Abstract
Cytomegalovirus (CMV) is a ubiquitous β-herpesvirus that establishes life-long latent infection in a high percentage of the population worldwide. CMV induces the strongest and most durable CD8+ T cell response known in human clinical medicine. Due to its unique properties, the virus represents a promising candidate vaccine vector for the induction of persistent cellular immunity. To take advantage of this, we constructed a recombinant murine CMV (MCMV) expressing an MHC-I restricted epitope from influenza A virus (IAV) H1N1 within the immediate early 2 (ie2) gene. Only mice that were immunized intranasally (i.n.) were capable of controlling IAV infection, despite the greater potency of the intraperitoneally (i.p.) vaccination in inducing a systemic IAV-specific CD8+ T cell response. The protective capacity of the i.n. immunization was associated with its ability to induce IAV-specific tissue-resident memory CD8+ T (CD8TRM) cells in the lungs. Our data demonstrate that the protective effect exerted by the i.n. immunization was critically mediated by antigen-specific CD8+ T cells. CD8TRM cells promoted the induction of IFNγ and chemokines that facilitate the recruitment of antigen-specific CD8+ T cells to the lungs. Overall, our results showed that locally applied MCMV vectors could induce mucosal immunity at sites of entry, providing superior immune protection against respiratory infections.
Author summary
Vaccines against influenza typically induce immune responses based on antibodies, small molecules that recognize the virus particles outside of cells and neutralize them before they infect a cell. However, influenza rapidly evolves, escaping immune recognition, and the fastest evolution is seen in the part of the virus that is recognized by antibodies. Therefore, every year we are confronted with new flu strains that are not recognized by our antibodies against the strains from previous years. The other branch of the immune system is made of killer T cells, which recognize infected cells and target them for killing. Influenza does not rapidly evolve to escape T cell killing; thus, vaccines inducing T-cell responses to influenza might provide long-term protection. We introduced an antigen from influenza into the murine cytomegalovirus (MCMV) and used it as a vaccine vector inducing killer T-cell responses of unparalleled strength. Our vector controls influenza replication and provides relief to infected mice, but only if we administered it through the nose, to activate killer T cells that will persist in the lungs close to the airways. Therefore, our data show that the subset of lung-resident killer T cells is sufficient to protect against influenza.
Introduction
Respiratory infections caused by influenza viruses usually are associated with mild-to-moderate disease symptoms but are linked with high morbidity and mortality in susceptible populations like the elderly, young children, patients with co-morbidities and immunocompromised patients. Influenza virus causes seasonal epidemics, with typically 3 to 5 million cases of severe illness worldwide, according to WHO reports [1], and influenza type A viruses (IAV) cause the more severe disease form. Vaccines against influenza are based on the induction of adaptive immunity that targets the projected yearly epidemics. While most vaccines are based on inactivated IAV formulations inducing anti-IAV IgG responses, live attenuated influenza vaccines (LAIV) are also used as formulations for i.n. administration. This is based on the assumption that the induction of local immunity may provide superior immune protection [2, 3]. However, it remains unclear whether this protection depends on local IgA responses, on cytotoxic T cell responses, or on their combined antiviral activity. Of note, functional T cell responses were shown to substantially contribute to antiviral IAV immunity [4–6]. In particular, cytotoxic influenza-specific CD8+ T lymphocytes (CTLs) promote viral clearance indirectly by secretion of pro-inflammatory cytokines such as IFNγ [7] and directly by perforin/Fas-mediated killing of infected epithelial cells in the bronchoalveolar space [8]. However, it remained unclear if T cell responses alone may control IAV, or if Ig responses were the crucial contributor to LAIV-mediated immune protection. We considered that this question could be addressed by developing a vaccine formulation that optimizes T cell responses against IAV while excluding the humoral ones.
CMV infection induces sustained functional T cell responses that are stronger in the long-term than the immune response to any other infectious pathogen [9]. Experiments in the mouse model have shown that defined CMV epitope-specific CD8+ T cells accumulate in tissues and blood and are maintained at stable high levels during mouse CMV (MCMV) latency [10]. This phenomenon was termed ‘‘Memory Inflation” [11]. While some MCMV derived peptides, as the ones derived from the IE3 (416RALEYKNL423) and M139 proteins (419TVYGFCLL426) induce inflationary responses, other peptides, such as the M45-derived (985HGIRNASFI993), induce conventional CD8+ T cell response [12]. Antigen-experienced CD8+ T cells are subdivided into CD62L- effector memory (CD8TEM) and CD62L+ central memory CD8+ T cells (CD8TCM). The antigen-specific CD8+ T cells during latent infection bear predominantly a CD8TEM phenotype and localize in secondary lymphoid or non-lymphoid organs [13]. They may provide immune protection against diverse viral targets [14–18], but also against bacterial [19] or tumor antigens [20, 21]. The exceptionally long-lasting cellular immunity to CMV antigens has raised the interest in CMV as a potential new vaccine vector [14]. Many studies have demonstrated of optimal design of such CMV-based vaccines display efficient protection against virus infection such as rhesus macaque CMV (RhCMV) based Ebola [22] and SIV [23] vaccines and MCMV based Ebola virus vaccines [15]. Erkes, D.A. and Qiu, Z. et al. demonstrated that CMV based vaccine vectors provide protection in suppressing tumors [24, 25].
Both CD8TEM and CD8TCM subsets recirculate between the blood, the lymphoid organs, and the peripheral tissues. A special subset of memory CD8+ T cells (CD8TRM) resides in non-lymphoid tissues such as lungs, the female reproductive tract (FRT), the skin, the brain or the small intestine [26–29]. These cells lose the capacity of recirculating, maintain themselves at the site of infection, and their phenotype and transcriptional profile differ from classical memory T cells [30]. The well-characterized CD8TRM cells express C-type lectin CD69 [26] and the integrin αEβ7, also known as CD103 [30]. They provide rapid and superior protection against pathogens at the site of infection [26, 30, 31]. A recent publication argued that a vaccine formulation adjuvanted by IL-1β enhances the immune control of IAV by improving mucosal T cell responses [32], but IL-1β improved both humoral and cellular responses in their study. Hence, the contribution of CD8TRM to IAV immune control remains unclear.
CD8TRM are found in the salivary glands of MCMV-infected animals [33], but not in their lungs [34]. We showed that i.n. infection with MCMV induces inflationary CD8+ T cell responses, but also that memory inflation is more pronounced in relative and absolute terms upon i.p. infection [35]. The i.n. administration of an MCMV vaccine vector induced CD8TRM responses in the lungs [29] and only i.n. immunization restricted the replication of respiratory syncytial virus (RSV) upon challenge [29], indicating that CD8TRM elicited by i.n. administration of MCMV vectors might provide immune protection against respiratory virus infections, yet this evidence remains correlative. Upon antigen encounter, CD8TRM cells quickly reactivate at the mucosal site and secrete cytokines and chemokines or support the release of inflammatory mediators by other immune cells [28, 36, 37]. Lung airway CD8TRM cells provide protection against respiratory virus infection through IFNγ and help to recruit circulating memory CD8+ T cells to the site of infection in an IFNγ-dependent way [36]. Therefore, to understand if CD8TRM cell may provide immune control of respiratory infections may help to refine strategies for tissue-targeted vaccine design.
In this study, we constructed an MCMV vector expressing the MHC-I restricted peptide 533IYSTVASSL541 (IVL533-541) [38] from IAV H1N1 hemagglutinin (HA)-MCMVIVL under the transcriptional control of the ie2 promotor. We investigated the potential of this recombinant virus to induce HA-specific CD8+ T cells that confer protection against a lethal IAV challenge. We showed that i.n., but not i.p. immunization with MCMVIVL resulted in robust protection against an IAV challenge. Protection following i.n. MCMVIVL immunization was associated with high levels of antigen-specific CD8TRM cells in the lungs, and targeted depletion of lung-CD8TRM cells revealed that the control of the IAV in the lungs depended on these cells.
Results
Generation of recombinant MCMV and its replication in vitro and in vivo
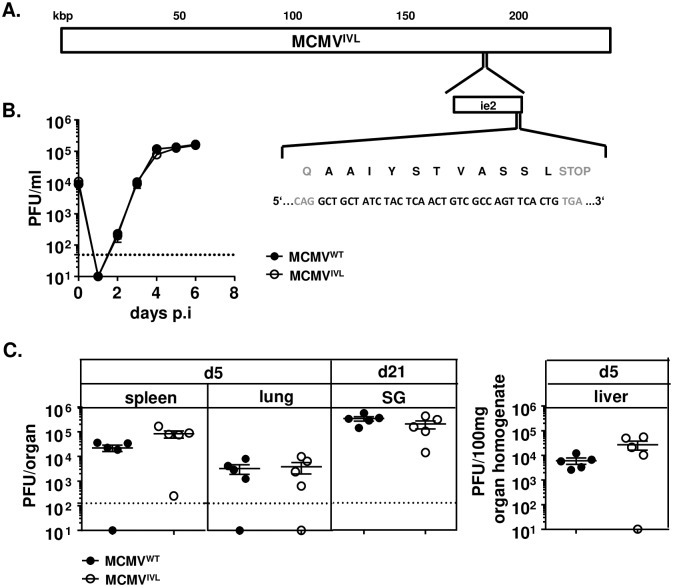
We showed recently that MCMV vector expressing a single optimally positioned MHC-I restricted antigenic epitope provides a more efficient immune protection than vectors expressing the full-length protein [21]. Therefore, we constructed an MCMV influenza vaccine vector by inserting the coding sequence for the H-2Kd MHC-I restricted peptide IYSTVASSL from the hemagglutinin (HA) of the H1N1 (PR8) IAV [38] into the C-terminus of the MCMV ie2 gene. The resulting recombinant virus was called MCMVIVL (Fig 1A). To test if the recombinant virus retained its capacity to replicate in host cells, virus replication was assessed by multi-step growth kinetics assays in NIH-3T3 cells in vitro and by ex vivo quantification of virus titers in livers, lungs and spleens 5 days post-infection (dpi) and in salivary glands 21 dpi. MCMVIVL showed identical replication properties as the MCMVWT, both in vitro (Fig 1B) and in vivo (Fig 1C), indicating that the insertion of the IVL533-541 epitope does not impair virus replication and spread.
Fig 1. Generation of the recombinant MCMV expressing the 533IYSTVASSL541 epitope.
The sequence of IAV epitope 533AAIYSTVASSL541 (IVL533-541) was introduced at the C-terminus of the MCMV ie2 gene, and the growth of the recombinant virus MCMVIVL was tested in vitro and in vivo. (A) The location of the ie2 gene within the MCMV genome at ~186-187kb position is shown; the insertion site of the peptide IVL533-541 and the corresponding nucleotide sequences are magnified. (B) MCMVIVL and wild-type MCMV (MCMVWT) growth at a multiplicity of infection of 0.1 was compared in NIH-3T3 cells. Virus titers in supernatants expressed as plaque-forming units (PFU) were established at indicated time points. Group means +/- standard error of the mean (SEM) are shown. The dashed line indicates the limit of detection. (C) BALB/c mice were i.p. infected with 2 x 105 PFU of MCMVIVL or MCMVWT virus. Spleen, lung and liver homogenates were assayed for virus titers 5 days post-infection (dpi). Salivary-gland (SG) homogenates were assayed 21 dpi. Each symbol represents one mouse. Group means +/- standard error of the mean (SEM) are shown. The dashed line indicates the limit of detection.
Intranasal immunization with MCMVIVL induces a lower magnitude of CD8+ T cell response compared with intraperitoneal immunization
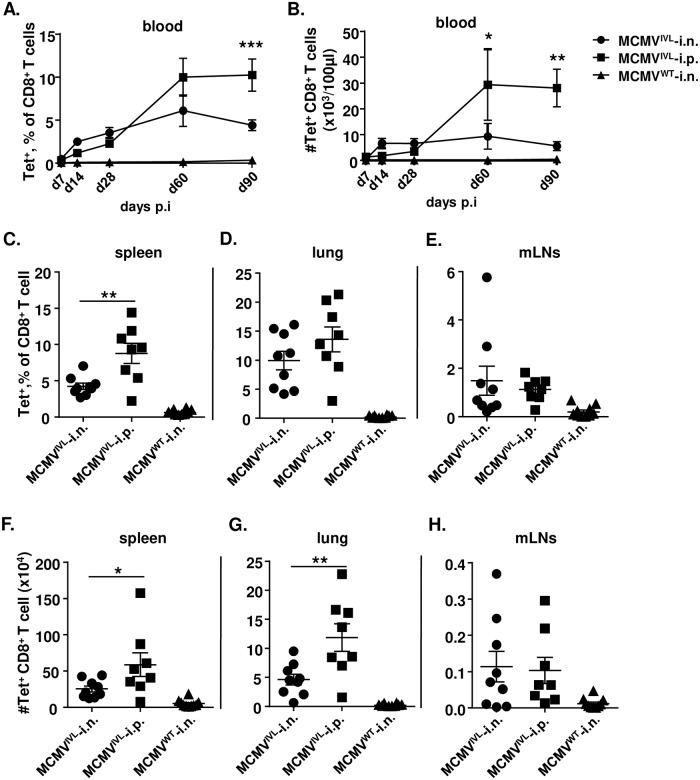
We have shown that mucosal infection with MCMV by the i.n. route induces memory inflation, although to a lower extent than upon the i.p. infection route [35]. To define if this pattern would hold true for the artificially incorporated influenza epitope as well, we compared the magnitude of the CD8+ T cell responses to MCMVIVL and MCMVWT induced via the i.n. and i.p. route, respectively. S1 Fig shows the gating strategy of flow cytometry analysis. The kinetics of antigen-specific CD8+ T cell responses in peripheral blood was determined by IVL-tetramer staining. While we did not observe striking difference at early times post immunization, i.p. immunization induced an overall higher magnitude of inflationary CD8+ T cell response during latency (Fig 2A and 2B). This pattern was observed both in relative terms (Fig 2A) and in absolute cell counts (Fig 2B). We next analyzed the IVL-specific CD8+ T cell responses in spleens, lungs and mediastinal lymph nodes (mLNs) at times of latency (>3months post infection (p.i)). Similarly, i.p. immunization induced higher levels of IVL-specific CD8+ T cells than the i.n. immunization in the spleen and lungs, both in relative (Fig 2C and 2D) and in absolute terms (Fig 2F and 2G). There were no significant differences in the mLNs (Fig 2E and 2H). In sum, mucosal (i.n.) immunization with MCMVIVL induces a systemic inflationary IVL-specific CD8+ T cell response, whereas the overall magnitude of the IAV-specific CD8+ T cell response is less pronounced compared to that induced in i.p. immunized mice.
Fig 2. Intranasal immunization of MCMVIVL induces lower magnitude of CD8+ T cell response than intraperitoneal immunization.
BALB/c mice were infected with 2 x 105 PFU MCMVIVL via the i.n. or i.p. route or with MCMVWT via the i.n. route. IVL-specific CD8+ T cell responses were analyzed by IVL-tetramers staining and flow cytometry. (A, B) Blood leukocytes were analyzed at indicated time points upon infection to define the (A) percentage of IVL-specific (Tet+) cells among CD8+ T cells and (B) cell counts of IVL-specific (Tet+) cells in peripheral blood. Two independent experiments were performed and results were pooled and shown as group means +/- SEM (n = 8–10). (E-H) IVL-specific CD8+ T cells in spleen, lungs and mLNs were quantified by IVL-tetramer staining 120 dpi as relative cell percentages among CD8+ T cells (C, D, E) or absolute cell counts (F, G, H) per organ. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 7–10. Group means +/- SEM are shown. Significance was assessed by Two-way ANOVA test (panels A, B) or one-way ANOVA test (Panels C-H). *P <0.05, **P<0.01, ***P<0.001.
Intranasal immunization with MCMVIVL facilitates the elimination of IAV is dependent on CD8+ T cell
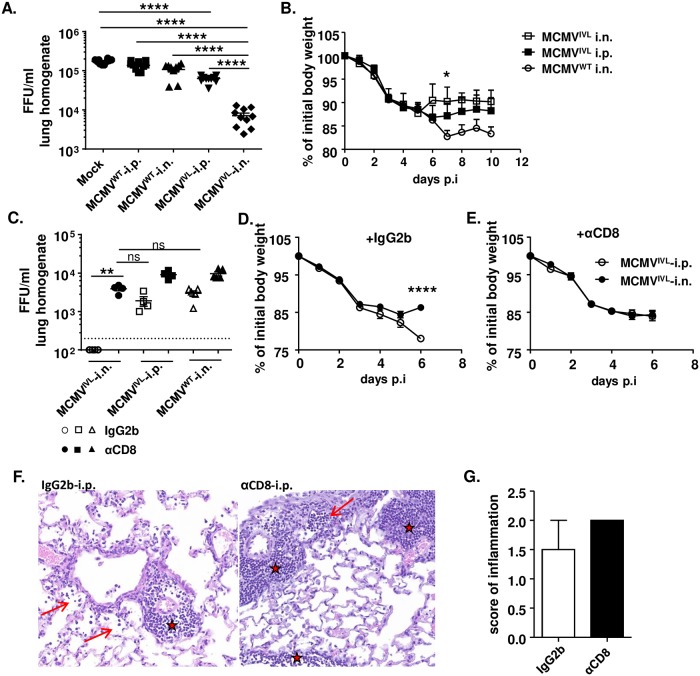
To test whether immunization with MCMVIVL protects against IAV infection, latently immunized BALB/c mice were i.n. challenged with IAV. IAV titers in the lungs were quantified 5 dpi. Viral loads in mice that were immunized with MCMVWT via either the i.p. or the i.n. route were comparable to those detected in mock-immunized mice (Fig 3A). In contrast, mice immunized with MCMVIVL via the i.n. route showed significantly lower IAV titers than in any other group. Interestingly, i.p. MCMVIVL immunization also resulted in reduced IAV loads, but to a lower extent than the i.n. immunization (Fig 3A). Similarly, animals immunized with MCMVWT suffered the most severe weight loss whilst i.n. immunization of MCMVIVL led to the least pronounced body weight loss. I.p. immunization with MCMVIVL displayed an intermediate level (Fig 3B). Numerous studies have reported that CD8+ T cells play an important role in protecting against influenza infection [39, 40] and it was reasonable to assume that our vector provided immune protection by eliciting CD8+ T cell responses. To formally prove that efficient immune control of IAV observed in the MCMVIVL (i.n.) immunized group depends on CD8+ T cells, we depleted these cells by systemic treatment of mice with a depleting anti-CD8α antibody (depletion efficiency is shown in S2A Fig) one day prior to IAV challenge and quantified viral titers in lungs 6 dpi. While the virus titer was below the detection limit in mice that were i.n. immunized with MCMVIVL and received isotype control antibodies, CD8+ T cell depletion indeed resulted in a significant increase of the IAV titer to levels comparable to the groups that were i.p. immunized with MCMVIVL and to control mice immunized with MCMVWT by i.n. route (Fig 3C). CD8+ T cell depletion also slightly increased virus titers in both control groups—MCMVIVL (i.p.) and MCMVWT (i.n.), but not as pronounced as in the MCMVIVL i.n. immunized group (Fig 3C). Similar to Fig 3B, animals of all experimental groups showed a comparable body weight loss post-challenge, whereas i.n. MCMVIVL immunized mice showed a faster recovery than the i.p. immunized mice (Fig 3D). Of note, this difference disappeared in the groups lacking CD8+ T cells (Fig 3E). Together, these data demonstrate that IAV-specific CD8+ T cells induced by the mucosal (i.n.) administration of MCMVIVL confer protection against IAV in the lungs of vaccinated mice.
Fig 3. Intranasal immunization with MCMVIVL facilitates the elimination of IAV.
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL or MCMVWT via the i.n. or i.p. route. Mock controls received 100 μl PBS by i.p. route. Once latency was established (> 3 months p.i), mice were challenged with IAV (PR8). (A) IAV titers in the lungs on day 5 post-challenge (i.n., 220 FFU) by focus-forming assay (FFA). Two independent experiments were performed and pooled data are shown, n = 10. (B) Body weight loss upon IAV challenge (i.n., 1100 FFU), n = 3–7. (C) CD8+ T cells were depleted systemically by i.p. injection of 200 μg anti-CD8α antibody (αCD8) or isotype control antibody (IgG2b) one day before PR8 challenge (i.n., 1100 FFU). Virus loads in the lung homogenates were quantified on day 6 post-challenge by FFA. Two independent experiments were performed and one cohort was shown. Each symbol represents one mouse, n = 5. Group means +/- SEM are shown. (D, E) Body weight loss upon IAV challenge (i.n., 1100 FFU) without (D) or with (E) systemic CD8+ T cell depletion. Two independent experiments were performed and pooled data are shown, n = 10. Group means +/-SEM are shown. (F) H&E staining of the lung tissue on day 5 post-challenge (i.n., 1100 FFU) with or without systemic depletion of CD8+ T cell. (G) The score of inflammation in the lungs upon IAV challenge (i.n., 1100 FFU). Bars indicate means, error bars are SEM. Significance was assessed by One-way ANOVA test or Two-way ANOVA test. *P<0.05, **P<0.01, ****P<0.0001, ns: no significant difference.
We further compared the lung pathology upon IAV challenge by histology. A moderate perivascular inflammation was observed in the lungs of most mice (stars) and to a lesser degree surrounding the bronchioles (arrows) (Fig 3F). The CD8+ T cell depleted group showed more severe pathology than isotype-treated controls, but the difference was not very pronounced (Fig 3G). Taken together, these data imply that intranasal immunization with the MCMVIVL vector can limit IAV growth in the lungs by inducing IAV-specific CD8+ T cell responses, whereas the clinical outcome is only moderately improved.
Intranasal immunization with MCMVIVL induces antigen-specific tissue-resident memory CD8+ T (CD8TRM) cells in the lungs
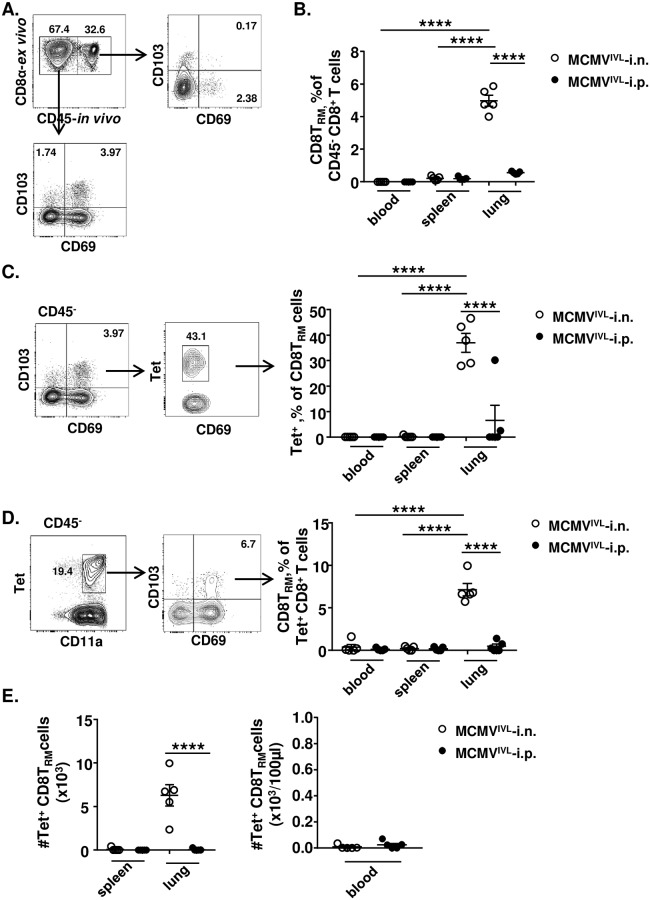
We assumed that intranasal MCMVIVL immunization may control the IAV replication by inducing CD8TRM cell responses in the lungs. In order to test this hypothesis, we identified the CD8TRM cell compartment by staining cells with the CD69 [26] and the CD103 [30] marker at times of MCMV latency (> 3 months p.i.). It is known that resident memory T cells reside in the mucosal tissue layer and are non-migratory [31]. In this study, intravenous (i.v.) injection of a fluorescent anti-CD45 antibody prior sampling allows for the discrimination of circulating leukocytes (fluorescence-positive) from emigrated or tissue-resident leukocytes (fluorescence-negative). The CD69+CD103+ cells from lungs were virtually absent from the CD45-labelled fraction (Fig 4A). Barely CD8TRM could be detected in the spleen and blood regardless of the route of immunization (Fig 4B). CD8TRM (CD69+CD103+) cells were solely induced in the lungs and the frequency was significantly higher when i.n. immunized with MCMVIVL than via the i.p. route (Fig 4B). Approximately forty percent of these lung CD8TRM cells were IVL-tetramer+ which is significantly higher than the i.p. immunization group (Fig 4C). Similar results were observed when gated reversibly (Fig 4D) and when analyze these tetramer+ CD8TRM cell numbers (Fig 4E). CD8TRM cells were also induced in the group that was i.n. immunized with MCMVWT, but not IVL-specific (S3A Fig). In addition, there was an overall higher percentage of the CD69+CD103-CD8+ T cells in the lungs of i.n. immunized mice (S3B Fig), although the absolute cell numbers did not show a significant difference (S3C Fig). Since CD69 and CD103 are imperfect markers of tissue residence, we validated if the CD69+CD103+ and CD69+CD103- populations in lungs are truly CD8TRM by staining them for Eomesodermin (Eomes) expression, because CD8TRM cells show low expression of Eomes. Low levels of Eomes were observed in CD69+CD103+ CD8 T cells, whereas CD69+CD103-CD8+ T cells showed high expression of Eomes which is consistent with primed CD8+ T cells (S3D Fig). Hence the data suggested the CD69+CD103- cell subset show distinct transcription profile from the CD8TRM cell. According to the expression of CD62L and KLRG1, IVL-specific CD8+ T cells are classified into three subsets: effector (TEFF: KLRG1+CD62L-); effector memory (TEM: KLRG1-CD62L-) and central memory (TCM: KLRG1-CD62L+) cells. The fractions of each subset in the mucosal (CD45-) and circulation (CD45+) in the blood, spleen and lung were analyzed (S4A & S4B Fig). While the fraction of CD8TCM cells remained relatively low in all compartments irrespectively of the route of administration, i.p. infection resulted in a response polarized towards TEFF cells, whereas i.n. immunization induced a larger fraction of TEM cells in all analyzed organs both in circulating and mucosal CD8+ T cell subsets (S4A & S4B Fig). Majority of the IVL-specific CD8TRM cells show an EM phenotype (S4C Fig).
Fig 4. Intranasal immunization with the MCMVIVL induces antigen-specific tissue-resident CD8+ T cells in the lungs.
BALB/c mice were i.n. (◌) or i.p. (●) infected with 2 x 105 PFU of MCMVIVL virus. During latency (> 3 months p.i), anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from peripheral blood, spleen and lungs, stained with antibodies against CD3, CD4, CD8α, CD11a, CD103, CD69, IVL-tetramer and measured by flow cytometry. (A) CD8TRM cells were gated as CD45-CD69+CD103+. (B) Percentage of CD8TRM cells among CD45- CD8+ T cells in the peripheral blood, spleen and lungs. (C) IVL-tetramer+ cells were gated on the CD8TRM cell subset and percentages of IVL-specific cells among CD8TRM cells in blood, spleen and lungs are shown. (D) CD8TRM cells were gated within the IVL-tetramer+ cell subset and the percentages of CD8TRM cells among tetramer+ CD8+ T cells in blood, spleen and lungs are shown. (E) The number of lung CD8TRM cells in spleen, lungs and blood of different groups. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA. **P<0.01, ****P<0.0001.
In summary, i.n. immunization with MCMVIVL induces an accumulation of IAV-specific CD8TRM response in the lungs. Moreover, antigen-specific CD8+ T cell responses induced via the mucosal route skew towards an effector memory phenotype.
Pulmonary CD8TRM cells improve viral clearance and the production of CD8+ T cell-recruiting chemokines during IAV infection
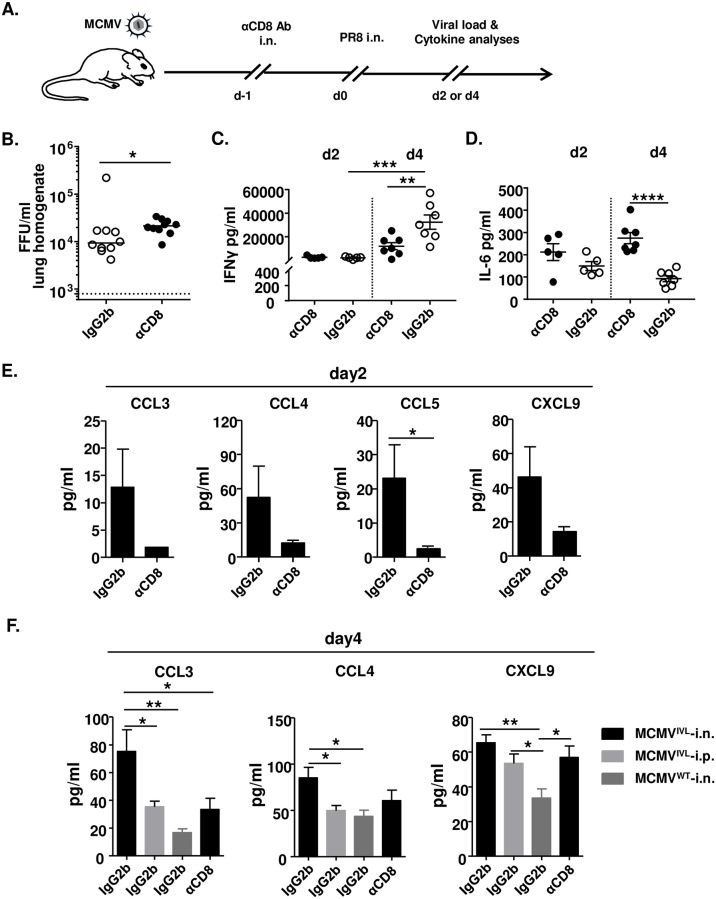
Resident memory T cells reside in the epithelial barrier of mucosal tissue [31] that is in close proximity to the airways. Hence, they reactivate rapidly to a virus challenge at the site of infection upon encountering cognate antigens [29, 31]. To define the relevance of lung CD8TRM cells in protection against IAV challenge, we specifically depleted the airways CD8+ T cells by i.n. administration of αCD8 antibodies one day before challenge (Fig 5A). Upon depletion, tetramer+ CD8TRM cell number reduced significantly while the circulating CD8+ T cell number did not change a lot in the lungs (S2B Fig). The local depletion did not affect the CD8+ T cell counts in the blood (S2C Fig). An earlier day was chosen (4 days post-challenge) to assess whether CD8TRM cell could rapidly confer protection. The IAV titers in the lungs of i.n. MCMVIVL immunized mice were quantified. Targeted depletion of pulmonary CD8TRM cells was associated with a significantly higher viral burden during IAV infection (Fig 5B). These data indicate that CD8TRM cells induced by i.n. immunization with MCMVIVL promote the clearance of IAV.
Fig 5. CD8TRM cells facilitate the elimination of IAV.
BALB/c mice were i.n. or i.p. immunized with 2 x 105 PFU MCMVIVL or i.n. with MCMVWT. During latency (> 3 months p.i), mice were treated with αCD8 or IgG2b antibodies and challenged with IAV (PR8) (i.n., 1100 FFU). Leukocytes were isolated from lungs on day 4 post-challenge for flow cytometric analysis. (A) Graphic representation of the mucosal CD8+ T cell depletion protocol. (B) IAV titers in the lungs on day 4 post-challenge of MCMVIVL i.n. immunized mice. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 10. Group medians are shown. (C-F) The concentration of inflammatory cytokines and chemokines were measured in the BALF on day 2 and day 4 post-IAV challenge. (C) The concentrations of IFNγ and (D) IL-6 in the BALF of each MCMVIVL i.n. immunized mice are shown as symbols. Group means +/- SEM are shown. (E) The concentration of CCL3, CCL4, CCL5 and CXCL9 in the BALF on day 2 post-challenge. (F) The concentration of CCL3, CCL4 and CXCL9 in the BALF on day 4 post-challenge. Two independent experiments were performed and pooled data are shown. n = 5–7. Bars indicate means, error bars are SEM. Significance was assessed by Mann-Whitney U test, One-way ANOVA test, or Two-way ANOVA test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Influenza virus infection can induce a vigorous cytokine storm in airways and lungs, which promotes the recruitment of inflammatory cell. IFNγ as a pivotal antiviral cytokine is expressed early after influenza virus infection [41]. It has been demonstrated that CD8TRM cells activate rapidly when they re-encounter the cognate antigen and provide protection by secreting cytokines such as IFNγ and granzyme B [42, 43]. Morabito et al. showed that intranasal immunization with an MCMV-based vaccine vector induced CD8TRM cells and IFNγ was secreted at the very early time upon challenge during RSV infection [29].
Therefore, we measured the production of IFNγ in the bronchoalveolar lavage fluid (BALF) early upon challenge. IFNγ levels were generally low on day 2 post-challenge and no difference could be observed between groups regardless of airway CD8+ T cells depletion (Fig 5C). On day 4, the IFNγ level was significantly increased compared to the level on day 2, but more IFNγ was induced in the control group than in the one lacking CD8TRM cell in the lungs (Fig 5C). IFNγ was also induced in the MCMVIVL i.p. immunization group and extremely low level of IFNγ could be detected in the MCMVWT control group (S5A Fig). These data suggest that primary cognate antigen immunization is needed for the rapid IFNγ secretion and that resident CD8+ T cells may not be the major IFNγ producer. In contrast to these effects, depletion of lung airway CD8+ T cells increased the concentration of IL-6 as compared to the group that was intranasally immunized with MCMVIVL and treated with isotype control antibodies (Fig 5D). Similarly, a higher concentration of IL-6 was also detected in the i.p. immunization group, whereas the MCMVWT control group displayed the highest IL-6 levels (S5B Fig). Very low levels of other cytokines could be detected in all groups, both on day 2 and 4 post-challenge and regardless of the depletion of the airway CD8+ T cell (S5C Fig), suggesting that the presence of pulmonary CD8TRM cells does not affect the Th1, Th2 and Th17 immune profile during early IAV infection.
It has been demonstrated that TRM cells help to recruit immune cells to the infection site through the induction of chemokines such as CCL3 and CXCL9 in the female reproductive tract (FRT), and CCL4 in the lungs, either by direct chemokine expression or by their induction in nearby cells, such as epithelial cells [28, 29].
To determine whether i.n. immunization with MCMVIVL induced inflammatory chemokines expression upon IAV challenge, a series of inflammatory chemokines were measured in the BALF on day 2 (Fig 5E) and day 4 (Fig 5F) upon IAV challenge. Airway depletion of CD8+ T cells reduced CCL3, CCL4, CCL5 levels on day 2. On day 4, CCL3 and CCL4 levels were significantly higher in the MCMVIVL i.n. group than in the MCMVIVL i.p. and in the MCMVWT i.n. immunization groups. Airway CD8+ T cell depletion reduced the level of CCL3 and CCL4 to values in the i.p. MCMVIVL immunization group (Fig 5F). CXCL9 levels were comparable between the MCMVIVL i.n. and i.p. immunization groups, but dramatically lower in the MCMVWT immunization group (Fig 5F), which was consistent with the low IFNγ level in the BALF, as IFNγ is known as an inducer of CXCL9, which then acts as a T cell-attracting chemokine. Together, these data indicate that CD8TRM cells induced by i.n. immunization with MCMVIVL promote the induction of the pro-inflammatory chemokines CCL3, CCL4, CCL5 and CXCL9, along with a reduction of IL-6 in the lungs.
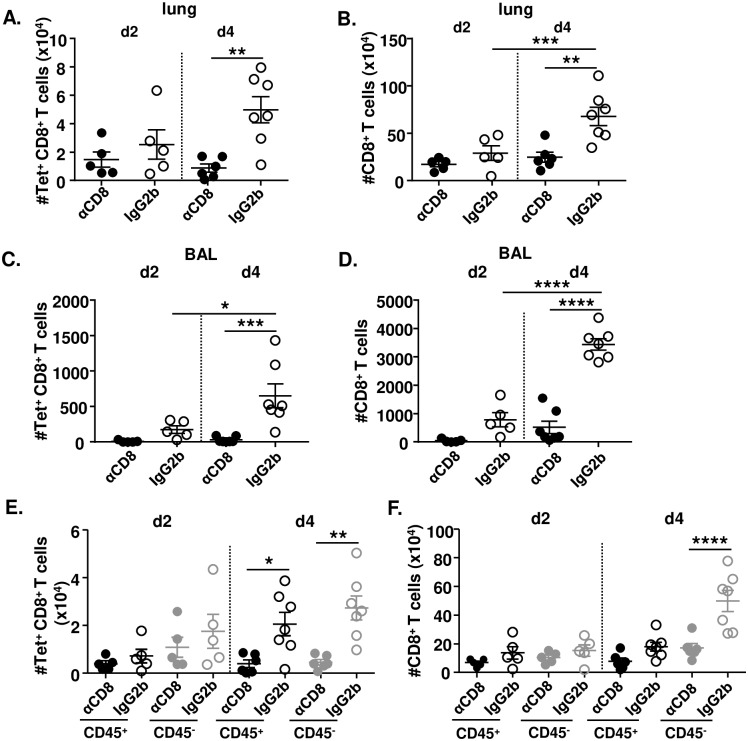
CD8TRM cells facilitate the expansion of CD8+ T cells in the lungs
Since i.n. immunization induced stronger chemokine responses in comparison to the i.p. immunization route, we decided to define whether CD8TRM cells induced by MCMVIVL promoted the accumulation of CD8+ T cells in the lungs. We first analyzed the total IVL-specific and CD8+ T cell numbers (CD45+ plus CD45-) in the MCMVIVL i.n. immunization group. IVL-specific and CD8+ T cell numbers increased from day 2 to day 4 post-challenge, but only in mice that were not depleted for airway CD8+ T cells (Fig 6A and 6B). Both IVL-specific and total CD8+ T cell counts increased significantly in the BALF of i.n. immunized mice by day 4 post IAV challenge (Fig 6C and 6D), However, in the mice which CD8+ T cells were depleted prior to challenge, very few IVL-tetramer+ CD8+ T cells (Fig 6C, filled dots) and CD8+ T cells (Fig 6D, filled dots) could be detected in the BALF, both on day 2 and at day 4 post-challenge. These data indicate that CD8+ T cells accumulate in the lungs and migrate to the lung tissue and bronchoalveolar space upon IAV challenge. In addition, IVL-specific CD8+ T cell counts in the lung tissue and BAL were slightly higher in the MCMVIVL i.n. group than in the i.p. immunized group (S6A and S6B Fig). This differs from results prior to IAV challenge, where significantly larger amounts of IVL-specific CD8+ T cells were detected in the i.p. group (Fig 2G).
Fig 6. CD8TRM cells facilitate the accumulation of CD8+ T cells in the lungs.
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. route. During latency (> 3 months p.i), mice were i.n. treated with αCD8 (black circle;grey circle) or IgG2b (black-lined circle;grey-lined circle) antibodies and challenged with IAV (PR8) (i.n., 1100 FFU) one day after. Anti-CD45 antibodies were injected intravenously 3–5 min before euthanasia. Leukocytes were isolated from lung tissue to analyze the CD8+ T cell response on day 2 and day 4 post-challenge. (A) Total cell counts of IVL-specific CD8+ T cells of both intravitally labeled (CD45+) or unlabeled (CD45-) in the lung tissue. (B) Total cell counts of CD8+ T cells of both CD45+ and CD45- in the lung tissue. (C) Cell counts of IVL-specific CD8+ T cells in the BAL. (D) Cell counts of total CD8+ T cells in the BAL. (E) IVL-specific CD8+ T cells or (F) Total CD8+ T cells that were intravitally labeled or remained unlabeled in the lung tissue were counted on day 2 or day 4 post IAV challenge. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA test. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
CD8+ T cells in the lung tissue were further analyzed by in vivo labeling with anti-CD45 antibodies in the presence or absence of airway CD8+ T cells. The IVL-specific CD8+ T cell population failed to expand upon airway CD8+ T cell depletion, with significantly lower numbers in CD45- subset on day 4. However, IAV-specific CD8+ T cell counts showed an increasing trend both in the CD45+ and in the CD45- subsets on day 4 post-challenge (Fig 6E). Airway CD8+ T cell depletion prevented also the expansion of total CD8+ T cells counts on day 4 (Fig 6F). Interestingly, in contrast to IVL-specific CD8+ T cells, only the CD45- fraction of the total CD8 pool expanded on day 4 (Fig 6F), suggesting that bystander CD8+ T cells were also accumulated within the lungs. The depletion effects were more pronounced later upon antibody administration. Hence, direct depletion of incoming cells appears an unlikely scenario. Together with the increased chemokines in the BAL, these data indicate that the increase of IVL-specific CD8+ T cells upon challenge is not due to in situ proliferation or differentiation but most probably by recruiting CD8+ T cells from circulating system.
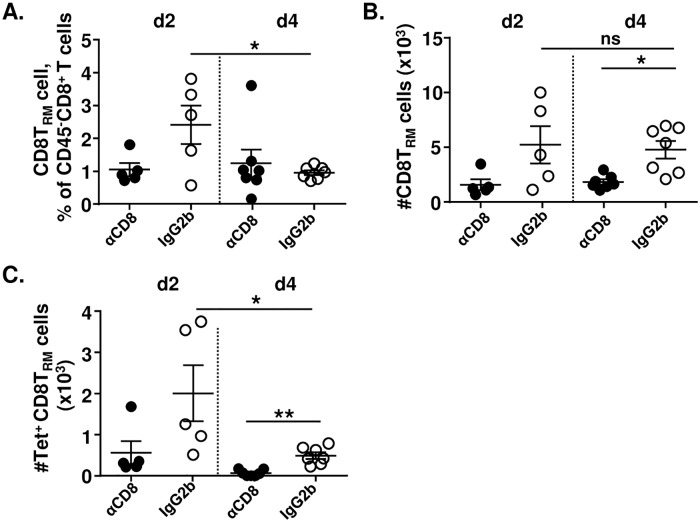
We also checked whether CD8TRM cells expanded upon IAV challenge that may contribute to the accumulation of CD8+ T cells. We found that the number of CD8TRM cells in the lungs did not expand from day 2 to day 4; if anything, their frequency decreased (Fig 7A and 7B). Likewise, IVL-Tetramer+ CD8TRM cell counts were also slightly reduced from day 2 to day 4 post-challenge (Fig 7C), although IVL-Tetramer+ CD8+ T cell counts increased at the same time (Fig 6A). It seems that the effect of the i.n. depletion was local, since the frequencies (S6C and S6D Fig) and counts (S6E and S6F Fig) of IVL-specific CD8+ T cells in the blood and spleen did not significantly differ upon αCD8 or isotype-control antibody i.n. administration. In addition, CD4+ T cell numbers showed no difference upon IAV challenge when airway CD8+ T cells were depleted compared with the control group (S7 Fig), suggesting that CD8TRM cell do not promote CD4+ T cells trafficking into the lungs. Therefore, our data indicated that CD8TRM cells confer protection by recruiting circulating CD8+ T cells upon IAV challenge.
Fig 7. CD8TRM cells do not expand upon IAV challenge.
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. route. During latency (> 3 months p.i), mice were i.n. treated with αCD8 (black circle) or IgG2b (white circle) antibodies and challenged with IAV (PR8) (i.n., 1100 FFU) one day after. Anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from lung tissue to analyze the CD8+ T cell response on day 2 and day 4 post-challenge. CD8TRM cells were gated within the CD45-unlabeled population. (A) Percentage of CD8TRM cells among CD45- CD8+ T cell. (B) Counts of CD8TRM cells in the lungs. (C) Counts of IVL-specific CD8TRM cells in the lungs. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA test. *P <0.05, **P <0.01, ns: no significant difference.
Discussion
Influenza-specific CD8+ T cells are known to contribute to virus elimination, as the clearance of influenza virus is delayed in T cell-deficient mice [5, 44]. However, previous evidence did not clarify whether vaccines solely inducing influenza-specific CD8+ T cell responses improve immune protection. To avoid confounding humoral immune responses and focus on the potential of optimally primed CD8+ T cells in protecting against influenza, we generated a new MCMV based vaccine vector. CMV vaccine vectors expressing exogenous antigenic peptides fused to a CMV gene induce CD8+ T cell responses of unparalleled strength [14, 15, 21, 29]. We show here that robust CD8+ T cell responses against a single MHC-I restricted epitope derived from the HA protein of IAV, promote the clearance of IAV from lungs, but only upon i.n. immunization. While some pathology was observed even in immunized mice, arguing that the protection was not complete, depletion assays confirmed that CD8+ T cells are crucial for the immune protection observed in our model. Morabito et al. showed that the volume of MCMV inoculum affects the magnitude of T cell responses [29]. Hence, a larger inoculum volume could have resulted in even stronger lung CD8+ T cell responses and protection. Remarkably, immunization with the same virus dose by the i.p. route induced even higher magnitude of CD8+ T cell responses, but conferred poor protection.
This conundrum was resolved once we noticed that only i.n. immunization induces TRM responses in the lung. CD8TRM cells act as sentinels in the host and form the first line of defense, providing rapid and effective protection to fight against pathogens invasion [27, 29, 31, 45]. Prior studies have revealed that direct delivery of vaccines to the target tissue is necessary for the generation of TRM cells [29, 46] and that sustained lung CD8TRM responses in MCMV-infected mice are generated by immunoproteasome-independent antigenic stimulation [47], akin to the CD8 expansions in memory inflation [21], arguing that their induction may share similar or overlapping mechanisms. Some prior studies have claimed that skin-resident CD8TRM cells may confer protection in an antigen-unspecific manner [48], whereas others argued that only the antigen-specific CD8TRM cells respond to cognate antigens [49]. MCMVWT induced robust CD8TRM responses in our model, but these were not specific for IAV, and did not provide any protection against IAV in our study. Site-specific anti-CD8α antibody administration depleted CD8TRM, and increased IAV titers in immunized mice, indicating that CD8TRM cells facilitated IAV elimination. Thus, the protection against IAV challenge requires antigen-specific CD8TRM cells in our model. However, the weight loss was not different in the early days upon challenge and TRM depletion affected chemokine expression and T cell influx at later time points.
Former studies have described that the inflationary MCMV-specific CD8+ T cells induced by intraperitoneal infection display an effector phenotype (KLRG1+CD62L-CD127-) [50, 51]. Mucosal immunization of MCMVIVL affects not only the magnitude but also the quality of CD8+ T cells skewing cells towards an effector memory phenotype. Since KLRG1 is assumed a marker for terminally differentiated cells which usually are short-lived [52], the low expression of KLRG1 may contribute to a longer life-span of these cells. Additionally, recent work has demonstrated that KLRG1- CD8+ TEM cells traffic and migrate more rapidly to non-lymphoid tissues than the KLRG1+ CD8+ TEFF cells, which mostly remain in the circulation [53, 54]. Accordingly, this may give us a clue that CD8TEM cells induced by mucosal immunization may rapidly migrate to the lungs and exert immune functionality there. Nonetheless, further studies need to be done to prove this hypothesis.
It has been assumed that CD8TRM cells have poor proliferative capacity upon challenge. Previous work has demonstrated that airway CD8+ T cells fail to expand in vivo upon intratracheal transfer [36] and that CD8TRM cells induced by MCMV infection display a limited proliferative capacity in salivary glands [55]. However, this is in contrast to two recent studies demonstrating that CD8TRM cells in the skin [49] and FRT [56] maintain the capacity of in situ proliferation upon cognate antigen stimulation. Such stimulation differentiates circulating effector memory CD8+ T cells into CD8TRM cells without displacing the pre-existing CD8TRM population [49]. In our study, CD8+ T cells accumulated in the lungs upon IAV challenge, but the CD8TRM population did not expand and the number of antigen-specific CD8TRM cells even displayed a reduction trend. This appears unrelated to apoptosis, because CD8TRM cells showed less caspase3 expression than circulating CD8+ T cells upon challenge. It is possible that CD8TRM cells downregulated CD103 from the cell surface upon activation, and this intriguing question needs to be addressed in future studies. Therefore, our data argued that either lung CD8TRM in general or CD8TRM induced by MCMV i.n. immunization in particular, may behave differently from CD8TRM in other organs. This distinction, however, goes beyond the scope of our current work and remains to be addressed in future studies.
Early upon IAV challenge, not only the IVL-specific CD8+ T cells but also the bystander CD8+ T cells in the lung tissue increased significantly, indicating that the accumulation was not due to in situ proliferation but probably due to recruitment from circulating system. In addition, IAV challenge expanded IVL-specific CD8+ T cell counts in the blood and spleen of i.n. immunized mice to levels observed in the i.p. immunized controls, although the levels were significantly lower in the i.n. immunization group before IAV challenge. Overall, these results indicated that i.n. immunization facilitated CD8+ T cell responses upon challenge, both locally in the lungs and systemically in the blood and spleen. It is unclear if this apparent alarming function of TRM cells is exclusive to the lung tissue.
We have shown in this study that concentration of CCL3, CCL4 and CXCL9 in the BALF of the MCMVIVL i.n. immunization group is significantly higher than in MCMVIVL i.p. or MCMVWT i.n. immunized mice. Intravital CD45 labeling showed that CD8+ T cells accumulating in the lungs are sequestered from the bloodstream, but not CD8TRM, arguing that circulating antigen-specific cells were attracted into the lungs under the presence of mucosa-resident CD8+ T cells. This is in line with the work of Schenkel et al. showing a rapid local induction of chemokines CXCL9 and CCL3/4 in the FRT upon re-infection, and recruitment of memory CD8+ T cells from the periphery [28]. Depletion of mucosal CD8+ T cells depressed chemokine levels in the BALF to levels seen in the i.p. immunization group. This, together with the high levels of IFNγ in the MCMVIVL i.n. immunization group and extremely low IFNγ in MCMVWT i.n. immunization points to a putative model where antigen-specific re-stimulation induces IFNγ, which drives chemokine responses that recruit CD8+ T cells from the bloodstream to the lungs. We observed a surprising lower level of IL-6 upon challenge in mice that controlled influenza, because IL-6 is known as a cytokine that is involved in controlling virus infection [57–61]. It is not clear if IL-6 reduction was a consequence of lower virus titers or of negative regulation by TRM. McMaster et al. showed a reduced IL-6 production accompanied with lower virus titer in the appearance of lung airway CD8TRM cells [36] and this was similar to a report by Lee et al. in a clinical study in human patients [62].
In summary, our data demonstrate that CD8TRM cells promote the induction of chemokines, which help to drive the recruitment of IVL-specific CD8+ T cells and facilitates the elimination of IAV. Furthermore, the optimal induction of CD8TRM cells in the lungs by the MCMV vector can be only achieved after i.n. vaccination. Therefore, immunization with an MCMV vector at the local site provided CD8+ T cell-based protection against IAV infection. Our results, therefore, demonstrate that CD8+ T cell induction, and CD8TRM in particular, contribute to vaccination outcomes in influenza infection independently of humoral immune responses, and the selection of the adequate immunization route plays a critical role in terms for promoting superior protective efficacy.
Materials and methods
Ethics statement
Mice were housed and handled in agreement with good animal practice as defined by EU directive EU 2010/63 and ETS 123 and the national animal welfare body ‘‘Die Gesellschaft für Versuchstierkunde /Society of Laboratory Animals (GV-SOLAS)”. Animal experiments were performed in accordance with the German animal protection law and were approved by the responsible state office (Lower Saxony State Office of Consumer Protection and Food Safety) under permit number: 33.9-42502-04-14/1709.
Mice
BALB/c mice were purchased from Janvier (Le Genest Saint Isle, France) and housed in the animal facility of the HZI Braunschweig under SPF conditions according to FELASA recommendations [63].
Cells
Bone marrow stromal cell line M2-10B4 (CRL-1972) and NIH-3T3 fibroblasts (CRL-1658) were purchased from American Type Culture Collection (ATCC). The cells were maintained in DMEM supplemented with 10% FCS, 1% L-glutamine, and 1% penicillin/streptomycin. C57BL/6 murine embryonic fibroblasts (MEFs) were prepared in-house and maintained as described previously [64].
Viruses
BAC-derived wild-type murine cytomegalovirus (MCMVWT clone: pSM3fr 3.3) [65] was propagated on M2-10B4 lysates and purified on a sucrose cushion as described previously [66]. Virus titers were determined on MEFs by plaque assay as shown elsewhere [67].
Recombinant MCMV was generated by the ‘‘en passant mutagenesis”, essentially as described previously [68, 69]. In brief, we generated a construct containing an antibiotic resistance cassette coupled with the insertion sequence and the restriction site Sce-I. This construct was flanked by sequences homologous to the target region of insertion within the MCMV BAC genome. Then, the fragment containing the insertion sequences was integrated into the MCMV BAC genome by homologous recombination. In a second step, Sce-I was induced to linearize the BAC followed by a second round of induced homologous recombination to re-circularize it and select for clones that discarded the antibiotic selection marker but retained the inserted sequence.
The PR8M variant of Influenza A/PR/8/34 was obtained from the strain collection at the Institute of Molecular Virology, Muenster, Germany. Virus stocks from chorioallantoic fluid of embryonated chicken eggs were generated as previously described [70].
Tetramers and antibodies
533IYSTVASSL541 (IVL533-541)-tetramer was bought from MBL (cat. NO.TS-M520-1), anti-CD8α depletion antibody (clone: YTS 169.4). Rat IgG2b isotype antibody (clone: LTF-2) was purchased from Bio X Cell. Antibodies for flow cytometry included anti-CD3-APC-eFluor780 (clone: 17A2; eBioscience), anti-CD4-Pacific Blue (clone: GK1.5; BioLegend), anti–CD8α-PerCP/Cy5.5 (clone: 53–6.7; BioLegend), anti-CD11a-PE/Cy7 (clone: 2D7; BD Bioscience), anti–CD44-Alexa Fluor 700 (clone: IM7; BioLegend), anti-CD45-APC-eFluor780 (clone:30-F11;Biolegend), anti-CD62L-eVolve 605 (clone: MEL-14; eBioscience), anti-CD127-PE & PE/Cy7 (clone: A7R34; BioLegend), anti-KLRG1-FITC & BV510 (clone: 2F1/KLRG1; BioLegend), anti-CD103-APC (clone: 2E7; BioLegend), anti-CD69-FITC (clone: H1.2F3; BioLegend) and anti-IFNγ-APC (clone: XMG1.2; BioLegend), anti-Eomes-PE & PE/Cy7 (clone: Dan11mag; eBioscience).
Virus in vitro infection
NIH-3T3 cell monolayers were infected with MCMVWT and MCMVIVL at an MOI of 0.1, incubated at 37°C for 1h, upon which the inoculum was removed, cells were washed with PBS, and supplied with fresh medium. Cells were incubated for 6 days; the supernatant was harvested every day and stored at -80°C until titration.
Virus in vivo infection
6 to 8 weeks old BALB/c female mice were infected with 2 x 105 PFU MCMVWT and MCMVIVL diluted in PBS. For i.p. infection, 100 μl virus dilution was injected. For i.n. infection, mice were first anesthetized with ketamine (10 mg/ml) and xylazine (1 mg/ml) in 0.9% NaCl (100 μl/10 g body weight), then administered with 20 μl of virus suspension onto nostrils [35]. For IAV challenge, BALB/c mice that were latently (> 3 months) immunized with MCMV were i.n. inoculated with 220 focus forming units (FFU) or with 1100 FFU of PR8M influenza virus as described previously [35].
Infectious virus quantification (MCMV)
MCMV virus from organ homogenates was titrated on MEFs with centrifugal enhancement as described previously [17].
Infectious virus quantification (IAV)
Mice were sacrificed by CO2 inhalation, whole lungs were excised and mechanically homogenized using a tissue homogenizer. Tissue homogenates were spun down and supernatants were stored at -70°C. Lung virus titers were determined by using the focus-forming assay (FFA), as described before [70] with minor modifications. Briefly, MDCK cells were cultured in MEM, supplemented with 10% FCS, 1% penicillin/streptomycin. Supernatants of lung tissue homogenates were serially diluted in DMEM, supplemented with 0.1% BSA and N-acetylated trypsin (NAT; 2.5 μg/ml) and added to the MDCK cell monolayers. After 1h, cells were overlaid with DMEM supplemented with 1% Avicel, 0.1% BSA and NAT (2.5 μg/ml). After 24h cells were fixed with 4% PFA and incubated with quenching solution (PBS, 0.5% Triton X-100, 20 mM Glycin). Cells were then treated with blocking buffer (PBS, 1% BSA, 0.5% Tween20). Focus forming spots were identified using primary polyclonal goat anti-H1N1 IgG (Virostat), secondary polyclonal rabbit anti-goat IgG conjugated with horseradish peroxidase (KPL) and TrueBlue™ peroxidase substrate (KPL). Viral titers were calculated as FFU per ml of lung tissue homogenate.
Isolation of lymphocytes from blood and organs
Blood, spleen and mLNs were prepared as described previously [35]. Lungs were perfused by injecting 5–10 ml PBS into the right heart ventricle. The lungs were cut into small pieces, resuspended in 1 ml RPMI1640 (0.5% FCS), and digested with 1 ml of RPMI1640 with DNase I (Sigma-Aldrich Chemie) and Collagenase I (ROCKLAND™) in a shaker at 37°C for 30 min. Digested tissue was passed through cell strainers and single cell suspensions were washed with RPMI1640, centrifuged at 500x g for 10 min. Subsequently, the cells were resuspended in 7 ml of 40% Easycoll solution (Biochrom), overlayed onto 6 ml of 70% Easycoll solution in a 15 ml Falcon and centrifuged at 25 min at 1000x g at room temperature. The interface layer was transferred to a 5 ml tube, washed, and resuspended in RPMI1640 (10% FCS).
Peptide stimulation
T cells were stimulated with peptides (1 μg/ml) in 85 μl RPMI 1640 for 1h at 37°C, supplemented with brefeldin A (10 μg/ml in 15 μl RPMI 1640) and incubated for additional 5h at 37°C. Cells incubated without any peptide in the same condition were used as negative controls. Cytokine responses were detected by intracellular cytokine staining.
Cell surface staining, intracellular cytokine staining for flow cytometry
Blood cells and lymphocytes from spleen, lung and mLNs were stained with IVL533-541-tetramer-PE and surface antibodies for 30 min, washed with FACS buffer and analyzed. For intracellular cytokine stainings, the cells were first stained with cell surface antibodies for 30 min, washed and fixed with 100 μl IC fixation buffer (eBioscience) for 5 min at 4°C. Following this, cells were permeabilized for 3 min with 100 μl permeabilization buffer (eBioscience) and incubated with anti-IFNγ antibody for 30 min. Afterwards, cells were washed with FACS buffer and acquired using an LSR-Fortessa flow cytometer (BD Bioscience).
In vivo cell labeling
Mice were intravenously (i.v.) injected with 3 μg anti-CD45-APC/eFluor780 (clone: 30-F11; BioLegend). Mice were euthanatized 3–5 min after injection, and blood, spleen and lungs were collected. Following their isolation from the respective compartment, lymphocytes were stained and analyzed as described above.
In vivo CD8+ T cell depletion
For systemic in vivo CD8+ T cell depletion, published protocols [71, 72] were adopted as follows. BALB/c mice were i.p. injected with 200 μg anti-CD8α (αCD8: clone: YTS 169.4) or isotype antibody (Rat IgG2b: clone: LTF-2; Bio X Cell) one day before IAV challenge. To deplete mucosal CD8+ T cells in the lungs, BALB/c mice were i.n. administered 10 μg αCD8 or IgG2b in 20 μl of PBS one day before IAV challenge [40].
Collection of bronchoalveolar lavage fluid (BALF)
Mice were sacrificed by CO2 inhalation, the chest cavity was opened and skin and muscle around the neck were gently removed to expose the trachea. A catheter was inserted and the lungs were carefully flushed with 1 mL PBS via the trachea. The BALF was transferred into a 1.5 ml tube and stored on ice. The BALF was centrifuged at 500x g at 4°C for 10 min. The supernatant was aliquoted and stored at -80°C until further analysis.
Cytokine and chemokine quantification
Mouse IFNγ enzyme-linked immunosorbent assay (ELISA) MAX™ kits (BioLegend) and the bead-based immunoassay LEGENDplex™ Mouse Inflammation Panel (13-plex, BioLegend) were used to quantify IFNγ and other cytokine levels in the BALF according to the manufacturer’s instructions. The bead-based immunoassay LEGENDplex Mouse Pro-inflammation Chemokine Panel (13-plex, BioLegend) was used to quantify multiple chemokine levels in the BALF.
Histopathology
Lungs were harvested from BALB/c mice that were latently infected with MCMVWT and MCMVIVL and challenged with IAV during latency. Lungs were fixed in 4% formalin, paraffin embedded, sliced and hematoxylin and eosin (H&E) stained according to standard laboratory procedures.
Statistics
One-way ANOVA analysis was used to compare multiple groups at single time points. Two-way ANOVA analysis was used to compare different groups at multiple time points. Comparisons between two groups were performed using Mann-Whitney U test (two-tailed). Statistical analysis was performed using GraphPad Prism 7.
Supporting information
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.p. or i.n. route. During latency (> 3 months p.i), Leukocytes from blood, spleen and lungs were stained with cell surface markers CD3, CD4, CD8, CD11a, CD69, CD103, KLRG1, CD62L, IVL-tetramer and analyzed by flow cytometry. For in vivo labeling, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Gating strategy of each cell subset is shown.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. route. (A) During latency (> 3 months p.i), mice were injected 200 μg αCD8 antibody (i.p.) to deplete total CD8+ T cells. Same amount of IgG2b antibody was given as isotype control. Leukocytes from blood, spleen and lungs were analyzed by flow cytometry and representative flow cytometric panels in blood, spleen and lungs on day 1 post-depletion are shown. (B-C) Mice were administered with 10 μg αCD8 antibody (i.n.) to deplete airway CD8+ T cells in the lungs or IgG2b as a control. (B) The number of IVL-tetramer+ CD8TRM cells and circulating CD8+ T cells (CD45+) in the lungs on day 1 post airway CD8+ T cell depletion. (C) The number of IVL-specific and total CD8+ T cells in the peripheral blood on day 1 post airway CD8+ T cell depletion. Bars indicate means, error bars are SEM.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVWT via the i.n. route. During latency (> 3 months p.i), leukocytes were isolated from lungs, stained with cell surface markers against CD4, CD8, CD69, CD103 before flow cytometry. (A) Representative dot plots of CD8TRM and IVL-specific CD8TRM cells. (B, C) BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. or i.p. route. (B) Percentage of CD69+CD103-CD8+ T cells in the lungs. (C) The number of CD69+CD103-CD8+ T cells in the lungs. (D) Eomes expression on different subsets of CD8+ T cells in the lungs. (E) Percentage of caspase3/7+ cells among CD8TRM and circulating CD8+ T (CD45+) cells. (F) Percentage of caspase3/7+ cells among tetramer+ CD8TRM and circulating CD8+ T (CD45+) cells. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–9. Group means +/- SEM are shown. Significance was assessed by Mann-Whitney U test. **P <0.01, ***P <0.001, ns: no significance.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.p. or i.n. route. During latency (> 3 months p.i), anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes from blood, spleen and lungs were stained with cell surface markers CD3, CD4, CD8, CD11a, KLRG1, CD62L, IVL-tetramer and analyzed by flow cytometry. TEFF cells are defined as KLRG1+CD62L-, TEM as KLRG1-CD62L-and TCM as KLRG1-CD62L+. (A) The percentages of each phenotype subset among CD45- tetramer+ CD8+ T cells in the lungs and spleen. (B) The percentages of each phenotype subset among CD45+ tetramer+ CD8+ T cells and tetramer+ CD8TRM cells in the lungs, spleen and blood. (C) The percentages of each phenotype subset among tetramer+ CD8TRM cells in the lungs. Two independent experiments were performed and pooled data are shown, n = 5. Each symbol represents one mouse. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA and Two-way ANOVA test. ****P <0.0001.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. or i.p. route or with MCMVWT via the i.n. route. During latency (> 3 months p.i), MCMVIVL (i.n.) immunized mice were administered with 10 μg αCD8 or 10 μg IgG2b antibody (i.n.). MCMVIVL (i.p.) and MCMVWT (i.n.) immunized mice were administered with 10 μg IgG2b antibody (i.n.). One day later, animals were challenged with IAV (PR8) (i.n., 1100 FFU). On day 2 and day 4 post-challenge, BALF was harvested and measured cytokines production by bio-plexing. The concentration of (A) IFNγ and (B) IL-6 in the BALF on day 4 post-challenge. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. (C) Cytokine concentrations in the BALF in different immunization group on day 2 and day 4 post-challenge. Bars indicate means, error bars are SEM. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Significance was assessed by One-way ANOVA test. *P <0.05, ***P <0.001.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. or i.p. route or with MCMVWT by the i.n. route. During latency (> 3 months p.i), mice were challenged with IAV (PR8) (i.n., 1100 FFU) one day after airway CD8+ T cell depletion. On day 4 post-challenge, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from lung, BAL, blood and spleen. (A-B) Count of IVL-specific CD8+ T cells in the lungs (A) and BAL (B). (C-D) Percentage of IVL-specific CD8+ T cells among CD8+ T cells in the blood (C) and spleen (D). (E-F) Count of IVL-specific CD8+ T cell in the blood (E) and spleen (F). Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA test. *P <0.05, **P <0.01.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. route. During latency (> 3 months p.i), mice were challenged with IAV (PR8) (i.n., 1100 FFU) one day after airway CD8+ T cell depletion. On day 2 and day 4 post-challenge, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from lungs and BAL. CD4+ T cell numbers in the lungs are shown. (A) The number of total CD4+ T cells. (B) The number of CD45- CD4+ T cells. (C) The number of CD4+ T cells in the BAL. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown.
(TIF)
Acknowledgments
We wish to thank Inge Hollatz-Rangosch and Ayse Barut from the Cicin-Sain lab and Tatjana Hirsch from the group of Prof. Dr. Dunja Bruder for excellent technical assistance. We thank Laura McKay and Frank Carbone for useful discussions, Ramon Arens for tetramer reagents and Prof. Dr. Dirk Busch for providing us streptamer reagents.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the European Research Council through the ERC Starting Grant 260934 to LCS and the Helmholtz Association through the Helmholtz EU Partnering Grant PIE-008 to LCS. XZ was supported by a scholarship from the Chinese Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Influenza (Seasonal) Fact sheet N°211". who.int. 2014; http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
- 2.Barria M.I., et al. , Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J Infect Dis, 2013. 207(1): p. 115–24. 10.1093/infdis/jis641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilyushina N.A., et al. , Live attenuated and inactivated influenza vaccines in children. J Infect Dis, 2015. 211(3): p. 352–60. 10.1093/infdis/jiu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichelberger M., et al. , Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med, 1991. 174(4): p. 875–80. 10.1084/jem.174.4.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender B.S., et al. , Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med, 1992. 175(4): p. 1143–5. 10.1084/jem.175.4.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yager E.J., et al. , Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med, 2008. 205(3): p. 711–23. 10.1084/jem.20071140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgarth N. and Kelso A., In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol, 1996. 70(7): p. 4411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topham D.J., Tripp R.A., and Doherty P.C., CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol, 1997. 159(11): p. 5197–200. [PubMed] [Google Scholar]
- 9.Sylwester A.W., et al. , Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med, 2005. 202(5): p. 673–85. 10.1084/jem.20050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtappels R., et al. , Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol, 2000. 74(24): p. 11495–503. 10.1128/jvi.74.24.11495-11503.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karrer U., et al. , Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol, 2003. 170(4): p. 2022–9. 10.4049/jimmunol.170.4.2022 [DOI] [PubMed] [Google Scholar]
- 12.Munks M.W., et al. , Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol, 2006. 177(1): p. 450–8. 10.4049/jimmunol.177.1.450 [DOI] [PubMed] [Google Scholar]
- 13.Podlech J., et al. , Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol, 2000. 74(16): p. 7496–507. 10.1128/jvi.74.16.7496-7507.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karrer U., et al. , Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol, 2004. 78(5): p. 2255–64. 10.1128/JVI.78.5.2255-2264.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuda Y., et al. , A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis, 2011. 5(8): p. e1275 10.1371/journal.pntd.0001275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen S.G., et al. , Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature, 2011. 473(7348): p. 523–7. 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekhtiarenko I., et al. , The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J Immunol, 2013. 190(7): p. 3399–409. 10.4049/jimmunol.1203173 [DOI] [PubMed] [Google Scholar]
- 18.Borkner L., et al. , Immune Protection by a Cytomegalovirus Vaccine Vector Expressing a Single Low-Avidity Epitope. J Immunol, 2017. 199(5): p. 1737–1747. 10.4049/jimmunol.1602115 [DOI] [PubMed] [Google Scholar]
- 19.Beverley P.C., et al. , A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J Immunol, 2014. 193(5): p. 2306–16. 10.4049/jimmunol.1302523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klyushnenkova E.N., et al. , A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother, 2012. 35(5): p. 390–9. 10.1097/CJI.0b013e3182585d50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekhtiarenko I., et al. , Peptide Processing Is Critical for T-Cell Memory Inflation and May Be Optimized to Improve Immune Protection by CMV-Based Vaccine Vectors. PLoS Pathog, 2016. 12(12): p. e1006072 10.1371/journal.ppat.1006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzi A., et al. , Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci Rep, 2016. 6: p. 21674 10.1038/srep21674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen S.G., et al. , Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science, 2013. 340(6135): p. 1237874 10.1126/science.1237874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erkes D.A., Wilski N.A., and Snyder C.M., Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity. Hum Vaccin Immunother, 2017. 13(8): p. 1778–1785. 10.1080/21645515.2017.1331795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Z., et al. , Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-cell Response and Protects Mice from Melanoma. Cancer Immunol Res, 2015. 3(5): p. 536–46. 10.1158/2326-6066.CIR-14-0044 [DOI] [PubMed] [Google Scholar]
- 26.Gebhardt T., et al. , Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol, 2009. 10(5): p. 524–30. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 27.Wakim L.M., et al. , The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol, 2012. 189(7): p. 3462–71. 10.4049/jimmunol.1201305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenkel J.M., et al. , Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol, 2013. 14(5): p. 509–13. 10.1038/ni.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morabito K.M., et al. , Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol, 2017. 10(2): p. 545–554. 10.1038/mi.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackay L.K., et al. , The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol, 2013. 14(12): p. 1294–301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 31.Jiang X., et al. , Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature, 2012. 483(7388): p. 227–31. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapuente D., et al. , IL-1beta as mucosal vaccine adjuvant: the specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol, 2018. 11(4): p. 1265–1278. 10.1038/s41385-018-0017-4 [DOI] [PubMed] [Google Scholar]
- 33.Smith C.J., et al. , Murine CMV Infection Induces the Continuous Production of Mucosal Resident T Cells. Cell Rep, 2015. 13(6): p. 1137–1148. 10.1016/j.celrep.2015.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann N.S., et al. , Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLoS Pathog, 2018. 14(4): p. e1006993 10.1371/journal.ppat.1006993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oduro J.D., et al. , Murine cytomegalovirus (CMV) infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J Gen Virol, 2016. 97(1): p. 185–95. 10.1099/jgv.0.000339 [DOI] [PubMed] [Google Scholar]
- 36.McMaster S.R., et al. , Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol, 2015. 195(1): p. 203–9. 10.4049/jimmunol.1402975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hombrink P., et al. , Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol, 2016. 17(12): p. 1467–1478. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- 38.Tamura M., et al. , Definition of amino acid residues on the epitope responsible for recognition by influenza A virus H1-specific, H2-specific, and H1- and H2-cross-reactive murine cytotoxic T-lymphocyte clones. J Virol, 1998. 72(11): p. 9404–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn K.J., et al. , Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity, 1998. 8(6): p. 683–91. [DOI] [PubMed] [Google Scholar]
- 40.Slutter B., et al. , Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity, 2013. 39(5): p. 939–48. 10.1016/j.immuni.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel C.E., Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology, 1991. 183(1): p. 1–11. 10.1016/0042-6822(91)90112-o [DOI] [PubMed] [Google Scholar]
- 42.Cheuk S., et al. , CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity, 2017. 46(2): p. 287–300. 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topham D.J. and Reilly E.C., Tissue-Resident Memory CD8(+) T Cells: From Phenotype to Function. Front Immunol, 2018. 9: p. 515 10.3389/fimmu.2018.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells M.A., Albrecht P., and Ennis F.A., Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol, 1981. 126(3): p. 1036–41. [PubMed] [Google Scholar]
- 45.Pizzolla A., et al. , Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol, 2017. 2(12). [DOI] [PubMed] [Google Scholar]
- 46.Zens K.D., Chen J.K., and Farber D.L., Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight, 2016. 1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morabito K.M., et al. , Memory Inflation Drives Tissue-Resident Memory CD8(+) T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus. Front Immunol, 2018. 9: p. 1861 10.3389/fimmu.2018.01861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ariotti S., et al. , T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science, 2014. 346(6205): p. 101–5. 10.1126/science.1254803 [DOI] [PubMed] [Google Scholar]
- 49.Park S.L., et al. , Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol, 2018. 19(2): p. 183–191. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- 50.Sierro S., Rothkopf R., and Klenerman P., Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol, 2005. 35(4): p. 1113–23. 10.1002/eji.200425534 [DOI] [PubMed] [Google Scholar]
- 51.Snyder C.M., et al. , Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity, 2008. 29(4): p. 650–9. 10.1016/j.immuni.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henson S.M. and Akbar A.N., KLRG1—more than a marker for T cell senescence. Age (Dordr), 2009. 31(4): p. 285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osborn J.F., et al. , Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8(+) T cells. Sci Immunol, 2017. 2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerlach C., et al. , The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity, 2016. 45(6): p. 1270–1284. 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thom J.T., et al. , The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep, 2015. 13(6): p. 1125–36. 10.1016/j.celrep.2015.09.082 [DOI] [PubMed] [Google Scholar]
- 56.Beura L.K., et al. , Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol, 2018. 19(2): p. 173–182. 10.1038/s41590-017-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshizaki K., et al. , Interleukin 6 and expression of its receptor on epidermal keratinocytes. Cytokine, 1990. 2(5): p. 381–7. [DOI] [PubMed] [Google Scholar]
- 58.Scheller J., et al. , The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta, 2011. 1813(5): p. 878–88. 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 59.Murphy E.A., et al. , Effect of IL-6 deficiency on susceptibility to HSV-1 respiratory infection and intrinsic macrophage antiviral resistance. J Interferon Cytokine Res, 2008. 28(10): p. 589–95. 10.1089/jir.2007.0103 [DOI] [PubMed] [Google Scholar]
- 60.Strestik B.D., et al. , The role of IL-5, IL-6 and IL-10 in primary and vaccine-primed immune responses to infection with Friend retrovirus (Murine leukaemia virus). J Gen Virol, 2001. 82(Pt 6): p. 1349–54. 10.1099/0022-1317-82-6-1349 [DOI] [PubMed] [Google Scholar]
- 61.Harker J.A., et al. , Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science, 2011. 334(6057): p. 825–9. 10.1126/science.1208421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee N., et al. , Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza A virus infection. Clin Infect Dis, 2007. 45(6): p. 723–31. 10.1086/520981 [DOI] [PubMed] [Google Scholar]
- 63.Mahler Convenor M., et al. , FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim, 2014. 48(3): p. 178–192. 10.1177/0023677213516312 [DOI] [PubMed] [Google Scholar]
- 64.Reddehase M.J., Podlech J., and Grzimek N.K., Mouse models of cytomegalovirus latency: overview. J Clin Virol, 2002. 25 Suppl 2: p. S23–36. [DOI] [PubMed] [Google Scholar]
- 65.Jordan S., et al. , Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2. J Virol, 2011. 85(19): p. 10346–53. 10.1128/JVI.00545-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dag F., et al. , Reversible silencing of cytomegalovirus genomes by type I interferon governs virus latency. PLoS Pathog, 2014. 10(2): p. e1003962 10.1371/journal.ppat.1003962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cicin-Sain L., Podlech J., Messerle M., Reddehase M. J., and Koszinowski U. H., Frequent coinfection of cells explains functional in vivo complementation between cytomegalovirus variants in the multiply infected host. Journal of Virology, 2005. 79: p. 9492–9502. 10.1128/JVI.79.15.9492-9502.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tischer B.K., Smith G.A., and Osterrieder N., En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol, 2010. 634: p. 421–30. 10.1007/978-1-60761-652-8_30 [DOI] [PubMed] [Google Scholar]
- 69.Dekhtiarenko I., Cicin-Sain L., and Messerle M., Use of recombinant approaches to construct human cytomegalovirus mutants. Methods Mol Biol, 2014. 1119: p. 59–79. 10.1007/978-1-62703-788-4_5 [DOI] [PubMed] [Google Scholar]
- 70.Blazejewska P., et al. , Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology, 2011. 412(1): p. 36–45. 10.1016/j.virol.2010.12.047 [DOI] [PubMed] [Google Scholar]
- 71.Salem M.L. and Hossain M.S., In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. Int J Immunopharmacol, 2000. 22(9): p. 707–18. 10.1016/s0192-0561(00)00033-3 [DOI] [PubMed] [Google Scholar]
- 72.Kruisbeek A.M., In vivo depletion of CD4- and CD8-specific T cells. Curr Protoc Immunol, 2001. Chapter 4: p. Unit 4 1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.p. or i.n. route. During latency (> 3 months p.i), Leukocytes from blood, spleen and lungs were stained with cell surface markers CD3, CD4, CD8, CD11a, CD69, CD103, KLRG1, CD62L, IVL-tetramer and analyzed by flow cytometry. For in vivo labeling, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Gating strategy of each cell subset is shown.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. route. (A) During latency (> 3 months p.i), mice were injected 200 μg αCD8 antibody (i.p.) to deplete total CD8+ T cells. Same amount of IgG2b antibody was given as isotype control. Leukocytes from blood, spleen and lungs were analyzed by flow cytometry and representative flow cytometric panels in blood, spleen and lungs on day 1 post-depletion are shown. (B-C) Mice were administered with 10 μg αCD8 antibody (i.n.) to deplete airway CD8+ T cells in the lungs or IgG2b as a control. (B) The number of IVL-tetramer+ CD8TRM cells and circulating CD8+ T cells (CD45+) in the lungs on day 1 post airway CD8+ T cell depletion. (C) The number of IVL-specific and total CD8+ T cells in the peripheral blood on day 1 post airway CD8+ T cell depletion. Bars indicate means, error bars are SEM.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVWT via the i.n. route. During latency (> 3 months p.i), leukocytes were isolated from lungs, stained with cell surface markers against CD4, CD8, CD69, CD103 before flow cytometry. (A) Representative dot plots of CD8TRM and IVL-specific CD8TRM cells. (B, C) BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. or i.p. route. (B) Percentage of CD69+CD103-CD8+ T cells in the lungs. (C) The number of CD69+CD103-CD8+ T cells in the lungs. (D) Eomes expression on different subsets of CD8+ T cells in the lungs. (E) Percentage of caspase3/7+ cells among CD8TRM and circulating CD8+ T (CD45+) cells. (F) Percentage of caspase3/7+ cells among tetramer+ CD8TRM and circulating CD8+ T (CD45+) cells. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–9. Group means +/- SEM are shown. Significance was assessed by Mann-Whitney U test. **P <0.01, ***P <0.001, ns: no significance.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.p. or i.n. route. During latency (> 3 months p.i), anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes from blood, spleen and lungs were stained with cell surface markers CD3, CD4, CD8, CD11a, KLRG1, CD62L, IVL-tetramer and analyzed by flow cytometry. TEFF cells are defined as KLRG1+CD62L-, TEM as KLRG1-CD62L-and TCM as KLRG1-CD62L+. (A) The percentages of each phenotype subset among CD45- tetramer+ CD8+ T cells in the lungs and spleen. (B) The percentages of each phenotype subset among CD45+ tetramer+ CD8+ T cells and tetramer+ CD8TRM cells in the lungs, spleen and blood. (C) The percentages of each phenotype subset among tetramer+ CD8TRM cells in the lungs. Two independent experiments were performed and pooled data are shown, n = 5. Each symbol represents one mouse. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA and Two-way ANOVA test. ****P <0.0001.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL via the i.n. or i.p. route or with MCMVWT via the i.n. route. During latency (> 3 months p.i), MCMVIVL (i.n.) immunized mice were administered with 10 μg αCD8 or 10 μg IgG2b antibody (i.n.). MCMVIVL (i.p.) and MCMVWT (i.n.) immunized mice were administered with 10 μg IgG2b antibody (i.n.). One day later, animals were challenged with IAV (PR8) (i.n., 1100 FFU). On day 2 and day 4 post-challenge, BALF was harvested and measured cytokines production by bio-plexing. The concentration of (A) IFNγ and (B) IL-6 in the BALF on day 4 post-challenge. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. (C) Cytokine concentrations in the BALF in different immunization group on day 2 and day 4 post-challenge. Bars indicate means, error bars are SEM. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Significance was assessed by One-way ANOVA test. *P <0.05, ***P <0.001.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. or i.p. route or with MCMVWT by the i.n. route. During latency (> 3 months p.i), mice were challenged with IAV (PR8) (i.n., 1100 FFU) one day after airway CD8+ T cell depletion. On day 4 post-challenge, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from lung, BAL, blood and spleen. (A-B) Count of IVL-specific CD8+ T cells in the lungs (A) and BAL (B). (C-D) Percentage of IVL-specific CD8+ T cells among CD8+ T cells in the blood (C) and spleen (D). (E-F) Count of IVL-specific CD8+ T cell in the blood (E) and spleen (F). Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown. Significance was assessed by One-way ANOVA test. *P <0.05, **P <0.01.
(TIF)
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL by the i.n. route. During latency (> 3 months p.i), mice were challenged with IAV (PR8) (i.n., 1100 FFU) one day after airway CD8+ T cell depletion. On day 2 and day 4 post-challenge, anti-CD45 antibodies were injected intravenously 3–5 min before mice euthanasia. Leukocytes were isolated from lungs and BAL. CD4+ T cell numbers in the lungs are shown. (A) The number of total CD4+ T cells. (B) The number of CD45- CD4+ T cells. (C) The number of CD4+ T cells in the BAL. Two independent experiments were performed and pooled data are shown. Each symbol represents one mouse, n = 5–7. Group means +/- SEM are shown.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.