Abstract
Background
Letermovir is a novel cytomegalovirus antiviral that is approved for prophylaxis in hematopoietic stem cell transplantation recipients
Methods
After obtaining informed consent, letermovir prophylaxis was started in a patient with a presumed late-onset primary, combined T- and B-cell immunodeficiency. Plasma CMV DNAemia was monitored with real-time polymerase chain reaction, and letermovir resistance analyses were performed using Sanger sequencing and Illumina MiSeq next-generation sequencing.
Results
A letermovir-resistant cytomegalovirus variant (C325Y mutation in UL56) emerged 17 weeks after start of prophylaxis. The letermovir-resistant variant was able to reactivate without drug selective pressure as this variant was again detected in plasma 20.6 weeks after stopping of letermovir.
Conclusions
This case indicates that the C325Y mutation in UL56 does not significantly alter fitness of cytomegalovirus in vivo.
Keywords: letermovir, resistance, viral fitness
Human cytomegalovirus (CMV) causes significant complications in immunocompromised patients, which can be prevented and treated with the antiviral drugs (val)ganciclovir, foscarnet, and cidofovir. However, treatment is often complicated by drug toxicity, antiviral resistance, and the need for intravenous (i.v.) drug administration [1, 2]. Letermovir, a novel CMV antiviral that was recently approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for prophylactic use in hematopoietic stem cell transplantation (HSCT) recipients, has several advantages over currently used CMV antivirals. Letermovir can be administrated either orally or intravenously and shows mild toxicity. In addition, there is no risk of cross-resistance with existing anti-CMV drugs due to a different mechanism of action [1, 3].
METHODS
After obtaining informed consent, letermovir prophylaxis was started in a patient with a presumed late-onset primary, combined T- and B-cell immunodeficiency. Plasma CMV-DNAemia was monitored by an internally controlled, in-house developed, dual target (UL54 and UL75), real-time polymerase chain reaction (PCR) using the 1st WHO International Standard for Human Cytomegalovirus for Nucleic Acid Amplification Techniques (code: 09/162, National Institute for Biological Standards and Control, Hertfordshire, Great Britain) as quantification standard. Cytomegalovirus-specific T-cell responses were analyzed with CMV ELISPOT (Oxford Immunotec Ltd., Abingdon, Great Britain) and CMV-QuantiFERON (QIAGEN GmbH, Hilden, Germany). Letermovir resistance analysis was performed by genotypic analysis of UL56 using Sanger sequencing and Illumina MiSeq (amplicon sequencing). Part of UL56 was amplified resulting in a PCR product, 1300-bp of which comprises the amino acids 231–369 described to be relevant for letermovir drug resistance. Amplification sensitivity was reached by performing a nested PCR including a first PCR with 25 cycles and a second PCR with 35 cycles. Ganciclovir, foscarnet, and cidofovir resistance analysis were performed by genotypic analysis of UL54 and UL97 using Sanger sequencing. All analyses were performed in ISO15189:2012 (or equivalent) accredited settings.
RESULTS
A 52-year-old female was referred to the Erasmus Medcial Center for analysis of a presumed primary immunodeficiency, after experiencing a recent episode of CMV colitis and CMV retinitis. Immunological analyses showed a combined B- and T-cell deficiency with total T-cell numbers of 200 cells/µL (lower reference value [LRV], 700 cells/µL), CD4+ T cells of 40 cells/µL (LRV, 300 cells/µL), CD8+ T cells of 140 cells/µL (LRV, 200 cells/µL), total B cells of 70 cells/µL (LRV, 100 cells/µL), and a severe hypogammaglobulinemia (immunoglobulin [Ig]G 1.5 g/L [LRV, 7.0 g/L], IgA 0.28 g/L [LRV, 0.76 g/L], IgM 0.21 g/L [LRV, 0.45 g/L]). She was extensively evaluated for a secondary immunodeficiency, including bone marrow aspiration and total-body positron emission tomography/computed tomography scan, which not revealed an underlying (hematological) malignancy. She tested negative for human immunodeficiency virus (HIV). Gene panel testing comprising over 350 primary immunodeficiency-associated genes was performed using whole exome sequencing [4]. No known pathogenic mutations were found. Because no secondary cause of the immunodeficiency was found, a late-onset primary, combined T- and B-cell immunodeficiency was assumed, and she was started on monthly i.v. Ig replacement therapy and 480 mg of co-trimoxazole prophylaxis once daily.
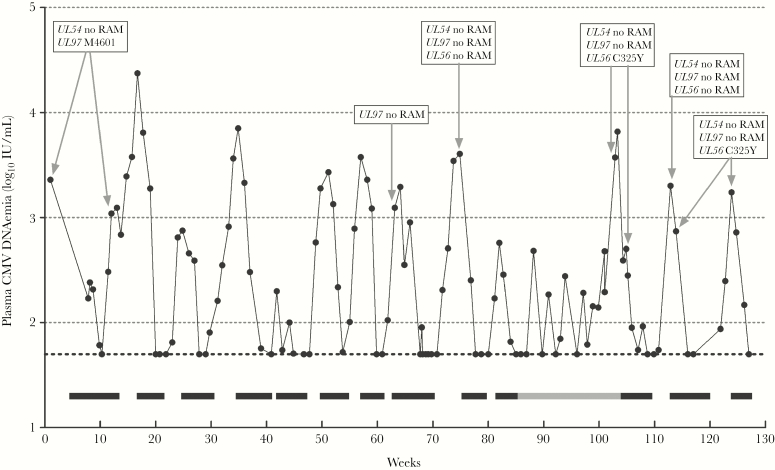
Within the next 2 years, the patient required antiviral therapy for multiple episodes of CMV end-organ disease and CMV reactivations. Because the CMV colitis and CMV retinitis relapse was unresponsive to ganciclovir, a switch to foscarnet was required. Genotypic resistance analyses of plasma and colonic biopsies showed the M460I mutation in UL97, which confers a 4-fold reduced susceptibility to ganciclovir [5]. During the next 18 months, the patient received a total of 10 months i.v. foscarnet for relapses of CMV colitis and retinitis and as pre-emptive therapy (Figure 1). To stimulate her T cells, interferon alpha-2a therapy was started, but it was halted after 3 months because of inefficacy. Cytomegalovirus Igs were combined with foscarnet to treat relapses of CMV end-organ disease. After these 18 months, no specific T-cell responses against CMV were detected (not reactive CMV-ELISPOT and CMV-QuantiFERON), indicating a persisting high risk for severe active CMV infections. Foscarnet i.v. treatments required hospitalization and were complicated by anemia, leukopenia, hypomagnesemia, and renal impairment. Therefore, we explored other treatment options. Cidofovir has considerable renal toxicity and cidofovir resistance mutations and can confer cross-resistance to foscarnet. Maribavir and brincidofovir are not approved by the FDA or EMA yet. Letermovir is FDA and EMA approved, has a mild toxicity profile, and does not confer cross-resistance to other CMV antivirals. Therefore, after obtaining informed consent, the patient was switched to off-label letermovir prophylaxis (480 mg orally once daily) as soon as plasma CMV DNAemia was <50 IU/mL (limit of quantification). During letermovir prophylaxis, several breakthroughs of CMV replication occurred (Figure 1). The patient did not experience CMV end-organ disease during or after letermovir prophylaxis failure. The first breakthrough coincided with an episode of vomiting and may have resulted in poor letermovir uptake. No causes could be identified for the other breakthroughs. Letermovir was continued, because plasma CMV DNAemia rapidly returned <50 IU/mL. However, 11.3 weeks after letermovir start, CMV breakthrough persisted and plasma CMV DNAemia increased to 3730 IU/mL at week 17 and 6580 IU/mL at week 17.4. Genotypic resistance analysis (Sanger sequencing) showed the C325Y mutation in UL56 in the sample obtained at week 17, which confers high-level resistance to letermovir [6, 7]. Resistance mutations against (val)ganciclovir, foscarnet, or cidofovir were not detected in this sample (Sanger sequencing of UL54 and UL97). The letermovir resistance mutation was confirmed with next-generation sequencing (NGS) (MiSeq; Illumina) showing the UL56 C325Y mutation at a frequency of 99.7%. The C325Y mutation was not detected by NGS (below cutoff of 1%) or Sanger sequencing in a sample obtained 2.5 months before start of letermovir, clearly indicating that the C325Y mutation was selected for during prophylaxis with letermovir. Letermovir was stopped and replaced with foscarnet. Ten days after start of foscarnet, the UL56 C325Y mutation was still detected at a frequency of 99.6% (NGS). Foscarnet was continued and plasma CMV DNAemia returned to <50 IU/mL. During the next 4 weeks, the patient did not receive any CMV antivirals. Plasma CMV DNAemia rose again to 2010 IU/mL, and no drug resistance mutations were detected in UL56 (Sanger, NGS), UL54 (Sanger), and UL97 (Sanger). Three days later foscarnet i.v. was restarted, and another 4 days later CMV load declined to 740 IU/mL. We were surprised to find that the C325Y mutation in UL56 was detected in this sample (Sanger, NGS 99.4% frequency), whereas no resistance mutations were detected in UL54 and UL97 (Sanger). Plasma CMV DNAemia declined to below 50 IU/mL. One month after stopping foscarnet, plasma CMV DNAemia rose to 1740 IU/mL and the C325Y mutation in UL56 was again detected by Sanger sequencing. Foscarnet was reinitiated and plasma CMV DNAemia declined to <50 IU/mL.
Figure 1.
Course of plasma cytomegalovirus (CMV) DNAemia during treatment with intravenous foscarnet (black bars) and oral letermovir (gray bar). Dotted horizontal line at 1.7 log10 is the lower limit of quantification (50 IU/mL). Boxes with gray arrows show the results from genotypic resistance analyses.
DISCUSSION
This is the first report on the off-label use of letermovir in a patient with presumed primary, combined immunodeficiency. Letermovir was well tolerated, but several breakthroughs of CMV replication occurred, with emerging antiviral resistance. Our patient was treated with a dose of 480 mg per day, which has proven to be safe and effective as CMV prophylaxis among HSCT recipients in a phase 3 clinical trial [3]. Nevertheless, 7.7% of the participants experienced a breakthrough of CMV replication during prophylaxis. Higher dosages could be more effective in preventing these breakthroughs, but data on this topic are sparse. The registration label reports healthy subjects receiving letermovir dosages ranging from 720 mg per day up to 1440 mg per day for up to 14 days with similar adverse events as the 480 mg per day dosage [8]. Turner et al [9] reported the use of higher dosages for off-label treatment of 4 transplant patients with CMV retinitis (720 and 960 mg per day), but resistance emerged in 2. It should be noted that there is a lack of safety data on sustained high doses of letermovir (>480 mg per day) in published literature. Currently, there is an urgent need for additional data on dosage and effectiveness in different patient groups.
There are an increasing number of reports of acquired letermovir resistance. Among HSCT recipients, UL56 mutations V236M [1, 3], C325W [3], C325F [10], and C325Y [11, 12] are reported; in solid organ transplants patients, the C325Y [9, 13] and C325F [9] are reported. In this case, the UL56 mutation C325Y emerged after 17 weeks of letermovir prophylaxis. Taken together, these data indicate that UL56 mutation at position C325 is the main acquired letermovir resistance mutation in vivo. We recommend tight monitoring of CMV DNAemia during prophylaxis with letermovir because timely resistance might occur. Only a single transition can result in the C325Y mutation reducing susceptibility by >3000-fold with minimal impact on viral replication in vitro [6, 7]. Our results indicate that CMV strains harboring the C325Y mutation in UL56 are able to reactivate in humans without selective drug pressure because the C325Y mutation was still detected in plasma during the second episode of CMV reactivation occurring 20.6 weeks after stopping letermovir. It is interesting to note that during the first episode of CMV reactivation, the UL56 C325Y mutation was not detected (9.6 weeks after stopping letermovir), although 1 week later the mutation was detected. In between these 2 samples, foscarnet was started. Although we cannot rule out a decreased foscarnet susceptibility for the variant harboring the UL56 C325Y mutation, it seems unlikely as previous in vitro studies showed no cross-resistance between letermovir and foscarnet, no foscarnet mutations were detected in this particular variant and the patient responded well to foscarnet treatment. We hypothesize that during this episode multiple CMV variants were reactivating and that the letermovir-resistant variant was the majority in the second sample.
CONCLUSIONS
Regardless of the apparent low barrier to resistance, letermovir can positively contribute to management of CMV infections. First, letermovir has a favorable safety profile compared with other CMV antivirals and is not associated with myelotoxicity and nephrotoxicity. Second, letermovir can be used orally so no hospitalization is needed nor an i.v. access. Finally, letermovir targets the CMV terminase complex instead of CMV deoxyribonucleic acid polymerase so no cross-resistance with the other approved CMV antivirals occurs [3], suggesting discussions about administration of combination therapies or cycling of therapies. However, it should be noted that without immune control, any antiviral therapy of CMV will eventually fail and acquired antiviral drug resistance will emerge [14]. More data are needed to provide insight of in vivo-detected mutations, interpretation of genotyping results, and the clinical relevance of the results. This can all be provided by a CMV registry. Moreover, generated knowledge can contribute to CMV consensus guidelines and treatment algorithms. Databases established as resistance surveillance and resistance interpretation for HIV and hepatitis C virus are successful and provide useful information. A similar approach for CMV would be highly recommended.
Acknowledgments
Author contributions. S. P., V. A. S. H. D., N. L., V. d. C., R. K., C. A. B. B., and J. J. A. v. K. contributed to the concept of the data. S. P., V. A. S. H. D., N. L., V. d. C., and J. J. A. v. K. contributed to data analyses. S. P. and J. J. A. v. K. wrote the original draft. S. P., V. A. S. H. D., N. L., V. d. C., R. K., C. A. B. B., and J. J. A. v. K. wrote, reviewed, and edited the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 17th European Meeting on HIV & Hepatitis, May 22–24, 2019, Rome, Italy.
References
- 1. Chemaly RF, Ullmann AJ, Stoelben S, et al. . Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 370:1781–9. [DOI] [PubMed] [Google Scholar]
- 2. Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23:689–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marty FM, Ljungman P, Chemaly RF, et al. . Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 4. Picard C, Al-Herz W, Bousfiha A, et al. . Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J Clin Immunol 2015; 35:696–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Komatsu TE, Pikis A, Naeger LK, Harrington PR. Resistance of human cytomegalovirus to ganciclovir/valganciclovir: a comprehensive review of putative resistance pathways. Antiviral Res 2014; 101:12–25. [DOI] [PubMed] [Google Scholar]
- 6. Chou S. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother 2015; 59:6588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldner T, Hempel C, Ruebsamen-Schaeff H, et al. . Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother 2014; 58:610–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Summary of product characteristics, PREVYMIS, INN-letermovir - European Medicines Agency - Europa. Available at: www.ema.europa.eu/en/documents/product-information/prevymis-epar-product-information_en.pdf. Accessed 5 september 2019. [Google Scholar]
- 9. Turner N, Strand A, Grewal DS, et al. . Use of letermovir as salvage therapy for drug-resistant CMV retinitis: a case series. Antimicrob Agents Chemother 2019; 63:e02337-18. doi:10.1128/AAC.02337-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knoll BM, Seiter K, Phillips A, Soave R. Breakthrough cytomegalovirus pneumonia in hematopoietic stem cell transplant recipient on letermovir prophylaxis. Bone Marrow Transplant 2019; 54:911–2. [DOI] [PubMed] [Google Scholar]
- 11. Frietsch JJ, Michel D, Stamminger T, et al. . In vivo emergence of UL56 C325Y cytomegalovirus resistance to letermovir in a patient with acute myeloid leukemia after hematopoietic cell transplantation. Mediterr J Hematol Infect Dis 2019; 11:e2019001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung S, Michel M, Stamminger T, Michel D. Fast breakthrough of resistant cytomegalovirus during secondary letermovir prophylaxis in a hematopoietic stem cell transplant recipient. BMC Infect Dis 2019; 19:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherrier L, Nasar A, Goodlet KJ, et al. . Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant 2018; 18:3060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herling M, Schröder L, Awerkiew S, et al. . Persistent CMV infection after allogeneic hematopoietic stem cell transplantation in a CMV-seronegative donor-to-positive recipient constellation: Development of multidrug resistance in the absence of anti-viral cellular immunity. J Clin Virol 2016; 74:57–60. [DOI] [PubMed] [Google Scholar]