Abstract
It is well recognized that the decrease of adiponectin associated with high-fat diet and lack of exercise accounts for the onset of insulin resistance, type 2 diabetes, the metabolic syndrome, and cardiovascular disease. Our research efforts have led to the identification of adiponectin receptors, AdipoR1 and AdipoR2, with the former shown to activate AMP kinase in the liver and the latter shown to activate peroxisome proliferator-activated receptor-α signaling thereby increasing fatty acid oxidation. Again, adiponectin upregulates mitochondrial function in the skeletal muscle thereby improving glucose/lipid metabolism and insulin resistance. These findings suggested that activation of adiponectin/AdipoR signaling could represent a viable therapeutic approach to lifestyle-linked diseases associated with prevalent obesity thus contributing to healthy longevity in humans. Indeed, they have led to the successful discovery of AdipoRon, a small-molecule AdipoR-activating compound. Thus far, AdipoRon has been found not only to improve insulin resistance in mice but to prolong their lifespan shortened by high-fat diet. Additionally, our structure-based drug discovery research has led to AdipoR being identified as an entirely novel structure having a zinc iron bound within its seven-transmembrane domain as well as an opposite orientation to that of G protein-coupled receptors. It is expected that increasing insight into AdipoR signaling will facilitate the structure-based optimization of candidate small-molecule AdipoR-activating compounds for human use as well as the development of molecularly targeted and calorie-limiting/exercise-mimicking agents for lifestyle-linked diseases.
Keywords: Adiponectin, AdipoR, Healthy longevity, Diabetes, Obesity, Drug development
Introduction
Obesity is shown to continue to rise in incidence affecting some 64,100 million people worldwide [1]. With insulin resistance as a basis, obesity is shown to induce the so-called metabolic syndrome consisting of diabetes mellitus, dyslipidemia and hypertension, thus accounting for an increased incidence of cardiovascular disease [2, 3]. Of note, in agreement with the observation that individuals with the metabolic syndrome represent a high-risk group for diabetes mellitus, it is shown that those with diabetes mellitus are drastically increasing in number and present a formidable social challenge worldwide. According to the report of the International Diabetes Federation (IDF), as of 2017, the number of individuals affected by diabetes mellitus stands at 425,000 million in the world, with an estimated prevalence of 8.8% among those 20 years of age or older but younger than 80 years of age (affecting one in 11 adults) in the world [4]; it is also estimated that the patient number will increase to as high as 629,000 million by 2045. Thus, there is an acute and pressing need to elucidate the mechanisms of onset of obesity, insulin resistance, diabetes and their complications, as well as to establish radical preventive and therapeutic measures for these conditions based on insights to be gained into their pathophysiology.
It is now well recognized that adipose tissue has a critical role as an organ secreting multiple hormones, i.e., adipokines, involved in the regulation of wide-ranging physiological functions [5–8]. Of these, adiponectin is currently drawing a great deal of attention as a multifunctional hormone [9–12]. Of note, given that the blood concentration of adiponectin is shown to be decreased with obesity in humans, adiponectin deficiency has come to be counted among the major causes of lifestyle-linked diseases [13, 14]. Moreover, decreases in adiponectin levels have been observed in obese mice, correlating with insulin sensitivity [15]. Our research efforts have led to the identification of the adiponectin receptors, AdipoR1 and AdipoR2, which are both shown to be membrane proteins involved in glucose/lipid metabolism and activated by adiponectin [16]. Of these, AdipoR1 has been shown to activate the AMP kinase pathway, and AdipoR2 to activate peroxisome proliferator-activated receptor (PPAR) signaling, thus improving glucose/lipid metabolism and exerting antidiabetic effects [17]. Thus, elucidating the mechanisms through which these adiponectin receptors become activated is thought likely to lead to refinements in available therapeutics as well as to the development of novel therapeutics for the metabolic syndrome and diabetes mellitus.
Of note, our research efforts have led to the discovery of AdipoRon, the world’s first-ever small-molecule AdipoR-activating compound to emerge from adiponectin research [18]. Our structure-based drug discovery research using X-ray-based crystal structure analysis has also led to the AdipoR structure being elucidated as one that contains an internal cavity within the seven-transmembrane helices coordinating a zinc ion, which our research laboratory was the first to identify as an entirely novel structure with an intracellular N-terminus and an extracellular C-terminus thus having an opposite orientation to that of commonly known G protein-coupled receptors (GPCRs) [19].
In the years to come, it is expected that these structural findings will facilitate the development of novel, innovative, safe, effective, and curative antidiabetic drugs for use in humans through optimization of the AdipoR agonists currently available.
Adiponectin and its role in improving glucose/lipid metabolism
Obesity is thought to result mainly from enlargement of adipocytes and lead to the onset of the metabolic syndrome. In addition to its known role in storing excess energy in the form of triglycerides, adipose tissue serves as an endocrine organ secreting “adipokines”, i.e., signaling molecules for leptin, tumor necrosis factor (TNF)-α, and free fatty acids (FFA) [20–24]. It has become clear that, with enlargement of adipocytes, TNF-α, FFA and other signaling molecules are produced in such large amounts that they interfere with insulin signaling in the skeletal muscle thus inducing insulin resistance.
On the other hand, of all adipokines, adiponectin is shown to improve insulin resistance, a glycoprotein with a molecular weight of 30 kDa, adiponectin consists of signal peptide, collagen, and globular domains [25–28].
While high-fat diet is shown to induce enlargement of adipocytes and increase insulin resistance thus leading to marked decreases in blood adiponectin levels in a mouse model of type 2 diabetes [29], adiponectin replenishment is shown to improve insulin resistance in mice with obesity induced by high-fat diet, demonstrating that adiponectin has a vital role to play in improving insulin resistance [13].
It is also reported that adiponectin not only promotes fatty acid oxidation in the skeletal muscle but enhances insulin sensitivity and inhibits gluconeogenesis in the liver thereby lowering blood glucose levels [14, 15, 30].
Furthermore, adiponectin-deficient mice are shown to be associated with insulin resistance, impaired glucose tolerance, dyslipidemia and hypertension and thus present with the metabolic syndrome, suggesting that adiponectin deficiency has a key role to play in their pathogenesis [31–34].
Altogether, these findings suggest that the decrease of adiponectin associated with obesity is among the major causes of impaired glucose tolerance, dyslipidemia and hypertension, i.e., main components of the metabolic syndrome.
Adiponectin is also found to increase the expression in the skeletal muscle of the PPAR-α-targeted acylCoA oxidase (ACO) genes involved in fatty acid oxidation and the uncoupling protein (UCP) gene involved in energy consumption. Again, adiponectin is found not only to increase the expression of PPAR-α [13], but to enhance endogenous PPAR-α ligand activity [35].
Furthermore, while adiponectin is shown to promote fatty acid oxidation in C2C12 cells, an in vitro model of skeletal muscle [36], part of this fatty acid oxidation-promoting mechanism is known to involve the activation and phosphorylation of AMPK-activated protein kinase (AMPK) [37], a molecule thought to be activated by exercise and promote non-insulin-dependent glucose uptake and fatty acid oxidation thereby providing the energy required for exercise.
It is quite interesting to note that adiponectin is shown to activate the AMPK pathway, as the finding suggests that fatty acid oxidation, glucose uptake and utilization in the skeletal muscle as promoted by adiponectin, as well as glucose lowering with adiponectin administration in vivo, may be accounted for at least in part by the activation of the AMPK pathway [36], in agreement with the report of another research group demonstrating that globular adiponectin activates the AMPK pathway [38].
Adiponectin receptors and their identification
The decrease of adiponectin as it is associated with obesity is known not only to increase risk factors for diabetes and dyslipidemia but to directly adversely affect the vascular wall [32, 39] thus causing the metabolic syndrome and associated macroangiopathy. Thus, our research efforts have been focused on the elucidation of the mechanism of action of adiponectin as being critical to the development of curative therapy for diabetes and the metabolic syndrome.
With a focus on the specific binding characteristics of adiponectin, our research has led to the successful identification of the adiponectin receptors, AdipoR1 and AdipoR2 [16], which are shown to be highly homologous (67% at the amino acid level) and structurally conserved across various species from yeast to humans, with the yeast AdipoR1 homolog (YOL002c) shown to play a critical role in fatty acid oxidation [40]. AdipoR1 is found to be abundantly in the skeletal muscle, while relatively ubiquitously expressed throughout the body; AdipoR2 is found to be predominantly expressed in the liver. While AdipoR1 and AdipoR2 were predicted to be seven-transmembrane receptors each with an internal N-terminus and an external C terminus, the topology of which is opposite to that reported for GPCRs. Again, our research has revealed that they are essential for adiponectin binding to cell surfaces in cultured cells [16].
Adiponectin receptors and their physiological and pathophysiological roles
Our research has revealed that the expression of both AdipoR1 and AdipoR2 is decreased in a mouse model of obesity/diabetes, demonstrating that their decreased expression accounts in part for the onset of diabetes [17]. Furthermore, our research has led to AdipoR1- and AdipoR2-deficient mice being genetically engineered toward elucidation of the pathophysiological roles of AdipoR1 and AdipoR2. Our research revealed that adiponectin binding and action are lost in AdipoR1/AdipoR2-double knockout mice, demonstrating that the AdipoRs are essential adiponectin receptors in the body [17].
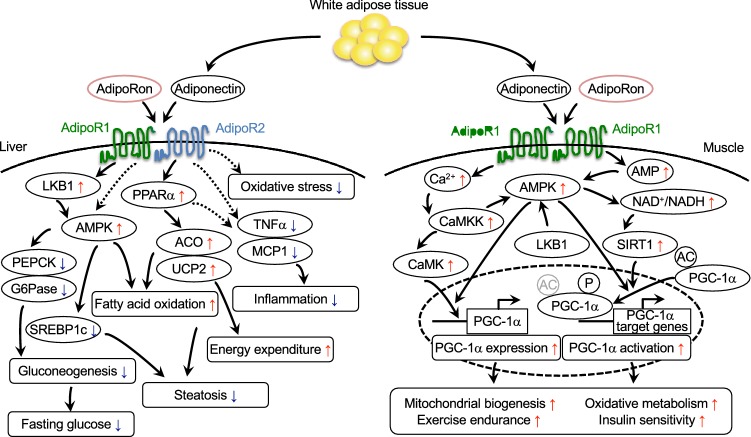
AdipoR1/AdipoR2-double knockout mice are associated with insulin resistance and impaired glucose tolerance, with the mechanisms of their onset accounted for by increased inflammation and oxidative stress leading to decreased gluconeogenesis and glucose uptake in such major metabolic organs as the liver, skeletal muscle and adipose tissue [17]. Increasing the expression of AdipoR1 leads to the activation of the AMPK pathway and increasing the expression of AdipoR2 leads to the activation of PPAR-α and promotes fatty acid oxidation and energy consumption and produces anti-inflammatory and anti-oxidative stress effects thereby improving impaired glucose tolerance (Fig. 1) [17].
Fig. 1.
Scheme illustrating the actions of adiponectin/AdipoR agonist, AdipoRon, in the liver and the muscle. Adiponectin and the AdipoR agonist, AdipoRon participate in the regulation of glucose and lipid metabolism through their critical effects within the liver and muscle. In the liver, adiponectin/AdipoRon activates not only the AMPK pathway via AdipoR1 but the PPARα pathways via AdipoR2, thus doubly ameliorating insulin resistance and steatosis [17, 18]. In the muscle, adiponectin/AdipoRon regulates PGC-1α through the AMPK-SIRT1 and the calcium-CaMKK pathways to promote mitochondrial biogenesis via AdipoR1 [18, 41]
Again, our research has shown that adiponectin/AdipoR1 signaling increases mitochondrial mass and function and improves glucose/lipid/energy metabolism and exercise endurance by exerting similar effects to those of exercise [41]. The expression of PPAR-γ coactivator-1α (PGC-1α) [42] was shown to be decreased by 75%, accompanied by decreases in mitochondrial mass and function as well as in the proportion of type 1 fiber, leading to decreased exercise endurance in the skeletal muscle of skeletal muscle-specific AdipoR1-deficient mice newly engineered at our laboratory [41], which showed evidence of impaired glucose tolerance and insulin resistance. Thus, adiponectin has an important dual role to play in the regulation of PGC-1α [41] not only by increasing the intracellular Ca2+ concentration via AdipoR1 thus elevating the expression of PGC-1α but by activating [41] the AMPK pathway/the longevity gene SIRT1 [43] thus contributing to the activation of PGC-1α (Fig. 1). In other words, our research has revealed that adiponectin/AdipoR1 signaling in the skeletal muscle is exercise mimicking.
Discovery of adiponectin receptor agonist (AdipoRon)
It is assumed that enhancing adiponectin/AdipoR signaling brings about qualitative changes in the metabolic capacity of an organism thereby normalizing its metabolic environment. Thus, adiponectin/AdipoR enhancers and AdipoR-activating drugs are likely to represent “exercise-mimicking agents”. When successfully developed, exercise-mimicking agents are strongly expected to represent not only radical therapeutic options for the metabolic syndrome, type 2 diabetes, and atherosclerosis but also effective treatments for patients with some medical or locomotor disease who may find it difficult to exercise.
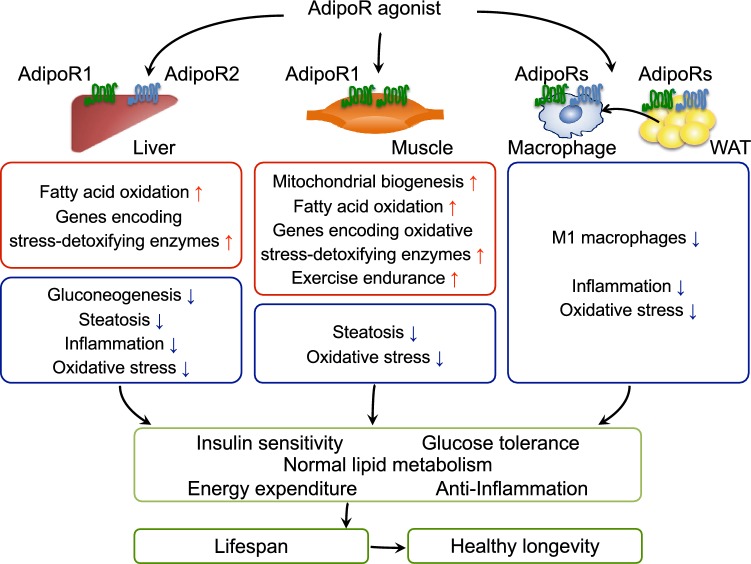
In our drug discovery research, an originally developed method has been employed in screening candidate compounds available at the Chemical Library, University of Tokyo Drug Discovery Open Innovation Center [currently Drug Discovery Initiative (DDI)] and other resources, which has led to the successful discovery of a small-molecule adiponectin receptor-activating compound (adiponectin receptor agonist; AdipoRon) [18], which has been shown to bind directly to AdipoR1 and AdipoR2, thus activating AMPK in C2C12 cells, a skeletal muscle cell model, and enhancing mitochondrial function [18]. Furthermore, AdipoRon has proven to be an oral compound capable of improving glucose/lipid metabolism thus exerting antidiabetic effects via AdipoRs (Fig. 2) [18]. AdipoR1 and AdipoR2 signaling mechanisms in macrophage and white adipose tissue are not well understood, future research efforts should elucidate the molecular mechanisms by which adiponectin/AdipoRs functions in these tissues.
Fig. 2.
Scheme illustrating the mechanisms by which a typical AdipoR agonist increases insulin sensitivity and glucose tolerance leading to healthy longevity. In the liver, AdipoRon is shown to suppress the expression of genes involved in gluconeogenesis, increase fatty acid oxidation, and reduce oxidative stress and inflammation [18]. In the muscle, a typical AdipoR agonists is assumed to increase mitochondrial biogenesis leading to increased exercise endurance, while at the same time increasing the levels of expression of genes involved in fatty acid combustion, oxidative phosphorylation, and oxidative stress reduction [18]. In white adipose tissue (WAT), AdipoRon is shown to reduce oxidative stress and inflammation as well as inhibit the accumulation of M1 macrophages. Enhanced activation of the AdipoR pathways is assumed to improve insulin resistance and normalize lipid metabolism [18]. Drugs targeted at the AdipoR pathways may have beneficial effects on obesity-linked diseases and healthy longevity
Role of the adiponectin receptor agonist AdipoRon in prolonging lifespan
Adiponectin activates the AMPK pathway via AdipoR1 thereby improving insulin resistance in the skeletal muscle [16, 17, 36, 41]. Again, adiponectin/AdipoR1 elevates the NAD+/NADH ratio in the skeletal muscle thus activating the longevity gene SIRT1 [41]. It is also shown that adiponectin upregulates via AdipoR2 the expression of ACO and UCP which are shown to be involved in fatty acid oxidation and energy consumption, respectively [16, 17]. An examination of the ACO and UCP genes each shown to be equipped with a PPAR response element (PPRE) revealed upregulated endogenous PPAR-α ligand activity, with the expression of PPAR-α also shown to be upregulated [16]. These results demonstrate that adiponectin/AdipoR2 signaling upregulates the expression of the catalase and superoxide dismutase (SOD) genes thereby reducing tissue oxidative stress [17].
Again, while caloric restriction is shown to prolong lifespan [44], AMPK, mechanistic target of rapamycin (mTOR) [45], and SIRT have been shown to be partly implicated in the mechanism involved. It is known that over-expressing the AMPK-α subunit in nematodes leads to prolongation of their lifespan [46, 47]. It is also reported that the AMPK pathway inhibits mTOR signaling and reduces protein synthesis thereby inhibiting cancer cell proliferation and neoangiogenesis. Furthermore, many reports are available to show that inhibiting mTOR signaling in yeasts, nematodes, and Drosophila leads to prolongation of their lifespan, as well as in mice whose lifespan is shown to be prolonged when treated with the mTOR inhibitor rapamycin [45]. Tissue oxidative stress is shown to be increased in obese organisms thus adversely affecting their aging process or lifespan, while over-expressing the oxidative stress-reducing catalase gene or SOD is shown to prolong their lifespan [47–49].
Of note, adiponectin/AdipoR signaling is shown to activate the AMPK/SIRT1 pathways and thus positively regulate the catalase and SOD genes thereby reducing tissue oxidative stress [43, 50], which has led us to examine AdipoR-deficient mice for their lifespan, which may be found to be shortened. Indeed, AdipoR1-/AdipoR2-deficient mice given high-fat diet have been found to have a shorter lifespan than their wild-type counterpart, and AdipoR1/AdipoR2-double knockout mice have the shortest of all [18]. Again, high-fat diet is shown to markedly shorten lifespan in a mouse model of obesity/type 2 diabetes compared to standard diet, while administration of the AdipoR agonist AdipoRon has been shown to reverse the shortening of lifespan associated with obesity in these mice despite being given high-fat diet [18]. Thus, all these observations combine to suggest a potential role for AdipoR-activating approaches in prolonging lifespan.
Therefore, AdipoR-targeted approaches have the potential to improve insulin resistance upstream, positively affect vessels, improve atherosclerosis, and effectively treat lifestyle-related diseases and their complications that are increasingly associated with obesity, thus bringing healthy longevity within reach [51]. This potential appears to provide the rationale for optimization of AdipoR agonists including AdipoRon for use in humans.
Structure and function analysis of AdipoR1 and AdipoR2
The mechanisms of activation of GPCRs have begun to be unraveled through structural analysis of their downstream GP complexes. While adiponectin receptors were predicted to contain seven-transmembrane helices with an intracellular N-terminus and an extracellular C-terminus whose orientation was opposite to that of GPCRs, the structures of AdipoRs remained unknown. This spurred efforts at our research laboratory to unravel their structures.
Our research efforts led to the development of antibodies capable of recognizing the structures of AdipoRs as well as to the crystallization of AdipoR/antibody fragment (Fv) complexes using lipid mesophases [52]. Based on the crystallized AdipoR/Fv complexes thus obtained, the structures of AdipoR1 and AdipoR2 were thus determined at 2.9 Å and 2.4 Å, respectively [19].
AdipoR1 and AdipoR2 have been shown to be quite similar in structure, with each consisting of an N-terminal intracellular region, a short intracellular helix, a seven-transmembrane domain, and a C-terminal extracellular region [19]. The Fv used for AdipoR structure recognition was shown to have recognized the N-terminal intracellular region in AdipoR1 and AdipoR2. The seven-transmembrane domains in AdipoR1 and AdipoR2 were each shown to have an opposite orientation to that of microbial rhodopsins or GPCRs which are each shown to have an N-terminal extracellular region [19]. Furthermore, the structural characteristics of the transmembrane helices in GPCRs, such as proline-induced kink, were not found in those in AdipoR1 and AdipoR2 [19]. It was thus concluded that the structures of AdipoR1 and AdipoR2 are distinct from those of GPCRs and therefore novel.
Again, a zinc ion has been shown to be present, bound within the seven-transmembrane domain in both the AdipoR1 and AdipoR2 structures [19], with the zinc-binding site being located at a distance of 4 Å from the intracellular layer of the membrane and with the zinc iron being coordinated by three His residues at a distance of 2.1–2.6 Å [19]. In addition, a water molecule has been found to be present between the zinc ion and the side-chain carboxyl group, and these three His and Asp residues of AdipoR1 and AdipoR2 have been found to be conserved in the homologues found across species [19]. Again, an examination of AdipoR1/AdipoR2 activity through substitution of the zinc ion-coordinating amino acid residues with Ala suggested that the zinc ion found within AdipoR1 may not be directly required for AMPK activation but may be in place to help maintain the AdipoR1 structure as such [19]. In contrast, it is shown that the zinc ion within AdipoR2 may not contribute to the maintenance of its structure but may directly affect AdipoR2 signaling.
Additionally, an internal cavity containing a zinc ion has been found within the seven-transmembrane domain in both the AdipoR1 and AdipoR2 structures, and unidentified electron densities have also been found to be present within the cavity [19]. Given the presence of the water molecule, this suggested that AdipoR1 and AdipoR2 may have hydrolytic activity with the electron densities possibly serving as substrates for this activity or their products [19]. Of note, Vailiaukaité-Brooks et al. recently reported that AdipoR1 and AdipoR2 exhibit extremely low ceramidase activity, while also noting that further research is required into their activity as well as their substrate specificity [53]. To demonstrate their hydrolytic activity, the substrates involved as well as their hydrolytic characteristics and products, remain yet to be elucidated. Again, given that, alongside their potential hydrolytic activity, AdipoR1 and AdipoR2 may also have further functions that remain to be identified, and further developments in adiponectin research are eagerly awaited [54].
Conclusions
It has now become clear that the decrease of adiponectin secreted from adipocytes and its receptors is among the major causes of obesity/diabetes. Our research has demonstrated that AdipoR-deficient mice are associated with insulin resistance and impaired glucose tolerance, thus revealing adiponectin/AdipoR signaling in the liver. Furthermore, our research has shown that, as with exercise, adiponectin and AdipoR1 enhance mitochondrial function and exercise endurance in the skeletal muscle thereby improving glucose/lipid metabolism. Subsequent research has also led to the discovery of a small-molecule compound that binds and activates AdipoRs, which has been shown not only to ameliorate diabetes but to reverse the shortening of lifespan associated with obesity thus prolonging lifespan. Again, with a focus on structure-based drug discovery, our research laboratory has successfully crystalized AdipoR1 and AdipoR2 and has been the first in the world to elucidate the structures of AdipoR1 and AdipoR2. Furthermore, our structural analysis of AdipoR1 and AdipoR2 has also revealed that AdipoR1 and AdipoR2 are distinct in structure and function from GPCRs and, therefore, represent a novel class of receptors. The elucidation of AdipoR1 and AdipoR2 structures is thought likely not only to provide insight into the mechanisms of AdipoR1/AdipoR2 signaling but to have significant implications for the optimization of the small-molecule AdipoR-activating compound AdipoRon for use in humans as well as for the development of first-in-class and best-in-class AdipoR-activating compounds (Fig. 3). It is expected that AdipoR-targeted therapy will prove an effective radical therapy for the metabolic syndrome and diabetes and bring within reach healthy longevity in humans.
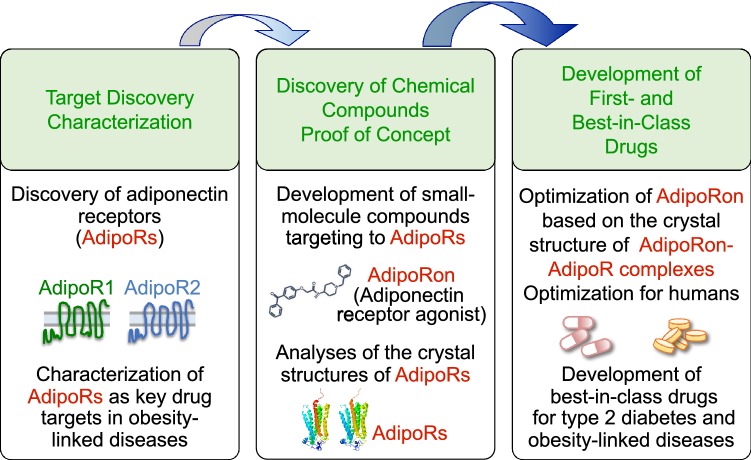
Fig. 3.
Strategy for the development of AdipoR agonists in the treatment of obesity-linked diseases such as type 2 diabetes. AdipoR1 and AdipoR2 were identified in 2003 and were shown to represent key drug targets in obesity-linked diseases. Of the small-molecule AdipoR-targeted compounds, AdipoRon, was reported in 2013, and the crystal structures of AdipoRs (© Protein Data Bank Japan [PDBj] licensed under CC-BY-4.0 International) were determined in 2015. Elucidation of the crystal structures of AdipoRon-AdipoR complexes should prove crucial for the development of first- and best-in-class drugs for type 2 diabetes and obesity-linked diseases [18, 19, 35, 41, 51]
Acknowledgements
A summary of this review was presented by the Lilly Award Lecture at the 62nd Japan Diabetes Society 2019, Sendai, Japan. We thank N. Ohuchi and K. Miyata for help in preparing the manuscript. This work was supported by JSPS KAKENHI Grant numbers JP16K15487, JP26000012, JP26293216, JP19K11639, JP19H01052, JP 18K10988 and by JST, PRESTO (JPMJPR13MF).
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest associated with this research.
Human or animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaboration NCDRF Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IDF Diabetes Atlas 8th Edition, International Diabetes Federation
- 3.Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev. 1997;13:3–13. doi: 10.1002/(SICI)1099-0895(199703)13:1<3::AID-DMR178>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754–1759. doi: 10.1161/ATVBAHA.111.241885. [DOI] [PubMed] [Google Scholar]
- 5.Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 6.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.S143. [DOI] [PubMed] [Google Scholar]
- 7.Rajala MW, Scherer PE. Minireview: the adipocyte at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 8.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.CIR.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 11.Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1–12. doi: 10.1016/j.cccn.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Chazenbalk G, Trivax BS, Yildiz BO, Bertolotto C, Mathur R, Heneidi S, Azziz R. Regulation of adiponectin secretion by adipocytes in the polycystic ovary syndrome: role of tumor necrosis factor-{alpha} J Clin Endocrinol Metab. 2010;95:935–942. doi: 10.1210/jc.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 14.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 15.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 18.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hato M, Ogasawara S, Hino T, Murata T, Iwata S, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Shirouzu M, Yamauchi T, Kadowaki T, Yokoyama S. Crystal structures of the human adiponectin receptors. Nature. 2015;520:312–316. doi: 10.1038/nature14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn CR. Triglycerides and toggling the tummy. Nat Genet. 2000;25:6–7. doi: 10.1038/75610. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 24.Lazar MA. The humoral side of insulin resistance. Nat Med. 2006;12:43–44. doi: 10.1038/nm0106-43. [DOI] [PubMed] [Google Scholar]
- 25.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 26.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 27.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 28.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 29.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 32.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 33.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 34.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 37.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 40.Karpichev IV, Cornivelli L, Small GM. Multiple regulatory roles of a novel Saccharomyces cerevisiae protein, encoded by YOL002c, in lipid and phosphate metabolism. J Biol Chem. 2002;277:19609–19617. doi: 10.1074/jbc.M202045200. [DOI] [PubMed] [Google Scholar]
- 41.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 42.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 43.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 44.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 48.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 49.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 50.Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech Dis. 2015;1:15013. doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okada-Iwabu M, Iwabu M, Ueki K, Yamauchi T, Kadowaki T. Perspective of small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Diabetes Metab J. 2015;39:363–372. doi: 10.4093/dmj.2015.39.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanabe H, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hosaka T, Hato M, Fujii Y, Nakamura Y, Ogasawara S, Hino T, Murata T, Iwata S, Okada-Iwabu M, Iwabu M, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Shirouzu M, Yamauchi T, Kadowaki T, Yokoyama S. Expression, purification, crystallization, and preliminary X-ray crystallographic studies of the human adiponectin receptors, AdipoR1 and AdipoR2. J Struct Funct Genom. 2015;16:11–23. doi: 10.1007/s10969-014-9192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vasiliauskaite-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, De Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 2017;544:120–123. doi: 10.1038/nature21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada-Iwabu M, Iwabu M, Yamauchi T, Kadowaki T. Structure and function analysis of adiponectin receptors toward development of novel antidiabetic agents promoting healthy longevity. Endocr J. 2018;65:971–977. doi: 10.1507/endocrj.EJ18-0310. [DOI] [PubMed] [Google Scholar]