Abstract
Objectives:
Using optic coherence tomography (OCT), to determine relationships with visual function (VF) and disability in patients with multiple sclerosis (MS) and a) ganglion cell-inner plexiform layer (GCIPL) b) peripapillary retinal nerve fiber layer (pRNFL); and c) Bruch’s membrane opening minimum rim width (BMO-MRW) thicknesses.
Methods:
Sixty-eight MS patients and 22 healthy controls (HCs) underwent spectral-domain OCT, and 100%-contrast visual acuity (VA), 2.5%-, and 1.25%-contrast letter-acuity (LA), and Expanded Disability Status Scale (EDSS) testing. Mixed-effects linear regression models, accounting for within-subject, inter-eye correlations, were used to assess relationships.
Results:
The MS cohort exhibited significantly lower BMO-MRW (p=0.01), pRNFL at 3.7 mm, 4.1 mm, and 4.7 mm diameters surrounding the optic disc (p<0.001 for all), and GCIPL (p<0.001) thicknesses than HCs. BMO-MRW thickness was associated with 100%-VA (p<0.001, R2=0.08), 2.5%-LA (p<0.001; R2=0.13), and 1.25%-LA (p=0.002; R2=0.11). All measured pRNFL thicknesses were associated with high- and low-contrast VF (all: p<0.001). GCIP thickness was more strongly associated with 100%-VA (p<0.001; R2=0.23), 2.5%-LA (p<0.001; R2=0.27), and 1.25%-LA (p<0.001; R2=0.21) than the other OCT measures assessed. All OCT measures were significantly, but weakly associated, with EDSS scores.
Conclusions:
BMO-MRW and pRNFL thicknesses are reduced and associated with VF and disability in MS, but GCIPL thickness is a stronger marker of visual impairment. Our findings corroborate the utility of OCT in providing valuable information regarding the MS disease process.
Keywords: optical coherence tomography, Bruch’s membrane minimum rim width, multiple sclerosis, visual function
Magnetic resonance imaging (MRI) has been the gold-standard technique for detecting inflammatory activity, as well as axonal and neuronal degeneration in multiple sclerosis (MS). While conventional MRI markers of inflammation, including the formation of new or enlarging T2 and contrast-enhancing lesions, are used to monitor MS, such parameters only modestly correlate with disability progression (1). Non-conventional MRI measures of neurodegeneration such as brain sub-structure volumetric derived gray matter volumes, for example, correlate better with disability in MS, but have been difficult to implement into routine clinical practice due to cost and technical challenges. Thus, imaging techniques that allow reliable and translatable quantification of neurodegeneration remain an unmet need.
Optical coherence tomography (OCT) is an inexpensive, high-resolution, non-invasive imaging technique that uses near-infrared light to reliably quantify retinal neurodegeneration (2). There is an abundance of evidence suggesting the utility of OCT in complementing MRI to monitor MS. Specifically, OCT-derived peri-papillary retinal nerve fiber layer (pRNFL) and ganglion cell + inner plexiform layer (GCIPL) thicknesses correlate with global disability and visual function in MS. GCIPL thickness may have superior structure-function relationships than pRNFL thickness, potentially related to better reproducibility and reliability (3). Moreover, rates of GCIPL atrophy correlate with rates of whole-brain and gray matter atrophy in MS and are accelerated in patients exhibiting inflammatory disease activity and/or disability progression (4).
Recent advances in OCT techniques have expanded the range of OCT measures that can be measured, potentially allowing more comprehensive retinal characterization. Novel software on Spectralis (Heidelberg Engineering, Heidelberg, Germany) spectral domain (SD) OCT now allows determination of Bruch’s membrane opening minimum rim width (BMO-MRW) thickness, which represents the minimum width measured from Bruch’s membrane opening to the termination of the neuroretinal rim (Fig 1). BMO-MRW has been suggested as an anatomical surrogate for the neuroretinal rim (5). BMO-MRW is reduced in glaucoma patients compared to healthy controls (6) and is thought to be more sensitive for the assessment of glaucomatous optic neuropathy (7) as well as being associated with lower visual function (8). While retinal ganglion cells represent a principal site of neurodegeneration in glaucoma as in MS (9), glaucoma likely has a different underlying pathophysiological mechanism than MS related optic neuropathy, including neuroretinal rim degeneration and progressive optic disc cupping (10). New Spectralis OCT software allows more comprehensive pRNFL thickness analysis at 4.1 mm and 4.7 mm diameters surrounding the optic disc, in addition to the traditionally utilized 3.5 mm diameter measurement. Whether these novel measures contribute additional utility beyond GCIP thickness in MS remains to be examined.
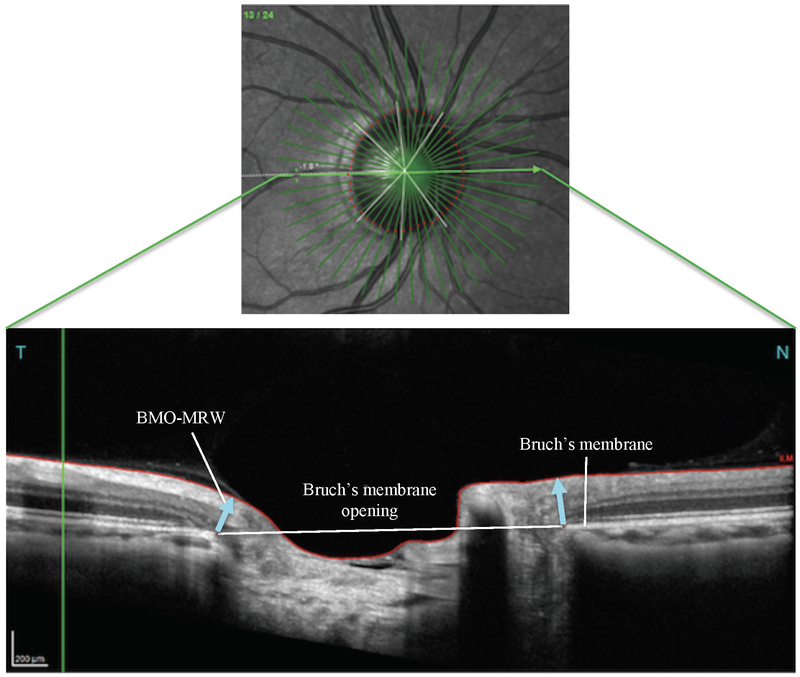
Fig. 1.
Panel A depicts a Spectralis optical coherence tomography (OCT) derived fundus image showing the optic disc with corresponding retinal microvasculature, as well as 24 radial slices of the peri-papillary region and 3 circular scans at 3.5 mm, 4.1 mm, and 4.7 mm diameters surrounding the optic disc (Panel A). The corresponding OCT image (Panel B) shows the Bruch’s membrane opening – minimum rim width (BMO-MRW) flanking the optic cup (blue arrows). Scans were acquired from a healthy participant
In this cross-sectional study, we sought to determine the relationships between Spectralis OCT-derived BMO-MRW and pRNFL thicknesses at varying diameters with visual function and global disability measures in MS, and compare these with GCIPL thickness structure-function correlations. We hypothesized that BMO-MRW, as well as pRNFL thicknesses at varying diameters, would be abnormal in MS, but might not exhibit benefit over the robustness of GCIPL thickness measurements in MS.
PATIENTS AND METHODS
Patients
MS patients and healthy controls (HCs) were recruited by convenience sampling from the Johns Hopkins MS Center, and written informed consent was obtained from all participants. All study protocols were approved by the Johns Hopkins University Institutional Review Board, and the study was performed in accordance with the tenets of the Declaration of Helsinki. MS diagnoses were based on the 2010 revised McDonald criteria (11). Patients were categorized according to the Lublin classification as having either relapsing remitting (RRMS), secondary progressive (SPMS), or primary progressive MS (PPMS) (12). Since the numbers of SPMS and PPMS patients recruited were relatively small, they were combined into a single group designated progressive MS (PMS). Participants with glaucoma, diabetes, uveitis, eye trauma or surgery, hypertension, refractive errors of greater than or equal to ±6 diopters, a history of optic neuritis (ON) within 6 months of OCT acquisition, or other ophthalmological or neurological disorders were excluded. Disease duration, age, ethnicity, sex, and history, date, and side of prior ON were recorded.
Optical Coherence Tomography
Spectralis SD-OCT, with automated macular segmentation (model OCT2; software version 6.4.7.0; Heidelberg Engineering, Heidelberg, Germany), as well as confocal scanning laser ophthalmoscopy that produces simultaneous high-resolution fundus images, was used to perform retinal imaging by experienced technicians. For each participant’s eye, an anatomic positioning system (APS) constructed a map of the retina using the center of the fovea and the center of Bruch’s membrane opening as anatomic landmarks (7). Thereafter, all scans were oriented based on this anatomic map, allowing more precise and accurate characterization of retinal structures (13). BMO-MRW and pRNFL measurements were obtained using the Optic Nerve Head - Radial, Circle scan protocol, consisting of 24 radial, 15-degree B-scans centered on BMO, with a radial automated real time (ART) of 25, as well as three circle scans at 3.5 mm, 4.1 mm, and 4.7 mm diameters surrounding the optic disc and circle ART of 100, as described elsewhere (14). GCIPL measures were derived from the Posterior Pole Horizontal scan protocol with an ART of 9, as described elsewhere (14). Mean measurements for all OCT parameters were calculated by averaging temporal, temporal superior, nasal superior, nasal, nasal inferior, and temporal inferior sectors. Each scan included in the study exhibited signal strength greater than 20 dB, and were devoid of artifact, in accordance with the OSCAR-IB criteria (15), as well as microcystoid changes.
Visual and Neurological Testing
High-contrast (100%) visual acuity at 4m and low-contrast letter acuity (2.5% and 1.25%) at 2m were assessed using retro-illuminated Early Treatment Diabetic Retinopathy Study and Sloan letter charts (Precision Vision, Lasalle, IL), respectively. The number of correctly identified letters was recorded for each eye (with a maximum score of 70 letters per chart). Visual assessments were administered in a darkened room by trained technicians using standard testing protocols (16). Participants used their habitual distance corrective lenses. To assess neurological function, Expanded Disability Status Scale (EDSS) scores were determined by Neurostatus-certified examiners.
Statistical Analyses
Statistical analyses were performed using Stata (version 12; StataCorp LP, College Station, TX). Comparisons between MS subtypes, as well as between MS and HCs, in regard to proportions of eyes with ON history and differences in sex were performed using the chi-square (χ2) test. The Wilcoxon rank-sum test was used to assess differences in age and disease duration between sub-types. The Shapiro-Wilk test was used to assess normality of distributions. Associations were then determined using multi-level, mixed-effects linear regression analyses, accounting for within-subject inter-eye correlations, and adjusting for age, sex, disease duration, and ON history. The HC and MS cohorts were compared using mixed-effects linear regression restrictive maximum likelihood models, accounting for within-subject inter-eye correlations, and adjusting for age and sex. Snijders/Bosker Level 1 R2 values were derived from this statistical model. Statistical significance was defined as p ≤ 0.05. Since this study was hypothesis-driven, correction for multiple comparisons was not performed (17).
RESULTS
Demographics/clinical characteristics
Sixty-eight people with MS (48 RRMS, 91 eyes; 20 PMS, 38 eyes) and 22 HCs fulfilled eligibility criteria and participated in the study. Seven eyes (5 RRMS and 2 PMS eyes) were excluded from the study due to inadequate OCT quality. Mean age, disease duration, and EDSS were significantly higher in PMS as compared to RRMS (p<0.001 for all; Table 1). Similarly, the RRMS cohort exhibited a significantly greater proportion of eyes with (ON) history (45%) as compared to PMS (25%; p=0.03). No significant differences were found between the RRMS and PMS cohorts in regards to sex or race. As compared to HCs, MS patients were significantly older, more likely to be female, and had a lower proportion of participants with race classified as other (p<0.001 for all). The summary of demographics and patient characteristics is outlined in Table 1.
Table 1.
Summary of demographics and clinical characteristics of patients with multiple sclerosis vs. healthy controls
| Characteristic | Overall MS n=68 (eyes=129) |
RRMS n=48 (eyes=91) |
PMS n=20 (eyes=38) |
HC n=22 (eyes=44) |
p-value RRMS vs. PMS |
p-value HC vs. MS |
|---|---|---|---|---|---|---|
| Mean age, years (SD) | 46.5 (12.5) | 41.6 (10.3) | 58.5 (8.7) | 33.4 (1.65) | <0.0011 | <0.0011 |
| Mean disease duration, years (SD) | 13.9 (10.9) | 11.2 (8.4) | 20.5 (13.4) | - | <0.0011 | - |
| Females (%) | 81 | 82 | 80 | 55 | 0.202 | <0.0012 |
| Race | ||||||
| Caucasian | 59 | 41 | 18 | 8 | ||
| African American | 8 | 6 | 2 | 3 | 0.893 | <0.0013 |
| Other | 1 | 1 | 0 | 11 | ||
| Patients with ON History (%) | 44 (65) | 37 (77) | 7 (35) | - | - | - |
| Eyes with ON History (%) | 54 (41) | 44 (47) | 10 (26) | - | 0.032 | - |
| Mean EDSS | 3.13 | 1.83 | 6.44 | - | <0.0011 | - |
Computed with Wilcoxon rank-sum test
Computed with chi-square test
Computed with Fisher exact test
RRMS = relapsing-remitting multiple sclerosis; PMS = progressive multiple sclerosis; HC = healthy controls; ON = optic neuritis; EDSS = expanded disability scale score
Bolded values represent statistically significant results
Differences in OCT measures between MS and HCs
Adjusting for age, sex and within-subject inter-eye correlations, BMO-MRW thickness was significantly lower in MS as compared to HCs (297μm vs 337μm; mean difference = 40μm; p=0.01) (Table 2). Similarly, pRNFL thickness at 3.5mm, 4.1mm, and 4.7mm surrounding the optic disc was significantly lower in MS patients relative to HCs, with mean differences between the cohorts of 17μm, 14μm and 11μm, respectively (p<0.001 for all). GCIPL thickness was significantly lower in patients with MS than HCs, with a mean difference of 14μm (p<0.001). In MS subtype analyses, BMO-MRW, pRNFL at 3.7mm, 4.1mm, and 4.7mm, as well as GCIPL thicknesses in RRMS and PMS were all similarly significantly lower than in HCs (data not shown).
Table 2.
Comparison of OCT-derived measures between patients with multiple sclerosis and healthy controls.
| HC (eyes = 44) |
MS (eyes = 131) |
HC vs. MS | HC vs. MS | |
|---|---|---|---|---|
| Mean, μm (SD) | Mean, μm (SD) | Mean difference, μm | p-value | |
| BMO-MRW | 337 (77) | 297 (66) | 40 | 0.01 |
| pRNFL – 3.5 mm | 105 (11) | 88 (17) | 17 | <0.001 |
| pRNFL – 4.1 mm | 90 (8) | 75 (13) | 14 | <0.001 |
| pRNFL – 4.7 mm | 78 (7) | 67 (11) | 11 | <0.001 |
| GCIPL | 80 (4) | 66 (11) | 14 | <0.001 |
MS = multiple sclerosis cohort; HC = healthy controls; BMO-MRW = Bruch’s membrane opening minimum rim width; pRNFL = peri-papillary retinal nerve fiber layer; GCIPL = ganglion cell + inner plexiform layer; mm = millimeters; μm = micrometers
p-values were derived from mixed-effect linear regression restrictive maximum likelihood models, accounting for within-subject inter-eye correlation and adjusting for age and sex.
Bolded values designate statistical significance
Relationships between OCT-derived retinal measurements, visual function, and EDSS
Adjusting for age, sex, disease duration, history of ON and within-subject, inter-eye correlations, BMO-MRW, pRNFL at 3.5mm, 4.1mm and 4.7mm, as well as average GCIP thicknesses were all significantly associated with 100%-VA, 2.5%-LA, 1.25%-LA, and EDSS scores across the MS cohort (Table 3). For all relationships described below, lower OCT-derived thicknesses were associated with lower visual function and higher disability scores. In general, across the OCT measures assessed, R2 relationships were numerically highest with 2.5%-LA scores, which is in accordance with prior data suggesting that 2.5%-LA may be the more sensitive for capturing visual dysfunction in MS (Fig 2). In general, of the OCT measures analyzed, R2 relationships across visual function measures were numerically highest with average GCIPL thickness. GCIPL thickness was associated with 100%-VA (p<0.001; R2=0.23), 2.5%-LA (p<0.001; R2=0.27), and 1.25%-LA (p<0.001; R2=0.21). Although the associations of BMO-MRW, pRNFL at 3.5mm, 4.1mm and 4.7mm, and average GCIP thicknesses with EDSS were all significant, they were weak and similar across OCT measures. In MS subtype analyses, similar associations between BMO-MRW, pRNFL at 3.7mm, 4.1mm, and 4.7mm diameters, as well as GCIPL thicknesses with visual function and EDSS scores were observed in RRMS and PMS, as compared to the entire MS cohort (data not shown).
Table 3.
Relationships between OCT-derived retinal measurements, visual function, and global disability scores in patients with multiple sclerosis.
| Scores | Average BMO-MRW |
pRNFL - 3.5mm |
pRNFL - 4.1mm |
pRNFL - 4.7mm |
Average GCIPL |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | |
| 100%-VA | <0.001 | 0.08 | <0.001 | 0.19 | <0.001 | 0.17 | <0.001 | 0.13 | <0.001 | 0.23 |
| 2.5%-LA | <0.001 | 0.13 | <0.001 | 0.21 | <0.001 | 0.19 | <0.001 | 0.15 | <0.001 | 0.27 |
| 1.25%-LA | 0.002 | 0.11 | <0.001 | 0.19 | <0.001 | 0.17 | <0.001 | 0.15 | <0.001 | 0.21 |
| EDSS | 0.02 | 0.05 | 0.001 | 0.10 | 0.001 | 0.10 | 0.001 | 0.08 | 0.002 | 0.06 |
VA = visual acuity; LA = letter acuity; EDSS = Expanded Disability Scale Score; BMO-MRW = Bruch’s membrane opening minimum rim width; pRNFL = peri-papillary retinal nerve fiber layer; GCIPL = ganglion cell + inner plexiform layer
p-values were derived from mixed-effect linear regression models, adjusting for within-subject inter-eye correlation, and accounting for age, sex, disease duration, and history of optic neuritis.
Bolded values indicate statistically significant results
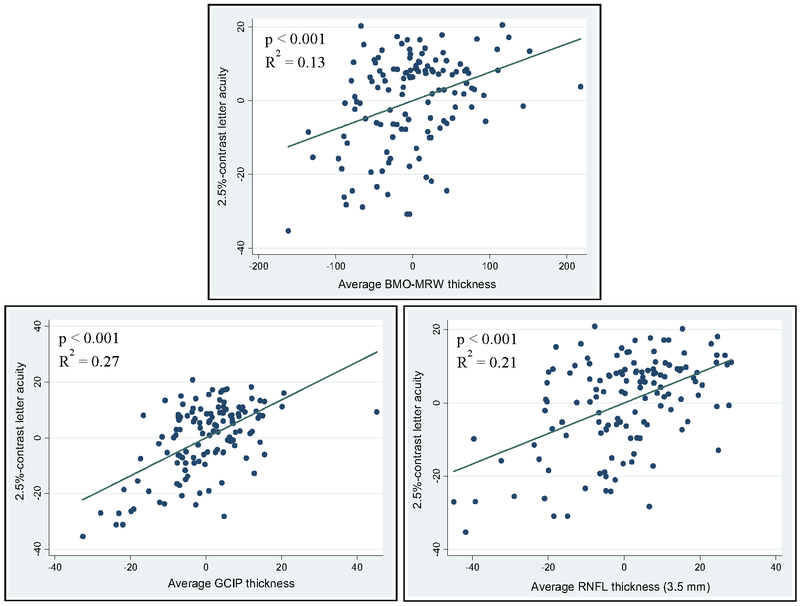
Fig. 2.
Adjusted variable plots comparing the strength of significant associations between OCT-derived average Bruch’s membrane opening minimum rim width (BMO-MRW; upper panel), average ganglion cell + inner plexiform layer (GCIP; lower left panel), and peri-papillary retinal nerve fiber layer (pRNFL; lower right panel) thicknesses and 2.5%-contrast letter acuity in MS, accounting for age, sex, disease duration, and history of optic neuritis. Consistent with a tighter distribution fit along the linear regression line, GCIP thickness exhibits the highest R2-value. p-values and R2-values were derived from mixed-effect linear regression models, adjusting for within-subject inter-eye correlation, and accounting for age, sex, disease duration, and history of optic neuritis
Discussion
The results of our study suggest associations between OCT-derived retinal thickness measures, and disability in MS, illustrating new, as well as confirming previously studied parameters by which OCT may be of value to track disease progression and disability in MS. Since the novel measures studied (BMO-MRW and pRNFL thicknesses at varying diameters around the optic disc) are related to conventional OCT measures such as standard pRNFL at 3.5mm and GCIPL thicknesses, it is not surprising that these new measures similarly correlate with visual function and disability scores in MS. However, the proposed benefit of BMO-MRW thickness in glaucoma over conventional OCT measures may not similarly be the case in MS. GCIPL thickness previously has been shown to be one of the most robust OCT measures in MS, with superior-structure function relationships, and potentially overcoming many of the known limitations of conventional pRNFL thickness measures. For example, GCIPL may not swell during ON and may also be less prone to astrogliosis, which primarily occurs in the RNFL and may lead to false increases in RNFL thickness (18). In our study, lower GCIPL thickness appeared to explain the greatest degree of variance in reduced 100%-VA, 2.5%-LA and 1.25%-LA scores across the MS cohort as compared to BMO-MRW and pRNFL thicknesses at various diameters. Conversely, BMO-MRW thickness may explain a smaller amount of variance in visual function among patients with MS. This may simply relate to the anatomy of this measurement. The BMO-MRW includes all retinal layers, including the pRNFL and GCIPL. Thus, decreased BMO-MRW thickness and its associations with visual function found in this study may be driven by retrograde neurodegeneration of the pRNFL and GCIPL layers, yet may at the same time, be partially diluted by the inclusion of the other retinal layers intrinsic to the BMO-MRW measurement. Among the pRNFL thickness measures assessed, relationships with visual function appeared to be strongest for pRNFL measurements closer to the optic disc, measured at 3.5 mm, the conventional pRNFL thickness measurement on the Spectralis OCT device. Interestingly, all of the OCT measures examined in the current study similarly and only weakly explained variance in EDSS scores in MS participants.
To the best of our knowledge, this is one of the first demonstrations that BMO-MRW thickness is significantly lower in MS compared to HCs and is associated with high- and low-contrast visual function in MS. Analogous to studies in patients with glaucoma, BMO-MRW thickness may be a surrogate measure for the number of axons entering the optic nerve head. However, based on our findings, BMO-MRW thickness in MS patients may be less robustly associated with VF compared to GCIPL thickness. This is likely due to retrograde degeneration of the constituent fibers of the optic nerve and a subsequent dropout of retinal ganglion cell axons (RNFL) and neurons (GCIPL) (19-22).
Across all cohorts, low-contrast LA (LCLA), and in particular 2.5% LA, generally exhibited a stronger relationship with all retinal measures examined as compared to 100% high-contrast VA (HCVA). This is consistent with previous findings, which have shown that relative to HCVA, LCLA is more closely associated with OCT-derived measures, (23) is a more sensitive measure of visual disability (24) and is more broadly associated with MS disability, as estimated by EDSS scores (25), as well as neuropsychological function (27). Our study findings, in conjunction with previous reports, support the concept that OCT and visual function, particularly LCLA, may be complementary tools in MS.
Our study has several limitations. Firstly, the study cohort was heterogeneous, and there were insufficient numbers of SPMS or PPMS patients to examine these cohorts separately. The number of HCs included in the study was relatively low, and the HC cohort was generally younger than those in the MS cohort, and more biased towards being male. There is a possibility that age-related pRNFL reduction in the MS cohort may have affected our results; however, all our analyses were adjusted for age and sex. Lack of obtaining best corrected visual acuity with refraction is another limitation (27). Since our study was cross-sectional and exploratory in nature, our results regarding BMO-MRW thickness being decreased and associated with disability in MS are preliminary. It is likely that BMO-MRW may secondarily decrease as a result of RNFL degeneration as it does in glaucoma. In order to elucidate the progression of anatomical changes in MS, future studies should focus on increasing the study sample size in a longitudinal setting across MS subtypes.
In summary, our findings suggest strong associations between a broad panel of OCT-derived measures and functional disability, particularly of VF in patients with MS. While novel OCT-derived parameters, BMO-MRW and pRNFL thicknesses at varying diameters surrounding the optic disc, seem to be reduced in and broadly associated with visual and global disability in MS, GCIPL thickness, in accordance with prior studies, appears to be the most accurate OCT-derived structural marker in MS. Our study findings provide further support for OCT measures: a) as outcome metrics in clinical trials of neuroprotective and/or remyelinating therapies, b) for monitoring disease progression, and C) for contributing clinically useful information regarding global aspects of the MS disease process.
Acknowledgments
FUNDING
This study was funded by Race to Erase MS [grant number not applicable (to S.S.)]; National Institute of Health [grant number 5R01NS082347-02 (to P.A.C.)]; National Multiple Sclerosis Society [grant number RG-1606-08768 (to SS)]; and Walters Foundation [grant number not applicable (to E.M.F.)].
Footnotes
DECLARATION OF CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Kappos L, Moeri D, Radue EW, Schoetzau A, Schweikert K, Barkhof F, Miller D, Guttmann CR, Weiner HL, Gasperini C, Filippi M. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999; 353:964–969. [DOI] [PubMed] [Google Scholar]
- 2.Syc SB, Warner CV, Hiremath GS, Farrell SK, Ratchford JN, Conger A, Frohman T, Cutter G, Balcer LJ, Frohman EM, Calabresi PA. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler 2010; 16:829–839. [DOI] [PubMed] [Google Scholar]
- 3.Saidha S, Syc BS, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN, Frohman EM, Calabresi PA. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler 2011; 17:1449–1463. [DOI] [PubMed] [Google Scholar]
- 4.Saidha, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, Prince JL, Pham D, Roy S, van Zijl P, Balcer LJ, Frohman EM, Reich DS, Crainiceanu C, Calabresi PA. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 2016; 78:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis AS, O’Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, Chauhan BC. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci 2012; 53:1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K, Lee J, Nouri-Mahdavi K, Caprioli J. Bruch’s membrane opening-minimum rim width and visual field loss in glaucoma: a broken stick analysis. Int J Ophthalmol 2018; 11:828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan BC, O’Leary N, Almobarak FA. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthal. 2013; 120:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollet-Villard F, Chiquet C, Romanet JP, Noel C, Aptel F. Structure-function relationships with spectral-domain optical coherence tomography retinal nerve fiber layer and optic nerve head measurements. Invest Ophthal Vis Sci 2014; 55:2953–2962. [DOI] [PubMed] [Google Scholar]
- 9.HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci 1995; 36:774–786. [PubMed] [Google Scholar]
- 10.Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol 2009; 57:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Motalban X, O’Connor P, Sandberg-Wellheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol 2011; 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol 2014; 72:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Park K, Kim J, Lee J. Reproducibility of Bruch Membrane Opening-Minimum Rim Width Measurements with spectral domain optical coherence tomography. J Glaucoma 2017; 26:1041–1050. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez NC, Antony B, He Y, Lang A, Nguyen J, Rothman A, Ogbuokiri E, Avornu A, Balcer LJ, Frohman E, Frohman TC, Bhargava P, Prince J, Calabresi PA, Saidha S. Analysis of agreement of retinal-layer thickness measures derived from the segmentation of horizontal and vertical spectralis OCT macular scans. Curr Eye Res 2017; 43:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talman LS, Bisker ER, Sackel DJ, Long DA Jr, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying GS, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ. Longitudinal study of vision and retinal nerve fiber layer thickness in MS. Ann Neurol 2010; 67:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender R and Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol 2001; 54:343–349. [DOI] [PubMed] [Google Scholar]
- 18.Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN, Frohman EM, Calabresi PA. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler 2011; 17:1449–63. [DOI] [PubMed] [Google Scholar]
- 19.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338:278–285. [DOI] [PubMed] [Google Scholar]
- 20.Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res 2008; 87:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010; 133:1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994; 14:445–451. [DOI] [PubMed] [Google Scholar]
- 23.Balcer LJ, Baier ML, Cohen JA. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003; 61:1367–1373. [DOI] [PubMed] [Google Scholar]
- 24.Ratchford J, Saidha S, Sotirchos E, Oh J, Seigo M, Eckstein C, Durbin MK, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome SD, Balcer LJ, Frohman EM, Calabresi PA. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013; 80:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balcer LJ, Baier ML, Cohen JA, Kooijmans MF, Sandrock A, Nano-Schiavi, Pfohl DC, Mills M, Bowen J, Ford C, Heidenreich FR, Jacobs DA, Markowitz CE, Stuart WH, Ying GS, Galetta SL, Maguire MG, Cutter GR. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003; 61:1367–1373. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen J, Rothman A, Fitzgerald K, Whetstone A, Syc-Mazurek S, Aquino J, Balcer LJ, Frohman EM, Frohman TC, Crainiceanu C, Beier M, Newsome SD, Calabresi PA, Saidha S. Visual pathway measures are associated with neuropsychological function in multiple sclerosis. Curr Eye Res 2018; 43:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pointer JS. Habitual vs optimal distance visual acuity. Ophthalmic Physiol Opt 2008; 28:457–466. [DOI] [PubMed] [Google Scholar]