Abstract
Matrix metalloproteases (MMPs) are a large family of zinc-dependent endopeptidases, involved in a diverse set of physiological and pathological processes, most notably in cancer. Current methods for imaging and quantifying MMP activity lack sufficient selectivity and spatiotemporal resolution to allow studies of specific MMP function in vivo. Previously, we reported a strategy for selective targeting of MMPs by engineering a functionally silent cysteine mutation that enables highly specific covalent modification by a designed activity-based probe (ABP). Here we describe the translation of that technology into a mouse model of breast cancer and subsequent demonstration of the utility of the approach for studies of MMP-14 activation in the tumor microenvironment. Using this approach, we find that MMP-14 is active in late stage tumors and is predominantly associated with stromal cell populations that have been activated by specific signaling molecules (e.g. TGFβ) produced by tumor cells. Our data demonstrates the applicability of this approach for studies of MMP function in whole organisms and identifies important regulatory mechanisms for MMP-14 activity in the tumor microenvironment.
Introduction
MMPs are a family of zinc-dependent endopeptidases, consisting of more than 25 members in humans.1 This large family of proteases is responsible for the degradation of most extracellular proteins and has long been associated with many types and stages of cancer.2, 3 Proteolysis by MMPs is essential for a variety of physiological activities4 and signaling events5, 6 and is tightly regulated at several levels. In addition to regulation at the transcriptional level, all MMPs are produced as latent pro-enzymes that require enzymatic activation for catalytic function.1 Their activity is regulated by compartmentalization and trafficking and is inhibited by endogenous tissue inhibitors of matrix metalloproteinases (TIMPs).7
MMPs promote tumor progression and regulate the tumor microenvironment through degradation of the extracellular matrix (ECM) as well as through additional signaling functions.6, 8 However, attempts to target MMPs for therapeutic purposes have been hampered by a general poor understanding of the specific roles of individual MMP members and inadequate target validation.3, 9 Contradicting roles have emerged for MMPs both as tumor promoters and tumor suppressors.10 Depending on the circumstances, different members of the MMPs regulate tumorigenesis during the process of microenvironment remodeling. Despite mounting evidence of elevated MMP activity at the interface of a tumor and its stroma,2, 8 our understanding of the specific roles, physical location and dynamics of proteolytic activation of individual MMPs remains elusive. Current methods to visualize MMP activity, such as fluorogenic substrates,11, 12 radiolabeled inhibitors13, 14 or fluorescent matrix proteins15 lack the specificity and spatiotemporal precision required to map the activity of individual MMPs in the context of a native tissue during tumor development.
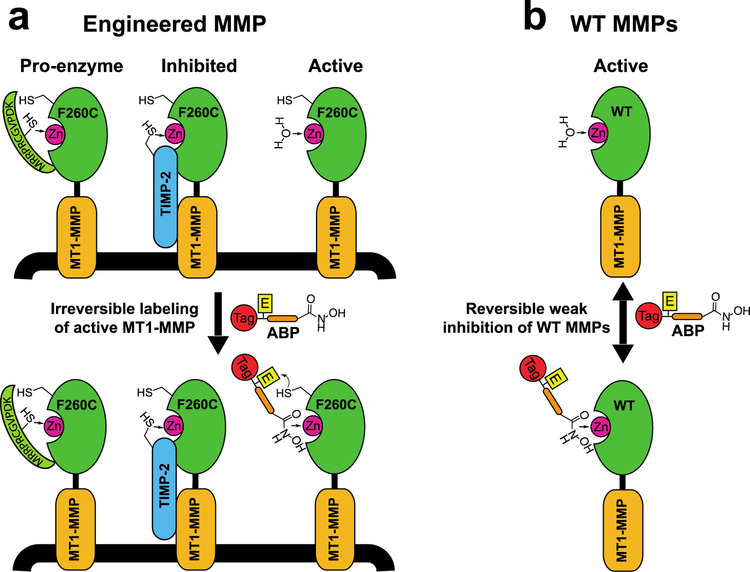
Our group previously reported the design and validation of a methodology to specifically image and selectively inhibit individual MMPs.16 This approach makes use of a combination of protein engineering and a corresponding activity-based probe (ABP) to selectively label active forms of MMPs in intact cells (Figure 1a). A proximity-induced interaction between an engineered cysteine and an electrophilic warhead on the ABP results in irreversible labeling and selective inhibition of the engineered ‘probe-sensitive’ MMP, while any non-specific interaction of the probe with wild type MMPs remains reversible and results in weak, reversible inhibition (Figure 1b). Since ABP binding relies on the accessibility of the catalytic zinc ion and the active site, this strategy provides a means to distinguish active enzyme from pro-enzyme forms or inhibited complexes in the context of live cells or intact tissues with high spatial resolution.16
Figure 1:
Specific labeling of active MMP-14 using protein engineering coupled with ABPs. a) Engineered MMP-14 contains a cysteine nucleophile that is irreversibly labeled by the ABP. Covalent binding occurs only with the active form of MMP-14 following affinity targeting of the catalytic zinc by the hydroxamate-containing ABP, but not with the proenzyme or the inhibited form in which the zinc is inaccessible. b) ABP labeling is selective to the probe sensitive MMP while any interactions with native MMPs result in reversible weak inhibition.
The membrane-anchored MMP-14 (MT1-MMP) is thought to be one the main proteases involved in the process by which tumor cells penetrate the basement membrane, a process that marks the transition to invasive carcinoma.2, 17, 18 High expression levels of MMP-14 by tumor cells, and cells within the tumor stroma are associated with rapid tumor progression and increased invasiveness.19–21 The coordinated compartmentalization of MMP-14 in invadopodia or podosomes, membrane protrusions specialized for ECM-adhesion and degradation22, is evident for both the invading tumor cells and stromal cells in the tumor microenvironment such as cancer-associated fibroblasts18, 23 (CAFs), macrophages24, 25 and other myeloid cells.26 However, the identity of soluble signaling molecules produced by malignant cancer cells and the surrounding stroma that induces expression and activation of MMP-14 remain largely undefined. Understanding the regulation, activity and function of MMP-14 is essential for defining the cell invasion programs of specific cancers. Here, we describe the construction of a new mouse model in which the native MMP-14 gene is engineered to express a cysteine mutant MMP-14 that can be targeted with a corresponding ABP through a covalent labeling reaction. This mutation was also crossed with MMTV-PyMT mice to generate a breast cancer model in which all cells express the probe-sensitive MMP-14. Using an activity-based probe, we were able to visualize MMP-14 activity patterns and cellular localization within components of the tumor microenvironment. We find elevated levels of expression and activation of MMP-14 primarily in stromal cells, while MMP-14 is expressed at the protein level within the tumor cells but remains largely inactive within the tumor bulk. The induction of MMP-14 activity within the stroma is orchestrated by soluble signals produced by cancer cells. We identify TGF-β1 as one of the potential cytokines that regulates this activity. Overall, our data demonstrate the applicability of this methodology to visualize and quantify the activity of a specific protease in the context of the complex tumor microenvironment. It also shows that, while MMP-14 is expressed at the protein level by both tumor and stromal derived immune cells, it is primarily activated in the immune cell populations as the result of response to tumor cell-derived cytokines.
Results and Discussion
Optimization of the synthesis of probes for cysteine mutant MMP-14.
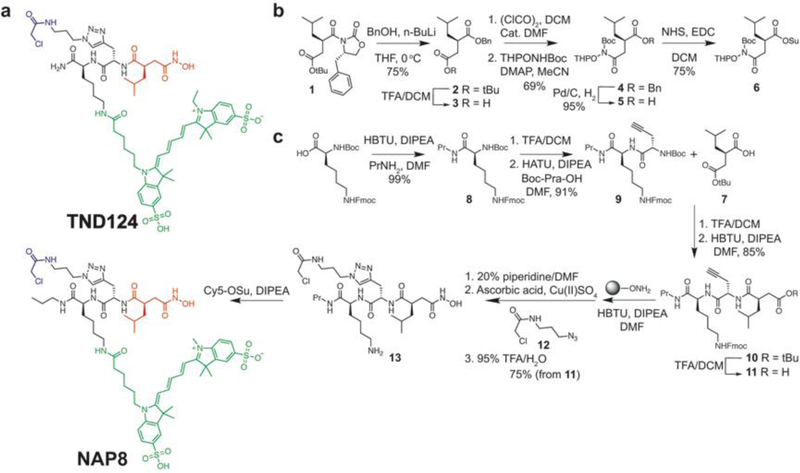
The ABP TND124 (Figure 2a) was previously designed and synthesized by our group to enable specific, activity-dependent labeling of a mutant MMP-14 containing a functional silent cysteine mutation near the active site. It is capable of irreversibly labeling engineered MMP mutants, MMP-12 T184C and MMP-14 F260C. These point mutations do not interfere with enzymatic activity, substrate specificity and protein stability as we have previously reported16. Its design was based on the scaffold of the broad-spectrum MMP inhibitor Ilomastat (GM6001) that employs an isobutylsuccinylhydroxamic acid motif as the affinity recognition element. Difficulties with the chemical stability of the isobutylsuccinylhydroxamic acid and several low yielding steps prompted us to modify the synthesis route. Specifically, our focus turned to issues arising from 1) low yields for the formation of the N-terminal amide 2) low chemical stability of the isobutylsuccinylhydroxamic acid intermediate and 3) considerable loss of material due to the nucleophilic potential of the hydroxamic acid and its cross-reactivity with the electrophilic warhead that is introduced in a click reaction. Replacing the terminal amide of TND124 with a propyl amide in NAP8 (Figure 2), using a solution phase amidation reaction instead of solid support, increased the yields for this step, and potentially improves its metabolic stability. The precursor t-butyl (R)-2-isobutylsuccinate 7 used in the synthesis of TND124 is intrinsically unstable with a high propensity to form a cyclic anhydride, leading to the formation of multiple byproducts both in basic and in acidic reaction conditions. To get around instability of 7 and avoid the cross-reactivity of the unprotected hydroxamate towards the electrophilic warhead of the probe, we sought to pursue a strategy that replaces 7 with a protected hydroxamate. Thus, we synthesized precursor 5, adopting a published strategy,27, 28 but quickly discovered that its instability was even greater than that of 7 (Fig. 2b). Preparing the activated ester derivative 6 slightly improved stability but still resulted in considerable byproduct formation and low reaction yields in the consequent coupling step. We determined that a hydroxylamine Wang resin could be used to both produce the final hydroxamate product and also serve as a protecting group while introducing the electrophilic warhead (Figure 2c). To improve reaction yields for the coupling of 7, we used HBTU and DIPEA because it balances mild activation with fast reaction time, minimizing byproducts and increasing reaction yields. To facilitate the click reaction on the solid support (CuAAC-SP), we used anhydrous CuSO4 dissolved in DMF as the source of Cu(II) ions along with ascorbic acid as the reducing agent. An overnight reaction with incubation at 37 °C with agitation increased the yields from 18% to 75% over four steps from 11 to 13. Collectively, these modifications of the synthesis route enable the synthesis of NAP8 at multi-milligram scale.
Figure 2:
Chemical structure and optimized synthesis of an ABP that targets the cysteine-mutant MMP-14. a) ABPs TND124 and NAP8 are designed based on the core scaffold of GM6001 and comprise its high affinity isobutylsuccinylhydroxamic acid motif (red) with the addition of a chloromethyl amide electrophilic warhead (blue) and a Cy5 fluorescent tag (green). NAP8 has an additional propylamide to improve its metabolic stability. b) Optimized synthesis strategy to prepare double-protected hydroxylamine derivative 6, synthesis procedures were adopted from Overkleeft et al.28 c) Improved synthesis scheme for NAP8, employing an on-resin click reaction to add the electrophile 12 in the penultimate reaction step.
Generation of a mouse line endogenously expressing probe-sensitive MMP-14.
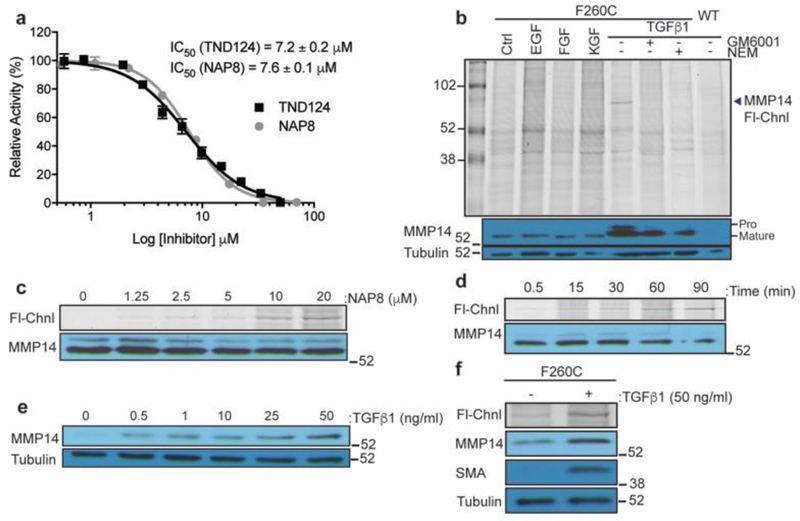
To confirm that the binding and inhibition properties of NAP8 do not differ from TND124 we incubated recombinant MMP-14 F260C active domain with each probe and compared the IC50 inhibition values (Figure 3a). As expected, both TND124 and NAP8 had nearly identical IC50 values towards the recombinant enzyme, roughly 100-fold higher than that of GM6001.29 MMP-14 is tightly regulated by various mechanisms including compartmentalization and trafficking, enzymatic activation, inhibition by small molecules or TIMPs, degradation and clearance.7, 30 Therefore, it is essential to achieve expression of the cysteine mutant MMP-14 from the endogenous gene locus to be able to make relevant conclusions about its activation and regulation. Using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology, we generated a knock in mouse in which the native mMmp-14 gene was replaced with the gene containing the F260C mutation. This was achieved by changing Thymidine to Guanine at the 6688th nucleotide of mMmp-14 cDNA (Figure S1a–c), followed by pronuclear microinjection of guide RNA (gRNA), single stranded oligo donor nucleotide (ssODN) and Cas-9 mRNA into a C57BL/6 embryo (conducted by Applied StemCell Inc, Milpitas, CA). After implantation and isolation of offspring, we identified heterozygous founders by PCR screening. These were used to generate homozygous mMmp14-F260C F1 mice using standard breeding schemes (Figure S1d). Importantly, we observed no detectable phenotypes associated with the presence of the homozygous mutation with growth and breeding indistinguishable from that of the wild type (WT) mice. This confirms that the cysteine mutation on MMP-14 is functionally silent as shown in our prior studies.16
Figure 3:
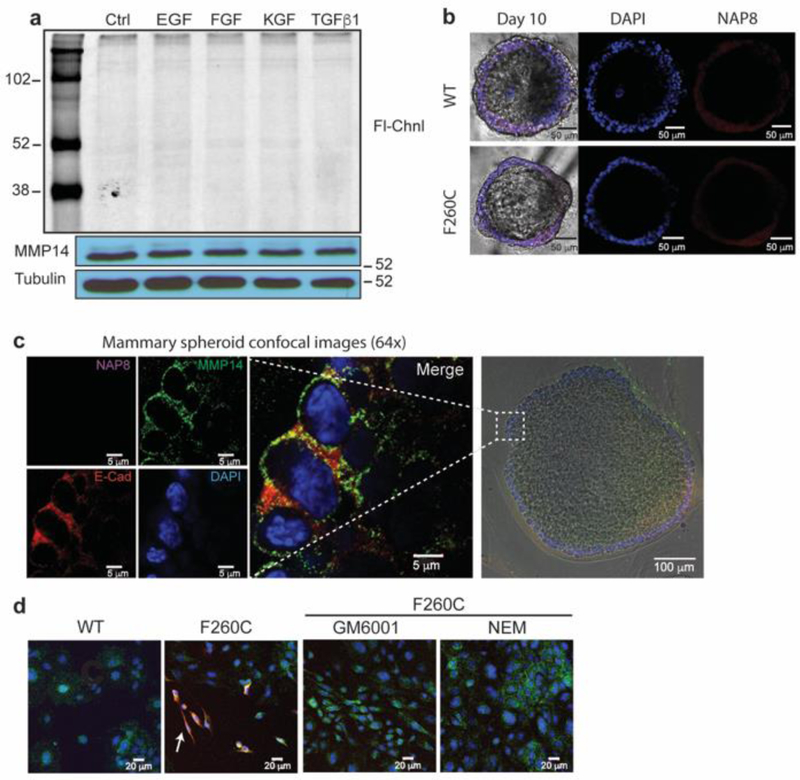
Active MMP-14 is selectively labeled by ABPs in stimulated fibroblasts. a) Recombinant MMP-14 F260C catalytic domain was incubated with TND124 or NAP8 over a range of concentrations. Enzyme activity was measured with the MMP FRET substrate MCA-PLGL-Dap(Dnp)-AR-NH2 (Anaspec). Activity is relative to a DMSO control. Each data point represents an average of three replicates, error bars ± SD. b) Primary mouse fibroblasts derived from the MMP14-F260C knock in mice and immortalized WT fibroblasts were serum starved for 12 hours followed by 24 hours incubation in the presence or absence of growth factors (all at 10 ng/ml), GM6001 (100 μM) and NEM (100 μM). Cell cultures were then incubated with NAP8 (10 μM) for 60 minutes and washed. Cell were lysed in sample buffer and resolved on SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14 and α-tubulin. Locations for pro-MMP14 (60kDa) and mature MMP14 (57kDa) are marked. c) Serum starved primary mMmp14-F260C fibroblasts were incubated with TGFβ1 for 24 hours then NAP8 over a range of concentrations as indicated. Cell lysates were analyzed by SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with MMP-14 antibody. d) Same as c) except cells were labeled with 10 μM NAP8 for the indicated incubation times. e) Serum starved WT fibroblasts were incubated with TGFβ1 over a range of concentrations for 24 hours. Western blot analysis was performed with antibodies for MMP-14 and α-tubulin. f) Serum starved primary mMmp14-F260C fibroblasts were incubated in the presence or absence of TGFβ1 (50 ng/ml) for 24 hours, then treated with NAP8 (10 μM). Cell lysates were analyzed by SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14, α-smooth muscle actin (SMA) and α-tubulin.
Engineered ABPs specifically label probe-sensitive MMP-14 in activated fibroblasts.
Using primary fibroblasts generated from the mMmp14-F260C mice, we performed labeling studies with the NAP8 probe to assess its overall selectivity and to determine the levels of MMP-14 activity in those cells. We first labeled WT and mMmp14-F260C fibroblasts after incubation with multiple paracrine factors such as epithelial growth factor (EGF), fibroblast growth factor (FGF), keratinocyte growth factor (KGF) and transforming growth factor β1 (TGFβ1) over 24 hours (Figure 3b). We then treated intact cells with NAP8 and resolved lysates by SDS-PAGE. Surprisingly, in the absence of growth factors, no specific labeling was observed. However, labeling of fibroblasts that were stimulated with TGFβ1 showed a selective fluorescent band that corresponds to the active MMP-14. The labeled protein was only observed in the cells expressing the mutant MMP-14 F260C and was blocked by pre-incubation with excess of the non-specific hydroxamate inhibitor Ilomastat (GM6001) or the general cysteine reactive molecule N-ethylmaleimide (NEM) suggesting that it results from labeling of the probe sensitive MMP-14 by the NAP8 probe. Consistent with this finding, western blot of MMP-14 protein levels showed increased expression in fibroblasts only when treated with TGFβ1 and not with other cytokines such as EGF, FGF and KGF. Notably, the labeled band for active MMP-14 in figure 3b appears to migrate slightly higher than the expected ~57kDa for the mature active protein. The intensity of labeling was dependent on both probe concentration (Figure 3c) and labeling time (Figure 3d) as expected for a covalent modifier. In addition, incubation with TGFβ1 increased the expression of MMP-14 in a dose dependent manner (Figure 3e). This increase in MMP-14 activity corresponds with the TGFβ-induced differentiation program of fibroblasts into myofibroblasts31 that is accompanied by the production of α smooth muscle actin (SMA; Figure 3f and S2).32
MMP-14 is active in primary macrophage cells.
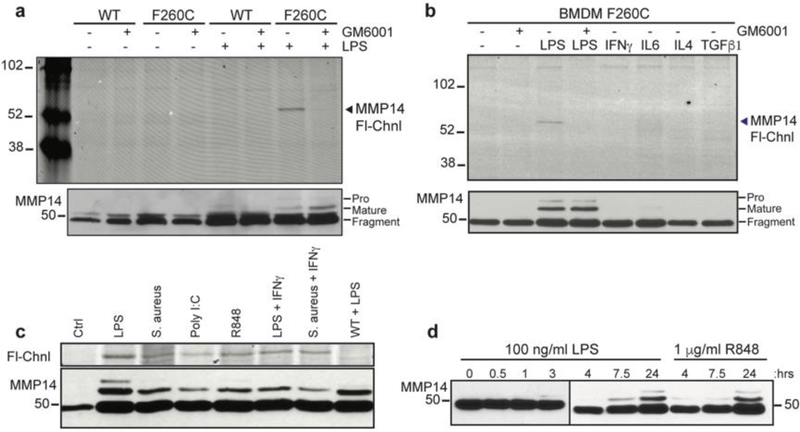
We next assessed the labeling of MMP-14 in bone marrow derived macrophages (BMDMs) isolated from either WT or mMmp14-F260C mice. As observed for primary fibroblasts, we found no probe labeling of MMP-14 in unstimulated BMDMs (Figure 4a). When we stimulated the cells with a range of cytokines including TGFβ1, IFN-γ, IL6 and IL4 we again found no labeling by the NAP8 or TND124 probes. However, we did observe labeling of active MMP-14 in BMDMs treated with LPS, and this labeling was blocked by pretreatment with GM6001 (Figure 4b). Western blot analysis revealed that the mature 57kDa form of MMP-14 accumulated only upon stimulation by LPS. Unstimulated cells and cells treated with the panel of cytokines showed accumulation of only a 44kDa fragment. Previous studies have identified the 44kDa fragment as the product of autocatalytic processing that takes place in the absence of TIMP-2. Sequential cleavages at Ala255 and Gly284 of the 57kDa species generates an inactive 18kDa soluble species and a membrane bound 44kDa species, missing the entire catalytic domain.30 LPS activates macrophages to their pro-inflammatory, M1 functional state33 and results in MMP-14 activation. Stimulation by immune-regulatory cytokines such as IFNγ and IL-6 (M1 activation) or IL-4 and TGFβ1 (M2 activation) do not result in increased activity of MMP-14 (Figure 4b). To test if this is an LPS-specific response we tested the effect of different agonists of toll-like receptors (TLRs). Stimulation of macrophages with multiple different pathogen-associated molecular patterns (PAMPs) triggered a uniform response resulting in activation of MMP-14 (Figure 4c). Sensing of pathogen-associated molecular patterns PAMPs is a general response that leads to the pro-inflammatory M1 activation, a state that has been implicated with generally higher proteolytic activity.31, 34 Priming of macrophages with PAMPs, either LPS or R-848, results in the increase in levels of the mature form of MMP-14 over 24 hours (Figure 4d), suggesting that activation of pro-inflammatory response through TLRs leads to an increase in levels of active MMP-14.
Figure 4:
Active MMP-14 is detected only in M1 activated macrophages. Primary bone marrow-derived macrophages (BMDMs) from the WT or mMmp14-F260C knock in mice were primed in the presence of a) LPS (100 ng/ml) and b) other pro-inflammatory cytokines; IFNγ (40 ng/ml), IL6 (50 ng/ml), IL4 (10 ng/ml), TGFβ1 (10 ng/ml) for 16 hours, and treated with GM6001 (10 μM) or DMSO vehicle control. Cell cultures were treated with TND124 (10 μM) and lysed cells were analyzed by SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14 and α-tubulin. Locations for pro-MMP-14, mature MMP-14 and an inactive fragment are marked. c) Primary mMmp14-F260C and WT BMDMs were primed with different TLR agonists; LPS (100 ng/ml), S. aureus (heat inactivated, 1×107 cells/ml), Poly I:C (1 μg/ml), R-848 (1 μg/ml), IFNγ (50 ng/ml) for 16 hours. Cell cultures were treated with TND124 (5 μM) and lysed cells were analyzed by SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with MMP-14 antibody. d) Western blot analysis for BMDMs primed with LPS (100 ng/ml) or R-848 (1 μM) over 24 hours.
MMP-14 activity is tightly regulated on the surface of mammary epithelial cells.
After determining that MMP-14 activity is found predominantly on activated fibroblasts and macrophage cells, we wanted to examine how the activity of this protease may be regulated on mammary epithelial cells during normal cell growth and morphogenesis. The development of the mammary gland is an ideal model to study the physiologic roles of MMPs. This complex organ undergoes continuous changes in structure and function from initial development through cycles of lactation and involution.35 MMPs play a crucial role in ECM remodeling that takes place during ductal growth and morphogenesis.6, 36 Mammary epithelial cells or epithelial organoids can be grown in three dimensional ex-vivo cultures (3D-cultures). These 3D–cultures recapitulate numerous features of glandular epithelium in vivo, including differentiation of myoepithelial and luminal cells and the formation of polarized spheroids with a hollow lumen.37, 38 We therefore generated mammary organoids by isolation of cells from the inguinal fat pads of WT or mMmp14-F260C virgin females. We treated organoid cultures with NAP8 and lysed cells for analysis by fluorescence scanning. Surprisingly, while the mature 57kDa MMP-14 species is present as confirmed by western blot, we could not detect labeling of active MMP-14 in these cells with our probe (Figure 5a). This suggests that while mature MMP-14 is present, its activity may be kept in check by endogenous inhibitors. Incubation with different growth factors, among these TGFβ1, for 24 hours did not increase the expression of MMP-14 nor change its activation state. To understand if chemical cues or stimuli are needed for MMP-14 activation, we seeded mammary organoids on collagen matrix (Matrigel) and allowed development and maturation of the spheroids over the course of 10 days. At day 10, we incubated spheroids with NAP8 to label active MMP-14 and then performed fluorescence microscopy (Figure 5b). Active MMP-14 was not detected on the surface of the mature spheroids, and no significant differences in fluorescence were observed between the WT spheroids and the mMmp14-F260C spheroids. To further assess the presence and specific localization of MMP-14 on mature spheroids, we fixed probe labeled spheroids on slides for immunofluorescence analysis. Anti-MMP-14 staining showed its localization in small micro-domains on the apical membrane, where it co-localizes with E-cadherin forming tight junctions between neighboring cells (Figure 5c). The lack of probe fluorescence indicates that MMP-14 is inactive on the surface of the spheroids, suggesting that growth and expansion of the spheroid into the matrix is independent of MMP-14 or alternatively, MMP-14 activity may be contributed from cell populations present in the stroma, that are not present in our 3D cultures. It is important to note that Matrigel contains an undefined mixture of proteins and can include hormones, growth factors or enzymes that may influence the activation or inhibition of MMP14 on the surface of the spheroids. When organoids were plated and cultured in 2D-cultures, prior to separation from populations of stromal cells, probe labeling revealed that active MMP-14 is present on sub-populations of cells other than the epithelial cells (Figure 5d and S3). These labeled cells have spindle morphology resembling that of mesenchymal cells such as fibroblasts or myeloid cells that reside in the stroma. Probe labeling of these cell populations was specific to the probe sensitive MMP-14 in the mMmp14-F260C organoids. Preincubation with excess of Ilumastat (GM6001) or NEM blocked labeling by NAP8.
Figure 5:
Active MMP-14 is not detected on the surface of primary epithelial cells. a) Primary mMmp14-F260C epithelial cells were serum starved for 12 hours followed by 24 hours incubation in the presence of absence of growth factors (10 ng/ml). Cell cultures were treated with NAP8 (10 μM) and lysed cells were analyzed by SDS-PAGE followed by in-gel fluorescence scanning for Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14 and α-tubulin b) Confocal microscopy of mammary spheroids in 3D-cultures. Primary organoids isolated from WT and mMmp14-F260C 14-week virgin females were cultured on Matrigel for 10 days. At day 10 spheroids were treated with NAP8 (10 μM) fixed, stained with the molecular marker DAPI and visualized by confocal microscopy (scale bar 50 μm). c) Magnified images (64X) of sections of mMmp14-F260C spheroids treated with NAP8 (10 μM, purple) and stained with antibodies for MMP-14 (green), E-cadherin (red) and DAPI (blue). The magnified section is marked with a white box (scale bar 5 μm) on the white light image of the spheroid (bottom right). d) Crude primary organoid extract, grown in 2D-cultures were pretreated with GM6001 (10 μM), NEM (100 μM) or DMSO and then treated with NAP8(10 μM). Cells were fixed and stained with anti-MMP-14 and DAPI and visualized by confocal microscopy. NAP8 (red), MMP14 (green), DAPI (blue), cells that have active MMP14 are marked with a white arrow (scale bar 20 μm).
Construction of a cancer model to study the pathophysiologic role of MMP-14.
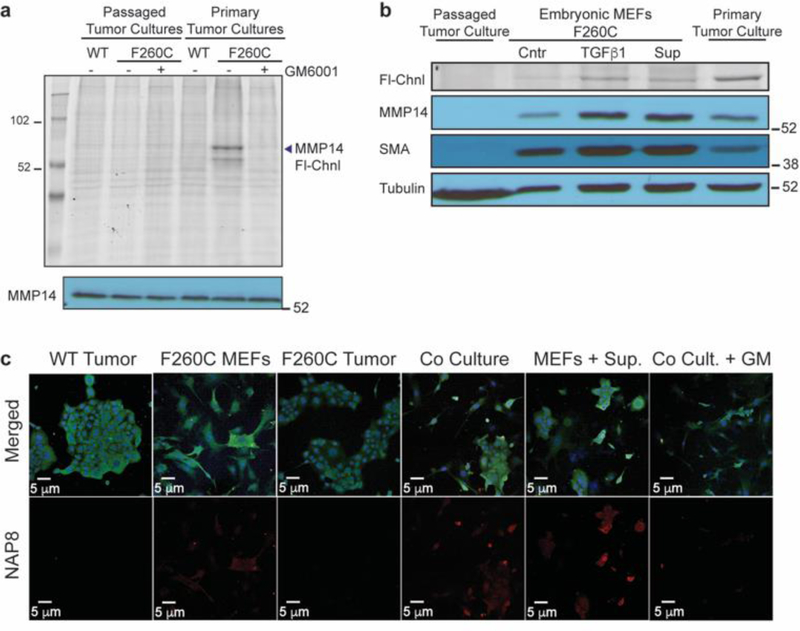
To better understand the role of MMP-14 in regulation of the tumor microenvironment, we crossed the mice expressing the probe sensitive MMP-14 with mice that express the Polyoma Virus middle T antigen under the direction of the mouse mammary tumor virus promoter (MMTV-PyMT). MMTV-PyMT+ females develop mammary tumors that become palpable typically at around 140 days of age and metastasize to the lung. This allowed us to harvest tumors from the mammary glands of mMmp14-F260CWTMMTV-PyMT+ and mMmp14-F260C+/+MMTV-PyMT+ females. After the initial digestion and dissociation of tumor tissue, we filtered cells and plated them for culture. Primary cell cultures treated with NAP8 show specific labeling of MMP-14 only in the cells derived from tumors from the mice expressing MMP14-F260C but not from mice expressing WT MMP-14, confirming the presence of active MMP-14 in the tumor microenvironment (Figure 6a). However, after multiple passages of the cells in culture, the MMP-14 activity was completely lost. While primary, non-neoplastic cells (i.e. stromal cells) reach replicative senescence and perish after 4–5 passages, transformed cancer cells proliferate and dominate the culture. After 10 passages the entire cell population consists of neoplastic epithelial cancer cells. This population of cells, although clearly expressing MMP-14, shows no active MMP-14 as assessed by probe labeling (Figure 6a). We therefore concluded that the observed labeled active MMP-14 from the primary tumor cultures originates from non-neoplastic cells that normally exist in the tumor microenvironment. These cell populations include fibroblasts, bone marrow derived cells, leukocytes and endothelial cells.26, 39 Activated fibroblasts, also known as cancer associated fibroblasts (CAFs) are myofibroblast populations present at the tumor-stroma interface.31
Figure 6:
The majority of MMP-14 activity originates from stromal cells in the microenvironment of late-stage carcinoma that respond to stimulation by tumor cells. a) Tumors harvested from the mammary glands of mMmp14(F260C)WTMMTV-PyMT+ and mMmp14(F260C)+/+MMTV-PyMT+ mice were cultured in 2D-cultures and treated with NAP8 (10 μM) at the first passage or after 10 passages. Whole cell lysates were analyzed by SDS-PAGE and scanned for in-gel fluorescence of Cy5 (Fl-Chnl). Western blot analysis was performed with MMP-14 antibody. b) Primary tumor cultures, passaged tumor cultures (neoplastic epithelial cells) and primary fibroblasts from mMmp14(F260C)+/+MMTV-PyMT+ mice were incubated with TGFβ1 (50 ng/ml) or supernatant of neoplastic epithelial cells (24 hours, serum free medium) for 24 hours, then treated with NAP8 (10 μM). Whole cell lysates were analyzed by SDS-PAGE and scanned for in-gel fluorescence of Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14, α-smooth muscle actin (SMA) and α-tubulin. c) 2D-cultures of mMmp14(F260C)WTMMTV-PyMT+ and mMmp14(F260C)+/+MMTV-PyMT+ passaged tumor cells, fibroblasts and co-cultures of passaged tumor cells and fibroblasts were pretreated with DMSO or GM6001 (10 μM), or pre-incubated with supernatant of neoplastic epithelial cells (24 hours, serum free medium), then treated with NAP8 (10 μM). Cells were fixed and stained with anti-MMP-14 and DAPI and visualized by confocal microscopy. NAP8 (red), MMP14 (green), DAPI (blue), each experiment was performed in three independent biological repeats (scale bar 20 μm).
To test our hypothesis, we compared the expression and activity profile of MMP-14 in primary tumor cultures to that of TGFβ1-stimulated myofibroblasts (Figure 6b). An increase in MMP-14 expression as well as probe labeling was observed in primary tumor cultures, similar to TGFβ1-stimulated fibroblasts. The expression of SMA in the primary tumor culture confirmed the presence of myofibroblasts. Although the total expression of MMP-14 was lower than that of fibroblasts, primary tumor cultures consists primarily of cancer cells that express lower levels of MMP-14, thus the observed increase results from a minority population of cells. Nevertheless, MMP-14 activity is highest in the primary tumor cultures as evidenced by probe labeling, presumably by stimulation through TGFβ1 signaling or in conjunction with additional factors. A similar enhancement in expression of MMP-14 was acheived by incubation of fibroblasts with tumor cell culture supernatant. Stimulation through soluble factors also resulted in increased activity of MMP-14, although not to the extent seen in the primary tumor cultures.
While TGFβ1 produced by tumor cells may act on fibroblasts to increase MMP-14 expression and activation, additional signals such as physical contact of tumor cells with the surrounding CAFs may be needed for substantial activation of MMP-14. To recapitulate the interactions between tumor cells and fibroblasts in the tumor microenvironment, we co-cultured tumor cells with embryonic fibroblasts on cover slides, followed by incubation with NAP8 to label active MMP-14 before cells were fixed and stained with an MMP-14 antibody and DAPI (Figure 6c). Similar to the biochemical results, epithelial cancer cells, although expressing MMP-14, do not show labeling by the probe. Embryonic fibroblasts that are not stimulated express high levels of MMP-14 but virtually all of this is in an inactive state. On the other hand, fibroblasts that are co-cultured with cancer cells have substantial levels of active MMP-14, as assessed by NAP8 labeling. This increase is likely due to signaling events that involve soluble factors given that fibroblasts that are cultured with the supernatant of cancer cells exhibit a similar induction of MMP-14 activity that can be blocked by excess GM6001.
Conclusions
MMP-14 is associated with multiple human cancers and is considered the major cell-associated protease necessary to confer invasive properties to normal or neoplastic cells.18 Its role in breaching the basement membrane during the early stages of invasion is well established, both in the physiologic and pathologic states.18, 40 However, its role in infiltration and migration through the interstitial tissue at the later stages of tumorigenesis is still subject to debate, as are the molecular cues that stimulate its activity. MMP-14 has been implicated in tissue-invasive activity but mounting evidence suggests that the majority of MMP-14 is expressed at the tumor-stroma interface rather than within the bulk of the tumor.8, 19, 21, 41, 42 Significant proteolytic activity is attributed to the stromal cell populations that are stimulated through the cross talk between the tumor and its microenvironment.26, 39, 43–45 While many of these studies make use of expression of mutants or deletion of MMP-14, there remains an insufficient understanding of its activation pattern in the context of complex multi-cellular tissues.3, 46, 47 Using an engineered probe-sensitive MMP-14 and a corresponding ABP, we were able to profile the activity levels of this protease in vivo in several cell types as well as in the components of the microenvironment of late stage breast cancer tumors.
Our findings support the notion that MMP-14 activity is tightly regulated in the absence of stimulatory signals. This regulation is well documented in the literature with TIMP-2 as the major endogenous inhibitor of MMP-14.6, 7, 36 The interaction between MMP-14 and TIMP-2 has been suggested to increase stability of MMP-14 and prevent autoproteolytic degradation.30, 48 While the autoproteolytic degradation product was the predominant form of MMP-14 observed in non-differentiated (M0) or alternatively activated (M2) macrophages, classically activated (M1) macrophages had increased levels of mature MMP-14 and active MMP-14 was detected by our probe only in classically activated (M1) macrophages. This finding is consistent with previous findings that M1 macrophages exhibit enhanced matrix degradation capabilities, and increased localization of MMP-14 in filopodia.24 The compartmentalization of MMP-14 in filopodia or invadopedia has also been associated with increased stability of MMP-14 and elevated collagenase activity.22 Interestingly, the activation of MMP-14 in M1 macrophages requires stimulation through TLRs with pro-inflammatory cytokines such as IFNγ and IL6 not eliciting MMP-14 activation.
MMP-14 activity was also low in non-stimulated fibroblasts. We have identified TGFβ1 as a paracrine factor that not only increases expression49 but also induces the activation of MMP-14. This activation correlates with the initiation of the myofibroblast differentiation program, which is accompanied by elevated MMP-14 protein levels. Interestingly, we found that labled MMP-14 migrated as a slightly higher molecular weight species in fibroblasts compared to macrophages. This difference in migration could be due to modifications of the enzyme that alter its regulatory activities in different cell types. Consistent with this hypothesis, various post translational modifications and protein-protein interactions have been attributed to the short cytoplasmic tail of MMP-14 which could result in the observed differences in migration in the gel50–54. While additional studies will be required to confirm such modifications and their biological significance, these data highlight the value of the active site probe for identifying the specific active form a target protease.
In primary tumor cultures, we observed high activity of MMP-14, however this activity originated from non-neoplastic fibroblasts and other stromal cell populations and not from transformed cancer cells. We found that MMP-14 activation is stimulated by soluble factors secreted by the tumor cells. One such effector is TGFβ1, which acts to differentiate fibroblasts in the stroma and stimulate expression and activation of MMP-14. TGFβ1 signaling has been suggested to promote tumor growth, progression and migration at later stages of multiple carcinomas.55–58 Santibanez et al. describe TGFβ signaling in the tumor microenvironment as a regulatory loop, where TGFβ is overproduced by late stage cancer cells causing increased expression of MMPs, which in turn mediate the activation of more latent TGFβ59. Thus, through balancing levels of MMPs and TIMPs, both TGFβ and MMPs contribute to tumor progression by the release of cytokines sequestered by the ECM. Similarly, TGFβ increases the expression of Furin, a protease known to activate Pro-MMP-14 as well as Pro-TGFβ but not the expression of TIMPs, thus potentially enhancing the proteolytic response32, 60. Fibroblasts that were co-cultured with tumor cells show similar patterns of MMP-14 activity, suggesting that CAFs similarly stimulated by tumor cells not only express more MMP-14 but also increase levels of active MMP-14. TGFβ1 and perhaps additional soluble factors are responsible for the activation of MMP-14 in fibroblasts that are cultured with supernatants of tumor cells. As tumor cells invade through the basement membrane and make contact with the three-dimensional collagenous matrix of the interstitial tissue, paracrine signaling leads to the recruitment of stromal cells, mostly fibroblasts and macrophages. These cells within the invasion front support tumor progression, help evade the immune system and sustain the flow of nutrients.2, 17, 61, 62 Our data supports this model in which the invading cancer cells mobilize and activate MMP-14 in these recruited stromal populations to negotiate the ECM barrier.39, 43
Herein we demonstrate the utility of a methodology that involves the coupling of protein engineering with ABP design to specifically and selectively label active MMP-14 in the context of live cells. Generation of mice endogenously expressing the probe sensitive cysteine mutant MMP-14 F260C followed by crossing of the mice into a model of breast cancer provides a means to selectively image MMP-14 in complex cellular environments. Specifically, it enables the dissection of specific roles of MMP-14 at various stages of tumorigenesis as well as the contribution of specific cell populations within the tumor microenvironment. We have shown that signaling cascades initiated by TGFβ1 or through TLRs lead to the activation of MMP-14 on fibroblasts and macrophages and furthermore that these are the cells that predominantly express active MMP-14. Further studies may elucidate additional signaling pathways that lead to the activation of MMP-14 on other cell types. A clear understanding of the activity profile of MMP-14 is paramount to determine specific cell populations to be targeted for therapeutic applications. In addition, this general methodology is applicable to additional MMP members as well as other large enzyme families that lack selective inhibitors and dynamic reporters of enzyme activity levels.
Materials and Methods
All materials and methods can be found in the Supporting Information.
Supplementary Material
Acknowledgements
This work was supported by funding by National Institutes of Health grant R01 CA179253 to M.B. M.T. was supported through a postdoctoral research fellowship by the German Research Foundation (DFG). N.A. was supported by an EMBO postdoctoral fellowship.
Footnotes
Supporting Information. Supplemental figures, detailed synthetic procedures and compound characterizations can be found in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nagase H., Visse R., and Murphy G. (2006) Structure and function of matrix metalloproteinases and TIMPs, Cardiovasc Res 69, 562–573. [DOI] [PubMed] [Google Scholar]
- 2.Rowe RG., and Weiss SJ. (2009) Navigating ECM barriers at the invasive front: the cancer cell-stroma interface, Annu Rev Cell Dev Biol 25, 567–595. [DOI] [PubMed] [Google Scholar]
- 3.Overall CM., and Kleifeld O. (2006) Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy, Nat Rev Cancer 6, 227–239. [DOI] [PubMed] [Google Scholar]
- 4.Page-McCaw A., Ewald AJ., and Werb Z. (2007) Matrix metalloproteinases and the regulation of tissue remodelling, Nat Rev Mol Cell Biol 8, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks WC., Wilson CL., and Lopez-Boado YS. (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity, Nat Rev Immunol 4, 617–629. [DOI] [PubMed] [Google Scholar]
- 6.Sternlicht MD., and Werb Z. (2001) How matrix metalloproteinases regulate cell behavior, Annu Rev Cell Dev Biol 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadler-Olsen E., Fadnes B., Sylte I., Uhlin-Hansen L., and Winberg JO. (2011) Regulation of matrix metalloproteinase activity in health and disease, FEBS J 278, 28–45. [DOI] [PubMed] [Google Scholar]
- 8.Kessenbrock K., Plaks V., and Werb Z. (2010) Matrix metalloproteinases: regulators of the tumor microenvironment, Cell 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav L., Puri N., Rastogi V., Satpute P., Ahmad R., and Kaur G. (2014) Matrix metalloproteinases and cancer - roles in threat and therapy, Asian Pac J Cancer Prev 15, 1085–1091. [DOI] [PubMed] [Google Scholar]
- 10.Decock J., Thirkettle S., Wagstaff L., and Edwards DR. (2011) Matrix metalloproteinases: protective roles in cancer, J Cell Mol Med 15, 1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Littlepage LE., Sternlicht MD., Rougier N., Phillips J., Gallo E., Yu Y., Williams K., Brenot A., Gordon JI., and Werb Z. (2010) Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression, Cancer Res 70, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherer RL., McIntyre JO., and Matrisian LM. (2008) Imaging matrix metalloproteinases in cancer, Cancer Metastasis Rev 27, 679–690. [DOI] [PubMed] [Google Scholar]
- 13.Furumoto S., Takashima K., Kubota K., Ido T., Iwata R., and Fukuda H. (2003) Tumor detection using 18F-labeled matrix metalloproteinase-2 inhibitor, Nucl Med Biol 30, 119–125. [DOI] [PubMed] [Google Scholar]
- 14.Schafers M., Riemann B., Kopka K., Breyholz HJ., Wagner S., Schafers KP., Law MP., Schober O., and Levkau B. (2004) Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo, Circulation 109, 2554–2559. [DOI] [PubMed] [Google Scholar]
- 15.Sameni M., Anbalagan A., Olive MB., Moin K., Mattingly RR., and Sloane BF. (2012) MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression, J Vis Exp. [DOI] [PMC free article] [PubMed]
- 16.Morell M., Nguyen Duc T., Willis AL., Syed S., Lee J., Deu E., Deng Y., Xiao J., Turk BE., Jessen JR., Weiss SJ., and Bogyo M. (2013) Coupling protein engineering with probe design to inhibit and image matrix metalloproteinases with controlled specificity, J Am Chem Soc 135, 9139–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotary K., Li XY., Allen E., Stevens SL., and Weiss SJ. (2006) A cancer cell metalloprotease triad regulates the basement membrane transmigration program, Genes Dev 20, 2673–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., Allen E., Chung D., and Weiss SJ. (2004) Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP, J Cell Biol 167, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabova L., Yamada SS., Birkedal-Hansen H., and Holmbeck K. (2005) Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland, J Cell Physiol 205, 123–132. [DOI] [PubMed] [Google Scholar]
- 20.Wiseman BS., Sternlicht MD., Lund LR., Alexander CM., Mott J., Bissell MJ., Soloway P., Itohara S., and Werb Z. (2003) Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis, J Cell Biol 162, 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cid S., Eiro N., Fernandez B., Sanchez R., Andicoechea A., Fernandez-Muniz PI., Gonzalez LO., and Vizoso FJ. (2018) Prognostic Influence of Tumor Stroma on Breast Cancer Subtypes, Clin Breast Cancer 18, e123–e133. [DOI] [PubMed] [Google Scholar]
- 22.Murphy DA., and Courtneidge SA. (2011) The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function, Nat Rev Mol Cell Biol 12, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao H., Eppinga RD., Razidlo GL., Krueger EW., Chen J., Qiang L., and McNiven MA. (2016) Stromal fibroblasts facilitate cancer cell invasion by a novel invadopodia-independent matrix degradation process, Oncogene 35, 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohensinner PJ., Baumgartner J., Kral-Pointner JB., Uhrin P., Ebenbauer B., Thaler B., Doberer K., Stojkovic S., Demyanets S., Fischer MB., Huber K., Schabbauer G., Speidl WS., and Wojta J. (2017) PAI-1 (Plasminogen Activator Inhibitor-1) Expression Renders Alternatively Activated Human Macrophages Proteolytically Quiescent, Arterioscler Thromb Vasc Biol 37, 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gocheva V., Wang HW., Gadea BB., Shree T., Hunter KE., Garfall AL., Berman T., and Joyce JA. (2010) IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion, Genes Dev 24, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murdoch C., Muthana M., Coffelt SB., and Lewis CE. (2008) The role of myeloid cells in the promotion of tumour angiogenesis, Nat Rev Cancer 8, 618–631. [DOI] [PubMed] [Google Scholar]
- 27.Altenburger JM., C. M, d’Orchymont H., Schirlin D., Schalk C., Tarnus C.,. (1992) Useful hydroxylamine derivatives for the synthesis of hydroxamic acids., Tetrahedron Letters, 33, 5055–5058. [Google Scholar]
- 28.Leeuwenburgh MA., Geurink PP., Klein T., Kauffman HF., van der Marel GA., Bischoff R., and Overkleeft HS. (2006) Solid-phase synthesis of succinylhydroxamate peptides: functionalized matrix metalloproteinase inhibitors, Org Lett 8, 1705–1708. [DOI] [PubMed] [Google Scholar]
- 29.Levy DE., Lapierre F., Liang W., Ye W., Lange CW., Li X., Grobelny D., Casabonne M., Tyrrell D., Holme K., Nadzan A., and Galardy RE. (1998) Matrix metalloproteinase inhibitors: a structure-activity study, J Med Chem 41, 199–223. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Barrantes S., Toth M., Bernardo MM., Yurkova M., Gervasi DC., Raz Y., Sang QA., and Fridman R. (2000) Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation, J Biol Chem 275, 12080–12089. [DOI] [PubMed] [Google Scholar]
- 31.Santibanez JF., Quintanilla M., and Bernabeu C. (2011) TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions, Clin Sci (Lond) 121, 233–251. [DOI] [PubMed] [Google Scholar]
- 32.Krstic J., and Santibanez JF. (2014) Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells, ScientificWorldJournal 2014, 521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass CK., and Natoli G. (2016) Molecular control of activation and priming in macrophages, Nat Immunol 17, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A., Sozzani S., Locati M., Allavena P., and Sica A. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes, Trends Immunol 23, 549–555. [DOI] [PubMed] [Google Scholar]
- 35.Richert MM., Schwertfeger KL., Ryder JW., and Anderson SM. (2000) An atlas of mouse mammary gland development, J Mammary Gland Biol Neoplasia 5, 227–241. [DOI] [PubMed] [Google Scholar]
- 36.Vu TH., and Werb Z. (2000) Matrix metalloproteinases: effectors of development and normal physiology, Genes Dev 14, 2123–2133. [DOI] [PubMed] [Google Scholar]
- 37.Debnath J., Muthuswamy SK., and Brugge JS. (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures, Methods 30, 256–268. [DOI] [PubMed] [Google Scholar]
- 38.Mroue R., and Bissell MJ. (2013) Three-dimensional cultures of mouse mammary epithelial cells, Methods Mol Biol 945, 221–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D., and Coussens LM. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment, Cancer Cell 21, 309–322. [DOI] [PubMed] [Google Scholar]
- 40.Rowe RG., and Weiss SJ. (2008) Breaching the basement membrane: who, when and how?, Trends Cell Biol 18, 560–574. [DOI] [PubMed] [Google Scholar]
- 41.Packard BZ., Artym VV., Komoriya A., and Yamada KM. (2009) Direct visualization of protease activity on cells migrating in three-dimensions, Matrix Biol 28, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang M., Lu S., Li XY., Xu J., Seong J., Giepmans BN., Shyy JY., Weiss SJ., and Wang Y. (2008) Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging, J Biol Chem 283, 17740–17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noel A., Jost M., and Maquoi E. (2008) Matrix metalloproteinases at cancer tumor-host interface, Semin Cell Dev Biol 19, 52–60. [DOI] [PubMed] [Google Scholar]
- 44.Mueller MM., and Fusenig NE. (2004) Friends or foes - bipolar effects of the tumour stroma in cancer, Nat Rev Cancer 4, 839–849. [DOI] [PubMed] [Google Scholar]
- 45.Tlsty TD., and Hein PW. (2001) Know thy neighbor: stromal cells can contribute oncogenic signals, Curr Opin Genet Dev 11, 54–59. [DOI] [PubMed] [Google Scholar]
- 46.Egeblad M., and Werb Z. (2002) New functions for the matrix metalloproteinases in cancer progression, Nat Rev Cancer 2, 161–174. [DOI] [PubMed] [Google Scholar]
- 47.Overall CM., and Lopez-Otin C. (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era, Nat Rev Cancer 2, 657–672. [DOI] [PubMed] [Google Scholar]
- 48.Zucker S., Drews M., Conner C., Foda HD., DeClerck YA., Langley KE., Bahou WF., Docherty AJ., and Cao J. (1998) Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP), J Biol Chem 273, 1216–1222. [DOI] [PubMed] [Google Scholar]
- 49.Bierie B., and Moses HL. (2006) TGF-beta and cancer, Cytokine Growth Factor Rev 17, 29–40. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto T., and Seiki M. (2009) Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity, Genes Cells 14, 617–626. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto T., and Seiki M. (2010) A membrane protease regulates energy production in macrophages by activating hypoxia-inducible factor-1 via a non-proteolytic mechanism, J Biol Chem 285, 29951–29964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J., Kasberg WC., Celo A., Liang Z., Quispe K., and Stack MS. (2017) Post-translational modification of the membrane type 1 matrix metalloproteinase (MT1-MMP) cytoplasmic tail impacts ovarian cancer multicellular aggregate dynamics, J Biol Chem 292, 13111–13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyalendo C., Michaud M., Beaulieu E., Roghi C., Murphy G., Gingras D., and Beliveau R. (2007) Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration, J Biol Chem 282, 15690–15699. [DOI] [PubMed] [Google Scholar]
- 54.Moss NM., Wu YI., Liu Y., Munshi HG., and Stack MS. (2009) Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices, J Biol Chem 284, 19791–19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhowmick NA., Neilson EG., and Moses HL. (2004) Stromal fibroblasts in cancer initiation and progression, Nature 432, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derynck R., Akhurst RJ., and Balmain A. (2001) TGF-beta signaling in tumor suppression and cancer progression, Nat Genet 29, 117–129. [DOI] [PubMed] [Google Scholar]
- 57.de Caestecker MP., Piek E., and Roberts AB. (2000) Role of transforming growth factor-beta signaling in cancer, J Natl Cancer Inst 92, 1388–1402. [DOI] [PubMed] [Google Scholar]
- 58.de Visser KE., and Kast WM. (1999) Effects of TGF-beta on the immune system: implications for cancer immunotherapy, Leukemia 13, 1188–1199. [DOI] [PubMed] [Google Scholar]
- 59.Quintanilla M., del Castillo G., Kocic J. and Santibanez JF., (2012) TGF-B and MMPs: A Complex Regulatory Loop Involved in Tumor Progression. In: Matrix Metalloproteinases: Biology, Functions and Clinical Implications, Nova Science Publishers, New York, USA. [Google Scholar]
- 60.Blanchette F., Day R., Dong W., Laprise MH., and Dubois CM. (1997) TGFbeta1 regulates gene expression of its own converting enzyme furin, J Clin Invest 99, 1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gal P., Varinska L., Faber L., Novak S., Szabo P., Mitrengova P., Mirossay A., Mucaji P., and Smetana K. (2017) How Signaling Molecules Regulate Tumor Microenvironment: Parallels to Wound Repair, Molecules 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotary KB., Allen ED., Brooks PC., Datta NS., Long MW., and Weiss SJ. (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix, Cell 114, 33–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.