Abstract
Background
The use of clinical signs, or end‐tidal anaesthetic gas (ETAG), may not be reliable in measuring the hypnotic component of anaesthesia and may lead to either overdosage or underdosage resulting in adverse effects because of too deep or too light anaesthesia. Intraoperative awareness, whilst uncommon, may lead to serious psychological disturbance, and alternative methods to monitor the depth of anaesthesia may reduce the incidence of serious events. Bispectral index (BIS) is a numerical scale based on electrical activity in the brain. Using a BIS monitor to guide the dose of anaesthetic may have advantages over clinical signs or ETAG. This is an update of a review last published in 2014.
Objectives
To assess the effectiveness of BIS to reduce the risk of intraoperative awareness and early recovery times from general anaesthesia in adults undergoing surgery.
Search methods
We searched CENTRAL, MEDLINE, Embase, and Web of Science on 26 March 2019. We searched clinical trial registers and grey literature, and handsearched reference lists of included studies and related reviews.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐RCTs in which BIS was used to guide anaesthesia compared with standard practice which was either clinical signs or end‐tidal anaesthetic gas (ETAG) to guide the anaesthetic dose. We included adult participants undergoing any type of surgery under general anaesthesia regardless of whether included participants had a high risk of intraoperative awareness. We included only studies in which investigators aimed to evaluate the effectiveness of BIS for its role in monitoring intraoperative depth of anaesthesia or potential improvements in early recovery times from anaesthesia.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We assessed the certainty of evidence with GRADE.
Main results
We included 52 studies with 41,331 participants; two studies were quasi‐randomized and the remaining studies were RCTs. All studies included participants undergoing surgery under general anaesthesia. Three studies recruited only participants who were at high risk of intraoperative awareness, whilst two studies specifically recruited an unselected participant group. We analysed the data according to two comparison groups: BIS versus clinical signs; and BIS versus ETAG. Forty‐eight studies used clinical signs as a comparison method, which included titration of anaesthesia according to criteria such as blood pressure or heart rate and, six studies used ETAG to guide anaesthesia. Whilst BIS target values differed between studies, all were within a range of values between 40 to 60.
BIS versus clinical signs
We found low‐certainty evidence that BIS‐guided anaesthesia may reduce the risk of intraoperative awareness in a surgical population that were unselected or at high risk of awareness (Peto odds ratio (OR) 0.36, 95% CI 0.21 to 0.60; I2 = 61%; 27 studies; 9765 participants). However, events were rare with only five of 27 studies with reported incidences; we found that incidences of intraoperative awareness when BIS was used were three per 1000 (95% CI 2 to 6 per 1000) compared to nine per 1000 when anaesthesia was guided by clinical signs. Of the five studies with event data, one included participants at high risk of awareness and one included unselected participants, four used a structured questionnaire for assessment, and two used an adjudication process to identify confirmed or definite awareness.
Early recovery times were also improved when BIS was used. We found low‐certainty evidence that BIS may reduce the time to eye opening by mean difference (MD) 1.78 minutes (95% CI ‐2.53 to ‐1.03 minutes; 22 studies; 1494 participants), the time to orientation by MD 3.18 minutes (95% CI ‐4.03 to ‐2.33 minutes; 6 studies; 273 participants), and the time to discharge from the postanaesthesia care unit (PACU) by MD 6.86 minutes (95% CI ‐11.72 to ‐2 minutes; 13 studies; 930 participants).
BIS versus ETAG
Again, events of intraoperative awareness were extremely rare, and we found no evidence of a difference in incidences of intraoperative awareness according to whether anaesthesia was guided by BIS or by ETAG in a surgical population at unselected or at high risk of awareness (Peto OR 1.13, 95% CI 0.56 to 2.26; I2 = 37%; 5 studies; 26,572 participants; low‐certainty evidence). Incidences of intraoperative awareness were one per 1000 in both groups. Only three of five studies reported events, two included participants at high risk of awareness and one included unselected participants, all used a structured questionnaire for assessment and an adjudication process to identify confirmed or definite awareness.
One large study (15,452 participants) reported a reduced time to discharge from the PACU by a median of three minutes less, and we judged the certainty of this evidence to be low. No studies measured or reported the time to eye opening and the time to orientation.
Certainty of the evidence
We used GRADE to downgrade the evidence for all outcomes to low certainty. The incidence of intraoperative awareness is so infrequent such that, despite the inclusion of some large multi‐centre studies in analyses, we believed that the effect estimates were imprecise. In addition, analyses included studies that we judged to have limitations owing to some assessments of high or unclear bias and in all studies, it was not possible to blind anaesthetists to the different methods of monitoring depth of anaesthesia.
Studies often did not report a clear definition of intraoperative awareness. Time points of measurement differed, and methods used to identify intraoperative awareness also differed and we expected that some assessment tools were more comprehensive than others.
Authors' conclusions
Intraoperative awareness is infrequent and, despite identifying a large number of eligible studies, evidence for the effectiveness of using BIS to guide anaesthetic depth is imprecise. We found that BIS‐guided anaesthesia compared to clinical signs may reduce the risk of intraoperative awareness and improve early recovery times in people undergoing surgery under general anaesthesia but we found no evidence of a difference between BIS‐guided anaesthesia and ETAG‐guided anaesthesia. We found six studies awaiting classification and two ongoing studies; inclusion of these studies in future updates may increase the certainty of the evidence.
Plain language summary
Bispectral index (BIS) for improving intraoperative awareness and early postoperative recovery in adults
Background
During surgery under general anaesthesia, the anaesthetist will adjust the amount of anaesthetic drugs to ensure that the patient remains unconscious. This adjustment is made according to clinical signs, such as the patient's heart rate or blood pressure, or end‐tidal anaesthetic gas (ETAG) for anaesthesia that is given as a gas, which is a measure of the amount of remaining gas after the patient breathes out. However, using these methods alone may increase the chance that the patient is given too little or too much anaesthetic. Intraoperative awareness, a distressing event in which a patient may become conscious enough to recall events during surgery, is very rare and may be caused by too little anaesthetic. Too much anaesthetic may lead to a longer time needed to reach full recovery. Bispectral index (BIS) is a measurement scale based on the electrical activity in the brain, and by using a monitor of brain activity during anaesthesia, the anaesthetist may use this scale to inform the amount of anaesthesia to give to the patient.
This is an update of a review which was previously published in 2014.
Study characteristics
The evidence is current to 26 March 2019. We found 52 studies with 41,331 participants. Six studies are awaiting classification (because we did not have sufficient information to assess them), and two studies are ongoing. All studies included people having surgery under general anaesthesia. Three studies included only people who were at high risk of intraoperative awareness, and two studies included only people who were not selected according to high risk of intraoperative awareness. Forty‐eight studies compared BIS‐guided anaesthesia with anaesthesia guided by clinical signs, and six studies compared BIS‐guided anaesthesia with ETAG‐guided anaesthesia.
Key results
We found low‐certainty evidence that BIS‐guided anaesthesia may reduce the risk of intraoperative awareness. However, events were rare and only five of 27 studies reported incidences. When BIS‐guided anaesthesia was used, we found three per 1000 fewer incidences of intraoperative awareness compared to nine per 1000 incidences when anaesthesia was guided by clinical signs. In addition, we found low‐certainty evidence that BIS may improve recovery ‐ the time for people to open their eyes was less, as was the time for orientation, and the time to be discharged from the post‐anaesthesia care unit.
We found no evidence of a difference in incidences of intraoperative awareness according to whether anaesthesia was guided by BIS or by ETAG, although, again, there were few incidences of awareness (1 per 1000 in each group). Only one study that compared BIS with ETAG‐guided anaesthesia measured recovery times; this low‐certainty evidence showed that discharge from the postanaesthesia care unit was earlier if anaesthesia was BIS‐guided. No studies that compared BIS with ETAG‐guided anaesthesia measured the time to eye opening or the time to orientation.
Certainty of the evidence
We used GRADE to downgrade the evidence for all outcomes to low certainty. The incidence of intraoperative awareness is so rare and, even though we found some large studies, we concluded that the evidence was still imprecise. In addition, we judged many studies to have limitations because of high or unclear risks of bias. For example, all of the anaesthetists were aware of using an additional BIS monitor and we could not be certain how this affected the anaesthetists' standard practice.
In addition, we noted that some studies did not report a clear definition of intraoperative awareness. Time points of measurement differed, and the methods used to identify intraoperative awareness also differed and we expected that some assessment tools were more comprehensive than others.
Conclusion
Intraoperative awareness is rare, and despite finding a large number of eligible studies, evidence for the effectiveness of using BIS to guide anaesthetic depth is imprecise. We found low‐certainty evidence that BIS‐guided anaesthesia compared to anaesthesia guided by clinical signs may reduce the risk of intraoperative awareness and improve early recovery times in people having surgery under general anaesthesia. We found no evidence of a difference between BIS‐guided anaesthesia and ETAG‐guided anaesthesia, and we also judged this evidence to be low certainty.
Summary of findings
Summary of findings 1. Bispectral index compared to clinical signs for improving intraoperative awareness and early postoperative recovery.
| BIS compared to clinical signs for intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants Setting: hospitals in: Australia; Bangladesh; Belgium; Canada; China; Croatia; Egypt; Finland; Germany; Greece; India; Iran; Israel; Japan; Saudi Arabia; South Korea; Spain; Sweden; Switzerland; Turkey; USA Intervention: BIS‐guided anaesthesia, with target values between 40 and 60 Comparison: anaesthesia guided by clinical sides | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with clinical sides | Risk with BIS | |||||
|
Occurrence of intraoperative awareness Time points of measure after surgery: 2 to 6 hours;12 hours; 1 day; 2 days; 3 days; 14 days; 30 days; or time point was not reported Measurement tools: simple questioning; interviews; or structured questionnaires |
Study population | Peto OR 0.36 (0.21 to 0.60) | 9765 (27 studies) | ⊕⊕⊝⊝ Lowa | Only 5 of 27 studies included incidences of awareness. Of these 5 studies: 4 used a structured questionnaire, and 1 used an interview method; 2 used an adjudication process to categorise incidences of awareness as 'confirmed' or 'definite'; participants in 1 study were at high risk of awareness, in 1 study were unselected, and in the remaining studies risk of awareness was not specified |
|
| 9 per 1,000 | 3 per 1,000 (2 to 6) | |||||
|
Time to eye opening (in minutes) |
‐ | MD 1.78 minutes lower (2.53 minutes lower to 1.03 minutes lower) | ‐ | 1494 (22 studies) | ⊕⊕⊝⊝ Lowb | |
|
Time to orientation (in minutes) |
‐ | MD 3.18 lower (4.03 lower to 2.33 lower) | ‐ | 273 (6 studies) | ⊕⊕⊝⊝ Lowc | |
|
Time to discharge from the PACU (in minutes) |
‐ | MD 6.86 lower (11.72 lower to 2.00 lower) | ‐ | 930 (13 studies) | ⊕⊕⊝⊝ Lowb | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BIS: bispectral index; CI: confidence interval; MD: mean difference; OR: odds ratio; PACU: postanaesthesia care unit | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate.The true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. We downgraded by one level for imprecision; whilst we noted a narrow CI, the effect was dominated by two large trials (with two different populations selected according to the likelihood of intraoperative awareness) and we found many studies with zero events in both arms. We conducted sensitivity analysis to explore alternative statistical models to account for zero events in both arms as well as rare events and found more conservative estimates when we used a random‐effects model, thus reducing our certainty in the estimate. bWe downgraded by one level for inconsistency owing to the substantial statistical heterogeneity in this effect, and by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. cWe downgraded by one level for imprecision as the evidence was from few studies with few participants, and by one level for study limitation owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout.
Summary of findings 2. BIS compared to ETAG for improving intraoperative awareness and early postoperative recovery.
| BIS compared to ETAG for improving intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants Setting: hospitals in: Canada; India; Sweden; and USA Intervention: BIS‐guided anaesthesia, with target values between 40 and 60 Comparison: ETAG‐guided anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ETAG | Risk with BIS | |||||
|
Occurrence of intraoperative awareness Time points of measure after surgery: 24 hours; 24 to 72 hours; 72 hours; 30 days; 18 hours after extubation in the ICU Measurement tools: structured interviews; or structured questionnaire |
Study population | Peto OR 1.13 (0.56 to 2.26) | 26,572 (5 studies) | ⊕⊕⊝⊝ Lowa | Only 3 of these studies included incidences of awareness. Of these 3 studies: all used a structured questionnaire; all used an adjudication process to categorise incidences of awareness as 'definite'; 2 studies included only participants who were at high risk for intraoperative awareness, and 1 study included participants who were unselected |
|
| 1 per 1,000 | 1 per 1,000 (1 to 3) | |||||
| Time to eye opening | ‐ | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | |
| Time to orientation | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | ||
|
Time to discharge from the PACU (in minutes) |
Median (IQR): 98 minutes (66 to 140 minutes) | Median (IQR) 3 minutes lower (2 minutes lower to 2 minutes lower) | ‐ | 15,452 (1 study) |
⊕⊕⊝⊝ Lowb | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BIS: bispectral index; CI: confidence interval; ETAG: end‐tidal anaesthetic gas; ICU: intensive care unit; IQR: interquartile range; OR: odds ratio; PACU: postanaesthesia care unit | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded by one level for imprecision; despite a large number of participants, events were very rare (one per 1000 in the intervention and the comparison group) and the confidence interval for this effect was wide. In addition, we downgraded by one level for study limitations owing to the inclusion of some studies with unclear and high risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. bWe downgraded by two levels for imprecision because the evidence was from one study in which the IQR of time spent in the PACU was wide in both groups.
Background
Description of the condition
The practice of anaesthesia is based on the concept of components of anaesthesia resulting from separate pharmacological actions of multiple agent administration (Kissin 1997). Many anaesthesiologists rely on somatic signs (motor responses, changes in respiratory pattern) and autonomic signs (tachycardia (abnormally rapid heart rate), hypertension (abnormally high blood pressure), lacrimation (flow of tears), sweating) to guide the dosages of anaesthetic agents in order to achieve the basic goals of anaesthetic management; that is unconsciousness (hypnotic effects), blockade of somatic motor responses, and suppression of autonomic responses to noxious stimulation. However, these clinical signs are not reliable measures of the conscious state of anaesthetized patients (Mahla 1997). The use of these clinical signs in judging the dosages of anaesthetic agents can lead to either overdosage or underdosage, which can result in adverse effects due to too deep or too light anaesthesia. Furthermore, there has been much concern regarding intraoperative awareness, which is an uncommon phenomenon occurring in about 0.1% to 0.2% of the general surgical population (Sebel 2004), but which can lead to a serious psychological disturbance called post‐traumatic stress disorder (PTSD), resulting in major depression and suicide. The incidence may approach 1% in surgical patients at high risk for intraoperative awareness such as patients with poor cardiac reserve, or undergoing cardiac surgery or caesarean section, where doses of anaesthetics have to be reduced to a light level of anaesthesia (Mashour 2012; Myles 2004). From a review of reported cases of intraoperative awareness, too light anaesthesia could account for 87% of the cases (Ghoneim 2009). Hence, strategies to provide optimal anaesthesia depth are required to avoid too light anaesthesia.
Description of the intervention
The bispectral index (BIS) is a dimensionless numerical scale for measuring brain electrical activity. It is derived from cerebral electrical activity (an electroencephalogram (EEG)) captured from the scalp surface at the forehead to reflect the sedative and hypnotic components of anaesthesia (Rampil 1998; Schneider 2010). Its value is a number within a range between 0 to 100, where 0 represents 'no detectable brain electrical activity' and 100 represents 'awake state'.
How the intervention might work
BIS has been recommended to guide doses of anaesthetics to achieve optimal depth of anaesthesia in individual patients. This is in order to avoid unnecessarily deep or too light anaesthesia due to overdosage or underdosage of the hypnotic medications during maintenance and recovery from anaesthesia (Schneider 2010; Sebel 2001). The recommended range of BIS is between 40 to 60 during maintenance of anaesthesia (Avidan 2011; Myles 2004) and 55 to 70 at 15 minutes prior to the end of surgery (Gan 1997).
Why it is important to do this review
Several studies have been conducted to assess the effect of BIS monitoring on the utilization of currently available anaesthetic agents, such as propofol, desflurane and sevoflurane (Gan 1997; Johansen 1998; Nelskyla 2001; Song 1997). A survey was conducted among anaesthesiologists regarding the routine use of BIS monitoring in anaesthesia (Johansen 1998). Although the majority of the respondents found that the monitor was easy to use, and it provided useful information, their comments revealed some ambivalence towards hypnotic titration using a BIS monitor. Most respondents felt that no changes occurred in their individual drug usage. Some respondents who reported a change in their practice felt that the hypnotic medication use might decrease, while analgesic and haemodynamic control agent use might increase. A previous study by Song and colleagues (Song 1997) reported increased use of mivacurium in the BIS‐targeted group. Badrinath 1999 reported an increase in the use of intraoperative opioids in the BIS‐guided group. The increased use of either a muscle relaxant or an opioid analgesic might relate to the ability to maintain 'lighter' planes of anaesthesia with BIS, to avoid movement and increased blood pressure or heart rate during the operation.
Since 1977, several articles and abstracts regarding the utility of BIS have been published by numerous medical researchers and academic institutions. It has been suggested that close titration of anaesthetic effect with the BIS monitor may improve some measures of patient outcomes and operating suite efficiency. However, the results are still contradictory across studies. Many studies (Anez 2001; Boztuğ 2006; Gan 1997; Kreuer 2003; Muralidhar 2008; Tufano 2000) have reported a significant improvement in anaesthetics delivery in terms of reduced anaesthetic consumption or requirements and improved recovery profiles, but some studies (Bruhn 2005; Kreuer 2005; Luginbuhl 2003; Zohar 2006) have failed to demonstrate these effects.
Nowadays, the impact of BIS monitoring on the incidence of intraoperative awareness is a matter of interest in anaesthesia practice. The optimisation of the depth of anaesthesia may avoid too light anaesthesia which may result in intraoperative awareness. However, because of the low incidence of intraoperative awareness in an unselected surgical population undergoing surgeries with low risk of intraoperative awareness, an extremely large number of patients would be needed to determine the effects of BIS on awareness (Mashour 2012; O'Connor 2001). Questions regarding the utility of BIS, particularly to assess whether it is beneficial in reducing incidences of intraoperative awareness and improving early postoperative recovery are important in the clinical practice of anaesthesia. Additional studies have been published since the last update of this Cochrane Review (Punjasawadwong 2014), and therefore this review includes the most up‐to‐date evidence for the effectiveness of BIS.
Objectives
To assess the effectiveness of bispectral index (BIS) to reduce the risk of intraoperative awareness and early recovery times from general anaesthesia in adults undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) or quasi‐randomized trials comparing the use of the bispectral index (BIS) with either clinical signs or end‐tidal anaesthetic gas (ETAG) as the standard practice in the titration of anaesthetic agents, regardless of the language of publication of the articles.
We did not include studies with publications that were retracted from journals. And we excluded articles that were only available as abstracts if they were published early than 2005.
Types of participants
We included men and women over 18 years of age undergoing any type of surgery under general anaesthesia. We included participants who were selected because they were at high risk of intraoperative awareness (using any criteria specified by the study authors), and participants who were unselected, or for whom the study investigators did not specify risk of awareness.
Types of interventions
We included studies with at least two arms, which used BIS to guide the dose of an intravenous anaesthetic, a hypnotic, or a volatile anaesthetic and compared it with standard practice, which was either clinical signs or ETAG to guide the anaesthetic dose.
Therefore, the review included the following two comparison groups.
BIS‐guided anaesthesia versus clinical signs‐guided anaesthesia.
BIS‐guided anaesthesia versus ETAG‐guided anaesthesia
We included only studies in which investigators aimed to evaluate the effectiveness of BIS for its role in monitoring intraoperative depth of anaesthesia or potential improvements in early recovery times from anaesthesia.
Types of outcome measures
We included fewer outcomes in the updated review (see Differences between protocol and review). For the primary outcome, we included any events (or lack of events) which were described using the term 'intraoperative awareness', regardless of the time of measure, whether formal collection tools were used or whether reported events were subject to an adjudication process.
in the event that study authors differentiated data for intraoperative awareness as definite or confirmed awareness and possible awareness, we included only data in the analysis that were defined as confirmed or definite awareness.
Primary outcomes
Occurrence of intraoperative awareness
Secondary outcomes
Time to eye opening
Time to orientation
Time to discharge from the postanaesthesia care unit (PACU)
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). We applied no restrictions to language or publication status. We sourced the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 3)
MEDLINE (Ovid SP: 1946 to 26 March 2019)
Embase (Ovid SP; 1974 to 26 March 2019)
Web of Science (SCI‐EXPANDED; 1900 to 26 March 2019)
We developed a subject‐specific search strategy in MEDLINE and other listed databases. The search strategy was developed in consultation with the Information Specialist for the Cochrane Anaesthesia Review Group. Search strategies can be found in: Appendix 1; Appendix 2; Appendix 3; Appendix 4.
We searched the following clinical trial registers for ongoing and unpublished trials.
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/; on 20 June 2019)
ClinicalTrials/gov (www.clinicaltrials.gov; on 7 June 2019)
Searching other resources
We carried out citation searching of identified included studies published since 2013 in Web of Science on 10 June 2019 (apps.webofknowledge.com). We conducted a search of grey literature using Opengrey on 20 June 2019 (www.opengrey.eu/). In addition, we scanned reference lists of relevant systematic reviews which were published since 2010.
Data collection and analysis
Two review authors (SL, and MP or LF) independently selected studies and extracted data from new included studies. We compared decisions at each stage. In cases of disagreement, we reassessed the respective studies to reach consensus.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence software to screen results of the search of titles and abstracts and identify potentially relevant studies (Covidence 2019). We sourced the full texts of all potentially relevant studies and considered whether they met the inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included these in the review only if they provided sufficient information and relevant results that included denominator figures for the intervention and control groups. Because of changes made to the review inclusion criteria (see Differences between protocol and review), we re‐evaluated all studies previously included in the review.
We recorded the number of papers retrieved at each stage and reported this information using a PRISMA flow chart (Figure 1). We did not report details of all studies excluded during the evaluation of full‐text articles; we reported in the review brief details of only closely related but excluded articles.
1.
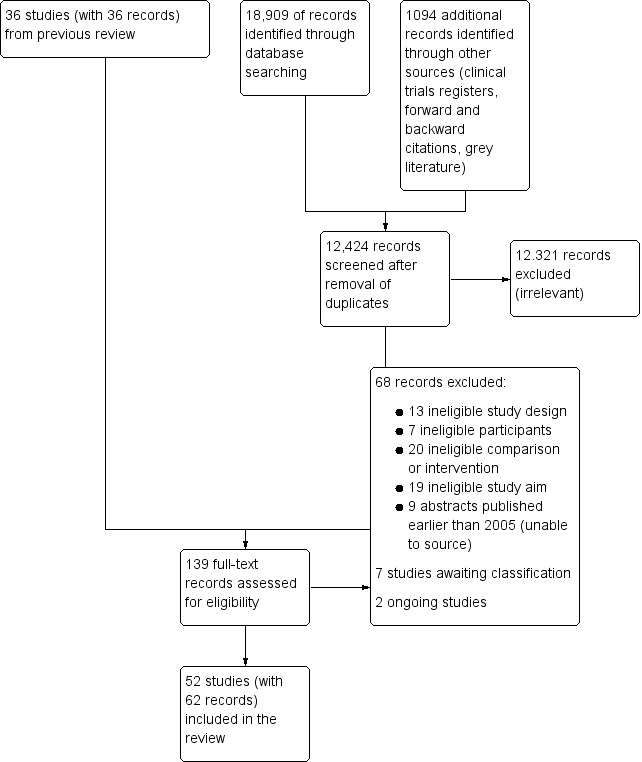
Study flow diagram. Search conducted in March 2019.
Data extraction and management
We used a data extraction form to collect information and outcome data from studies (Appendix 5). We collected the following information.
Methods: type of study design, setting; dates of study; funding sources and study author declarations of interest.
Participants: number randomized to each group; number of losses; number analysed in each group; baseline characteristics (age, gender, American Society of Anesthesiologists (ASA) physical status or other measure of health status; body mass index (BMI); weight; height; type of surgery; and duration of anaesthesia).
Intervention: details of BIS target values; details of control group; anaesthetic agents; experience of anaesthetist.
Outcomes: data for all reported review outcomes to include study author definitions, measurement tools and time points.
We considered the applicability of information from individual studies and generalizability of the data to our intended study population.
In multi‐arm studies, we did not collect data on any groups that were not eligible for inclusion in the review.
Assessment of risk of bias in included studies
We assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We considered the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel, and outcomes assessors (performance and detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other risks of bias.
For each domain, two review authors (SL, and MP or LF) judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using three measures ‐ high, low, or unclear risk of bias. We recorded this in 'Risk of bias' tables and presented summary 'Risk of bias' figures (Figure 2; Figure 3).
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Blank spaces indicate that we did not conduct 'Risk of bias' assessments because studies did not report review outcomes.
Measures of treatment effect
We collected dichotomous data for intraoperative awareness. We collected continuous data for recovery outcomes, which were time to eye opening, time to orientation, and time to discharge from the postanaesthesia care unit (PACU).
We reported dichotomous data as Peto odds ratios (OR) to compare groups and continuous data as a mean difference (MD). We reported 95% confidence intervals (CIs).
Unit of analysis issues
The review included multi‐arm studies, in which more than one type of anaesthesia was included as separate study groups, or in which comparison groups included a clinical signs group and an ETAG‐guided group.
For multi‐arm studies that included study groups with different types of anaesthesia, we combined data for these groups for dichotomous data (occurrence of intraoperative awareness), and we selected the anaesthetic group which had the most conservative result for continuous data (recovery times). In subgroup analysis, we included each group separately according to type of anaesthetic agent.
We reported data separately for different comparison groups, and therefore there was no unit of analysis considerations for multi‐arm studies that included study groups with clinical signs and ETAG‐guided anaesthesia.
Dealing with missing data
We did not re‐include missing data by using imputation methods; we used the number of analysed participants as reported by study authors. In the previous version of the review (Punjasawadwong 2014), we contacted study authors to obtain missing data; owing to time limitations in the preparation of this update, we did not contact study authors in the case of missing data.
We did not recalculate the standard deviations (SDs) for studies reporting continuous outcomes as medians with ranges or interquartile ranges (IQR). In this update, we reported data with median values in the notes section of the relevant table in Characteristics of included studies.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, interventions, and outcomes, and used the data collected from the full‐text reports (as stated in Data collection and analysis). We explored clinical and methodological heterogeneity through subgroup analysis. We assessed statistical heterogeneity by calculating the Chi² test or the I² statistic and judged any heterogeneity above an I² value of 50% and a Chi² P value less than or equal to 0.05 to indicate moderate to substantial statistical heterogeneity (Higgins 2011).
In addition to looking at statistical results, we considered point estimates and overlap of CIs. If CIs overlap, then results are more consistent. However, combined studies may show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source the published protocols for each of our included studies by using the results from our clinical trial register searches. We compared published protocols with published study results to assess the risk of selective reporting bias. In addition, we appraised reporting bias through visual assessment of funnel plots for outcomes for which we found more than 10 studies (Egger 1997). We only included figures of funnel plots in the review in which we identified possible reporting bias from visual inspection.
Data synthesis
We presented a statistical summary of treatment effects in the absence of significant clinical or methodological heterogeneity. We used the statistical calculator in ReMan 5 to perform meta‐analysis (Review Manager 14).
For the occurrence of intraoperative awareness, we used the Peto method to pool ORs across studies; this method accounted for the extremely rare events for this outcome. For continuous outcomes, we calculated the mean difference (MD); we used a random‐effects model to account for potential variability in types of surgeries between studies (Borenstein 2010).
We calculated CIs at 95% and used a P value less than or equal to 0.05 to judge whether a result was statistically significant. We considered imprecision in the results of analyses by assessing the CI around an effects measure; a wide CI would suggest a higher level of imprecision in our results. A small number of studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
In subgroup analysis, we evaluated the type of anaesthetic agent which was used in the maintenance of anaesthesia (propofol, desflurane, isoflurane, sevoflurane). We conducted subgroup analysis for outcomes in which we found more than 10 studies (Higgins 2011).
Sensitivity analysis
We explored the potential effect of decisions made as part of the review process. In each sensitivity analysis, we compared the effect estimate with the main analysis. We reported these effect estimates only if they indicated a difference in interpretation of the effect. We performed the following sensitivity analyses.
We excluded studies that we judged to have a high or unclear risk of selection bias (for sequence generation).
We excluded studies that we judged to have a high risk of attrition bias because of a loss of more than 10% participants, or loss which was unbalanced between groups, or which was unexplained.
We found a large number of studies that reported intraoperative awareness with zero events in both arms. We used the Peto OR in the primary analysis of intraoperative awareness but made a post‐hoc decision to evaluate the effect of these zero‐event data by using alternative statistical methods. In sensitivity analysis, we used risk ratios (RR) with both a Mantel‐Haenszel and Inverse Variance method; we also evaluated these methods using a fixed‐effect and a random‐effects model. We did not use a risk difference method, because this method is unsuitable when events are rare (Bradburn 2007).
'Summary of findings' table and GRADE
One review author (SL) used the GRADE system to assess the certainty of the body of evidence associated with the following outcomes (Guyatt 2008).
Occurrence of intraoperative awareness
Time to eye opening
Time to orientation
Time to discharge from the PACU
The GRADE approach appraises the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias.
We constructed 'Summary of findings' tables using GRADE profiler software for two comparisons (gradepro.org).
BIS‐guided anaesthesia versus clinical signs‐guided anaesthesia
BIS‐guided anaesthesia versus ETAG‐guided anaesthesia
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
Results of the search
After the removal of duplicates from the search results, we screened 12,424 titles and abstracts, which included forward and backward citation searches, clinical trials registers and grey literature. We re‐evaluated previously included studies alongside 103 articles sourced as full‐text reports and, therefore, assessed eligibility of 139 articles. See Figure 1.
Included studies
See Characteristics of included studies.
We included 52 studies with 41,331 participants (Ahmad 2003; Alimian 2016; Anez 2001; Arbabpour 2015; Assare 2002; Avidan 2008; Avidan 2011; Başar 2003; Boztuğ 2006; Bresil 2013; Bruhn 2005; Ellerkmann 2010; Fakhr 2014; Gan 1997; Georgakis 2000; Guo 2015; Ibraheim 2008; Jain 2016; Kabukcu 2012; Kamal 2009; Kamali 2017a; Karaca 2014; Khoshrang 2016; Kim 2003; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Masuda 2002; Morimoto 2002; Mozafari 2014; Muralidhar 2008; Myles 2004; Nelskyla 2001; Paventi 2001; Payas 2013; Persec 2012; Puri 2003; Rahul 2015; Raksakietisak 2016; Recart 2003; Savli 2005; Shafiq 2012; Siampalioti 2015; Song 1997; Sudhakaran 2018; Tufano 2000; White 2004; Wong 2002; Zhang 2011; Zhang 2016; Zohar 2006). Two studies were quasi‐randomized (Anez 2001; Shafiq 2012); the remaining studies were randomized controlled trials (RCTs). We included three studies for which we could only source the abstract and this limited the details of study characteristics that we were able to extract (Georgakis 2000; Kabukcu 2012; Raksakietisak 2016). We sourced the full text of all the remaining studies. We did not seek translation of five studies (Arbabpour 2015; Kim 2003; Masuda 2002; Morimoto 2002; Savli 2005), and details of study characteristics and outcome data in these studies were limited to data in the English abstract or tables within the main text.
This review includes 22 new studies (Alimian 2016; Arbabpour 2015; Bresil 2013; Fakhr 2014; Georgakis 2000; Guo 2015; Jain 2016; Kabukcu 2012; Kamali 2017a; Karaca 2014; Khoshrang 2016; Kim 2003; Mozafari 2014; Payas 2013; Persec 2012; Rahul 2015; Raksakietisak 2016; Savli 2005; Shafiq 2012; Siampalioti 2015; Sudhakaran 2018; Zhang 2016). The remaining studies were previously included in Punjasawadwong 2014.
Study population
All studies included participants undergoing surgery under general anaesthesia. In one study, participants were undergoing procedures using regional anaesthesia combined with general anaesthesia (Ellerkmann 2010).
General anaesthesia was maintained by propofol, or by sevoflurane, desflurane or isoflurane, and in one study, halothane was used (Jain 2016). Only three studies used laryngeal masks (LMA); these were during surgical procedures with a duration of less than one hour (Anez 2001; Assare 2002; Zohar 2006).
Five studies included more than one comparison group according to the type of anaesthetic agent (Luginbuhl 2003; Muralidhar 2008; Siampalioti 2015; Song 1997; Tufano 2000). The type of anaesthetic agents was at the discretion of the attending anaesthetists in four studies (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004); in Avidan 2008 and Avidan 2011 agents were only volatile. We were uncertain of the type of anaesthetic agent in Arbabpour 2015.
Three studies recruited only participants who were at high risk of intraoperative awareness (Avidan 2008; Avidan 2011; Myles 2004), whilst two studies specifically recruited an unselected participant group (Mashour 2012; Zhang 2011). In general, we found most studies did not report whether the population group was at risk. However, in the findings of the 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia (NAP5 2014), some factors may increase risk of intraoperative awareness: female gender; age (younger adults); obesity; seniority of anaesthetists (junior trainees); history of accidental awareness; out of hours operating; emergencies; type of surgery (obstetric, cardiac, thoracic, neurosurgery); and use of neuromuscular blockade. We collected information on studies that had at least one risk factor and reported this information in Appendix 6. However, we did not think this information alone was sufficient to categorise these studies as having participants at high risk of awareness.
Study setting
All studies were conducted in a hospital setting and seven were multi‐centre studies (Avidan 2008; Avidan 2011; Bruhn 2005; Mashour 2012; Myles 2004; Rahul 2015; Zhang 2011).
Interventions and comparisons
All studies included an intervention group in which a BIS monitor was used to guide anaesthesia. In six studies, this was compared with ETAG‐guided anaesthesia (Avidan 2008; Avidan 2011; Jain 2016; Mashour 2012; Muralidhar 2008; Sudhakaran 2018); one of these studies included comparisons with both ETAG‐guided anaesthesia, and anaesthesia guided by clinical signs (Sudhakaran 2018). One study described that the control group participants had anaesthesia guided by both clinical signs and end‐tidal concentration in the form of minimum alveolar concentration;(MAC) (Shafiq 2012); we categorised this study as belonging to our first comparison group (BIS versus clinical signs). The remaining studies included comparisons with only clinical signs. We could not be certain of other monitoring methods used in the included studies; our information was limited to the details reported in published reports. Therefore, it is possible that some studies in which anaesthesia was guided by clinical signs may also have been guided by ETAG. Similarly, because it was not always clearly reported, we could not be certain whether audible alarms were used for ETAG‐guided anaesthesia.
The large number of participants in this review was dominated by five large studies; two of these studies compared BIS with clinical signs and in total included 7772 participants (Myles 2004; Zhang 2011), and three studies compared BIS with ETAG and had 29,642 participants (Avidan 2008; Avidan 2011; Mashour 2012).
Most studies defined clinical signs as using parameters such as heart rate or systolic blood pressure. One study used a standardised scoring system (PRST: systolic blood pressure, heart rate, sweating and tears) to evaluate depth of anaesthesia (Rahul 2015).
BIS target values varied in each study but all were within a range of 40 to 60.
Only four studies reported the experience of the attending anaesthetist, which was described as a quote: "experienced anaesthesiologist" (Ellerkmann 2010), "supervised by a faculty anaesthetist" (Gan 1997), greater than one year (Başar 2003), and greater than five years (Wong 2002). The remaining studies did not describe the experience of the attending anaesthetist.
Outcomes
Five studies did not report outcomes relevant to the review (Ahmad 2003; Bresil 2013; Jain 2016; Payas 2013; Raksakietisak 2016). The remaining studies reported measured at least one of the review outcomes.
For the measurement of intraoperative awareness, we noted that studies did not report a clear definition of intraoperative awareness. In addition, the time point of measurement varied between studies and included one or more measurement taken from as early as the time in the PACU to several days, and up to 30 days, after anaesthesia. In addition, the methods or tools used to collect the information were not always reported, and were described in limited terms such as 'an interview', or in more comprehensive terms (for example, by including a specific standardised questionnaire such as structured modified Brice questionnaire (Brice 1970).
Funding
Five studies reported financial support or included study authors who had received fees (for example for consultancy work), from companies involved in the manufacture of BIS monitors (Ahmad 2003; Bruhn 2005; Gan 1997; Myles 2004; Wong 2002). Twenty‐one studies were either not funded or funded from independent sources (Alimian 2016; Avidan 2008; Avidan 2011; Bresil 2013; Fakhr 2014; Kamali 2017a; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Mozafari 2014; Nelskyla 2001; Payas 2013; Persec 2012; Rahul 2015; Raksakietisak 2016; Recart 2003; Sudhakaran 2018; White 2004; Zohar 2006). We could not ascertain funding sources from the remaining studies.
Excluded studies
We excluded 68 studies during full‐text review and the reasons for these exclusions are described in Figure 1.
In order to improve usability of the review, we have only reported details of 19 of these 68 excluded studies in the review (Aceto 2015; Aimé 2006; Chan 2013; Chiu 2007; Hachero 2001; Kamali 2017b; Karwacki 2014; Kaval 2015; Kerssens 2009; Nitzschke 2014; Panagopoulou 2000; Quesada 2016; Radtke 2013; Rüsch 2018; Samarkandi 2004; Shahrbazi 2008; Struys 2001; Vretzakis 2005; Zhou 2018). We excluded these 19 studies because their study aims did not match the review aim. See Characteristics of excluded studies.
Of these 19 studies, five studies were included in the previous version of the review (Aimé 2006; Chiu 2007; Hachero 2001; Samarkandi 2004; Struys 2001); the decision to exclude these five studies was due to a change in the review criteria (see Differences between protocol and review).
We did not include in the review studies that were excluded during previous versions of the review (Punjasawadwong 2007; Punjasawadwong 2014).
Studies awaiting classification
Six studies are awaiting classification (Aksun 2007; Croci 2014; CTRI/2018/03/012457; Golmohammadi 2014; Jeong 2002; Qu 2011). We were unable to source the full text of two studies from current library sources and the abstracts contained insufficient information to assess eligibility (Aksun 2007; Qu 2011). Croci 2014 was published only as an abstract and, similarly this contained insufficient information to assess eligibility. We found one completed study in a clinical trial register but the clinical trial register did not include study results and therefore, we await publication of the full report (CTRI/2018/03/012457). Two studies require translation to assess eligibility (Golmohammadi 2014; Jeong 2002).
Ongoing studies
We found two ongoing studies (Martins 2013; NCT03571945). One study is a protocol, previously included in the review as 'awaiting classification' (Martins 2013); because the status is recorded in the clinical trial register as unknown we have assumed that it is still ongoing. This study compares BIS with clinical signs in people undergoing coronary artery bypass graft. The other ongoing and multi‐centre study aims to recruit 2000 participants and will compare BIS‐guided anaesthesia with anaesthesia guided by ETAG in participants undergoing elective surgery lasting more than 30 minutes (NCT03571945).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
We only conducted 'Risk of bias' assessments on studies that measured or reported review outcomes. Blank spaces in Figure 2 and Figure 3 indicate that these studies did not measure or report review outcomes.
Allocation
Two quasi‐randomized studies were at high risk of selection bias because of methods used for sequence generation and allocation concealment (Anez 2001; Shafiq 2012). In addition, we noted differences between the number of participants allocated to each group and differences between groups in one study which we also judged to have a high risk of bias for sequence generation (Zhang 2011).
Twenty‐two studies reported insufficient detail of methods to randomize participants and we judged these studies to have an unclear risk of bias for sequence generation (Alimian 2016; Arbabpour 2015; Assare 2002; Başar 2003; Georgakis 2000; Ibraheim 2008; Kamal 2009; Kamali 2017a; Karaca 2014; Kim 2003; Masuda 2002; Morimoto 2002; Muralidhar 2008; Nelskyla 2001; Paventi 2001; Rahul 2015; Recart 2003; Savli 2005; Tufano 2000; White 2004; Zhang 2016; Zohar 2006). The remaining studies reported adequate methods to randomize participants and we judged these to have a low risk of bias for sequence generation.
Four studies reported adequate methods to conceal allocation and we judged these to have a low risk of bias (Avidan 2011; Boztuğ 2006; Gan 1997; Myles 2004). The remaining studies did not report sufficient methods to conceal allocation to enable us to judge the risk of bias for allocation concealment.
Blinding
Whilst some studies reported that participants were blinded to group allocation, we based our judgement for risk of performance bias according to whether relevant personnel were blinded; this was because we expected that knowledge of group assignment was unlikely to influence participants. In all studies, it was not feasible to blind anaesthetists to the two methods used to guide anaesthesia in this review. Therefore we judged all studies to have a high risk of performance bias. As well as the risk that anaesthetists may alter their methods of providing anaesthesia depending on the group to which they are allocated, this type of study has a performance bias risk related to 'learning contamination' bias. Learning contamination bias is the risk of changing clinical practice in the parallel control or unmonitored group by using the information from the BIS group (Roizen 1994).
Intraoperative awareness is a self‐reported outcome; however, we did not consider the blinding of participants to influence the measurement of intraoperative awareness. We expected that most studies used an anaesthetist who interviewed the participants postoperatively (either informally or using a standardised questionnaire) and, in this review, we classed the interviewer as the outcome assessor as the terms used to ask questionnaires may be subject to bias. Nineteen studies reported that outcome assessors were blinded (Avidan 2008; Avidan 2011; Bruhn 2005; Fakhr 2014; Gan 1997; Kamali 2017a; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Myles 2004; Paventi 2001; Recart 2003; Siampalioti 2015; White 2004; Wong 2002; Zhang 2011; Zohar 2006). The remaining studies did not report whether outcome assessors were blinded.
Incomplete outcome data
In four studies, we noted a loss of more than 10% participants or that the loss of participants was imbalanced between groups (Gan 1997; Mashour 2012; Morimoto 2002; Zhang 2011); we judged these studies to have a high risk of attrition bias. We could not be certain whether all participants were accounted for in two studies; therefore, we judged these studies to have an unclear risk of attrition bias (Arbabpour 2015; Fakhr 2014). The remaining studies had no apparent participant loss, or the loss of participants was fewer than 10%, and we judged these studies to have a low risk of attrition bias.
Selective reporting
Two studies were prospectively registered with a clinical trial register (Avidan 2011; Mashour 2012). We judged Mashour 2012 to have a low risk of reporting bias because outcomes in the published report were the same as those in the clinical trial register documents. We could not be certain whether a risk of reporting bias was evident in Avidan 2011 because several outcomes listed in the clinical trial register documents were not included in the published report.
Four studies were retrospectively registered with a clinical trial register (Alimian 2016; Avidan 2008; Khoshrang 2016; Persec 2012), and we could not be certain whether registration was prospective or retrospective in one study (Siampalioti 2015); it was not feasible to use these documents to effectively assess risk of reporting bias. The remaining studies did not report clinical trial registration or study protocol publication, and therefore, it was similarly not feasible to effectively assess risk of reporting bias.
Other potential sources of bias
We were unable to assess risks of other sources of bias in those studies for which we did not seek translation (Arbabpour 2015; Kim 2003; Masuda 2002; Morimoto 2002; Savli 2005), or in studies that were reported only as abstracts (Georgakis 2000; Kabukcu 2012); therefore, we used an unclear judgement for other risks of bias. Similarly, we used an unclear judgement in two studies in which study characteristics were poorly reported (for example, with no baseline characteristics table) (Kamali 2017a; Tufano 2000).
We noted some important baseline imbalances between groups in three studies and we could not be certain of the influence of these imbalances on the outcome data (Avidan 2008; Persec 2012; Rahul 2015).
Effects of interventions
1. BIS versus clinical signs
Occurrence of intraoperative awareness
Twenty‐nine studies measured intraoperative awareness (Anez 2001; Assare 2002; Bruhn 2005; Ellerkmann 2010; Fakhr 2014; Guo 2015; Ibraheim 2008; Kabukcu 2012; Kamal 2009; Kamali 2017a; Karaca 2014; Kim 2003; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mozafari 2014; Myles 2004; Paventi 2001; Persec 2012; Puri 2003; Rahul 2015; Recart 2003; Song 1997; Sudhakaran 2018; White 2004; Wong 2002; Zhang 2011; Zhang 2016; Zohar 2006). In two studies, data were measured but were unclearly reported or were not reported (Fakhr 2014; Paventi 2001).
Events were rare, and only five of these studies included incidences of awareness (Kamali 2017a; Mozafari 2014; Myles 2004; Puri 2003; Zhang 2011). Two of these studies were large, multi‐centre trials ‐ one included only participants at high risk of intraoperative awareness (Myles 2004), and one included an unselected population (Zhang 2011). Four of these five studies used a structured questionnaire to collect data on awareness, and one study reported that participants were interviewed (with no additional details). Two of these five studies used an adjudication process to judge whether descriptions of awareness were possible or confirmed, and we included data only for confirmed reports of awareness.
We found that BIS‐guided anaesthesia may reduce the risk of intraoperative awareness in a surgical population that were at unselected or at high risk of awareness (Peto odds ratio (OR) 0.36, 95% confidence interval (CI) 0.21 to 0.60; I2 = 61%; 27 studies; 9765 participants; low‐certainty evidence; Analysis 1.1). We used GRADE to downgrade the certainty of the evidence by two levels. We downgraded by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies, it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. We downgraded by one level for imprecision; whilst we noted a narrow CI, the effect was dominated by two large trials (with two different populations selected according to the likelihood of intraoperative awareness) and we found many studies with zero events in both arms. We conducted sensitivity analysis to explore alternative statistical models to account for zero events in both arms as well as rare events and found more conservative estimates when we used a random‐effects model, thus reducing our certainty in the estimate. See Table 1.
1.1. Analysis.

Comparison 1: BIS versus clinical sides, Outcome 1: Occurrence of intraoperative awareness
Recovery time to eye opening
Twenty‐seven studies measured time to eye opening (Anez 2001; Başar 2003; Boztuğ 2006; Bruhn 2005; Ellerkmann 2010; Gan 1997; Georgakis 2000; Ibraheim 2008; Kamal 2009; Karaca 2014; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Masuda 2002; Morimoto 2002; Myles 2004; Nelskyla 2001; Paventi 2001; Puri 2003; Recart 2003; Savli 2005; Shafiq 2012; Siampalioti 2015; Tufano 2000; White 2004; Wong 2002; Zohar 2006). In two studies, time was measured but not reported (Georgakis 2000; Zohar 2006). We did not combine data in analysis from studies that reported time to eye opening as median values (Myles 2004; Paventi 2001; Tufano 2000).
For Siampalioti 2015, a multi‐arm study, we included data only for participants in which sevoflurane was used for anaesthesia; the effect for these participants showed a more conservative estimate.
We found that BIS‐guided anaesthesia may reduce time to eye opening by mean difference (MD)1.78 minutes (95% CI ‐2.53 to ‐1.03 minutes; I2 = 83%; 22 studies; 1494 participants; low‐certainty evidence; Analysis 1.2). We used GRADE to downgrade the certainty of the evidence by one level for inconsistency owing to the substantial statistical heterogeneity in this effect, and by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies, it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See Table 1.
1.2. Analysis.

Comparison 1: BIS versus clinical sides, Outcome 2: Time to eye opening (minutes)
Recovery time to orientation
Eight studies measured time to orientation (Fakhr 2014; Kamal 2009; Nelskyla 2001; Paventi 2001; Savli 2005; Song 1997; White 2004; Wong 2002). In Fakhr 2014, data were measured but not reported. We did not combine data in analysis from studies that reported time to orientation as median values (Paventi 2001). In Song 1997, data were included separately according to the type of volatile anaesthetic (sevoflurane and desflurane); we included data for participants who were given sevoflurane as this presented a more conservative finding.
We found that BIS‐guided anaesthesia may reduce time to orientation by MD 3.18 minutes (95% CI ‐4.03 to ‐2.33 minutes; I2 = 41%; 6 studies; 273 participants; low‐certainty evidence; Analysis 1.3). We used GRADE to downgrade the certainty of the evidence by one level for imprecision as the evidence was from few studies with few participants, and by one level for study limitation owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See Table 1.
1.3. Analysis.

Comparison 1: BIS versus clinical sides, Outcome 3: Time to orientation (minutes)
Time to discharge from the postanaesthesia care unit (PACU)
Seventeen studies measured time to discharge from the PACU (Alimian 2016; Anez 2001; Arbabpour 2015; Boztuğ 2006; Bruhn 2005; Fakhr 2014; Gan 1997; Kamal 2009; Khoshrang 2016; Masuda 2002; Morimoto 2002; Myles 2004; Recart 2003; Song 1997; White 2004; Wong 2002; Zohar 2006). We did not include data for one study in which time was reported as median values (Myles 2004), nor for three studies in which data were not reported or were reported unclearly (Arbabpour 2015; Fakhr 2014; Khoshrang 2016). In Song 1997, data were included separately according to the type of volatile anaesthetic (sevoflurane and desflurane); we included data for participants who were given sevoflurane as this presented a more conservative finding.
We found that BIS‐guided anaesthesia may reduce time to discharge from the PACU by MD 6.86 minutes (95% CI ‐11.72 to ‐2.00; I2 = 79%; 13 studies; 930 participants; low‐certainty evidence; Analysis 1.4). We used GRADE to downgrade the certainty of the evidence by one level for inconsistency owing to the substantial statistical heterogeneity in this effect, and by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See Table 1.
1.4. Analysis.

Comparison 1: BIS versus clinical sides, Outcome 4: Time to discharge from the PACU (minutes)
From visual inspection of a funnel plot for these outcome data, we noted the possibility of publication bias (Figure 4).
4.

Funnel plot of comparison: 1 BIS versus clinical sides, outcome: 1.4 Time to discharge from the PACU (minutes).
Subgroup analysis
Type of agent used to guide anaesthesia
We did not include Myles 2004 in this subgroup analysis because the type of anaesthetic was at the discretion of the attending anaesthetists, and therefore may include all types.
Occurrence of intraoperative awareness: the effect for propofol was consistent with our primary analysis, showing a reduction in intraoperative awareness when propofol was guided by BIS (Peto OR 0.24, 95% CI 0.10 to 0.60; I2 = 0%; 10 studies; 5784 participants). The evidence for isoflurane was inconsistent and showed no evidence of a reduction in intraoperative awareness when isoflurane was guided by BIS (Peto OR 0.58, 95% CI 0.26 to 1.28; I2 = 74%; 4 studies; 637 participants). None of the studies in which desflurane or sevoflurane was given reported events. Analysis 2.1.
2.1. Analysis.

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 1: Occurrence of intraoperative awareness
Recovery time to eye opening: this subgroup analysis included both propofol and sevoflurane groups in Siampalioti 2015. The effect for time to eye opening was consistent with our primary analysis, showing a reduction in time to eye opening for BIS‐guided anaesthesia with: propofol (MD ‐2.13 minutes, 95% CI ‐3.82 to ‐0.43 minutes; I2 = 89%; 8 studies; 680 participants); isoflurane (MD ‐2.45 minutes, 95% CI ‐4.80 to ‐0.09 minutes; I2 = 73%; 3 studies; 150 participants); and sevoflurane (MD ‐1.52 minutes, 95% CI ‐2.60 to ‐0.44 minutes; I2 = 83%; 8 studies; 392 participants). We found no evidence of a difference in time to eye opening when desflurane was used (MD ‐0.51 minutes, 95% CI ‐1.44 to 0.42 minutes; I2 = 38%; 4 studies; 322 participants). Analysis 2.2.
2.2. Analysis.

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 2: Time to eye opening (minutes)
Recovery time to orientation: we did not conduct subgroup analysis for this outcome because the primary analysis included too few studies.
Recovery time to discharge from the PACU: this subgroup analysis included both sevoflurane and desflurane anaesthetic groups in Song 1997. We noted differences between subgroups in this analysis (Chi² = 57.54, df = 13, P < 0.00001). Whilst studies in which propofol was used showed a reduction in time to discharge from the PACU which was consistent with the primary analysis (MD ‐5.42 minutes, 95% CI ‐9.36 to ‐1.48 minutes; I2 = 0%; 4 studies; 398 participants), we found that the evidence when volatile agents were used was inconsistent: desflurane (MD ‐14.76 minutes, 95% CI ‐29.61 to 0.09 minutes; I2 = 88%; 4 studies; 272 participants); isoflurane (MD ‐14.00 minutes, 95% CI ‐34.12 to 6.12 minutes; 1 study; 60 participants); sevoflurane (MD ‐5.99 minutes, 95% CI ‐13.34 to 1.36 minutes; I2 = 83%; 5 studies; 230 participants). Analysis 2.4.
2.4. Analysis.

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 4: Time to discharge from the PACU stay (minutes)
Sensitivity analysis
Unclear or high risk of selection bias for sequence generation
Occurrence of intraoperative awareness: only 14 studies included in the primary analysis had a low risk of selection bias (Bruhn 2005; Ellerkmann 2010; Guo 2015; Kabukcu 2012; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mozafari 2014; Myles 2004; Persec 2012; Puri 2003; Song 1997; Sudhakaran 2018; Wong 2002). When we excluded the remaining studies, analysis demonstrated no evidence of an effect (Peto OR 0.60 (95% CI 0.29 to 1.23; 14 studies; 3654 participants); however, we noted that this sensitivity analysis included only three studies with event data, of which one small study had findings which were inconsistent with other studies and which we could not explain (Mozafari 2014).
Time to eye opening: only 10 studies included in the primary analysis had a low risk of selection bias (Boztuğ 2006; Bruhn 2005; Ellerkmann 2010; Gan 1997; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Puri 2003; Siampalioti 2015; Wong 2002). When the remaining studies were excluded, we found no difference in the interpretation of the effect.
Time to orientation: only two studies included in the primary analysis had a low risk of selection bias (Song 1997; Wong 2002). When we excluded the remaining studies, we found no difference in the interpretation of the effect.
Time to discharge from the PACU: only five studies included in the primary analysis had a low risk of selection bias (Boztuğ 2006; Bruhn 2005; Gan 1997; Song 1997; Wong 2002). When we excluded the remaining studies, we found no evidence of an effect (MD ‐1.62 minutes (95% CI ‐5.96 to 2.72); 519 participants).
Unclear or high risk of attrition bias
Occurrence of intraoperative awareness: we excluded one study with a high risk of attrition bias (Zhang 2011). This did not alter the interpretation of the effect.
Time to eye opening: we excluded two studies with a high risk of attrition bias (Gan 1997; Morimoto 2002). This did not alter the interpretation of the effect.
Time to orientation: no studies included in the primary analysis had an unclear or high risk of attrition bias.
Time to discharge from the PACU: we excluded two studies with a high risk of attrition bias (Gan 1997; Morimoto 2002). This did not alter the interpretation of the effect.
Zero event data
Analysis of the occurrence of intraoperative awareness included 22 studies with zero events in both arms. We evaluated alternative statistical tools and methods using the calculator in Review Manager 14. We report the effect of these sensitivity analyses in Appendix 7. Based on a fixed‐effect model, using a RR with either Mantel‐Haenszel or Inverse Variance did not alter the interpretation of the effect. Although we evaluated the effect using a random‐effects model, this model is less appropriate for evidence of rare events (Higgins 2011). Based on a random‐effects model, we found a more conservative estimate which indicated no evidence of a difference in intraoperative awareness when the Mantel‐Haenszel method was used (RR 0.32, 95% CI 0.10 to 1.01; I2 = 62%), and when the Inverse Variance method was used (RR 0.32, 95% CI 0.10 to 1.00; I2 = 60%).
2. BIS versus ETAG
Occurrence of intraoperative awareness
Five studies measured and reported intraoperative awareness (Avidan 2008; Avidan 2011; Mashour 2012; Muralidhar 2008; Sudhakaran 2018). This surgical population included participants who were at high risk of intraoperative awareness (Avidan 2008; Avidan 2011), who were unselected (Mashour 2012), or for whom risk of awareness was not specified (Muralidhar 2008; Sudhakaran 2018).
Events were rare and only three of these studies included incidences of awareness. Of the three studies with event data, all used a structured Brice questionnaire to collect data on awareness, and all used an adjudication process to categorise incidences of awareness as possible or definite; we included in analysis data for definite awareness.
We found no evidence of a difference in incidences of intraoperative awareness according to whether anaesthesia was guided by BIS or by ETAG (Peto OR 1.13, 95% CI 0.56 to 2.26; I2 = 37%; 26,572 participants; low‐certainty evidence; Analysis 3.1). We used GRADE to downgrade the certainty of the evidence by one level for imprecision; despite a large number of participants, events were very rare (one per 1000 in the intervention and the comparison group) and the confidence interval for this effect was wide. In addition, we downgraded by one level for study limitations owing to the inclusion of some studies with unclear and high risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See Table 2.
3.1. Analysis.

Comparison 3: BIS versus ETAG, Outcome 1: Occurrence of intraoperative awareness
Recovery time to eye opening
No studies reported time to eye opening.
Recovery time to orientation
No studies reported time to orientation.
Time to discharge from the PACU
One study measured and reported time to discharge from the PACU (Mashour 2012). Study authors reported a reduction in readiness for discharge from the PACU when BIS‐guided anaesthesia was used, with a median interquartile range (IQR)) duration of 95 minutes (64 to 138 minutes) for participants having BIS‐guided anaesthesia (6076 participants) compared to a median (IQR) duration of 98 minutes (66 to 140 minutes) for participants having ETAG‐guided anaesthesia (9376 participants). We used GRADE to downgrade this evidence to low certainty. We downgraded by two levels for imprecision because the evidence was from one study in which the IQR of time spent in the PACU was wide in both groups.
Subgroup analysis
We did not conduct subgroup analysis for the comparison BIS versus ETAG because we found too few studies in the primary analysis.
Sensitivity analysis
Unclear or high risk of selection bias for sequence generation
Occurrence of intraoperative awareness: all studies in the primary had a low risk of selection bias (Mashour 2012).
Time to discharge from the PACU: data for this outcome were from a single study which we judged to have a low risk of selection bias (Mashour 2012).
Unclear or high risk of attrition bias
Occurrence of intraoperative awareness: we excluded one study with a high risk of attrition bias (Mashour 2012). This did not alter the interpretation of the effect.
Time to discharge from the PACU: data for this outcome was from a single study which we judged to have a high risk of attrition bias (Mashour 2012).
Zero event data
Analysis of the occurrence of intraoperative awareness included two studies with zero events in both arms. We evaluated alternative statistical tools and methods using the calculator in Review Manager 14. We report the effect of these sensitivity analyses in Appendix 7. We found that alternative statistical tools did not alter the interpretation of the effect for this outcome.
Discussion
Summary of main results
We found 52 studies that compared bispectral index (BIS)‐guided anaesthesia with either clinical signs or end‐tidal anaesthetic gas (ETAG). Studies included participants undergoing any type of surgery under general anaesthesia. Three studies included only participants who were at high risk of intraoperative awareness, and two studies included only unselected participants. Whilst some studies included participants who had one or more factor that may increase the risk of intraoperative awareness (according to NAP5 2014), we did not categorise these studies as at high risk of intraoperative awareness.
We included two comparison groups in the review: BIS‐guided anaesthesia compared with anaesthesia guided by clinical signs, and BIS‐guided anaesthesia compared with anaesthesia guided by ETAG.
We found low‐certainty evidence that BIS‐guided anaesthesia compared to clinical signs may reduce the incidence of intraoperative awareness. However, incidences of awareness were rare. Incidences, when BIS was used, were only six per 1000 fewer than in the clinical signs group. We found low‐certainty evidence that early recovery times may be reduced when BIS was used; the time to eye opening, to orientation, and to discharge from the PACU was shorter for all studies in which BIS was used.
For BIS‐guided anaesthesia compared to ETAG‐guided anaesthesia, we found no evidence of a difference in the incidence of intraoperative awareness. Again, we found few incidences of intraoperative awareness (1 per 1000 in both groups) and we judged this evidence to be low certainty. Only one study in which BIS was compared with ETAG‐guided anaesthesia measured the time to discharge from the PACU, and this study reported median values which showed a reduction in time to discharge from the PACU for BIS‐guided anaesthesia (low‐certainty evidence). No studies comparing BIS‐ to ETAG‐guided anaesthesia measured or reported the time to eye opening and the time to orientation.
Overall completeness and applicability of evidence
We identified 52 studies with 41,331 participants. All participants were undergoing surgery under general anaesthesia. Most studies did not specify whether participants were selected according to their risk of intraoperative awareness, and we noted that some characteristics of included participants and the methods used in their anaesthesia indicated at least one risk factor for awareness. These factors, identified in the most recent NAP5 audit (NAP5 2014), included female gender, obesity, type of surgery (obstetric, cardiac, thoracic, neurosurgery), and the use of neuromuscular blockade. Whilst experience of the anaesthetist may be relevant to the risk of intraoperative awareness, we found that this was poorly reported in studies, and no studies reported that attending anaesthetists in the trials had a junior level of experience.
Most studies compared BIS with clinical signs to monitor the depth of anaesthesia with only six studies comparing BIS with ETAG.
We attempted to account for differences between studies in terms of the type surgery by using a random‐effects model in the analysis of the recovery time points. However, we noted a moderate to substantial statistical heterogeneity in most of our primary analyses. We expected that this was inevitable because of the broad inclusion criteria regarding type of surgery. These differences in surgery type may increase the duration of general anaesthesia and the subsequent recovery times, and we believed that this statistical heterogeneity was unavoidable in the review.
Quality of the evidence
We used GRADE to downgrade the certainty of the evidence for all outcomes in this review to low. Whilst we found a large number of studies that compared BIS with anaesthesia guided by clinical signs, incidences of intraoperative awareness were so infrequent with most studies reporting no events in either group. We reported the effect estimate as Peto odds ratio (OR) which accounted for rare events, but when we used alternative statistical methods using random‐effects models, we found more conservative estimates, thus we believed the evidence was imprecise. Similarly, although evidence comparing BIS to ETAG, included a larger number of participants, events were from only three studies and were infrequent.
We also used GRADE to downgrade the certainty of the evidence owing to study limitations. We used the 'Risk of bias' tool to assess some studies as having an unclear risk of bias, often because these studies reported no information on which to base a confident judgement. This study design prohibits blinding of the attending anaesthetists and therefore, we judged all studies to have a high risk of performance bias. We expected that some anaesthetists who were assigned a BIS monitor may continue to base their judgements of a patient's depth of anaesthesia on standard clinical practices rather than BIS, or that they may alter their standard anaesthetic practice in other ways when using the BIS monitor.
We also noted that intraoperative awareness was often not clearly defined in studies and without a precise definition we could not be certain that the incidences (or lack of incidences) were comparable in the analyses. Similarly, the time points at which intraoperative awareness was measured (for example in the postanaesthesia care unit (PACU), or on the first, second, or third postoperative day, or up to 30 days postoperatively) were not comparable, nor the methods of data collection (for example, using a simple question or using a recognised data collection tool such as the Brice questionnaire, and whether reports of awareness were evaluated for being possible or definite).
Potential biases in the review process
We conducted a thorough search in the update and used two review authors to assess study eligibility, extract data, and assess risk of bias in included studies; therefore, we reduced potential bias in the review process.
In updating this review, we made minor changes to the review inclusion criteria. We decided to exclude studies that did not aim to address our review question. As a result of this decision, we excluded five previously included studies. As these five studies, and other similar studies identified during the search, did not address our review aims, it was not expected that this decision affected data for the relevant outcomes in the review. In addition, we re‐evaluated the previous review outcomes. In order to improve the focus of the review, we reduced the number of outcomes and included only those that measured the success of anaesthesia in terms of a reduction in the risk of intraoperative awareness and an optimum early recovery; for usability, we selected only three measures of recovery (time to eye opening, time to orientation, and time to discharge from the PACU). The decision to reduce the number of outcomes may introduce bias into the review process, however, we believed that this decision improved the usability of the review.
In addition, we were unable to source all texts from current British Library sources, and we did not seek translation of some studies, and therefore the review includes six studies awaiting classification. We could not be certain whether these studies included relevant data for the review.
For the primary outcome (the occurrence of intraoperative awareness), we found many studies with zero events in both arms. We limited the choice of appropriate meta‐analytic tools to those available in Review Manager 14, and used Peto OR in the primary analysis. We conducted sensitivity analysis using alternative statistical methods in Review Manager 14. Alternative methods, such as Bayesian meta‐analysis, may offer a more robust method to account for rare events as well as studies with zero events in both arms (Cheng 2016).
Agreements and disagreements with other studies or reviews
A recent meta‐analysis conducted by Gao and colleagues (Gao 2018), combined data from the five largest studies in this review (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004; Zhang 2011). Whilst their findings demonstrated that intraoperative awareness did not appear to be associated with BIS monitoring, this result combined studies of both ETAG‐ and clinical signs‐guided comparison groups. Similarly, the Cochrane Review by Messina and colleagues also combined both comparison groups (Messina 2016). We noted that Messina 2016 gave greater emphasis to the definition of different types of awareness, and the methods by which data were collected. As we had not specified this criteria, and the primary outcome in our review was for a broader criteria for intraoperative awareness, we subsequently included more small studies in the analysis. We do not think that the result in Messina 2016 is comparable with our own findings.
In relation to recovery measures, our findings are consistent with other systematic reviews which indicate that anaesthesia guided by BIS monitoring improves early postoperative recovery with a shorter time to eye opening and to orientation demonstrated in Chiang 2018 and Oliveira 2017, and a reduced time in the PACU for participants undergoing ambulatory surgery in Liu 2004.
Authors' conclusions
Implications for practice.
BIS‐guided anaesthesia may reduce the risk of intraoperative awareness in surgical patients at high risk or unselected for risk of awareness compared to using clinical signs as the guide for anaesthetic depth. Bispectral index (BIS)‐guided anaesthesia may also reduce early recovery parameters of the time to eye opening, the time to orientation, and the time to discharge from the postanaesthesia care unit (PACU). We found no evidence to indicate whether BIS‐guided or end‐tidal anaesthetic gas (ETAG)‐guided anaesthesia affected the incidence of intraoperative awareness, and evidence from only one study comparing BIS with ETAG that indicated a shorter length of stay in the PACU with BIS‐guided anaesthesia. However, we considered the certainty of evidence to be low for each of these outcomes.
Implications for research.
Despite some large multi‐centre studies included in this review, the incidences of awareness are so infrequent that imprecision is inevitable. We hope that future research will continue to contribute evidence towards the evaluation of the effectiveness of BIS‐monitoring to reduce incidences of intraoperative awareness. We would recommend future studies to consider the need to report clearly their definition of awareness, and to use measurement tools such as the Brice questionnaire with appropriate adjudication of whether reports of intraoperative awareness are possible or definite.
What's new
| Date | Event | Description |
|---|---|---|
| 2 July 2020 | Amended | Number of participants for the outcome 'Time to discharge from PACU' corrected in Summary of findings table 2 and Abstract. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 20 September 2019 | New citation required but conclusions have not changed | We updated the review and made the following amendments.
|
| 20 September 2019 | New search has been performed |
|
| 30 June 2018 | Amended | We corrected a typo in 'what's new' section (the following line: 'the result of our updated review published in 2014 seems contradictory to the result in a recent review published in 2016 by Messina et al' was repeated. |
| 11 September 2017 | Amended | We made the following corrections to the published review:
|
| 10 June 2014 | New citation required and conclusions have changed |
|
| 10 June 2014 | New search has been performed |
|
| 3 May 2009 | New search has been performed |
|
Acknowledgements
For acknowledgements in previous versions of the review, see Punjasawadwong 2007 and Punjasawadwong 2014.
For this 2019 update, we would like to thank Lars H Lundstrøn (Content Editor), Jing Xie (Statistical Editor), Michel Struys and Paul S Myles (Peer Reviewers), Stephana Cherak (Consumer Referee), Janne Vendt (Information Specialist) Jane Cracknell and Teo Quay (Managing Editors), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review
We would like to thank review authors for their contributions to previous versions of the review (Aram Phongchiewboon and Nutchanart Bunchungmongkoi). We would like to thank study authors who provided additional information on their studies (Dr Jan Jakobsson, Dr Tong J Gan, and Dr Paul Manberg). We would like to thank people who supported the review by searching databases or establishing search strategy terms in the previous versions of the review (Denna N Braaksma, Ms Chompunuch Boonyawan, Anupa Shah, and Karen Hovhannisyan). We would like to thank people who provided comment on or translation of studies in the previous versions of the review (Valeria Salerno, Dr Andrea Casati, Dr Sandro Salzano, Prof Martha Delgado, Dr Cesar Carcamo, Dr Idoris Cordero, Ivan Sola, Dr Tomoki Hashimoto, Dr Simonia Vecchi). For the review published in 2007, we would like to thank Dr Mathew Zacharias (Content Editor), Prof Nathan Pace (Statistical Editor), Dr Michel Struys and Dr Chris Pomfrett (Peer Reviewers), Dr Janet Wale (Consumer Editor), and Jane Cracknell for co‐ordinating the updated review. For the review published in 2014, We would like to thank Dr Stephan C Kettner and Dr Mathew Zacharias (Content Editors), Dr Cathal Walsh (Statistical Editor), Dr Melissa Giraldo‐Duque, Dr Maurizio Solca and Dr Paul Myles (Peer Reviewers).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Electroencephalography] explode all trees
#2 MeSH descriptor: [Monitoring, Physiologic] explode all trees
#3 MeSH descriptor: [Monitoring, Intraoperative] explode all trees
#4 ((intraoperat* or perioperat* or peroperat* or intra‐operat* or peri‐operat* or per‐operat*) NEAR monitor*)
#5 (BIS or bispectral*)
#6 (electroencephalogra* or "electro encephalogra*" or electrocorticograph* or "electro corticograph*" or eeg*)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Anesthesia and Analgesia] explode all trees
#9 MeSH descriptor: [Anesthesia] explode all trees
#10 MeSH descriptor: [Anesthetics, General] explode all trees
#11 MeSH descriptor: [Anesthesia, General] explode all trees
#12 anaesth* or anesth*
#13 #8 or #9 or #10 or #11 or #12
#14 #7 and #13
#15 #14 in Trials
Appendix 2. MEDLINE (Ovid SP) search strategy
1 exp Electroencephalography/
2 monitoring, physiologic/
3 exp monitoring, intraoperative/
4 ((intraoperat* or perioperat* or peroperat* or intra operat* or peri operat* or per operat*) adj10 monitor*).mp.
5 (BIS or bispectral*).mp.
6 (electroencephalogra* or electro encephalogra* or electrocorticograph* or electro corticograph* or eeg*).mp.
7 1 or 2 or 3 or 4 or 5 or 6
8 "Anesthesia and Analgesia"/
9 exp Anesthesia/
10 exp Anesthetics, General/
11 exp Anesthesia, General/
12 an?esth*.mp.
13 8 or 9 or 10 or 11 or 12
14 7 and 13
15 ((randomized controlled trial or controlled clinical trial).pt. or random*.ab. or placebo.ab. or drug therapy.fs. or trial.ab. or groups.ab. or clinical trials as topic.sh. or random allocation.sh.) not (exp animals/ not humans.sh.)
16 14 and 15
Appendix 3. Embase (Ovid SP) search strategy
1 exp electroencephalography/
2 exp physiologic monitoring/
3 exp intraoperative monitoring/
4 ((intraoperat* or perioperat* or peroperat* or intra operat* or peri operat* or per operat*) adj10 monitor*).mp.
5 (BIS or bispectral*).mp.
6 (electroencephalogra* or electro encephalogra* or electrocorticograph* or electro corticograph* or eeg*).mp.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp anesthesia/
9 exp general anesthesia/
10 exp anesthetic agent/
11 an?esth*.mp.
12 8 or 9 or 10 or 11
13 7 and 12
14 (randomized controlled trial/ or randomization/ or placebo/ or crossover procedure/ or double blind procedure/ or single blind procedure/ or (crossover* or cross over*).ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or (placebo* or allocat* or trial* or random* or groups).ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti,ab.))
15 13 and 14
Appendix 4. Web of Science search strategy
#1 TS=((intraoperat* or perioperat* or peroperat* or "intra operat*" or "peri operat*" or "per operat*") NEAR/10 monitor*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#2 TS=(BIS or bispectral*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#3 TS=(electroencephalogra* or "electro encephalogra*" or electrocorticograph* or "electro corticograph*" or eeg*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#4 #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#5 TS=(anaesth* or anesth*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#6 #5 AND #4 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#7 TS=((controlled OR clinical OR comparative) NEAR/3 (trial* or stud*)) OR TS=random* OR TS=placebo* OR TS=((single or double or triple or treble) NEAR/3 (mask* or blind*)) OR TS=multicenter OR TS=(crossover OR cross‐over) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#8 #7 AND #6 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
Appendix 5. Data extraction form
| Completed by: | |
| Date: | |
| Study ID | |
| Methods | |
| Participants |
Total number of randomized participants: Country: Setting: Inclusion criteria: Exclusion criteria: Type of surgery: Overall duration of anaesthesia, if reported: Experience of anaesthetist (in years or qualifications): Baseline characteristics Intervention group (BIS)
Comparison group
|
| Interventions | Intervention group (BIS)
Comparison group
|
| Outcomes |
Outcomes measured/reported by study authors: Outcomes relevant to the review: |
| Notes |
Funding/declarations of interest: Study dates: Notes: |
Outcome data
| Name of outcome: | |
| Time point of measurement: | |
| Intervention group | |
| Number of events | Total number of participants in the group |
| Control group | |
| Number of events | Total number of participants in the group |
| Name of outcome: | Length of stay | |
| Intervention group | ||
| Mean | SD | Total number of participants in the group |
| Control group | ||
| Mean | SD | Total number of participants in the group |
'Risk of bias' table
| Domain | High/Low/ Unclear |
Judgement |
| Random sequence generation (selection bias) | ||
| Allocation concealment (selection bias) |
||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessors (detection bias) | ||
| Incomplete outcome data (attrition bias) | ||
| Selective reporting (reporting bias) |
||
| Other bias |
Appendix 6. Factors that increase the risk of intraoperative awareness
| Factors that increase the risk of interoperative awareness (NAP5 2014) | Study ID |
| Female gender | Kamali 2017a; Luginbuhl 2003; Nelskyla 2001; Savli 2005; Song 1997; White 2004 |
| Obesity | Ibraheim 2008; Siampalioti 2015 |
| Type of surgery | Obstetric: Kamali 2017a; Luginbuhl 2003; Nelskyla 2001; Savli 2005; Song 1997; White 2004 Cardiac: Kabukcu 2012; Kim 2003; Muralidhar 2008; Puri 2003 Neurosurgery: Boztuğ 2006; Karaca 2014; |
| Neuromuscular blockade | Ahmad 2003; Alimian 2016; Anez 2001; Boztuğ 2006; Fakhr 2014; Georgakis 2000; Guo 2015; Ibraheim 2008; Jain 2016; Kamal 2009; Kamali 2017a; Karaca 2014; Khoshrang 2016; Kim 2003; Luginbuhl 2003; Nelskyla 2001; Paventi 2001; Payas 2013; Persec 2012; Puri 2003; Raksakietisak 2016; Recart 2003; Shafiq 2012; Siampalioti 2015; Song 1997; Sudhakaran 2018; Tufano 2000; White 2004; Wong 2002; Zhang 2016 |
Appendix 7. Sensitivity analysis on statistical models: occurrence of intraoperative awareness
| BIS versus clinical signs | ||
| Statistical tool | Effect estimate using fixed‐effect model | Effect estimate using random‐effects model |
| Peto OR (9765 participants) | 0.36, 95% CI 0.21 to 0.60; I2 = 61% | n/a |
| RR, M‐H (9765 participants) | 0.35, 95% CI 0.20 to 0.62; I2 = 62% | 0.32, 95% CI 0.10 to 1.01; I2 = 62% |
| RR, IV (9765 participants) | 0.43, 95% CI 0.23 to 0.80; I2 = 60% | 0.32, 95% CI 0.10 to 1.00; I2 = 60% |
| BIS versus ETAG | ||
| Statistical tool | Effect estimate using fixed‐effect model | Effect estimate using random‐effects model |
| Peto OR | 1.13, 95% CI 0.56 to 2.26; I2 = 37% | n/a |
| RR, M‐H | 1.13, 95% CI 0.56 to 2.26; I2 = 32% | 1.19, 95% CI 0.45 to 3.14; I2 = 32% |
| RR, IV | 1.06, 95% CI 0.51 to 2.21; I2 = 31% | 1.19, 95% CI 0.45 to 3.11; I2 = 31% |
CI: confidence interval; IV: inverse variance; M‐H: Mantel‐Haenszel; OR: odds ratio; RR: risk ratio
Data and analyses
Comparison 1. BIS versus clinical sides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Occurrence of intraoperative awareness | 27 | 9765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.21, 0.60] |
| 1.2 Time to eye opening (minutes) | 22 | 1494 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐2.53, ‐1.03] |
| 1.3 Time to orientation (minutes) | 6 | 273 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐4.03, ‐2.33] |
| 1.4 Time to discharge from the PACU (minutes) | 13 | 930 | Mean Difference (IV, Random, 95% CI) | ‐6.86 [‐11.72, ‐2.00] |
Comparison 2. BIS versus clinical signs: subgroup by type of anaesthetic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Occurrence of intraoperative awareness | 26 | 7302 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.1.1 Propofol | 10 | 5784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.10, 0.60] |
| 2.1.2 Desflurane | 7 | 474 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.1.3 Isoflurane | 4 | 637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.26, 1.28] |
| 2.1.4 Sevoflurane | 7 | 407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.2 Time to eye opening (minutes) | 22 | 1544 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.40, ‐0.95] |
| 2.2.1 propofol | 8 | 680 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.82, ‐0.43] |
| 2.2.2 desflurane | 4 | 322 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.44, 0.42] |
| 2.2.3 isoflurane | 3 | 150 | Mean Difference (IV, Random, 95% CI) | ‐2.45 [‐4.80, ‐0.09] |
| 2.2.4 sevoflurane | 8 | 392 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐2.60, ‐0.44] |
| 2.3 Time to orientation (minutes) | 7 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐3.70, ‐2.61] |
| 2.3.1 propofol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.3.2 desflurane | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐4.23, ‐0.97] |
| 2.3.3 isoflurane | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐5.92, ‐1.28] |
| 2.3.4 sevoflurane | 5 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐3.80, ‐2.60] |
| 2.4 Time to discharge from the PACU stay (minutes) | 13 | 960 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐10.68, ‐1.84] |
| 2.4.1 propofol | 4 | 398 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐9.36, ‐1.48] |
| 2.4.2 desflurane | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐14.76 [‐29.61, 0.09] |
| 2.4.3 isoflurane | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐14.00 [‐34.12, 6.12] |
| 2.4.4 sevoflurane | 5 | 230 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐13.34, 1.36] |
2.3. Analysis.

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 3: Time to orientation (minutes)
Comparison 3. BIS versus ETAG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Occurrence of intraoperative awareness | 5 | 26572 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.56, 2.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 99 Country: USA Setting: hospital; single centre Inclusion criteria: undergoing gynaecological laparoscopy; with written informed consent Exclusion criteria: not reported Type of surgery: gynaecologic laparoscopy Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: neuromuscular blocking agents to facilitate tracheal intubation and intraoperative paralysis in all participants; type of agent and dose, plus agents for reversal, was at discretion of attending anaesthetist. Sufentenil administered before induction, and given in supplemental doses for BP or HR increases > 20% despite BIS value of 50 to 60 or end‐tidal sevoflurane concentration of 2%. Dexmethasone given after induction as an antiemetic. Thirty minutes before end of surgery, metochlorpropamide and ephedrine were given as additional antiemetics, and ketorolac for opiate‐sparing analgesia |
|
| Outcomes |
Outcomes measured/reported by study authors: successful fast track rate (using modified Aldrete Score, main outcome); mean concentration of sevoflurane (%); mean dose of sufentanil; mean dose of rocuronium; mean duration of phase II recovery room stay (time to discharge); pain in phase II recovery area; nausea/vomiting in phase II recovery area Outcomes relevant to the review: none |
|
| Notes |
Funding/declarations of interest: supported in part by Aspect Medical Systems, USA Study dates: not specified Note:
|
|
Alimian 2016.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Country: Iran Setting: hospital; single centre Inclusion criteria: 15 to 53 years of age; scheduled for laparoscopic surgery in women's field; ASA I or II Exclusion criteria: < 18 years old; history of COPD; kidney dysfunction with creatinine > 2 mg/dL or liver dysfunction; neurological diseases; difficult airway (by direct laryngoscopy or fibreoptic); history of analgesics, anticonvulsants and antidepressants; untreated hypertension; heart failure; drug allergy; undergoing emergency surgery Type of surgery: laparoscopic gynaecology Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with fentanyl and midazolam. Induction with propofol and cisatracurium. Then propofol and cisatracurium every 30 minutes and fentanyl every 40 minutes. Reversal with neostigmine and atropine. |
|
| Outcomes |
Outcomes measured/reported by study authors: time to discharge from recovery; nausea and vomiting; pain in recovery Outcomes relevant to the review: time to discharge from recovery |
|
| Notes |
Funding/declarations of interest: funded by Research Deputy of Iran University of Medical Sciences Study dates: March 2015 to October 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "triple‐blinded randomized trial". However, method used to randomize participants to group is not described. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered with clinical trials register (IRCT2015122919715N2). It is not feasible to used this registration document to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Anez 2001.
| Study characteristics | ||
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: Spain Setting: hospital; single centre Inclusion criteria: ASA status I or II; scheduled for general, vascular, or orthopaedic surgery under GA Exclusion criteria: using psychotropic medication; contraindications to medications used in the study, to use of an LMA, or for whom GA is not indicated; ASA status > II Type of surgery: vascular (venous) or orthopaedic outpatient surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Atropine, alfentanil, propofol, rocuronium. Use of LMA. |
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative awareness (assessed after full recovery from anaesthetic); propofol consumption; recovery (time to eye opening; time in the recovery room) Outcomes relevant to the review: intraoperative awareness, time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study used sequential randomization (quasi‐randomization). The rational for this 'sequence' was to avoid any contamination or influence of the 'BIS guided anaesthesia' on the 'standard anaesthesia' administered subsequently |
| Allocation concealment (selection bias) | High risk | Investigators did not conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of only one participant |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or published protocol. It is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Arbabpour 2015.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 68 Country: Iran Setting: hospital; single centre Inclusion criteria: women undergoing caesarean section Exclusion criteria: not specified in English abstract Type of surgery: caesarean section Baseline characteristics not reported in English abstract |
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Outcomes |
Outcomes measured/ reported by study authors: record times (time to extubation, time to discharge from recovery unit); complications during recovery Outcomes relevant to the review: time to discharge from the PACU (see note below) |
|
| Notes |
Funding/declarations of interest: not reported in English abstract Study dates: not reported in English abstract Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants are randomly assigned; no additional details in English abstract |
| Allocation concealment (selection bias) | Unclear risk | Not specified in English abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear from the English abstract whether all participants were accounted for in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or published protocol. It is not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | We did not seek full translation of this study report, and we could not be certain whether the study included other sources of bias |
Assare 2002.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: Sweden Setting: hospital; single centre Inclusion criteria: ASA I or II; scheduled for elective knee arthroscopy; informed consent Exclusion criteria: not reported Type of surgery: elective arthroscopy (ambulatory surgery) Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with cyclizine. Induction with fentanyl and propofol according to clinical need. Muscle relaxants were not used and LMA placed in all participants. For maintenance sevoflurane was combined with oxygen in nitrous oxide. Lidocaine prior to incision and with adrenaline at the start of surgery, and with fentanyl at the end of surgery. All participants were given paracetamol and lornoxicam for postoperative analgesia. |
|
| Outcomes |
Outcomes measured/reported by study authors: sevoflurane consumption; recovery (time to removal of laryngeal mask; time to state birth and name; time to readiness to discharge); fentanyl consumption; rescue analgesics; PONV; intraoperative awareness (postoperative interview; details of interview structure or time not reported) Outcomes relevant to the Review: intraoperative awareness; time to readiness to discharge |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or publication of a protocol and it was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Avidan 2008.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 2000 Country: USA Setting: single centre Inclusion criteria: ≥ 18 years of age; scheduled for surgery under GA with isoflurane, sevoflurane or desflurane; at high risk of intraoperative awareness with at least one criterion (preoperative long‐term use of anticonvulsant agents, opiates, benzodiazepines, or cocaine; cardiac ejection fraction < 40%; history of anaesthesia awareness; history of difficult intubation or anticipated difficult intubation; ASA status IV or V; aortic stenosis; end‐stage lung disease; marginal exercise tolerance not resulting from musculoskeletal dysfunction; pulmonary hypertension; planned open‐heart surgery; daily alcohol consumption) or two minor criteria (preoperative use of beta‐blockers; COPD; moderate exercise tolerance not resulting from musculoskeletal dysfunction; smoking ≥ 2 packs of cigarettes per day; obesity defined as BMI > 30 kg/m²) Exclusion criteria: the surgical procedure or positioning of the participant prevented BIS monitoring or if the surgery required a wake‐up test; dementia: unable to provide informed consent; history of stroke with residual neurological deficits Risk of awareness: participants at high risk for this complication (see inclusion criteria) Baseline characteristics Intervention group (BIS)
Comparison group (ETAG)
|
|
| Interventions | Intervention group (BIS)
Comparison group (ETAG)
|
|
| Outcomes |
Outcomes measured/reported by study authors: definite intraoperative awareness and possible intraoperative awareness (assessed using Brice questionnaire at 3 time points: 24 hours after anaesthesia; between 24 hours and 72 hours; and 30 days); BIS values; ETAG concentrations Outcomes relevant to the review: definite intraoperative awareness |
|
| Notes |
Funding/declarations of interest: study authors report that manufacturer of the BIS monitor (Aspect Medical Systems) had no role in the study design, data collection, data analysis, or manuscript preparation. No study monitors or other means of support were provided by Aspect Medical Systems. Supported by a grant form the Barnes‐Jewish Hospital Foundation (to Dr. Avidan) Study dates: September 2005 to October 2006. Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "2000 patients underwent prerandomization electronically in blocks of 100, with 50 patients assigned to a BIS‐guided protocol and 50 to an ETAG‐guided protocol." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted 59 losses, but these were relatively balanced between groups, and amounted to a loss of fewer than 10%. ITT analysis was planned |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered on clinical trials register (NCT00281489). It is not feasible to assess the risk of reporting bias from these reports. In addition, we noted a retrospectively published protocol (Avidan 2009) |
| Other bias | Unclear risk | All patients classed as high risk although a significant difference was noted between the amount of patients with a neurological pre‐existing medical condition in the ETAG group and the BIS group. |
Avidan 2011.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 6041 Country: USA and Canada Setting: hospital; multi‐centre (3 centres) Inclusion criteria: ≥ 18 years of age; scheduled for elective surgery under GA and at high risk of intraoperative awareness (see below) Exclusion criteria: people with dementia; unable to provide written informed consent; history of stroke with residual neurological deficits. Also, the surgical procedure or positioning of the participant prevented BIS monitoring or if the surgery required a wake‐up test Type of surgery: elective surgery (type not specified) Criteria used for risk of intraoperative awareness: ≥ 1 major risk factor (preoperative long‐term use of anticonvulsant agents, opiates, benzodiazepines, or cocaine; a cardiac ejection fraction < 40%; a history of anaesthesia awareness; a history of difficult intubation or anticipated difficult intubation; ASA physical status class IV or class V; aortic stenosis; end‐stage lung disease; marginal exercise tolerance not resulting from musculoskeletal dysfunction; pulmonary hypertension; planned open‐heart surgery; daily alcohol consumption) or two minor criteria (preoperative use of beta‐blockers; chronic obstructive pulmonary disease; moderate exercise tolerance not resulting from musculoskeletal dysfunction; smoking ≥ 2 packets cigarettes per day; obesity, defined as a BMI > 30 kg/m²) Baseline characteristics (for analysed participants) Intervention group (BIS)
Comparison group (ETAG)
|
|
| Interventions |
Intervention group (BIS)
Comparison group (ETAG)
Both groups: GA with isoflurane, sevoflurane or desflurane |
|
| Outcomes |
Outcomes measured/reported by study authors: incidence of definite and possible intraoperative awareness Outcomes relevant to the review: intraoperative awareness assessed using Brice questionnaire and using Michigan Awareness Classification Instrument (assessed within 72 hours of surgery and at 30 days after extubation) |
|
| Notes |
Funding/declarations of interest: funded by the Foundation for Anesthesia Education and Research and the American Society of Anesthesiologists; grant from the Winnigpeg Regional Health Authority and the University of Manitoba Department of Anesthesia, and from departmental support Study dates: May 2008 to May 2010 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...6100 prerandomization designations were generated electronically in blocks of 100, divided equally between the groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "Labels indicating BIS group to ETAC group were sealed in opaque, numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." Anaesthetists were able to see the ETAG values in each group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 in the BIS group and 50 in the ETAG group were lost to follow‐up. Losses were relatively balanced between groups and were < 10%. A modified ITT analysis were performed |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered on clinical trials register (NCT00682825). We noted additional secondary outcomes which were not included in the primary report (e.g. PTSD, dreams, mortality, haemodynamic variables, dose and concentration). In addition, we noted a retrospectively published protocol (Avidan 2009). |
| Other bias | Low risk | We identified no other sources of bias |
Başar 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: Turkey Setting: hospital; single centre Inclusion criteria: informed consent; ASA I or II Exclusion criteria: renal, hepatic or neurological dysfunction; use of benzodiazepines, anticonvulsants, alcohol, opioids or other psychotropic drugs Type of surgery: open abdominal surgery Experience of anaesthetist (in years or qualifications): anaesthesia residents ≥ 1 year's experience Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with atropine and diazepam. Induction with fentanyl, thiopental, and rocuronium to facilitate intubation. Maintenance with sevoflurane in nitrous oxide/oxygen (50%/50%). Signs of inadequate anaesthesia managed by increasing concentration of sevoflurane. |
|
| Outcomes |
Outcomes measured/reported by study authors: mean sevoflurane exposure (aged‐adjusted MAC);
amount of sevoflurane used; immediate recovery times (time to open eyes on verbal command; time to motor respond to verbal command); Aldrete score at 10 minutes: bradycardia; hypotension or hypertension requiring treatment Outcomes relevant to the review: time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Boztuğ 2006.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Country: Turkey Setting: hospital; single centre Inclusion criteria: 18 to 75 years of age; ASA I or II patients undergoing craniotomy. Exclusion criteria: any medication interaction with the central nervous system (antidepressant drugs, anti‐seizure drugs) or cardiopulmonary system (antihypertensive drugs, beta blockers); or a need for postoperative ventilation or other psychotropic drugs Type of surgery: supratentorial craniotomy Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with thiopental and fentanyl, followed by cisatracurium to facilitate intubation. Maintenance with sevoflurane 0.8% to 1.5% with mix of oxygen/air (50%/50%). Cisatracurium administered if needed. |
|
| Outcomes |
Outcomes measured/reported by study authors: average end tidal concentrations of sevoflurane; recovery times (from end of surgery to first spontaneous breathing; from end of surgery to eye opening; from end of surgery to extubation; time to reach Aldrete score of 9 or 10; PACU stay); haemodynamic variables Outcomes relevant to the review: duration of PACU stay |
|
| Notes |
Funding/declarations of interest: supported by the Akdeniz University Faculty of Medicine Research Application Center Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated sequence of number was used |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope technique was used; insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors did not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "3 patients were excluded from the study due to disconnection of BIS probe (2) or artefact contamination (1)." Study authors do not report missing outcome data is managed, or to which group the missing participants belonged, however number of losses is < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or published protocol. It is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Bresil 2013.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 70 Country: Denmark Setting: hospital; single centre Inclusion criteria: both genders; ASA I and II; 1 to 65 years of age (see note below); undergoing elective ENT surgery (tonsillectomy, adenotomy, myringotomy, laryngoscopy, bronchoscopy and oesophagoscopy, myringo‐tympanoplasty) Exclusion criteria: patient refusal; presence of psychiatric conditions; use of psychopharmacological, antiepileptic or anti‐arrhythmic medication; chronic use of opioids; intake of > 21 units of alcohol per week Type of surgery: elective ENT Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs):
|
|
| Interventions | Intervention group (BIS):
Comparison group (clinical signs):
Both groups: no muscle relaxants, gas anaesthetic or premedication with benzodiazepine was used. Induction with remifentanil and propofol. Lidocaine used during laryngoscopy. Maintenance with propofol, remifentanil. |
|
| Outcomes |
Outcomes measured/ reported by study authors: time to extubation; volumes of propofol and remifentanil; hypotension, bradycardia; haemodynamic variables Outcomes relevant to the review: none |
|
| Notes |
Funding/declarations of interest: department funding only. Study authors report no conflicts of interest Study dates: January 2010 to March 2012 Notes:
|
|
Bruhn 2005.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 142 Country: Germany Setting: multi‐centre; 4 university anaesthesia departments. Inclusion criteria: 18 to 80 years of age; written informed consent; undergoing minor surgery expected to last ≥ 1 hour Exclusion criteria: a history of any disabling central nervous or cerebrovascular diseases; hypersensitivity to opioids or substance abuse; a treatment with opioids or any psychoactive medication Type of surgery: minor surgery expected to last ≥ 1 hour Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with remifentanil and propofol, cisatracurium for tracheal intubation. Maintenance with remifentanil and desflurane. No neuromuscular blocking agents were given intraoperatively. |
|
| Outcomes |
Outcomes measured/reported by study authors: Desflurane consumption (end tidal concentrations); recovery times (time to open eyes; time to be extubated; time to stating name; time to arrive in PACU; time to discharge from the PACU); occurrence of intraoperative recall (interviewed on POD1 and POD3) Outcomes relevant to the review: intraoperative awareness; time to discharge from the PACU |
|
| Notes |
Funding/declarations of interest: supported by funding from Baxter, Inc., Germany Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After enrolment the patients were randomized by drawing lots from a closed box" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recovery times were recorded by a blinded investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Ellerkmann 2010.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: Germany Setting: hospital; single centre Inclusion criteria: 18 to 80 years of age; ASA status I to III; undergoing orthopaedic surgery expected to last ≥ 1 hour in which regional anaesthesia for intra‐ and postoperative pain control for surgery to the upper or lower extremity was used in combination with GA Exclusion criteria: history of any disabling central nervous or cerebrovascular diseases; hypersensitivity to opioids or substance abuse; a treatment with opioids or any psychoactive medication Type of surgery: minor orthopaedic surgery Experience of anaesthetist (in years or qualifications): an experienced anaesthetist Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with remifentanil and propofol. Cisatracurium for intubation. Maintenance with propofol and remifentanil. No neuromuscular blocking agents were given intraoperatively. An additional bolus dose of propofol could be given in the presence of an unexpected somatic intraoperative response. |
|
| Outcomes |
Outcomes measured/reported by study authors: propofol consumption; remifentanil consumption; recovery (time to eye opening; time to extubation; time to reach Aldrete scores); BIS values; intraoperative awareness (in recovery room; POD 1 and POD 3. NOTE: study authors do not report whether this was assessed using an interview, a questionnaire, or was voluntarily reported by participants) Outcomes relevant to the review: intraoperative awareness; time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized by drawing lots from a closed box |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were aware of group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Due to insufficient regional anaesthesia or EEG data loss, 3 participants in each of the BIS and standard practice groups had to be excluded from further investigation. Although this loss was 10% of participants, it was balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Fakhr 2014.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 68 Country: Iran Setting: hospital; single centre Inclusion criteria: > 60 years of age; ASA status I to III; scheduled for elective abdominal surgery; normal healthy patients with mild systemic disease or patients with severe systemic disease with no immediate danger of death Exclusion criteria: people with psychotic disorders; dementia; previous cerebrovascular accident; head trauma; drug abuse Type of surgery: abdominal surgery Baseline characteristics not reported. Study authors report that there were quote: "no significant differences in age, sex, height, weight and physical status between the control and intervention groups" |
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with fentanyl, midazolam, propofol, and atracurium. Then isoflurane 1% or 2%, nitrous oxide, and oxygen. Neostigmine and atropine given at end of anaesthesia |
|
| Outcomes |
Outcomes measured/ reported by study authors: recovery times (time to extubation; time to orientation; time to transfer to PACU; time up to discharge from PACU); intraoperative awareness (on the following day; asked if any memory of the surgery room and its events; asked if they heard anything during surgery) Outcomes relevant to the review: intraoperative awareness (not reported by study authors, see below); time to orientation (not reported); duration of time in PACU |
|
| Notes |
Funding/declarations of interest: funded by the Research Council of Hamedan University of Medical Sciences. Study authors declare no conflicts of interest Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization used |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments made by an anaesthetist who was not aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study report included the total number of randomized participants, but did not report how many were randomized to each group, and did not report number of analysed participants. Therefore, it was not possible to effectively assess risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Gan 1997.
| Study characteristics | ||
| Methods | RCT, multi‐centre | |
| Participants |
Total number of randomized participants: 268 Country: USA Setting: multi‐centre; 4 institutions Inclusion criteria: 18 to 80 years of age; ASA I to III; scheduled for general surgical procedures expected to last at least 1 hour. Exclusion criteria: known neurological disorders; uncontrolled hypertension; baseline systolic BP < 106 HR < 55; other serious medical conditions that would interfere with cardiovascular response assessment. Type of surgery: general surgical procedures ≥ 1 hour Experience of anaesthetist (in years or qualifications): anaesthesia supervised by a faculty anaesthetist. Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with propofol and alfentanil, then with 50% nitrous oxide. If necessary a neuromuscular blocking agent was given to facilitate tracheal intubation. After intubation or insertion of LMA, additional neuromuscular blocking agents only administered if surgically indicated. |
|
| Outcomes |
Outcomes measured/reported by study authors: normalized propofol infusion rate (µg/kg/hour); mean propofol used (mg); normalized alfentanil infusion rate (µg/kg/min); time to open eyes (min); time to respond to command (minutes); time to be extubated; time to be eligible to discharge from the PACU; number of unwanted somatic and haemodynamic responses; intraoperative global assessment score; % of patients arrived fully oriented to the postanaesthesia care unit (PACU); overall global nursing impression score Outcomes relevant to the review: recovery (time to eye opening); time to be ready for discharge from the PACU |
|
| Notes |
Funding/declarations of interest: sponsored in part by a grant from Aspect Medical Systems (MS, USA). Some study authors received fees from Aspect Medical Systems Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of treatments was determined in blocks of 10 using a random number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignment to the study condition was determined using sequential coded envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were assessed continuously by a recovery room nurse who blinded to the intraoperative treatment group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty‐eight patients were excluded from efficacy analysis due to protocol violations for various reasons." As a result, there were 125 participants in the clinical signs group and 115 participants in the BIS group. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Georgakis 2000.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: unknown Setting: hospital; single centre Inclusion criteria: undergoing varicose vein surgery, ASA status I or II Exclusion criteria: not specified in abstract Type of surgery: varicose vein surgery Baseline characteristics not reported in abstract |
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: propofol using TIVA, fentanyl, vecuronium, and maintained with propofol in nitrous oxide/oxygen (60%/40%). Additional increments of fentanyl and vecuronium as required |
|
| Outcomes |
Outcomes measured/ reported by study authors: recovery (time to extubation, time to eye opening, time to response to command); propofol consumption; depth of anaesthesia (assessed using areas outside of target BIS values) Outcomes relevant to the review: time to eye opening (see below) |
|
| Notes |
Funding/declarations of interest: funding not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not reported whether participants are aware of group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration of pre published protocol. It is not feasible to assess risk of reporting bias without these documents |
| Other bias | Unclear risk | Study report is an abstract and therefore, it is not feasible to assess other risks of bias from this short report |
Guo 2015.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Country: China Setting: hospital; single centre Inclusion criteria: people with severe burns undergoing elective escharectomy within 1 week of injury; ASA status II to III Exclusion criteria: drug allergies; apparent heart, lung, liver or kidney dysfunction; BMI > 30 kg/m² Type of surgery: elective escharectomy Baseline characteristics Intervention group (BIS)
Comparison group:
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: remifentanil, and propofol via TCI, cisatracurium for tracheal intubation. Cisatracurium to maintain muscle relaxation. During induction dopamine given if MAP decreased by > 20%. Ephedrine, dopamine, atropine, esmolol, and urapidil were administered when necessary. |
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; target concentrations of remifentanil and propofol; BIS values; intraoperative awareness (time point of measurement, and method of data collection was not reported) Outcomes relevant to the review: intraoperative awareness |
|
| Notes | Funding/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: December 2011 to December 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not reported whether participants are aware of group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration of pre published protocol. It is not feasible to assess risk of reporting bias without these documents |
| Other bias | Low risk | We identified no other sources of bias |
Ibraheim 2008.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Country: Saudi Arabia Setting: hospital; single centre Inclusion criteria: morbidly obese (BMI > 35 kg/m²); ASA I or II; scheduled to undergo gastric band procedures Exclusion criteria: renal, hepatic or neurological dysfunction; use of benzodiazepines, anticonvulsants, alcohol, opioids or other psychotropic drugs Type of surgery: gastric banding procedures Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with fentanyl, propofol, and succinylcholine. Maintenance with sevoflurane 2% mixed with oxygen and air. Atracurium neuromuscular blockade maintained, and reversal with neostigmine and glycopyrrolate. |
|
| Outcomes |
Outcomes measured/reported by study authors: sevoflurane consumption during maintenance (mL/hour); recovery (time to awakening ‐ opening eyes on verbal command; time to extubation; time to achieve Aldrete score of 9); pain scores; intraoperative awareness (at time of discharge from PACU, and 24 hours after surgery, participants asked whether they dreamt or recalled any intraoperative events) Outcomes relevant to the review: intraoperative awareness; time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were aware of group allocation. It is not feasible for anaesthetists to be blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded study personnel recorded recovery times. However, study authors did not report whether outcome assessors who assessed intraoperative awareness were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Jain 2016.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 62 Country: India Setting: hospital; single centre Inclusion criteria: ASA status I or II; receiving halothane‐based GA Exclusion criteria: refusal to participate; psychiatric patients; chronic users of psychoactive medication; known or suspected EEG abnormality; abnormal liver function; conduction abnormalities; lost to follow‐up due to surgery exceeding 6‐hour duration; change of anaesthetic plan Type of surgery: study authors stated that surgery mostly comprised of open cholecystectomy and abdominal hysterectomy Baseline characteristics Intervention group (BIS)
Comparison group (ETAG)
|
|
| Interventions | Intervention group (BIS)
Comparison group (ETAG)
Both groups: premedication with IV glycopyrrolate and nalbuphine. Induction with propofol, intubation facilitated with rocuronium. Maintenance with 60% nitrous oxide and halothane, and use of rocuronium as required. Reversal of neuromuscular blockade with neostigmine and glycopyrrolate. |
|
| Outcomes |
Outcomes measured/ reported by study authors: duration of surgery; duration of anaesthesia; time to extubation Outcomes relevant to the review: none |
|
| Notes |
Funding/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: not reported Note:
|
|
Kabukcu 2012.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 70 Country: unknown Setting: hospital; single centre Inclusion criteria: scheduled for open heart surgery Exclusion criteria: unknown Type of surgery: open heart surgery Baseline characteristics not reported in abstract. Study authors reported no statistical differences between groups |
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with fentanyl and etomidate, and vecuronium to facilitate tracheal intubation. Maintenance with propofol and remifentanil |
|
| Outcomes |
Outcomes measured/reported by study authors: consumption of anaesthetic agents: intraoperative awareness (time point and method of assessment is not specified); haemodynamic variables; BIS values Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants are randomized to groups; no additional information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Study report is an abstract and therefore, it is not feasible to assess other risks of bias from this short report |
Kamal 2009.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: Egypt Setting: hospital; single centre Inclusion criteria: informed consent; 45 to 60 years of age; ASA I to III; scheduled for elective moderate abdominal surgical procedures; expected durations at least 2 hours. Exclusion criteria: a history of any disabling central nervous or cerebrovascular disease; hypersensitivity to opioids; substance abuse; treatment with opioids or any psychoactive medication; a BMI > 40 Type of surgery: abdominal surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with propofol and fentanyl, then atracurium. Maintenance with sevoflurane and nitrous oxide/oxygen (50%/50%), and intermittent boluses of atracurium. Hypotension treated with IV fluid replacement or decrease in sevoflurane concentration, or finally by ephedrine or phenylephrine. Bradycardia treated with reduction in sevoflurane or with atropine. Residual neuromuscular blockade was reversed with glycopyrrolate and neostigmine |
|
| Outcomes |
Outcomes measured/reported by study authors: recovery times (time to eye opening; time to extubation; time to orientation; time to arrival in the PACU; time to discharge from the PACU); sevoflurane consumption; propofol and fentanyl consumption; end‐tidal concentration of sevoflurane; incidence of intraoperative awareness (POD 1, POD 2, and POD 3; interviewed about any recall of events, sounds, feeling surgical instruments or dressings, or dreaming) Outcomes relevant to the review: intraoperative awareness; time to orientation; time to discharge from the PACU |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2006 to July 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly selected and assigned into two groups of 30 patients each. Method of randomization is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded. However, these losses were fewer than 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Kamali 2017a.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 214 Country: Iran Setting: hospital; single centre Inclusion criteria: scheduled for non‐emergency caesarean section; gestational age of 37 to 42 weeks; lack of any systemic disorder; ASA status I or II; 15 to 45 years of age; no chronic drug abuse; no prior history of heart, liver or kidney disorder; maximum surgery duration of 60 minutes; undergoing surgery by one surgeon Exclusion criteria: intubation for more than 35 seconds; pre‐eclampsia or chronic hypertension; morbid obesity; ASA status > II; systemic or mental disorder; duration of surgery > 90 minutes Type of surgery: non‐emergency caesarean Baseline characteristics are not reported |
|
| Interventions | Intervention group (BIS):
Comparison group (clinical signs)
Both groups: induction with thiopental and succinylcholine. Maintenance with nitrous oxide/oxygen (50%/50%) and 1% isoflurane, and atracurium if required. Fentanyl given after delivery |
|
| Outcomes |
Outcomes measured/ reported by study authors: intraoperative awareness (interview and questionnaire at 12 hours and 24 hours after surgery); haemodynamic variables Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: support from Arak University of Medical Sciences. Study authors declare no conflicts of interest Study dates: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to group allocation. However, it was not feasible to blind anaesthetists to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Awareness was evaluated by a trainee anaesthetist, to ensure blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration (ChiCTR‐TRC‐1200239); it is not feasible to effectively assess risk of reporting bias from this document |
| Other bias | Unclear risk | Study authors do not report baseline characteristics table, and we could not be certain whether characteristics were equivalent between groups |
Karaca 2014.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 82 Country: Turkey Setting: hospital; single centre Inclusion criteria: ASA I to II; 20 to 60 years of age; undergoing elective surgery for supratentorial mass lesions under general anaesthesia Exclusion criteria: cardiac failure; renal failure; anaemia; ischaemic heart disease; liver disease; gastrointestinal system disease; diabetes mellitus; hypothalamus pituitary gland disorders; diuretic use; hypoalbuminaemia; hyperglycaemia; electrolyte imbalance; alcohol consumption; requiring hormone replacement therapy; pregnancy or lactating women; psychiatric condition Type of surgery: neurosurgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Vecuronium to facilitate intubation. Maintenance with propofol, remifentanil and use of rocuronium |
|
| Outcomes |
Outcomes measured/ reported by study authors: intraoperative awareness (participants were asked quote: "the last event they recalled about the surgery"; time point of assessment is not specified); recovery times (time to eye opening; time to spontaneous breathing; Aldrete scores at 20 minutes); TOF values; fluid balance; bleeding volume; consumption of propofol, remifentanil, and rocuronium; duration of surgery; haemodynamic variables Outcomes relevant to the review: intraoperative awareness; time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of quote: "closed envelope method" for randomization; no additional details |
| Allocation concealment (selection bias) | Unclear risk | See above; insufficient details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were aware of group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Khoshrang 2016.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 96 Country: Iran Setting: hospital; single centre Inclusion criteria: 15 to 65 years of age Exclusion criteria: personality disorder; neurological disorders; prior history of head trauma; drug abuse; drugs that affect the central nervous system; craniofacial anomalies; abnormal forehead; uncontrolled blood pressure (SBP > 150 mmHg and DBP > 105 mmHg); insulin‐dependent diabetes; BMI > 33; emergency operation; ASA class ≥ II Type of surgery: open renal surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with propofol, fentanyl, and then atracurium. Maintenance, propofol and remifentanil. Nitrous oxide and oxygen used for inhalation anaesthesia with atracurium IV |
|
| Outcomes |
Outcomes measured/reported by study authors: recovery times (time to: eye opening; verbal response to verbal stimulation; extubation; stay in the PACU); first‐time to narcotic usage; total dosage of IV narcotics Outcomes relevant to the review: time to eye opening; length of stay in the PACU (see notes) |
|
| Notes |
Funding/declarations of interest: quote: "no financial relationship with any organization". Study authors report no conflicts of interest Study dates: October 2014 to October 2015 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors use block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of discharge from PACU based on Aldrete score ≥ 9, by an anaesthetist who was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study was retrospectively registered with a clinical trials register (IRCT2015042111766N2). It is not feasible to use this document to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Kim 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: Korea Setting: hospital; single centre Inclusion criteria: scheduled for elective CABG Exclusion criteria: not specified Type of surgery: elective CABG Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: anaesthesia with propofol, fentanyl, and vecuronium. Participants were given 12 words during surgery, which they were asked if they recalled |
|
| Outcomes |
Outcomes measured/ reported by study authors: intraoperative awareness (interview on second postoperative day); consumption of propofol, fentanyl, and morphine Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: not reported in abstract Study dates: not specified Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not specify whether participants were aware of group allocation. Not feasible to blind anaesthetists from group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of only one participant in the BIS group |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | We did not seek full translation of this study report, and we could not be certain whether the study included other sources of bias |
Kreuer 2003.
| Study characteristics | ||
| Methods | RCT parallel design | |
| Participants |
Total number of randomized participants: 80 Country: Germany Setting: hospital; single centre Inclusion criteria: 18 to 80 years of age; ASA I to III; scheduled to undergo minor orthopaedic surgery expected to last at least one hour. Exclusion criteria: disabling central nervous or cerebrovascular diseases; hypersensitivity to opioid; substance abuse; treatment with opioids or any psychoactive medication Type of surgery: minor orthopaedic surgery lasted ≥ 1 hour Overall duration of anaesthesia, if reported: 121.2 (± 40.9) minutes (BIS); 108.2 (± 44.2) minutes (clinical signs) Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with diazepam. Induction with remifentanil and propofol, then cisatracurium to facilitate tracheal intubation. Propofol TCI and remifentanil for maintenance. Metamizol for postoperative pain relief. |
|
| Outcomes |
Outcomes measured/reported by study authors: normalized propofol infusion rate; normalized remifentanil infusion rate; time to open eyes; time to be extubated; time to arrive in PACU; intraoperative awareness (study authors did not report time point or method of assessment); number of patients receiving intervention to treat intraoperative hypotension; haemodynamic variables Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: departmental support only Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ".. patients were randomized by drawing lots from a closed box." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recovery times and propofol consumption were recorded by a blinded investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Kreuer 2005.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Country: Germany Setting: hospital Inclusion criteria: men or women; 18 to 80 years of age; ASA physical status I to III; scheduled for minor orthopaedic surgery expected to last ≥ 1 hour Exclusion criteria: history of any disabling central nervous or cerebrovascular disease; hypersensitivity to opioids or substance abuse; treatment with opioids or any psychoactive medication Type of surgery: minor orthopaedic surgery expected to last at least 1 hour Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam, induction with remifentanil and propofol and atracurium to facilitate tracheal intubation. Maintenance with remifentanil and desflurane. Hypotension treated with IV fluid replacement, then IV vasopressor. Bradycardia treated with atropine. Use of metamizol for postoperative analgesia. |
|
| Outcomes |
Outcomes measured/reported by study authors: time taken (spontaneous eye opening; extubation; arrival in PACU); BIS values; desflurane consumption; end‐tidal desflurane concentrations; infusion rates of remifentanil; MAP; use of vasopressors and atropine; intraoperative recall (interview on first and third postoperative day), Outcomes relevant to the Review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: department funding only Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ".. patients were randomized by drawing lots from a closed box." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors did not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recovery times were recorded by a blinded investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Luginbuhl 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 160 Country: Switzerland Setting: hospital; single centre Inclusion criteria: scheduled for gynaecological surgery lasting > 15 minutes; under GA Exclusion criteria: central nervous system disease (i.e. history of cerebrovascular disease or epilepsy); taking EEG‐affecting drugs; ASA status > III Type of surgery: gynaecological surgery Baseline characteristics Intervention group (BIS desflurane)
Intervention group (BIS propofol)
Comparison group (clinical signs desflurane)
Comparison group (clinical signs propofol)
|
|
| Interventions | Intervention group (BIS propofol)
Intervention group (BIS desflurane)
Comparison group (clinical signs propofol)
Comparison group (clinical signs desflurane)
All groups: premedication with midazolam or lorazepam. Intubation facilitated with vecuronium; ventilation with mix of oxygen and air. Remifentanil at discretion of attending anaesthetist. Muscle relaxants and opioids administered according to clinical criteria |
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative data (HR, BP etc.); anaesthetic drug use; inadequate hypnosis with potential for explicit recall (BIS > 60 for > 3 minutes; BIS > 65 for > 4 minutes); haemodynamic variables; recovery (Aldrete score; extubation times); patient satisfaction (to include nausea and vomiting) Outcomes relevant to the Review: intraoperative awareness (described as quote: "explicit recall of events during anaesthesia"; time point or method of measurement was not reported) |
|
| Notes |
Funding/declarations of interest: funding from Research Fund of the Department of Anesthesiology, University Hospital of Bern, Switzerland Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patients were randomized into four groups by drawing lots from sealed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients, the PACU nurses and the nurses on the ward were blinded to the allocation of the patients". However, it is not feasible to blind the anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients, the PACU nurses and the nurses on the ward were blinded to the allocation of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Mashour 2012.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 21,601 Country: USA Setting: multi‐centre; 3 hospitals within one medical centre Inclusion criteria: > 18 years of age; anaesthesia using inhalational or intravenous technique; availability for follow‐up interviews Exclusion criteria: intracranial procedures; adhesive allergy; psychosis; history of traumatic brain injury Type of surgery: any surgical case that did not involve the forehead Risk of awareness: unselected population Baseline characteristics Intervention group (BIS)
Comparison group (ETAG)
|
|
| Interventions | Intervention group (BIS)
Comparison group (ETAG)
|
|
| Outcomes |
Outcomes measured/reported by study authors: definite intraoperative awareness (using modified Brice interview; single interview 28 to 30 days after surgery via telephone. In the event of a reported incident, participant had another more detailed interview); anaesthetic consumption; time to readiness to discharge from the PACU; PONV; BIS values; MAC values Outcomes relevant to the review: intraoperative awareness; time to discharge from the PACU |
|
| Notes |
Funding/declarations of interest: supported by the Cerebral Function Monitoring grant; National Institutes of Health; Department of Anesthesiology, University of Michigan Medical School Study dates: May 2008 to May 2010 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a random‐number, computer‐generated block scheme based on even or odd operating room number" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unaware of group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, postoperative interviewers, and all case reviewers were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 9,460 patients randomized to the BIS intervention and successfully interviewed, 3,384 or 36% did not have BIS data recorded because of technical issues described in Materials and Methods.This population was used for secondary analysis only as a post hoc control group because it had neither intervention;" |
| Selective reporting (reporting bias) | Low risk | Prospectively registered with clinical trials register (NCT00689091); outcomes relevant to the review were reported according to this prospectively published document |
| Other bias | Low risk | We identified no other sources of bias |
Masuda 2002.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 46 Country: Japan Setting: hospital; single centre Inclusion criteria: without hypertension or obesity; ASA I to II; 18 to 65 years of age Exclusion criteria: not reported Type of surgery: laparotomy; laparoscopy; surgery on extremities; arthroscopy; surface; head; neck Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Outcomes |
Outcomes measured/reported by study authors: propofol infusion rate; propofol consumption; recovery (time to discharge from the PACU); intraoperative responses (definition not described in English abstract) Outcomes relevant to the review: time to discharge from the PACU |
|
| Notes |
Funding/declarations of interest: unknown Study dates: unknown Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | It was uncertain whether study authors reported prospective clinical trials registration, therefore it is not feasible to assess risk of reporting bias |
| Other bias | Unclear risk | Insufficient information reported in English abstract to assess risks of other bias |
Morimoto 2002.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: Japan Setting: hospital; single centre Inclusion criteria: participants undergoing various surgical procedures under sevoflurane with nitrous oxide anaesthesia; ASA I or II; surgery scheduled to last 2 to 6 hours; 18 to 70 years of age Exclusion criteria: unknown Type of surgery: various Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Outcomes |
Outcomes measured/reported by study authors: sevoflurane consumption; fentanyl and vecuronium required; recovery (time to eye opening; time to extubation; time to discharge from recovery room) Outcomes relevant to the review: time to discharge from recovery room |
|
| Notes |
Funding/declarations of interest: unknown Study dates: unknown Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not feasible to blind anaesthetists to group allocations |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants were excluded: 11 participants excluded because surgery was either longer than 6 hours or shorter than 2 hours, and 3 patients excluded because of mechanical dysfunction of BIS. It is unclear whether these losses were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | It was uncertain whether study authors reported prospective clinical trials registration, therefore it is not feasible to assess risk of reporting bias |
| Other bias | Unclear risk | Insufficient information reported in English abstract to assess risks of other bias |
Mozafari 2014.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 333 Country: Iran Setting: hospital; single centre Inclusion criteria: ASA status I to III; 18 to 65 years of age; scheduled for elective abdominal surgery under GA Exclusion criteria: cardiopulmonary disorders; history of head trauma; cerebrovascular accident; psychotic disorders; dementia; depression; history of drug or substances abuse; lack of sufficient fluency in Persian language Type of surgery: abdominal ("most frequent surgery was laparoscopy, cholecystectomy") Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with sufentanil, thiopental, and atracurium. Maintenance with isoflurane or halothane with nitrous oxide |
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative awareness (using questionnaire; time point of assessment is not specified); haemodynamic parameters Outcomes relevant to the review: intraoperative awareness (see note below) |
|
| Notes |
Funding/declarations of interest: supported by Research Council of Hamadan University of Medical Sciences Study dates: not specified Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of permutated block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are aware of group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Muralidhar 2008.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: India Setting: hospital; single centre Inclusion criteria: undergoing elective CABG Exclusion criteria: poor ventricular function < 40%; left ventricular aneurysms; renal or hepatic dysfunction; requiring extra corporeal circulation; preoperative or intraoperative intra‐aortic balloon pump; presence of unstable angina; carotid stenosis; cerebrovascular accident; excessive alcohol intake; drug abuse Type of surgery: elective off‐pump CABG Baseline characteristics Intervention group (BIS isoflurane)
Intervention group (BIS propofol)
Comparison group (ETAG isoflurane)
Comparison group (ETAG propofol)
|
|
| Interventions | Intervention group (BIS isoflurane)
Intervention group (BIS propofol)
Comparison group (ETAG isoflurane)
Comparison group (propofol ‐ no BIS)
All groups: anti‐hypertensive and anti‐anginal medication continued until the morning of surgery. Premedication with diazepam. Induction with midazolam, fentanyl and thiopentone. Pancuronium bromide for intubation. Haemodynamic parameters maintained within 20% baseline with dopamine, phenylephrine, and glyceryl trinitrate, as required. Perioperative analgesic using rectal diclofenac |
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative awareness (structured interview in the ICU, 18 hours after extubation); volume of anaesthetic agents; time to extubation; length of ICU and hospital stay Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information regarding the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly divided into four groups by a sealed envelope technique.." Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Myles 2004.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 2463 Country: Australia Setting: hospital; multi‐centre Inclusion criteria: 18 years of age or older; scheduled for surgery under GA; at least one of risk factors for awareness, i.e. caesarean section, high‐risk cardiac surgery, acute trauma with hypovolaemia, rigid bronchoscopy, significant impairment of cardiovascular status, severe end‐stage lung disease, past history of awareness, unplanned awake intubation, known or suspected heavy alcohol intake, chronic benzodiazepine or opioid use, or current protease inhibitor therapy Exclusion criteria: inadequate comprehension of English language; traumatic brain injury; memory impairment; psychosis; known or suspected EEG abnormality; not expected to be available for postoperative interview Type of surgery: minor (208 participants), intermediate (457 participants), major (1808 participants) Risk of awareness: see inclusion criteria Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Outcomes |
Outcomes measured/reported by study authors: confirmed intraoperative awareness (interviews using a structured questionnaire at 3 time points: 2 to 6 hours after surgery; 24 to 36 hours postoperatively; and 30 days postoperatively); possible awareness; recovery times (eye opening; eligibility for discharge from the PACU); hypnotic drug administration; hypotension; anxiety and depression; patient satisfaction; major complications; 30 day mortality Outcomes relevant to the review: confirmed intraoperative awareness; time in the PACU (only for participants who were transferred to the PACU); time to eye opening (only for participants who were transferred to the PACU) |
|
| Notes |
Funding/declarations of interest: funded by project grants from: the Austrailian and New Zeland College of Anaesthetists; the Alfred Hospital Research Trust; Royal Hobart Hospital Research Foundation; the Centre for Encouragement of Philanthropy in Australia. One author (P Myles) was funded by an Australian National Health and Medical Research Council Practioner's Fellowship. Loan of equipment and some unrestricted funding from Aspect Medical Systems, and one author (K Leslie) received support for travel and conference expenses from Aspect Medical Systems. Study sponsors had no involvement in study design, data analysis or data interpretation Study dates: September 2000 to December 2002 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random group allocation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Follow‐up was undertaken by a blinded observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 participants were withdrawn because of cancellation of surgery, withdrawal of consent, GA was not used; or participants were under age |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with clinical trials register or details of pre published protocol. Therefore, It is not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Nelskyla 2001.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 62 Country: Finland Setting: hospital; single centre Inclusion criteria: females; ASA status I or II; 18 to 50 years of age; normal body weight; scheduled for gynaecological laparoscopy Exclusion criteria: procedures that involved tubal ligation Type of surgery: gynaecologic laparoscopy Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with diazepam. Then glycopyrrolate and fentanyl, induction with propofol, with rocuronium to facilitate intubation and maintained throughout anaesthesia. Manual ventilation of lungs with 50% nitrous oxide in air and 1.5% sevoflurane. Residual neuromuscular blockade reversal with neostigmine and glycopyrrolate. At the end of anaesthesia, participants were given ketoprofen |
|
| Outcomes |
Outcomes measured/reported by study authors: PONV; volume of anaesthetic agents; recovery times (time to extubation; spontaneous eye opening; response to commands; orientation; tolerate oral fluids; able to sit; able to walk; home readiness); postoperative analgesics; pain Outcomes relevant to the review: time to spontaneous eye opening; time to orientation |
|
| Notes |
Funding/declarations of interest: supported by Helsinki University Central Hospital Clinical Research Funds Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information regarding adequate sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Paventi 2001.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 90 Country: Italy Setting: hospital; single centre Inclusion criteria: participants scheduled for abdominal surgery under GA expected to last > 30 minutes; 18 to 75 years of age Exclusion criteria: history of neurologic disease; medication affecting central nervous system; alcohol and drug abuse Type of surgery: general abdominal surgery > 30 minutes Baseline characteristics not reported by group. Mean age: 42 to 48 years; mean weight: 60 to 71 kg; mean height: 160 to 172 cm; mean duration of anaesthesia: 74 to 102 minutes |
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with diazepam. Induction with remifentanil and TPS, and vecuronium to facilitate tracheal intubation and for maintaining neuromuscular blockade during surgery. Maintenance with sevoflurane and remifentanil. Reversal of residual neuromuscular blockade if needed. Postoperative analgesia achieve with tramadol and ketorolac by elastomeric pump started 50 minutes before end of surgery |
|
| Outcomes |
Outcomes measured/reported by study authors: consumption of anaesthetic drugs; recovery (time to spontaneous breathing; time to extubation; time to eye opening; time to orientation); BIS levels; cost; intraoperative awareness (interview one hour after surgery about any memory in the operating room) Outcomes relevant to the review: intraoperative awareness (not clearly reported); recovery times (orientation; time to eye opening) |
|
| Notes |
Funding/declarations of interest: not reported Study date: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to study groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All recovery parameters were assessed by the same research coordinator not involved in treatment of the patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Payas 2013.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 100 Country: Turkey Setting: hospital; single centre Inclusion criteria: ASA status II to III; 30 to 65 years of age; having a cardiac problem but no previous history of cardiac surgery; scheduled for elective open cholecystectomy under GA Exclusion criteria: ASA status III with decompensated heart failure or history of myocardial infarction in the last 6 months; liver failure; chronic renal insufficiency; history of neurological and psychiatric diseases; respiratory system diseases; alcohol and drug use; history of allergy Type of surgery: elective open cholecystectomy Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam, induction with fentanyl, etomidate, and rocuronium for tracheal intubation and to maintain neuromuscular blockade. Maintenance with nitrous oxide/oxygen (50%/50%) and desflurane |
|
| Outcomes |
Outcomes measured/ reported by study authors: duration of anaesthesia; total opioid dose; total dose of neuromuscular blockade; extubation duration; time to reach an Aldrete recovery score of ≥ 9; haemodynamic variables; BIS values Outcomes relevant to the review: none |
|
| Notes |
Funding/declarations of interest: study authors report quote: "Financial disclosure: N/A". Study authors report no conflicts of interest Study dates: not reported Note:
|
|
Persec 2012.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 45 Country: Croatia Setting: hospital; single centre Inclusion criteria: ≥ 18 years of age; ASA status II or III Exclusion criteria: memory impairment; psychosis; known or suspected electroencephalograph abnormality; chronic use of psychoactive medication; surgery lasting > 6 hours Type of surgery: major abdominal surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with midazolam, fentanyl, and vecuronium to facilitate tracheal intubation. For maintenance 1.5 to 2 MAC of sevoflurane, nitrous oxide in 50% oxygen, fentanyl and vecuronium |
|
| Outcomes |
Outcomes measured/ reported by study authors: BIS values; haemodynamic variables; surgery time; extubation time; intraoperative recall (interview on first postoperative day); adverse events or side effects Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: no financial support and study authors report no conflicts of interest Study dates: February 2011 to July 2011 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are aware of group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trials registration (NCT01470898). It was not feasible to use this report to assess risk of reporting bias |
| Other bias | Unclear risk | Although study authors described no statistically significant differences between groups, we noted that study authors reported baseline characteristics using median values which may indicate data that is skewed. We noted that the control group had a higher median and range values for ASA status; this may indicate an important clinical difference between groups |
Puri 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Country: India Setting: hospital; single centre Inclusion criteria: 18 to 70 years of age; undergoing either CAGB or valve replacement under cardiopulmonary bypass (CPB) Exclusion criteria: neurological disorders; poor ventricular function; New York Heart Association grade IV; diabetes mellitus; impaired renal or hepatic function Type of surgery: CAGB or valve replacement under CPB Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with diazepam. Induction with morphine, midazolam and thiopental. Vecuronium to facilitate tracheal intubation. Maintenance with isoflurane, 66% nitrous oxide in oxygen, and morphine. Isoflurane discontinued once skin suturing completed |
|
| Outcomes |
Outcomes measured/reported by study authors: number of haemodynamic disturbances (hypertension; tachycardia; hypotension; bradycardia); recovery endpoint (time from switching off anaesthetic vaporizer to opening eyes or response to verbal commands); time to tracheal extubation
awareness (interview on first postoperative day) Outcomes relevant to the review: intraoperative awareness; time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note: during the search in 2019, we identified an abstract by the same author team (Puri 1999) which also compared BIS with clinical signs in people undergoing CABG. The number of randomized participants in each study differed but we could not be certain whether Puri 1999 was an interim publication of Puri 2003. We did not include Puri 1999 as a separate study in this review; we added this reference as an associated reference to Puri 2003. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Rahul 2015.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 160 Country: Bangladesh Setting: hospital; multi‐centre (2 hospitals) Inclusion criteria: either gender; 18 to 65 years of age; ASA status I or II; undergoing surgery under GA Exclusion criteria: < 18 years of age or > 65 years of age; ASA status III or IV Type of surgery: mixed surgeries (urology; orthopaedics; ENT; gynaecological; dental; general) Baseline characteristics Intervention group (BIS)
Comparison group:
|
|
| Interventions | Intervention group:
Comparison group:
Both groups: premedication with midazolam and fentanyl. Induction with propofol and vecuronium. Maintenance with sevoflurane and nitrous oxide/oxygen (60%/40%). |
|
| Outcomes |
Outcomes measured/ reported by study authors: PRST scores; BIS scores; duration of anaesthesia; intraoperative awareness (interview at 24 hours postoperatively according to Modified Brice Questionnaire) Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: study authors declare no financial or competing interests Study dates: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants are blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | We noted an imbalance between groups in types of surgery, e.g. more participants in the BIS group had gynaecological surgery. We were uncertain whether these differences could influence results |
Raksakietisak 2016.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 34 Country: Thailand Setting: hospital; single centre Inclusion criteria: 18 to 80 years of age; undergoing spinal surgery with neurophysiology monitoring Exclusion criteria: not specified Type of surgery: spinal surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs):
Both groups: TIVA via TCI propofol, fentanyl, and atracurium |
|
| Outcomes |
Outcomes measured/ reported by study authors: TCI propofol levels; extubation time Outcomes relevant to the review: none |
|
| Notes |
Funding/declarations of interest: funding from Siriraj Research Development Fund Study dates: January 2014 to January 2016 Notes:
|
|
Recart 2003.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: USA Setting: hospital; single centre Inclusion criteria: undergoing laparoscopic general surgery procedures (e.g. cholecystectomy; gastric bypass/banding; hernia repair) Exclusion criteria: history of central nervous system disease; chronic use of psychoactive medication; clinical significant cardiovascular, renal, hepatic or endocrinology disorders Type of surgery: laparoscopic general surgery procedures (e.g. cholecystectomy, gastric bypass/banding, hernia repair) Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with propofol and fentanyl, and rocuronium to facilitate tracheal intubation. Maintenance with desflurane 4% combined with air and oxygen, titrated in 1% to 2% increments. Fentanyl given to maintain stable haemodynamics, and labetalol as required. Residual neuromuscular block antagonized with neostigmine and glycopyrrolate. |
|
| Outcomes |
Outcomes measured/reported by study authors: end‐tidal concentrations of desflurane; fentanyl, rocuronium, labetalol use; recovery (time to eye opening; time to extubation; time to obey commands; time in PACU; time to reach Aldrete score of 10; time to reach fast‐track score of > 12); intraoperative awareness (assessed at discharge from the PACU and at 24 hours postoperatively); pain scores; PONV Outcomes relevant to the review: intraoperative awareness; recovery (time in the PACU) |
|
| Notes |
Funding/declarations of interest: supported in part by an educational grant from Alaris Medical Systems; salary support from the Margaret Milam McDermot Distinguished Chair of Anesthesiology Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " Emergence times were determined .......by a blinded observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other risks of bias |
Savli 2005.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: Turkey Setting: hospital; single centre Inclusion criteria: scheduled for radical mastectomy; ASA I or II; 18 to 50 years of age Exclusion criteria: not specified in English abstract Type of surgery: radical mastectomy Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs):
|
|
| Interventions | Intervention group (BIS):
Comparison group (clinical signs):
Both groups: sevoflurane and nitrous/oxide (70%/30%) |
|
| Outcomes |
Outcomes measured/ reported by study authors: recovery times (time to extubation; time to eye opening; time to orientation; and time to reaching Aldrete score of 9); dose of sevoflurane Outcomes relevant to the review: time to eye opening; time to orientation |
|
| Notes |
Funding/declarations of interest: not specified Study dates: not specified Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized; no additional information in English abstract |
| Allocation concealment (selection bias) | Unclear risk | Not specified in English abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified in English abstract. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in English abstract |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses; data taken from English abstract, and from tables within the main text |
| Selective reporting (reporting bias) | Unclear risk | Not specified in English abstract |
| Other bias | Unclear risk | It is not feasible to fully assess other risks of bias from the English abstract only |
Shafiq 2012.
| Study characteristics | ||
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: Pakistan Setting: hospital; single centre Inclusion criteria: ≥ 60 years of age; ASA status I or II; no significant organ damage; requiring GA with endotracheal intubation and controlled mode ventilation; undergoing general and gynaecological surgeries expected to last 2 to 6 hours Exclusion criteria: history of psychiatric illness; alcohol abuse; altered state of mind; requiring head, neck or laparoscopic surgeries Type of surgery: general and gynaecological Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs/ETAG)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs/ETAG)
Both groups: premedication with midazolam. Induction with propofol, fentanyl and atracurium to facilitate tracheal intubation. Maintenance with nitrous oxide/oxygen (60%/40%) and isoflurane, and intermittent doses of atracurium. For hypertension, adjustments made accordingly to fentanyl or muscle relaxant. Ephedrine or phenylephrine for hypotension, and glycopyrrolate for bradycardia |
|
| Outcomes |
Outcomes measured/ reported by study authors: haemodynamic variables; time to eye opening; time to extubation; time to transfer to PACU; postanaesthesia recovery score Outcomes relevant to the review: time to eye opening |
|
| Notes |
Funding/declarations of interest: not specified Study dates: January 2008 to December 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized trial using slips of paper labelled as BIS group or control group which were taken from an envelope. |
| Allocation concealment (selection bias) | High risk | No method used to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It was not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Siampalioti 2015.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 100 Country: Greece Setting: hospital; single centre Inclusion criteria: scheduled for elective bariatric surgery; super obese with BMI > 50 kg/m²; aged 21 to 60 years of age Exclusion criteria: severe cardiopulmonary disease; significant renal dysfunction; liver dysfunction; history of hyper‐ or hypothyroidism; serious psychiatric or neurologic disorders; recall during GA; allergy to local anaesthetics; history of substance abuse; contra‐indications for placement of thoracic epidural catheter; refusal to participate Type of surgery: bariatric surgery Baseline characteristics Intervention group (BIS ‐ propofol)
Intervention group (BIS ‐ sevoflurane)
Comparison group (clinical signs ‐ propofol)
Comparison group (clinical signs ‐ sevoflurane)
|
|
| Interventions | Intervention (BIS ‐ propofol)
Intervention (BIS ‐ sevoflurane)
Comparison (clinical signs ‐ propofol)
Comparison (clinical signs ‐ sevoflurane)
All groups: doses of all anaesthetic drugs were based on either ideal body weight or corrected body weight. Neuromuscular blockade (cisatracurium) given by continuous infusion, with reversal using neostigmine and atropine |
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; recovery times (time to eye opening, time to extubation, time to reach specified recovery scores); pain Outcomes relevant to the review: time to eye opening |
|
| Notes |
Funding/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ""Both the anesthesiologist performing the assessment and the patients were blinded to the general anesthetic used and the BIS monitoring" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study is registered with a clinical trial register (NCT01279499). However, because the trial does not report a study date, and the clinical trial register has not been updated, it is not possible to assess with registration was retrospective or prospective. It is not feasible to assess the risk of reporting bias without this information |
| Other bias | Low risk | We identified no other sources of bias |
Song 1997.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Country: USA Setting: hospital, single centre Inclusion criteria: outpatients scheduled for tubal ligation Exclusion criteria: neurologic disease; cardiovascular or metabolic diseases; impaired renal or hepatic function; body weight > 100% above the ideal; history of alcohol or drug abuse Type of surgery: laparoscopic tubal ligation Baseline characteristics Intervention group (BIS desflurane)
Intervention group (BIS sevoflurane)
Comparison group (desflurane clinical signs)
Comparison group (sevoflurane clinical signs)
|
|
| Interventions | Intervention group (BIS desflurane)
Intervention group (BIS sevoflurane)
Comparison group (desflurane clinical signs)
Comparison group (sevoflurane clinical signs)
All groups: midazolam, then induction with fentanyl and propofol. Succinylcholine to facilitate tracheal intubation, and lidocaine for topical anaesthesia. Intermittant doses of mivacurium as required. Supplemental doses of fentanyl to treat persistent elevations in HR (> 100 bpm) or MAP (> 20% baseline). Ketorolac and droperidol given 10‐15 minutes before end of surgery for analgesia |
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; mean BIS values; end‐tidal concentrations and volumes of anaesthetic agents; fentanyl requirement; mivacurium requirement; peak airway pressure; coughing or bucking; recovery times (verbal response, extubation, orientation, PACU stay, oral fluid intake, home readiness); intraoperative awareness (questioned at time of hospital discharge and at telephone interview 24 hours after surgery) Outcomes relevant to the review: intraoperative awareness; time to orientation; time in PACU |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "" Patients were randomly assigned to one of four study groups according to a computer‐generated random numbers table." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Sudhakaran 2018.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 68 Country: India Setting: hospital; single centre Inclusion criteria: undergoing lumbar spine surgery; ASA I or II; both genders; 20 to 60 years of age Exclusion criteria: psychiatric illness; clinically significant cardiovascular, respiratory, hepatic or renal disease; long‐term drug or alcohol abuse Type of surgery: lumbar spine surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
Comparison group (ETAG)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Comparison group (ETAG)
All groups: induction with morphine and propofol, and vecuronium. Maintenance with desflurane in nitrous oxide/oxygen (50%/50%) and vecuronium. Diclofenac and ondansetron 15 minutes before end of procedure, and bupivacaine prior to skin closure. Residual neuromuscular blockade was reversed with neostigmine and glycopyrrolate |
|
| Outcomes |
Outcomes measured/ reported by study authors: recovery times (time to emergence; time extubation; time to name recall; fast track time); postoperative analgesic requirements; intraoperative awareness (interview at 24 hours postoperatively using Modified Brice Questionnaire); PONV Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: no funding. Study authors report no conflicts of interest Study dates: July 2011 to December 2012 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Participants selected a sealed envelope; insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses |
| Selective reporting (reporting bias) | Unclear risk | Although we identified a clinical trial register report that described a similar study, we did not clarify this with the study authors and could not use this document to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Tufano 2000.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 160 Country: Italy Setting: hospital; single centre Inclusion criteria: 18 to 70 years of age; scheduled for abdominal surgery under GA with sevoflurane or anaesthesia; surgery expected to last > 60 minutes Exclusion criteria: history of drug or alcohol abuse; neurological or psychiatric disorders Type of surgery: abdominal surgery Baseline characteristics were not reported |
|
| Interventions | Intervention group (propofol BIS)
Intervention group (sevoflurane BIS)
Comparison group (propofol clinical signs)
Comparison group (sevoflurane clinical signs)
All groups: premedication with atropine. Use of cisatracurium, ventilation with nitrous oxide in oxygen (60%/40%), and fentanyl |
|
| Outcomes |
Outcomes measured/reported by study authors: consumption of propofol or sevoflurane; fentanyl consumption; recovery (time to spontaneous breathing; time to extubation; time to eye opening; time to respond to simple commands); incidence of undesirable intraoperative responses Outcomes relevant to the review: time to eye opening |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Study authors did not report baseline characteristics table and we could not be certain whether groups were comparable |
White 2004.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Country: USA Setting: hospital; single centre Inclusion criteria: healthy outpatients scheduled to undergo laparoscopic gynaecological surgery under GA Exclusion criteria: known neurological or psychiatric disorders; currently using anticonvulsants or other centrally‐active medications; clinically significant cardiovascular, respiratory, hepatic, renal or metabolic diseases; long‐term drug or alcohol abuse; body weight > 50% above the ideal body weight Type of surgery: gynaecological laparoscopic surgery Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: premedication with midazolam. Induction with propofol and fentanyl, succinylcholine to facilitate intubation. Desflurane for maintenance, with 60% nitrous oxide in oxygen. Cisatracurium for neuromuscular blockade. Esmolol to treat increases in HR. Neuromuscular reversal with neostigmine and glycopyrrolate. Ketorolac for pain, and ondansetron for emesis |
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; end‐tidal concentrations and desflurane consumption; recovery times (eyes opening; extubation; following commands; orientation; sitting up; tolerating oral fluids; standing up; ambulation; fit for discharge; actual discharge); fast‐track score; modified Aldrete score on arrive in PACU; quality of recovery score; PONV; intraoperative recall (questioned at time of discharge and at telephone interview 24 hours after surgery) Outcomes relevant to the review: intraoperative awareness; time to eye opening; time to orientation; time to discharge from the PACU |
|
| Notes |
Funding/declarations of interest: supported by endowment funds from the Margaret Milam McDermott Distinguished Chair in Anesthesiology and the White Mountain Institute, Los Altos, California (the lead author is the president of this nonprofit organisation) Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "".......the times at which patients were able to open their eyes,...were assessed...by a third investigator who was unaware of the monitoring group.. " |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Wong 2002.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 68 Country: Canada Setting: hospital; single centre Inclusion criteria: ASA status I to III; > 60 years of age; scheduled for elective orthopaedic knee or hip replacement Exclusion criteria: significant cardiopulmonary diseases or other end‐organ disease; depression or psychiatric disorders; dementia; previous CVA; head trauma; inadequate command of English; drugs and all alcohol abuse; preoperative baseline of MMSE score < 24 Type of surgery: elective orthopaedic knee or hip replacement Experience of anaesthetist (in years or qualifications): ≥ 5 years experience of providing anaesthetic patient care Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with propofol, fentanyl and midazolam. Rocuronium to facilitate intubation. Maintenance with isoflurane and 60% to 70% nitrous oxide. Additional rocuronium if required. Reversal with neostigmine and glycopyrrolate |
|
| Outcomes | Outcomes measured/reported by study authors: end‐tidal concentrations and consumption of isoflurane; recovery times (awakening; orientation; discharge from PACU); BIS values; MMSE scores; intraoperative awareness (interview 72 hours after surgery and at 14 days after surgery) Outcomes relevant to the review: intraoperative awareness; time to orientation; time in PACU | |
| Notes |
Funding/declarations of interest: supported in part by a grant from Aspect Medical, Newton, Massachusetts, USA Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization with concealed varying block sizes was performed with computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ""The Aldrete score was assessed at 15 min intervals by a research nurse blinded to the group assignment ......." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ""....., eight patients (three from the SP group, and five from the BIS group) were excluded from the analysis for protocol violations." The missing outcome data seem to balance across intervention group |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration or published protocol not reported. It was not feasible to assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Zhang 2011.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of analysed participants: 5309 Country: China Setting: multi‐centre; 13 hospitals Inclusion criteria: over 18 years of age; without any apparent mental defects; patients scheduled for total intravenous anaesthesia (TIVA) Exclusion criteria: unable to be interviewed after surgery; unable to communicate in Mandarin Chinese; undergoing awake intubation; undergoing intraoperative arousal test. Type of surgery: neurosurgery; craniofacial and cervical surgery; heart surgery; gynaecologic and obstetric surgery; chest and abdominal surgery; urinary surgery; spine and limb surgery; other surgeries. Risk of awareness: unselected for risk of awareness Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: no premedication. Initiation with midazolam, and induction and maintenance with propofol. Other types of anaesthetics (analgesics and muscle relaxants) were at the discretion of the attending anaesthetists. |
|
| Outcomes |
Outcomes measured/reported by study authors: confirmed intraoperative awareness and possible intraoperative awareness and dreaming (using a structured questionnaire on POD 1 and POD 4) Outcomes relevant to the review: confirmed intraoperative awareness |
|
| Notes |
Funding/declarations of interest: not reported Study dates: November 2008 to November 2010 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Despite using computer‐generated random numbers, we are uncertain whether sequence generation was adequately conducted because the information of group allocation was not available in 54 cases. Furthermore, using the baseline characteristics table as a guide, there was an unequal number of participants in each group, and baseline differences between groups in gender and ASA scores which indicated the possibility of poor methods of randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to group allocation. However, it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ""Interviewers and patients were blinded to the group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ""Fifty‐four cases were withdrawn because the information of group allocation was unavailable and another 21 patients were excluded due to age younger than 18 years old (11/10) and a further six patients were excluded because of failure to be interviewed (2/2), one patient died postoperatively, operation was cancelled in one case after anaesthesia induction." |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Zhang 2016.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 72 Country: China Setting: hospital; single centre Inclusion criteria: severe burns; escharotomy + dermatoplasty under GA during early stages (within 7 days after burn); 18 to 65 years of age; BMI > 20 kg/m² or > 30 kg/m²; no history of primary hypertension Exclusion criteria: preoperative heart, lung, liver, kidney and other viscera insufficiently; elective surgery; other serious complications such as MI or cerebral infarction Type of surgery: escharotomy + dermatoplasty Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: atropine before surgery; induction with midazolam, etomidate, sufentanil, and rocuronium. Maintenance with propofol and remifentanil, and rocuronium |
|
| Outcomes |
Outcomes measured/ reported by study authors: haemodynamic variables; doses of propofol and remifentanil; recovery (time to spontaneous breathing; time to directional force; time to extubation); intraoperative awareness (time of measure and method of collection was not reported) Outcomes relevant to the review: intraoperative awareness |
|
| Notes |
Funding/declarations of interest: funding not specified. Study authors declare no conflicts of interest Study dates: August 2013 to August 2015 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were quote: "randomly divided"; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were aware of group allocation. it is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
Zohar 2006.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Country: Israel Setting: hospital; single centre Inclusion criteria: geriatric (more than 65 years of age); undergoing short elective transurethral surgical procedures Exclusion criteria: a history of unstable cardiovascular, pulmonary, hepatic, renal, neurologic, psychiatric or metabolic diseases Type of surgery: short elective transurethral surgical procedures Baseline characteristics Intervention group (BIS)
Comparison group (clinical signs)
|
|
| Interventions | Intervention group (BIS)
Comparison group (clinical signs)
Both groups: induction with fentanyl and propofol. Use of LMA. Maintenance with sevoflurane which was increased in response to signs of an inadequate “depth of anaesthesia” (e.g. movement in response to surgical stimulation). Rescue fentanyl given for sustained increase in respiratory rate. Muscle relaxants were not used |
|
| Outcomes |
Outcomes measured/reported by study authors: anaesthetic requirement (sevoflurane MAC during maintenance (MAC/hour); amount of propofol needed at induction; amount of fentanyl needed at induction; fentanyl 'rescue' dose required); recovery times (time to spontaneous eye opening (study authors do not report data for this outcome); time to remove laryngeal mask airway (LMA) device (study authors do not report data for this outcome); time to responding to simple verbal commands; time to correctly state name, age, and personal identification number; time to achieve fast‐track ability (main outcome); time from awakening from anaesthesia to achieve post anaesthesia care unit (PACU) discharge eligibility); the occurrence of any side effects; the occurrence of need for therapeutic interventions; the occurrence of intraoperative recall awareness (questioned at time of discharge from PACU); patients' satisfaction scores Outcomes relevant to the review: time from awakening from anaesthesia to achieve PACU discharge eligibility; occurrence of intraoperative recall awareness; time to eye opening (not reported) |
|
| Notes |
Funding/declarations of interest: quote: "no industry related funding" Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not report whether participants were blinded to group allocation. It is not feasible to blind anaesthetists to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ""Early recovery endpoints were recorded...by a blinded observer..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or a published protocol. Therefore, it is not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | We identified no other sources of bias |
AAI: auditory‐evoked potential; AEP: auditory evoked potential; ASA: American Society of Anesthesiologists; BIS: bispectral index; BMI: body mass index; BP: blood pressure; bpm: beats per minute; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; CVA: cardiovascular accident; EEG: electroencephalography; ENT: ear, nose, throat; ETAC: end‐tidal anaesthetic concentration; ETAG: end‐tidal anaesthetic gas; ETVAC: end‐tidal concentration of the volatile anaesthetic; GA: general anaesthesia; HR: heart rate; IQR: interquartile range; ITT: intention to treat; IV: intravenous(ly); LMA: laryngeal mask airway; M/F: male/female; MAC: minimum alveolar concentration; MAP: mean arterial pressure; MMSE: mini mental state examination; n: number of participants; N/A: not applicable; PACU: postanaesthesia care unit; POD: postoperative day; PONV: postoperative nausea and vomiting; PRST: systolic blood pressure, heart rate, sweating, tears; PTSD: post‐traumatic stress disorder; RCT: randomized controlled trial; RSI: rapid sequence induction; SBP: systolic blood pressure; SD: standard deviation; TBSA: total burn surface area; TCI: target controlled infusion; TIVA: total intravenous anaesthesia; TNG: topical nitroglycerin; TOF: train‐of‐four
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aceto 2015 | The aim of the study was to evaluate whether BIS‐guided sevoflurane may achieve a lower MAC value, and to search for a MAC threshold for preventing arousal. We excluded this study because it did not meet the review criteria |
| Aimé 2006 | The study was included in a previous version of the review (Punjasawadwong 2014). The aim of the study was to evaluate the economic impact of hypnosis with sevoflurane and we excluded this study because it did not meet the review criteria |
| Chan 2013 | The aim of the study was to evaluate the effects of BIS‐guided anaesthesia on postoperative delirium and cognitive decline and we excluded this study because it did not meet the review criteria |
| Chiu 2007 | The study was included in a previous version of the review (Punjasawadwong 2014). The aim of the study was to evaluate the impact of the use of BIS monitoring on propofol requirements and haemodynamic stability during cardiopulmonary bypass. We excluded this study because it did not meet the review criteria |
| Hachero 2001 | The study was included in a previous version of the review (Punjasawadwong 2014). The aim of the study was to evaluate analgesic requirements when BIS monitoring was used. We excluded this study because it did not meet the review criteria |
| Kamali 2017b | The aim of the study was to evaluate the effects of BIS‐guided anaesthesia on the time of extubation in the ICU following CABG. We excluded this study because it did not meet the review criteria |
| Karwacki 2014 | The aim of the study was to optimise the dosage of anaesthetic agents using BIS‐guided anaesthesia. We excluded this study because it did not meet the review criteria |
| Kaval 2015 | The aim of the study was to evaluate the effects of BIS‐guided anaesthesia on the time of extubation in the ICU following CABG. We excluded this study because it did not meet the review criteria |
| Kerssens 2009 | The aim of the study was to evaluate the effect of BIS‐guided anaesthesia on memory function and physiologic stress response to surgery. We excluded this study because it did not meet the review criteria |
| Nitzschke 2014 | The study was in the list of studies awaiting classification in the previous version of the review (Punjasawadwong 2014). Participants undergoing on‐pump cardiac surgery. We excluded this study because it was a sequential two‐arm clinical study and was not randomized |
| Panagopoulou 2000 | RCT, parallel design. Participants undergoing ENT procedures with anaesthesia titrated to BIS (target values 40 to 60) or by clinical signs. Study is available only as an abstract and does not include the number of participants randomized or analysed in each group. We excluded this study because we did not expect that a publication of the full text is likely, since that abstract was published in 2000. |
| Quesada 2016 | The study was in the list of studies awaiting classification in the previous version of the review (Punjasawadwong 2014). Participants were undergoing echobronchial ultrasound under sedation and we excluded the study because the participant group was not eligible |
| Radtke 2013 | The aim of the study was to evaluate the effects of BIS‐guided anaesthesia on postoperative delirium in elderly people and we excluded this study because it did not meet the review criteria |
| Rüsch 2018 | The aim of the study was to evaluate the induction of anaesthesia guided by BIS or a weight‐based manual administration for the incidence of hypotension. We excluded this study because it did not meet the review criteria |
| Samarkandi 2004 | The study was included in a previous version of the review (Punjasawadwong 2014). The aim of the study was to evaluate the effects of BIS monitoring on anaesthetic requirements and the need for circulatory support. We excluded this study because it did not meet the review criteria |
| Shahrbazi 2008 | The aim of the study was to evaluate the effect of BIS monitoring on serum cortisol levels in the people undergoing CABG. We excluded this study because it did not meet the review criteria |
| Struys 2001 | The study was included in a previous version of the review (Punjasawadwong 2014). The study compared the use of a closed‐loop system that included BIS with a manually‐controlled system and we excluded this study because it did not meet the review criteria |
| Vretzakis 2005 | The aim of the study was to evaluate decision making processes when the value of BIS is known during anaesthesia. We excluded this study because it did not meet the review criteria |
| Zhou 2018 | The aim of the study was to evaluate the effect of BIS monitoring on postoperative attention network dysfunction in elderly surgical patients. We excluded this study because it did not meet the review criteria |
BIS: bispectral index; CABG: coronary artery bypass graft; ENT: ear, nose and throat; ICU: intensive care unit; MAC: minimum alveolar concentration; RCT: randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Aksun 2007.
| Methods | RCT, parallel design |
| Participants |
Number of randomized participants: 40 Type of surgery: cholecystectomy |
| Interventions |
|
| Outcomes | Drug consumption; recovery times (type of recovery is not specified in English abstract) |
| Notes | We were unable to source the full text of this study from current library sources and the abstract contained insufficient information to assess eligibility |
Croci 2014.
| Methods | RCT, parallel design |
| Participants |
Number of randomized participants: 480 Inclusion criteria: women undergoing gynaecological laparoscopy surgery: ASA status I or II |
| Interventions |
|
| Outcomes | PONV; desflurane consumption; cost |
| Notes | Study published only as an abstract. We could not be certain from the information in the abstract by what methods anaesthesia was guided in the control group |
CTRI/2018/03/012457.
| Methods | RCT |
| Participants |
Estimated number of randomized participants: 402 Inclusion criteria: ASA I and II; 15 to 65 years of age; either gender; undergoing elective surgical procedures requiring general anaesthesia Exclusion criteria: history of preoperative long‐term use of anticonvulsant agents, opiates, benzodiazepines, cocaine or daily alcohol consumption; pre‐existing renal hepatic and cardiac disease; history of difficult intubation or anticipated difficult intubation; ASA status III, IV or IV; surgical procedure or positioning of the patient prevents BIS monitoring; people with dementia; unable to provide informed consent; history of stroke with residual neurological deficits Country: India |
| Interventions | BIS‐guided anaesthesia; ETAG‐guided anaesthesia |
| Outcomes | Time to recovery; time to extubation |
| Notes | Completed study in clinical trials register. We await publication of full report to assess eligibility |
Golmohammadi 2014.
| Methods | It is unclear if the study is an RCT |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: morbidly obese adult patients undergoing elective laparoscopic cholecystectomy |
| Interventions | BIS‐guided isoflurane anaesthesia; and standard clinical practice |
| Outcomes | Isoflurane consumption; recovery (time to extubation; time to awakening) |
| Notes | Requires translation from Persian. We could not be certain from the English abstract whether this study was an RCT or a cohort study |
Jeong 2002.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: scheduled for gastric resection under GA Exclusion criteria: kidney or liver function abnormalities; hypertension; diabetes; surgery expected to take < 150 minutes |
| Interventions | Intervention group (BIS), n = 20; versus control group (not specified), n = 20 |
| Outcomes | Concentrations of sevoflurane: BIS values: recovery times (time to response, time to extubation, time to reach 10 points on recovery scale, time to discharge from PACU) |
| Notes | Requires translation from Korean. We could not be certain from the English abstract of the control group methods to monitor depth of anaesthesia |
Qu 2011.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: participants undergoing TIVA anaesthesia; no additional details |
| Interventions | BIS‐guided anaesthesia; no BIS |
| Outcomes | Intraoperative recall awareness |
| Notes | We were unable to source the full text of this study from current sources. The English abstract does not include denominator figures for each group and we require the full text in order to include this study |
ASA: American Society of Anesthesiologists: BIS: bispectral index; ETAG: end‐tidal anaesthetic gas; GA: general anaesthesia; PACU: postanaesthesia care unit; PONV: postoperative nausea and vomiting; RCT: randomized controlled trial; TIVA: total intravenous anaesthesia
Characteristics of ongoing studies [ordered by study ID]
Martins 2013.
| Study name | Influence of processed EEG monitoring in the anesthetic management and its cost in off‐pump coronary surgery: a research protocol |
| Methods | RCT |
| Participants | Participants undergoing CABG without CPB |
| Interventions | BIS visible; BIS not visible (BIS monitor is hidden and monitoring of anaesthetic depth is based on clinical signs associated with the monitoring of expiratory fraction of halogenated anaesthetic agent) |
| Outcomes | Anaesthetic depth; cost |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | We were unable to source the full text of this article. We have not been able to identify any completed trials for which this protocol may be associated, and therefore we assume that the study is ongoing. To populate this tables, we have used information included in the previous version of the review (Punjasawadwong 2014). |
NCT03571945.
| Study name | Incidence of intraoperative awareness in Indian patient population |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 2000 Inclusion criteria: either gender; 18 to 65 years of age; ASA status I or II; GA; elective surgery with a duration of > 30 minutes; consenting to follow‐up Exclusion criteria: uncompensated systemic co‐morbidity; cardiac and neurosurgical procedures; head and neck surgery; obstetric surgery; emergency surgery; anticipated difficult airway; H/O brain injury; EEG abnormality; neuropsychiatry disorders; substance abuse (opioids, alcohol, recreational drugs, benzodiazepine); pacemakers and electronic implants; obesity (BMI > 30kg/m²); adhesive allergy Setting: India; multi‐centre |
| Interventions | BIS versus ETAG |
| Outcomes | Incidence of intraoperative awareness; BIS score; ETAG concentration; MAC concentration; recovery (time to open eyes; time to extubation); haemodynamic variables; postoperative sedation; PONV; postoperative analgesia |
| Starting date | 10 October 2018 |
| Contact information | Amitabh Dutta (duttaamiatbh@yahoo.co.in); Nitin Sethi (nitinsethi77@yahoo.co.in) |
| Notes | Estimated primary completion date: July 2020 |
ASA: American Society of Anesthesiologists; BIS: bispectral index; BMI: body mass index; CABG: coronary artery bypass graft; CPB: cardiopulmonary bypass; EEG: electroencephalography; ETAG: end‐tidal anaesthetic gas; GA: general anaesthesia; H/O: heterotopic ossification; MAC: minimum alveolar concentration; PONV: postoperative nausea and vomiting; RCT: randomized controlled trial
Differences between protocol and review
Differences between the previous review and the updated review
Title: we changed the title of review because it did not sufficiently describe the review objectives in relation to the primary outcome (intraoperative awareness) and the changes that we made to the outcomes (see below).
Review authors: we added three new authors (Sharon Lewis, Michael Pritchard, and Lizzy Fawcett), and we removed two authors who did not wish to be included in the current update (Aram Phongchiewboon and Nutchanart Bunchungmongkoi).
Objectives: we changed the objectives to reflect the changes that we made to the outcomes (see below).
Types of studies: we specified that we did not include studies with publications that were retracted from journals. We excluded abstracts with limited information that were published prior to 2005.
Types of interventions: we included only studies in which investigators aimed to evaluate the effectiveness of BIS for its role in monitoring intraoperative depth of anaesthesia or potential improvements in early recovery times from anaesthesia. The BIS scale is based on a measure of electrical brain activity and some studies sought to use the BIS monitor for purposes other than the objective of this review, for example to reduce the risk of postoperative cognitive dysfunction. However, in this updated review, we did not excluded studies that did not measure or report review outcomes. For clarity in this section, we specified that the review included two comparison groups.
Types of outcome measures: we re‐evaluated the previous review outcomes. In order to improve the usability, manageability, and focus of the review (Methodological Expectation of Cochrane Intervention Reviews), we reduced the number of outcomes. We removed the measure of the consumption of anaesthetics and other drugs, and the measure of cost. We believed that these proxy measures were less important considerations to the anaesthetist, whose aim is to provide a good quality anaesthetic with an appropriate depth of anaesthesia without risk of intraoperative awareness and that provides optimum early recovery. The previous review included six measurements of early recovery (time to: eye opening, response to verbal command, extubation, orientation, discharge from the PACU, and readiness to home discharge). To improve usability we included only the time to eye opening, the time to orientation, and the time to discharge from the PACU. In addition, we provided clarity on the type of data collected for the occurrence of intraoperative awareness.
Search methods: although we used the same databases for searches, we used different search platforms which were more readily available.
Selection of studies: we used Covidence software to (Covidence) to manage the results of the searches. We re‐evaluated all studies included in previous versions of the review against the updated criteria. We noted that one study (previously called Leslie 2005a) was an associated report of Myles 2004and we merged these studies to avoid double counting participants. In addition, we excluded five studies which no longer met the review criteria (Aimé 2006; Chiu 2007; Hachero 2001; Samarkandi 2004; Struys 2001).
Data extraction and management: we used an amended template for collecting data which was more familiar to the new review authors who were responsible for data extraction in this review. In order to improve transparency, we added additional detail to the tables in Characteristics of included studies, and we created a summary table of factors that increased the risk of intraoperative awareness (Appendix 6). We did not include a summary table of anaesthetic practices in each study; we provided this information in the Characteristics of included studies.
Assessment of risk of bias in included studies: we altered the domains in which risk of bias decisions were previously assessed, in order to use the current standard risk of bias domains. We re‐evaluated risk of bias judgements in the previously included studies to ensure a consistent decision‐making process for previously included and new included studies; we made judgements which were based on recommendations in Higgins 2011.
Dealing with missing data: we did not perform intention‐to‐treat analysis in the review. We re‐evaluated the decision to re‐calculate median value data because we believed that these re‐calculations may not provide a true value owing to the potential of skewed data; therefore, we did not include outcome data for recovery times in analysis for three studies (Myles 2004; Paventi 2001;Tufano 2000).
Assessment of reporting bias: we assessed risk of reporting bias against published protocols or clinical trial register documents, and specified that we only assessed funnel plots for risk of reporting bias for outcomes in which we had more than 10 studies.
Subgroup analyses: we conducted subgroup analyses only on the maintenance type of anaesthesia. Rather than using subgroup analysis to distinguish between comparisons of clinical signs and ETAG, we treated these as separate comparisons in the review.
Sensitivity analysis: we did not perform sensitivity analysis on missing data using best‐ and worst‐case scenarios. We made a post‐hoc decision to re‐analyse the data for intraoperative awareness using different statistical methods; this accounted for the evidence including many studies with zero events in both arms, and the rate of rare events.
'Summary of findings' table and GRADE: we added detail to the Methods section to describe the use of GRADE in the review
Contributions of authors
Contributions of authors in the current version of the review.
Sharon Lewis (SL), Michael Pritchard (MP), Lizzy Fawcett (LF), Yodying Punjasawadwong (YP)
Conceiving of the review: YP
Co‐ordinating the review: SL
Undertaking manual searches: SL, Janne Vendt (Information Specialist, Cochrane Anaesthesia Review Group)
Screening search results: SL, MP, LF
Organizing retrieval of papers: SL, LF, MP
Screening retrieved papers against inclusion criteria: SL, LF, MP
Appraising quality of papers: SL, LF, MP
Abstracting data from papers: SL, MP, LF
Managing data for the review: SL
Entering data into Review Manager 5 (Review Manager 2014): SL, MP, LF
Analysing RevMan statistical data: SL
Interpreting data: SL, LF, MP, YP
Making statistical inferences: SL, YP
Writing the review: SL
Securing funding for the review: Cochrane Anaesthesia Review Group
Taking responsibility for reading and checking the review before submission: SL
Sources of support
Internal sources
The Faculty of Medicine, Chiang Mai University, Thailand
External sources
NIHR Cochrane Incentive Awards Scheme, 2018, UK
Declarations of interest
Sharon Lewis: none known
Michael Pritchard: none known
Lizzy Fawcett: none known
Yodying Punjasawadwong: none known
Edited (no change to conclusions)
References
References to studies included in this review
Ahmad 2003 {published data only}
- Ahmad S, Yilmaz M, Marcus RJ, Glisson S, Kinsella A. Impact of bispectral index monitoring on fast tracking of gynecologic patients undergoing laparoscopic surgery. Anesthesiology 2003;98(4):849-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alimian 2016 {published data only}
- Alimian M, Safaeian R, Zaman B, Faiz H, Azizi A. Comparing patient recovery after laparoscopic surgery for women using bispectral index and standard clinical method. Journal of Babol University of Medical Sciences 2016;18(11):14-21. [Google Scholar]
Anez 2001 {published data only}
- Anez C, Papaceit J, Sala JM, Fuentes A, Rull M. The effect of encephalogram bispectral index monitoring during total intravenous anesthesia with propofol in outpatient surgery. Revista Espanola de Anestesiologia Reanimacion 2001;48(6):264-9. [MEDLINE: ] [PubMed] [Google Scholar]
Arbabpour 2015 {published data only}
- Arbabpour R, Ganjifard M, Tabiee S, Saadatjoo SA. Effect of bi-spectral index on recovery and postoperative complications in patients undergoing caesarean section. Journal of Birjand University of Medical Sciences 2015;22(2):94-103. [Google Scholar]
Assare 2002 {published data only}
- Assare H, Anderson RE, Jakobsson J. Sevoflurane requirements during ambulatory surgery: a clinical study of bispectral index and auditory evoked potential guided anaesthesia. Ambulatory Surgery 2002;9:207-11. [PII:S0966-6532(02)00004-5] [Google Scholar]
Avidan 2008 {published data only}
- Avidan MS, Zhang L, Burnside BA, Fink KJ, Searleman AC, Selvidge JA, et al. Anesthesia awareness and the bispectral index. New England Journal of Medicine 2008;358(11):1097-108. [PMID: ] [DOI] [PubMed] [Google Scholar]
Avidan 2011 {published data only}
- *.Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, et al. Prevention of intraoperative awareness in a high-risk surgical population. New England Journal of Medicine 2011;365(7):591-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Avidan MS, Palanca BJ, Glick D, Jacobsohn E, Villafranca A, O'Connor, M, et al. Protocol for the BAG-RECALL clinical trial: a prospective, multi-center, randomized, controlled trial to determine whether a bispectral index-guided protocol is superior to an anesthesia gas-guided protocol in reducing intraoperative awareness with explicit recall in high risk surgical patients. BMC Anesthesiology 2009;9(8):1-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafranca A, Thomson IA, Grocott HP, Avidan MS, Kahn S, Jacobsohn E. The impact of bispectral index versus end-tidal anesthetic concentration-guided anesthesia on time to tracheal extubation in fast-track cardiac surgery. Anesthesia and Analgesia 2013;116(3):541-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Başar 2003 {published data only}
- Başar H, Ozcan S, Buyukkocak U, Akpinar S, Apan A. Effect of bispectral index monitoring on sevoflurane consumption. European Journal of Anaesthesiology 2003;20(5):396-400. [PMID: ] [PubMed] [Google Scholar]
Boztuğ 2006 {published data only}
- Boztuğ N, Bigat Z, Akyuz M, Demir S, Ertok E. Does using the bispectral index (BIS) during craniotomy affect the quality of recovery? Journal of Neurosurgical Anesthesiology 2006;18(1):1-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bresil 2013 {published data only}
- Bresil P, Nielsson MS, Malver LP, Kraemer AK, Schjørring O, Dethlefsen C, et al. Impact of Bispectral Index for monitoring propofol remifentanil anaesthesia. A randomised clinical trial. Acta Anaesthesiologica Scandinavica 2013;57(8):978-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bruhn 2005 {published data only}
- Bruhn J, Kreuer S, Bischoff P, Kessler P, Schmidt GN, Grzesiak A, et al. Bispectral index and A-line AAI index as guidance for desflurane-remifentanil anaesthesia compared with a standard practice group: a multicentre study. British Journal of Anaesthesia 2005;94(1):63-9. [PMID: 15516347 ] [DOI] [PubMed] [Google Scholar]
Ellerkmann 2010 {published data only}
- Ellerkmann RK, Soehle M, Kreuer S. The Entropy Module® and Bispectral Index® as guidance for propofol-remifentanil anaesthesia in combination with regional anaesthesia compared with a standard clinical practice group. Anaesthesia and Intensive Care 2010;38(1):159-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fakhr 2014 {published data only}
- *.Fakhr AA, Asadi HK, Shahrzad ME. Facilitate in elderly waking after surgery with depth of anesthesia monitoring. Turk Geriatri Dergisi 2014;17(1):86-9. [Google Scholar]
- Fakhr AA, Salehi I, Emani B, Mozaffari H. Evaluation of continuous electroencephalogram recording for assessing and control of depth of anesthesia in elderly patients. Journal of Zanjan University of Medical Sciences and Health Services 2012;20(82):no pagination. [Google Scholar]
Gan 1997 {published data only}
- Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, et al. Bispectral Index monitoring allows faster emergence and improved recovery from propofol, alfentanil and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology 1997;87(4):808-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Georgakis 2000 {published data only}
- Georgakis P, Panagopoulou O, Kalosakas K, Foniadaki D, Katsouli I. Effects of BIS monitoring on recovery characteristics, propofol consumption and depth of anaesthesia during TIVA with propofol and fentanyl. European Journal of Anaesthesiology 2000;17(Suppl 19):23. [Google Scholar]
Guo 2015 {published data only}
- Guo Z, Hao J, Jia X, Qi Z. Observation of anesthesia depth in severe burn patients with target controlled infusion of propofol and remifentanil by bispectral index. Medical Journal of Chinese People's Liberation Army 2012;37(4):304-6. [Google Scholar]
- *.Guo ZG, Jia XP, Wang XY, Li P, Su XJ, Hao JH. Bispectral index for monitoring anesthetic depth in patients with severe burns receiving target-controlled infusion of remifentanil and propofol. Genetic Molecular Research 2015;14(3):7597-604. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibraheim 2008 {published data only}
- Ibraheim O, Alshaer A, Mazen K, El-Dawlaty A, Turkistani A, Alkathery K, et al. Effect of bispectral index (BIS) monitoring on postoperative recovery and sevoflurane consumption among morbidly obese patients undergoing laparoscopic gastric banding. Middle East Journal of Anesthesiology 2008;19(4):819-30. [PMID: ] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain N, Mathur PR, Khan S, Khare A, Mathur V, Sethi S. Effect of bispectral index versus end-tidal anesthetic gas concentration-guided protocol on time to tracheal extubation for halothane-based general anesthesia. Anesthesia Essays and Researches 2016;10(3):591-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabukcu 2012 {unpublished data only}
- Kabukcu HK, Sahin N, Ozkaloglu K, Golbasi Y, Titiz TA. Bispectral index monitoring in open heart surgery. In: British Journal of Anaesthesia. 15th WFSA World Congress of Anaesthesiologists Predio Ferial de Buenos Aires Argentina edition. 2012.
Kamal 2009 {published data only}
- Kamal NM, Omar SH, Radwan KG, Youssef A. Bispectral index monitoring tailors clinical anesthetic delivery and reduces anesthetic drug consumption. Journal of Medical Sciences 2009;9:10-6. [DOI: 10.3923/jms.2009.10.16 ] [Google Scholar]
Kamali 2017a {published data only}
- Kamali A, Shokrpour M, Pazoki S. Assessment of the effect of bispectral index (BIS) monitoring on awareness during anesthesia in patients candidates for non emergency cesarean section. IIOAB Journal 2017;8(1):98-102.
Karaca 2014 {published data only}
- Karaca İ, Akçıl FE, Dilmen ÖK, Köksal GM, Tunalı Y. The effect of BIS usage on anaesthetic agent consumption, haemodynamics and recovery time in supratentorial mass surgery. Turkish Journal of Anaesthesiology and Reanimation 2014;42(3):117–22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoshrang 2016 {published data only}
- *.Khoshrang H, Nemati M, Mokhtari G, Pourreza F, Roshan ZA, Najmabadi AD, et al. Comparative efficacy of bispectral index monitoring and clinical assessment in the recovery of patients undergoing open renal surgery: a randomized, double-blind study. Annals of Anesthesiology & Critical Care 2016;1(1):e8799. [Google Scholar]
- Khoshrang H, Nemati M, Mokhtari G, Pourreza F. Comparative efficacy of bispectral index monitoring and clinical assessment in the recovery of patients undergoing open renal surgery: a randomized, double-blind study. Regional Anesthesia and Pain Medicine 2016;41(5 Suppl 1):e90. [Google Scholar]
Kim 2003 {published data only}
- Kim SC, Kim CS, Cho HS, Lee SM. The usefulness of the bispectral index during propofol and fentanyl anesthesia for coronary bypass surgery under cardiopulmonary bypass. Korean Journal of Anesthesiology 2003;44(3):370-5. [Google Scholar]
Kreuer 2003 {published data only}
- Kreuer S, Biedler A, Larsen R, Altmann S, Wilhelm W. Narcotrend monitoring allows faster emergence and a reduction of drug consumption in propofol-remifentanil anesthesia. Anesthesiology 2003;99(1):34-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kreuer 2005 {published data only}
- Kreuer S, Bruhn J, Stracke C, Aniset L, Silomon M, Larsen R, et al. Narcotrend or bispectral index monitoring during desflurane-remifentanil anesthesia: a comparison with a standard practice protocol. Anesthesia and Analgesia 2005;101(2):427-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luginbuhl 2003 {published data only}
- Luginbuhl M, Wuthrich S, Petersen-Felix S, Zbinden AM, Schnider TW. Different benefit of bispectral index (BIS) in desflurane and propofol anesthesia. Acta Anaesthesiologica Scandinavica 2003;47:165-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mashour 2012 {published data only}
- *.Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner CR, Ramachandran SK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology 2012;117(4):717-25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA, Tremper KK, Avidan MS. Protocol for the "Michigan Awareness Control Study": a prospective, randomized, controlled trial comparing electronic alerts based on bispectral index monitoring or minimum alveolar concentration for the prevention of intraoperative awareness. BMC Anesthesiology 2009;9(7):1-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masuda 2002 {published data only}
- Masuda T, Yamada H, Takada K, Sagata Y, Yamaguchi M, Tomiyama Y, et al. Bispectral index monitoring is useful to reduce total amount of propofol and to obtain immediate recovery after propofol anesthesia. Masui 2002;51(4):394-9. [MEDLINE: ] [PubMed] [Google Scholar]
Morimoto 2002 {published data only}
- Morimoto Y, Oka S, Mii M, Shinjo Y, Yamashita A, Gohara T, et al. Efficacy of bispectral index monitoring in improving anesthetic management, economics, and use of the operating theater. Masui 2002;51(8):862-8. [MEDLINE: ] [PubMed] [Google Scholar]
Mozafari 2014 {published data only}
- Mozafari H, Fakhr AA, Salehi I, Moghimbigi A. The ability of bispectral-guided management compared to routine monitoring for reflecting awareness rate in patients undergoing abdominal surgery. Iranian Red Crescent Medical Journal 2014;16(9):e13584. [PMCID: PMC4270681] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muralidhar 2008 {published data only}
- Muralidhar K, Banakal S, Murthy K, Garg R, Rani GR, Dinesh R. Bispectral index-guided anaesthesia for off-pump coronary artery bypass grafting. Annals of Cardiac Anaesthesia 2008;11(2):105-10. [MEDLINE: 18603750 ] [DOI] [PubMed] [Google Scholar]
Myles 2004 {published data only}
- Leslie K, Myles PS, Forbes A, Chan MT, Short TG, Swallow SK. Recovery from bispectral index-guided anaesthesia in a large randomized controlled trial of patients at high risk of awareness. Anaesthesia and Intensive Care 2005;33(4):443-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- *.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet 2004;363(9423):1757-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nelskyla 2001 {published data only}
- Nelskyla K, Yli Hankala A, Puro H, Korttila K. Bispectral index (BIS) of EEG improves recovery but not home-readiness after gynaecological laparoscopy. European Journal of Anaesthesiology 2000;17(Suppl 19):13-4. [Google Scholar]
- *.Nelskyla KA, Yli-Hankala AM, Puro PH, Korttila KT. Sevoflurane titration using bispectral index decreases postoperative vomiting in phase II recovery after ambulatory surgery. Anesthesia and Analgesia 2001;93(5):1165-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paventi 2001 {published data only}
- Paventi S, Santevecchi A, Metta E, Annetta MG, Perilli V, Sollazzi L. Bispectral index monitoring in sevoflurane and remifentanil anesthesia. Analysis of drugs management and immediate recovery. Minerva Anestesiologica 2001;67(6):435-9. [MEDLINE: ] [PubMed] [Google Scholar]
Payas 2013 {published data only}
- Payas A, Kaygusuz K, Duger C, Isbir AC, Kol IO, Gursoy S, et al. The effects of bispectral Index and neuromuscular blockade monitoring on the depth of anaesthesia and recovery in cardiac patients under desflurane anaesthesia. Turk Anestezi Ve Reanimasyon Dergisi 2013;41(6):211-5. [PMID: ] [DOI] [PMC free article] [PubMed]
Persec 2012 {published data only}
- Persec J, Persec Z, Kopljar M, Sojcic N, Husedzinovic I. Effect of bispectral index monitoring on extubation time and analgesic consumption in abdominal surgery: a randomised clinical trial. Swiss Medical Weekly 2012;142:w13689. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puri 2003 {published data only}
- *.Puri GD, Murthy SS. Bispectral index monitoring in patients undergoing cardiac surgery under cardiopulmonary bypass. European Journal of Anaesthesiology 2003;20(6):451-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Puri GD, Shiva MS, Batra Y. Effect of bispectral index (BIS) monitoring on haemodynamic stability and recovery from anesthesia in patients, undergoing cardiac surgery under cardiopulmonary bypass. British Journal of Anaesthesia 1999;82(Suppl 1):44-5. [Google Scholar]
Rahul 2015 {published data only}
- Rahul R, Sowmya MJ, Rangalakshmi S, Kumar BN, Karthik GS. Monitoring depth of anaesthesia using PRST score and bispectral index. Journal of Evolution of Medical and Dental Sciences 2015;4(25):4282-92. [Google Scholar]
Raksakietisak 2016 {published data only}
- Raksakietisak M, Plailaharn N, Kratayjan W, Songarj P. Awakening in spine surgery patients having neurophysiologic monitoring: a comparison study between clinical signs and bispectral index (BIS) guided target controlled infusion (TCI) of propofol. Anesthesia and Analgesia 2016;123(3 (Suppl 2)):576-7. [Google Scholar]
Recart 2003 {published data only}
- Recart A, Gasanova I, White PF, Thomas T, Ogunnaike B, Hamza M, et al. The effect of cerebral monitoring on recovery after general anesthesia: a comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesthesia and Analgesia 2003;97(6):1667-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savli 2005 {published data only}
- Savli S, Adalig B, Ozalp G, Tuncel G, Kadiogullari N. Effects of bispectral index (BIS) monitorisation on sevoflurane consumption and recovery. Anestezi Dergisi 2005;13(2):96-100. [Google Scholar]
Shafiq 2012 {published data only}
- Shafiq F, Naqvi H, Ahmed A. Effects of bispectral index monitoring during anaesthesia on isoflurane consumption, hemodynamic variables and recovery profiles in elderly Pakistani patients. British Journal of Anaesthesia 2012;2:ii154-5. [Google Scholar]
- *.Shafiq F, Naqvi H, Ahmed A. Effects of bispectral index monitoring on isoflurane consumption and recovery profiles for anesthesia in an elderly asian population. Journal of Anaesthesiology Clinical Pharmacology 2012;28(3):348-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siampalioti 2015 {published data only}
- Siampalioti A, Karavias D, Zotou A, Kalfarentzos F, Filos K. Anesthesia management for the super obese: is sevoflurane superior to propofol as a sole anesthetic agent? A double-blind randomized controlled trial. European Review for Medical and Pharmacological Sciences 2015;19(13):2493-500. [PubMed] [Google Scholar]
Song 1997 {published data only}
- Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology 1997;87:842-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sudhakaran 2018 {published data only}
- Sudhakaran R, Makkar JK, Jain D, Wig J, Chabra R. Comparison of bispectral index and end-tidal anaesthetic concentration monitoring on recovery profile of desflurane in patients undergoing lumbar spine surgery. Indian Journal of Anaesthesia 2018;62(7):516-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tufano 2000 {published data only}
- Tufano R, Palomba R, Lambiase G, Giurleo LG. The utility of bispectral index monitoring in general anesthesia. Minerva Anestesiologica 2000;66(6):389-93. [MEDLINE: ] [PubMed] [Google Scholar]
White 2004 {published data only}
- White PF, Ma H, Tang J, Wender RH, Sloninsky A, Kariger R. Does the use of electroencephalographic bispectral index or auditory evoked potential index monitoring facilitate recovery after desflurane anesthesia in the ambulatory setting? Anesthesiology 2004;100(4):811-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong J, Song D, Blanshard H, Grady D, Chung F. Titration of isoflurane using BIS index improves early recovery of elderly patients undergoing orthopedic surgeries. Canadian Journal of Anaesthesia 2002;49(1):13-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang C, Xu L, Ma YQ, Sun YX, Li YH, Zhang L, et al. Bispectral index monitoring prevent awareness during total intravenous anesthesia: a prospective, randomized, double-blinded, multi-center controlled trial. Chinese Medical Journal 2011;124(22):3664-9. [MEDLINE: : 22340221 ] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Y, Yang S, Liu J, Wang Y, Gao B, Cui W, et al. Narcotrend and bispectral index for monitoring intraoperative anesthetic depth in patients with severe burns. International Journal of Clinical and Experimental Medicine 2016;9(11):22020-6. [Google Scholar]
Zohar 2006 {published data only}
- Zohar E, Luban I, White PF, Ramati E, Shabat S, Fredman B. Bispectral index monitoring does not improve early recovery of geriatric outpatients undergoing brief surgical procedures. Canadian Journal of Anesthesia 2006;53(1):20-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aceto 2015 {published data only}
- Aceto P, Perilli V, Lai C, Sacco T, Modesti C, Luca E, et al. Minimum alveolar concentration threshold of sevoflurane for postoperative dream recall. Minerva Anestesiologica 2015;81(11):1201-9. [PMID: ] [PubMed] [Google Scholar]
Aimé 2006 {published data only}
- Aimé I, Verroust N, Masson-Lefoll C, Taylor G, Laloë P, Liu N, et al. Does monitoring bispectral index or spectral entropy reduce sevoflurane use? Anesthesia and Analgesia 2006;103(6):1469-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chan 2013 {published data only}
- Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. Journal of Neurosurgical Anesthesiology 2013;25(1):33-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiu 2007 {published data only}
- Chiu CL, Ong G, Majid AA. Impact of bispectral index monitoring on propofol administration in patients undergoing cardiopulmonary bypass. Anaesthesia and Intensive Care 2007;35(3):342-7. [PMID: 17591126 ] [DOI] [PubMed] [Google Scholar]
Hachero 2001 {published data only}
- Hachero A, Alamo F, Caba F, Echevarria M, Merino S, Gomez P, et al. Influence of bispectral index monitoring on fentanyl requirements during total intravenous anesthesia for major gynecological surgery. Revista Espanola de Anestesiologia Reanimacion 2001;48(8):364-9. [PMID: ] [PubMed] [Google Scholar]
Kamali 2017b {published data only}
- Kamali A, Rostami A, Modir H, Shokrpour M. Studying the effects of BIS monitoring (depth of anesthesia) in early extubation of patients candidated for non-emergency CABG. Biomedical and Pharmacology Journal 2017;10(1):199-206. [Google Scholar]
Karwacki 2014 {published data only}
- Karwacki Z, Niewiadomski S, Rzaska M, Witkowska M. The effect of bispectral index monitoring on anaesthetic requirements in target-controlled infusion for lumbar microdiscectomy. Anaesthesiology Intensive Therapy 2014;46(4):284-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaval 2015 {published data only}
- Kaval E, Zeyneloglu P, Camkiran A, Sezgin A, Pirat A, Arslan G. Bispectral index-guided anesthesia on time to tracheal extubation after onpump cardiac surgery. Critical Care 2015;1:S172-3. [Google Scholar]
Kerssens 2009 {published data only}
- Kerssens C, Gaither JR, Sebel PS. Preserved memory function during bispectral index-guided anesthesia with sevoflurane for major orthopedic surgery. Anesthesiology 2009;111(3):518-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nitzschke 2014 {published data only}
- Nitzschke R, Wilgusch J, Kersten JF, Trepte CJ, Haas SA, Reuter DA, et al. Bispectral index guided titration of sevoflurane in on-pump cardiac surgery reduces plasma sevoflurane concentration and vasopressor requirements: a prospective, controlled, sequential two-arm clinical study. European Journal of Anaesthesiology 2014;31(9):482-90. [DOI] [PubMed] [Google Scholar]
Panagopoulou 2000 {published data only}
- Panagopoulou O, Georgakis P, Tsamandouraki K, Papanastasiou R, Katsouli I. Titration of sevoflurane using BIS for day-case surgery. European Journal of Anaesthesiology 2000;17(14):no pagination. [Google Scholar]
Quesada 2016 {published data only}
- Quesada N, Júdez D, Martínez Ubieto J, Pascual A, Chacón E, De Pablo F, et al. Bispectral index monitoring reduces the dosage of propofol and adverse events in sedation for endobronchial ultrasound. Respiration 2016;92(3):166-75. [DOI] [PubMed] [Google Scholar]
Radtke 2013 {published data only}
- Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. British Journal of Anaesthesia 2013;110(Suppl 1):i98-i105. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rüsch 2018 {published data only}
- Rüsch D, Arndt C, Eberhart L, Tappert S, Nageldick D, Wulf H. Bispectral index to guide induction of anesthesia: a randomized controlled study. BMC Anesthesiology 2018;18(66):1-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samarkandi 2004 {published data only}
- Samarkandi AH, Abdel-Meguid ME, Abdullah KM, Riad W. Bispectral index monitoring and titration of anaesthetics during off-pump coronary artery bypass surgery. Egyptian Journal of Anaesthesia 2004;20(4):357-61. [Google Scholar]
Shahrbazi 2008 {published data only}
- Shahrbazi S, Jouibar R, Allahyari E, Chohedri AH. Impact of bispectral index monitoring versus clinical judgment as a guide for conduction of anesthesia on serum cortisol level in coronary artery bypass graft surgery. Iranian Journal of Medical Sciences 2008;33(3):155-9. [Google Scholar]
Struys 2001 {published data only}
- Struys MM, De Smet T, Versichelen LF, Van De Velde S, Van den Broecke R, Mortier EP. Comparison of closed-loop controlled administration of propofol using Bispectral Index as the controlled variable versus "standard practice" controlled administration. Anesthesiology 2001;1:6-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vretzakis 2005 {published data only}
- Vretzakis G, Argiriadou H, Mikroulis D, Bitzikas G, Ferdi E, Papziogas B, et al. Influence of bispectral index monitoring on decision making during cardiac anesthesia. Journal of Clinical Anesthesia 2005;17(7):509-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2018 {published data only}
- Zhou Y, Li Y, Wang K. Bispectral index monitoring during anesthesia promotes early postoperative recovery of cognitive function and reduces acute delirium in elderly patients with colon carcinoma: A prospective controlled study using the attention network test. Medical Science Monitor 2018;24:7785-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aksun 2007 {published data only}
- Aksun M, Aydin O, Aran G, Atasoy N, Savaci S. The effects of bispectral index (BIS) monitorization on recovery from sevoflurane and desflurane anesthesia. Anestezi-Derg Anestezi-Dergisi 2007;15(1):14. [EMBASE: 2007166072] [Google Scholar]
Croci 2014 {published and unpublished data}
- Croci M, Hudecova S, Fracassi S, Greco S. Control of the postoperative nausea and vomiting using a bispectral index-guide anesthesia and its economic impact: 1AP1-8. European Journal of Anaesthesiology 2014;31(Suppl 52):5. [Google Scholar]
CTRI/2018/03/012457 {published data only}
- CTRI/2018/03/012457. A study to compare the time to recover from general anaesthesia in patients monitored by measuring the electrical activity of brain with patients monitored by measuring the concentration of exhaled anaesthetic gases [Comparison of recovery times from inhalational general anaesthesia using BIS monitoring versus end tidal agent concentration monitoring]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012457 (first received 12 March 2018).
Golmohammadi 2014 {published data only}
- Golmohammadi M, Abasgholizadeh M. Bispectral index monitoring in isoflurane anesthesia in laparoscopic cholecystectomy of morbid obese patients. Tehran University Medical Journal 2014;72(7):471-9. [Google Scholar]
Jeong 2002 {published data only}
- Jeong SW, Park CH, Jeong CY. The effect of bispectral index monitoring on anesthetic use and recovery in long duration sevoflurane anesthesia. Korean Journal of Anesthesiology 2002;43(4):401-6. [Google Scholar]
Qu 2011 {published data only}
- Qu X-X, Ma H-C, Yuan Y, Feng C-S. Effect of bispectral index on intraoperative awareness under total intravenous anesthesia. Journal of Jilin University Medicine Edition 2011;37:308-11. [Google Scholar]
References to ongoing studies
Martins 2013 {published and unpublished data}
- Martins A, Pereira A, Seabra H, Gomes D, Fonte Boa A, Lima F. Influence of processed EEG monitoring in the anesthetic management and its cost in off-pump coronary surgery: a research protocol. Revista Portuguesa De Cirurgia Cardio-Toracica E Vascular 2013;20(2):67-71. [PMID: 24730013 ] [PubMed] [Google Scholar]
NCT03571945 {published data only}
- NCT03571945. Incidence of intraoperative awareness in Indian patient population [Impact of bi-spectral index guided inhalation anaesthesia on the incidence of intraoperative awareness in Indian patient population: a prospective, randomised, multi-centric study]. clinicaltrials.gov/ct2/show/record/NCT03571945 (first received 28 June 2018).
Additional references
Badrinath 1999
- Badrinath S, Avramov MN, Papaioannou BS, Ivankovich AD. The impact of EEG-bispectral index monitoring on drug usage and recovery during ambulatory anesthesia. Anesthesia and Analgesia 1999;88 Suppl:51. [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods 2010;1(2):97-111. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Statistics in Medicine 2007;26(1):53-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brice 1970
- Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. British Journal of Anaesthesia 1970;42(6):535-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheng 2016
- Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open 2016;6(8):e010983. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2018
- Chiang MH, Wu SC, Hsu SW, Chin JC. Bispectral Index and non-Bispectral Index anesthetic protocols on postoperative recovery outcomes. Minerva Anestesiologica 2018;84(2):216-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, Version accessed 2 January 2019.Available at www.covidence.org.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote [Computer program]
- Endnote X5. Thomson Reuters. Philadelphia (PA): Thomson Reuters, 2011.
Gao 2018
- Gao WW, He YH, Liu L, Yuan Q, Wang YF, Zhao B. BIS monitoring on intraoperative awareness: a meta-analysis. Current Medical Science 2018;38(2):349-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghoneim 2009
- Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesthesia and Analgesia 2009;108(2):527-35. [MEDLINE: 19151283 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johansen 1998
- Johansen JW. Provider survey of bispectral index utility. Anesthesia and Analgesia 1998;86 Suppl:212. [Google Scholar]
Kissin 1997
- Kissin I. A concept for assessing interactions of general anesthetics. Anesthesia and Analgesia 1997;85(1):204-10. [PMID: 9212148 ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2004
- Liu SS. Effects of bispectral index monitoring on ambulatory anesthesia: a meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology 2004;101(2):311-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mahla 1997
- Mahla ME. Electroencephalogram in the OR. Seminars in Anesthesia 1997;16(1):3-13. [Google Scholar]
Messina 2016
- Messina AG, Wang M, Ward MJ, Wilker CC, Smith BB, Vezina DP, et al. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD007272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NAP5 2014
- Pandit JJ, Andrade J, Bogod DG, Hitchman JM, Jonker WR, Lucas N, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. British Journal of Anaesthesia 2014;113(4):549-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Connor 2001
- O'Connor MF, Daves SM, Tung A, Cook RI, Thisted R, Apfelbaum J. BIS monitoring to prevent awareness during general anesthesia. Anesthesiology 2001;94(3):520-2. [PMID: 11374615 ] [DOI] [PubMed] [Google Scholar]
Oliveira 2017
- Oliveira CR, Bernardo WM, Nunes VM. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Brazilian Journal of Anesthesiology 2017;67(1):72-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rampil 1998
- Rampil IJ. A Primer for EEG signal processing in anesthesia. Anesthesiology 1998;89(4):815-7. [PMID: 9778016 ] [DOI] [PubMed] [Google Scholar]
Review Manager 14 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roizen 1994
- Roizen MF, Toledano A. Technology assessment and the "learning contamination" bias. Anesthesia and Analgesia 1994;79(3):410-2. [PMID: 8067542 ] [DOI] [PubMed] [Google Scholar]
Schneider 2010
- Schneiderder G. Monitoring anesthetic depth. In: Mashour GA, editors(s). Consciousness, Awareness, and Anesthesia. 1st edition. New York: Cambridge University Press, 2010:122-30. [Google Scholar]
Sebel 2001
- Sebel PS. Can we monitor depth of anesthesia. In: International Anesthesia Research Society Review Course Lectures. 2001:95-7.
Sebel 2004
- Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, et al. The incidence of awareness during anesthesia: a multicenter United States study. Anesthesia and Analgesia 2004;99(3):833-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Punjasawadwong 2002
- Punjasawadwong Y, Phongchiewboon A, Bunchungmonkol N, Braaksma DN. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Punjasawadwong 2007
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003843.pub2] [DOI] [PubMed] [Google Scholar]
Punjasawadwong 2014
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003843.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


