Abstract
Normothermic Machine Perfusion presents a novel platform for per-transplant assessment and reconditioning of kidney grafts. Maintaining the metabolic activity of a preserved graft at physiologic levels requires an adequate oxygen supply, typically delivered by crystalloid solutions supplemented with red blood cells. In this study, we explored the feasibility of using a synthetic hemoglobin-based oxygen carrier (HBOC) in human kidney normothermic perfusion. 14 discarded human kidneys were perfused for 6 hours at mean temperature of 37°C using a pressure-controlled system. Kidneys were perfused with a perfusion solution supplemented with either HBOC (n=7) or PRBC (n=7) to increase oxygen carrying capacity. Renal artery resistance, oxygen extraction, metabolic activity, energy stores, and histological features were evaluated. Throughout perfusion, kidneys from both groups exhibited comparable behavior regarding vascular flow (p=0.66), oxygen consumption (p=0.88), and reconstitution of tissue adenosine triphosphate tissue (p=0.057). Lactic acid levels were significantly higher in kidneys perfused with PRBC (p=0.007). Histological findings were comparable between groups and there was no evidence of histological damage caused by the HBOC. This feasibility experiment demonstrates that a hemoglobin-based oxygen carrier solution can offer a logistically more convenient off-the-shelf alternative to packed red blood cells in normothermic machine perfusion of human kidneys.
1. Introduction
Kidney transplantation remains the optimal treatment for most patients with end-stage renal disease. As the number of patients with chronic renal failure increases globally, the gap between supply and demand for suitable kidney allografts has expanded.1 To expand the pool of transplantable kidneys, hypothermic machine perfusion (HMP) has been employed, as an alternative to static cold storage (SCS). HMP has been found to decrease delayed graft function and allow for evaluation of flow and pressure within the potential allograft prior to transplantation in humans.2–5 However, cold storage technology is limited in the amount of data it is able to provide, as the allograft is held at 4C and kept metabolically inactive.3,4,6–8 Extended periods of cold ischemia have been shown to increase the risk of early graft dysfunction and reduce long-term survival of the allograft.9–12 Furthermore, low perfusion temperatures offer little opportunity to improve organ quality, thereby potentially limiting the maximum utilization of marginal kidneys. Normothermic Ex Vivo Kidney Perfusion (NEVKP) has recently generated increasing interest as a new modality for assessing and improving kidney function prior to transplant. The approach relies on oxygenated perfusion of the kidney on a perfusion platform that limits cold ischemia, allows real time measurement of urine output, creatinine clearance, lactate clearance, and assessment of renal perfusion characteristics under more physiologic conditions than that under cold perfusion. Furthermore, the technology may permit discrimination of which of the kidneys currently being discarded can provide patients with and acceptable post-transplant outcome. 13–17
Published reports of NEVKP to date have relied exclusively on the human red blood cell-based perfusates, whether in the form of whole blood, leukocyte depleted blood, or packed red blood cells (PRBC), as the oxygen carrier of the system, in an attempt to recapitulate the optimal physiologic environment.18 The use of blood-based components in Normothermic Machine Perfusion (NMP), although effective, comes with several logistic impediments including scarce availability, high utilization cost and short shelf life. Moreover, there are inherent drawbacks related to red blood cell hemolysis especially in centrifugal/rotatory pump perfusion systems,19 as well the risks of infection transmission and graft rejection due to potential presence of undetected inflammatory components. 20–23
Organs undergoing ex vivo perfusion at normothermic temperatures require adequate oxygen delivery to meet metabolic demand. Over the past two decades, new generations of acellular synthetic oxygen carrying agents or what is also known a hemoglobin-based oxygen carrier (HBOC) have been suggested as a promising alternative to PRBC not only in human transfusions,24,25 but also in ex vivo organ normothermic and sub-normothermic perfusion procedures for organs other than kidney.26–28 The promise of these agents lies in their long shelf half-life, ease of use, easy accessibility, and negligible infectious risks. In this study, we describe our experience using a HBOC solution in human kidney normothermic machine perfusion.
2. Materials and Methods
2.1. Donor kidney selection
This study was declared exempt by the Massachusetts General Hospital institutional review board and approved by New England Donor Services (NEDS). 14 human kidneys were obtained from NEDS after being declined for transplantation by all centers. No age or preservation time limits were applied. Kidneys donated after circulatory death (DCD) as well as kidneys donated after brain death (DBD) were accepted. Demographics of the kidney donors were entered into a database created for this study. Recorded information included donor age, gender, donor type (DBD/DCD), cause of death, duration of cold ischemia time (CIT), duration of warm ischemic time (WIT) if applicable, reason for discard, Kidney Donor Profile Index (KDPI), biopsy results if available, baseline creatinine, and terminal creatinine. When applicable, DCD kidneys were preserved on HMP pumps (Life Port Kidney Transporter from Organ Recovery Systems®, Itasca, IL). Otherwise organs were transported from their respective donor hospitals in SCS (table 1).
Table 1.
Kidney donor demographics data.
| (HBOC NEVKP) n=7 | (PRBC NEVKP) n=7 | ||
|---|---|---|---|
| Donor type | 4 DCD | 3 DBD | 7 DBD |
| Age (years)* | 57 (50–61) | 31 (31–67) | 67 (66–72) |
| Sex ratio (M:F) | 3:1 | 3:0 | 5:2 |
| KDPI (%)* | 93 (76–96) | 54 (54–99) | 100 (99–100) |
| Terminal creatinine (mg/dL)* | 0.8 (0.57–1.1) | 4.3 (1.7–4.3) | 1.9 (1.3–2.2) |
| WIT (min)* | 20 (16–24) | n/a | n/a |
| CIT (h)* | 21.7 (19.4–24.7) | 11.4 (11.4–17.6) | 24.7 (17.7–28.8) |
| Cold storage medium | 4 HMP | 3HMP | 1 SCS, 6 HMP |
| Cause of death | |||
| • Stroke | 2 | 1 | 4 |
| • Hypoxic brain injury | -- | 2 | 2 |
| • Cardiac arrest | 1 | -- | 1 |
| • Other | 1 | -- | -- |
| Reason for decline | |||
| • Technical/anatomical | 1 | -- | -- |
| • HMP parameters | 1 | -- | -- |
| • Donor medical history | 1 | 1 | 5 |
| • Histology | 1 | 2 | 2 |
median (IQR); HBOC, hemoglobin-based oxygen carrier; PRBC, packed red blood cells; NEVKP, normothermic ex-vivo kidney perfusion; DCD, donor after cardiac death; DBD, donor after brain death; KDPI, Kidney donor profile index; WIT, warm ischemia time; CIT, cold ischemia time; SCS, static cold storage; HMP, hypothermic machine perfusion.
2.2. Graft preparation
After arrival at our lab, the donor kidney was kept in ice-cold University of Wisconsin preservation solution (UW solution) while the extrarenal fat was removed. The renal artery and ureter were dissected and cannulated. An appropriate 10 or 12 French cannula was chosen based on renal artery size and secured in place with ligatures. Prior to perfusion, the kidney was flushed with 500cc of cold Lactated Ringer’s solution. 7 kidneys were perfused with a HBOC solution and 7 kidneys perfused with a PRBC based solution.
2.3. Ex-vivo normothermic machine perfusion
NEVKP was performed for 6-hours using a modified pressure-controlled commercial perfusion system (Organ Assist, Groningen, Netherlands). Perfusion temperature was set at 37∘C, inflow arterial pressure was set at 70mmHg. A physiologic pH of 7.34–7.45 was achieved by titration with sodium bicarbonate (20–50 mmol) and adjustment of the gas flow rate as needed.
In both groups, a perfusate total volume of 2000mL perfusate was used, consisting of a base of 1500cc of Williams E Media supplemented with dexamethasone (8 mg/L), insulin (5 U/l), heparin (1000U/l), sodium bicarbonate 8.4% (titrated to physiologic pH), calcium chloride to ionized calcium of 1.1–1.4mmol/L. Creatinine (1000 µmol/L) was added to evaluate creatinine clearance. The hemoglobin component of the solution was provided by either 500 cc of Hemopure ((HBOC)-201, HBO2 Therapeutics LLC, Cambridge, MA, USA) in the HBOC group, or 500 cc of blood group-compatible PRBC. The perfusate was oxygenated with a carbogen mixture of 95% O2 / 5% CO2, achieving maximum partial oxygen pressure of >500 mmHg and un-depleted oxygen outflow (>200 mmHg). Ringer lactate was used to replace urine and evaporation. Glucose levels were maintained at 100–200 mg/dL by supplementing dextrose accordingly throughout perfusion.
The combination of HBOC-201 and William E media, was previously shown to be applicable for NEVKP in animal models,29 and achieved a hemoglobin concentration of 3.5 g/dL, that demonstrated sustained low methemoglobin levels while providing reliable oxygen delivery and carbon dioxide clearance.28 Based on our optimization experiments we refrained from including additional oncotic elements such as albumin, due to the adverse effect on renal physiology during NMP.
2.4. Perfusion assessment
During the 6-hour perfusion period, 1 ml perfusate samples were taken at hourly intervals from the efferent and afferent sides of the oxygenator (reflecting the arterial inflow and venous outflow respectively). Samples were tested for pH, partial pressure of oxygen, partial pressure of carbon dioxide, oxygen saturation, electrolytes, glucose and creatinine levels. Sample analysis was performed using an i-STAT Blood Analyzer (Abbott Point of Care, Princeton, NJ, USA). Additionally, 6 mL of perfusate were drawn off the circuit hourly for complete biochemistry analysis. Samples were centrifuged at 12,000 rpm for 8 minutes and aliquots of the supernatant were stored at –80 °C). Kidney weight was recorded before and after perfusion.
Oxygen consumption (mLO2/minute/gram) was calculated as following: (oxygen solubility coefficient (mlO2/mmHgO2/ml) x (arterial partial oxygen pressure (mmHg) x renal artery flow (ml/min) – venous partial oxygen pressure (mmHg) x renal artery flow (ml/min))) + hemoglobin concentration (g/dL)/100 x hemoglobin oxygen-binding capacity (mlO2/gram) x (arterial oxygen saturation (%) /100 x renal artery flow (ml/min) – venous oxygen saturation (%) /100 x renal artery flow (ml/min)) /kidney weight. The calculations took into consideration the differences in HBOC and RBC, Hb content and O2 transport properties.
2.5. Urine
The ureteral outflow was drained in a collection tube and urine production was regularly measured during perfusion in addition to sample collection. Urine creatinine and sodium were measured simultaneously with perfusate creatinine and sodium measurements. Creatinine clearance was calculated as urine creatinine × urine production rate/perfusate creatinine. Fractional excretion of sodium (FENa) was calculated as urine sodium × serum creatinine/(serum sodium × urine creatinine) × 100.
2.6. Histological evaluation
Cortical biopsies were taken at baseline (end of CIT) and end of perfusion, fixed in 10% formalin, stained with Hematoxylin & Eosin (H&E) and evaluated by two blinded pathologists to calculate the Remuzzi score30 and characterize changes associated with NEVKP in both groups. Perfusion related histologic assessment was based on the following morphological parameters of tubular injury: loss of brush borders/Tubular dilation, epithelial vacuolation, epithelial desquamation/cellular-casts, coagulation necrosis and loss of tubular integrity (i.e. epithelial necrosis, destruction of basement membrane). These parameters were scaled before and after perfusion 0–3 (0, none; 1, mild; 2, moderate; 3, sever). The average score from 10 high-power fields was used as an overall assessment.
2.7. Evaluation of renal energy charge
Four tissue biopsies were collected from each kidney at 2-hour intervals. Prior to tissue homogenization, biopsies were pulverized in liquid nitrogen. Metabolic cofactors (ATP, ADP, AMP and energy charge) were analyzed with a LC-MS/MS (Sciex TripleTOF 6600 Quadrupole Time-Of-Flight; AB Sciex, Foster City, CA).
2.8. Quality assessment
A Quality Assessment Score (QAS) reported by Hosgood et al. was employed to classify the potential transplantability of the kidney grafts following NEVKP. 31 The scoring system was developed using receiver operating characteristic (ROC) curves to determine thresholds of renal inflow and urine production. These thresholds were combined with a macroscopic grade to give an overall assessment score on a scale of 1 to 5. Macroscopic grades I: excellent perfusion (global pink appearance), II: moderate perfusion (patchy appearance) and III: poor perfusion (global mottled and purple/black appearance) were assigned scores of 1, 2 and 3 respectively. Kidneys with a mean renal artery flow below 50 mL/min/100g and urine output below 43 mL/h were given an additional score of 1 for each. Therefore, overall the assessment scores ranged from 1, indicating the least injured, to 5, the most severely injured.
2.9. Statistical analysis
Data analysis was performed using Prism 7.03 for PC (GraphPad software, Inc., La Jolla, CA). The results are expressed as numerical values and percentages for categorical variables and as medians with interquartile range for continuous variables, unless stated otherwise. Group medians of continuous data were compared using the Mann-Whitney U test at each hourly time point. Multivariate curve comparison was also performed using nonlinear regression analysis followed by one-way ANOVA test. Statistical significance was assumed if p < 0.05.
3. Results
3.1. Donor characteristics
14 human kidneys were included in this study. 4 DCD and 3 DBD kidneys were perfused with HBOC medium, the overall median terminal creatinine value for this group was 1.2 (0.8–4.3) mg/dL. All kidneys perfused with a PRBC based medium were from DBD donors with a median terminal creatinine of 1.9 (1.36–2.2) mg/dL. The median relative CIT for all kidneys was 20 (range 18–25) hours. Median donor age was 53 (31– 61) years for the HBOC group and 67 (66–72) years for the PRBC group (table 1). Overall, the two groups were comparable regarding reason for organ decline, male: female ratio, or the mode of cold preservation (table 1).
3.2. Perfusion assessment
Both experimental arms demonstrated a comparable increase in renal artery flow, reaching medians of 312 (277–364) mL/min in the HBOC group and 347 (273‐406) mL/min in the PRBC group, at 6 hours of EVNKP. This increase in vascular flow corresponded with a decrease in vascular resistance approximating physiologic levels (normal = 0.60–0.70 mmHg⋅min⋅mL−1) by the third hour of perfusion (figure 1). Although kidneys perfused with PRBC showed an initial higher vascular resistance the difference was not significance (p=0.66).
Figure 1.

Resistance and flow during 6 hours of normothermic ex-vivo kidney perfusion. A: Renal artery resistance showed no statistical difference between both groups throughout perfusion (p=0.66); B: Renal artery flow followed a similar pattern in the HBOC and PRBC groups (p=0.57). Total flow peaked at 240 mins to median 348 mL/min (244 – 358 mL/min) and 333 mL/min (235 – 358 mL/min) in both groups respectively. The red lines represent the kidneys perfused with a PRBC based solution and the black dotted lines represent the kidneys perfused with HBOC based solution.
pH and bicarbonate levels were sustained within a physiologic range (pH 7.35–7.457) with minimal exogenous bicarbonate supplementation required. Lactic acid levels measured at each time point, were significantly higher in kidneys perfused with PRBC (p=0.007) (figure 3 E). There was no difference between groups in renal function parameters including urine production rate, creatinine clearance, urine-to-perfusate creatinine ratio. The median total urine output was 398 (157‐560) mL in the HBOC group and 299 (55‐539) mL in the PRBC group (p=0.78). Increased urine production trend significantly correlated with the decreasing perfusate creatinine levels in both groups (p=0.02) (figure 3 A,C,D). FENa, used as an additional parameter of renal tubule functin, did not reveal a difference between groups (p=0.23) (figure 3 B).
Figure 3.
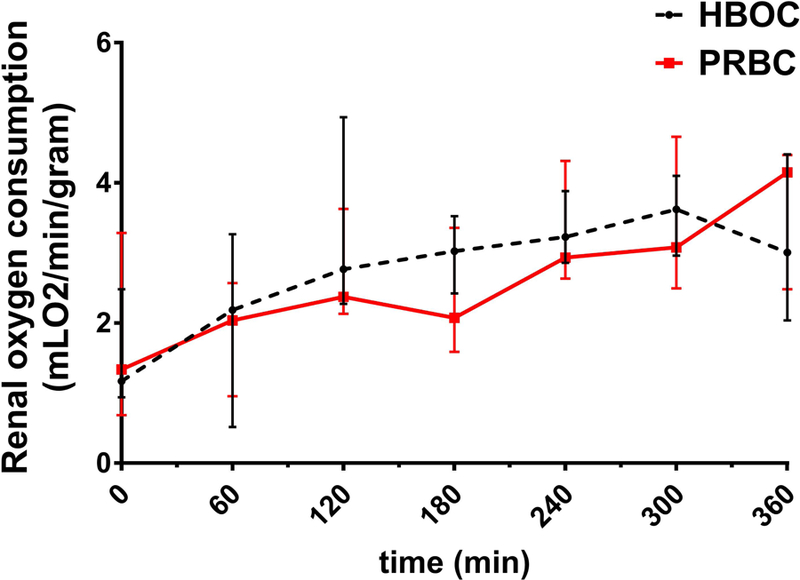
Oxygen consumption during 6 hours of normothermic ex-vivo kidney perfusion. No statistical difference was observed in oxygen consumption levels between both groups (p=0.88). The calculations of oxygen consumption were made based on the HBOC and PRBC hemoglobin content and oxygen transport properties as well as the kidney weight, renal blood flow. The red line represents the kidneys perfused with a PRBC based solution and the black dotted line represents the kidneys perfused with HBOC based solution.
Baseline weight decreased in the all HBOC perfused grafts 20 (3 – 46)g; While only four kidneys in the PRBC group lost weight (8, 12, 24, 60)g, the remaining three gained weight following NMP (9, 32, 44)g.
3.3. Oxygen Consumption
Oxygen delivery/extraction was stable showing no high variability across all kidneys. There was no significant difference in oxygen consumption patterns between groups (p=0.88), as represented in (figure 3).
3.4. Histological Evaluation
Histological assessment revealed no significant differences between groups at baseline (p= 0.11) Remuzzi scores ranged from (2 to 12) in the HBOC perfused group and from (3 to 12) in the PRBC group. (table 2) In both groups there was also no significant correlation between the Remuzzi score and WIT (p=0.34), CIT (p=0.17), terminal creatinine (p=0.28), and donor age (p=0.70).
Table 2.
Baseline Remuzzi Scores in both groups, calculated by two consultant pathologist who were blinded to the donor types. Four different parameters were assessed in the scoring system; Glomerular global sclerosis, tubular atrophy, interstitial fibrosis and vascular lesions (8). The score ranged from a minimum of 0 (indicating the absence of renal lesions) to 3 (severe). The sum of the four parameters was then calculated. A score of 0–3 indicated mild changes, 4–6 moderate and 7–12 severe.
| HBOC | PRBC | ||
|---|---|---|---|
| Donor Type | Remuzzi Score | Donor Type | Remuzzi Score |
| DCD | 2 | DBD | 3 |
| DCD | 4 | DBD | 4 |
| DCD | 7 | DBD | 5 |
| DCD | 12 | DBD | 6 |
| DBD | 4 | DBD | 6 |
| DBD | 5 | DBD | 10 |
| DBD | 6 | DBD | 12 |
The PRBC perfused group demonstrated greater vacuolization and loss of tubular integrity when comparing pre and post perfusion samples (p=0.023). There was no difference in epithelial shedding between groups (p=0.24). However, there was significantly more coagulation necrosis and overall loss of tubular integrity in the PRBC group post 6-hour perfusion compared to the HBOC group. (p=0.01 and p=0.02, respectively). (figure 4) (table 3)
Figure 4.

Histological evaluation: 10x Photomicrographs of cortical biopsies, taken at baseline (end of CIT) and end of 6 hours of machine perfusion, fixed in 10% formalin, stained with Hematoxylin & Eosin (H&E) and evaluated by two blinded pathologists. A: Pre-prefusion with HBOC; B: Post-perfusion with HBOC; C: Pre-perfusion with PRBC; D: Post-perfusion with PRBC. Histology reveals glomerular tufts irregularly engorged with accumulated luminal red blood cells (black arrows), loss of the epithelial brush border, frequent cytoplasmic vacuolations (blue arrows), and rupture of some tubules with interstitial edema (red arrows).
Table 3.
Histology findings before and after 6 hours of normothermic ex-vivo kidney perfusion (NEVKP) assessed by hematoxylin-eosin. Biopsies scored on a scale of (0–3) and represented as median (range) for 10 high-power fields.
| Criteria | Baseline | p | After 6h NEVKP | p | |||
|---|---|---|---|---|---|---|---|
| HBOC n=7 | PRBC n=7 | HBOC n=7 | PRBC n=7 | ||||
| Tubular dilation | 0.8 (0.1 – 2.4) | 0.5 (0.2–2.2) | 0.23 | 1.1 (0.5 – 2) | 1.4 (1 – 2.1) | 0.17 | |
| Epithelial vacuolization | 0 (0 – 0.8) | 0.1 (0 – 0.6) | 0.53 | 0.5 (0 – 1.3) | 1.6 (1.5 – 2.4) | 0.03 | |
| Epithelial desquamation | 1 (0.9 – 1.7) | 1.2 (0.3 – 1.9) | 0.74 | 1 (0.5 – 1.6) | 1.4 (1 – 2.2) | 0.24 | |
| Coagulation necrosis | 1.1 (0.5 – 1.4) | 1 (0.2 – 1.7) | 0.99 | 1 (0.2 – 1) | 2 (0.9 – 2.5) | 0.01 | |
| Tubular integrity | 1 (0.2 – 1.5) | 1.3 (0.5 – 1.5) | 0.61 | 1.5 (1– 2) | 2.3 (1.8 – 3) | 0.02 | |
3.5. Renal Energy Charge
Tissue concentration of ATP was used as an indicator of the energy status of the grafts. The overall renal energy charge was similar in both groups (p=0.057). ATP:ADP ratio and ATP:AMP ratio dropped in both groups after the first hour of perfusion. Followed by a steady rise in ATP generation. At 6 hours NEVKP, the median ATP content was 14.6 (8.9‐30.2) μg/mL in the HBOC group and 10.5 (9.4‐26.2) μg/mL in the PRBC group.
The ATP:ADP ratio after perfusion did not significantly differ from the starting value in both groups (p=0.34), however, there was a significant fluctuation in the ratio within the perfusion period. When comparing pre and post perfusion ATP:AMP ratios an opposing trend was seen between both groups; increasing in the PRBC group (p=0.03) and decreasing in the HBOC group (p=0.01). Nevertheless, ATP:AMP remained significantly higher in HBOC perfused kidneys following the first hour of perfusion (p=0.03).
3.6. Quality Assessment
Assessment was performed at the end of perfusion. Based on the macroscopic appearance in the HBOC group, 4 kidneys were graded as II while 3 as I. In the PRBC group, 3 kidneys were graded as II, 2 as III and the other 2 as I, using the QAS system. Two researchers independently assessed the macroscopic appearance. There was no discordance between their observations. Regarding the renal blood flow, all kidneys were graded as 0 by achieving a mean renal blood flow ≥50 mL/min/100g. With respect to urine output, 3 kidneys in the HBOC group and 3 in the PRBC group, were given a grade of 1 (mean urine output <43 mL/h) while the rest were graded 0. The overall assessment scores are listed in table 4.
Table 4.
Quality Assessment Score (QAS): Macroscopic grades 1: excellent perfusion (global pink appearance), 2: moderate perfusion (patchy appearance) and 3: poor perfusion (global mottled and purple/black appearance). Kidneys with a mean renal artery flow below 50 mL/min/100g and urine output below 43 mL/h were given an additional score of 1 for each. Overall the assessment score ranged from 1: least injured, to 5: most severely injured. According to QAS, 13 out the 14 kidneys perfused in this study were potentially transplantable.
| Kidney | Macro (1–2-3) | Flow (50 mL/min/100g) | Urine (43 mL/h) | Total |
|---|---|---|---|---|
| K01 | 1 | 0 | 1 | 2 |
| K02 | 2 | 0 | 0 | 2 |
| K03 | 2 | 0 | 0 | 2 |
| K04 | 2 | 0 | 0 | 2 |
| K05 | 1 | 0 | 0 | 1 |
| K06 | 2 | 0 | 1 | 3 |
| K07 | 1 | 0 | 1 | 2 |
| K08 | 1 | 0 | 0 | 1 |
| K09 | 2 | 0 | 0 | 2 |
| K10 | 3 | 0 | 0 | 3 |
| K11 | 3 | 0 | 1 | 4 |
| K12 | 1 | 0 | 0 | 1 |
| K13 | 2 | 0 | 1 | 3 |
| K14 | 2 | 0 | 1 | 3 |
A sub-analysis of hemodynamic behavior, oxygen consumption, ATP reconstitution, creatinine clearance, and urine and lactate production, revealed no significant difference between DBD and DCD kidneys perfused with HBOC (supplemental figure 1). There were also no clear differences on the same parameters when comparing DBD only kidneys in HBOC and PRBC groups. However, lactate levels were rising at a significantly higher rate in the PRBC group following the first 3 hours of perfusion (p=<0.005) (supplemental figure 2).
4. Discussion
While a growing body of data supports the use of NEVKP to condition and provide real time evaluative data on the kidney allograft, more research is needed to define the appropriate perfusion conditions and protocols to maximize application of this nascent technology; the optimal preservation medium is one of the most important factors in question.18,32–34 In this study, we describe for the first time, the efficacy of 6-hour NEVKP using an HBOC solution relative to a human red cell-based solution and demonstrate that HBOC is non inferior to PRBC in ex vivo normothermic kidney preservation with 6 hours of perfusion.
Two studies have reported comparable if not superior outcomes when using a HBOC vs PRBC-based solutions in NMP of human livers. Both studies, reported significantly higher oxygen extraction using HBOC vs RBCs. Their results also demonstrated that the HBOC could be effectively flushed out of organs prior to transplantation. 26,27
Second generation HBOCs like HBOC-201 (Hemopure) demonstrate reduced vasoactivity, longer intravascular retention, superior oxygenation and minimal toxicity. 24,35 The effect of HBOC on nitric oxide levels was not evaluated in this study but will be assessed in future investigations. There are reports of modest vasoconstriction during isolated organ machine perfusion with HBOC, nevertheless, this did not seem to impede achieving protocol targets in terms of perfusion, flow, and pressure.36 The effects of vasoconstriction - if present - may have been minimized by the excess oxygen delivery in most perfusion systems. In addition, HBOCs readily release oxygen bound to hemoglobin, as oxygen has fewer physical diffusion barriers and shorter diffusion paths with HBOCs. 36,37 The accumulation of methemoglobin is one of the potential drawbacks of using HBOCs. However, the amount of HBOC reaching the recipient circulation in a clinical setting would be negligible, as the perfusion solution would be washed out prior to transplantation.26,27,38,39
The combination of the perfusion parameters, urine output and macroscopic and microscopic assessment provides an overall measure of kidney quality during NEVKP. Renal artery flow is usually used as an index of vascular integrity. Perfused kidney grafts autoregulate renal flow according to intrarenal resistance. As the perfusion pressure is kept constant during NEVKP, a low renal blood flow indicates a high intrarenal resistance and therefore increased vascular injury or interstitial edema. Although this study did not show a difference in flow between groups, kidneys in the PRBC group demonstrated a higher evidence of vacuolization and loss of tubular integrity. Vacuolization has been previously reported in normothermic perfused kidneys,40 however, the clinical significance of this observation remains questionable.41 Regarding tubular injury, the higher degree of tubular integrity loss with red-cell based perfusate could be suggestive of more damage compared to HBOC. In addition, there was no specific histological evidence of damage caused by HBOC infusion.
Previous studies indicate that NEVKP allows for mitochondrial and cellular homeostasis prior to reperfusion.42 The preconditioning effects achieved through active replenishment of ATP stores may help renal grafts mitigate the injurious effects of reperfusion.43 Restoration of mitochondrial phosphorylation in pre-damaged grafts following cold storage is fundamentally dependent on the amount of oxygen delivered to the cells.44 Due to lower oxygen affinity and lessened viscosity of an HBOC-201 solution (Hemopure units are 1 × 10‐8 the size of an RBC) compared to a red cell based solution, the former is able to deliver significantly higher oxygen levels, thereby, achieving a superior cellular ATP content within perfused tissues. 27 We recorded sustained ATP production in the HBOC group following the first hour of perfusion, which was associated with a consistent increase in renal energy charge throughout the perfusion. Although, not reaching significance likely due to small sample size, our findings highlight that prolonged perfusion durations (>1hour) may be required to achieve optimal renal energy resuscitation.
Although, in this study, we maintained the perfusion temperature at 37°C from the start, HBOC-201 has the capacity to function in a wide temperature range, from hypothermic to normothermic (4°−37°C).26–28,36 This presents an opportunity to explore the effects of gradual rewarming. To date, gradual rewarming studies have been performed without an oxygen carrier as there was no available agent that could be applied in temperatures below 37°C. Therefore, an important line of future investigation will investigate the efficacy of HBOC-201 in gradual rewarming perfusion protocols.
Red blood cells have been reported to lose structural integrity over time during ex-vivo roller pump machine perfusion, resulting in unavoidable hemolysis and decrease in oxygen carrying capacity.19 In our study, the acellular HBOC-201 solution, resulted in more stable lactate levels throughout NEVKP, when compared to the significant rise in lactate observed in kidneys perfused with PRBC; Although hemolysis was not quantified due to equipment limitation, it was suggested by the red stained supernatant observed in the centrifuged perfusate samples of the latter group.
Hosgood and colleagues have utilized a one-hour NEVKP model to develop a large data set from which the Quality Assessment Score was produced. The scoring system allows for pretransplant evaluation of marginal kidneys, based on combining macroscopic graft assessment with thresholds of renal blood flow and urine output. In a clinical series of kidneys transplanted after NMP, higher overall perfusion assessment scores reflected a greater degree of pretransplant injury, and were associated with reduced early graft function and lower glomerular filtration rate at 12 months.31 Employing the same scoring model in our study, we found that 13 out of the total 14 kidneys, had an overall score range of 1 to 3 (7 in the HBOC group and 6 in the PRBC group). Based on Hosgood et al interpretations, these grafts could have been utilized for transplantation.
In congruence with previous NEVKP studies, we adopted a research design relying on comparable groups of singly isolated kidneys for NEVKP. We do acknowledge the small sample size of our study as a potential limitation. An additional study constraint is the lack of transplantation or a simulated reperfusion component that would enable controlled evaluation of immediate post-transplant kidney function. Future studies will need to address this to fully investigate the potential of our findings, before moving forward with a phase 1 safety trial testing the clinical function of currently discarded kidneys demonstrating favorable perfusion characteristics.
To conclude, our work demonstrates that 6-hour normothermic perfusion using HBOC of kidneys discarded for human transplantation is feasible and doesn’t result in inferior outcomes compared to PRBC. Furthermore, we demonstrate there is little deterioration in functional and histological characteristics of kidney grafts during perfusion with a HBOC. These findings provide the first evidence in favor of using acellular synthetic oxygen carrying agents during normothermic ex vivo perfusion of human kidney grafts.
Supplementary Material
Figure 2.

Biochemical evaluation during 6 hours of normothermic ex-vivo kidney perfusion. There was no significant difference between the HBOC and PRBC groups in A: urine production (p=0.69), B: fractional excretion of sodium FENa (p=0.23), C: perfusate creatinine (p=0.53) and D: urine creatinine (p=0.30); Increase in urine production significantly correlated a decrease in perfusate creatinine levels in both groups (p=0.02); E: Lactic acid levels at each time point, were significantly higher in the PRBC group (p=0.007); The red lines represent the kidneys perfused with a PRBC based solution and the black lines represent the kidneys perfused with HBOC based solution.
Figure 5.
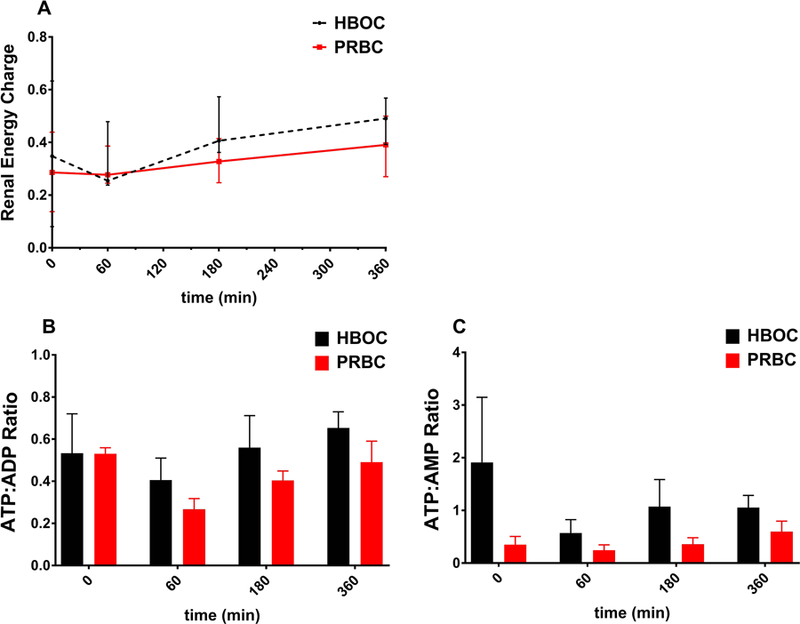
Energy charge evaluation during 6 hours of normothermic ex-vivo kidney perfusion. A: The overall renal energy charge was similar in both groups (p=0.057); B: ATP:ADP ratio after perfusion did not significantly differ from the starting value in both groups (p=0.34); C: ATP:AMP increased in the PRBC group (p=0.03) and decreased in the HBOC group (p=0.01). ATP:AMP remained significantly higher in HBOC perfused kidneys following the first hour of perfusion(p=0.03); Red represents the kidneys perfused with a PRBC based solution and black represents the kidneys perfused with HBOC based solution.
Acknowledgments
Support from the New England Organ Bank (discarded kidney perfusion grant; 2017–2018), the US National Institutes of Health (grants R01DK096075, R01DK107875 and R01DK114506), and the Department of Defense (DoD RTRP W81XWH-17–1-0680) is gratefully acknowledged. Appreciation is extended to the Mass Spectrometry Core Facility at Shriners Hospital for Children for processing our samples and performing the ATP analysis. We thank HBO2 Therapeutics LLC for partial financial support and providing the Hemoglobin Based Oxygen Carrier used in this study (Hemopure).
Abbreviations:
- ADP
Adenosine diphosphate
- ALT
Alanine aminotransferase
- AMP
Adenosine monophosphate
- ATP
Adenosine triphosphate
- CIT
Cold ischemia time
- DBD
Donation after Brain Death
- DCD
Donation after Circulatory Death
- FENa
Fractional Excretion of Sodium
- HBOC
Hemoglobin-based oxygen carrier
- HMP
Hypothermic machine perfusion
- KDPI
Kidney Donor Profile Index
- MP
Machine perfusion
- NEDS
New England Donor Services
- NEVKP
Normothermic ex vivo kidney perfusion
- NMP
Normothermic machine perfusion
- PRBC
Packed red blood cells
- QAS
Quality Assessment Score
- ROC
Receiver operating characteristic
- SCS
Static cold storage
- UW solution
University of Wisconsin solution
- WIT
Warm ischemia time
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Uygun has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Dr. Uygun’s interests are managed by the MGH and Partners HealthCare in accordance with their conflict of interest policies. The other authors have no conflicts of interest to disclose.
The study was in part supported by HBO2 therapeutics LLC.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of this article.
References
- 1.Garcia GG, Harden P, Chapman J, World Kidney Day Steering Committee 2012. The global role of kidney transplantation. Lancet 2012;379(9820):e36–38. doi: 10.1016/S0140-6736(12)60202-5 [DOI] [PubMed] [Google Scholar]
- 2.Tozzi M, Franchin M, Soldini G, et al. Impact of static cold storage VS hypothermic machine preservation on ischemic kidney graft: inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. International Journal of Surgery 2013;11:S110–S114. doi: 10.1016/S1743-9191(13)60029-1 [DOI] [PubMed] [Google Scholar]
- 3.Moers C, Smits JM, Maathuis M-HJ, et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. New England Journal of Medicine 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289 [DOI] [PubMed] [Google Scholar]
- 4.Wight J, Chilcott J, Holmes M, Brewer N. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol Assess 2003;7(25):1–94. [DOI] [PubMed] [Google Scholar]
- 5.Levy MN. Oxygen consumption and blood flow in the hypothermic, perfused kidney. American Journal of Physiology-Legacy Content 1959;197(5):1111–1114. doi: 10.1152/ajplegacy.1959.197.5.1111 [DOI] [PubMed] [Google Scholar]
- 6.O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg 2013;100(8):991–1001. doi: 10.1002/bjs.9169 [DOI] [PubMed] [Google Scholar]
- 7.Watson CJE, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant 2010;10(9):1991–1999. doi: 10.1111/j.1600-6143.2010.03165.x [DOI] [PubMed] [Google Scholar]
- 8.Wight JP, Chilcott JB, Holmes MW, Brewer N. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transplant 2003;17(4):293–307. [DOI] [PubMed] [Google Scholar]
- 9.Quiroga I, McShane P, Koo DDH, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant 2006;21(6):1689–1696. doi: 10.1093/ndt/gfl042 [DOI] [PubMed] [Google Scholar]
- 10.Summers DM, Watson CJE, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015;88(2):241–249. doi: 10.1038/ki.2015.88 [DOI] [PubMed] [Google Scholar]
- 11.Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 2013;381(9868):727–734. doi: 10.1016/S0140-6736(12)61685-7 [DOI] [PubMed] [Google Scholar]
- 12.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant 2007;7(7):1797–1807. doi: 10.1111/j.1600-6143.2007.01852.x [DOI] [PubMed] [Google Scholar]
- 13.Yong C, Hosgood SA, Nicholson ML. Ex-vivo normothermic perfusion in renal transplantation: past, present and future. Curr Opin Organ Transplant 2016;21(3):301–307. doi: 10.1097/MOT.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 14.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am J Transplant 2016;16(11):3282–3285. doi: 10.1111/ajt.13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013;13(5):1246–1252. doi: 10.1111/ajt.12179 [DOI] [PubMed] [Google Scholar]
- 16.Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011;92(7):735–738. doi: 10.1097/TP.0b013e31822d4e04 [DOI] [PubMed] [Google Scholar]
- 17.Hosgood SA, Nicholson ML. Ex vivo normothermic perfusion of declined human kidneys after inadequate in situ perfusion. Am J Transplant 2014;14(2):490–491. doi: 10.1111/ajt.12568 [DOI] [PubMed] [Google Scholar]
- 18.Hamar M, Selzner M. Ex-vivo machine perfusion for kidney preservation. Curr Opin Organ Transplant 2018;23(3):369–374. doi: 10.1097/MOT.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 19.Sakota D, Sakamoto R, Sobajima H, et al. Mechanical damage of red blood cells by rotary blood pumps: selective destruction of aged red blood cells and subhemolytic trauma. Artif Organs 2008;32(10):785–791. doi: 10.1111/j.1525-1594.2008.00631.x [DOI] [PubMed] [Google Scholar]
- 20.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med 1996;334(26):1685–1690. doi: 10.1056/NEJM199606273342601 [DOI] [PubMed] [Google Scholar]
- 21.Chawla SC, Lal S. Blood safety and rational use of blood. J Indian Med Assoc 1994;92(1):22–23. [PubMed] [Google Scholar]
- 22.Senay S, Toraman F, Gunaydin S, Kilercik M, Karabulut H, Alhan C. The impact of allogenic red cell transfusion and coated bypass circuit on the inflammatory response during cardiopulmonary bypass: a randomized study. Interact Cardiovasc Thorac Surg 2009;8(1):93–99. doi: 10.1510/icvts.2008.183608 [DOI] [PubMed] [Google Scholar]
- 23.Moritz ED, Winton CS, Tonnetti L, et al. Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med 2016;375(23):2236–2245. doi: 10.1056/NEJMoa1600897 [DOI] [PubMed] [Google Scholar]
- 24.Levy JH, Goodnough LT, Greilich PE, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg 2002;124(1):35–42. [DOI] [PubMed] [Google Scholar]
- 25.Mozzarelli A, Ronda L, Faggiano S, Bettati S, Bruno S. Haemoglobin-based oxygen carriers: research and reality towards an alternative to blood transfusions. Blood Transfus 2010;8(Suppl 3):s59–s68. doi: 10.2450/2010.010S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laing RW, Bhogal RH, Wallace L, et al. The Use of an Acellular Oxygen Carrier in a Human Liver Model of Normothermic Machine Perfusion. Transplantation 2017;101(11):2746–2756. doi: 10.1097/TP.0000000000001821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matton Alix PM, Burlage Laura C, van Rijn Rianne, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transplantation 2017;24(4):528–538. doi: 10.1002/lt.25005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontes P, Lopez R, van der Plaats A, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant 2015;15(2):381–394. doi: 10.1111/ajt.12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahboub P, Ottens P, Seelen M, et al. Gradual Rewarming with Gradual Increase in Pressure during Machine Perfusion after Cold Static Preservation Reduces Kidney Ischemia Reperfusion Injury. PLoS One 2015;10(12). doi: 10.1371/journal.pone.0143859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med 2006;354(4):343–352. doi: 10.1056/NEJMoa052891 [DOI] [PubMed] [Google Scholar]
- 31.Hosgood SA, Barlow AD, Hunter JP, Nicholson ML. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. British Journal of Surgery 2015;102(11):1433–1440. doi: 10.1002/bjs.9894 [DOI] [PubMed] [Google Scholar]
- 32.Jochmans I, Nicholson ML, Hosgood SA. Kidney perfusion: some like it hot others prefer to keep it cool. Curr Opin Organ Transplant 2017;22(3):260–266. doi: 10.1097/MOT.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 33.Kaths JM, Echeverri J, Chun YM, et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation: Transplantation 2017;101(4):754–763. doi: 10.1097/TP.0000000000001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiRito JR, Hosgood SA, Tietjen GT, Nicholson ML. The future of marginal kidney repair in the context of normothermic machine perfusion. Am J Transplant 2018;18(10):2400–2408. doi: 10.1111/ajt.14963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrales P, Tsai AG, Intaglietta M. Increased tissue PO2 and decreased O2 delivery and consumption after 80% exchange transfusion with polymerized hemoglobin. Am J Physiol Heart Circ Physiol 2004;287(6):H2825–2833. doi: 10.1152/ajpheart.00654.2004 [DOI] [PubMed] [Google Scholar]
- 36.Fontes PA. The Evolution of Oxygen Carrier Solutions for Machine Perfusion. Transplantation 2017;101(11):2657–2658. doi: 10.1097/TP.0000000000001857 [DOI] [PubMed] [Google Scholar]
- 37.Ortiz D, Barros M, Yan S, Cabrales P. Resuscitation from hemorrhagic shock using polymerized hemoglobin compared to blood. Am J Emerg Med 2014;32(3):248–255. doi: 10.1016/j.ajem.2013.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anbari KK, Garino JP, Mackenzie CF. Hemoglobin substitutes. Eur Spine J 2004;13 Suppl 1:S76–82. doi: 10.1007/s00586-004-0737-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vries Y, Matton A, W. N. Nijsten M, et al. Pretransplant Sequential Hypo- and Normothermic Machine Perfusion of Suboptimal Livers Donated after Circulatory Death Using a Hemoglobin-based Oxygen Carrier Perfusion Solution. American Journal of Transplantation December 2018. doi: 10.1111/ajt.15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaths JM, Spetzler VN, Goldaracena N, et al. Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation. J Vis Exp 2015;(101):e52909. doi: 10.3791/52909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas M, Sonnenday CJ, Cicone JS, Rabb H, Montgomery RA. Isometric tubular epithelial vacuolization in renal allograft biopsy specimens of patients receiving low-dose intravenous immunoglobulin for a positive crossmatch. Transplantation 2004;78(4):549–556. [DOI] [PubMed] [Google Scholar]
- 42.Hoyer DP, Gallinat A, Swoboda S, et al. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014;98(9):944–950. doi: 10.1097/TP.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 43.Pegg DE, Foreman J, Hunt CJ, Diaper MP. The mechanism of action of retrograde oxygen persufflation in renal preservation. Transplantation 1989;48(2):210–217. [DOI] [PubMed] [Google Scholar]
- 44.Manekeller S, Minor T. Possibility of conditioning predamaged grafts after cold storage: influences of oxygen and nutritive stimulation. Transplant International 19(8):667–674. doi: 10.1111/j.1432-2277.2006.00320.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


