Abstract
Staphylococcus aureus is one of the most common pathogens for hospital-acquired and community-acquired infections. Methicillin-resistant S. aureus (MRSA) formed biofilms in wounds are difficult to treat with conventional antibiotics. By targeting FabB/FabF of bacterial fatty acid synthases, platensimycin (PTM) was discovered as a promising natural antibiotic against MRSA infections. In this study, PTM and its previously synthesized sulfur-Michael derivative PTM-2t, could reduce over 95% biofilm formation from S. aureus ATCC 29213 when used at 2 μg/mL in vitro. Topical application of ointments containing PTM or PTM-2t (2 × 4 mg/day/mouse) was successfully used to treat MRSA infections in a BABL/c mouse wound burn model. As a potential prodrug lead, PTM-2t showed improved in vivo efficacy in a mouse peritonitis model, compared to PTM. Our study suggests that PTM and its analogue may be used topically or locally to treat bacterial infections. In addition, the use of prodrug strategies might be instrumental to improve the poor pharmacokinetics properties of PTM.
Keywords: Prodrug, Platensimycin, Antibiotic, Staphylococcus aureus, Skin infection, Peritonitis
Graphical Abstract
1. INTRODUTCTION
Since Staphylococcus aureus was first identified in 1880, it has become one of the most common pathogens for hospital-acquired infections and later community-acquired infections. S. aureus related infections such as bacteremia, peritonitis, burn wound infection have become a prevalent healthy problem.1–5 Due to the formation of methicillin-resistant S. aureus (MRSA) biofilm, burn wound infections have become difficult to treat with conventional antibiotics.6–9 Therefore drug leads with anti-Staphylococcal biofilm activities are extremely desired.10–11
Platensimycin (PTM), discovered from soil bacteria Streptomyces platensis about a decade ago by Singh and co-workers in Merck Research Laboratory, showed strong antibacterial activities against MRSA and other Gram-positive pathogens with no effects of toxicity (Figure 1).12 It was regarded as a milestone discovery, due to its unprecedented molecular structure and novel mode of action against FabB/FabF from bacterial fatty acid biosynthesis, as well as the powerful whole-cell screening strategy using 250,000 crude natural product extracts.13 While the poor pharmacokinetics (PK) of PTM, namely its rapid renal clearance,14 limits its clinical application as potential antibiotics, the generation of dozens of PTM analogues through total synthesis, semi-synthesis and biosynthesis, has revealed critical new insights into its structure-activity relationships.14–25
Figure 1.
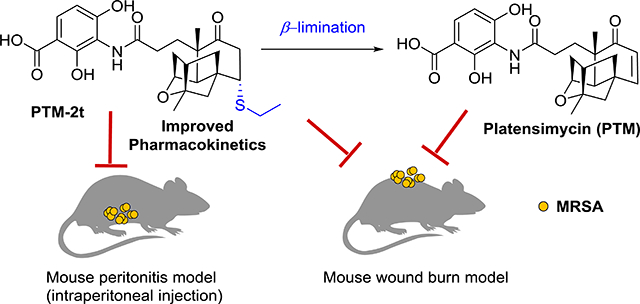
The design of the β-elimination prodrug strategy to target FabB/FabF in the fatty acid biosynthesis pathway in Staphylococcus aureus, using the PTM-inspired thioether analogue PTM-2t.
We recently reported a series of PTM thioether-analogues with promising antibacterial activities against MRSA, through a biomimetic sulfur-Michael addition strategy.23 Those sulfur analogues could undergo a β-elimination reaction, which resulted in PTM in an aqueous buffer. We hypothesized that they may be used as prodrug forms of PTM, as they could be easily converted into PTM inside cells. It would be fascinating to explore if these PTM prodrugs have improved PK properties in animal models compared to those of PTM. In addition, we hypothesized that PTM may be a useful antibiotic against local or topical infections, thus bypassing any potential renal clearance.14 In the current work, PTM and one of its sulfur analogues (PTM-2t) were discovered to prevent biofilm formation by S. aureus in vitro and also were shown to be effective against MRSA skin infections in mice. PTM-2t was also effective against MRSA infection in a mouse model of acute peritonitis.
2. EXPERIMENTAL SECTION
2.1. Hepatic microsome stability assay
Microsome stability was evaluated as described previously.14 Briefly, 1 μM compound was added to 1 mg/mL hepatic microsomes (mouse) in 100 mM potassium phosphate buffer (pH 7.4) at 37 °C with continuous shaking. Reactions were performed in the absence or presence of 1 mM NADPH. Twenty microliter aliquots were removed and quenched with 150 μL of CH3CN at six time points: 0, 5, 10, 20, 40 and 60 min. Resultant samples were centrifuged through a 0.45 μm filter and analyzed by LC-MS/MS. The data were log-transformed and reported as half-lives (min). The data of intrinsic clearance were described as ln2 * 1/T1/2 * incubation volume in mL/protein in incubation in mg * 1000 mL/1 mL.
2.2. Plasma Binding
Plasma protein binding was determined using equilibrium dialysis. All samples were tested in triplicate using the RED Rapid Equilibrium Dialysis Device (Thermo Scientific). Initial drug concentration in the plasma chamber was 5 μM and phosphate buffered saline (PBS) was added to the receiver chamber. The plate was covered and allowed to shake in a 37 °C incubator for 6 hours. Twenty-five microliters were sampled from the plasma and PBS chambers, diluted with either blank PBS or plasma to achieve a 1:1 ratio of plasma: PBS for all samples. The concentration of drug in the plasma and PBS chambers were determined by LC-MS/MS. The fraction bound was calculated as ([plasma] – [PBS]) / [plasma].
2.3. Prevention of Biofilm Formation
The effects of PTM and PTM-2t on preventing biofilm formation were evaluated using the microtiter dish biofilm formation assay.30–31 Briefly, S. aureus ATCC 29213 and MRSA, collected from local hospitals in central China, were cultured in tryptone soybean broth (TSB) supplemented with 1% glucose in a 96-well tissue-culture plate with compound (0.0125–8 μg/mL). After 24 h, the biofilms were washed by sterile water and stained using 0.1% (w/v) crystal violet. The biofilm mass was dissolved using 95% ethanol. The intensity of crystal violet was measured at 540 nm.
2.4. Animals
All animal studies were carried out at the Department of Laboratory Animals in Central South University (CSU). The protocols for the mice skin infections and acute mouse peritonitis with PTM and PTM-2t were reviewed and approved by CSU, Laboratory Animal Welfare Ethics committee (No. 2018sydw0153). Animals were acclimatized for 5–6 days prior to initiating the study. The mice had free access to chow and water. All mice were purchased from Hunan Silaikejingda Experimental Animal Company Limited (Changsha, China).
2.5. Mouse Skin Infection with MRSA
Mouse MRSA skin infection study was performed as previously described.32–33 Briefly, the animal study was performed with 7̶week old female BABL/c mice, 20–22 g. The mice were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital, and the back hair of the mice were shaved and washed with povidone iodine-propanol solution. A copper bar (12×12 mm), heated in boiling water for 10 min, was placed on the paraspinal site of each mouse for 40 s without pressure. Only the weight of the block was used to create the burns. A small gauze was placed over each burn and then infected with 1 × 108 colony-forming units (CFU) of MRSA. The infected mice were left for 48 h before an open wound formed at the site of infection. The mice were then divided into four groups with each group of five infected mice being treated. PTM and PTM-2t were formulated into 2% (w/w) ointment consisting of 2% (w/v) carbomer in double distilled water. Mupirocin (2% w/w) and vehicle alone were used as controls. All mice were treated twice a day for 7 days. Exactly 24 h after the final treatment, mice were euthanized and the area around the wound was slightly swabbed with 70% ethanol. The wound area (1 cm2) was excised, homogenized, and plated onto TSB agar plates to count viable bacteria. Other skin biopsy specimens removed from the wounds were stored in 4% paraformaldehyde solution to be used for hematoxylin and eosin (HE) staining.
2.6. Mouse Peritonitis Model
The mouse peritonitis model was described previously.34–35 In brief, all animal studies were performed with female C57BL/6J mice, 18–20 g with 7–8 weeks old. Mice were infected with 0.5 mL MRSA suspension (2–3×107) including 5% (w/v) mucin via intraperitoneal injection. At 1 h and 5 h post-infection, mice were treated with PTM-2t (10 mg/kg and 50 mg/kg) or PTM (10 mg/kg), with saline or vancomycin (50 mg/kg) as controls. Mice survival was observed for 14 days after infection.
2.7. Statistical Analysis
Two-sample comparisons were made using a Mann-Whitney rank sum test with P<0.05 considered as significant.
3. RESULTS
3.1. Microsome stability and plasma protein binding of PTM and PTM inspired thioether-analogues
In vitro metabolic stability of PTM and its thioether and thioester analogues were first compared in mouse liver microsomes (Table 1). The half-life (T1/2) of PTM thioester analogues, including PTM-2a to PTM-2r, ranged between 1.4 — 9.5 min, while the PTM thioether derivatives PTM-2s to PTM-2t, had a half-life of 10.4 or 15.0 min, respectively. PTM-2t was slightly more stable than PTM, which had a half-life of 13.6 min, while PTM could survive longer than PTM-2t in human liver microsome, with a T1/2 of 51 min (Table 2). The difference might be explained by the differential binding of PTM and PTM-2t to human plasma proteins (Table 2).
Table 1.
In vitro metabolic stability in mouse liver microsome of PTM and PTM-inspired thioether-analogues.
| Entry | R | T1/2 (min) | Intrinsic Clearance (μL/min/mg) | Entry | R | T1/2 (min) | Intrinsic Clearance (μL/min/mg) |
|---|---|---|---|---|---|---|---|
| Sunitinib | - | 13.1 | 53 | 2j | 3-CF3C6H4 | 3.2 | 217 |
| PTM | - | 13.6 | 51 | 2k | 2-CF3C6H4 | 5.7 | 122 |
| 2a | Ph | 1.4 | 495 | 2l | 4-NO2C6H4 | 1.5 | 462 |
| 2b | 4-ClC6H4 | 1.6 | 433 | 2m | 3,5-diFC6H4 | 1.6 | 433 |
| 2c | 4-MeOC6H4 | 1.3 | 533 | 2n | 3,5-diCF3C6H4 | 7.4 | 94 |
| 2d | 4-MeC6H4 | 1.4 | 495 | 2o | -thiophene | 1.6 | 433 |
| 2e | 4-FC6H4 | 2.8 | 248 | 2p | -naphthyl | 1.7 | 408 |
| 2f | 3-FC6H4 | 1.6 | 433 | 2q | -CH3 | 9.5 | 73 |
| 2g | 2-FC6H4 | 1.8 | 385 | 2r | 4-FBn | 1.4 | 495 |
| 2h | 4-BrC6H4 | 1.5 | 462 | 2s | 4-CH3C6H4 | 10.4 | 67 |
| 2i | 4-CF3C6H4 | 1.6 | 433 | 2t | -CH2CH3 | 15.0 | 46 |
Table 2.
In vitro metabolic stability, intrinsic clearance in human liver microsomes and plasma protein binding of PTM and PTM-2t.
| Compd. | T1/2(min) | Intrinsic Clearance (μL/min/mg) | Plasma Protein Binding (%) |
|
|---|---|---|---|---|
| Free | Bound | |||
| PTM | 51.0 | 14.0 | 34.0 | 66.0 |
| PTM-2t | 22.0 | 32.0 | 65.0 | 35.0 |
3.2. Prevention of biofilm formation by PTM and PTM-2t
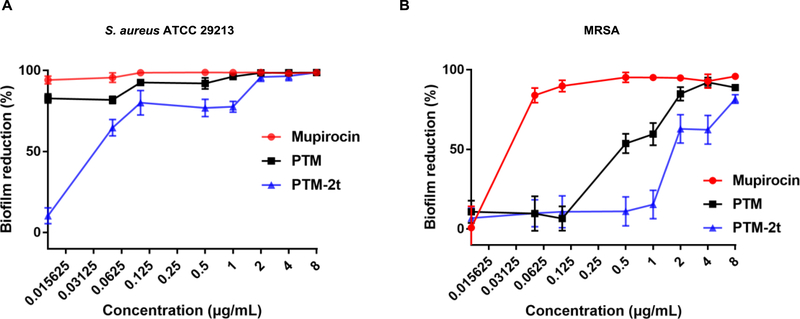
Since biofilms play a major role in S. aureus infections, the anti-biofilm activities of PTM and PTM-2t were next investigated. PTM and PTM-2t dose-dependently inhibited the biofilm formation from S. aureus ATCC 29213 and MRSA, in comparison to the clinically used antibiotic mupirocin (Figure 2). After exposure to 2 μg/mL of PTM or PTM-2t for 24 h, biofilm formation from S. aureus ATCC 29213 was reduced over 95%. PTM reduced more biofilm from MRSA than PTM-2t. The potent in vitro anti-biofilm activities indicated that PTM and PTM-2t may be useful antibiotics against MRSA skin infections.
Figure 2.
Prevention of biofilm formation by PTM and PTM-2t. (A) S. aureus ATCC 29213; (2) MRSA. Results were expressed as reduction in biofilm mass. Values are from three independent experiments in triplicates.
3.3. In vivo antibacterial activity of PTM and PTM-2t in MRSA induced skin infection in mouse
We also evaluated the effects of PTM and PTM-2t on MRSA-induced mice skin infections. An ointment containing 4 mg of PTM or PTM-2t were used to treat the MRSA-infected female BABL/c mice, twice a day for 7 days. Compared to mupirocin, the applications of PTM and PTM-2t, were highly effective in reducing the number of viable S. aureus in the infected skin area. Compared to the untreated control mice (4.3×108 CFU/g), PTM or PTM-2t reduced viable S. aureus to 2.0×106 CFU/g and 8.6 ×106 CFU/g of mouse skin, which was very comparable to mupirocin (2.5×106 CFU/g) (Figure 3B). The skin of the MRSA infected mice showed secondary scald.36 On the ninth day after burn injury and infection, the detachment of the epidermis from the dermis was observed. Hematoxylin and eosin staining revealed that the dermis of the infected mice was partly damaged (Figure 3D). The untreated mice showed partially destroyed hair follicles, incomplete fat layer and the presence of a large number of inflammatory cells in the muscle layer; in contrast, the treated mice showed relatively healed skin structure.
Figure 3.
The antibacterial activity of PTM and PTM-2t in MRSA induced skin infection in mouse. (A) Therapeutic efficacy of PTM and PTM-2t in a mouse skin infection model, Mupirocin was used as the positive control. The infected mice were treated twice a day for 7 days. (B) The total bacterial loads in the skin lesions were determined. Statistical analysis was calculated by the Mann-Whitney test. P values of (*P<0.05) (**P<0.01) were considered as significant. P values (**P<0.01) were: vehicle versus 2% mupirocin, 0.0022; vehicle versus 2% PTM, 0.0043; vehicle versus 2% PTM-2t, 0.0022. (C) Wounds of BALB/c mice with treated and untreated with compounds at day 4, day 7 and day 9. (D) HE staining histological appearance of S. aureus-infected skin lesion on day 10. Biopsy specimens were taken immediately after the termination of the experiment, fixed in formalin, and embedded in paraffin. The biopsy specimens were stained with hematoxylin and eosin. Each point represents data from a single mouse. Mean values are presented, n=6. Abbreviations: D: Dermis; F: Fat layer; I: Inflammatory cells.
3.4. Therapeutic efficacy of PTM-2t on MRSA infected mouse peritonitis model
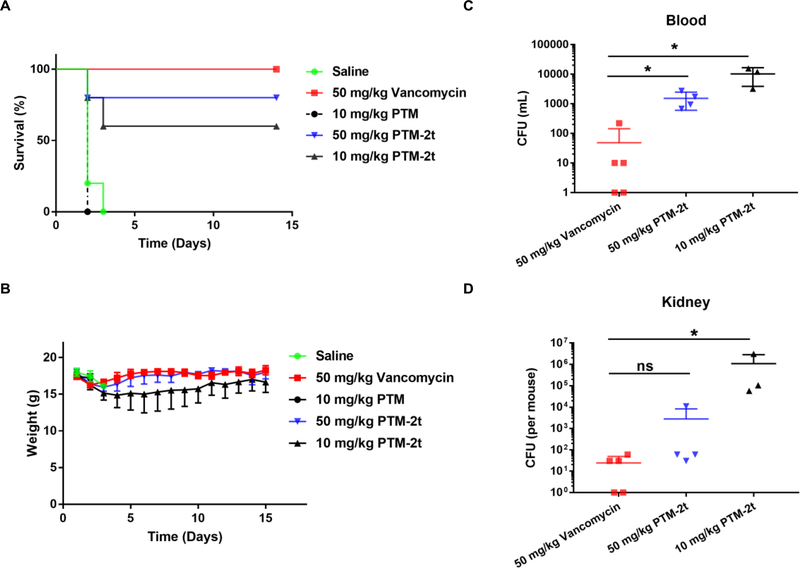
The in vivo efficacy of PTM-2t, a potential prodrug of PTM, was further investigated in MRSA induced mouse peritonitis model, using PTM and vancomycin as controls. The application of 2.5 ×107 CFU/mL of MRSA (0.5 mL) led to 100% mice mortality within 48 h through intraperitoneal (i. p.) injection. Therefore, the C57BL/6J mice were given at the lethal dose of MRSA by i.p. injection, and subsequently they were treated with two i.p. injections of PTM, PTM-2t and vancomycin at 1 h and 5 h post infection. Compared to PTM, a dose of 10 mg/kg of PTM-2t could cure 60% mice, while a high dose of 50 mg/kg of PTM-2t could improve mice survival to 80% (Figure 4A). Next the MRSA strain in blood and kidney of the survived mice were subsequently examined (Figure 4C–D). Only a few MRSA were present in the blood or kidney of the mice treated by vancomycin, while a significant number of MRSAs was still present in the mice treated by PTM-2t, especially in the treatment group of 10 mg/kg.
Figure 4.
Therapeutic efficacy of PTM-2t on MRSA infected mice. Vancomycin or saline were used as controls. At 1 h and 5 h post-infection, mice were treated with PTM at 10 mg/kg, PTM-2t at dose of 50 mg/kg and 10 mg/kg. The survival (A) and weights (B) of the infected mice were tracked for 14 days. (C) and (D) Quantitative comparison of blood and kidney bacterial counts of mice treated with vancomycin versus those of mice treated with PTM-2t at 14 days after administration. Each point represents data from a single mouse. Mean values are presented, n=5.
4. DISCUSSION
Effective treatment and management of S. aureus biofilm infection would be a major clinical breakthrough, especially in burn wound infections.37–38 In this study, PTM and its thioether analogue PTM-2t, were shown to inhibit the S. aureus biofilm formation and were able to treat MRSA skin infection. Although the clinical development of PTM has been hampered by its poor PK properties, the topical application of PTM, with novel mode of action against major pathogens, would open a new avenue for this fascinating natural product. In addition, the accumulating efforts in the last decade has generated many PTM analogues, with comparable or improved antibacterial activities in vitro. It would be very interesting to compare them with PTM in various animal models, for their efficacy in topical or local treatments against various bacterial infections.
The PTM prodrug PTM-2t showed improved in vivo efficacy against MRSA infection in an acute mouse peritonitis model, even though PTM and PTM-2t were both relatively stable in the hepatic microsomal assay. The rapid renal clearance of PTM might have decreased its efficacy, resulting in the need for continuous infusion to achieve in vivo efficacy against MRSA infection in mouse models.12 In contrast, the in vivo efficacy of PTM-2t (10 mg/kg or 50 mg/kg) suggested that it has improved PK properties and it is bio-converted to PTM. This study also suggests that prodrugs, drug delivery systems or optimal formulation of PTM, might be instrumental for their effective delivery in situ, in order to achieve the maximal clinical benefits. We are currently persuing these strategies and will report them in due course.
In conclusion, PTM and PTM-2t were demonstrated to be effective topical antibiotics against MRSA skin infection in mice. The thioether analogue PTM-2t served as a prodrug and exhibited improved in vivo efficacy in systemic MRSA infection, compared to PTM. Since S. aureus is one of the major pathogens globally, the further development of PTM analogues as potential drug leads is warranted.
ACKNOWLEDGMENTS
Current research is financially supported by NSFC grants 81473124 (to Y.H.), the Chinese Ministry of Education 111 Project B0803420 (to Y.D.), U. S. National Institutes of Health Grants GM114353 (to B.S.), the Fundamental Research Funds for the Central Universities of Central South University (CSU) 2018zzts241 (to M.S.).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Hsu LY; Wijaya L; Koh TH Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010, 376 (9743), 767–768. [DOI] [PubMed] [Google Scholar]
- (2).Moran GJ; Krishnadasan A; Gorwitz RJ; Fosheim GE; McDougal LK; Carey RB; Talan DA Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006, 355 (7), 666–674. [DOI] [PubMed] [Google Scholar]
- (3).Wernitz MH; Swidsinski S; Weist K; Sohr D; Witte W; Franke KP; Roloff D; Ruden H; Veit SK Effectiveness of a hospital-wide selective screening program for methicillin-resistant Staphylococcus aureus (MRSA) carriers at hospital admission to prevent hospital-acquired MRSA infections. Clin Microbiol Infect. 2005, 11 (6), 457–465. [DOI] [PubMed] [Google Scholar]
- (4).Brennan L; Lilliebridge RA; Cheng AC; Giffard PM; Currie BJ; Tong SY Community-associated methicillin-resistant Staphylococcus aureus carriage in hospitalized patients in tropical northern Australia. J Hosp Infect. 2013, 83 (3), 205–211. [DOI] [PubMed] [Google Scholar]
- (5).Henderson A; Nimmo GR Control of healthcare- and community-associated MRSA: recent progress and persisting challenges. Br Med Bull. 2018, 125 (1), 25–41. [DOI] [PubMed] [Google Scholar]
- (6).Norbury W; Herndon DN; Tanksley J; Jeschke MG; Finnerty CC Infection in Burns. Surg Infect (Larchmt). 2016, 17, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).D’Avignon LC; Hogan BK; Murray CK; Loo FL; Hospenthal DR; Cancio LC; Kim SH; Renz EM; Barillo D; Holcomb JB; Wade CE; Wolf SE Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns 2010, 36, 773–779. [DOI] [PubMed] [Google Scholar]
- (8).Wang Y; Beekman J; Hew J; Jackson S; Issler-Fisher AC; Parungao R; Lajevardi SS; Li Z; Maitz P Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018, 123, 3–17. [DOI] [PubMed] [Google Scholar]
- (9).Percival SL; McCarty SM; Lipsky B Biofilms and wounds: an overview of the evidence. Adv Wound Care. (New Rochelle) 2015, 4 (7), 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Davies D Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003, 2 (2), 114–122. [DOI] [PubMed] [Google Scholar]
- (11).Chen M; Yu Q; Sun H Novel strategies for the prevention and treatment of biofilm related infections. Int J Mol Sci. 2013, 14 (9), 18488–18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang J; Soisson SM; Young K; Shoop W; Kodali S; Galgoci A; Painter R; Parthasarathy G; Tang YS; Cummings R; Ha S; Dorso K; Motyl M; Jayasuriya H; Ondeyka J; Herath K; Zhang C; Hernandez L; Allocco J; Basilio A; Tormo JR; Genilloud O; Vicente F; Pelaez F; Colwell L; Lee SH; Michael B; Felcetto T; Gill C; Silver LL; Hermes JD; Bartizal K; Barrett J; Schmatz D; Becker JW; Cully D; Singh SB Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 2006, 441 (7091), 358–361. [DOI] [PubMed] [Google Scholar]
- (13).Young K; Jayasuriya H; Ondeyka JG; Herath K; Zhang C; Kodali S; Galgoci A; Painter R; Brown-Driver V; Yamamoto R; Silver LL; Zheng Y; Ventura JI; Sigmund J; Ha S; Basilio A; Vicente F; Tormo JR; Pelaez F; Youngman P; Cully D; Barrett JF; Schmatz D; Singh SB; Wang J Discovery of FabH/FabF inhibitors from natural products. Antimicrob Agents Chemother. 2006, 50 (2), 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dong LB, Rudolf JD, Lin L, Ruiz C, Cameron MD, Shen B In vivo instability of platensimycin and platencin: synthesis and biological evaluation of urea- and carbamate-platensimycin. Bioorg. Med. Chem 2017, 25 (6), 1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Nicolaou KC; Tang Y; Wang J; Stepan AF; Li A; Montero A Total synthesis and antibacterial properties of carbaplatensimycin. J Am Chem Soc. 2007, 129 (48), 14850–14851. [DOI] [PubMed] [Google Scholar]
- (16).Nicolaou KC; Stepan AF; Lister T; Li A; Montero A; Tria GS; Turner CI; Tang Y; Wang J; Denton RM; Edmonds DJ Design, synthesis, and biological evaluation of platensimycin analogues with varying degrees of molecular complexity. J Am Chem Soc. 2008, 130 (39), 13110–13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang J; Lee V; Sintim HO Efforts towards the identification of simpler platensimycin analogues--the total synthesis of oxazinidinyl platensimycin. Chemistry 2009, 15 (12), 2747–2750. [DOI] [PubMed] [Google Scholar]
- (18).Jang KP; Kim CH; Na SW; Jang DS; Kim H; Kang H; Lee E 7-Phenylplatensimycin and 11-methyl-7-phenylplatensimycin: more potent analogs of platensimycin. Bioorg Med Chem Lett. 2010, 20 (7), 2156–2158. [DOI] [PubMed] [Google Scholar]
- (19).Dong LB; Rudolf JD; Shen B A mutasynthetic library of platensimycin and platencin analogues. Org Lett. 2016, 18 (18), 4606–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rudolf JD; Dong LB; Shen B, Platensimycin and platencin: Inspirations for chemistry, biology, enzymology, and medicine. Biochem Pharmacol 2017, 133, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Qiu L; Tian K; Pan J; Jiang L; Yang H; Zhu X; Shen B; Duan Y; Huang Y A facile semi-synthetic approach towards halogen-substituted aminobenzoic acid analogues of platensimycin. Tetrahedron 2017, 73 (6), 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Qiu L; Tian K; Wen Z; Deng Y; Kang D; Liang H; Zhu X; Shen B; Duan Y; Huang Y Biomimetic stereoselective sulfa-Michael addition leads to platensimycin and platencin sulfur analogues against methicillin-resistant Staphylococcus aureus. J. Nat. Prod 2018, 81 (2), 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Deng Y; Kang D; Shi J; Zhou W; Sun A; Ju J; Zhu X; Shen B; Duan Y; Huang Y The semi-synthesis, biological evaluation and docking analysis of the oxime, hydrazine and hydrazide derivatives of platensimycin. Med. Chem. Comm 2018, 9 (5), 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Deng Y; Su M; Kang D; Liu X; Wen Z; Li Y; Qiu L; Shen B; Duan Y; Huang Y Semi-synthesis of platensimycin derivatives with antibiotic activities in mice via Suzuki-Miyaura cross-coupling reactions. J Med Chem. 2018, 61 (24), 11341–11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Tian K, Deng Y, Qiu L, Zhu X, Shen B, Duan Y, Huang Y Semisynthesis and Biological Evaluation of Platensimycin Analogues with Varying Aminobenzoic Acids. ChemistrySelect. 2018, 3, 12625–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Walther R; Rautio J; Zelikin AN Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv Drug Deliv Rev. 2017, 118, 65–77. [DOI] [PubMed] [Google Scholar]
- (27).Rautio J; Kumpulainen H; Heimbach T; Oliyai R; Oh D; Jarvinen T; Savolainen J Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008, 7 (3), 255–270. [DOI] [PubMed] [Google Scholar]
- (28).Beaumont K; Webster R; Gardner I; Dack K Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: challenges to the discovery scientist. Curr Drug Metab. 2003, 4 (6), 461–485. [DOI] [PubMed] [Google Scholar]
- (29).Stella VJ; Nti-Addae KW Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev. 2007, 59 (7), 677–694. [DOI] [PubMed] [Google Scholar]
- (30).de Breij A; Riool M; Kwakman PHS; de Boer L; Cordfunke RA; Drijfhout JW; Cohen O; Emanuel N; Zaat SAJ; Nibbering PH; Moriarty TF Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release 2016, 222, 1–8. [DOI] [PubMed] [Google Scholar]
- (31).Malachowa N; Kobayashi SD; Braughton KR; Deleo FR Mouse model of Staphylococcus aureus skin infection. Methods. Mol. Biol 2013, 1031, 109–116. [DOI] [PubMed] [Google Scholar]
- (32).Simonetti O; Lucarini G; Orlando F; Pierpaoli E; Ghiselli R; Provinciali M; Castelli P; Guerrieri M; Di Primio R; Offidani A; Giacometti A; Cirioni O Role of daptomycin on burn wound healing in an animal methicillin-resistant Staphylococcus aureus infection model. Antimicrob. Agents. Chemother 2017, 61 (9), e00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Kalita S; Kandimalla R; Devi B; Kalita B; Kalita K; Deka M; Kataki AC; Sharma A; Kotoky J Dual delivery of chloramphenicol and essential oil by poly-ɛ-caprolactone-pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Advances. 2017, 3 (7), 1749–1758. [Google Scholar]
- (34).Gomez-Zorrilla S; Calatayud L; Juan C; Cabot G; Tubau F; Oliver A; Dominguez MA; Ariza J; Pena C Understanding the acute inflammatory response to Pseudomonas aeruginosa infection: differences between susceptible and multidrug-resistant strains in a mouse peritonitis model. Int. J. Antimicrob. Agents 2017, 49 (2), 198–203. [DOI] [PubMed] [Google Scholar]
- (35).Kalita S; Kandimalla R; Bhowal AC; Kotoky J; Kundu S Functionalization of beta-lactam antibiotic on lysozyme capped gold nanoclusters retrogress MRSA and its persisters following awakening. Sci Rep. 2018, 8 (1), 5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Jackson DM The diagnosis of the depth of burning. Br J Surg. 1953, 40 (164), 588–596. [DOI] [PubMed] [Google Scholar]
- (37).Olsen I Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis 2015, 34 (5), 877–886. [DOI] [PubMed] [Google Scholar]
- (38).Akiyama H; Huh WK; Yamasaki O; Oono T; Iwatsuki K Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol. 2002, 147 (5), 879–885. [DOI] [PubMed] [Google Scholar]