Abstract
L-type calcium channels regulate a diverse array of cellular functions within excitable cells. Of the four molecularly defined subclasses of L-type Ca channels, two are expressed ubiquitously in the mammalian nervous system (CaV1.2α1 and CaV1.3α1). Despite diversity at the molecular level, neuronal L-type channels are generally assumed to be functionally and pharmacologically similar, i.e., high-voltage activated and highly sensitive to dihydropyridines. We now show that CaV1.3α1 L-type channels activate at membrane potentials ∼25 mV more hyperpolarized, compared with CaV1.2α1. This unusually negative activation threshold for CaV1.3α1 channels is independent of the specific auxiliary subunits coexpressed, of alternative splicing in domains I–II, IVS3–IVS4, and the C terminus, and of the expression system. The use of high concentrations of extracellular divalent cations has possibly obscured the unique voltage-dependent properties of CaV1.3α1 in certain previous studies. We also demonstrate that CaV1.3α1 channels are pharmacologically distinct from CaV1.2α1. The IC50for nimodipine block of CaV1.3α1 L-type calcium channel currents is 2.7 ± 0.3 μm, a value 20-fold higher than the concentration required to block CaV1.2α1. The relatively low sensitivity of the CaV1.3α1 subunit to inhibition by dihydropyridine is unaffected by alternative splicing in the IVS3–IVS4 linker. Our results suggest that functional and pharmacological criteria used commonly to distinguish among different Ca currents greatly underestimate the biological importance of L-type channels in cells expressing Cav1.3α1.
Keywords: CaV1.3α1, L-type, voltage-gated calcium channel, dihydropyridine, low threshold, R-type, clone, Xenopus oocytes, HEK 293
L-type calcium channels couple excitation to contraction in muscle, to gene expression in neurons, to hormone secretion in endocrine cells, and to transmitter release in hair cells (Beam et al., 1989; Ashcroft et al., 1994; Fuchs, 1996;Finkbeiner and Greenberg, 1998). Four genes encode L-type CaV1α1 subunits in mammals (Ertel et al., 2000). CaV1.2α1(α1C) and CaV1.3α1(α1D) are the two most widely expressed L-type channel subunits, and together they are thought to underlie L-type currents in neurons (Hell et al., 1993). Despite reports of functional diversity among L-type currents in select cells, notably endocrine cells, hippocampal neurons, and hair cells (Smith et al., 1993; Avery and Johnston, 1996; Kavalali and Plummer, 1996; Platzer et al., 2000), L-type channels are often assumed to be high voltage-activated and dihydropyridine-sensitive (Ertel et al., 2000). These defining criteria are used to establish the involvement of L-type channel in Ca signaling in a variety of cell types, including neurons. Functional studies of recombinant CaV1.2α1subunits have confirmed that they form prototypic, high voltage-activated, long-lasting, dihydropyridine-sensitive L-type currents (Mori et al., 1993). However, comparable studies of CaV1.3α1 are limited (Williams et al., 1992; Ihara et al., 1995; Bell et al., 2001). We report that CaV1.2α1 and CaV1.3α1 L-type channels are significantly different both at the functional and pharmacological level, contrary to current classification schemes (Ertel et al., 2000). Our results suggest that functional and pharmacological criteria currently used to establish the relative contributions of different Ca channels to various signaling pathways will greatly underestimate the biological importance of L channels in cells that express CaV1.3α1. Their unique voltage dependence of activation also implies that CaV1.3α1 L-type Ca channels mediate cellular processes that depend on calcium influx at relatively hyperpolarized membrane potentials (Liljelund et al., 2000).
MATERIALS AND METHODS
Cloning. Full-length CaV1.3α1 cDNAs were constructed from overlapping rat superior cervical ganglia library- and RT-PCR-derived cDNAs. Library screening and RT-PCR methods have been described previously (Lin et al., 1997). The Expand High Fidelity PCR system (Roche Molecular Biochemicals, Indianapolis, IN) was used for PCR amplification. Library-derived cDNA clones, pRD9901, pRD9902, pRD9904 and pRD9905 were in pBluescript SK vector (Stratagene, La Jolla, CA). pRD9906 and pRD9907 were in pGEM-T vector (Promega, Austin, TX), derived from RT-PCR with primer pairs RDU2715 (5′-ACT TCA TCA TCC TTT TCA TCT GTG G-3′) and RDD3955 (5′-TGA CGA AGC CCA CGA AGA TAT TC-3′), and RDU6165 (5′-CTC TCC CAT TGG CTA TGA CTC AC-3′) and RDD6945 (5′-GCT ACA AGG TGG TGA TGC AAA TC-3′), respectively. The (ATG)7 repeat in the 5′-untranslated region (UTR) was removed by PCR from a library-derived clone, RD9901, with primers RDU2 (5′-ATA TCG ATG CTA GCT GTT CGT GGA AAT GCA GCA TCA T-3′) and RDD711 (5′-AAT CCA GTA AGT TCC ATC CGT TCC-3′), leaving one ATG as the single translation initiation site. ClaI and NheI sites were introduced in the 5′-UTR to facilitate further cloning work. Standard cloning methods were used to generate four full-length CaV1.3α1 cDNA clones containing different combinations of three alternative splice sites (+/Δ exon 11, +/Δ exon 32, and exon 42a/exon 42). Exons are numbered according to the method of Yamada et al. (1995), except for exon 42a, which was present in one of our CaV1.3α1 cDNA clones and in the human genomic clone AC012467 that localizes to the CaV1.3α1 gene (3p14.3). The cDNAs were cloned into either pcDNA6 (Invitrogen, Carlsbad, CA) or into pBluescript (Stratagene) for expression. Unless indicated otherwise, the CaV1.3α1variant used in these studies has the following combination of alternatively spliced exons: +exon11, Δexon32, and +exon42a (GenBank accession number AF370009).
Other clones.CaV1.2α1 cDNA was provided by Dr. Y. Mori (National Institute for Physiological Science, Okazaki, Japan; Mikami et al., 1989); CaVβ1b cDNA was provided by Dr. K. P. Campbell (University of Iowa, Iowa City, IA; Pragnell et al., 1991); CaVβ2a and CaVβ4 cDNAs were provided by Dr. E. Perez-Reyes (Loyola University, Marywood, IL; Castellano and Perez-Reyes, 1994), and CaVβ3 and CaVα2δ were cloned in our laboratory. The cDNAs were inserted in either pcDNA3 (Invitrogen) or pBluescript (Stratagene).
Transient expression and recording from Xenopusoocytes. Calcium channels were transiently expressed inXenopus oocytes, and a two-microelectrode voltage clamp was used for recordings, essentially as described previously (Lin et al., 1999). Thirty nanograms of CaV1.3α1 or CaV1.2α1 together with 15 ng of CaVβ cRNA were injected into defolliculated Xenopus oocytes in a volume of 46 nl. Currents were recorded from oocytes 7–9 d after RNA injection. Fifteen to 30 min before recording, oocytes were injected with 46 nl of a 50 mm1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) solution to reduce activation of the endogenous calcium-activated chloride current (Lin et al., 1997). The estimated concentration of BAPTA inside the oocyte is 4.5 mm, assuming uniform dispersion from the point of injection and based on a cell radius of 0.5 mm. L-type calcium channel currents were recorded using 3 m KCl-filled electrodes with 0.7–1 and 0.4–0.7 MΩ resistances for voltage and current electrodes, respectively. The standard recording solution contained (in mm): 5 BaCl2, 85 tetraethylammonium hydroxide (TEAOH), 5 KCl, and 5 HEPES, adjusted to pH 7.4 with methanesulphonic acid. When 2 mm BaCl2 or 5 mm CaCl2 was substituted for 5 mm BaCl2, other components of the recording solutions remained the same. When 10, 20, and 40 mm BaCl2 were used, tetraethylammonium was reduced to 75, 55, and 42.5 mm, respectively. The use of 3m KCl agar bridges for grounding reduced junction potentials arising from changes in extracellular divalent cation concentration to <2 mV; therefore, no correction was made. Data were leak-subtracted on-line using a P/4 protocol (pClamp V6.0; Axon Instruments). Voltage steps were applied every 12 sec from a holding potential of −80 mV unless indicated otherwise. Peak current–voltage plots were fit with the following combination of the Goldman–Hodgkin–Katz (GHK) and Boltzmann equation to estimate activation midpoints: I = {P1 ×P22 × Vm × (1 − exp (−(Vm − Vrev ×P2))/(1 − exp (−(Vm ×P2)))}/(1 + exp (−(Vm − V1/2)/k)), where Vm is the membrane potential; Vrev is the reversal potential; V1/2 is the activation midpoint;P1 is the permeability of the ion × [Ca2+]i × RT;P2 is zF/RT (z, the valency of the ion; F, the Faraday constant; R, the gas constant; and T, the temperature in degrees Kelvin); andk is Boltzmann constant.
Transient transfection and recording from tsA-201 cells.tsA-201 cells (large T-antigen transformed HEK293 cells) were transfected with 2.7 μg of a mix of CaV1.3α1, CaVβ3, and CaVα2δ in a molar ratio of 1:1:1, together with 0.3 μg of enhanced green florescent protein cDNA in a 60 mm tissue culture dish with 3 ml of growth media. Cells were transfected with the various cDNAs when 80% confluent, using LipofectAMINE-PLUS (Life Technologies, Gaithersburg, MD). Cells were trypsinized 36 hr later and plated on poly-d-lysine-coated coverslips for recording. Growth media contained DMEM supplemented with 10% FBS, 5 U/ml penicillin, and 5 μg/ml streptomycin, and cells were cultured at 37°C in 5% CO2.
CaV1.3α1 currents were recorded from the majority of fluorescing tsA-201 cells (∼80%). Whole-cell voltage-clamp recording was performed with the Axopatch 200A (Axon Instruments). Recording patch pipettes were fire-polished to a resistance of 2–6 MΩ. Sylgard (Dow Corning, Midland, MI) was used to decrease the pipette capacitance. The internal solution contained (in mm): 135 CsCl, 4 MgCl2, 4 ATP, 10 HEPES, 10 EGTA, and 1 EDTA, adjusted to pH 7.2 with TEAOH. The bath solution contained: 135 mm CholineCl, 1 mmMgCl2, 5 mmBaCl2 or 2 mmCaCl2 and 10 mm HEPES, adjusted to pH 7.2 with TEAOH. Series resistance was compensated 80%, with a 10 μs lag time. A voltage error of 4 mV attributable to the liquid junction potential was corrected for. Signals were sampled at 20 kHz and low pass-filtered at 2 kHz. Data were leak-subtracted on-line using a P/4 protocol and analyzed using pClamp V7.0 (Axon Instruments).
Pharmacology. Bay K 8644 (mixed enantiomer), nimodipine (a gift from Bayer), and nitrendipine (Sigma, St. Louis, MO) were dissolved in polyethylene glycol 400 at a concentration of 10 mm. (Stock solutions were stored at 4°C in the dark for up to 1 month.). The extracellular solution was exchanged at a rate of 5–10 ml/min.
RESULTS
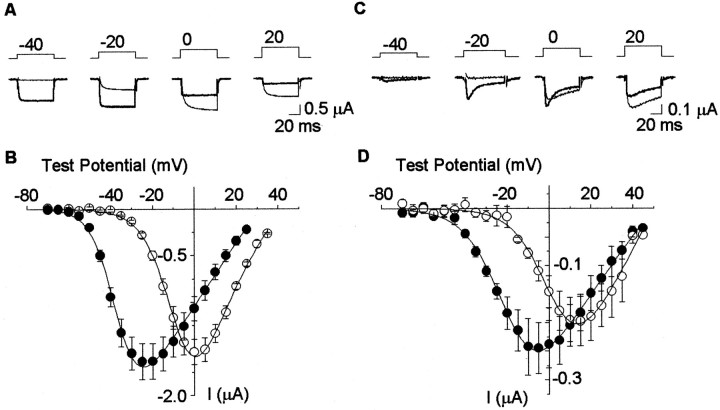
We constructed a full-length CaV1.3α1 cDNA clone derived from rat sympathetic neurons. Figure1 compares current–voltage relationships of L-type Ca channel currents recorded in Xenopus oocytes expressing either CaV1.3α1 or CaV1.2α1 together with the Ca channel CaVβ1bsubunit (5 mm barium as charge carrier). CaV1.3α1 and CaV1.2α1 currents show little or no inactivation during a 60 msec depolarizing pulse, a property typical of L-type currents recorded with barium as the charge carrier (Fig. 1A). Maximum currents in oocytes expressing CaV1.2α1 and CaV1.3α1 subunits were comparable in amplitude (−1.5 ± 0.1 μA, n = 6; and −1.6 ± 0.2 μA, n = 6, respectively). However, CaV1.3α1currents activate at voltages significantly more negative compared with CaV1.2α1. With 5 mm barium as charge carrier, CaV1.2α1 generates prototypic, high voltage-activated L-type Ca channel currents that begin to activate at approximately −35 mV and peak at ∼0 mV. In contrast, CaV1.3α1currents activate at approximately −55 mV and reach ∼60% of peak current at −40 mV, the very foot of the CaV1.2α1 current–voltage plot (Fig. 1B). The large difference in the voltage dependence of activation between CaV1.2α1 and CaV1.3α1 currents was also observed with 5 mm calcium as the charge carrier (Fig. 1C, D). Interestingly, CaV1.3α1 currents showed more pronounced calcium-dependent inactivation compared with CaV1.2α1 (Fig.1C).
Fig. 1.
Two classes of L-type Ca channel activate at different voltages. A, Individual current traces measured from Xenopus oocytes transiently expressing Cav1.3α1 (thick traces) and Cav1.2α1 (thin traces), together with CaVβ1b, using 5 mm barium as the charge carrier. Currents were activated in response to voltage steps as indicated from a holding potential of −80 mV. B, Averaged, peak current–voltage plots for Cav1.3α1 (●) and Cav1.2α1 (○) channels using 5 mm barium as charge carrier. C, D, same as in A and B, except recordings were obtained using 5 mm calcium as the charge carrier. V1/2 from peak current–voltage plot: with 5 mm barium as the charge carrier, Cav1.3α1 and CaVβ1b, −36.8 ± 0.8 mV (n = 6); Cav1.2α1 and CaVβ1b, −8.8 ± 0.8 mV (n = 6); with 5 mm calcium as the charge carrier, −20.0 ± 2.2 mV (n = 4) and 3.3 ± 2.0 mV (n = 4), respectively. Values are mean ± SE.
There are reports that native L-type Ca currents in select cells can activate at relatively hyperpolarized membrane potentials (Smith et al., 1993; Avery and Johnston, 1996; Kavalali and Plummer, 1996;Platzer et al., 2000), but this is not a feature generally associated with L-type Ca channels (Ertel et al., 2000). Perhaps more perplexing, earlier studies of cloned CaV1.3α1 subunits derived from other tissues did not identify this channel as unique with regard to its voltage dependence of activation (Williams et al., 1992; Ihara et al., 1995; Bell et al., 2001).
A conspicuous difference in our studies of cloned CaV1.3α1 channels compared with others is the use of relatively low concentrations of divalent cations (5–10-fold lower than those typically used previously, 20 and 40 mm barium; Williams et al., 1992;Ihara et al., 1995; Bell et al., 2001). Voltage-gated ion channels, including Ca channels, are strongly influenced by the extracellular concentration of divalent cations as a result of surface charge screening effects (Frankenhaeuser and Hodgkin, 1957; Kostyuk et al., 1982). This led us to test whether higher divalent cation concentrations might obscure the negative activation threshold of CaV1.3α1 channels.
Figure 2 demonstrates that an increase in the extracellular concentration of barium from 2 to 40 mm increases the peak current approximately threefold, but more significantly, the voltage dependence of channel activation shifts toward more depolarized membrane potentials (∼25 mV). Depolarizations to −40 mV evoke sizeable Cav1.3α1 currents with 2 mm extracellular barium, whereas the same depolarization barely activates the channels in 40 mm barium (Fig.2A). A comparison of averaged, normalized peak current–voltage plots reveals an incremental shift in the voltage dependence of Cav1.3α1channel activation with increasing concentrations of barium. These data suggest that CaV1.3α1L-type Ca channels appear to be high-voltage activating when high concentrations of extracellular divalent cations are used.
Fig. 2.
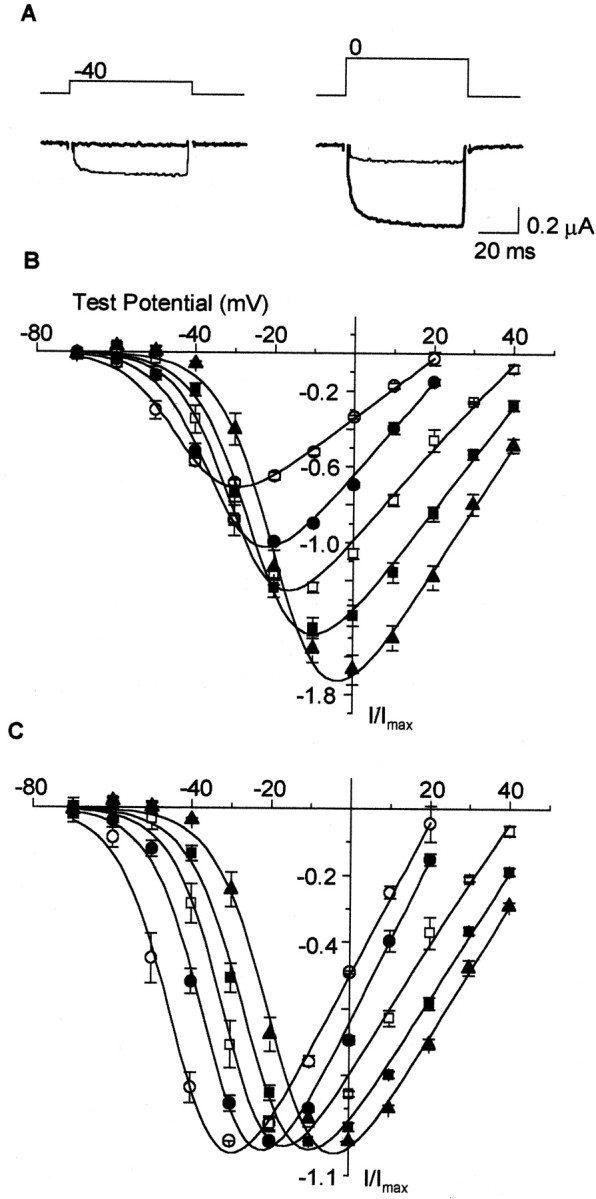
An increase in extracellular divalent cation concentration shifts the voltage dependence of CaV1.3α1 activation to depolarized potentials. A, Individual current traces measured fromXenopus oocytes transiently expressing CaV1.3α1 together with CaVβ1b, using 2 mm barium (thin trace) or 40 mm barium (thick trace) as the charge carrier. B, C, CaV1.3α1 channel currents recorded with 2 mm barium (○), 5 mm barium (●), 10 mm barium (■), 20 mm barium (▪), and 40 mm barium (▴). Values plotted are averaged peak currents measured from each cell normalized to the maximum peak currents recorded with 5 mm barium (B) and averaged peak currents normalized to maximum peak current recorded at each barium concentration (C). V1/2 from peak current–voltage plots: 2 mm barium, −42.7 ± 1.6 mV (n = 5); 5 mm barium, −33.8 ± 0.8 mV (n = 5); 10 mm barium, −29.6 ± 1.8 mV (n = 5); 20 mm barium, −24.7 ± 1.2 mV (n = 4); and 40 mm barium, −18.9 ± 1.6 mV (n = 4).
The data presented in Figure 2 offer one explanation to account for differences between our findings and those reported previously. However, functional diversity exists among Ca channels containing the same CaVα1 subunit because of the potential to associate with different auxiliary subunits and because each CaVα1gene is subject to extensive alternative splicing. Either of these factors at least has the potential to influence the voltage range over which CaV1.3α1 L-type Ca channels activate.
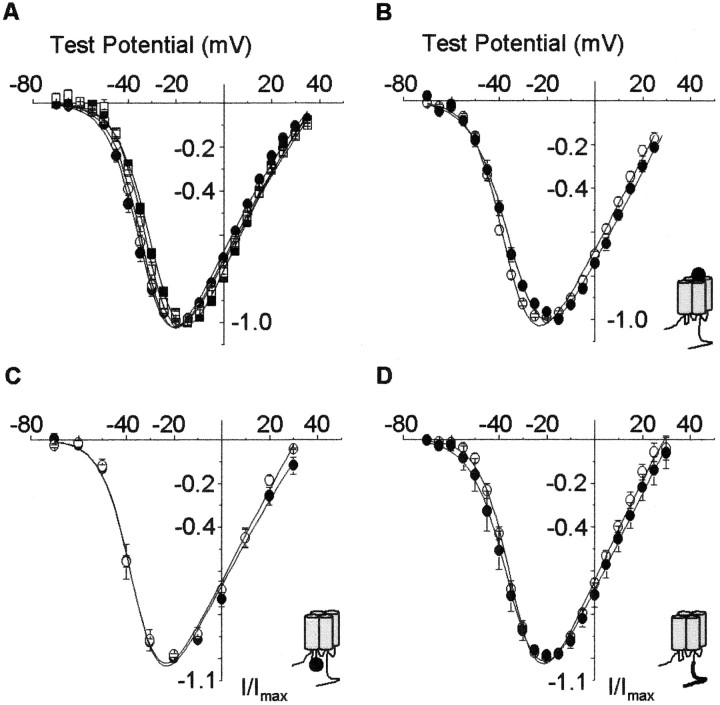
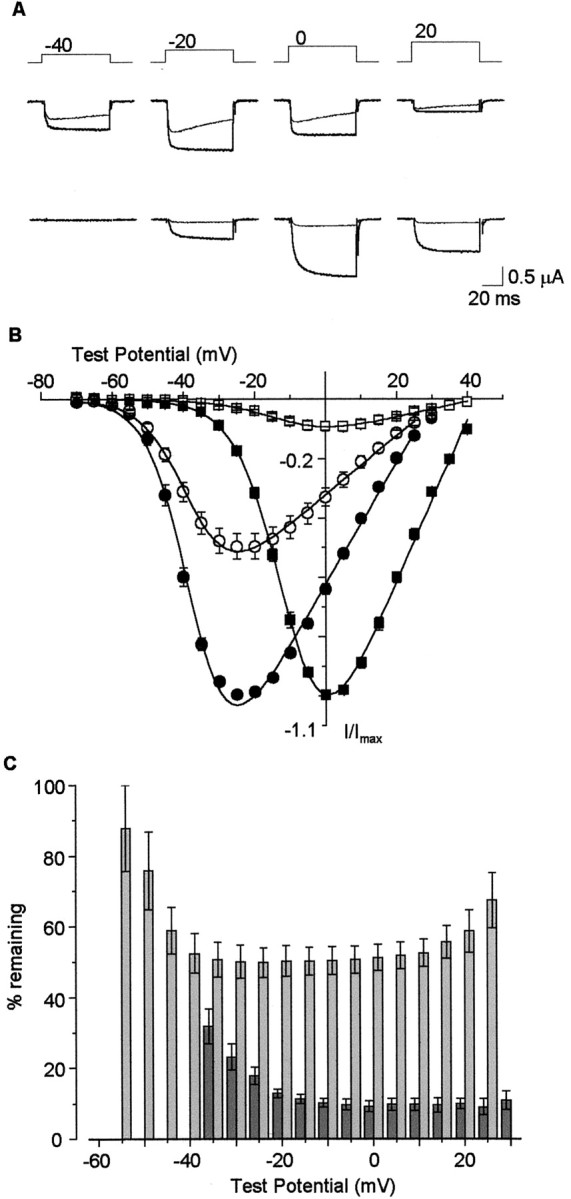
At least four different Cavβ subunits are expressed in mammals. Each Cavβ subunit can associate with multiple Cavα1 subunits, including L-type Cav1α1 subunits,in vitro and in vivo (Pichler et al., 1997;Walker and De Waard, 1998). Cavβ subunits have been shown to differentially modulate channel properties (Walker and De Waard, 1998). Therefore, we analyzed four Cav1.3α1/Cavβ subunit combinations. All four Cavβ subunits facilitated Ca channel current amplitudes compared with Cav1.3α1 alone (see legend to Fig. 3A), consistent with studies of other Cavα1 subunits (Walker and De Waard, 1998). A comparison of normalized current–voltage plots show that all four Cav1.3α1–Cavβ subunit combinations activate at membrane potentials that are at least 20 mV more hyperpolarized than CaV1.2α1 (Fig.3A). We conclude that the association of Cav1.3α1 with any one of four different Cavβ subunits does not account for the large difference in channel activation thresholds that exists between Cav1.2α1 and Cav1.3α1.
Fig. 3.
CaV1.3α1 channels open at relatively hyperpolarized voltages independent of several factors that potentially affect the voltage dependence of activation. Shown are comparisons of normalized, averaged peak current–voltage plots of CaV1.3α1 coexpressed with CaVβ1b (●), CaVβ2a (○), CaVβ3(▪), or CaVβ4 (■) subunits (A); with (●) or without (○) exon 32, which encodes a 15-amino acid sequence, PSDSENIPLPTATPG, in domain IVS3–IVS4 coexpressed with CaVβ1b(B); with (●) or without (○) exon 11, which encodes a 20-amino acid sequence, CWWKRRGAAKTGPSGCRRWG, in loop I–II coexpressed with CaVβ1b(C); and with exon 42 (●) or exon 42a (○) coexpressed with CaVβ1b(D), Ca channel currents were recorded with 5 mm barium as the charge carrier. Maximum, peak current amplitudes induced by coexpressing Cav1.3α1with different CaVβ subunits were −1.3 ± 0.1 μA (n = 7; CaVβ1b), −2.8 ± 0.2 μA (n = 8; CaVβ2a), −1.0 ± 0.1 μA (n = 7; CaVβ3), and −1.0 ± 0.5 μA (n = 4; CaVβ4). Cav1.3α1 alone induced currents with amplitudes of −0.43 ± 0.05 μA (n = 6). Average V1/2 values were: A, −33.0 ± 1.0 mV (n = 7; CaVβ1b), −32.1 ± 0.8 mV (n = 8; CaVβ2a), −28.4 ± 0.5 mV (n = 7; CaVβ3), and −30.0 ± 1.0 mV (n = 4; CaVβ4);B, −35.7 ± 0.5 mV (n = 8; Δexon 32) and −31.7 ± 0.7 mV (n = 5; +exon 32); C, −36.1 ± 1.6 mV (n = 8; Δexon 11) and −35.3 ± 0.6 mV (n = 5; +exon 11); and D, −33.9 ± 1.5 mV (n = 3; +exon42) and −32.9 ± 0.4 mV (n = 5; +exon 42a). Values are mean ± SE.
CaVα2δ, another auxiliary subunit that associates with Cavα1, has the potential to modulate the voltage dependence of channel activation (Wakamori et al., 1999; Platano et al., 2000). We therefore compared peak current–voltage relationships of Ca channels recorded from oocytes expressing Cav1.3α1 and Cavβ1b in the absence and presence of CaVα2δ. The presence of the CaVα2δ subunit did not influence the voltage dependence of Cav1.3α1 current activation (V1/2: Cav1.3α1 and Cavβ1b, −35.3 ± 0.6 mV, n = 6; Cav1.3α1, Cavβ1b, and CaVα2δ, −36.8 ± 1.2 mV, n = 6) (see Fig.4).
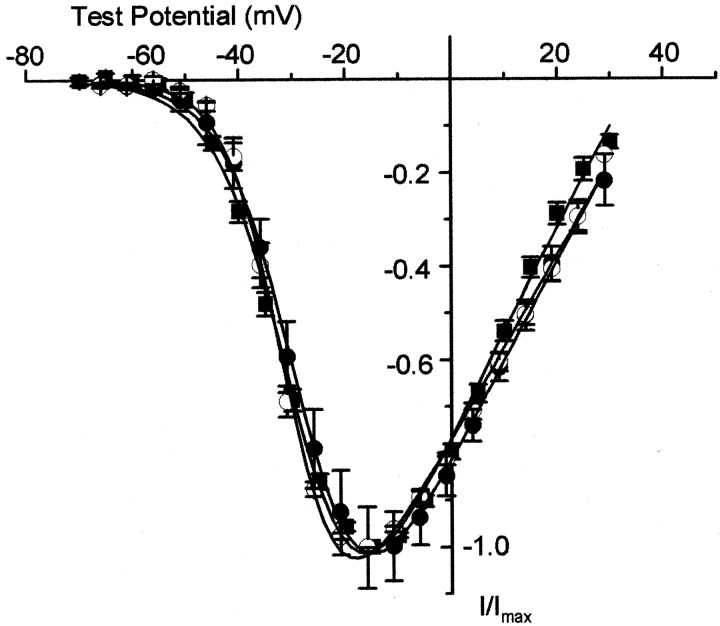
Fig. 4.
CaV1.3α1 channels open at relatively hyperpolarized voltages independent of expression system. Shown are normalized, averaged peak-CaV1.3α1channel current–voltage plots recorded from Xenopusoocytes expressing CaV1.3α1 and CaVβ3 (5 mm Ba, ▪) and tsA-201 cells expressing CaV1.3α1, CaVβ3, and CaVα2δ (5 mm Ba, ●; and 2 mm Ca, ○). Maximum peak currents were −1.0 ± 0.1 μA (n = 7, ▪), −1.2 ± 0.1 nA (n = 3, ●), and −3.3 ± 1.0 nA (n = 4, ○). Average V1/2 values were −28.4 ± 0.5 mV (n = 7, ▪), −26.8 ± 0.7 mV (n = 3, ●), and −31.0 ± 0.8 mV (n = 4; ○), respectively. Values are mean ± SE.
Nascent Cavα1 subunit RNAs, including Cav1.3α1, are subject to extensive alternative splicing (Perez-Reyes et al., 1990; Hui et al., 1991; Williams et al., 1992; Ertel et al., 2000). Alternative splicing occurs throughout the coding region of Cavα1 subunit genes and in some cases has been shown to influence the voltage dependence of Ca channel activation (Lin et al., 1997). Although the extent of alternative splicing in Cav1.3α1 has yet to be determined, we have characterized three regions of the gene that contain exons whose expression is regulated in a tissue-specific manner (Xu and Lipscombe, 2000). Exon 32 encodes 15 amino acids within domain IVS3–IVS4, and exon 11 encodes 20 amino acids in the I–II intracellular loop. Both these exons are alternatively spliced. Exons 42 and 42a in the C terminus are expressed in a mutually exclusive manner. The presence of exon 42a predicts a Cav1.3α1 subunit containing C termini 500 amino acids shorter than exon 42-containing subunits (GenBank accession number AF370010). We constructed four splice variants of Cav1.3α1 that differ in the expression of each exon and compared their properties (Fig.3B–D). The current–voltage relationships of all Cav1.3α1 splice variants were virtually indistinguishable, and all activated at voltages that were ∼20 mV more hyperpolarized than that of Cav1.2α1.
Finally, we studied the properties of Cav1.3α1 channel currents transiently expressed in a mammalian kidney cell line together with CaVα2δ and Cavβ3 (5 mmbarium as charge carrier). Cav1.3α1 L-type currents expressed in tsA-201 cells are indistinguishable from those recorded inXenopus oocytes (Fig. 4). The current–voltage curve of Cav1.3α1 L-type channel currents transiently expressed in tsA-201 cells with 2 mm calcium as the charge carrier is also shown to indicate the probable activation threshold of this current in neurons. Note that the position of the Cav1.3α1 current–voltage curve with 2 mm calcium is comparable with that recorded in 5 mm barium (Fig. 4). This is consistent with previous studies showing that calcium is more effective at shifting the gating of voltage-gated ion channels compared with barium (Hille, 1992).
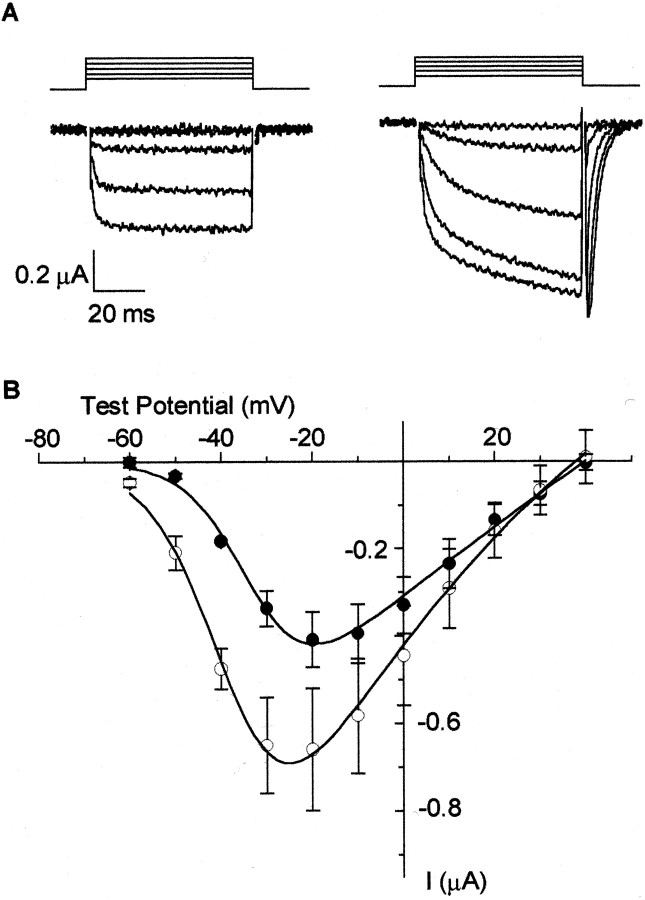
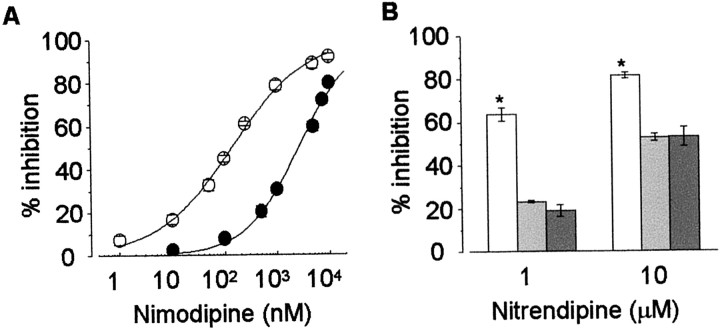
Figures 1-4 demonstrate that L-type Ca channels are not all high voltage-activated as often assumed. However, in addition to biophysical criteria, L-type Ca channels are also, and more often, defined by their high sensitivity to dihydropyridine agonists and antagonists (Bean, 1989; Ertel et al., 2000). We therefore studied the pharmacological sensitivity of Cav1.3α1and Cav1.2α1 to dihydropyridines. Bay K 8644 augmented Cav1.3α1 L-type currents and induced an ∼10 mV shift in the voltage dependence of activation toward more hyperpolarized voltages compared with control (Fig.5). Bay K 8644 also slowed channel activation and deactivation kinetics, consistent with its effects on other Cav 1α1 L-type currents (Grabner et al., 1996). Cav1.3α1 L-type channel currents were also inhibited by the dihydropyridine antagonist nimodipine. The degree of nimodipine-induced inhibition of Cav1.3α1 channel currents, however, differed significantly compared with Cav1.2α1. At 1 μm, nimodipine inhibited ∼90% of the Cav1.2α1 channel current at voltages between −20 and +40 mV, whereas at the same concentration, peak Cav1.3α1 currents were inhibited ∼50% at most (Fig. 6). Nimodipine was less effective at inhibiting both classes of L-type Ca channels at threshold voltages (Fig. 6C), consistent with a state-dependent blocking mechanism (Bean, 1984; Hess et al., 1984). Inhibition of peak Cav1.2α1 currents was, nonetheless, significantly greater compared with Cav1.3α1 at all membrane potentials (Fig. 6B,C). An examination of individual current traces shows that nimodipine induces an acceleration of the decay of the residual Cav1.3α1 current (Fig.6A). However, even after a 60 msec depolarization, ∼30% of the Cav1.3α1current remained unblocked in the presence of 1 μm nimodipine. Figure7A summarizes the pharmacological differences between Cav1.3α1 and Cav1.2α1 channels revealed by nimodipine. Cav1.3α1 channels are ∼20-fold less sensitive to inhibition by nimodipine compared with Cav1.2α1 (Fig.7A). Even at 10 μm, nimodipine did not completely inhibit the Cav1.3α1 current. The relatively low sensitivity of Cav1.3α1 to inhibition by dihydropyridine was confirmed using a second antagonist, nitrendipine (Fig. 7B). At 1 and 10 μm, nitrendipine reduced peak Cav1.2α1 currents by 64 ± 3% (n = 4) and 81 ± 1% (n = 4), respectively, but at these same concentrations only inhibited Cav1.3α1currents by 23 ± 1% (n = 8) and 53 ± 2% (n = 8), respectively. The pharmacological sensitivity of Cav1.3α1 channels to dihydropyridine antagonists was unaffected by the presence or absence of exon 32 in the putative extracellular IVS3–IVS4 linker (Fig.7B). Finally, we tested the effectiveness of the classic N-type Ca channel toxin ω-conotoxin GVIA to inhibit Cav1.3α1 channels. Others have reported that Cav1.3α1 channels are reversibly inhibited by high concentrations of this toxin (Williams et al., 1992). ω-Conotoxin GVIA did not inhibit Cav1.3α1 channel currents at concentrations up to 1 μm (99.0 ± 1.6%; n = 6, compared with control).
Fig. 5.
CaV1.3α1 currents were enhanced by Bay K 8644. A, CaV1.3α1 currents in the absence (left) and presence (right) of 1 μm Bay K 8644. Currents were activated from a holding potential of −80 mV. B, Averaged, peak current–voltage plots of CaV1.3α1 channels recorded with 5 mm barium in control (●) and in the presence of 1 μm Bay K 8644 (○). Maximum peak currents were −0.4 ± 0.1 μA (n = 3, ●) and −0.7 ± 0.1 μA (n = 3, ○). Average V1/2 values were −32.2 ± 1.1 mV in control (n = 3, ●) and −39.0 ± 2.5 mV in the presence of Bay K 8644 (n = 3, ○).
Fig. 6.
CaV1.3α1 currents are partially inhibited by dihydropyridines. A, CaV1.3α1 (top) and CaV1.2α1 (bottom) in the absence (thick traces) and presence (thin traces) of 1 μm nimodipine. Currents were activated by voltage steps as indicated, from a holding potential of −80 mV. B, Comparison of normalized, averaged peak current–voltage plots of CaV1.3α1 and CaV1.2α1 channels in the absence (●, ▪) and presence (○, ■) of 1 μm nimodipine, respectively.C, Peak CaV1.3α1 (light gray) and peak CaV1.2α1 (dark gray) currents remaining in the presence of nimodipine, plotted as percentage of control currents at various test potentials. Values are mean ± SE; n = 6 for CaV1.3α1 and n = 3 for CaV1.2α1.
Fig. 7.
CaV1.3α1 and CaV1.2α1 channels are pharmacologically distinct. A, Dose–response curve of nimodipine inhibition of CaV1.3α1 (●) and CaV1.2α1 (○) channel currents. Data were fit to the Hill equation: (Icontol −Inimodipine)/Icontrol= 1/(1 + (IC50/Cnimodipine)h), where IC50 is the concentration of nimodipine required to inhibit 50% of peak current, and h is the Hill coefficient. CaV1.3α1, IC50 = 2.7 ± 0.3 μm;h = 0.85 ± 0.04 (n = 5); CaV1.2α1, IC50 = 139 ± 12 nm;h = 0.63 ± 0.05 (n = 5). B, Inhibition of CaV1.3α1 (Δexon 32, light gray, n = 8; +exon 32, dark gray, n = 6) and CaV1.2α1 (white,n = 4) by nitrendipine are significantly different at concentrations of 1 and 10 μm. Values are mean ± SE.
DISCUSSION
Cav1.3α1 L-channels open at relatively hyperpolarized membrane potentials
Our results suggest that calcium channels that contain the Cav1.3α1 subunit are not typical high voltage-activated L-type channels, and our study highlights the large difference in activation thresholds (∼25 mV) between the two major classes of L-type channels expressed in neurons. This difference appears to stem from intrinsic differences between Cav1.2α1 and Cav1.3α1 genes, because it is independent of auxiliary subunits, alternative splicing in at least three different domains of the protein, and expression system (Figs. 3, 4). Given the unusually hyperpolarized activation threshold of Cav1.3α1 channels observed in our studies, it is perhaps surprising that this unique property has not been highlighted in previous studies of other Cav1.3α1 clones (Williams et al., 1992; Ihara et al., 1995; Bell et al., 2001). Although we cannot exclude the possibility that specific variants of Cav1.3α1 derived from other tissues or cell lines differ from our clones with respect to activation thresholds, we suggest that the use of high concentrations of extracellular divalent cations in certain studies may have obscured the unusually hyperpolarized activation range of Cav1.3α1 channels. For example, if our data are compared with those of Williams et al. (1992)under similar recording conditions (40 mm barium; Fig. 2), the Cav1.3α1 L-type current–voltage relationships are comparable. With 40 mmbarium, the Cav1.3α1current–voltage relationship is shifted to ∼20 mV more depolarized. Cav1.3α1 L-type currents expressed in HEK293 cells (Bell et al., 2001; 20 mm barium) and Chinese hamster ovary cells (Ihara et al., 1995; 40 mmbarium), however, activate at membrane potentials more depolarized compared with our data and with that of Williams et al. (1992), even after considering the effect of surface charge screening. The reasons for these differences are not apparent, given that Bell et al. (2001)used the same clone as that of Williams et al. (1992), and our results suggest that different expression systems do not influence the voltage dependence of Cav1.3α1L-type channel activation (Fig. 4).
Is there evidence that native L-type Cav1.3α1 currents activate at hyperpolarized membrane potentials? Our studies of cloned Cav1.3α1 channels suggest that they activate at membrane potentials significantly more hyperpolarized than any Cav1α1 or Cav2α1 channel described to date. Cav1.3α1-containing L-type channels begin to activate at approximately −55 mV in the presence of 5 mm barium or 2 mm calcium, not as hyperpolarized as members of the T-type family but significantly more hyperpolarized relative to Cav2.3α1-containing (α1E) channels (Perez-Reyes et al., 1998). Consistent with our studies of cloned channels, certain cells that express high levels of Cav1.3α1, such as hair cells and endocrine cells, contain native L-type Ca channels that activate at membrane potentials close to −50 mV (Smith et al., 1993;Zidanic and Fuchs, 1995; Kollmar et al., 1997). Perhaps most significantly, there is a selective loss of a low threshold-activating component of the whole cell Ca current in inner hair cells in Cav1.3α1-deficient mice (Platzer et al., 2000).
Although complicated by the presence of multiple classes of Ca channels with overlapping properties, there is evidence for the presence of low threshold-activating L-type Ca channels in neurons. For example, a component of low threshold-activating Ca current in CA3 pyramidal neurons of the hippocampus is reduced by high concentrations of dihydropyridines (Avery and Johnston, 1996). Furthermore, L-type Ca currents are thought to underlie spontaneous calcium oscillations that drive intrinsic activity in immature Purkinje cells of the cerebellum (Liljelund et al., 2000). Ca channels that drive spontaneous electrical activity are thought typically to activate at hyperpolarized membrane potentials (Bean, 1989). There is also evidence for the presence of two functionally distinct classes of L-type Ca channels from single-channel recordings in hippocampal neurons that differ significantly in their activation thresholds (Kavalali and Plummer, 1994, 1996). It would be interesting to test whether Cav1.2α1- and Cav1.3α1-containing channels underlie these different channels, because both these genes are expressed in the hippocampus (Hell et al., 1993). Our clone was isolated from sympathetic neurons, suggesting that a low-threshold L-type current exists in these cells. There is a low threshold-activating dihydropyridine- and conotoxin-resistant Ca current in sympathetic neurons that has been described in both whole-cell and single-channel recordings (Boland et al., 1994; Elmslie et al., 1994; Elmslie, 1997). It would be interesting to know whether at least part of the resistant current in sympathetic neurons originates from the activity of Cav1.3α1-containing channels (see below).
Cav1.3α1 calcium channels are less sensitive to dihydropyridine antagonists
High sensitivity to dihydropyridines is the universal indicator for the involvement of L-type calcium channels. However, at concentrations typically assumed to inhibit the majority of L-type Ca channels, ∼50% of the peak Cav1.3α1 channel current remains unblocked in the presence of dihydropyridines (Figs. 6, 7). Support for the presence of native L-type channels in neurons that are incompletely inhibited by dihydropyridine antagonists is not simple to extract from available studies because of the prevalence of multiple classes of non-dihydropyridine-sensitive Ca channels in these cells. However, native L-type Ca currents in hair cells that express a relatively high density of Cav1.3α1 channels are incompletely inhibited by high concentrations of dihydropyridines (Zidanic and Fuchs, 1995; Platzer et al., 2000).
An additional feature of the dihydropyridine block of Cav1.3α1 channels is the altered inactivation kinetics of the residual Ca channel current. Although our study was not designed to address that mechanism, the dihydropyridine-induced inactivation profile is consistent with a state-dependent blocking model described for both native and cloned L-type Ca channels (Bean, 1984; Berjukow et al., 2000). It is of interest, however, that high concentrations of dihydropyridines induce residual Cav1.3α1 L-type currents to inactivate during brief depolarizations, because similar low to mid threshold-activating and -inactivating and drug-resistant Ca channel currents have been described in a variety of neurons (Randall and Tsien, 1995; Avery and Johnston, 1996; Tottene et al., 1996). Such drug-resistant currents (or R-type currents) revealed by a mixture of Ca channel blockers that includes dihydropyridine antagonists have been attributed to the presence of non-L-type Cav2.3α1(α1E) subunits (Randall and Tsien, 1995;Tottene et al., 1996). Cav2.3α1 currents activate at somewhat hyperpolarized membrane potentials, inactivate with a relatively fast time course, and are resistant to various Ca channel blockers, including dihydropyridine antagonists (Ellinor et al., 1993; Randall and Tsien, 1995). However, the prevalence of significant drug-resistant Ca current in neurons of Cav2.3α1-deficient mice suggests that other Cavα1subunits in addition to Cav2.3α1 contribute to this current (Wilson et al., 2000). On the basis of their similarities overall, it is likely that Cav1.3α1-containing channels underlie a significant fraction of the resistant Ca current described in neurons in the presence of Ca channel blockers.
Functional implications
L-type Ca channels have been implicated in regulating gene expression, cell survival, and synaptic plasticity in neurons (Galli et al., 1995; Norris et al., 1998; Mao et al., 1999; Weisskopf et al., 1999) and in supporting exocytosis in hair cells and pancreatic β cells (Ashcroft et al., 1994; Fuchs, 1996). However, our studies suggest that functional and pharmacological criteria currently used to distinguish among different Ca currents probably greatly underestimate the biological importance of L-type Ca channels in neurons expressing Cav1.3α1. The unique functional properties of Cav1.3α1 compared with Cav1.2α1 highlighted in the present study combine with evidence of distinct subcellular localization patterns (Hell et al., 1993) to imply that these two classes of L-type Ca channels may couple to different signaling pathways. Because of their unique properties, Cav1.3α1 channels are likely to be important for mediating Ca influx in response to relatively small membrane depolarizations, and our preliminary analysis of the voltage dependence of steady-state inactivation suggests that Cav1.3α1 L-type channels possess a significant window current at membrane potentials close to −50 mV. Such properties may be important for sustaining spontaneous rhythmic firing in neurons, traditionally attributed to the activity of low-threshold, T-type Ca channels (Bean, 1989; Ertel et al., 2000). Consistent with this, the dihydropyridine antagonist nimodipine partially suppresses spontaneous intracellular calcium oscillations and slows rhythmic firing in postnatal cerebellar Purkinje cells (Liljelund et al., 2000). More direct evidence for the involvement of Cav1.3α1 L-type Ca channels in driving rhythmic activity in excitable cells comes from studies of Cav1.3α1-deficient mice whose phenotype includes compromised sinoatrial node function (Platzer et al., 2000). A selective inhibitor of the Cav1.3α1 L-type current would greatly aid in establishing its contribution to the whole-cell Ca current in neurons and consequently its importance in coupling to downstream signaling pathways.
In summary, analysis of recombinant neuron-derived Cav1.3α1 channels has permitted functional and pharmacological characterization of this class of Ca current, which has been lacking. The unique properties of Cav1.3α1 contrast with the standard view of L-type Ca channels as high voltage-activated and highly sensitive to dihydropyridines and suggests that new functional roles for Cav1.3α1 L-type channels will be forthcoming.
Note added in proof. Properties similar to those described here were reported recently for a Cav1.3α1 subunit isolated from human pancreas (Koschak et al., 2001).
Footnotes
This work was supported by National Institutes of Health Grants NS29967 and NS01927. We thank Dr. Yasuo Mori for providing Cav1.2α1 cDNA, Drs. Kevin Campbell and Derrick Witcher for Cavβ1b cDNA, and Dr. Edward Perez-Reyes for Cavβ2a and Cavβ4 cDNAs. We thank Christopher Thaler and Annette Gray for their helpful comments on this manuscript.
Correspondence should be addressed to Diane Lipscombe, Department of Neuroscience, Brown University, 192 Thayer Street, Providence, RI 02912. E-mail: Diane_Lipscombe@brown.edu.
REFERENCES
- 1.Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55:54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 2.Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beam KG, Tanabe T, Numa S. Structure, function, and regulation of the skeletal muscle dihydropyridine receptor. Ann NY Acad Sci. 1989;560:127–137. doi: 10.1111/j.1749-6632.1989.tb24090.x. [DOI] [PubMed] [Google Scholar]
- 4.Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci USA. 1984;81:6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 6.Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nurnberg B, Dolphin AC. Biophysical properties, pharmacology, and modulation of human, neuronal L-type (α1D, CaV1.3) voltage-dependent calcium currents. J Neurophysiol. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- 7.Berjukow S, Marksteiner R, Gapp F, Sinnegger MJ, Hering S. Molecular mechanism of calcium channel block by isradipine. Role of a drug-induced inactivated channel conformation. J Biol Chem. 2000;275:22114–22120. doi: 10.1074/jbc.M908836199. [DOI] [PubMed] [Google Scholar]
- 8.Boland LM, Morrill JA, Bean BP. ω-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J Neurosci. 1994;14:5011–5027. doi: 10.1523/JNEUROSCI.14-08-05011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellano A, Perez-Reyes E. Molecular diversity of Ca2+ channel beta subunits. Biochem Soc Trans. 1994;22:483–488. doi: 10.1042/bst0220483. [DOI] [PubMed] [Google Scholar]
- 10.Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 11.Elmslie KS, Kammermeier PJ, Jone SW. Reevaluation of Ca2+ channel types and their modulation in bullfrog sympathetic neurons. Neuron. 1994;13:17–28. doi: 10.1016/0896-6273(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 12.Elmslie KS. Identification of the single channels that underlie the N-type and L-type calcium currents in bullfrog sympathetic neurons. J Neurosci. 1997;17:2658–2668. doi: 10.1523/JNEUROSCI.17-08-02658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels [letter, comment]. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 14.Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- 15.Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol (Lond) 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs PA. Synaptic transmission at vertebrate hair cells. Curr Opin Neurobiol. 1996;6:514–519. doi: 10.1016/s0959-4388(96)80058-4. [DOI] [PubMed] [Google Scholar]
- 17.Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci. 1995;15:1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabner M, Wang Z, Hering S, Striessnig J, Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996;16:207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- 19.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 21.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 22.Hui A, Ellinor PT, Krizanova O, Wang JJ, Diebold RJ, Schwartz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the alpha 1 subunit of the voltage-dependent calcium channel. Neuron. 1991;7:35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- 23.Ihara Y, Yamada Y, Fujii Y, Gonoi T, Yano H, Yasuda K, Inagaki N, Seino Y, Seino S. Molecular diversity and functional characterization of voltage-dependent calcium channels (CACN4) expressed in pancreatic beta-cells. Mol Endocrinol. 1995;9:121–130. doi: 10.1210/mend.9.1.7760845. [DOI] [PubMed] [Google Scholar]
- 24.Kavalali ET, Plummer MR. Selective potentiation of a novel calcium channel in rat hippocampal neurones. J Physiol (Lond) 1994;480:475–484. doi: 10.1113/jphysiol.1994.sp020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavalali ET, Plummer MR. Multiple voltage-dependent mechanisms potentiate calcium channel activity in hippocampal neurons. J Neurosci. 1996;16:1072–1082. doi: 10.1523/JNEUROSCI.16-03-01072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc Natl Acad Sci USA. 1997;94:14883–14888. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 28.Kostyuk PG, Mironov SL, Doroshenko PA, Ponomarev VN. Surface charges on the outer side of mollusc neuron membrane. J Membr Biol. 1982;70:171–179. [Google Scholar]
- 29.Liljelund P, Netzeband JG, Gruol DL. L-Type calcium channels mediate calcium oscillations in early postnatal purkinje neurons. J Neurosci. 2000;20:7394–7403. doi: 10.1523/JNEUROSCI.20-19-07394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Haus S, Edgerton J, Lipscombe D. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 1997;18:153–166. doi: 10.1016/s0896-6273(01)80054-4. [DOI] [PubMed] [Google Scholar]
- 31.Lin Z, Lin Y, Schorge S, Pan JQ, Beierlein M, Lipscombe D. Alternative splicing of a short cassette exon in α1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci. 1999;19:5322–5331. doi: 10.1523/JNEUROSCI.19-13-05322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 33.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 34.Mori Y, Niidome T, Fujita Y, Mynlieff M, Dirksen RT, Beam KG, Iwabe N, Miyata T, Furutama D, Furuichi T, Mikoshiba K. Molecular diversity of voltage-dependent calcium channel. Ann NY Acad Sci. 1993;707:87–108. doi: 10.1111/j.1749-6632.1993.tb38045.x. [DOI] [PubMed] [Google Scholar]
- 35.Norris CM, Halpain S, Foster TC. Reversal of age-related alternations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Reyes E, Wei XY, Castellano A, Birnbaumer L. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem. 1990;265:20430–20436. [PubMed] [Google Scholar]
- 37.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 38.Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. Beta subunit heterogeneity in neuronal L-type Ca2+ channels. J Biol Chem. 1997;272:13877–13882. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]
- 39.Platano D, Qin N, Noceti F, Birnbaumer L, Stefani E, Olcese R. Expression of the alpha(2)delta subunit interferes with prepulse facilitation in cardiac L-type calcium channels. Biophys J. 2000;78:2959–2972. doi: 10.1016/S0006-3495(00)76835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 41.Pragnell M, Sakamoto J, Jay SD, Campbell KP. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 42.Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith PA, Ashcroft FM, Fewtrell CM. Permeation and gating properties of the L-type calcium channel in mouse pancreatic beta cells. J Gen Physiol. 1993;101:767–797. doi: 10.1085/jgp.101.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakamori M, Mikala G, Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J Physiol (Lond) 1999;517:659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- 47.Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 49.Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, Lipscombe D. Splice variants of the L-type Ca channel α1D subunit. Soc Neurosci Abstr. 2000;26:101. [Google Scholar]
- 51.Yamada Y, Masuda K, Li Q, Ihara Y, Kubota A, Miura T, Nakamura K, Fujii Y, Seino S, Seino Y. The structures of the human calcium channel alpha 1 subunit (CACNL1A2) and beta subunit (CACNLB3) genes. Genomics. 1995;27:312–319. doi: 10.1006/geno.1995.1048. [DOI] [PubMed] [Google Scholar]
- 52.Zidanic M, Fuchs PA. Kinetic analysis of barium currents in chick cochlear hair cells. Biophys J. 1995;68:1323–1336. doi: 10.1016/S0006-3495(95)80305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]