Abstract
Tacrolimus trough and dose requirements vary dramatically between individuals of European and African American ancestry. These differences are less well described in other populations. We conducted an observational, prospective, multi-center study from which 2595 kidney transplant recipients of European, African, Native American, and Asian ancestry were studied for tacrolimus trough, doses and genetic determinants of metabolism. We studied the well-known variants and conducted a CYP3A4/5 gene wide analysis to identify new variants. Daily doses, and dose-normalized troughs were significantly different between the four groups (p<0.001). CYP3A5*3 (rs776746) was associated with higher dose-normalized tacrolimus troughs in all groups but occurred at different allele frequencies and had differing effect sizes. The CYP3A5*6 (rs10264272) and *7 (rs413003343) variants were only present in African Americans. CYP3A4*22 (rs35599367) was not found in any of the Asian ancestry samples. We identified seven suggestive variants in the CYP3A4/5 genes associated with dose-normalized troughs in Native Americans (p=1.1×10−5 to 8.8×10−6) and one suggestive variant in Asian Americans (p=5.6×10−6). Tacrolimus daily doses and dose-normalized troughs vary significantly among different ancestry groups. We identified potential new variants important in Asians and Native Americans. Studies with larger populations should be conducted to assess the importance of the identified suggestive variants.
1. Introduction
The incidence of end stage kidney disease is increasing worldwide and kidney transplantation is the optimal treatment option due to better outcomes relative to dialysis 1. Tacrolimus is a potent immunosuppressant that is used in >90% of transplants to prevent acute rejection and maintain graft function 1. Tacrolimus has a narrow therapeutic index and troughs are therapeutically monitored to reduce toxicity and improve efficacy 2. Tacrolimus has high inter-individual pharmacokinetic variability 3. It is well known that tacrolimus troughs and dose requirements vary between recipients of European and African ancestry, African Americans have significantly lower tacrolimus trough concentrations in comparison with European Americans and require higher tacrolimus doses to achieve similar troughs concentrations 4–6. Little is known about tacrolimus trough and dose requirements in other populations although some data suggest that Native American transplant recipients require lower tacrolimus doses possibly due to decreased oral clearance 7,8, others found that there were no difference between tacrolimus doses between European Americans and Native Americans 9.
Cytochromes P450 (CYP) 3A4 and 5 are the main drug metabolizing enzymes for tacrolimus and the genes encoding for these proteins contain important genetic variants 10–12. These variants differ by ancestry and for some significantly different minor allele frequencies (MAF) 13,14. The CYP3A5 variant, CY3A5*3 (rs776746), is a loss of function variant and has been well-studied with tacrolimus pharmacokinetics 15,16. CYP3A5*6 (rs10264272) and *7 (rs413003343) are reduced or loss of function variants which are observed exclusively in individuals of African ancestry 17. CYP3A4*22 (rs35599367) is also a reduced function variant which occurs primarily in European ancestry and is associated with variation in tacrolimus pharmacokinetics 18–22. We have shown in our work that up to 50% of variability in tacrolimus pharmacokinetic is explained by CYP3A genetic variants and clinical factors 23–25. Identifying the influential variants in each population is important in developing accurate precision medicine dose models for tacrolimus therefore we previously conducted genome wide association studies (GWAS) of tacrolimus troughs in kidney transplant recipients of European and African ancestry to identify ancestry specific genetic variants 24,25. We were able to find only one study in Native Americans7 on the pharmacogenomics of tacrolimus.
The evidence for the association between genetic variation and tacrolimus disposition is strong and a Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline provides recommendations on tacrolimus directed dosing using the CYP3A5*3, *6, and *7 variants 26. Individuals who carry one or no loss of function alleles are CYP3A5 protein expressers and should receive significantly higher tacrolimus doses. The guideline does not however address the effect of clinical factors, the CYP3A4*22 variant, and variants that may be present in populations other than European Americans and African Americans.
In the current study, we compared tacrolimus daily dose requirements and troughs in European American, African American, Native Americans and Asian American transplant recipients. Additionally, we evaluated the association of well-known tacrolimus variants (CYP3A5*3, *6, *7 and CYP3A4*22) with troughs in the four populations. We also conducted a CYP3A4 and CYP3A5 gene wide analysis to identify new variants possibly present in transplant recipients of Asian or Native American ancestry.
2. Methods
2.1. Study Design and Patient Selection
We studied transplant recipients enrolled in the Deterioration of Kidney Allograft Function (DeKAF) and GEN03 genomic studies. These are multicenter, observational studies, which prospectively followed kidney transplant recipients from 2005 to 2016 at seven study sites in the United States and Canada. They are registered at www.clinicaltrials.gov (NCT00270712 and NCT01714440). Participants were enrolled at time of transplant and signed informed consents approved by the institutional review boards of the enrolling centers. Transplant recipients were selected for this analysis if they were >18 years old, received oral immediate release tacrolimus as maintenance immunosuppression, had tacrolimus trough concentrations measured as part of clinical care in the first 6 months posttransplant and genome wide association (GWA) genomic data available. Ancestry of each individual was determined using principal components (PC) of ancestry computed from the GWAS panel and through knowledge of self-reported ancestry. When using top 3 ancestry PCs, patients were classified as either European, African, Native, or Asian American ancestry (Supplemental Figures 1 and 2). In the first group, most self-identified as Caucasian/European American. In the second, most self-identified as Black/African American. In the third, most self-identified as Asian/Asian American. In the fourth cluster, most self-identified as Native American or as Hispanic/Latino ethnicity. The Native Americans (as per self-report) and Hispanic/Latino ethnicity (as per self-report) were indistinguishable by PC and were therefore analysed as one group. There was concordance with self-reported ancestry and PC defined ancestry, however, when discordance was raised, PC defined ancestry was used in that individual to reduce genetic confounding.
2.2. Tacrolimus Trough Concentrations and Doses
Tacrolimus troughs and corresponding doses in the first 6 months posttransplant were obtained as part of routine clinical care and taken from the medical record for analysis. Two tacrolimus troughs were obtained per week in the first 8 weeks and two troughs were obtained per month in months 3, 4, 5 and 6 for a maximum of 24 troughs per patient. Largely, the target trough concentrations were 8 to 12 ng/mL in the first 3 months, then 6 to 10 ng/mL in 3 to 6 months posttransplant. Dose normalized trough concentrations were determined by the ratio of trough concentration (ng/ml) and total daily dose (mg).
2.3. Genotyping
Recipient DNA obtained at time of transplant from peripheral blood lymphocytes. DNA was isolated from lymphocytes by centrifugation after red blood cell lysis. Genotyping was conducted on an exome-plus Affymetrix Transplant Array chip (Affymetrix, Santa Clara, CA) with ~800,000 high quality genomic markers after quality control and >34M markers after imputation using the 1000 Genomes phase 3 and Genome of the Netherlands v5 27–31. Data quality control was carried out with PLINK software (version 1.90b1a) 32. Genotypes were phased using SHAPEIT2 33; and imputed with IMPUTE234. Imputed variants with information score more than 0.8 were considered of good quality and used in the analysis. Genotypes were subjected to a 95% call rate threshold. Samples with very high heterozygosity and suspected contamination were confirmed and removed if high heterozygosity could not be resolved. Individual variants were excluded if they were monomorphic or had low MAF (<0.5%). Approximately, 49,000 measured and imputed variants from the CYP3A4 and CYP3A5 genes were taken from the GWA panel and used in this analysis. After removing variants in linkage disequilibrium (LD), the effective number of variants was ~10,000. CYP3A4 and CYP3A5 region was defined between positions 95,000,000 and 105,000,000 on chromosome 7.
2.4. Statistical Analysis
An ANOVA was used to assess the difference in tacrolimus doses and troughs among the populations and a p-value <0.05 was considered statistically significant. The association between natural log (ln) transformed dose-normalized tacrolimus troughs and known variants, CYP3A5*3, *6, *7 and 3A4*22, were tested in each of the 4 populations using linear mixed-effects models. A log transformation was used to ensure that the outcome was normally distributed. Dose-normalized trough concentrations initially started low and rose quickly until day 9 after transplant and then plateaued in the early weeks after transplant as we have previously described 35,36. Therefore, a simple spline method was used to model the effect of time on all trough concentrations, with the change in slope occurring at day 9. The models included a random intercept and slope for days posttransplant and were adjusted for age, gender and enrolling center. An interaction analysis was then conducted to compare the effect sizes of CYP3A5*3 among different groups in relation to the European American group. We also conducted a gene wide association analysis of the CYP3A4 and CYP3A5 genes in the Native and Asian American populations. The analysis was adjusted for age, gender, enrolling centre, and CYP3A5*3, and the level of significance was set at a p-value of <5×10-6. The p-value for gene wide association test was adjusted based on a Bonferroni correction using the effective number of single nucleotide polymorphisms (SNPs) (~10,000) which took into account SNPs in linkage disequilibrium 37. In our previous studies, the known genetic and clinical variables explained ~50% of variation of tacrolimus concentrations. Given the 77 recipients in the Native American group and the significance level of 5×10−6, we have 78% power to identify a variant that can explain 20% additional variation; for the 91 Asians American, we have 82% power to identify a variant explaining 18% additional variation. Analyses were conducted with R software version 3.5 (www.r-project.org) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
We studied 1966 European American, 461 African American, 77 Native American and 91 Asian American ancestry transplant recipients (Table 1). Tacrolimus total daily doses, trough concentrations and dose-normalized trough concentrations by ancestry are shown in Table 2 and were significantly different in the four populations (p <0.001). Native Americans and European Americans received the lowest median tacrolimus daily dose (5 mg) and the African Americans received the highest median daily dose (8 mg) (p <0.0001). Moreover, Native Americans had the highest dose-normalized tacrolimus trough concentration (1.73 ng/ml per total daily dose), and the African Americans had the lowest troughs (0.78 ng/ml per total daily dose) (p <0.0001). Tacrolimus troughs over the 6 months were generally similar between the populations except for African Americans who had significantly lower median trough concentrations (p <0.0001).
Table 1.
Recipient Demographics and Characteristics by Ancestry
| Native Americans (N=77) | Asia Americans (N=91) | African Americans (N=461) | European Americans (N=1966) | |
|---|---|---|---|---|
| Male Gender, n (%) | 41 (53.25%) | 56 (61.54%) | 287 (62.26%) | 1232 (62.67%) |
| Age at transplant (years), mean (s.d.) | 49.44 (±14.84) | 45.23 (±14.29) | 47.25 (±11.95) | 51.24 (±13.20) |
| Weight (kg), mean (s.d.) | 78.57 (±16.63) | 67.37 (±13.38) | 85.72 (±18.64) | 83.03 (±19.55) |
| SPK, n (%) | 6 (7.79%) | 1 (1.10%) | 17 (3.69%) | 148 (7.53%) |
| Diabetes before transplant, n (%) | 38 (49.35%) | 22 (24.18%) | 163 (35.36%) | 704 (35.83%) |
| Living donor, n (%) | 38 (49.35%) | 44 (48.35%) | 144 (31.24%) | 1336 (67.96%) |
| Antibody induction | ||||
| Monoclonal | 27 (35.06%) | 39 (42.86%) | 209 (45.34%) | 746 (37.95%) |
| Polyclonal | 46 (59.74%) | 47 (51.65%) | 240 (52.06%) | 1109 (56.41%) |
| Combination | 2 (2.60%) | 2 (2.20%) | 7 (1.52%) | 53 (2.70%) |
| None | 2 (2.60%) | 3 (3.30%) | 5 (1.08%) | 58 (2.95%) |
| Steroid present at day 14 | 51 (66.23%) | 59 (64.84%) | 269 (58.35%) | 1266 (64.43%) |
SPK: simultaneous pancreas-kidney transplant
Table 2.
Tacrolimus Doses and Concentrations by Ancestry in the First 6 Months Posttransplant
| Native American (N=77, obs= 1370) | Asian Ancestry (N=91, obs=1747) | European Ancestry (N=1966, obs=34594) | African American (N=461, obs= 8187) | P-value | |
|---|---|---|---|---|---|
| Trough Concentration (ng/ml) | 8.3 (6.5–10.3) | 8.4 (6.7–10.6) | 8.4 (6.5–10.3) | 6.9 (5–9) | <0.0001* |
| Total Daily Dose (mg) | 5.0 (3.0–8.0) | 6.0 (3.5–8.0) | 5.0 (4.0–8.0) | 8.0 (6.0–12.0) | <0.0001* |
| Dose-Normalized Trough Concentration (ng/ml per total daily dose in mg) | 1.73 (1.06–2.67) | 1.50 (0.98–2.53) | 1.56 (1.02–2.40) | 0.78 (0.53–1.2) | <0.0001* |
N=number of patients; Obs = number of tacrolimus troughs; Numbers are represented as median (interquartile ranges);
ANOVA test was used for comparison
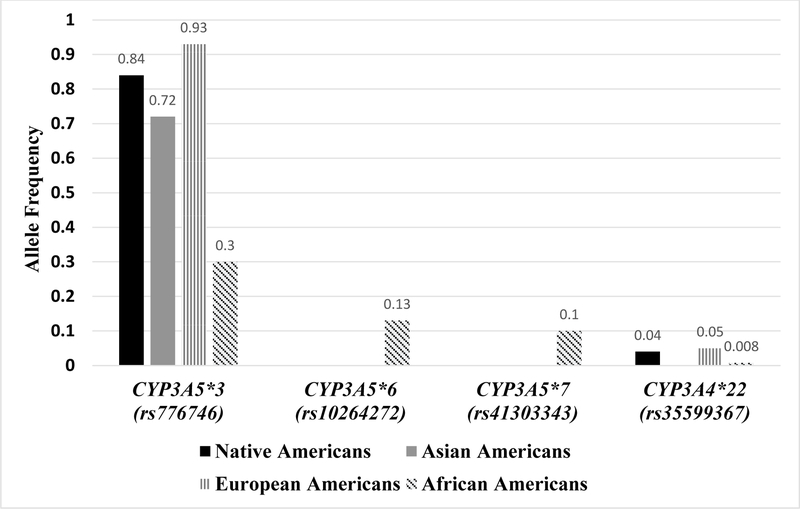
Alleles frequencies (AF) of the four well-known variants, CYP3A5*3, *6, *7, and CYP3A4*22, are shown in Figure 1. The CYP3A5*3 variant, was present in all four groups, and was most common in European ancestry (AF=0.93) followed by Native American (AF=0.84) and Asian American (AF=0.72), while the African Americans had the lowest frequency (AF=0.30). The CYP3A5 *6 and *7 variants were exclusively present in African Americans. The CYP3A4*22 variant allele frequency was ≤ 0.05 and was not observed in those of Asian ancestry.
Figure 1.
Allele Frequencies of Known Variants among the Four Ancestry Groups
Each of the tested variants was associated with higher dose-normalized trough concentrations (Table 3). The CYP3A5*3 variant was associated with higher dose-normalized tacrolimus troughs in all populations (p = 3×10−9 to 5×10−121). The size of the effect varied where the largest effect of the *3 allele was in European Americans. Native Americans, Asian Americans, and African Americans had similar effect sizes for CYP3A5*3 (Table 3) but were significantly different to the European Americans (p<0.001). In European Americans, each additional copy of *3 allele resulted in 1.86 times increase in dose-normalized troughs. In the other ancestry groups, the magnitude of increase in dose-normalized troughs of one *3 allele was similar to each other (1.6 in Native Americans, 1.55 in Asian American, and 1.54 in African Americans). In African Americans the *6 and *7 variants also were associated with an increase (each *6 and *7 allele increased dose-normalized troughs by 1.35 and 1.58 times, respectively) in dose-normalized troughs. Additionally, CYP3A4*22 was associated with higher dose-normalized tacrolimus troughs but only in the European Americans. The effect size of CYP3A4*22 variant was about one-half that of the CYP3A5*3 variant and was associated with 1.34 times increase in dose-normalized troughs.
Table 3.
Known CYP3A5 and CYP3A4 Variants and Associations with Dose-Normalized Tacrolimus Trough Concentrations by Ancestry
| CYP3A5*3 (rs776746) | CYP3A5*6 (rs10264272) | CYP3A5*7 (rs41303343) | CYP3AA*22 (rs35599367) | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size1 (95%CI) | P-value | Effect Size (95%CI) | P-value | Effect Size (95%CI) | P-value | Effect Size (95%CI) | P-value | |
| Native Americans2 | 0.47 (0.31–0.62) | 3×10−9 | NE | NE | −0.07 (−0.36– 0.22) | 0.63 | ||
| Asian Americans2 | 0.44 (0.30–0.57) | 2.1×10−10 | NE | NE | NE | |||
| European Americans3 | 0.624 (0.57–0.67) | 5.0×10−121 | NE | NE | 0.28 (0.22–0.34) | 3.3×10−22 | ||
| African Americans3 | 0.43 (0.36–0.49) | 2.0×10−37 | 0.30 (0.22 −0.39) | 6.1×10−12 | 0.46 (0.36–0.55) | 9.6×10−22 | 0.26 (−0.06–0.56) | 0.11 |
Models were adjusted for time posttransplant, age, gender, and enrolling center and each variant was adjusted for the other known variants.
CI: confidence interval.
NE - not evaluable due to either absence or low allele frequency of the variant in the population
Statistically significant difference between European American and other ancestry groups (p<0.001)
Gene wide association significance set at p-value< 5×10−6
Genome wide association significance set at p-value < 5×10−8
Effect size is the effect of one variant allele on ln of dose-normalized troughs; for example, in European Americans, the effect size of CYP3A5*3 is 0.62, which means that dose-normalized troughs are 1.86 [e0.62=1.86] times greater if recipient carries one *3 allele than carriers of no *3 alleles, and 3.46 [(e0.62) x (e0.62)=3.46] times greater if a carrier of two *3 alleles compared to those with no *3 alleles.
In the CYP3A4 and CYP3A5 gene wide analysis in the Native American and Asian American recipients, no additional new variants were associated with tacrolimus dose-normalized trough concentrations (Table 4) after correcting for multiple testing. However, we found seven suggestive variants in the Native American group with (minor allele frequency) MAF > 0.05 (p=1.1×10−5 to 8.8×10−6). Six of those variants were located near to each other and 4 were in complete LD (D’=1). In Asian Americans, one variant, rs6950190 with a MAF of 0.384, had a suggestive association with tacrolimus dose normalized dose troughs (p=5.6×10−6). The suggestive variants were imputed SNPs with a notable imputation quality (information score = 0.861–0.986).
Table 4:
Variants with Suggestive Associations with Dose-Normalized Tacrolimus Troughs in Native Americans and Asian Americans1
| Variant | Effect size | P-value (95%CI) | MAF | Location of SNP |
|---|---|---|---|---|
| Native Americans | ||||
| rs139190940_TG | 0.31 (0.17–0.44) | 8.8×10−6 | 0.18 | inter-genic region on chromosome 7, located downstream of SDHAF3 (succinate dehydrogenase complex assembly factor 3) and upstream of an uncharacterized gene LOC105375416 |
| rs73238872_G2 | 0.27 (0.14–0.40) | 6.6×10−5 | 0.19 | |
| rs878502_C2 | 0.27 (0.14–0.40) | 6.6×10−5 | 0.19 | |
| rs28369152_C2 | 0.27 (0.14–0.40) | 6.6×10−5 | 0.19 | |
| rs28413832_C2 | 0.27 (0.14–0.40) | 6.6×10−5 | 0.19 | |
| rs2158498_G | 0.30 (0.17–0.44) | 1.1×10−5 | 0.19 | |
| rs151269855_G | 0.53 (0.27–0.79) | 8.5×10−5 | 0.07 | downstream of LOCLOC105375416 at position 97,215,572 on chromosome 7 |
| Asian Americans | ||||
| rs6950190_C | 0.38 (0.22–0.55) | 5.6×10−6 | 0.38 | intronic SNP in DYNC1I1 (dynein cytoplasmic 1 intermediate chain 1) on chromsome 7 at 95,558,358 |
CI: confidence intervals; MAF: minor allele frequency; SNP: single nucleotide polymorphism
Longitudinal linear mixed effects model included a random intercept and random slopes for days after transplant and adjusted for CYP3A5*3, age, gender and transplant center
Variants are in linkage disequilibrium with each other, D’ = 1.0, p <0.0001.
4. Discussion
In the present study we evaluated the differences between tacrolimus troughs, doses and dose normalized troughs between four populations; European Americans, African Americans, Native Americans, and Asian Americans. African Americans received significantly higher doses, but achieved lower trough concentrations when compared to the 3 other populations. Our findings are in accordance with previous studies, including our own, which reported that African American patients require higher tacrolimus doses than European patients to achieve similar therapeutic trough concentrations 35,38–43. Two studies reported that Native Americans required lower tacrolimus doses than European Americans 7,8, however, in our current analysis of Native Americans tacrolimus doses and troughs were not different although they had the highest dose-normalized troughs of all groups. Our findings are supported by a small study that also found no difference between doses required to maintain therapeutic tacrolimus troughs in Native Americans, Hispanics and Non-Hispanic whites9. Direct comparisons of our trough and dose data to published work is difficult since targeted concentrations are generally higher than contemporary trough targets, doses are reported in mg/kg, and few studies report dose-normalized troughs, which would allow for direct comparison.
We evaluated the four variants (CYP3A5*3, *6, *7 and CYP3A4*22) known to be strongly associated with tacrolimus metabolism and their frequency in the four populations. The CYP3A5*3 loss of function variant was present in all four populations but the AFs were significantly different (Figure 1). The African Americans had the lowest AF of CYP3A5*3 (0.30) whereas the European population had the highest AF (0.93). African Americans also carried two additional loss of function variants (CYP3A5*6 and *7) which were not observed in our other populations. Despite African Americans carrying three common CYP3A5 loss of function variants they still cumulatively carried fewer loss of function CYP3A variants than the other populations, which accounts for their significantly higher rate of tacrolimus metabolism, lower troughs and higher dose requirements compared to other groups. The AFs of CYP3A5*3, *6, and *7 in our study are similar to previous reports for African ancestry, European, and Asian populations 44,45. Although infrequent, others have reported that individuals of Latin American ancestry also carry CYP3A5*6 (AF=0.037) and CYP3A5*7 (AF=0.025) as do individuals from the Middle East who carry CYP3A5*6 (AF=0.019) 45. In a study of 94 Native Americans in Montana, United States, by Fohner et al,46 the AF of CYP3A5*3 was 0.92 which is slightly higher than what we observed (0.84). They also found that CYP3A5*6, and *7 were not present 46. In another study of 24 adult Native Americans kidney transplant recipients, CYP3A5*3 had an a higher AF (0.94) 7.
CYP3A5*3 is the most studied CYP3A5 variant in association with tacrolimus and has been evaluated in Europeans18,21,47–52 African Americans5,24,35,53, Asian 52,54–60, and Native Americans7. Most studies have compared dose-normalized troughs in carriers of only the CYP3A5*3 allele to those who are not carriers. It is well established that carriers of CYP3A5*3 have significantly higher dose-normalized trough concentrations. We also found that the CYP3A5*3 variant had a large effect size in all four populations. However, the magnitude of the CYP3A5*3 effect varied by ancestry with the largest effect in the European American population. The effect size was lower but similar in the other three populations compared with European Americans in this study. This effect size may be explained by additional, yet unrecognized, variants that are important in the other populations; thereby reducing the overall effect of the CYP3A5*3 allele. The different effect sizes among the ancestry groups suggests that dosing models for each group will need to be developed for each population and that generalizability of an effect size will likely not be possible. We previously developed a tacrolimus dosing model for African Americans 61 and propose that dosing models such as this will be needed for each ancestry population for precise dosing predictions.
Tacrolimus is also metabolized by the CYP3A4 enzyme and is likely the predominant enzyme in those of European ancestry since they commonly carry the CYP3A5*3/*3 genotype and therefore do not express functional CYP3A5 enzyme. The CYP3A4*22 is the only variant in the CYP3A4 gene that has been consistently associated with tacrolimus metabolism18–20,62–65. CYP3A4*22 has a low MAF and an effect size about one-half relative to the CYP3A5*3 variant which is most likely because CYP3A4*22 is not a complete loss of function variant with a smaller reduction on tacrolimus clearance 62. We observed CYP3A4*22 in our European American, African American and Native American ancestry recipients (MAF≤0.05) whereas it was not found in our Asian American group, which is consistent with other published data 46,66,67. CYP3A4*22 was only significantly associated with tacrolimus in our European ancestry population. The lack of effect in our other populations may be due to its low AF and it will require a larger sample size to identify the effect. Because of the smaller effect size some propose that CYP3A4*22 may not be important clinically 68,69 which may be true when the variant occurs alone or in the absence of other variants. We recently described 4 patients who carried both CYP3A5*3/*3 and CYP3A4*22/*22 genotypes who had significantly reduced tacrolimus metabolism and very low dose requirements26. Therefore, when it is combined with other reduced or loss of function alleles, the effect can be quite profound. The current tacrolimus CPIC guidelines do not include recommendations regarding the CYP3A4*22 variant however, future updates should consider adding. The association of tacrolimus with ABCB1 variants is conflicting.53,70 In one study carriers of CYP3A4*22 the effect of ABCB1 was associated with a small but strong effect on tacrolimus in renal transplant patients71.
We previously conducted GWAS on tacrolimus troughs and found in European Americans that the CYP3A5*3 and CYP3A4*22 variants were important determinants of metabolism and in African Americans the CYP3A5*3, *6 and *7 were important 24,25. A recent study, using an extreme tacrolimus trough phenotype sampling model with targeted next-generation sequencing, identified potentially new variants associated with tacrolimus disposition. Numerous variants in the CYB5R2 gene were associated with extreme tacrolimus troughs in African Americans, including the potential loss of function variant rs6173305772. We hypothesized that genetic variants beyond CYP3A5*3 may also explain additional tacrolimus trough variability in Asian American and Native American transplant recipients. Therefore, we conducted an exploratory gene wide association analysis in the CYP3A4 and CYP3A5 genes and found no variants that were significantly associated at a gene wide significance level; however, several variants were suggestive and worthy of further investigation. Seven variants with modest MAF (0.07 to 0.19) were suggestive in the Native American group, of which 4 of the variants were in complete LD with each other. These variants were in intergenic regions so the mechanism of their effect is unclear. Genetic variants in strong LD (r2≥ 0.8) to those suggestive SNPs were identified, however, none of them were reported to have any function. Identifying the function of those variants might be aided by using gene-tissue expression or cell-line validation techniques that we have developed for tacrolimus metabolism73; however, that was beyond the scope of the present study. In the Asian Americans, one variant, rs6950190, in the dynein cytoplasmic 1 intermediate chain 1 gene (DYNC1I1) with a MAF of 0.384 also had a suggestive association with tacrolimus dose-normalized troughs. The DYNC1I1 gene, is involved in limb development and its association with metabolism is uncertain 74. One limitation to the current study is the lack of adherence and dietary information which could affect tacrolimus trough concentrations, however, when nonadherence was suspected, the trough concentration around that time was excluded. Because of the large number of variants studied, the power for discovery is low and these variants must be validated in other populations.
5. Conclusion
There are significant differences in tacrolimus daily doses and dose-normalized troughs by ancestry. Native American had highest dose normalized tacrolimus trough concentrations and African Americans had the lowest dose-normalized troughs due to higher frequency of CYP3A5 expressers. Genetic variants that influence tacrolimus metabolism also differ across populations. CYP3A5*3 is an important predictor of tacrolimus exposure in the four populations studied however its effect size on metabolism varied among the populations. In addition to CYP3A5*3, CYP3A5*6 and *7 are important predictors of tacrolimus metabolism in African Americans, and CYP3A4*22 is important in those of European ancestry. No additional variants at a gene wide significance level were identified for Asian or Native American kidney transplant recipients but several were suggestive and should be further evaluated. Larger populations of Asian and Native American ancestry recipients are needed to assess importance of suggestive variants.
Supplementary Material
Acknowledgments
The authors wish to thank the research subjects for their participation in this study. We acknowledge the dedication and hard work of our coordinators at each of the DeKAF Genomics and GEN03 clinical sites: University of Alberta, Nicoleta Bobocea, Tina Wong, Adrian Geambasu and Alyssa Sader; University of Manitoba, Myrna Ross and Kathy Peters; University of Minnesota, Mandi DeGrote, Monica Myers and Danielle Berglund; Hennepin County Medical Center, Lisa Berndt; Mayo Clinic, Tom DeLeeuw; University of Iowa, Wendy Wallace and Tammy Lowe; University of Alabama, Jacquelin Vaughn, Valencia Stephens and Tena Hilario. We also acknowledge the dedicated work of our research scientists Marcia Brott and Amutha Muthusamy. This study was supported by NIH/NIAID grants 5U19-AI070119, K01AI130409 and 5U01-AI058013.
Abbreviations:
- CYP
Cytochromes P450
- MAF
Minor allele frequencies
- GWAS
Genome wide association studies
- CPIC
Clinical Pharmacogenetics Implementation Consortium
- DeKAF
Deterioration of Kidney Allograft Function
- GWA
genome wide association
- AF
Alleles frequencies
- LD
Linkage disequilibrium
- DYNC1I1
Dynein cytoplasmic 1 intermediate chain 1 gene
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
The data with participant consent, that support the findings of this study are openly available in dbGaP at https://www.ncbi.nlm.nih.gov/gap.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of this article.
References
- 1.Jouve T, Noble J, Rostaing L, Malvezzi P. Tailoring tacrolimus therapy in kidney transplantation. Expert Rev Clin Pharmacol 2018;11(6):581–588. [DOI] [PubMed] [Google Scholar]
- 2.Phupradit A, Vadcharavivad S, Ingsathit A, et al. Impact of POR and CYP3A5 Polymorphisms on Trough Concentration to Dose Ratio of Tacrolimus in the Early Post-operative Period Following Kidney Transplantation. Ther Drug Monit 2018;40(5):549–557. [DOI] [PubMed] [Google Scholar]
- 3.Hu C, Yin W-J, Li D-Y, et al. Evaluating tacrolimus pharmacokinetic models in adult renal transplant recipients with different CYP3A5 genotypes. Eur J Clin Pharmacol 2018;74(11):1437–1447. [DOI] [PubMed] [Google Scholar]
- 4.Oetting WS, Wu B, Schladt DP, et al. Attempted validation of 44 reported SNPs associated with tacrolimus troughs in a cohort of kidney allograft recipients. Pharmacogenomics 2018;19(3):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asempa TE, Rebellato LM, Hudson S, Briley K, Maldonado AQ. Impact of CYP3A5 genomic variances on clinical outcomes among African American kidney transplant recipients. Clin Transplant 2018;32(1). [DOI] [PubMed] [Google Scholar]
- 6.Andrews PA, Sen M, Chang RW. Racial variation in dosage requirements of tacrolimus. Lancet (London, England) 1996;348(9039):1446. [DOI] [PubMed] [Google Scholar]
- 7.Chakkera HA, Chang YH, Bodner JK, et al. Genetic differences in Native Americans and tacrolimus dosing after kidney transplantation. Transplant Proc 2013;45(1):137–141. [DOI] [PubMed] [Google Scholar]
- 8.Grover A, Frassetto LA, Benet LZ, Chakkera HA. Pharmacokinetic differences corroborate observed low tacrolimus dosage in native American renal transplant patients. Drug Metab Dispos 2011;39(11):2017–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eghtesad B, Malhotra D, Hecker WP, et al. Use of tacrolimus as the primary immunosuppression after renal transplant in native Americans and Hispanics. Transplant Proc 1998;30(4):1232–1233. [DOI] [PubMed] [Google Scholar]
- 10.Kamdem LK, Streit F, Zanger UM, et al. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 2005;51(8):1374–1381. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Hebert MF, Isoherranen N, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos 2006;34(5):836–847. [DOI] [PubMed] [Google Scholar]
- 12.Lloberas N, Hesselink DA, van Schaik RH, et al. Detection of a rare CYP3A4 variant in a transplant patient characterized by a tacrolimus poor metabolizer phenotype. Pharmacogenomics 2018;19(4):305–310. [DOI] [PubMed] [Google Scholar]
- 13.Tang JT, Andrews LM, van Gelder T, et al. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol 2016;12(5):555–565. [DOI] [PubMed] [Google Scholar]
- 14.Quaranta S, Chevalier D, Allorge D, et al. Ethnic differences in the distribution of CYP3A5 gene polymorphisms. Xenobiotica 2006;36(12):1191–1200. [DOI] [PubMed] [Google Scholar]
- 15.Komine N, Satoh S, Saito M, et al. Influence of CYP3A5 genetic differences in tacrolimus on quantitative interstitial fibrosis and long-term graft function in kidney transplant recipients. Int Immunopharmacol 2018;58(February):57–63. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmgenomics Pers Med 2018;11:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hustert E, Haberl M, Burk O, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001;11(9):773–779. [DOI] [PubMed] [Google Scholar]
- 18.Lloberas N, Elens L, Llaudó I, et al. The combination of CYP3A4*22 and CYP3A5*3 single-nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet Genomics 2017;27(9):313–322. [DOI] [PubMed] [Google Scholar]
- 19.Elens L, Capron A, van Schaik RH, et al. Impact of CYP3A4*22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: Toward updated genotype-based dosage guidelines. Ther Drug Monit 2013;35(5):608–616. [DOI] [PubMed] [Google Scholar]
- 20.Pallet N, Jannot A-S, El Bahri M, et al. Kidney Transplant Recipients Carrying the CYP3A4*22 Allelic Variant Have Reduced Tacrolimus Clearance and Often Reach Supratherapeutic Tacrolimus Concentrations. Am J Transplant 2015;15(3):800–805. [DOI] [PubMed] [Google Scholar]
- 21.Andreu F, Colom H, Elens L, et al. A New CYP3A5*3 and CYP3A4*22 Cluster Influencing Tacrolimus Target Concentrations: A Population Approach. Clin Pharmacokinet 2017;56(8):963–975. [DOI] [PubMed] [Google Scholar]
- 22.Scheibner A, Remmel R, Schladt D, et al. Tacrolimus Elimination in Four Patients With a CYP3A5*3/*3 CYP3A4*22/*22 Genotype Combination. Pharmacotherapy 2018;38(7):e46–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. Tacrolimus Population Pharmacokinetics and Multiple CYP3A5 Genotypes in Black and White Renal Transplant Recipients. J Clin Pharmacol 2018;58(9):1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oetting WS, Schladt DP, Guan W, et al. Genomewide Association Study of Tacrolimus Concentrations in African American Kidney Transplant Recipients Identifies Multiple CYP3A5 Alleles. Am J Transplant 2016;16(2):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oetting WS, Wu B, Schladt DP, et al. Genome-wide association study identifies the common variants in CYP3A4 and CYP3A5 responsible for variation in tacrolimus trough concentration in Caucasian kidney transplant recipients. Pharmacogenomics J 2018;18(3):501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birdwell KA, Decker B, Barbarino JM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther 2015;98(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Genetics & Translational Research in Transplantation Network (iGeneTRAiN). Design and Implementation of the International Genetics and Translational Research in Transplantation Network. Transplantation 2015;99(11):2401–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YR, van Setten J, Verma SS, et al. Concept and design of a genome-wide association genotyping array tailored for transplantation-specific studies. Genome Med 2015;7(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbs RA, Boerwinkle E, Doddapaneni H, et al. A global reference for human genetic variation. Nature 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudmant PH, Rausch T, Gardner EJ, et al. An integrated map of structural variation in 2,504 human genomes. Nature 2015;526(7571):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genome of the Netherlands Consortium LC, Menelaou A, Pulit SL, et al. Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat Genet 2014;46(8):818–825. [DOI] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaneau O, Howie B, Cox AJ, Zagury J-F, Marchini J. Haplotype Estimation Using Sequencing Reads. Am J Hum Genet 2013;93(4):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howie B, Marchini J, Stephens M. Genotype Imputation with Thousands of Genomes. G3: Genes|Genomes|Genetics 2011;1(6):457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson PA, Oetting WS, Brearley AM, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation 2011;91(3):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant 2012;12(12):3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M-X, Yeung JMY, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet 2012;131(5):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation 1998;65(4):515–523. [DOI] [PubMed] [Google Scholar]
- 39.Mancinelli L, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: A comparison across ethnic groups. Clin Pharmacol Ther 2001;69(1):24–31. [DOI] [PubMed] [Google Scholar]
- 40.Laftavi MR, Pankewycz O, Patel S, et al. African American renal transplant recipients (RTR) require higher tacrolimus doses to achieve target levels compared to white RTR: Does clotrimazole help? Transplant Proc 2013;45(10):3498–3501. [DOI] [PubMed] [Google Scholar]
- 41.Dirks NL, Huth B, Yates CR, Meibohm B. Pharmacokinetics of immunosuppressants: a perspective on ethnic differences. Int J Clin Pharmacol Ther 2004;42(12):701–718. [DOI] [PubMed] [Google Scholar]
- 42.Narayanan M, Pankewycz O, El-Ghoroury M, et al. Outcomes in African American kidney transplant patients receiving tacrolimus and mycophenolic acid immunosuppression. Transplantation 2013;95(4):566–572. [DOI] [PubMed] [Google Scholar]
- 43.Beermann KJ, Ellis MJ, Sudan DL, Harris MT. Tacrolimus dose requirements in African-American and Caucasian kidney transplant recipients on mycophenolate and prednisone. Clin Transplant 2014;28(7):762–767. [DOI] [PubMed] [Google Scholar]
- 44.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 2002;54(10):1271–1294. [DOI] [PubMed] [Google Scholar]
- 45.CPIC® Guideline for Tacrolimus and CYP3A5 – CPIC. Supplemental Table S3; Frequencies of CYP3A5 alleles in major race/ethnic groups.
- 46.Fohner A, Muzquiz LI, Austin MA, et al. Pharmacogenetics in American Indian populations: analysis of CYP2D6, CYP3A4, CYP3A5, and CYP2C9 in the Confederated Salish and Kootenai Tribes. Pharmacogenet Genomics 2013;23(8):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billing H, Höcker B, Fichtner A, et al. Single-nucleotide polymorphism of CYP3A5 impacts the exposure to tacrolimus in pediatric renal transplant recipients: A pharmacogenetic substudy of the TWIST trial. Ther Drug Monit 2017;39(1):21–28. [DOI] [PubMed] [Google Scholar]
- 48.Andrews LM, Hesselink DA, van Gelder T, et al. A Population Pharmacokinetic Model to Predict the Individual Starting Dose of Tacrolimus Following Pediatric Renal Transplantation. Clin Pharmacokinet 2018;57(4):475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen MJ, Bergmann TK, Brøsen K, Thiesson HC. The Pharmacogenetics of Tacrolimus in Corticosteroid-Sparse Pediatric and Adult Kidney Transplant Recipients. Drugs R D 2017;17(2):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hesselink DA, Bouamar R, Elens L, van Schaik RHN, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet 2014;53(2):123–139. [DOI] [PubMed] [Google Scholar]
- 51.Hendijani F, Azarpira N, Kaviani M. Effect of CYP3A5*1 expression on tacrolimus required dose for transplant pediatrics: A systematic review and meta-analysis. Pediatr Transplant 2018;22(6):e13248. [DOI] [PubMed] [Google Scholar]
- 52.CPIC® Guideline for Tacrolimus and CYP3A5 – CPIC. Supplemental Material.
- 53.Haufroid V, Wallemacq P, VanKerckhove V, et al. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: Guidelines from an experimental study. Am J Transplant 2006;6(11):2706–2713. [DOI] [PubMed] [Google Scholar]
- 54.Han N, Ha S, Yun HY, et al. Population pharmacokinetic-pharmacogenetic model of tacrolimus in the early period after kidney transplantation. Basic Clin Pharmacol Toxicol 2014;114(5):400–406. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, Guo Y, Ye W, et al. RS11212617 is associated with metformin treatment response in type 2 diabetes in Shanghai local Chinese population. Int J Clin Pract 2014;68(12):1462–1466. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J-J, Liu S-B, Xue L, Ding X-L, Zhang H, Miao L-Y. The genetic polymorphisms of POR*28 and CYP3A5*3 significantly influence the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Int J Clin Pharmacol Ther 2015;53(09):728–736. [DOI] [PubMed] [Google Scholar]
- 57.Xing J, Zhang X, Fan J, Shen B, Men T, Wang J. Association between interleukin-18 promoter variants and tacrolimus pharmacokinetics in Chinese renal transplant patients. Eur J Clin Pharmacol 2015;71(2):191–198. [DOI] [PubMed] [Google Scholar]
- 58.Li CJ, Li L, Lin L, et al. Impact of the CYP3A5, CYP3A4, COMT, IL-10 and POR genetic polymorphisms on tacrolimus metabolism in Chinese renal transplant recipients. PLoS One 2014;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen P, Li J, Li J, et al. Dynamic effects of CYP3A5 polymorphism on dose requirement and trough concentration of tacrolimus in renal transplant recipients. J Clin Pharm Ther 2017;42(1):93–97. [DOI] [PubMed] [Google Scholar]
- 60.Luo X, Zhu LJ, Cai NF, Zheng LY, Cheng ZN. Prediction of tacrolimus metabolism and dosage requirements based on CYP3A4 phenotype and CYP3A5∗3 genotype in Chinese renal transplant recipients. Acta Pharmacol Sin 2016;37(4):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanghavi K, Brundage RC, Miller MB, et al. Genotype-guided tacrolimus dosing in African-American kidney transplant recipients. Pharmacogenomics J 2017;17(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okubo M, Murayama N, Shimizu M, Shimada T, Guengerich FP, Yamazaki H. CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J Toxicol Sci 2013;38(3):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuypers DRJ, de Loor H, Naesens M, Coopmans T, de Jonge H. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenet Genomics 2014;24(12):597–606. [DOI] [PubMed] [Google Scholar]
- 64.Elens L, van Schaik RH, Panin N, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 2011;12(10):1383–1396. [DOI] [PubMed] [Google Scholar]
- 65.Elens L, van Gelder T, Hesselink DA, Haufroid V, van Schaik RH. CYP3A4*22 : promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 2013;14(1):47–62. [DOI] [PubMed] [Google Scholar]
- 66.McClain CA, Bernhardt MB, Berger A, et al. Pharmacogenetic association with neurotoxicity in Hispanic children with acute lymphoblastic leukaemia. Br J Haematol 2018;181(5):684–687. [DOI] [PubMed] [Google Scholar]
- 67.Reference SNP (refSNP) Cluster Report: rs35599367.
- 68.Lunde I, Bremer S, Midtvedt K, et al. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur J Clin Pharmacol 2014;70(6):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moes DJARAR, Swen JJ, Den Hartigh J, et al. Effect of CYP3A4*22, CYP3A5*3, and CYP3A combined genotypes on cyclosporine, everolimus, and tacrolimus pharmacokinetics in renal transplantation. CPT Pharmacometrics Syst Pharmacol 2014;3(2):e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akbas SH, Bilgen T, Keser I, et al. The Effect of MDR1 (ABCB1) Polymorphism on the Pharmacokinetic of Tacrolimus in Turkish Renal Transplant Recipients. Transplant Proc 2006;38(5):1290–1292. [DOI] [PubMed] [Google Scholar]
- 71.Vanhove T, Annaert P, Lambrechts D, Kuypers DRJ. Effect of ABCB1 diplotype on tacrolimus disposition in renal recipients depends on CYP3A5 and CYP3A4 genotype. Pharmacogenomics J 2017;17(6):556–562. [DOI] [PubMed] [Google Scholar]
- 72.Dorr CR, Wu B, Remmel RP, et al. Identification of genetic variants associated with tacrolimus metabolism in kidney transplant recipients by extreme phenotype sampling and next generation sequencing. Pharmacogenomics J November 2018. [DOI] [PMC free article] [PubMed]
- 73.Dorr CR, Remmel RP, Muthusamy A, et al. CRISPR/Cas9 Genetic Modification of CYP3A5 *3 in HuH-7 Human Hepatocyte Cell Line Leads to Cell Lines with Increased Midazolam and Tacrolimus Metabolism. Drug Metab Dispos 2017;45(8):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delgado S, Velinov M. 7q21.3 Deletion involving enhancer sequences within the gene DYNC1I1 presents with intellectual disability and split hand-split foot malformation with decreased penetrance. Mol Cytogenet 2015;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.