Abstract
Angiotensin processing peptidases (carboxypeptidase A (CPA) and chymase) are stored in cardiac mast cell (MC) secretory granules in large quantity and are co-released into the extracellular environment after activation/degranulation. In the human heart, chymase is primarily responsible for angiotensin II (Ang II) generation from the alternate substrate angiotensin-(1–12) (Ang-(1–12)). We investigated the individual and combined hydrolytic specificity of CPA and chymase enzymes (1:1 and 1:⅓ ratio) in the processing of the human Ang-(1–12) (hAng-(1–12)) substrate. To determine the Km and Vmax, the CPA and re-combinant human chymase (rhChymase) enzymes were incubated with increasing concentrations of hAng-(1–12) substrate (0–300 μM). We found that CPA alone sequentially metabolized hAng-(1–12) substrate into angiotensin-(1–9) (Ang-(1–9), 53%), Ang II (22%) and angiotensin-(1–7) (Ang-(1–7), 11%) during a 15min incubation. In the presence of rhChymase alone, 125I-hAng-(1–12) was directly metabolized into Ang II (89%) and no further hydrolysis of Ang II was detected. In the presence of both CPA + rhChymase enzymes (1:1 or 1:⅓ ratio), the amount of Ang II formation from 125I-hAng-(1–12) within a 5 min incubation period were 68% or 65%, respectively. In the presence of both (CPA + rhChymase), small amounts of Ang-(1–9) and Ang-(1–7) were generated from 125I-hAng-(1–12). The Km and Vmax values were 150 ± 5 μM and 384 ± 23 nM/min/mg of CPA and 40 ± 9 μM and 116 ± 20 nM/min/mg of rhChymase. The catalytic efficiency (Vmax/Km ratio) was higher for rhChymase/hAng-(1–12) compared to CPA/hAng-(1–12). Compared to CPA, chymase has a much higher affinity to hydrolyze the hAng-(1–12) substrate directly into Ang II. In addition, Ang II and Ang-(1–7) are the end products of chymase and CPA, respectively. Overall, our findings suggest that the Ang II generation from hAng-(1–12) is primarily mediated by chymase rather than CPA.
Keywords: Mast cell protease, Carboxypeptidase A, Chymase, Angiotensin-(1–12), Angiotensin I, Angiotensin II, Metabolism, Renin-angiotensin system, Angiotensinogen
1. Introduction
Mast cells (MCs) are the immune cells having abundance of electron-dense secretory granules filled with large amounts of preformed powerful chemical compounds called MC mediators. In addition to these mediators, MC secretory granules also store a number of MC-specific proteases including tryptase, chymase and carboxypeptidase A (CPA). Chymase and CPA proteases are stored in remarkably high amounts in human MC secretory granules (4.5 and 16 pg per MC in adult foreskin, respectively) [1,2]. MCs are present throughout the connective and mucosal tissues of the body. MCs involvement in the pathophysiology of cardiovascular disorders continues to receive attention among the scientific community [3–5]. Increased numbers of cardiac MCs have been found in adversed myocardial remodeling of animal models as well as human heart tissues with coronary spasm, idiopathic dilated, ischemic cardiomyopathy and at the inflammatory sites in other diseases [6–12].
Based on the content of neutral proteases in secretary granules, two subtypes of MCs have been recognized in human tissues: tryptase-positive MCs (MCT) and MCTC, which is positive for tryptase in combination with chymase, CPA and cathepsin G [1,13]. A single α-form of chymase has been found in human MCs [14]. Whereas human tissues contain several types of CPA (CPA1 to CPA6) that have diverse functions ranging from catabolism to protein maturation [15–18]. CPA1 and CPA2 are pancreatic exopeptidases. CPA3 (originally called MC-CPA) has only been detected in MC and MC-like cell lines [17]. CPA4, previously reported as CPA3 by Huang et al. [16] was renamed by a gene nomenclature committee in the order of their discovery. Both CPA4 and CPA5 have not been well studied. CPA6 is broadly expressed in many tissues and has been linked to Duane syndrome [19,20].
Various studies show the existence of additional angiotensin substrates upstream of Ang I and increased expression of angiotensin processing enzymes in human cardiovascular and car-diometabolic diseased tissues [21–26]. The existence of an extended form of Ang I, the dodecapeptide angiotensin-(1–12), which serves as a primary substrate for Ang II formation, and the co-existence of hydrolytic proteases in human atrial appendage tissue radically altered our understanding of Ang II production [22]. Our research clearly established that chymase is primarily responsible for direct Ang II generation from the alternate substrate Ang-(1–12) in addition to Ang I in human and rodents [22–24,27,28]. Compared to Ang I, an increased level of Ang-(1–12) was detected in rat tissues [29]. Another study shows the expression of Ang-(1–12) was higher in spontaneously hypertensive rat heart and kidney tissues compared to corresponding normotensive Wistar-Kyoto rats [30].
MC proteases (chymase and CPA) are stored in their fully active form but they have no functional effects as long as they are confined within the MC. Activation and degranulation of MCs lead to the massive release of proteases into the extracellular environment, which might have a major impact on MC-driven cardiovascular disease development and progression [31,32]. Since CPA is also released along with chymase from the MC into the extracellular environment after activation/degranulation, we investigated the individual and combined effects of CPA and rhChymase in the processing of human Ang-(1–12) (hAng-(1–12)) substrate. Our findings show that CPA and rhChymase hydrolyze the hAng-(1–12) substrate differently. Compared to CPA, rhChymase has a higher substrate affinity and catalytic efficiency for hAng-(1–12) to generate Ang II.
2. Materials and Methods
2.1. Reagents
All custom-made angiotensin peptides (hAng-(1–12), Ang I, Ang-(1–9), Ang II, and Ang-(1–7); purity >98%) were purchased from GenScript USA Inc. (Piscataway, NJ). CPA and recombinant human chymase (rhChymase) enzymes were purchased from Sigma-Aldrich Co. (St. Louis, MO). 125I was purchased from Perki-nElmer Life and Analytical Sciences, Inc. (Waltham, Massachusetts). All other chemicals used in this study were of analytical grade and were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Atlanta, GA).
2.2. Radiolabeling of hAng-(1–12) peptide and HPLC purification
hAng-(1–12) peptide {DRVYIHPFHLVI} was radiolabeled with 125I at the Tyrosine 4th position using oxidant chloramine-T and purified on a C18 column by reverse-phase high performance liquid chromatography (HPLC), as previously described [23].
2.3. Hydrolysis of 125I-hAng-(1–12) substrate by CPA and rhChymase enzymes
Metabolic products of 125I-hAng-(1–12) by CPA and rhChymase enzymes were analyzed by HPLC. Briefly, in a 200 μL reaction volume highly purified radiolabeled human 125I-Ang-(1–12) substrate (~50 fmoles, specific activity 3900 cpm/fmol, purity ≥98%) was incubated with CPA (0.325 μg/mL), rhChymase (0.325 μg/mL) or a combination of CPA + rhChymase (1:1 or 1:⅓ ratio) in 50 mM Tris-HCl buffer solution containing 150 mM NaCl (pH 8.0) for 15 min (5 min for combination experiments) at 37 °C. The enzymatic re-actions were stopped by adding an equal volume of 1% phosphoric acid and centrifuged at 28,000 g for 10 min. The 125I-hAng-(1–12) products were separated by HPLC on a C18 column using a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/min at 32 °C. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). The eluted 125I-hAng-(1–12) products were monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC). Products were identified by comparison of retention times of synthetic (125I) standard Ang peptides and the data were analyzed with Shimadzu LCSolution (Kyoto, Japan) acquisition software.
2.4. Km and Vmax of CPA and rhChymase for hAng-(1–12) substrate
To determine the Km and Vmax of cardiac CPA and rhChymase enzymes for hAng-(1–12) substrate, CPA (0.325 μg/mL) or rhChymase (0.325 μg/mL) were incubated with increasing concentrations (0–300 μM) of non-radiolabeled hAng-(1–12) substrate in 200 μL of 50 mM Tris-HCl buffer solution containing 150 mM NaCl (pH 8.0) at 37 °C for 20 min. The hydrolytic products generated from the hAng-(1–12) substrate by CPA and rhChymase were separated by HPLC connected to UV-detector as described above and eluted fractions were monitored as the absorbance at 215 nm. The concentrations of hAng-(1–12) products were determined using a standard curve of angiotensin synthetic peptides. The Km and Vmax of CPA and rhChymase for hAng-(1–12) substrate were calculated using the Michaelis-Menten equation.
2.5. Statistical analysis
Experiments were repeated three or more times. Data were analyzed using GraphPad Prism 7.0 software (San Diego, CA) and are presented as mean ± SEM.
3. Results
As shown in Fig. 1, a highly pure (purity ≥98%) radiolabeled 125I hAng-(1–12) substrate was used in CPA and rhChymase-mediated hydrolysis studies. The purity of 125I-hAng-(1–12) was routinely checked on the HPLC to make sure that the radiolabeled substrate was not degraded at the time it was used for enzymatic hydrolysis.
Fig. 1.
HPLC Chromatogram of Purified 125I-hAng-(1–12). The HPLC chromatogram shows the 125I-hAng-(1–12) substrate was highly pure (purity ≥98%).
Fig. 2 illustrates the HPLC chromatogram of the hydrolytic products generated from 125I-hAng-(1–12) substrate by CPA or rhChymase. We found that CPA sequentially metabolized the 125I-hAng-(1–12) into Ang-(1–9) (53% (major product)), Ang II (22%) and Ang-(1–7) (11%) (Fig. 2A). rhChymase directly metabolized the 125I-hAng-(1–12) substrate into Ang II (89%) and no further hydrolysis of Ang II was detected (Fig. 2B). Ang II (68%) was the major product generated from hAng-(1–12) when the substrate was incubated with both CPA + rhChymase (1:1 ratio) for 5 min (Fig. 3A). In addition to Ang II, a small amount of Ang-(1–9) and Ang-(1–7) products (11% and 10%, respectively) were also detected in the reaction mixture. When CPA and rhChymase were incubated with hAng-(1–12) substrate at 1:⅓ ratio for 5 min, Ang II was still a major product (65%) (Fig. 3B). Ang-(1–9) and Ang-(1–7) production from 125I-hAng-(1–12) amounted to only 27% and 7%, respectively (Fig. 3B). To determine Ang-(1–9) hydrolysis by rhChymase, human 125I-hAng-(1–12) substrate was first incubated with CPA (0.325 μg/mL) for 5 min, next the CPA activity was stopped by adding 50 μM of benzylsuccinate [19], and then this reaction mixture was further incubated with rhChymase (0.325 μg/mL) for an additional 5 min. In these experiments, we found that the hydrolytic product of CPA “Ang-(1–9)” was not metabolized by rhChymase (data not shown).
Fig. 2.
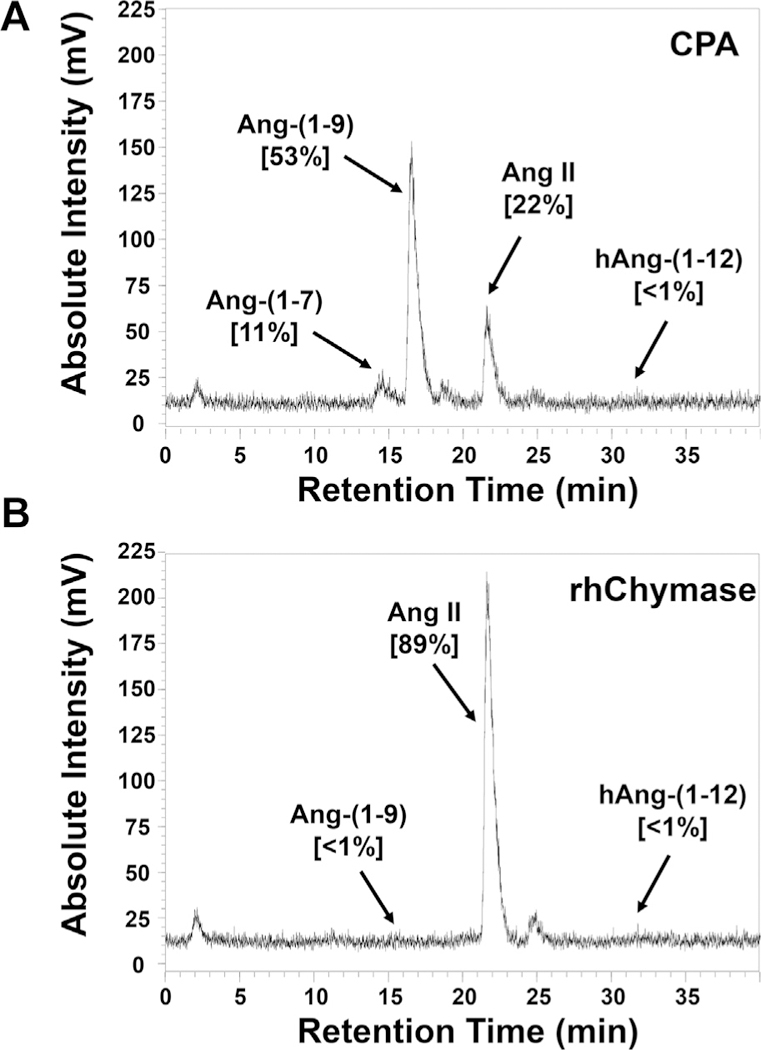
Hydrolysis of 125I-hAng-(1–12) by CPA or rhChymase alone. The HPLC chromatogram shows the 125I-Ang products generated from 125I-hAng-(1–12) substrate by (A) CPA or (B) rhChymase. Results are representative of three or more separate experiments.
Fig. 3.
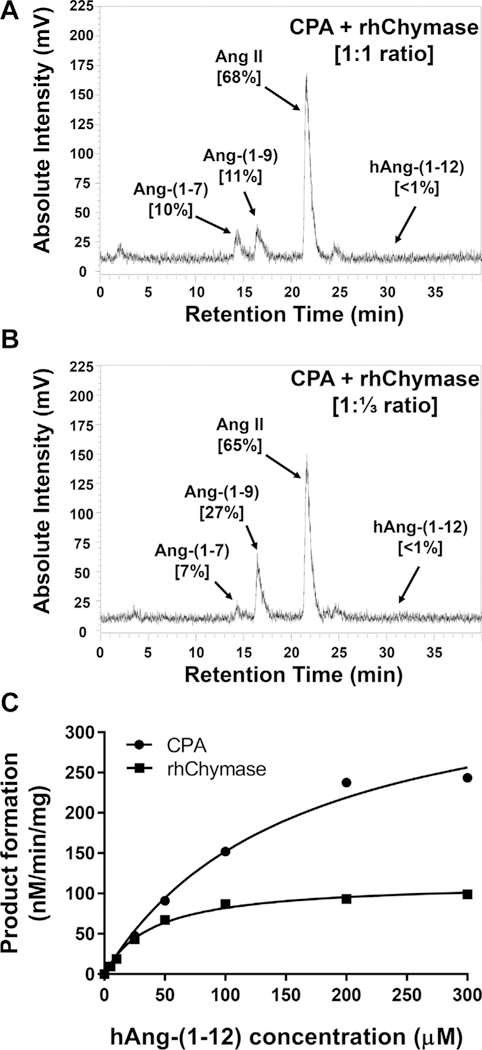
Hydrolysis of 125I-hAng-(1–12) by CPA + rhChymase (1:1 or 1:⅓ ratio). The HPLC chromatogram shows the 125I-Ang products generated from 125I-hAng-(1–12) substrate by a combination of CPA + rhChymase (A) 1:1 ratio or (B) 1:⅓ ratio. (C) Kinetics (Km and Vmax) of CPA and rhChymase for hAng-(1–12) substrate. Results are representative of three or more separate experiments described in the Materials and Methods.
Kinetic analysis (Km, Vmax and catalytic efficiency) of CPA and rhChymase enzymes for hAng-(1–12) substrate was also determined. A representative curve showing the hydrolytic products generated by CPA and rhChymase with increasing concentrations of hAng-(1–12) substrate is shown in Fig. 3C. The Km and Vmax were 150 ± 5 μM and 384 ± 23 nM/min/mg of CPA/hAng-(1–12) and 40 ± 9 μM and 116 ± 20 nM/min/mg of rhChymase/hAng-(1–12) reactions, respectively (Table 1). The catalytic efficiency (the ratio of Vmax/Km ratio) was higher for rhChymase/hAng-(1–12) (2.97 ± 0.1) compared to CPA/hAng-(1–12) (2.56 ± 0.1).
Table 1.
Enzyme Kinetics (Km, Vmax and Vmax/Km) of CPA and rhChymase for hAng-(1–12) Substrate.
| Enzyme Kinetics | CPA | rhChymase |
|---|---|---|
| Km (μM) | 150 ± 5 | 40 ± 9 |
| Vmax (nM/min/mg) | 384 ± 23 | 116 ± 20 |
| Vmax/Km ratio (Catalytic efficiency) | 2.56 ± 0.1 | 2.97 ± 0.1 |
Results are representative of three or more separate experiments. Details of the kinetic analysis were described in Materials and Methods.
4. Discussion
The discovery of an Ang I upstream precursor (Ang-(1–12)), which serves as an alternate substrate for biologically active Ang II formation in the human heart, radically altered our understanding of Ang II production [22]. Our laboratory has done pioneer work in unraveling the complexity of the biotransformation pathways that account for the formation of the Ang II hormone from angiotensinogen [24–26,33–37]. Although chymase-mediated Ang II formation from Ang I has a long-standing history in humans [38,39], the clinical importance of the Ang II-forming pathways from hAng-(1–12)/MC proteases axis is largely unknown. To date, the specific role of MC CPA in hAng-(1–12) substrate hydrolysis has not been demonstrated. In this study, we investigated the individual and combined effects of two MC proteases to hydrolyze the hAng-(1–12) substrate.
Incubation of CPA with hAng-(1–12) substrate for 15 min at 37 °C, yielded the generation of Ang-(1–9), Ang II and Ang-(1–7) and no further hydrolysis was detected. rhChymase rapidly generates Ang II from hAng-(1–12) and no further hydrolysis was detected. In the presence of both CPA and rhChymase (1:1 and 1:⅓ ratio), we found that hAng-(1–12) was rapidly metabolized into Ang II within 5 min. In both conditions, relatively small amounts of Ang-(1–9) and Ang-(1–7) products were generated. Although hAng-(1–12) is rapidly metabolized into Ang II directly, our current investigation shows that CPA-mediated Ang-(1–9) production has negligible substrate affinity for rhChymase enzyme to generate Ang II. These findings clearly indicate that hAng-(1–12) is primarily hydrolyzed by chymase into Ang II. Furthermore, the enzyme kinetic results confirm the specificity and primacy of chymase over CPA to generate directly Ang II product from hAng-(1–12) substrate.
The classical view of the biochemical pathways for the formation of biologically active angiotensin peptides continues to undergo significant revision as new data uncovers the existence of important alternate non-renin dependent mechanisms of Ang II formation from the novel dodecapeptide Ang-(1–12) by MC proteases. In contrast to rodents, humans only have the α-form of chymase (a chymotryptic serine endopeptidase) stored in MC secretory granules in large amounts [14]. The MC CPA resembles bovine pancreatic CPA in cleaving C-terminal aromatic and aliphatic amino acid residues [2]. CPAs cleave newly exposed C-terminal residues after endopeptidase cleavage by chymase, thereby sequentially degrading common proteins and peptides substrates [40,41]. Our recent findings suggest that the CPA has a low affinity to cleave the newly exposed C-terminal amino acid of Ang II generated from hAng-(1–12) hydrolysis by rhChymase. Once Ang-(1–9) is cleaved by CPA from hAng-(1–12), chymase has negligible substrate affinity to hydrolyze the Phe8-His9 bond of the Ang-(1–9) to generate Ang II product (Fig. 4).
Fig. 4.
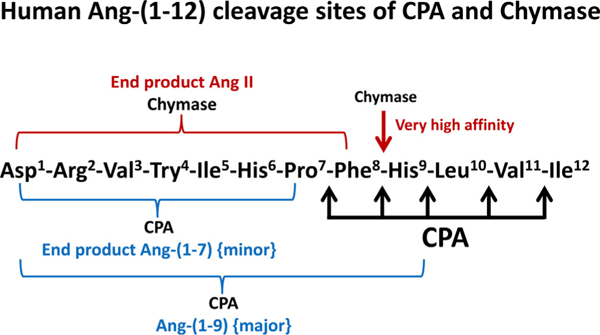
Human Ang-(1–12) Cleavage Sites of CPA and rhChymase. CPA sequentially cleaves the C-terminal end amino acids (Ile, Val, Leu, His and Phe) of hAng-(1–12). Chymase is an endopeptidase that cleaves one Phe8-His9 bond of the hAng-(1–12) substrate.
The hydrolytic potential of an enzyme depends on the amino acid sequence of the peptides/proteins at the cleavage site. In Table 2, we document the characteristic differences between chymase and CPA enzymes. CPA1 and CPA2 isolated from rat mesenteric arterial bed perfusate (identical with their pancreatic counterparts), hydrolyze rat Ang-(1–12) substrate differently [42]. CPA1 has negligible affinity to hydrolyze the C-terminal amino acid of the rat Ang-(1–12) sequence. However, CPA2 rapidly hydrolyzes the rat Ang-(1–12) into Ang I. Further hydrolysis of Ang I into Ang-(1–9) by CPA2 is negligible. The amino acids sequence of the rat and hAng-(1–12) are different (rat sequence, Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12 and human sequence, Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Val11-Ile12).In humans, -Val11-Ile12 is present at the C-terminal end, whereas in the rat, -Leu11-Tyr12 exists at the C-terminus [24]. Both hAng-(1–12) and Ang I substrates have high affinity for human cardiac chymase to generate Ang II. We showed for the first time that rat cardiac chymase has a much higher substrate affinity for rat Ang-(1–12) substrate compared to Ang I to generate Ang II [33]. Our current findings show that CPA hydrolyzes the hAng-(1–12) substrate into Ang-(1–9), Ang II and Ang-(1–7) products. No intermediate products (Ang-(1–11) and Ang I) were detected in the reaction mixture suggesting that CPA sequentially cleaves the hAng-(1–12) C-terminal three peptide bonds (Val11-Ile12, Leu10-Val11 and His9-Leu10) rapidly. Once Ang-(1–9) is generated (after the cleavage of C-terminal His9-Leu10 bond of Ang I) by CPA, the formation of Ang II and Ang-(1–7) is markedly delayed, indicating that CPA has less substrate affinity for Ang-(1–9) to generate Ang II, and an even further decrease for Ang II to generate Ang-(1–7) (Phe8-His9 and Pro7-Phe8 bonds, respectively).
Table 2.
Characteristics of human chymase and CPA.
| Chymase | CPA |
|---|---|
| - Single α-form of chymase is present in human MC. | - Six different isoforms of CPA (CPA-1 to CPA-6) is present in human tissues. |
| - Belongs to serine endopeptidase of family S1. | - Belongs to Zinc-metallocarboxypeptidase family. |
| - High affinity to hydrolyze Phe-His bond. | - Sequentially hydrolyzes aliphatic amino acids and aromatic amino acids from C-terminus. Low affinity for His. |
| - α-Chymase has high affinity for both Ang I and hAng-(1–12) substrates to directly generate Ang II. | - High affinity for hAng-(1–12)/Ang I to generate Ang-(1–9) sequentially. |
| - α-Chymase has low/negligible affinity to hydrolyze Ang-(1–9) into Ang II. | - Low affinity to hydrolyze Ang-(1–9) into Ang II, and subsequently Ang II into Ang-(1−7). |
| - Enzyme content in human adult foreskin (4.5 μg/106 MC). | - Enzyme content in adult foreskin (16 μg/106 MC). |
CPA3 (the MC CPA) shares significant homology with the other CPA subfamilies. CPA3 resembles the pancreatic CPA1 in cleaving the C-terminal end of aromatic (Phe, Tyr and Trp) and aliphatic (Ala, Leu, Ile and Val) amino acids. CPA3 functions together with endo-peptidases (chymases and tryptases) secreted from mast cells to degrade proteins and peptides, including Ang I [41,43]. CPA3 may be involved in host defense against certain parasites, snake venom toxins, and the vasoconstricting peptide endothelin 1 [44–46]. CPA3 is upregulated, making it a potential diagnostic parameter in allergic inflammation and/or autoimmune disease models [47–50]. CPA3 mRNA was not detected in normal human tissues (including lung, heart, and kidney) but its expression could be induced in disease [16]. Our studies clearly show that both chymase and Ang-(1–12) substrate were predominantly expressed intracellularly in human atrial cardiac myocytes obtained from diseased patients [22]. The expression and precise role of CPA in diseased hearts remains to be established.
Overall, our study suggests that Ang II generation from hAng-(1–12) substrate was primarily mediated by chymase rather than CPA. Our studies also suggest that selective inhibition of chymase may provide greater benefit in the management of adverse cardiac remodeling than the MC CPA therapeutic approaches.
Acknowledgment
This work was supported by grant from the National Heart, Blood, Lung Institute of the National Institutes of Health (P01 HL-051952).
Footnotes
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- [1].Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB, Human mast cell carboxypeptidase. Selective localization to MCTC cells, J. Immunol. 147 (1991) 247–253. [PubMed] [Google Scholar]
- [2].Goldstein SM, Kaempfer CE, Kealey JT, Wintroub BU, Human mast cell carboxypeptidase. Purification and characterization, J. Clin. Investig. 83 (1989) 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu H, Melendez GC, Levick SP, Janicki JS, Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype, Am. J. Physiol. Heart Circ. Physiol. 302 (2012) H811–H817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP, Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells, Cardiovasc. Res. 92 (2011) 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Espinosa E, Valitutti S, New roles and controls of mast cells, Curr. Opin. Immunol. 50 (2018) 39–47. [DOI] [PubMed] [Google Scholar]
- [6].Stewart JA Jr., Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell’Italia LJ, Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog, J. Mol. Cell. Cardiol. 35 (2003) 311–319. [DOI] [PubMed] [Google Scholar]
- [7].Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS, Cardiac mast cells: the centrepiece in adverse myocardial remodelling, Car-diovasc. Res. 89 (2011) 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G, Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy, Circulation 97 (1998) 971–978. [DOI] [PubMed] [Google Scholar]
- [9].Forman MB, Oates JA, Robertson D, Robertson RM, Roberts LJ 2nd, Virmani R, Increased adventitial mast cells in a patient with coronary spasm, N. Engl. J. Med. 313 (1985) 1138–1141. [DOI] [PubMed] [Google Scholar]
- [10].Rathod S, Raj A, Wanikar I, Quantitative analysis of mast cell count and density in chronic periodontal disease, J. Indian Soc. Periodontol. 22 (2018) 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hodges K, Kennedy L, Meng F, Alpini G, Francis H, Mast cells, disease and gastrointestinal cancer: a comprehensive review of recent findings, Transl. Gastrointest. Cancer 1 (2012) 138–150. [PMC free article] [PubMed] [Google Scholar]
- [12].Fischer M, Harvima IT, Carvalho RF, Moller C, Naukkarinen A, Enblad G, Nilsson G, Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion, J. Clin. Investig. 116 (2006) 2748–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ahn K, Takai S, Pawankar R, Kuramasu A, Ohtsu H, Kempuraj D, Tomita H, Iida M, Matsumoto K, Akasawa A, Miyazaki M, Saito H, Regulation of chymase production in human mast cell progenitors, J. Allergy Clin. Immunol. 106 (2000) 321–328. [DOI] [PubMed] [Google Scholar]
- 14.Caughey GH, Zerweck EH, Vanderslice P, Structure, chromosomal assignment, and deduced amino acid sequence of a human gene for mast cell chymase, J. Biol. Chem. 266 (1991) 12956–12963. [PubMed] [Google Scholar]
- 15.Pejler G, Knight SD, Henningsson F, Wernersson S, Novel insights into the biological function of mast cell carboxypeptidase A, Trends Immunol. 30 (2009) 401–408. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Reed CP, Zhang JS, Shridhar V, Wang L, Smith DI, Carboxy-peptidase A3 (CPA3): a novel gene highly induced by histone deacetylase inhibitors during differentiation of prostate epithelial cancer cells, Cancer Res. 59 (1999) 2981–2988. [PubMed] [Google Scholar]
- 17.Reynolds DS, Gurley DS, Austen KF, Cloning and characterization of the novel gene for mast cell carboxypeptidase A, J. Clin. Investig. 89 (1992) 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds DS, Gurley DS, Stevens RL, Sugarbaker DJ, Austen KF, Serafin WE, Cloning of cDNAs that encode human mast cell carboxypeptidase A, and comparison of the protein with mouse mast cell carboxypeptidase A and rat pancreatic carboxypeptidases, Proc. Natl. Acad. Sci. U. S. A. 86 (1989) 9480–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons PJ, Callaway MB, Fricker LD, Characterization of carboxypeptidase A6, an extracellular matrix peptidase, J. Biol. Chem. 283 (2008) 7054–7063. [DOI] [PubMed] [Google Scholar]
- 20.Lyons PJ, Fricker LD, Substrate specificity of human carboxypeptidase A6, J. Biol. Chem. 285 (2010) 38234–38242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua Y, Nair S, Proteases in cardiometabolic diseases: pathophysiology, molecular mechanisms and clinical applications, Biochim. Biophys. Acta 1852 (2015) 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM, Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue, PLoS One 6 (2011), e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, Ferrario CM, Chymase mediates angiotensin-(1–12) metabolism in normal human hearts, J. Am. Soc. Hypertens. 7 (2013) 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’italia LJ, An evolving story of angiotensin-II-forming pathways in rodents and humans, Clin. Sci. (Lond.) 126 (2014) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ, Intracrine angiotensin II functions originate from noncanonical pathways in the human heart, Am. J. Physiol. Heart Circ. Physiol. 311 (2016) H404–H414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ, Ferrario CM, Novel cardiac intracrine mechanisms based on Ang-(1–12)/chymase Axis require a revision of therapeutic approaches in human heart disease, Curr. Hypertens. Rep. 19 (2017) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND, Ferrario CM, Angiotensin-(1–12): a chymase-mediated cellular angiotensin II substrate, Curr. Hypertens. Rep. 16 (2014), 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM, Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes, PLoS One 6 (2011), e15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K, Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system, Biochem. Biophys. Res. Commun. 350 (2006) 1026–1031. [DOI] [PubMed] [Google Scholar]
- 30.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM, Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats, Am. J. Physiol. Heart Circ. Physiol. 294 (2008) H2614–H2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pejler G, Ronnberg E, Waern I, Wernersson S, Mast cell proteases: multi-faceted regulators of inflammatory disease, Blood 115 (2010) 4981–4990. [DOI] [PubMed] [Google Scholar]
- 32.Xing D, Zhang R, Li S, Huang P, Luo C, Hei Z, Xia Z, Gan X, Pivotal role of mast cell carboxypeptidase A in mediating protection against small intestinal ischemia-reperfusion injury in rats after ischemic preconditioning, J. Surg. Res. 192 (2014) 177–186. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, Ferrario CM, Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme, Biochem. Biophys. Res. Commun. 478 (2016) 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrario CM, New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I meta-bolism, Hypertension 55 (2010) 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrario CM, Cardiac remodelling and RAS inhibition, Ther. Adv. Cardiovasc. Dis. 10 (2016) 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A, Tallant EA, Angiotensin-(1–7): a new hormone of the angiotensin system, Hypertension 18 (1991). III126–133. [DOI] [PubMed] [Google Scholar]
- 37.Ferrario CM, Flack JM, Pathologic consequences of increased angiotensin II activity, Cardiovasc. Drugs Ther. 10 (1996) 511–518. [DOI] [PubMed] [Google Scholar]
- 38.Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A, Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart, J. Clin. Investig. 91 (1993) 1269–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz JN, Chymase: the other ACE? Am.J. Physiol. Renal. Physiol. 298 (2010) F35–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokkonen JO, Kovanen PT, Proteolytic enzymes of mast cell granules degrade low density lipoproteins and promote their granule-mediated uptake by macrophages in vitro, J. Biol. Chem. 264 (1989) 10749–10755. [PubMed] [Google Scholar]
- 41.Kokkonen JO, Vartiainen M, Kovanen PT, Low density lipoprotein degradation by secretory granules of rat mast cells. Sequential degradation of apolipoprotein B by granule chymase and carboxypeptidase A, J. Biol. Chem. 261 (1986) 16067–16072. [PubMed] [Google Scholar]
- 42.Pereira HJ, Souza LL, Costa-Neto CM, Salgado MC, Oliveira EB, Carboxy-peptidases A1 and A2 from the perfusate of rat mesenteric arterial bed differentially process angiotensin peptides, Peptides 33 (2012) 67–76. [DOI] [PubMed] [Google Scholar]
- 43.Lundequist A, Tchougounova E, Abrink M, Pejler G, Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II, J. Biol. Chem. 279 (2004) 32339–32344. [DOI] [PubMed] [Google Scholar]
- 44.Sanglas L, Aviles FX, Huber R, Gomis-Ruth FX, Arolas JL, Mammalian metallopeptidase inhibition at the defense barrier of Ascaris parasite, Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ, Mast cells can enhance resistance to snake and honeybee venoms, Science 313 (2006) 526–530. [DOI] [PubMed] [Google Scholar]
- 46.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ, Mast cells promote homeostasis by limiting endothelin-1-induced toxicity, Nature 432 (2004) 512–516. [DOI] [PubMed] [Google Scholar]
- 47.Benoist C, Mathis D, Mast cells in autoimmune disease, Nature 420 (2002) 875–878. [DOI] [PubMed] [Google Scholar]
- 48.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV, Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma, J. Allergy Clin. Immunol. 125 (2010) 1046–1053 e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, Grammer LC, Tan BK, Chandra RK, Conley DB, Kern RC, Fujieda S, Schleimer RP, Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps, J. Allergy Clin. Immunol. 130 (2012) 410–420, e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo XJ, Wang YY, Zhang HY, Jin QQ, Gao CR, Mast cell tryptase and carboxypeptidase A expression in body fluid and gastrointestinal tract associated with drug-related fatal anaphylaxis, World J. Gastroenterol. 21 (2015) 13288–13293. [DOI] [PMC free article] [PubMed] [Google Scholar]


