Highlights
-
•
Children with ASD, including those with minimal language, showed EEG evidence of semantic processing.
-
•
In children with ASD, semantic processing was characterized by delayed speed of processing and limited integration with mental representation.
-
•
There was variability in ERP patterns across children with ASD, suggesting that semantic processing may be a deficit for some individuals.
Keywords: EEG, ERP, Language, Semantics, Autism, ASD
Abstract
25% of children with autism spectrum disorder (ASD) remain minimally verbal (MV), despite intervention. Electroencephalography can reveal neural mechanisms underlying language impairment in ASD, potentially improving our ability to predict language outcomes and target interventions. Verbal (V) and MV children with ASD, along with an age-matched typically developing (TD) group participated in a semantic congruence ERP paradigm, during which pictures were displayed followed by the expected or unexpected word.
An N400 effect was evident in all groups, with a shorter latency in the TD group. A late negative component (LNC) also differentiated conditions, with a group by condition by region interaction. Post hoc analyses revealed that the LNC was present across multiple regions in the TD group, in the mid-frontal region in MVASD, and not present in the VASD group. Cluster analysis identified subgroups within the ASD participants. Two subgroups showed markedly atypical patterns of processing, one with reversed but robust differentiation of conditions, and the other with initially reversed followed by typical differentiation. Findings indicate that children with ASD, including those with minimal language, showed EEG evidence of semantic processing, but it was characterized by delayed speed of processing and limited integration with mental representations.
1. Introduction
There is substantial variability in the language outcomes of children with autism spectrum disorder (ASD). Most individuals with ASD evidence some level of language impairment, with only a small minority showing fully intact language development (Anderson et al., 2007; Pickles et al., 2014). Approximately 25–30% of children with ASD remain minimally verbal, using at most single words and fixed phrases to communicate (Pickles et al., 2014; Tager-Flusberg and Kasari, 2013). Even with intensive intervention, many children continue to have significant expressive language impairments. While pre-intervention language abilities, non-verbal cognitive abilities and joint attention have been consistently identified as predictors of treatment response (Perry et al., 2010), individual outcomes are highly variable and difficult to predict (Eapen et al., 2013; Howlin et al., 2009; Stahmer et al., 2011).
While united by their limited expressive language, there seems to be significant heterogeneity in receptive language ability across the minimally verbal population. Some children are only able to demonstrate understanding of a few words, while others have receptive language abilities far greater than their expressive language (Plesa Skwerer et al., 2015; Rapin et al., 2009). Accurate characterization of receptive language in minimally verbal children is important both scientifically and clinically. This information is necessary for research that seeks to understand the causes and developmental courses of language impairment in ASD and related disorders. Clinically, understanding the limits of a child’s receptive language will influence treatment planning, and facilitate targeted interventions. One of the major barriers to measuring abilities in the minimally verbal population is the limited availability of appropriate assessments (Kasari et al., 2013). Standardized assessments rely on the child’s ability to selectively attend to the task, to understand directions and to provide overt behavioral responses. These requirements are challenging for many minimally verbal children and are likely interfere with the acquisition of accurate results (Tager-Flusberg, 1999). A recent study investigating multiple methods for assessing receptive language in minimally verbal children with ASD found significant heterogeneity across participants and methods, highlighting the difficulties in obtaining reliable measurements in this population (Plesa Skwerer et al., 2015). Additionally, although standardized assessments can document the degree of language impairment, they are inadequate to reveal neurodevelopmental differences underlying the observed deficits.
Electroencephalography (EEG) represents a promising methodology to measure neural correlates of language processing in the minimally verbal ASD population (Tager-Flusberg and Kasari, 2013). Event-Related Potentials (ERPs) reflect the time-locked electrophysiological response to stimuli. ERPs measure the synchronized post-synaptic activity of large groups of neurons, reflecting cognitive processing in response to the presentation of a stimulus. ERP components are classified according to their polarity (positive or negative deflection) and the latency of their peak. Due to the exquisite temporal specificity of ERPs, they offer a method for tracking language manipulations through time with millisecond resolution. Such an instantaneous and continuous measure is well suited for evaluating the inherently incremental nature of language processing (Kutas and Federmeier, 2011). Additionally, electrophysiology is minimally invasive, does not rely on children’s ability to understand directions and requires no overt behavioral response, making suitable for use with young children and developmental populations (Jeste and Nelson, 2009).
In typical development, lexical-semantic processing has been extensively explored using ERP paradigms. These paradigms tap into knowledge of word meanings by presenting semantically congruent or incongruent information, which elicits a negative peak around 400 ms (N400) after the stimulus that is larger to the mismatch condition (e.g. Kutas and Hillyard, 1980; Bentin et al., 1985). This N400 represents lexical-semantic processing (Hagoort, 2003), where the priming effect reflects facilitated access to word meanings and conceptual representations (Friedrich and Friederici, 2010). In young children (3–4 years old), semantic violations may elicit an initial negative peak around 400 ms, along with a negative slow wave that persists through 800 ms, possibly reflecting initial semantic processing followed by integration with mental representations (Silva-Pereyra et al., 2005).
The N400 has been used as a biomarker to differentiate minimally conscious patients from those in a vegetative state (Rohaut et al., 2015), to index the severity of comprehension deficits in adults with aphasia (Chang et al., 2016), and to assess receptive language in individuals with limited speech (Connolly et al., 1999). The N400 effect has been demonstrated consistently in children as young as 14 months (Friederici, 2005), making it a good candidate for investigating semantic processing in minimally verbal children who may have young developmental ages. In addition, the magnitude of the N400 response has been found to relate to language ability in children with language impairment. Larger N400 amplitude was associated with better nonsense word repetition in a study of children with specific language impairment (Kaganovich et al., 2016), and with better phonological and lexical development in children with developmental language disorder (Kornilov et al., 2015).
There have been a limited number of studies on semantic processing in children and adults with ASD, primarily focused on individuals with IQs in the average range. These studies have yielded mixed findings, some showing reduced or absent N400 responses in individuals with ASD, while others have found an intact N400 response or other ERP response. Relatively intact N400 effects have been demonstrated in adults with ASD, using a sentence congruence paradigm (Fishman et al., 2011), as well as in children (Méndez et al., 2009) and adults (Coderre, 2017) in the context of single-word priming paradigms.
Two studies have found altered, but not absent semantic processing in individuals with ASD. Ribeiro et al. (2013) investigated semantic processing in seven children with high functioning autism, using a semantic congruency sentence task. They found that there was no N400 response in the children with ASD, but instead the conditions were differentiated by a late positive potential (LPP). Pijnacker et al. (2010) also found absent N400 responses in adults with ASD, but a larger LPP to semantically incongruous sentences.
Two additional studies found that children with ASD failed to show any ERP evidence of semantic processing. Dunn and Bates (2005) investigated semantic processing in 18 children with ASD using an in-versus-out-of-category words task (not a priming task) and found that N400 responses in the ASD group did not differ by condition. McCleery et al. (2010) compared verbal and nonverbal semantic integration in 14 young children with high functioning autism (mean age 5.8 years). They found that the N400 effect was absent in the children with ASD during a picture-word priming paradigm, but was present when the pictures were instead paired with environmental sounds.
Only one study to date has examined ERP correlates of semantic processing in minimally verbal children with ASD. Ten participants with ASD (4–7 years old) and ten age-matched typically developing children participated in a picture-word priming paradigm. Participants were minimally verbal based on parent report of daily language use (no language, single words or occasional phrases). The ERP paradigm consisted of a color photo displayed on a screen, followed by a spoken word that matched or mismatched the picture, followed by a second spoken word that was either semantically related or unrelated to the first word. There was no N400 effect in the ASD group overall, although there was substantial inter-individual variability with half of the children showing an intact N400 response based on visual inspection of the waveforms (Cantiani et al., 2016).
The mixed findings from this small body of research are likely related to multiple factors. First, the paradigms used to elicit the N400 effect varied across studies, including picture and single word priming paradigms, sentence congruency paradigms, and an in-versus-out-of-category task that required participants to make a judgment on the fit of the word without having a direct prime. Additionally, these studies did not investigate variability within the ASD group, or the association between ERP measures and language ability. Within group variation is likely an important factor contributing to mixed findings. Finally, with the exception of Cantiani et al., all of the studies reviewed here include only highly verbal individuals with ASD. Research that seeks to understand the mechanisms of language impairment in ASD must include participants with a wide range of abilities, including those with minimal language. By using ERP measures to examine language processing across a wide range of children with ASD, we can potentially identify biomarkers of language impairment. These markers can inform our understanding of the mechanisms of language impairment, inform prognosis, and serve as measures of treatment response.
To begin to answer these questions, we investigated ERP correlates of semantic processing in both MV and verbal children with ASD, and an age and sex-matched TD control group. We first asked whether neural correlates of semantic processing differentiated minimally verbal children from verbal children with ASD, and from TD children. We then examined variability across participants with ASD, evaluated whether ERP correlates were associated with behavioral measures of language, and investigated subgroups within the ASD participants. We hypothesized that as a group, minimally verbal children with ASD would show reduced ERP evidence of semantic processing compared with verbal children with ASD and TD children. Further, we predicted that there would be substantial heterogeneity within the ASD groups, and that degree of abnormality/reduction in ERP correlates of semantic processing would be associated with more impaired language functioning.
2. Methods
2.1. Participants
A total of 58 children (5–11 years old) were recruited: minimally verbal children with ASD (MVASD, N = 20), verbal children with ASD (VASD, N = 20) and typically developing children (TD, N = 18). Groups were matched on chronological age and sex. Children in the ASD groups had a previous clinical diagnosis of ASD, made through the California State Regional Center, independent clinical psychologists, child psychiatrist, and/or developmental pediatricians. Diagnosis was confirmed by the research team using the Autism Diagnostic Observation Schedule (ADOS) and Social Communication Questionnaire (SCQ). “Minimally verbal” was defined as receiving a Module 1 ADOS (see “Assessments” section below for more information), indicating that the child used no language, or primarily single words to communicate. Exclusionary criteria included other neurological abnormalities (including active epilepsy), birth-related complications and uncorrected vision or hearing impairment. Additional exclusionary criteria for the TD group included history of developmental delays, need for special services in school, diagnosis of psychiatric conditions, or a first-degree relative with an ASD diagnosis. All components of this study were approved by the University of California Los Angeles Institutional Review Board. Informed consent was collected from the parents of all participants. Child assent was collected from children who had sufficient language and cognitive abilities to demonstrate understanding of the study procedures.
All TD participants provided sufficient EEG data to be included in analyses. In the ASD groups, 7 participants (5 VASD, 2 MVASD) were unable to provide sufficient high quality EEG data due to behavioral dysregulation. Groups did not significantly differ on chronological age (F(2, 49) = 2.09, p = 0.13) or sex ratio (X2 = 1.07, p = 0.59). As expected, groups significantly differed on verbal IQ (F(2, 49) = 102.55, p < 0.001), non-verbal IQ (F(2, 49) = 37.57, p < 0.001) and receptive language (F(2, 49) = 64.40, p < 0.001). VASD and MVASD groups significantly differed from each other and from the TD group on all measures (all p-values < 0.01). See Table 1 for participant characteristics by group. Table 2 displays age distribution by group.
Table 1.
Participant Characteristics.
| Group | N | Sex Ratio male/female | Age (months) M (SD) | Verbal IQ M (SD) | Non-Verbal IQ M (SD) | Receptive Lang M (SD) |
|---|---|---|---|---|---|---|
| TD | 18 | 13/5 | 91.61 (24.50) | 118.28 (16.19) | 115.11 (16.50) | 121.67 (11.25) |
| VASD | 15 | 13/2 | 88.67 (22.04) | 75.97 (24.71) | 86.52 (32.81) | 78.57 (27.93) |
| MVASD | 18 | 12/4 | 92.42 (22.53) | 27.17 (14.32) | 42.89 (19.69) | 36.82 (21.50) |
Abbreviations: TD - typically developing, VASD - verbal ASD, MVASD - minimally verbal ASD.
Table 2.
Number of Participants at Each Age by Group.
| 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | 11 years | |
|---|---|---|---|---|---|---|---|
| TD | 5 | 2 | 3 | 2 | 4 | 1 | 1 |
| VASD | 5 | 4 | 1 | 2 | 1 | 1 | 1 |
| MVASD | 4 | 1 | 3 | 5 | 3 | 1 | 1 |
Abbreviations: TD - typically developing, VASD - verbal ASD, MVASD - minimally verbal ASD.
2.2. Assessments
2.2.1. ASD diagnosis and language level
The Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al., 2012) was administered to all ASD participants by study staff to confirm diagnosis and to determine minimally verbal status. The ADOS modules (1–3) are chosen based on the participant’s language level. Module 1 is designed for participants with no or minimal language, module 2 is used for participants with phrase speech and module 3 is used for participants with fluent language. All participants in the MVASD group received a module 1 ADOS, while participants in the VASD group received a module 2 or 3 ADOS. Diagnosis was further confirmed with the Social Communication Questionnaire (SCQ; Rutter et al., 2003). The SCQ is a parent report measure with questions based on the Autism Diagnostic Interview-Revised, which assesses the degree of social communication impairment. Scores over a certain threshold indicate a high likelihood of an ASD diagnosis. All participants in the ASD groups had SCQ scores above the ASD threshold, while no TD participants had elevated scores.
2.2.2. Cognitive and language abilities
Cognitive abilities were assessed primarily with the Differential Abilities Scale-Second Edition (DAS-II; (Elliot, 2007). For participants who were not able to achieve a score on the DAS-II, the Mullen Scales of Early Learning (MSEL; (Mullen, 1995) was used instead (N = 10, all MVASD). From these measures, ratio scores for full scale IQ (FSIQ), non-verbal IQ (NVIQ) and verbal IQ (VIQ) were calculated for each child, based on the age-equivalent score and chronological age. Ratio scores were used to account for the scores of children who performed outside of the standardized norms for their chronological age. For children who were tested with the DAS-II, NVIQ and VIQ were calculated from the protocol-specific sub-scores. For children who were administered the MSEL, VIQ was calculated using the average of the Receptive Language and Expressive Language subscale scores, and NVIQ was calculated using the average of the Visual Reception and Fine Motor subscale scores (Akshoomoff, 2006). There is high convergent validity between the MSEL and the DAS-II, supporting the combination of assessments through standardized scores (Bishop et al., 2011). Receptive vocabulary was assessed with the Peabody Picture Vocabulary Test, 4th Edition (PPVT-4; Dunn and Dunn, 2007) in all participants.
2.3. Procedure
Participants with ASD first participated in an EEG net familiarization and desensitization procedure (DiStefano et al., 2018; Roesler et al., 2013). Prior to the day of testing, parents of participants were interviewed regarding their child’s preferences and interests so that the testing environment could be made as comfortable as possible with the child’s preferred reinforcers available (e.g. a favorite snack). Pictures of a child undergoing EEG testing were sent ahead of the session for parents to review with their child. On the day of testing, behavioral strategies were used to acclimate participants to the testing environment, including modeling, incremental practice and positive reinforcement. Participants first underwent the EEG recording, followed by behavioral testing. All study procedures were completed in a single visit.
2.4. Stimuli
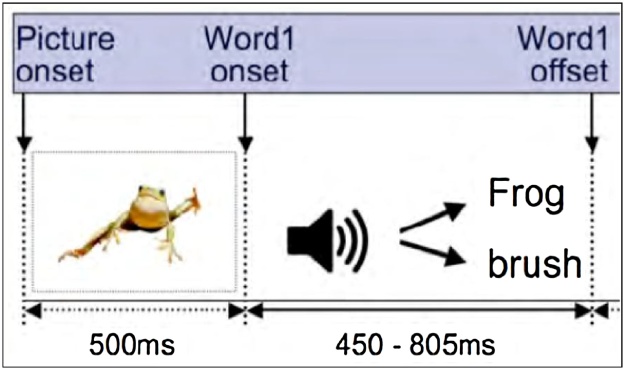
This study employed a picture-word matching paradigm, adapted from the paradigm reported by Cantiani et al. (2016a,b). Vocabulary included 60 basic nouns taken from the MacArthur-Bates Communicative Development InventorieS-2nd edition. Visual stimuli were presented on a white background on the compute monitor and consisted of 60 color photographs of animals (e.g. bird, dog) and inanimate objects (e.g. doll, bike). Visual stimuli were paired with auditory stimuli consisting of either the correct spoken word (match) or a word neither semantically nor phonologically related to the picture (mismatch) (Fig. 1). Auditory stimuli were produced by a female native speaker of English and digitally recorded at a sampling rate of 44.1 kHz (16 bit; mono). Words varied in duration from 450 to 805 ms, and were presented via two speakers situated 30 degrees to the right and left of midline, in front of the participant. See Cantiani et al. (2016a,b) for more details on the creation of the paradigm.
Fig. 1.
Lexical-Semantic Knowledge ERP Paradigm.
ERP paradigm experimental design. Visual stimuli (color photograph) appeared on the screen first, and remained throughout the trial. Auditory stimuli (matching or mismatching word) onset 500 ms into the trial. Paradigm was adapted from Cantiani et al. (2016).
Each block consisted of 30 trials (50% mismatch). Four blocks were presented, for a total of 120 trials. Across the experiment, each picture was presented twice, once in each condition. Each trial began with a picture presented on the screen for 2000 ms. A word that either matched or mismatched the picture was presented after a 500 ms delay, overlapping with the picture on the screen. Participants were asked to look at the picture and listen to the words. No behavioral responses were required and the experiment lasted approximately 6 min. The entire session was video recorded to enable later removal of trials where the participants were not attending to the screen.
2.5. EEG acquisition and processing
EEG was recorded using a 128-channel HydroCel Geodesic Sensor Net, with impedances kept below 100 kOhms in all electrodes. Recordings were sampled at 500 Hz with an online filter of 0.1–100 Hz and then digitized with a 12-bit National Instruments board. Raw EEG data were referenced online to vertex (Cz). Recordings were processed off-line using NetStation 4.4.5 software (Electrical Geodesics, Inc.). EEG was digitally filtered using a 0.3–30 Hz bandpass filter, segmented into 1000 ms epochs starting at 100 ms before the auditory stimulus onset, and baseline corrected using mean voltage during the 100 ms pre-stimulus baseline period. Data were then processed using an automated artifact detection tool that rejected channels if the amplitude difference (max-min) was greater than 150 mV. The purpose of this automated detection is to remove channels that have grossly noisy data, usually from excessive electrode movement, net manipulation or drift. Following this automatic artifact detection, each trial was visually inspected to remove any remaining channels that contained EMG, eye-blink, or eye-movement artifacts from further analysis. Trials with more than 15% bad channels were rejected. Bad channels in the data of trials containing fewer than 15% bad electrodes were replaced using a spherical spline interpolation algorithm (Srinivasan et al., 1996). The data were then averaged for each participant for each condition and re-referenced to an average reference.
After artifact rejection, only subjects with more than 10 artifact-free trials per condition were accepted for further analysis. Although there was variability across subjects with regard to total number of good trials, the choice of using a minimum threshold of 10 trials per condition is based on prior literature using this convention (e.g. de Haan and Nelson, 1999; Jeste et al., 2012; Olvet and Hajcak, 2009).
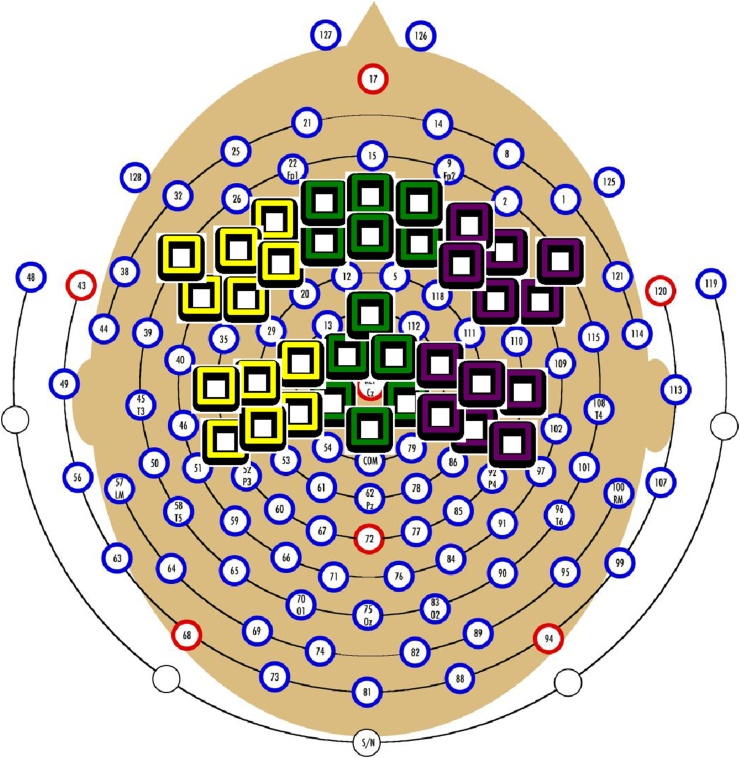
The number of artifact-free trials significantly differed across groups for both the match (F(2,49) = 5.12, p = 0.10) and mismatch conditions (F(2,49) = 6.83, p = 0.002). VASD and MVASD did not differ from each other in number of trials retained (match t(32) = −0.44, p = 0.66; mismatch t(32) = 0.57, p = 0.57), while significantly more trials were retained in the TD group (match t(50) = 3.20, p = 0.002; mismatch t(50) = 3.69, p = 0.001). In the TD group, an average of 31.75 trials were retained per condition. Average trials retained were 21.57 in the VASD group and 21.36 in the MVASD group. Regions of interest were generated with clusters of electrodes in right, middle and left frontal and central regions (see Fig. 2). Regions were chosen based on research demonstrating that in young children, the N400 effect is maximal over anterior regions (e.g. Friedrich and Friederici, 2010; Silva-Pereyra et al., 2005).
Fig. 2.
Electrode Groupings.
Six regions of interest were chosen across left/midline/right and frontal/central areas.
Time windows for the components were chosen based on prior literature and visual inspection of data from both groups, with a window wide enough to include the peaks for each participant. Mean amplitude and fractional area latency were calculated for the N400 in the 400–550 ms window. To capture the long negative wave described in previous research with this age group, we also calculated the mean amplitude in the 550–900 ms window. We refer to this as the late negative component (LNC). In addition, difference scores for the N400 and LNC amplitude variables were calculated using the difference of the mismatch value from the match value.
2.6. Analysis
Repeated measures ANOVAs were used to examine lexical-semantic processing across groups. Separate analyses were performed for the N400 amplitude and latency, as well as the LNC amplitude. For N400 and LNC amplitude, models included the between-subjects factor group (MVASD, VASD, TD) and within-subjects factors region (6 regions: left/midline/right, frontal/central) and condition (match, mismatch). For N400 latency, the model included the between-subjects factor group and within-subjects factor region. Post-hoc contrasts were conducted based on the results of the omnibus ANOVA analyses for the significance of the main effects and interaction terms. False Discovery Rate (FDR) correction was used to correct for multiple testing involving all 40 contrasts considered from the three models (for N400 amplitude, latency and LNC amplitude). P-values reported below are the FDR-corrected values, and effect sizes reported correspond to Cohen’s f, where effect sizes of 0.10, 0.25, and 0.40 are generally regarded as small, moderate, and large, respectively (Cohen, 1999). Table 3 presents means and standard deviations corresponding to LNC contrasts.
Table 3.
Means and Standard Deviations of LNC Amplitude by Region and Group.
| Condition | ROI | LNC Mean Amplitude M(SD) |
||
|---|---|---|---|---|
| TD | VASD | MVASD | ||
| Match | LF | −2.41 (4.23) | −2.83 (6.93) | 2.11 (9.71) |
| MF | −1.34 (4.44) | −4.54 (6.27) | 0.20 (9.63) | |
| RF | −0.76 (3.33) | −5.76 (7.43) | −2.05 (7.32) | |
| LC | −1.27 (2.12) | 1.51 (5.88) | 1.30 (5.59) | |
| MC | −0.30 (2.52) | 1.43 (2.82) | 1.27 (4.69) | |
| RC | 1.60 (2.33) | 0.38 (3.45) | −.34 (3.47) | |
| Mismatch | LF | −3.93 (3.60) | −2.18 (4.69) | −2.81 (5.34) |
| MF | −6.52 (5.62) | −2.38 (8.78) | −5.74 (6.58) | |
| RF | −4.48 (3.72) | −2.74 (7.61) | −4.23 (5.52) | |
| LC | −0.43 (2.55) | 0.55 (3.15) | −0.61 (3.97) | |
| MC | −1.92 (3.99) | −0.43 (4.24) | −0.66 (3.74) | |
| RC | −0.75 (1.90) | −0.27 (4.15) | −0.14 (3.08) | |
Abbreviations: TD – typically developing, VASD – verbal ASD, MVASD – minimally verbal ASD, ROI – region of interest, LF – left frontal, MF – mid frontal, RF – right frontal, LC – left central, MC – mid central, RC – right central.
Variability within the ASD groups was investigated using correlational analyses and cluster analysis. First, partial correlations controlling for age were used to explore the relationship between ERP variables and behavioral measures of language and cognition. Next, k-means cluster analysis was used to identify subgroups within the ASD participants and the profile for each cluster was described in terms of both ERP results and behavioral measures. Analyses were carried out using IBM SPSS Statistics for Mac, Version 24 (IBM Corp., 2016).
3. Results
3.1. Group comparisons
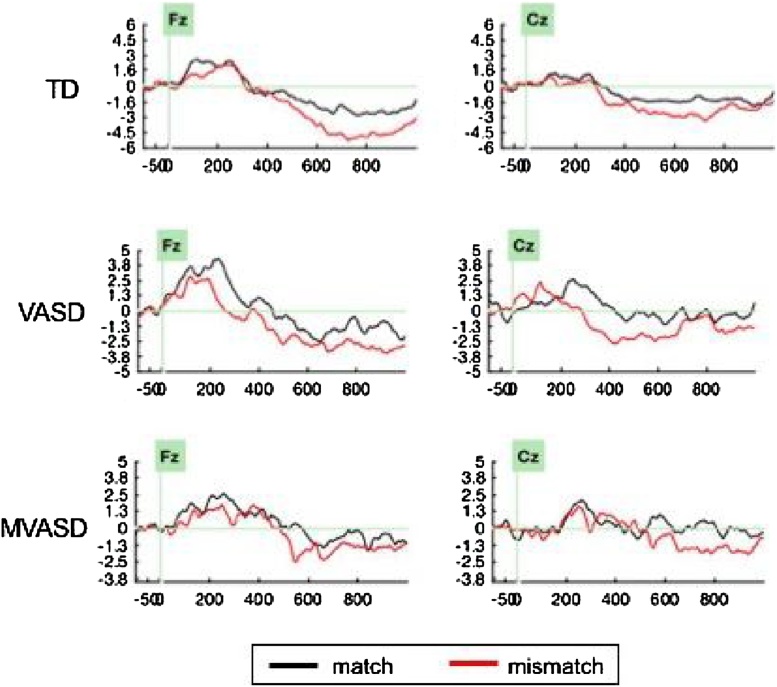
Fig. 3 shows grand average ERP results by group. Although the averaged waveform appears to indicate a single, slow negativity, the individual results showed two distinct negative components (see Fig. 4). The first negativity peaked around 450 ms, consistent with the canonical N400, while the second negativity occurred between 600 and 900 ms, corresponding to the LNC. Note that even though the two distinct negativities are visible for most subjects, they become a single, wide negativity in the grand average, due to misalignment in the latency of N400 across subjects. Hence, the grand average ERP results for groups should be interpreted only for the group specific condition difference for LNC. Indeed the significantly lower LNC corresponding to mismatch compared to the match condition is visible in the TD and MVASD groups in the mid frontal region.
Fig. 3.
Grand Average ERPs by Group and Region.
Grand average ERP results by group, shown for mid-frontal and mid-central electrodes.
Fig. 4.
Sample Individual ERP Waveforms.
Two examples of individual waveforms (top: TD, bottom: ASD [verbal participant], showing separate N400 and LNC components.
Analysis of the N400 amplitude yielded a main effect of condition (F(1) = 6.06, p = 0.018, ES = 0.34). Main effects of group and region and their interaction were not found significant for N400 amplitude. There was a significant condition difference (more negative in the mismatch condition) in N400 amplitude across groups and regions (t(50) = 2.57, p = 0.045, ES = 0.36, match M(SD) = −0.18(2.90), mismatch M(SD) = −1.24(3.11)). N400 latency showed a main effect of group (F(2) = 13.27, p = <0.001, ES = 0.81), with no main effect of region or region by group interaction. There was a significant group difference for N400 latency, with the latency being shorter in the TD group (M(SD) = 457.6(11.72)) compared to VASD (t(31) = −4.85, p < 0.001, ES = 0.87, M(SD) = 475.43(7.66)) and MVASD (t(34) = −3.93, p = 0.01, ES = 0.67, M(SD) = 475.72(13.11)) across regions. N400 latency did not differ between VASD and MVASD groups (t(31) = 0.71, p = 0.99).
LNC analysis showed a group by condition by region interaction (F(10) = 2.5, p = 0.026, ES = 0.32). Within groups, LNC mean amplitude significantly differed between conditions (mismatch more negative than match) in the mid frontal, right frontal and right central regions in the TD group (t(17) = 4.57, t(17) = 4.78, t(17) = 3.63, p < 0.001, p < 0.001, p = 0.016, ES = 1.11, ES = 1.16, ES = 0.88, respectively), but only in the mid frontal region in the MVASD group (t(17) = 2.94, p = 0.045, ES = 0.71). The condition difference was not found significant in the VASD group. Group differences for the match-mismatch condition difference were significant in the right frontal region, with the TD group demonstrating a higher condition difference than the VASD group (t(31) = 2.86, p = 0.045, ES = 0.51).
3.2. Relationship of ERP variables to behavioral language measures
Partial correlations controlling for chronological age were used to examine the association of ERP variables to non-verbal IQ, receptive and expressive language within the ASD and TD groups. There were no significant relationships between any ERP variable (N400 amplitude, N400 latency, LNC amplitude) and cognitive or language measures in either group (all p-values > 0.05).
3.3. Individual differences
K-means cluster analysis was used to explore variability within the ASD participants. N400 amplitude difference between conditions, N400 latency and LNC amplitude difference between conditions were used as clustering variables. N400 variables were averaged across regions, while the right frontal region was used for the LNC amplitude (as this region robustly differentiated conditions in the TD group). Three, four and five cluster solutions were tested. The four-cluster solution resulted in the best fit, as it had the highest average silhouette value (0.52) (Table 4).
Table 4.
K-Means Cluster Results.
| Cluster | One – Inverse Response | Two – Mixed Response | Three – Minimal Response | Four – Moderate Response |
|---|---|---|---|---|
| N | 4 | 2 | 11 | 16 |
| N400 Latency (ms) | 475.96 | 445.93 | 485.80 | 467.60 |
| N400 Amplitude (μV) | −3.10 | −2.14 | 1.57 | 1.83 |
| LNC Amplitude (μV) | −17.34 | 2.98 | 0.8 | 2.53 |
The first cluster, referred to as the “inverse response” group, showed a large differentiation between match and mismatch conditions, but with a greater (more negative) response to the match condition. All four participants in this cluster were in the verbal ASD group. The second cluster, referred to as the “mixed” group showed moderate differentiation between conditions, with an inverse response for the N400 (match more negative) but the expected response for the LNC. Both participants in this cluster were minimally verbal, with low receptive and expressive language scores (ratio scores < 25). Clusters three and four (referred to as minimal and moderate response) showed the expected direction of differentiation between conditions (mismatch more negative than match), but varied in terms of the magnitude of the response, particularly in regard to the LNC. These were the largest clusters and had a mix of verbal abilities. One-way ANOVA analyses showed that the clusters significantly varied across all three clustering variables (N400 amplitude F(3,32) = 3.44, p = 0.03; N400 latency F(3,32) = 46.74, p < 0.001; LNC Amplitude F(3,32) = 11.15, p < 0.001).
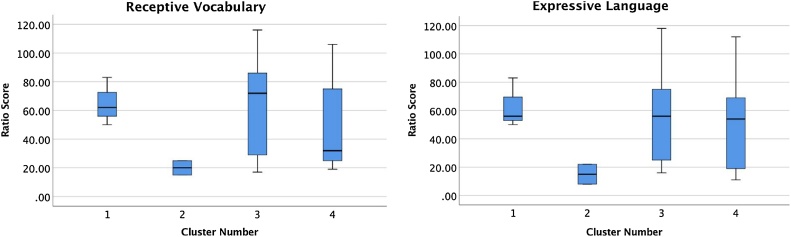
Table 5 shows receptive vocabulary (PPVT) and expressive language (cognitive assessment specific sub-test) by cluster grouping. Due to the exploratory nature of this analysis and the small group sizes, no statistical comparisons were performed (Fig. 5).
Table 5.
Language and Cognitive Skills By Cluster.
| Cluster | One – Inverse Response | Two – Mixed Response | Three – Minimal Response | Four – Moderate Response |
|---|---|---|---|---|
| Receptive Vocabulary | 65.00 (9.64) | 20.00 (5.00) | 62.78 (12.72) | 50.62 (8.68) |
| Expressive Language | 63.00 (10.15) | 15.00 (7.00) | 56.33 (12.43) | 49.08 (9.13) |
All scores are M(SD).
Fig. 5.
Boxplots of Receptive and Expressive Language Scores by Cluster.
Boxplots showing receptive and expressive language scores, by cluster.
4. Discussion
This study examined neural correlates of semantic processing in children with ASD, both minimally verbal and verbal, and compared them to age-matched typically developing children. We employed a passive picture-word priming paradigm, using common nouns. Findings indicate that children with ASD (including those with minimal language) showed some EEG evidence of semantic processing, but altered compared to typically developing children. Both ASD groups showed the expected N400 effect to semantically incongruent information, but with a longer N400 latency compared with the TD group. Additionally, verbal children with ASD did not show a late negative slow wave, while minimally verbal children showed a negative slow wave only in one region. Contrary to our hypotheses, our analyses did not show that verbal children with ASD showed more intact semantic processing than minimally verbal children. Within the full ASD group, there was substantial heterogeneity in performance, both in terms of behavioral assessments of language, and ERP variables. Our findings are somewhat inconsistent with research demonstrating an absent N400 in children with ASD (Cantiani et al., 2016; Dunn and Bates, 2005; McCleery et al., 2010), although other studies have found intact N400 effects (Coderre, 2017; Fishman et al., 2011; Méndez et al., 2009). The inconsistent findings across studies seem likely due to the variability across children with ASD, combined with relatively small sample sizes.
Our results suggest that as a group, semantic processing is not absent in children with ASD, but it is characterized by delayed speed of processing and limited integration with mental representations. Longer N400 latencies suggest that semantic information may be processed at a slower rate in children with ASD, compared with typically developing children. This finding is consistent with findings of delayed N400 in children with language impairment (e.g. Cummings and Ceponiene, 2010). Previous research has demonstrated that the latency and amplitude of the N400 decrease linearly with age across childhood, attributed to increased efficiency of lexical access and the semantic integration process (Holcomb et al., 1992). Our findings indicate that children with ASD may lag behind their same-age peers in the development of efficient lexical access.
Additionally, there were notable differences in the late negative slow wave between groups. While typically developing children evidenced a negative slow wave that was more pronounced to the mismatch condition and present over mid frontal, right frontal, and right central regions, minimally verbal children with ASD showed an amplitude difference only evident in the mid frontal region, while verbal children with ASD did not show any effect. This is contrary to our hypothesis, which was that verbal children with ASD would show more intact ERP evidence of semantic processing than minimally verbal children. This may be due in part to the substantial variability in ERP results across ASD participants, obscuring our ability to detect significant differences in the verbal ASD group. Additionally, while the groups were age matched overall, the verbal ASD group had more participants in the 5–6 year old age range, while the minimally verbal ASD group had more participants in the 7–9 year old age range. These minor variations in chronological age, along with the wide age range represented, may have contributed to the unexpected result. Alternatively, verbal children with ASD may be employing compensatory mechanisms to process semantic information, supporting their language development in the face of semantic deficits. Previous research has suggested that high functioning children with ASD may rely on enhanced perceptual processing in order to compensate for inefficient higher level semantic processing (Harris et al., 2006; Kamio et al., 2006).
Previous research with young children has suggested that while the initial N400 component indexes preliminary semantic processing and lexical retrieval, the negative slow wave is associated with integration of the stimuli with mental representations (Silva-Pereyra et al., 2005). Silva-Pereyra et al. hypothesized that this late negativity was similar to the ‘sentence closure’ effect reported in adults, in which secondary semantic processing following the end of a sentences generates a negativity around 700 ms (Friederici et al., 1999). The largely absent negative slow wave in children with ASD suggests that while they do process the preliminary semantic information, they have considerable difficulty integrating that information with broader mental representations.
Within the ASD groups, there was substantial variability across all measures. Although there were no direct correlations between ERP indices and behavioral measures of language, cluster analysis identified multiple subgroups within the ASD participants. Two subgroups showed markedly atypical patterns of processing, one with reversed but robust differentiation of conditions (all verbal participants), and the other with initially reversed followed by typical differentiation (both minimally verbal participants). The remaining subgroups showed the expected direction of differentiation between conditions (mismatch more negative than match), but varied in terms of the magnitude of the response, particularly in regard to the late negativity. Participants in the last two subgroups were split relatively evenly between verbal and minimally verbal participants, suggesting that these profiles are not specifically to expressive language impairment in ASD. While this subgroup analysis is only descriptive given the sample size, it is encouraging that meaningful clusters emerged, with potential links to behavioral characteristics. The substantial heterogeneity within the ASD population stems, in part, from the fact that a range of etiologies and neural mechanisms contribute to the ASD phenotype (Chahrour et al., 2016; De Rubeis and Buxbaum, 2015; Jeste and Geschwind, 2014; Masi et al., 2017). Given this variability, it is unlikely that the language impairments observed in ASD derive from a single underlying deficit. Instead, deficits in a variety of linguistic and cognitive processes likely combine in multiple ways to give rise to the minimally verbal phenotype. The “mixed response” cluster identified here, characterized by a reversed N400 response followed by a standard LNC response included only minimally verbal participants, both with equally impaired receptive and expressive language. Although replication with a larger sample size is necessary to draw clear conclusions, it is possible that this cluster represents a specific subgroup within the minimally verbal ASD population who evidence impaired semantic processing related to global language impairment. In contrast, the “inverse response” cluster, characterized by robust but reversed differentiation, contained only verbal participants. A reverse N400 has been observed in studies employing masked priming techniques, which prevents participants from using expectancy based strategies (Wentura and Frings, 2005). In these studies, participants demonstrate a larger N400 response to the related word compared to the unrelated word. This has been linked to a process called “center-surround inhibition” (Carr and Dagenbach, 1990), which assumes that a masked prime can only weakly activate the corresponding node. The surrounding nodes are then inhibited, in order to facilitate access to the activated prime node. In a word priming paradigm, this results in a larger amplitude N400 to the related word, reflecting the additional cognitive resources necessary to access the inhibited surrounding nodes (Carr and Dagenbach, 1990; Frings et al., 2008; Bermeitinger et al., 2008). Although not directly tested in the current study, it is possible that the reverse N400 effect observed in some children with ASD reflects a higher allocation of cognitive resources in order to activate semantic representations associated with the initial picture stimulus, thus inhibiting access to related semantic concepts. If that were the case, one hypothesis would be that these participants would demonstrate deficits in visual processing abilities. Future research is needed to further explore whether this reverse N400 effect is replicated in other samples of children with ASD, and if it is associated with visual processing deficits.
4.1. Limitations
Although the average number of trials retained in all groups were well above the threshold of 10 trials per condition, significantly more trials were retained in the typically developing group. This is a consistent concern in electrophysiology and imaging research in children with disabilities, due to the difficulties inherent in tolerating the study procedures. While we addressed EEG data quality by including only participants with a certain number of good trials, it is possible that the smaller number of trials in ASD participants introduced additional noise into the data, thus obscuring potential ERP results.
4.2. Future directions
Given the challenges in predicting individual treatment outcomes (Eapen et al., 2013; Howlin et al., 2009; Stahmer et al., 2011), there is a great need for measures which are sensitive to individual differences and relate to core deficits in ASD. Performance on semantic-lexical ERP tasks may be a meaningful stratification biomarker, relating to underlying social communication and language deficits. Future research will evaluate whether neural correlates of semantic processing are associated with response to intervention, and examine whether they can help target treatments to specific language processing profiles.
Declarations of interest
None.
Acknowledgements
The authors would like to thank April Benasich and Chiara Cantiani, for their assistance in developing the ERP paradigm, and Elizabeth Baker and Andrew Marin for their assistance in data collection.
Footnotes
This work was supported by an Autism Speaks Meixner Postdoctoral Fellowship in Translational Research(9292, PI: DiStefano, Mentor: Jeste), National Institutes of Health Autism Center of Excellence (2P50HD055784-08, PI: Bookheimer, Co-I: Jeste), National Institute of General Medical Sciences (R01GM111378-01A1, PI: Senturk).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100640.
Contributor Information
Charlotte DiStefano, Email: cdistefano@mednet.ucla.edu.
Damla Senturk, Email: dsenturk@mednet.ucla.edu.
Shafali Spurling Jeste, Email: sjeste@mednet.ucla.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Akshoomoff N. Use of the mullen scales of early learning for the assessment of young children with autism spectrum disorders. Child Neuropsychol. 2006;12:269–277. doi: 10.1080/09297040500473714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.K., Lord C., Risi S., DiLavore P.S., Shulman C., Thurm A. Patterns of growth in verbal abilities among children with autism spectrum disorder. J. Consult. Clin. Psychol. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Bentin S., McCarthy G., Wood C.C. Event-related potentials, lexical decision and semantic priming. Electroencephalogr. Clin. Neurophysiol. 1985;60(4):343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Bermeitinger C., Frings C., Wentura D. Reversing the N400: event-related potentials of a negative semantic priming effect. Neuroreport. 2008;19(15):1479–1482. doi: 10.1097/WNR.0b013e32830f4b0b. [DOI] [PubMed] [Google Scholar]
- Bishop S.L., Guthrie W., Coffing M., Lord C. Convergent validity of the mullen scales of early learning and the differential ability scales in children with autism spectrum disorders. Am. J. Intellect. Dev. Disabil. 2011;116(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C., Choudhury N.A., Yu Y.H., Shafer V.L., Schwartz R.G., Benasich A.A. From sensory perception to lexical-semantic processing: an ERP study in non-verbal children with autism. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161637. e0161637–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr T.H., Dagenbach D. Semantic priming and repetition from masked words: evidence for a center-surround attentional mechanism in perceptual recognition. J. Exp. Psychol. Learn. Mem. Cogn. 1990;16(2):341–350. [PubMed] [Google Scholar]
- Chahrour M., O’Roak B.J., Santini E., Samaco R.C., Kleiman R.J., Manzini M.C. Current perspectives in autism spectrum disorder: from genes to therapy. J. Neurosci. 2016;36(45):11402–11410. doi: 10.1523/JNEUROSCI.2335-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-T., Lee C.-Y., Chou C.-J., Fuh J.-L., Wu H.-C. Predictability effect on N400 reflects the severity of reading comprehension deficits in aphasia. Neuropsychologia. 2016;81(C:117–128. doi: 10.1016/j.neuropsychologia.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Coderre E.L. Linguistic and non-linguistic semantic processing in individuals with autism spectrum disorders: an ERP study. J. Autism Dev. Disord. 2017;47(3):795–812. doi: 10.1007/s10803-016-2985-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences, 1988. In: Connolly J.F., Mate-Kole M.C., Joyce B.M., editors. Vol. 80. Routledge Academic; New York, NY: 1999. pp. 1309–1315. (Global Aphasia: An Innovative Assessment Approach). Arch. Phys. Med. Rehabil. [Google Scholar]
- Cummings A., Ceponiene R. Verbal and nonverbal semantic processing in children with developmental language impairment. Neuropsychologia. 2010;48(1):77–85. doi: 10.1016/j.neuropsychologia.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. PMID: 10442879. [DOI] [PubMed] [Google Scholar]
- De Rubeis S., Buxbaum J.D. Recent advances in the genetics of autism spectrum disorder. Curr. Neurol. Neurosci. Rep. 2015;15(6):36. doi: 10.1007/s11910-015-0553-1. [DOI] [PubMed] [Google Scholar]
- DiStefano C., Dickinson A., Baker E., Jeste S.S. EEG data collection in children with ASD_ the role of state in data quality and spectral power. Res. Autism Spectr. Disord. 2018;57:132–144. doi: 10.1016/j.rasd.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M.A., Bates J.C. Developmental change in neutral processing of words by children with autism. J. Autism Dev. Disord. 2005;35(3):361–376. doi: 10.1007/s10803-005-3304-3. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn D.M. PsychCorp. 4th Edition. 2007. Peabody Picture Vocabulary Test. Bloomington MN. [Google Scholar]
- Eapen V., Crnčec R., Walter A. Exploring links between genotypes, phenotypes, and clinical predictors of response to early intensive behavioral intervention in autism Spectrum disorder. Front. Hum. Neurosci. 2013;7:567. doi: 10.3389/fnhum.2013.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot C.D. 2nd edition. Harcourt Assessment; San Antonio, TX: 2007. Differential Ability Scales. [Google Scholar]
- Fishman I., Yam A., Bellugi U., Lincoln A., Mills D. Contrasting patterns of language-associated brain activity in autism and Williams syndrome. Soc. Cogn. Affect. Neurosci. 2011;6(5):630–638. doi: 10.1093/scan/nsq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. Neurophysiological markers of early language acquisition: from syllables to sentences. Trends Cogn. Sci. 2005;9(10):481–488. doi: 10.1016/j.tics.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Steinhauer K., Frisch S. Lexical integration: sequential effects of syntactic and semantic information. Mem. Cogn. 1999;27(3):438–453. doi: 10.3758/bf03211539. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. Maturing brain mechanisms and developing behavioral language skills. Brain Lang. 2010;114(2):66–71. doi: 10.1016/j.bandl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Frings C., Bermeitinger C., Wentura D. Center-surround or spreading inhibition. Exp. Psychol. 2008;55(4):234–242. doi: 10.1027/1618-3169.55.4.234. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. NeuroImage. 2003;20:S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Harris G.J., Chabris C.F., Clark J., Urban T., Aharon I., Steele S. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61(1):54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holcomb P.J., Coffey S.A., Neville H.J. Visual and auditory sentence processing: a developmenal analysis using event-related brain potentials. Dev. Neuropsychol. 1992;8(2-3):203–241. [Google Scholar]
- Howlin P., Magiati I., Charman T. Systematic review of early intensive behavioral interventions for children with autism. Am. J. Intellect. Dev. Disabil. 2009;114(1):23–41. doi: 10.1352/2009.114:23;nd41. [DOI] [PubMed] [Google Scholar]
- IBM Corp, Released 2016. IBM SPSS Statistics for Mac, Version 24. Armonk, NY: IBM Corp. Jeste S.S., Geschwind D.H. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature (Publishing Group) 2014;10(2):74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste S.S., Nelson C.A. Event related potentials in the understanding of autism spectrum disorders: an analytical review. J. Autism Dev. Disord. 2009;39(3):495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste S.S., Hirsch S., Vogel-Farley V., Norona A., Navalta M.-C., Gregas M.C. Atypical face processing in children with tuberous sclerosis complex. J. Child Neurol. 2012;28(12):1569–1576. doi: 10.1177/0883073812465122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaganovich N., Schumaker J., Rowland C. Atypical audiovisual word processing in school-age children with a history of specific language impairment: an event-related potential study. J. Neurodev. Disord. 2016:1–22. doi: 10.1186/s11689-016-9168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Robins D., Kelley E., Swainson B., Fein D. Atypical lexical/semantic processing in high-functioning autism spectrum disorders without early language delay. J. Autism Dev. Disord. 2006;37(6):1116–1122. doi: 10.1007/s10803-006-0254-3. [DOI] [PubMed] [Google Scholar]
- Kasari C., Brady N., Lord C., Tager-Flusberg H. Assessing the minimally verbal school-aged child with autism spectrum disorder. Autism Res. 2013;6(6):479–493. doi: 10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilov S.A., Magnuson J.S., Rakhlin N., Landi N., Grigorenko E.L. Lexical processing deficits in children with developmental language disorder: an event-related potentials study. Dev. Psychopathol. 2015;27(02):459–476. doi: 10.1017/S0954579415000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu. Rev. Psychol. 2011;62(1):621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Hillyard S.A. Reading senseless sentences: brain potentials reflect semantic incongruity. Science (New York, N.Y.) 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P.C., Risi S., Gotham K., Bishop S. Western Psychological Services. Second Edition. 2012. Autism Diagnostic Observation Schedule. Torrance CA. [Google Scholar]
- Masi A., DeMayo M.M., Glozier N., Guastella A.J. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci. Bull. 2017;33(2):183–193. doi: 10.1007/s12264-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J.P., Ceponiene R., Burner K.M., Townsend J., Kinnear M., Schreibman L. Neural correlates of verbal and nonverbal semantic integration in children with autism spectrum disorders. J. Child Psychol. Psychiatry. 2010;51(3):277–286. doi: 10.1111/j.1469-7610.2009.02157.x. [DOI] [PubMed] [Google Scholar]
- Méndez M., Sans O., Abril B., Valdizan J.R. 13. Event-related potentials (N 400) in autistic children. Clin. Neurophysiol. 2009;120(4):e136. [Google Scholar]
- Mullen E.M. American Guidance Service; Circle Pines, MN: 1995. Mullen Scales of Early Learning: AGS Edition. [Google Scholar]
- Olvet D.M., Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Perry A., Cummings A., Geier J.D., Freeman N.L., Hughes S., Managhan T. Predictors of outcome for children receiving intensive behavioral intervention in a large, community-based program. Res. Autism Spectr. Disord. 2010;5(1):592–603. [Google Scholar]
- Pickles A., Anderson D.K., Lord C. Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J. Child Psychol. Psychiatry. 2014;55(12):1354–1362. doi: 10.1111/jcpp.12269. [DOI] [PubMed] [Google Scholar]
- Pijnacker J., Geurts B., van Lambalgen M., Buitelaar J., Hagoort P. Exceptions and anomalies: an ERP study on context sensitivity in autism. Neuropsychologia. 2010;48(10):2940–2951. doi: 10.1016/j.neuropsychologia.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Plesa Skwerer D., Jordan S.E., Brukilacchio B.H., Tager-Flusberg H. Comparing methods for assessing receptive language skills in minimally verbal children and adolescents with autism spectrum disorders. Autism. 2015:1–14. doi: 10.1177/1362361315600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I., Dunn M.A., Allen D.A., Stevens M.C., Fein D. Subtypes of language disorders in school-age children with autism. Dev. Neuropsychol. 2009;34(1):66–84. doi: 10.1080/87565640802564648. [DOI] [PubMed] [Google Scholar]
- Ribeiro T.C., Valasek C.A., Minati L., Boggio P.S. Altered semantic integration in autism beyond language. Neuroreport. 2013;24(8):414–418. doi: 10.1097/WNR.0b013e328361315e. [DOI] [PubMed] [Google Scholar]
- Roesler C.P., Flax J., MacRoy-Higgins M., Fermano Z., Morgan-Byrne J., Benasich A.A. Sensory desensitization training for successful net application and EEG/ERP acquisition in difficult to test children. Commun. Disord. Q. 2013;35(1):14–20. [Google Scholar]
- Rohaut B., Faugeras F., Chausson N., King J.-R., Karoui, El I., Cohen L., Naccache L. Probing ERP correlates of verbal semantic processing in patients with impaired consciousness. Neuropsychologia. 2015;66(C:279–292. doi: 10.1016/j.neuropsychologia.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services. 2003. Social Communication Scales. Torrance, CA. [Google Scholar]
- Silva-Pereyra J., Rivera-Gaxiola M., Kuhl P.K. An event-related brain potential study of sentence comprehension in preschoolers: semantic and morphosyntactic processing. Cogn. Brain Res. 2005;23(2–3):247–258. doi: 10.1016/j.cogbrainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Nunez P.L., Tucker D.M., Silberstein R.B., Cadusch P.J. Spatial sampling and filtering of EEG with spline laplacians to estimate cortical potentials. Brain Topogr. 1996;8(4):355–366. doi: 10.1007/BF01186911. [DOI] [PubMed] [Google Scholar]
- Stahmer A.C., Schreibman L., Cunningham A.B. Toward a technology of treatment individualization for young children with autism spectrum disorders. Brain Res. 2011;1380:229–239. doi: 10.1016/j.brainres.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. A psychological approach to understanding the social and language impairments in autism. Int. Rev Psychiatry. 1999;11(4):325–334. doi: 10.1080/09540269974203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H., Kasari C. Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Res. 2013;6(6):468–478. doi: 10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentura D., Frings C. Repeated masked category primes interfere with related exemplars: new evidence for negative semantic priming. J. Exp. Psychol. Learn. Mem. Cogn. 2005;31(1):108–120. doi: 10.1037/0278-7393.31.1.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.