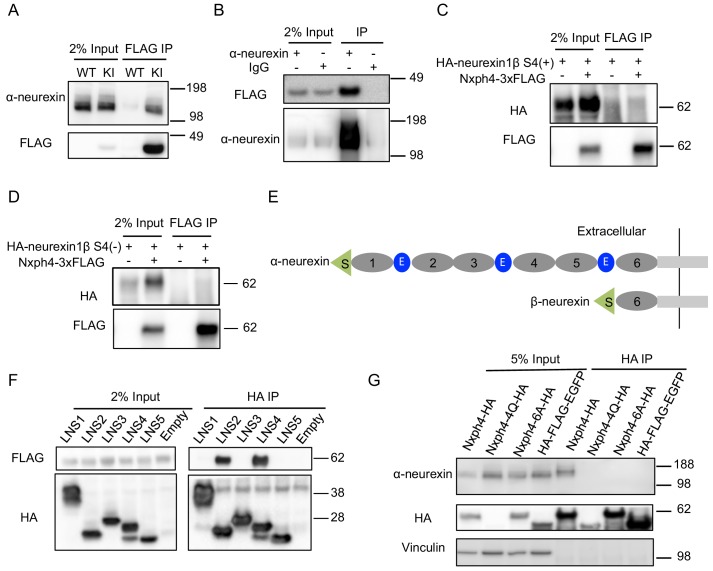
Figure 4. Nxph4 forms a complex with α-neurexin in vivo.
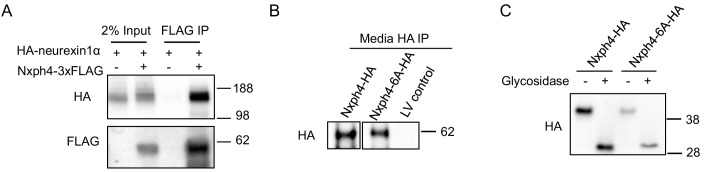
(A) Synaptosomes (tissue used: olfactory bulb, hypothalamus, midbrain, hindbrain, and the cerebellum) from Nxph4-3xFLAG KI or WT (negative control) mice were precipitated with an antibody against FLAG. Bound proteins as well as 2% input were analyzed by immunoblotting with anti-FLAG and anti-α-neurexin antibodies as indicated. (B) Brain lysates from Nxph4-3xFLAG KI mice were precipitated with an anti-α-neurexin antibody. Elution and 2% input were analyzed by immunoblotting with anti-α-neurexin and anti-FLAG antibodies. IgG was used as negative control. (C, D) Nxph4-3xFLAG and HA-neurexin1β S4(+) (with the insertion of splicing site 4, C) or HA-neurexin1β S4(-) (without the insertion of splicing site 4, D) were co-expressed in HEK293T cells. Cell lysates were precipitated with an anti-FLAG antibody. Bound proteins were analyzed by immunoblot showing pulling down of Nxph4-3xFLAG but not HA-neurexin1β. Cells transfected with HA-neurexin1β alone were used as negative control. (E) Schematic drawing of the extracellular domain structure of α- and β-neurexins. α-neurexin contains 6 LNS domains interspersed by 3 EGF-like repeats. β-neurexin has a single LNS6 domain. S: signal peptide; 1–6: LNS1-6; E: EGF-like domain. (F) Nxph4-3xFLAG was co-expressed with individual α-neurexin specific LNS domains in HEK293T cells. Culture media was precipitated by an anti-FLAG antibody. LNS2 and LNS4 were co-precipitated with Nxph4-3xFLAG. (G) Cultured primary cortical neurons overexpressing wild type or mutant Nxph4-HA were subjected to co-IP with an anti-HA antibody. Elution and 5% input were analyzed by immunoblotting with anti-HA, anti-α-neurexin, and anti-vinculin antibodies. Wild type and Nxph4 mutants were fused with mCherry. Nxph4-4Q-HA was not detectable in 5% input and was only detected as a faint band in the IP sample.