Abstract
To ensure a safe drinking water supply, it is necessary to protect water quality. To classify the suitability of the Orós Reservoir (Northeast of Brazil) water for human consumption, a Water Quality Index (WQI) was enhanced and refined through a Principal Component Analysis (PCA). Samples were collected bi-monthly at seven points (P1 – P7) from July 2009 to July 2011. Samples were analysed for 29 physico-chemical attributes and 4 macroinvertebrate metrics associated with the macrophytes Pistia stratiotes and Eichhornia crassipes. PCA allowed us to reduce the number of attributes from 33 to 12, and 85.32 % of the variance was explained in five dimensions (C1 – C5). Components C1 and C3 were related to water-soluble salts and reflect the weathering process, while C2 was related to surface runoff. C4 was associated with macroinvertebrate diversity, represented by ten pollution-resistant families. C5 was related to the nutrient phosphorus, an indicator of the degree of eutrophication. The mean values for the WQIs ranged from 49 to 65 (rated as fair), indicating that water can be used for human consumption after treatment. The lowest values for the WQI were recorded at the entry points to the reservoir (P3, P1, P5, and P4), while the best WQIs were recorded at the exit points (P6 and P7), highlighting the reservoir’s purification ability. The proposed WQI adequately expressed water quality, and can be used for monitoring surface water quality.
Keywords: Macroinvertebrates, Biological Monitoring Working Party (BMWP), Biological metrics, Surface water, Semi-arid, Aquatic macrophytes
Introduction
Water is essential for sustaining life on Earth (Giriyappanavar and Patil 2013). However, it has been altered significantly from its natural state, and human activities can affect its availability for various uses, both in quantity and in quality (Magesh and Chandrasekar 2013). Faced with this reality, and coupled with the limited availability of freshwater for human consumption on our planet (Pal et al. 2013), the quality of the available water must be monitored. This is particularly important in arid and semi-arid regions (Aenab et al. 2012), which are characterised by irregular rainfall, both spatially and temporally, and with high rates of evapotranspiration (Andrade et al. 2010).
In tropical semi-arid regions, such as the northeastern part of Brazil where rivers are ephemeral or intermittent, reservoirs are the main source of water; monitoring their water quality is therefore essential. Water quality indices developed for temperate regions, based on physical and chemical attributes, have been used to assess water quality in this area (Santos et al. 2014; Batista et al. 2014). We believe, however, that further investigation is needed to obtain an improved understanding of the quality of the waters of the Orós reservoir.
Water quality monitoring is traditionally carried out by environmental agencies who analyse physical (temperature, pH and transparency), chemical (BOD, DO, total phosphorus, total ammonia, nitrate, calcium, magnesium, sodium, potassium, bicarbonate and phosphate) and microbiological attributes (total and faecal coliforms) (Vasanthavigar et al. 2010) of water. Water quality indices (WQIs) are tools that use an integrative methodology to convert a large set of data into a single number to express the water quality (Lumb et al. 2011); they can be calculated using physical, chemical and microbiological data collected by environmental agencies (Hurley et al. 2012).
WQIs are easier and quicker for the general public to understand than a large amount of complicated environmental data presented in reports. They can therefore be very useful in water resource and watershed management (Yisa and Jimoh 2010); they can also reduce the cost of analyses by highlighting attributes that are less important for water quality, thereby allowing us to omit them. Although WQIs have been used for many decades in other parts of the world, Brazilian researchers have only started to develop and apply them in the past decade (Almeida and Schwarzbold 2003; Andrade et al. 2005).
Generally, WQIs are prepared using physical and chemical attributes. However, Yan et al. (2014) have suggested that biological attributes obtained from studies of the structure of communities of organisms that act as bioindicators of water quality, such as macroinvertebrates, fish, macrophytes, phytoplankton and zooplankton, should be included. According to Baptista (2008), these organisms react to disturbances in the environments in which they live, whether of natural or anthropogenic origin. Additionally, Ferreira et al. (2011) observed that, when disruption is severe, the more resistant bioindicator organisms may become dominant, while the more sensitive become rare or absent.
In the last decade, bioindicators have been widely used in many countries, such as the UK, Spain, China, Australia, the USA and Canada (Morse et al. 2007). In the world, the USA is at a more advanced stage in the use of macroinvertebrates and other groups of organisms in water quality assessment (Hurley et al. 2012).
Brazilian legislation, by means of the National Policy for Water Resources (Law 9433/97) and Brasil (2001), provides for water quality to be assessed with biological indicators (Buss and Borges 2008). Current legislation, according to Oliveira et al. (2008), represents an important advance, but biomonitoring of waterbodies is still not mandatory. Brazilian environmental laws and regulatory processes only require that water quality assessments are based on physical, chemical and bacteriological parameters. As a result, macroinvertebrate metrics have only been used in the recent past as bioindicators of water quality in Brazil, especially in the Amazon region (Silveira et al. 2005; Uherek and Gouveia 2014).
Traditionally, physical, chemical and biological indicators have been treated separately by WQIs. Also, to date, few indices have been proposed for the reservoirs in this semi-arid region of Brazil. One noteworthy example is the index proposed by Andrade et al. (2005), in which only chemical attributes were considered. Using a cross-sectional view of the processes that determine water quality, the aim of this study therefore was to develop a WQI for the waters of artificial reservoirs, which would consider the physical and chemical attributes and the biological metrics of macroinvertebrates of a semi-arid tropical region. As a result of this study, we will have a new method for evaluating water quality, in which physical, chemical and biological attributes will be integrated in a single WQI.
Materials and methods
Description of the study area
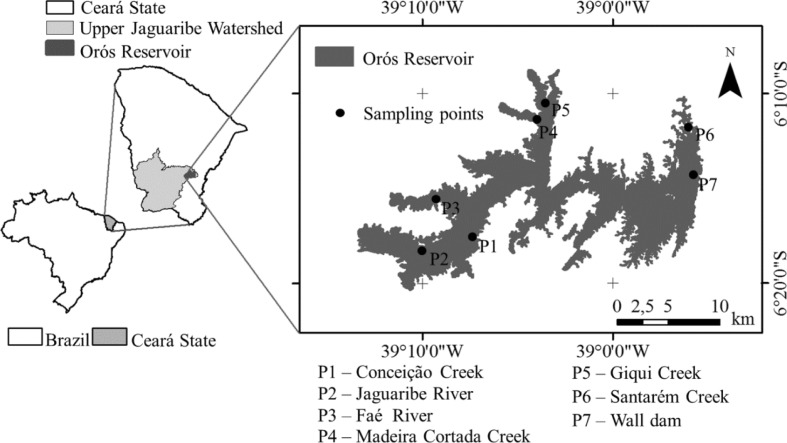
This study was carried out in the Orós reservoir, which is in the watershed of the Upper Jaguaribe River, in the semi-arid region of the state of Ceará, Brazil (6° 8′ 3″ S–6° 20′ 26″ S and 38° 54′ 56″ W–39° 13′ 28″ W). The reservoir has a total water storage capacity of 1.94 billion m3, and a contributing area of 25,000 km2 (DNOCS 2014).
Using the Köppen classification, the climate in the region is BSw'h', otherwise known as semi-arid hot with summer/autumn rains and a monthly average temperature greater than 18 °C. The average rainfall is 750 mm year−1, with a potential evaporation of 1988 mm year−1 and insolation of 2945 h year−1. Rainfall in the region is characterised by a high spatial and temporal variability, with the main limitation being the irregularity of the regime rather than the actual amount of annual rainfall.
The geology of the area is dominated by crystalline basement rocks with a predominance of homogeneous and heterogeneous migmatites, gneiss and quartzite (Radambrasil 1981). According to Embrapa (2006), the soils of the watershed fall into seven classes, with neosols (31.9 %) and argisols (29.06 %) being the most prevalent.
The resident population in the Upper Jaguaribe watershed is approximately 600,000 inhabitants. On average, 85.95 % of households have a piped water supply; however, only 11.22 % are connected to a sewage system (IPECE 2012).
Agriculture, livestock and manufacturing are the main sources of income (Lopes et al. 2014). Fish farming and subsistence farming have been developed by communities located around the Orós reservoir (Fig. 1). Batista et al. (2014) state that 42.38 % of the permanent farmland area is used for pasture, 24.05 % for poultry farming, 2.08 % for maize cultivation, 15.83 % for rice cultivation and 7.89 % for other crops and uses; only 7.77 % is covered by natural vegetation.
Fig. 1.
Fish farming and livestock around the Orós reservoir
Sampling and analysis
The data used to develop the proposed WQI were obtained from seven sampling sites (P1, Conceição; P2, River Jaguaribe; P3, River Faé; P4, Madeira Cortada; P5, Giqui; P6, Santarém; and P7, Upstream) in the Orós reservoir. Six of these sites correspond to the confluences of the major tributaries (P1 to P6), while the other site (P7) is located near the spillway of the reservoir, as shown in Fig. 2. All the sampling points were accurately georeferenced with a Garmin GPS. The sampling points were chosen so that the inputs from the six major tributaries to the reservoir were represented.
Fig. 2.
Geographical position of the reservoir and the locations of the sampling points
The water samples were taken at a depth of 30 cm from the surface using a range of specific containers: Samples to be analysed for phytoplankton were collected in 1-L flasks containing formalin and lugol; samples for dissolved oxygen determination were collected in separate flasks; and samples for the remaining attributes were collected in 1.5-L properly decontaminated bottles. The samples were placed into isothermal boxes and taken to the laboratory, where they were either processed immediately or properly stored. pH, temperature, Secchi transparency and electrical conductivity (EC) were measured in the field.
Sampling was carried out nine times from July 2009 to July 2011. Samples from each sampling point were tested for 33 attributes (physical, chemical, microbiological and biological) (Table 1), giving a total of 2772 tests (7 points × campaigns × 33 attributes).
Table 1.
Attributes tested, methodologies and references
| Attributes | Methodologies | References |
|---|---|---|
| Temperature (°C) | Mercury filament thermometer 0–60 °C | APHA (2005) |
| Turbidity (uT) | Turbidimetric | |
| Apparent colour (uH) | Colourimetric | |
| Electrical conductivity (dS m−1) | Conductivity meter | |
| Secchi transparency (m) | Secchi disk | |
| pH | pH meter | |
| Total solids, TS (mg L−1) | Drying at 103–105 °C | |
| Total volatile solids, TVS (mg L−1) | Ignition at 500–550 °C | |
| Total fixed solids, TFS (mg L−1) | ||
| Total suspended solids, TSS (mg L−1) | Vacuum filtration with fibreglass membrane 0.45-μm porosity | |
| Total dissolved solids, TDS (mg L−1) | Drying at 103–105 °C | |
| Inorganic suspended solids, ISS (mg L−1) | Vacuum filtration with fibreglass membrane 0.45-μm porosity | |
| Volatile suspended solids, VSS (mg L−1) | Ignition at 500–550 °C | |
| Dissolved oxygen (mg L−1) | Winkler method (Azide modification)—iodometry | |
| DBO5 (mg L−1) | Standard flasks—iodometry | |
| Total phosphorus, TP (mg L−1) | Spectrophotometry—ascorbic acid | |
| Soluble orthophosphate, SOP (mg L−1) | ||
| Sulphates (mg L−1) | Turbidimetric | |
| Chlorides (mg L−1) | Argentometric titration | |
| Potassium (mg L−1) | Photometry—flame emission | |
| Sodium (mg L−1) | ||
| Calcium (mg L−1) | Titration | |
| Magnesium (mg L−1) | ||
| Escherichia coli (NMP/100 mL) | Colilert | |
| Thermotolerant coliforms (NMP/100 mL) | Multiple tubes in an A1 medium | |
| Phytoplankton—qualitative and quantitative | Bright field microscopy of slides prepared from sediment obtained by centrifugation at 1500 rpm for 5–10 min to estimate the density of cyanobacteria and identification of phytoplankton using dichotomous identification keys | |
| Total ammonia nitrogen, TAN (mg L−1) | Spectrophotometric—macro-Kjeldahl distillation followed by direct Nesslerization | |
| Ammonia (mg L−1) | ||
| Nitrate (mg L−1) | Spectrophotometric—sodium salicylate | Rodier (1975) |
| Chlorophyll a (μg L−1) | Spectrophotometric—hot extraction with methanol | Jones (1979) |
| ricF(ec) | Richness of macroinvertebrate families associated with the roots of the macrophyte Eichhornia crassipes | Brusca and Brusca (2007); Mugnai et al. (2010) |
| ricF(ps) | Richness of macroinvertebrate families associated with the roots of the macrophyte Pistia stratiotes | |
| Abundance (Abec) | Abundance of macroinvertebrates associated with the roots of the macrophyte Eichhornia crassipes | |
| Abundance (Abps) | Abundance of macroinvertebrates associated with the roots of the macrophyte Pistia stratiotes |
The testing frequency for water quality attributes is generally based on the population being supplied or on the volume of water distributed (CESTEB 2011). To ensure that the data were representative of the range of possible environmental conditions, the samples were collected in both the wet and dry seasons, following the standards of the National Water Quality Assessment Program (PNQA) in Brazil (ANA 2012).
In this study, the richness of the macroinvertebrate families associated with the roots of two aquatic macrophyte species, Pistia stratiotes and Eichhornia crassipes, was used to represent the biological attributes. At each of the seven sampling points, samples of P. stratiotes and E. crassipes were taken in triplicate. Samples were collected in a delta net with a mesh aperture of 500 μm and were stored in properly labelled plastic containers with hydrated ethyl alcohol (80 %).
In the laboratory, the macrophyte roots were washed to remove any macroinvertebrates. These were separated and fixed in hydrous ethyl alcohol (70 %) for later identification with the help of various identification keys (Brusca and Brusca 2007; Mugnai et al. 2010).
The richness of the invertebrate families was then calculated for each sampling point, and scores were allocated to the families based on the Biological Monitoring Working Party (BMWP) index proposed by Armitage et al. (1983) and later adapted by Mustow (2002), Wyżga et al. (2013) and others. This classification assigns scores to each family of macroinvertebrates that are present. The BMWP classifies organisms at the family taxonomic level and allocates values ranging from 1 to 10 (Alba-Tercedor et al. 2002). Families sensitive to high levels of organic pollutants receive higher values, while resistant families receive lower values (Rossaro et al. 2007).
Selection of attributes
We used factor analysis/principal component analysis (FA/PCA) to identify the most important attributes in explaining the variability of water quality. There were four stages to this analysis:
-
(i)
Preparation of the correlation matrix—used to eliminate the problem of using different scales and units to measure the attributes
-
(ii)
Extraction of factors for each component—used to determine the factors that represented the variability of water quality in the Orós reservoir, with the least possible loss of information contained in the total data
-
(iii)
Extraction of the number of components and communality of each variable—using the criteria proposed by Kaiser (1958) and Norusis (1990), only components with eigenvalues greater than 1 were considered. The commonality expresses the variance for each variable.
-
(iv)
Factor transformation—using studies of Hair Júnior et al. (2005), the varimax orthogonal rotation method was used, in which the attributes are given weights close to 1 or 0, and intermediate values that could make the interpretation difficult are eliminated. Statistical analyses were carried out using SPSS version 16.0 as it is capable of carrying out all the analyses relevant to principal component analysis.
The WQI was calculated as the sum of the individual values of each attribute (q i) weighted by the importance of this attribute in the evaluation of the total variability of the water quality (w i). This method has already been successfully used by Almeida and Schwarzbold (2003) and Lopes et al. (2008). The general formula used was
| 1 |
where
- WQI
water quality index (dimensionless)
- Π
multiplier
- qi
relative quality of the ith attribute
- wi
relative weighting of the ith attribute
- i
attribute number
For the limits of q i (Table 2), we used the recommendations of water quality for human consumption proposed by Armitage et al. (1983), Junqueira and Campos (1998), Mustow (2002), Lamparelli (2004), Brasil (2004, 2005, 2011), WHO (2006), Boyacioglu (2010), Rubio-Arias et al. (2014) and Rekha et al. (2013). The weighting (w i) for each attribute of water quality used in the WQI was defined by the component that explained the greatest proportion of the total variance.
Table 2.
Limits of the attributes used in the WQI for calculation of the q i
| Attribute | Intervals of q i | |||||
|---|---|---|---|---|---|---|
| 100–90 (excellent) | 90–70 (good) | 70–50 (fair) | 50–25 (bad) | 25–0 (very bad) | References | |
| Ca2+ (mg L−1) | 10 < X <75 | 75 < X <200 | 200 < X <500 | >500 | >500 | BRASIL (2004), WHO (2006) |
| Mg2+ (mg L−1) | 10 < X <30 | 30 < X <150 | 150 < X <500 | >500 | >500 | BRASIL (2004), WHO (2006) |
| Cl− (mg L−1) | 0 < X <250 | 250 < X <400 | 400 < X <600 | 600 < X <1000 | >1000 | BRASIL (2004, 2005, 2011), WHO (2006) |
| Col A uH | 0 < X <5 | 5 < X <10 | 10 < X <15 | 15 < X ≤20 | 20 < X ≤200 | BRASIL (2004, 2011) |
| TSS (mg L−1) | 0 < X <5 | 5 < X <15 | 15 < X <25 | 25 < X <50 | 50 < X <100 | Boyacioglu (2010) |
| Tur (uT) | 0 | 0 < X <2.5 | 2.5 < X <5 | 5 < X <10 | 10 < X <100 | Brasil (2004, 2011), Rubio-Arias et al. (2014), Rekha et al. (2013) |
| K + (mg L−1) | 0 < X <50 | 50 < X <100 | 100 < X <200 | >200 | >200 | WHO (2006) |
| Na+ (mg L−1) | 0 < X <50 | 50 < X <100 | 100 < X <200 | 200 < X <400 | 400 < X <600 | BRASIL (2004,2011), WHO (2006) |
| SO4 (mg L−1) | 0 < X <100 | 100 < X <200 | 200 < X <250 | 250 < X <1000 | >1000 | BRASIL (2004,2011), WHO (2006) |
| ricF (ec) BMWP | ≥ 86 | 64 < X ≤85 | 37 < X ≤63 | 17 < X ≤36 | ≤16 | Armitage et al.(1983), Junqueira and Campos (1998), Mustow (2002) |
| ricF (ps) BMWP | ≥ 86 | 64 < X ≤85 | 37 < X ≤63 | 17 < X ≤36 | ≤16 | Armitage et al.(1983), Junqueira and Campos (1998), Mustow (2002) |
| Total phosphorous (mg L−1) | <0.008 | 0.008 < X <0.019 | 0.019 < X <0.052 | 0.052 < X <0.120 | 0.120 < X <1.2 | Lamparelli (2004), Boyacioglu (2010) |
Ca +2 ion calcium, Mg +2 ion magnesium, Cl − ion chloride, Col A apparent Colour, TSS total suspended solids, Tur turbidity, K + potassium, Na +1 sodium, SO 4 sulphate, ricF (ec) richness of macroinvertebrate families associated with the roots of the macrophyte Eichhornia crassipes, ricF (ps) richness of macroinvertebrate families associated with the roots of the macrophyte Eichhornia crassipes
Definition of the weightings (w i) assigned to each attribute of water quality used in the WQI was established from the results of the principal component analysis. In this procedure, the eigenvalues of the components, and how much of each attribute was explained by its respective components taken from the PCA, were used. The equation used to calculate w i was
| 2 |
where
- wi
weight assigned to the ith variable making up the WQI
- F1 and F2
eigenvalue of the main components
- Pi
explicability of the ith variable by principle component
- Pj
explicability of the jth variable by principle components
- i and j
indices from 0 to 6 for the attributes
- n
number of variables involved in the PCA
The calculated WQI can have a value between 0 and 100. We divided the values into five classes (Table 3).
Table 3.
Ranges of water quality for the WQI
| Value of WQI | Water quality | Restrictions on use for human consumptiona |
|---|---|---|
| 90–100 | Excellent | With disinfection |
| 70–90 | Good | Simplified treatment |
| 50–70 | Fair | Conventional treatment |
| 25–50 | Bad | Advanced treatment |
| 0–25 | Very Bad | Unsuitable |
The Student’s t test was applied at a significance level of 5 % to compare the means (1) of the WQIs between collection points and (2) for the wet and dry periods.
Results and discussion
After the factor analysis/principal component analysis (FA/PCA), we were able to bring down the number of attributes from 33 to 12 (Table 4). A test for model adequacy (KMO) resulted in a value of 0.61. The five components explained 29.60, 21.42, 14.20, 11.70 and 8.38 % of the total variance in the data, respectively, with five dimensions explaining 85.32 % of the variance.
Table 4.
Factor loading matrix of water quality attributes for the Orós reservoir, CE
| Attribute | Component | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | ||
| 01 | Ca+2 (mg L−1) | 0.930 | −0.094 | 0.133 | −0.059 | 0.061 |
| 02 | Mg+2 (mg L−1) | 0.912 | −0.041 | 0.082 | 0.044 | −0.096 |
| 03 | Cl− (mg L−1) | 0.843 | 0.021 | 0.253 | −0.079 | −0.091 |
| 04 | Apparent colour (uH) | −0.071 | 0.971 | −0.033 | 0.026 | 0.074 |
| 06 | Turbidity (uT) | 0.035 | 0.939 | 0.131 | 0.050 | 0.032 |
| 05 | Total suspended solids (mg L−1) | −0.006 | 0.902 | −0.180 | 0.096 | 0.050 |
| 07 | K+ (mg L−1) | 0.371 | −0.054 | 0.881 | −0.062 | 0.050 |
| 08 | Na+ (mg L−1) | 0.388 | −0.039 | 0.883 | −0.073 | 0.145 |
| 09 | SO4 −2 (mg L−1) | −0.337 | 0.007 | 0.674 | 0.190 | −0.476 |
| 10 | Richness of the macroinvertebrate family associated with the macrophyte Eichhornia crassipes | 0.006 | −0.046 | −0.129 | 0.837 | 0.074 |
| 11 | Richness of the macroinvertebrate family associated with the macrophyte Pistia stratiotes | −0.069 | 0.100 | 0.104 | 0.748 | −0.173 |
| 12 | Total phosphorus | −0.148 | 0.079 | 0.061 | −0.060 | 0.946 |
| Eigenvalue | 3.55 | 2.57 | 1.70 | 1.40 | 1.00 | |
| Variance (%) | 29.60 | 21.42 | 14.20 | 11.70 | 8.38 | |
| Accumulated variance (%) | 29.60 | 51.03 | 65.23 | 76.94 | 85.32 | |
| KMO | 0.61 | |||||
The reduction in attributes with little loss in the explicability of the variance is interesting because it means that the number of laboratory tests can be reduced, thereby saving time and resources (Zeng and Rasmussen 2010); this is especially useful in countries where financial resources are limited (Debels et al. 2012).
Table 4 shows that the principal components C1 and C3 explain 29.6 and 14.20 % of the total variability of the data, respectively, and are associated with the chemical attributes represented by calcium (Ca+2, 0.930), magnesium (Mg+2, 0.912), chloride (Cl−, 0.843), potassium (K+, 0.881), sodium (Na+, 0.883) and sulphate (S04 −2, 0.674). These results create two components related to the soluble salts in the water (C1 and C3) that can be explained by the presence of crystalline and carbonate rocks of the Orós group that are found in the Upper Jaguaribe watershed.
These salts may be related to the weathering process, as verified by Meireles et al. (2007) and Andrade et al. (2007), and to domestic sewage inputs (Pal et al. 2013). Evaporation also contributes negatively to water quality in reservoirs and is aggravated by the lack of water renewal in the dry season (Palácio et al. 2011).
Similarly, Deepak and Singh (2013), in studies conducted in Dhar, India, claim that the high levels of salts in water bodies are related to inputs of domestic and industrial effluents. Kumar and James (2013) also noted that increases in these elements are due mainly to industrial and agricultural activities and to the lack of basic sanitation.
The second component, C2, explains 21.42 % of the variability in the data and is associated with the physical attributes of apparent colour (0.971), total suspended solids (0.902) and turbidity (0.939), which basically reflect surface runoff. These results suggest that the reservoir has been receiving a large input of suspended matter from soil erosion and degradation of the riparian vegetation, residues of agricultural fertilisers, and excessive loads of domestic sewage and solids disposed of improperly on the reservoir shores (Lopes et al. 2014).
Batista et al. (2014) carried out an evaluation of the trophic state of the Orós reservoir and concluded that sediments transported by surface runoff played a key role in reducing water transparency and that there was seasonal variation in the trophic status of the reservoir waters. Trophic levels are higher in the dry season because of the lower volume of stored water.
These findings are consistent with those from other studies carried out by Lopes et al. (2014) and Santos et al. (2014) in the Orós reservoir, in which they point to weathering, surface runoff and human activity as being responsible for most deterioration in water quality. These authors suggest that intervention is necessary in order to reduce inputs of waste, which would then improve the trophic state of the water in the reservoir.
Table 4 also shows that component C4 explains 11.70 % of the total variability in the data. C4 is related to the biological attributes, as represented by the richness of families associated with the aquatic macrophytes E. crassipes (0.837) and P. stratiotes (0.748) (Table 5).
Table 5.
Macroinvertebrate family richness and scores assigned to each family
| Level of macroinvertebrates | Abundance | Scorea | |||
|---|---|---|---|---|---|
| Class | Order | Family | Pistia stratiotes | Eichhornia crassipes | |
| Malacostraca | Decapoda | Atydae | 198 | 71 | 8 |
| Insecta | Odonata | Libellulidae | 31 | 17 | 5 |
| Perilestidae | 93 | 44 | 5 | ||
| Coleoptera | Dyticidae | 19 | 45 | 5 | |
| Hydrophilidae | 97 | 85 | 4 | ||
| Elmidae | 19 | 53 | 4 | ||
| Diptera | Chironomidae | 6 | 22 | 2 | |
| Gastropoda | Mesogastropoda | Ampularidae | 1 | 36 | 3 |
| Neotaenioglossa | Thiaridae | 823 | 1550 | 3 | |
| Basommatophora | Planorbidae | 207 | 256 | 3 | |
| Abundance | 2179 | 1494 | |||
aBMWP and adaptations
Three classes and eight orders were found (Decapoda, Araneae, Odonata, Coleoptera, Diptera, Mesogastropoda, Neotaenioglossa and Basommatophora) distributed over ten families (Atyidae, Libellulidae, Perilestidae, Dyticidae, Hydrophilidae, Elmidae, Chironomidae, Ampularidae, Thiaridae and Planorbidae). All of the families were associated with the two species of aquatic macrophytes. The presence of these families is clear evidence of environments with poor water quality (Wyżga et al. 2013).
Results from the biological component showed that resistant families were present at all of the sampling points. Results were highest at point P2, showing that the environmental conditions at this site are unfavourable to families that are sensitive to environmental changes. These results are consistent with those of Ngodhe et al. (2014) and Ogrena and Huckins (2014), who also used macroinvertebrate biological metrics as indicators of water quality. Ogrena and Huckins (2014), in their studies of the Manistee watershed, found that bioindicators responded accurately to the quality of the local water.
The low diversity and families of macroinvertebrates, and in particular the Thiaridae family, associated with the two species of macrophyte are indicators of poor water quality.
Integrating biological attributes of macroinvertebrates with physical and chemical attributes made it possible to distinguish the degree of water quality deterioration at the different sampling points of the Orós reservoir; it also indicated the main agents responsible for the loss of environmental quality. Results confirm that the macroinvertebrate community is sensitive to changes in the aquatic environment.
Similar results were obtained by Mustow (2002) in biomonitoring studies carried out in Thai rivers. The author defends the inclusion of macroinvertebrate metrics, stating that they provide a rapid means for assessing water quality, with the added benefit of significantly lower costs.
Piedras et al. (2006) reported that the deterioration of water quality resulted in low macroinvertebrate diversity and that it also prevented the development of certain macroinvertebrate groups. Melo and Hepp (2008) also considered that biological metrics of richness, abundance and uniformity were capable of providing relevant information about the conservation of water bodies and that impacted environments tend to have limited biological diversity, with only a few dominating species.
Component C5 explains 8.38 % of the total variance and is related to a single nutrient, total phosphorous. C5 has a weighting of 0.946 (Table 4) and is an indicator of phosphorus enrichment. Phosphorus-rich waters favour the development of algae and the consequent eutrophication of the aquatic environment. Rabee et al. (2011), in studies conducted on the River Tigris in Iraq, found that phosphorus was the main factor responsible for eutrophication of the river and that it contributed to the excessive proliferation of microalgae in water bodies. Guedes et al. (2012) also observed drinking water quality deterioration due to phosphorus in the River Pomba in Minas Gerais, as did Silva (2013) in his study of phytoplankton communities in the Orós reservoir.
Other factors, such as fish farming and evaporation, may be related to increases in nutrients and consequent decreases in water quality. Mallasen et al. (2012), in their study of water quality in the Orós reservoir, found that phosphorus enrichment was due to an increase in fish rearing using net cages, because the feed for the fish is very rich in phosphorus. We can therefore assume that the water quality of the Orós reservoir is strongly influenced by the nature of the rocks and soil types in the region, and by anthropogenic activities that promote nutrient inputs to water bodies (livestock, fish farming and the erosion of agricultural areas).
The largest weightings for the WQI were recorded by attributes related to water hardness (Table 6). The higher factor weighting values indicate the most significant attributes for each factor. Ca+2, Mg+2 and Cl− had the highest weightings (>0.10); the index displays high sensitivity to variability of these ions. In contrast, the biological attributes related to the richness of macroinvertebrate families and to total phosphorus had lower weightings, indicating little sensitivity to variations in these attributes.
Table 6.
Weightings (w i) for the respective attributes of the WQI
| Attribute | Weighting |
|---|---|
| Calcium (Ca+2) | 0.113 |
| Magnesium (Mg+2) | 0.106 |
| Chloride (Cl−) | 0.105 |
| Apparent colour | 0.093 |
| Total suspended solids | 0.090 |
| Turbidity | 0.089 |
| Potassium (K+) | 0.084 |
| Sodium (Na+) | 0.083 |
| Sulphate (SO4 −2) | 0.082 |
| Richness of the family associated with Eichhornia crassipes | 0.055 |
| Richness of the family associated with Pistia stratiotes | 0.054 |
| Total Phosphorus | 0.046 |
| Total | 1.000 |
Similarly, Andrade et al. (2005) found that weightings were highest for attributes related to the water salt concentration (Na+, sodium absorption ratio (SAR) and EC), and lower weightings were related to the presence of organic compounds, pH and NO3 −. In their study of water quality in the River Odzi, Jonnalagadda and Mhere (2001) found that weightings were highest for pH and BOD.
Values of WQI for surface water in the Orós reservoir
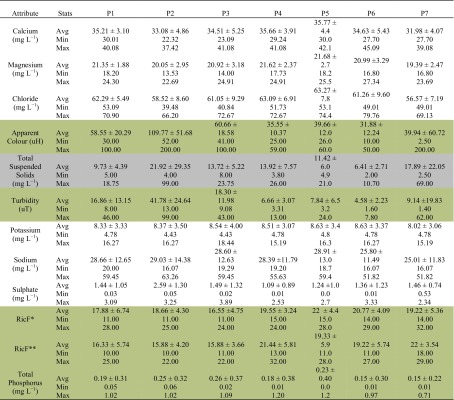
Mean values of the attributes chosen to represent the water quality of the reservoir are presented in Table 7. It is noteworthy that the mean values for Ca+2, Mg+2, Cl−, total suspended solids, K+, Na+ and SO4 −2 are within the acceptable limits for human consumption. Colour, turbidity, total phosphorus and macroinvertebrate family richness associated with the two aquatic macrophytes were considered unfit for human consumption, based on the limits suggested by Brazilian and international legislation (Table 2).
Table 7.
Statistics of water quality attributes of the Orós reservoir
White background: excellent quality; light grey: good quality; light brown: very bad
RicF* richness of the macroinvertebrate family associated with the aquatic macrophyte Eichhornia crassipes, RicF** richness of the macroinvertebrate family associated with the aquatic macrophyte Pistia stratiotes
Table 8 shows the values of q i calculated from the results of the attribute analyses. Table 9 shows that values of the WQI at the sampling points are similar, indicating that there was little spatial or temporal variability in the water quality of the Orós reservoir (ts = 0.001, p > 0.05). Furthermore, there were no significant differences between the dry or rainy seasons (ts = 0.001, p > 0.05).
Table 8.
Water quality index values for surface waters of the Orós reservoir
| Collection | P1 | P2 | P3 | P4 | P5 | P6 | P7 | Avg. | C.V. |
|---|---|---|---|---|---|---|---|---|---|
| August 2009 | 61.49 | 59.08 | 57.71 | 59.98 | 61.09 | 65.55 | 62.22 | 61.02 | 4.12 |
| October 2009 | 56.70 | 54.18 | 55.43 | 62.62 | 65.85 | 67.38 | 67.95 | 61.44 | 9.63 |
| December 2009 | 55.68 | 41.21 | 52.94 | 63.31 | 65.40 | 64.06 | 65.57 | 58.31 | 15.49 |
| February 2010 | 57.48 | 43.89 | 58.98 | 58.85 | 57.11 | 64.69 | 51.6 | 56.08 | 11.78 |
| April 2010 | 56.85 | 52.67 | 55.99 | 67.02 | 65.58 | 63.68 | 68.09 | 61.41 | 9.98 |
| June 2010 | 47.03 | 50.15 | 48.56 | 41.24 | 44.97 | 53.68 | 60.85 | 49.50 | 12.84 |
| September 2010 | 52.64 | 55.00 | 51.84 | 64.64 | 60.69 | 65.80 | 79.94 | 61.51 | 16.03 |
| January 2011 | 47.15 | 51.00 | 48.89 | 61.09 | 52.46 | 69.12 | 72.73 | 57.49 | 17.80 |
| March 2011 | 58.92 | 42.29 | 55.88 | 62.10 | 61.48 | 65.33 | 62.96 | 58.42 | 13.22 |
| Average | 54.88 | 49.94 | 54.02 | 60.09 | 59.40 | 64.37 | 65.76 | ||
| C.V. | 9.13 | 12.40 | 6.85 | 12.44 | 11.70 | 6.75 | 12.08 |
C.V. Coefficient of Variation
Table 9.
Comparison of average WQI values for the rainy and dry seasons for the Orós reservoir, CE
| Sampling point | Statistic | Season | |
|---|---|---|---|
| Dry | Rainy | ||
| P1 | Average | 54.70 ± 5.34 | 55.09 ± 5.37 |
| C.V. | 9.76 | 9.74 | |
| P2 | Average | 51.92 ± 6.77 | 47.46 ± 10.82 |
| C.V. | 13.05 | 25.54 | |
| P3 | Average | 53.29 ± 3.49 | 54.93 ± 7.78 |
| C.V. | 6.55 | 7.78 | |
| P4 | Average | 58.35 ± 9.71 | 62.26 ± 5.53 |
| C.V. | 16.65 | 5.53 | |
| P5 | Average | 59.60 ± 8.51 | 59.15 ± 9.54 |
| C.V. | 14.29 | 9.54 | |
| P6 | Average | 63.29 ± 5.50 | 65.70 ± 3.61 |
| C.V. | 8.69 | 3.61 | |
| P7 | Average | 67.30 ± 7.59 | 63.84 ± 14.23 |
| C.V. | 11.28 | 26.93 | |
| Average | 58.35 ± 5.57 | 58.35 ± 3.63 | |
| C.V. | 9.54 | 8.66 | |
C.V. Coefficient of Variation
The mean WQI values at the sampling points ranged from 49 to 65 (rated as normal), indicating that water is suitable for human consumption as long as the water is treated. Water treatments proposed for the waters of the Orós reservoir follow the recommendations of Brasil (2005) and involve removal and/or inactivation of refractory constituents that influence the colour, odour, taste, toxicity or pathogenic activity by conventional treatment processes.
Almeida and Schwarzbold (2003) applied the NSF WQI to the Cria Montenegro Stream (RS) and found that water quality was low. Andrade et al. (2005) obtained WQI values of between 72 and 89 for the Trussu River Valley, CE, which indicate that the water may be used for human consumption. Lopes et al. (2008) also applied a WQI to the River Acaraú and obtained values ranging from 60 to 80. Franco and Hernandez (2012), in their study of water quality in the Coqueiro catchment in São Paulo, obtained values ranging from 38 (acceptable) to 92 (excellent). Melo Junior et al. (2003), found values ranging from 59 to 85 (fair to good) for a stretch of the River Açu, in Rio Grande do Norte.
WQI values were highest at sampling points P7 (65.76) and P6 (64.37), located near the spillway of the reservoir. These high values highlight the purification capacity of the reservoir (Ostroumov 2005; Wei et al. 2009), as these points are furthest from the sediment and nutrient inputs from runoff.
Water quality was worst at P2 (49.94), P3 (54.02), P1 (54.88), P5 (59.40) and P4 (60.09); these sampling points are located at the upper end of the reservoir. The low results are mainly due to the very high values for apparent colour, turbidity and total phosphorus, and to the low levels of macroinvertebrate family richness. Sampling point P2 is located close to the inflow of the River Jaguaribe, the largest tributary with the largest nutrient inputs. The apparent colour, turbidity, macroinvertebrate richness and total phosphorus contribute the most to water quality deterioration.
Inputs of sewage and household waste from the town of Iguatu, approximately 20 km from the reservoir, are thought to make a significant contribution to the contamination of the Orós reservoir, and, in particular, upstream of sampling point P2. Iguatu has a population of 96,495, and data from IPECE (2014) indicate that only 11.25 % of the population is connected to an urban sewage system. Batista et al. (2014) agreed and classified P2 as hypereutrophic, and P1 and P3 as supereutrophic, due to the low values for transparency, and high total phosphorus levels. Overall, the reservoir was classified as hypereutrophic because of sediments carried by surface runoff.
Using the WQI, water quality is classified as fair, meaning that it can be used for human consumption. However, it should be noted that not all the attributes included in Table 7 are within acceptable limits. This suggests that the WQI should be adapted in order to detect the sensitivity of attributes such as total phosphorus and macroinvertebrate communities.
Conclusions
Using factor analysis/principal component analysis, we reduced the number of water quality attributes from 33 to 12; these 12 attributes explained 85.32 % of the total variance.
The five components from factor analysis/principal component analysis highlighted that weathering runoff and nutrient inputs, resulting from human activities such as agriculture, livestock, sewage discharge and household waste, were the main factors responsible for water quality deterioration in the Orós reservoir.
The proposed WQI, based on physical and chemical attributes and a macroinvertebrate metric, showed that water quality in the Orós reservoir is classified as ‘fair’, meaning that it is suitable for human consumption, as long as advanced treatment is carried out.
WQI values were highest at points P7 and P6, located near the spillway of the reservoir, reflecting the purification capacity of the reservoir.
The worst water quality was recorded at points P2, P3, P1, P4 and P5; these sampling points are located at the upper end of the reservoir, and the poor water quality reflects nutrient inputs, especially total phosphorus.
This study presents a more holistic view of the water quality of the Orós reservoir due to the inclusion of macroinvertebrate metrics in the physical and chemical attributes.
The low diversity of macroinvertebrates associated with the two species of macrophytes, and the dominance of pollution-resistant families indicate degradation of the water quality of the Orós reservoir.
Acknowledgments
The authors wish to thank CNPq INCTSal and CAPES for their financial support of this research.
References
- Aenab AM, Singh SK, Al-Rubaye AAM. Evaluation of Tigris River by water quality index analysis using C++ Program. Journal of Water Resources and Protection. 2012;4:523–527. doi: 10.4236/jwarp.2012.47061. [DOI] [Google Scholar]
- Alba-Tercedor J, Jáimez-Cuellar P, Álvarez M, Avilés J, Bonada N, Casas J, Mellado A, Ortega M, Pardo I, Prat N, Rieradevall M, Robles S, Sáinz-Cantero CE, Sánchez-Ortega A, Suárez ML, Toro M, Vidal-Abarca MR, Vivas S, Zamora-Munoz C. Caracterización del estado ecológico de ríos mediterrâneos ibéricos mediante el índice IBMWP (antes BMWP) Limnetica. 2002;21:175–185. [Google Scholar]
- ANA - Agência Nacional de Águas (2012). Panorama da qualidade das águas superficiais do Brasil. Brasília, DF
- Almeida MAB, Schwarzbold A. Seasonal evaluation of the quality of the water in Arroio da Cria, Montenegro, RS, applying a water quality index (WQI) Revista Brasileira de Recursos Hídricos. 2003;8:81–97. [Google Scholar]
- Andrade EM, Palácio HAQ, Crisóstomo LA, Souza IH, Teixeiras AS. A proposed water quality index: the case of Trussu River, Ceará, Brazil. Revista Ciência Agronômica. 2005;36:135–142. [Google Scholar]
- Andrade EM, Araújo LFP, Rosa MF, Disney W, Alves AB. Surface water quality indicators in low Acaraú Basin, Ceará, Brazil, using multivariable analysis. Revista Engenharia Agrícola Jaboticabal. 2007;27:683–690. doi: 10.1590/S0100-69162007000400011. [DOI] [Google Scholar]
- Andrade EM, Lopes FB, Palácio HAQ, Aquino DN, Alexandre DMB. Land use and groundwater quality: the case of Baixo Acaraú irrigated perimeter, Brazil. Revista Ciência Agronômica. 2010;41:208–215. doi: 10.1590/S1806-66902010000200006. [DOI] [Google Scholar]
- APHA -American Public Health Association. American Water Works Association (AWWA) and Water Environment Federation (WEF) Standard methods for the examination of water and wastewater. 21. Washington: American Public Health Association; 2005. [Google Scholar]
- Armitage PD, Moss D, Wright JF, Furse MT. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Research. 1983;17:333–347. doi: 10.1016/0043-1354(83)90188-4. [DOI] [Google Scholar]
- Baptista DF. The use of macroinvertebrates in biomonitoring aquatic ecosystems. Revista Oecologia Brasileira. 2008;12:425–441. [Google Scholar]
- Batista AA, Meireles ACM, Andrade EM, Izídio NSC, Lopes FB. Seasonal and spatial variation of the trophic state index of the Orós reservoir, Ceará, Brazil. Revista Agroambiente On-line. 2014;8:39–48. [Google Scholar]
- Boyacioglu H. Utilization of the water quality index method as a classification tool. Environmental Monitoring and Assessment. 2010;167:115–124. doi: 10.1007/s10661-009-1035-1. [DOI] [PubMed] [Google Scholar]
- Brasil - Conselho Nacional do Meio Ambiente . Resolução n° 274, de 29 de novembro de 2000. Define os critérios de balneabilidade em águas brasileiras. Brasília: Diário Oficial da União; 2001. [Google Scholar]
- Brasil - Conselho Nacional do Meio Ambiente . Resolução n° 518, de 25 de março de 2004. Estabelece os padrões de aceitação para consume humano. Brasília: Diário Oficial da União; 2004. [Google Scholar]
- Brasil - Conselho Nacional do Meio Ambiente . Resolução n° 357, de 17 de março de 2005. Estabelece os padrões de qualidade de água. Brasília: Diário Oficial da União; 2005. [Google Scholar]
- Brasil - Ministério da Saúde . Portaria n° 2914, de 12 de dezembro de 2011. Estabelece os padrões de qualidade de água para consume humano e de potabilidade. Brasília: Diário Oficial da União; 2011. [Google Scholar]
- Brusca RC, Brusca GJ. Invertebrados. Rio de Janeiro: Editora Guanabara Koogan; 2007. [Google Scholar]
- Buss DF, Borges EL. Application of Rapid Bioassessment Protocols (RBP) for benthic macroinvertebrates in Brazil: Comparison between sampling techiniques and mesh sizes. Neotropical Entomology. 2008;37:288–295. doi: 10.1590/S1519-566X2008000300007. [DOI] [PubMed] [Google Scholar]
- CETESB - Companhia Ambiental do Estado de São Paulo . Guia nacional de coleta e preservação de amostras: água, sedimentos, comunidades aquáticas e efluentes líquidos. Companhia Ambiental do Estado de São Paulo; Organizadores: Carlos Jesus Brandão … [et al.] Brasília: ANA; 2011. [Google Scholar]
- Comitesinos – Comitê de Preservação, Gerenciamento e Pesquisa da Bacia do Rio dos Sinos . Utilização de um índice de qualidade da água no rio dos Sinos. Porto Alegre: Comitesinos; 1990. p. 112p. [Google Scholar]
- Debels P, Figueroa R, Urrutia R, Barra R, Niell X. Evaluation of water quality in the Chillan River (central Chile) using physicochemical parameters and a modified water quality index. Environmental Monitoring and Assessment. 2012;110:301–322. doi: 10.1007/s10661-005-8064-1. [DOI] [PubMed] [Google Scholar]
- Deepak S, Singh NU. Water quality index for Ground Water (GWQI) of Dhar town MP, India. International Research Journal of Environmental Science. 2013;2:72–77. [Google Scholar]
- DNOCS – Departamento Nacional de Obras Contra as Secas. (2014). Barragem Orós. Available at: http://www.dnocs.gov.br/barragens/oros/oros.htm Accessed 08 jun 2014.
- EMBRAPA – Centro Nacional de Pesquisa de Solo. (2006). Sistema brasileiro de classificação de solos. (2th edition). Rio de Janeiro.
- Ferreira WR, Paiva LT, Callisto M. Development of a benthic multimetric índex for biomonitoring of a neotropical watershed. Brazilian Journal of Biology. 2011;71:15–25. doi: 10.1590/S1519-69842011000100005. [DOI] [PubMed] [Google Scholar]
- Franco RAM, Hernandez FBT. Water quality of Coqueiro stream watershed, northwest of São Paulo State. Water Resources and Irrigation Management. 2012;1:61–69. [Google Scholar]
- Guedes HAS, Silva DD, Elesbon AAA, Ribeiro CBM, Matos AT, Soares JHP. Application of multivariate statistical analysis in the study of water quality in the Pomba River (MG) Revista Brasileira de Engenharia Agrícola e Ambiental. 2012;16:558–563. doi: 10.1590/S1415-43662012000800014. [DOI] [Google Scholar]
- Giriyappanavar BS, Patil RR. Water quality assessment of Gullava Reservoir of Belgaum District using NSF-WQI. International Journal of Scientific Research. 2013;2:2277–8179. [Google Scholar]
- Hair Júnior JF, Anderson RE, Tathan RL, Black WC. Análise Multivariada de Dados. 5. Porto Alegre: Bookman; 2005. [Google Scholar]
- Hurley T, Sadiq R, Mazumder A. Adaptation and evaluation of the Canadian Council of Ministers of the Environment water quality index (CCME WQI) for use as an effective tool to characterize drinking source water quality. Water Research. 2012;46:3544–3552. doi: 10.1016/j.watres.2012.03.061. [DOI] [PubMed] [Google Scholar]
- IPECE - Instituto de Pesquisa e Estratégia Econômica do Ceará. (2012). Anuário Estatístico do Ceará 2010. Available at: http://www2.ipece.ce.gov.br/publicacoes/anuario/anuario2010/index.htm. Accessed 02 jun 2014.
- IPECE - Instituto de Pesquisa e Estratégia Econômica do Ceará. (2014). Perfil Básico Municipal- Iguatu. Available at: http://www.ipece.ce.gov.br/publicacoes/perfil_basico/pbm-2012/Iguatu.pdf. Accessed 03 jun 2014.
- Jones JG. A guide to methods for stimating microbial numbers and biomass in fresh waters. London: FreshWaters Biological Association; 1979. [Google Scholar]
- Jonnalagadda SB, Mhere G. Water quality of the Odzi river in the eastern highlands of Zimbabwe. Water Research. 2001;35:2371–2376. doi: 10.1016/S0043-1354(00)00533-9. [DOI] [PubMed] [Google Scholar]
- Junqueira VM, Campos SCM. Adaptation of the “BMWP” method for water quality evaluation to rio das Velhas Watershed (Minas Gerais, Brazil) Acta Limnologica Brasiliensia. 1998;10:125–135. [Google Scholar]
- Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23:187–200. doi: 10.1007/BF02289233. [DOI] [Google Scholar]
- Kumar PJS, James EJ. Development of water quality index (WQI) model for the ground water in Tirupur district. South Indian and Chinese Journal of Geochemistry. 2013;32:261–268. doi: 10.1007/s11631-013-0631-5. [DOI] [Google Scholar]
- Lamparelli, M. C. (2004). Trophic status in São Paulo State water bodies: evaluation of monitoring methodologies. 238 p. Tese (Doutorado em Ciências), Universidade de São Paulo, São Paulo.
- Lumb A, Sharma TC, Bibeault JF. A review of genesis and evolution of water quality index (WQI) and some future directions. Water Quality Exposure and Health. 2011;3:11–24. doi: 10.1007/s12403-011-0040-0. [DOI] [Google Scholar]
- Lopes FB, Teixeira AS, Andrade EM, Aquino DN, Araújo LFP. Map of Acaraú River water quality by the use of WQI and GIS. Revista Ciência Agronômica. 2008;39:392–402. [Google Scholar]
- Lopes FB, Andrade EM, Meireles ACM, Becker H, Batista AA. Assessment of the water quality in a large reservoir in semiarid region of Brazil. Revista Brasileira de Engenharia Agrícola Ambiental. 2014;18:437–445. doi: 10.1590/S1415-43662014000400012. [DOI] [Google Scholar]
- Magesh NS, Chandrasekar N. Evaluation of spatial variations in groundwater quality by WQI and GIS technique: a case study of Virudunagar District, Tamil, India. Arabian Journal of Geosciences. 2013;3:1883–1898. doi: 10.1007/s12517-011-0496-z. [DOI] [Google Scholar]
- Mallasen M, Carmo CF, Tucci A, Barros HP, Rojas NET, Fonseca FS, Yamashita EY. Qualidade da água em Sistema de piscicultura em tangues-rede no reservatório de Ilha Solteira. São Paulo Boletim do Instituto de Pesca. 2012;38:15–30. [Google Scholar]
- Melo Junior G, Costa CEFS, Cabral Neto CI. Avaliação hidroquímica e da qualidade das águas de um trecho do rio Açu, Rio Grande do Norte. Revista de Geologia. 2003;16:27–36. [Google Scholar]
- Melo AS, Hepp LU. Statistical tools to analyze data from biomonitoring studies. Revista Oecologia Brasiliensis. 2008;12:463–486. [Google Scholar]
- Meireles ACM, Frischkorn H, Andrade EM. Seasonal quality changes in Waters from Edson Queiroz dam, Acaraú basin, in the semiarid of Ceará. Revista Ciência Agronômica. 2007;38:25–31. [Google Scholar]
- Morse JC, Bae YJ, Munkhjargal G, Narumon S, Tanida K, Vshivkova TS, Wang B, Yang L, Yule CM. Freshwater biomonitoring with macroinvertebrates in East Asia. Frontiers in Ecology and the Environment. 2007;5:33–42. doi: 10.1890/1540-9295(2007)5[33:FBWMIE]2.0.CO;2. [DOI] [Google Scholar]
- Mugnai R, Nessimian JL, Baptista DF. Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro. Rio de Janeiro: Technical Books; 2010. [Google Scholar]
- Mustow SE. Biological monitoring of rivers in Thailand: use and adaptation of the BMWP score. Hydrobiologia. 2002;479:229–229. doi: 10.1023/A:1021055926316. [DOI] [Google Scholar]
- Ngodhe SO, Raburu PO, Achieng A. The impact of water quality on species diversity and richness of macroinvertebrates in small water bodies in lake Victoria Basin, Kenya. Journal of Ecology and the Natural Environment. 2014;6:32–41. doi: 10.5897/JENE2013.0403. [DOI] [Google Scholar]
- Norusis MJ. SPSS base system user’s guide. Chicago: SPSS Inc; 1990. p. 520. [Google Scholar]
- Ogrena SA, Huckins CJ. Evaluation of suitability and comparability of stream assessment indices using macroinvertebrate data sets from the Northern Lakes and Forests Ecoregion. Ecological Indicators. 2014;40:117–126. doi: 10.1016/j.ecolind.2014.01.018. [DOI] [Google Scholar]
- Oliveira RBS, Castro CM, Baptista DF. Developing multimetric índices for aquatic ecosystems bioassessment. Revista Oecologia Brasileira. 2008;12:425–441. [Google Scholar]
- Ostroumov SA. On some issues of maintaining water quality and self-purification. Water Resources. 2005;32:337–346. doi: 10.1007/s11268-005-0039-7. [DOI] [Google Scholar]
- Pal A, Ri AK, Zaidi J. Water quality index (WQI) of three historical lakes in Mahoba District of Bundelkhand Region, Uttar Pradesh, India. Asian Journal of Science and Technology. 2013;4:048–053. [Google Scholar]
- Palácio HAQ, Araujo Neto JR, Meireles ACM, Andrade EM, Santos JCN, Chaves LCG. Similarity and determining factors in the salinity of surface waters of Ceara by multivariate techniques. Revista Brasileira de Engenharia Agrícola e Ambiental. 2011;15:395–402. doi: 10.1590/S1415-43662011000400011. [DOI] [Google Scholar]
- Piedras SRN, Bager A, Moraes PRR, Isoldi LA, Ferreira OGL, Heemann C. Macroinvertebrados bentônicos como indicadores de qualidade de água na barragem Santa Bárbara, Pelotas, RS, Brasil. Ciência Rural. 2006;36(494–500):2006. [Google Scholar]
- Rabee AM, Abdul-Kareem BM, Al-Dhamin AS. Seasonal variations of some ecological parameters in Tigris River water at Baghdad Region, Iraq. Journal of Water Resource Protection. 2011;3:262–267. doi: 10.4236/jwarp.2011.34033. [DOI] [Google Scholar]
- Radambrasil, Projeto. Folhas SA-24 Fortaleza e SB-24 Jaguaribe, Geologia, geomorfologia, solos, vegetação e uso potencial da terra. Rio de Janeiro, 1981. (Levantamento de Recursos Naturais, 2 e 3).
- Rekha VB, George AV, Rita M. A comparative study of water quality index (WQI) of Peruvanthanam and Valiyathodu sub-watersheds of Manimala river basin, Kerala, South India. Journal of Environmental Science, Toxicology and Food Technology. 2013;3:2319–2399. [Google Scholar]
- Rodier J. L’analyse de L’éaux; naturelles, eaux residuals, eaux de mer. 5. Paris: Dunod; 1975. p. 629. [Google Scholar]
- Rossaro B, Marziali L, Cardoso AC, Solimini A, Free G, Giacchini R. A biotic index using benthic macroinvertebrates for Italian Lakes. Ecological Indicators. 2007;7:412–429. doi: 10.1016/j.ecolind.2006.04.002. [DOI] [Google Scholar]
- Rubio-Arias H, Contreras-Caraveo M, Quintana RM, Saucedo-Teran RA, Pinales-Munguia A. An overall water quality índex (WQI) for a man-made aquatic reservoir in Mexico. International Journal of Environmental Research and Public Health. 2014;9:35–44. doi: 10.3390/ijerph9051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JCN, Andrade EM, Araujo Neto JR, Meireles ACM, Palácio HAQ. Land use and trophic state dynamics in a tropical semi-arid reservoir. Revista Ciência Agronômica. 2014;45:35–44. doi: 10.1590/S1806-66902014000100005. [DOI] [Google Scholar]
- Silva, M. D. Diagnóstico da comunidade fitoplanctônica de um reservatório no semiárido nordestino. 2013. 113 f. Dissertação (Mestrado em Ecologia e Recursos Naturais) - Centro de Ciências, Universidade Federal do Ceará, Fortaleza.
- Silveira MP, Baptista DF, Buss DF, Nessimian JL, Egler M. Application of biological measures for stream integrity assessment in south-east Brazil. Environmental Monitoring and Assessment. 2005;101:117–128. doi: 10.1007/s10661-005-9141-1. [DOI] [PubMed] [Google Scholar]
- Uherek CB, Gouveia FBP. Biological monitoring using macroinvertebrates as bioindicators of water quality of Maroaga stream in the maroaga cave system, Presidente Figueredo, Amazon, Brazil. International Journal of Ecology. 2014;2014:1–7. doi: 10.1155/2014/308149. [DOI] [Google Scholar]
- Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R. Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin Tamilnadu, India. Environmental Monitoring and Assessment. 2010;171:595–609. doi: 10.1007/s10661-009-1302-1. [DOI] [PubMed] [Google Scholar]
- Wei G, Yang Z, Cui B, Li B, Chen H, Bai J, Dong S. Impact of dam construction on water quality and water self-purification capacity of the lancing river, China. Water Resources Management. 2009;23:1763–1780. doi: 10.1007/s11269-008-9351-8. [DOI] [Google Scholar]
- WHO - World Health Organization . Guidelines for drinking water quality: first addendum to volume 1, Recommendations. Geneva: WHO; 2006. [Google Scholar]
- Wyżga B, Oglęcki P, Hajdukiewicz H, Zawiejska J, Radecki-Pawlik A, Skalski T, Mikuś P. Interpretation of the invertebrate-based BMWP-PL index in a gravel-bed river: insight from the Polish Carpathians. Hydrobiologia. 2013;712:71–88. doi: 10.1007/s10750-012-1280-0. [DOI] [Google Scholar]
- Yan J, Liu J, Ma M. In situ variations and relationships of water quality index with periphyton function and diversity metrics in Baiyangdian Lake of China. Ecotoxicology. 2014;33:987–995. doi: 10.1007/s10646-014-1199-5. [DOI] [PubMed] [Google Scholar]
- Yisa J, Jimoh T. Analytical studies on water quality index of River Landzu. American Journal of Applied Sciences. 2010;7:453–458. doi: 10.3844/ajassp.2010.453.458. [DOI] [Google Scholar]
- Zeng X, Rasmussen TC. Application of multivariate statistical techniques in the assessment of water quality in the Southwest New Territories and Kowloon, Hong Kong. Environmental Monitoring and Assessment. 2010;137:17–27. doi: 10.1007/s10661-010-1366-y. [DOI] [PubMed] [Google Scholar]