Abstract
The classic approach to measure the spiking response of neurons involves the use of metal electrodes to record extracellular potentials. Starting over 60 years ago with a single recording site, this technology now extends to ever larger numbers and densities of sites. We argue, based on the mechanical and electrical properties of existing materials, estimates of signal-to-noise ratios, assumptions regarding extracellular space in the brain, and estimates of heat generation by the electronic interface, that it should be possible to fabricate rigid electrodes to concurrently record from essentially every neuron in the cortical mantle. This will involve fabrication with existing yet nontraditional materials and procedures. We further emphasize the need to advance materials for improved flexible electrodes as an essential advance to record from neurons in brainstem and spinal cord in moving animals.
eTOC blurb
Understanding cognition can, in principle, require simultaneous records of spikes from every neuron in cortex. Can this be achieved? The results from back-of-the-envelope calculations show that such measurements may be obtained using electrodes fabricated with existing yet nontraditional materials and procedures.
Electrical recordings are the sine qua non for the measurement of computations in the nervous system. It is thus of fundamental interest to ask if, as a matter of principle, one can record spikes from all neurons in a mammalian brain. And, if not, from what fraction of the brain can one record? In this regard, the discussions that led to the United States BRAIN Initiative were convened with a working title of ÒThe Brain Activity MapÓ (Alivisatos et al., 2012). The prospects for such a dynamic map (Buzsáki et al, 2015), with a corresponding connectome (Denk and Horstmann, 2004) to map all chemical and resistive connections among neurons, forms the basis for modeling and ultimately understanding brain function (Kleinfeld et al., 2011; Plaza et al., 2014; Rubinov et al., 2015). Here we discuss the physical limits to obtain the brain-wide activity map in a mammal. The corresponding connectivity map could be obtained via transmission electron microscopy (Kasthuri et al., 2015) or focused ion beam milling combined with scanning electron microscopy (Knott et al., 2008; Hayworth et al., 2019). As demonstrated for nonmammalian species (Kornfeld and Denk, 2018), there are no fundamental barriers beyond the constraints of experimental time and the current need for substantial human curating.
Both optical and electrical approaches have been taken to measure neuronal activity across many sites, albeit the most pervasive is optical imaging using a fluorescent indicator of intracellular Ca 2+ concentration (Grienberger and Konnerth, 2012). While no doubt useful, [Ca 2+] transients are only an approximate reflection of spiking (Theis et al., 2016). The advent of genetically expressible voltage sensors may obviate this problem (Platisa and Pieribone, 2018). Yet currently available sensors, while impressive for recording local network activity in vivo (Adam et al., 2019), appear insufficiently sensitive for brain-wide imaging and require sparsely labeled brain regions to prevent cross-talk from obfuscating the desired signals. Lastly, the photon budget to simultaneously image large numbers of neurons, and the technology to image at all depths throughout cortex without deleterious aberrations (Ji, 2017; Liu et al., 2019), remains an elusive goal. Our focus here is on a classical approach, that of measuring extracellular electrical potentials (Lemon, 1984). These signals depend on current flow through the extracellular space during the propagation of an action potential. While the signal-to-noise ratio can be quite high, it is important to recall that isopotential neurons, e.g., electrically compact cells, will not generate an extracellular signal (Jack et al., 1974). This contingency, however, seems unlikely to apply to neurons that are present in neocortex.
A corollary of our query is that of sufficiency. That is, from what fraction of all cells does one need to record to determine how few neurons are needed to quantize the neuronal basis of perception or behavior? This question is bound up in the issue of the dimensionality of neuronal computations (Ganguli and Sompolinsky, 2012; Gao et al., 2017; Stringer et al., 2019), i.e., how fine a sensory field is perceived, or how finely controlled is motion within a motor act? Further, the question of sufficiency is likely to be widely variable for different parts of the brain (Lehky et al., 2014; Schoelvinck et al., 2015). Thus we focus on the reductionist issue of fundamental physical limits, practical limits, and numbers.
Theory, together with transport and mechanical parameters for known materials, places physical limits on the dimensions of wires. For example, how stiff does a sensor carrier need to be before it buckles in the insertion process? What force is required to insert a probe of a given cross-section? How thick a wire does one require to propagate a signal without appreciable attenuation at action potential frequencies? For concreteness, we discuss a cylindrical probe of diameter dshaft with recording sites distributed along the length of the shank. We consider the case of neocortex with dimensions appropriate for mouse through marmoset. We ask if, in principal, enough multi-electrode shanks could be inserted into the cortex to reliably record spikes from all neurons. Conversely, if we set an upper limit for displaced brain tissue, this same calculation can be used to estimate the fraction of neurons whose spiking could be detected with available probes.
We first establish the essential parameters for the electrical measurement process. We then consider physical arguments for the size and density of electrode carriers and electrodes that will enable electrical recordings with high signal-to-noise ratios. Lastly, we discuss potential physiological constraints, including the thermal load from the associated electronics.
1. Neuroelectric Scales
The electrical capture length for extracellular recording from a neuron, denoted D, is key. The average density of neurons in murine cortex is ρ = 1×10 5 /mm 3 (Petersen, 2007; Tsai et al., 2009), which roughly translates to a cube that is on edge per neuron. The range of extracellular spike detection is an issue of signal amplitude relative to that of background spiking activity. While detection of spikes more than 100 µm from the originating soma has been demonstrated for the largest cortical pyramidal neurons (Buzsáki, 2004), the detection length is less well defined for smaller neurons. A conservative estimate of the range of detection is the electronic length for a propagating action potential. This length is set by the ratio of current flow through the membrane, which is dominated by sodium conductance during an action potential, to current flow that is along the axis of the axon (Jack et al., 1974). Typically, , which matches the neuronal spacing . Thus we take D = 20 µm as the maximum length between a neuron and an electrode for reliable recording. Clearly, there are significant variations in neuronal size and D will depend on cell geometry and levels of channel expression.
The second scale is the bandwidth of neuronal signaling, which we denote as fAP. The spectral density of the action potential extends to 10 kHz (Fee et al., 1996), so that we assign fAP = 10 kHz. We note in passing that the collective activation of sodium channels in the axon hillock has a frequency components up 40 kHz (Naundorf et al., 2006), yet these are not essential to the detection of a spike.
2. Metrology
What is the minimum distance between electrode shanks? We put the issue of displacement of brain tissue by the electrodes aside for the moment. Ideally, the shanks should be spaced at nominally 2D ≈ 40 µm, so that each neuron is, on average, within one electronic length of a shank. The shanks are chosen to be arranged in a triangular array with a spacing of 2D = 40 µm (Figure 1a).
Figure 1. Schematic of the proposed stiff electrode.
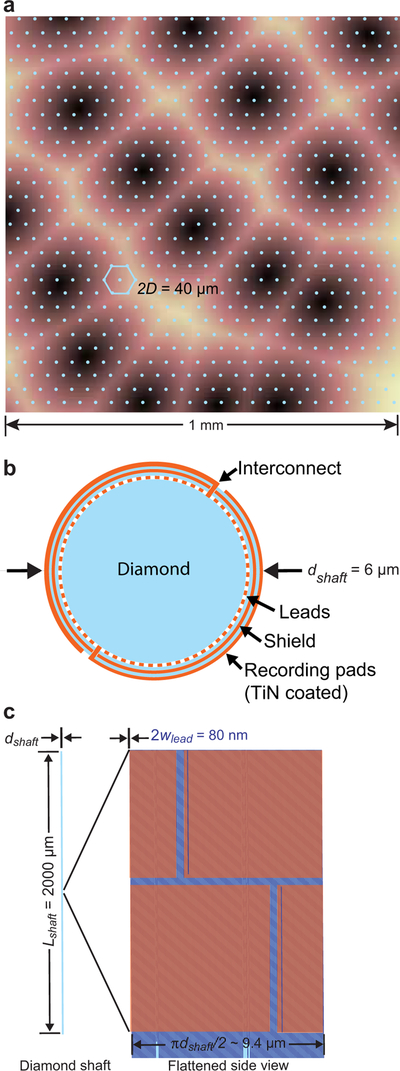
(a) The proposed triangular grid of electrodes spaced on top of primary vibrissa sensory cortex. Image from Knutsen et al. (2016). (b) The electrode is constructed of a diamond shaft that is covered with five layers. First is the leads and the mineral insulation between leads, second is mineral insulation, third is a conduction shield, fourth is a second is mineral insulation, and fifth is the electrode pads. Each pad is connected to one lead via a thru-hole interconnects that pierce the shield. Illustration is not to scale. (c) The multi-electrode shaft with an expanded view of the face to show the arrangement of pads.
With the above parameters for detection range and bandwidth, we can estimate limits to the diameter of the electrode. This shank should have the smallest feasible tissue displacement, contain recording sites of sufficient density to resolve the signals from the full density of neurons, and have a mechanical strength sufficient to insert into cortex.
3. Design Considerations for Rigid Probes
With the knowledge of the necessary insertion force, we can now estimate the diameter of our canonical shank. With additional information, we can further estimate the number and size of the electrode pads and leads.
A critical limit for the fabrication of the thinnest usable rigid probe is the buckling force at the time of insertion of the electrode into cortex. This constrains the dimensions of the shank. The ideal material for a rigid shank, i.e., the stiffest and thus most resistant to buckling, is diamond, with Young’s modulus E = 1.2 N/µm 2. By comparison, E = 0.2 N/µm 2 for silicon. We choose a recording depth of Lshaft = 2000 µm, which is appropriate for mouse through marmoset cortex. Recent measurements of the force required to insert cylindrical electrodes into mouse cortex, ex vivo, with diameters ranging from 7.5 to 100 µm, yield a penetration force of Fi = F0 (1 + d / d0), with F0 = 8×10 −6 N and d0 = 1 µm (Obaid et al., 2018). Note that the penetration force reported for a 100 µm diameter probe is about 6-times less than that was reported for inserting electrodes in monkey cortex (Reitboech, 1983). Lastly, the sustaining force to insert the electrode did not exceed the penetrating force down to at least 1500 µm of insertion depth (Obaid et al., 2018).
Our dimensional estimates (Box 1) for diamond shanks supports the use of cylindrical electrodes that are at least 4 µm in diameter. We choose the larger value of dshaft = 6 µm, based on electrical considerations (Box 2), for which the volume fraction of the brain occupied by the electrodes, denoted Θ, is Θ ≈ 0.02. This value for the volume fraction is ten-fold less than the mean extracellular volume fraction (Tonnesen et al., 2018).
Box 1. Dimensional estimates for a rigid electrode array.
- What is the shank diameter required for insertion? Standard formula for the threshold force, F, at the onset of buckling of a solid cylindrical beam (Timoshenko and Young, 1945) give
with the beam taken to be rigidly supported on one end. We equate F with Fi, approximated as Fi ≈ ( F0 / d0) dshaft, take E as the Young’s modulus for diamond, and estimate a minimum diameter of dshaft = 4 µm to insert a diamond shaft into cortex without the electrode buckling. A larger value may be required to penetrate the dura, recalling that , and/or to accommodate sufficient numbers of electrode pads. - What is the volume fraction of the brain occupied by the electrodes? Geometry sets this fraction at
This fraction is Θ = 0.009 for dshaft = 4 µm and Θ ≈ 0.02 for dshaft = 6 µm. The fraction must be small compared to the volume fraction of extracellular space, denoted α. Super-resolution imaging data from hippocampal slice shows that the fractional volume varies across anatomical locations (Tonnesen et al., 2018); it is α = 0.05 in stratum pyramidalis, the cell layer, up in α = 0.36 in fibrous regions, and has a mean value of . These values can change by 1.5-fold with neuromodulator concentration (Ding et al., 2016). Data from in vivo reports give similar values for (Sykov‡ and Nicholson, 2008). - How many recording pads are required to cover the shank? The number of cells probed by one shank is
which yields N = 280. We set the number of recording pads equal to the target number of neurons to minimize, but not obviate, complications from spike sorting (Hill et al., 2011; Barnett et al., 2017). The surface of the shank is covered with one layer of lead wires, to bring signals to the surface of the brain. The overlaying layer is a mineral insulator, followed by a ground plane to shield the leads from the electrodes and a second mineral insulator layer. The final, or fifth layer, contains the recording pads. Thru-holes connect each pad to the underlying wire (Figure 1b,c). - What is the size of the leads and pads? We choose to wrap the leads for the N electrodes along the entire circumference of the first layer. We assume an equal lead width and separation distance of wlead. This gives
which yields wlead = 35 nm. Each electrode pad occupies an area with dimensions wide by 2 Lshaft/N = 14 µm high, in which space remains for the inclusion of insulating gaps between pads. This corresponds to an area of Apad = 126 µm2.
Box 2. Electrical noise and shunting for a rigid electrode array.
-
How much thermal noise is generated by the recording pads? There are two types of electrode interfaces, Faradaic and non-Faradaic, that transform electrical signals between the ionic conduction in solution and the electronic conduction in gold or other metals (Bard and Faulkner 2000). Non-Faradaic electrodes, also known as ideal polarizable electrodes, refer to interfaces for which no electrochemical, i.e., reduction-oxidation, reactions take place. Faradaic electrodes make use of reduction-oxidation processes at the extracellular solution to metal interface. Faradaic electrodes are characterized by a shot noise component that is associated with the electrochemical processes.
For electrophysiology, non-Faradaic electrodes are preferred. In this case, the electrical noise from the electrodes is solely thermal in origin and is associated primarily with resistances of the double-layer at the extracellular solution to metal interface, as well as any access resistances to the bulk electrolyte and tissue. Under the assumption that the resistance of the double-layer dominates the losses, the root-mean-square (RMS) thermal noise is
which is derived by equating the noise energy with the equipartition energy 1 / 2 kBT, where kB is the Boltzman constant and T is the temperature. A large electrode capacitance is clearly preferred based on noise considerations. The capacitance given byThe double layer is characterized by a thickness tdouble = 0.3 nm and relative dielectric constant ϵdouble = 2, for which Cpad = 7 pF. The addition of a coating of titanium nitride (TiN) to the face of the electrode will dramatically increase the value of the surface area of the pad. The increase in area exceeds one hundred-fold at the onset on testing (Jun et al., 2017) and diminishes to a worst case of twenty-fold under chronic, in vivo recording conditions (TDH, unpublished observations). Thus Cpad is conservatively replaced by Cpad, TiN = 150 pF and the estimated RMS noise is over the bandwidth associated with the double layer.
The noise will be reduced if the spectral bandwidth of the neuronal activity, fAP, is less than the bandwidth, denoted fpad, associated with the electrode and double layer. This bandwidth is found from input resistance between the neuron and the electrode times the capacitance, where the input resistance is estimated as the spreading resistance over a separation distance D, i.e.,
where we have corrected for the volume fraction, (Weissberg, 1963). The resistivity of cerebral spinal fluid in neocortex, denoted , is estimated to be in the range 2–4 Ω m (Logothetis et al., 2007). Thus Rinput ≈ 60 kΩ so that fpad = 1/2 πRinput Cpad, TiN = 18 kHz, or 4-times fAP. Thus the filtered noise level will be . - How much thermal noise is generated by the leads to the recording pads? The resistance of a gold, 35 nm thick by 35 nm wide wire is Rlead = 30 kΩ. The corresponding RMS thermal noise, also referred to as Johnson noise, is
We estimate . The noise from the leads and electrode pads are, for our design, nominally equal. - What is the expected noise from passive tissue? The electrode thermal noise must be compared with the variability in the extracellular signal that arises from background neuronal activity in cortex. The spreading resistance between a signal and a reference electrode spaced a distance Δd apart is . The corresponding thermal noise is
For a reference spaced Δd =10 mm from the main electrodes,. This is negligible compared to the noise of the electrodes. -
Is the capacitive coupling between electrodes leads deleterious? The coupling between neighboring leads and leads and electrode pads can both attenuate and corrupt the measured signal. For our geometry (Figure 1b) and interposed short and long leads,
where the sum accounts for coupling to all leads. The coupling between the leads and the pads is prevented by the incorporation of a ground plane with via holes between the layer of pads and that of leads (Figure 1b).We use ϵdielectric = 5 for diamond and estimate Clead = 0.1 pF. This value is negligible compared to both the capacitance of the pad and the capacitance of the gate of a field effect transistor (FET) at the input of an ultra-low noise amplifier (Wattanapanitch et al., 2007). The roll-off frequency for attenuation of high frequency components of the signal is for a lumped parameter model, or over two orders-of-magnitude larger than fAP. Lastly, as a design rule, the expression for C takes on a simple scaling in the limit of large N, i.e.,
where 436.00 in Dwight (1961) was used. - What is the expected signal strength? The signal strength from the electrode can be attenuated by the capacitance divider formed between the electrode capacitance and the input capacitance of the first-stage amplifier. Thus
The input capacitance of an an ultra-low noise amplifier is less than 1 pF (Wattanapanitch et al., 2007). Thus ΔVmeas/ΔVcell ≈ 1. The maximum voltage for extracellular signals ranges from ΔVcell = 300 µV (Hill et al., 2011) to ΔVcell = 1 mV (Lemon, 1984), so a conservative upper bound is ΔVmeas < 300 µV. High values of Cpad, TiN values are clearly preferred to maximize the input signal as well as reduce thermal noise from the electrode.
The mechanical and electrical properties of currently available materials allow, in principle, for spikes to be recorded from all neurons in a slab equivalent to that of the cortical mantle of mouse or marmoset (Boxes 1 and 2). The proposed design, constrained by neuronal density and the extracellular volume, consists of electrode shafts arranged on a triangular lattice, spaced 2D = 40 µm. Each multi-site electrode shaft has 280 pads, 9 µm in width with a 14 µm pitch, on a shaft that is 6 µm in diameter and 2000 µm in length. The pads are joined to insulated leads by thru-hole interconnects. We next ask if the geometry and size of the electrodes are consistent with signal-to-noise and bandwidth constraints for the detection of neuronal spikes.
The estimated RMS noise, which is dominated by the thermal noise of the electrode pad, is . Thus the estimated peak signal-to-RMS-noise ratio of the probe should exceed 50:1 for an action potential amplitude of ΔVmeas = 300 µV. Note that the measured fluctuations in vivo will further contain nonstationary contributions from active sources, including spiking by distant neurons and other fast ionic processes. A RMS value of was reported for regularly spiking neurons in primary vibrissa cortex (Fee et al., 1996). Including these physiological sources, the estimated signal-to-RMS-noise ratio of the probe drops to 12:1.
Our estimates are conservative. The minimum diameter of the electrode required to forestall buckling may be reduced by sharpening the electrode tip (Obaid et al., 2018). Further, a guide tube may be used to reduce the unsupported length of the electrode. Past work also suggests the utility of dissolvable supports (Weltman et al., 2016) and microfluidic injection systems (Vitale et al., 2018). Lastly, the noise of the implanted electrode pads will decrease with improvements in the stability of coatings. In addition to TiN, poly(3,4-ethylenedioxythiophene (PEDOT) (Charkhkar et al., 2016; Ludwig et al., 2011; Rivnay et al., 2015), sputtered Pt (Whalen III et al., 2006), and IrOx (Negi et al., 2010) are other materials to consider.
3.1. Comparison with the state-of-the-art rigid probes
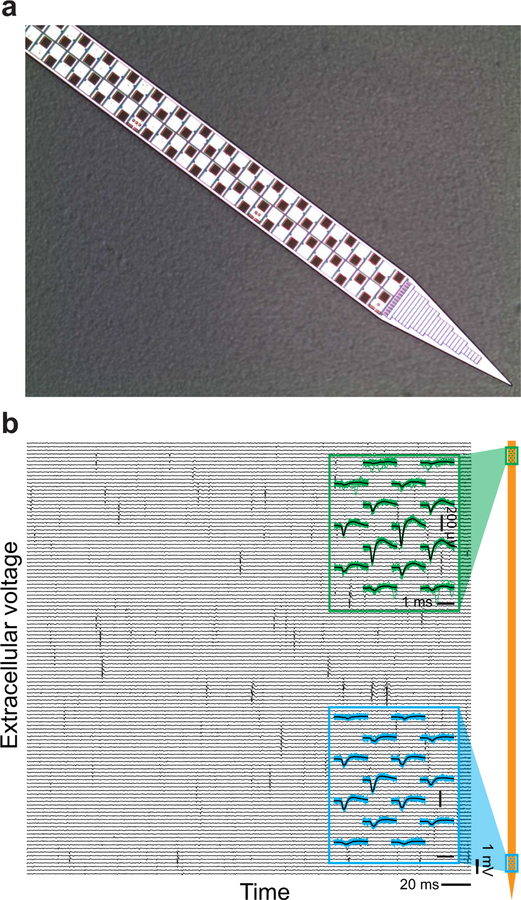
The status of current high-density density probes was reviewed by Steinmetz, Koch, Harris and Caradini (2018). Prominent among recent probes is the Neuropixels probe (Jun et al., 2017) (Figure 2), with a cross-section of 20 µm by 70 µm, about 50-times larger than that in our theoretical example (Figure 1a), and with electrodes on only one face. If we maintain the same filling-factor of Θ = 0.02, this suggests that Neuropixels probes can be placed as close as 2D = 165 µm and, for a λAP approximately 20 µm capture distance, record spikes from 0.01 to 0.02 of all neurons. The initial use of Neuropixels probes in mouse cortex yielded 0.6 to 1.0 neurons per site across 100 sites (Steinmetz et al., 2018), which is consistent with recording the majority of neurons within the capture distance of a single probe.
Figure 2. A state-of-the-art rigid probe.
(a) Photograph of the distal 68 sites of the 960 sites on a shank of a Neuropixels probe. The shank is 10 mm long, 70 µm wide, and 24 µm thick, with 12 µm by 12 µm TiN recording sites pitched at 2 per 20 µm of shank length. (b) Two example recordings from Neuropixels probe pads. The probe was chronically implanted in rat prefrontal cortex one day prior to data acquisition. Blue traces and green traces are 30 raw traces in the vicinity of a spike near the top (green) and bottom (blue) of the probe; black lines are average of those traces. Adapted from Jun et al. (2017).
4. Considerations for Flexible Shanks
We shift our attention to electrodes with flexible shafts or, simply, soft wires. Though less mature than rigid electrodes, flexible electrodes have, in the ideal case, the advantage of bending with changes in local brain shape and size over the course of the day. This is essential for recording in flexible structures, like the hindbrain. Further, increasing evidence suggests that more flexible electrodes promote more stable neural interfaces (Xie et al., 2014; Fu et al., 2016; Luan et al., 2017; Yang et al 2019). Flexible electrodes can be driven into the brain with retractable support rods and thus provide a strategy to record in hindbrain, spinal cord, and even cortex where a rigid connection between the brain and the world is deleterious. As a matter of principle, the wires can be sufficiently fine to occupy a negligible volume fraction (Box 2).
We estimate that the same 280 electrode pads per shank target may be accommodated by a flexible rectangular shank with a 10 µm width and 1.5 µm thickness. Most estimates of the rigid probe (Box 1) apply to flexible shanks except for several points (Box 3).
Box 3. Estimates for a flexible electrode array.
What is the minimal thickness of a flexible probe? While mineral-based materials work as insulators for rigid shanks, they can easily crack on a flexible shank. Polymers are typically used as insulators for flexible probes and a thickness of approximately 500 nm is typically required. Taking dlead = 40 nm, 300 leads can readily fit in two 10-µm-wide lead layers and three insulator layers, yielding an overall thickness of 1.5 µm and cross-sectional area of 15 µm 2 (Figure 3a).
What is the shuttle diameter required for insertion? The shuttle should have a cross section similar to that of the rigid probe (Felix et al., 2013; Zhao et al., 2019) (Box 1), i.e., 6 µm diameter for diamond.
How should the array be transiently attached the shuttle for insertion? Temporarily attaching the flexible probe to the shuttle can be achieved with geometrical anchors (Luan et al., 2017), or water soluble adhesives (Felix et al., 2013; Zhao et al., 2019).
What is the tissue displacement? The total displacement for the flexible probe is half of that for the rigid probe, or Θ = 0.01. The shuttle will transiently triple the volume occupied by the flexible electrode alone. Yet this increase in negligible if the shanks are inserted sequentially. On the other hand, there is potential for tissue damage during the process of insertion per se (Na et al., 2019; Zhao et al., 2019; Ferro et al. 2018; Joo et al 2019).
4.1. Comparison with the state-of-the-art flexible probes
The thinnest multi-channel flexible probes are currently fabricated with thicknesses of approximately 1 µm. To achieve the highest contact density, e-beam lithography is most promising for its approximately 10-nm spatial resolution (Luan et al., 2017; Wei et al., 2018; Yang et al., 2019) (Figure 3). This and related approaches (Chung et al., 2019) provide proof of the viability of flexible electrodes, although the demonstrated channel count per shank is about 20-times less than proposed array. In principle, the resolution of electron beam lithography should permit the fabrication process at a much higher electrode count but is limited by currently available back-end electronics.
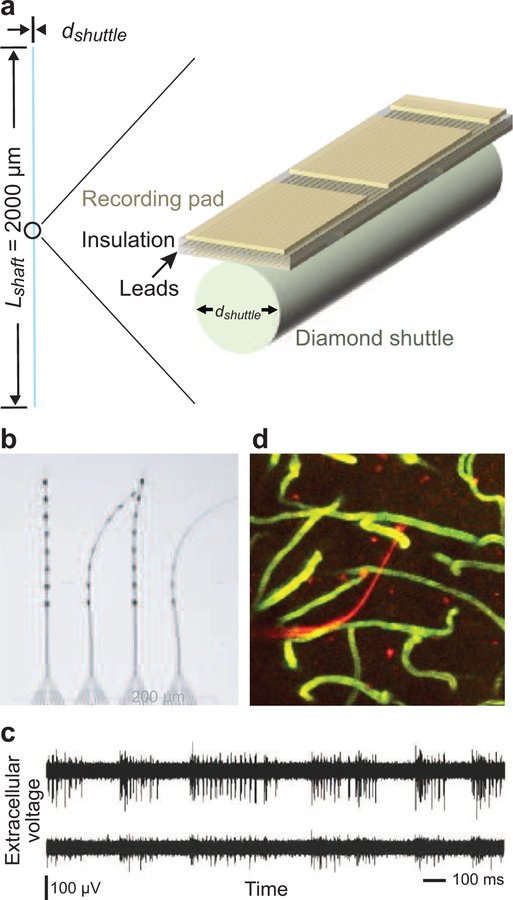
Figure 3. A state-of-the-art flexible probe.
(a) The electrode is constructed of gold pads and epoxy insulators. A diamond shank provides temporary rigidity for insertion. (b) A flexible multi-channel neural probe fabricated by electron beam lithography, with a shank width of 8 µm, thickness of 0.8 µm, and electrode pad size of 5 µm by 15 µm. (c) Representative electrical traces from the probe in panel b. (d) Three dimensional reconstruction of vasculature by in vivo two-photon microscopy (Kleinfeld et al., 1998) around a probe (red). Data obtained two months after implantation and shown as a maximum projection 100Ð320µm below the pia. Adapted from Wei et al. (2018).
It is also important to note that even brittle materials can be rendered flexible if they are sufficiently thin. Bending stiffness scales roughly with the cube of the film thickness. For the example of silicon, this allows a reduction in bending stiffness from roughly 2 Nm for a standard 500 µm thick silicon to only 5 µNm for silicon thinned to a thickness of 12 µm. The resulting nearly six-order-of-magnitude increase in mechanical compliance allows many of the properties of more flexible materials to be achieved. For the particular case of silicon, this permits the design of flexible probes that incorporate electronics to be produced in state-of-the-art fabrication lines.
5. Interface Electronics and Power Limits
The signal from each electrode needs to be electronically buffered, i.e., transformed from a high impedance source to a low-impedance driver, amplified, and digitized. This task is performed by a FET placed at the top of the electrode. This transistor and the associated downstream amplification and digitization electronics will generate heat that must be dissipated in the air above the animal. The channel densities that we propose are high, i.e., or twenty million channels across the readily accessible 8 mm by 10 mm cortical mantle in mice.
How much power can be safely dissipated? Recent studies on the suppression of neuronal activity by heating through the absorption of light (Owen et al., 2019) suggest that the threshold thermal load is roughly 0.06 W/mm2; similar conclusions are found for light-induced vasodilation (Rungta et al 2017). This is likely to be an underestimate as heating was local in these studies, yet it provides a guide and suggests the need for an electronic interface that dissipates less than 0.2 µW per channel. Recalling that thermal conduction by diamond is 30,000 × that of air, essentially all of the heat will flow into the brain unless steps are taken block this flow. For example, a gap between the electrode and the electronics that is comprised of epoxy-silica should reduce the conduction of heat into the brain by a factor of 0.4.
5.1. Feasibility of ultra-low power amplification
Amplifiers for neuronal recording whose power dissipation is close to the fundamental physical limits set by input-referred thermal noise in transistors have already been built (Wattanapanitch et al., 2007; Sarpeshkar 2010; Wattanapanitch et al., 2011). Such amplifiers have a power dissipation that is inversely proportional to the square of the input-referred noise as well as linear in the required bandwidth; 22.8 in Sarpeshkar (2010). A relevant design was configured for an input-referred noise of 3.1 µV RMS and a 5.3 kHz bandwidth and realized with a gain of nearly 100, an input capacitance significantly less than 1 pF, and a ”1/f” noise that is negligible for spike recording. The concomitant measured dissipation was 7.6 µW per channel. This implies a dissipation of per channel, or 1.5 W/mm 2, using the 6 µV RMS noise floor of our electrodes. This value, however, exceeds the target value for power dissipation.
A novel technique known as adaptive power biasing permits the power dissipation of a large, multi-channel electrode to be reduced by over an order of magnitude (Sarpeshkar 2010; Wattanapanitch et al., 2011). The statistics of multi-channel arrays are such that their input-referred noise varies over a probability distribution. Thus one low-noise amplifier can measure, calibrate, digitally store, and bias all other amplifiers in a multi-channel system such that each channel only dissipates power that is appropriate to the noise floor of the associated electrode pad. The method is effective precisely because the mean power dissipation of an amplifier in a multi-channel array, which is inversely proportional to the square of the input-referred noise, is significantly lower than that of an array of amplifiers designed for a fixed input noise; e.g., our estimated mean RMS noise floor of 6 µV. Thus, for our electrode array, we estimate that we could dissipate less than 0.15 W/mm2, on average. This value is less than the target value for power dissipation, and thus supports the feasibility our approach.
Additional decrements on power dissipation can occur by increasing the acceptable noise floor toward the value with background activity, i.e., , mindful of the quadratic decrement in power dissipation with increasing amplifier noise. Further improvements may also be achieved with lower supply voltages and, as in the case of Neuropixels probes (Jun et al., 2017), by disabling unused channels.
5.2. Digitization
What of the power costs of digitization? Electronics with adaptive or programmable gain and analog-to-digital converters (ADCs) can provide a large dynamic range of operation, i.e., peak signal-relative to noise, and good bit precision while maintaining low power dissipation. As an example (Wattanapanitch et al., 2011), capacitively coupled ADCs with programmable gain can readily digitize amplified action potentials that range in amplitude from 10 µV, i.e., at the noise-floor and thus amplified with the highest gain, to 3 mV, i.e., amplified with the lowest gain, with 8-bit precision over a 12-bit dynamic range. Well known techniques for ultra low power subthreshold digital design can ensure that switching power dissipation is minimized, so that the lionÕs share of power dissipation originates from the neural amplifiers and not the ADCs (Sarpeshkar 2010).
A final point is data transmission. The bit rate per square millimeter for 8-bits of digitization depth and is . From all of cortex, the rate is about 5 THz. This is well within the capacity of a single fiber optic transmission line (Stark et al., 2001). Nonetheless, one may wish to transmit only segments of the data with potential spikes. Each action potential will contribute to multiple electrode pads, so that the detection of action potentials is a source localization problem. The fraction of active electrodes at a given time can be estimated for the case of asynchronous activity and action potentials that contribute to a subset, denoted n, of the electrodes on a shank, where each spike occurs at an average rate of r and lasts for Δt. The fraction of time that an electrode is active is then ; we assume a spread of ± 100 µm (Figure 2), implying n = 14 for a 14 µm pitch (Figure 1c), and take Δt = 2 ms and r = 10/s. We find p = 0.25, implying that the bit rate per square millimeter can drop to 16 GHz and the bit rate for the cortical mantle drops to nearly 1 THz. The data rate can be reduced further with optimized compression schemes.
6. Biomechanics of Probe Insertion
Compression of the brain during the insertion of either rigid or flexible shanks could pose complications. First, it is simply unknown if neocortex can be compressed to 0.98 of its original volume. This presumably can occur given the ability of the brain to accommodate tumors of roughly 0.1-times the cortical volume before there are clinical indicators (Jalali et al., 2010). It is entirely possible that the compression would lead solely to changes in the volume of ventricles. Second, even if the brain can perform normally in a reduced volume, the act of inserting electrodes can lead to dysfunction from the compression of the pia and upper layers of cortex during insertion. Established procedures may obviate this issue, such as the use of a weak vacuum to stabilize the surface of the brain onto a mesh of guide tubes (Ventakachalam et al., 1999) and vibro-cutting of the dura and pia (Gilleland et al., 2015). Other factors should be considered as well, particularly the insertion speed (Rousche and Normann, 1992; Maynard et al., 1997; Nicolelis et al., 2003; Rennaker et al., 2005; Felix et al. 2013) and possible enzymatic treatment of the pia (Paralikar and Clement 2008).
The vasculature could pose challenges in gaining access to all locations, as the pial surface of cortex is covered with venules and arterioles. These two classes of vessels overlap with each other but not within a class. Together, they roughly cover 0.1 of the surface of mouse cortex. It may be possible to penetrate through vessels with a sharp tip at the end of the shank and have the vessel seal; such tests remain unreported. While sparse blockage to surface arterioles is tolerated, blockage of a penetrating vessel will lead to an approximately 500 µm diameter infarct (Shih et al., 2013). A second issue is the potential lesions of microvessels, which in mice occupy close to 0.01 of the cortical volume, have mean diameter of 2.5 µm, and have no directional bias (Blinder et al., 2013). We reanalyzed published data (Blinder et al., 2013) and estimate that the probability of encountering a microvessel varies as with insertion depth, z, and zo = 300 µm. Nonetheless, it is likely, based on experience with silicon shaft electrodes, that microvessels splay and are spared from damage with a slowly inserted shaft. Further, sparse blockage of microvessels is tolerated (Shih et al., 2013).
7. Discussion
The essence of our analysis is that basic physical estimates suggest the utility of moving toward a program of all-cortex, if not all brain, spike-based electrical imaging through the adoption of non-traditional materials and the extension of current fabrication processes. Practical limits exist in terms of fabrication processes. For example, one can only pull a wire to be so thin, or reliably deposit a layer of insulation above a minimum thickness. Economics will limit the choice of materials. Electronic heating and power considerations, while likely to be challenging, do not appear to be insurmountable with respect to fundamental limits. All told, our analysis points to the adoption of an engineering-based feasibility study for rigid electrodes.
Soft electrodes represent an emerging technology (Xie et al., 2014; Luan et al., 2017; Zhao et al., 2017; Chung et al., 2019). Beyond issues of scientific investigation, particularly in subcortical regions, they may have extensive utility in brain-machine interfaces that involve the brainstem and spinal cord. Our analysis points to the adoption of a discovery-based program to explore soft materials for flexible electrodes.
7.1. Epilog
The practical problems that must be overcome for electrode technology to reach its full potential appear daunting but can be addressed and likely surmounted. The insertion of a dense array of shanks, one shank every 40 µm, will pose significant challenges, as the pitch is one tenth that of the ”Utah” array (Maynard et al., 1997) (Blackrock Microsystems). Signal processing at the head of the shank will definitely be required. Automation for craniotomies (Jeong et al., 2012) and automated shank insertion will likely be required. The vasculature will likely occupy some of the space assumed for this array of shanks. Lastly, while spikes appear to be the major currency of neuronal computation, the brain-wide measurement and understanding of subcellular electrodynamics, certainly down to the level of fine dendrites (HŠusser et al., 2000; Moore et al., 2017; Ranganathan et al., 2018), represents an important albeit futuristic goal.
Highlights.
Physical limits do not preclude simultaneous recordings of all spikes in neocortex.
Future electrodes need nontraditional materials and fabrication procedures.
Challenges for dense recording include heat dissipation from interface electronics.
8. Acknowledgements
These investigations were initiated in response to the National Science Foundation workshop on ”Physical, Engineering and Biological Limits to Bra in Measurements” in 2014 and a related workshop at the HHMI Janelia Research Campus in 2017. We thank X. Ji for help with the analysis of the vasculature, D. P. Arovas, G. E. Blonder, T. J. Gardner and U. V. Nägerl for discussions, and B. Friedman for critique of an early version of the manuscript. This work was supported by the Cold Spring Harbor Laboratory Crick-Clay Professorship, the Howard Hughes Medical Institute, NEI grant EY028397, NHLBI grant HL140153, NINDS grants NS107466, NS109361, and NS0905905, and ONR grant N00014-19-1-2434.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
All authors declare no conflict of interest.
12 References
- Adam Y, Kim JJ, Lou S, Zhao Y, Brinks D, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Farhi SL, Madisen L, Harvey CD, Zeng H, Arlotta P, Campbell RE, and Cohen AE (2019). All-optical electrophysiology reveals brain-state dependent changes in hippocampal subthreshold dynamics and excitability. Nature 569, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, and Yuste R (2012). The brain activity map project and the challenge of functional connectomics. Neuron 74, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, and Faulkner LR (2000) Electrochemical Methods: Fundamentals and Applications, Second edition, Wiley Press, New York. [Google Scholar]
- Barnett AH, Magland JF, and Greengard LF (2017). Validation of neural spike sorting algorithms without ground-truth information. Journal of Neuroscience Methods 264, 65–77. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, and Kleinfeld D (2013). The murine cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nature Neuroscience 16, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2004). Large-scale recording of neuronal ensembles. Nature Neuroscience 7, 446–451. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Stark E, BerŽnyi A, Khodagholy D, Kipke DR, Yoon E, and Wise KD (2015). Tools for probing local circuits: High density silicon probes combined with optogenetics. Neuron 86, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkhkar H, Knaack GL, McHail DG, Mandal HS, Peixoto N, Rubinson JF, Dumas TC, and Pancrazio JJ (2016). Chronic intracortical neural recordings using microelectrode arrays coated with PEDOTÐTFB. Acta Biomaterialia 32, 57–67. [DOI] [PubMed] [Google Scholar]
- Chung JE, Joo HR, Fan JL, Liu DF, Barnett AH, Chen S, Geaghan-Breiner C, Karlsson MP, Karlsson M, Lee KY, Liang H, Magland JF, Pebbles JA, Tooker AC, Greengard LF, Tolosa VM, and Frank LM (2019). High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, and Horstmann H (2004). Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. Public Library of Science: Biology 2, e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, and Nedergaard M (2016). Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwight HB (1961) Tables on Integrals ad Other Mathematical Data, 4th Edition. The Macmillian Company, NY. [Google Scholar]
- Fee MS, Mitra PP, and Kleinfeld D (1996). Variability of extracellular spike waveforms of cortical neurons. Journal of Neurophysiology 76, 3823–3833. [DOI] [PubMed] [Google Scholar]
- Felix SH, Shah KG, Tolosa VM, Sheth HJ, Tooker AC, Delima TL, Jadhav SP, Frank LM, and Pannu SS (2013). Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. Journal of Visualized Experiments 79, e50609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro MD, Proctor CM, Gonzalez A, Zhao E, Slezia A, Pas J, Dijk G, Donahue MJ, Williamson A, Malliaras GG, Giocomo L, and Melosh NA (2018) NeuroRoots, a bio-inspired, seamless Brain Machine Interface device for long-term recording. BioRxiv 460949.
- Fu T-M, Hong G Zhou T, Schuhmann TG, Viveros RD and Lieber CM (2016) Stable long-term chronic brain mapping at the single-neuron level. Nature Methods 13, 875–882. [DOI] [PubMed] [Google Scholar]
- Ganguli S, and Sompolinsky H (2012). Compressed sensing, sparsity, and dimensionality in neuronal information processing and data analysis. Annual Review of Neuroscience 35, 485–508. [DOI] [PubMed] [Google Scholar]
- Gao P, Trautmann E, Yu B, Santhanam G, Ryu S, Shenoy K and Ganguli S (2017). A theory of multineuronal dimensionality, dynamics and measurement. BioRxiv 214262. [Google Scholar]
- Gilleland CL, Falls AT, Noraky J, Heiman MG, and Yanik MF (2015) Computer-assisted transgenesis of caenorhabditis elegans for deep phenotyping. Genetics 201, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, and Konnerth A (2012). Imaging calcium in neurons. Neuron 73, 862–885. [DOI] [PubMed] [Google Scholar]
- Hayworth KJ, Peale D, Januszewski M, Knott GW, Lu Z, Xu CS, and Hess HF (2019). GCIB-SEM: A path to 10 nm isotropic imaging of cubic millimeter volumes. BioRxiv 563239.
- HŠusser M, Spruston N, and Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 290, 739–744. [DOI] [PubMed] [Google Scholar]
- Hill DN, Mehta SB, and Kleinfeld D (2011). Quality metrics to accompany spike sorting of extracellular signals. Journal of Neuroscience 31, 8699–8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Noble D, and Tsien RW (1974). Electric Current Flow in Excitable Cells (Oxford: Clarendon press; ). [Google Scholar]
- Jalali R, Mallick I, Dutta D, Goswami S, Gupta T, Munshi A, Deshpande D, and Sarin R (2010). Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. International Journal of Radiation Oncology 77, 974–979. [DOI] [PubMed] [Google Scholar]
- Jeong DC, Tsai PS, and D. Kleinfeld D (2012). Prospect for feedback guided surgery with ultra-short pulsed laser light. Current Opinions in Neurobiology 22, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N (2017). Adaptive optical fluorescence microscopy. Nature Methods 14, 374–380. [DOI] [PubMed] [Google Scholar]
- Joo HR, Fan JL, Chen S Pebbles JA, Liang H, Chung JE, Yorita AM, Tooker AC, Tolosa VM, Geaghan-Breiner C, Roumis DK, Liu DF, Haque R, and Frank LM (2019) A microfabricated, 3D-sharpened silicon shuttle for insertion of flexible electrode arrays through dura mater into brain. Journal of Neural Engineering, in press. [DOI] [PMC free article] [PubMed]
- Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza MB, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydõn, ., Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, HŠusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun W.-l., Svoboda K, Carandini M, Harris KD, Koch C, OÕKeefe J, and Harris TD (2017). Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasthuri N, Hayworth KJ, Berger DR, Schalek RL, Conchello JA, Knowles-Barley S, Lee D, V‡zquez-Reina A, Kaynig V, Jones TR, Roberts M, Morgan JJ, Tapia JC, Seung HS, Roncal WG, Vogelstein JT, Burns R, Sussman DL, and Lichtman JW (2015). Saturated reconstruction of a volume of neocortex. Cell 162, 648–661. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Bharioke A, Blinder P, Bock DD, Briggman KL, Chklovskii DB, Denk W, Helmstaedter M, Kaufhold JP, Lee W-CA, Meyer H-S, Micheva KD, Oberlaender M, Prohaska S, Reid RC, Smith SJ, S. Takemura S, Tsai PS, and Sakmann B (2011). Large-scale automated histology in the pursuit of connectomes. Journal of Neuroscience 31, 16125–16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Mitra PP, Helmchen F, and Denk W (1998). Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proceedings of the National Academy of Sciences USA 95, 15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G, Marchman H, Wall D, and Lich B (2008). Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. Journal of Neuroscience 28, 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen PM, Mateo C, and Kleinfeld D (2016). Precision mapping of vibrissa representation within murine primary somatosensory cortex. Philosophical Transactions of the Royal Society B 371, e20150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld J, and Denk W (2018). Progress and remaining challenges in high-throughput volume electron microscopy. Current Opinion in Neurobiology 50, 261–267. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Kiani R, Esteky H, and Tanaka K (2014). Dimensionality of object representations in monkey inferotemporal cortex. Neural Computation 26, 2135–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R (1984). Methods for Neuronal Recording in Conscious Animals (Chichester: John Wiley and Sons; ). [Google Scholar]
- Liu R, Li Z, Marvin JS, and D. Kleinfeld D (2019) Direct wavefront sensing enables functional imaging of infragranular axons and spines. Nature Methods 16, 615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Kayser C, and Oeltermann A (2007) In vivo measurement of cortical impedance spectrum in monkeys: Implications for signal propagation. Neuron 55, 809–823. [DOI] [PubMed] [Google Scholar]
- Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, Lin S, Kazmi S, Fowler RA, Holloway S, Dunn AK, Chitwood RA, and Xie C (2017). Ultraflexible nanoelectronic probes form reliable, glial scarÐfree neural integration. Science Advances 3, e1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig KA, Langhals NB, Joseph MD, Sarah M Richardson-Burns SM, Hendricks JL, and Kipke DR (2011) PEDOT polymer coatings facilitate smaller neural recording electrodes. Journal of Neural Engineering 8, e014001, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard EM, Nordhausen CT, and Normann RA (1997). The Utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalography and Clinical Neurophysiology 102, 228–239. [DOI] [PubMed] [Google Scholar]
- Moore JJ, Ravassard PM, David Ho D, Acharya L, Kees AL, Vuong C, and Mehta MR (2017). Dynamics of cortical dendritic membrane potential and spikes in freely behaving rats. Science 355, eaaj1497. [DOI] [PubMed] [Google Scholar]
- Na K, Sperry ZJ, Lu J, Voeroeslakos M, Parizi SS, Bruns TM, Yoon E, and Seymour JP (2019). Novel diamond shuttle to deliver flexible bioelectronics with reduced tissue compression. BioRxiv 435800. [DOI] [PMC free article] [PubMed]
- Naundorf B, Wolf F, and Volgushev M (2006). Unique features of action potential initiation in cortical neurons. Nature 440, 1060–1063. [DOI] [PubMed] [Google Scholar]
- Negi S, Bhandari R, Rieth L, and Solzbacher F (2010). In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomedical Materials 5, e015007. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, and Wise SP (2003) Chronic, multisite, multielectrode recordings in macaque monkeys. Proceedings of the National Academy of Sciences USA 100, 11041–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A, Wu Y-W, Hanna M, Nix W, Ding J, and Melosh N (2018). Ultra-sensitive measurement of brain penetration with microscale probes for brain machine interface considerations. BioRxiv 454520
- Owen SF, Liu MH, and Kreitzer AC (2019) Thermal constraints on in vivo optogenetic manipulations. Nature Neuroscience 22, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralikar KJ,and Clement RS (2008) Collagenase-aided intracortical microelectrode array insertion: Effects on insertion force and recording performance. IEEE Transactions on Biomedical Engineering 55, 2258–2267. [DOI] [PubMed] [Google Scholar]
- Petersen CC (2007). The functional organization of the barrel cortex. Neuron 56, 339–355. [DOI] [PubMed] [Google Scholar]
- Platisa J, and Pieribone VA (2018). Genetically encoded fluorescent voltage indicators: are we there yet? Current Opinion in Neurobiology 50, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza SM, Scheffer LK, and Chklovskii DB (2014). Toward large-scale connectome reconstructions. Current Opinion in Neurobiology 25, 201–210. [DOI] [PubMed] [Google Scholar]
- Ranganathan GN, Apostolides PF, Harnett MT, Xu NL, Druckmann S, and Magee JC (2018) Active dendritic integration and mixed neocortical network representations during an adaptive sensing behavior. Nature Neuroscience 21, 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitboech HJ (1983). Fiber microelectrodes for electrophysiological recordings. Journal of Neuroscience Methods 83, 249–265. [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Street S, Ruyle AM, and Sloan AM (2005). A comparison of chronic multi-channel cortical implantation techniques: manual versus mechanical insertion. Journal of Neuroscience Methods 142, 169–176. [DOI] [PubMed] [Google Scholar]
- Rivnay J, Leleux P, Ferro M, Sessolo M, Williamson A, Koutsouras DA, Khodagholy D, Ramuz M, Strakosas X, Owens RM, Benar C, Badier J-M, Bernard C, and Malliaras GG (2015). High-performance transistors for bioelectronics through tuning of channel thickness. Science Advances 1, e1400251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousche PJ, and Normann RA (1992). A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Annals of Biomedical Engineering 20, 413–422. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Ypma RJF, Watson C, and Bullmore ET (2015). Wiring cost and topological participation of the mouse brain connectome. Proceeding of the National Academy of Sciences USA 112, 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta RL, Osmanski B-F, Boido D, Tanter M, and Charpak S (2017) Light controls cerebral blood flow in naive animals. Nature Communications 8, e14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpeshkar R (2010) Ultra Low Power Bioelectronics: Fundamentals, Biomedical Applications, and Bio-inspired Systems Cambridge University Press, Cambridge, UK. [Google Scholar]
- Schšlvinck ML, Saleem AB, Benucci A, Harris KD, and Carandini M (2015). Cortical state determines global variability and correlations in visual cortex. Journal of Neuroscience 35, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, and Kleinfeld D (2013). The smallest stroke: Occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nature Neuroscience 16, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JB, Mitra PP, and Sengupta A (2001) Capacity of nonlinear wavelength division multiplexing fiber optic transmission line. Journal of Optical Fiber Technology 7, e275288. [Google Scholar]
- Steinmetz NA, Koch C, Harris KD, and Carandini M (2018). Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Current Opinion in Neurobiology 50, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Marius Pachitariu M, Steinmetz N, Carandini M and Harris KD (2019). High-dimensional geometry of population responses in visual cortex. Nature 571 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, and Nicholson C (2008). Diffusion in brain extracellular space. Physiological Reviews 88, 1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis L, Berens P, Froudarakis E, Reimer J, Roson MR, Baden T, Euler T, Tolias AS, and Bethge M (2016). Benchmarking spike rate inference in population calcium imaging. Neuron 90, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoshenko S, and Young DH (1945) Theory of Structures McGraw Hill Book Company, New York. [Google Scholar]
- Tonnesen J, KrishnaInavalli VVG, and Nägerl V (2018). Super-resolution imaging of the extracellular space in living brain tissue. Cell 172, 1108–1121. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold J, Blinder P, Friedman B, Drew P, Karten HJ, Lyden PD, and Kleinfeld D (2009). Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of cell nuclei and microvessels. Journal of Neuroscience 18, 14553–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventakachalam S, Fee MS, and Kleinfeld D (1999). Ultra-miniature headstage with 6-channel drive and vacuum-assisted micro-wire implantation for chronic recording from neocortex. Journal of Neuroscience Methods 90, 37–46. [DOI] [PubMed] [Google Scholar]
- Vitale F, Vercosa DG, Rodriguez AV, Pamulapati SS, Seibt F, Lewis E, Yan S, Badhiwala K, Adnan M, Royer-Carfagni G, Beierlein M, Kemere C, Pasquali M, and Robinson JT (2018). Fluidic microactuation of flexible electrodes for neural recording. Nano Letters 18, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapanitch W, Fee MS and Sarpeshkar R (2007). An energy-efficient micropower neural recording amplifier. IEEE Transactions on Biomedical Circuits and Systems 1, 136–147. [DOI] [PubMed] [Google Scholar]
- Wattanapanitch W and Sarpeshkar R (2011) A low-power 32-channel digitally programmable neural recording integrated circuit. IEEE Transactions on Biomedical Circuits and Systems 5, 592–602. [DOI] [PubMed] [Google Scholar]
- Wei X, Luan L, Zhao Z, Li X, Zhu H, Potnis O, and Xie C (2018). Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Advanced Science 5, e1700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg HL (1963) Effective diffusion coefficient in porous media. Journal of Applied Physics 34, 2636–2640. [Google Scholar]
- Weltman A, Yoo J, and Meng E (2016). Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines 7, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen JJ III, Young J, Weiland JD, and Searsonb JC (2006). Electrochemical characterization of charge injection at electrodeposited platinum electrodes in phosphate buffered saline. Journal of The Electrochemical Society 153, C834–C839. [Google Scholar]
- Xie C, Liu J, Fu T-M, Dai X, Zhou W, and Lieber CM (2014). Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nature Materials 14, 1286–1292. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhou T, Zwang TJ, Hong G, Zhao Y, Viveros RD, Fu T-M, Gao T and Lieber CM (2019). Bioinspired neuron-like electronics. Nature Materials 18, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Li X, He F, Wei X, Lin S, and Xie C (2019). Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. Journal of Neural Engineering 16, e3035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Luan L, Wei X, Zhu H, Li X, Lin S, Siegel JJ, Chitwood RA, and Xie C (2017). Nanoelectronic coating enabled versatile multifunctional neural probes. Nanoscience Letters 16, 4588–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]