PC2 mutant amygdala extracts. Extracts from WT animals (open circles) and PC2 mutant animals (filled circles) were fractionated using a linear gradient of 8–36% acetonitrile (dashed line). The fractions were assayed for OFQ/N immunoreactivity. The arrow indicates the elution time of synthetic OFQ/N. The profile shown is representative of four separate determinations.
In addition, we assayed the HPLC fractions using an RIA directed against the C terminus of the OFQ/N proprotein (Mathis et al., 2001). In the WT and heterozygote hypothalamus, we found two major peaks of C-terminus immunoreactivity eluting at 62 and 66 min, with synthetic C-terminal peptide eluting at 62 min (Fig.8A). As shown previously, OFQ/N elutes at 31 min (Fig. 5A). In the mutant animals, the major peak of both OFQ/N and C-terminal immunoreactivity eluted at 71 min, and the relative amounts of immunoreactivity eluting at 62 and 66 min decreased markedly (Fig. 8B). These results support the notion that a moiety eluting at 71 min contains both the C-terminal and OFQ/N epitope and is most likely pOFQ/N. The peaks eluting at 62 and 66 min appear to be the full-length C-terminal peptide and a derivative containing an intact internal RRR sequence.
Fig. 8.
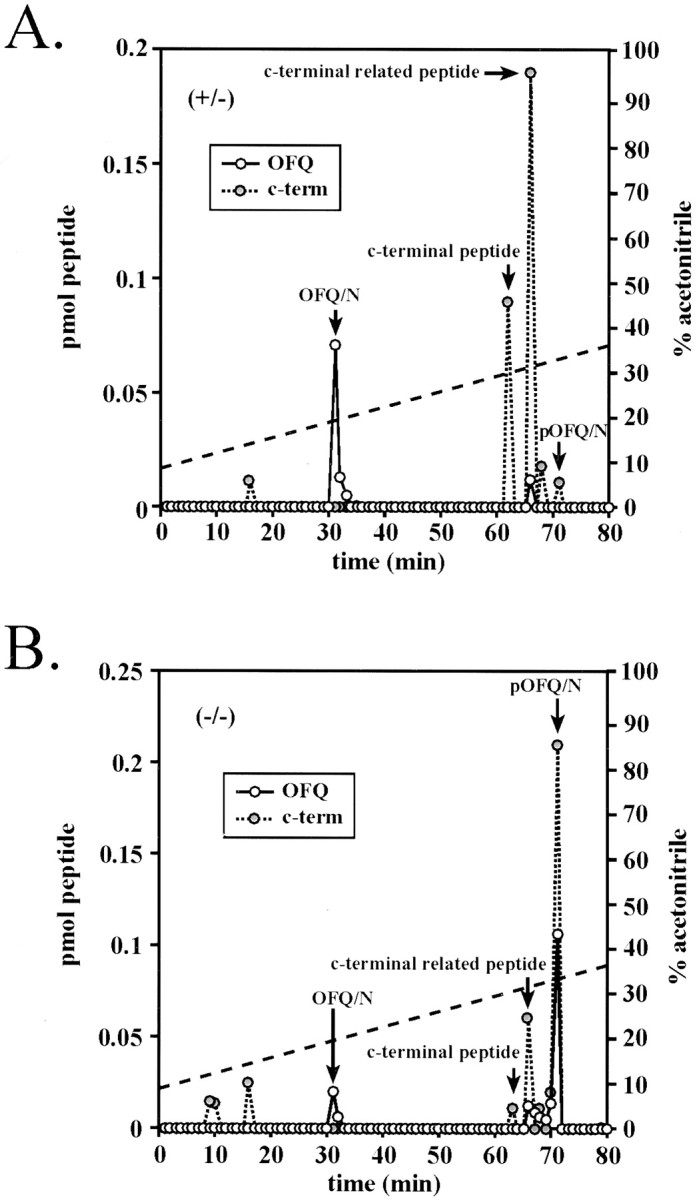
Fractionation of pOFQ/N C-terminal (c-term)-containing peptides in WT and PC-2-deficient mice. Extracts were fractionated as described in Figure 5, and the fractions were assayed for OFQ/N and C-terminal peptide immunoreactivity. The elution positions of synthetic peptides are indicated by the arrows. The dashed lineindicates the percentage of acetonitrile. A, Heterozygote;B, PC null.
DISCUSSION
The proteolytic processing patterns of POMC-derived peptides have been studied previously in the rat hypothalamus (Emeson and Eipper, 1986). Those studies demonstrated that POMC processing in the hypothalamus resembled the processing pattern in the neurointermediate pituitary rather than the anterior pituitary (Eipper and Mains, 1981;Emeson and Eipper, 1986). The exception to this was the lack of acetylation needed to produce α-N-acetylated endorphins and MSH. Thus, in the rat, almost all of the hypothalamic β-LPH was converted to β-endorphin 1–31-related molecules, and approximately one-third of this population was carboxy-shortened. We found essentially the same result in the WT mouse hypothalamus (Figs. 3, 4).
Zhou and Mains (1994) found that overexpression of PC2 in AtT-20 anterior pituitary cells resulted in enhanced proteolytic cleavages at specific, paired-basic sites. In the case of PC2, it enhanced the conversion of β-LPH to γ-LPH and β-endorphin and produced carboxy-shortened β-endorphin 1–27. As predicted, we found no carboxy-shortened β-endorphins in the hypothalamus of mice lacking a functional PC2; thus, PC1 does not cleave at the β-endorphin C-terminal KK site in vivo. We found that lack of a functional PC2 greatly diminished (by two-thirds) the conversion of β-LPH to γ-LPH and β-endorphin in vivo. We did not find any increase in unprocessed POMC in brain tissues from the mutant mice, unlike our studies of pituitary POMC processing in PC2 mutant mice (Allen et al., 1999).
The results obtained by examining pOFQ/N processing in specific brain tissues were in contrast to these observations. In both the hypothalamus and the amygdala, we found that lack of a functional PC2 resulted in an increase in unprocessed OFQ/N-containing material, including pOFQ/N. Also, in contrast to the POMC pathway, there was a reduction in the OFQ/N immunoreactivity in both the hypothalamus and amygdala of the mutant animals. The decrease in the production of mature OFQ/N suggests that PC2 is much more active than PC1 in cleaving the KR sites bounding OFQ/N (Fig. 1B). The PC2-deficient mice used here have been examined for DYN processing in the brain (Berman et al., 2000). These studies showed a substantial reduction in DYN A8, DYN A17, and DYN B13, and it was concluded that PC2 was critical for DYN peptide production.
The presence of PC2 activity completes the conversion of β-LPH to β-endorphin and carboxy-shortens some of the β-endorphin 1–31 to β-endorphin 1–27 in vitro. PC2 is autoactivated in the acidic environment of a late post-Golgi compartment (Lamango et al., 1999), and it is after early cleavages by PC1 that PC2 performs its functions. Our studies confirm the in vitro studies using POMC pituitary cells as a model; however, in vivo, PC1 (presumably) was able to convert a substantial amount of β-LPH to γ-LPH and β-endorphin, as seen in the mutant animals (Fig.2A). In fact, the processing profile resembles the pattern found in the mouse anterior pituitary (Eipper and Mains, 1980). This general scenario holds true with regard to some of the pOFQ/N cleavages at KR sites (Fig. 1), but unlike the β-endorphin immunoreactivity, we found a reduction in OFQ/N immunoreactivity in the mutant, as well as relative increases in unprocessed prohormone and a putative biosynthetic intermediate. Thus, the effects of a nonfunctional PC2 on propeptide processing are different, depending on which proprotein pathway is examined. What still remains unclear is what prevents PC1 from completely cleaving at all of the KR sites examined in our studies. There is no sequence homology bounding the KR sites examined here in either pOFQ/N or POMC; thus, site-specific amino acid sequence homology does not explain why conversion stops.
Very recently an inhibitor of PC1 called proSAAS has been cloned and characterized (Fricker et al., 2000). The expression of proSAAS in AtT-20 cells results in a substantial reduction in the rate of endogenous POMC processing. We know that there are activators of PCs, because the 7B2 protein is essential for the activation and maturation of PC2 (Lamango et al., 1999). Because there are specific inhibitors and activators for PCs, their existence may provide the answer to the incomplete conversion at specific KR sites in POMC and pOFQ/N. PC1 and PC2 show temporal and spatial expression during development (Marcinkiewicz et al., 1993; Zheng et al., 1994); thus, it will also be interesting to examine the effects of PC2 deficiency on pOFQ/N and POMC processing during fetal development.
We have shown that the lack of functional PC2 produces aberrant processing in two neuroendocrine prohormone pathways. Not only is PC2 required for site-specific cleavages, but it also appears to play a role in the conversion of certain intermediates in vivo. In the case of pOFQ/N, PC2 appears to affect the amount of end product peptide produced. We have also shown that expression of a nonfunctional PC2 can affect processing in a prohormone-specific manner. The ability to generate multiple biological activities from a given prohormone is an efficient way to create physiological diversity without the use of gene products encoding each peptide separately. It has been shown previously that PC1 and PC2 can be regulated by a variety of modulators, such as neurotransmitters, steroid hormones, and hypothalamic releasing factors (Day et al., 1992). The combination of such regulation and potentially selective expression of inhibitors and activators for the PCs creates a new level of complexity in producing bioactivities from prohormones.
Footnotes
This work was supported in part by National Institutes of Health Grants DA11282 (R.G.A.), DA08562 and DA10703 (D.K.G.), and DA08622 (J.E.P.). We thank D. F. Steiner and the University of Chicago Diabetes Research and Training Center for providing the PC2 null mouse strain. We also thank Dan Austin and Harlene Finn.
Correspondence should be addressed to Dr. Richard G. Allen, Oregon Health Sciences University, The Center for Research on Occupational and Environmental Toxicology, 3181 Southwest Sam Jackson Park Road, L606, Portland, OR 97201. E-mail: allenr@ohsu.edu.
REFERENCES
- 1.Allen RG, Pellegrino MJ, Peng, B, Pintar JE (1999) Altered POMC processing in SPC2 KO mice. Program Abstr Endocr Soc Annu Meet 270.99.
- 2.Berman Y, Mzhavia N, Polonskaia A, Furuta M, Steiner D, Pintar J, Devi L. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J Neurochem. 2000;75:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- 3.Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, δ, or κ opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 4.Day R, Schafer M, Watson SJ, Chretien M, Seidah NG. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. 1992;6:485–497. doi: 10.1210/mend.6.3.1316544. [DOI] [PubMed] [Google Scholar]
- 5.Eipper BA, Mains RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980;1:1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Eipper BA, Mains RE. Further analysis of post-translational processing of β-endorphin in rat intermediate pituitary. J Biol Chem. 1981;256:5689–5695. [PubMed] [Google Scholar]
- 7.Emeson RB, Eipper BA. Characterization of pro-ACTH/endorphin-derived peptides in rat hypothalamus. J Neurosci. 1986;6:837–849. doi: 10.1523/JNEUROSCI.06-03-00837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci. 2000;20:639–648. doi: 10.1523/JNEUROSCI.20-02-00639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatfield JM, Allen RG, Stack J, Ronnekleiv O. Post-translational processing of pro-opiomelanocortin (POMC)-derived peptides during fetal monkey pituitary development II: β-endorphins, β-melanotropins. Dev Biol. 1988;126:164–172. doi: 10.1016/0012-1606(88)90250-3. [DOI] [PubMed] [Google Scholar]
- 11.Houtani T, Nishi M, Takeshima H, Sato K, Sakuma S, Kakimoto S, Ueyama T, Noda T, Sugimoto T. Distribution of nociceptin/orphanin FQ precursor protein and receptor in brain and spinal cord: a study using in situ hybridization and X-Gal histochemistry in receptor-deficient mice. J Comp Neurol. 2000;424:489–508. [PubMed] [Google Scholar]
- 12.Jenck F, Moreau J-L, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker H-P, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK (1999) Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA 10444–10449. [DOI] [PMC free article] [PubMed]
- 14.Lamango NS, Apletalina E, Liu J, Lindberg I. The proteolytic maturation of prohormone convertase 2 (PC2) is a pH-driven process. Arch Biochem Biophys. 1999;362:275–282. doi: 10.1006/abbi.1998.1033. [DOI] [PubMed] [Google Scholar]
- 15.Mains RE, Eipper BA. Differences in the post-translational processing of β-endorphin in rat anterior and intermediate pituitary. J Biol Chem. 1981;256:5683–5688. [PubMed] [Google Scholar]
- 16.Mains RE, Eipper BA, Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci USA. 1977;74:3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcinkiewicz M, Day R, Seidah NG, Chretien M. Ontogeny of the prohormone convertases PC1 and PC2 in the mouse hypophysis and their colocalization with corticotropin and α-melanotropin. Proc Natl Acad Sci USA. 1993;90:4922–4926. doi: 10.1073/pnas.90.11.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis JP, Ross GC, Pellegrino MJ, Jimenez C, Pasternak GW, Allen RG. Carboxyl terminal peptides derived from prepro-orphanin FQ/nociceptin (ppOFZ/N) are produced in the hypothalamus and possess analgesic bioactivities. Brain Res. 2001;895:89–94. doi: 10.1016/s0006-8993(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 19.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Monsarrat B, Mazarguil G, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 20.Pan ZZ, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron. 2000;26:515–522. doi: 10.1016/s0896-6273(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 21.Quigley DI, McDougall J, Darland T, Zhang GE, Ronnekliev O, Grandy DK, Allen RG. Orphanin FQ is the major OFQ1–17-containing peptide produced in the rodent and monkey hypothalamus. Peptides. 1998;19:133–139. doi: 10.1016/s0196-9781(97)00268-4. [DOI] [PubMed] [Google Scholar]
- 22.Reinscheid RK, Nothacker H-P, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 23.Rouille Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva AA, Jr, Chan SJ, Steiner DF. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol. 1995;16:322–361. doi: 10.1006/frne.1995.1012. [DOI] [PubMed] [Google Scholar]
- 24.Seidah NG, Chretien M. Proprotein and prohormone convertase of the subtilisin family. Trends Endocrinol Metab. 1992;3:133–140. doi: 10.1016/1043-2760(92)90102-7. [DOI] [PubMed] [Google Scholar]
- 25.Sossin WS, Fisher JM, Scheller RH. Cellular and molecular biology of neuropeptide processing and packaging. Neuron. 1989;2:1407–1417. doi: 10.1016/0896-6273(89)90186-4. [DOI] [PubMed] [Google Scholar]
- 26.Thorne BA, Thomas G. An in vitro characterization of the cleavage site specificity of the insulin cell prohormone processing enzymes. J Biol Chem. 1990;265:8436–8443. [PubMed] [Google Scholar]
- 27.Vaughan CW, Ingram SL, Christie MJ. Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci. 1997;17:996–1003. doi: 10.1523/JNEUROSCI.17-03-00996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M, Streck R, Seidah N, Pintar J. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci. 1994;14:4656–4673. doi: 10.1523/JNEUROSCI.14-08-04656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]
- 31.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]