Abstract
OBJECTIVE:
To examine the role that intrinsic functional networks, specifically the default mode network, have on metacognitive accuracy for individuals with moderate to severe traumatic brain injury (TBI).
METHOD:
A sample of 44 individuals (TBI n = 21; healthy controls (HC) n = 23) were included in the study. All participants underwent an MRI scan and completed neuropsychological testing. Metacognitive accuracy was defined as a participant’s ability to correctly judge their item-by-item performance on an abstract reasoning task. Metacognitive values were calculated using the signal detection theory approach of area under the receiver operating characteristic curve (AUROC). Large-scale subnetworks were created using Power’s 264 Functional Atlas. The graph theory metric of network strength was calculated for six subsystem networks to measure functional connectivity.
RESULTS:
There were significant interactions between head injury status (TBI or HC) and internetwork connectivity between the anterior DMN and salience network on metacognitive accuracy (R2 = 0.13, p = 0.047) and between the posterior DMN and salience network on metacognitive accuracy (R2 = 0.15, p = 0.038). There was an interpretable interaction between head injury status and internetwork connectivity between the attention network and salience network on metacognitive accuracy, R2 = 0.13, p = 0.067. In all interactions, higher connectivity predicted better metacognitive accuracy in the TBI group, but this relationship was reversed for the HC group.
CONCLUSION:
Enhanced connectivity to both anterior and posterior regions within the DMN facilitates metacognitive accuracy post injury. These findings are integrated into a larger literature examining network plasticity in TBI.
Keywords: traumatic brain injury (TBI), metacognition, resting state functional connectivity, DMN
Brain Injury and Metacognition
Traumatic brain injury (TBI) is accompanied by a variety of symptoms, including changes in physical symptoms, cognition, and mood. Metacognition, the ability to evaluate one’s own cognitive processes in the moment of task completion, is one commonly disrupted cognitive process following TBI (Flavell, 1979; Chiou & Hillary, 2012; Kennedy, 2001). Deficits in metacognition are linked to a host of functional deficits in the areas of insight and planning, including disengagement in rehabilitation and problems understanding one’s own functional limitations (Flashman & McAllister, 2012; Ownsworth & Fleming, 2005). Metacognitive impairments have been consistently observed after TBI (Chiou & Hillary, 2012; Kennedy, 2001; O’Keeffe, Dockree, Moloney, Carton, & Robertson, 2007), but what is less clear is how the pathophysiology of TBI directly interacts with the neural substrates that give rise to metacognitive capacity. Previous literature has demonstrated a link between gray matter volume in frontal brain regions and metacognitive accuracy in both healthy individuals (Fleming, Weil, Nagy, Dolan, & Rees, 2010; McCurdy et al., 2013) as well as individuals who have experienced a traumatic brain injury (Grossner, Bernier, Brenner, Chiou, & Hillary, 2018). While this literature is still emerging, there is a scarcity of evidence for the role of distributed functional networks in metacognition. The goal of this study is to use functional connectivity modeling combining fMRI and graph theory to examine how frontal regions, including those not typically involved in volitional cognitive processes (e.g., medial frontal cortex), contribute to metacognitive accuracy and network plasticity following neurological disruption. Here we define network plasticity as differences in network functioning between healthy control and TBI groups, as we assume that changes in connectivity between these groups result from brain injury.
Frontal Systems and Metacognition
There is longstanding evidence that frontal systems are critical for the implementation of metacognitive capacity. This has been visible in both structural brain imaging studies (Sinanaj, Cojan, & Vuilleumier, 2015; Valk, Bernhardt, Bockler, Kanske, & Singer, 2016; Fleming & Dolan, 2012) and more recent functional imaging studies (Schmitz, Rowley, Kawahara, & Johnson, 2006; De Martino, Fleming, Garrett, & Dolan, 2013; Paul et al., 2015). Given that the frontal lobes are affected in moderate and severe TBI approximately 70% of the time, irrespective of injury mechanism (Hillary, Moelter, Schatz, & Chute, 2001; Stuss & Gow, 1992), the link between TBI and metacognitive deficit is not surprising. Most recently, research from our laboratory demonstrated that metacognition was associated with gray matter volume in dorsolateral frontal region and posterior regions of the cingulate cortex, angular gyrus, and supramarginal gyrus (Grossner et al., 2018). These findings were well-aligned with a broader literature demonstrating that regional gray matter volumes in the frontal pole (Fleming & Dolan, 2012, Fleming, Weil, Nagy, Dolan & Rees, 2010), prefrontal cortex (Sinanaj et al., 2015), and anterior cingulate cortex (Valk et al., 2016) predict metacognitive ability.
While studies examining lesions and structural gray matter volume have begun to draw clear links between specific neural substrates that give rise to metacognition, the interactive relationships within and between distributed networks where these substrates are embedded remains unclear. In functional imaging studies, multiple efforts have reported an important role of the anterior prefrontal cortex (aPFC) and anterior cingulate cortex (ACC) in metacognitive accuracy both during task (De Martino et al., 2013; Fleming, Huijgen, & Dolan, 2012) and at rest (Baird, Smallwood, Gorgolewski, & Margulies, 2013; see Fleming & Dolan, 2012 for review). Additionally, it has been demonstrated that metacognitive accuracy may depend on how the aPFC and ACC are communicating and integrating information related to performance monitoring (Fleming & Dolan, 2012). This has led to a more formalized theory regarding the role of frontoparietal control network (FPCN) in self-awareness in healthy individuals as well as those who have suffered a traumatic brain injury (Ham et al., 2014), where the FPCN has been defined as the lateral frontal gyri, anterior insula, and inferior parietal lobules. In a sample of individuals who have sustained a TBI, those who were low in “performance-monitoring,” or correctly identifying their own errors, demonstrated reduced connectivity within the FPCN. Interestingly, lesion location did not have an association with performance-monitoring ability (Ham et al., 2014); this finding has important implications for understanding the distributed nature of metacognition.
The salience network, an intrinsic network that includes areas of the anterior cingulate cortex (ACC) and anterior insula, may also play an important role in metacognition. Ham and colleagues (2014) defined the salience network as a sub-network of the FPCN and demonstrated that it is associated with metacognitive ability. Others have shown that this network is associated with performance monitoring, particularly driven by the ACC (Oliveira, McDonald, & Goodman, 2007). The anterior insula has also been shown to be related to error detection while engaged in goal-directed behavior (Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010). These components of self-monitoring and error detection are central to intact metacognitive ability. Lastly, because the salience network integrates other networks and functions and controls changes of activity in other networks (Sridharan, Levitin, & Menon, 2008), it is hypothesized that this network may assist in mediating metacognitive ability.
While task-positive networks have been linked to engagement of metacognitive processes, networks that monitor internal states, such as the default mode network (DMN), which is an integrated network including medial PFC, posterior cingulate cortex (PCC), and precuneus, have more recently received attention as a moderator of metacognition (Andrews-Hanna, Smallwood, & Spreng, 2015; Baird et al., 2013, Chua, Schacter, Rand-Giovanetti, & Sperling, 2006). The DMN, described in work by Raichle and colleagues, has traditionally been viewed as a task-negative network that is associated with self-referential thoughts (2001). One contemporary view of the DMN posits that it may play a critical role in monitoring internal states and self-referential thought (Mason et al., 2007), which is an extension of work over two decades old that links medial prefrontal cortex to insight, decision making, and self-regulation (Bechara, Damasio, Damasio, & Anderson, 1994; Damasio, 1996). Given this history, the role of the medial PFC in metacognition is particularly intriguing.
While the DMN is a functionally connected network, it spans a number of regions that maintain distinct roles in information processing. The anterior area of the DMN that includes the ventromedial PFC (vmPFC) has been linked to perception and judgment (Uddin, Clare Kelly, Biswal, Castellanos, & Milham, 2009) as well as what others have called “attention demanding” self-referential behaviors (Gusnard, Akbudak, Shulman, & Raichle, 2001). The posterior area of the DMN, which includes the PCC and precuneus, may be related to episodic memory retrieval (Uddin et al., 2009). Moreover, portions of medial frontal and dorsomedial PFC regions may reflect processing present mental states and internally driven thoughts about the self (Gusnard et al. 2001), whereas the integrated function between anterior medial PFC and PCC is associated with integrating memories of prior events and judgments about the self (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). Several of the capacities related to self-reflection and monitoring are crucial for metacognition and are therefore an important focus of this study as we aim to uncover potential connectivity changes in medial PFC after significant neurological disruption.
Goals for the Present Study
The present study is a follow-up to our recently published work examining changes in gray matter volume that predict metacognition after moderate and severe TBI (Grossner et al., 2018), with the goal of extending this analysis to functional neural networks. First, we aim to examine positive functional connectivity focusing on positive correlations between subnetworks associated with metacognitive ability. Specifically, we are interested in whether connectivity within and between frontal regions supporting distinct networks (i.e., lateral PFC in fronto-parietal control network (FPCN) and the medial PFC of the DMN), predict metacognitive accuracy differently in healthy control and TBI groups. Second, we aim to examine the potentially dissociable roles of the anterior and posterior areas of the DMN and their contributions to metacognitive capacity after injury. To do so, we examine patterns of neural connectivity established using blood-oxygen-level dependent (BOLD) resting state fMRI methods with focus on intrinsic functional networks purported to play a role in metacognition and metacognitive ability.
Hypotheses
Based upon prior literature, we hypothesized that connectivity between and within two critical functional networks, the DMN and FPCN, would modulate metacognitive accuracy in both the TBI and healthy control groups. Specifically, we predicted that there would be an association with metacognition and connectivity between anterior portions of the DMN and FPCN and the salience network, a critical network for attentional capture and shifting. We also anticipated that intranetwork connectivity for the anterior DMN and FPCN would facilitate metacognitive ability. Second, we hypothesized a dissociation between anterior and posterior DMN in the contribution to metacognition. Specifically, the anterior DMN (medial frontal regions) would better predict metacognitive accuracy related to online monitoring of performance compared to the posterior DMN (posterior cingulate regions), which is more often linked to semantic processing and memory functioning. To test these hypotheses, we examined functional network connectivity strength between and within networks during resting state using graph theory methods.
Methods
Participants
The participants were 21 individuals with moderate to severe TBI and 23 healthy control (HC) individuals. See Table 1 for participant demographics. Participants were included in the TBI group if they had a moderate to severe brain injury as defined by a Glasgow Coma Scale (GCS) of 3–12 (Teasdale & Jennett, 1974). Individuals with GCS scores of 13–15 were included if they had positive neuroimaging findings at the time of injury. Deficits in individuals with this kind of complicated mild TBI have demonstrated similar deficits to those sustaining moderate to severe TBI (Tayim, Flashman, Wright, Roth, & McAllister, 2016). This sample was extracted from two larger studies that included both longitudinal and cross-sectional data. From this sample, 12 participants were from the cross-sectional study and 32 were from the longitudinal study. In these data, all participants were exposed to the metacognitive task (defined below) a single time (i.e., first measurement point for subjects from longitudinal work) and were included only if they had T1-weighted structural images, viable resting state data, and scores and confidence judgments for the Matrix Reasoning metacognitive task. All of the data was collected in the same visit.
Table 1.
Demographic information for participants in the traumatic brain injury (TBI) and healthy control (HC) groups.
| Age (years) Mean(SD) |
Education (years) Mean(SD) |
Gender | GCS Mean(SD) |
Time Post Injury (mos) Mean(SD) |
|
|---|---|---|---|---|---|
| TBI | 32.9 (14.0) | 13.0 (1.7) | 12 M, 9 F | 7.3 (4.7) | 49.9 (77.0) |
| HC | 36.9 (12.1) | 13.6 (1.8) | 13 M, 10 F | -- | -- |
All participants were recruited through a telephone screen to determine that they met all inclusion and exclusion criteria. Exclusion criteria included left-handedness, history of neurodegenerative disease, psychiatric illness or substance use, and contraindications for MRI safety. Participants signed all consent forms, which were approved through The Pennsylvania State University IRB. Participants were compensated for their participation in the study and travel expenses.
Procedure
All TBI and HC participants underwent the same testing procedure. The testing session was comprised of consenting, mock scan, a 90-minute MRI protocol that included structural scans, resting state, and task, and a brief neuropsychological battery that assessed various measures of cognition that are often impaired following brain injury, including domains of attention, memory, and executive functioning.
The primary measure used to quantify metacognition was a modified Matrix Reasoning task from the Wechsler Adult Intelligence Scale - Third Edition (WAIS-III), completed outside of the scanner. This task presented the participants with a figure with a missing piece and the participant was to choose the missing piece from five choices. This task was modified from its original version into “ordered” and “random” versions of the task. The ordered version is anchored, in that it increases in difficulty from the easiest to the most difficult question. The random version loses this anchoring and randomizes the difficulty level from item to item. Each participant received both versions of this task, and the administration was counterbalanced across subjects to eliminate ordering effects. After answering each task question, participants were asked, “How confident are you of your choice?” and responded on a 6-point Likert scale ranging from “completely certain” to “completely uncertain” (Chiou, Arnett, Carlson, Cosentino, & Hillary, 2011; Chiou & Hillary, 2012; Grossner et al, 2018). The confidence ratings are shown in Figure 1.
Figure 1.
Example of retrospective confidence judgments choices used following each item of the modified Matrix Reasoning task.
Quantifying Metacognition
Metacognition values were computed from the performance scores and retrospective confidence judgments collected from the modified Matrix Reasoning task that was administered to all participants. For this analysis, the “random” version of the task was used, as this version was previously shown to be most sensitive to differences following brain injury (Grossner et al., 2018). Metacognitive accuracy was statistically quantified using a Type II area under the receiver operating characteristic (AUROC) curve. This method is ideal for quantifying metacognition because it incorporates both the classification of correct and incorrect responses based on the participant’s reported confidence level (metacognitive sensitivity) as well as the participant’s overall confidence rating, i.e., consistently responding confidently or unconfidently (metacognitive bias) (Fleming & Lau, 2014). This calculation was completed using Matlab code detailed in Fleming & Lau (2014) and is available in the Supplementary Materials.
MRI Acquisition
Data were collected on one of two scanners at one of the following sites: Penn State Hershey Medical Center Department of Radiology on a Siemens Magnetom Trio 3T scanner (n=20; 17 TBI and 3 HC), or on an identical Siemens Magnetom Trio 3T scanner in University Park at the Social, Life, and Engineering Sciences Imaging Center (n=24; 4 TBI and 20 HC). Significant efforts were made to monitor data fidelity and reliability. Data collection was consistent with previous studies in our laboratory (Roy et al., 2017; Roy, Campbell, Bernier, & Hillary, 2016; Bernier et al., 2017; Grossner et al., 2018).
Structural Data
Anatomical structural scans were collected using an MPRAGE sequence at a spatial resolution of 1 × 1 × 1 mm voxels, repetition time (TR) of 2,300 ms, echo time (TE) of 2.98 ms, and flip angle of 9 degrees. Slices were collected interleaved. Images were preprocessed using Statistical Parameter Mapping (SPM) version 8 (2008, Wellcome Department of Cognitive Neurology, London UK; http://www.fil.ion.ucl.ac.uk/spm).
Resting State fMRI Scan
Participants in the scanner were instructed to fixate on a white cross in the center of a black screen and they were asked to remain awake. This scan ran for 10 minutes. Slices were collected interleaved with a spatial resolution of 3 × 3 × 4 mm voxels, TR of 2,000 ms, and TE of 30 ms. The first 5 volumes for each participant were removed before analysis, leaving a remaining 145 volumes per participant. Images were preprocessed using Statistical Parameter Mapping (SPM) version 8 (2008, Wellcome Department of Cognitive Neurology, London UK; http://www.fil.ion.ucl.ac.uk/spm).
Data Preprocessing
Each participant’s data underwent an identical preprocessing pipeline. All preprocessing steps used SPM8. Using ArtRepair, bad slices were repaired using the art-slice procedure. Volumes were then slice-time corrected and realigned. Despiking eliminated artifacts using ArtRepair’s despike filter. Co-registration was done in SPM8 using each participant’s T1 weighted image and the mean functional image. This co-registered image was segmented in SPM8 to normalize the image into the Montreal Neurological Institute (MNI) space. These images were then resliced to 3mm isotropic. To improve signal-to-noise ratio and reduce effects of anatomical differences, we applied a 6mm isotropic smoothing filter to the normalized data. To address artifactual signal, time series from cerebrospinal fluid (CSF) and cerebral white matter were sampled, averaged, and treated as regressors of no interest. The time series was then bandpass filtered 0.01 Hz to 0.12 Hz using the functional connectivity toolbox (CONN; https://www.nitrc.org/projects/conn). Finally, as a step to address framewise motion, volume repair was performed using ArtRepair and individuals with movement presented in greater than 20% of volumes were removed from the analysis. We did not use global signal regression in our preprocessing as it has been shown to introduce false negative connectivities into the data (Murphy et al., 2009; Saad et al., 2012).
Network Creation (Subsystems)
For this analysis, subsystems were created in order to determine which smaller functional areas may be associated with metacognition. We first used a functionally defined atlas (Power et al., 2011) to define 264 regions of interest (ROIs) in the brain. From these regions, six subsystem networks were created, including two default mode networks (anterior and posterior), the attention network, the salience network, the fronto-parietal control network, and a “residual” network. The DMN was separated into anterior and posterior networks, as defined by Uddin and colleagues (2009). The attention network was comprised of the superior frontal gyrus, cingulate gyrus, and middle temporal gyrus. This network is associated with the involvement and completion of goal-directed tasks (Rueda, Checa, & Cómbita, 2012; Posner & Petersen, 1990). The salience network included the anterior cingulate cortex (ACC) and anterior insula. This network aids in integrating and guiding other networks and functions (Menon, 2015). Additionally, the fronto-parietal control network (FPCN) is useful in initiating and modulating cognitive control functions (Zanto & Gazzaley, 2013). This network was comprised of inferior frontal and inferior parietal regions. The “residual” network in this analysis was comprised of visual, sensory, and auditory areas. Table 2 indicates all subsystems and their included brain regions. Visualizations of these networks are located in Figure 2. For analyses, we examined within-network functional connectivity, henceforth “intranetwork” connections, and between-network connectivity, henceforth “internetwork” connections.
Table 2.
Subsystems created from Power’s 264 ROIs.
| Attention Network |
Anterior DMN | Salience Network | Residual | Fronto-parietal Network |
Posterior DMN |
|---|---|---|---|---|---|
| Middle Temporal | Middle Frontal | Insular Cortex | Sensory-motor | Precentral Gyrus | Posterior Cingulate |
| Lateral Occipital | Superior Frontal | Anterior Cingulate | Auditory | Superior Parietal | Precuneus |
| Ventral Frontal | Visual | Lateral Prefrontal | Temporal Pole |
Figure 2.
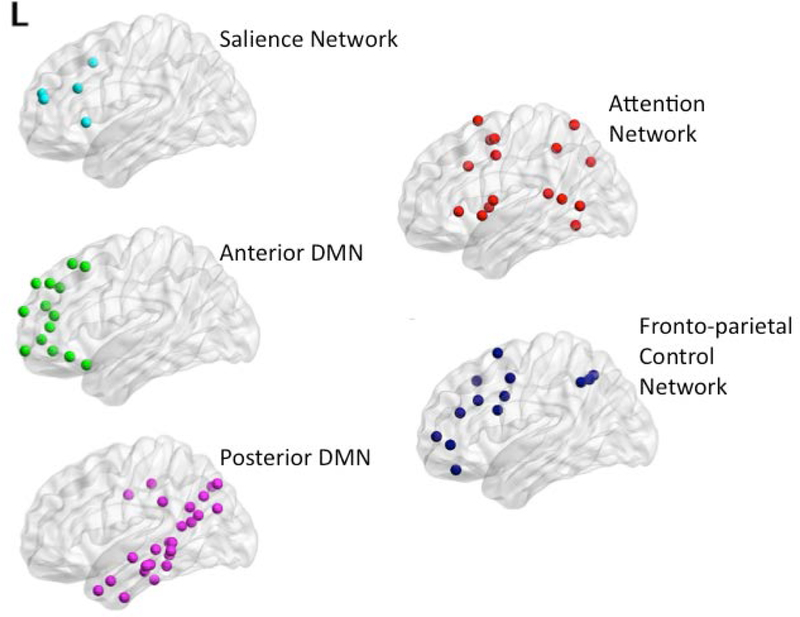
Visualizations of subsystems created with BrainNet Viewer (Xia et al., 2013, http://www.nitrc/org/projects/bnv/). L indicates the left side of the brain.
Network Strength
Network strength was the graph theory metric used to define functional connectivity for whole brain analyses and intra- and inter-network connectivity. An edge list was created for each participant using a script written in R version 3.1.1, which calculated Pearson’s R correlation values and p-values for all 264 ROIs in the functional atlas [e.g., N ROIs will produce N*(N − 1)/2 undirected connections]. Each ROI was comprised of the average blood oxygen-level dependent (BOLD) signal from 27 voxels to create a spherical ROI at each coordinate. Multiple comparisons were controlled for using a false discovery rate (FDR) of 0.05. Correlations that survived the correction were maintained and the remainder were set to 0. Edges were defined as the correlation between two ROIs (or nodes). Network strength examined the total number of edges by the correlation value of those edges within a network (e.g., each of the defined subsystems).
Data Analysis Plan
Primary analyses were cross-sectional, comparing connectivity in the subnetworks of interest between the TBI group and the healthy control groups. Additionally, we examined interactions between head injury status and connectivity on metacognitive accuracy for six network comparisons focusing on both intra- and inter-network connectivity: 1) between the anterior DMN and salience network, 2) between the posterior DMN and salience network, 3) between the attention network and salience network, 4) between the FPCN and salience network, 5) within the anterior DMN, and 6) within the FPCN. Lastly, we created a “residual” network comprised largely of primary motor and sensory regions to serve as a control, anticipating little association between these regions and metacognitive ability.
Results
Differences in Metacognitive Accuracy Between Groups
Previous work from our laboratory indicated that there was a significant difference in metacognitive accuracy between the TBI group and a healthy control group (Grossner et al., 2018). The present subsample of data did not demonstrate a significant difference in metacognitive accuracy between groups, p = 0.20, but consistent with the previous study, healthy control participants did show higher metacognitive accuracy (M = 0.70, SD = 0.09) than did the TBI group (M = 0.66, SD = 0.13), with the TBI sample showing greater performance variability overall.
Differences in Neuropsychological Performance Between Groups
There were no significant differences between the TBI group and the healthy control group on neuropsychological measures of attention (Digit Span - Forward), processing speed (Trails A, VSAT - Letters and Symbols), executive functioning (Trails B), and working memory (Digit Span - Backward). See Table 3 for a summary of behavioral performance measures.
Table 3.
Summary of neuropsychological performance measures. Of note, Trails A and B are measures in seconds, with larger values indicating worse performance.
| TBI Mean (SD) | HC Mean (SD) | P | |
|---|---|---|---|
| Digit Span - Forward | 10.29 (2.26) | 11.27 (2.27) | 0.161 |
| Digit Span - Backward | 6.86 (2.33) | 7.045 (2.30) | 0.791 |
| VSAT - Letter | 56.10 (13.01) | 59.26 (10.81) | 0.384 |
| VSAT- Symbol | 57.62 (13.94) | 55.78 (16.05) | 0.683 |
| Trails A (seconds) | 29.45 (13.79) | 23.48 (7.92) | 0.101 |
| Trails B (seconds) | 64.95 (22.30) | 56.24 (23.58) | 0.231 |
Whole Brain Network Metrics
Whole brain graph theory metrics were conducted to describe the global brain network properties to provide context for the networks observed in this sample (see Hallquist & Hillary, 2018). There was an interpretable difference between average path length for the TBI group (M = 1.91, SD = 0.06) and the healthy control group (M = 1.95, SD = 0.07), [t(42) = −1.91, p = 0.059, d = 0.61]. This reduction in path length attributed to increased connectivity has been observed elsewhere in TBI (Castellanos et al., 2011). Figure 3 illustrates the shift in the overall weighted degree distribution, with the frequency of the most highly connected nodes more likely to occur in TBI cases. There were no significant differences between TBI and healthy control groups for global brain metrics of global, local, or weighted transitivity, p > 0.10. Descriptive statistics for global metrics are shown in Table 4.
Figure 3:
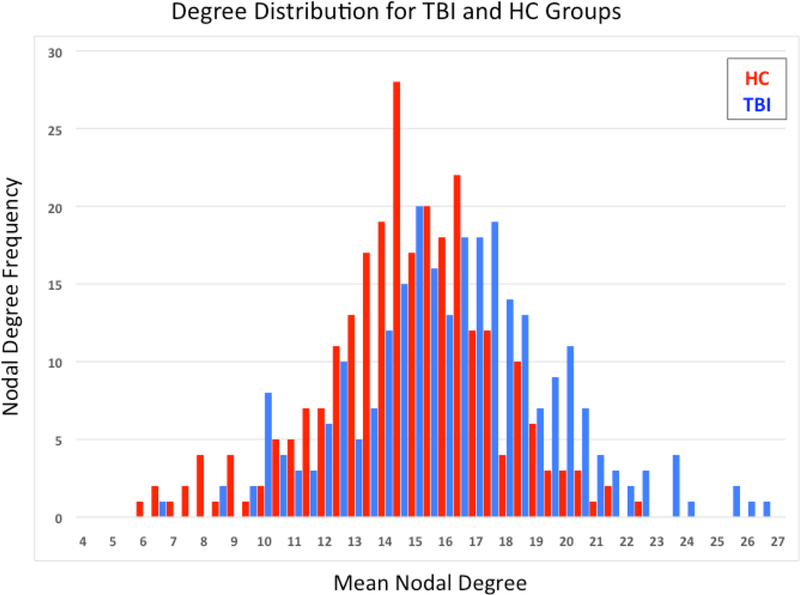
Degree distribution for each sample. Note the right skewed distribution for the TBI sample with the most highly connected nodes appearing in this sample.
Table 4.
Summary of whole brain network metrics
| Average Path Length mean(SD) |
Local Transitivity mean(SD) |
Global Transitivity mean(SD) |
Weighted Transitivity mean(SD) |
|
|---|---|---|---|---|
| TBI | 1.91 (0.06) | 0.43 (0.05) | 0.44 (0.06) | 0.46(0.05) |
| HC | 1.95 (0.07) | 0.41 (0.04) | 0.42 (0.04) | 0.44 (0.04) |
Differences in Intranetwork Connectivity Between Groups
When comparing strength of intranetwork connectivity between the TBI and HC groups, there was a significant difference in the attention network, which includes areas of the middle temporal cortex, lateral occipital cortex, and ventral frontal cortex, with the TBI group demonstrating higher connectivity (M = 72.03, SD = 15.90) than the HC group (M = 61.87, SD = 13.40), [t(39) = 2.28, p = 0.028, d = 0.69]. There were no other significant differences in intranetwork connectivity between the HC and TBI groups for the anterior DMN, (p = 0.504), posterior DMN (p = 0.088), FPCN (p = 0.363), or salience network (p = 0.809).
Interactions Between Head Injury Status and Connectivity on Metacognitive Accuracy
Internetwork Connectivity
We examined the interactions between the TBI and HC groups and both inter- and intranetwork connectivity on metacognitive accuracy. There was a significant interaction between head injury status and internetwork connectivity between the anterior DMN and salience network on metacognitive accuracy (R2 = 0.13, p = 0.047). Mirroring this finding, there was a significant interaction between head injury status and internetwork connectivity between the posterior DMN and salience network on metacognitive accuracy (R2 = 0.15, p = 0.038). Figures 4 and 5 illustrate these two findings. There was also an interpretable interaction between head injury status and internetwork connectivity between the attention network and salience network on metacognitive accuracy (R2 = 0.13, p = 0.067; See Figure 6). Of note, this significant interaction occurs in the absence of a main effect of connectivity on metacognitive accuracy within TBI where r-values were 0.29 for all correlations, p > 0.10. Within the healthy control group, there was an interpretable main effect of interconnectivity between posterior DMN and salience network on metacognitive accuracy (r = −0.39, p = 0.064). There were no significant main effects for metacognition and interconnectivity between anterior DMN and salience network, or between the attention and salience networks, with r-values ranging from −0.34 to −0.31, p > 0.10. Simple main effects testing yielded non-significant effects within groups (p > 0.05), but there was a meaningful difference between the TBI and healthy control groups, as evidenced by the negative relationship in the healthy control group and the positive relationship in the TBI group. Finally, there was no significant interaction between the FPCN and salience network (R2 = 0.08, p = 0.328). For all significant interactions, there were positive slopes for the relationship between connectivity and metacognitive accuracy in the TBI group, but negative slopes in the HC group.
Figure 4.
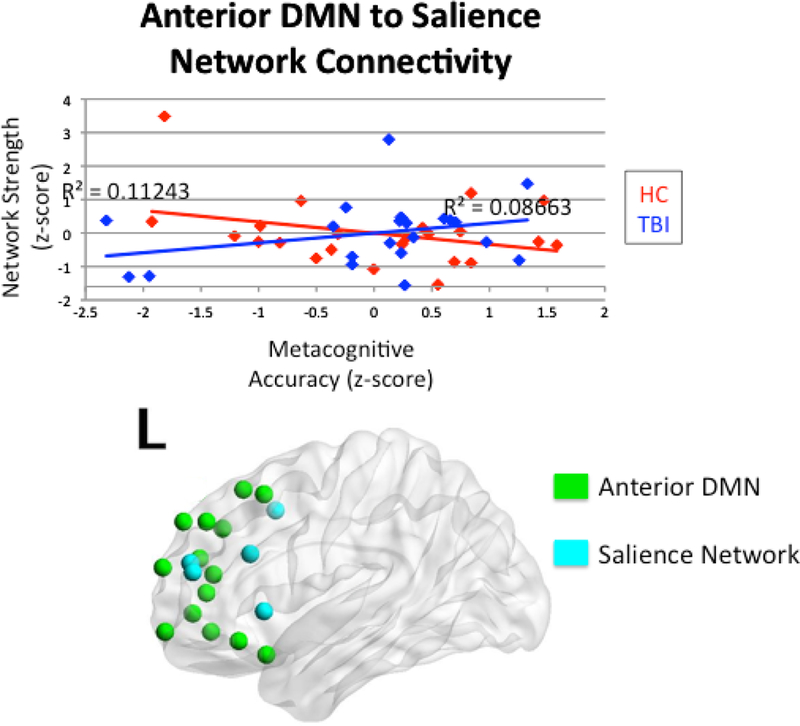
Interaction between head injury status and internetwork connectivity between the anterior DMN and salience network on metacognitive accuracy.
Figure 5.
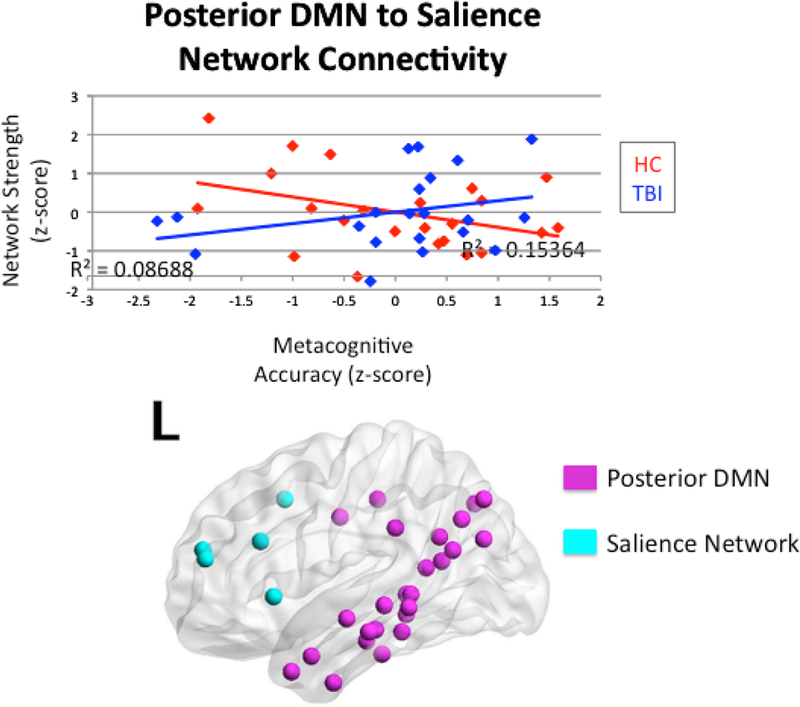
Interaction between head injury status and internetwork connectivity between the posterior DMN and salience network on metacognitive accuracy.
Figure 6.
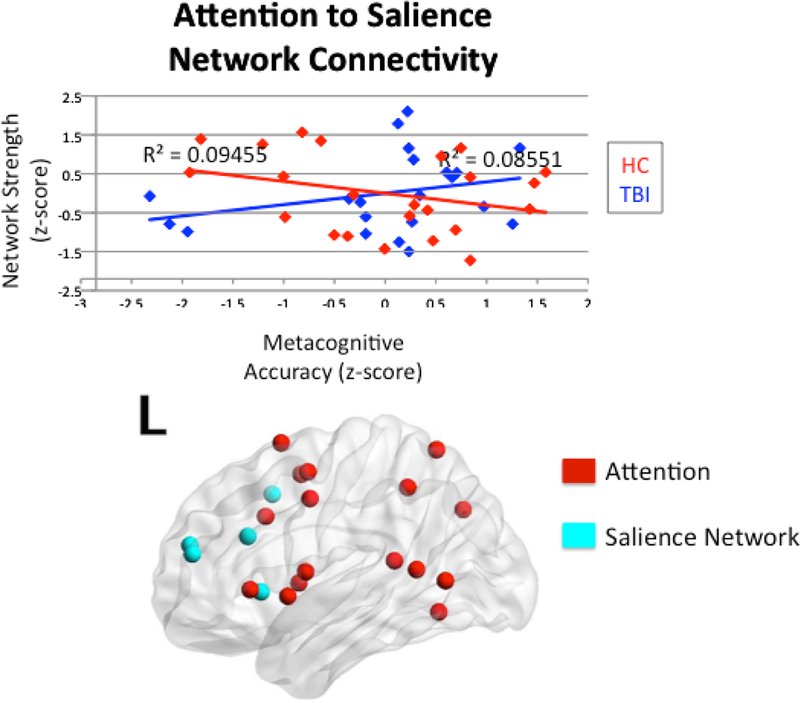
Interaction between head injury status and internetwork connectivity between the attention network and salience network on metacognitive accuracy.
As a quality check for the influence of MRI scanner on the results, analyses were repeated using the scanner as a covariate. Results remained consistent, with similar interactions between head injury status and internetwork connectivity between the anterior DMN and salience network on metacognitive accuracy (R2 = 0.27, p = 0.054), between head injury status and internetwork connectivity between the posterior DMN and salience network on metacognitive accuracy (R2 = 0.31, p = 0.026), and an interpretable interaction between head injury status and internetwork connectivity between the attention network and salience network on metacognitive accuracy (R2 = 0.28, p = 0.076). We therefore do not anticipate that the use of distinct MRI scanners in this study accounts for the findings.
Intranetwork Connectivity
Additionally, we examined intranetwork connectivity between HC and TBI groups within the anterior DMN and within the FPCN. There were no significant interactions between head injury status and metacognition and connectivity within the anterior DMN (R2 = 0.10, p = 0.161), or within the FPCN (R2 = 0.06, p = 0.431) on metacognitive accuracy.
As above, additional analyses were conducted to control for scanner type. Consistent with the prior results, analyses did not yield significant interactions between head injury status and connectivity within the anterior DMN (R2 = 0.27, p = 0.106), or within the FPCN (R2 = 0.21, p = 0.539) on metacognitive accuracy.
Control Analysis – “Residual” Network
In order to guarantee that the finding regarding increased connectivity in TBI was not generally related to metacognitive performance, we conducted an identical interaction analysis using the “residual” networks serving as a control. As predicted, the result of this analysis was not significant (R2 = 0.07, p = 0.250).
Discussion
In this study, we examined network plasticity in intrinsic functional networks, with focus on the DMN, and metacognitive accuracy following moderate to severe traumatic brain injury. To date, the DMN has been linked to metacognition including potentially dissociable roles for the medial PFC during online evaluation (Bechara, Damasio, Damasio, & Anderson, 1994; Damasio, 1996) and the PCC, which may be more involved in self-referential processing in the context of memory (Andrews-Hanna, Smallwood, & Spreng, 2015; Baird et al., 2013, Chua, Schacter, Rand-Giovanetti, & Sperling, 2006). We hypothesized that, after TBI, frontal regions including the FPCN and anterior DMN (as opposed to posterior DMN, including PCC), would be the most affected by injury and therefore influence the evaluative process of metacognition.
Findings from this study partially converge with previous work, revealing an important role of anterior DMN and salience network in modulating metacognitive accuracy after TBI. However, FPCN connectivity did not dissociate the groups and did not predict performance (see below). Moreover, connectivity between the salience network and posterior DMN showed significant interaction effects between the groups when predicting metacognitive capacity. With the exception of one analysis (in the healthy control sample), the first-order correlations between connectivity and metacognitive performance in the TBI were generally non-significant (e.g., small effects of r~0.30 in TBI). However, in all analyses, higher connectivity in the TBI group was associated with better metacognitive accuracy and the opposite was found in the healthy control group. Primary findings are further explicated in the following Discussion.
Corroborating Volumetric Change via Functional Connectivity and Hyperconnectivity
The observed association between the anterior DMN and salience network and metacognitive accuracy mirrors previous findings from our laboratory in a partially overlapping sample. In this prior work, we observed an association between gray matter volume loss in orbitofrontal and dorsolateral PFC regions and metacognitive accuracy (Grossner et al., 2018). The findings in Grossner and colleagues (2018) were the inspiration for the current analysis, with the current results revealing that gross neural network differences observable using resting state functional connectivity (RSFC) and BOLD methods may reflect underlying changes in brain morphometry. While counterintuitive (gray matter volume reduction leads to increased connectivity), this finding is consistent with a “less wiring, more firing” hypothesis observed in aging (see Daselaar et al., 2015) and other clinical samples (Hillary & Grafman, 2017).
If one focuses on the strength of connections in the networks analyzed, findings show that TBI results in stronger connections between subnetworks post-injury and increased connectivity in these specific networks is generally associated with improved metacognitive performance. The finding that injury results in increased network connectivity is consistent with prior literature regarding “hyperconnectivity” in TBI (Caeyenberghs, Verhelst, Clemente, & Wilson, 2017; Hillary et al., 2014; Sharp et al., 2011; Palacios et al., 2013) and, in fact, may be a common response to neurologic disruption more generally (Hillary et al., 2015).
Differentiating Anterior and Posterior DMN in Metacognition
One important goal was to dissociate the relative roles of anterior and posterior DMN in supporting metacognition post injury. It has been demonstrated that the anterior DMN, and in particular dorsomedial PFC, is associated with mentalizing and social cognition (Gallagher & Frith, 2003; Frith & Frith, 2003; Hampton, Bossaerts, & O’Doherty, 2008). However, the relationship between metacognition and posterior regions of the DMN is less clear (Andrews-Hanna et al., 2015), so the finding that posterior DMN connectivity was an important predictor of metacognition is intriguing. Of note, the PCC is a functional hub of the posterior DMN and has been thought to play an important role in self-referential processing, which may contribute to the current finding linking the posterior DMN with metacognitive accuracy. There is also evidence from the electrophysiological literature implicating the PCC as the source of the error positivity, an event related potential component that has been observed during tasks related to metacognition, such as the conscious awareness of error detection (O’Connell et al., 2007). To determine if the PCC specifically was driving the posterior DMN findings, we removed the PCC components from the posterior DMN subsystem and conducted a post-hoc analysis. Without the inclusion of the PCC in the posterior DMN subsystem, the relationship was reduced but remained comparable (R2 = 0.12, p = 0.093 excluding PCC compared to R2 = 0.15, p = 0.038 including PCC), indicating a broader role of posterior DMN regions in this relationship. Overall, this finding is consistent with at least two studies revealing the role of the PCC, specifically in metacognition (Kim & Cabeza, 2009; Chua et al., 2006), but the link between the anatomical constituents of posterior DMN and metacognition likely requires additional study.
Fronto-Parietal Control Network and Metacognition
At odds with the hypotheses, there was no relationship between the FPCN and metacognitive accuracy. In a post-hoc analysis to further understand the role of the FPCN and metacognition, associations between and within the FPCN and metacognition were examined in only the healthy control group. There was a significant relationship between internetwork connectivity between the FPCN and the anterior DMN and metacognition in the healthy control group (R2 = 0.24, p = 0.018). Significant relationships were not found for internework connectivity between the FPCN and the salience network (R2 = 0.13, p = 0.091) or within the FPCN (R2 = 0.04, p = 0.376).
We did observe a relationship between metacognition and internetwork connectivity between the FPCN and anterior DMN in the healthy control population, which may be attributable to more traditional “monitoring” of goal directed behavior. However, previous research has demonstrated a link between metacognitive ability and FPCN connectivity in a TBI population (Ham et al., 2014), interpreting the involvement of FPCN connectivity as associated with the ability to correct errors “on-line.” The method used by Ham and colleagues is different than the one utilized in our study, where we focused on case-control group comparison, rather than comparing those who succeeded at the metacognitive task and those who did not. Additionally, Ham and colleagues (2014) included regions of the dorsal anterior cingulate cortex and bilateral insulae within the FPCN, which are commonly thought of as part of the salience network. The present analysis defined the FPCN according to Power and colleagues (2011), which included areas of the precentral gyrus, lateral PFC, and superior parietal cortex, but excluded the insula. In the current analysis, the salience network did demonstrate a significant interaction between healthy control and TBI groups with respect to internetwork connectivity with the DMN and was associated with metacognitive ability. Therefore, the differences in findings between these studies warrant further investigation into the role of the FPCN, and in particular involvement of the insular cortex within this network on metacognitive accuracy.
DMN Connectivity and Broader Cognitive Capacity
The DMN maintains multi-modal structures that support a range of human behaviors, so we do not interpret the current findings to mean that the DMN is solely dedicated to metacognition. However, to determine if network connectivity defined in this study holds specific relationships with metacognition or if observed network plasticity is more generally predictive of broad cognitive performance, we conducted post-hoc analyses (i.e., between anterior DMN and salience network, as well as between posterior DMN and salience network) using neuropsychological outcome measures of visual processing speed (VSAT) and auditory attention (Digit Span - forward). Results revealed no significant correlations between measures of processing speed and anterior DMN to salience connectivity (p = 0.163) or posterior DMN to salience connectivity (p = 0.137). Similarly, there were no significant associations between an auditory attention measure and anterior DMN to salience connectivity (p = 0.729) or posterior DMN to salience connectivity (p = 0.326). This demonstrates that the association between network connectivity and metacognition is not simply due to an association between anterior and posterior DMN networks and general cognitive performance. Moreover, the “residual” network, which was comprised of sensory-motor, auditory, and visual areas to serve as a control, was not associated with metacognitive accuracy. Together these data indicate that alterations in specific networks (as opposed to whole-brain alterations) contribute to metacognitive capacity following TBI. Finally, the TBI and healthy control groups performed comparably on a measure of executive functioning (Trails B; p = 0.231), indicating that the findings attributed to metacognition are unlikely to be explained by differences in higher-order cognitive functions.
As a final caveat, our task manipulation for obtaining a value of metacognitive accuracy removes difficulty anchors, creating a task that is more sensitive to metacognitive deficits (Chiou et al., 2012; Grossner et al., 2018). We did not see this same association between network connectivity and metacognition using the “ordered” version of the matrix reasoning task, where the design of the task is retained and item difficulty increases incrementally over the course of the task (ps > 0.342).
Limitations and Future Directions
While this study represents one of the few efforts to examine metacognitive accuracy and functional brain connectivity in moderate and severe TBI, there are several limitations that can be addressed in future work. First, the sample size was relatively small and heterogeneous. While not unlike the broader functional imaging literature examining moderate and severe TBI, our sample included individuals who had various amounts of time following their injury before participating in this study, ranging from 2 months to 22 years. Future work might gain nuanced perspective regarding the role of the age and time-post-injury on DMN involvement in metacognition by recruiting larger samples that vary along these demographic/clinical dimensions. Additional differences in TBI participants include differences in type, intensity, and duration of rehabilitation following injury. Despite these differences, this sample is representative of the typical moderate and severe TBI population and we anticipate that the current findings generalize well to this clinical population. Lastly, our sample included participants who were scanned on two different scanners, which were identical, but housed in separate locations. Analyses were conducted with scanner as a covariate to ensure that the results remained consistent when controlling for scanner type. Including multiple scanners into a study has the potential to interfere with data quality, but we have demonstrated that this effect is unlikely to account for the current findings.
Future studies may want to more closely examine areas of the DMN and FPCN to determine precisely what brain regions contribute to metacognition and how these networks may work together or separately to aid in metacognitive ability. Because of the overlap between the salience network, regions of the DMN, and the FPCN as defined in this study, it remains a challenge to definitively parcellate these networks. Future studies may examine these networks independently in order to preserve specificity of networks. Overall, network neuroscience offers unique opportunities to examine large-scale network plasticity and how network alterations after injury implement complex cognitive processes such as metacognition.
Supplementary Material
Public Significance Statement:
TBI is a public health concern, with millions of people sustaining head injuries every year. This study examines the metacognitive deficit in individuals who have a TBI, as well as provides insight into brain networks that are impacted by injury and contribute to this ability.
Acknowledgements
This research was supported by the National Center for Advancing Translational Sciences, NIHUL Tr000127 through the Clinical Translational Science Institute, the Social Life and Engineering Sciences Imaging Center, and the Social Sciences Research Institute at the Pennsylvania State University, University Park, PA 16801, USA.
References
- Akturk AO & Sahin I (2011). Literature review on metacognition and its measurement. Procedía: Social and Behavioral Sciences, 15, 3731–3736. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2015). The default network and selfgenerated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, & Margulies DS (2013). Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. The Journal of Neuroscience, 33, 16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasiao AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15, [DOI] [PubMed] [Google Scholar]
- Bernier RA, Roy A, Venkatesan UM, Grossner EC, Brenner EK, & Hillary FG (2017). Dedifferentiation does not account for hyperconnectivity after traumatic brain injury. Frontiers in Neurology, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos NP, Leyva I, Buldú JM, Bajo R, Paúl N, Cuesta P, ... & Del-Pozo F(2011). Principles of recovery from traumatic brain injury: Reorganization of functional networks. Neuroimage, 55, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Verhelst H, Clemente A, & Wilson PH (2017). Mapping the functional connectome in traumatic brain injury: What can graph metrics tell us? Neurimage, 160, 113–123. [DOI] [PubMed] [Google Scholar]
- Chiou KS, Carlson RA, Arnett PA, Cosentino SA, & Hillary FG (2011). Metacognitive monitoring in moderate and severe traumatic brain injury. Journal of the International Neuropsychological Society, 17, 720–731. [DOI] [PubMed] [Google Scholar]
- Chiou KS & Hillary FG (2012). Benefits of order: The influence of item sequencing on metacognition in moderate and severe traumatic brain injury. Journal of the International Neuropsychological Society, 18, 379–383. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, & Sperling RA (2006). Understanding metamemory: Neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage, 29, 1150–1160. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2015. April;25(4):983–90. doi: 10.1093/cercor/bht289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Fleming SM, Garrett N, & Dolan RJ, (2013). Confidence in value-based choice. Nature Neuroscience, 16, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London B, 351, 1413–1420. [DOI] [PubMed] [Google Scholar]
- Flashman LA & McAllister TW (2002). Lack of awareness and its impact in traumatic brain injury. NeuroRehabilitation, 17, 285–296. [PubMed] [Google Scholar]
- Flavell JH (1979). Metacognition and cognitive monitoring: A new area of cognitive- developmental inquiry. American Psychologist, 34, 906–911. [Google Scholar]
- Fleming SM & Dolan RJ (2012). The neural basis of metacognitive ability. Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences, 367, 1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Huijgen J, & Dolan RJ (2012). Prefrontal contributions to metacognition in perceptual decision making. Journal of Neuroscience, 32, 6117–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM & Lau HC (2014). How to measure metacognition. Frontiers in Human Neuroscience, 8, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, & Rees G (2010). Relating introspective accuracy to individual differences in brain structure. Science, 329, 1541–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U & Frith CD (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 358, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL & Frith CD (2003). Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences, 7, 77–83. [DOI] [PubMed] [Google Scholar]
- Grossner EC, Bernier RA, Brenner EK, Chiou KS, Hillary FG (2018). Prefrontal gray matter volume predicts metacognitive accuracy following traumatic brain injury. Neuropsychology, 32, 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, & Raichle ME (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. PNAS, 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham TE, Bonnelle V, Hellyer P, Jilka S, Robertson IH, Leech R, & Sharp DJ (2014). The neural basis of impaired self-awareness after traumatic brain injury. Brain, 137, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, & O’Doherty JP (2008). Neural correlates of mentalizing-related computations during strategic interactions in humans. Proceedings of the National Academy of Sciences, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M & Hillary FG (2018). Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small world. Network Neuroscience, 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG & Grafman JH (2017). Injured brains and adaptive networks: The benefits and costs of hyperconnectivity. Trends in Cognitive Science, 21, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Moelter ST, Schatz P, & Chute DL (2001). Seatbelts contribute to location of lesion in moderate to severe closed head trauma. Archives of Clinical Neuropsychology, 16, 171–181. [PubMed] [Google Scholar]
- Hillary FG, Rajtmajer SM, Roman CA, Medaglia JD, Slocomb-Dluzen JE, Calhoun VD, ... & Wylie GR (2014). The rich get richer: Brain injury elicits hyperconnectivity in core subnetworks. PloS One, 9, e104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Roman CA, Venkatesan U, Rajtmajer SM, Bajo R, & Castellanos ND(2015). Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology, 29, 59. [DOI] [PubMed] [Google Scholar]
- O’Connell RD, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, & Foxe JJ (2007). The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. European Journal of Neuroscience, 25, 2571–2579. [DOI] [PubMed] [Google Scholar]
- O’Keeffe F, Dockree P, Moloney P, Carton S, & Robertson IH (2007). Awareness of deficits in traumatic brain injury: A multidimensional approach to assessing metacognitive knowledge and online-awareness. Journal of the International Neuropsychological Society, 13, 38–49. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, & Vendrell P (2013). Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurology, 70, 845–851. [DOI] [PubMed] [Google Scholar]
- Kennedy MRT (2001). Retrospective confidence judgments made by adults with traumatic brain injury: Relative and absolute accuracy. Brain Injury, 15, 469–487. [DOI] [PubMed] [Google Scholar]
- Kim H & Cabeza R (2009). Common and specific brain regions in high-versus low-confidence recognition memory. Brain Research, 1282, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, & Macrae CN (2007). Wandering minds: The default mode network and stimulus-independent thought. Science, 315, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2015). Salience network. Brain Mapping: An Encyclopedia Reference, 2, 597–611. [Google Scholar]
- McCurdy LY, Maniscalco B, Metcalfe J, Liu KY, de Lange FP, & Lau H (2013). Anatomical coupling between distinct metacognitive systems for memory and visual perception. Journal of Neuroscience, 33, 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Bim RM, Handwerker DA, Jones TB, & Bandettini PA (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduce? Neuroimage, 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, & Goodman D (2007). Performance monitoring in the anterior cingulate is not all error related: Expectancy deviation and the representation of action-outcome associations. Journal of Cognitive Neuroscience, 19, 1994–2004. [DOI] [PubMed] [Google Scholar]
- Ownsworth T & Fleming J (2005). The relative importance of metacognitive skills, emotional status, and executive function in psychosocial adjustment following acquired brain injury. Journal of Head Trauma Rehabilitation, 20, 315–332. [DOI] [PubMed] [Google Scholar]
- Paul EJ, Smith JD, Valentin VV, Turner BO, Barbey AK, & Ashby FG (2015). Neural networks underlying the metacognitive uncertainty response. Cortex, 71, 306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, ... & Petersen SE (2011). Functional network organization of the human brain. Neuron, 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States, 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Bernier RA, Wang J, Benson M, French JJ Jr., Good DC, & Hillary FG (2017). The evolution of cost-efficiency in neural networks during recovery from traumatic brain injury. PLoS ONE, 12: e0170541. 10.137/journal.pone.0170541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Campbell C, Bernier RA, & Hillary FG (2016). An evolutionary computation approach to examine functional brain plasticity. Frontiers in Neuroscience, 10, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, & Cox RW (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Rowley HA, Kawahara TN, & Johnson SC (2006). Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia, 44, 762–773. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, ... & Leech R (2011). Default mode network functional and structural connectivity after traumatic brain injury. Brain, 134, 2233–2247. [DOI] [PubMed] [Google Scholar]
- Sinanaj I, Cojan Y, & Vuilleumier P (2015). Inter-individual variability in metacognitive ability for visoumotor performance and underlying brain structures. Consciousness and Cognition, 36, 327–337. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Science USA, 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT & Gow CA (1992). “Frontal dysfunction” after traumatic brain injury. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 5, 272–282. [Google Scholar]
- Tayim FM, Flashman LA, Wright MJ, Roth RM, & McAllister TW (2016). Recovery of episodic memory subprocesses in mild and complicated mild traumatic brain injury at 1 and 12 months post injury. Journal of Clinical and Experimental Neuropsychology, 38, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, & Ridderinkhof KR (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function, 214, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, Bernhardt BC, Bockler A, Kanske P, & Singer T (2016). Substrates of metacognition on perception and metacognition on higher-order cognition relate to different subsystems of the mentalizing network. Human Brain Mapping, 37, 3388–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A & Cartwright-Hatton S (2004). A short form of the Metacognitions Questionnaire: Properties of the MCQ-30. Behavioural Research and Therapy, 42, 385–396. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, & He Y (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 8: e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP & Gazzaley A (2013). Fronto-parietal network: Flexible hub of cognitive control. Trends in Cognitive Sciences, 17, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


