Abstract
Background:
Evidence shows cytokine dysregulation in children with developmental disabilities. Less clear is the association between immune activity during the perinatal period and child development.
Methods:
We examined the relationship between newborn concentrations of immune markers and child development. Within Upstate KIDS, a population-based birth cohort (2008–2010, upstate New York), we assayed immune markers, which are postulated to have neuro-modulatory effects, in newborn dried blood spots (NDBS, n=3038). Mothers completed the Ages & Stages Questionnaire© (ASQ) for their children repeatedly through age 36 months. At 30 and 36 months, mothers also reported whether their children received any developmental services. We used generalized linear mixed models adjusted for maternal and child characteristics to test associations.
Results:
Sixteen immune markers were associated with failing ASQ in unadjusted models. After full adjustment (for gestational age, mode of delivery, parity, pregnancy smoking, etc.), we observed that higher levels of 4 markers, including platelet-derived growth factor-AA (PDGF-AA, OR 0.77, 95% CI 0.67, 0.89), plasminogen activator inhibitor-1 (OR 0.80, 95% CI 0.68, 0.94), stromal cell derived factor-1 (OR 0.85, 95% CI 0.73, 0.98), and macrophage inflammatory protein-1beta (OR 0.87, 95% CI 0.77, 0.98) were associated with lower odds of ASQ failure. The associations did not exist if correction for multiple comparisons was performed, except for PDGF-AA. Analyses with developmental service use revealed similar null findings.
Conclusions:
Immune marker concentrations in NDBS may not be associated with developmental delay in the general population. Newborn concentrations of growth factor PDGF-AA may be protective of developmental delay in childhood.
Keywords: immune markers, development, population-based, longitudinal, neonates
Introduction
Children with developmental disabilities such as autism spectrum disorder (ASD) have elevated levels of cytokines such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α).1 Pro-inflammatory cytokines have been suggested to explain the association between prenatal infection and children’s risk of neuropsychiatric disorders because these cytokines can pass through the placenta and the blood brain barrier and lead to aberrant neural growth and plasticity.2 Inflammatory processes are among the factors that underlie abnormal brain development, cognitive impairments, and behavioral disturbances in extremely preterm children.3 To the extent that early pathogenic immune processes are involved in the etiology of developmental disabilities1 as well as poor neuropsychological functioning in children born very preterm,3 it is anticipated that other aspects of child development could also be impacted by immune system activation. Prospective studies among preterm infants that assessed immune markers (i.e., before the emergence of behavioral and cognitive impairments) suggest that low-grade inflammation adversely impacts child brain development.2 However, few studies examined this association in term infants.4,5 Moreover, we do not yet know which markers of immune activity are most important for brain development, nor whether higher or lower levels of immune markers are associated with better or worse developmental outcomes.
Several cytokines are involved in central nervous system signaling to produce neurochemical and neuro-immune alterations that might translate into behavioral and cognitive abnormalities in children.6 These signals induce processes such as migration and activation of inflammatory cells, and other vascular responses, which could in turn increase peripheral levels of cytokines and acute phase reactants. Chemokines, e.g., monocyte chemotactic protein-1 (MCP-1), may also play a role in brain development because of their neuromodulatory role, neuro-transmitter-like effects, and their involvement in regulation of neurogenesis and neuronal migration.7 Both decreased and increased levels of circulatory growth factors such as platelet-derived growth factor-AA (PDGF-AA) and PDGF-BB are reported in children with developmental disabilities.8,9
This study examines the prospective association between immune markers as measured in newborn dried blood spots (NDBS) and failing screening for developmental skills in a large sample of U.S. children drawn from the general population. Neonatal blood specimens obtained from archived NBDS offer a simple and cost effective source to assess various biomarkers.10 Immune markers including cytokines, chemokines, and acute phase reactants in newborns may reflect the immune system activation during perinatal period and serve as a proxy for perinatal immune disruption in neonates.
Methods
Participants
We used data from the Upstate KIDS Study, a population-based birth cohort in upstate New York, 2008–2010.11 Recruitment was based on birth certificate indication of infertility treatment and plurality. Infertility treatments included two broad categories: (a) assisted reproductive technology-specific treatment including in vitro fertilization or intracytoplasmic sperm injection, and (b) ovulation induction via oral or injectable medications with or without intrauterine insemination. All live births conceived with infertility treatment and all of multiple gestations were recruited. Singletons not conceived by infertility treatment were also recruited at a 3:1 ratio to those conceived by treatment, while frequency matching on region of birth. There were 3905 mothers of singletons and 1084 mothers of twins who were enrolled in the study. Here, we included singletons and a randomly selected twin in each pair (n=4989) and excluded triplets and quadruplets (n=134) due to small number. Parents consented to the use of residual NDBS for 3038 (60%) infants at eight months. Characteristics between parents who consented and those who did not were similar.12
The New York State Department of Health and the University of Albany Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health. All participants provided written informed consent.
Measurements
NDBS were collected as part of the Newborn Screening Program 2–3 days after birth. As required by the Program, the hospitals shipped the NDBS within the first 24 hours of collection to the State’s laboratory. NDBS were stored at 4°C and later retrieved for blood spot analyses. Punches of the residual spots were extracted and handled in a similar manner as previously described.13 Briefly, blood spots were extracted overnight (18–20 hr) at 4°C with 60 μL of elution buffer in low-binding 96-well round bottom plates (catalog #1830–9600, USA Scientific, Ocala, FL) on an orbital shaker (Titer Plate Shaker, model # 1830–9600, USA Scientific, Ocala, FL) at 500±50 rpm. The elution buffer was comprised of Phosphate Buffered Saline (PBS, Lonza catalog #17–516Q) with 0.1% BSA (Sigma-Aldrich, catalog #A-4503) and EDTA-free protease inhibitors (1 tablet/10 ml, Roche Applied Science, catalog #04693159001, Mannheim, Germany). Eluants from the extraction of each 3.2 mm punch were frozen at −80°C until analysis. Immune markers collected from the blood spots were measured as part of the Kit A, Obesity, and Millipore’s Milliplex Panels II and III (R&D Systems, Minneapolis, and EMD Millipore, Billerica) using a Luminex100 analyzer with xPONENT 3.1 software (Luminex System, Austin, TX, USA).
We assayed 24 immune markers with possible neuro-modulatory roles.14–17 Markers included basic fibroblast growth factor, IL-8, IL-1 receptor antagonist (IL-1ra), IL-1 alpha (IL-1α), MCP-1, macrophage inflammatory protein-1 alpha (MIP-1α), MIP-1β, vascular endothelial growth factor (VEGF), 6Ckine, cutaneous T-cell-attracting chemokine (CTACK), IL-16, IL-20, MCP-2, MIP-1d, stromal cell derived factor-1 (SDF-1), thymus and activation regulated chemokine (TARC), soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 (sVCAM-1), cathepsin D, myeloperoxidase, PDGF-AA, plasminogen activator inhibitor type-1 (PAI-1), neural cell adhesion molecule, and c-reactive protein (CRP). Assays of IL-6, IL-5, IL-33, and TNF-α resulted in zero values for more than 50% of the population and, therefore, were not further considered in the analysis. Intra-assay coefficients of variation are listed in Table 1. Instrument reported values for markers below the limit of detection (including samples where the instrument could not detect the presence of the analyte) were used without censoring.18 Batch effects in the marker values were removed using COMBAT, a statistical program commonly used to remove batch-to-batch measurement error.
Table 1.
Immune markers in newborns dried blood spots. Upstate KIDS Study
| Immune markers | n | Intra-assay CV(%) | Median (Q1, Q3) (ng/mL) | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| 1 | Basic fibroblast growth factor (bFGF) | 2770 | 9.5 | 48.6 (41.9, 55.0) | 0 | 407.0 |
| 2 | Interleukin 8 (IL-8) | 2692 | 11.5 | 23.8 (16.3, 35.0) | 0 | 1390.8 |
| 3 | Interleukin 1 receptor antagonist (IL-1ra)a | 2566 | 10.1 | 2.0 (1.3, 2.8) | 0.9 | 1.3E+05 |
| 4 | Interleukin 1-alpha (IL-1α) | 2705 | 14.0 | 12.3 (6.2, 21.8) | 0 | 171.6 |
| 5 | Monocyte chemotactic protein 1 (MCP-1) | 2653 | 6.3 | 83.4 (62.7, 108.7) | 7.2 | 741.0 |
| 6 | Macrophage inflammatory protein 1 alpha (MIP-1α) | 1439 | 15.8 | 42.0 (0, 75.3) | 0 | 2845.4 |
| 7 | Macrophage inflammatory protein 1 beta (MIP-1β) | 2661 | 10.4 | 13.4 (9.3, 18.6) | 0.1 | 141.8 |
| 8 | Vascular endothelial growth factor (VEGF) | 2657 | 8.4 | 26.4 (20.2, 34.2) | 2.6 | 1929.5 |
| 9 | 6Ckine (CCL21) | 2434 | 7.9 | 430.0 (368.2, 507.3) | 85.8 | 2462.0 |
| 10 | Cutaneous T-cell-attracting chemokine (CTACK) (CCL27) | 2510 | 14.9 | 34.3 (27.3, 43.3) | 0 | 166.2 |
| 11 | Interleukin 16 (IL-16) | 2606 | 12.9 | 444.4 (336.7, 594.6) | 1.1 | 2158.0 |
| 12 | Interleukin 20 (IL-20) | 2671 | 19.8 | 245.2 (129.7, 385.4) | 0 | 2107.8 |
| 13 | Monocyte chemoattractant protein 2 (MCP-2) | 2758 | 16.6 | 10.2 (7.9, 13.9) | 0 | 81.9 |
| 14 | Macrophage inflammatory proteins 1 d (MIP-1d) | 2700 | 9.8 | 357.0 (264.5, 497.9) | 44.8 | 2136.8 |
| 15 | Stromal cell derived factor 1 (SDF-1)(CCL12)a | 2579 | 12.3 | 1.2 (0.8, 1.7) | 0 | 5328.085 |
| 16 | Thymus and activation regulated chemokine (TARC) (CCL-17) | 2820 | 12.7 | 23.9 (15.9, 35.4) | 0 | 171.3 |
| 17 | Soluble intercellular adhesion molecule 1 (sICAM-1) | 2012 | 12.9 | 22.4 (0, 22.4) | 0 | 2807.2 |
| 18 | Soluble vascular cell adhesion molecule 1 (sVCAM-1)a | 2603 | 6.8 | 2.7 (2.3, 3.3) | 0.7 | 8.6 |
| 19 | Cathepsin Da | 2455 | 12.0 | 2.0 (1.5, 2.7) | 0 | 24510.8 |
| 20 | Myeloperoxidase (MPO)a | 2237 | 13.6 | 22.5 (11.2, 39.3) | 0 | 1.0E+06 |
| 21 | Platelet-derived growth factor-AA (PDGF-AA) | 2584 | 7.3 | 19.1 (15.2, 23.5) | 3.4 | 50.6 |
| 22 | Plasminogen activator inhibitor type 1 (PAI-1) | 2565 | 9.3 | 241.9 (186.2, 299.6) | 0.9 | 1044.7 |
| 23 | Neural cell adhesion molecule (NCAM)a | 2585 | 6.9 | 1.1 (0.9, 1.3) | 0.4 | 3.44 |
| 24 | c-reactive protein (CRP)a | 2820 | 8.6 | 1.4 (0.6, 3.4) | 0.02 | 54.0 |
mg/L
At ages 4–6, 8, 12, 18, 24, 30, and 36 months, mothers reported on their children’s development using the age appropriate Ages & Stages Questionnaire© (ASQ) (postal survey).19 The ASQ is a parent-completed developmental screening instrument recommended for the early identification of developmental delays in five domains: fine motor, gross motor, communication, personal-social functioning, and problem-solving ability.19 The ASQ applies images and extensive instructions to make it comprehensible for parents and encourages them to participate in activities with their children to obtain an accurate developmental assessment and then respond to questions about their children’s development. We implemented the ASQ-2nd edition up to 12 months, and the 3rd edition from 18 months onwards. Each questionnaire item was scored as “yes”=10 points, “sometimes”=5 points, and “not yet”=0 points. The scores were summed for each domain (0–60 points) and domain specific fails were defined if scores were 2 standard deviations below the mean for the child’s age based on ASQ scores in the U.S. norm sample.19
Study personnel called parents to follow-up when the child failed any of the five domains or parental concern was noted. Trained specialists implemented an age-appropriate follow-up ASQ for the domain(s) that failed or discussed concerns. We defined age at fail as the time of the initial screen fail (regardless of the time of the follow-up). When follow-up was incomplete, the child remained as having failed for that ASQ domain. Overall ASQ fail was defined as if the child failed any of the five domains of ASQ. Failure in screening by ASQ at 36 months is shown to predict IQ score at age 5 and 6 years in the general population.20
At 30 and 36 months, parents were asked if their child has received in the past 6 months or is currently receiving any of the developmental services through the Early Intervention (EI) program, Committee on Preschool Special Education (CPSE) or through private services. The positive and negative predictive values of maternal reported use of developmental services compared against Early Intervention Program linkage were high for the 478 infants.21
Data on maternal age (continuous, years), parity (nulliparity, yes/no), plurality (singleton, twin), infant’s sex (boy, girl), gestational age (continuous, weeks), mode of delivery (cesarean section, through birth canal), and birthweight (continuous, grams) was obtained from vital records. A self-administered maternal questionnaire at four months postpartum was used to obtain information on race/ethnicity (Non-Hispanic White, Non-White or other), highest acquired education level (less than high school, high school equivalent, some college, college graduate, graduate/professional school), private insurance (yes/no), and pregnancy smoking (yes/no) and alcohol consumption (yes/no). Mexican, Mexican American, Chicana, Puerto Rican, Cuban, and Other Central or South American were categorized together as Hispanic. Maternal body mass index (BMI) (continuous) was calculated using pre-pregnancy weight and height as provided in the vital records and in the maternal questionnaire (if missing in vital record).
Statistical analyses
Missing values of immune markers were imputed using a full Markov Chain Monte Carlo data imputation and 10 datasets were created (Table 1). For imputations, all markers were transformed for normality using a Box-Cox transformation, then imputed conditional on the values of other markers. The immune marker values were back-transformed after imputations.
We used generalized linear mixed models with logit link function and unstructured covariance matrices to estimate the odds ratios (OR) and 95% confidence intervals (CI) of ASQ failure per standard deviation change in levels of the immune markers. All models included a child-level random intercept to account for repeated ASQ measures. We examined the normality of residuals and transformed the values of immune markers using logarithm or square root-transformation, where appropriate. If any association existed with overall ASQ fail, the association between that immune marker and ASQ domain specific fails were also tested. We tested a non-linearity in the association between immune markers and ASQ failure using restricted cubic splines. We also calculated an inflammation score by summing the concentrations of markers for an individual, with above median values for pro-inflammatory markers assigned 1 point and −1 point for anti-inflammatory markers (IL-1ra). Biomarkers with both pro and anti-inflammatory effects (e.g., MCP-1) were assigned 1 point if their production was induced by pro-inflammatory markers. This method, which considers the action of markers, has been described as a summary measure of inflammatory markers in other epidemiologic studies to limit the number of statistical tests.22,23 The association between inflammatory scores and failing the ASQ was also examined using generalized linear mixed models. Similar analyses were run with the reported use of developmental services at ages 30 and/or 36 months as the outcome. Additionally, we used an alternative approach, in which, we selected the immune markers associated with children’s ASQ fail (two-sided P < 0.2) and examined the association with any ASQ and domain specific fails while these immune markers were mutually adjusted in one model (in total, 10 immune markers).
We selected confounders a priori considering maternal lifestyle factors, inflammation, and children neurodevelopment.24–26 using a Directed Acyclic Graph. Models were adjusted for a child’s sex, and maternal age, education, race, pregnancy smoking and alcohol drinking, pre-pregnancy BMI, private insurance, parity, plurality, mode of delivery, gestational age, and birthweight. Infertility treatment was not associated with children’s development after accounting for plurality;21 however, models were also adjusted for infertility treatment to account for the design. Models included the discrete ‘time’ variable indicating the wave of ASQ assessment.
Missing values of parity were imputed using multiple imputations through creating 10 data sets based on a Bernoulli distribution with probabilities dependent on their observed distributions by infertility treatment type. Other covariates had <1% missing and therefore simple imputation was used to replace missing values. We examined the interaction with plurality to determine if associations in twins and singletons differed. Testing for interaction with sex was grounded on evidence showing vulnerability of male fetus to maternal immune system activation.27 We performed correction for multiple comparisons using False Discovery Rate. We used SAS version 9.3 for analyses (SAS Institute Inc., Cary, NC).
Results
Table 2 summarizes participants’ characteristics. Thirty-two percent of infants were conceived by infertility treatment. From ages 4 to 36 months, 6–11% of children failed the screening for one or more domains of ASQ (Supplementary Table 1). The percentage of screen fail in 5 domains of ASQ varied between 2–4%, which is lower than the 5% in norm population. Among the 2092 children with available data on the use of services, mothers reported using developmental services for 370 children (18%). Correlations between markers were generally low (average r <0.4).
Table 2.
Participants’ characteristics (n=3038)
| Child characteristics | N | Mean (SD)a |
|---|---|---|
| Sex, % | ||
| Male | 1566 | 52 |
| Female | 1472 | 48 |
| Missing | 0 | 0 |
| Gestational age, week | 3038 | 39 (34, 41) |
| Birthweight, gram | 3038 | 3195 (682) |
| Mode of delivery, % | ||
| Cesarean section | 1421 | 48 |
| Vaginal delivery | 1617 | 53 |
| Missing | 0 | 0 |
| Maternal Characteristics | ||
| Age, year | 3038 | 31.1 (5.9) |
| Parity, % | ||
| Nulliparous | 1387 | 46 |
| Other | 1628 | 54 |
| Missing | 0 | 0 |
| Race/ethnicity, % | ||
| Non-Hispanic White | 2541 | 84 |
| Not White or Other | 497 | 16 |
| Missing | 0 | 0 |
| Educational levels, % | ||
| Less than high school | 128 | 4 |
| High school equivalent | 326 | 11 |
| Some college | 869 | 29 |
| College graduate | 747 | 24 |
| Graduate/professional school | 968 | 32 |
| Missing | 0 | 0 |
| Private health insurance, % | ||
| Yes | 2413 | 80 |
| No | 623 | 20 |
| Missing | 0 | 0 |
| Smoking in pregnancy, % | ||
| Yes | 346 | 11 |
| No | 2691 | 89 |
| Missing | 0 | 0 |
| Alcohol consumption during pregnancy, % | ||
| Yes | 413 | 14 |
| No | 2624 | 86 |
| Missing | 1 | 0.0 |
| Pre-pregnancy body mass index | 3032 | 27.0 (6.8) |
| Infertility treatment, % | ||
| Assisted Reproductive Technology | 475 | 16 |
| Ovulation Induction/Intrauterine Insemination | 498 | 16 |
| No | 2064 | 68 |
| Missing | 1 | 0 |
Numbers are mean (standard deviation) for continuous normally distributed variables (birthweight, maternal age and pre-pregnancy body mass index), median (90% range) for continuous variables with skewed distribution (gestational age), and percentage for categorical variables.
Observations counted from singletons (n=2400) and one randomly selected twin of each pair (n=638).
In unadjusted analyses, we observed associations between several immune markers and children’s failing developmental screening (Figure 1). Except for sVCAM-1 (OR per standard deviation 1.25, 95% CI 1.11, 1.40), higher levels of immune markers were associated with lower odds of failing any ASQ in unadjusted models. In marker-specific models adjusted for maternal and child factors, most associations were attenuated and became imprecise (Figure 1). Higher levels of PDGF-AA, PAI-1, SDF-1, and MIP-1β were associated with lower odds of failing overall ASQ after full adjustment (Table 3). When corrected for multiple comparisons, only the association with PDGF-AA remained. A higher inflammation score was associated with lower odds of failing ASQ (OR 0.97, 95% CI 0.96, 0.98); but the effect attenuated and became imprecise after adjustment for confounders (adjusted OR 0.99, 95% CI 097, 1.00). The results of restricted cubic splines confirmed a linear association between immune markers and ASQ failure.
Figure 1.
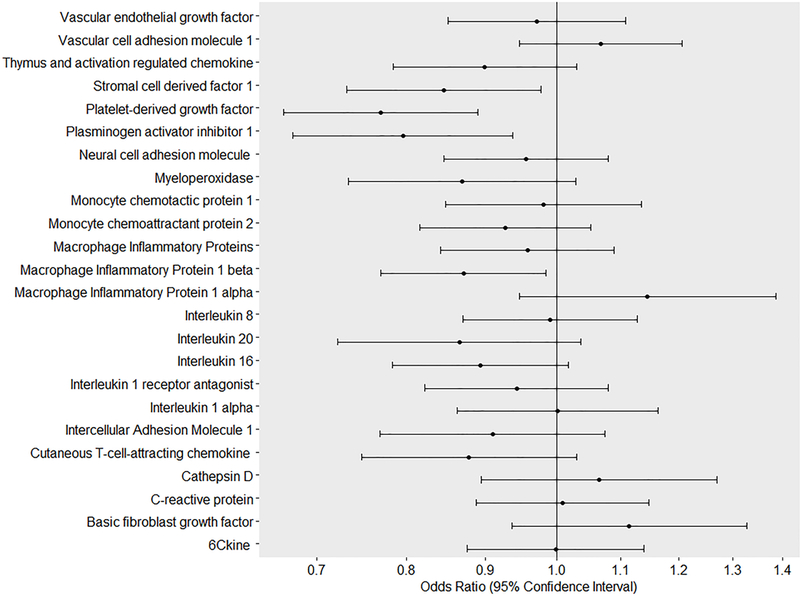
Immune markers in newborns and developmental delay through age three years (n=3038).
Legend: Lines denote adjusted odds ratios (95%confience intervals), respectively. Models adjusted for a child’s sex, maternal age, maternal educational levels, maternal race, maternal history of smoking in pregnancy and drinking alcohol, maternal pre-pregnancy body mass index, having private insurance, parity, history of infertility treatment, plurality, mode of delivery, gestational age, and birthweight. Odds ratios refer to the effect estimates per standard deviation change in levels of immune marker (in logarithmic scale or square-root). Developmental delay was defined if children had scores two standard deviations below the mean for their age through age three years based on the Ages and Stages Questionnaire scores.
Table 3.
Immune markers in newborns and developmental delay through age 3 years (n=3038)
| Exposure | Developmental delay: Odds ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|
| Overall ASQ | Fine motor | Gross motor | Communication | Personal-social | Problem solving | |
| PDGF-AA | ||||||
| Unadjusted | 0.81 (0.72, 0.91) | 0.79 (0.66, 0.94) | 0.73 (0.59, 0.90) | 0.77 (0.65, 0.91) | 0.85 (0.73, 1.01) | 0.80 (0.66, 0.96) |
| Adjusted | 0.77 (0.67, 0.89) | 0.88 (0.74, 1.05) | 0.72 (0.52, 1.01) | 0.80 (0.61, 1.04) | 0.92 (0.77, 1.09) | 0.92 (0.77, 1.11) |
| PAI-1 | ||||||
| Unadjusted | 0.82 (0.72, 0.92) | 0.83 (0.69, 1.01) | 0.70 (0.55, 0.87) | 0.78 (0.66, 0.92) | 0.83 (0.70, 0.98) | 0.82 (0.68, 0.99) |
| Adjusted | 0.80 (0.68, 0.94) | 0.91 (0.72, 1.14) | 0.75 (0.56, 0.99) | 0.84 (0.67, 1.05) | 0.89 (0.74, 1.06) | 0.92 (0.77, 1.10) |
| SDF-1 | ||||||
| Unadjusted | 0.87 (0.78, 0.97) | 0.94 (0.80, 1.10) | 0.84 (0.70, 1.01) | 0.83 (0.71, 0.97) | 0.93 (0.80, 1.08) | 0.87 (0.74, 1.03) |
| Adjusted | 0.85 (0.73, 0.98) | 0.99 (0.83, 1.17) | 0.90 (0.74, 1.10) | 0.90 (0.75, 1.07) | 1.00 (0.82, 1.18) | 0.95 (0.79, 1.13) |
| MIP-1β | ||||||
| Unadjusted | 0.84 (0.75, 0.93) | 0.87 (0.74, 1.01) | 0.85 (0.71, 1.02) | 0.82 (0.70, 0.95) | 0.88 (0.75, 1.03) | 0.92 (0.79, 1.08) |
| Adjusted | 0.87 (0.77, 0.98) | 0.98 (0.81, 1.18) | 1.03 (0.85, 1.25) | 0.94 (0.77, 1.14) | 1.00 (0.84, 1.20) | 1.04 (0.87, 1.25) |
Platelet-derived growth factor-AA (PDGF-AA), Plasminogen activator inhibitor-1 (PAI-1), Stromal cell derived factor 1 (SDF-1), Macrophage inflammatory protein 1 beta (MIP-1β)
Odds ratios refer to the effect estimates per standard deviation change in levels of immune marker (in logarithmic scale or square-root).
Developmental delay was defined as a failure in five domains of Ages and Stages Questionnaire (ASQ) through age 3 years.
Models adjusted for a child’s sex, maternal age, maternal educational levels, maternal race, maternal history of smoking in pregnancy and drinking alcohol, maternal pre-pregnancy body mass index, having private insurance, parity, history of infertility treatment, plurality, mode of delivery, gestational age, and birthweight.
Unadjusted analyses with developmental service use revealed similar findings, showing associations between higher levels of PAI-1 and lower odds of receiving developmental services and higher levels of MIP-1β and lower odds of receiving services. Results became close to null and imprecise in fully adjusted models (Table 4). Supplementary Table 2 and 3 show the associations of covariates with 2 selected immune markers, CRP (results became null after adjustment) and PDGF-AA (results changed minimally after adjustment), to reflect the effect of adjustment for covariates.
Table 4.
Immune markers in newborns and use of developmental services at 30 and 36 months (n=2092).
| Any service use reported by
parents at 30 and 36 months (yes=370) |
||
|---|---|---|
| Exposure | OR (95%CI) | |
| Unadjusted | Adjusted | |
| PDGF-AA | ||
| 0.92 (0.82, 1.03) | 0.97 (0.87, 1.09) | |
| PAI-1 | ||
| 0.89 (0.80, 0.99) | 0.92 (0.83, 1.03) | |
| SDF-1 | ||
| 0.98 (0.88, 1.10) | 1.02 (0.91, 1.15) | |
| MIP-1β | ||
| 0.87 (0.79, 0.96) | 0.95 (0.86, 1.06) | |
Platelet-derived growth factor-AA (PDGF-AA), Plasminogen activator inhibitor-1 (PAI-1), Stromal cell derived factor 1 (SDF-1), Macrophage inflammatory protein 1 beta (MIP-1β)
Odds ratios refer to the effect estimates per standard deviation change in levels of immune marker (in logarithmic scale or square-root).
Models adjusted for a child’s sex, maternal age, maternal educational levels, maternal race, maternal history of smoking in pregnancy and drinking alcohol, maternal pre-pregnancy body mass index, having private insurance, parity, history of infertility treatment, plurality, mode of delivery, gestational age, and birthweight.
In analyses of ASQ domain-specific fails (Table 3), the association between PDGF-AA and developmental delay was accounted for by gross motor skills. Higher PAI-1 was also associated with lower odds of delay in the domain of gross motor skills. When we ran a model mutually adjusted for a selection of ten immune markers (MIP-1α, MIP-1β s, CTACK, IL-16, IL-20, SDF-1, TARC, myeloperoxidase, PAI-1, and PDGF-AA), we observed no associations between immune markers and failing any ASQ domains.
Comment
Principal findings
We used residual NDBS to examine the prospective association of 24 immune markers with child development in a large population-based sample. Neonates with higher levels of 4 immune markers, i.e., PDGF-AA, PAI-1, SDF-1, and MIP-1β, were less likely to fail developmental screening up to age three years, mainly gross motor skills. The associations did not exist if correction for multiple comparisons was performed, except for the association between PDGF-AA and developmental delay. The absence of associations involving the majority of immune markers measured in NDBS with developmental screening and maternal report of receiving developmental services in children suggest that the association between neonatal immune markers and child development are influenced largely by confounding factors. Important were a child’s sex, gestational age, mode of delivery, and parity.
Interpretation
In addition to their well-known role in immune system response, immune molecules are shown to signal the central nervous system and initiate crucial brain processes during brain development.6 Several cytokines have receptors in the brain regions, e.g., the hippocampus and amygdala.28 Production and action of these proteins are tightly regulated in cascades and therefore, extremely high and low levels could be detrimental to the developing brain. Several studies have attempted to show the programming effect of immune system dysregulation on brain development by measuring immune markers during gestation.4,5
It is postulated that adverse pregnancy events with a substantial cytokine response, e.g., severe infections, disrupt normal brain development in the offspring. This disruption likely occurs through increased production of immune markers. Findings regarding the impact of low-grade inflammation during gestation, reflected by a slight elevation of maternal CRP, on children’s brain development remained limited to children with ASD. For example, Brown et al. showed that higher levels of maternal CRP during first and early second trimester of pregnancy was associated with a diagnosis of ASD in children.24 In contrast, a similar study found that maternal CRP levels in mid-pregnancy were lower in mothers of ASD children compared with controls.25 Koks et al. reported that the observed association between maternal CRP and children’s autistic traits was largely explained by sociodemographic and maternal health-related factors.26 Our findings provide further support that confounding may explain the inconsistencies across studies on CRP and child development, even though previous studies analyzed maternal specimens. In unadjusted associations, higher levels of several neonatal markers were associated with smaller odds of failing developmental screening. Adjusted models with ASQ and developmental service use revealed that most of these associations were explained by confounding. We have previously shown that maternal BMI is associated with increased inflammation and separately, that maternal obesity is associated with higher odds of failing gross motor development.29,30 While studies on neonatal samples universally consider gestational age at birth as a confounder,31,32 information on socioeconomic characteristics and maternal health-related factors such as BMI are not available in many studies.
Measurements of immune markers in maternal biospecimens are indirect indicators of fetal exposures to immune system response. When using newborn samples, several considerations are relevant. First, we observed that some immune markers such as IL-6 and TNF-α had very low levels in neonatal samples. Second, when interpreting the results on neonatal immune markers, the effect of parturition should also be considered. Around birth, an acute surge in levels of glucocorticoids and prostaglandins might suppress immune response and therefore, impact immune system activation in newborns. Finally, brain processes during the early postnatal period could have obscured the influences of early immune response on child development. Therefore, a comparison of our findings, with studies of maternal inflammation during gestation should be done cautiously.
We found that neonates with higher levels of PAI-1, PDGF-AA, SDF-1, and MIP-1β were less likely to fail a developmental screening. PDFG-AA is a protein crucial for the myelination process in the central nervous system because of its key signaling role for regulation of differentiation of oligodendrocyte. It is hypothesized that decreased levels of PDGF-AA underlie the neurodevelopmental sequels in children maple syrup urine disease.8 This growth factor also decreases in the cerebral spinal fluid with the progression of multiple sclerosis.33 Because of the complex biological relation between immune molecules and their pleiotropic effects and relation with neurotransmitters and glucocorticoids in normal development, their role might be different from their involvement in various disease and injury paradigms. Also, we measured the immune markers in infancy and before children could display signs of developmental delay. Therefore, levels of immune proteins might be an indicator of what is expected for normal development, as opposed to the presence of pathology, where the levels represent a status that is the body’s reaction to the disease condition. Future studies are needed to further explain the directionality of this association by exploring the physiological relevance of chemokines such as PDGF-AA for early brain development. Furthermore, other environmental factors with the nurturing effect on brain development that occur during the prenatal and postnatal period along with potential interventions should be considered.
Strength of the study
This study has several strengths including large sample size, repeated measurement of children’s development, and assessment of more than 24 immune markers. Upstate KIDS prospectively collected data on various important confounders.
Limitation of the data
However, we faced limitations. First, our analysis did not include important cytokines with neuro-inflammatory roles such as IL-6, IL-8, IL-17 or TNF-α. Also, we acknowledge that the ASQ is a screening rather than a diagnostic tool. Nevertheless, the ASQ is a validated instrument that has been shown to identify children between 0–5 years of age with developmental delay.19 ASQ has adequate psychometric properties (75% sensitivity and 81% specificity) and modest agreement with other instruments,34 which make it a reliable, cost-effective, and feasible method to capture children’s developmental abilities in large population-based studies. Furthermore, our analysis with maternal reports of the use of early developmental services revealed similar results as the analysis with ASQ. Another limitation is that Upstate KIDS experienced loss to follow-up, which motivated the use of generalized linear mixed effects models that are robust to loss to follow-up under the missing at random assumption. Immune markers were measured in archived NDBS and degradation of these markers was possible. However, we did not expect the degradation to be associated with child development and to introduce bias. We did not perform a comparison of concentrations of immune markers in NDBS and serum or plasma. Nonetheless, NDBS provide a robust and convenient sample for immunoassay analysis of immune markers in whole blood, if stored at low temperature (4°C or less).35
Conclusions
Concentrations of immune markers in newborns were not associated with developmental outcomes in early childhood, except that children with higher levels of neonatal PDGF-AA were less likely to fail a developmental screening. Null associations indicate that the measurement of immune markers in NDBS may not be a strong early indicator of developmental delay in children from the general population. Neonatal concentrations of growth factor PDGF-AA may be protective of developmental delay in childhood.
Supplementary Material
Acknowledgement
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; contracts #HHSN275201200005C, #HHSN267200700019C). We thank all of the Upstate KIDS Study participants and staff for their important contributions.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Mitchell RH, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. Journal of American Academy of Child and Adolescent Psychiatry. 2014;53(3):274–296. [DOI] [PubMed] [Google Scholar]
- 2.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatric Research. 1997;42(1):1–8. [DOI] [PubMed] [Google Scholar]
- 3.Silveira RC, Procianoy RS. High plasma cytokine levels, white matter injury and neurodevelopment of high risk preterm infants: Assessment at two years. Early Human Development. 2011;87(6):433–437. [DOI] [PubMed] [Google Scholar]
- 4.Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, Buka SL, Goldstein JM. Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(26):6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghassabian A, Albert P, Horing M, Yeung E, Cherkerzian S, Goldstein RB, Buka SL, Goldstein JM, Gilman SE. Gestational cytokine concentrations and neurocognitive development at 7 years. Translational Psychiatry. 2018; 13;8(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nature Reviews Neuroscience. 2007;8(3):221–232. [DOI] [PubMed] [Google Scholar]
- 7.Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neuroscience & Biobehavioral Reviews. 2014;42:93–115. [DOI] [PubMed] [Google Scholar]
- 8.Scaini G, Tonon T, de Souza CFM, Schuk PF, Ferreira GC, Neto JS, Amorin T, Schwartz IVD, Streck EL. Serum markers of neurodegeneration in maple syrup urine disease. Molecular Neurobiology. 2017;54(7):5709–5719. [DOI] [PubMed] [Google Scholar]
- 9.Kajizuka M, Miyachi T, Matsuzaki H, Iwata K, Shinmura C, Suzuki K, Suda S, Tsuchiya KJ, Matsumoto K, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Takei N, Mori N. Serum levels of platelet-derived growth factor bb homodimers are increased in male children with autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(1):154–158. [DOI] [PubMed] [Google Scholar]
- 10.Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. Journal of Visualized Experiments. 2014(83):50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck Louis GM, Hediger ML, Bell EM, Kus CA, Sundaram R, McLain AC, Yeung E, Hills EA, Thoma ME, Druschel CM. Methodology for establishing a population-based birth cohort focusing on couple fertility and children’s development, the upstate kids study. Paediatric and Perinatal Epidemiology. 2014;28(3):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung EH, Louis GB, Lawrence D, Kannan K, McLain AC, Caggana M, Druschel C, Bell E. Eliciting parental support for the use of newborn blood spots for pediatric research. BMC Medical Research Methodology. 2016;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung EH, McLain AC, Anderson N, Lawrence D, Boghossian NS, Druschel C, Bell E. Newborn adipokines and birth outcomes. Paediatric and Perinatal Epidemiology. 2015;29(4):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology. 2005;57(1):67–81. [DOI] [PubMed] [Google Scholar]
- 15.Clark-Raymond A, Meresh E, Hoppensteadt D, Fareed J, Sinacore J, Garlenski B, Halaris A. Vascular endothelial growth factor: Potential predictor of treatment response in major depression. World Journal of Biological Psychiatry. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 16.Oglodek EA, Szota AM, Just MJ, Mos DM, Araszkiewicz A. The mcp-1, ccl-5 and sdf-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disorders. Pharmacological Reports. 2015;67(1):85–89. [DOI] [PubMed] [Google Scholar]
- 17.Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. Ng2 cells in white matter but not gray matter proliferate in response to pdgf. Journal of Neuroscience. 2013;33(36):14558–14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schisterman EF, Vexler A, Whitcomb BW, Liu A. The Limitations due to Exposure Detection Limits for Regression Models. American journal of epidemiology. 2006;163(4):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squires J, Bricker D. Ages & stages questionnaires [r], (asq-3 [tm]): A parent-completed child-monitoring system. Brookes Publishing Company. 2009. [Google Scholar]
- 20.Charkaluk ML, Rousseau J, Calderon J, et al. Ages and Stages Questionnaire at 3 Years for Predicting IQ at 5–6 Years. Pediatrics. 2017;139(4). [DOI] [PubMed] [Google Scholar]
- 21.Yeung EH, Sundaram R, Bell EM, Druschel C, Kus C, Ghassabian A, Bello S, Xie Y, Buck Louis GM. Examining infertility treatment and early childhood development in the upstate kids study. JAMA Pediatrics. 2016;170(3):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellanda LC, Duncan BB, Vigo A, Rose K, Folsom AR, Erlinger TP. Low birth weight and markers of inflammation and endothelial activation in adulthood: the ARIC study. International Journal of Cardiology. 2009;134(3):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopkins MH, Owen J, Ahearn T, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prevention Research. 2011;4(10):1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal c-reactive protein and autism in a national birth cohort. Molecular Psychiatry. 2014;19(2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zerbo O, Traglia M, Yoshida C, Heuer LS, Ashwood P, Delorenze GN, Hansen RL, Kharrazi M, Van de Water J, Yolken RH, Weiss LA, Croen LA. Maternal mid-pregnancy c-reactive protein and risk of autism spectrum disorders: The early markers for autism study. Translational Psychiatry. 2016;6(4):e783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koks N, Ghassabian A, Greaves-Lord K, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Maternal c-reactive protein concentration in early pregnancy and child autistic traits in the general population. Paediatric and Perinatal Epidemiology. 2016;30(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makinson R, Lloyd K, Rayasam A, et al. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain, Behavior and Immunity. 2017;66:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szelenyi J, Vizi ES. The catecholamine cytokine balance: Interaction between the brain and the immune system. The Annals of the New York Academy of Sciences. 2007;1113:311–324. [DOI] [PubMed] [Google Scholar]
- 29.Yeung EH, Sundaram R, Ghassabian A, Xie Y, Buck Louis G. Parental obesity and early childhood development. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broadney MM, Chahal N, Michels KA, McLain AC, Ghassabian A, Lawrence DA, Yeung EH. Impact of parental obesity on neonatal markers of inflammation and immune response. International Journal of Obesity (Lond). 2017;41(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen PR, Agerbo E, Skogstrand K, Hougaard DM, Meyer U, Mortensen PB. Neonatal levels of inflammatory markers and later risk of schizophrenia. Biological Psychiatry. 2015;77(6):548–555. [DOI] [PubMed] [Google Scholar]
- 32.Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, Hansen RL, Kharrazi M, Croen LA. Neonatal cytokines and chemokines and risk of autism spectrum disorder: The early markers for autism (ema) study: A case-control study. Journal of Neuroinflammation. 2014;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harirchian MH, Tekieh AH, Modabbernia A, Aghamollaii V, Tafakhori A, Ghaffarpour M, Sahraian MA, Naji M, Yazdankhah M. Serum and csf pdgf-aa and fgf-2 in relapsing-remitting multiple sclerosis: A case-control study. European Journal of Neurology. 2012;19(2):241–247. [DOI] [PubMed] [Google Scholar]
- 34.Schonhaut L, Armijo I, Schonstedt M, Alvarez J, Cordero M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics. 2013;131(5):e1468–1474. [DOI] [PubMed] [Google Scholar]
- 35.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Nørgaard-Pedersen B, Hougaard DM. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. Journal of Immunological Methods. 2008;336(1):78–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


