SUMMARY
Lightly stroking the lips or gently poking some skin regions can evoke mechanical itch in healthy human subjects. Sensitization of mechanical itch and persistent spontaneous itch are intractable symptoms in chronic itch patients. However, the underlying neural circuits are not well defined. We identified a subpopulation of excitatory interneurons expressing Urocortin 3::Cre (Ucn3+) in the dorsal spinal cord as a central node in the pathway that transmits acute mechanical itch and mechanical itch sensitization as well as persistent spontaneous itch under chronic itch conditions. This population receives peripheral inputs from Toll-like receptor 5-positive (TLR5+) Aβ low-threshold mechanoreceptors, and is directly innervated by inhibitory interneurons expressing neuropeptide Y::Cre (NPY+) in the dorsal spinal cord. Reduced synaptic inhibition and increased intrinsic excitability of Ucn3+ neurons lead to chronic itch sensitization. Our study sheds new light on the neural basis of chronic itch and unveils novel avenues for developing mechanism-specific therapeutic advancements.
Graphical Abstract
eTOC Blurb
Pan et al. identify a microcircuit in the dorsal spinal cord that transmit mechanically-evoked itch. Sensitization of this pathway is required for chronic itch development.
INTRODUCTION
Itch is a distinct sensory modality within the somatosensory system. Itch can be generated acutely, but it is also produced chronically in many dermatological or systemic diseases. Under physiological conditions, acute itch has evolved as a threat detection system to remove potentially harmful stimuli, such as parasites coming in contact with the skin (mechanical itch) or mosquitoes bites triggering pruritogen release in the skin (chemical itch). Over the past decade, intensive studies have revealed the neural circuits that transmit chemical itch (Bautista et al., 2014; Dong and Dong, 2018). Distinct populations of primary C-pruriceptors expressing MAS-related GPR A3 (MrgprA3), MrgprD and natriuretic peptide B (Nppb), sense a variety of pruritogens and transmit chemical itch signals to the dorsal spinal cord (Han et al., 2013; Liu et al., 2012; Liu et al., 2009; Mishra and Hoon, 2013). Excitatory interneurons (INs) expressing natriuretic peptide receptor A (Npra) or gastrin-releasing peptide receptor (GRPR) in the superficial dorsal spinal cord are essential to process chemical itch information. Inhibitory INs in the dorsal spinal cord expressing the transcription factor Bhlhb5 and the neuropeptide dynorphin are reported to modulate chemical itch (Huang et al., 2018; Kardon et al., 2014; Ross et al., 2010).
Mechanical itch was first described in healthy human subjects by Edward Titchener in 1909. As the skin was explored with a fine hair, well-defined itchy points were identified at low stimulation intensity (Tirtchener, 1909). Later psychophysiological studies have described "itchy skin" or acute alloknesis (mechanical itch sensitization) as an area of skin surrounding a histamine puncture (Bickford, 1938; Graham et al., 1951; Lamotte, 1988). Recent studies reporting mechanical itch assessment in human and mice have revealed that mechanical itch is distinct from chemical itch (Akiyama et al., 2012; Akiyama et al., 2015; Fukuoka et al., 2013). Mechanical itch is modulated by touch-dome Merkel cells in the skin (Feng et al., 2018) and inhibitory INs expressing neuropeptide Y::Cre (referred to as NPY+) in the dorsal spinal cord (Bourane et al., 2015). These findings provided compelling evidence to explain why tactile stimuli cannot elicit itch on most skin areas under physiological conditions. Strikingly, these inhibitory pathways do not modulate chemical itch (Bourane et al., 2015; Feng et al., 2018). Despite the progress, the precise identity of the neural circuit that transmits mechanical itch remains unknown.
Under pathological conditions, patients with chronic itch commonly have both peripheral and central sensitization of acute itch pathways, which enables a pruritogen to elicit a stronger itch response than normal (chemical itch sensitization), light touch to evoke an itch sensation on non-itchy skin areas (mechanical itch sensitization), and results in persistent spontaneous itch (Ikoma et al., 2006; LaMotte et al., 2014; Yosipovitch and Bernhard, 2013). It has been hypothesized that acute itch transmission neurons are sensitized to provide a stronger itching signal in chronic itch (Bautista et al., 2014). However, we know little about the cellular and molecular mechanisms underlying the transition from acute to chronic itch.
In this study, we have identified a subpopulation of excitatory INs expressing Urocortin 3::Cre (referred to as Ucn3+) in the dorsal spinal cord that acts as a central node in the mechanical itch-transmission circuit. Subsequent anatomical and functional assays showed that Ucn3+ INs receive inputs from Toll-like receptor 5-positive (TLR5+) Aβ low-threshold mechanoreceptors (LTMRs) and are directly gated by spinal NPY+ INs. Disinhibition and enhanced intrinsic excitability of spinal Ucn3+ INs are essential for the transition from acute to chronic itch.
RESULTS
Identification of mechanical itch-transmission neurons in the spinal cord
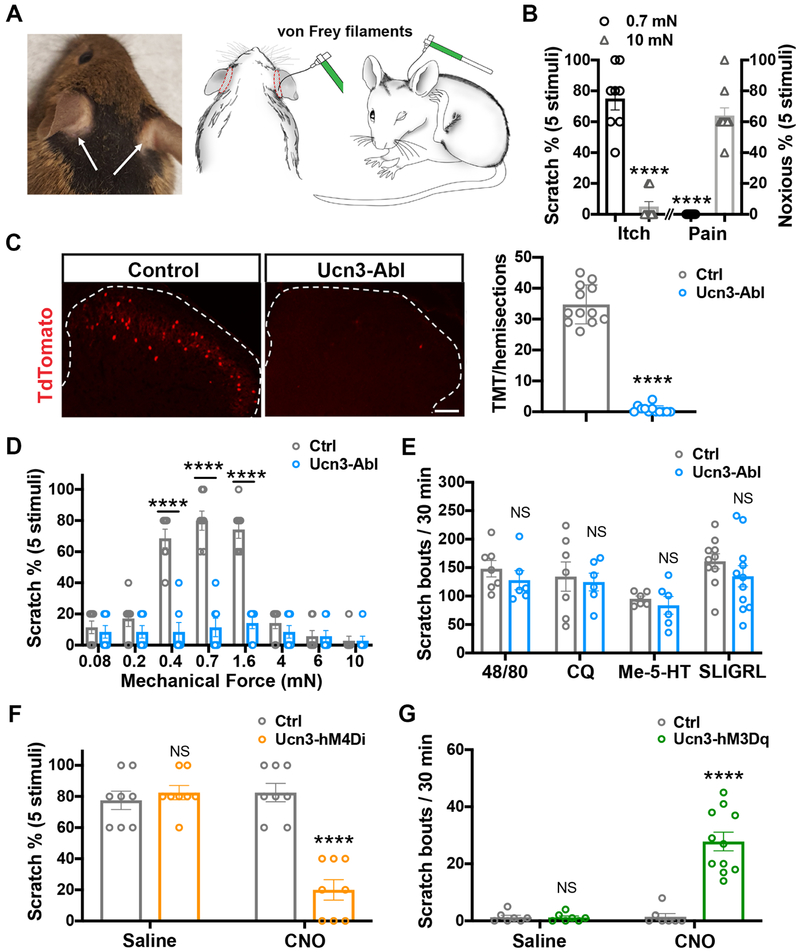
To measure acute mechanical itch in mice, we developed a new behavioral assay in which a von Frey monofilament was used to poke the shaved skin of mice at varying levels of mechanical force (Figure 1A). Light punctate stimuli (0.7 mN) applied to the shaved skin behind the ears in wild-type (WT) naïve mice evoked hindpaw scratching bouts directed to the stimulus site, while noxious mechanical stimuli (10 mN) did not evoke scratching responses (Figure 1B). Instead, noxious mechanical stimuli (10 mN) evoked forelimb wiping or head-shaking responses (Figure 1B). Light punctate stimuli applied to other skin areas, such as the back, nape, head, or cheek, rarely evoked hindpaw scratching responses. Interestingly, the observed acute mechanical itch response was independent of chemical itch pathways. Blockade of histamine receptors or ablating the key elements of chemical itch circuits in the spinal cord, including Npra+ and GRPR+ INs, did not affect acute mechanical itch (Figure S1A-S1C). Nav1.8-Cre is expressed in numerous functional classes of afferents, including C-LTMRs (Shields et al., 2012). Next, we used Nav1.8-Cre; Rosa26LSL-DTR mice to ablate Nav1.8+ neurons by expressing human diphtheria toxin (DTX) receptor (DTR) in these neurons. Tyrosine hydroxylase (TH), a marker of C-LTMRs (Li et al., 2011), was undetectable in Nav1.8-ablated mice validating the loss of C-LTMRs (Figure S1D). We found that acute mechanical itch remained intact after ablation of Nav1.8+ sensory neurons (Figure S1E), suggesting that C-LTMRs may not transmit mechanical itch.
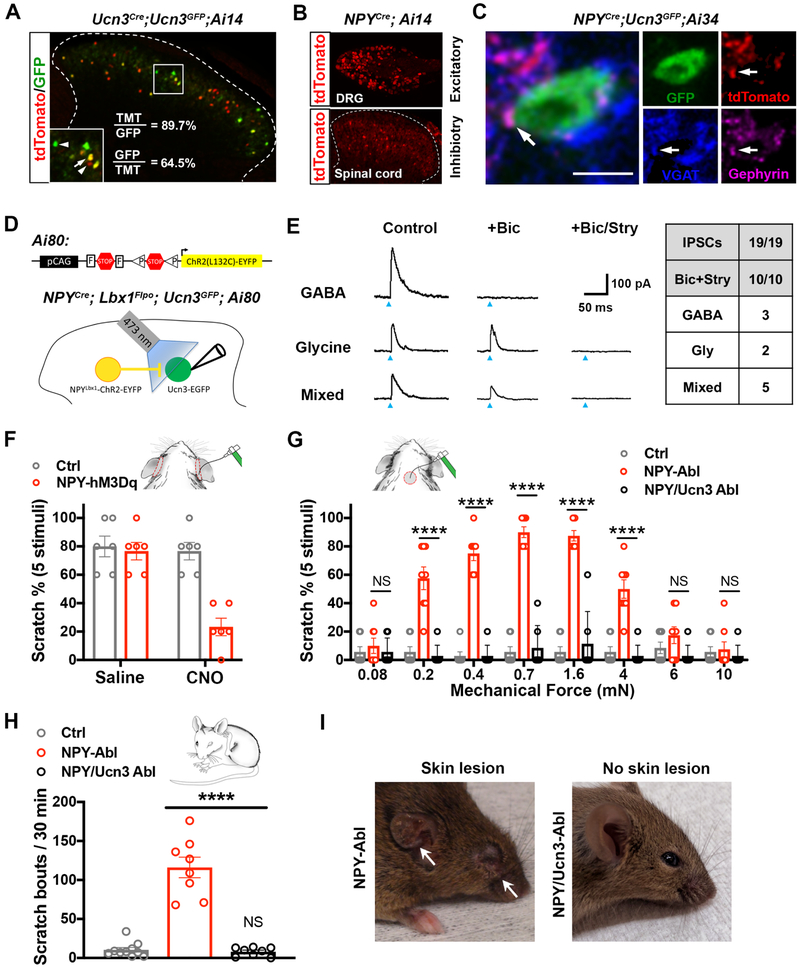
Figure 1. Spinal Ucn3+ neurons transmit acute mechanical itch.
(A) Image showing acute mechanical itch test in mice. White arrows indicate the areas on the shaved skin behind the ears that were poked with von Frey filaments.
(B) Measurement of acute mechanical itch in WT mice. Innocuous mechanical stimuli, 0.7 mN, n = 8; Noxious mechanical stimuli, 10 mN, n = 10; ****, p < 0.0001, Student’s unpaired t test. See also Figure S1A-S1E.
(C) Spinal ablation of Ucn3+ neurons in adult mice. Bar graph represents quantified data for tdTomato (TMT) signals in control and ablated animals. n = 12 sections from 3 mice in each group; p < 0.0001, Student’s unpaired t test. Scale bar: 100 μm. See also Figure S2.
(D) Acute mechanical itch in Ucn3+ neuron-ablated (Ucn3-Abl) and control (Ctrl) groups. n = 7 in each group; ****, p < 0.0001, Student’s unpaired t test. See also Table S1 and Figure S3.
(E) Scratching responses after the administration of compound 48/80 (Control: n = 7; Ucn3-Abl: n= 6), chloroquine (CQ. Control: n = 7; Ucn3-Abl: n= 6), Me-5-HT (n = 6 in each group) and SLIGRL (Control: n = 10; Ucn3-Abl: n= 11). NS: no significant difference, Student’s unpaired t test. See also Figure S1F.
(F) Acute mechanical itch test following saline or CNO injection in mice expressing hM4Di receptors in spinal Ucn3+ neurons (Ucn3-hM4Di) and control littermates (Ctrl). n = 8 in each group; ****, p < 0.0001, Student’s unpaired t test. See also Figure S4A-S4D.
(G) Spontaneous scratching responses following saline or CNO injection in mice expressing hM3Dq receptors in spinal Ucn3+ neurons (Ucn3-hMD3q) and control littermates (Ctrl). n = 11 in CNO-treated groups, n = 7 in saline-treated groups. ***, p < 0.0001, Student’s unpaired t. See also Figure S4E-S4G.
To identify the mechanical-itch transmission neurons in the spinal cord, we focused on neurons in the LTMR-innervated zone (laminae vIIiIV) and ablated nine individual subpopulations of spinal neurons using genetic or pharmacological strategies (Table S1). To genetically ablate the neurons in the LTMR-innervated zone of dorsal spinal cord, we expressed the human DTR in spinal neurons by crossing the Cre mouse line of interest with Lbx1Flpo mice and Tauds-DTR mice (Duan et al., 2014). Cre-dependent reporter Ai14 was used to evaluate ablation efficiency. Following ablation, we performed behavioral screening to identify spinal Ucn3+ neurons are mechanical itch-transmission neurons.
The neuropeptide Ucn3 is expressed in different brain regions, including amygdala, hypothalamus, and brainstem (Lewis et al., 2001). By crossing Ucn3::Cre with Ai14 (referred to as Ucn3tdTomato mice), we found that a small population of neurons were labeled by tdTomato in the dorsal spinal cord (Figure 1C). Ucn3 mRNA was not expressed in the dorsal spinal cord at detectable levels in adult (P60) Ucn3tdTomato mice (Figure S2A). In comparison, tdTomato fluorescence signals in the hypothalamus were highly overlapped with Ucn3 mRNA (Figure S2B). We selectively labeled Ucn3+ neurons in the dorsal spinal cord by crossing Ucn3::Cre; Lbx1Flpowith a Cre and Flpo double reporter line Ai65, which enabled the expression of tdTomato protein in spinal Ucn3+ neurons (referred to as Ucn3Lbx1). Immunohistochemistry staining of tdTomato protein showed that no axonal projections from Ucn3Lbx1 neurons were detected in the major brain targets of spinal projection neurons, including thalamus, parabrachial nucleus and periaqueductal gray (Figure S2C), suggesting that Ucn3Lbx1 neurons represent a subpopulation of spinal INs.
Using this intersectional genetic strategy, we ablated ~97.7 % (control: 34.8 ± 1.7; ablated: 0.8 ± 0.4) of Ucn3+ INs in the dorsal spinal cord (Figure 1C), without ablating Ucn3+ INs in the brain (Figure S2D). In our behavioral test, we found that mechanical itch responses to low force stimuli (0.4-1.6 mN) were nearly abolished in spinal Ucn3+ IN-ablated mice (Ucn3-Abl) compared to control littermates (Figure 1D). However, Ucn3-Abl mice showed normal locomotion coordination, touch, thermal sensation, acute and chronic pain (Figure S3). Importantly, pruritogen-induced chemical itch responses were unchanged in Ucn3-Abl mice (Figure 1E). Consistently, pruritic chemical compound 48/80 did not induce the expression of c-Fos protein in spinal Ucn3+ INs (Figure S1F).
To rule out potential secondary effects on spinal circuits by neuronal ablation, we used intersectional strategies to transiently silence or activate spinal Ucn3+ INs by crossing Ucn3::Cre;Lbx1Flpo and a Cre- and Flpo-dependent hM4Di designer receptors exclusively activated by a designer drug (DREADD) strain Rosa26CAG-ds-hM4Di (Ray et al., 2011) or hM3Dq DREADD strain Rosa26CAG-ds-hM3Dq(Sciolino et al., 2016), respectively (Figure S4A). Forty minutes after silencing spinal Ucn3+ INs by activating hM4Di with clozapine N-oxide (CNO), light punctate stimuli (0.7 mN)-evoked mechanical itch responses were greatly attenuated (Figure 1F). However, touch, pain and thermal sensation remained intact (Figure S4B-S4D). Strikingly, acute activation of spinal Ucn3+ INs by CNO triggered spontaneous scratching behaviors in Ucn3::Cre; Lbx1Flpo; Rosa26CAG-ds-hM3Dq mice (Figure 1G), whereas touch, pain and thermal sensation were unaffected (Figure S4E-S4G). Together, our findings demonstrate that Ucn3+ INs selectively transmit mechanical itch in the dorsal spinal cord.
Ucn3+ INs represent a subpopulation of excitatory INs in the dorsal spinal cord
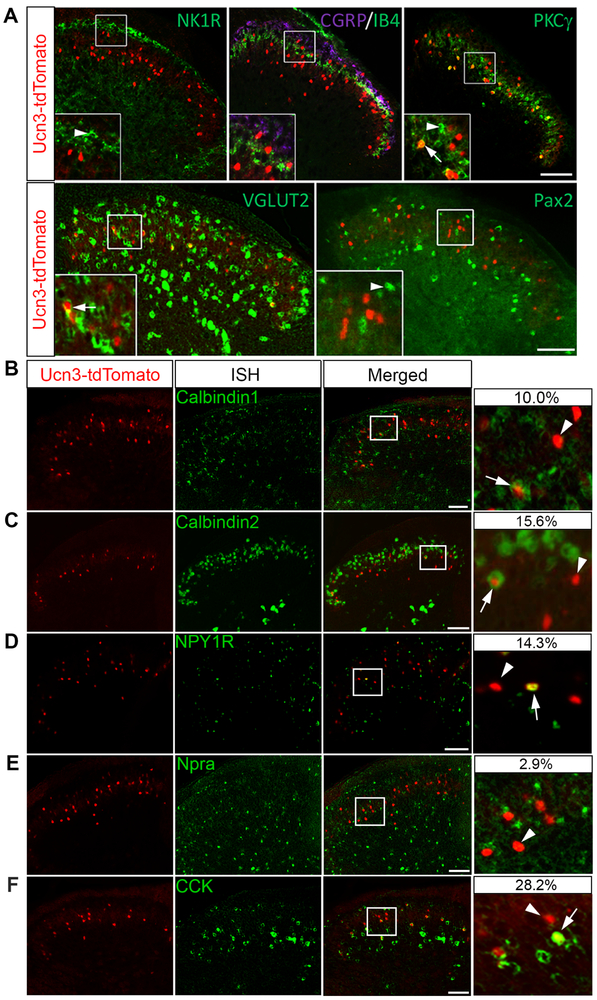
To characterize the cellular identity of spinal Ucn3+ INs, we performed immunohistochemical double staining with lamina-specific markers using adult Ucn3tdTomato mice. Ucn3-tdtomato+ INs were found ventral to projection neurons expressing Neurokinin 1 receptor (NK1R) in lamina I (Figure 2A). A minor portion of Ucn3-tdTomato+ INs (24.3%, 74/304) was intermingled with calcitonin gene-related peptide (CGRP)/isolectin B4 (IB4) terminals in lamina II outer layer (IIo) to the dorsal part of lamina II inner layer (dIIi) (Figure 2A). Most Ucn3-tdTomato+ INs (73.7%, 224/304) were located in the ventral portion of the inner lamina II (vIIi) to the dorsal lamina III (dIII). One fourth of Ucn3-tdTomato+ INs (25.1%, 104/415) overlapped with protein kinase C γ (PKCγ) (Figure 2A), the marker of a subset of spinal excitatory INs in laminae vIIi-dIII that receives Aβ-LTMR inputs (Neumann et al., 2008). According to neurotransmitter phenotypes, the vast majority of Ucn3-tdTomato+ INs were glutamatergic, with 95.9% (353/368) expressing the vesicular glutamate transporter 2 (VGLUT2) mRNA, but none (0 of 288) expressing paired box gene 2 (Pax2) protein (Figure 2A), which is a marker of inhibitory INs in the superficial spinal cord (Cheng et al., 2005). Ucn3-tdTomato+ INs co-expressed several markers of spinal excitatory neurons at varying degrees, including Calbindin 1, Calbindin 2, NPY receptor NPY1R and cholecystokinin (CCK) (Figure 2B-2D and 2F). But few tdTomato+ neurons overlapped with Npra (Figure 2E). Our results indicated that Ucn3::Cre targets a subset of spinal excitatory INs enriched in laminae II-III.
Figure 2. Characterization of Ucn3+ INs in the dorsal spinal cord.
(A) Double staining of Ucn3-tdTomato signals (red) with markers of different spinal laminae: NK1R, CGRP, IB4, PKCγ, and markers of neurotransmitter phenotype: VGLUT2 (in situ hybridization), Pax2. Insets represent higher magnification of the boxed areas. Arrows indicate double-labeled cells. Arrowheads indicate cells expressing different markers but not tdTomato. n = 9-12 sections from 3-4 mice. Scale bar: 100 μm.
(B-E) Double staining of Ucn3-tdTomato with Calb1, Calb2, NPY1R, Npra or CCK mRNA by in situ hybridization, and the signals were overlapped with tdTomato. Arrows indicate double-positive cells for indicated mRNA and tdTomato, and arrowheads show cells positive for tdTomato alone. The percentage is calculated as double positive neurons over total number of Ucn3-tdTomato+ neurons: Calbindin 1 (36/369), Calbindin 2 (105/675), NPY1R (69/482), Npra (10/354), and CCK (62/220). Right panels show magnified images from their respective insets. n = 9-15 sections from 3 mice. Scale bar: 75 μm.
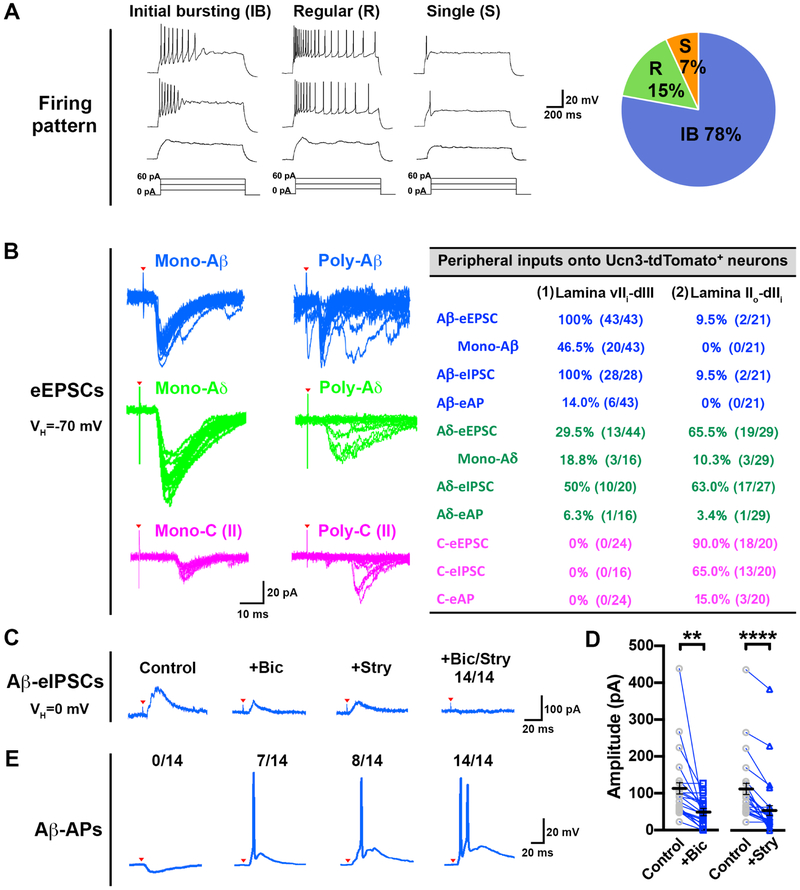
To further characterize spinal Ucn3+ INs, we examined the firing pattern and sensory inputs of Ucn3-tdTomato+ INs. The majority of Ucn3-tdTomato+ INs exhibited initial bursting firing in response to depolarizing current pulses (Figure 3A). To examine the sensory inputs onto Ucn3+ INs, we used two different slice preparations to separately record Aβ or Aδ/C inputs (Figure S5D), and identified two types of Ucn3+ INs in the dorsal spinal cord based on their location and sensory inputs. Type 1 Ucn3+ INs, located in laminae vIIidIII, receive sensory inputs from Aβ-LTMRs (100%, 43/43), in which 46.5% (20/43) are monosynaptic. We detected Aδ-inputs in 29.5% (13/44) of type 1 Ucn3+ INs (Figure 3B). In comparison, type 2 Ucn3+ INs are located in laminae IIo-dIIi and mainly receive Aδ (65.5%, 19/29) and/or C (90.0%, 18/20) inputs (Figure 3B). Strikingly, all type 1 Ucn3+ INs receive Aβ-inputs with feed-forward inhibition and most (86.0%, 37/43) do not generate Aβ-evoked action potentials (APs) in naïve mice (Figure 3B).
Figure 3. Firing pattern and sensory input of spinal Ucn3+ Ins.
(A) Firing properties of Ucn3-tdTomato+ INs in naïve Ucn3tdTomato mice. The majority of tdTomato+ INs (78%, 169/217, 31 mice) display an initial bursting (IB) firing pattern upon current injection. R: regular firing pattern. S: single firing pattern.
(B) Left: typical traces of Aβ, Aδ or C-evoked EPSCs showing Aβ, Aδ or C-fiber inputs onto Ucn3-tdTomato+ INs. Red arrowheads indicate stimulation artifacts. Right: the table is a summary of inputs in 137 Ucn3-tdTomato+ INs located in laminae vIIi-dIII (type 1) or IIo-dIIi (type 2) from 31 naïve Ucn3tdTomato mice. See also Figure S5.
(C) A typical trace of Aβ-eIPSCs in a recorded Ucn3-tdTomato+ INs with strong inhibition before bicuculine/strychnine treatment (control). Bath application of bicuculine (n = 23), or strychnine (n = 23) reduced the amplitude of Aβ-eIPSCs. Bath application of bicuculline plus strychnine (n = 14) abolished Aβ-eIPSCs in the recorded neurons. Red arrowheads indicate stimulation artifacts.
(D) Amplitude of Aβ-eIPSCs generated in Ucn3-tdTomato+ INs before (control) and after bath application of bicuculline (n = 23) or strychnine (n = 23).
(E) Typical traces of Aβ-evoked action potentials (Aβ-eAPs) in the recorded Ucn3-tdTomato+ INs. In the 14 recorded neurons, none generated Aβ-eAPs before bicuculine/strychnine treatment (control). After bicuculine treatment, Aβ-stimuli evoked APs in 7 out of 14 neurons. After strychnine treatment, Aβ-stimuli evoked APs in 8 out of 14 neurons. Following bath application of bicuculline plus strychnine, Aβ-eAPs were generated in all neurons. Red arrowheads indicate stimulation artifacts.
Two inhibitory neurotransmitters, γ-amino butyric acid (GABA) and glycine, mediate fast synaptic inhibition during sensory processing in the spinal cord (Zeilhofer et al., 2012). To further assess synaptic inhibition onto Ucn3+ INs, we specifically blocked GABAA receptors (GABAARs) and/or glycine receptors (GlyRs). We tested 23 Ucn3-tdTomato+ INs without Aβ-evoked APs in laminae vIIi-dIII (Figure 3C). There was a decrease in the amplitude of Aβ-evoked post-synaptic inhibitory currents (Aβ-eIPSCs) during the blockade of GABAARs by bicuculline or GlyRs by strychnine (Figure 3C and 3D). We treated 14 out of 23 recorded neurons with a combination of bicuculline and strychnine and found that Aβ-eIPSCs were completely abolished after blocking both GABAARs and GlyRs (14/14) (Figure 3C and 3D). Aβ stimulation therefore consistently evoked APs in all (14/14) neurons following blockade of both GABAARs and GlyRs (Figure 3E).
Ucn3+ INs receive inputs from TLR5+ Aβ-LTMRs
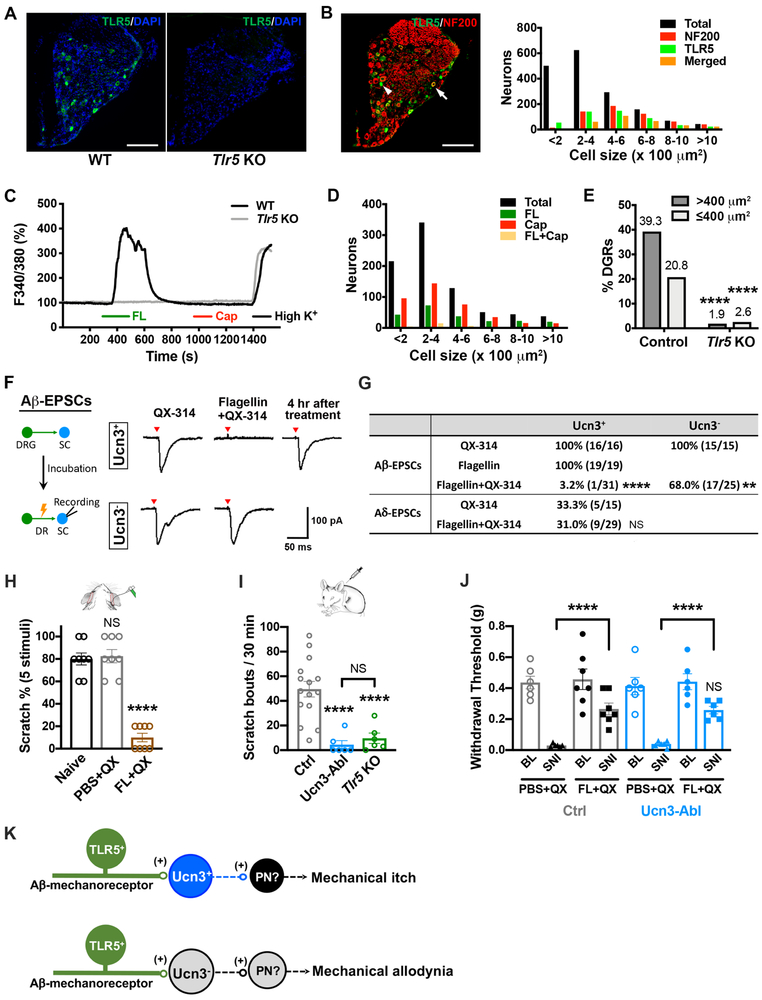
We next examined the type of Aβ-LTMRs that innervates Ucn3+ mechanical itch transmission neurons. Tick bites transmit bacteria B. burgdorferi bacteria, which release flagellin in infected patients manifesting itch (~ 40% patients) or pain (~10 % patients) (Ni et al., 2014). Toll-like receptor 5 (TLR5) is an innate immune receptor that recognizes bacterial flagellin from invading bacteria (Akira et al., 2006; Hayashi et al., 2001). Recently, a study based on single-cell RNA profiling revealed that TLR5 is expressed in several subpopulations of DRG neurons: NF1 Aδ-LTMRs, NF2/3 Aβ-LTMRs, NF5 proprioceptors, and PEP2 TRPV1low nociceptors, among which TLR5 is expressed in NF1 and NF2 subsets at a relatively high level (Usoskin et al., 2015). TLR5+ Aβ-LTMRs has been reported to transmit mechanical allodynia (Xu et al., 2015). We examined the expression of TLR5 protein in the DRG. TLR5 immunoreactivity was observed in 29.1% (492/1693) of DRG neurons (Figure 4A), including 52.3% (298/570) of NF200+ neurons (Figure 4B). The specificity of the antigen recognition was confirmed by testing the antibody in the Tlr5 KO DRGs (Figure 4A). The most widely used classification of DRG neurons is based on cell body size. Based on this classification, clear, medium-to-large cells are categorized as myelinated A-LTMR (> 400 μm2), while dark, small cells are classified as unmyelinated C-nociceptors (≤ 400 μm2) (Lawson et al., 1984; Willis and Coggeshall, 2004). Size frequency analysis showed that 60.2% (296/492) of TLR5+ neurons are medium-to-large cells, while 39.8% (196/492) of TLR5+ cells are of small cells. Consistently, 60.6% (298/492) of TLR5+ neurons express NF200. Our immunohistochemistry results showed that TLR5 is expressed in heterogeneous subpopulations of sensory neurons and a large portion of TLR5+ neurons are labeled by NF200.
Figure 4. TLR5+ Aβ-LTMRs connect to spinal Ucn3+ INs transmitting mechanical itch.
(A) Immunohistochemical detection of TLR5 expression (green) in the lumber DRG of WT and Tlr5 KO mice. Blue indicates DAPI staining. Scale bar: 100 μm.
(B) Double immunohistochemistry images showing the overlap between neuronal sub-population expressing TLR5 and NF200 in the lumber DRG. Right panel shows the size frequency analysis of overlap of TLR5 and NF200. A total of 1693 neurons from 4 WT mice were counted. Scale bar: 100 μm.
(C) Changes in [Ca2+]i are indicated as the ratio of Fura-2 signal at wavelengths 340 and 380 nm. Represented traces showing elevation of [Ca2+]i in two medium-to-large cells (> 400 μm2) in response to flagellin (FL), capsaicin (Cap), and high K+ (KCl). See also Figure S6A and S6B.
(D) Distribution of DRG cells responsive to flagellin, capsaicin or both. See also Figure S6C.
(E) Percentage of medium-to-large (>400 μm2) and small (≤ 400 μm2) neurons responsive to flagellin. A total of 819 neurons from 3 WT mice and 971 neurons from 3 Tlr5 KO mice, ****, p < 0.001, Student’s unpaired t test.
(F, G) Representative traces and summary of Aβ- and Aδ-evoked EPSCs after QX-314, or flagellin, or flagellin plus QX-314 incubation in Ucn3-tdTomato+ or Ucn3-tdTomato− INs from 22 mice. Four hours after flagellin plus QX-314 incubation, Aβ-EPSCs were detected in all recorded neurons (9/9, from 3 mice). SC: spinal cord slice; DR: dorsal root. See also Figure S6D and S7C
(H) Acute mechanical itch test 45 min after the co-administration of flagellin (FL) and QX-314 (QX) to the nape of WT mice. Naïve mice or PBS+QX-administered mice were tested as control groups. n = 8 in each group; ****, p < 0.0001, one-way ANOVA with Tukey post hoc analysis.
(I) Flagellin-induced spontaneous scratching in control (n = 15), Tlr5 KO (n = 6) and Ucn3-Abl mice (n = 6). Scratching events were recorded in a 30 minutes-bout following administration of flagellin in the nape. ****, p < 0.0001, one-way ANOVA with Tukey post hoc analysis.
(J) Assessment of neuropathic pain employing spared nerve injury model in control and Ucn3-Abl mice with or without inhibition of TLR5+ sensory neurons. n = 6 in each group; ****, p < 0.0001, NS, no significance, two-way ANOVA with Tukey post hoc analysis. BL: base line, before nerve injury; SNI: 7 day after spared nerve injury; QX: QX-314; FL: flagellin.
(K) Schematic showing proposed neural pathways that transmit mechanical pain and itch. Distinct TLR5+ Aβ-LTMRs transmit mechanical itch or mechanical allodynia through two discrete spinal networks: spinal Ucn3+ INs in laminae vIIi-dIII are the transmission neurons for mechanical itch, whereas Ucn3− neurons in laminae vIIi-dIII are proposed to transmit mechanical allodynia. See also Figure S6 and S7A, S7C.
We next tested whether flagellin can activate TLR5+ sensory neurons. We assayed the elevation of intracellular Ca2+ concentration ([Ca2+]i) in response to a variety of chemical stimuli in acutely dissociated DRG neurons from 4-week old mice. We perfused these neurons with flagellin, followed by capsaicin, and high potassium (KCl) (Figure 4C). Size frequency analysis revealed 47.0% (103/219) of flagellin-responding cells were medium-to-large cells and 53.0% (116/219) were small cells (Figure 4D). Of total recorded WT control DRG neurons, 39.3% (103/262) of medium-to-large cells and 20.8% (116/557) of small cells responded to flagellin (Figure 4E). Comparatively, in DRG neurons from Tlr5 KO, flagellin generated Ca2+ response in 1.9% (6/310) of medium-to-large cells, and 2.6% (17/660) of small cells (Figure 4E). It is notable that the percentage of medium-to-large cells responding to flagellin in Ca2+ imaging experiments was inconsistent with the expression of TLR5 protein in DRG tissues. Immunocytochemistry showed that 26.3% (373/1418) of small cells in the acutely dissociated DRG cultures expressed NF200, indicating these small cells were also A-LTMRs (Figure S6A and S6B). Additionally, we observed that a minor subset of small cells (20/557) responded to both flagellin and capsaicin (Figure 4D and S6C).
To assess whether TLR5+ sensory neurons synapse onto spinal Ucn3+ INs to transmit mechanical itch, we traced the monosynaptic inputs of spinal Ucn3+ INs using an intersectional genetic approach (Bourane et al., 2015). Injection of EnvA pseudotyped G-deleted-mCherry rabies virus into the lumbar dorsal spinal cords of P42 Ucn3::Cre; Lbx1Flpo; Rosa26CAG-ds-HTB mice infected ~ 32.2% spinal Ucn3+ INs (Figure S7A and S7B). The mCherry signals were observed in both the dorsal spinal cord and the lumbar DRG 5 days after virus injection (Figure S7B and S7C). Triple immunostaining with TLR5 and NF200 in the DRG showed that 89.1% (41/46) of mCherry+ neurons expressed TLR5 (Figure S7C). We also detected that 67.4% (31/46) of mCherry+ neurons expressed NF200+ (Figure S7C), in which the vast majority (29/31) are TLR5+ (Figure S7C). Our results suggest that myelinated TLR5+ LTMRs monosynaptically connect to spinal Ucn3+ neurons.
To examine the functional connection from TLR5+ LTMRs to Ucn3+ INs, we silenced TLR5+ sensory neurons by the selective delivery of a charged, membrane-impermeable sodium channel blocker lidocaine N-ethyl-lidocaine (QX-314) to TLR5+ sensory neurons, via the large pore ion channels activated specifically by flagellin (Xu et al., 2015). After incubating the DRG/dorsal root-contained spinal cord slice with flagellin plus QX-314, we tested the sensory inputs of spinal Ucn3+ INs and found that Aβ-evoked EPSCs were completely abolished in most of type 1 Ucn3-tdTomato+ INs at laminae vIIi-dIII (30/31) following the treatment of QX-314 plus flagellin, while incubating either QX-314 (0/16) or flagellin (0/19) alone did not affect Aβ-evoked EPSCs in Ucn3+ neurons (Figure 4F and 4G). The effect of flagellin plus QX-314 lasted for 2-4 hrs (Binshtok et al., 2007), we then recorded 9 Ucn3+ INs 4 hours after treatment and detected Aβ-evoked EPSCs in all 9 neurons (Figure 4F). Our results suggest that type 1 Ucn3+ INs predominantly receive sensory inputs from TLR5+ Aβ-LTMRs. Although ~30% of type 1 Ucn3+ INs receive Aδ-inputs (Figure 3B), incubating flagellin plus QX-314 did not affect Aδ-evoked EPSCs (Figure 4F and 4G). Consistent with our recording results, TLR5 immunoreactivity was very sparse in laminae I-II in the dorsal spinal cord, but dense throughout laminae vIIi-V (Figure S6D), where Aβ-fibers innervate.
We further tested the function of TLR5+ sensory neurons in mechanical itch. We intradermally injected flagellin plus QX-314 in the skin behind the ears to silence TLR5+ sensory neurons. Forty-five minutes after injection, the injected skin was poked by light punctate stimuli (0.7 mN). We found that mechanical itch responses were abolished in mice with co-application of flagellin and QX-314 (Figure 4H). Moreover, intradermal injection of flagellin alone in the skin behind the ears triggered spontaneous scratching responses in the first 30 min following injection in WT control mice (Figure 4I). In contrast, flagellin-evoked spontaneous scratching responses were greatly decreased in the Ucn3-Abl mice and Tlr5 null mice (Figure 4I).
To further assess the function of TLR5+ sensory neurons, we next investigated the effect of silencing TLR5+ sensory neurons in neuropathic pain. Following spared nerve injury, the mice developed long-lasting mechanical allodynia. Intradermal injection of flagellin plus QX-314 in the receptive field of mechanical allodynia on the lateral hindpaw increased the threshold of mechanical sensitivity in response to von Frey filaments 7 days after nerve injury, while QX-314 alone did not affect mechanical sensitivity in injured mice (Figure 4J). In comparison, injection of flagellin plus QX-314 did not affect the mechanical sensitivity before nerve injury (baseline) (Figure 4J). Additionally, ablation of spinal Ucn3+ INs did not influence the analgesic effect of silencing TLR5+ sensory neurons, suggesting that the TLR5+-Ucn3+ pathway is dispensable to transmit mechanical allodynia. In the neural circuits transmitting mechanical allodynia, pain transmission neurons in laminae vIIi-dIII receive synaptic inputs from Aβ-LTMRs (Cheng et al., 2017; Duan et al., 2014; Lu et al., 2013). We then recorded Ucn3-tdTomato− neurons in laminae vIIi-dIII, and detected Aβ-eEPSCs in recorded neurons. With the treatment of flagellin plus QX-314, 32.0% (8/25) of Ucn3-tdTomato− neurons lost Aβ-evoked responses (Figure 4F and 4G). Taken together, our results support the hypothesis that there could be two distinct subtypes of TLR5+ Aβ-LTMRs: one innervates type 1 Ucn3+ INs in laminae vIIi-dIII transmitting mechanical itch, while another innervates to Ucn3− pain transmission neurons in laminae vIIi-dIII to transmit mechanical allodynia (Figure 4K).
Spinal NPY+ INs gate mechanical itch by inhibiting Ucn3+ INs
We previously demonstrated that spinal NPY+ inhibitory INs gate mechanical itch (Bourane et al., 2015). To assess whether spinal NPY+ INs exert the gate-keeper role by preventing the activation of Ucn3+ INs, we examined synaptic inhibition from NPY+ INs to Ucn3+ INs in the dorsal spinal cord. Firstly, we performed anterograde circuit-tracing studies using a floxed synaptophysin-tdTomato reporter (Ai34), wherein synapse-localized synaptophysin-tdTomato is expressed following Cre-mediated recombination. To investigate the monosynaptic connections to Ucn3+ INs, we used a transgenic GFP reporter strain Ucn3::GFP to label Ucn3+ INs. To test whether the GFP reporter line targeted Ucn3::Cre+ INs in the dorsal spinal cord, we crossed Ucn3GFP with Ucn3::Cre and Ai14 reporter line. Double fluorescence signals of GFP and tdTomato showed that most of GFP+ cells (89.7%, 271/302) are tdTomato+, and 64.5% (271/420) of tdTomato+ cells co-express GFP (Figure 5A). We then crossed Ucn3::GFP with NPY::Cre and Ai34. The NPY::Cre targets not only spinal inhibitory neurons, but also glutamatergic primary sensory neurons in the DRG (Figure 5B). To investigate the innervations from spinal NPY+ inhibitory INs to Ucn3GFP INs, we performed quadruple immunostaining in the dorsal spinal cord of NPY::Cre; Ucn3::GFP;Ai34 mice (P42) with markers of inhibitory synapse, including the vesicular GABA transporter (VGAT, labeling presynapse) and gephyrin (labeling postsynapse). In most Ucn3GFP INs (90.9%, 100/110), we detected inhibitory synaptic connections from NPY+ INs to Ucn3GFP neurons (Figure 5C). To further confirm the monosynaptic inputs to Ucn3+ in situ hybridization, we performed retrograde tracing combined with ISH staining to detect NPY mRNA 5 days after rabies virus injection in the dorsal spinal cord of adult (P42) Ucn3:;Cre;Lbx1Flpo;Rosa26ds-HTB mice (Figure S7B). We found that more than half of presynaptic mCherry+ neurons (9/16) in the dorsal spinal was observed to express NPY mRNA (Figure S7B). Since vast majority of spinal NPY+ INs are inhibitory (Bourane et al., 2015), our study confirmed that spinal NPY+ INs monosynaptically connect to Ucn3+ INs in the dorsal spinal cord.
Figure 5. Spinal NPY+ INs synapse onto Ucn3+ INs to gate mechanical itch.
(A) Spinal cord section from triple transgenic mice Ucn3::Cre; Ucn3::GFP; Ai14 showing the overlap between Ucn3GFP INs and Ucn3-tdTomato+ INs. The percentage is indicative of overlap between the labeled population of Ucn3+ INs by Ucn3::Cre and Ucn3::GFP. Arrows indicate a double-positive cell and arrowheads indicate either tdTomato+ or GFP+ neurons. n = 14 sections from 3 mice. Scale bar: 75 μm.
(B) Sections from NPY::Cre; Ai14 showing NPY+ neurons labeled with tdTomato. Spinal NPY+ INs are inhibitory as opposed to excitatory NPY+ neurons in the DRG. Scale bar: 100 μm.
(C) Spinal section from NPY::Cre; Ucn3::GFP; Ai34 probed for VGAT, gephyrin and tdTomato. The existence of inhibitory synaptic puncta between NPY+ INs and Ucn3GFP INs is marked by inhibitory presynaptic terminal of NPY+ INs (labeled by tdTomato), gephyrin and VGAT (shown with an arrow). n = 21 sections from 3 mice. Scale bar: 5 μm. See also Figure S7A and S7B.
(D) Schematic representing the adapted experimental approach to optogenetically activate NPY pre-synaptic terminals and simultaneously record from Ucn3GFP INs. A quadruple cross NPY::Cre; Lbx1Flpo; Ucn3::GFP; Ai80 was employed.
(E) Typical traces of blue light-evoked (473nm) GABAergic (top), glycinergic (middle) and mixed (bottom) IPSCs in Ucn3GFP INs, respectively. Blue arrowheads indicate 1 ms of light (473nm) stimulation. Right panel shows summarized table of light-evoked IPSCs in Ucn3GFP INs. n = 5 mice.
(F) Acute mechanical itch test following saline or CNO injection in NPY::Cre;Lbx1Flpo;Rosa26CAG-ds-hM3Dq mice (NPY-hM3Dq) and control littermates (Ctrl). n = 6 in each group; ****, p < 0.0001, Student’s unpaired t test.
(G) Measure of mechanical itch after applying von Frey filaments to the shaved nape of the neck in NPY+ IN-ablated mice (NPY-Abl), NPY/Ucn3 dual ablated mice (NPY/Ucn3-Abl) and control littermates (Ctrl). Ctrl: n = 7; NPY-Abl: n = 8; NPY/Ucn3-Abl: n = 7. ****, p < 0.0001; NS, no significant difference, one-way ANOVA with Tukey post hoc analysis.
(H) Number of spontaneous scratching events over a 30-min period. Ctrl: n = 10; NPY-Abl: n = 10; NPY/Ucn3-Abl: n = 6. ***, p < 0.001; NS, no significant difference, one-way ANOVA with Tukey post hoc analysis.
(I) Skin lesions (white arrows) on the cheek of NPY-Abl mice 2 weeks after DTX injection (10/10 mice). No lesions on the skin of NPY/Ucn3-Abl mice 2 weeks after DTX injection (7/7 mice).
To further detect the functional inhibition from NPY+ INs to Ucn3+ INs, we expressed CatCh (the L132C mutant of channelrhodopsin ChR2) in the spinal NPY+ INs by crossing NPY::Cre;Lbx1Flpo;Ucn3::GFP with Ai80. The mouse strain Ai80 is a Cre- and Flpo-dependent (Daigle et al., 2018). In our strategy, spinal NPY+ inhibitory INs were activated by shining 473 nm light and Ucn3GFP INs were recorded concurrently (Figure 5D). Blue light (1 ms of 473 nm)-evoked IPSCs were detected in all recorded Ucn3GFP INs (19/19) (Figure 5E). Since Ucn3+ INs receive mixed GABAergic and glycinergic inhibition (Figure 3B), we next tested inhibitory transmitters associated with NPY+ IN-imposed inhibitory gate on Ucn3+ INs. Bicuculline (10 μM) alone and bicuculline plus strychnine (2 μM) were used to identify inhibitory synaptic transmitters. In all 10 Ucn3+ INs that we tested, bicuculline plus strychnine completely abolished light-evoked IPSCs (10/10) (Figure 5E). Interestingly, bicuculline alone completely abolished light-evoked IPSCs in 3 recorded neurons (Figure 5E), while light-evoked IPSCs were not affected in 2 recorded neurons following bicuculline treatment (Figure 5E). In the other 5 neurons, bicuculine partially attenuated the amplitude of light-evoked IPSCs (Figure 5E). Thus Ucn3+ INs receive GABAergic and/or glycinergic inhibition from NPY+ INs.
We next investigated whether synaptic inhibition from NPY+ INs to Ucn3+ INs is required to gate mechanical itch. We examined acute mechanical itch in NPY::Cre; Lbx1Flpo; Rosa26CAG-ds-hM3Dq mice. Selective activation of spinal NPY+ INs by administration of CNO attenuated acute mechanical itch responses to light punctate stimuli on the skin behind the ears (Figure 5F). Ablation of spinal NPY+ INs led to sensitized mechanical itch on non-itchy skin areas, such as the nape (Figure 5G), as well as persistent spontaneous scratching (Figure 5H) and skin lesions (Figure 5I), mimicking the major symptoms of chronic itch (Yosipovitch and Bernhard, 2013). To examine if Ucn3+ INs mediate mechanical itch sensitization following ablation of NPY+ INs, we ablated both NPY+ and Ucn3+ INs in the dorsal spinal cord using the intersectional genetic strategy. We found that sensitization of mechanical itch was completely abolished in mice after ablating both spinal NPY+ and Ucn3+ INs (Figure 5G). Moreover, spontaneous scratching and skin lesions seen following ablation of NPY+ INs were completely abolished in mice after ablating both NPY+ and Ucn3+ INs in the dorsal spinal cord (Figure 5H and 5I). Our results indicate that NPY+ inhibitory INs innervate Ucn3+ INs to prevent mechanical itch transduction, providing further evidence that Ucn3+ INs play an essential role in transmitting mechanical itch.
Sensitization of mechanical itch circuit contributes to chronic itch
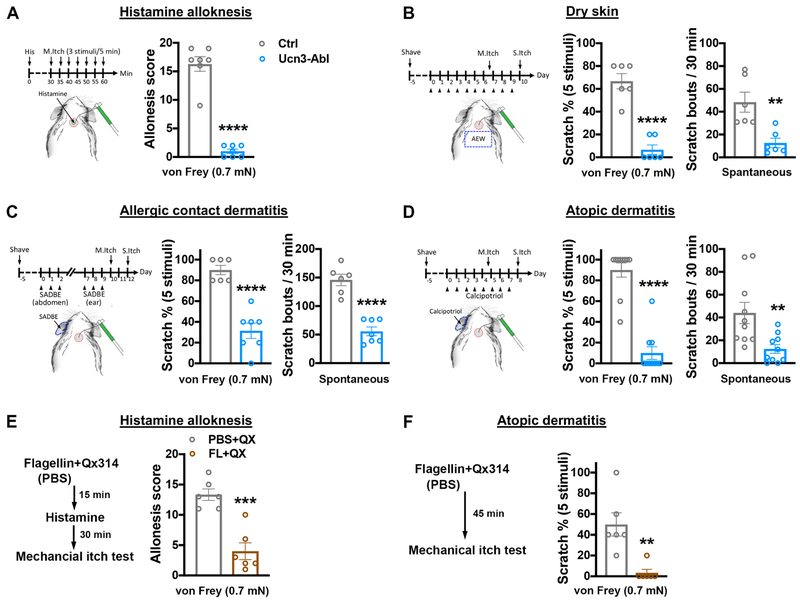
Mechanical itch sensitization and persistent spontaneous itch are hallmarks of chronic itch (Ikoma et al., 2006; LaMotte et al., 2014; Yosipovitch and Bernhard, 2013). We assessed the role of spinal Ucn3+ INs in mechanical itch sensitization and persistent spontaneous itch in mouse chronic itch models. Intradermal injection of histamine evokes acute mechanical alloknesis on non-itchy skin surrounding the injection site in healthy humans (Lamotte, 1988), which mimics central sensitization under chronic itch conditions (Andersen et al., 2018). We tested the function of spinal Ucn3+ INs in a mouse model of histamine-induced acute alloknesis (Akiyama et al., 2012). In control mice, light punctate stimuli by a weak von Frey filament (0.7 mN) near the site of histamine injection (0.5-1 cm distance) on the nape reliably evoked scratching responses 30-60 minutes after histamine injection (Figure 6A). Ablating spinal Ucn3+ INs abolished scratching responses evoked by light punctate stimuli (Figure 6A). We next examined the role of spinal Ucn3+ INs in three mouse models of chronic itch, including a dry skin model (Miyamoto et al., 2002), a SADBE-treated allergic contact dermatitis model (Fu et al., 2014) and a calcipotriol-treated atopic dermatitis model (Li et al., 2006). During early stages of chronic itch development, light punctate stimuli by a weak von Frey filament (0.7 mN) on the surrounding nape area of treated skin (0.5-1 cm distance) elicited reliable scratching responses in control mice (mechanical itch sensitization, Figure 6B-6D). Ablating spinal Ucn3+ INs abolished mechanical itch sensitization in all three chronic itch models (Figure 6B-6D). Following chronic itch treatment, those mice eventually developed persistent spontaneous itch several days after the induction of mechanical itch sensitization (Figure 6B-6D). We found that spontaneous scratching responses were greatly attenuated in Ucn3-Abl mice (Figure 6B-6D). In contrast, blockade of chemical itch pathways by ablating spinal Npra+ INs did not affect histamine-induced alloknesis and calcipotriol-induced mechanical itch sensitization (Figure S8A and S8B).
Figure 6. Spinal Ucn3+ INs are essential for mechanical itch sensitization in chronic itch.
(A) Mechanical alloknesis following acute histamine injection. The total scratching events following mechanical stimuli through 0.7 mN von Frey filament in 30-60 min after histamine injection indicates alloknesis score. Schematic showing the histamine injection site on nape and surrounding site (~ 0.5 cm distance) for mechanical stimuli. Control (Ctrl): n = 6; Ucn3+ neuron-ablated (Ucn3-Abl): n = 6. ****, p < 0.001, Student’s unpaired t test. See also Figure S8A.
(B-D) Measurement of mechanical itch sensitization and persistent spontaneous itch in three chronic itch models: dry skin (B), allergic contact dermatitis (C) and atopic dermatitis (D). Schematic showing chronic itch models and behavioral procedures. The circle with diagonal lines on the nape indicates the site of mechanical itch stimuli (M.itch). Scratching percentage indicates the scratching events in 5 mechanical itch stimuli through 0.7 mN von Frey filament to the indicated areas on the nape. In spontaneous itch tests (S.itch), the number of spontaneous scratching events over a 30-min period is counted. Dry skin: n = 6 in each group; allergic contact dermatitis: Ctrl, n = 6; Ucn3-Abl, n = 7; atopic dermatitis: n = 10 in each group. **, p < 0.01, ****, p < 0.001; Student’s unpaired t test. See also Figure S8B.
(E-F) Measurement of acute histamine alloknesis (E) and mechanical itch sensitization in aptopic dermatitis (F) with or without the pharmacological silencing of the TLR5+ sensory neurons. Schematics showing the employed protocol to silence TLR5+ sensory neurons through administration of flagellin and QX-314 in the nape. PBS+QX-treated group as control. n = 6 in each group. **, p < 0.01, ***, p < 0.001; Student’s unpaired t test.
We next asked whether TLR5+-Ucn3+ pathway is required to transmit mechanical itch sensitization in chronic itch. We investigated the role of TLR5+ sensory neurons in the histamine-induced alloknesis and the calcipotriol-induced mechanical itch sensitization. In the histamine-induced alloknesis test, we intradermally injected flagellin plus QX-314 in the nape of mice. Fifteen minutes later, we injected histamine in the surrounding skin of the nape to trigger mechanical alloknesis. We then started to test alloknesis 30 minutes after histamine injection. We found that flagellin plus QX-314 largely reduced touch-induced scratching at the injection site of flagellin plus QX-314 on the nape, compared to the control group that was injected with QX-314 alone (Figure 6E). Next, we tested whether TLR5+ sensory neurons are required for mechanical itch sensitization in calcipotriol-induced chronic itch. We intradermally injected flagellin plus QX-314 in the nape after 5-day calcipotriol treatment. Forty-five minutes after injection, we tested mechanical itch sensitization and found that flagellin plus QX-314 dramatically attenuated touch-induced scratching at the injected site of flagellin plus QX-314 on the nape (Figure 6F). Our results suggest that the neural pathway though TLR5+-LTMRs to Ucn3+ INs is necessary for mechanical itch sensitization.
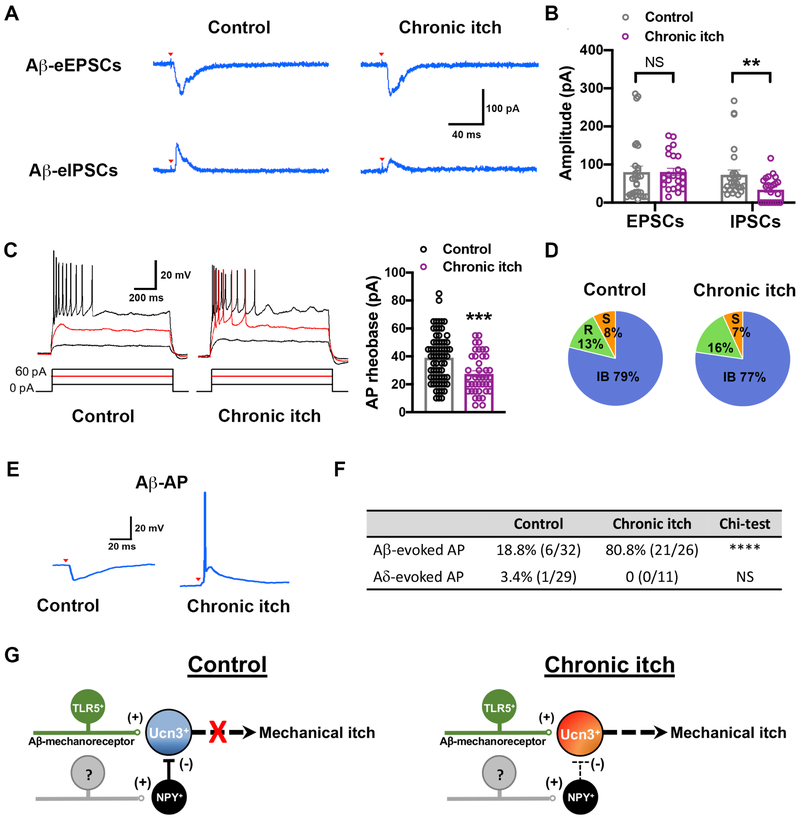
To further define the role of spinal Ucn3+ INs in mechanical itch sensitization, we determined whether Aβ-inputs can excite Ucn3+ INs in chronic itch. The 5-day calcipotriol treatment on the dorsal hindpaw developed mechanical itch sensitization (Figure S8C). Ucn3-tdTomato+ INs in laminae vIIidIII were recorded in calcipotriol-treated chronic itch mice. We found that the mean amplitude of Aβ-evoked inhibitory post-synaptic currents (IPSCs) of Ucn3-tdTomato+ INs was reduced by 47.1% (control: 73.2 ± 12.5 pA; chronic itch: 38.7 ± 8.1 pA), while the mean amplitude of Aβ-evoked EPSCs did not change in the chronic itch mice (control: 80.1 ± 15.5 pA; chronic itch: 80.5 ± 9.6 pA) (Figure 7A and 7B). To test whether chronic itch conditions altered intrinsic excitability, we performed current-clamp recordings of APs evoked by injecting currents in spinal cord slice from control and chronic itch mice. The threshold to generate APs (rheobase) was significantly decreased in Ucn3-tdTomato+ INs following calcipotriol treatment (control: 39.9 ± 2.3 pA; chronic itch: 30.3 ± 2.6 pA) (Figure 7C), indicating increased intrinsic excitability in Ucn3+ INs under chronic itch condition. However, the firing patterns were unchanged in chronic itch group (Figure 7D). Due to reduced synaptic inhibition and enhanced intrinsic excitability, most Ucn3-tdTomato+ INs (21/26) generated Aβ-evoked APs in chronic itch mice (Figure 7E and 7F). In contrast, sensitization of Aδ-evoked APs in Ucn3-tdTomato+ INs following calcipotriol treatment was not detected, suggesting Aδ-LTMRs may not be required for transmitting mechanical itch sensitization (Figure 7F).
Figure 7. Sensitization of spinal mechanical itch circuit in chronic itch.
(A, B) Typical traces and statistics results of of Aβ-evoked EPSCs/IPSCs in Ucn3-tdTomato+ INs in control and calcipotriol-treated (chronic itch) mice. In control mice, Aβ-stimuli successfully evoked IPSCs in all neurons (n = 28) whereas Aβ-stimuli evoked IPSCs in only 15 out of 24 neurons in chronic itch mice. Aβ-stimuli evoked EPSCs in Ucn3-tdTomato+ INs were unaffected in chronic itch mice. Red arrowheads indicate stimulation artifacts. EPSCs: control, n = 28; chronic itch, n = 24; p = 0.98. IPSCs: control, n = 28; chronic itch, n = 24; *, p < 0.05. Student’s unpaired t test. See also Figure S8C.
(C) Action potential (AP) induction upon current injection of Ucn3-tdTomato+ INs in control and calcipotriol-treated chronic itch mice. Right panel shows average AP rheobase in control and chronic itch mice. control, n = 66; chronic itch, n = 41. ***, p < 0.05. Student’s unpaired t test.
(D) Firing pattern in Ucn3-tdTomato+ INs in control mice (n = 65) and calcipotriol-treated chronic itch mice (n = 54).
(E) Representative traces for Aβ-evoked APs in Ucn3-tdTomato+ INs in control and calcipotriol-treated chronic itch mice.
(F) Summarized table of Aβ- and Aδ-evoked APs in control and chronic itch mice. ****, p < 0.0001; NS, no significant difference, Chi-square test.
(G) Schematic illustrating the sensory circuits that transmit mechanical itch in control and chronic itch condition. “(+)”: excitatory synapses. “(−)”: inhibitory synapses.
DISCUSSION
The cellular basis for itch processing is dependent on specialized spinal neurons
The dorsal horn of the spinal cord receives inputs from sensory nerve fibers, which terminate in a modality-specific fashion within particular spinal laminae (Todd, 2010). Previous anatomical and physiological experiments provide strong support for lamina vIIi-IV of the dorsal spinal cord being an important site that receives and processes LTMR information (Abraira and Ginty, 2013). Although both mechanical itch and chemical itch generate a similar desire to scratch, our studies demonstrate that these two forms of itch are transmitted through distinct neural circuits in the spinal cord. Ucn3+ excitatory INs in lamina vIIi-dIII are predominantly innervated by Aβ-LTMRs but not nociceptive C/Aδ afferents. Ablating or silencing spinal Ucn3+ excitatory INs suppressed mechanical itch but not chemical itch, touch, pain and temperature sensations, suggesting that these neurons represent a unique subpopulation of excitatory INs in LTMR-innervated zone for mechanical itch processing. Moreover, ablation of spinal Ucn3+ INs largely attenuated mechanical itch sensitization and persistent spontaneous itch under chronic itch conditions, suggesting the importance of spinal Ucn3+ INs in initiating and maintaining chronic itch. How do Ucn3+ INs transmit mechanical itch? Our studies suggest that Ucn3+ INs in lamina vIIi-dIII could be the central node that link Aβ-LTMRs and mechanical itch projection neurons in lamina I via a ventral-to-dorsal excitatory polysynaptic circuit. The identification of the downstream projection circuits is the future direction in this field.
In contrast to the abolition of mechanical itch, ablation of Ucn3+ INs did not affect touch, chemical itch, pain and temperature sensations. Previous studies indicated that noxious heat and pain stimuli activate neurons located predominantly in lamina I/IIo (Braz et al., 2014; Craig, 2003; Todd, 2010). The enrichment of Ucn3+ INs in lamina vIIi-dIII may explain why pain and temperature sensation remain intact, although we do not rule out a redundant role of type 2 Ucn3+ INs in lamina IIo-dIIi for temperature and pain sensations.
Our previous studies showed that spinal NPY+ INs gate mechanical itch (Bourane et al., 2015). In the present study, we found that NPY+ INs directly innervate Ucn3+ INs to prevent low-threshold mechanical stimuli from activating Ucn3+ mechanical itch transmission neurons. In contrast, spinal Npra+ INs (Mishra and Hoon, 2013) and downstream GRPR+ INs (Sun and Chen, 2007; Sun et al., 2009) transmit chemical itch, which is gated by spinal NPY− Bhlhb5-dependent inhibitory INs (Huang et al., 2018; Kardon et al., 2014; Ross et al., 2010), suggesting that distinct inhibitory INs modulate different forms of itch in the spinal cord. Although the NPY-NPY1R signal cascade has been reported to modulate mechanical itch and chemical itch (Gao et al., 2018), we detected few Ucn3+ neurons expressing NPY1R. Moreover, NPY1R agonist ([Leu31,Pro34]-NPY, I μM) did not attenuate AP induction in Ucn3+ neurons following current injection (data not shown). Our results suggest that the NPY-NPY1R signal cascade may not play the major role in regulating the activation of Ucn3+ neurons. However, we do not exclude the possibility that the NPY-NPY1R signal cascade might play a role in modulating mechanical itch in the polysynaptic circuit between Ucn3+ INs and mechanical itch projection neurons in the dorsal spinal cord.
Mechanisms underlying the transition from acute to chronic itch
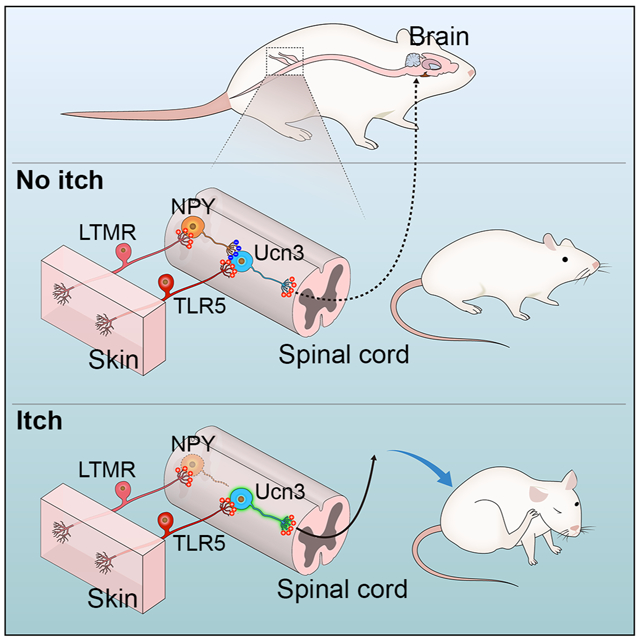
Our results reveal a feed-forward inhibition circuit for mechanical itch that involves TLR5+ Aβ-LTMRs, excitatory Ucn3+ INs and inhibitory NPY+ INs (Figure 7G). Normally, the mechanical itch circuit remains dormant due to NPY+ IN-mediated fast synaptic inhibition. Under chronic itch conditions, this gate is opened allowing light touch to elicit itch sensation on non-itchy skin areas (Figure 7G). In this hypothesis, a key question is how monosynaptic excitatory inputs to Ucn3+ INs can be gated by disynaptic inhibitory inputs? A recent study indicated that distinct glutamate receptor kinetics and electrical filtering by potassium channels contribute to the temporal integration of excitation/inhibition on spinal SOM+ INs with delayed-firing patterns in a feed-forward inhibition pathway for mechanical pain (Zhang et al., 2018). However, Ucn3+ INs do not fit this model because most Ucn3+ INs display initial-bursting firing patterns (Figure 3A). Our whole-cell recordings showed that the latency of monosynaptic Aβ-input onto Ucn3+ INs is 4.4 ms, which is much longer than the latency of monosynaptic Aβ-input onto NPY+ INs (3.2 ms) (Figure S5E), suggestive of a distinct subtype of Aβ-LTMRs that may innervate spinal NPY+ INs (Figure 7G). Given the average synaptic delay of a single synapse is ~0.2-0.5 ms (Sabatini and Regehr, 1999), the inhibition from NPY+ INs is sufficient to suppress the delayed excitatory inputs onto Ucn3+ INs to achieve gate control in this non-canonical feed-forward inhibition microcircuit. Consistent with this, loss of Merkel cells-type I slow-adapting (SAI) Aβ-LTMRs function selectively leads to mechanical itch sensitization in xerotic skin (Feng et al., 2018), indicating SAI Aβ-LTMRs might selectively innervate NPY+ INs in the spinal cord to gate mechanical itch. Our study highlights the clinical implications of chronic itch sensitization in the nervous system and show that increased excitability of dorsal spinal neurons, including enhanced intrinsic excitability and reduced GABAergic/glycinergic inhibition, may be one of the major features of chronic itch. Consistent with our findings, a recent preclinical study showed that restoring the inhibitory tone in the dorsal spinal cord by GABAA receptor agonists or GABAergic cell transplantation can relieve chronic itch sensitization and skin lesions in an atopic dermatitis model (Cevikbas et al., 2017), suggesting that dormant GABAergic/glycinergic inhibition acts as a common factor in chronic itch.
Taken together, we have identified a feed-forward inhibition microcircuit in the dorsal spinal cord that processes mechanical itch. As the key cellular node in this circuit, Ucn3+ excitatory INs receive inputs from TLR5+ Aβ-LTMRs with feed-forward inhibition via NPY+ inhibitory INs. Under pathological conditions, sensitized Ucn3+ INs are essential in initiating and maintaining chronic itch. Thus, our findings indicate spinal Ucn3+ INs as a valid cellular target for future therapeutic interventions against chronic itch, without affecting normal touch and temperature sensations in patients.
STAR★ METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
This study did not generate new unique reagents. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Bo Duan (bduan@umich.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at University of Michigan following NIH guidelines. Both male and female mice were used for all experiments. Mice were group housed at room temperature with ad libitum access to standard lab mouse pellet food and water on a 12hr light/12hr dark cycle. The mouse lines used in the present study were: Ucn3::Cre (KF43, Gensat), NPY::Cre (RH26, Gensat) (Bourane et al., 2015), Rosa26LSL-tdTomato (Ai14, #007914, JAX) (Madisen et al., 2010), Lbx1Flpo (Duan et al., 2014), Rosa26CAG-FSF-LSL-hM4Di (Rosa26CAG-ds-hM4Di) (Ray et al., 2011), Rosa26CAG-FSF-LSL-hM3Dq (Rosa26CAG-ds-hM3Dq) (Sciolino et al., 2016), Rosa26CAG-LSL-FSF-HTB (Rosa26ds-HTB) (Bourane et al., 2015), Nav1.8-Cre (Stirling et al., 2005), TauLSL-FSF-DTR (Tauds-DTR) (Duan et al., 2014) and Rosa26LSL-DTR (#007900, JAX) (Buch et al., 2005), Tlr5 KO (#008377, JAX) (Feuillet et al., 2006), Ai34 (#012570, JAX), Ai65 (#021875, JAX) and Ai80 (#025109, JAX) (Daigle et al., 2018). Other mice strains that were used for intersection ablation and behavioral screening are listed in Supplementary Table S1.
Ucn3::Cre or NPY::Cre mice were crossed with Ai14 reporter mice (Ucn3tdTomato or NPYtdTomato) to label the Ucn3::Cre-derived (referred to as Ucn3+) or NPY::Cre-derived (referred to as NPY+) neurons. We ablated DTR-expressing neurons as previously described (Duan et al., 2014). 6-10 weeks old mice were intraperitoneally injected with diphtheria toxin (DTX, 50 μg/kg; MilliporeSigma, St. Louis, MO) at day 1 and day 4. In most strains, we performed behavioral or histochemical experiments 4 weeks after DTR injection. For NPY-ablated mice, we performed behavioral tests 7-14 days after DTX injection before the animals developed severe skin lesions. Rosa26CAG-ds-hM4Di or Rosa26CAG-ds-hM3Dq mice were crossed with Ucn3::Cre mice and Lbx1Flpo mice, then clozapine N-oxide (CNO, 5 mg/kg, MilliporeSigma, St. Louis, MO) was injected to acutely silence or activate Ucn3+ neurons. Behavioral tests were performed 40 min after CNO injection.
To ablate spinal GRPR+ neurons, mice were given a single intrathecal injection of either bombesin-saporin (400 ng in 10 μl sterile saline, Advanced targeting Systems, San Diego, CA), which selective ablated spinal GRPR+ neurons but not Neuromedin B receptor+ neurons (Mishra et al., 2012), or blank-saporin (400 ng in 10 μl sterile saline, Advanced targeting Systems, San Diego, CA). To ablate spinal Npra+ neurons, mice were given a single intrathecal injection of either Nppb-saporin (5 μg in 10 μl sterile saline, Advanced targeting Systems, San Diego, CA) or blank-saporin (5 μg in 10 μl sterile saline, Advanced targeting Systems, San Diego, CA).
METHOD DETAILS
In Situ Hybridization and Immunohistochemistry
In situ hybridization (ISH) procedures were performed to detect mRNA expression as described previously (Duan et al., 2014). For double staining analyses of tdTomato fluorescence coupled with ISH, the tdTomato fluorescent signal was first photographed under a fluorescent microscope (Leica DMi8, Germany) prior to performing ISH. After ISH the pseudo fluorescent signals were converted from bright field images and then merged onto the tdTomato images by Photoshop software (Adobe Photoshop CS6). Quantitative analysis was determined by analyzing 3-4 spinal cords (3-5 sections each) per genotype. Only cells with clearly visible nuclei were scored. Immunohistochemistry was performed to detect protein expression on spinal cord sections using rabbit anti-NK1R (1:1000, #S8305, MilliporeSigma, St. Louis, MO), rabbit anti-α-CGRP (1:1000, #T-4032, Peninsula Lab, San Carlos, CA), Alexa Fluor 488-conjugated Isolectin GS-IB4 (10 μg/ml, #I21411, Thermo Fisher Scientific, Waltham, MA), rabbit anti-PKCγ (1:400, #sc-211, Santa Cruz, Dallas, TX), Pax2 (1:500, #71-6000, Thermo Fisher Scientific, Waltham, MA), anti-goat tdTomato (1:500, #AB8181-200, SICGEN, Cantanhede, Portugal), anti-guinea pig gephyrin (1:1000, #147 318, Synaptic Systems, Goettingen, Germany), anti-mouse VGAT (1:200, #131 011, Synaptic Systems, Goettingen, Germany), anti-rabbit TH (1:500, #AB152, MilliporeSigma, Burlington, MA), TLR5 (1:100, # sc-517439, Santa Cruz, Dallas, TX) or rabbit anti-c-Fos (1:500, #ABE457, MilliporeSigma, Burlington, MA), which were diluted in 0.2% of Triton X-100 and 10% of normal goat serum in PBS. ISH signals and IHC fluorescent were observed under the fluorescent microscope.
Cell Counting
L4/L5 lumbar DRG were dissected from four WT and three Tlr5 KO mice. Three to four WT or mutant DRGs were used to prepare eight adjacent sections at 12-μm thickness. Each set was processed for immunostaining. Only cells containing nuclei and showing levels of expression or staining clearly above background were counted.
Behavioral Testing
For acquisition of behavioral data, the experimenter was blinded to the genotype of the animals. Littermate mice (B6J/129 mixed genetic background) of either sex were used in all behavioral tests. As described previously (Bourane et al., 2015; Cheng et al., 2017; Duan et al., 2014), after three to five ‘habituation’ sessions (30 min per day) in the behavioral testing apparatus, acute somatosensory measures were recorded on four consecutive days in the given order: rotarod, light touch (day 1); von Frey, acute mechanical itch and Hargreaves (day 2), hot plate and cold plate (day 3); pinprick, and Randall-Selitto (day 4). The interval between different tests was at least for 2 h. Following acute behavioral tests, separate groups of mice were used for inflammatory pain, neuropathic pain, acute chemical itch and chronic itch measurements.
Acute mechanical itch
To test acute mechanical itch, the fur behind the ears (pale yellow) or the fur on the nape of the neck was shaved 5 days before tests. Mice were habituated for 15 min for 2 consecutive days in behavioral testing apparatus (IITC, Woodland Hills, CA). On the testing day, mice were placed in the plastic enclosures and habituated for 15 min. Mice then received five separate mechanical stimuli for ~1 sec at 3-5 sec intervals at randomly selected sites on the skin behind the ears. Mechanical stimuli were delivered with von Frey filaments ranging from 0.08 mN to 10 mN (North coast medical, Morgan Hill, CA). The scratching response of hind paw towards the poking site was considered as a positive response. The percentage of positive responses in 5 stimuli was measured to indicate acute mechanical itch score. Noxious mechanical stimuli (10 mN) triggered forelimb wiping/head shaking responses in WT mice and the responses in 5 stimuli was recorded to calculate the noxious score.
Acute chemical itch
To test acute chemical itch, the fur on the nape of the neck was shaved 5 days before tests. Mice were placed in the behavioral testing apparatus for three to five ‘habituation’ sessions. On the testing day, mice were habituated in the behavioral testing apparatus for 15 min. The behavior of mice was then video recorded 15 min before (baseline), and 30 min after chemical injection. Compound 48/80 (100 μg, MilliporeSigma, St. Louis, MO), chloroquine (200 μg, MilliporeSigma, St. Louis, MO), Me-5-HT (50 μg, MilliporeSigma, St. Louis, MO) or SLIGRL (100 μg, Bachem, Switzerland) in 50 μl of sterile saline was injected intradermally into the nape. Scratching bouts were counted for 30 min after injection.
Histamine-induced alloknesis
The fur on the nape of the neck was shaved 5 days before treatment. On testing day, histamine (50 μg, MilliporeSigma, St. Louis, MO) in 10 μl saline was injected intradermally in the middle of the nape (Akiyama et al., 2012). 30 min after injection, mice received three separate innocuous mechanical stimuli for ~1 sec delivered using a von Frey filament (0.7 mN) at 3-5 sec intervals at randomly selected sites oriented radially 0.5-1 cm away from the injection site. The scratching response of hind paw toward the poking site was considered as a positive response. The alloknesis score was counted as the positive responses in three stimuli. Mice repeatedly received the test for 30 min at a 5-min interval (30 min-60 min after injection, totally seven tests). The overall alloknesis score was calculated as the sum of individual alloknesis scores from seven tests.
Chronic dry skin model
The fur on the nape of the neck and rostral back was shaved 5 days before treatment. The rostral back of mice was treated with a mixture of acetone/ether (1:1, MilliporeSigma, St. Louis, MO) for 15 seconds followed by distilled water for 25 seconds (Acetone-Ether-Water, AEW). After twice daily AEW treatment for seven consecutive days, mechanical itch sensitization was measured. On the testing day to measure mechanical itch sensitization, mice were habituated for 15 min in the behavioral testing apparatus. Mice then received five separate innocuous mechanical stimuli for ~1 sec delivered using a von Frey filament (0.7 mN) at 3-5 sec intervals at randomly selected sites on the nape surrounding the AWE-treated site (0.5-1 cm distance). The scratching response of hind paw toward the poking site was considered as positive response. The percentage of positive responses in 5 stimuli was measured to indicate mechanical itch sensitization score. Mice then received twice daily AEW treatment for three consecutive days. On the testing day, to measure spontaneous scratching, mice were habituated for 15 min in the behavioral testing apparatus. The behavior of mice was then video recorded for 30 min. The video recording was subsequently played back and spontaneous scratching bouts in 30 min were counted.
Allergic contact dermatitis model
The fur on the nape of the neck and abdominal skin was shaved 5 days before treatment. Mice were sensitized by the topical application of 25 μl of 1% squaric acid dibutylester (SADBE, MilliporeSigma, St. Louis, MO) in acetone to abdominal skin once daily for three consecutive days, as described previously (Fu et al., 2014). Five days later, the left ear skin was challenged with a topical application of 25 μl of 1% SADBE in acetone for three days. On the testing day to measure mechanical itch sensitization, mice were habituated for 15 min in the behavioral testing apparatus. Mice then received five separate innocuous mechanical stimuli for ~1 sec delivered using a von Frey filament (0.7 mN) at 3-5 sec intervals at randomly selected sites on the nape surrounding the treated ear (0.5-1 cm distance). The percentage of positive responses in 5 stimuli was measured to indicate mechanical itch sensitization score. Two days after testing mechanical itch sensitization, spontaneous scratching in 30 min was measured.
Atopic dermatitis model
The fur on the nape of the neck was shaved 5 days before treatment. Mice were topically treated once daily with 2 nmol of calcipotriol (MC903, Tocris Bioscience, UK) for five days on left ear skin in 20 μl of ethanol. On the testing day to measure mechanical itch sensitization, mice were habituated for 15 min in the behavioral testing apparatus. Mice then received five separate innocuous mechanical stimuli for ~1 sec delivered using a von Frey filament (0.7 mN) at 3-5 sec intervals at randomly selected sites on the nape surrounding the treated ear (0.5-1 cm distance). The percentage of positive responses in 5 stimuli was measured to indicate mechanical itch sensitization score. After test, mice received once daily calcipotriol treatment for three consecutive days. Spontaneous scratching in 30 min was then measured. To develop atopic dermatitis in hindpaw, mice were topically treated once daily with 2 nmol of calcipotriol for five days on the dorsal skin of right hindpaw, On the testing day to measure mechanical itch sensitization, mice were habituated for 15 min in the behavioral testing apparatus. Mice then received five separate innocuous mechanical stimuli for ~1 sec delivered using a von Frey filament (0.7 mN) at 3-5 sec intervals at randomly selected sites on the dorsal skin of hindpaw.
Drug administration
To block histamine receptors, antagonist of H1 receptor diphenhydramine (50 mg/kg, MilliporeSigma, St. Louis, MO) and antagonist of H4 receptor JNJ7777120 (30 mg/kg, MilliporeSigma, St. Louis, MO) were administered orally as a 20 min pretreatment in a volume of 300 μl sterile saline. Oral administration of 300 μl saline was used as control.
Flagellin (0.9 μg, MilliporeSigma, St. Louis, MO) was dissolved in 20 μl saline for intradermal injection on the skin behind the ears to evoke scratching responses. Flagellin (0.9 μg, MilliporeSigma, St. Louis, MO) and QX-314 (1%, MilliporeSigma, St. Louis, MO) were dissolved in 10 μl distilled PBS for intradermal injection to silence TLR5+ Aβ-LTMRs. QX-314 (1%) was dissolved in 10 μl distilled PBS as control.
For Formalin test, 50 ul of formalin (5%, MilliporeSigma, St. Louis, MO) was injected into the plantar region of the hindpaw and licking, biting or flinching was recorded for an hour.
Surgery
We established spared nerve injury model of neuropathic pain as previously described (Duan et al., 2014). Briefly, von Frey and dynamic allodynia were tested in the lateral plantar region of the left. We performed von Frey and dynamic allodynia test of adult mice before surgery (Day 0), 7 days and 14 days after surgery. To develop inflammatory pain in mice, we injected 20 μl of complete Freund’s adjuvant (CFA, MilliporeSigma, St. Louis, MO) into the mice plantar of the left. Hargreaves test, von Frey test and dynamic allodynia test were performed the next day of CFA injection.
C-Fos Induction
Compound 48/80 (40 μg in 20 μl saline) was injected intradermally into the dorsal to induce c-Fos expression in Ucn3tdTomato mice. Elizabethan collars (Harvard Apparatus, MA, USA) were used to avoid biting-triggered c-Fos induction after compound 48/80 injection. Two hours later, mice were euthanized by isoflurane and perfused with 4% of paraformaldehyde in PBS. The lumber spinal cord was then dissected, post-fixed for 2 hours at 4°C, embedded in OCT and then sagittally sectioned for further c-Fos immunostaining.
Rabies virus tracing
Adult Ucn3::Cre;Lbx1Flpo;Rosa26ds-HTB mice (P42) were anesthetized by isoflurane and laminectomy was performed at the lumber spinal cord (L3-L4). After removing the dura mater with a fine needle and exposing the spinal cord, a fine glass capillary held by the nanoliter injector (WPI, Sarasota, FL) with stereotaxic device (David Kopf Instruments, Tujunga, CA) was inserted into the right side of the dorsal spinal cord. Focal injections of EnvA-pseudotyped, G-deleted-mCherry rabies virus (300 nl; ~1×109 unit per ml, gift from Dr. Zhigang He at Boston Children’s Hospital) were made into the dorsal spinal cord to target Ucn3+ neurons in laminae II-III (250-300 μm depth from the surface) with the control of a micro-controller (Micro4, Sarasota, FL). Mice were perfused 5 days after injection and processed for further ISH or immunohistochemistry staining.
Acute DRG dissociation
As described previously (Lou et al., 2013), mice at P30 were killed by CO2 inhalation and DRGs from T10-L6 were collected in Ca2+ and Mg2+-free Hank’s buffered salt solution (HBSS). DRGs were subsequently treated with papain (1.5 mg/ml, MilliporeSigma, St. Louis, MO) and collagenase/dispase (1 mg/ml, MilliporeSigma, St. Louis, MO) at 37 °C for 10 and 15 min, respectively. Digested DRGs were washed twice with growth medium (Dulbecco’s modified Eagle’s medium (DMEM)-F12 (Thermo Fisher Scientific, Waltham, MA) supplemented with GlutaMAX (Thermo Fisher Scientific, Waltham, MA) and 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA), triturated using fire-polished Pasteur pipettes and plated in a droplet of growth medium on a glass coverslip pre-coated with poly-D-lysine (20 μg/ml, MilliporeSigma, St. Louis, MO) and laminin (20 μg/ml, MilliporeSigma, St. Louis, MO). To allow neurons to adhere, coverslips were kept for 2 h at 37 °C in a humidified 5% incubator.
Ca2+ imaging
Calcium imaging was performed on an inverted microscope (Olympus IX73, Japan) under a 60× lens as previously described (Gong et al., 2016). Acutely dissociated DRG neurons were incubated with 1 μm Fura-2-acetoxymethyl ester (Thermo Fisher Scientific, Waltham, MA) for 20 min at 37°C, washed three times, and incubated in standard extracellular solution (10 mM HEPES [pH 7.4], 5 mM KCl, 145 mM NaCl, 1.2 mM MgCl2, 2.5 mM CaCl2, and 10 mM glucose) for 30 min. Images were acquired with a Roper CoolSnap CCD camera and processed with MetaFluor software (Molecular Devices, San Jose, CA). Flagellin (5 nM), capsaicin (500 nM), and KCl (50 mM) was dissolved in standard extracellular solution.
Electrophysiology
Chronic itch treatment
Calcipotriol (2 nmol in 20 μl ethanol) was dropped on the dorsal surface of right hindpaw of mice (from P17- P23) once daily for seven days. After treatment, light punctate stimuli on the treated dorsal hindpaw by a weak von Frey filament (0.7 mN) elicited biting responses indicative of mechanical itch sensitization. Vehicle (20 μl ethanol) treatment did not influence neuronal excitability and synaptic transmission in Ucn3-tdTomato or NPY-tdTomato neurons. We thus combine vehicle-treated mice and naïve mice as the control group.
Spinal cord slice preparation
As described previously (Cheng et al., 2017), to prepare parasagittal spinal cord slices attached with full length of dorsal root and dorsal root ganglia (DRG), mice (P23-30) were deeply anesthetized with isoflurane (VetOne, Boise, Idaho), decapitated and the lumber spinal cord was rapidly removed and placed in ice-cold modified artificial cerebrospinal fluid (ACSF) containing (in mM): 80 NaCl, 2.5 KCl, 1.25 NaH2PO4 0.5 CaCl2, 3.5 MgCl2, 25 NaHCO3, 75 sucrose, 1.3 sodium ascorbate and 3.0 sodium pyruvate, with pH at 7.4 and osmolality at 310-320 mOsm, bubbled with 95% O2 and 5% of CO2. Spinal cord slices (350-480 μm) attached with dorsal roots and DRGs were cut sagittally by a VT1200s vibratome (Leica, Germany), as illustrated in Figure S5C. To eliminate Aβ-inputs to dorsal horn neurons in some experiments (distinguish Aδ/C inputs from poly Aβ inputs), slices (~350 μm) were prepared as illustrated in Figure S5D. The slice was then incubated for about 1 hour at 33°C in oxygenated (95% O 2 and 5% CO2) cutting solution which contains (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 25 D-glucose, 1.3 sodium ascorbate and 3.0 sodium pyruvate, with pH at 7.2 and osmolality at 310-320 mOsm. All chemicals were purchased from MilliporeSigma (St. Louis, MO). For flagellin plus QX-314 treatment, spinal cord slice with DRG/dorsal root was incubated for 1 hour at 33 μC in oxygenated cutting solution with flagellin (5 nM) and QX-314 (60 μM).
Whole-cell patch clamp recordings
After incubation, spinal cord slices were placed in a recording chamber and perfused with oxygenated recording solution at a rate of 5ml/min at room temperature. Whole-cell recording experiments were then performed on Ucn3-tdTomato or NPY-tdTomato dorsal horn neurons. Borosilicate glass pipettes (Sutter instrument, Novato, CA) with resistance of 3-6 MΩ were then filled with internal solution that contains (in mM): 130 potassium gluconate, 5 KCl, 4 Na2ATP, 0.5 NaGTP, 20 HEPES, 0.5 EGTA, pH 7.28 with KOH, and measured osmolality at 310-320 mOsm. Data were acquired by pClamp 10.0 software (Molecular Devices, San Jose, CA) with MultiClamp 700B patch-clamp amplifier and Digidata 1550B (Molecular Devices, San Jose, CA). Responses were low-pass filtered on-line at 2 kHz, and digitized at 5 kHz.
Optogenetics
Spinal cord slices were transversely cut from NPY::Cre; LBx1Flpo;Ucn3::GFP;Ai80D mice, placed under a 60X water-immersion objective on an upright fluorescent microscope (Scientifica SliceScope) equipped with a digital camera (U-CMAD3, Olympus). To avoid desensitization of ChR2 (L132C), Ucn3GFP neurons were visualized by 6% intensity of the 473nm light. The ion channel ChR2 (L132C) was stimulated by flashing 100% intensity of 473 nm light (1 ms pulses; 2.3 mW) on the whole slice through the light path of the microscope using a light-emitting diode (LED, pE-300, CooLED) under computer control. A total of 5 mice were used for recording experiments.
Characterization of firing pattern
Neurons were classified based on the responses to continuously injected depolarizing currents. Tonic neurons exhibited continuous discharge over the entire period of current injection of supra-threshold currents, with relatively constant inter-spike intervals. Adapting tonic spiking neurons have sustained firings after continuous injection of supra-threshold depolarizing current, with relatively increased intervals. Initial bursting neurons discharged at the beginning of the continuous injection of supra-threshold depolarizing current. Single neurons discharged once or twice immediately after the supra-threshold depolarizing current injection, but never discharged again during the following sustained current injection.
Dorsal root stimulation
Aβ, Aδ and C-fiber-mediated synaptic inputs onto the spinal dorsal horn neurons were screened by 25 μA, 50 μA and 500 μA stimulus (pulse width 0.1 ms), respectively (Figure S5A and S5B). Different responses of dorsal horn neurons to primary afferent inputs were recorded under different recording conditions. Firstly, evoked excitatory postsynaptic currents (eEPSCs) were detected by holding membrane potential at −70mV, which minimized evoked inhibitory postsynaptic currents (eIPSCs) (Yoshimura and Jessell, 1990). Whether a neuron receives Aβ, Aδ or C-fiber inputs directly (mono-eEPSC) or indirectly (poly-eEPSC) were determined under this recording condition. Monosynaptic inputs for Aβ, Aδ or C fibers were determined by high frequency stimulation at 20, 2 or 1 Hz, respectively (Torsney and MacDermott, 2006). Transduction velocity was also used to determine monosynaptic inputs: Aβ, 2.16-4.06 m/s; Aδ, 0.92-1.04 m/s; C, 0.18-0.62 m/s. Secondly, eIPSCs were recorded by holding membrane potential at 0 mV when eEPSCs were minimized. Bicuculline (10 μM, MilliporeSigma, St. Louis, MO) and/or strychnine (2 μM, MilliporeSigma, St. Louis, MO) were used to disinhibit dorsal horn neurons. Thirdly, dorsal root stimulation-evoked IPSP, EPSP, or APs were detected by current clamp recording at the resting membrane potential. Action potential firing patterns were determined by current clamp recording at the resting membrane potential.
QUANTIFICATION AND STATISTICAL ANALYSIS
Results are expressed as mean ± SEM. Statistical analysis was performed in Prism 7 (GraphPad). A threshold of P < 0.05 was accepted as statistically different and P > 0.05 considered non-significant. For locomotion coordination, touch, itch, acute pain and inflammatory pain assessment, data were subjected to Student’s t tests. For dual ablation experiments, data were subjected to one-way ANOVA with Tukey’s post hoc analysis. For SNI-induced neuropathic pain, time-course measurements were assessed by Bonferroni’s post hoc ANOVA. For statistical analysis of incidence of electrophysiological results, data were analyzed with Chi-square (χ2) test. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications (Bourane et al., 2015; Duan et al., 2014).
DATA AND CODE AVAILABILITY
This study did not generate/analyze datasets or code.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-NK1R | MilliporeSigma | Cat# S8305 |
| Rabbit anti-α-CGRP | Peninsula Lab | Cat# T- 4032 |
| Alexa Fluor 488-conjugated Isolectin GS-IB4 | ThermoFisher | Cat# I21411 |
| Rabbit anti-PKCγ | Santa Cruz | Cat# sc-211 |
| Rabbit anti-Pax2 | ThermoFisher | Cat# 71-6000 |
| Rabbit anti-c-Fos | MilliporeSigma | Cat# ABE457 |
| Goat anti-tdTomato | SICGEN | Cat# AB8181-200 |
| Guinea pig anti-gephyrin | Synaptic Systems | Cat# 147 318 |
| Mouse anti-VGAT | Synaptic Systems | Cat# 131 011 |
| Rabbit anti-TH | MilliporeSigma | Cat# AB152 |
| Mouse anti-TLR5 | Santa Cruz | Cat# sc-517439 |
| Rabbit anti-NF200 | MilliporeSigma | Cat# AB1989 |
| Chicken anti-NF200 | Abcam | Cat# ab4680 |
| Goat anti-Rabbit lgG (H+L) Alexa Fluor plus 647 | ThermoFisher | Cat# A32733 |
| Goat anti-Guinea Gig IgG (H+L) Alexa Fluor plus 647 | ThermoFisher | Cat# A21450 |
| Goat anti-Mouse IgG (H+L) Alexa Fluor plus 488 | ThermoFisher | Cat# A32723 |
| Donkey anti-Goat IgG (H+L) Alexa Fluor plus 568 | ThermoFisher | Cat# A11057 |
| DyLight 405 AffiniPure Donkey Anti-Mouse IgG (H+L) | Jackson ImmunoResearch | Cat# 715-475-150 |
| Bacterial and Virus Strains | ||
| EnvA-pseudotyped, G-deleted-mCherry rabies virus | Generated by He lab | Addgene #32636 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Diphtheria toxin | MilliporeSigma | Cat# D0564 |
| Clozapine N-oxide | MilliporeSigma | Cat# C0832 |
| Bombesin-saporin | Advanced targeting Systems | Cat# IT-40 |
| Nppb-saporin | Advanced targeting Systems | Cat# IT-69 |
| Compound 48/80 | MilliporeSigma | Cat# C2313 |
| Chloroquine | MilliporeSigma | Cat# C6628 |
| Me-5-HT | MilliporeSigma | Cat# SML0640 |
| SLIGRL | Bachem | Cat# 4033483 |
| SADBE | MilliporeSigma | Cat# 339792 |
| Calcipotriol | Tocris Bioscience | Cat# 2700 |
| Diphenhydramine | MilliporeSigma | Cat# D3630 |
| JNJ7777120 | MilliporeSigma | Cat# J3770 |
| Complete Freund’s adjuvant | MilliporeSigma | Cat# 344289 |
| Capsaicin | Tocris Bioscience | Cat# 0462 |
| Histamine | MilliporeSigma | Cat# H7250 |
| Bicuculline | MilliporeSigma | Cat# B7561 |
| Strychnine | MilliporeSigma | Cat# 57-24-9 |
| Flagellin from Salmonella typhimurium | MilliporeSigma | Cat# SRP8029 |
| QX-314 | MilliporeSigma | Cat# L5783 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Tg(Ucn3-cre)KF43Gsat/Mmucd | Gensat | 032078-UCD |
| Mouse: B6.FVB(Cg)-Tg(Npy-cre)RH26Gsat/Mmucd | Gensat | 037423-UCD |
| Mouse: B6;129S-Lbx1 < tm1(flpo)Gou > (Lbx1-Flpo) | Generated by Goulding Lab | Duan et al., 2014 |
| Mouse: B6;129S6-Gt(ROSA)26Sortm9(CAG-mCherry,-CHRM4*)Dym/J (Rosa26CAG-FSF-LSL-hM4Di) | Jackson Laboratory | 029040 |
| Mouse: B6. Cg-Gt(ROSA)26Sortm3.2(CAG-EGFP,-CHRM3*/mCherry/Htr2a)Pjen/J (Rosa26CAG-FSF-LSL-hM3Dq | Jackson Laboratory | 026942 |
| Mouse: Rosa26CAG-LSL-FSF-HTB | Generated by Goulding Lab | Bourane et al., 2015 |
| Mouse: Nav1.8-Cre | Generated byWood lab | Stirling et al., 2005 |
| Mouse: B6.129-Tau < tm1(LSL.FSF.DTR)Gou > (Tau-loxP-STOP-loxP-FRT-STOP-FR T-DTR | Generated by Goulding Lab | Duan et al., 2014 |
| Mouse: ROSA26iDTR (Rosa26LSL-DTR) | Jackson Laboratory | 007900 |
| Mouse: Ai14 | Jackson Laboratory | 007914 |
| Mouse: Ai34 | Jackson Laboratory | 012570 |
| Mouse: Ai80 | Jackson Laboratory | 025109 |
| Mouse: B6.129S1-Tlr5tm1Flv/J (Tlr5 KO) | Jackson Laboratory | 008377 |
| Software and Algorithms | ||
| Prism 7 | GraphPad | RRID:SCR_002798 |
| MetaFluor | Molecular Devices | RRID:SCR_014294 |
| pClamp 10.0 | Molecular Devices | RRID:SCR_011323 |
Highlights.
Spinal Ucn3+ neurons transmit both mechanical itch and persistent spontaneous itch.
Spinal Ucn3+ neurons receive inputs from TLR5+ LTMRs.
Spinal NPY+ inhibitory interneurons gate Ucn3+ neurons.
Sensitization of mechanical itch pathway contributes to chronic itch.
ACKNOWLEDGMENTS:
This work is supported by startup funds to B.D. from the Department of MCDB, Neuroscience Scholar Program, University of Michigan and in part by National Institutes of Health (NIH) grant R01NS109170 (B.D.). We thank Dr. Qiufu Ma for Tac2-Cre knock-in mice, Dr. Zhou-Feng Chen for GRP-Cre knock-in mice, Dr. Bradford Lowell for VGLUT3-Cre knock-in mice, Dr. John Wood for Nav1.8-Cre mice, Dr. Martyn Goulding for Lbx1Flpo, Tauds-DTR , and Rosa26ds-HTB mice, Dr. Susan Dymecki for Rosa26CAG-ds-hM4Di mice, Dr. Patricia Jensen for Rosa26CAG-ds-hM3Dq mice, Dr. Hongkui Zeng for Ai14, Ai34 (shared by Dr. Dawen Cai), Ai65, and Ai80 (shared by Dr. Qiufu Ma) mice. We thank Drs. Zhigang He, Chen Wang and Yuanyuan Liu for sharing EnvA-pseudotyped, G-deleted-mCherry rabies virus. We thank Dr. Brian Pierchala for sharing chicken anti-NF200 antibody. We are grateful to Drs. John Kuwada, Rich Hume, Haoxing Xu, Bing Ye and Hongzhen Hu for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments from other members of the Duan laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS: Authors declare no competing interests.
REFERENCES
- Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, and Takeuchi O (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, and Carstens E (2012). Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol 132, 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Delahanty J, Carstens MI, and Carstens E (2015). A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain 156, 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HH, Akiyama T, Nattkemper LA, van Laarhoven A, Elberling J, Yosipovitch G, and Arendt-Nielsen L (2018). Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain 159, 1185–1197. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, and Hoon MA (2014). Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 17, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford RG (1938). Experiments relating to the itch sensation, its peripheral mechanism and central pathways. . Clin Sci 3, 377–386. [Google Scholar]
- Binshtok AM, Bean BP, and Woolf CJ (2007). Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449, 607–610. [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, and Goulding M (2015). Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, and Basbaum AI (2014). Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, and Waisman A (2005). A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2, 419–426. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Braz JM, Wang X, Solorzano C, Sulk M, Buhl T, Steinhoff M, and Basbaum AI (2017). Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J Allergy Clin Immunol 140, 454–464 e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia-Campmany L, Ren X, Vong L, Lowell BB, et al. (2017). Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat Neurosci 20, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, and Ma Q (2005). Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci 8, 1510–1515. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003). Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26, 1–30. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. (2018). A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, and Dong X (2018). Peripheral and Central Mechanisms of Itch. Neuron 98, 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Luo J, Yang P, Du J, Kim BS, and Hu H (2018). Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360, 530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, and Alexopoulou L (2006). Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A 103, 12487–12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K, Qu L, Shimada SG, Nie H, and LaMotte RH (2014). Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett 579, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M, Miyachi Y, and Ikoma A (2013). Mechanically evoked itch in humans. Pain 154, 897–904. [DOI] [PubMed] [Google Scholar]
- Gao T, Ma H, Xu B, Bergman J, Larhammar D, and Lagerstrom MC (2018). The Neuropeptide Y System Regulates Both Mechanical and Histaminergic Itch. J Invest Dermatol 138, 2405–2411. [DOI] [PubMed] [Google Scholar]
- Gong J, Yuan Y, Ward A, Kang L, Zhang B, Wu Z, Peng J, Feng Z, Liu J, and Xu XZS (2016). The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor. Cell 167, 1252–1263 e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DT, Goodell H, and Wolff HG (1951). Neural mechanisms involved in itch, itchy skin, and tickle sensations. J Clin Invest 30, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. (2013). A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, and Aderem A (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- Huang J, Polgar E, Solinski HJ, Mishra SK, Tseng PY, Iwagaki N, Boyle KA, Dickie AC, Kriegbaum MC, Wildner H, et al. (2018). Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci 21, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, and Schmelz M (2006). The neurobiology of itch. Nat Rev Neurosci 7, 535–547. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, et al. (2014). Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte RH (1988). Psychophysical and neurophysiological studies of chemically induced cutaneous pain and itch. The case of the missing nociceptor. Prog Brain Res 74, 331–335. [PubMed] [Google Scholar]
- LaMotte RH, Dong X, and Ringkamp M (2014). Sensory neurons and circuits mediating itch. Nat Rev Neurosci 15, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Harper AA, Harper EI, Garson JA, and Anderton BH (1984). A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol 228, 263–272. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, et al. (2001). Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A 98, 7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. (2011). The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, and Chambon P (2006). Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A 103, 11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, and Dong X (2012). Mechanisms of itch evoked by beta-alanine. J Neurosci 32, 14532–14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. (2009). Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, and Ma Q (2013). Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci 33, 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, and Xiong L (2013). A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123, 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Holzman S, and Hoon MA (2012). A nociceptive signaling role for neuromedin B. J Neurosci 32, 8686–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, and Hoon MA (2013). The cells and circuitry for itch responses in mice. Science 340, 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Nojima H, Shinkado T, Nakahashi T, and Kuraishi Y (2002). Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol 88, 285–292. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, and Basbaum AI (2008). Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci 28, 7936–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni XB, Jia N, Jiang BG, Sun T, Zheng YC, Huo QB, Liu K, Ma L, Zhao QM, Yang H, et al. (2014). Lyme borreliosis caused by diverse genospecies of Borrelia burgdorferi sensu lato in northeastern China. Clin Microbiol Infect 20, 808–814. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, and Dymecki SM (2011). Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. (2010). Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, and Regehr WG (1999). Timing of synaptic transmission. Annu Rev Physiol 61, 521–542. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, and Jensen P (2016). Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep 15, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, and Dib-Hajj SD (2012). Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, and Nassar MA (2005). Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain 113, 27–36. [DOI] [PubMed] [Google Scholar]
- Sun YG, and Chen ZF (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, and Chen ZF (2009). Cellular basis of itch sensation. Science 325, 1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirtchener EB (1909). A Text-Book of Psychology. Part I. The Macmillan Co, New York. [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, and MacDermott AB (2006). Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26, 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Willis W, and Coggeshall R (2004). Sensory Mechanisms of the Spinal Cord, Vol 1 (Kluwer Achademic/Plenum Publishers; ). [Google Scholar]
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, and Ji RR (2015). Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 21, 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, and Jessell T (1990). Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol 430, 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G, and Bernhard JD (2013). Clinical practice. Chronic pruritus. N Engl J Med 368, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, and Yevenes GE (2012). Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev 92, 193–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Zhang YQ, Goulding M, Wang YQ, and Ma Q (2018). Timing Mechanisms Underlying Gate Control by Feedforward Inhibition. Neuron 99, 941–955 e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets or code.