Abstract
A new optical contrast agent has been developed by exposing dye-loaded microbubbles to a rapidly-cooled thermal treatment to homogenize the dye distribution across the surface. Ultrasound causes these microbubbles to oscillate in size which changes the self-quenching efficiency of the dye molecules creating a “blinking” signal. We demonstrate for the first time that these microbubbles can reproducibly generate second, third, and even fourth harmonic fluorescence intensity modulations in addition to the fundamental frequency of the driving ultrasound. Detecting these harmonic signals could produce a higher signal-to-noise ratio for fluorescence imaging in medical applications by allowing fundamental frequency interference and artifacts to be filtered out.
The main challenge of using photons in the visible light spectrum for biological imaging and medical applications is the high degree of photon scattering that occurs in the tissue [1]. Most optical information about deep tissue comes from the degree of absorption or differential absorption of photons at certain wavelengths of light [2]. However, significant tissue scattering prevents most photons from having a straight line migration [3]. Existing imaging techniques use photons that are produced external to the body and are introduced from the body surface. The photons flood the region of interest and surrounding tissue [4]. These photons must travel down through the tissue of interest and back up to the body surface where they can be detected by an optical sensor. However, it is very difficult to distinguish which photons have actually passed through the region of interest and which photons have traveled entirely through the surrounding tissue.
In contrast to photons, ultrasound energy can be focused tightly in deep tissue [5] and can be pinpointed to a region of choice at depths greater than 1 mm. Using the focused ultrasound to generate fluorescent light from inside the tissue of interest at a known location would help overcome the localization issue of tissue scattering and help recover spatial information.
The production of light within tissue can be accomplished through the use of fluorescent dyes. This takes advantage of the high sensitivity of fluorescence imaging and allows for the use of dyes that are environmentally sensitive in wavelength or intensity [6–8]. The fluorescence generated can be used to provide information about tissue chemical state, such as oxygenation and pH which can be markers for cancer and other diseases [9–12]. Combining the chemical analysis abilities of fluorescent dyes with the spatial precision of focused ultrasound could allow small regions of tissue to be analyzed within the body potentially as deep as the effective focusing depth for ultrasound which can be several centimeters [13].
To use ultrasound to manipulate fluorescent light, we have previously developed a new microbubble-based imaging contrast agent that takes advantage of focused ultrasound pressure waves to produce an intensity-modulated fluorescent signal within the ultrasound focal zone [14, 15]. Because of the compressibility of gas, microbubbles undergo radial size oscillations in response to the compression and rarefaction portions of an ultrasound pulse [16–19]. These microbubbles have a stabilizing lipid coating on the surface which can be loaded with a self-quenching fluorescent dye. As the microbubble expands and contracts the distance between individual fluorescent dye molecules on the surface increases and decreases. This effectively modulates the self-quenching efficiency of the dyes resulting in an intensity modulation of the fluorescent light production and creating a “blinking” signal. The blinking occurs at the same 2.25 MHz frequency as the driving ultrasound, but only in the ultrasound focal zone [14, 15].
Microbubbles have been shown to have oscillation behavior that occurs in addition to the fundamental driving frequency. Passive hydrophone monitoring of microbubbles exposed to ultrasound as well as light-scattering techniques have shown that microbubbles also oscillate at harmonic frequencies which result from asymmetric oscillations [20, 21]. However, harmonics have not yet been reproducibly observed using this new modulated fluorescence technique. Designing microbubbles that are capable of producing modulated fluorescence signals at harmonics above the fundamental driving frequency could increase the signal-to-noise ratio of the detected signals, akin to phase inversion ultrasound imaging [22]. These harmonics would preferentially be produced by the microbubbles, allowing for interference or artifacts from the fundamental frequency to be filtered or canceled out.
In our previous work, the morphology of the lipid and dye on the microbubble surface was manipulated using several thermal treatment techniques to improve the production of fluorescence modulations at the fundamental driving frequency [15]. These thermal treatments are evaluated here to determine their effects on the production of harmonic blinking signals. The microbubbles were prepared and treated with the heating followed by cooling procedure described in Schutt et al. [15]. Microbubbles were premade and surface loaded with DiI dye (Biotium, Inc. CA) (Figure 1a). The transition temperature of the distearoyl phosphatidylcholine lipid shell (Avanti Polar Lipids, Inc. AL) was 56°C so the microbubbles were heated to 75°C to ensure that the lipid and dye partitions would melt and distribute themselves over the microbubble surface by Brownian motion [23]. The microbubbles were separated into five different groups. Each group was treated with a different thermal protocol as described in Table 1.
Fig. 1.
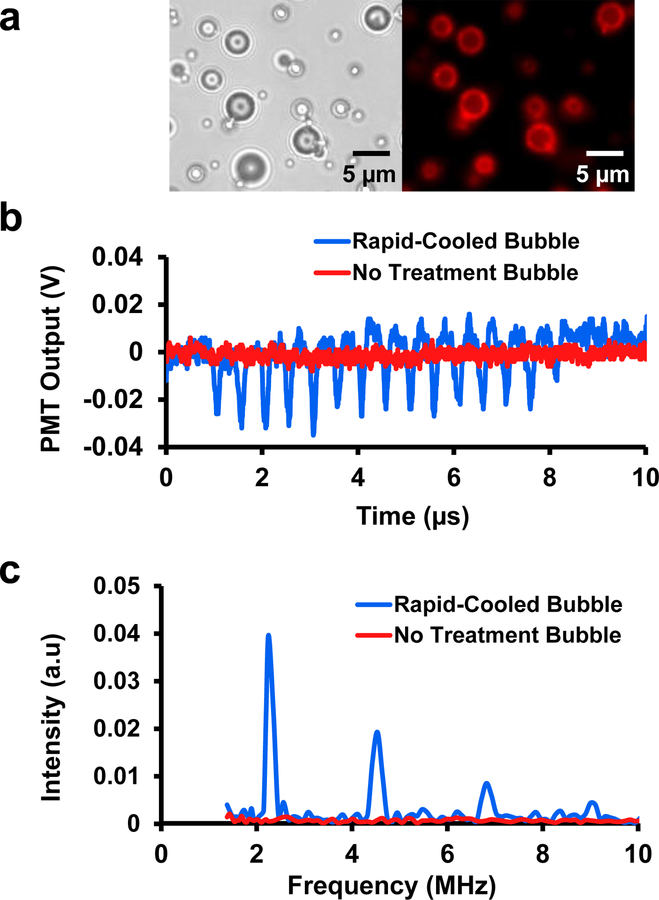
Dye-loaded microbubble images and fluorescence signals acquired from microbubbles. (a) Brightfield (left) and fluorescence (right) images of fabricated lipid-coated microbubbles with self-quenching dye loaded in the lipid shell. (b) Example acquired time domain fluorescence signals from rapidly-cooled (Group 5) and untreated microbubbles (Group 1). The signal from the rapidly-cooled bubble that was heated for 3 minutes at 75°C and cooled for 5 minutes at 0°C shown in blue displays a fluorescence modulation that is not seen in a typical untreated bubble shown in red. (c) Frequency spectra of the acquired fluorescence signals shown in panel b. The rapidly-cooled microbubble signal displays clear peaks at the second, third, and fourth harmonic frequencies of 4.5, 6.75, and 9 MHz. The untreated microbubble signal does not show any clear peak in the frequency spectra at either the fundamental or harmonic frequencies.
Table 1.
Heat treatments used on the microbubble samples to distribute dye across the microbubble surface. The terms “rapid” and “slow” are used in reference to the rate of degrees/minute at which the lipids pass through their transition temperature.
| Group | Heat Treatment | Heat Time | Heat Temp. | Cooling Time | Cooling Temp. |
|---|---|---|---|---|---|
| 1 | No treatment | Washed microbubbles were maintained at room temperature | |||
| 2 | Slow-cooling, short time | 1 min | 75°C | 1 min | 45°C |
| 3 | Rapid-cooling, short time | 1 min | 75°C | 1 min | 0°C |
| 4 | Slow-cooling | 3 min | 75°C | 5 min | 45°C |
| 5 | Rapid-cooling | 3 min | 75°C | 5 min | 0°C |
A custom acousto-optic based detection system using a photomultiplier tube was designed to detect the fluorescence modulation signals and oscillation of the microbubbles [14, 21]. Ultrasound at 2.25 MHz was pulsed at a 1 Hz repetition rate with 15 sine wave cycles per pulse and a pressure of 1.0 MPa. The microbubbles were introduced into the field of view that contained the focal zone of both the optical objective and the ultrasound by injecting fluid with a highly diluted sample of microbubbles into the tank. The microbubbles were significantly diluted so that only one unconstrained microbubble would, on average, be passing through this field of view at any given time. The photomultiplier tube signal was monitored directly and recorded using custom-written LabView data collection software for analysis of harmonic signals when the microbubbles were in a non-scattering media. The 10 µs time period over which the oscillation data was collected did not allow the microbubbles to undergo any significant translations within the field of view including translations caused by the flow speed of the injected stream or any translations that occurred as a result of the acoustic radiation force that the microbubbles likely experienced. Three complete batches of microbubbles were manufactured and tested on three different days. Microbubbles from all five treatment groups were shown to oscillate in size when exposed to the ultrasound [15]. Collected fluorescence signals were scanned to look for second, third, and fourth harmonic peaks at 4.5, 6.75, and 9 MHz, respectively. To be considered a detected signal, the intensity of the peak at these frequencies had to be at least 1.25 times higher than the background level. The signals that met these criteria were tagged and counted for statistical analysis. Statistical significance between treatment conditions was determined using a chi-squared test with post hoc comparisons between groups using the Bonferroni adjustment (p < 0.05).
The use of the thermal treatment techniques to manipulate the lipid distribution in the microbubble shell resulted in microbubbles that produced detectable harmonics in the fluorescence intensity signals. An example of these harmonic signals is shown in Figure 1b and 1c. The rapidly-cooled (Group 5) microbubble displays clear peaks at the second, third, and fourth harmonic frequencies. These peaks are lower in intensity than the peak at the fundamental driving frequency at 2.25 MHz. The untreated microbubble (Group 1) does not show any clear peak in the frequency spectra at either the fundamental or harmonic frequencies.
The percentage of microbubbles that displayed detectable harmonic fluorescence intensity modulations is shown for the different temperature treatment groups in Figure 2.
Fig. 2.
Percentage of microbubbles produced under the different thermal treatment processes that displayed a detectable second, third, and fourth harmonic fluorescence modulation signal. These data combine all the signals acquired from three microbubble batches for all five temperature treatments (combined bubble count data shown in Table 2). The letters above each column indicate statistically significant differences for nonzero groups using a chi-square test with post hoc comparisons between treatments within a harmonic group using the Bonferroni adjustment (p < 0.05). The fourth harmonic signals were relatively rare to observe and did not lend themselves to reliable statistics. The Group 5 rapidly-cooled microbubbles (3 minutes 75°C, 5 minutes 0°C) had a higher percentage displaying harmonic signals than other treatments, especially in the second harmonic.
A summary of this data is shown in Table 2. The Group 5 rapidly-cooled microbubbles (3 minutes 75°C, 5 minutes 0°C) displayed the highest incidence of harmonic signals of the five different heat treatment protocols. The microbubbles that underwent this rapid-cooling treatment had significantly higher percentages of second and third harmonics than other treatments. The rapid-cooling phase of the treatment was able to solidify the lipids into place, preserving the homogeneous distribution of lipid and dye created by the heating phase [15]. The slower cooling rate investigated allowed the lipids and dye to once again partition into isolated islands before eventual solidification [15]. Rapid-cooling with shorter time intervals of heating and cooling (Group 3) was less effective at generating harmonic signals than the rapid-cooling technique using longer time intervals (Group 5) since the shorter heating time was likely insufficient to redistribute the lipids and dye homogenously [15]. The homogeneous distribution created by the rapid-cooling technique (Group 5) allowed the dye molecules to separate sufficiently from one another during the ultrasound-induced microbubble expansion phase to change their self-quenching efficiency, resulting in a significant increase in detectable fluorescence.
Table 2.
Summary of the absolute numbers of microbubbles studied under different thermal treatments that displayed detectable harmonic signal production.
| Treatment Group | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 2nd Harmonic No Modulation | 627 | 482 | 427 | 427 | 317 |
| 2nd Harmonic Modulation | 1 | 8 | 4 | 7 | 35 |
| 3rd Harmonic No Modulation | 628 | 489 | 427 | 424 | 340 |
| 3rd Harmonic Modulation | 0 | 1 | 4 | 10 | 12 |
| 4th Harmonic No Modulation | 628 | 490 | 428 | 433 | 345 |
| 4th Harmonic Modulation | 0 | 0 | 3 | 1 | 7 |
Microbubbles that displayed fourth harmonics were rare, making statistical comparisons unreliable. However, 11 confirmed observations show that fourth harmonic signals can be generated and detected. There were no observed instances of fifth harmonic signals. Figure 3 shows the batch-to-batch variability for the different thermal treatment protocols showing the reproducibility of the harmonic signal generation.
Fig. 3.
Batch-to-batch variability of the percentage of microbubbles displaying detectable harmonic fluorescence modulation for each temperature treatment shows the reproducibility of the harmonic signal generation. Data are shown as the mean fluorescence modulation percentage and standard deviation of three batches of microbubbles prepared and analyzed on separate days.
The dominant effect in creating the harmonic signals is likely from the nonlinear oscillatory behavior of the gas microbubbles. Microbubbles are known to undergo asymmetric oscillations in response to ultrasound which causes the production of harmonics [24]. The self-quenching of the dye also has a nonlinear dependence on the distance between individual dye molecules [14] which can produce very rapid changes in fluorescence intensity as the distance between dye molecules changes. The occurrence of harmonic signals likely comes from the frequency with which the distance between dye molecules is changing which is dictated by the oscillation behavior of the microbubble.
Using the second, third, and fourth harmonic fluorescence intensity-modulated signals for detection may enable a higher signal-to-noise ratio than detecting the fundamental 2.25 MHz driving frequency because signals at 4.5, 6.75, and 9 MHz are only produced at significant intensities by the microbubbles themselves, and would have virtually no acoustic background noise to contend with. Acoustic harmonic signals generated by microbubbles are used in this manner in ultrasonography to improve signal to noise ratios for imaging purposes [20, 25]. Detection of the harmonics would also reduce the potential for interference from other acousto-optic effects. The modulated-fluorescence harmonic signals described in this work could also be exploited to avoid any possible electrical noise from the ultrasound signal generator or other possible effects from the driving ultrasound frequency interacting with tissues in the body. Future work will look to further increase the yield of microbubbles that can display modulated fluorescence harmonic signals by cooling the microbubbles down from the heating temperature at a faster rate to help solidify the dye molecules in the most distributed state possible, as well as incorporating additional lipid components into the lipid monolayer to increase fluidity.
In conclusion, we detected for the first time, to the best of our knowledge, harmonic oscillations in the fluorescence intensity signal generated reproducibly by microbubble-based contrast agents. The microbubbles created by the rapid-cooling (Group 5) treatment had the most homogeneous surface dye distribution of the different treatments tested [15]. These microbubbles were significantly better at translating the asymmetric oscillations of the microbubble into detectable fluorescence intensity modulations at harmonic frequencies than microbubbles that had more partitioned lipid and dye distributions. This was likely because of a more favorable separation distance between dye molecules [15]. The rapid cooling (Group 5) treatment increased the percentage of microbubbles displaying second harmonic fluorescence intensity modulations by 50 times compared to the untreated microbubbles and showed third and fourth harmonic modulations which were not observed in the untreated microbubbles. Our previous research has shown that the rapid-cooling treatment increased the percentage of microbubbles displaying fundamental frequency fluorescence modulations by 12 times, compared to the untreated microbubbles [15]. The rate of cooling and duration of heating are important factors in producing microbubbles with the maximum detectable harmonic fluorescence modulation behavior.
Acknowledgements.
Support was provided by Grant Numbers T32 CA121938 and R25 CA153915 from the National Cancer Institute and from the UCSD Cancer Center Specialized Support Grant P30 CA23100. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Cheong W-F, Prahl SA, and Welch AJ, “A review of the optical properties of biological tissues,” Quant. Elec., IEEE Journal of 26, 2166–2185 (1990). [Google Scholar]
- 2.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, and Butler J, “Noninvasive in vivo characterization of breast tumors using photon migration spectroscopy,” Neoplasia 2, 26–40 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladkova ND, Petrova GA, Nikulin NK, Radenska Lopovok SG, Snopova LB, Chumakov YP, Nasonova VA, Gelikonov VM, Gelikonov GV, and Kuranov RV, “In vivo optical coherence tomography imaging of human skin: norm and pathology,” Skin Res. & Tech 6, 6–16 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Li A, Kwong R, Cerussi A, Merritt S, Hayakawa C, and Tromberg B, “Method for recovering quantitative broadband diffuse optical spectra from layered media,” Applied optics 46, 4828–4833 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Zanelli CI, DeMarta S, Hennige CW, and Kadri MM, “Beamforming for Therapy with High Intensity Focused Ultrasound (HIFU) Using Quantitative Schlieren,” IEEE Ultrasonics Symposium, 1233–1238 (1993). [Google Scholar]
- 6.Niu C-G, Gui X-Q, Zeng G-M, and Yuan X-Z, “A ratiometric fluorescence sensor with broad dynamic range based on two pH-sensitive fluorophores,” Analyst 130, 1551–1556 (2005). [DOI] [PubMed] [Google Scholar]
- 7.McEvoy AK, McDonagh CM, and MacCraith BD, “Dissolved oxygen sensor based on fluorescence quenching of oxygen-sensitive ruthenium complexes immobilized in sol-gel-derived porous silica coatings,” Analyst 121, 785–788 (1996). [Google Scholar]
- 8.Zhang G, Palmer GM, Dewhirst MW, and Fraser CL, “A dual-emissive-materials design concept enables tumour hypoxia imaging,” Nature mat 8, 747–751 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Veen RL, Amelink A, Menke-Pluymers M, van der Pol C, and Sterenborg HJ, “Optical biopsy of breast tissue using differential path-length spectroscopy,” Phys. Med. Biol 50, 2573–2581 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Vaupel P, Schlenger K, Knoop C, and Hockel M, “Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements,” Cancer Res 51, 3316–3322 (1991). [PubMed] [Google Scholar]
- 11.Janssen HL, Haustermans KM, Balm AJ, and Begg AC, “Hypoxia in head and neck cancer: how much, how important?,” Head Neck 27, 622–638 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Helmlinger G, Yuan F, Dellian M, and Jain RK, “Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation,” Nat. med 3, 177–182 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Bai J, Li F, Du Y, Wen S, Hu K, Xu G, Ma P, Yin N, and Chen W, “Study of a “biological focal region” of high-intensity focused ultrasound,” Ultrasound in med. & bio 29, 749–754 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Benchimol MJ, Hsu MJ, Schutt CE, Hall DJ, Mattrey RF, and Esener SC, “Phospholipid/carbocyanine dye-shelled microbubbles as ultrasound-modulated fluorescent contrast agents,” Soft matter 9, 2384–2388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schutt C, Ibsen S, Benchimol M, Hsu M, and Esener S, “Manipulating Nanoscale Features on the Surface of Dye-Loaded Microbubbles to Increase their Ultrasound-Modulated Fluorescence Output,” Small 10, 3316–3324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch SH, Wan M, Dayton PA, and Ferrara KW, “Optical observation of lipid-and polymer-shelled ultrasound microbubble contrast agents,” Applied physics letters 84, 631–633 (2004). [Google Scholar]
- 17.Chin CT, Lancée C, Borsboom J, Mastik F, Frijlink ME, de Jong N, Versluis M, and Lohse D, “Brandaris 128: A digital 25 million frames per second camera with 128 highly sensitive frames,” Review of scientific instr 74, 5026–5034 (2003). [Google Scholar]
- 18.Chomas JE, Dayton P, May D, and Ferrara K, “Threshold of fragmentation for ultrasonic contrast agents,” J. biomed. optics 6, 141–150 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Postema M, van Wamel A, Folkert J, and de Jong N, “High-speed photography during ultrasound illustrates potential therapeutic applications of microbubbles,” Medical physics 32, 3707 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Burns PN, “Instrumentation for contrast echocardiography,” Echocardio 19, 241–258 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Hsu MJ, Eghtedari M, Goodwin AP, Hall DJ, Mattrey RF, and Esener SC, “Characterization of individual ultrasound microbubble dynamics with a light-scattering system,” J. biomed. optics 16, 0670021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt T, Hohl C, Haage P, Honnef D, Mahnken A, Krombach G, Piroth W, and Günther R, “Phase-inversion tissue harmonic imaging compared to fundamental B-mode ultrasound in the evaluation of the pathology of large and small bowel,” European radiology 15, 2021–2030 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Borden MA, Martinez GV, Ricker J, Tsvetkova N, Longo M, Gillies RJ, Dayton PA, and Ferrara KW, “ Lateral phase separation in lipid-coated microbubbles,” Langmuir 22, 4291–4297 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Burns PN, Wilson SR, and Hope Simpson D, “Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast,” Investigative radiology 35, 58 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Burns PN, Powers JE, Hope Simpson D, Uhlendorf V, and Fritzsch T, “Harmonic contrast-enhanced Doppler as a method for the elimination of clutter: in vivo duplex and color studies,” Radiology 189, 285 (1993).8372207 [Google Scholar]