Abstract
Systemic mastocytosis (SM) in felines is a rare neoplasm defined by increased growth and accumulation of immature mast cells (MC) in various organs including the spleen. Although in many cases splenectomy is an effective approach, relapses may occur. In these patients, treatment options are limited. Recent data suggest that various Kit tyrosine kinase inhibitors (TKI) interfere with growth of neoplastic MC in humans. In the current study, we examined the effects of four TKI, imatinib, midostaurin, nilotinib, and dasatinib, on growth of spleen-derived feline neoplastic MC in three SM patients. Expression of Kit in neoplastic MC was confirmed by flow cytometry and/or Western blotting. In all three cases, a 12-bp internal tandem duplication in exon 8, resulting in a four amino acid-insertion between residues Thr418 and His419 in Kit, was detectable. As assessed by 3H-thymidine incorporation experiments, all four TKI were found to inhibit the growth of feline neoplastic MC in a dose-dependent manner. The growth-inhibitory TKI effects were found to be associated with morphologic signs of apoptosis in MC. In conclusion, various Kit-targeting TKI can inhibit the in vitro growth and survival of feline neoplastic MC in SM.
Keywords: Feline, Mast cell, Mastocytosis, Tyrosine kinase inhibitor, c-Kit mutation
1. Introduction
Mast cells (MC) neoplasms are characterized by uncontrolled growth and accumulation of tissue MC in various organ systems. In felines, three major categories of the disease have been defined: cutaneous mastocytosis, intestinal mastocytosis, and systemic mastocytosis (SM) (Liska et al., 1979; Carpenter et al., 1987; Molander-McCrary et al., 1998; Allan et al., 2000; Litster and Sorenmo, 2006). The prognosis in these patients varies, depending on the spread of disease, organs affected, and response to conventional therapy (Confer et al., 1978; Liska et al., 1979; Guerre et al., 1979; Molander-McCrary et al., 1998; Litster and Sorenmo, 2006). Whenever possible, surgical intervention with resection of tumor lesions is recommended (Liska et al., 1979; Guerre et al., 1979; Litster and Sorenmo, 2006). In SM, splenectomy is regarded standard therapy, and is often followed by long-term disease-free survival (Liska et al., 1979; Guerre et al., 1979).
However, not all patients can be cured by splenectomy. Other patients are not eligible or cannot tolerate surgery. Without splenectomy, however, the prognosis in feline SM is grave. These patients are candidates for euthanasia or are treated with experimental cytostatic or experimental (targeted) drugs (Lachowicz et al., 2005; Litster and Sorenmo, 2006). Recently, the Kit-targeting drug imatinib (STI571) has been applied to patients with feline SM, and in some of these patients, the drug was found to produce a response with partial regression of MC tumor lesions (Lachowicz et al., 2005; Isotani et al., 2006).
The tyrosine kinase receptor Kit is a major regulator of differentiation and survival of normal and neoplastic MC in various species (Galli et al., 1993; Valent, 1994; Akin and Metcalfe, 2004; Metcalfe, 2008). In many cases, mutations or duplications in the Kit gene are detectable and lead to autonomous activation of the receptor, with consecutive spontaneous growth of MC (Furitsu et al., 1993; Nagata et al., 1995; Longley et al., 1996; Zemke et al., 2002; Riva et al., 2005). Recently, a duplication in Kit (within exon 8) has also been reported for feline neoplastic MC (Isotani et al., 2006). Based on these observations and on the clinical response seen in first pilot studies, Kit is now regarded as a potential therapeutic target in MC neoplasms in various species (Valent et al., 2004; Lachowicz et al., 2005; Isotani et al., 2006; Droogendijk et al., 2006; Hoffmann et al., 2008). However, in humans, the most common Kit mutation in SM (encoding the D816V substitution) confers resistance against imatinib (Ma et al., 2002; Akin et al., 2003; Gleixner et al., 2006).
During the past few years, a number of more potent Kit-targeting TKI, such as nilotinib (AMN107), dasatinib, and midostaurin (PKC412), have been developed and have been introduced in clinical trials in human patients with advanced SM (Gotlib et al., 2005; Gleixner et al., 2006, 2007a; Shah et al., 2006; Verstovsek et al., 2008). In the current study, we examined the in vitro effects of four TKI on growth and survival of feline neoplastic MC obtained from the spleen of patients with advanced SM. In addition, we provide evidence that an exon 8 ITD mutation is a recurrent genetic defect in feline SM.
2. Materials and methods
2.1. TKI and other reagents
Imatinib (STI571), midostaurin (PKC412), and nilotinib (AMN107) were kindly provided by Dr. Elisabeth Buchdunger, Dr. Johannes L. Roesel, and Dr. Paul W. Manley (Novartis Pharma AG, Basel, Switzerland). Dasatinib (BMS-354825) was kindly provided by Dr. Francis Y. Lee (Bristol-Meyers-Squibbs, New Brunswick, NJ). Stock solutions of TKI were prepared by dissolving in dimethylsulfoxide (DMSO) (Merck, Darmstadt, Germany). Fetal calf serum (FCS) was purchased from PAA Laboratories (Pasching, Austria), Iscovés modified Dulbecco’s medium (IMDM) from Gibco Life Technologies (Gaithersburg, MD), and 3H-thymidine from Amersham (Buckinghamshire, UK). The PE-labeled monoclonal anti-Kit antibody 104D2 (IgG1) and an isotype-matched antibody were from Becton Dickinson Bioscience (San Jose, CA).
2.2. Isolation of primary neoplastic feline MC
In three feline patients with SM who underwent splenectomy (n = 2) or euthanasia (n = 1), splenic tissue was obtained. Patients’ characteristics are shown in Table 1. Tissue samples were split, one part prepared for histopathological and immunohistochemical analysis, and the second part was used for MC isolation. In particular, tissue fragments were cut into small pieces and dispersed using collagenase type II (Worthington, Lakewood, NJ) following a published protocol (Valent et al., 1989; Rebuzzi et al., 2007). Isolated cells were recovered by filtration through nytex cloth and placed in FCS-containing tubes. After washing, cells were examined for viability (trypan blue exclusion) and for the percentage of MC by Wright–Giemsa staining.
Table 1. Patients’ characteristics and IC50 values obtained with TKI.
| Age | Sex | Breed | Diagnosis | IC50 |
||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib | Imatinib | Midostaurin | Nilotinib | |||||
| Cat #1 | 13 | m/n | European shorthair | Systemic mastocytosis | 1–10 | 50–250 | 100–250 | 10–100 |
| Cat #2 | 10 | f/s | European shorthair | Systemic mastocytosis | 10–100 | 1–10 | 10–50 | 50–100 |
| Cat #3 | 19 | m/n | European shorthair | Systemic mastocytosis | 1–5 | 100–1000 | 200–500 | 50–100 |
m/n, male/neutered; f/s, female/spayed; IC50 values are expressed in nM (range from three experiments each).
2.3. Histopathology and immunocytochemistry
Histologic analysis was performed on paraffin-embedded, formalin-fixed spleen sections. For immunocytochemical (ICC) evaluation, primary MC were spun on cytospin slides and fixed in acetone. The ICC technique was performed as described previously (Agis et al., 2006) using a polyclonal rabbit-anti-human Kit antibody (Dako, Glostrup, Denmark).
2.4. Evaluation of expression and phosphorylation of Kit in neoplastic MC by flow cytometry, Western blotting, and immunoprecipitation (IP)
To confirm surface expression of Kit in isolated neoplastic MC, flow cytometry was performed as described using the anti-Kit antibody 104D2 (Rebuzzi et al., 2007). Flow cytometry was conducted using a FACScan (Becton Dickinson Bioscience). In one of the three patients, neoplastic feline MC were examined for expression of total Kit and phosphorylated Kit by Western blotting and IP analysis as reported (Gleixner et al., 2006). Before being examined, cells were cultured in control medium, or in medium containing TKI (imatinib, midostaurin, nilotinib, or dasatinib, each 1 µM) at 37 °C and 5% CO2 for 4 h. IP was performed on cell lysates (107 cells) using anti-Kit antibody 1C1 kindly provided by Dr. Hans-Jörg Bühring (University of Tübingen, Germany) and protein G Sepharose-beads (Amersham). Then, immunoprecipitates were separated by 10% SDS polyacrylamid gel and transferred to a polyvinylidenedifluoride (PVDF) membrane (Amersham). For detection of phosphorylated Kit, anti-phospho-tyr-mAb 4G10 (1:1000) (Upstate Biotechnology, Lake Placid, NY) was applied. Antibody-reactivity was made visible by sheep anti-mouse IgG or donkey anti-rabbit IgG and Lumingen PS-3 detection reagent (all from Amersham).
2.5. Analysis of feline neoplastic MC for Kit exon 8 tandem duplication
Genomic DNA was extracted from paraffin-embedded tumor samples obtained from the three feline patients, using a published protocol (Zemke et al., 2002). Standard PCR reactions were prepared in a 25-µl total volume using the following primer pairs: 5′ AGCAGTCTGACGTGTGACCGT 3′ and 5′ GTAGTATGCCACTCACGAGACT 3′, with 1 µl extracted DNA, 2 pmol of each primer, and 0.5 U of Taq polymerase (Invitrogen, Carlsbad, CA). Cycling conditions were as follows: 94 °C for 4 min; 40 cycles at 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min; and 72 °C for 5 min. Amplified products were visualized by agarose gel electrophoresis on a 2% agarose gel after ethidium bromide staining. PCR products were purified by QIAEX II gel extraction kit (QIAGENgen, Mississauga, ON, Canada), cloned into plasmids using the TA Cloning kit (Invitrogen, Carlsbad, CA), and then sequenced with ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
2.6. Measurement of 3H-thymidine uptake and evaluation of apoptosis
To determine growth-inhibitory drug effects, primary neoplastic MC were incubated with various concentrations of TKI, i.e. imatinib, midostaurin, nilotinib, and dasatinib (each 1 nM-10 µM), or control medium in 96-well culture plates (TPP, Trasadingen, Switzerland) at 37 °C for 48 h. After incubation, 0.5 µCi 3H-thymidine was added (37 °C, 12 h). Cells were then harvested on filter membranes (Packard Bioscience, Meriden, CT) in a Filtermate 196 harvester (Packard Bioscience). Filters were air-dried, and the bound radioactivity was counted in a β-counter (Top-Count NXT, Packard Bioscience). All experiments were performed in triplicates.
Effects of the TKI on apoptosis were analyzed by morphologic examination. In these experiments, neoplastic MC were incubated with TKI (imatinib, midostaurin, nilotinib, dasatinib, each 0.5 or 1 μM), or control medium in 24 well culture plates in medium plus 10% FCS for 24 or 48 h. The percentage of apoptotic cells was quantified on Wright–Giemsa-stained cytospin preparations. Apoptosis was defined by conventional morphologic criteria (Van Cruchten and Van Den Broeck, 2002).
3. Results and discussion
3.1. Expression of Kit in neoplastic feline MC
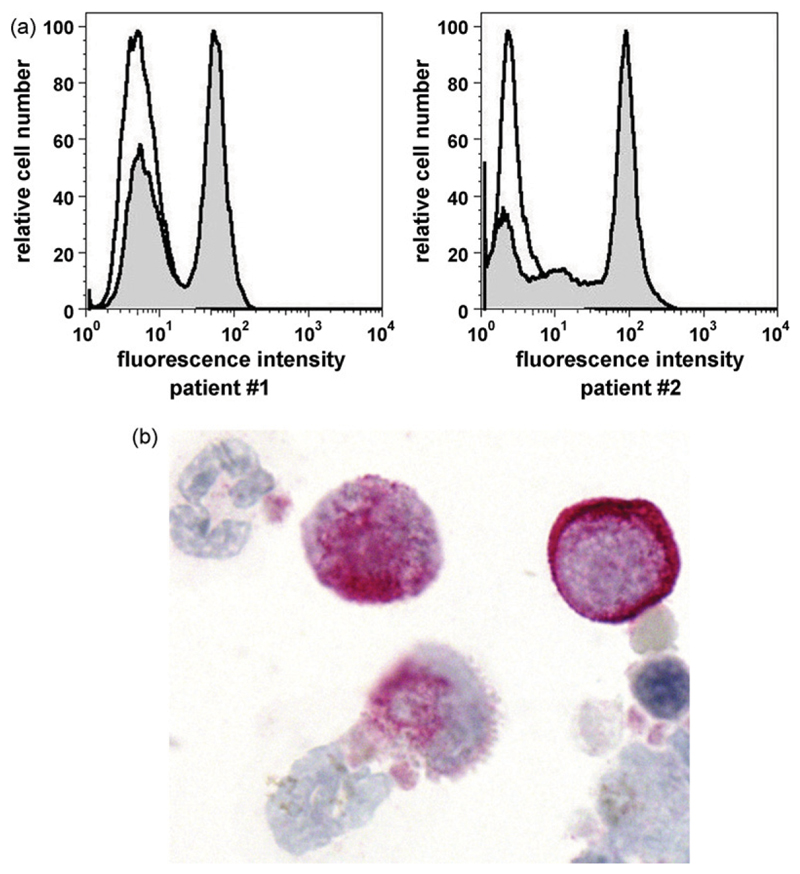
Recent data suggest that Kit is a potential target in MC neoplasms (Valent et al., 2004; Gotlib, 2006; Isotani et al., 2006; Hoffmann et al., 2008; Metcalfe, 2008; Jensen et al., 2008; Valent, 2008). Most of these data refer to human neoplastic MC, however. In the present study, we have analyzed expression of Kit in feline neoplastic MC. In two of the three feline patients examined, flow cytometry experiments were performed on isolated neoplastic MC. As expected, neoplastic MC were found to express the Kit protein on their cell surface (Fig. 1A). Interestingly, a subset of cells in the tumor cell samples was found to stain negative for Kit in these experiments (Fig. 1A), although most cells were found to be MC by Giemsa staining. In particular, as assessed by Giemsa staining the purity of MC was found to be 97% (patient #1), 99% (patient #2), and 81% (patient #3). This discrepancy may be explained by a lack of Kit on a subset of neoplastic (immature) MC. Expression of Kit in MC could also be confirmed by immunocytochemistry in two of two patients examined (Fig. 1B). All in all, these data show that neoplastic feline MC express Kit, confirming the data of Morini and colleagues (Morini et al., 2004).
Fig. 1.
Expression of Kit/CD117 in feline neoplastic mast cells. (A) In two patients with systemic mastocytosis (left image: feline patient #1 and right image: feline patient #2 in Table 1), primary neoplastic mast cells were stained with a PE-labeled anti-Kit antibody (grey graph) or an IgG1 isotype control antibody (open graph). Expression of Kit was analyzed by flow cytometry on a FACScan. (B) Immunocytochemical detection of Kit in neoplastic feline mast cells. A cytospin preparation of primary neoplastic mast cells prepared in feline patient #3 was stained with a polyclonal anti-Kit antibody as described in the text. As visible, neoplastic mast cells were found to stain positive for Kit.
3.2. Detection of a tandem duplication in Kit exon 8 in feline neoplastic MC
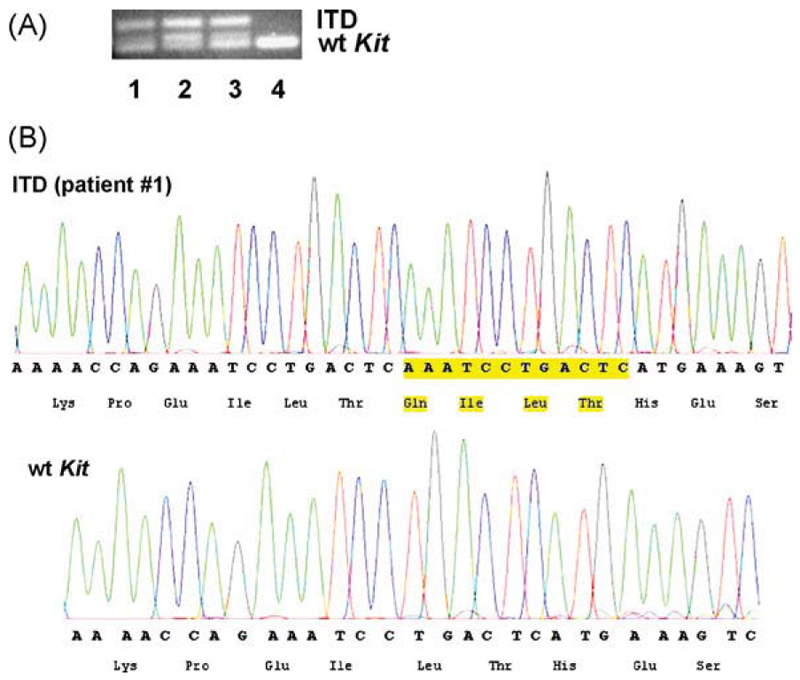
So far, recurrent Kit mutations (duplications, point mutations) have been described in human mastocytosis as well as in canine mast cell neoplasms (Furitsu et al., 1993; Nagata et al., 1995; Longley et al., 1996; London et al., 1999; Zemke et al., 2002; Downing et al., 2002; Valent et al., 2005). In cats, the data reported so far concerning Kit mutations are conflicting. Whereas Dank et al. (2002) did not report Kit mutations in feline MC tumors, Isotani et al. (2006) reported on the presence of an exon 8 ITD in Kit in a feline mastocytoma patient. In the present study, we were able to show that neoplastic MC in all three feline patients examined displayed an exon 8 Kit ITD. In particular, in all three patients, sequence analysis performed on neoplastic MC confirmed the presence of the expected 85 bp PCR product of wild-type Kit. In addition, in all three patients, we detected a second PCR product of 97 bp containing the 12 bp internal tandem duplication (AAATCCTGACTC), resulting in a four amino acid-insertion between residues Thr418 and His419 within the fifth immunoglobulin-like domain (in exon 8) of Kit (Fig. 2; Table 2). Genomic DNA obtained from normal cat tissue did not show an ITD in exon 8 of Kit (Fig. 2). These data strongly suggest that the exon 8 Kit ITD mutation is a recurrent defect in feline SM. However, as only three patients and only one (major european) breed were examined, we cannot exclude with certainty that other recurrent mutations in Kit or in other genes may also be detectable in feline MC neoplasms.
Fig. 2.
Kit mutation analysis in feline neoplastic mast cells. Genomic DNA from feline neoplastic mast cells was extracted, amplified using primers specific for a Kit exon 8 internal tandem duplication (ITD), purified, cloned into plasmids, sequenced, and analyzed by PCR (A) as well as by chromatography (B). (A) Shows ethidium bromide-stained agarose gel electrophoresis results from amplification products from the three feline mastocytoma patients examined (lanes #1, #2, and #3) as well as from a normal cat genomic DNA that served as control (lane #4). The identity of the products was all confirmed by sequencing. A chromatogram of the Kit ITD (obtained in patient #1) compared to wild-type Kit is shown in (B).
Table 2. Alignment of ITD in cat mast cell tumors comparing with wild-type c-Kit.
| Cat | MCT | wt | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTC--- | ---------A | TGAAAGTCTC |
| Cat | MCT | No.1 | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTCAAA | TCCTGACTCA | TGAAAGTCTC |
| ********** | ********** | ********* | ******* | * | ********** | |||
| Cat | MCT | wt | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTC--- | ---------A | TGAAAGTCTC |
| Cat | MCT | No.2 | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTCAAA | TCCTGACTCA | TGAAAGTCTC |
| ********** | ********** | ********* | ******* | * | ********** | |||
| Cat | MCT | wt | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTC--- | ---------A | TGAAAGTCTC |
| Cat | MCT | No.3 | CTCTCTTTTC | CTGCAGCAAA | ACCAGAAATC | CTGACTCAAA | TCCTGACTCA | TGAAAGTCTC |
| ********** | ********** | ********* | ******* | * | ********** |
MCT, mast cell tumor; wt, wild-type.
3.3. Kit-targeting TKI suppress the in vitro growth of neoplastic feline MC
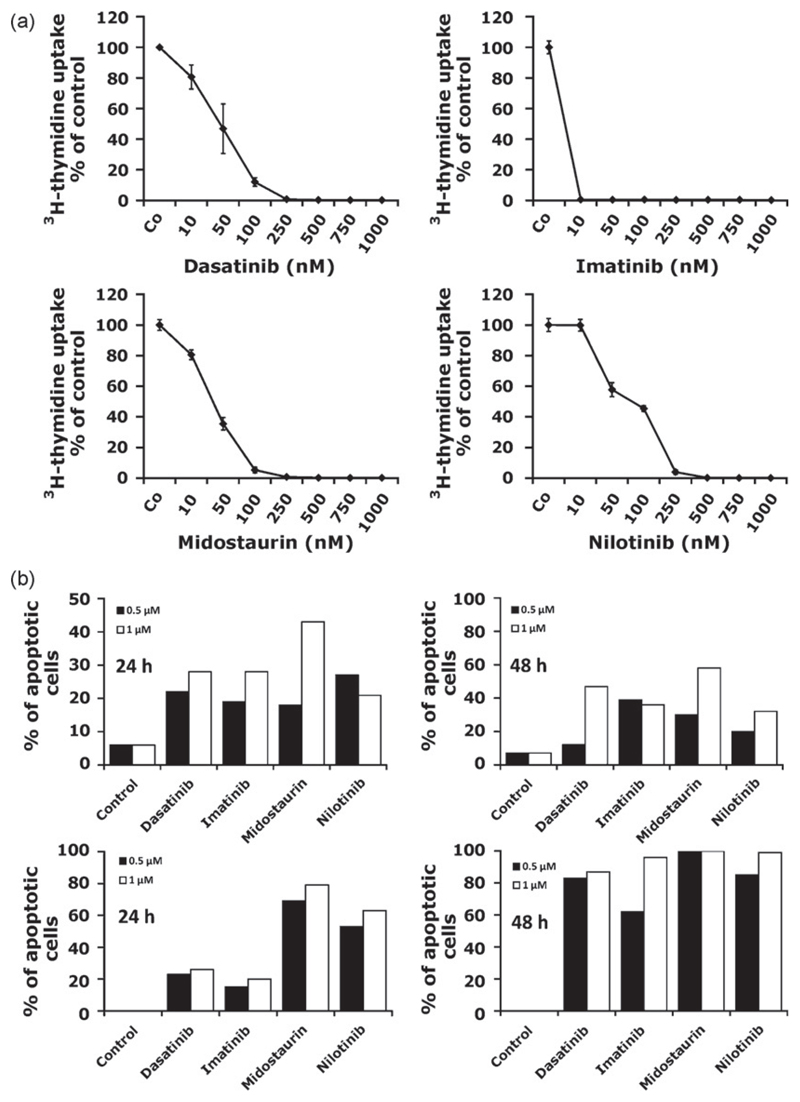
As assessed by 3H-thymidine uptake experiments, all four Kit TKI applied (imatinib, midostaurin, nilotinib, dasatinib) were found to suppress the spontaneous in vitro growth of neoplastic feline MC (Fig. 3; Table 1). The effects of the TKI on growth of MC were found to be dose-dependent, with IC50 values varying from patient to patient (Table 1). Thus, variable IC50 values were obtained when the three TKI were compared with each other and with imatinib. Likewise, imatinib was found to induce MC growth arrest at very low concentrations in patient #2 (<10 nM), whereas in patient #3, imatinib produced a relatively high IC50 (higher nM range) (Table 1). Similarly, dasatinib produced growth arrest in neoplastic MC at low concentrations in patients #1 and #2, whereas in patient #3, the IC50 for dasatinib was rather high (Table 1). By contrast, in one patient (#2 in Table 1), all TKI including imatinib showed a good response at relatively low IC50 values. Interestingly, in this cat, the IC50 for dasatinib was higher than that obtained for imatinib (Table 1). In control experiments, DMSO did not modulate (inhibit) growth of feline neoplastic MC (not shown). Fig. 3A shows typical examples for the dose-dependent effects of the various TKI on neoplastic feline MC. The reason for the different drug potencies (IC50) found in the patients remains unknown. One possibility might be that additional targets (apart from Kit) that exert distinct drug-binding profiles and drug-inhibition profiles (IC50 values) were expressed by neoplastic feline MC. In fact, it is well established that the TKI used not only interact with one or two targets in neoplastic cells, but with many different target kinases (Hantschel et al., 2007; Rix et al., 2007). An alternative explanation would be that these TKI have a different drug-transport kinetics into and out of neoplastic MC which could explain the different response profiles.
Fig. 3.
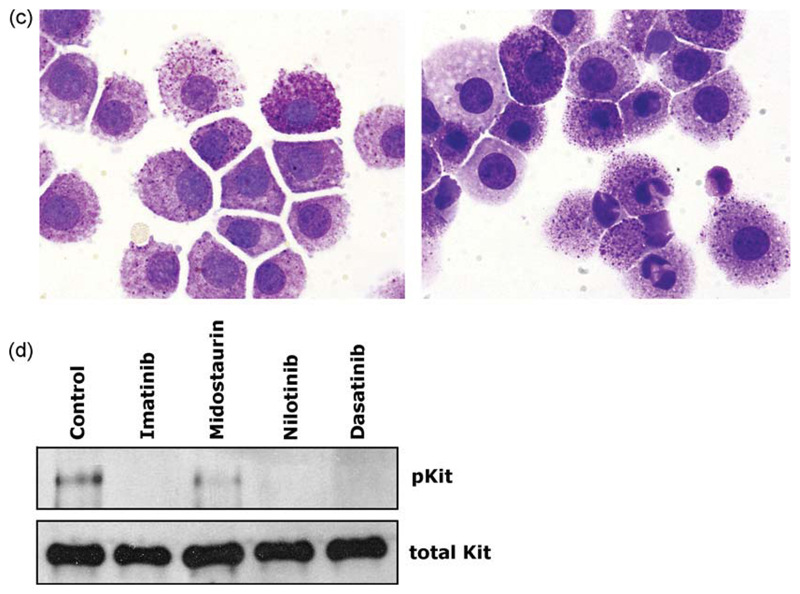
TKI inhibit growth and survival of feline neoplastic mast cells. (A) Feline neoplastic mast cells obtained from patient #2 were cultured in control medium (Co) or in the presence of various concentrations of dasatinib, imatinib, midostaurin, and nilotinib (as indicated) at 37 °C for 48 h. Then, cells were examined for 3H-thymidine uptake. Results show the percentage of 3H-thymidine uptake compared to medium control (=100%) and represent the mean ± SD of triplicates. (B) TKI-induced apoptosis in feline neoplastic mast cells. Neoplastic mast cells isolated from patient #2 (upper panel) and patient #3 (lower panel) were cultured in control medium (control) or with various TKI (dasatinib, imatinib, nilotinib, and midostaurin) at 0.5 μM (black bars) or 1 μM (open bars) for 24 h (left graph) or 48 h (right graph). Then, the numbers (percentage) of apoptotic cells were assessed by light microscopy. (C) Wright–Giemsa-stained mast cells in patient #2 after incubation in control medium (left panel) or in dasatinib at 1 μM for 24 h (right panel). As visible dasatinib induced apoptosis in neoplastic feline mast cells. (D) TKI-induced dephosphorylation of Kit in feline neoplastic mast cells. Cells isolated from primary neoplastic mast cells were incubated with the tyrosine kinase inhibitors imatinib, nilotinib, midostaurin, and dasatinib (each 1 μM) at 37 °C for 4 h. Then, the expression of phosphorylated Kit (pKit) and of total Kit was analyzed by Western blotting and immunopreciptiation (IP) as described in the text.
3.4. TKI-induced growth arrest in neoplastic feline MC is associated with apoptosis
A number of previous studies have shown that various TKI including dasatinib and midostaurin induce apoptosis in human neoplastic MC (Shah et al., 2006; Gleixner et al., 2006, 2007a, 2007b). To explore the mechanism of drug-induced inhibition of growth in MC, we examined the effects of the four TKI on survival of neoplastic feline MC in the present study. In these experiments, we were able to show that all four TKI applied induce apoptosis in neoplastic feline MC at 0.5 μM and 1 μM (Fig. 3B and C). Fig. 3C shows an example for the apoptosis-inducing effect of dasatinib on neoplastic feline MC (patient #2). As visible, the TKI-induced typical signs of apoptosis in MC, with nuclear chromatin condensation, fragmentation of nuclei, and cell size shrinkage. These data suggest that Kit, and possibly also other TKI targets, are important survival factors in neoplastic feline MC.
3.5. Effects of TKI on expression of phosphorylated Kit in neoplastic feline MC
The TK receptor Kit is expressed on normal and neoplastic MC and is considered essential for MC growth and survival (Galli et al., 1993; Valent, 1994; Akin and Metcalfe, 2004; Metcalfe, 2008). In MC neoplasms, Kit is frequently phosphorylated in neoplastic MC in a ligand/factor-independent manner (Furitsu et al., 1993; Nagata et al., 1995; Longley et al., 1996; Valent et al.,2005; Gleixner et al., 2007b). To confirm that Kit is also phosphorylated and is a relevant target of TKI in neoplastic feline MC, we performed Western blot and IP analysis in one patient with SM. As expected, we found that Kit is constitutively phosphorylated in neoplastic feline MC (Fig. 3D). Inaddition, all TKI applied, i.e. imatinib, midostaurin, nilotinib, and dasatinib (each at 1 μM) were found to down regulate expression of phosphorylated Kit without influencing the expression of total Kit in neoplastic MC (Fig. 3D). These data confirm that Kit is a relevant target in feline SM and suggest that TKI-induced apoptosis and growth arrest through targeting of Kit TK activity in feline neoplastic MC.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vetimm.2009.05.007.
Acknowledgements
This study was supported by Von Fircks-Fonds and a cooperation-grant of the Clinic for Internal Medicine and Infectious Diseases, Veterinary University of Vienna; by the Austrian Federal Ministry for Science and Research, GENAU-C.h.i.l.d. grant #GZ 200.136/1-VI/1/2005, and by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF), grants #P21173-B13 and #SFB-F01820.
References
- Agis H, Krauth MT, Bohm A, Mosberger I, Mullauer L, Simonitsch-Klupp I, Walls AF, Horny HP, Valent P. Identification of basogranulin (BB1) as a novel immunohistochemical marker of basophils in normal bone marrow and patients with myeloproliferative disorders. Am J Clin Pathol. 2006;125:273–281. doi: 10.1309/M9FQ-MQGF-6616-7N2X. [DOI] [PubMed] [Google Scholar]
- Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, Metcalfe DD. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114:13–19. doi: 10.1016/j.jaci.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Allan R, Halsey TR, Thompson KG. Splenic mast cell tumour and mastocytaemia in a cat: case study and literature review. N Z Vet J. 2000;48:117–121. doi: 10.1080/00480169.2000.36176. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Andrews L, Holzworth J. Tumours and tumour-like lesions. In: Holzworth J, editor. Diseases of the Cat. Vol. 1. WB Saunders; Philadelphia, USA: 1987. pp. 406–596. [Google Scholar]
- Confer AW, Langloss JM, Cashell IG. Long-term survival of two cats with mastocytosis. J Am Vet Med Assoc. 1978;172:160–161. [PubMed] [Google Scholar]
- Dank G, Chien MB, London CA. Activating mutations in the catalytic or juxtamembrane domain of c-kit in splenic mast cell tumors of cats. Am J Vet Res. 2002;63:1129–1133. doi: 10.2460/ajvr.2002.63.1129. [DOI] [PubMed] [Google Scholar]
- Downing S, Chien MB, Kass PH, Moore PE, London CA. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c-kit in mast cell tumors of dogs. Am J Vet Res. 2002;63:1718–1723. doi: 10.2460/ajvr.2002.63.1718. [DOI] [PubMed] [Google Scholar]
- Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL. Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial. Cancer. 2006;107:345–351. doi: 10.1002/cncr.21996. [DOI] [PubMed] [Google Scholar]
- Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y, Matsuzawa Y, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil BK. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993;142:965–974. [PMC free article] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, Gruze A, Samorapoompichit P, Manley PW, Fabbro D, Pickl WF, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C, Lee FY, Aichberger KJ, Manley PW, Fabbro D, Pickl WF, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007a;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Rebuzzi L, Mayerhofer M, Gruze A, Hadzijusufovic E, Sonneck K, Vales A, Kneidinger M, Samorapoompichit P, Thai-wong T, Pickl WF, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Exp Hematol. 2007b;35:1510–1521. doi: 10.1016/j.exphem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gotlib J. KIT mutations in mastocytosis and their potential as therapeutic targets. Immunol Allergy Clin North Am. 2006;26:575–592. doi: 10.1016/j.iac.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C, Kajiguchi T, Ruan J, Lilleberg SL, Durocher JA, Lichy JH, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816 V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre R, Millet P, Groulade P. Systemic mastocytosis in a cat: remission after splenectomy. J Small Anim Pract. 1979;20:769–772. doi: 10.1111/j.1748-5827.1979.tb06691.x. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Rix U, Schmidt U, Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL, Ellmeier W, Valent P, Superti-Furga G. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann KM, Moser A, Lohse P, Winkler A, Binder B, Sovinz P, Lackner H, Schwinger W, Benesch M, Urban C. Successful treatment of progressive cutaneous mastocytosis with imatinib in a 2-year-old boy carrying a somatic KIT mutation. Blood. 2008;112:1655–1657. doi: 10.1182/blood-2008-03-147785. [DOI] [PubMed] [Google Scholar]
- Isotani M, Tamura K, Yagihara H, Hikosaka M, Ono K, Washizu T, Bonkobara M. Identification of a c-kit exon 8 internal tandem duplication in a feline mast cell tumor case and its favorable response to the tyrosine kinase inhibitor imatinib mesylate. Vet Immunol Immunopathol. 2006;114:168–172. doi: 10.1016/j.vetimm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz JL, Post GS, Brodsky E. A phase I clinical trial evaluating imatinib mesylate (Gleevec) in tumor-bearing cats. J Vet Intern Med. 2005;19:860–864. doi: 10.1892/0891-6640(2005)19[860:apicte]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Liska W, MacEwan E, Zaki F, Garvey M. Feline systemic mastocytosis: a review and results of splenectomy in seven cases. J Am Anim Hosp Assoc. 1979;15:589–597. [Google Scholar]
- Litster AL, Sorenmo KU. Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J Feline Med Surg. 2006;8:177–183. doi: 10.1016/j.jfms.2005.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp Hematol. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, Duffy T, Jacobs P, Tang LH, Modlin I. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, McMahon G, Longley BJ. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander-McCrary H, Henry C, Potter K, Tyler J, Buss M. Cutaneous mast cell tumours in cats: 32 cases (1991–1994) J Am Anim Hosp Assoc. 1998;34:281–284. doi: 10.5326/15473317-34-4-281. [DOI] [PubMed] [Google Scholar]
- Morini M, Bettini G, Preziosi R, Mandrioli L. C-kit gene product (CD117) immunoreactivity in canine and feline paraffin sections. J Histochem Cytochem. 2004;52:705–708. doi: 10.1177/002215540405200515. [DOI] [PubMed] [Google Scholar]
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuzzi L, Willmann M, Sonneck K, Gleixner KV, Florian S, Kondo R, Mayerhofer M, Vales A, Gruze A, Pickl WF, Thalhammer JG, et al. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007;115:320–333. doi: 10.1016/j.vetimm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Rix U, Hantschel O, Dürnberger G, Remsing-Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Köcher T, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- Riva F, Brizzola S, Stefanello D, Crema S, Turin L. A study of mutations in the c-kit gene of 32 dogs with mastocytoma. J Vet Diagn Invest. 2005;17:385–388. doi: 10.1177/104063870501700416. [DOI] [PubMed] [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816 V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- Valent P. The riddle of the mast cell: kit(CD117)-ligand as the missing link? Immunol Today. 1994;15:111–114. doi: 10.1016/0167-5699(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Valent P, Akin C, Sperr WR, Mayerhofer M, Födinger M, Fritsche-Polanz R, Sotlar K, Escribano L, Arock M, Horny HP, Metcalfe DD. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005;46:35–48. doi: 10.1080/10428190400010775. [DOI] [PubMed] [Google Scholar]
- Valent P, Ashman LK, Hinterberger W, Eckersberger F, Majdic O, Lechner K, Bettelheim P. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood. 1989;73:1778–1785. [PubMed] [Google Scholar]
- Valent P, Ghannadan M, Akin C, Krauth MT, Selzer E, Mayerhofer M, Sperr WR, Arock M, Samorapoompichit P, Horny HP, Metcalfe DD. On the way to targeted therapy of mast cell neoplasms: identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur J Clin Invest. 2004;34(S2):41–52. doi: 10.1111/j.0960-135X.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- Valent P. Systemic mastocytosis. Cancer Treat Res. 2008;142:399–419. doi: 10.1007/978-0-387-73744-7_18. [DOI] [PubMed] [Google Scholar]
- Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Tefferi A, Cortes J, O'Brien S, Garcia-Manero G, Pardanani A, Akin C, Faderl S, Manshouri T, Thomas D, Kantarjian H. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14:3906–3915. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Vet Pathol. 2002;39:529–535. doi: 10.1354/vp.39-5-529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.