Abstract
Previous electrophysiological studies suggested that the initiation of behavioral sensitization to cocaine and amphetamine involves a transient increase in AMPA receptor responsiveness in the ventral tegmental area (VTA). To test this, we used in vivomicrodialysis to examine the effects of intra-VTA administration of AMPA (10 μm) and NMDA (100 μm) on dopamine (DA) and glutamate efflux in the VTA and the nucleus accumbens (NAC), an important target of VTA DA neurons. We compared rats treated for 5 d with saline or 5 mg/kg amphetamine and withdrawn for 3 or 10–14 d. After 3 d of withdrawal, intra-VTA AMPA increased both NAC and VTA DA levels to a greater extent in the amphetamine group, whereas NMDA produced similar effects in the saline and amphetamine groups. This enhanced responsiveness to AMPA was no longer evident in rats tested 10–14 d after the last injection. In addition, intra-VTA AMPA but not NMDA increased both VTA and NAC glutamate levels in rats tested 3 d after the last injection of amphetamine but not in saline controls. After 10–14 d, the responsiveness of glutamate levels to AMPA was no longer evident in the NAC but persisted in the VTA. Additional studies indicated that the glutamate effect in the NAC may involve increased responsiveness of DA receptors within the NAC. These findings establish an in vivo animal model with which to explore the consequences of repeated drug administration for AMPA receptor plasticity in the VTA. They also indicate that repeated amphetamine leads to potentiated interactions between DA and glutamate transmission.
Keywords: amphetamine, AMPA receptors, behavioral sensitization, microdialysis, nucleus accumbens, plasticity, ventral tegmental area
Repeated amphetamine administration leads to a progressive augmentation of many behavioral effects of the drug (behavioral sensitization). Sensitization is of interest as a model for drug-induced neuroplasticity in neuronal circuits important for addiction. In rodents, sensitization is observed as a progressive augmentation of locomotor activity that may relate to an increase in the incentive to obtain drugs (Robinson and Berridge, 1993; Lorrain et al., 2000). There is also evidence of sensitization in human drug users (Satel et al., 1991) and normal subjects (Strakowski and Sax, 1998).
Previous studies have shown that amphetamine sensitization is initiated by drug actions within the ventral tegmental area (VTA; Kalivas and Weber, 1988; Vezina and Stewart, 1990). The VTA contains dopamine (DA) neurons, which project to the nucleus accumbens (NAC) and other corticolimbic regions and are thought to constitute the anatomical substrate for drugs of abuse. Glutamate projections to the VTA are important in determining the activity of VTA DA neurons (White, 1996), and considerable evidence suggests that sensitization is triggered by changes in glutamate transmission within the VTA (Wolf, 1998).
Previous electrophysiological studies demonstrated an increase in the responsiveness of VTA DA neurons to the excitatory effects of AMPA in rats tested shortly after discontinuing repeated amphetamine or cocaine administration (White et al., 1995; Zhang et al., 1997). By increasing excitatory drive, enhancement of AMPA transmission could account for the transient increase in DA cell activity thought to be critical in “transferring” sensitization to forebrain regions such as the NAC that are important in its maintenance and expression (Wolf, 1998). We therefore hypothesized that the induction of behavioral sensitization is associated with a transient long-term potentiation (LTP)-like potentiation of AMPA transmission onto VTA DA neurons. This hypothesis is supported by recent studies in brain slices demonstrating that VTA DA neurons exhibit LTP and long-term depression (LTD) and that amphetamine modulates LTD (Bonci and Malenka, 1999; Overton et al., 1999; Jones et al., 2000; Thomas et al., 2000).
Although in vitro studies are essential for characterizing synaptic plasticity in the VTA, it is well established that the induction of sensitization involves complex neuronal circuitry (Wolf, 1998). It is therefore desirable to develop an animal model, preferably in awake rats, to study AMPA receptor plasticity after chronic drug administration. On the basis of previous results demonstrating enhanced electrophysiological responsiveness of VTA DA neurons to AMPA (above), we predicted that increased AMPA receptor responsiveness should be detectable in microdialysis experiments as an increase in the ability of AMPA to drive DA cells and thus elicit DA release in the NAC. A dual-probe procedure allowed us to monitor the ability of a low dose of AMPA, administered directly into the VTA, to modulate extracellular levels of DA and glutamate both locally within the VTA and within the ipsilateral NAC. We found evidence of potentiated AMPA transmission within the VTA, as expected, and also found striking changes in the ability of DA cell activity to modulate glutamate transmission in the NAC and VTA.
MATERIALS AND METHODS
Subjects. Male Sprague Dawley rats (Harlan, Indianapolis, IN), weighing 200–225 gm at the beginning of the experiment, were used. After 3 d of adaptation, one group of animals was treated with amphetamine (5 mg/kg, i.p.), and another group was treated with saline (1 mg/kg, i.p.) once a day for 5 consecutive days. This amphetamine regimen produces robust behavioral sensitization that is evident throughout the withdrawal period examined in the present study (Wolf and Jeziorski, 1993). Probes were implanted 48 hr after the last injection, and the microdialysis experiment was performed 24 hr after the probe implantation surgery. The animals, maintained on a 12 hr light/dark cycle, were housed two or three per cage before surgery and individually after surgery. Food and water were available ad libitum. All experimental procedures were performed in strict accordance with the National Institutes of HealthGuide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Surgery. Before surgery, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.; Abbott Laboratories, North Chicago, IL) and then placed in a stereotaxic frame. Microdialysis probes were implanted ipsilaterally in the right NAC and VTA at the following coordinates relative to bregma and the dura: anteroposterial (AP), 1.7; lateral (L), 1.0; and dorsoventral (DV), −8 (for the right NAC); and AP, −5.8; L, 0.5; and DV, −9 (for the right VTA). NAC probes had 2 mm of exposed membrane, whereas VTA probes had 1 mm of exposed membrane. These coordinates were determined according to the atlas of Paxinos and Watson (1997). Dialysis probes were prepared using 23 gauge stainless steel and silica capillary tubing. The dialysis fiber (inner diameter, 0.22 mm; outer diameter, 0.31 mm; 2 mm long; Dasco) was prepared from a polyacrilonitrile-sodium methalyl sulfonate copolymer with a molecular weight cutoff of ≥15,000 Da.
In vivo microdialysis. Twenty-four hours after probe implantation, saline- or amphetamine-pretreated rats were connected to a microperfusion pump (CMA 100; CMA Microdialysis, North Chelmsford, MA) with a plastic syringe (1 ml vol; Becton Dickinson, Mountain View, CA). The NAC and VTA were perfused simultaneously using artificial CSF with the following composition (in mm): NaCl, 145; KCl, 2.7; MgCl2, 1; CaCl2, 1.2; and Na2HPO4, 2, pH 7.40. The perfusion rate was set at 2 μl/min with a collection time for each sample of 30 min, such that 60 μl of perfusate was collected. The sample was immediately split for separate HPLC analysis of glutamate and DA; 40 μl was used to determine DA levels, and 20 μl was used for glutamate analysis. However, treatment group numbers for DA and glutamate levels are not always equivalent, primarily because of problems with chromatography for some glutamate samples. In a second study (see Fig. 6), additional saline- and amphetamine-treated rats were implanted with a single probe in the right NAC to investigate the effects of local administration of DA and DA agonists (SKF 38393 and quinpirole) on extracellular glutamate levels.
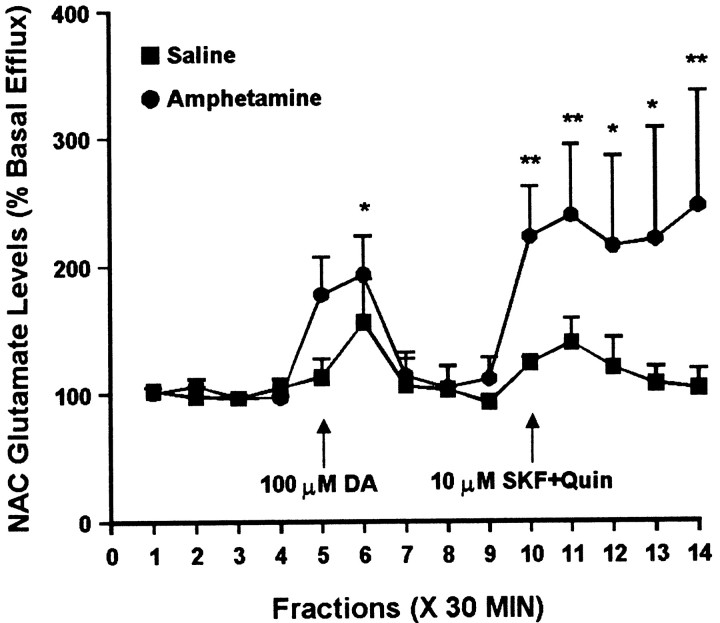
Fig. 6.
Effect of intra-NAC administration of 100 μm DA and a combination of 10 μm SKF 38393 and 10 μm quinpirole on glutamate efflux from the NAC of amphetamine- and saline-pretreated rats tested 3 d after the last injection. DA was administered for 30 min during sample5. The combination of SKF 38393 and quinpirole was administered for 30 min during sample 10, after recovery from the effects of DA perfusion. One-way ANOVA with time as the repeated measure indicated a significant change in glutamate efflux over time within the amphetamine group (F(13,52) = 2.26; p< 0.05) but not the saline group (F(13,39) = 1.36; p= 0.22). Within the amphetamine group, contrast analysis revealed a statistically significant difference between the weighted means of the baseline samples (1–4) and the post-DA samples (5, 6) (p < 0.05, indicating a significant effect of DA), no difference between baseline samples (1–4) and “post-DA baseline samples” (7–9) (p = 0.76, indicating recovery of baseline after termination of DA perfusion), and a significant difference between baseline samples (1–4) and post-SKF+Quin samples (11–14) (p < 0.001, indicating a significant effect of the drug combination). Contrast analysis comparing the weighted mean of the baseline samples with individual postdrug samples indicated significantly higher efflux of glutamate for samples 6 and 10–14(**p < 0.01; *p < 0.05). Basal glutamate levels were 16.5 ± 1.0 pg/μl in the saline group (n = 4) and 13.4 ± 0.8 pg/μl in the amphetamine group (n = 5) and did not differ significantly between groups.
Measurement of DA levels in microdialysis samples. The perfusate was assayed for DA content by reverse-phase HPLC coupled with electrochemical detection. The mobile phase was composed of 75 mm NaH2PO4, 1.5 mm SDS, 25 μm EDTA, 15% acetonitrile, and 12.5% methanol; this was adjusted to pH 5.6 with sodium hydroxide. The mobile phase was delivered at a flow rate of 1 ml/min (model 582, Solvent Delivery Module; ESA, Chelmsford, MA) through a HR-80 column (C18, 4.6 × 80 mm, 3 μm; ESA). DA was detected using a coulometric detector (Coulochem II, 5200A; ESA) coupled to a dual electrode analytical cell (model 5014B; ESA) tailored for use in the analysis of microdialysates. The potential applied to the working electrode was 150 mV; under these conditions, the limit of detection of DA was ∼2–3 fmol. Data were taken by a chart recorder (model RYYT; Bioanalytical Systems, West Lafayette, IN) and quantified using DA standards at different concentrations.
Measurement of glutamate levels in microdialysis samples.Analysis of amino acids was performed as described previously (Xue et al., 1996). An internal standard (carboxymethylcysteine; Sigma, St. Louis, MO) was added after collection, and dialysates were frozen (−80°C) for 1–7 d before analysis. Precolumn derivitization witho-pthalaldehyde and β-mercaptoethanol was performed by an autoinjector (SIL-10A; Shimadzu Scientific Instruments, Columbia, MD) as described by Donzanti and Yamamoto (1988). Samples in the autoinjector were maintained at 14°C by a Peltier thermoelectric sample cooler. The sample and reagent were allowed to react for 2 min. Then a portion of the mixture was injected onto a Primesphere 5 μm C18-HC column (100 × 4.6 mm; Phenomonex) fitted with a Primesphere guard column (30 × 4.6 mm). The mobile phase was 0.1m phosphate buffer containing 0.01m EDTA, pH 6.35. Acetonitrile was used as the organic eluent, with a gradient profile of 13–28%. Amino acid derivatives were detected using an RF-10A fluorescence detector with excitation and emission wavelengths set at 325 and 425 nm, respectively. Data were taken by a personal computer using EZChrom 1-2 software and quantified on the basis of peak area by comparison with standards injected throughout the run. Alanine was measured as a control (nontransmitter) amino acid. Data were used only if alanine levels remained relatively stable during the experiment (i.e., the first and last samples were within 15% of the mean).
Histological analysis. At the completion of microdialysis experiments, the animals were given an overdose of pentobarbital and perfused through the heart with PBS followed by 10% formalin and isotonic saline. Brain coronal slices (100 μm) were stained with cresyl violet, and probe placement was determined by light microscopy in the NAC and VTA. Rats were included in data analysis only if at least 80% of the active region of the microdialysis membrane was located within the anatomical borders of the targeted site. Probe locations were nearly identical to those described and illustrated in our previous studies (Xue et al., 1996; Wolf and Xue, 1998, 1999). NAC probes were located mainly in the shell, although some sampled both core and shell. Most VTA probes were located between AP −5.8 to −5.3 and L 0.6–0.9. Any rat with the VTA probe ≥1 mm lateral to the midline was not included. In our previous electrophysiological studies showing that VTA DA neurons recorded from amphetamine-sensitized rats exhibit increased responsiveness to glutamate and AMPA, recordings were made from 0.2 to 1.0 L at approximately the same AP coordinates used in the present study (White et al., 1995; Zhang et al., 1997). Thus, microdialysis probes in the present study were sampling the same area of VTA recorded in previous electrophysiological studies.
Data analysis. Basal DA and glutamate levels for each rat were defined as the average of the first four samples obtained before AMPA administration in the VTA (or other experimental manipulation). Data are expressed as percentage of baseline. All statistical analysis was done with SuperAnova software (Abacus Concepts, Berkeley, CA). Within-group analyses were conducted by one-way ANOVA with time as the repeated measure to determine whether a treatment had a significant effect within each experimental group (saline and amphetamine). If a significant effect was found, specific samples within a group were compared by contrast analysis (protected t tests that enabled comparison of the weighted mean of baseline samples with an experimental sample or with the weighted mean of a group of experimental samples). Saline and amphetamine groups were compared using two-way ANOVA (group × time) with repeated measures on one variable (time). Post hoc between-group comparisons of specific samples were performed using t tests with Bonferroni corrections. In all cases, significance was set atp < 0.05.
Drugs. (+)-Amphetamine sulfate was purchased from Sigma-Aldrich. Amphetamine dose refers to the salt weight. DA and AMPA were obtained from Sigma-Aldrich. SKF 38393, quinpirole, and NMDA were purchased from Research Biochemicals-Sigma (Natick, MA).
RESULTS
Effect of intra-VTA administration of AMPA or NMDA on DA efflux in the NAC
Previous electrophysiological studies showed that the excitatory effect of AMPA on VTA DA cell activity was greater in rats that had received repeated amphetamine or cocaine injections, compared with saline controls, and that this effect was evident at a short withdrawal time (3 d) but not a longer withdrawal time (10–14 d; Zhang et al., 1997). One goal of the present study was to determine whether similar results could be obtained with microdialysis using DA efflux in the NAC as a measure of mesoaccumbens DA cell activity. Figure1 shows results from rats treated repeatedly with saline or amphetamine and tested 3 d after the last injection. Local administration of 10 μm AMPA in the ipsilateral VTA elicited an increase in DA efflux from the NAC of both experimental groups, with peak levels reached 30–60 min after the onset of AMPA perfusion. However, the magnitude of the increase in DA efflux was considerably greater in the amphetamine group. Figure2 shows results from different saline and amphetamine groups tested 10–14 d after the last injection. At this later withdrawal time, DA efflux in the NAC was increased to a similar extent in both groups by intra-VTA AMPA. In electrophysiological studies, the increased responsiveness seen after 3 d of withdrawal was specific to AMPA, because responses to intra-VTA NMDA were not different between the saline and amphetamine groups (Zhang et al., 1997). Similarly, we found no difference between the saline and amphetamine groups in the effect of intra-VTA administration of 100 μm NMDA on DA efflux in the ipsilateral NAC (Fig.3). This concentration of NMDA was chosen on the basis of a previous microdialysis study (Karreman et al., 1996).
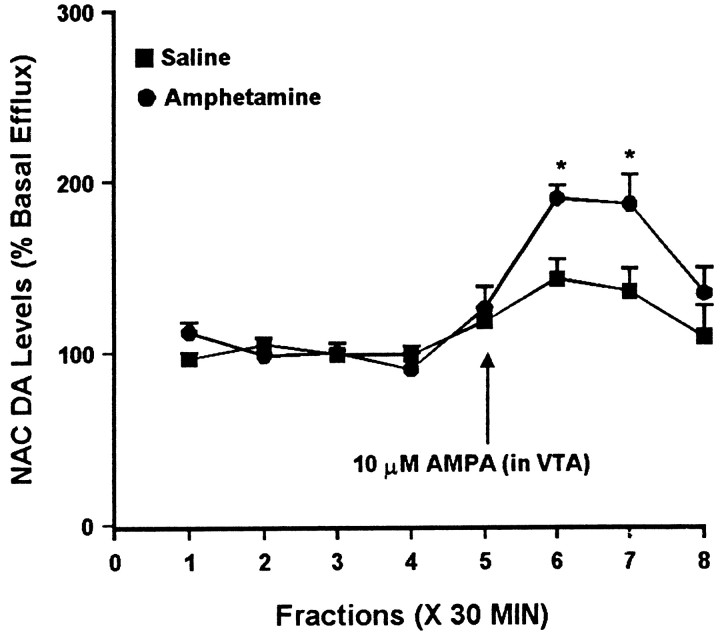
Fig. 1.
Effect of local administration of 10 μm AMPA into the VTA on DA efflux from the ipsilateral NAC of amphetamine- and saline-pretreated rats tested 3 d after the last injection. In this and all subsequent figures, the first four samples were used to calculate basal efflux, and values are expressed as a percentage of the mean basal efflux. AMPA was administered locally into the VTA for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in DA efflux over time in both the saline group (F(7,42) = 4.36; p< 0.01) and the amphetamine group (F(7,28) = 15.86; p< 0.001). Contrast analysis indicated significant differences between the weighted means of baseline samples (1–4) and post-AMPA samples (5–8) for both groups (p < 0.001). In the saline group, individual post-AMPA samples 6 and 7differed significantly from baseline (p < 0.001). In the amphetamine group, individual samples 5(p < 0.05), 6 and7 (p < 0.001), and8 (p < 0.01) differed from baseline. Two-way ANOVA with time as the repeated measure indicated a significant difference between the amphetamine and saline groups (group × time effect, F(7,70) = 3.11; p < 0.01). Post hoccomparisons of individual samples between the saline and amphetamine groups revealed significantly higher DA efflux in the amphetamine group for samples 6 and 7(*p < 0.05, t tests with Bonferroni corrections). In this and all subsequent figures, the results are expressed as mean ± SEM. Basal DA levels were 63.2 ± 5.3 fmol/sample in the saline group (n = 7) and 50.1 ± 6.5 fmol/sample in the amphetamine group (n = 5) and did not differ significantly between groups.
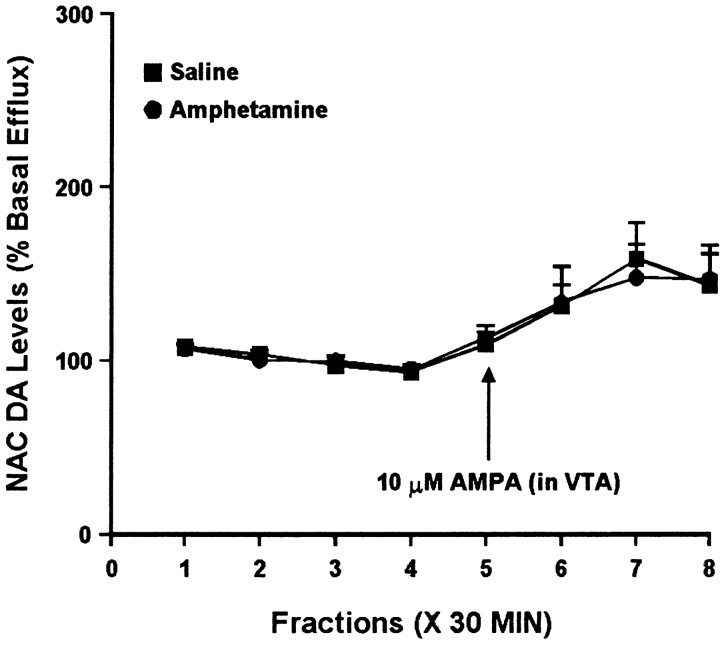
Fig. 2.
Effect of local administration of 10 μm AMPA into the VTA on DA efflux from the ipsilateral NAC of amphetamine- and saline-pretreated rats tested 10–14 d after the last injection. AMPA was administered locally into the VTA for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in DA efflux over time in both the saline group (F(7,35) = 5.08; p < 0.001) and the amphetamine group (F(7,28) = 4.70; p< 0.01). Contrast analysis indicated significant differences between the weighted means of baseline samples (1–4) and post-AMPA samples (5–8) for both groups (p < 0.001). Two-way ANOVA with time as the repeated measure indicated no significant difference between the amphetamine and saline groups (group × time effect,F(7,63) = 0.11; p = 0.99). Basal DA levels were 34.1 ± 6.2 fmol/sample in the saline group (n = 6) and 37.6 ± 3.6 fmol/sample in the amphetamine group (n = 5) and did not differ significantly between groups.
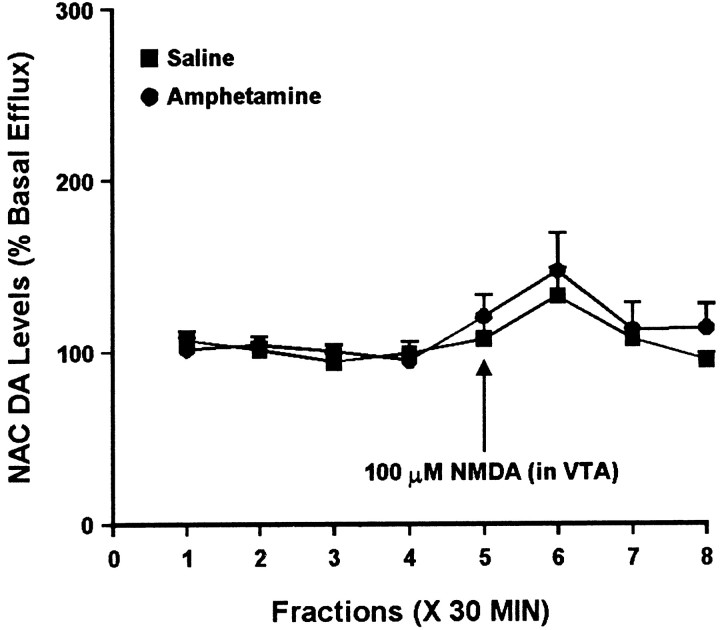
Fig. 3.
Effect of local administration of 100 μm NMDA into the VTA on DA efflux from the ipsilateral NAC of amphetamine- and saline-pretreated rats tested 3 d after the last injection. NMDA was administered locally into the VTA for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a trend toward a significant change in DA efflux over time in both the saline group (F(7,28) = 2.32; p= 0.053) and the amphetamine group (F(7,28) = 2.31; p= 0.054). Two-way ANOVA with time as the repeated measure indicated no significant difference between the amphetamine and saline groups (group × time effect, F(7,56) = 0.41; p = 0.89). Basal DA levels were 46.5 ± 4.5 fmol/sample in the saline group (n = 5) and 40.9 ± 4.9 fmol/sample in the amphetamine group (n = 5) and did not differ significantly between groups.
Effect of intra-VTA administration of AMPA or NMDA on glutamate efflux in the NAC
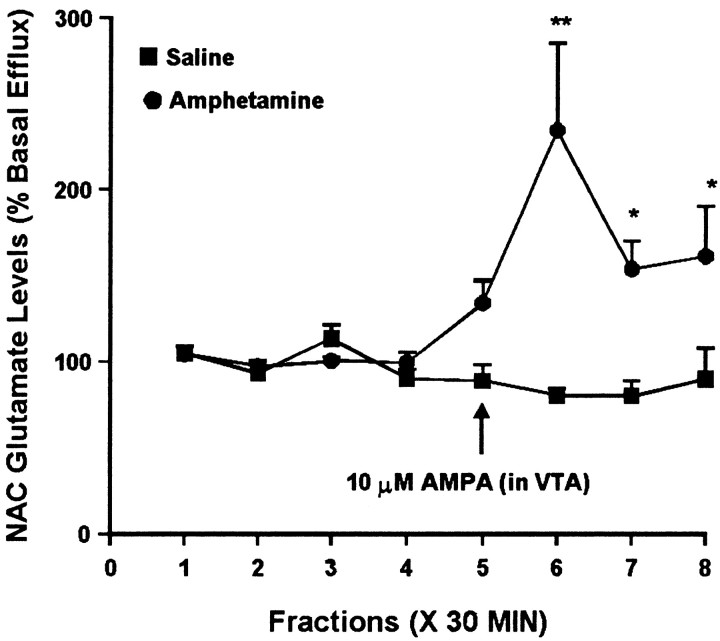
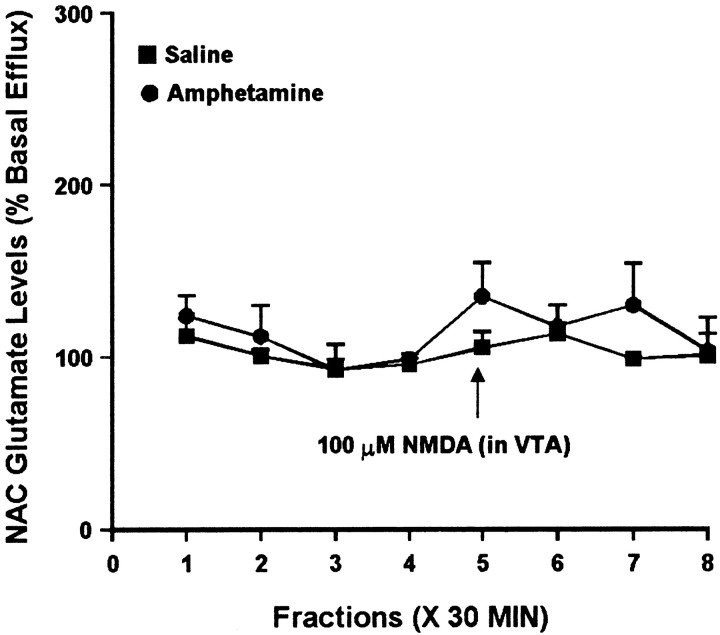
Because some studies have reported modulation of NAC glutamate efflux by DA agonists, it was of interest to see whether the difference in NAC DA levels observed in saline and amphetamine groups after 3 d of withdrawal was associated with a difference in NAC glutamate levels. Thus, NAC samples obtained at this withdrawal time were split to also enable analysis of extracellular glutamate levels. Administration of 10 μm AMPA into the VTA had no effect on NAC glutamate efflux in the saline group. However, in the amphetamine group, intra-VTA AMPA elicited a robust increase in extracellular glutamate levels in the ipsilateral NAC (Fig.4). Intra-VTA NMDA did not significantly alter glutamate efflux in the NAC of either group (Fig.5).
Fig. 4.
Effect of local administration of 10 μm AMPA into the VTA on glutamate efflux from the ipsilateral NAC of amphetamine- and saline-pretreated rats tested 3 d after the last injection. AMPA was administered locally into the VTA for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in glutamate efflux over time in the amphetamine group (F(7,56) = 4.85; p< 0.001) but not the saline group (F(7,35) = 1.38; p= 0.24). Within the amphetamine group, contrast analysis indicated significant differences between the weighted means of baseline samples (1–4) and post-AMPA samples (5–8) (p < 0.001). Contrast analysis comparing the weighted mean of baseline samples to individual post-AMPA samples indicated significant increases for samples6–8 (**p < 0.001; *p < 0.05). Basal glutamate levels were 22.1 ± 3.5 pg/μl in the saline group (n = 6) and 23.8 ± 3.3 pg/μl in the amphetamine group (n = 9) and did not differ significantly between groups.
Fig. 5.
Effect of local administration of 100 μm NMDA into the VTA on glutamate efflux from the ipsilateral NAC of amphetamine- and saline-pretreated rats tested 3 d after the last injection. NMDA was administered locally into the VTA for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated no significant change in glutamate efflux over time in the saline group (F(7,21) = 0.82; p= 0.59) or the amphetamine group (F(7,21) = 0.92; p= 0.51). Two-way ANOVA with time as the repeated measure indicated no significant difference between the amphetamine and saline groups (group × time effect, F(7,42) = 0.47; p = 0.85). Basal glutamate levels were 21.9 ± 4.8 pg/μl in the saline group (n = 4) and 5.7 ± 0.8 pg/μl in the amphetamine group (n = 4) and did not differ significantly between groups.
A number of mechanisms could explain the ability of intra-VTA AMPA to influence extracellular glutamate levels in the NAC of the amphetamine group. To determine whether this effect might involve a change in responsiveness to DA receptor stimulation within the NAC, we investigated the effects of DA agonists, applied directly to the NAC by reverse dialysis, on glutamate efflux in saline- and amphetamine-pretreated rats (Fig. 6). Local administration of DA (100 μm) for 30 min elicited a significant increase in glutamate efflux from the NAC of amphetamine-treated rats, whereas the saline group exhibited a trend toward increased glutamate efflux that did not attain statistical significance. After recovery from the effects of DA perfusion, the NAC of the same rats was perfused with a combination of the D1 receptor agonist SKF 38393 and the D2 receptor agonist quinpirole, each at a concentration of 10 μm. The combined administration of D1 and D2 agonists elicited a sustained increase in glutamate efflux from the NAC of amphetamine-treated rats, whereas no significant increase was observed in the NAC of control rats (Fig. 6).
Effect of intra-VTA administration of AMPA or NMDA on DA and glutamate efflux in the VTA
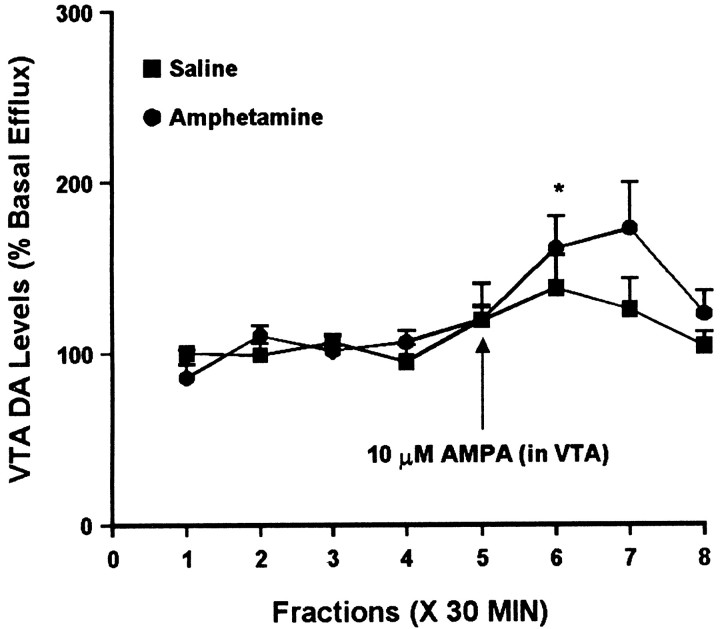
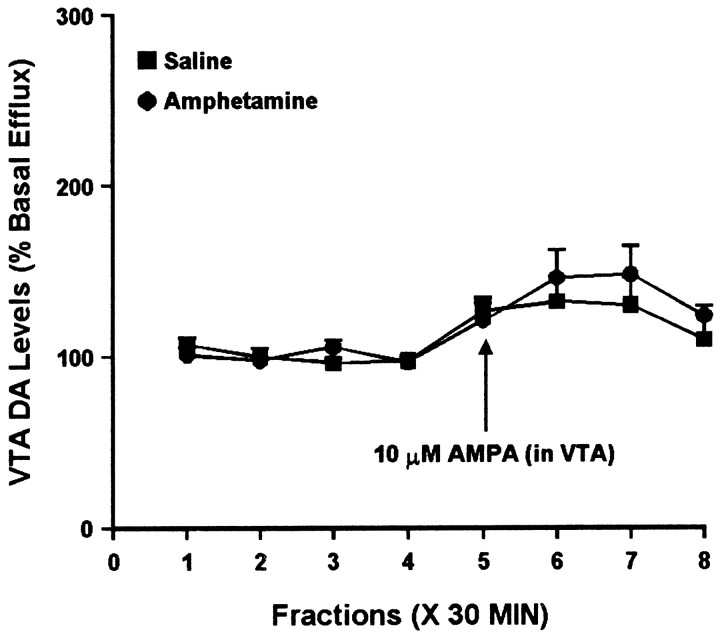
To determine whether AMPA regulated somatodendritic DA efflux differently in the saline and amphetamine groups, we compared the effects of intra-VTA AMPA (10 μm AMPA for 30 min) on extracellular DA levels in the VTA. Figure7 shows results from experiments performed 3 d after the last injection. One-way ANOVA indicated a significant increase in DA levels over time in the amphetamine group, whereas the saline group showed a similar trend that did not attain statistical significance. This suggests an augmented response in the amphetamine group that parallels the effect observed in the NAC (see above). However, two-way ANOVA failed to show a significant difference between the saline and amphetamine groups. In different groups of rats tested 10–14 d after the last injection, both saline- and amphetamine-treated rats showed a significant increase in DA levels as a result of AMPA administration, similar in magnitude to that observed in the saline group at the 3 d withdrawal time. Two-way ANOVA did not indicate a difference between the amphetamine and saline groups (Fig. 8).
Fig. 7.
Effect of local administration of 10 μm AMPA on DA efflux from the VTA of amphetamine- and saline-pretreated rats tested 3 d after the last injection. AMPA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in DA efflux over time within the amphetamine group (F(7,28) = 5.38; p< 0.001) but not the saline group (F(7,21) = 1.43; p= 0.25). Contrast analysis within the amphetamine group revealed a statistically significant difference between the weighted means of the baseline samples (1–4) and the post-AMPA samples (5–8) (p < 0.001). Contrast analysis comparing the weighted mean of the baseline samples with individual post-AMPA samples (5–8) indicated a significant increase in sample 6 (*p< 0.001). Basal DA levels were 31.7 ± 2.9 fmol/sample in the saline group (n = 4) and 37.0 ± 4.1 fmol/sample in the amphetamine group (n = 5) and did not differ significantly between groups.
Fig. 8.
Effect of local administration of 10 μm AMPA on DA efflux from the VTA of amphetamine- and saline-pretreated rats tested 10–14 d after the last injection. AMPA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in DA efflux over time within the saline group (F(7,35) = 2.46; p< 0.05) and the amphetamine group (F(7,42) = 5.92; p< 0.001). Contrast analysis within the saline group revealed a statistically significant difference between the weighted mean of the baseline samples (1–4) and individual post-AMPA samples (5–7) (p < 0.05). Within the amphetamine group, there was a significant difference for samples 5–8 (p < 0.05). However, two-way ANOVA did not indicate a difference between the two groups (F(7,77) = 0.59;p = 0.77). Basal DA levels were 20.8 ± 1.8 fmol/sample in the saline group (n = 6) and 18.3 ± 1.7 fmol/sample in the amphetamine group (n = 7) and did not differ significantly between groups.
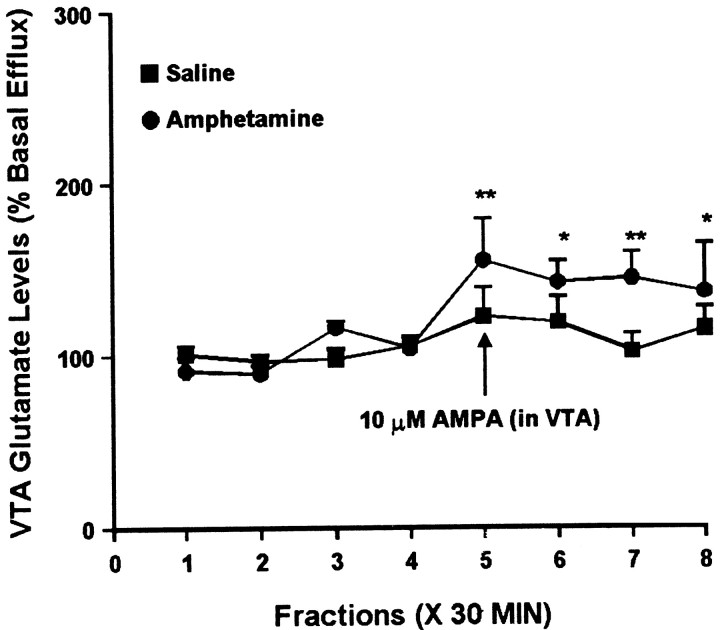
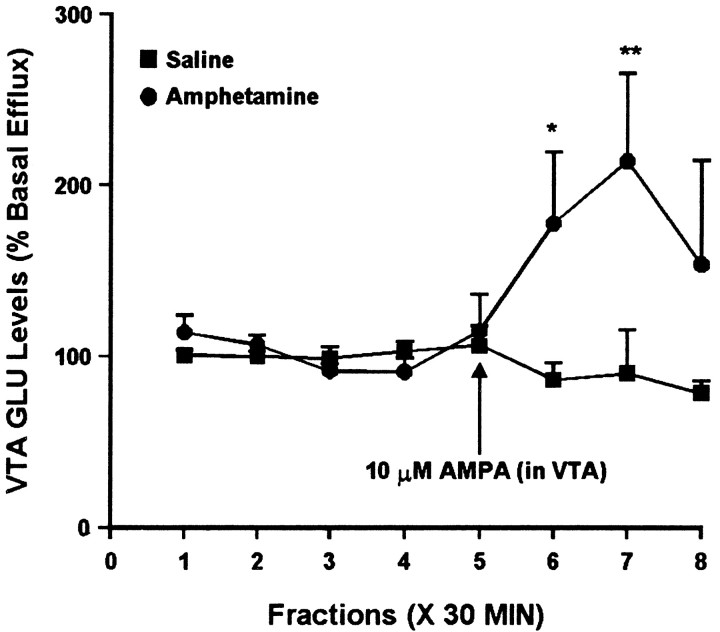
The same samples were analyzed for glutamate to determine the effect of intra-VTA AMPA administration on glutamate efflux in the VTA. In studies performed 3 d after the last injection, a small difference was observed between the saline and amphetamine groups. One-way ANOVAs indicated that intra-VTA AMPA had no effect on glutamate efflux in the VTA of saline-treated rats but did significantly alter glutamate efflux in the amphetamine group. However, two-way ANOVA did not indicate a significant group difference (Fig. 9). After 10–14 d of withdrawal, intra-VTA AMPA was without effect in the saline group but produced a robust increase in the amphetamine group (Fig. 10).
Fig. 9.
Effect of local administration of 10 μm AMPA on glutamate efflux from the VTA of amphetamine- and saline-pretreated rats tested 3 d after the last injection. AMPA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in glutamate efflux over time within the amphetamine group (F(7,49) = 3.32; p< 0.01) but not the saline group (F(7,42) = 1.72; p= 0.13). Within the amphetamine group, contrast analysis revealed a statistically significant difference between the weighted means of the baseline samples (1–4) and the post-AMPA samples (5–8) (p < 0.001). Contrast analysis comparing the weighted mean of the baseline samples with individual post-AMPA samples (5–8) indicated significantly higher efflux of glutamate for samples5–8 (**p < 0.01; *p < 0.05). Basal glutamate levels were 18.8 ± 2.7 pg/μl in the saline group (n = 7) and 18.0 ± 2.3 pg/μl in the amphetamine group (n = 8) and did not differ significantly between groups.
Fig. 10.
Effect of local administration of 10 μm AMPA on glutamate efflux from the VTA of amphetamine- and saline-pretreated rats tested 10–14 d after the last injection. AMPA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in glutamate efflux over time within the amphetamine group (F(7,35) = 2.59; p< 0.05) but not the saline group (F(7,35) = 0.78; p= 0.61). Within the amphetamine group, contrast analysis revealed a statistically significant difference between the weighted means of the baseline samples (1–4) and the post-AMPA samples (5–8) (p < 0.01). Contrast analysis comparing the weighted mean of the baseline samples with individual post-AMPA samples (5–8) indicated significantly higher efflux of glutamate for samples 6(*p < 0.05) and 7(**p < 0.001). Basal glutamate levels were 16.5 ± 2.4 pg/μl in the saline group (n = 6) and 11.5 ± 1.3 pg/μl in the amphetamine group (n = 6) and did not differ significantly between groups.
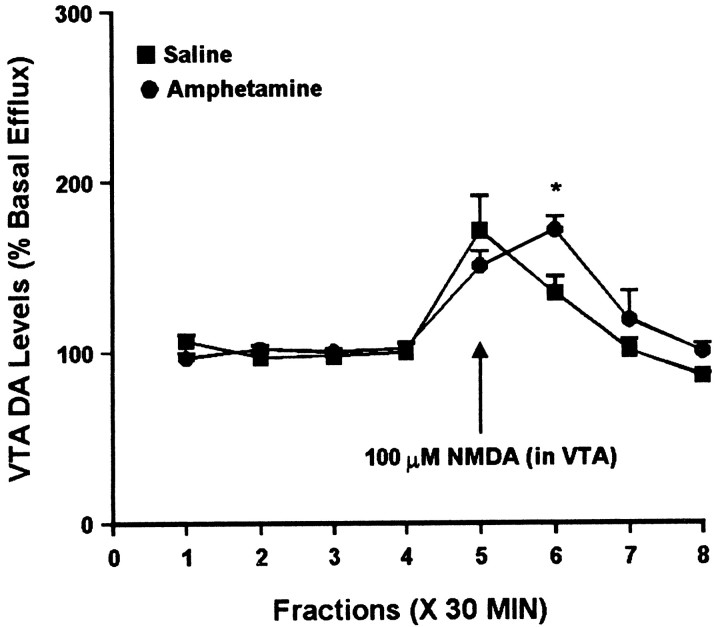
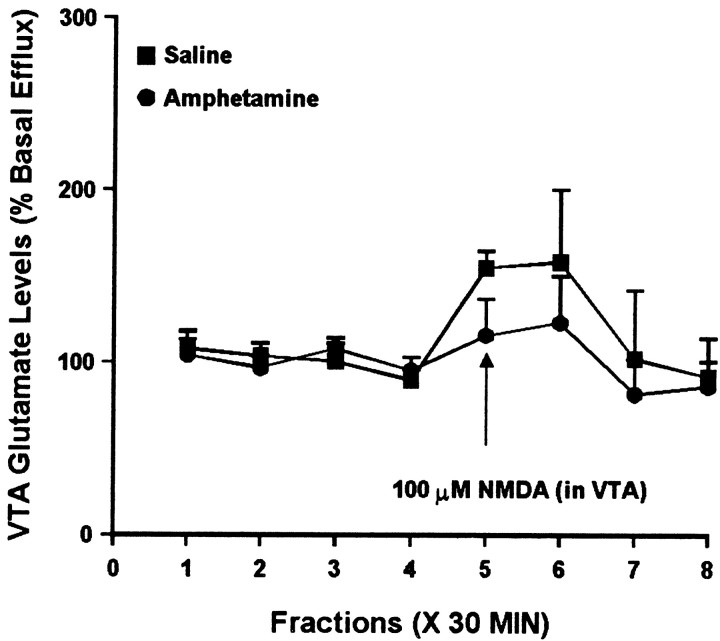
Intra-VTA administration of NMDA increased VTA DA efflux to the same magnitude in both groups, with a slightly delayed effect observed in the amphetamine group (Fig. 11). Analysis of the same samples for glutamate suggested a trend toward increased glutamate efflux in both groups, although one-way ANOVA failed to indicate a statistically significant change over time in either group (Fig. 12). Two-way ANOVA indicated no difference between the groups.
Fig. 11.
Effect of local administration of 100 μm NMDA on DA efflux from the VTA of amphetamine- and saline-pretreated rats tested 3 d after the last injection. NMDA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated a significant change in DA efflux over time within the saline group (F(7,28) = 9.87; p< 0.001) and the amphetamine group (F(7,28) = 14.52; p< 0.001). For both groups, contrast analysis revealed a statistically significant difference between the weighted means of the baseline samples (1–4) and the post-AMPA samples (5–8) (p < 0.01). Two-way ANOVA with time as the repeated measure indicated a significance difference between the amphetamine group and the saline group (group × time effect, F(7,56) = 2.32; p < 0.05). Post hoccomparisons of individual samples between the saline and amphetamine groups revealed significantly higher DA efflux in the amphetamine group for sample 6 (*p < 0.05,t tests with Bonferroni corrections). Basal DA levels were 23.5 ± 4.7 pg/μl in the saline group (n = 5) and 30.1 ± 4.9 pg/μl in the amphetamine group (n = 5) and did not differ significantly between groups.
Fig. 12.
Effect of local administration of 100 μm NMDA on glutamate efflux from the VTA of amphetamine- and saline-pretreated rats tested 3 d after the last injection. NMDA was administered for 30 min during sample 5. One-way ANOVA with time as the repeated measure indicated no significant change in glutamate efflux over time within the saline group (F(7,28) = 1.73;p = 0.14) or the amphetamine group (F(7,21) = 1.17; p= 0.36). Two-way ANOVA with time as the repeated measure indicated no significance difference between the amphetamine group and the saline group (group × time effect,F(7,49) = 0.45; p = 0.86). Basal glutamate levels were 10.4 ± 1.8 pg/μl in the saline group (n = 5) and 12.7 ± 2.2 pg/μl in the amphetamine group (n = 4) and did not differ significantly between groups.
DISCUSSION
There is increasing evidence that regulation of AMPA transmission in the VTA is important for neuroadaptations related to sensitization. Using in vivo microdialysis, we have demonstrated that repeated amphetamine administration profoundly alters the ability of VTA AMPA receptors to influence DA and glutamate transmission both locally in the VTA and downstream in the NAC. Although multiple approaches will be necessary to characterize AMPA receptor plasticity in the VTA, the availability of a microdialysis model is an important advance. First, all neuronal circuits are intact, which is important because brain regions other than the VTA participate in the development of sensitization (Kalivas and Alesdatter, 1993; Wolf et al., 1995; Khan and Shoaib, 1996; Bedingfield et al., 1997; Karler et al., 1997). Second, studies are performed in awake rats, avoiding concerns about effects of anesthetics on glutamate transmission (Moghaddam and Bolinao, 1994).
Mesoaccumbens DA neurons are more sensitive to intra-VTA AMPA after repeated amphetamine
Intra-VTA AMPA produced a greater increase in NAC DA levels in rats treated for 5 d with amphetamine and tested after 3 d of withdrawal. Similar to results in naïve rats (Karreman et al., 1996), saline-treated rats administered a threshold dose of AMPA (10 μm) directly into the VTA showed a small increase (40–50%) in NAC DA levels. However, the same dose of AMPA elicited a more robust increase (100%) in amphetamine-treated rats. This augmented response to AMPA was transient, because it was not present 10–14 d after the last injection. It was specific for AMPA, because intra-VTA NMDA administration produced a trend toward increased NAC DA levels that did not differ between groups. Thus, our microdialysis data are in complete agreement with previous electrophysiological studies using the same amphetamine regimen. In those studies, AMPA (applied by microiontophoresis) was more effective at increasing DA cell firing rates after repeated amphetamine treatment; the effect was observed 3 but not 10–14 d after the last injection, and there was no change in responsiveness to NMDA (Zhang et al., 1997).
Together, microdialysis and electrophysiological findings support the hypothesis that the excitatory drive to VTA DA neurons is increased shortly after discontinuation of repeated stimulant administration (Wolf, 1998). Many other findings support a link between increased DA cell activity and induction of sensitization. For example, augmented responses to psychostimulant challenge (i.e., a state resembling sensitization) can be produced by repeated electrical stimulation of the VTA (Ben-Shahar and Ettenberg, 1994), electrical kindling of the prefrontal cortex (Schenk and Snow, 1994), and pharmacological disinhibition of VTA DA cells (Steketee and Kalivas, 1991). The transient nature of the increased responsiveness to AMPA suggests that this alteration, like other sensitization-related changes in the VTA, is an early step in a cascade leading to more persistent alterations in the NAC and other forebrain regions (Wolf, 1998). In fact, the present findings are the first to demonstrate a link between chronic stimulant-induced alterations in DA cell excitability at the level of the VTA and a change in DA (and glutamate) output in the NAC.
The mechanism by which repeated amphetamine administration alters AMPA receptor responsiveness in the VTA is unclear. Acutely, amphetamine produces a long-lasting increase in extracellular glutamate levels in the VTA (Xue et al., 1996; Wolf and Xue, 1998, 1999; Wolf et al., 2000) and blocks LTD in VTA DA neurons via stimulation of D2-like receptors (Jones et al., 2000; Thomas et al., 2000). Perhaps increased glutamate levels, combined with a loss of “braking” effects normally provided by LTD, promotes strengthening of excitatory synapses on VTA DA neurons via LTP (Jones et al., 2000; Thomas et al., 2000; Wolf, 2001).
Alterations in DA and glutamate levels in the VTA after repeated amphetamine treatment
Amphetamine- but not saline-treated rats showed a significant increase in VTA DA levels in response to intra-VTA AMPA. This is likely related to the increased NAC DA levels produced by intra-VTA AMPA in the amphetamine group, because both effects were evident only after 3 d of withdrawal and were not produced by intra-VTA NMDA. Insofar as somatodendritic DA release is related to DA cell activity, an increase in AMPA-driven DA cell firing could account for increased DA levels in both the VTA and NAC.
Intra-VTA AMPA increased VTA glutamate levels in amphetamine- but not saline-treated rats at both withdrawal times, although the effect appeared more pronounced after 10–14 d of withdrawal. What mechanism underlies this effect? The VTA contains glutamate nerve terminals but not cell bodies. A direct effect of AMPA on glutamate release from these terminals is unlikely, given scant evidence of presynaptic AMPA receptors (Takumi et al., 1999). At the 3 d withdrawal time, it is possible that increased VTA glutamate levels are related to the AMPA-induced increase in VTA DA levels (via a local effect) or the AMPA-induced increase in NAC DA levels (via a polysynaptic pathway), because both of the latter effects are also seen at this withdrawal time. However, the saline and amphetamine groups did not differ in VTA or NAC DA levels after 10–14 d of withdrawal. Alternatively, the glutamate response in the amphetamine group may be unrelated to a difference in DA levels per se but instead may reflect a change in VTA systems that respond to DA. Consistent with this, systemic cocaine elevated VTA glutamate levels via a D1 receptor-dependent mechanism in cocaine-treated rats but not saline-treated rats (Kalivas and Duffy, 1998). This effect was observed 21 d after the last cocaine injection and could therefore be related to our findings at both early and late withdrawal times. On the other hand, we have reported decreased glutamate efflux during intra-VTA perfusion of a D1 agonist in saline-treated rats and attenuation of this effect by repeated amphetamine administration (Wolf and Xue, 1998). Differences in experimental conditions (the timing of probe implantation and composition of perfusion solution) may contribute to divergent findings with D1 agonists (Wolf and Xue, 1998). The timing of probe implantation in the present study is most similar to that of Kalivas and Duffy (1998).
If intra-VTA AMPA does increase VTA glutamate levels in the amphetamine group through a mechanism involving DA release and D1 receptor stimulation, that mechanism is likely to be complex. One possibility is that it involves GABA transmission. Whereas D1 receptors augment GABAB transmission in the VTA of control animals, D1 receptors suppress GABAB transmission after chronic cocaine or morphine (Bonci and Williams, 1996). We have found that glutamate efflux in the VTA of amphetamine-pretreated rats is under strong inhibitory control by GABABreceptors (Giorgetti and Wolf, 2000). If D1 receptor stimulation suppresses this inhibitory mechanism, its loss could contribute to an increase in glutamate levels.
Repeated amphetamine alters dopaminergic regulation of NAC glutamate levels
Intra-VTA AMPA increased glutamate levels in the NAC of amphetamine- but not saline-treated rats. A possible explanation is that AMPA activated polysynaptic circuits originating in the VTA that ultimately increase the activity of glutamate projections to the NAC. This is difficult to test because of the complexity of reciprocal circuits connecting VTA and its targets (Carr and Sesack, 2000). Alternatively, it could reflect direct effects of DA on glutamate efflux from nerve terminals in the NAC, because intra-VTA AMPA also increased NAC DA levels in the amphetamine group. Testing this by application of DA antagonists to the NAC is not feasible, because DA antagonists increase DA efflux via autoreceptors and feedback loops (Moghaddam and Bunney, 1990). Therefore, we examined the effect of intra-NAC perfusion of DA itself or the combination of D1 and D2 agonists and found that both treatments increased NAC glutamate efflux in the amphetamine group but not the saline group. These results suggest that DA receptors in the NAC are involved in mediating the increased glutamate efflux produced by intra-VTA AMPA and that these receptors are more sensitive after repeated amphetamine treatment. Consistent with this, intra-VTA NMDA failed to increase NAC DA levels in either group and also failed to increase NAC glutamate levels.
Our results are somewhat consistent with studies reporting an enhanced ability of a systemic cocaine injection to increase NAC glutamate levels in cocaine-sensitized rats (Pierce et al., 1996; Reid and Berger, 1996; Bell et al., 2000), although we did not observe this phenomenon in amphetamine-sensitized rats (Xue et al., 1996). In untreated rats, direct NAC application of amphetamine or quinpirole (1–100 μm) decreased glutamate efflux (Kalivas and Duffy, 1997). On the other hand, Dalia et al. (1998) found no change in glutamate levels after perfusing NAC with D1 or D2 agonists alone, whereas combined administration of SKF 38393 (20 mm) and quinpirole (20 or 50 mm) or administration of 10 mm amphetamine increased glutamate levels. Neither study examined rats previously exposed to psychostimulants. However, a recent study showed that AMPA receptor antagonists blocked reinstatement of drug-seeking behavior produced by either DA or AMPA administration into the NAC (Cornish and Kalivas, 2000).
Conclusions
Shortly after discontinuation of repeated amphetamine administration, there is an enhancement of the ability of VTA AMPA receptors to regulate DA and glutamate transmission in the VTA and the NAC. This transient alteration in AMPA transmission at the level of the VTA may represent a switching mechanism that enables downstream changes more directly involved in behavioral manifestations of chronic drug exposure.
Footnotes
This work was supported by United States Public Health Service Grants DA09621 and DA13006, by Independent Scientist Award DA00453, and by a grant from the National Alliance for Research on Schizophrenia and Depression (M.E.W.). We are grateful to Dr. Matthew P. Galloway for helpful discussions.
Correspondence should be addressed to Dr. Marina E. Wolf, Department of Neuroscience, Finch University of Health Sciences/The Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064-3095. E-mail: marina.wolf@finchcms.edu.
M. Giorgetti's present address: Department of Psychiatry, University of California San Francisco, San Francisco, CA 94143-0984.
REFERENCES
- 1.Bedingfield JB, Calder LD, Thai DK, Karler R. The role of the striatum in the mouse in behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 1997;56:305–310. doi: 10.1016/s0091-3057(96)00331-0. [DOI] [PubMed] [Google Scholar]
- 2.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shahar O, Ettenberg A. Repeated stimulation of the ventral tegmental area sensitizes the hyperlocomotor response to amphetamine. Pharmacol Biochem Behav. 1994;48:1005–1009. doi: 10.1016/0091-3057(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 4.Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine or morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- 6.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20 2000. RC89(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalia A, Uretsky NJ, Wallace LJ. Dopaminergic agonists administered into the nucleus accumbens: effects on extracellular glutamate and locomotor activity. Brain Res. 1998;788:111–117. doi: 10.1016/s0006-8993(97)01518-7. [DOI] [PubMed] [Google Scholar]
- 9.Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- 10.Giorgetti M, Wolf ME. Modulation of dopamine and glutamate efflux in ventral tegmental area (VTA) and nucleus accumbens (NAC) by GABAB and AMPA receptors in the VTA: effect of repeated amphetamine treatment. Soc Neurosci Abstr. 2000;26:789. [Google Scholar]
- 11.Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalivas PW, Alesdatter JE. Involvement of N-methyl-d-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- 13.Kalivas PW, Duffy P. Dopamine regulation of extracellular glutamate in the nucleus accumbens. Brain Res. 1997;761:173–177. doi: 10.1016/s0006-8993(97)00464-2. [DOI] [PubMed] [Google Scholar]
- 14.Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- 16.Karler R, Bedingfield JB, Thai DK, Calder LD. The role of the frontal cortex in the mouse in behavioral sensitization to amphetamine. Brain Res. 1997;757:228–235. doi: 10.1016/s0006-8993(97)00221-7. [DOI] [PubMed] [Google Scholar]
- 17.Karreman M, Westerink BHC, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- 18.Khan MA, Shoaib M. Neuroanatomical localization of the effects of (+)-HA966 on locomotor activity after cocaine injections to the nucleus accumbens of rats. Brain Res. 1996;719:198–202. doi: 10.1016/0006-8993(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 20.Moghaddam B, Bolinao ML. Glutamatergic antagonists attenuate ability of dopamine uptake blockers to increase extracellular levels of dopamine: implications for tonic influence of glutamate on dopamine release. Synapse. 1994;18:337–342. doi: 10.1002/syn.890180409. [DOI] [PubMed] [Google Scholar]
- 21.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 22.Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. NeuroReport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Compact Ed 3. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- 24.Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther. 1996;278:384–392. [PubMed] [Google Scholar]
- 25.Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. NeuroReport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 27.Satel SL, Southwick SM, Gawin FH. Clinical features of cocaine-induced paranoia. Am J Psychiatry. 1991;148:495–498. doi: 10.1176/ajp.148.4.495. [DOI] [PubMed] [Google Scholar]
- 28.Schenk S, Snow S. Sensitization to cocaine's motor activating properties produced by electrical kindling of the medial prefrontal cortex but not of the hippocampus. Brain Res. 1994;659:17–22. doi: 10.1016/0006-8993(94)90858-3. [DOI] [PubMed] [Google Scholar]
- 29.Steketee JD, Kalivas PW. Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region. J Pharmacol Exp Ther. 1991;259:916–924. [PubMed] [Google Scholar]
- 30.Strakowski SM, Sax KW. Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry. 1998;44:1171–1177. doi: 10.1016/s0006-3223(97)00454-x. [DOI] [PubMed] [Google Scholar]
- 31.Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann NY Acad Sci. 1999;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci. 2000;20:5582–55856. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine; lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- 34.White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- 35.White FJ, Hu X-T, Zhang X-F, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- 36.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 37.Wolf ME. The neuroplasticity of addiction. In: Shaw C, McEachern J, editors. Toward a theory of neuroplasticity. Taylor & Francis; Philadelphia: 2001. pp. 359–372. [Google Scholar]
- 38.Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- 39.Wolf ME, Xue C-J. Amphetamine and D1 receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem. 1998;70:198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolf ME, Xue C-J. Amphetamine-induced glutamate efflux in the rat ventral tegmental area is prevented by MK-801, SCH 23390, and ibotenic acid lesions of the prefrontal cortex. J Neurochem. 1999;73:1529–1538. doi: 10.1046/j.1471-4159.1999.0731529.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolf ME, Dahlin SL, Hu X-T, Xue C-J, White FJ. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-d-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- 42.Wolf ME, Xue C-J, Li Y, Wavak D. Amphetamine increases glutamate efflux in the rat ventral tegmental area by a mechanism involving glutamate transporters and reactive oxygen species. J Neurochem. 2000;75:1634–1644. doi: 10.1046/j.1471-4159.2000.0751634.x. [DOI] [PubMed] [Google Scholar]
- 43.Xue C-J, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. J Neurochem. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X-F, Hu X-T, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]