Abstract
Purpose
Heart transplantation causes denervation of the donor heart, but the consequences for cardiovascular homeostasis remain to be fully understood. The present study investigated cardiovascular autonomic control at supine rest, during orthostatic challenge and during isometric exercise in heart transplant recipients (HTxR).
Methods
A total of 50 HTxRs were investigated 7–12 weeks after transplant surgery and compared with 50 healthy control subjects. Continuous, noninvasive recordings of cardiovascular variables were carried out at supine rest, during 15 min of 60° head-up tilt and during 1 min of 30% of maximal voluntary handgrip. Plasma and urine catecholamines were assayed, and symptoms were charted.
Results
At supine rest, heart rate, blood pressures and total peripheral resistance were higher, and stroke volume and end diastolic volume were lower in the HTxR group. During tilt, heart rate, blood pressures and total peripheral resistance increased less, and stroke volume and end diastolic volume decreased less. During handgrip, heart rate and cardiac output increased less, and stroke volume and end diastolic volume decreased less. Orthostatic symptoms were similar across the groups, but the HTxRs complained more of pale and cold hands.
Conclusion
HTxRs are characterized by elevated blood pressures and total peripheral resistance at supine rest as well as attenuated blood pressures and total peripheral resistance responses during orthostatic challenge, possibly caused by low-pressure cardiopulmonary baroreceptor denervation. In addition, HTxRs show attenuated cardiac output response during isometric exercise due to efferent sympathetic denervation. These physiological limitations might have negative functional consequences.
Keywords: Heart transplantation, Autonomic cardiovascular control, Catecholamines, Denervation
Introduction
Heart transplantation (HTx) remains the treatment of choice for end-stage heart failure, offering improved survival and quality of life for the recipients (Lund et al. 2017; Alraies and Eckman 2014). Normally, the heart as well as peripheral vessels are intimately controlled by the autonomic nervous system, ensuring immediate compensatory responses to all homeostatic aberration, which occurs—for instance—during orthostatic challenge and isometric exercise. However, cardiac transplantation results in surgical denervation of the donor heart, with complete loss of both efferent and afferent autonomic connections.
Denervation results in altered cardiovascular control and performance, including impaired cardiovascular reflex responses (Banner et al. 1990; Idema et al. 1994; Doering et al. 1996; Raczak et al. 1999; Awad et al. 2016). Although the functional consequences of these alterations have not been thoroughly investigated, denervation might contribute importantly to several important phenomena among HTx recipients (HTxRs). First, a tendency towards hypertension and peripheral vasoconstriction is well known among HTxRs (Idema et al. 1994; Bennett and Ventura 2017), but the underlying mechanisms remain to be fully understood, including whether there is a causal link between denervation and hypertension development. Second, being in an upright position might conceivably be difficult for HTxRs, as the baroreceptor reflex-mediated increase in heart rate during transition from supine to upright position is absent in a denervated heart. Still, symptoms of orthostatic intolerance do not appear to be more common among HTxRs than healthy controls; however, whether this is due to enhanced peripheral vessel responses has not been well addressed (Banner et al. 1990; Doering et al. 1991, 1996; Fitzpatrick et al. 1993). Third, HTxRs show reduced exercise capacity (Nytrøen and Gullestad 2013), but it is not known to what extent factors other than attenuated heart rate acceleration and myocardial contractility might contribute to this phenomenon; in particular, the potential role of altered peripheral vessel responses during exercise has not been focused on in previous reports.
Cardiovascular responses to orthostatic challenge have been extensively studied in healthy adults (Dambrink and Wieling 1987; Sprangers et al. 1991; Toska and Walloe 2002). The autonomic reflex adjustments during transition to upright position are characterized by altered afferent signaling from low-pressure cardiopulmonary baroreceptors as well as high-pressure arterial baroreceptors, normally resulting in increased heart rate due to enhanced sympathovagal balance to the heart as well as increased total peripheral resistance due to enhanced peripheral sympathetic activity. Cardiovascular responses to isometric exercise, on the other hand, are not dependent on afferent information from the heart, but are mainly due to afferent signaling from the working muscle combined with a “central command” in the brain (Kamiya et al. 2000). This results in a gradual increase in the set point of the barostat, normally enhancing sympathetic nervous activity to the heart as well as peripheral vasculature.
In a study of HTxRs, an experimental approach in which participants undergo both an orthostatic test (head-up tilt) and an isometric exercise test (handgrip) might be beneficial, as this combined setup might differentiate between effects of afferent and efferent cardiac denervation. Orthostatic responses are presumably affected by both afferent denervation of low-pressure cardiopulmonary baroreceptors and efferent denervation of the sinoatrial node and myocardium, whereas isometric exercise responses are affected by efferent denervation only. To the best of our knowledge, no previous study of HTxRs has applied a similar experimental approach. In addition, previous reports of orthostatic responses (Banner et al. 1990; Doering et al. 1991, 1996; Fitzpatrick et al. 1993) and isometric exercise responses (Roca et al. 1991; Brunner-La Rocca et al. 1998) in HTxRs are hampered by a small number of participants.
Thus, the aim of the present study was to investigate cardiovascular autonomic control at supine rest, during orthostatic challenge and during isometric exercise in a relatively large group of HTxRs and compare them to healthy control subjects. In particular, the study was designed to delineate the characteristics of peripheral vascular responses in HTxRs. We hypothesized that HTxRs would have increased total peripheral resistance during supine rest, enhanced total peripheral resistance responses to orthostatic challenge and unaltered responses to isometric exercise.
Materials and methods
Design
This study is part of the AccHEART project (Autonomic Cardiovascular Control after Heart Transplantation; ClinicalTrials ID: NCT01759966), which addresses autonomic denervation and reinnervation in a population-based prospective cohort of HTxRs. The present study reports results from the first encounter (baseline) only, compared with a group of healthy controls. AccHEART has been approved by the Norwegian National Committee for Ethics in Medical research. Participation was based upon informed consent.
Participants
All patients at the Department of Cardiology, Oslo University Hospital receiving a heart transplant between January 2013 and December 2015 were screened for eligibility. To be included, we required the age of the recipient to be between 16 and 70 years and the transplant surgery to have been performed during the last 7–12 weeks (Table 1). This time window was chosen to ensure no interference from cardiovascular, neuroendocrine and inflammatory responses related to the surgical procedure per se, and at the same time avoid any reinnervation processes to have taken place. Exclusion criteria included dysfunction of the allograft, other chronic medical conditions, ECG abnormalities, other acute medical complications, and non-compliance. Drug usage was not considered an exclusion criterion; all eligible patients received immunosuppressive therapy (cyclosporine A/tacrolimus/everolimus, mycophenolate mofetil, corticosteroids) and statins, and the majority also received cardiovascular pharmaceuticals.
Table 1.
Criteria for inclusion and exclusion in AccHeart
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Heart transplant recipients (HTxR) |
Completed heart transplantation during the last 7–12 weeks Age > 16 years and < 70 years |
Peri- or postoperative complications causing permanent dysfunction of the allograft (such as hyperacute rejection episodes, severe myocardial ischemia, etc.) Other chronic medical conditions, such as: Diabetes with HbA1C > 7.5% (mean value during 6 months prior to HTx) and/or manifest diabetic complications Renal failure with plasma creatinine > 200 µmol/l ECG abnormalities (scattered ectopic beats and minor conduction problems are allowed) Medical instability, non-compliance, multiorgan recipients, active infection or permanently bed-ridden patients |
| Healthy controls | Age and sex matching the patients |
Other chronic diseases (such as diabetes mellitus) Permanent use of pharmaceuticals (including hormone drugs) Pregnancy |
In addition, a volunteer group of healthy controls having the same distribution of sex and age as the HTxR group was recruited from the hospital staff and the general community population.
Investigational program
All participants attended a 3-day investigational program at the Oslo University Hospital, Norway. The program was executed between 7.30 and 11.00 a.m. and was carried out in a fixed sequence. It included a clinical examination, autonomic cardiovascular control assessment, blood, and urine sampling, and questionnaire charting. All participants were instructed to abstain from tobacco products and caffeine 48 h prior to attendance, and to fast overnight. They were maintained on immunosuppressive medications, while all other drugs were paused on the morning of testing. They brought morning spot urine in a sterile container, and were instructed to apply a local anesthetic ointment (EMLA®, AstraZeneca) on both antecubital areas 1 h before arriving.
Autonomic cardiovascular assessment
The Task Force Monitor® (Model 3040i, CNSystems Medizintechnic, Graz, Austria) is a combined hardware and software device for noninvasive continuous recording of cardiovascular variables (Fortin et al. 2006). In the present study, recordings were performed (1) during 5-min supine rest; (2) during 15 min of 60° head-up tilt; (3) during 60 s of 30% of maximal voluntary handgrip. The handgrip procedure was repeated twice.
The duration of orthostatic challenge in the tilt procedure was shorter than what is usual in clinical settings (15 vs 30 min), to avoid syncope and other manifestations of failing cardiovascular homeostasis. Prior to initiation of the autonomic tests, participants were asked to perform maximum left-sided handgrip for 10 s, using an electronic device that provides a continuous display of the force (GRIP-IT s/n: 120521, Load Indicator System AB, Askim, Sweden). Based on the maximum value, the 30% level was calculated, and the participants used a couple of minutes to familiarize with this force.
Instantaneous heart rate (HR) was obtained from the electrocardiogram. Continuous arterial blood pressure was measured noninvasively beat-to-beat by finger plethysmography (Parati et al. 1989). The finger blood pressure values were automatically calibrated every fifth minute against conventional oscillometric upper arm measurements of arterial blood pressure. Impedance cardiography with electrodes placed on the neck and lower thorax was used to obtain a continuous recording of the temporal derivate of the transthoracic impedance (dZ/dt) (Denniston et al. 1976).
All primary cardiovascular variables (beat-to-beat recordings) were manually inspected and artifacts (such as non-sine node beats) were removed. Thereafter, beat-to-beat stroke volume (SV) was calculated from the impedance signal. Cardiac output (CO) was calculated as SV times HR, and total peripheral resistance (TPR) was calculated as mean blood pressure divided by CO. Flow-dependent variables were indexed according to body surface area (BSA), estimated from the formula BSA = 0.0235 × height (cm)0.42246 × weight (kg)0.51456.
For each individual, the median values of all cardiovascular variables were computed in the following epochs: (1) from 30 to 270 s. after start of supine rest; (2) from 30 to 270 s. after 60° head-up tilt; and (3) from 57 to 60 s. After initiation of each handgrip procedure, the mean value across the two most representative series of cardiovascular recordings (based on manual inspection) were used in subsequent analyses.
The epochs were defined in accordance with previously established routines at our laboratory (Wyller et al. 2007, 2008). For supine rest and tilt, the epochs correspond to the approximate steady-state situations before and after the application of orthostatic challenge, avoiding periods of mentally evoked autonomic activity and reflexive autonomic adjustments immediately before and after infliction of orthostatic challenge. For handgrip, the short (3 s) epoch was chosen to obtain the most extreme (maximal) value in each individual.
The cardiovascular response to tilt and handgrip was defined as the delta values (i.e., tilt values—supine rest values and handgrip values—supine rest values, respectively). Finally, mean values across all individuals in each group (HTxRs and healthy controls) were computed.
Laboratory assays
Blood samples for catecholamine analysis were obtained from venous puncture in vacutainer tubes treated with ethylene glycol tetra acetic acid and glutathione from Sigma-Aldrich (St. Louis, USA). They were immediately put on ice and centrifuged (2500 rpm, 10 min, 4 °C) within 15 min. Both plasma epinephrine and norepinephrine were analyzed by high-performance liquid chromography (HPLC) (Agilent Technologies, Santa Clara, CA, USA) with a reversed-phase C-18 column (Chromsystem, München, Germany) and electrochemical detector (Antec, Leyden Decade II SCC, Zoeterwoude, The Netherlands) using a commercial kit from Chromsystem. The intra- and inter-assay coefficients of variation (CV) were 3.9% and 10.8%, respectively. Urine samples for catecholamine analysis were acidified to pH ~ 2.5 after collections, and thereafter analyzed by the same HPLC system as for plasma catecholamines. The intra- and inter-assay CVs were 3.9 and 5.2%, respectively. N-terminal pro-brain natriuretic peptide (NT-pBNP) in plasma was assayed at the accredited laboratory at Oslo University Hospital, Norway using routine procedures.
Questionnaire
The autonomic symptom profile (ASP) is a validated inventory for assessing autonomic symptoms (Suarez et al. 1999). A composite score reflecting orthostatic symptoms was constructed from 8 single items from the ASP, addressing experiences of dizziness in specific situations (such as rising suddenly from supine position, taking a shower, etc.). The total sum score is from 0 to 8; higher values reflect more pronounced orthostatic problems. In addition, complaints of pale and cold hands were charted on a 1–5 Likert scale. A few complementary questions addressing personal symptoms and demographic data were added.
Other variables
Background clinical information regarding the HTxR group was obtained from patients’ medical records. Activity level (steps/day) was charted using the activPAL accelerometer device (PAL Technologies, Glasgow, Scotland) for 7 consecutive days (thus including weekend days in all individuals) (Grant et al. 2006). Supine blood pressures were measured by a standard oscillometric device. Cardiac ejection fraction was obtained from transthoracic echocardiography, using the Simpson biplane method.
Statistics
Statistical analyses were carried out using SPSS statistical software (SPSS Inc., Chicago, IL, USA). Previously, studies with identical experimental setup have been conducted at our institution; in the present study, power estimation suggested that a total of 50 participants in each group would support a power of about 80% to detect a medium effect size (Cohen’s d ≈ 0.5). Results are presented with mean (standard deviation) or median (interquartile range) for continuous data, depending on distribution. Categorical data are reported with frequencies. Statistical tests of differences between HTxRs and healthy controls were performed applying Student t test, Mann–Whitney U test, Chi square test or Fisher’s exact test as appropriate. Relationships between selected variables within the HTxR group were explored by Pearson correlation analyses. A p value ≤ 0.05 was considered statistical significant, and all tests were carried out two-sided. As several variables are strongly intercorrelated, p values were not adjusted for test multiplicity.
Results
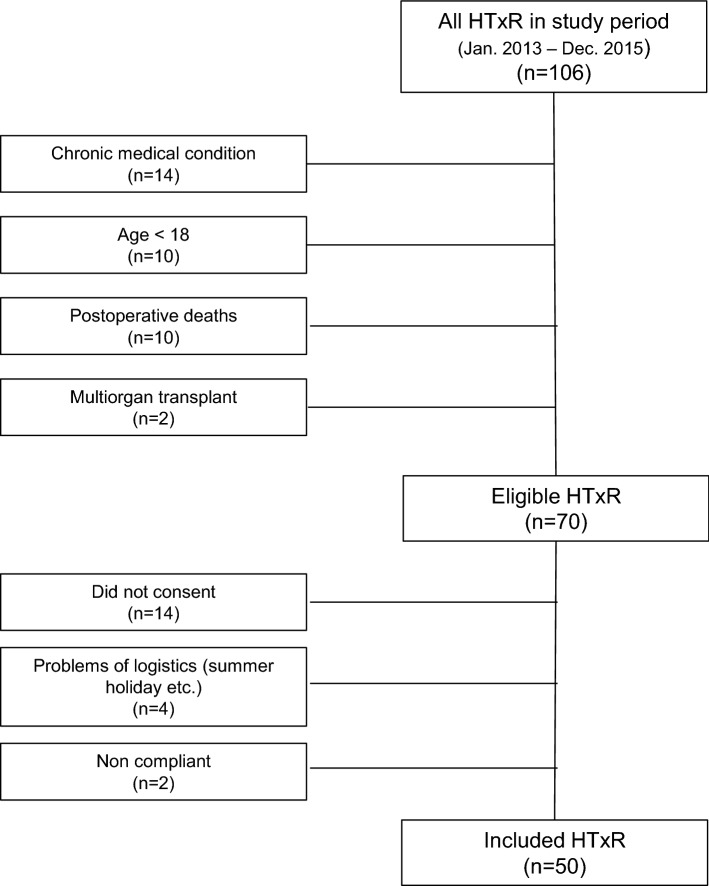
A total of 106 patients received a heart transplant during the study period and was screened for eligibility in AccHEART. A total of 70 HTxRs were found to be eligible, of which 50 were finally enrolled after a mean (standard deviation) time of 2.5 (0.4) months after HTx surgery (Fig. 1). The remaining non-attending transplant recipients (n = 20) were comparable to the study participants regarding demographics and pre- and postoperative characteristics (Table 2).
Fig. 1.
Flowchart of HTxR inclusion in AccHEART
Table 2.
Characteristics of eligible heart transplant recipients during the study perioda, included vs. not included
| Included in AccHEART | Not included in AccHEART | p valueb | |
|---|---|---|---|
| Number | 50 | 20 | n.a. |
| Sex—no. (%) | |||
| Male | 35 (70) | 13 (65) | 0.684 |
| Female | 15 (30) | 7 (35) | |
| Ethnicity—no. (%) | |||
| Norwegian | 46 (92) | 17 (85) | 0.378 |
| Not Norwegian | 4 (8) | 3 (15) | |
| Age—years, mean (SD) | 48.2 (13.0) | 47.9 (13.4) | 0.924 |
| Heart failure prior to HTx—years, mean (SD) | 5.8 (5.5) | 7.1 (4.5) | 0.364 |
| Waiting list for HTx—months, mean (SD) | 3.1 (3.4) | 4.1 (3.9) | 0.305 |
| Causes of heart failure—no. (%) | |||
| Ischemic heart disease | 13 (26) | 7 (35) | 0.495 |
| Dilated cardiomyopathy | 23 (46) | 9 (45) | |
| Other | 14 (28) | 4 (20) | |
| Donor age—years, mean (SD) | 36.4 (13.4) | 42.4 (16.6) | 0.120 |
| Graft ischemic time—min, mean (SD) | 172 (80) | 145 (85) | 0.229 |
SD standard deviation, HTx heart transplant
aFrom January 2013 until December 2015. All HTx recipients at the Oslo University Hospital (the only transplant center in Norway) were screened for eligibility in AccHEART
bBased upon Chi square test, Fisher’s exact test or Student t test, as appropriate
The included HTxRs underwent either primary single-organ orthotopic HT (n = 46) or re-transplantation (n = 4). The bicaval surgical technique with a small left atrial cuff and cavoatrial anastomoses was applied to all cases. They were maintained on immunosuppressive therapy, consisting of cyclosporine A (CsA) and mycophenolate mofetil in the majority of male recipients and tacrolimus and mycophenolate mofetil in the majority of female recipients. In patients with renal dysfunction (n = 13), a combination of low-dose CsA and the mTOR inhibitor everolimus was initiated. In addition, corticosteroids and statins were given to all HTxRs, and a total of 15 patients were on beta-blocking agents. Routine surveillance myocardial biopsies (each week during the first 8 weeks and then after 10 and 12 weeks) revealed an episode of acute rejection in a total of 14 HTxRs prior to inclusion in the AccHEART study. At the time of inclusion, acute rejection was revealed in five of the recipients (2R in 3 patients and 1R in 2 patients); all without symptoms and with normal heart function.
The HTxRs did not differ significantly from the healthy controls regarding sex, age, ethnicity and body mass index (Table 3). However, glomerular filtration rate and steps per day were lower, and HbA1c was higher in the HTxR group.
Table 3.
Background characteristics of study participants
| HTx recipients | Healthy controls | p valuea | |
|---|---|---|---|
| Number | 50 | 50 | n.a. |
| Sex—no. (%) | |||
| Male | 35 (70) | 35 (70) | 1.000 |
| Female | 15 (30) | 15 (30) | |
| Ethnicity—no. (%) | |||
| Norwegian | 46 (92) | 48 (96) | 0.678 |
| Not Norwegian | 4 (8) | 2 (4) | |
| Age—years, mean (SD) | 48.2 (13.0) | 47.8 (12.4) | 0.861 |
| Body mass index—kg/m2, mean (SD) | 24.8 (3.8) | 25.2 (3.0) | 0.586 |
| Glomerular filtration rate, estimated—ml/min/1.73 m2, mean (SD) | 55.8 (16.6) | 92.2 (19.0) | < 0.001 |
| HbA1c—%, mean (SD) | 5.7 (0.7) | 5.2 (0.3) | < 0.001 |
| Steps per day—number, mean (SD) | 5409 (2345) | 8577 (3158) | < 0.001 |
| Rejection episodes—no. (%) | n.a. | n.a. | |
| No rejection | 31.00 (62) | ||
| Grade 1 | 11.00 (22) | ||
| Grade 2 or more | 8.00 (16) | ||
Numbers in bold indicate a p value < 0.05
SD standard deviation, HTx heart transplant
aBased upon Chi square test, Fisher’s exact test, or Student t test, as appropriate
Systolic blood pressure (measured supine using standard oscillometric technique) was slightly, but significantly increased in the HTxR group, whereas diastolic blood pressure was identical, as were left ventricular ejection fraction (Table 4). Levels of catecholamines were similar across groups, except for a slightly lower urine norepinephrine:creatinine ratio among HTxRs. Plasma NT-pBNP was significantly higher in the HTxR group, and showed a negative correlation with estimated glomerular filtration rate (Pearson’s r = − 0.34, p = 0.017). Orthostatic symptom score was identical across groups, whereas HTxRs complained more of pale and cold hands.
Table 4.
Cardiovascular markers, neuroendocrine markers and symptoms in HTx recipients and healthy controls
| HTx recipients | Healthy controls | p valuea | Adj. p valueb | |
|---|---|---|---|---|
| Cardiovascular markers | ||||
| Systolic blood pressure, supine—mmHg, mean (SD) | 131.5 (14.9) | 125.4 (14.6) | 0.040 | 0.082 |
| Diastolic blood pressure, supine—mmHg, mean (SD) | 78.9 (8.4) | 78.1 (9.1) | 0.638 | 0.960 |
| Left ventricular ejection fraction—%, mean (SD) | 58.5 (5.4) | 58.2 (4.6) | 0.799 | 0.164 |
| Neuroendocrine markers | ||||
| Plasma NT-pBNP—ng/l, median (IQR) | 901 (926) | 51 (66) | < 0.001 | < 0.001 |
| Plasma norepinephrine—pmol/l, median (IQR) | 2072 (1844) | 2041 (1139) | 0.697 | 0.375 |
| Urine norepinephrine:creatinine ratio—nmol/mmol, median (IQR) | 9.0 (7.2) | 12.2 (8.3) | 0.046 | 0.178 |
| Plasma epinephrine—pmol/l, median (IQR) | 577 (405) | 563 (373) | 0.563 | 0.577 |
| Urine epinephrine:creatinine ratio—nmol/mmol, median (IQR) | 1.5 (1.2) | 1.7 (1.2) | 0.175 | 0.665 |
| Symptoms | ||||
| Orthostatic symptoms—total score, mean (SD) | 0.7 (0.8) | 0.5 (0.8) | 0.206 | n.a.c |
| Pale and cold hands—score, mean (SD) | 1.6 (1.2) | 1.3 (0.9) | 0.038 | n.a.c |
Numbers in bold indicate a p value < 0.05
NT-pBNP N-terminal pro-brain natriuretic peptide, SD standard deviation, IQR interquartile range, HTx heart transplant
aBased upon Chi square test, Fisher’s exact test, Student t test, or Mann–Whitney U test, as appropriate
bAdjusted according to group differences in activity levels (steps/day)
cNot applicable, as the statistical prerequisites for linear regression-based adjustment were not met
At supine rest during the autonomic experiments, heart rate, blood pressures and total peripheral resistance index were significantly higher, and stroke index and end diastolic volume index were significantly lower in the HTxR group compared with healthy controls (Table 5). Cardiac index was identical across the two groups. During tilt, heart rate, blood pressures and total peripheral resistance index increased less, and stroke index and end diastolic volume index decreased less in the HTxR group, whereas the cardiac index response did not differ. During handgrip, heart rate and cardiac index increased less, and stroke index and end diastolic volume index decreased less in the HTxR group, whereas the total peripheral resistance index response did not differ.
Table 5.
Cardiovascular variables at supine rest, and responses to head-up tilt and handgrip in HTx recipients and healthy controls. Mean (95% confidence interval)
| Supine rest | Response to tilt, 60° head up | Response to handgrip, 30% max force | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTx recipients | Healthy controls | p valuea | Adj. p valueb | HTx recipients | Healthy controls | p valuea | Adj. p valueb | HTx recipients | Healthy controls | p valuea | Adj. p valueb | |
| Heart rate—beats/min | 81.6 (78.7 to 84.6) | 56.0 (53.7 to 58.3) | < 0.001 | < 0.001 | 1.9 (0.7 to 3.1) | 14.6 (12.2 to 16.9) | < 0.001 | < 0.001 | − 0.3 (− 0.5 to − 0.1) | 5.4 (3.9 to 7.0) | < 0.001 | < 0.001 |
| Systolic blood pressure—mmHg | 117.9 (113.9 to 121.9) | 109.0 (105.1 to 112.9) | 0.002 | 0.019 | 5.8 (1.3 to 10.3) | 11.2 (8.1 to 14.3) | 0.050 | 0.117 | 15.1 (12.3 to 18.0) | 17.0 (14.8 to 19.2) | 0.307 | 0.144 |
| Mean arterial blood pressure—mmHg | 90.2 (87.2 to 93.2) | 83.2 (80.1 to 86.3) | 0.002 | 0.020 | 7.6 (3.7 to 11.4) | 14.6 (11.9 to 17.3) | 0.003 | 0.018 | 13.5 (10.9 to 16.2) | 16.1 (14.0 to 18.2) | 0.133 | 0.063 |
| Diastolic blood pressure—mmHg | 78.9 (76.0 to 81.7) | 72.4 (69.4 to 75.3) | 0.002 | 0.024 | 8.4 (5.0 to 11.9) | 15.5 (12.7 to 18.2) | 0.002 | 0.013 | 11.8 (9.1 to 14.5) | 14.8 (12.6 to 17.0) | 0.085 | 0.046 |
| Stroke index—ml/m2 | 29.5 (27.8 to 31.1) | 42.4 (40.0 to 44.7) | < 0.001 | < 0.001 | − 0.8 (− 1.9 to 0.4) | − 10.2 (− 12.3 to − 8.0) | < 0.001 | < 0.001 | 0.3 (− 0.1 to 0.8) | − 1.7 (− 2.7 to − 0.8) | < 0.001 | 0.018 |
| Cardiac index—l/min/m2 | 2.4 (2.3 to 2.5) | 2.4 (2.2 to 2.5) | 0.906 | 0.314 | 0.01 (− 0.09 to 0.10) | − 0.13 (− 0.28 to 0.02) | 0.126 | 0.363 | 0.01 (− 0.02 to 0.05) | 0.10 (0.03 to 0.17) | 0.025 | 0.119 |
| Total peripheral resistance index—mmHg/l/min/m2 | 10.8 (9.9 to 11.6) | 9.2 (8.5 to 9.9) | 0.006 | 0.009 | 0.9 (0.3 to 1.5) | 1.8 (1.2 to 2.5) | 0.044 | 0.199 | 1.5 (1.2 to 1.8) | 1.3 (1.0 to 1.7) | 0.536 | 0.748 |
| End diastolic volume index—ml/m2 | 51.5 (49.0 to 54.0) | 68.6 (65.0 to 72.1) | < 0.001 | < 0.001 | 1.0 (− 1.2 to 3.1) | − 11.2 (− 14.6 to − 7.9) | < 0.001 | < 0.001 | 0.1 (− 0.5 to 0.7) | − 2.5 (− 3.9 to − 1.1) | 0.001 | 0.079 |
Numbers in bold indicate a p value < 0.05
HTx heart transplant
aBased upon Student t test. A Bonferroni-correction due to multiple statistical tests suggests a level of significance of 0.05/26 = 0.002
bAdjusted according to group differences in activity levels (steps/day)
Within the HTxR group, total peripheral resistance index during supine rest showed a strong negative correlation to body weight and to dosage of prednisolone; in partial correlation analyses controlling for body weight, the associations between prednisolone and TPRI became negligible and non-significant (Table 6). In addition, urine norepinephrine:creatinine ratio was positively associated with mean arterial blood pressure response during tilt (Table 6). HTxRs receiving diuretics tended to have lower supine mean arterial blood pressure, and an attenuated response of mean arterial blood pressure and total peripheral resistance index during tilt (Table 7).
Table 6.
Correlations (Pearson’s r) between cardiovascular variables of interest and possible confounding factors within the HTx recipient group
| Supine: mean arterial blood pressure, mmHg | Supine: total peripheral resistance index, mmHg/l/min/m2 | Tilt response: mean arterial blood pressure, mmHg | Tilt response: total peripheral resistance index, mmHg/l/min/m2 | Handgrip response: cardiac index, l/min/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | r | p value | |
| Glomerular filtration rate, estimated, ml/min/1.73 m2 | 0.19 | 0.185 | 0.06 | 0.710 | 0.10 | 0.512 | 0.11 | 0.483 | − 0.16 | 0.303 |
| HbA1c, % | 0.00 | 0.991 | − 0.25 | 0.081 | 0.05 | 0.740 | 0.07 | 0.652 | − 0.25 | 0.100 |
| Steps per day, number | 0.11 | 0.534 | 0.10 | 0.549 | − 0.15 | 0.409 | − 0.05 | 0.760 | − 0.02 | 0.910 |
| NT-pBNP, ng/l | 0.04 | 0.793 | − 0.02 | 0.877 | − 0.22 | 0.147 | − 0.12 | 0.428 | 0.07 | 0.644 |
| Urine Norepinephrine:creatinine ratio, nmol/mmol | − 0.26 | 0.080 | 0.08 | 0.596 | 0.33 | 0.028 | 0.20 | 0.183 | − 0.11 | 0.488 |
| Cyclosporine dosagea, mg/day | 0.20 | 0.255 | − 0.05 | 0.787 | 0.05 | 0.767 | 0.10 | 0.557 | 0.10 | 0.604 |
| Tacrolimus dosageb, mg/day | − 0.09 | 0.777 | − 0.18 | 0.573 | 0.47 | 0.145 | 0.55 | 0.081 | 0.14 | 0.659 |
| Prednisolone dosagec, mg/day | 0.17 | 0.243 | − 0.52 | < 0.001 | − 0.20 | 0.193 | − 0.12 | 0.443 | − 0.07 | 0.652 |
| Controlled for weight (partial corr.) | − 0.03 | 0.842 | ||||||||
| Metoprolol dosaged, mg/day | 0.05 | 0.870 | 0.38 | 0.196 | − 0.24 | 0.457 | − 0.28 | 0.382 | − 0.30 | 0.343 |
| Valganciclovir dosagee, mg/day | 0.13 | 0.558 | 0.01 | 0.955 | − 0.12 | 0.602 | − 0.15 | 0.508 | 0.12 | 0.613 |
Numbers in bold indicate a p value < 0.05
NT-pBNP N-terminal pro-brain natriuretic peptide, HTx heart transplant
aUsed by a total of 36 HTx recipients
bUsed by a total of 13 HTx recipients
cUsed by a total of 50 HTx recipients
dUsed by a total of 14 HTx recipients
eUsed by a total of 22 HTx recipients
Table 7.
Cardiovascular variables of interest in subgroups of HTx recipients according to medication usage. Mean (95% confidence intervals)
| Diureticsa | Calcium blockerb | Betablockerc | All HTx recipientsd | All healthy controlsd | ||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 40) | No (n = 10) | Yes (n = 13) | No (n = 37) | Yes (n = 15) | No (n = 25) | |||
| Supine: mean arterial blood pressure, mmHg | 88.9 (85.6 to 92.3) | 95.7 (89.3 to 102.1) | 94.1 (89.5 to 98.6) | 88.9 (85.2 to 92.6) | 92.1 (86.6 to 97.6) | 89.4 (85.7 to 93.1) | 90.2 (87.2 to 93.2) | 83.2 (80.1 to 86.3) |
| Supine: total peripheral resistance index, mmHg/l/min/m2 | 10.7 (9.7 to 11.7) | 11.1 (10.0 to 12.3) | 10.3 (8.9 to 11.7) | 10.9 (9.9 to 12.0) | 11.1 (9.0 to 13.3) | 10.6 (9.7 to 11.5) | 10.8 (9.9 to 11.6) | 9.2 (8.5 to 9.9) |
| Tilt response: mean arterial blood pressure, mmHg | 6.6 (2.6 to 10.6) | 12.1 (− 1.3 to 25.4) | 3.1 (− 3.0 to 9.1) | 8.8 (4.2 to 13.5) | 7.3 (0.5 to 14.1) | 7.7 (2.8 to 12.5) | 7.6 (3.7 to 11.4) | 14.6 (11.9 to 17.3) |
| Tilt response: total peripheral resistance index, mmHg/l/min/m2 | 0.7 (0.02 to 1.3) | 1.9 (− 0.01 to 3.9) | 1.2 (− 0.3 to 2.6) | 0.8 (0.1 to 1.5) | 1.0 (− 0.1 to 2.0) | 0.9 (0.1 to 1.7) | 0.9 (0.3 to 1.5) | 1.8 (1.2 to 2.5) |
| Handgrip response: cardiac index, l/min/m2 | 0.01 (− 0.03 to 0.05) | 0.04 (− 0.08 to 0.17) | 0.06 (− 0.03 to 0.16) | − 0.003 (− 0.04 to 0.4) | − 0.01 (− 0.08 to 0.06) | 0.02 (− 0.02 to 0.07) | 0.01 (− 0.02 to 0.05) | 0.10 (0.03 to 0.17) |
Numbers in bold indicate a p value < 0.05
HTx heart transplant
aIncludes bumetanide (n = 37), furosemide (n = 2) and thiazide (n = 5)
bIncludes nifedipine (n = 10) and amlodipine (n = 3)
cIncludes metoprolol (n = 14) and carvedilol (n = 1)
dShown for clarity
Discussion
The most important results of this study are: (1) at supine rest, HTxRs have higher blood pressures and total peripheral resistance than healthy controls. (2) During orthostatic challenge, HTxRs have attenuated blood pressure and total peripheral resistance responses. (3) During isometric exercise, HTxRs have preserved blood pressure and total peripheral resistance responses, but an attenuated cardiac output response.
The tendencies towards hypertension and peripheral vasoconstriction in HTxRs are well known (Idema et al. 1994; Bennett and Ventura 2017). In the present study we also observed more frequent complaints of pale and cold hands in the HTxR group, suggesting a direct link between altered peripheral vasoconstriction and patient well-being. While the underlying mechanisms remain to be fully understood, a main causal factor might be the usage of immunosuppressive medication, in particular calcineurin inhibitors and corticosteroids (Idema et al. 1994; Bennett and Ventura 2017). For instance, cyclosporine (CyA) has been reported to increase circadian sympathetic activity (Scherrer et al. 1990), to interfere with pressor responses of vasoconstrictor hormones (Lustig et al. 1987), to alter the prostacyclin–thromboxane A2 balance in favor of vasoconstriction (Kahan 1989) and to induce vessel wall inflammation (Reeves et al. 1986). Interestingly, in the present study, there were no significant correlations between calcineurin inhibitors dosage and cardiovascular variables, and the association between glucocorticoid dosage and resting vasoconstriction disappeared when controlling for body weight. Thus, the tendencies towards hypertension and vasoconstriction in HTxRs might have other explanations. We speculate that the permanent denervation of low-pressure cardiopulmonary baroreceptors in the heart might be a contributing factor. Tonic stimulation of these receptors in the normal heart reflexively reduces sympathetic outflow to the peripheral vasculature (Triedman et al. 1993; Goldstein 2001); thus, a lack of afferent impulses would presumably cause a permanent enhancement of sympathetic vasomotion. This hypothesis should be scrutinized in further research.
The attenuated response of blood pressure and peripheral vasoconstriction to orthostatic challenge might at first seem surprising. Since arterial baroreceptors are preserved in HTxRs, we hypothesized that the inability to increase heart rate and cardiac output during orthostasis due to efferent cardiac denervation would lead to a compensatory response of total peripheral resistance, which would be larger than in healthy controls. The preserved vasoconstrictive response we found during handgrip suggests the efferent sympathetic pathways to peripheral vessels are intact. However, it is clear in the literature that low-pressure cardiopulmonary baroreceptors in the heart play an important role in modulating the peripheral sympathetic response to orthostasis, so the lack of afferent signals from these receptors during volume depletion might attenuate the response of efferent sympathetic outflow (Triedman et al. 1993; Goldstein 2001). The usage of diuretics among some HTxRs might add to this effect. Surprisingly, the substantial alterations of orthostatic homeostasis after HTx did not increase orthostatic symptoms. This is in line with previous findings and suggests that the subjective experiences of orthostatic intolerance might be more specifically related to certain phenomena, such as reflex-mediated (“vasovagal”) hypotension which presupposes normal cardiac innervation and, therefore, does not exist early after HTx.
The attenuated cardiac output response to handgrip in the HTxR group might be directly explained from cardiac sympathetic denervation, which inhibits the normal increase in heart rate as well as myocardial contractility during isometric exercise (Kamiya et al. 2000). In addition, in the present study, cardiac output was associated with end diastolic volume index (Pearson’s r = 0.78, p < 0.001), suggesting that usage of diuretics and thereby reduced diastolic filling might contribute to lower cardiac output response. The functional impact of this particular homeostatic aberration remains to be fully characterized, but reduced exercise capacity is a logical assumption. This hypothesis deserves further investigations; interestingly, handgrip strength is a marker of frailty in heart failure patients (Chung et al. 2014).
The elevated heart rate at supine rest as well as the lack of heart rate response under both experimental conditions in the HTxR group confirms complete efferent cardiac denervation, as assumed early after HTx (Robson et al. 1989; Doering et al. 1991; Awad et al. 2016). Cardiac sympathetic reinnervation over time has been demonstrated in previous reports, but the speed of reinnervation process, as well as the eventual presence of parasympathetic and afferent reinnervation, remain to be fully investigated and should be a focus of future research. The group differences in stroke volume and end diastolic volume are likely explained by the differences in heart rate and thereby diastolic filling time (Elstad et al. 2001); accordingly, heart rate, stroke index and end diastolic volume index were strongly intercorrelated in the present study.
Taken together, the findings reported in the present study are likely responsible in part for reduced exercise capacity in heart transplant recipients (Nytrøen and Gullestad 2013). Specifically, in addition to the well-recognized effects of the limited response of heart rate to buffering the effects of posture and increasing cardiac output during exercise in transplant patients, increased resting peripheral resistance and the subsequent inability to modulate resistance normally is also likely to affect both isometric and isotonic exercise responses. That said, level of activity has a well-known effect on autonomic cardiovascular control (O’Sullivan and Bell 2000). Thus, the lower number of steps per day in the HTxR group than the control group might be a cause for as well as an effect of autonomic alterations. Of note, group differences regarding total peripheral resistance response to orthostatic challenge and cardiac output response to isometric exercise became non-significant when p values were adjusted for differences in steps per day.
Strengths and limitations
Strengths of the present study include the relatively large number of HTxRs as compared to other studies in the field, and the population-based nature of patient recruitment from a nationwide transplant center. We cannot totally exclude selection bias, but the similarity of baseline characteristics between the included and non-included group of eligible patients suggests wide generalizability of the results. The time window for investigations of the HTxRs (7–12 weeks after transplant surgery) was chosen to avoid interference from compensatory responses related to the surgical procedure per se, and at the same time avoid any reinnervation processes to have taken place. While the data suggest that the latter presumption holds, we cannot completely rule out that long-lasting effects from the surgical procedure and subsequent intensive care might have influenced the results. Cardiac transplantation is an effective treatment against chronic heart failure; as the patient group was investigated 7–12 weeks after transplant surgery, we find it likely that previous autonomic abnormalities related to a failing heart would have normalized prior to inclusion. However, it is possible that “hangover” effects from the patients’ previous health condition or another underlying disease process might have biased the results.
Missing data were not imputed; however, the numbers of missing data points were few and considered negligible. p values were not adjusted for test multiplicity; however, the computed confidence intervals support the conclusions drawn from the statistical tests (i.e., the central estimate of one group is not included in the confidence interval for the central estimate of the other group when a statistically significant difference is suggested from the p value).
Conclusion
HTxRs are characterized by elevated blood pressures and total peripheral resistance at supine rest, as well as attenuated blood pressure and total peripheral resistance responses during orthostatic challenge; both these effects might be caused by low-pressure cardiopulmonary baroreceptor denervation. In addition, HTxRs show attenuated cardiac output response during isometric exercise due to efferent sympathetic denervation. These physiological limitations might have negative functional consequences in HTxRs.
Acknowledgements
We thank the HTx nurses Anne Relbo Authen and Ingelin Grov for help in patient coordination; Hamsana Chaudrakumar and the bioengineers for blood sampling and laboratory assistance; Steinar Kumarasamy Aune, Knut Andresen and Elisabeth Bjørkelund for practical support; Henrik Brun and Arne Kristian Andreassen for discussion on study design and results; Terje Rootwelt for institutional support; and finally all the enthusiastic participants. This study was founded by the Health South–East Hospital Trust, Norway.
Abbreviations
- AccHEART
Autonomic cardiovascular control after heart transplantation
- ANS
Autonomic nervous system
- ASP
Autonomic symptom profile
- CO
Cardiac output
- CsA
Cyclosporine A
- CV
Coefficient of variation
- HPLC
High-performance liquid chromography
- HR
Heart rate
- HTxR
Heart transplant recipients
- NT-pBNP
N-terminal pro-brain natriuretic peptide
- SV
Stroke volume
- TPR
Total peripheral resistance
Author contributions
SN and AHC collected clinical data, contributed to study design and participated in data analyses. KR, KN, LG, AF, ET, GD and JPS supervised data analyses and contributed to study design. VBBW conceived of the study, contributed to study design and participated in data analyses. All the authors contributed to data interpretation and drafting of the manuscript. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Funding was provided by Helse Sør-Øst RHF.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the procedures performed in the present study were in accordance with the ethical standards of the Norwegian National Committee for Ethics in Medical research and with the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014;6:1120–1128. doi: 10.3978/j.issn.2072-1439.2014.06.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad M, et al. Early denervation and later reinnervation of the heart following cardiac transplantation: a review. J Am Heart Assoc. 2016;5:e004070. doi: 10.1161/JAHA.116.004070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner NR, et al. Altered cardiovascular and neurohumoral responses to head-up tilt after heart-lung transplantation. Circulation. 1990;82:863–871. doi: 10.1161/01.CIR.82.3.863. [DOI] [PubMed] [Google Scholar]
- Bennett AL, Ventura HO. Hypertension in patients with cardiac transplantation. Med Clin N Am. 2017;101:53–64. doi: 10.1016/j.mcna.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Brunner-La Rocca HP, et al. Relative frequency of functional sympathetic and parasympathetic reinnervation after heart transplantation. J Heart Lung Transplant. 1998;17:725–728. [PubMed] [Google Scholar]
- Chung CJ, et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J Card Fail. 2014;20:310–315. doi: 10.1016/j.cardfail.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambrink JH, Wieling W. Circulatory response to postural change in healthy male subjects in relation to age. Clin Sci (Lond) 1987;72:335–341. doi: 10.1042/cs0720335. [DOI] [PubMed] [Google Scholar]
- Denniston JC, et al. Measurement of cardiac output by electrical impedance at rest and during exercise. J Appl Physiol. 1976;40:91–95. doi: 10.1152/jappl.1976.40.1.91. [DOI] [PubMed] [Google Scholar]
- Doering LV, et al. Evidence of time-dependent autonomic reinnervation after heart transplantation. Nurs Res. 1991;48:308–316. doi: 10.1097/00006199-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Doering LV, et al. Hemodynamic adaptation to orthostatic stress after orthotopic heart transplantation. Heart Lung. 1996;25:339–351. doi: 10.1016/S0147-9563(96)80076-8. [DOI] [PubMed] [Google Scholar]
- Elstad M, et al. Respiratory sinus arrhythmia: opposite effects on systolic and mean arterial pressure in supine humans. J Physiol. 2001;536:251–259. doi: 10.1111/j.1469-7793.2001.t01-1-00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AP, et al. Vasovagal reactions may occur after orthotopic heart transplantation. J Am Coll Cardiol. 1993;21:1132–1137. doi: 10.1016/0735-1097(93)90235-S. [DOI] [PubMed] [Google Scholar]
- Fortin J, et al. Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med. 2006;36:1185–1203. doi: 10.1016/j.compbiomed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Goldstein D. The autonomic nervous system in health and disease. New York: Marcel Dekker Inc; 2001. [Google Scholar]
- Grant PM, et al. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med. 2006;40:992–997. doi: 10.1136/bjsm.2006.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idema RNM, et al. Abnormal diurnal variation of blood pressure, cardiac output, and vascular resistance in carida transplant recipients. Circulation. 1994;90:2797–2803. doi: 10.1161/01.CIR.90.6.2797. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Cyclosporine. N Engl J Med. 1989;321:1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kamiya A, et al. Muscle sympathetic nerve activity (MSNA) during high-intensity, isometric leg exercise in humans. Environ Med. 2000;44:49–52. [PubMed] [Google Scholar]
- Lund LH, et al. The Registry of the International Society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Lustig S, et al. Mechanisms of cyclosporine A hypertension. Transplant Proc. 1987;19:1262–1264. [PubMed] [Google Scholar]
- Nytrøen K, Gullestad L. Exercise after heart transplantation: an overview. World J Transplant. 2013;3:78–90. doi: 10.5500/wjt.v3.i4.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Bell C. The effects of exercise training on human cardiovascular reflexcontrol. J Auton Nerv Syst. 2000;81:16–24. doi: 10.1016/S0165-1838(00)00148-X. [DOI] [PubMed] [Google Scholar]
- Parati G, et al. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–655. doi: 10.1161/01.HYP.13.6.647. [DOI] [PubMed] [Google Scholar]
- Raczak G, et al. Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J Heart Lung Transplant. 1999;18:399–406. doi: 10.1016/S1053-2498(98)00071-0. [DOI] [PubMed] [Google Scholar]
- Reeves RA, et al. Loss of nocturnal decline in blood pressure after cardiac transplantation. Circulation. 1986;73:401–408. doi: 10.1161/01.CIR.73.3.401. [DOI] [PubMed] [Google Scholar]
- Robson SC, et al. Isometric exercise in the denervated heart: a Doppler echocardiographic study. Br Heart J. 1989;61:224–230. doi: 10.1136/hrt.61.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca J, et al. Left ventricular dynamics and plasma catecholamines during isometric exercise in patients following cardiac transplantation. Eur Heart J. 1991;12:928–936. [PubMed] [Google Scholar]
- Scherrer U, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323:693–699. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]
- Sprangers RL, et al. Circulatory responses to stand up: discrimination between the effects of respiration, orthostasis and exercise. Clin Physiol. 1991;11:221–230. doi: 10.1111/j.1475-097X.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Suarez GA, et al. The autonomic symptom profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523–528. doi: 10.1212/WNL.52.3.523. [DOI] [PubMed] [Google Scholar]
- Toska K, Walloe L. Dynamic time course of hemodynamic responses after passive head-up tilt and tilt back to supine position. J Appl Physiol 1985. 2002;92:1671–1676. doi: 10.1152/japplphysiol.00465.2000. [DOI] [PubMed] [Google Scholar]
- Triedman JK, et al. Mild hypovolemic stress alters autonomic modulation of heart rate. Hypertension. 1993;21:236–247. doi: 10.1161/01.HYP.21.2.236. [DOI] [PubMed] [Google Scholar]
- Wyller VB, et al. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin Physiol Funct Imaging. 2007;26:1–8. doi: 10.1111/j.1475-097X.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- Wyller VB, et al. Sympathetic cardiovascular control during orthostatic stress and isometric exercise in adolescent chronic fatigue syndrome. Eur J Appl Physiol. 2008;102:623–632. doi: 10.1007/s00421-007-0634-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.